Abstract
Background
Altered functional brain connectivity has been proposed as an intermediate phenotype between genetic risk loci and clinical expression of schizophrenia. Genetic high-risk groups of healthy subjects are particularly suited for the investigation of this proposition because they can be tested in the absence of medication or other secondary effects of schizophrenia.
Methods
Here we applied dFC analysis to functional MRI data in order to reveal the reconfiguration of brain networks during a cognitive task. We recruited healthy carriers of common risk variants using the recall-by-genotype design. We assessed 197 individuals: 99 individuals (52 female, 47 male) with low polygenic risk scores (Schizophrenia risk profile scores, SCZ-PRS) and 98 individuals (52 female, 46 male) with high SCZ-PRS from both tails of the SCZ-PRS distribution from a genotyped population cohort, the Avon Longitudinal Study of Parents And Children (ALSPAC) (N=8169). We compared groups both on conventional brain activation profiles, using the general linear model of the experiment, and on the neural Flexibility Index (FI) which quantifies how frequent a brain region’s community affiliation changes over experimental time.
Results
Behavioral performance and standard brain activation profiles did not differ significantly between groups. High SCZ-PRS was associated with reduced FI and network modularity across n-back levels. The whole-brain FI and that of the fronto-parietal working memory network was associated with n-back performance. We identified a dynamic network phenotype related to high SCZ-PRS.
Conclusions
Such neurophysiological markers can become important for the elucidation of biological mechanisms of schizophrenia and particularly the associated cognitive deficit.
Keywords: population study, genetics, imaging, schizophrenia, brain flexibility, temporal modularity
1. Introduction
Schizophrenia is a highly heritable and highly polygenic disorder. Liability is conferred by the cumulative effect of a large number of variants, most of which individually have small effects (1-3). Within a given population, an individual’s relative liability to the disorder can be described by their polygenic risk score or schizophrenia risk profile score (SCZ-PRS) which represents the relative, weighted burden of common risk alleles carried by that individual. SCZ-PRS can be estimated based on statistically significant associations found in genome-wide association studies (GWAS), but most commonly the threshold for capturing maximal liability is much less stringent (e.g. P<0.05) (4-6). The SCZ-PRS can then be utilized to explore the cumulative impact of genetic risk for the disease over phenotypical dimensions; a more powerful approach than the examination of the effects of single variants (7-12). The power of the polygenic model of schizophrenia is evidenced by recent studies using SCZ-PRS revealing association with negative symptoms and anxiety (13) and childhood and adolescent psychopathology (14). To identify the biological mechanisms that link the genetic architecture of a complex disorder to the clinical outcome, one should examine brain-based phenotypes via the hypothesis that these intermediate phenotypes are more direcly linked to the genetic mechanisms (13-15).
A characteristic cognitive finding in schizophrenia is the impairment of working memory function (14). Many fMRI studies have demonstrated altered prefrontal cortex recruitment during working memory processing in schizophrenia, particularly in the dorsolateral prefrontal cortex (DLPFC) (16,17) which has been closely linked to poorer functional outcomes (18) although alterations in several other areas of the working memory network have also been proposed (19,20). Recent theoretical work has related the coordination of temporal dynamics of BOLD activity via functional connectivity to the extraction of a “community architecture” of functional brain networks (21). The framework of the generation of communities posits a grouping of functionally coupled to each other brain areas compared to the rest of the brain. These network topologies of clustered brain regions of interest (ROIs) can be seen as a stable representation of the functional partitioning of the static brain network. Research findings demonstrated increased connectivity strength and higher clustering coefficient in healthy controls and a high number of smaller modules in SZs (22,23). However, this type of analysis has been limited by the requirement to integrate data across long time windows, which makes it impossible to capture the dynamic components of functional connectivity, which are likely to be even more affected in mental disorders.
Recent developments in dynamic functional network neuroscience (24,25) have addressed this issue by introducing and validating novel methods to untangle the temporal evolution of community affiliations over dynamic brain networks (26,27). Unlike the static network analysis, dynamic network analysis allows the quantification of integrated functional alterations of the network community partitioning over experimental time like the cognitive workload over a working memory task. The dynamic network neuroscience method is further supported biologically for both normal and disordered brain network dynamics compared to the static approach (27-30). Novel results supporting this dynamic approach have included prediction of learning (27) and executive function (29) via the temporal variability of the neural community in healthy volunteers and more robust evidence for disconnected brain areas in schizophrenia (31,32,43). Thus, it appears that the temporal dynamics and the related functional connectivity across brain areas describe the capability of human brain connectome to reconfigure in a flexible way supporting daily cognitive demands. This adjustment of the brain connectome is maybe a feature that can be linked to the genetic risk of schizophrenia and which can only be explored with functional cognitive activation rather than resting state paradigms.
In this study, we apply an holistic data-driven approach to first construct dynamic functional connectivity brain graphs during the cognitive workload levels of the n-back task (33,34). The n-back task is a well-established experimental paradigm to study the neural substrates of the cognitive deficits in subjects with genetic risk for schizophrenia (35,36). We analyze neurovascular signals from brain areas (which are the nodes in a brain network modeled via a graph) and their time-dependent functional interactions (dynamic edges of a dynamic brainnetwork) to untangle spatio-temporally community partitions whose affiliations change during working memory performance. We quantify the abrupt changes of temporal communities affiliations using the network flexibility index (FI), a measure of how frequent a brain region changes its allegiance over time (27) (Fig. 1). We test whether these alterations in dynamic community structure are related to genetic liability to schizophrenia by comparing two healthy adult groups with low and high SCZ-PRS. We hypothesize that the dynamic community structure is modulated by the genetic liability for schizophrenia.
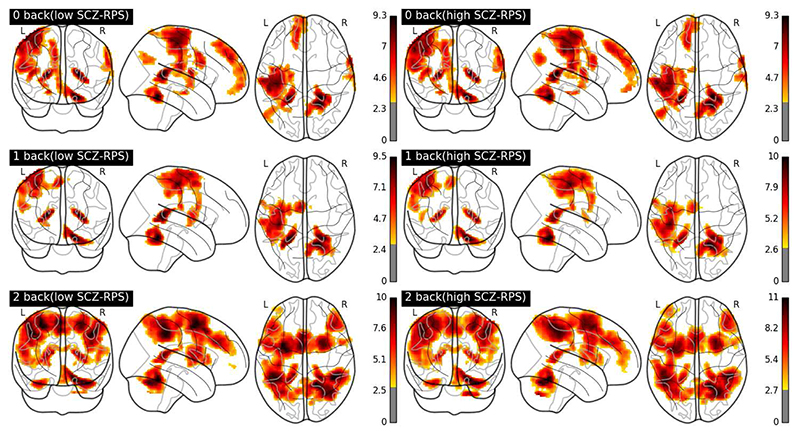
Fig. 1.
Glass brain activity maps of maximum intensity projection of the absolute value of BOLD activity across tasks and in both groups. Colors bars represents Z-statistics for each contrast of parameter estimates.
2. Material and Methods
2.1. Participants, Data Acquisition, and Preprocessing
The protocol was approved by the Central Bristol Research Ethics Committee (13/SW/0170) and all participants provided written informed consent. Participant recruitment was based on the stratification of the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort by polygenic risk for schizophrenia (37,38). We compared 197 healthy individuals of low (N = 99, 52 female, 47 male) or higher (N = 98, 52 female, 46 male) SCZ-PRS with structural and functional magnetic resonance imaging (fMRI) while performing a standard N-back memory task. Empirically, our SCZ-PRS low and high groups were in the lowest 5th and highest 10th percentiles groups, respectively and more extreme than 87% of the sample population (area between 2 z’s, calculated with pnorm(z)). There was a 3 standard-deviation difference between the two groups making them extremely distinct from each other. Population stratification was assessed by multidimensional scaling analysis and compared with Hapmap II (release 22) European descent (CEU), Han Chinese, Japanese, and Yoruba reference populations; all individuals with non-European ancestry were removed. By the nature of the birth cohort study, groups were age matched, and we documented absence of IQ difference in our paper describing the sample and general procedures (39). Further details regarding the selection of these groups and WISC-III IQ measures can be found in our recent paper (39) and in section 1 in the Supplementary Material. The two groups didn’t differ at age (low SCZ-PRS 22 years and 1 month ± 10 months, high SCZ-PRS 22 years and 2 months ± 8 months with a p-value = 0.33, Wilcoxon rank-sum test).
BOLD fMRI was acquired for all subjects while performing an established n-back working memory task (39,40). Investigators were blind to PRS status during data acquisition and during pre-processing stage. Details on the task, acquisition parameters, quality measures and pre-processing steps can be found in the Supplementary Materials.
2.2. Construction of dynamic functional connectivity graph (dFCG) and the Detection of Dynamic Functional Communities
For the dynamic functional connectivity analysis, we first decomposed BOLD ROI activity into 4 frequency scales 0.125~0.25 Hz (Scale 1), 0.06~0.125 Hz (Scale 2), 0.03~0.06 Hz (Scale 3), and 0.015~0.03 Hz (Scale 4) and then we applied a sliding time window with a length of 15 volumes (41) with no gap between the windows (27,29) over the 90 time series ( = number of ROIs of the AAL atlas) representative BOLD time series. The frequency decomposition of BOLD activity is important because different frequency bands could be sensitive to different functional interactions. Subsequently, we adopted wavelet coherence to estimate the functional connectivity between each pair of brain nodes using the MATLAB (MathWorks) package (42,27,29). For each subject, this estimation yielded 73 functional connectivity graphs (FCGs) of dimension ROIs x ROIs [90 x 90] describing the functional connectivity in each time window (temporal segment or “slice”) during the n-back task performance yielding a dynamic functional connectivity graph (dFCG) with size [73 x 90 x 90] for each subject. The dFCGs were represented as multi-layer brain networks with interslice connections. Applying a multi-layer community detection algorithm via Louvain greedy heuristic algorithm for maximizing the Q quality function, we partitioned the temporal nodes into temporal modules (26,27,29). For further details regarding the surrogates and the optimization procedure related to the detection of multi-layer communities see supp.material. Quality Q function of modularity quantifies how well the nodes-ROIs are temporally connected within the same module (within scatter) compared to the rest of the temporal modules (between scatter) (26,27,29). The outcome of a graph-clustering algorithm is to divide a dFCG into modules (also called groups, clusters or communities) which describe a set of ROIs that are grouped together. The quality Q function demands the optimal selection of two parameters called γ1 and ωjir (for further details see p.13-18 in supp.material). After optimizing this set of variables independently per subject, we calculated a time-dependent network flexibility matrix (27), FI (Flexibility Index), whose binary elements, FIij, indicate whether a node i changes its community (1) or not (0) at the transition j between two consecutive time windows. We first estimated nodal FI, then a whole-brain network flexibility estimate by averaging across nodal FI, and finally the mean FI within and between five established brain networks a (see supp. materials).
We estimated the mean FI within five established brain networks. The original 90 ROIs were grouped into the following brain networks: Default Mode Network (DMN); Fronto-Parietal (FP); Cingulo-Opercular (CO); Occipital (O); and SensoriMotor (SM), according to meta-analysis of cognitive fMRI studies (41).
2.3. Prediction of Behavioral Performance via nodal FI
We quantified the predictive power of nodal FI versus the BOLD activity (as per the GLM approach) to model behavioral performance. For further details see the Supplementary Material.
3. Results
3.1. Task Performance in N-Back Memory Task
As expected for an n-back task, we found a main effect of load condition with lower proportion of correct responses for higher load conditions (p = 0.00034, Wilcoxon Sign Rank Sum Test; see S7). We found no interaction between load (n-back levels) and group (low-high SCZ-PRS) (F(1,196) = 38.76, p > 0.671, 2-way ANOVA, factors: n-back levels, groups) and no significant group difference in percentage of correct responses between low and high RPS group (see supp. materials; p > 0.563, Wilcoxon Sign Rank Sum Test; see S7).
3.2. Brain Bold Activity Profile across Tasks and Groups
Fig.1 illustrates the maximum intensity projection of the absolute value of BOLD activity group-averaged for each working memory level. It shows the activation of the fronto-parietal network and the increment of the intensity from n-back level 0 to 2, using parameter estimates from a conventional GLM analysis (see suppl. Methods). We applied Wilcoxon Rank Sum Test per voxel adjusted for multiple comparisons with false discovery rate (q < 0.05). There were no significant group differences in activation level at any of the working memory loads.
3.3. Dynamic Network Community Partitioning Triggerd by Working Memory Task
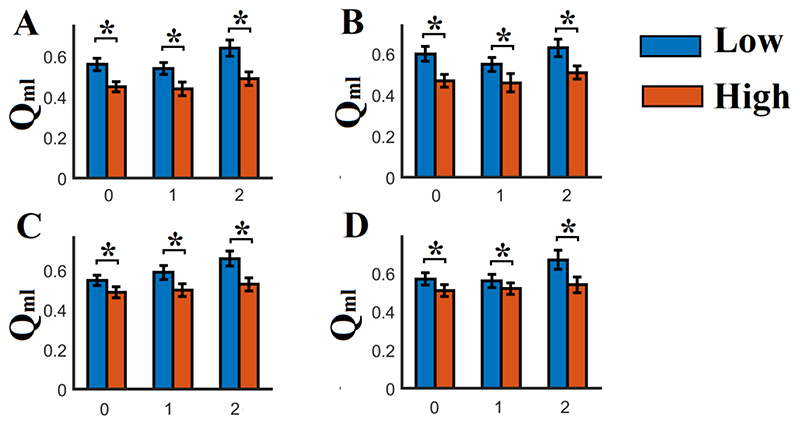
For each subject, frequency sub-band and n-back memory task, the resulting dFCG were segmented into time-resolved functional modules employing a multilayer-community detection algorithm which optimizes a multi-layer version of the modularity index Qml. Higher values denote more cohesive temporal modules.
Group-averaged multi-slice (multi-temporal segments) temporal modularity Qml and number of temporal modules across n-back levels and frequency ranges are illustrated in Fig.2 and S8. Qml quantifies how well the clusters/modules are separated across space and time. High values of Qml (> 0.5) signify that the nodes within each cluster are strongly connected compared to nodes from different clusters/modules.
Fig. 2.
Group-averages (low and high RPS groups) of the modularity Qml across n-back levels and frequency scales (A-D, showing scales 1 – 4: 0.125~0.25 Hz (Scale 1), 0.06~0.125 Hz (Scale 2), 0.03~0.06 Hz (Scale 3), and 0.015~0.03 Hz (Scale 4))
(* Wilcoxon Rank Sum Test, p< 0.01 ; Low : Low SCH RPS ; High : High SCH RPS ; 0 – 2 denotes the 3 n-back levels)
Here, the value of Qml was > 0.5 in both groups and n-back levels deviating from the distribution of Qml produced via the randomization of dynamic networks (p < 0.001 via surrogate analysis), supports the rationale of the detection of highly cohesive temporal modules. Furthermore, we quantified the module evolution of each ROI throughout the experimental time via the time-dependent FI.
The group with low SCZ-PRS demonstrated significantly higher Qml and higher number of temporal modules across n-back levels and frequency ranges compared to the group with higher RPS (Fig.2 and S8; * Wilcoxon Rank Sum Test, p < 0.0001, Bonferroni corrected p’ < p/12 where 12 = n-back levels x 4 frequency ranges). Both results can be seen as indicating more cohesive (Qml) and specialized functional brain networks (more temporal brain modules) for the group with low SCZ-PRS compared to the group with high SCZ-PRS.
3.4. Flexibility analysis
In line with our hypothesis, the dynamic network community FI during the working memory task was significantly lower in the high SCZ-PRS compared to the low SCZ-PRS group. These significant differences were observed across subnetworks,frequency ranges and n-back levels.
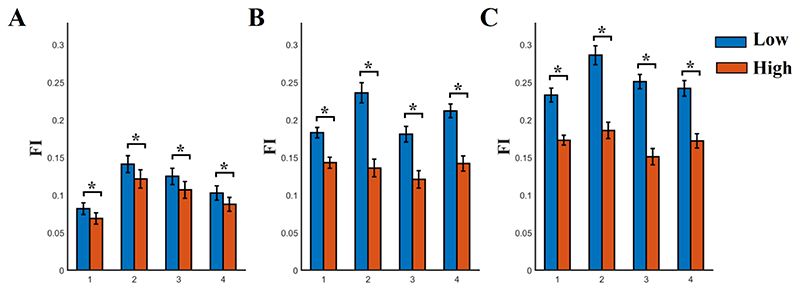
Fig.3 illustrates the whole-brain group-averaged brain network FI across n-back levels and frequency scales. We applied a Wilcoxon Rank Sum Test between the two groups independently for n-back level and frequency range (p < 0.001, Bonferroni Corrected p’< p/12 where 12 denotes the comparisons between the four frequency scales and the 3 n-back levels). FI was consistently higher for group with low SCZ-PRS compared to the group with high SCZ-PRS. Group-differences in terms FI were consistent across various widths of temporal windows (see S14).
Fig. 3. Whole-brain network Flexibility Index (FI) estimated across nodal FI for each n-back level and frequency scale.
A) 0 n-back level, B) 1 n-back level, C) 2 n-back level
FI was significantly higher for the group with low SCZ-PRS compared to high SCZ-PRS across frequency scales and n-back levels
(* Wilcoxon Rank Sum Test, p < 0.001, Bonferroni Corrected p’< p/12 where 12 denotes the 3 working memory levels multiplied by the 4 frequency scales
1 – 4 denotes the 4 frequency scales)
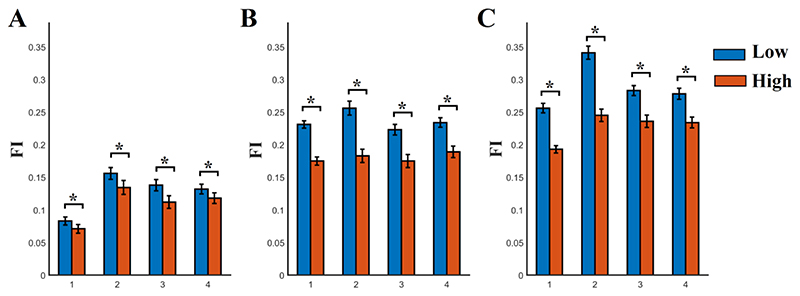
We further constrained the estimation of FI across the three subnetworks most implicated in working memory processes by the literature, the FP, the CO and the DMN29. Fig.4 demonstrates the group-averaged FI for those three subnetworks for each scale frequency and n-back levels.
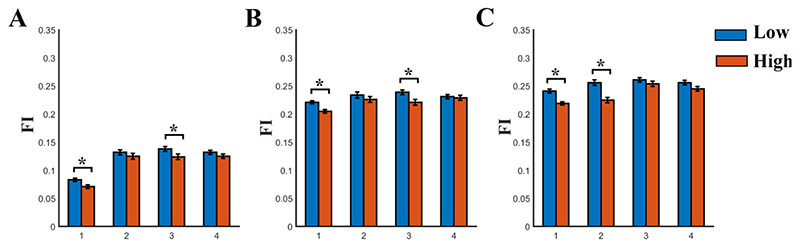
Fig. 4. Sub-network Flexibility Index (FI) estimated across nodes within the fronto-parietal, cingulo-opercular and default-mode networks for each n-back level and frequency scale.
A) N-back level 0, B) N-back level 1, C) N-back level 2. FI was significantly higher for both groups between 0-back/1-back, 1-back/2-back and 0-back/2-back in all the frequency scales 1-4. Both groups demonstrated significant higher increment of FI from 0-back to 1-back, from 1-back to 2-back and from 0-back to 2-back in all the frequency scales. This increment was significantly higher for the group with low SCZ-PRS compared to the group with high SCZ-PRS.
(* Wilcoxon Rank Sum Test, p < 0.001, Bonferroni Corrected p’< p/12 where 12 denotes the comparisons between the four frequency scales).
Firstly, we applied a Wilcoxon Rank Sum Test between the two groups independently for n-back levels and frequency ranges. FI was consistently higher for the group with low SCZ-PRS compared to the group with high SCZ-PRS (p < 0.001, Bonferroni Corrected p’< p/12 where 12 denotes the comparisons between the four frequency scales and the three n-back levels).
Secondly, we applied a Wilcoxon Rank Sum Test between the n-back levels independently for every frequency range and separately for both groups (p < 0.001, Bonferroni Corrected p’< p/8 where 8 denotes the 2 comparisons between the 2 pairs of n-back levels and the 4 frequency scales). This analysis revealed a significant effect of the cognitive workload. FI increased from 0-back to 1-back and from 1-back to 2-back in both groups and in frequency scales 1-4 (Fig.4).
Third, we further estimated the delta difference of FI (ΔFI) between 1-0 n-back level and 2-1 n-back level independently for every subject and frequency scale and we applied a Wilcoxon Rank Sum Test between the two groups independently for the 2 pairs and the 4 frequency ranges. ΔFI was consistently higher for the group with low SCZ-PRS compared to the group with high SCZ-PRS from 0 to 1 n-back level and from 1 to 2 n-back level in every frequency scale. RPS (p < 0.001, Bonferroni Corrected p’< p/8 where 8 denotes the comparisons between the 4 frequency scales and 2 three n-back levels).
Conversely, as illustrated in Fig.5, group effects were weaker for the FI in the combined O and SM networks (significant only for frequency bands 1 and 3 for 0 n-back, bands 1 and 3 for 1 n-back and bands 1 and 2 for 2 n-back). Importantly, there was no group effect on the FI increment across load levels, as demonstrated by non-significant Wilcoxon Rank Sum Tests for the delta differences (all p’s > 0.67).
Fig. 5.
Sub-network Flexibility Index (FI) estimated across nodes within the occipital and sensorimotor networks for each n-back level and frequency scale. (same as in Fig.4)
The ROI topologies of FI for the rest of the studied frequency ranges are shown in the supplementary material (S.9-S.12).
3.5. Prediction of Behavioral Performance via Bold Activity and Nodal FI
Our analysis revealed whole-brain FI estimates as a better predictor of behavioral performance compared to BOLD activity. For further details see supp.material and STable 1. The range of R2 across the N-back levels of the multivariate analysis was:
0.63 – 0.83 for low SCZ-PRS and 0.59 – 0.83 for high SCZ-PRS following a multilinear modelling of whole brain FI (nodal FI) and behavioural performance
0.24 – 0.42 for low SCZ-PRS and 0.23 – 0.43 for high SCZ-PRS following a multilinear modelling of nodal FI from the FP network and behavioural performance
0.21 – 0.42 for low SCZ-PRS and 0.20 – 0.42 for high SCZ-PRS following a multilinear modelling of BOLD activation levels of the whole brain and behavioural performance
0.14 – 0.29 for low SCZ-PRS and 0.14 – 0.29 for high SCZ-PRS following a multilinear modelling the FP network and behavioural performance
4. Discussion
We report working memory fMRI data from a large multi-modal neuroimaging study of mechanisms of genetic risk for schizophrenia, in which power was enhanced by selecting a subset of individuals from a large birth cohort on the basis of high or low numbers of risk alleles for schizophrenia. This design, based on recall-by genotype from population cohorts (39), is particularly suited to highly polygenic disorders such as schizophrenia. Importantly, the high SCZ-PRS group was comparable to the low SCZ-PRS for several potential confounds like the head movement and data quality measures, supporting the validity of our research.
We applied innovative methodologies tailored to dynamic network neuroscience applied to BOLD time series acquired during an n-back task. Our key finding is the alteration of dynamic reconfiguration of brain modules in people with high SCZ-PRS in a working memory task (43). Specifically, we found a lower number of dynamic modules (Fig. S8) and a less coherent dynamic network expressed via the lower Qml for the high SCZ-PRS group (Fig.2).The brain Flexibility Index (FI) across n-back tasks was defined as an index that measures the rate (probability) of transition of ROI assignment over consecutive temporal segments across experimental time. FI increases when a ROI changes its cluster assignment many times between consecutive temporal windows. The thus-defined FI was lower in three well-described brain networks and across the working memory levels in the high SCZ-PRS compared to the low SCZ-PRS group (Fig.3). We used a multi-linear regression analysis using behavioral performance as the dependent variable and both BOLD activity (Fig.1) and the nodal FI (S9-S12) as the independent sets. Our analysis revealed that nodal FI had higher predictive value compared to GLM-based analysis of BOLD activity and showed the importance of the whole network to this prediction compared to only the task-related FP network (S.Table 1.a-b vs S.Table 1.c-d).
It is important to underline here that the estimation of FI within the combined fronto-parietal, cingulo-opercular and default mode brain networks revealed significant effects of increased cognitive workload. Subnetwork FI increased in both groups from 0-back to 2-back in frequency scales 1-4 but this increment was significantly higher for low SCZ-PRS compared to high SCZ-PRS. Our findings support the role of the FPN, CON, and DMN in working memory and n-back task performance, and indicate that the subnetwork FI differs between low and high SCZ-PRS.
In this study of the effect of polygenic schizophrenia risk on brain function during a working memory task we found reduced network modularity and decreased global brain network flexibility for the group with high SCZ-PRS. Our data establish the first evidence that the genetic risk for schizophrenia can alter the reconfiguration of dynamic network communities in individuals with a high burden of common schizophrenia risk alleles. A previous study established that these temporal neural assemblies are altered in patients with schizophrenia during a working memory task (29).
Aberrant oscillatory activity, temporal discoordination and altered excitatory–inhibitory balance of neural networks have been proposed as central mechanisms in the pathophysiology of schizophrenia (44). Based on the widespread alterations of topological brain networks observed in schizophrenia (45), we postulated that these alterations in the temporal functional communities in individuals carrying a high load of genetic risk variants would also be distributed across the brain rather than showing a regional impairment (44,45). This expectation was confirmed by our findings of a globally decreased FI in the group with high SCZ-PRS. Our findings are also in accordance with global electrophysiological effects (measured with MEG/EEG) in patients with schizophrenia (46,47). Our network-wise analytic pathway highlighted a system-wide and not a regionally confined decrease in whole brain network flexibility (48,49) in the high genetic risk group compared to the low SCZ-PRS.
A common cognitive deficit in schizophrenia is working memory impairment (50), which may contribute significantly to the development of thought disorder (51). A recent study revealed fronto-parietal modulation during a working memory task in first episode schizophrenia (52) but underscored the notion of brain connectivity, its dynamic changes and the importance of analyzing the whole brain connectivity profile and not only the task-related subnetwork.
The human brain is functionally organized into intrinsic networks that relatively stable during both resting state and task execution [53,54]. A recent large fMRI-working-memory (WM) study revealed two distinct functional networks that accounted for differences in WM performance of the individuals. A network centered at parietal brain areas, which was relevant to individual differences in WM task performance, and a network centered at frontal brain areas, which was relevant to attentional task demands [55]. A recent study following a similar methodology as ours, applied to effects of WM training, reported a decreased temporal modularity for 1- and 2-back tasks compared to the resting-state condition in the pre-training period [56]. A possible explanation is that network segregation lowest in high demanding n-back tasks compared to a less demanding task or resting-state [57,58]. This was also the reasoning behind our use of a demanding task to elicit dynamic connectivity differences between groups.
Behavioral performance was better predicted by nodal FI compared to estimates of BOLD activation height, revealing the functional importance of the dynamically changing network connectivity. This finding supports the power of dynamic connectivity analysis with the incorporation of network neuroscience for the understanding of mechanisms of cognition. Our results conform to those of several studies in healthy individuals that indicate that executive functions are linked to the reconfiguration of large-scale clustered brain networks rather than the activity of isolated brain areas (27-29).
As per the convention of quantitative neuroimaging studies we have used concepts of inferential statistics and significance testing. We are aware of the recent critique of these concepts (59), especially in the context of researcher degrees-of-freedom, which is still an issue in the neuroimaging literature. Furthermore, our data set came from a multimodal imaging study in which participants underwent a series of MRI, MEG and behavioral experiments (35), which is an increasingly common scenario in the age of large imaging cohort studies. One way of addressing the multiple testing across series of experiments is through pre-specified analysis plans. The GLM analysis reported here (Results, section 3.2) was included in our analysis plan, but not the further analyses of community structure and flexibility index (sections 3.3 and 3.4). These used analytic approaches that were developed after the original analytic plan and should therefore be regarded as exploratory. One problem with the pre-specification of analysis plans in neuroimaging is that, with the rapid progress of analytical methods especially in the area of functional connectivity, we need to remain open to methodological innovation and the improved capture of the richness of fMRI time series information (25).
We probe connectome alterations during an n-back task following a replicated body of research that revealed context-dependent alterations in temporal coherence across working memory networks in those with familial genetic liability for SCZ (such as siblings) [60,61]. In the present study, we use an advanced, dynamic assessment of working memory-dependent connectomics to study genetic risk in an unbiased, whole brain approach, taking into account transitory functional brain community affiliations across working memory loads.
SCZ-PRS are likely to reflect biological substrates that differ between and across participants. As PRS methodologies improve to incorporate pathway based approaches [62], we hope to further establish specific SCZ biological pathways (e.g. voltage-gated calcium channels, FMRP binding proteins, glutamate receptor complexes) that may preferentially influence our associations. We anticipate that these advances will enable future studies to parse heterogeneity in genetic risk profiles in order to establish discrete mechanisms linking SCZ genetic risk and downstream neurobiological processes.
5. Conclusions
We provide evidence for a novel endophenotype (63) associated with polygenic risk for schizophrenia, manifesting as reduced modularity and reduced flexibility of the brain’s functional connectome during a working memory task. Our findings suggest that these alterations may be risk factors related to the common genetic susceptibility associated with schizophrenia, rather than downstream of the clinical phenotype. These alterations of dynamic network properties may explain some of the clinical and cognitive vulnerability of high-risk groups.
Supplementary Material
Acknowledgements
Supported by a MRC grant MR/K004360/1 (Behavioural and Neurophysiological Effects of Schizophrenia Risk Genes: A Multi-locus, Pathway Based Approach) and the MRC/EPSRC funded UK MEG Partnership Grant (MR/K005464/1) and the MRC Centre for Neuropsychiatric Genetics and Genomics (MR/L010305/1). SD is also supported by a MARIE-CURIE COFUND EU-UK Research Fellowship. DJ is supported by a Wellcome Trust New Investigator Award and Wellcome Trust Strategic Award (104943/Z/14/Z). SZ & GDS are supported by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. GDS works in the Medical Research Council Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council (MC_UU_00011/1). We are grateful to Professor Peter Holmans for comments on the manuscript. The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe.
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Footnotes
Author Contributions
DL, KDS, DKJ, SZ, GDS, MO, MO’D and JH designed research; GP and SID performed experiments;
SID designed analysis pipeline and analyzed data; TLM and KET contributed to data analysis.
SID wrote the paper.
All of the authors discussed the results and made critical revisions to the manuscript.
Competing financial interests
The authors declare no relevant competing financial interests.
Disclosure
The authors have nothing to disclose.
References
- 1.Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purcell SM, Wray NR, Stone JL, Vissher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton E, Geisler D, Lee PH, Hass J, Turner JA, Liu J, et al. Prefrontal inefficiency is associated with polygenic risk for schizophrenia. Schizophr Bull. 2013 December 10; doi: 10.1093/schbul/sbt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walton E, Turner J, Gollub RL, Manoach DS, Yendiki A, Ho BC, et al. Cumulative genetic risk and prefrontal activity in patients with schizophrenia. Schizophr Bull. 2013;39:703–711. doi: 10.1093/schbul/sbr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, et al. Phenotypic Manifestation of Genetic Risk for Schizophrenia During Adolescence in the General Population. JAMA Psychiatry. 2016;73(3):221–228. doi: 10.1001/jamapsychiatry.2015.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golov AK, Kondratyev NV, Kostyuk GP, Golimbet AVE. Novel Approaches for Identifying the Molecular Background of Schizophrenia. Cells. 2020;9(1):246. doi: 10.3390/cells9010246. Published 2020 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallet J, Le Strat Y, Dubertret C, Gorwood P. Polygenic Risk Scores Shed Light on the Relationship between Schizophrenia and Cognitive Functioning: Review and Meta-Analysis. J Clin Med. 2020;9(2):341. doi: 10.3390/jcm9020341. Published 2020 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dezhina Z, Ranlund S, Kyriakopoulos M, Williams SCR, Dima D. A systematic review of associations between functional MRI activity and polygenic risk for schizophrenia and bipolar disorder. Brain Imaging Behav. 2019;13(3):862–877. doi: 10.1007/s11682-018-9879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reay WR, Cairns MJ. Pairwise common variant meta-analyses of schizophrenia with other psychiatric disorders reveals shared and distinct gene and gene-set associations. Transl Psychiatry. 2020;10:134. doi: 10.1038/s41398-020-0817-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rees E, Owen MJ. Translating insights from neuropsychiatric genetics and genomics for precision psychiatry. Genome Med. 2020;12:43. doi: 10.1186/s13073-020-00734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kida S, Kato T. Microendophenotypes of psychiatric disorders: phenotypes of psychiatric disorders at the level of molecular dynamics, synapses, neurons, and neural circuits. Curr Mol Med. 2015;15(2):111–118. doi: 10.2174/1566524015666150303002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Womer FY, Leng H, Chang M, Yin Z, Wei Y, et al. The Relationship Between Cognitive Dysfunction and Symptom Dimensions Across Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. Front Psychiatry. 2019;10:253. doi: 10.3389/fpsyt.2019.00253. Published 2019 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manoach DS, Press DZ, Thangaraj V, Searl M, Goff GC, Halpern E, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 16.Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, et al. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res. 2006;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt CL, Eichele T, Melle I, Sundet K, Server A, Agartz I, et al. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. Br J Psychiatry. 2014;204:290–298. doi: 10.1192/bjp.bp.113.129254. [DOI] [PubMed] [Google Scholar]
- 21.Rubinov M, Sporns O, van Leeuwen C, Breakspear M. Symbiotic relationship between brain structure and dynamics. BMC Neurosci. 2009;10:55. doi: 10.1186/1471-2202-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander-Bloch A, Lambiotte R, Roberts B, Giedd J, Gogtay N, Bullmore ED, et al. The discovery of population differences in network community structure: New methods and applications to brain functional networks in schizophrenia. Neuroimage. 2012;59(4):3889–3900. doi: 10.1016/j.neuroimage.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Q, Plis SM, Erhardt EB, Allen EA, Sui J, Kiehl KA, et al. Modular organization of functional network connectivity in healthy controls and patients with schizophrenia during the resting state. Front Syst Neurosci. 2012;5:103. doi: 10.3389/fnsys.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calhoun VD, Miller R, Pearlson G, Adali T. The Chronnectome: Time-Varying Connectivity Networks as the Next Frontier in fMRI Data Discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preti MG, Bolton TA, Ville DVD. The dynamic functional connectome: State-of-the-art and perspectives. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.12.061. In press. [DOI] [PubMed] [Google Scholar]
- 26.Mucha PJ, Richardson T, Macon K, Porter MA, Onnela JP. Community structure in time-dependent, multiscale, and multiplex networks. Science. 2010;328(5980):876–878. doi: 10.1126/science.1184819. [DOI] [PubMed] [Google Scholar]
- 27.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci USA. 2011;108(18):7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun U, Schäfer A, Bassett DS, Rausch F, Schweiger JI, Bilek E. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci USA. 2015;112(37):11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: An updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 33.Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, et al. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. Neuroimage. 2012;60(3):1746–1758. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- 34.Cao H, Plichta MM, Schäfer A, Haddad L, Grimm O, Schneider M, et al. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. Neuroimage. 2014;84:888–900. doi: 10.1016/j.neuroimage.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 36.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62(4):379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 37.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: The ‘Children of the 90s’; the index offspring of The Avon Longitudinal Study of Parents and Children (ALSPAC) International Journal of Epidemiology. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International Journal of Epidemiology. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lancaster TM, Dimitriadis SI, Tansey KE, Perry G, Ihssen N, Jones DK, et al. Structural and Functional Neuroimaging of Polygenic Risk for Schizophrenia: A Recall-by-Genotype-Based Approach. Schizophr Bull. 2019 Mar 7;45(2):405–414. doi: 10.1093/schbul/sby037. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 41.Leonardi N, Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage. 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys. 2004;11(5/6):561–566. [Google Scholar]
- 43.Forbes NF, et al. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- 44.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 45.Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16(3):159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- 46.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75(6):963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Yang GJ, Murray JD, Wang XJ, Glahn DC, Godfrey D Pearlson, Repovs G, et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci USA. 2016;113(2):E219–E228. doi: 10.1073/pnas.1508436113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita M, Kawato M, Imamizu H. Predicting learning plateau of working memory from whole-brain intrinsic network connectivity patterns. Sci Rep. 2015;5:7622. doi: 10.1038/srep07622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2008;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- 51.Arnsten AF. The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937–03. Cereb Cortex. 2013;23:226–2281. doi: 10.1093/cercor/bht195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielsen JD, Madsen KH, Wang Z, Liu Z, Friston KJ, Zhou Y. Working Memory Modulation of Frontoparietal Network Connectivity in First-Episode Schizophrenia. Cereb Cortex. 2017 Jul 1;27(7):3832–3841. doi: 10.1093/cercor/bhx050. [DOI] [PubMed] [Google Scholar]
- 53.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole MW, Ito T, Bassett DS, Schultz DH. Activity flow over resting-state networks shapes cognitive task activations. Nat Neurosci. 2016;19:1718–1726. doi: 10.1038/nn.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egli T, Coynel D, Spalek K, Fastenrath M, Freytag V, Heck A, et al. Identification of two distinct working memory-related brain networks in healthy young adults. 2018;5(1):ENEURO.0222-17.2018. doi: 10.1523/ENEURO.0222-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finc K, Bonna K, He X, Lydon-Staley DM, Kühn S, Duch W, et al. Dynamic reconfiguration of functional brain networks during working memory training. Nat Commun. 2020;11:2435. doi: 10.1038/s41467-020-17747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen JR, D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, et al. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron. 2016;92:544–554. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schizophrenia Working Group of the Psychiatric GC. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider M, Walter H, Moessnang C, Schäfer A, Erk S, Mohnke S, Romanczuk-Seiferth N. Altered DLPFC-hippocampus connectivity during working memory: Independent replication and disorder specificity of a putative genetic risk phenotype for schizophrenia. Schizophrenia bulletin. 2017;43(5):1114–1122. doi: 10.1093/schbul/sbx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Archives of general psychiatry. 2011;68(12):1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- 62.Choi Shing Wan, O’Reilly Paul F. “PRSice-2: Polygenic Risk Score software for biobank-scale data.”. Gigascience. 2019;8(7):giz082. doi: 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, et al. Phenotypicmanifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73:221–228. doi: 10.1001/jamapsychiatry.2015.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.