Abstract
Chronic hepatitis B, C and D virus (HBV, HCV and HDV) infections are a major cause of liver disease and cancer worldwide. Despite employing distinct replication strategies, the three viruses are exclusively hepatotropic, and therefore depend on hepatocyte-specific host factors. The sodium taurocholate co-transporting polypeptide (NTCP), a transmembrane protein highly expressed in human hepatocytes that mediates the transport of bile acids, plays a key role in HBV and HDV entry into hepatocytes. Recently, NTCP has been shown to modulate HCV infection of hepatocytes by regulating innate antiviral immune responses in the liver. Here, we review the current knowledge of the functional role and the molecular and cellular biology of NTCP in the life cycle of the three major hepatotropic viruses, highlight the impact of NTCP as an antiviral target and discuss future avenues of research.
Keywords: Liver cell biology, Bile acid transport, Host factor, Anti-viral therapy, Hepatocytes
Introduction
Every year, viral hepatitis is estimated to cause around 1.3 million deaths worldwide, mainly through chronic liver disease and hepatocellular carcinoma (HCC). Approximately, 95% of these deaths are caused by hepatitis B and C viruses (HBV, HCV) [1]. Despite the availability of an effective vaccine for HBV, 250 million people are chronically infected by the virus worldwide [2]. An estimated 5% of HBV patients are co-infected with hepatitis D virus (HDV), a satellite virus hijacking HBV envelope proteins to assemble its infectious viral particles. HDV co-infection worsens the outcome of HBV infection, and treatment of HBV–HDV co-infected patients is less effective [3, 4]. Moreover, around 70 million people are living with chronic HCV infection and, despite the existence of effective curative strategies, the incidence of HCV is still increasing [3].
Remarkable progress has recently been made for treatment of HCV infection. The development and approval of direct acting antivirals (DAAs) specifically targeting viral proteins now allows for HCV cure, but these therapies remain inaccessible for the majority of HCV patients [5]. For chronic HBV infection, two therapeutic approaches are used to suppress viral replication: pegylated interferon and nucleos(t)ide analogues (NUCs). While these treatments allow control of HBV infection, viral eradication is rare and, in most cases, lifelong therapy is required [6]. For patients with chronic HBV/HDV co-infection, the current treatment options are limited to interferon-alpha (IFNα) and its pegylated derivative. Furthermore, although current antivirals decrease the risk of HCC, they are not sufficient to eliminate the risk [7, 8]. To effectively combat these hepatotropic viruses, it is necessary to improve existing therapies and uncover new strategies for prevention and treatment of viral hepatitis.
Alternative strategies against chronic HBV and HCV infection include host-targeting agents (HTA), which target host cell factors required for viral replication. HTAs have been shown to be promising candidates for the prevention and treatment of infections by various pathogens, including HBV and HCV [9–11]. This approach requires a profound understanding of the viral life cycle and the virus-host interactions involved. Indeed, the identification of the human sodium taurocholate co-transporting polypeptide (NTCP) as a functional receptor for HBV/HDV infection [12, 13] opened perspectives for new antiviral strategies. Several entry inhibitors for treatment of HBV infection targeting NTCP are now in development [14–19]. Furthermore, this crucial discovery has allowed the development of novel infectious model systems that will enable an improved understanding of the complete HBV/HDV viral life cycle [20]. However, the regulatory role of NTCP in HCV host cell infection, and its potential immunomodulatory activities in hepatocytes, should not be overlooked. The aim of this review is to summarize what is known about the interactions of NTCP with three major hepatitis viruses during infection, to describe the molecular mechanisms, and to highlight possible applications in research and therapy.
Sodium taurocholate co-transporting polypeptide, a bile acid transporter
The circulation of bile and bile components between human intestine enterocytes and liver parenchymal cells is known as the enterohepatic circulation (EHC) [21]. In the liver, bile acids are mainly involved in cholesterol metabolism and elimination of toxic compounds [22]. Interestingly, bile acids have also been shown to inhibit interferon (IFN) signaling pathways, resulting in reduced expression of IFN-stimulated genes (ISG) [23, 24]. In hepatocytes, bile acid homeostasis is maintained by the interplay between uptake, synthesis and secretion of bile acids. The major hepatic uptake transporter for conjugated bile acids in humans is sodium taurocholate co-transporting polypeptide (NTCP) [25]. NTCP is predominantly expressed at the hepatic basolateral membrane and is involved in the recycling of bile acids from portal blood to hepatocytes in a sodium-dependent manner [21]. NTCP is a member of the solute carrier family SLC10 and is encoded by SLC10A1 [26, 27]. SLC10A1 mRNA is translated into a 349 amino acid glycosylated phosphoprotein with seven or nine transmembrane domains [21, 28–31]. While the exact function of some SLC10 family members remains unknown, all of them are thought to be sodium-dependent transporters [21]. Interestingly, bile acid transport through NTCP can be blocked by small molecules already in clinical use, such as cyclosporine A (CsA, an immunosuppressive drug used in transplantation) or ezetimibe (used for hypercholesterolemia) [16, 32].
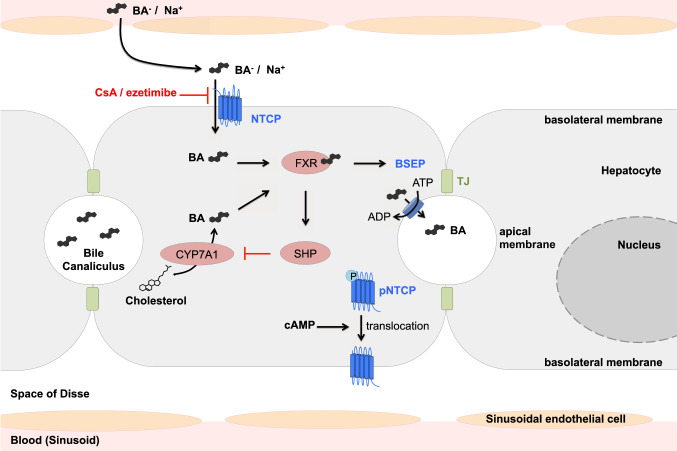
Hepatic bile acid metabolism is tightly regulated, including at the transcriptional level (see Fig. 1) [33]. Upon bile acid activation, the nuclear factor Farnesoid X Receptor (FXR) indirectly downregulates several target genes through transcriptional induction of the small heterodimer partner (SHP) [34, 35], including the first and rate-limiting enzyme in bile acid biosynthesis, cholesterol 7α-hydroxylase (CYP7A1) [36, 37]. FXR also directly activates the expression of the bile salt export pump (BSEP, ABCB11), which is expressed at the apical membrane and secretes conjugated bile acids into the bile canaliculus in an ATP-dependent manner [38, 39]. FXR does not directly interact with the promoter of human SLC10A1 but induces the expression of different factors to indirectly repress slc10a1 expression in rat and mouse, although mechanisms of transcriptional regulation of human NTCP remain unknown [40–42]. In hepatic inflammation, the cytokines tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 downregulate mRNA levels of SLC10A1 and reduce the transporter protein expression [43–45]. The downregulation of NTCP expression in the human liver has been implicated in several cholestasis pathologies. The reduction of NTCP expression could explain impaired hepatic bile acid uptake, resulting in cholestasis and jaundice. Several studies have shown a downregulation of bile salt transporters in primary biliary cirrhosis [46, 47]. Interestingly, a recent study showed a suppression of NTCP expression via cyclin D1 in hepatocellular carcinoma (HCC) [48]. These data may explain the low expression level of NTCP in HCC-derived cell lines, such as Huh7 and clones or HepG2.
Fig. 1.
Model of the functional role of NTCP in hepatic bile acid transport and metabolism. Transport of bile acids from portal blood into hepatocytes via NTCP depends on a sodium gradient and is inhibited by CsA or ezetimibe. Secretion into the bile canaliculus via bile salt export pump (BSEP) in an ATP-dependent manner and synthesis from cholesterol are regulated by bile acid-mediated activation of FXR. cAMP mediates dephosphorylation and membrane translocation of NTCP. NTCP sodium taurocholate co-transporting polypeptide, BSEP bile salt export pump, FXR Farnesoid X Receptor, SHP small heterodimer partner, CYP7A1 cholesterol 7α-hydroxylase, BA bile acid, TJ tight junction, CsA cyclosporin A, cAMP cyclic adenosine monophosphate
The localization and membrane expression of NTCP is controlled by post-translational mechanisms [49]. For example, cyclic adenosine monophosphate (cAMP) plays a role in stimulating the dephosphorylation and membrane translocation of NTCP (see Fig. 1) [50–52]. Sequencing analysis of NTCP revealed the existence of several ethnic-dependent single nucleotide polymorphisms (SNPs) which may alter NTCP activities [53]. For example, mutation S267F, found in 7.5% of allele frequencies in Chinese Americans, is associated with an almost complete loss of bile acid uptake function. However, no pathologies have been described resulting from these NTCP polymorphisms and their clinical roles remain controversial [54]. Besides its major role in the bile acid uptake system, Yan et al. described the crucial role of NTCP on HBV and HDV entry [12]. For the time being, NTCP remains the only described HBV and HDV entry receptor.
NTCP is a host factor for HBV/HDV infection
Hepatitis B virus is the prototypic member of the Hepadnaviridae family of small-enveloped hepatotropic DNA viruses. Its envelope consists of three different forms of the HBV surface protein (HBsAg)—the small (S), middle (M) and large (L) proteins. Importantly, the preS1-domain of L envelope protein is known to bind to the hepatocyte cell surface and is required for HBV and HDV entry [55]. The HBV capsid is comprised of HBV core protein (HBcAg) and encapsidates a partially double-stranded relaxed circular DNA (rcDNA) genome of 3.2 kilobases. Upon infection of hepatocytes, genomic rcDNA is converted into covalently closed circular DNA (cccDNA), a minichromosome-like structure that persists in the nucleus as a central transcription template for all viral RNAs [56]. The presence of cccDNA in the nucleus is thought to be responsible for viral rebound after withdrawal of NUC therapy that targets reverse transcription, a late step in the HBV life cycle. Therefore, removal of cccDNA from HBV-infected hepatocytes will be essential to achieve the goal of HBV cure [57].
HDV is a defective hepatotropic virus which depends on HBV surface proteins for assembly of infectious virions and viral entry [58]. The HDV genome is a negative single-stranded circular RNA of nearly 1700 nucleotides containing one functional open reading frame, which encodes the hepatitis delta protein (HDAg) expressed in small and large form. Replication of HDV RNA and transcription of HDAg mRNA in the nucleus depends on host cell polymerases, including DNA-dependent RNA polymerase II. Both forms of the delta protein are then produced and reimported in the nucleus where they bind to genomic RNA to form the ribonucleoprotein (RNP), which is then exported into the cytoplasm and is associated with HBV envelope proteins to form a mature HDV virion [59]. Thus, HDV enters hepatocytes using the same pathways as HBV, and depends on the same host factors for host cell binding and entry. HDV is therefore a useful surrogate model for HBV entry.
The first step of viral infection is virion binding to attachment factors and receptors at the host cell surface. This specific interaction between viral surface proteins and host entry receptors often determines the tissue tropism and host range of the virus [60]. HBV and its satellite virus HDV share HBV envelope proteins and are known to exclusively infect human, chimpanzee and tree shrew (Tupaia belangeri) hepatocytes, suggesting the involvement of species- and liver-specific cell surface factors in the common entry process of these viruses [20]. Two elements of the HBV envelope proteins are necessary for interaction with these factors. One determinant of infectivity resides in the surface-exposed cysteine-rich antigenic loop (AGL), a polypeptide located in the S domain of all three envelope proteins [61, 62]. The second known infectivity determinant is a receptor-binding site in the N-terminal pre-S1 domain of the L-HBsAg [55]. This domain is post-translationally modified by addition of myristic acid [63], and this myristoylation is essential for virion infectivity [64, 65]. A synthetic myristoylated peptide comprising the N-terminal amino acids 2–78 of the pre-S1 domain prevents HBV infection [66].
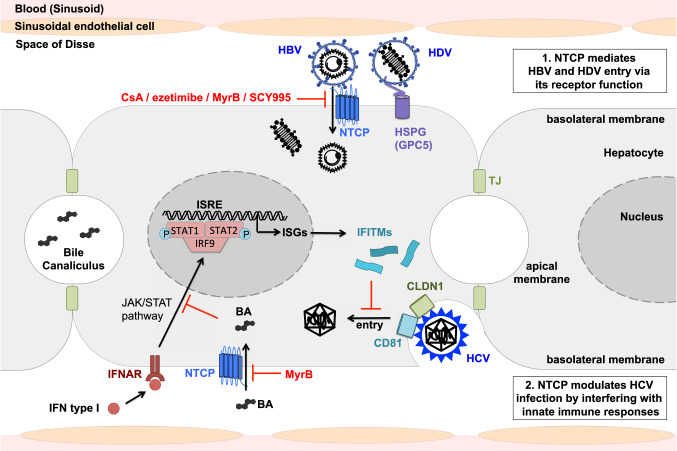
As for many viruses [67, 68], HBV/HDV infection requires the initial attachment to the glycosaminoglycan (GAG) side chains of heparan sulfate proteoglycans (HSPGs) [69]. Both the antigenic loop of all HBV envelope proteins and the preS1-region of HBsAg-L are involved in this interaction [69, 70]. Indeed, glypican-5 (GPC5), a member of the glypican family of HSPGs, acts as an entry factor for HBV and HDV (see Fig. 2) [71]. After this initial step of HBV/HDV attachment to HSPGs, the virions bind to a high-affinity receptor via the preS1-domain [72], allowing uptake into hepatocytes. Despite the discovery of several preS1-interacting proteins that did not affect HBV infectivity [73–78], the identity of the HBV/HDV entry receptor remained unclear until 2012, when Yan et al. identified NTCP as a functional receptor for HBV and HDV infection. Using a labeled preS1 peptide as a bait in Tupaia hepatocytes, a mass spectrometry purification of preS1-bound proteins, and validation in human hepatocytes, it was shown that NTCP specifically interacts with the HBV receptor-binding domain preS1, allowing viral entry [12]. Zhong et al. showed that Tupaia NTCP mediates entry of woolly monkey HBV, indicating that NTCP orthologs act as a common cellular receptor for known primate hepadnaviruses [79]. Differential gene expression patterns between non-susceptible undifferentiated and susceptible differentiated HepaRG cells validated the role of NTCP as a specific receptor for HBV and HDV [13]. Moreover, silencing of NTCP in primary Tupaia hepatocytes (PTH) or differentiated HepaRG cells inhibits HBV and HDV infection [12, 13]. Exogenous expression of NTCP directly renders non-susceptible hepatoma cell lines susceptible to HBV and HDV infection, while entry inhibitors derived from the preS1 peptide efficiently inhibit this infection [12]. In addition, the S267F mutant of NTCP, conferring a loss of bile acid uptake function, is significantly associated with resistance to chronic hepatitis B and decreased risk of cirrhosis and liver cancer development, supporting the role of NTCP as cellular receptor for HBV in human infection [80–82]. However, S267F homozygote patients can still be infected by HBV, suggesting the existence of alternative receptors allowing viral entry in the absence of functional NTCP [83].
Fig. 2.
Model of interactions between NTCP and the entry of HBV, HDV, and HCV in hepatocytes. After initial attachment to HSPG including GPC5, HBV and HDV virions bind to the receptor NTCP through the preS1-domain of the large envelope protein. NTCP inhibitors CsA and ezetimibe block viral entry like preS1-derived MyrB and CsA-derived SCY995. NTCP modulates HCV infection by interfering with innate immune responses. Bile acids interfere with the IFN signaling pathway and thereby favor HCV entry. Inhibition of NTCP-mediated bile acid import into hepatocytes promotes inhibition of HCV entry through the upregulation of ISGs including IFITMs. HBV hepatitis B virus, HCV hepatitis C virus, HDV hepatitis D virus, HSPG heparan sulfate proteoglycan, GPC5 glypican-5, NTCP sodium taurocholate co-transporting polypeptide, MyrB myrcludex B, CsA cyclosporin A, SCY995 synthesized CsA derivative 995, IFN interferon, IFNAR IFN-α/β receptor, JAK Janus kinase, STAT signal transducer and activator of transcription, IRF9 Interferon regulatory factor 9, ISRE IFN-sensitive response element, ISG IFN-stimulated gene, IFITM IFN-induced transmembrane protein, CLDN1 Claudin 1, CD81 cluster of differentiation 81, BA bile acid, TJ tight junction
Interestingly, expression of human (but not mouse) NTCP in non-susceptible hepatocarcinoma cells confers limited susceptibility to infection. For robust infection, addition of dimethyl sulfoxide (DMSO) to culture medium is essential [13]. The fact that human hepatoma cell lines HepG2 and Huh7 are not susceptible to HBV and HDV infection without exogenous expression of NTCP is consistent with reports that NTCP expression is reduced in human hepatocellular carcinoma cells [48, 84]. NTCP expression rapidly decreases over time following isolation of cultured PTHs, which supports observations that primary human hepatocytes (PHH) remain susceptible to HBV infection in vitro only for a few days after isolation [12, 85]. Considering the predominant expression of NTCP in the liver, this receptor is likely to contribute to the hepatotropism of both viruses [12]. In addition, NTCP protein sequences vary among mammalian species, which might contribute to the narrow species tropism of HBV and HDV infection. For example, monkey NTCP does not support HBV and HDV infection despite a high protein sequence homology to human NTCP. Replacing amino acids 157–165 of nonfunctional monkey NTCP with the human counterpart conferred susceptibility to both HDV and HBV infection [12]. The fact that hepatocytes from cynomolgus and rhesus macaques and pigs become fully susceptible to HBV upon hNTCP expression indicates that NTCP is the key host factor limiting HBV infection in these species [86].
As a key host factor enabling HBV and HDV infection in vitro, the discovery of NTCP has been crucial for the development of novel animal models supporting virus infection. Indeed, only chimpanzees and tree shrews can experimentally support HBV and HDV infections [87]. The state-of-the-art mouse model for the study of HBV/HDV consists of liver-engrafted humanized chimeric uPa/SCID or FRG mice, which support virus entry and replication, but lack an efficient immune system, limiting the study of virus–host interactions [87]. The recent development of human NTCP-expressing transgenic mice opened perspectives for the development of novel immune-competent animal models for the investigation of HDV infection and HDV-induced pathogenesis in vivo [88]. As HBV infection is limited in mouse cells expressing hNTCP, probably due to the lack of a key host factor [89], it should be noted that hNTCP-transgenic mice are not susceptible to HBV infection. Recently, an elegant study demonstrated that vector-mediated expression of hNTCP in the hepatocytes of rhesus macaques conferred susceptibility to HBV infection, providing a robust and relevant model for the study of HBV infection, including its interaction with adaptive immunity and the understanding of viral clearance [90].
Overall, NTCP was identified as the long-sought preS1-specific HBV receptor contributing to HBV liver tropism and species specificity [13]. Targeting the interactions between the HBV preS1-domain and its receptor NTCP required for HBV/HDV entry is a promising strategy to block viral entry for both viruses.
NTCP as a therapeutic target for HBV/HDV infection
Even before the identification of NTCP as HBV/HDV receptor, entry inhibitors derived from the HBV preS1 were shown to efficiently inhibit HBV infection in vitro and in vivo [91, 92]. One of these compounds, the myristoylated preS1-derived peptide (also called Myrcludex B or MyrB), efficiently prevents HBV dissemination in vivo and hinders amplification of the cccDNA pool in infected human hepatocytes [14]. MyrB is the first HBV/HDV entry inhibitor targeting NTCP to reach clinical trials [93], where it was shown to have a good safety profile with a mild and reversible elevation of serum bile acid salts [93, 94]. Phase IIa clinical studies revealed a marked antiviral effect of MyrB, as measured by HDV RNA, HBV DNA and improvement of biochemical disease activity (ALT), when used in combination with IFN therapy, although there was no significant decrease in HBsAg levels. In monotherapy, however, MyrB did not show significant antiviral activity [94]. Further studies are necessary to confirm these results obtained in small patient cohorts [95].
Importantly, the identification of NTCP as the first HBV/HDV entry receptor has accelerated the discovery and development of several new potential entry inhibitors. Binding of myristoylated preS1-derived peptide to NTCP was shown to interfere with the physiological bile acid transport function of NTCP, indicating that NTCP-inhibiting drugs might be able to block HBV infection [96]. In a study evaluating FDA-approved therapeutics with documented inhibitory effect on NTCP cellular function against HDV entry, three of these molecules (irbesartan, ezetimibe, and ritonavir) inhibited HDV infection in vitro [97]. The inhibitory effect of ezetimibe on HBV infection had already been described previously without understanding its interactions with NTCP [98]. In 2014, Watashi et al. evaluated the effect of compounds on the early phase of the HBV life cycle to identify cyclosporine A as an HBV entry inhibitor targeting NTCP [15]. In the same year, Nkongolo et al. characterized the effect of cyclosporine A, a cholestasis-inducing drug inhibiting NTCP bile acid transport [32, 97, 98], against HBV/HDV infection and found that inhibition of entry resulted from interference with the NTCP receptor [16]. The screening of FDA/EMA-approved drugs or small molecules for interaction with NTCP allowed the identification of several additional potential HBV/HDV entry inhibitors targeting NTCP [18, 19]. All of these NTCP-targeting HBV/HDV entry inhibitors concomitantly inhibit the transporter function of NTCP and impair bile acid uptake into hepatocytes, increasing the risk of adverse effects. NTCP-deficient mice and a patient with NTCP deficiency were shown to exhibit an elevated level of serum bile acids and to develop related pathologies including growth retardation and hypercholanemia [101, 102].
Two different strategies to selectively inhibit HBV entry without impairing bile acid uptake have been suggested recently. Shimura et al. showed that cyclosporine A derivatives SCY450 and SCY995 inhibit HBV/HDV entry without interfering with the NTCP transporter activity (see Fig. 2) [17]. Tsukuda et al. identified an oligomeric flavonoid, proanthocyanidin (PAC) and its analogs, as a new class of entry inhibitors, which directly target the preS1-domain of the HBV large envelope protein and thereby prevent its attachment to NTCP. By directly targeting HBV particles, PAC impairs HBV infectivity without affecting the NTCP-mediated bile acid transport activity [103]. Further studies are required to determine if these novel inhibitory strategies will show efficacy in vivo and in clinical studies in co-treatment with NUC therapy.
NTCP is a host factor for HCV infection
Hepatitis C virus is an enveloped single-stranded positive-sense RNA virus in the Flaviviridae family. The host-cell-derived lipid envelope contains the two viral envelope glycoproteins, E1 and E2 [104]. Within the envelope, an icosahedral capsid contains the RNA genome of 9.6 kilobases. Like HBV and HDV, attachment of HCV to hepatocytes is mediated by HPSGs on the host cell surface [105–107]. Following attachment, the envelope glycoprotein E2 mediates interactions with a series of specific cellular entry factors, including CD81 and claudin-1 (see Fig. 2) [108–111]. HCV is internalized via endocytosis in a clathrin- and dynamin-dependent process [112]. Following fusion with early endosomal membranes, the HCV genome is released into the cytosol, where it is translated into a polyprotein cleaved by viral and host proteases. The HCV genome is replicated directly into RNA without passing through a DNA intermediate [113]. Therefore, HCV entry and replication steps are very distinct from those described for HBV/HDV. Nonetheless, the mutual hepatotropism of these three viruses mediated by tissue-specific factors suggests a possible overlap in usage of common hepatocyte-specific host factors like NTCP.
Following establishment of the pivotal role of NTCP for HBV and HDV entry into hepatocytes, a recent study identified a role for NTCP in HCV infection (see Fig. 2). Exogenous overexpression or silencing of NTCP increased or decreased HCV infection in vitro, respectively [114]. Unlike HBV, however, no direct interaction between HCV envelope proteins and NTCP was identified. Instead, the bile acid transporter function of NTCP was found to be important for HCV entry [114]. Bile acids are known to modulate cellular antiviral responses by inhibiting interferon (IFN) type I signaling and thereby decreasing the expression of IFN-stimulated genes (ISGs) [23, 24]. NTCP was shown to regulate HCV infection by inducing the bile acid-mediated repression of ISG expression in hepatocytes, including IFITM1, IFITM2 and IFITM3 [114]. These transmembrane proteins are known to restrict the entry of several viruses, including HCV [115]. IFITM1 blocks the interaction between HCV and its receptors [116], whereas IFITM2 and IFITM3 inhibit entry at a post-endocytosis step by blocking the release of virions into the cytoplasm [117]. NTCP facilitates HCV infection by modulating innate antiviral responses via its bile acid transport function. As bile acids have been shown to enhance HCV replication [118], it is likely that NTCP expression and activity modulates HCV infection through a multimodal mechanism of action. Interestingly, MyrB-mediated inhibition of NTCP blocks the import of bile acids, which in turn stimulates the expression of ISGs, inhibiting HCV entry and infection [114]. However, it still needs to be determined whether the inhibition of NTCP-mediated bile acid entry affects the HBV life cycle through similar mechanisms as described for HCV. The potential of NTCP-targeting antivirals to enhance innate antiviral responses and to engage the host immune system to clear infection may be a useful property for the treatment of all hepatotropic viruses, including HBV, HCV and HDV.
Conclusions
The discovery of NTCP as the first HBV/HDV receptor was a milestone in the study of the life cycle of these viruses. This landmark discovery enabled significant progress in understanding HBV/HDV entry and virus–host interactions. Moreover, based on this discovery, novel infectious model systems based on transduced cell lines stably expressing NTCP have been developed which allow detailed study of the early steps of the viral life cycle. By allowing the study of authentic infection in cell lines, these model systems will help to understand the formation and degradation of HBV cccDNA, which is a key target to achieve the ultimate goal of HBV cure. Robust human NTCP-expressing animal model systems will enable the in vivo validation of virus–host interactions and antiviral therapies. Moreover, NTCP has been established as an antiviral target, and several molecules targeting NTCP are in clinical development with the goal to improve current therapies in the future. The recent discovery of NTCP as a host-dependency factor in HCV infection underscores its essential role in virus–hepatocyte interactions.
Acknowledgements
This work was supported by Inserm, the University of Strasbourg, the European Union (ERC-2014-AdG-671231-HEPCIR, Infect-ERA hepBccc, EU H2020 Hep-CAR 667273), ANRS (2015/1099), the French Cancer Agency (ARC IHU201301187) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R03AI131066. CCC acknowledges fellowships from the Canadian Institutes of Health Research (201411MFE-338606-245517) and the Canadian Network on Hepatitis C. ERV is the recipient of an ANRS fellowship (ECTZ50121).
Author contributions
CFE, LH, CCC, ERV, CS, TFB wrote the manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no conflicting interests to disclose.
References
- 1.World Health Organization (2016) Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. World Health Organization. http://www.who.int/iris/handle/10665/246177
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2017) Global hepatitis report 2017. World Health Organization. License: CC BY-NC-SA 3.0 IGO. http://www.who.int/iris/handle/10665/255016
- 4.Sultanik P, Pol S. Hepatitis delta virus: epidemiology, natural course and treatment. J Infect Dis Ther. 2016;4:2–7. doi: 10.4172/2332-0877.1000271. [DOI] [Google Scholar]
- 5.Chung RT, Baumert TF. Curing chronic hepatitis C—the arc of a medical triumph. New Engl J Med. 2014;370:1576–1578. doi: 10.1056/NEJMp1401833. [DOI] [PubMed] [Google Scholar]
- 6.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE, Xiong S, Brosgart CL, Chen S, Gibbs CS, Zoulim F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, Van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S, Loglio A, Siakavellas S, Keskın O, Gatselis N, Hansen BE, Lehretz M, De Revilla J, Savvidou S, Kourikou A, Vlachogiannakos I, Galanis K, Yurdaydin C, Berg T, Colombo M, Esteban R, Janssen HLA, Lampertico P. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444–1453. doi: 10.1002/hep.29320. [DOI] [PubMed] [Google Scholar]
- 8.Baumert TF, Jühling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med. 2017;15:1–10. doi: 10.1186/s12916-017-0815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumert TF, Verrier ER, Nassal M, Chung RT, Zeisel MB. Host-targeting agents for treatment of hepatitis B virus infection. Curr Opin Virol. 2015;14:41–46. doi: 10.1016/j.coviro.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Mailly L, Xiao F, Lupberger J, Wilson GK, Aubert P, Duong FHT, Calabrese D, Leboeuf C, Fofana I, Thumann C, Bandiera S, Lütgehetmann M, Volz T, Davis C, Harris HJ, Mee CJ, Girardi E, Chane-Woon-Ming B, Ericsson M, Fletcher N, Bartenschlager R, Pessaux P, Vercauteren K, Meuleman P, Villa P, Kaderali L, Pfeffer S, Heim MH, Neunlist M, Zeisel MB, Dandri M, McKeating JA, Robinet E, Baumert TF. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33:549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel MB, Baumert TF. Clinical development of hepatitis C virus host-targeting agents. Lancet. 2017;389:674–675. doi: 10.1016/S0140-6736(17)30043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e000049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Fälth M, Stindt J, Königer C, Nassal M, Kubitz R, Sültmann H, Urban S. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Volz T, Allweiss L, Ben ḾBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, Iwamoto M, Nakajima S, Tsukuda S, Borroto-Esoda K, Sugiyama M, Tanaka Y, Kanai Y, Kusuhara H, Mizokami M, Wakita T. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP) Hepatology. 2014;59:1726–1737. doi: 10.1002/hep.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nkongolo S, Ni Y, Lempp FA, Kaufman C, Lindner T, Esser-Nobis K, Lohmann V, Mier W, Mehrle S, Urban S. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J Hepatol. 2014;60:723–731. doi: 10.1016/j.jhep.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Shimura S, Watashi K, Fukano K, Peel M, Sluder A, Kawai F, Iwamoto M, Tsukuda S, Takeuchi JS, Miyake T, Sugiyama M, Ogasawara Y, Park S, Tanaka Y, Kusuhara H, Mizokami M, Sureau C, Wakita T. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J Hepatol. 2017;66:685–692. doi: 10.1016/j.jhep.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donkers JM, Zehnder B, Van Westen GJP, Kwakkenbos MJ, Ijzerman AP, Elferink RPJO, Beuers U, Urban S, van de Graaf SFJ. Reduced hepatitis B and D viral entry using clinically applied drugs as novel inhibitors of the bile acid transporter NTCP. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-15338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneko M, Futamura Y, Tsukuda S, Kondoh Y, Sekine T, Hirano H, Fukano K, Ohashi H, Saso W, Morishita R, Matsunaga S, Kawai F, Ryo A, Park S-Y, Suzuki R, Aizaki H, Ohtani N, Sureau C, Wakita T, Osada H, Watashi K. Chemical array system, a platform to identify novel hepatitis B virus entry inhibitors targeting sodium taurocholate cotransporting polypeptide. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20987-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verrier ER, Colpitts CC, Schuster C, Zeisel MB, Baumert TF. Cell culture models for the investigation of Hepatitis B and D Virus infection. Viruses. 2016;8:1–10. doi: 10.3390/v8090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Döring B, Lütteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–168. doi: 10.1016/B978-0-12-394316-3.00004-1. [DOI] [PubMed] [Google Scholar]
- 22.Esteller A. Physiology of bile secretion. World J Gastroenterol. 2008;14:5641–5649. doi: 10.3748/wjg.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podevin P, Rosmorduc O, Conti F, Calmus Y, Meier PJ, Poupon R. Bile acids modulate the interferon signalling pathway. Hepatology. 1999;29:1840–1847. doi: 10.1002/hep.510290617. [DOI] [PubMed] [Google Scholar]
- 24.Graf D, Haselow K, Münks I, Bode JG, Häussinger D. Inhibition of interferon-a-induced signaling by hyperosmolarity and hydrophobic bile acids. Biol Chem. 2010;391:1175–1187. doi: 10.1515/BC.2010.108. [DOI] [PubMed] [Google Scholar]
- 25.Slijepcevic D, van de Graaf FJ. Bile acid uptake transporters as targets for therapy. Dig Dis. 2017;35:251–258. doi: 10.1159/000450983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geyer J, Wilke T, Petzinger E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn-Schmiedeberg’s Arch Pharmacol. 2006;372:413–431. doi: 10.1007/s00210-006-0043-8. [DOI] [PubMed] [Google Scholar]
- 27.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na +/bile acid cotransporter. J Clin Invest. 1994;93:1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic adenosine monophosphate. Hepatology. 1998;92:1629–1636. doi: 10.1002/hep.510280624. [DOI] [PubMed] [Google Scholar]
- 29.da Silva TC, Polli JE, Swaan PW. The solute carrier family 10 (SLC10): beyond bile acid transport. Mol Aspects Med. 2013;34:252–269. doi: 10.1016/j.mam.2012.07.004.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu N-J, Iwata S, Cameron AD, Drew D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature. 2011;478:408–411. doi: 10.1038/nature10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol. 2011;201:169–203. doi: 10.1007/978-3-642-14541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Z, Ekins S, Polli JE. Structure activity relationship for FDA approved drugs as inhibitors of the human sodium taurocholate co-transporting polypeptide (NTCP) Mol Pharm. 2013;10:1008–1019. doi: 10.1021/mp300453k.Structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang JYL. Bile acid regulation of hepatic physiology III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G349–G356. doi: 10.1152/ajpgi.00417.2002. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin B, Jones SA, Price RR, Watson MA, Mckee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/S1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 35.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/S1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 36.Jonker JW, Stedman CAM, Liddle C, Downes M. Hepatobiliary ABC transporters: physiology, regulation and implications for disease. Front Biosci. 2009;14:4904–4920. doi: 10.2741/3576. [DOI] [PubMed] [Google Scholar]
- 37.Chiang JYL. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 39.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 40.Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 41.Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol. 2004;286:752–761. doi: 10.1152/ajpgi.00456.2003. [DOI] [PubMed] [Google Scholar]
- 42.Geier A, Martin IV, Dietrich CG, Balasubramaniyan N, Strauch S, Suchy FJ, Gartung C, Trautwein C, Ananthanarayanan M. Hepatocyte nuclear factor-4a is a central transactivator of the mouse Ntcp gene. Am J Physiol Gastrointest Liver Physiol. 2008;295:226–233. doi: 10.1152/ajpgi.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Zimmerman TL, Thevananther S, Lee H-Y, Kurie JM, Karpen SJ. Interleukin-1b-mediated suppression of RXR:RAR transactivation of the Ntcp promoter Is JNK-dependent. J Biol Chem. 2002;277:31416–31422. doi: 10.1074/jbc.M204818200. [DOI] [PubMed] [Google Scholar]
- 44.Siewert E, Dietrich CG, Lammert F, Heinrich PC, Matern S, Gartung C, Geier A. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochem Biophys Res Commun. 2004;322:232–238. doi: 10.1016/j.bbrc.2004.07.102. [DOI] [PubMed] [Google Scholar]
- 45.Le Vee M, Lecureur V, Stieger B, Fardel O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-a or interleukin-6. Drug Metab Dispos. 2009;37:685–693. doi: 10.1124/dmd.108.023630.pump. [DOI] [PubMed] [Google Scholar]
- 46.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall H-U, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717–727. doi: 10.1016/S0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 47.Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol. 2003;39:693–702. doi: 10.1016/S0168-8278(03)00410-0. [DOI] [PubMed] [Google Scholar]
- 48.Kang J, Wang J, Cheng J, Cao Z, Chen R, Li H, Liu S, Chen X, Sui J, Lu F. Down-regulation of NTCP expression by cyclin D1 in hepatitis B virus-related hepatocellular carcinoma has clinical significance. Oncotarget. 2017;8:56041–56050. doi: 10.18632/oncotarget.10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anwer MS. Role of protein kinase C isoforms in bile formation and cholestasis. Hepatology. 2014;60:1090–1097. doi: 10.1002/hep.27088.Role. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grüne S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinases in the activation of hepatic Na +/taurocholate cotransport by cyclic AMP. J Biol Chem. 1993;268:17734–17741. [PubMed] [Google Scholar]
- 51.Webster CRL, Blanch C, Anwer MS. Role of PP2B in cAMP-induced dephosphorylation and translocation of NTCP. Am J Physiol Gastrointest Liver Physiol. 2002;283:G44–G50. doi: 10.1152/ajpgi.00530.2001. [DOI] [PubMed] [Google Scholar]
- 52.Anwer MS, Gillin H, Mukhopadhyay S, Balasubramaniyan N, Suchy FJ, Ananthanarayanan M. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J Biol Chem. 2005;280:33687–33692. doi: 10.1074/jbc.M502151200. [DOI] [PubMed] [Google Scholar]
- 53.Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+ -taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004;279:7213–7222. doi: 10.1074/jbc.M305782200. [DOI] [PubMed] [Google Scholar]
- 54.Pan W, Song I-S, Shin H-J, Kim M-H, Choi Y-L, Lim J, Kim W-Y, Lee S-S, Shin J-G. Genetic polymorphisms in Na+ -taurocholate co-transporting polypeptide (NTCP) and ileal apical sodium-dependent bile acid transporter (ASBT) and ethnic comparisons of functional variants of NTCP among Asian populations. Xenobiotica. 2011;41:501–510. doi: 10.3109/00498254.2011.555567. [DOI] [PubMed] [Google Scholar]
- 55.Sureau C, Guerra B, Lanford RE. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993;67:366–372. doi: 10.1128/jvi.67.1.366-372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- 57.Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA—the holy grail to hepatitis B cure. J Hepatol. 2016;64:S41–S48. doi: 10.1016/j.jhep.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcellt RH, Gerin JL. Delta agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. PNAS. 1980;77:6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sureau C, Negro F. The hepatitis delta virus: replication and pathogenesis. J Hepatol. 2016;64:S102–S116. doi: 10.1016/j.jhep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abou Jaoude G, Sureau C. Role of the antigenic loop of the hepatitis B virus envelope proteins in infectivity of hepatitis delta virus. J Virol. 2005;79:10460–10466. doi: 10.1128/JVI.79.16.10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abou Jaoude G, Sureau C. Entry of hepatitis delta virus requires the conserved cysteine residues of the hepatitis B virus envelope protein antigenic loop and is blocked by inhibitors of thiol-disulfide exchange. J Virol. 2007;81:13057–13066. doi: 10.1128/JVI.01495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Persing DH, Varmus HE, Ganem DON. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987;61:1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gripon P, Seyec JLE, Rumin S, Guguen-Guillouzo C. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology. 1995;213:292–299. doi: 10.1006/viro.1995.0002. [DOI] [PubMed] [Google Scholar]
- 65.Bruss V, Hagelstein J, Gerhardt E, Galle PR. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology. 1996;218:396–399. doi: 10.1006/viro.1996.0209. [DOI] [PubMed] [Google Scholar]
- 66.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spillmann D. Heparan sulfate: Anchor for viral intruders? Biochimie. 2001;83:811–817. doi: 10.1016/S0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- 68.Bartlett AH, Park PW. Proteoglycans in host–pathogen interactions: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2015;12:e5. doi: 10.1017/S1462399409001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- 70.Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology. 2013;57:985–994. doi: 10.1002/hep.26125. [DOI] [PubMed] [Google Scholar]
- 71.Verrier ER, Colpitts CC, Bach C, Heydmann L, Weiss A, Renaud M, Durand SC, Habersetzer F, Durantel D, Abou-Jaoudé G, López Ledesma MM, Felmlee DJ, Soumillon M, Croonenborghs T, Pochet N, Nassal M, Schuster C, Brino L, Sureau C, Zeisel MB, Baumert TF. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology. 2016;63:35–48. doi: 10.1002/hep.28013. [DOI] [PubMed] [Google Scholar]
- 72.Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol. 2008;10:122–133. doi: 10.1111/j.1462-5822.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 73.Neurath AR, Kent SBH, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986;46:429–436. doi: 10.1016/0092-8674(86)90663-X. [DOI] [PubMed] [Google Scholar]
- 74.Neurath BAR, Strick N, Sproul P. Search for hepatitis B virus cell receptors reveals binding sites for interleukin 6 on the virus envelope protein. J Exp Med. 1992;175:461–469. doi: 10.1084/jem.175.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pontisso P, Ruvoletto MG, Tiribelli C, Gerlich WH, Ruop A, Alberti A. The preS1 domain of hepatitis B virus and IgA cross-react in their binding to the hepatocyte surface. J Gen Virol. 1992;73:2041–2045. doi: 10.1099/0022-1317-73-8-2041. [DOI] [PubMed] [Google Scholar]
- 76.Ryu CJ, Cho D, Gripon P, Kim HS, Guguen-Guillouzo C, Hong HJ. An 80-kilodalton protein that binds to the Pre-S1 domain of hepatitis B virus. J Virol. 2000;74:110–116. doi: 10.1128/JVI.74.1.110-116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Falco S, Ruvoletto MG, Verdoliva A, Ruvo M, Raucci A, Marino M, Senatore S, Cassani G, Alberti A, Pontisso P, Fassina G. Cloning and expression of a novel hepatitis B virus-binding protein from HepG2 cells. J Biol Chem. 2001;276:36613–36623. doi: 10.1074/jbc.M102377200. [DOI] [PubMed] [Google Scholar]
- 78.Li D, Wang XZ, Ding J, Yu J-P. NACA as a potential cellular target of hepatitis B virus PreS1 protein. Dig Dis Sci. 2005;50:1156–1160. doi: 10.1007/s10620-005-2724-4. [DOI] [PubMed] [Google Scholar]
- 79.Zhong G, Yan H, Wang H, He W, Jing Z, Qi Y, Fu L, Gao Z, Huang Y, Xu G, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of tupaia hepatocytes. J Virol. 2013;87:7176–7184. doi: 10.1128/JVI.03533-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng L, Zhao Q, Li Q, Li M, Li C, Xu T, Jing X, Zhu X, Wang Y, Li F, Liu R, Zhong C, Pan Q, Zeng B, Liao Q, Hu B, Hu Z, Huang Y, Sham P, Liu J, Xu S, Wang J, Gao Z, Wang Y. The p.Ser267Phe variant in SLC10A1 is associated with resistance to chronic hepatitis B. Hepatology. 2015;61:1251–1260. doi: 10.1002/hep.27608. [DOI] [PubMed] [Google Scholar]
- 81.Lee HW, Park HJ, Jin B, Dezhbord M, Kim DY, Han KH, Ryu WS, Kim S, Ahn SH. Effect of S267F variant of NTCP on the patients with chronic hepatitis B. Sci Rep. 2017;7:1–7. doi: 10.1038/s41598-017-17959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.An P, Zeng Z, Winkler CA. The loss-of-function S267F variant in HBV receptor NTCP reduces human risk to HBV infection and disease progression. J Infect Dis. 2018 doi: 10.1093/infdis/jiy355/5037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu H, Liu J, Lin Y-L, Luo W, Chu Y-J, Chang C-L, Jen C-L, Lee M-H, Lu S-N, Wang L-Y, You S-L, Yang H-I, Chen C-J. The rs2296651 (S267F) variant on NTCP (SLC10A1) is inversely associated with chronic hepatitis B and progression to cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B. Gut. 2016;65:1514–1521. doi: 10.1136/gutjnl-2015-310686. [DOI] [PubMed] [Google Scholar]
- 84.Zollner G, Wagner M, Fickert P, Silbert D, Fuchsbichler A, Zatloukal K, Denk H, Trauner M. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005;25:367–379. doi: 10.1111/j.1478-3231.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 85.Gripon P, Diot C, Theze N, Fourel I, Loreal O, Brechot C, Guguen-Guillouzo C. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J Virol. 1988;62:4136–4143. doi: 10.1128/jvi.62.11.4136-4143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lempp FA, Wiedtke E, Qu B, Roques P, Chemin I, Vondran FWR, Le Grand R, Grimm D, Urban S. Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology. 2017;66:703–716. doi: 10.1002/hep.29112. [DOI] [PubMed] [Google Scholar]
- 87.Allweiss L, Dandri M. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J Hepatol. 2016;64:S17–S31. doi: 10.1016/j.jhep.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 88.He W, Ren B, Mao F, Jing Z, Li Y, Liu Y, Peng B, Yan H, Qi Y, Sun Y, Guo J-T, Sui J, Wang F, Li W. Hepatitis D virus infection of mice expressing human sodium taurocholate co-transporting polypeptide. PLoS Pathog. 2015;11:1–17. doi: 10.1371/journal.ppat.1004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lempp FA, Mutz P, Lipps C, Wirth D, Bartenschlager R, Urban S. Evidence that hepatitis B virus replication in mouse cells is limited by the lack of a host cell dependency factor. J Hepatol. 2016;64:556–564. doi: 10.1016/j.jhep.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 90.Burwitz BJ, Wettengel JM, Mück-Häusl MA, Ringelhan M, Ko C, Festag MM, Hammond KB, Northrup M, Bimber BN, Jacob T, Reed JS, Norris R, Park B, Moller-Tank S, Esser K, Greene JM, Wu HL, Abdulhaqq S, Webb G, Sutton WF, Klug A, Swanson T, Legasse AW, Vu TQ, Asokan A, Haigwood NL, Protzer U, Sacha JB. Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques. Nat Commun. 2017;8:1–10. doi: 10.1038/s41467-017-01953-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol. 2005;79:1613–1622. doi: 10.1128/JVI.79.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen J, Dandri M, Mier W, Lütgehetmann M, Volz T, von Weizsäcker F, Haberkorn U, Fischer L, Pollok J-M, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 93.Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T, Alexandrov A, Haag M, Schwab M, Urban S, Haefeli WE. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65:483–489. doi: 10.1016/j.jhep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 94.Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, Lehr T, Lempp FA, Wedemeyer H, Haag M, Schwab M, Haefeli WE, Blank A, Urban S. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol. 2016;65:490–498. doi: 10.1016/j.jhep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 95.Wedemeyer H, Bogomolov P, Blank A, Allweiss L, Dandri-Petersen M, Bremer B, Voronkova N, Schöneweis K, Pathil A, Burhenne J, Haag M, Schwab M, Haefeli W-E, Wiesch JSZ, Alexandrov A, Urban S. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol. 2018;68:S3. doi: 10.1016/S0168-8278(18)30224-1. [DOI] [Google Scholar]
- 96.König A, Döring B, Mohr C, Geipel A, Geyer J, Glebe D. Kinetics of the bile acid transporter and hepatitis B virus receptor Na +/taurocholate cotransporting polypeptide (NTCP) in hepatocytes. J Hepatol. 2014;61:867–875. doi: 10.1016/j.jhep.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 97.Blanchet M, Sureau C, Labonté P. Use of FDA approved therapeutics with hNTCP metabolic inhibitory properties to impair the HDV lifecycle. Antivir Res. 2014;106:111–115. doi: 10.1016/j.antiviral.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antivir Res. 2013;97:195–197. doi: 10.1016/j.antiviral.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Azer SA, Stacey H. Differential effects of Cyclosporin A on the transport of bile acids by human hepatocytes. Biochem Pharmacol. 1993;46:813–819. doi: 10.1016/0006-2952(93)90489-J. [DOI] [PubMed] [Google Scholar]
- 100.Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. Inhibition of bile acid transport across Na +/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab Dispos. 2006;34:1575–1581. doi: 10.1124/dmd.105.008748.basolateral. [DOI] [PubMed] [Google Scholar]
- 101.Slijepcevic D, Kaufman C, Wichers CGK, Gilglioni EH, Lempp FA, Duijst S, de Waart DR, Oude Elferink RPJ, Mier W, Stieger B, Beuers U, Urban S, van de Graaf SFJ. Impaired uptake of conjugated bile acids and hepatitis B virus Pres1-binding in Na+ -taurocholate cotransporting polypeptide knockout mice. Hepatology. 2015;62:207–219. doi: 10.1002/hep.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vaz M, Paulusma CC, Huidekoper H, De RuM, Lim C, Koster J, Ho-Mok K, Bootsma AH, Groen AK, Schaap FG, Oude Elferink RPJ, Waterham HR, Wanders RJA. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology. 2015;61:260–267. doi: 10.1002/hep.27240. [DOI] [PubMed] [Google Scholar]
- 103.Tsukuda S, Watashi K, Hojima T, Isogawa M, Iwamoto M, Omagari K, Suzuki R, Aizaki H, Kojima S, Sugiyama M, Saito A, Tanaka Y, Mizokami M, Sureau C, Wakita T. A new class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology. 2017;65:1104–1116. doi: 10.1002/hep.28952. [DOI] [PubMed] [Google Scholar]
- 104.Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, Cygan A, Price AA, Yost SA, Bohannon CD, Jacob J, Grakoui A, Marcotrigiano J. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509:381–384. doi: 10.1038/nature13117.Structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barth H, Schäfer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H, Kinoshita-Toyoda A, Toida T, van Kuppevelt TH, Depla E, von Weizsäcker F, Blum HE, Baumert TF. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem. 2003;278:41003–41012. doi: 10.1074/jbc.M302267200. [DOI] [PubMed] [Google Scholar]
- 106.Shi Q, Jiang J, Luo G. Syndecan-1 serves as the major receptor for attachment of hepatitis C virus to the surfaces of hepatocytes. J Virol. 2013;87:6866–6875. doi: 10.1128/JVI.03475-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lefevre M, Felmlee DJ, Parnot M, Baumert TF, Schuster C. Syndecan 4 is involved in mediating HCV entry through interaction with lipoviral particle-associated apolipoprotein E. PLoS One. 2014;9:3–10. doi: 10.1371/journal.pone.0095550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–942. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 109.Owen DM, Huang H, Ye J, Gale M., Jr Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low density lipoprotein receptor. Virology. 2010;394:99–108. doi: 10.1016/j.virol.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeisel MB, Koutsoudakis G, Schnober EK, Haberstroh A, Blum HE, Cosset F-L, Wakita T, Jaeck D, Doffoel M, Royer C, Soulier E, Schvoerer E, Schuster C, Stoll-Keller F, Bartenschlager R, Pietschmann T, Barth H, Baumert TF. Scavenger receptor class B Type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46:1–3. doi: 10.1002/hep.21994. [DOI] [PubMed] [Google Scholar]
- 111.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset F-L, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoël M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farquhar MJ, Hu K, Harris HJ, Davis C, Brimacombe CL, Fletcher SJ, Baumert TF, Rappoport JZ, Balfe P, McKeating JA. Hepatitis C virus induces CD81 and Claudin-1 endocytosis. J Virol. 2012;86:4305–4316. doi: 10.1128/JVI.06996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dubuisson J, Cosset F-L. Virology and cell biology of the hepatitis C virus life cycle—An update. J Hepatol. 2014;61:S3–S13. doi: 10.1016/j.jhep.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 114.Verrier ER, Colpitts CC, Bach C, Heydmann L, Zona L, Xiao F, Thumann C, Crouchet E, Gaudin R, Sureau C, Cosset F-L, McKeating JA, Pessaux P, Hoshida Y, Schuster C, Zeisel MB, Baumert TF. Solute carrier NTCP regulates innate antiviral immune responses targeting hepatitis C virus infection of hepatocytes. Cell Rep. 2016;17:1357–1368. doi: 10.1016/j.celrep.2016.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith SE, Weston S, Kellam P, Marsh M. IFITM proteins—cellular inhibitors of viral entry. Curr Opin Virol. 2014;4:71–77. doi: 10.1016/j.coviro.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilkins C, Woodward J, Lau DT-Y, Barnes A, Joyce M, McFarlane N, McKeating J, Tyrrell DL, Gale M., Jr IFITM1 is a tight junction protein that inhibits hepatitis C virus entry. Hepatology. 2013;57:461–469. doi: 10.1002/hep.26066.IFITM1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Narayana SK, Helbig KJ, Mccartney EM, Eyre NS, Bull RA, Eltahla A, Lloyd AR, Beard MR. The interferon-induced transmembrane proteins, IFITM1, IFITM2, and IFITM3 inhibit hepatitis C virus entry. J Biol Chem. 2015;290:25946–25959. doi: 10.1074/jbc.M115.657346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang K-O, George DW. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J Virol. 2007;81:9633–9640. doi: 10.1128/JVI.00795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]