Abstract
The inflammatory response is a complex regulated effector mechanism of the innate immune system that is initiated after tissue injury or infection. The NLRP3 inflammasome is an important initiator of inflammation by regulating the activation of caspase-1, the maturation of pro-inflammatory cytokines and the induction of pyroptotic cell death. Numerous studies demonstrate that the NLRP3 inflammasome could be modulated by lipids, existing a relation between lipids and the activation of different inflammatory processes. In this review we will summarize how the mechanism of NLRP3 inflammasome activation is regulated by different lipids and how these lipids control specific cellular localization of NLRP3 during activation. Although being a cytosolic protein, NLRP3 interacts with lipids accessible in neighbor membranes. Also, the modulation of NLRP3 by endogenous lipids has been found causative of different metabolic diseases and bacterial-pathogenic lipids lead to NLRP3 activation during infection. The understanding of the modulation of the NLRP3 inflammasome by lipids has resulted not only in a better knowledge about the mechanism of NLRP3 activation and its implication in disease, but also opens a new avenue for the development of novel therapeutics and vaccines, as NLRP3 could be modulated by synthetic lipids used as adjuvants.
Keywords: Inflammation, inflammasome, non-canonical inflammasome, caspase, pyroptosis, interleukin-1, cardiolipin, phosphatidylinositol-4-phosphate, phosphatidylcholine, cholesterol, 25-hydroxycholesterol, ceramide, sphingosine, saturated fatty acid, polyunsaturated fatty acid, oleic acid, linoleic acid, lipopolysaccharide, lipophosphoglycan, ornithine lipid, cationic lipid
1. Introduction
1.1. Canonical and non-canonical NLRP3 inflammasome activation
The nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3 (NLRP3) inflammasome is a multiprotein complex induced in response to different pathogens- and damage-associated molecular patterns (PAMPs and DAMPs, respectively) such as extracellular ATP, uric acid or cholesterol crystals, insoluble particles like silica, alum or amyloid deposition among others [1–5].
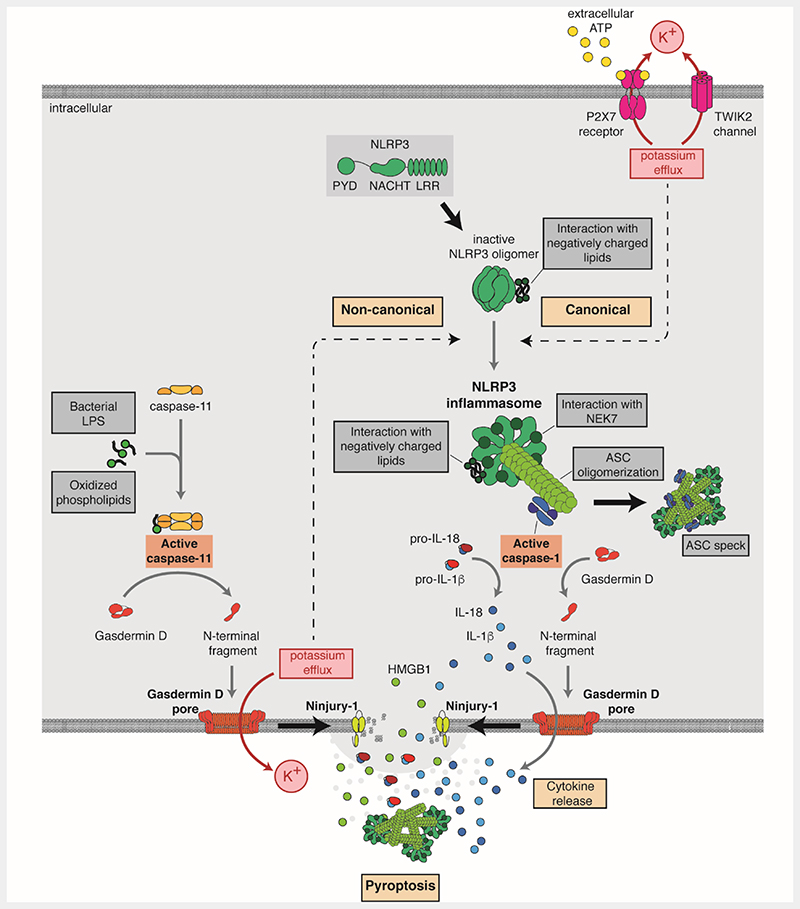
The NLRP3 protein belongs to the family of intracellular pattern recognition receptor. It is mainly expressed in myeloid cells, such as macrophages. Toll-like receptors (TLRs) engagement or tumor necrosis factor (TNF) receptor activation upregulate the expression levels of NLRP3 and produce specific post-transcriptional modifications, including deubiquitination and a series of phosphorylation/dephosphorylation events [6,7]. This process is called “priming”. After priming and before inflammasome activation, NLRP3 is present in inactive oligomeric cages that keeps the receptor in a stable closed structure [8–10]. PAMPs and DAMPs do not bind directly to NLRP3, but induce intracellular events that produce a conformational change in NLRP3 to initiate the activation of the inflammasome. One events can be a decrease in intracellular K+, such as in situations where the cell encounters pore forming toxins (i.e. nigericin and melittin) [11,12] or when ionic channels are activated (i.e. activation of purinergic P2X7 receptor or the two-pore domain K+ channel TWIK2 by ATP) [13,14], or following the phagocytosis of crystals or particles [15]. Other events, such as the production of reactive oxygen species by compound targeting mitochondria also lead to NLRP3 activation but independently of K+ efflux [16] (Figure 1). During the activation, NLRP3 localizes to cellular compartments with exposed negatively charged lipids such as phosphatidylinositol-4-phosphate in dispersed trans-Golgi network vesicles, where it adopts a stable oligomeric structure interacting with never in mitosis gene a-related kinase 7 (NEK7) [8,17–20]. This active NLRP3 oligomer seeds the nucleation of apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) (Figure 1). ASC binds to PYD domains in the NLRP3 oligomer and form homo-oligomeric fibers by subsequent PYD-PYD homo-typic domain interaction among ASC proteins. These ASC fibers nucleate each other interacting by their caspase activation and recruitment domains (CARD) forming a large ASC oligomer, where the inflammatory caspase-1 is recruited and activated [21–24]. Caspase-1 cleaves different protein substrates in the cell, including gasdermin D (GSDMD) and the pro-cytokines pro-interleukin (IL)-1β and pro-IL-18 to their bioactive forms (Figure 1). The excision of the amino-terminal fragment of GSDMD by caspase-1 cleavage allows its oligomerization in the plasma membrane to form pores of approximately 22 nm. The pores of GSDMD present a negative electrostatic conduit that preferentially allows the release of mature IL-1β and IL-18, that present a basic surface after caspase-1 processing [25,26]. GSDMD pores in the plasma membrane could be repaired by the endosomal sorting complexes required for transport machinery. This kind of release of mature IL-1β and IL-18 from living cells is called hyperactive state of the macrophage [26,27]. However, if GSDMD continues forming pores in the plasma membrane, repairing mechanisms are not efficient enough to seal the damaged membrane and GSDMD pores lead to a specific type of necrotic and inflammatory cell death called pyroptosis, characterized by ninjurin-1 protein dependent plasma membrane rupture and cell lysis [28–31]. A common marker of pyroptosis is the release of intracellular content such as high-mobility group box 1 (HMGB1) protein and glycolytic cytosolic enzyme lactate dehydrogenase (LDH), a tetrameric enzyme that does not directly permeate through GSDMD pores. The release of intracellular content amplifies the inflammatory response, since known pro-inflammatory molecules, including HMGB1, NLRP3 inflammasome oligomers or mitochondrial DNA, are released during pyroptosis [31–34].
Figure 1.
Schematic representation of lipids involved in the canonical and non-canonical NLRP3 activation pathways. In the canonical activation, NLRP3 activation is caused by the K+ efflux that can be induced by cholesterol crystals, sphingosine and saturated fatty acids (SFAs) such as palmitic and stearic acids and can be inhibited by unsaturated fatty acids (UFAs). Interaction with cholesterol and negatively charged lipids such as cardiolipin, and phosphatidylinositols in organelles membranes favors NLRP3 activation and interaction with ASC and caspase-1. Active caspase-1 cleaves gasdermin D (GSDMD), pro-IL18, and pro-IL1β causing pore membrane formation and cytokine release. In the non-canonical pathway, bacterial lipopolysaccharides (LPS), lipophosphoglycans (LPG) or ornithine lipids (OL) or host-derived oxidized phospholipids (oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine, oxPAPC) activate caspase-11, which cleaves GSDMD, inducing membrane pore formation. The consequent K+ efflux activates in turn the NLRP3 inflammasome. Both canonical and non-canonical pathways culminate in Ninjury-1-mediated lytic cell death. The NLRP3 inflammasome could be also activated by cationic lipids through an unknown mechanism.
Caspase-11 in mice (and its orthologues caspase-4/5 in humans) is another inflammatory caspase that functions as an intracellular sensor of different lipids. Caspase-11/4/5 are activated after binding to bacterial lipopolysaccharide (LPS) or certain endogenous oxidized phospholipids [35–38]. Meanwhile caspase-11/4/5 can cleave GSDMD and induce pyroptosis, it cannot cleave pro-IL-1β nor pro-IL-18. In that situation, the NLRP3 inflammasome is then activated, likely in response to K+ efflux from GSDMD pores; this process is known as the non-canonical NLRP3 inflammasome activation [39,40] (Figure 1). In the non-canonical NLRP3 inflammasome the maturation of IL-1β and IL-18 is dependent on caspase-1 and occurs in a second stage, while GSDMD cell permeabilization and pyroptosis mainly depends on caspase-11/4/5 activation and occurs before caspase-1 is activated (Figure 1). In contraposition, in the canonical NLRP3 inflammasome the maturation of cytokines and GSDMD-mediated pyroptosis both depend on caspase-1 activation.
1.2. NLRP3 inflammasome in inflammation and disease
The NLRP3 inflammasome is an effector mechanism of the innate immune system important to establish an immune response, its activation is important for the optimal elimination of bacterial but also viral infections by activating the adaptive immune system[41]. The NLRP3 inflammasome is also important to promote tissue repair in the intestinal barrier and so suppress colorectal cancer in mouse model (for reviews see [42,43]). However, an exacerbate activation of the NLRP3 inflammasome has been linked to the development of other types of cancer and various pathologies, including rare conditions and chronic, metabolic or degenerative diseases (for reviews see [42–45]). The implication of the NLRP3 inflammasome in disease come from two main observations: (i) the benefits of molecular inhibition and genetical depletion of NLRP3 in pre-clinical models of diseases; and (ii) the identification of disease-causing gain-of-function mutations in human patients suffering Cryopyrin associated-periodic syndromes (CAPS) [42–44,46]. Over 200 different variants of NLRP3 have been associated to CAPS [42] and these mutations result in a constitutively open conformation of the receptor that favor its activation [47]. Also, patient monocytes carrying NLRP3 mutations constitutively release IL-1β and knock-in of mutated NLRP3 in mice causes a constitutive inflammatory phenotype [48–51]. Different DAMPs, including endogenous lipids (as will be reviewed in the following sections), have been involved in the activation of NLRP3 in disease. NLRP3 knock-out mice present a protected phenotype in models of gout, rheumatoid arthritis, atherosclerosis, diabetes, non-alcoholic fatty liver disease, myocardial infarction, inflammatory bowel disease, nephropathy, graft-versus-host disease, contact hypersensitivity, multiple sclerosis/experimental autoimmune encephalitis, traumatic brain injury, stroke and Alzheimer’s disease and several cancer type [42–45]. Also, blocking NLRP3 with specific inhibitors ameliorates the symptoms of these different conditions in pre-clinical models [43–45], establishing that NLRP3 is a good druggable candidate target for the development of novel anti-inflammatory therapies. In fact, some small-molecules inhibitors of NLRP3 are already in phase I clinical trials and in particular the β-sulfonyl nitrile compound OLT1177 (Dapansutrile) has satisfactory reported safety results in humans and efficacy in the reduction of joint pain in a phase IIa clinical trial for patients with monoarticular monosodium urate crystal-gout flares, and improvement of left ventricular ejection fraction in a phase Ib trial for systolic heart failure [52,53]. However, future clinical studies are needed with Dapansutrile and other NLRP3 inhibitors to confirm the clinical potential of these new drugs in different human diseases.
1.3. Lipid metabolism and inflammation
Numerous studies demonstrated that there is a link between modifications occurring in the lipid metabolic pathway and the activation of inflammatory processes [54,55]. Such a remodeling of the lipid composition is observed and characterizes pathologies like atherosclerosis and diabetes. How activation of inflammatory factors contributes to those diseases is largely unknown. In the following section we will review how lipids can activate NLRP3 or contribute to its compartmental location (summarized in Table 1). Activation of NLRP3 by endogenous lipids can be deleterious and cause inflammatory diseases, whereas a normal lipid-mediated regulation is important for immune-defense. Moreover, activation of NLRP3 by synthetic lipids has turned out to be extremely advantageous for the formulation of safe adjuvant-free vaccine.
Table 1. Lipids origin and how they modulate the NLRP3 inflammasome.
| Lipid | Origin | Relation to NLRP3 inflammasome | References |
|---|---|---|---|
| Cardiolipin | Mitochondria | Favors NLRP3 proximity with ASC and caspase-1 | [56,62] |
| Phosphatidylinositol-4-phosphate | Trans-Golgi network | Stabilize the active open conformation of the NLRP3 oligomers | [8,17] |
| Cholesterol | Endoplasmatic reticulum | Enables NLRP3 transport and association to ASC | [58] |
| Cholesterol; 25-hydroxycholesterol | Cholesterol metabolites | Activates NLRP3 inflammasome | [81,82] |
| Cholesterol crystals | Oxidized low-density lipoproteins | Activate NLRP3 inflammasome | [69] |
| Oxidized Phosphatidilcholine | Lipid droplets/foam cells | Activates the non-canonical NLRP3 inflammasome | [70] |
| Activates the NLRP3 inflammasome | [71] | ||
| Inhibits the non-canonical NLRP3 inflammasome | [71] | ||
| Ceramide | Diet | Activates the NLRP3 inflammasome | [85] |
| Sphingosine | Diet | Activates the NLRP3 inflammasome | [86] |
| Saturated fatty acids | Diet | Activate the NLRP3 inflammasome | [89–91] |
| Polyunsaturated fatty acids | Diet | Inhibit the NLRP3 inflammasome | [87,88] |
| Cationic lipids lipopolyamines | Synthetic | Activate NLRP3 inflammasome | [122,124] |
| Ornithine lipid | Gram negative bacteria | Activate the non-canonical NLRP3 inflammasome | [104] |
| Penta- and hexa-acylated LPS | Gram negative bacteria | Activate the non-canonical NLRP3 inflammasome | [36,95] |
| Lipid IVa | LPS synthesis precursor | Activates the non-canonical NLRP3 inflammasome | [36] |
| Dephosphorilated tetra-acylated LPS | Gram negative bacteria | Inhibit the non-canonical NLRP3 inflammasome | [36] |
| Lipophosphoglycans | Leishmania parasites | Activate the non-canonical NLRP3 inflammasome | [92] |
Deciphering the role and the mechanism of lipid in NLRP3 inflammasome activation might lead to novel strategies for vaccine formulation as well as for the treatment of diseases for which perturbed lipid metabolism is an underlying cause.
2. Regulation of NLRP3 inflammasome by endogenous lipids
2.1. Spatially regulated-NLRP3 interaction with endogenous lipids
The cellular localization of NLRP3 in macrophages has been reported to be in the cytosol and associated with different intracellular organelles, as mitochondria-associated membranes (MAMs), the trans-Golgi network, the endoplasmic reticulum (ER), or the microtubule-organizing center [8,17,18,56–58]. The association of NLRP3 with such a variety of organelles and its importance to activate the inflammasome appears conflictual. The presence of exposed negatively charged lipids in the organelles determines the association to NLRP3. The different cell types used might results in differences in the exposed negatively charged lipid on the organelles and could explain the discrepancies between the different studies. However, it must be noted that mitochondrial membranes are particularly difficult to purify from ER membrane, and studies evaluating the association with mitochondria are actually looking at both mitochondria and mitochondria-associated membrane from the ER. However, organelle membranes are an intricated and highly dynamic network and, despite the apparent incongruences, it is plausible that NLRP3 associates with most of the aforementioned membranes at different stages. Altogether, the studies reviewed in the following paragraphs suggest that, in the priming step, NLRP3 is expressed and could be transported through the ER (and MAMs), whereas the oligomerization to inactive cage structure might occurs in the TGN during the activation step, before moving to the microtubules-organizing center (MTOC) for inflammasome assembly.
2.1.1. Cholesterol in the endoplasmic reticulum is involved in NLRP3 activation
It has been described that depletion of cholesterol in the ER but not in the plasma membrane of murine macrophages strongly affects ATP- and nigericin-induced NLRP3 association with ASC, resulting in inhibition of caspase-1 activation and IL-1β and IL-18 release [58]. Interestingly, NLRP3 signal was restored by replenishment of cells with”cholesterol [58]. The reduction of NLRP3 activation was not due to a defective priming, as TNF-α secretion and the expression of NLRP3, ASC, caspase-1 and pro-IL-1β were not affected by depletion of cholesterol in the ER. Moreover, other inflammasomes (NLRC4 and AIM2) were normally activated by their stimuli [58]. The authors suggest that depletion of cholesterol in the ER affects the transport of NLRP3 to the ASC. However, further studies have to be conducted to confirm this hypothesis. Indeed, the authors did not investigate the effect of ER cholesterol depletion in NLRP3 and ASC localization in the ER, MAMs or cytosol [58]. The reduced ASC-NLRP3 interaction might be due to defective NLRP3 transition to the open/active conformation needed to interact with ASC [12]. It would be interesting to know whether cholesterol is needed for NLRP3 association with the ER and whether the characteristic of cholesterol enriched membrane helps NLRP3 conformational changes like it has been suggested for TLR4 [59]. Assays to monitor conformational changes of NLRP3 during activation, as using bioluminescence resonance energy transfer (BRET) NLRP3 sensors, may reveal whether NLRP3 conformational change is affected by cholesterol depletion [47,60]. The role of cholesterol in NLRP3 activation might open the way for the understanding of diseases involving altered cholesterol trafficking, such as the Niemann-Pick disease type C1, a neurodegenerative lysosomal disorder characterized by dysfunction of NPC1 and significant dysregulation of innate immunity [61], among others.
2.1.2. Cardiolipin favors NLRP3 inflammasome formation
Cardiolipin (CL) is a four-acylated phospholipid found in the mitochondrial membrane. CL liposomes induce oligomerization of recombinant NLRP3 and caspase-1 and both proteins are also able to bind to CL lipid strips [56,62]. Iyer and co-worker suggest that CL is needed for a functional NLRP3 inflammasome assembly in macrophages [62]. According to Elliott and co-workers, the interaction of CL with NLRP3 and caspase-1 in the mitochondrial membrane would create a platform for the formation of the NLRP3 inflammasome, which occurs once NLRP3 is activated[56]. Cardiolipin is normally located in the inner mitochondrial membrane, however, when cells are treated with LPS, cardiolipin is found in the external membrane of purified mitochondria [56]. The authors demonstrated that LPS-induced CL exposure was dependent on reactive oxygen species (ROS) production[56]. It is known that other ROS-inducing signals may cause CL exposure, strongly suggesting that priming with other TLR activators might induce the same effects [63]. Elliot and co-workers demonstrated that NLRP3 and caspase-1 migrate to mitochondria/MAMs fraction following priming and that ASC is recruited following NLRP3 activation [56]. They suggest that the localization of NLRP3 and caspase-1 with mitochondria and MAMs is due to the LPS-induced exposure of CL [56]. However, they did not investigate whether depleting CL decreases the amount of NLRP3 or caspase-1 in the mitochondrial fraction [56], therefore, this interesting hypothesis will need further investigation. Also, other studies observed proximity of NLRP3 to MAMs but not colocalization of NLRP3 with mitochondria was observed [17,58].
2.1.3. Phosphatidylinositol phosphate binds to NLRP3 for inflammasome activation
Following stimulation with nigericin and other stimuli, NLRP3 has been found to oligomerize into small puncta in the dispersed trans-Golgi network (dTGN) and then to associate with microtubule before assembly with ASC and caspase-1 [8,17,18]. Andreeva and co-workers showed that in wild-type and NLRP3-/- reconstituted with human NLRP3 immortalized murine macrophages primed with LPS, inactive NLRP3 oligomerizes into a cage-like structure and co-localizes with the TGN [8,17]. Chen & Chen also show that NLRP3 is co-purified with TGN membrane fraction before Nig-treatment[17]. Subsequent stimulation leading to NLRP3 activation induces the disassembly of the TGN in the dTGN and the formation of NLRP3 inflammasome at the MTOC [8,17]. A K+ efflux is required for NLRP3 activation by nigericin, whereas molecules such as imiquimod can activate NLRP3 independently of K+ efflux [62]. Intriguingly, dTGN formation by both nigericin and imiquimod was independent of K+ efflux, even when NLRP3 constitutively localizes with the TGN[17]. Also NLRP3 inhibition by MCC950 impaired NLRP3 activity but did not affect NLRP3 association to the dTGN and Nig-induced dTGN formation, demonstrating that dTGN formation is caused by NLRP3 stimuli but is upstream of NLRP3 activation [8]. The K+ dependence was instead reflected in the recruitment of NLRP3 to dTGN, being K+ efflux necessary for the NLRP3 requirement to the dTGN induced by nigericin but not by imiquimod[17].
NLRP3 is recruited to the dTGN due to the interaction of its polybasic region (residues KKKK 127-130 in mouse NLRP3, KMKK 129-132 in human NLRP3, found between the PYD and NACHT domains) with the negatively charged phosphatidylinositol 4-phosphate (PI4P) [8,17]. An in vitro assay with lipidic strips showed that a purified NLRP3 fragment containing the KKKK motif as well as inactive NLRP3 cages bind to several phosphorylated phosphatidylinositols and phosphatidic acid (PA), but not the unphosphorylated phosphatidylinositols [8,17]. However, only a phosphatase selective for phosphatidylinositol 4-phosphate (PI4P) inhibited nigericin-induced NLRP3 oligomerization and recruitment to dTGN. Moreover, after nigericin treatment, NLRP3 colocalizes with the pleckstrin homology (PH) domain of oxysterol binding protein (OSBP-PH), one of the best characterized PI4P-binding domains and the replacement of KKKK with OSBP-PH makes NLRP3 constitutively recruited to the TGN, although active only after nigericin-induced dTGN formation, demonstrating that PI4P specifically recruits NLRP3 to the TGN [17].. However, the elimination of the polybasic sequence KMKK in the human NLRP3 did not affect the activation of the inflammasome, suggesting that human NLRP3 could require a longer polybasic patch [64].Interestingly, Andreeva and co-workers also show that the mutation of the polybasic region disrupt the oligomerization of NLRP3 into cages, and this is rescued by addition of a OSBP linker, demonstrating that NLRP3 oligomerization is mediated by its association with TGN membranes [8]. Finally, the insertion of a OSBP linker in a mutant unable to form cages did not rescue dTGN formation and NLRP3 activity, demonstrating that cage formation is necessary for these events [8].
2.2. Activation of NLRP3 inflammasome by endogenous lipids and implication in disease
Whereas the lipids described so far are involved in the spatial regulation of the NLRP3 inflammasome for its activation, some lipids are able to activate the NLRP3 inflammasome independently of the presence of canonical stimuli. In condition such as atherosclerosis and rheumatoid arthritis, cholesterol and oxidized phospholipids, such as the ones derived from 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (PAPC), known as oxPAPC, accumulate in blood vessels inside foam cells (cells characterized by presenting intracellular lipid droplets) [65,66]. Advanced microscopic techniques have revealed that microscopic crystals and lipid droplets containing cholesterol and oxPAPC appear in mice at the early stage of atherosclerosis in concomitance with the appearance of inflammatory cells infiltration [3,67], suggesting that these lipids contribute to the early phases of inflammation. Cholesterol is a ubiquitous component of all cellular membranes and it plays essential roles in membrane structure and function. Cells capture exogenous cholesterol from circulating lipoproteins via LDL receptor-mediated endocytosis [66]. Disruption of cholesterol homeostasis results in the accumulation of free cholesterol and in the formation of cholesterol crystals [68]. Whereas cholesterol is reported to promote canonical NLRP3 inflammasome activation, oxPAPC activates or inhibits both canonical and non-canonical NLRP3 inflammasome depending on cell type and location [69–71].
2.2.1. Phosphatidylcholine
The ability of oxPAPC to modulate NLRP3 activation has been reported to depend on the cell type. OxPAPC is found in apoptotic and necrotic cells and in damaged tissues [72]. Oxidation of PAPC generates a large variety of products, such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC), and epoxy-isoprostane-PC 1-palmitoyl-2-(5,6 epoxyisoprostanoyl)-sn-glycero-3- phosphocholine (PEIPC), 1-(Palmitoyl)-2-(5-keto-6-octene-dioyl) phosphatidylcholine (KOdiA-PC) and lyso-PC among others [73,74]. Zanoni and co-workers have shown that 100 μg/mL of commercial mix of oxPAPC (around 120 μM) as well as the purified components KOdiA-PC, POVPC, and PGPC induced IL-1β secretion in primed dendritic cells (DCs) derived from bone-marrow (BMDC) or purified from mice spleen [35]. By contrast, bone marrow derived macrophages (BMDM) were not able to secrete IL-1β in response to oxPAPC mix [35]. The activation occurred even when DCs were primed with TLR ligands other than LPS, running out of the possibility that oxPAPC acts as a transporter of LPS to activate caspase-11 and the non-canonical NLRP3 inflammasome [35]. The secretion of IL-1β induced by oxPAPC was dependent on NLRP3, ASC, caspase-1 and caspase-11, suggesting that oxPAPC is an activator of the non-canonical NLRP3 inflammasome pathway in DCs. Similar to LPS, oxPAPC induced caspase-11 oligomerization, interacted with immobilized catalytically inactive caspase-11(C254A) as LPS does, and biotin-oxPAPC, as biotin-LPS, bound endogenous caspase-11 from DCs and immortalized macrophages. However, important differences with respect to LPS-caspase-11 activation have been observed. First of all, oxPAPC did not induce pyroptosis and neither the caspase-11 catalytic activity nor K+ efflux was required for oxPAPC-induced IL-1β secretion. Moreover, mutations in the caspase-11 CARD domain that prevents LPS binding did not impair oxPAPC binding, while the isolated caspase-11 catalytic domain (but not the CARD) retained the ability to bind oxPAPC [35], suggesting that oxPAPC binds to caspase-11 at a different region from LPS. Finally, differently from LPS, biotin-oxPAPC was able to capture not only caspase-11 but also caspase-1 and, very surprisingly, oxPAPC inhibited LPS-induced caspase-11 enzymatic activity observed by spectrofluorimetry [35], which made the authors suggest that oxPAPC may lead to the formation of a hetero-complex between caspase-11 and caspase-1 in DCs [35].
Another study showed that high concentration of oxPAPC induced IL-1β release in primed BMDCs, but independently of caspase-11 [71]. The authors showed that DCs had a basal IL-1β secretion induced by LPS or Pam3CSK4 priming which was increased by an elevated oxPAPC concentration (100 μg/mL). The release of IL-1β required 18h of incubation with oxPAPC and was independent of caspase-11 and dependent on TLR4, even in Pam3CSK4 primed DCs [71], a pattern reminiscent of the alternative inflammasome pathway observed in human monocytes, which rely on TLR4 and caspase-8 [75].
When LPS was delivered intracellularly with 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), caspase-11 dependent IL-1β release via the non-canonical NLRP3 inflammasome activation was completely blocked by co-transfection with 1 μg of oxPAPC [71], suggesting that oxPAPC is an inhibitor and not an activator of caspase-11 and the non-canonical NLRP3 activation. A 2-fold excess of oxPAPC over LPS was enough to completely block LPS-induced caspase-11 activation in BMDC and BMDM, and a 10-fold excess protected mice from acute LPS-induced septic shock in vivo [71]. Chu and co-workers demonstrated that 170-fold less concentrated oxPAPC (0.6 μg/mL) with respect to the concentration shown to activate caspase-11 in DCs, was sufficient to inhibit LPS-induced caspase-11 activation in BMDM [71]. They showed that oxPAPC but not PAPC inhibits DOTAP delivered or electroporated LPS- and bacterial outer membrane vesicles (OMVs)-induced pyroptosis and non-canonical NLRP3 activation, completely abrogating GSDMD cleavage and HMGB1, LDH, IL-1β and IL-1α release in human and murine primary macrophages and THP1 cell line [71]. The protective activity of oxPAPC was independent of TLR4 as it was observed also in TLR4-deficient BMDM and mice. oxPAPC captured tagged mouse caspase-11 and its orthologue human caspase-4 from lysates of transfected HEK293 cells and reduced biotin-LPS binding to tagged-caspase-4 in transfected HEK293 cells and to endogenous caspase-11 in lysates from BMDM [71]. This strongly suggests that oxPAPC binds to cytosolic caspase-11 and caspase-4 preventing LPS binding and caspase activation. Moreover, both oxPAPC and LPS are suggested to bind to the same region of caspase-11, although through different amino acids [71].
In BMDM, oxPAPC reduced LPS-driven caspase-11 activation when administered simultaneously or previous LPS delivery, whereas the inhibition of LPS-induced caspase-11 activation in DCs is observed only when oxPAPC is co-delivered [71]. Moreover, DCs presented a reduced uptake of oxPAPC with respect to BMDM, which may redirect the oxPAPC towards TLR4 on the cell membrane and explain why oxPAPC induces TLR4-dependent IL-1β secretion in BMDCs but not in BMDM [71][35,71]In line with the ability of extracellular oxPAPC to activate the NLRP3 inflammasome, Yeon and co-workers reported the ability of some oxPAPC components to activate NLRP3 in BMDM [76]. They observed that 1 hour treatment of BMDM with 100 μg/mL of POVPC, KOdiA-PC or PGPC was sufficient to induce NLRP3-dependent caspase-1 activation and IL-1β and IL-18 secretion in BMDM [76]. They also observed NLRP3 activation by POVPC in two different mouse models of air pouch and peritonitis [76]. The authors did not investigate caspase-11 involvement. Similar to the activation in DCs shown by Zanoni and co-workers [35], the activation of NLRP3 in BMDM was not dependent on K+ efflux, but was instead dependent on the increase in intracellular Ca2+ and on mitochondrial ROS production [76].
All together these studies suggest that high concentration of extracellular oxPAPC may activate the alternative NLRP3 pathway, whereas intracellular delivered oxPAPC is an antagonist of caspase-11 [77]. It must be noted that POVPC, KOdiA-PC and PGPC represent less than 10% of oxPAPC and that circulating concentrations of oxPAPC in human plasma are close to the ones required for LPS inhibition, whereas it is very unlikely to find high concentrations of 100 μg/mL physiologically [77], although local concentrations of oxPAPC may be much higher.
Another product of PAPC degradation, the lyso-PC (LPC), has been reported to have inflammatory properties. Treatment of THP1 or human umbilical vein endothelial cells (HUVECs) cells for 24h with 1 μg/mL of synthetic C16:0-LPC has been reported to lead to pro-caspase-1 induction and activation and caspase-1-dependent foam cell formation [67]. LPC induced IL-1β secretion in LPS-primed THP1. In endothelial cells IL-1β was not detectable although HMGB1 release was observed. LPC also induced weak IL-1β and LDH release in not primed cells, which was blocked by antibodies neutralizing TLR2, and inhibitors of cathepsin B and K+, but not by the ROS inhibitor N-acetyl cysteine [67]. IL-1β secretion was also observed in primary microglial cells and monocytes treated with C16:0 and C18:0 but not C18:1 LPC [78,79].
2.2.3. Cholesterol
Cholesterol crystals induced caspase-dependent IL-1β release in human peripheral blood mononuclear cells (PBMCs) and monocytes as well as in THP1 cells primed with LPS or modified low-density lipoproteins (LDL) [3,80]. The release was abrogated by silencing NLRP3 and ASC in human macrophages and absent in macrophages from Nlrp3-/- and Pycard-/- mice, demonstrating the ability of cholesterol crystal to activate the NLRP3 inflammasome [3,80]. The activation occurs following cholesterol crystal phagocytosis and lysosomal rupture, being partially decreased in macrophages deficient in cathepsin B or L [3,80]. Moreover, intraperitoneal injection of cholesterol crystal in mice induced IL-1α, IL-1β and IL-1 receptor dependent neutrophil recruitment, which was decreased in NLRP3 and cathepsin deficient mice [69]. Oxidized LDL can prime macrophages and at larger time can transform into cholesterol crystals and activate the NLRP3 inflammasome [69].
Oxysterols are cholesterol metabolites produced by enzymatic or radical processes. Among them, 25-hydroxycholesterol (25-HC) is the most studied as it is involved in metabolism and antiviral and cell-survival processes [81]. Jang and co-workers found that 10 hours treatment of primed BMDMs or microglial cells with 25-HC ranging from 10 to 100 μM induced NLRP3 inflammasome dependent caspase-1 activation and IL-1β secretion [82]. 25-HC-induced IL-1β release was also observed in primary glial cells and THP-1 [82]. Stereotaxic injection of 25-HC 100 μM in the corpus callosum of mouse brain resulted in the production of IL-1β and recruitment of glial cells; both events were absent in Nlrp3-/- mice [82]. The activation of NLRP3 by 25-HC in the brain might explain cerebral inflammation in childhood cerebral adrenoleukodystrophy (CCALD). In cells derived from CCALD patients, the gene for the enzyme cholesterol 25-hydroxylase (CH25H) was upregulated and 25-HC accumulated in cells supernatants [82]. Very long chain fatty acids (VLCFA) also accumulate in this condition and are considered a biomarker of the disease. However, Jang and co-workers did not observe any induction neither of the NLRP3 inflammasome nor of the NF-κB pathway by VLCFA [82].
In contrast to the reported pro-inflammatory properties of 25-HC, the canonical activation of NLRP3 treatment induced more IL-1β secretion in macrophages from CH25H-/- mice with respect to wild type mice [83]. CH25H gene is upregulated by LPS treatment in a IFN-α receptor-dependent manner and results in an increase of 25-HC in BMDMs supernatants [83]. Although pro-IL-1β was transiently overproduced after LPS treatment in CH25H-/-, the increase in IL-1β secretion cannot be simply ascribed to priming enhancement, as also IL-18 secretion and caspase-1 activation were enhanced while other cytokines depending solely on NF-κB activation, as IL-6, were not affected. Moreover, Casp1, Nlrp3 and Pycard genes were not upregulated in CH25H deficient cells. Intriguingly, the increase in caspase-1 activation was not specific to the NLRP3 activator extracellular ATP, but occurred also with flagellin (a specific NLRC4 inflammasome activator) and poly-dA:dT nucleotide (a specific AIM2 inflammasome activator) [83], suggesting that it CH25H gene could regulates different inflammasomes.
3. NLRP3, lipids, diabetes and obesity
Elevated levels of free fatty acids (FFAs) play a causal role in the progression of metabolic diseases, including obesity, atherosclerosis, type 2 diabetes mellitus, and metabolic syndrome [84].
3.1. Ceramide and sphingosine
A study from Vandanmagsar and co-workers focused on the role on NLRP3 activation in obesity-induced insulin resistance [85]. They show that in high fat diet-induced obese mice IL-18 concentrations significantly increased in the serum and caspase-1 was activated in adipose tissue and liver, with both events being blocked in mice deficient on NLRP3 [85]. Since obesity causes increase in tissue and circulating levels of free fatty acids, the authors tested the involvement of ceramide by treating LPS-primed BMDM with 100 μM of this fatty acid. This high concentration of ceramide induced an NLRP3-dependent IL-1β secretion and caspase-1 cleavage [85]. Another study has shown that lower concentrations of ceramide (20 and 40 μM) did not induce IL-1β release in LPS-primed peritoneal murine macrophages [86]. By contrast, 20 μM of sphingosine, the immediately downstream metabolite of ceramide, was able to induce NLRP3-dependent IL-1β secretion [86]. The same concentration of the water-soluble sphingosine analog FTY720, used in vivo as anti-sclerotic and anti-tumoral, induced IL-1β secretion from LPS-primed macrophages and in a in vivo model of peritonitis [86][86]The authors showed that activation of NLRP3 was dependent on K+ efflux and not on P2X7 receptor activation [86]. Sphingosine also induced lysosomal membrane rupture and cathepsin B leakage to the cytosol, but this process did not correlate with IL-1β secretion, although there is a requirement for an acidic lysosome for IL-1β secretion [86].
3.2. Saturated vs unsaturated fatty acids
Other fatty acids have been observed to induce NLRP3 inflammasome activation and may be involved in obesity-related inflammation. Albumin-conjugated saturated fatty acids (SFAs) such as palmitic and stearic acids activate TLRs and induce K+-dependent NLRP3 inflammasome and caspase-1 activation with consequent IL-1β release in human and murine monocytes and macrophages. By contrast, unsaturated fatty acids (UFAs) such as oleic (OA) and linoleic (LA) acids, among others, prevent SFAs-induced NLRP3 activation [87–91].OA and LA were also shown to inhibit nigericin-, ATP- and monosodium urate (MSU)-induced IL-1β release in human monocytes and THP1 [88]. Interestingly OA and LA were not able to inhibit the activation of the NLRC4, AIM2 or NLRP7 inflammasomes and did not affect pro-IL-1β and NLRP3 expression [87,88], indicating UFAs as specific NLRP3 inhibitors. However, other reports showed that OA and LA were not able to inhibit nigericin-induced IL-1β release in murine macrophages while ω-3 were effective inhibitors of the NLRP3 inflammasome [87,90]. The ability of UFAs to inhibit the canonical NLRP3 activation induced by nigericin or extracellular ATP remain controversial, while their ability to inhibit SFAs-induced NLRP3 activation seems to lie in their interfering in upstream events such as SFAs internalization, crystallization and SFAs-induced PC accumulation and plasma membrane disruption (leading to intracellular K+ efflux); which are at the fundament of the mechanism of the canonical NLRP3 activation by SFAs, but dispensable to activate the NLRP3 inflammasome in response to other canonical stimuli such as nigericin and ATP [89,90].
4. Pathogenic lipids and NLRP3 activation
4.1. Lipopolysaccharides and lipophosphoglycans
Pathogenic lipids such as E. coli LPS and Leishmania lipophosphoglycans (LPG) once delivered into the cytosol of cells induce GSDMD-dependent pore formation through binding and activation of caspase-11 (caspase-4/5 in humans) and the subsequent non-canonical NLRP3 inflammasome activation [39,92]. Exacerbated caspase-11 and NLRP3 activation by elevated amounts of circulating LPS causes septic shock and death. Mice deficient on caspase-11 or GSDMD are indeed completely protected from sepsis, which is not the case of mice deficient on the LPS receptor TLR4 [36,40]. On the other hand, caspase-11 activation has been shown to be important to defend the host against bacterial infections [93].
Pathogens with pore-forming toxins such as Listeria monocytogenes or Vibrio Cholerae or their purified toxins such as Listeriolysin O and Cholera Toxin B (CTB) favor LPS entry to the cell [93]. In experimental conditions, purified LPS or LPG can be delivered into the cytosol using electroporation or transfection agents such as DOTAP, Lipofectamine or Fugene [92,94,95]. Moreover, the endogenous HMGB1 protein is able to bind LPS and deliver it to caspase-11 through the receptor for advanced glycation end-products (RAGE)-mediated internalization. Depletion of hepatocyte-released HMGB1 protected mice from both purified LPS- and bacteria-induced sepsis, suggesting that LPS requires binding to circulating HMGB1 to initiate lethal responses in vivo [96]. However, also whole bacteria and OMVs are able to enter the cell by endocytosis and escape towards the cytosol, likely helped by GBPs through a poorly understood mechanism [97,98].
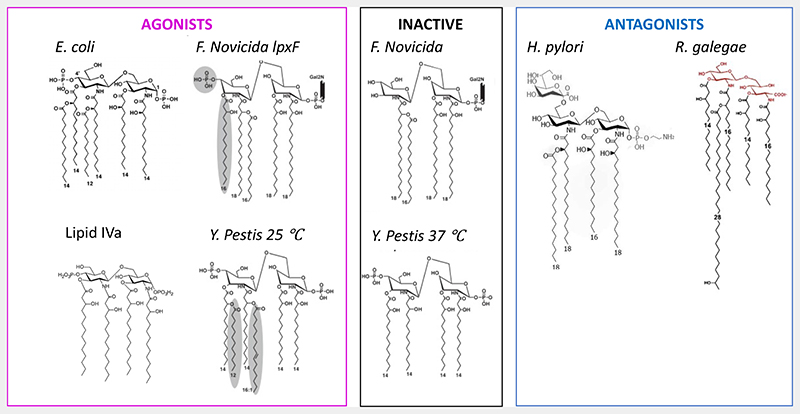
Interestingly, LPS from other bacteria such as S. minnesota RE595, S. typhimurium, F. novicida, Y. pestis, C. rodentium and S. flexneri among others, are also activators of caspase-11 [36,93], whereas LPS from H. pylori or R. galegae inhibit caspase-11 activation induced by E. coli LPS [36]. This might be either an escape strategy of bacteria that have altered their LPS structure to avoid caspase-11 activation or a selective immune response of the host immune system.
The polysaccharide moiety of LPS is not required for caspase-11 activation by LPS since synthetic E. coli Lipid A, which is devoid of it, is able to activate [36]. However, the polysaccharide moiety becomes important for LPS transport to the cytosol. For instance, polysaccharide region is specifically bound by CTB, which is able to transport LPS from B4 but not B5 or B8 E. coli strains [36]. Therefore, a proper structure-activity relationship study must be conducted with electroporated lipid A to determine lipid A structural requirement for caspase-11/-5/-4 activation and inhibition. The few studies conducted so far have shown that F. novicida and Y. pestis LPS lose their agonistic activity when modified to be tetra-acylated (Fig. 2), also the tetra-acylated LPS from H. pylori is an antagonist suggesting that caspase-11 is activated only by penta- and hexa- acylated LPS [95]. However, R. galegae LPS has five chains and is an antagonist of caspase-11, although one chain presents an OH group at ω-2 position which might decrease its hydrophobicity (Fig. 2). Moreover, the tetra-acylated F. novicida activates caspase-4 and the tetra-acylated LPS synthesis precursor lipid IVa is a weak activator of caspase-11 (Fig. 2) [36,99]. Furthermore, the modified tetra-acylated LPS from F. novicida, together with one acyl chain, lost the negatively charged phosphates group (Fig. 2) [95]. This might be responsible for the loss of activity. The lack of negatively charged phosphate groups also characterizes Lipid A from H. pylori and R. galegae LPS, which are caspase-11 antagonists (Fig. 2) [36]. Overall, this indicates a specific, not yet identified, structural requirement for LPS to activate or inhibit caspase-11/4/5 that may reside in both chains or polar heads of LPS Lipid A. Nevertheless, molecules with a very different structure from Lipid A like oxPAPC and LPG from Leishmania activate caspase-11, although their ability to bind caspase-11 has not been demonstrated and others non-identified molecules might be involved in the mechanism of caspase-11/4/5 activation [35,92].
Figure 2. Representative lipid A structures from agonists, inactive and antagonists LPS. Adapted from [36,95].
Finally, it must be noted that whether LPS interact with caspase-11/4/5 as a monomer, like it occurs for TLR4 [100], it is not known. LPS supramolecular organization in bacterial membrane, OMVs, liposomes or micelles might act as a platform for caspase-11/4/5 oligomerization [101,102]. In this case, the Lipid A polar head would mediate the binding, while the composition of acyl chain might affect the fluidity of the platform and then caspase-11/4/5 oligomerization and conformation. Even the ability of LPS to interact with caspase-11/4/5 is a matter of debate. The interferon-induced guanylate-binding proteins (GBPs) are proposed to be the very first sensor of cytosolic LPS[94,97]. In epithelial cells, GBPs have been observed to bind LPS, surround bacteria and mediate caspase-4 recruitment and activation by transforming bacteria in a platform for activation[101,102]. However, IFN-γ signaling and GBPs enhance but are not necessary for caspase-11/-4 activation by bacteria and transfection agents-delivered LPS in human and murine macrophages [97,103]. Like observed for CD14 and TLR4 [100], GBPs might favor LPS extraction from its membrane and its presentation to caspase-11/4/5.
4.2. Ornithine Lipids
[104]OLs are components of bacteria such as Vibrio cholerae and Pseudomonas aeruginosa among others, whose synthesis increases when bacteria grow in a phosphorous-depleted medium, maintaining bacterial membrane integrity and pathogenicity in the absence of LPS [105–108].
Peritoneal murine macrophages stimulated with OL purified from A. xylosoxidans and from F. meningosepticum secreted TNFα and IL-1β [109,110]. However, these results contradicted another study that showed OL from F. meningosepticum as an inhibitor of LPS-induced immune response in vivo [111]. The use of synthetic OL by our group allowed to run out of LPS contamination, which might be present in bacteria-extracted OLs, and elucidated that OL is a partial TLR4 and NLRP3 inflammasome activator, which competes with LPS for binding its receptors, reducing LPS-induced immune response while inducing a weak activation when administered alone [104]. Preliminary results showed GSDMD cleavage induced by OL in murine macrophages from wild type and from NLRP3-deficient mice, but not from double knock-out Casp1/11-/-, which suggests that OL may activate caspase-11 and induce non-canonical NLRP3 inflammasome activation [104]. Interestingly, preparation of OL as nanoparticle in aqueous solution was important for the induction of IL-1β but not TNFα secretion, the latter being maintained by OL dissolved in organic solvents [109], suggesting a mechanism similar to crystal-induced NLRP3 activation.
5. Activation of NLRP3 inflammasome by synthetic lipids
5.1. Cationic lipids
Cationic lipids are positively charged amphiphilic molecules made of a cationic polar head, a hydrophobic domain (comprising alkyl chains or cholesterol), and a linker connecting the polar head group with the nonpolar tail [112]. Cationic lipids may be permanently charged or acquire their charge at acid pH, the latter are usually less toxic and are also called ionizable lipids [113]. The pioneering work of Felgner [114] has opened the field of lipid-mediated delivery of hydrophilic molecules (nucleic acids, peptides and proteins). The ability of cationic lipid nanoparticles to transport proteins or transfect DNA and RNA can be exploited in gene therapy, anticancer or anti-viral immunotherapies, where intracellular delivery of an antigen, gene-editing tools, mRNA or silencing RNA can block or promote specific signals [113].
The identification of an efficient, safe and stable lipid nanoparticle (LNP) requires years of optimization and in vivo screening; however, once completed, the genetic cargo can be easily modified which makes of LNP an highly flexible tool. The use of a cationic lipid has been approved in 2018 for the treatment of hereditary transthyretin-mediated amyloidosis with siRNA (Onpattro) [115].
Cationic lipids have also been used as an efficient adjuvant for a DNA vaccine encoding glycoprotein 160 of human immunodeficiency virus type-1 (HIV-1)[116] for a prophylactic vaccine against dust mite allergy [117] and as an improved alum-based vaccine formulation enhancing adjuvant activity and the synthesis of antigen-specific immunoglobulins [118]. mRNA-containing LNPs that consist of a mixture of phospholipids, cholesterol, PEGylated lipids, and cationic or ionizable lipids initiate the production and release of IL-1 by NLRP3 inflammasome activation and have been exploited for two leading vaccines against coronavirus disease in 2019 (COVID-19) [113,119].
Safety and immunostimulatory effects have to be considered when developing cationic lipid-based nanoparticles for therapeutic applications [113]. The identification of synthetic modifications for nucleic acid cargo has significantly reduced RNA-associated inflammation but an open eye has to be kept on the carrier. Cationic lipids were thought to be inert for the immune system but they are instead able to activate TLRs and in some cases the NLRP3 inflammasome [120–124]. Such an immune stimulation may be detrimental for gene therapy [113,125]. On the other hand, it is advantageous for making adjuvant-free vaccines [124].
LPN made with saturated ionizable lipids with chain length from C12 to C18 and various geometry of polyamine head (lipopolyamines) are able to activate TLR4 and TLR2 receptors and at the same time to induce an NLRP3-dependent secretion of IL-1β in not-primed murine and human macrophages [122,124,126,127]. The TLR and inflammasome stimulations, together with the antigen transport, resulted in both humoral and cellular immunity in mice vaccinated against ovalbumin without the need of adjuvants [124].
A study from Li and co-workers evaluated the influence of the spacer in lysine type lipopolyamines on NLRP3 activation. C14 lipids with a spacer of 5 (Lys5C14) or 7 (Lys7C14) induced the most potent NLRP3- and caspase1-dependent IL-1β release after 18 h of incubation with primed THP1 or primary human macrophages differentiated from human blood monocytes. The most active lipids in activating the NLRP3 inflammasome were also the best internalized by the cells and induced a lysosomal rupture, probably responsible for NLRP3 activation, whereas no ROS production was observed [127]. LDH release from transfected cells was modest and induced after longer timing of stimulation. Lipids that did not induce IL-1β release still induce LDH release, suggesting an NLRP3-independent toxicity of the lipids [127].
Lys3C16, which differs from Lys3C14 just for a change in hydrophobic tail length by two hydrocarbons, is still able to induce IL-1β release in a NLRP3- and caspase-1-dependent manner [126]. However, phagocytosis inhibition blocked both silica particles- and Lys3C14-but not Lys3C16-induced IL-1β release [126,127]. Lys3C16 did not induce lysosomal rupture and localized with the plasma membrane indicating a different but not yet identified mechanism of NLRP3 inflammasome activation with respect to Lys3C14 lipid [126].
Recent results demonstrate that several LPN containing ionizable/cationic lipids are highly inflammatory and possibly cytotoxic [128]. Side effects and symptoms observed in humans after LNP injection might be a consequence of the inflammatory response triggered by the production of cytokines such as IL-1β and IL-6 [129]. A better understanding of the structural requirements for immunostimulatory effect of cationic/ionizable lipids will contribute to get a balance between inflammatory and adjuvant properties. Intriguingly, saturated ionizable amino lipids are in the formulation of adjuvant-free vaccines such as the one used for the SARS-CoV-2 [113]. Whether these cationic lipids activate the innate immune system and the NLRP3 inflammasome, presenting adjuvanticity function, is an open question that surely merits further investigation.
6. Conclusion and open questions
Some of the best-characterized activators of innate immunity are lipids, such as the Gram-negative bacterial cell wall component (LPS). The present review highlights how endogenous host-derived lipids (oxidized lipids, cholesterol, cardiolipin, phosphatidylinositol-4-phosphate, fatty acids) and also synthetic lipids (cationic and ionizable lipids) regulate inflammasome activation and how it leads to the progression of inflammatory diseases.
Membrane proteins are usually surrounded by an annulus of lipid molecules that provide a tight protein packing into the lipid membrane. Both lipid packing and nature of the lipid contribute to regulate the protein activity. The fluidity and the thickness of the lipid membrane modulate the protein activity but some specific lipids (non-annular lipids) can achieve tight and specific interactions with the protein and act as cofactors essential to protein function [130]. In both cases such interaction requires that protein and lipid come in close contact. However this dogmatical view does not explain how and why the activity of a lipid-free multimeric structure located in the cytosol is regulated by endogenous lipids.
The lipid-regulation of NLRP3 inflammasome reveals a new mechanism in which the activated protein cannot find the specific lipid in its own membrane, as described for most membrane proteins, but has to interact with neighbor membranes (mitochondria, TGN, ER) to find it. Such a spatially regulated process brings in contact specific lipids (cardiolipin, PI4P, cholesterol) with the NLRP3 inflammasome. For instance, association with the ER allows NLRP3 to bind the cholesterol required for its activity. On the other hand, lipids such as PI4P or CL recruit the NLRP3 to a location required for its activity or for interaction with caspase-1 and ASC. It must be noted that not all the described models fit together and further investigation, paraphs with improved methods for membrane purification, is needed. Finally, accumulation of oxidized lipids (oxPAPC, oxLDL) into the cytosol are observed in pathogenic conditions. These lipids are able to activate the NLRP3 inflammasome independently of the presence of canonical stimuli, causing an exacerbated activation of the immune system and inflammatory diseases.
Regulation of the innate signaling pathways of cytosolic proteins by cell membranes and lipid-protein interactions can be spatially and temporally regulated and opens a new field of investigation that has been largely overlooked. Deciphering the role of lipids in inflammasome activation will undoubtedly contribute to the development of therapeutics to limit inflammation-related pathologies and to get a balance between inflammatory and adjuvant properties in vaccines.
Funding
MP was supported by a Fond National de la Recherche Scientifique postdoctoral fellowship (CR 32774874) and Juan de la Cierva-Formación postdoctoral fellowship (FJC2018-036217-I). The work in PP lab was supported by grant PID2020-116709RB-I00 funded by MCIN/AEI/ 10.13039/501100011033, Fundación Séneca grants 20859/PI/18, 21081/PDC/19 and 0003/COVI/20, the EU Horizon 2020 project PlasticHeal (grant 965196) and European Research Council grant ERC-2019-PoC 899636.
Footnotes
Competing interests
PP declares that he is an inventor in a patent (PCT/EP2020/056729), co-founder of Viva in vitro diagnostics SL and consultant of Glenmark Pharmaceuticals. The other authors declare no competing interests.
References
- [1].Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- [2].Mayor A, Tardivel A, Martinon F, Pe V, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- [3].Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunology. 2008;9:847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Patel MN, Carroll RG, Galván-Peña S, Mills EL, Olden R, Triantafilou M, et al. Inflammasome Priming in Sterile Inflammatory Disease. Trends in Molecular Medicine. 2017;23:165–80. doi: 10.1016/j.molmed.2016.12.007. [DOI] [PubMed] [Google Scholar]
- [7].Baker PJ, De Nardo D, Moghaddas F, Tran LS, Bachem A, Nguyen T, et al. Posttranslational Modification as a Critical Determinant of Cytoplasmic Innate Immune Recognition. Physiological Reviews. 2017;97:1165–209. doi: 10.1152/physrev.00026.2016. [DOI] [PubMed] [Google Scholar]
- [8].Andreeva L, David L, Rawson S, Shen C, Pasricha T, Pelegrin P, et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell. 2021;184:6299–6312.:e22. doi: 10.1016/J.CELL.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hochheiser Iv, Pilsl M, Hagelueken G, Moecking J, Marleaux M, Brinkschulte R, et al. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature. 2022;604:184–9. doi: 10.1038/S41586-022-04467-W. [DOI] [PubMed] [Google Scholar]
- [10].Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–4. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- [11].Martín-Sánchez F, Martínez-García JJ, Muñoz-García M, Martínez-Villanueva M, Noguera-Velasco JA, Andreu D, et al. Lytic cell death induced by melittin bypasses pyroptosis but induces NLRP3 inflammasome activation and IL-1β release. Cell Death & Disease 2017 8:8. 2017;8:e2984. doi: 10.1038/cddis.2017.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tapia-Abellán A, Angosto-Bazarra D, Alarcón-Vila C, Baños MC, Hafner-Bratkovič I, Oliva B, et al. Sensing low intracellular potassium by NLRP3 results in a stable open structure that promotes inflammasome activation. Science Advances. 2021;7 doi: 10.1126/sciadv.abf4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, et al. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity. 2018;49:56–65.:e4. doi: 10.1016/J.IMMUNI.2018.04.032/ATTACHMENT/8AC7A927-EB8A-4FEA-86D6-9B17F81F3326/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pelegrin P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochemical Pharmacology. 2021;187:114385. doi: 10.1016/J.BCP.2020.114385. [DOI] [PubMed] [Google Scholar]
- [15].Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity. 2013;38:1142–53. doi: 10.1016/J.IMMUNI.2013.05.016/ATTACHMENT/19286918-F229-415F-AF54-38EB37527F06/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Groß CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, et al. K + Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity. 2016;45:761–73. doi: 10.1016/J.IMMUNI.2016.08.010. [DOI] [PubMed] [Google Scholar]
- [17].Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–6. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Magupalli VG, Negro R, Tian Y, Hauenstein Av, di Caprio G, Skillern W, et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science (1979) 2020;369 doi: 10.1126/SCIENCE.AAS8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–7. doi: 10.1038/NATURE16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–43. doi: 10.1038/S41586-019-1295-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. Journal of Experimental Medicine. 2018;215:827–40. doi: 10.1084/jem.20172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell. 2014;156:1193–206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Magupalli VG, Negro R, Tian Y, Hauenstein AV, Di Caprio G, Skillern W, et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science (1979) 2020;369:eaas8995. doi: 10.1126/science.aas8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schmidt FI, Lu A, Chen JW, Ruan J, Tang C, Wu H, et al. A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. J Exp Med. 2016;213:771–90. doi: 10.1084/jem.20151790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607–11. doi: 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The poreforming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44.:e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956–60. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- [28].Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nature Reviews Immunology. 2020;20:143–57. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- [29].Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020;180:941–955.:e20. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- [30].Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–63. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131–6. doi: 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- [32].Torre-Minguela C, Gómez AI, Couillin I, Pelegrín P. Gasdermins mediate cellular release of mitochondrial DNA during pyroptosis and apoptosis. The FASEB Journal. 2021;35:1–16. doi: 10.1096/fj.202100085R. [DOI] [PubMed] [Google Scholar]
- [33].Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nature Immunology. 2014;15:738–48. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- [34].Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC has extracellular and “prionoid” activities that propagate inflammation. Nature Immunology. 2014;15:727–37. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science (New York, NY) 2016;352:1232–6. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science (1979) 2013;341:1246–9. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- [37].Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. European Journal of Immunology. 2015;45:2911–7. doi: 10.1002/EJI.201545523. [DOI] [PubMed] [Google Scholar]
- [38].Baker PJ, Boucher D, Bierschenk D, Tebartz C, Whitney PG, D DB, et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45:2918–26. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- [39].Kayagaki N, Warming S, Lamkanfi M, Walle L Vande, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- [40].Kayagaki N, Stowe IB, Lee BL, Rourke KO, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- [41].Deets KA, Vance RE. Inflammasomes and adaptive immune responses. Nature Immunology. 2021;22:412–22. doi: 10.1038/s41590-021-00869-6. [DOI] [PubMed] [Google Scholar]
- [42].Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197–214. doi: 10.1038/S41568-019-0123-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Missiroli S, Perrone M, Boncompagni C, Borghi C, Campagnaro A, Marchetti F, et al. Targeting the NLRP3 Inflammasome as a New Therapeutic Option for Overcoming Cancer. Cancers. 2021;13:2297. doi: 10.3390/CANCERS13102297. 2021;13:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nature Reviews Drug Discovery. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- [45].Swanson KV, Deng M, Ting JP-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews Immunology. 2019;19:477–89. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].de Torre-Minguela C, Mesa del Castillo P, Pelegrín P. The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases. Frontiers in Immunology. 2017;8:43. doi: 10.3389/fimmu.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tapia-Abellán A, Angosto-Bazarra D, Martínez-Banaclocha H, de Torre-Minguela C, Cerón-Carrasco JPJP, Pérez-Sánchez H, et al. MCC950 closes the active conformation of NLRP3 to an inactive state. Nature Chemical Biology. 2019;15:560–4. doi: 10.1038/s41589-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mensa-Vilaro A, Teresa Bosque M, Magri G, Honda Y, Martínez-Banaclocha H, Casorran-Berges M, et al. Late onset cryopyrin-associated periodic syndrome due to myeloid-restricted somatic NLRP3 mosaicism. Arthritis Rheumatol. 2016;68:3035–41. doi: 10.1002/art.39770. [DOI] [PubMed] [Google Scholar]
- [49].Bertoni A, Carta S, Baldovini C, Penco F, Balza E, Borghini S, et al. A novel knock-in mouse model of cryopyrin-associated periodic syndromes with development of amyloidosis: Therapeutic efficacy of proton pump inhibitors. J Allergy Clin Immunol. 2020;145:368–378.:e13. doi: 10.1016/j.jaci.2019.05.034. [DOI] [PubMed] [Google Scholar]
- [50].Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, et al. Inflammasome-Mediated Disease Animal Models Reveal Roles for Innate but Not Adaptive Immunity. Immunity. 2009;30:875–87.:S1074-7613(09)00233-7 [pii] doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–74.:S1074-7613(09)00232-5 [pii] doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Klück V, Jansen TLTA, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. The Lancet Rheumatology. 2020;2:e270-80. doi: 10.1016/S2665-9913(20)30065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wohlford GF, Van Tassell BW, Billingsley HE, Kadariya D, Canada JM, Carbone S, et al. Phase 1B, Randomized, Double-Blinded, Dose Escalation, Single-Center, Repeat Dose Safety and Pharmacodynamics Study of the Oral NLRP3 Inhibitor Dapansutrile in Subjects With NYHA II-III Systolic Heart Failure. J Cardiovasc Pharmacol. 2020;77:49–60. doi: 10.1097/FJC.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alarcón-Vila C, Pizzuto M, Pelegrín P. Purinergic receptors and the inflammatory response mediated by lipids. Current Opinion in Pharmacology. 2019;47:90–6. doi: 10.1016/j.coph.2019.02.004. [DOI] [PubMed] [Google Scholar]
- [55].Wymann MP, Schneiter R. Lipid signalling in disease. Nature Reviews Molecular Cell Biology. 2008;9:162–76. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- [56].Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, et al. Cutting Edge: Mitochondrial Assembly of the NLRP3 Inflammasome Complex Is Initiated at Priming. The Journal of Immunology. 2018;200:3047–52. doi: 10.4049/jimmunol.1701723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ohto U, Kamitsukasa Y, Ishida H, Zhang Z, Murakami K, Hirama C, et al. Structural basis for the oligomerization-mediated regulation of NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/PNAS.2121353119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].de la Roche M, Hamilton C, Mortensen R, Jeyaprakash AA, Ghosh S, Anand PK. Trafficking of cholesterol to the ER is required for NLRP3 inflammasome activation. Journal of Cell Biology. 2018;217:3560–76. doi: 10.1083/JCB.201709057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ruysschaert JM, Lonez C. Role of lipid microdomains in TLR-mediated signalling. Biochimica et Biophysica Acta - Biomembranes. 2015;1848:1860–7. doi: 10.1016/j.bbamem.2015.03.014. [DOI] [PubMed] [Google Scholar]
- [60].Angosto-Bazarra D, Molina-López C, Peñín-Franch A, Hurtado-Navarro L, Pelegrín P. Techniques to Study Inflammasome Activation and Inhibition by Small Molecules. Molecules. 2021;26:1704. doi: 10.3390/molecules26061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Platt N, Speak AO, Colaco A, Gray J, Smith DA, Williams IM, et al. Immune dysfunction in Niemann-Pick disease type C. Journal of Neurochemistry. 2016;136:74–80. doi: 10.1111/jnc.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, et al. Mitochondrial cardiolipin is required for NLRP3 inflammasome activation. Immunity. 2013;39:311–23. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pizzuto M, Pelegrin P. Cardiolipin in Immune Signaling and Cell Death. Trends in Cell Biology. 2020;30:892–903. doi: 10.1016/j.tcb.2020.09.004. [DOI] [PubMed] [Google Scholar]
- [64].Tapia-Abellán A, Angosto-Bazarra D, Alarcón-Vila C, Baños MC, Hafner-Bratkovič I, Oliva B, et al. Sensing low intracellular potassium by NLRP3 results in a stable open structure that promotes inflammasome activation. Science Advances. 2021;7 doi: 10.1126/sciadv.abf4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. Journal of Lipid Research. 2009;50:S207. doi: 10.1194/JLR.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aguilar-Ballester M, Herrero-Cervera A, Vinué Á, Martínez-Hervás S, González-Navarro H. Impact of cholesterol metabolism in immune cell function and atherosclerosis. Nutrients. 2020;12:1–19. doi: 10.3390/nu12072021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Corrêa R, Silva LFF, Ribeiro DJS, das Almeida RN, de Santos IO, Corrêa LH, et al. Lysophosphatidylcholine Induces NLRP3 Inflammasome-Mediated Foam Cell Formation and Pyroptosis in Human Monocytes and Endothelial Cells. Frontiers in Immunology. 2020;10:2927. doi: 10.3389/fimmu.2019.02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Grebe A, Latz E. Cholesterol Crystals and Inflammation. Curr Rheumatol Rep. 2013;15:313. doi: 10.1007/S11926-012-0313-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zanoni I, Tan Y, di Gioia M, Broggi A, Ruan J, Shi J, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–6. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, Monack DM, et al. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nature Communications. 2018;9:996. doi: 10.1038/s41467-018-03409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. Journal of Experimental Medicine. 2004;200:1359–70. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. Journal of Biological Chemistry. 2002;277:38503–16. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- [74].Watson AD, Subbanagounder G, Welsbie DS, Faull KF, Navab M, Jung ME, et al. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. Journal of Biological Chemistry. 1999;274:24787–98. doi: 10.1074/jbc.274.35.24787. [DOI] [PubMed] [Google Scholar]
- [75].Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–46. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- [76].Yeon SH, Yang G, Lee HE, Lee JY. Oxidized phosphatidylcholine induces the activation of NLRP3 inflammasome in macrophages. Journal of Leukocyte Biology. 2017;101:205–15. doi: 10.1189/jlb.3vma1215-579rr. [DOI] [PubMed] [Google Scholar]
- [77].Oskolkova Ov, Afonyushkin T, Preinerstorfer B, Bicker W, von Schlieffen E, Hainzl E, et al. Oxidized Phospholipids Are More Potent Antagonists of Lipopolysaccharide than Inducers of Inflammation. The Journal of Immunology. 2010;185:7706–12. doi: 10.4049/jimmunol.0903594. [DOI] [PubMed] [Google Scholar]
- [78].Liu-Wu Y, Hurt-Camejo E, Wiklund O. Lysophosphatidylcholine induces the production of IL-1β by human monocytes. Atherosclerosis. 1998;137:351–7. doi: 10.1016/S0021-9150(97)00295-5. [DOI] [PubMed] [Google Scholar]
- [79].Stock C, Schilling T, Schwab A, Eder C. Lysophosphatidylcholine Stimulates IL-1β Release from Microglia via a P2X 7 Receptor-Independent Mechanism. The Journal of Immunology. 2006;177:8560–8. doi: 10.4049/jimmunol.177.12.8560. [DOI] [PubMed] [Google Scholar]
- [80].Rajamäki K, Lappalainen J, Öörni K, Välimäki E, Matikainen S, Kovanen PT, et al. Cholesterol Crystals Activate the NLRP3 Inflammasome in Human Macrophages: A Novel Link between Cholesterol Metabolism and Inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cao Q, Liu Z, Xiong Y, Zhong Z, Ye Q. Multiple Roles of 25-Hydroxycholesterol in Lipid Metabolism, Antivirus Process, Inflammatory Response, and Cell Survival. Oxidative Medicine and Cellular Longevity. 2020;2020 doi: 10.1155/2020/8893305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jang J, Park S, Jin Hur H, Cho HJ, Hwang I, Pyo Kang Y, et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nature Communications. 2016;7 doi: 10.1038/ncomms13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reboldi A, Dang Ev, McDonald JG, Liang G, Russell DW, Cyster JG. 25-hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science (1979) 2014;345:679–84. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Alegría Ezquerra E, Castellano Vázquez JM, Alegría Barrero A. Obesity, Metabolic Syndrome and Diabetes: Cardiovascular Implications and Therapy. Revista Española de Cardiología. 2008;61:752–64. doi: 10.1016/S1885-5857(08)60212-1. [DOI] [PubMed] [Google Scholar]
- [85].Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine. 2011;17:179–89. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Luheshi NM, Giles JA, Lopez-Castejon G, Brough D. Sphingosine regulates the NLRP3-inflammasome and IL-1β release from macrophages. European Journal of Immunology. 2012;42:716–25. doi: 10.1002/eji.201142079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity. 2013;38:1154–63. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- [88].L’homme L, Esser N, Riva L, Scheen A, Paquot N, Piette J, et al. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. Journal of Lipid Research. 2013;54:2998–3008. doi: 10.1194/jlr.M037861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gianfrancesco MA, Dehairs J, L’homme L, Herinckx G, Esser N, Jansen O, et al. Saturated fatty acids induce NLRP3 activation in human macrophages through K + efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2019;1864:1017–30. doi: 10.1016/j.bbalip.2019.04.001. [DOI] [PubMed] [Google Scholar]
- [90].Karasawa T, Kawashima A, Usui-Kawanishi F, Watanabe S, Kimura H, Kamata R, et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38:744–56. doi: 10.1161/ATVBAHA.117.310581. [DOI] [PubMed] [Google Scholar]
- [91].Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT-H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology. 2011;12:408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].de Carvalho RVH, Andrade WA, Lima-Junior DS, Dilucca M, de Oliveira Cv, Wang K, et al. Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome. Cell Reports. 2019;26:429–437.:e5. doi: 10.1016/j.celrep.2018.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, et al. Caspase-11 protects against bacteria that escape the vacuole. Science (1979) 2013;339:975–8. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Santos JC, Dick MS, Lagrange B, Degrandi D, Pfeffer K, Yamamoto M, et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. The EMBO Journal. 2018;37 doi: 10.15252/embj.201798089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science (1979) 2013;341:1250–3. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity. 2018;0:1–14. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Santos JC, Broz P. Sensing of invading pathogens by GBPs: At the crossroads between cell-autonomous and innate immunity. Journal of Leukocyte Biology. 2018;104:729–35. doi: 10.1002/JLB.4MR0118-038R. [DOI] [PubMed] [Google Scholar]
- [98].Meunier E, Dick MS, Dreier RF, Schürmann N, Broz DK, Warming S, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–70. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- [99].Lagrange B, Benaoudia S, Wallet P, Magnotti F, Provost A, Michal F, et al. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat Commun. 2018;9 doi: 10.1038/S41467-017-02682-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gay NJ, Gangloff M. Structure and function of {Toll} receptors and their ligands. Annual Review of Biochemistry. 2007;76:141–65. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- [101].Santos JC, Boucher D, Schneider LK, Demarco B, Dilucca M, Shkarina K, et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat Commun. 2020;11 doi: 10.1038/S41467-020-16889-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wandel MP, Kim BH, Park ES, Boyle KB, Nayak K, Lagrange B, et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat Immunol. 2020;21:880–91. doi: 10.1038/S41590-020-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Brubaker SW, Brewer SM, Massis LM, Napier BA, Monack DM. A Rapid Caspase-11 Response Induced by IFNγ Priming Is Independent of Guanylate Binding Proteins. IScience. 2020;23 doi: 10.1016/J.ISCI.2020.101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pizzuto M, Hurtado-Navarro L, Molina-Lopez C, Soubhye J, Gelbcke M, Rodriguez-Lopez S, et al. Ornithine Lipid Activates Both TLR4 and the non-canonical NLRP3 Inflammasome. BioRxiv. 2022:2022.01.28.477396. doi: 10.1101/2022.01.28.477396. [DOI] [Google Scholar]
- [105].Kim SK, Park SJ, Li XH, Choi YS, Im DS, Lee JH. Bacterial ornithine lipid, a surrogate membrane lipid under phosphate-limiting conditions, plays important roles in bacterial persistence and interaction with host. Environmental Microbiology. 2018;20:3992–4008. doi: 10.1111/1462-2920.14430. [DOI] [PubMed] [Google Scholar]
- [106].Barbosa LC, Goulart CL, Avellar MM, Bisch PM, Von Kruger WMA. Accumulation of ornithine lipids in Vibrio cholerae under phosphate deprivation is dependent on VC0489 (OlsF) and PhoBR system. Microbiology (United Kingdom) 2018;164:395–9. doi: 10.1099/mic.0.000607. [DOI] [PubMed] [Google Scholar]
- [107].Vences-Guzmán MÁ, Geiger O, Sohlenkamp C. Ornithine lipids and their structural modifications: From A to E and beyond. FEMS Microbiology Letters. 2012;335:1–10. doi: 10.1111/j.1574-6968.2012.02623.x. [DOI] [PubMed] [Google Scholar]
- [108].Córdoba-Castro LA, Salgado-Morales R, Torres M, Martínez-Aguilar L, Lozano L, Vences-Guzmán MÁ, et al. Ornithine Lipids in Burkholderia spp. Pathogenicity. Frontiers in Molecular Biosciences. 2021;7:457. doi: 10.3389/fmolb.2020.610932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Okemoto K, Hanada K, Nishijima M, Kawasaki K. The preparation of a lipidic endotoxin affects its biological activities. Biological and Pharmaceutical Bulletin. 2008;31:1952–4. doi: 10.1248/bpb.31.1952. [DOI] [PubMed] [Google Scholar]
- [110].Kawai Y, Akagawa K. Macrophage activation by an ornithine-containing lipid or a serine-containing lipid. Infection and Immunity. 1989;57:2086–91. doi: 10.1128/iai.57.7.2086-2091.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kawai Y, Kaneda K, Morisawa Y, Akagawa K. Protection of mice from lethal endotoxemia by use of an ornithine-containing lipid or a serine-containing lipid. Infection and Immunity. 1991;59:2560–6. doi: 10.1128/iai.59.8.2560-2566.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lonez C, Vandenbranden M, Ruysschaert J-M. Cationic liposomal lipids: from gene carriers to cell signaling. Prog Lipid Res. 2008;47:340–7. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [113].Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Advanced Drug Delivery Reviews. 2020;159:344. doi: 10.1016/J.ADDR.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated {DNA}-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nature Nanotechnology. 2019;14:1084–7. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- [116].S S, F J, A H, K KI, H K, I N, et al. Human immunodeficiency virus type-1-specific immune responses induced by DNA vaccination are greatly enhanced by mannan-coated diC14-amidine. Eur J Immunol. 1997;27:3121–9. doi: 10.1002/EJI.1830271207. [DOI] [PubMed] [Google Scholar]
- [117].Jacquet A, Vanderschrick JF, Vandenbranden M, Elouahabi A, Magi M, Garcia L, et al. Vaccination with the recombinant allergen ProDer p 1 complexed with the cationic lipid DiC14-amidine prevents allergic responses to house dust mite. Molecular Therapy. 2005;11:960–8. doi: 10.1016/j.ymthe.2004.12.024. [DOI] [PubMed] [Google Scholar]
- [118].Wilmar A, Lonez C, Vermeersch M, Andrianne M, Pérez-Morga D, Ruysschaert JM, et al. The cationic lipid, diC14 amidine, extends the adjuvant properties of aluminum salts through a TLR-4- and caspase-1-independent mechanism. Vaccine. 2012;30:414–24. doi: 10.1016/j.vaccine.2011.10.071. [DOI] [PubMed] [Google Scholar]
- [119].Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23:532–42. doi: 10.1038/S41590-022-01160-Y. [DOI] [PubMed] [Google Scholar]
- [120].Pizzuto M, Gangloff M, Scherman D, Gay NJNJ, Escriou V, Ruysschaert J-MJ-M, et al. Toll-like receptor 2 promiscuity is responsible for the immunostimulatory activity of nucleic acid nanocarriers. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2016;247:182–93. doi: 10.1016/j.jconrel.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lonez C, Irvine KL, Pizzuto M, Schmidt BI, Gay NJ, Ruysschaert J-M, et al. Critical residues involved in Toll-like receptor 4 activation by cationic lipid nanocarriers are not located at the lipopolysaccharide-binding interface. Cellular and Molecular Life Sciences. 2015;72:3971–82. doi: 10.1007/s00018-015-1915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lonez C, Bessodes M, Scherman D, Vandenbranden M, Escriou V, Ruysschaert JM. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine: Nanotechnology, Biology, and Medicine. 2014;10:775–82. doi: 10.1016/j.nano.2013.12.003. [DOI] [PubMed] [Google Scholar]
- [123].Lonez C, Lensink MF, Vandenbranden M, Ruysschaert JM. Cationic lipids activate cellular cascades. Which receptors are involved? Biochimica et Biophysica Acta - General Subjects. 2009;1790:425–30. doi: 10.1016/j.bbagen.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [124].Pizzuto M, Bigey P, Lachagès A-M, Hoffmann C, Ruysschaert J-M, Escriou V, et al. Cationic lipids as one-component vaccine adjuvants: A promising alternative to alum. Journal of Controlled Release. 2018;287:67–77. doi: 10.1016/j.jconrel.2018.08.020. [DOI] [PubMed] [Google Scholar]
- [125].Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Human Gene Therapy. 1997;8:2019–29. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- [126].He J, Li T, Próchnicki T, Horvath G, Latz E, Takeoka S. Membrane fusogenic lysine type lipid assemblies possess enhanced NLRP3 inflammasome activation potency. Biochemistry and Biophysics Reports. 2019;18:100623. doi: 10.1016/j.bbrep.2019.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li T, He J, Horvath G, Próchnicki T, Latz E, Takeoka S. Lysine-containing cationic liposomes activate the NLRP3 inflammasome: Effect of a spacer between the head group and the hydrophobic moieties of the lipids. Nanomedicine: Nanotechnology, Biology, and Medicine. 2018;14:279–88. doi: 10.1016/j.nano.2017.10.011. [DOI] [PubMed] [Google Scholar]
- [128].Samaridou E, Heyes J, Lutwyche P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Advanced Drug Delivery Reviews. 2020;154–155:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- [129].Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunological Reviews. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta. 2003;1612:1–40. doi: 10.1016/S0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]