Abstract
Background
Asthma is an inflammatory disease of the airway showing a strong time of day rhythm. Airway hyperresponsiveness is a dominant feature of asthma, but it is not known if this is under clock control. The circadian clock powerfully regulates inflammation. The clock protein REV-ERBα is known to play a key role as a repressor of the inflammatory response.
Objectives
To determine if allergy mediated airway hyperresponsiveness is gated by the clock protein, REV-ERBα.
Methods
After exposure to the intra-nasal house dust mite allergen challenge model at either dawn or dusk, airway hyper-responsiveness to methacholine was measured invasively in mice.
Main Results
Wild-type mice showed marked time-of-day differential responses of airway hyper-responsiveness (maximal at dusk, start of the active phase), both in vivo and ex vivo in precision cut lung slices. Hyper-responsive time of day effects were abolished in mice lacking the clock gene Rev-erbα, indicating that time-of-day effects on asthma responses are likely mediated via the circadian clock. We suggest that muscarinic receptors 1 and 3 (Chrm 1, 3) may play a role in this pathway.
Conclusions
We identify a novel circuit regulating a core process in asthma, potentially involving circadian control of muscarinic receptor expression, in a REV-ERBα dependent fashion.
Keywords: Body clock, airway hyperresponsiveness, muscarinic
Introduction
Asthma, is a chronic inflammatory disease of the airways and displays strong circadian rhythmicity (1–4). Asthma-associated mortality is strongly time-of-day dependent, peaking overnight between midnight and 08:00 (5). Airway hyper-responsiveness (AHR), a cardinal feature of asthma (6, 7) is increased sensitivity of the airways to bronchoconstrictor challenge, such as methacholine; clinically, AHR is useful in diagnosing asthma. There is diurnal variation in AHR in asthma, with a peak around 04:00, the time of maximal disease expression (8–13). Potential causes for this diurnal change in AHR in asthma remain undefined; but may be important for improved asthma treatment.
Circadian rhythms are generated by a molecular clock, expressed in virtually all cells. A central clock in the suprachiasmatic nucleus of the brain synchronises peripheral tissue clocks via neural and humoral mediators. The cellular circadian molecular clock consists of a positive arm — CLOCK and BMAL1 heterodimers — driving transcription of 2 inhibitory arms — PER/CRY and REV-ERBα/REV-ERBβ, which feedback to inhibit BMAL1/CLOCK heterodimer transactivation function (16). The circadian clock powerfully regulates inflammation (17–19). REV-ERBα plays a key role as a repressor of the inflammatory response (20).
Here we explored the biology of REV-ERBα and address whether this protein acts as a circadian mediator, gating AHR following allergic challenge. Using the house dust mite (HDM (19)) mouse model for allergic airways disease (20), and an in-vitro lung slice model, we investigated the role of airway smooth muscle in the circadian gating of AHR. We find that time-of-day effects in AHR following allergen challenge are ablated in REV-ERBα-deficient mice, as is rhythmic expression of key muscarinic receptor sub-classes, mediating cholinergic smooth-muscle responses. Thus, we identify a pathway linking the core cellular clock, through REV-ERBα to airway reactivity, smooth muscle tone, and airway narrowing.
Methods
Animals
All experimental procedures were carried out in accordance with the Animals (Scientific Procedures) Act, 1986. Rev-erbα-/- mice were provided by Ueli Schibler (University of Geneva) (21). Wild type control C57/Bl6J mice and Rev-erbα-/- mice were individually housed in 12:12 light/dark cycles. Zeitgeber time (ZT) 0 is when lights are turned on in the animal house, and ZT 12 is when lights are switched off. Female C57/Bl6 mice aged 8-12 weeks were used in all experiments.
Asthma Protocol-House Dust Mite (HDM)
Mice were exposed intra-nasally (i.n), to 25μg of HDM (Citeq Biologics; Batch No. 15J02) protein in 25μl of PBS under anaesthesia for 5 days/week for 3 weeks (22). Control mice received i.n PBS. One group of mice received HDM/PBS at ZT11 (just before lights off/start of active phase); a second group received HDM/PBS at ZT23 (just before lights on/start of rest phase).
Measurement of AHR
Airway resistance was measured 24 h after the final HDM exposure, in response to increasing concentrations of methacholine (3-100mg/ml, Sigma, UK), using flexiVent small animal ventilator (SciReq (23)) as previously described (24).
Collection of Serum
Blood samples (BD®Microtainer, Becton, Dickinson and Company) were placed on ice for one hour, then centrifuged to derive serum (5minutes, 7000rpm).
Bronchoalveolar Lavage (BAL) and Lung Digest
BAL was performed immediately after measurement of AHR (25). The right inferior and post-caval lobes were taken for lung digest (26). Lung cells were analysed by flow cytometry (24).
Histology
Following BAL, the left lung was taken for histology (26). For H&E stained slides a semi-quantitative scoring system graded the size of lung infiltrates (27). Goblet cells were counted on Periodic Acid-Schiff (PAS) stained lung sections using an arbitrary scoring system (28).
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
RNA was extracted from the right middle lobe (ReliaPrep™ RNA Miniprep Systems (Promega, #Z6011)) and reverse-transcribed (GoScript™ Reverse Transcription System (Promega, #A5001), before qPCR analysis (KAPA SYBR FAST qPCR Master Mix (2×) Universal Kit (KAPA Biosystems, #KK4601)). Relative gene expression was determined via normalization to Gapdh. Primers used: Qiagen-ADRB1 (QT00258692), ADRB2 (QT00253967), Chrm 1 (QT00282527), Chrm 2 (QT00290297), Chrm 3, GAPDH (QT01658692)and primer sequences-Nr1d1 (F GTCTCTCCGTTGGCATGTCT, R CCAAGTTCATGGCGCTCT) and Bmal1 (F CCAAGAAAGTATGGACACAGACAAA, R GCATTCTTGATCCTTCCTTGGT).
ELISA
Serum was analysed for anti-HDM IgE as per manufacturer’s instructions (Thermo Fisher, #EMIGHE and Chrondrex, Inc #3037).
Bioplex
BAL fluid was analysed using Bio-Plex Pro ™ Mouse Chemokine Panel 33-Plex (Bio-Rad, #12002231), on Bio-Rad Bio-Plex 200 system.
Lung Slice Model
Precision-cut ectopic lung slices (175 μm) were prepared (29). Slices placed on cell culture inserts (Millicell) were imaged using Nikon Long-Term Time Lapse microscope (Eclipse Ti Inverted). Airways were imaged in response to methacholine (0-100μM). Airway size was quantified using ImageJ software (v1.41o). Airway contraction was measured as percentage decrease from baseline.
Statistical Analysis
Linear mixed effects modelling was used to determine how AHR changes with increasing doses of nebulised methacholine.
Other data was analysed by 1-way ANOVA, followed by Tukey’s multiple comparison test and represented as median (± IQR). IgE data and change in AHR after 75mg/ml methacholine was analysed by Mann Whitney U tests. Serum IgE, AHR and PCR data is represented as mean (± SEM).
For the precision-cut lung slice model, methacholine dose response curves were fitted to a three-parameter sigmoidal dose-response curve. An extra sum-of-squares F-test was used to test whether one curve could adequately fit the data for ZT11 and ZT23.
Results
AHR varies by time of day of HDM allergen challenge
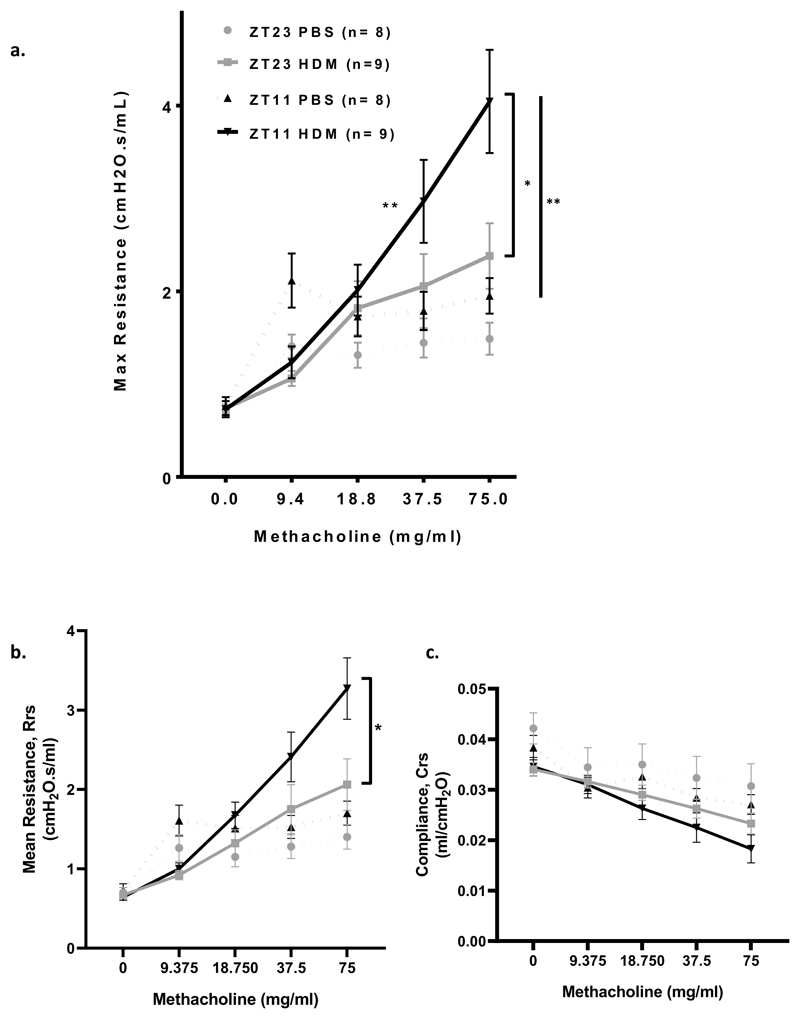
The time of day at which wild-type (WT) mice are challenged with HDM, significantly impacts the resultant AHR (Fig 1, a). WT mice were challenged with HDM at either ZT11 (just before lights off and the start of the active phase for mice), or at ZT23 (just before lights on and the start of the rest phase in mice). WT mice challenged with HDM at ZT11 (and in which maximal airway resistance was recorded 24 hours later at ZT11) showed a significant increase in the slope of the methacholine dose response curve compared to mice challenged at ZT23 (Fig 1, a, P= 0.005), indicating a significant time of challenge effect and suggesting increased sensitivity of the airway to the effects of methacholine after HDM challenge at ZT11 compared to at ZT23. This was also the case when airway resistance was measured as area under the curve (Rrs) (Supplementary Fig 1, a, P=0.005)
Fig 1. HDM challenge at ZT11 (just before start of the active phase) results in a significant increase in AHR compared to challenge at ZT23.
a. Airway hyperresposiveness to increasing doses of methacholine was measured as maximum airway resistance (mean ± SEM) using Flexivent in WT mice at ZT11 and ZT23. The slope of the dose response curve for methacholine is significantly increased after HDM challenge at ZT11 compared to at ZT23 (*P= 0.005), mixed linear modelling. WT mice challenged with HDM exhibited increased airway resistance after 75mg/ml methacholine at both ZT11 and ZT23 compared to control mice treated with PBS (‡P =0.007 for ZT11 and P = 0.055 for ZT23), Mann Whitney U. Maximal airway resistance was significantly higher after 75mg/ml of methacholine in WT mice challenged with HDM at ZT11 compared to at ZT23 (†P=0.05), Mann Whitney U. Baseline airway resistance was higher in control (PBS treated) mice at ZT11 compared to at ZT23.
b. Airway hyperresponsiveness to increasing doses of methacholine was measured as mean reistance (Rrs) (mean ± SEM) in WT mice at ZT11 and ZT23. WT mice challenged at ZT11 exhibited significantly increased mean resistance after 75mg/ml methacholine, compared to at Zt23 (*P=0.03) Mann Whitney U.
c. Compliance (Crs) to increasing doses of methacholine was measured (mean ± SEM) using FlexiVent in WT mice at ZT11 and ZT23. There was a reduction in compliance in both HDM and PBS challenged mice. There was no time of day difference. Mann Whitney U.
Solid lines-HDM treated; dotted lines-PBS treated mice. Grey lines-ZT23 and black lines indicate ZT11.
WT mice challenged with HDM exhibited increased airway resistance after 75mg/ml methacholine at both ZT11 and ZT23 compared to control mice treated with PBS (P =0.007 for ZT11 and P = 0.055 for ZT23). Maximal airway resistance was significantly higher after 75mg/ml of methacholine in WT mice challenged with HDM at ZT11 compared to at ZT23 (Fig 1, a, P=0.05). This was also the case for mean resistance (Rrs) (Fig 1, b, P=0.03). There were no differences in lung compliance between groups (Fig 1, c).
Airway and lung inflammation reveal no time of challenge differences
Next, we examined BAL to determine whether time of day differences in AHR were associated with airway inflammatory changes. There was a significant increase in BAL total cells from mice treated with HDM at ZT11, compared to controls, but not at ZT23. There was no difference by time of challenge (Table 1). BAL eosinophils significantly increased following HDM challenge at both ZT11 and at ZT23 compared to control. No time of challenge differences were seen for differential BAL cell types. BAL macrophages significantly reduced at ZT23 after HDM challenge. HDM-specific IgE significantly increased after HDM challenge in WT mice; there was no time of challenge difference, indicating similar sensitisation, and acquisition of adaptive immunity (Supplementary Fig1, b).
Table 1. Bronchoalveolar lavage fluid differential cell counts after HDM challenge at either ZT11 or ZT23 reveal no time of challenge differences.
| BAL Immune Cell type | Challenge | ZT11 | ZT23 | Adjusted P value |
|---|---|---|---|---|
| Eosinophils % of total cells Median (IQR) | PBS | 2.36 (0.55-6.28) | 3.1 (2.49-4.39) | 1.0 |
| HDM | 25.5 (19.6-50.8)* | 46.5 (18-50.2)* | 1.0 | |
| Neutrophils % of total cells Median (IQR) | PBS | 8.7 (1.35-11.58) | 3.29 (.05-5.62) | 1.0 |
| HDM | 25.65 (14.53-48.45) | 32 (15.6-50.8) | 1.0 | |
| Macrophages % of total cells Median (IQR) | PBS | 28.6 (17.4-46.05) | 67 (63.35-73.6) | 1.0 |
| HDM | 7.03 (5.45-12.45) | 5.52 (1.11-9.59)* | 1.0 | |
| Lymphocytes % of total cells Median (IQR) | PBS | 36.5 (26.48-48.33) | 20.0 (14.58-22.13) | 1.0 |
| HDM | 21.7 17.35-27.15) | 17.3 (11.72-19.9) | 1.0 | |
| Total Cells ×105/ml Median (IQR) | PBS | 0.75 (0.46-1.32) | 1.134 (0.72-2.83) | 0.97 |
| HDM | 4.36 (3.48-15.2)** | 4.09 (2.73-8.12) | 0.94 |
PBS v HDM, p≤ 0.05
PBS v HDM P≤0.01
BAL total cells increased after HDM at ZT11, compared to controls, but not at ZT23. There was no difference by time of challenge (P≤ 0.01). BAL eosinophils significantly increased following HDM challenge at both ZT11 and at ZT23 compared to PBS control (P≤0.05 and P≤ 0.05) No time of challenge differences were seen for differential BAL cell types. There was a significant reduction in BAL macrophages at ZT23 after HDM compared to PBS (P≤ 0.05). Asterixes indicate difference between HDM and PBS challenge. Median ± IQR; 1 way ANOVA, followed by Tukey multiple comparison adjustment, n=9-11 per treatment group.
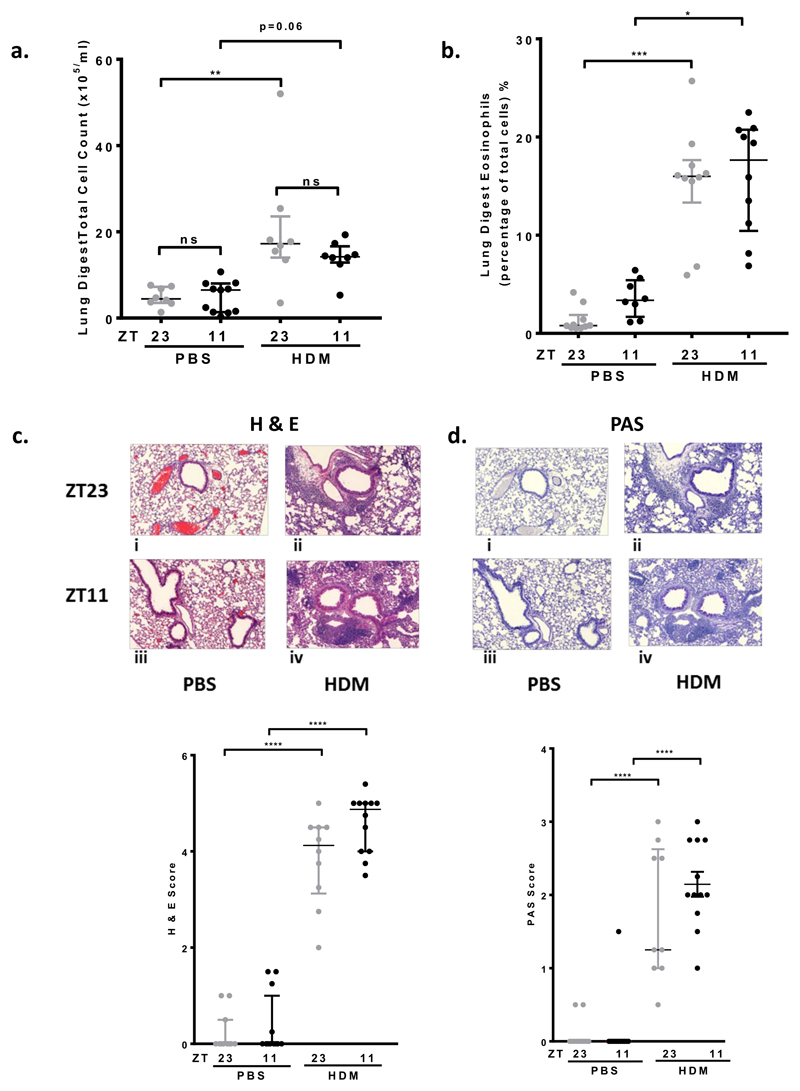
Next we analysed inflammatory cells present in lung digests. There was an increase in total immune cell content after HDM challenge, but this only reached significance at ZT23. There was no time of HDM challenge difference (Fig2, a). There was a significant increase in lung eosinophils after HDM challenge, but again no time of challenge difference (Fig2, b).
Fig 2. Eosinophilic lung inflammation increases after HDM challenge; but no real time of challenge differences.
a. Total cell count from lung digests in WT mice. There was an increase in total cell count after HDM challenge, compared to control (** P < 0.01 at ZT23, and P=0.06 at ZT11). There was no time of challenge difference after PBS or HDM challenge. Data is presented as median ± IQR. 1 way ANOVA followed by Tukey’s multiple comparison test, (n=8-11 per treatment group).
b. Eosinophils in lung digests from WT mice significantly increased after HDM challenge (*** P < 0.001 at ZT23 and P < 0.05 at ZT11); there was no time of challenge difference after PBS or HDM challenge. Data is presented as median ± IQR. 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=8-11 per treatment group).
c. HDM challenge at ZT11 and ZT23 caused predominantly eosinophilic inflammation around the bronchioles and blood vessels (haemoatoxylin and eosin (H&E) staining, i-iv) compared to PBS challenge (**** P < 0.0001 at ZT23 and ZT11). There was no time of challenge difference after PBS or HDM challenge. Data is presented as median ± IQR. 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=10-12 per treatment group).
d. Periodic Shift Staining (PAS) shows increased mucus present on the bronchial epithelium in the lungs of WT mice treated with HDM at both ZT11 and ZT23. There was no PAS staining seen in PBS treated mice (i-iv). There was significantly increased PAS scores in HDM challenged mice (**** P < 0.001 at ZT23 and ZT11), compared to controls, but no time of challenge differences. Data is presented as median ± IQR. 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=10-12 per treatment group).
Histology revealed a significant increase in eosinophil infiltration around the bronchioles and blood vessels within the lung following HDM challenge, compared to control mice. However, there was no time of challenge difference (Fig2, c). There was increased mucus in PAS stained lung sections after HDM challenge compared to control, but no time of challenge difference in PAS scores (Fig2, d).
REV-ERBα is a negative repressor of AHR
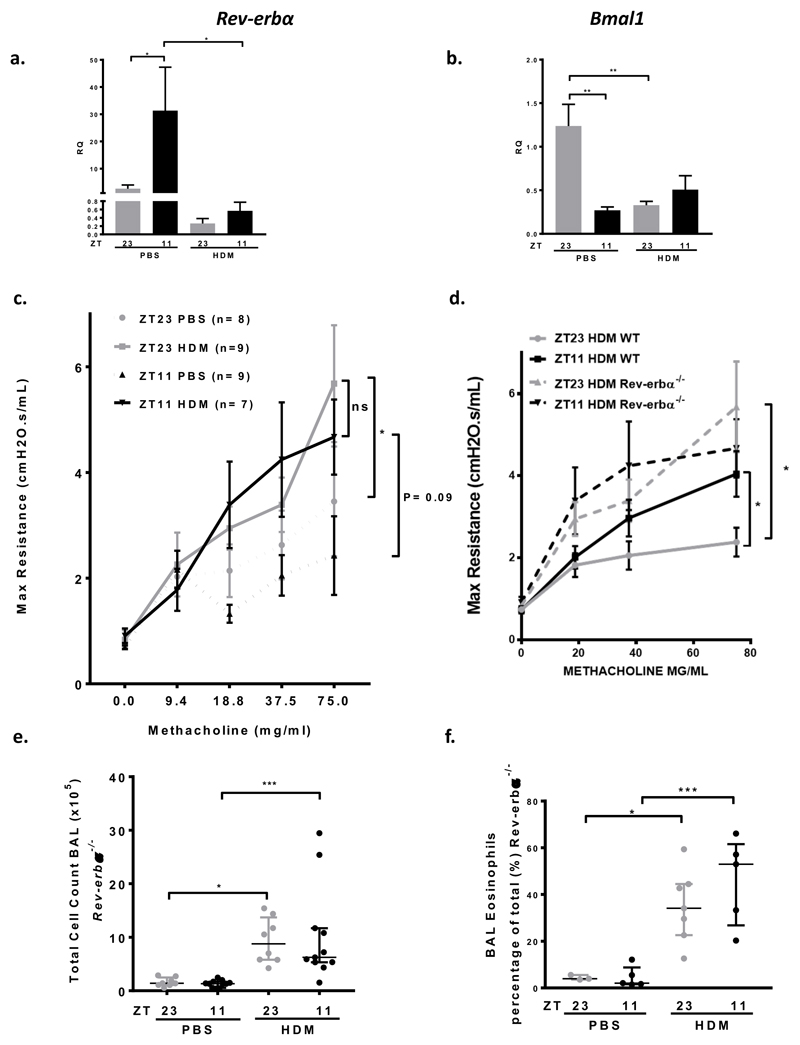
We then investigated the function of two components of the molecular clock within the lungs of WT mice. We focussed on BMAL1, the only non-redundant clock component, and the major element of the positive arm of the clock. BMAL1 has been implicated in the circadian control of inflammation (22–27). We also studied REV-ERBα, a component of the negative arm of the clock, and a known regulator of inflammation (16, 18), and itself repressed by inflammation. Bmal1 expression is in antiphase to Rev-erbα expression in PBS treated mice (Fig3, a, b). There was a significant time of day difference in Rev-erbα expression at baseline, with high levels of Rev-erbα expression at ZT11, close to the predicted circadian peak of expression, and low levels of expression at ZT23 (Fig3, a). After HDM challenge there is reduced expression of both Rev-erbα and Bmal1, and a loss of the time of day of expression within the lung (Fig3, a, b).
Fig 3. REV-ERBα is a negative repressor of AHR.
a. Expression of Rev-erbα in WT murine lung tissue, relative to the expression of Gapdh. There was a time of day difference in the expression of Rev-erbα in PBS challenged mice; Rev-erbα expression at ZT11 was significantly greater than at ZT 23 (* P < 0.05). This time of day difference was lost after HDM challenge; there was a reduction in expression of Rev-erbα at both challenge times (* P < 0.05 at ZT 11). 1 way ANOVA, followed by Tukey’s multiple comparison test. Data is presented as mean ± SEM (n=5-7 per treatment group, in duplicate). Grey bars-ZT23, Black bars ZT11.
b. Expression of Bmal1 in WT murine lung tissue, relative to the expression of Gapdh. There was a time of day difference in the expression of Bmal1 in PBS challenged mice; Bmal1 expression at ZT23 was significantly greater than at ZT11 (** P < 0.01), in anti-phase to Rev-erbα expression. This time of day difference was lost after HDM challenge; there was a reduction in expression of Bmal1 at both challenge times (** P < 0.01 at ZT23). 1 way ANOVA, followed by Tukey’s multiple comparison test. Data is presented as mean ± SEM (n=7-11 per treatment group, in duplicate).
c. Airway hyperresponsiveness to increasing doses of methacholine was measured in Rev-erbα-/- mice, as maximum airway resistance using Flexivent. There was no difference in the slopes of the methacholine dose response curves between Rev-erbα-/- mice challenged with HDM at either ZT11 or ZT23 (mixed linear modelling). There was no difference in the maximal AHR measured after 75mg/ml of methacholine in Rev-erbα-/- mice after challenge at ZT11 and ZT23 (Mann Whitney U). Mice challenged with HDM exhibited increased airway resistance after 75mg/ml methacholine at ZT11 (P=0.09) and at ZT23 (* P=0.03) compared to control (PBS challenged) mice, Mann Whitney U test. In Rev-erbα-/- mice baseline airway resistance is higher in control (PBS treated) mice at ZT23 compared to ZT11. This is in anti-phase to WT mice (Fig 1). Data is presented as mean± SEM. Grey lines-ZT23, HDM challenged, grey dotted line-ZT23, PBS control, black line-ZT11, HDM challenged and black dotted line-ZT11, PBS control.
d. AHR was significantly increased in Rev-erbα-/- mice after challenge with HDM compared to WT controls. After HDM challenge at ZT23 there was a significant increase in the maximal response to methacholine in Rev-erbα-/- mice compared to WT mice (* P < 0.05) mean ± SEM (n=7-9 per treatment group), 1 way ANOVA, followed by Tukey multiple comparison adjustment. Grey lines-ZT23 HDM challenged WT, black line-ZT11, HDM challenged WT, grey dotted line-HDM challenged ZT23 Rev-erbα-/-, and black dotted line-HDM challenged ZT11 Rev-erbα-/-.
e. Total cells recovered from BAL fluid from Rev-erbα-/- mice significantly increased after HDM challenge at both times, compared to control, PBS challenged mice (* P < 0.05 ZT23, ** P < 0.01 ZT11). There was no time of challenge difference in total cells in BAL in either PBS challenged or HDM challenged groups. 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=8-12 per treatment group). Data is presented as median ± IQR.
f. BAL eosinophils (measured as a percentage of the total) significantly increased after HDM challenge at both times in Rev-erbα-/- mice, compared to PBS challenged control mice (* P < 0.05 ZT23, *** P < 0.001 ZT11). There was no time of challenge difference in percentage eosinophils in BAL in either PBS challenged or HDM challenged groups. 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=8-12 per treatment group). Data is presented as median ± IQR.
The change in Rev-erbα expression seen after HDM challenge, taken with the previous work showing a role for REV-ERBα in lung inflammation prompted us to investigate HDM responses in Rev-erbα-/- mice. There is an increase in AHR to 75mg/ml methacholine, after HDM challenge in Rev-erbα-/- mice compared to control (P< 0.03 at ZT23 and P= 0.09 at ZT11), but no time of challenge effect (in contrast to WT mice) (Fig3, c and Supplementary Fig2, a). There was no difference in the slope of the methacholine dose response curves between Rev-erbα-/- mice challenged with HDM at either ZT11 or ZT23, in contrast to WT mice. We also noted a higher baseline AHR at ZT23 compared to ZT11 in PBS treated Rev-erbα-/- mice (Fig3, c); although this was not significant, this trend was in anti-phase to the effect seen in WT mice (Fig 1).
Furthermore, there was an increase in maximal effect of methacholine in Rev-erbα-/- mice compared to WT mice for both PBS (Supplementary Fig2, b) and HDM challenged mice (Fig3, d). This suggests that loss of REV-ERBα causes an exaggerated and clock-time independent AHR in response to methacholine challenge.
Airway and lung inflammation reveal no time of challenge differences in Rev-erbα-/- mice
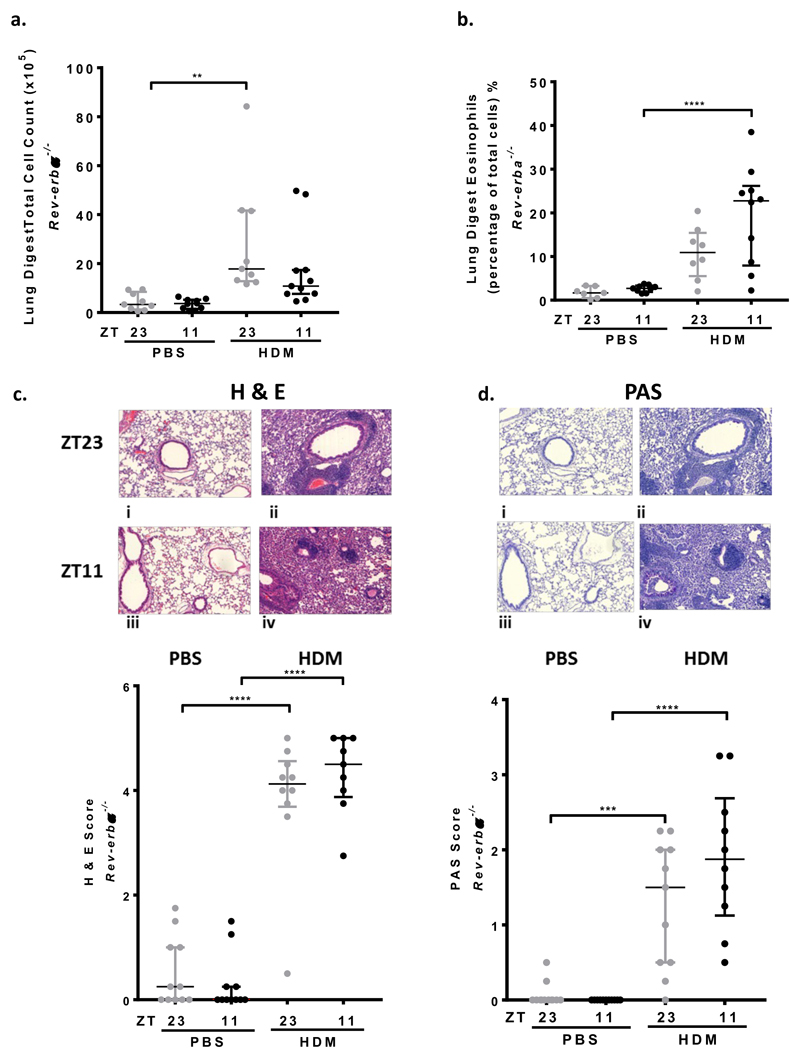
There was a significant increase in the total number of cells in BAL and in the percentage of eosinophils following HDM challenge in Rev-erbα-/- mice, but with no time of challenge effect in either, as previously seen in WT mice (Fig3, e, f). There was a significant increase in total cells in the lung digest following HDM challenge in Rev-erbα-/- mice at ZT23, compared to control mice but no time of challenge difference (Fig4, a). Lung eosinophils also increased after HDM challenge (only reaching significance at ZT11) and there was no time of challenge differences (Fig4, b).
Fig 4. Lung inflammation in Rev-erbα-/- mice after HDM challenge show no time of challenge difference.
a. Total cell count from lung digests increased after HDM challenge, compared to control, although only reached significance at ZT23 (** P < 0.01). There was no time of challenge difference. Data is presented as median ± IQR (n=8-11 per treatment group) and analysed using a 1 way ANOVA, followed by Tukey’s multiple comparison test.
b. Eosinophils in lung digests increased after HDM challenge, only reaching significance at ZT11 (**** P < 0.0001); there is no time of challenge difference. Data is presented as median ± IQR (n=8-11 per treatment group) and analysed using a 1 way ANOVA, followed by Tukey’s multiple comparison test.
c. HDM challenge at ZT11 and ZT23 caused predominantly eosinophilic inflammation around the bronchioles and blood vessels (haemoatoxylin and eosin staining (H&E)) compared to PBS challenge, histology sections i-iv and scatter plot, **** P < 0.0001 at ZT23 and ZT11. There was no significant time of challenge effect in PBS or HDM challenged groups. Data is presented as median ± IQR and analysed using a 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=9-11 per treatment group).
d. Periodic Shift Staining (PAS) shows increased mucus present on the bronchial epithelium in the lungs of WT mice treated with HDM at both ZT11 and ZT23. There was no PAS staining seen in PBS treated mice (i-iv) and scatter plot, *** P < 0.01 at ZT23 and **** P<0.0001 at ZT11). There was no time of challenge differences after PBS or HDM challenge. Data is presented as median ± IQR; data analysed using 1 way ANOVA, followed by Tukey’s multiple comparison test, (n=9-11 per treatment group).
Histological analysis showed increased H&E staining around the bronchioles, blood vessels and within the interstitial spaces as well as increased PAS staining, at both challenge times after HDM challenge, with no time of day effect in the Rev-erbα-/- mice (Fig4, c, d). HDM specific IgE following HDM challenge in Rev-erbα-/- mice was increased, but no time of day difference was seen (Supplementary Fig2, c).
Genotype comparison of WT versus Rev-erbα-/- mice
BAL individual cell counts measured as a percentage of the total cell count, reveal no significant genotype differences (Supplementary Fig 3, a). Cytokine and chemokine analysis revealed no time of challenge differences (data not shown) and only CXCL13 showed a genotype difference (Table 2).
Table 2. Cytokine/chemokine detection in bronchoalveolar lavage fluid (BALF) after HDM challenge at ZT11 and ZT23 in WT and Rev-erbα-/- mice.
| BAL cytokines/chemokines increased in response to HDM | ZT11 (pg/ml) Mean (± SEM) | Adjusted P value |
ZT23 (pg/ml) Mean (± SEM) | Adjusted P value |
||
|---|---|---|---|---|---|---|
| WT | Rev-erbα-/- | WT | Rev-erbα-/- | |||
| ENA79/CXCL5 | 1708 (175.5) | 2193 (273.5) | 0.68 | 2097 (299) | 2012 (424.7) | 1.00 |
| IP-10/CXCL10 | 1418 (498.6) | 2017 (549.6) | 0.88 | 1793 (593.6) | 1798 (626.6) | 1.00 |
| SDF-1a/CXCL-12 | 59.33 (9.92) | 158.7 (69.29) | 0.53 | 72.58 (16.32) | 120.2 (40.5) | 0.98 |
| BCA-1/CXCL13 | 2158 (448) | 3088 (780.7) | 0.76 | 2025 (310.6) | 4670 (1179) | 0.03 |
| SCYB16/CXCL16 | 298.2 (51.95) | 157.9 (25.75) | 0.14 | 250.9 (37.11) | 204.8 (48.24) | 0.87 |
| RANTES/CCL5 | 49.91 (14.27) | 55.28 (27.11) | 0.055 | 36.95 (6.43) | 25.62 (7.14) | 0.56 |
| MCP3/CCL-7 | 12.65 (2.99) | 12.48 (3.98) | 1.0 | 20.25 (3.26) | 16.06 (4.82) | 0.86 |
| CCL-17 | 4430 (1681) | 5023 (1789) | 0.61 | 6006 (2560) | 3318 (1693) | 0.49 |
| MIP3b/CCL-19 | 94.13 (16.04) | 110.3 (26.16) | 0.96 | 99.79 (14.22) | 133.6 (30.55) | 0.76 |
| MIP-3a/CCL20 | 129.3 (32.64) | 102.9 (30.86) | 0.84 | 117.9 (17.49) | 165.2 (53.54) | 1.00 |
| MDC/CCL-22 | 347.8 (63.46) | 274.3 (81.15) | 0.93 | 498.1 (111.3) | 291.5 (77.17) | 0.31 |
| Eotaxin 2/CCL24 | 16520 (3078) | 25488 (8946) | 0.69 | 24546 (5616) | 15254 (9173) | 0.17 |
| IL-1b | 76.98 (14.17) | 82.18 (13.68) | 0.99 | 97.59 (9.86) | 89.1 (18.32) | 0.97 |
| IL-6 | 17.82 (3.41) | 12.48 (2.23) | 0.68 | 19.04 (3.17) | 17.74 (4.64) | 0.99 |
| IL-16 | 178.8 (21.3) | 175.2 (23.79) | 1.00 | 239.7 (36.24) | 279.8 (69.31) | 0.90 |
Cytokines/chemokines that increased significantly in BALF after intranasal HDM challenge are shown in the table. Cytokine/chemokine concentrations (pg/ml) for WT and Rev-erbα-/- mice after HDM challenge at ZT11 and ZT23 are shown as mean ± SEM. BCA-1/CXCL13 showed a genotype effect and was increased in Rev-erbα-/- mice at ZT23 compared to WT mice (* P < 0.03). P values are given in the table. 1 way ANOVA, followed by Tukey multiple comparison adjustment, n=8-12 per treatment group.
REV-ERBα action is through airway smooth muscle muscarinic receptor regulation
Since we did not find a convincing correlation between inflammatory parameters and AHR in our models, we next investigated bronchiolar smooth muscle function.
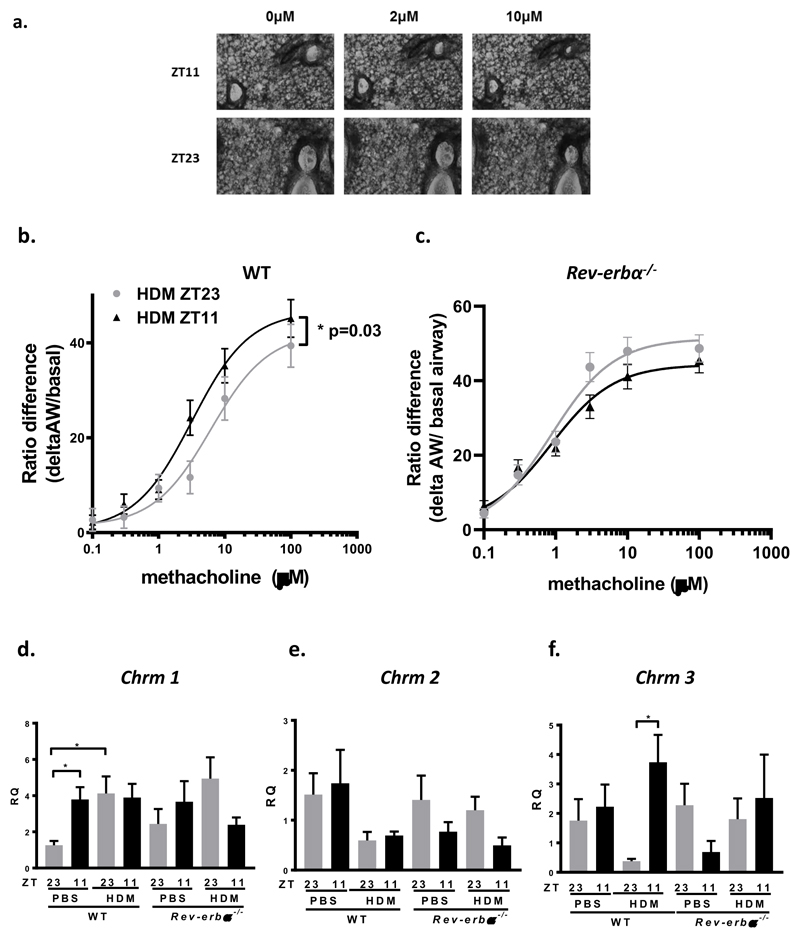
Using precision cut lung sections in organotypic culture we quantified airway contraction in response to methacholine. We found a significant increase in the maximal effect to methacholine at ZT11 compared to at ZT23 (P=0.03) and a reduction in the EC50 for methacholine in HDM challenged lung slices at ZT11 (3.2μM) compared to ZT23 (6.2μM) (Fig5, a, b). We repeated these experiments in lung slices from HDM challenged Rev-erbα-/- mice. We found no time of day differences to methacholine challenge and similar EC50s (ZT11, EC50= 9.3μM and ZT23, EC50= 8.5μM) (Fig5, c). There were no changes by time of day in PBS treated lung slices. We then investigated muscarinic receptor expression in the lungs. After saline challenge, we found Chrm 1 was more highly expressed at ZT11 rather than at ZT23, perhaps accounting for the physiological differences in AHR at baseline by time of day in WT mice (Fig5, d). We also found Chrm 3 expression was higher after HDM challenge at ZT11, but not at ZT23, potentially explaining the time of challenge difference in AHR. These time of day effects were lost in Rev-erbα-/- mice (Fig5, f). Chrm 2 expression showed no time of day or genotype differences after PBS or HDM challenge (Fig5, e). We also measured the expression of the muscle contractile apparatus genes smooth muscle actin (Acta), myosin light chain kinase (mylk1) and smooth muscle myosin (sm-mhc); none of these demonstrated a time of day response (Supplementary Fig4, a, b, c). Similarly, the beta adrenoceptors (Adrb1 and Adrb2) although reduced in response to HDM challenge, importantly also showed no time of day effects (Supplementary Fig4d, e).
Fig 5. Time of HDM challenge determines magnitude of contraction of airways to methacholine in lung slice model; muscarinic receptor expression is regulated by time of day and also by HDM challenge and REV-ERBα.
a. Representative images of precision cut lung slice experiment showing contraction of airways to increasing concentrations of methacholine in mice challenged with HDM at ZT11 or ZT23.
b. Concentration-response curves to increasing concentrations of methacholine. There is a significant increase in the maximal effect to methacholine in mice challenged with HDM at ZT11 compared to ZT23 (* P= 0.03). The EC50 for WT mice challenged at ZT11 was 3.2μM, and 6.2μM at ZT23.
c. Concentration-response curves to increasing concentrations of methacholine in Rev-erbα-/- mice challenged with HDM at ZT11 and ZT23. There was no difference in the effects of methacholine by time of day. The EC50 for ZT11 was 9.3μM and for ZT23 was 8.5μM.
Methacholine dose response curves were fitted to a three parameter sigmodal dose-response curve. An extra sum-of-squares F-test was used to test whether one curve could adequately fit the data for ZT11 and ZT23. Black triangles-HDM challenge at ZT11 and grey circles-HDM challenge at ZT23
d. Quantitative PCR for muscarinic receptor Chrm1. Chrm1 expression is significantly increased in PBS challenged mice at ZT11 compared to ZT 23 (* P < 0.05). This time of day difference is not apparent after HDM challenge. There is a significant increase in expression of Chrm1 after HDM challenge at ZT23 compared to control, PBS challenged mice (* P < 0.05). There was no time of challenge difference seen in Rev-erbα-/- mice (n=5-9 per treatment group, in duplicate).
e. Quantitative PCR for muscarinic receptor Chrm2. Chrm2 expression showed no time of challenge differences in WT or Rev-erb-/- mice (n=7-10 per treatment group, in duplicate). There were no time of genotype differences.
f. Quantitative PCR for muscarinic receptor Chrm3. Chrm3 expression is significantly increased after HDM challenge at ZT11 compared to ZT 23 (* P < 0.05). There is no time of day difference in Rev-erbα-/- mice (n=5-9 per treatment group, in duplicate).
All data presented as mean ± SEM and analysed by 1 way ANOVA, followed by Tukey’s multiple comparison test. All QPCR data is compared to expression of the housekeeping gene Gapdh in WT PBS challenged mice at ZT23. Black bars indicate challenge at ZT11 and grey bars indicate challenge at ZT23.
Discussion
We show that AHR is determined by time of day, an effect regulated through REV-ERBα. Allergen challenge at ZT11 (just before lights off/beginning of the active phase in mice; equivalent to early morning in humans) significantly increases the magnitude of AHR compared to allergen challenge at ZT23 (just before lights on/beginning of the rest phase in mice, equivalent to late afternoon/early evening in humans). This effect is abolished in Rev-erbα-/- mice, suggesting that AHR is regulated, or gated, by REV-ERBα. Despite the marked changes in AHR only modest changes in inflammatory mediators and cells were seen in the lungs, suggesting dissociation between the inflammatory response, and the airway constriction. Even ex-vivo the airways retain a time of day signature in response to methacholine, an effect which was lost in Rev-erbα-/- mice, prompting our analysis of the muscarinic receptor types. This revealed both time of day, and also REV-ERBα dependent changes in expression, especially of the M3 receptor in whole lung.
Nocturnal exacerbations of asthma, hospital admissions and deaths, remain an unmet medical need. The immune system lies under strong circadian control (28, 29), and lung inflammatory responses are strongly regulated by the circadian clock, and specifically by REV-ERBα (16, 18). Since asthma symptoms in humans peak in early morning at around 6am, we focussed on this time point (ZT11) and its anti-phasic time point ZT23 in our mouse studies. These time points have also been shown to be important for the lung innate immune response and in food allergy (26, 30) and also in our own work in human asthma (1). Using direct flexivent measurement of AHR and a physiologically relevant allergen, HDM, we found higher AHR at ZT11. In nocturnal mice, this time-point is equivalent to the transition from the rest-phase to activity, and is biologically comparable to early morning in humans.
The allergic inflammatory process recruits many specialised cells to the lung, resulting in a changed immune environment. We characterised the immune cell repertoire, both in BAL, and lung digests, and also measured inflammatory and immune mediators. Overall, the effects of time of day were dissociated from the consistent and marked changes in AHR, suggesting possible non-immune cell involvement. We acknowledge that there could have been time of day differences at other time points. Our sampling was undertaken at 24 hours after the final allergen challenge, when AHR was predicted to be greatest (20).
The orphan nuclear receptor REV-ERBα has recently emerged as a major regulator of the lung immune response, mediating time of day changes to acute inflammatory challenges (16, 18). Moreover, REV-ERBα plays important roles in non-immune cells, regulating energy metabolism, and muscle function (31, 32). For these reasons we repeated the HDM challenges in Rev-erbα-/- mice and showed that the time of day AHR effect was abolished. Interestingly, we also found that HDM challenge in wild-type mice had a major inhibitory effect on Rev-erbα expression, identifying inflammation acting through both transcriptional, and post-translational mechanisms to repress Rev-erbα expression (18). Again, we saw no differences in immune cells infiltrating into the lungs between wild-type and Rev-erbα-/- mice, despite the loss of temporal control of AHR. This again suggests a non-immune cell, and non-inflammatory effector mechanism.
To examine the airway responses directly, we removed the lungs of HDM sensitised animals, prepared precision cut lung slices for organotypic culture, and measured airway responses to methacholine. Here, we saw an increase in the maximal effect to methacholine at ZT11, indicating greater methacholine sensitivity. Furthermore, when we repeated these experiments in Rev-erbα-/- mice, the time of day difference was abolished. This correlates with the in-vivo measurements, and indicates a lung-intrinsic mechanism of action. Methacholine acts on muscarinic receptors, with little effect on nicotinic receptors (33). Therefore, we examined the expression of muscarinic receptors, and identified major changes in both type M1 and M3 muscarinic receptors. Importantly, we found no changes in the expression of genes involved in the contractile apparatus of airway muscle, or in adrenoceptors, suggesting that the changes in muscarinic receptors in the lung by time of day were specific. The increase in M1 receptor expression at ZT11 in the PBS treated group suggests that this receptor is important for conferring time of day constrictor tone to the airway under basal conditions. Furthermore, Chrm1 contains transcription factor binding sites for the clock proteins BMAL:CLOCK and RORβ (34); suggesting that Chrm1 is under direct clock control. In contrast Chrm3 only acquires a time of day effect after HDM-inflammation, with peak expression at ZT11. Strikingly, this time of day change in Chrm3 expression is completely lost in the Rev-erba-/- mouse, providing an attractive explanation for the loss of temporal gating in AHR we observed. However, according to the circadian data-base of rhythmic gene expression (Circa DB, 35), the expression of Chrm3, oscillates in healthy mouse lung with maximal expression at 6pm (ZT11) and nadir of expression at 6am (ZT23). In our study, we identified similar time of day differences in Chrm3 expression, but in our case, these differences were only apparent following stimulation with HDM and were not observed in baseline conditions. One potential explanation may be that our assay was insufficiently sensitive to detect low level changes in gene transcription in un-stimulated conditions. Bioinformatic analysis revealed no evidence of clock transcription factor binding sites in Chrm3 (34), and we therefore postulate that Chrm3 transcription may be under indirect clock control.
We acknowledge that the M3 receptor is not only expressed by airway smooth muscle cells but by multiple other cell types (36) including endothelial cells and inflammatory cells. However, given the immediate and directly visualised contraction of the airway to methacholine during the lung slice experiments, the likely mechanism of action of methacholine is through the muscarinic receptors present in the airway smooth muscle. Although we have focussed on time of day changes in muscarinic receptors, it should be noted that the parasympathetic nervous system as a whole displays marked circadian rhythmicity (37). It is therefore likely that in vivo the diurnal variation in AHR would be affected by both neural and humoral circadian rhythms, as well as in rhythmic changes in receptor expression.
To our knowledge this is the first time that the molecular clock has been shown to be important in gating AHR. Furthermore, the discovery that muscarinic receptors might play a role is important for the treatment of asthma (38). The cholinergic system is functionally linked to the circadian system (37). Tiotropium bromide, a long-lasting M3 muscarinic-receptor antagonist is licensed for asthma (39). In the future, a short-acting drug antagonising both M1 and M3 might prevent AHR in asthma and its administration at the peak of receptor expression could significantly increase its efficacy, leading to novel chronotherapeutic approaches.
Supplementary Material
Clinical Implication.
These insights suggest the importance of considering timing of drug administration in clinic trials, and in clinical practice; chronotherapy.
Capsule Summary.
REV-ERBα gates airway hyperresponsiveness by time of day; future asthma therapies should aim to dose anti-muscarinic agents at the most efficacious time of day (chronotherapy) and modulate the molecular clock.
Sources of Funding
HJD is supported by an Asthma UK Senior Clinical Academic Development Award (AUK-SCAD-2013-229), the JP Moulton Charitable Foundation, a North West Lung Centre Charity Project Grant and the University of Manchester’s Dean’s Prize for Clinicians.
KK is supported by the JP Moulton Charitable Trust.
PM is supported by MRC-DTP funding (AA07 P117413)
RJM is funded by Wellcome Trust Grant (107849/Z/15/Z) and Medical Research Council grant MR/P023576/1)
JG is a Career Development Fellow Versus Arthritis (20629) and holds a Medical Research Council grant MR/S002715/1
CML is a Wellcome Senior Fellow in Basic Biomedical Sciences (107059/Z/15/Z)
ASIL is a Wellcome Investigator Wellcome Trust (107849/Z/15/Z)
DWR is a Wellcome Investigator Wellcome Trust (107849/Z/15/Z) and holds a Medical Research Council Programme grant (MR/P023576/1)
JFB is funded by a MRC by a Medical research Council Grant (MR/L006499/1)
Footnotes
Conflict of Interest Statement
HJD, KK, PM, NB, RM, JEG, JFB, LG, CML, ASIL and DWR have no conflict of interests to declare.
References
- 1.Durrington HJ, Gioan-Tavernier GO, Maidstone RJ, Krakowiak K, Loudon ASI, Blaikley JF, Fowler SJ, Singh D, Simpson A, Ray DW. Time of Day Affects Eosinophil Biomarkers in Asthma: Implications for Diagnosis and treatment. Am J Respir Crit Care Med. 2018;198:1578–1581. doi: 10.1164/rccm.201807-1289LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durrington HJ, Farrow SN, Loudon AS, Ray D. The Circadian Clock and Asthma. Thorax. 2013:1–3. doi: 10.1136/thoraxjnl-2013-203482. 0. [DOI] [PubMed] [Google Scholar]
- 3.Turner-Warwick M. Nocturnal asthma: a study in general practice. J R Coll Gen Pract. 1989;39:239–243. [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland ER. Nocturnal asthma. J Allergy Clin Immunol. 2005;116:1179–1186. doi: 10.1016/j.jaci.2005.09.028. quiz 1187. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane GM, Clark JH. A survey of asthma mortality in patients between ages 35 and 64 in the Greater London hospitals in 1971. Thorax. 1975;30:300–305. doi: 10.1136/thx.30.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BTS/SIGN British Guideline on the Management of Asthma 2016.
- 7.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. Available from: www.ginasthma.org.
- 8.De Vries K, Goei JT, Booy-Noord H, Orie NGM. Changes during 24 hours in the lung function and histamine hyperreactivity of the bronchial tree in asthmatic and bronchitic patients. Int Arch Allergy. 1962;20:93–101. doi: 10.1159/000229248. [DOI] [PubMed] [Google Scholar]
- 9.Reinberg A, Gervais P, Morin M, et al. Circadian rhythms in the threshold of bronchial response to acetylcholine in healthy and asthmatic subjects. Chronobiology. 1974:174–77. [Google Scholar]
- 10.Bonnet R, Jorres R, Heitman U, et al. Circadian rhythm in airway responsiveness and airway tone in patients with mild asthma. J Appl Physiol. 1991;71(4):1598–1605. doi: 10.1152/jappl.1991.71.4.1598. [DOI] [PubMed] [Google Scholar]
- 11.Kondo S, Abe K. Priority of peak circadian variation of bronchial responsiveness tp the trough of circadian variation of bronchial calibre in asthmatic children. Chest. 1993;100:640–43. doi: 10.1378/chest.100.3.640. [DOI] [PubMed] [Google Scholar]
- 12.Van Aalderen WMC, postma DS, Koeter GH, et al. Circadian change in bronchial responsiveness and airflow obstruction in asthmatic children. Thorax. 1989;44:803–7. doi: 10.1136/thx.44.10.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gervais P, Reinberg PA, Gervais C, Smolensky M, DeFrance O. Twenty-four-hour rhythm in the bronchial hyperreactivity to house dust in asthmatics. J Allergy Clin Immunol. 1977;59:207–213. doi: 10.1016/0091-6749(77)90151-8. [DOI] [PubMed] [Google Scholar]
- 14.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellet MM, et al. Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A. 2013;110(24):9897–9902. doi: 10.1073/pnas.1120636110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbs JE, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(2):582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(31):12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pariollaud M, Gibbs JE, Hopwood TW, et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J Clin Invest. 2018;128(6):2281–2296. doi: 10.1172/JCI93910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cates EC, Fattouh R, Johnson JR, Llop-Guevara A, Jordana M. Modeling responses to respiratory house dust mite exposure. Contrib Microbiol. 2007;14:42–67. doi: 10.1159/000107054. [DOI] [PubMed] [Google Scholar]
- 20.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32(9):402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haspel JA, Chettimada S, Shaik RS, Chu JH, Raby BA, Cernadas M, Carey V, Process V, Hunninghake GM, Ifedigbo E, Lederer JA, et al. Circadian rhythm reprogramming during lung inflammation. Nat Commun. 2014;5:4753. doi: 10.1038/ncomms5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, Sajol G, Schutz R, Weaver R, Yu H, Castro M, et al. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol. 2018;11:97–111. doi: 10.1038/mi.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartney JM, Robichaud A. In: Mouse Models of Allergic Disease Methods in Molecular Biology (Methods and Protocols) Allen I, editor. Vol. 1032. Humana Press; Totowa, NJ: 2013. Assessment of Airway Hyperresponsiveness in Mouse Models of Allergic Lung Disease Using Detailed Measurements of Respiratory Mechanics. [DOI] [PubMed] [Google Scholar]
- 24.Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci USA. 2016;113:10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Hunter L, Wu G, Maidstone R, Mizoro Y, Vonslow R, Fife M, Hopwood T, Begley N, Saer B, Wang P, et al. Genome-wide effect of pulmonary airway epithelial cell-specific Bmal1 deletion. FASEB J. 2019;33(5):6226–6238. doi: 10.1096/fj.201801682R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Druzd D, Matveeva O, Ince L, et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity. 2017;46(1):120–132. doi: 10.1016/j.immuni.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W, Holtkamp S, Hergenhan SM, et al. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity. 2018;49(6):1175–1190.:e7. doi: 10.1016/j.immuni.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanabe K, Kitagawa E, Wada M, Haraguchi A, Orihara K, Tahara Y, Nakao A, Shibata S. Antigen exposure in the late light period induces severe symtoms of food allergy in an OVA-allergic mouse model. Scientific Reports. 2015;5:14424. doi: 10.1038/srep14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123–7. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayeuf-Louchart A, Thorel Q, Delhaye S, Beauchamp J, Duhem C, Danckaert A, Lancel S, Pourcet B, Woldt E, Boulinguiez A, Ferri L, et al. Rev-erb-α regulates atrophy-related genes to control skeletal muscle mass. Sci Rep. 2017;7:14383. doi: 10.1038/s41598-017-14596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coates AL, Wanger J, Cockcroft DW, Culver BH, Carlsen K-H, Diamant Z, Gauvreau G, Hall GL, Hallstrand TS, Horvath I, de Jongh FHC, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. ERJ. 2017;49:1601526. doi: 10.1183/13993003.01526-2016. [DOI] [PubMed] [Google Scholar]
- 34.Daily K, Patel VR, Rigor P, Xie X, Baldi P. MotifMap: integrative genome-wide maps of regulatory motif sites for model species. BMC Bioinformatics. 2011;12:495. doi: 10.1186/1471-2105-12-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CircaDB. http://circadb.hogeneschlab.org/
- 36.Schaum N, Karkanias J, Neff NF, et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562:367–372. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behavioural Brain Research. 2011;221:466–480. doi: 10.1016/j.bbr.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 38.Gosens R, Gross N. The mode of action of anticholinergics in asthma. European Respiratory Journal. 2018;52:1701247. doi: 10.1183/13993003.01247-2017. Global Initiative for Asthma. 2016 GINA report, global strategy report for asthma management and prevention. Global Initiative for Asthma; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.