Abstract
Aim
We conducted a two-sample Mendelian randomization (MR) study to assess the associations of genetically predicted circulating vitamin C levels with cardiovascular diseases (CVDs).
Methods and results
Ten lead single-nucleotide polymorphisms associated with plasma vitamin C levels at the genome-wide significance level were used as instrumental variables. Summary-level data for 15 CVDs were obtained from corresponding genetic consortia, the UK Biobank study, and the FinnGen consortium. The inverse-variance-weighted method was the primary analysis method, supplemented by the weighted median and MR-Egger methods. Estimates for each CVD from different sources were combined. Genetically predicted vitamin C levels were not associated with any CVD after accounting for multiple testing. However, there were suggestive associations of higher genetically predicted vitamin C levels (per 1 standard deviation increase) with lower risk of cardioembolic stroke [odds ratio, 0.79; 95% confidence interval (CI), 0.64, 0.99; P = 0.038] and higher risk of atrial fibrillation (odds ratio, 1.09; 95% CI, 1.00, 1.18; P = 0.049) in the inverse-variance-weighted method and with lower risk of peripheral artery disease (odds ratio, 0.76, 95% CI, 0.62, 0.93; P = 0.009) in the weighted median method.
Conclusion
We found limited evidence with MR techniques for an overall protective role of vitamin C in the primary prevention of CVD. The associations of vitamin C levels with cardioembolic stroke, atrial fibrillation, and peripheral artery disease need further study.
Keywords: Cardiovascular disease, Mendelian randomization, Vitamin C
Introduction
Vitamin C as an antioxidant has been proposed to alleviate oxidative stress and affect vascular remodelling, endothelial function, and lipid peroxidation, thereby potentially having a protective role in cardiovascular disease (CVD).1–3 Observational data have indicated that a high circulating level or intake of vitamin C is associated with a reduced risk of CVD and corresponding mortality.4–8 Several biological processes and signalling pathways have been proposed to be involved in the potential therapeutic effects of vitamin C on CVD.9 However, the cardio-protective effect of vitamin C has not been validated in randomized controlled trials (RCTs) of supplementation with vitamin C alone or together with other antioxidative vitamins.3,10,11 Thus, any causal relationship between vitamin C and CVD remains unestablished given potential confounding in previous observational findings and certain limitations of RCTs (e.g. a small sample size, imbalanced baseline characteristics, combined supplementation of vitamin C with other nutrients, and low compliance to intervention).
Utilizing genetic variants as instrumental variables for an exposure (e.g. plasma vitamin C levels) allows the Mendelian randomization (MR) design to more plausibly investigate causal inferences by minimizing residual confounding and other biases. Here, we conducted a two-sample MR study to assess the associations of genetically predicted circulating vitamin C levels with risk of a wide range of CVDs.
Methods
Outcome data sources
We included 15 cardiovascular endpoints with numbers of cases ranging from 3373 (large artery stroke) to 139 364 (coronary artery disease). Summary-level data for these outcomes were obtained from large genetic consortia,12–16 the UK Biobank study,17 and the FinnGen consortium.18 Detailed descriptions on data sources are presented in Table 1.
Table 1. Information on outcome data sources.
| Data source | Cardiovascular disease | Cases | Controls | Population | Covariates adjusted in GWAS |
|---|---|---|---|---|---|
| GWAS meta-analysis (Nielsen et al.) | Atrial fibrillation | 60 620 | 970 216 | European | Birth year, sex, genotype batch, and 1-4 principal components |
| CARDIoGRAMplusC4D plus UKBB | Coronary artery disease | 122 733 | 424 528 | Mixed | NA |
| HERMES consortium | Heart failure | 47 309 | 930 014 | European | Age and sex, and principal components in individual studies where applicable |
| MEGASTROKE consortium | Stroke | 40 585 | 406 111 | European | Age and sex |
| Ischaemic stroke | 34 217 | NA | |||
| Large artery stroke | 3373 | 406 111 | |||
| Small vessel stroke | 5386 | 406 111 | |||
| Cardioembolic stroke | 7193 | 406 111 | |||
| ISGC | Intracerebral hemorrhage | 3223 | 3725 | European | Age, sex, and principal components |
| The UK Biobank study (UKBB) | Aortic aneurysm | 2261 | 365 300 | European | Age, sex, and 10 genetic principal components |
| Aortic valve stenosis | 3528 | 364 033 | |||
| Stroke | 12 036 | 355 525 | |||
| Intracerebral hemorrhage | 1504 | 366 057 | |||
| Subarachnoid hemorrhage | 1292 | 366 269 | |||
| Ischaemic stroke | 6566 | 360 995 | |||
| Transient ischaemic attack | 4813 | 362 748 | |||
| Venous thromboembolism | 16 412 | 351 149 | |||
| Peripheral vessel disease | 4593 | 362 968 | |||
| The FinnGen consortium | Aortic aneurysm | 1919 | 167 843 | European | Age, sex, the first 10 genetic principal components, and genotyping batch |
| Atrial fibrillation | 17 325 | 97 214 | |||
| Coronary artery disease | 16 631 | 160 268 | |||
| Heart failure | 9576 | 159 286 | |||
| Stroke | 14 171 | 133 027 | |||
| Intracerebral hemorrhage | 1224 | 163 533 | |||
| Subarachnoid hemorrhage | 1019 | 163 508 | |||
| Ischaemic stroke | 8046 | 164 286 | |||
| Transient ischaemic attack | 6729 | 164 286 | |||
| Venous thromboembolism | 6913 | 169 986 | |||
| Peripheral vessel disease | 5323 | 167 843 |
CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; GWAS, genome-wide association study; HERMES; Heart Failure Molecular Epidemiology for Therapeutic Targets; ISGC, International Stroke Genetic Consortium; NA, not available. The UK Biobank was included in Consortium (Nielsen et al.), HERMES consortium, and ISGC.
Instrument selection
Eleven lead single-nucleotide polymorphisms (SNPs) associated with plasma vitamin C levels at the genome-wide significance level (P<5 x 10−8) were identified from a meta-analysis of genome-wide association studies (GWASs) including up to 52 018 individuals of European descent.19 These SNPs explained approximately 1.87% of variance in circulating vitamin C levels.19 Rs33972313 in the SLC23A1 gene region encodes the sodium-dependent vitamin C transporter 1 and explained most of the variance in circulating vitamin C levels. One additional effect allele of this variant is associated with an 11% higher plasma vitamin C level.20 Rs174547 in FADS1 gene region was excluded from analyses due to pleiotropic effects on plasma phospholipid fatty acids,21 leaving 10 SNPs leveraged as instrumental variables (Table 2). Rs13028225 was not available in FinnGen data of SNP-CVD associations and was replaced by rs17655123 in high linkage disequilibrium (r2 = 0.88) with rs13028225. Likewise, rs4074995 (r2 = 0.83) and rs3809260 (r2 = 0.98) were used as the proxy SNPs for rs10051765 and rs2559850, respectively, in the analysis of data from the International Stroke Genetics Consortium. Association estimates in the vitamin C GWAS were adjusted for age, sex, the first 10 genetic principal components, and study centre (where applicable).
Table 2. Information on instrumental variables.
| SNP | Chr | Position | Nearby gene | EA | NEA | EAF | Beta | SE | P-value |
|---|---|---|---|---|---|---|---|---|---|
| rs6693447 | 1 | 2330190 | RĒR1 | T | G | 0.551 | 0.039 | 0.006 | 6.25E-10 |
| rs13028225 | 2 | 220031255 | SLC23A3 | T | C | 0.857 | 0.102 | 0.009 | 2.38E-30 |
| rs33972313 | 5 | 138715502 | SLC23A1 | C | T | 0.968 | 0.360 | 0.018 | 4.61E-90 |
| rs10051765 | 5 | 176799992 | RGS14 | C | T | 0.342 | 0.039 | 0.007 | 3.64E-09 |
| rs7740812 | 6 | 52725787 | GSTA5 | G | A | 0.594 | 0.038 | 0.006 | 1.88E-09 |
| rs117885456 | 12 | 96249111 | SNRPF | A | G | 0.087 | 0.078 | 0.012 | 1.70E-11 |
| rs2559850 | 12 | 102093459 | CHPT1 | A | G | 0.598 | 0.058 | 0.006 | 6.30E-20 |
| rs10136000 | 14 | 105253581 | AKT1 | A | G | 0.283 | 0.040 | 0.007 | 1.33E-08 |
| rs56738967 | 16 | 79740541 | MAF | C | G | 0.321 | 0.041 | 0.007 | 7.62E-10 |
| rs9895661 | 17 | 59456589 | BCAS3 | T | C | 0.817 | 0.063 | 0.008 | 1.05E-14 |
Chr, chromosome; EA, effect allele; EAF, effect allele frequency; NEA, non-effect allele; SE, standard error; SNP, single-nucleotide polymorphism.
Statistical analysis
The multiplicative random-effects inverse-variance-weighted method22 was used as the main statistical method to assess the association between genetically predicted circulating vitamin C levels and CVDs. MR estimates for each CVD outcome from different data sources were combined using the fixed-effects meta-analysis method. We used two supplementary analyses, the weighed median approach23 and MR-Egger regression,24 to examine the robustness of the results and possible pleiotropy. The weighted median method can provide consistent causal estimates provided that ≥50% of the weight comes from valid SNPs.23 MR-Egger regression can detect horizontal pleiotropy by P-value for its intercept and generate estimate after correction for pleiotropy.24 The I2 statistic was calculated to assess the degree of heterogeneity25 among estimates of SNPs in each analysis. All reported odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of CVDs were scaled to 1 standard deviation (SD) increase in genetically predicted circulating levels of vitamin C. The Bonferroni method was used to adjust for multiple testing (15 CVDs). Associations with P-values of <0.003 were regarded as significant associations and associations with P-values between 0.05 and 0.003 were deemed as suggestive associations. All analyses were two-sided and performed using the mrrobust package26 in Stata/SE 15.0.
Results
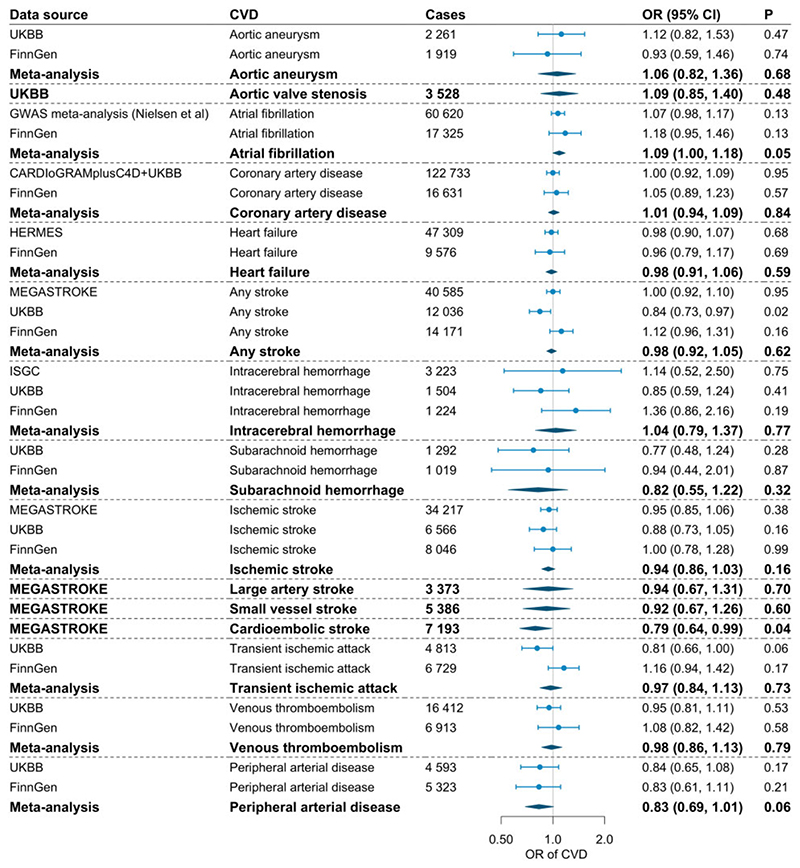
The associations of genetically predicted circulating vitamin C levels (per 1 SD increase) with the 15 CVDs in the main analysis are presented in Figure 1. We observed suggestive inverse associations of genetically predicted vitamin C levels with risk of any stroke in UK Biobank (OR, 0.84; 95% CI, 0.73, 0.97; P= 0.018) and cardioembolic stroke in MEGASTROKE (OR, 0.79; 95% CI, 0.64, 0.99; P = 0.038). However, the association for any stroke was not replicated in the MEGASTROKE and FinnGen consortia and did not persist in the meta-analysis. There was no association between genetically predicted vitamin C and atrial fibrillation in the GWAS meta-analysis by Nielsen et al. or in the FinnGen consortium, but the meta-analysis results revealed a suggestive positive association (OR 1.09, 95% CI, 1.00, 1.18; P= 0.049). Genetically predicted vitamin C levels were not associated with the other studied CVDs in the main analysis.
Figure 1.
Associations of genetically predicted circulating vitamin C levels with risk of cardiovascular disease. CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; CVD, cardiovascular disease; HERMES; Heart Failure Molecular Epidemiology for Therapeutic Targets; ISGC, International Stroke Genetic Consortium; LB, lower bound of 95% confidence interval; OR, odds ratio; UKBB, UK Biobank; UB, upper bound of 95% confidence interval. The UK Biobank was included in the GWAS meta-analysis for atrial fibrillation (Nielsen et al.), HERMES consortium, and ISGC.
Results of the supplementary analyses based on the weighted median and MR-Egger methods showed no association of genetically predicted vitamin C levels with any CVD (Table 3), with the exception for a suggestive inverse association with peripheral artery disease in the FinnGen consortium (OR, 0.71, 95% CI, 0.52, 0.97; P = 0.030) and the meta-analysis (OR, 0.76, 95% CI, 0.62, 0.93; P = 0.009). We observed the modest heterogeneity in several analyses; however, the P-values for the intercept in corresponding MR-Egger regression were >0.05 (Table 3). In a supplementary analysis using rs33972313 in the SLC23A1 gene region as instrumental variable, the associations of genetically predicted vitamin C levels with peripheral artery disease (OR, 0.67, 95% CI, 0.46, 1.00; P = 0.048) and other CVDs (data not shown) were consistent with the findings from the analyses based on all instrumental variables.
Table 3. Supplementary analyses of the associations of genetically predicted circulating vitamin C with cardiovascular disease.
| Data source | Cardiovascular disease | Cases | Controls | I2 (%) | Weighted median method | MR-Egger regression | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | Pintercept | |||||
| UKBB | Aortic aneurysm | 2261 | 365 330 | 0 | 1.05 | 0.71, 1.56 | 0.801 | 0.96 | 0.58, 1.59 | 0.880 | 0.443 |
| FinnGen | Aortic aneurysm | 1919 | 167 843 | 30 | 0.80 | 0.48, 1.33 | 0.384 | 0.57 | 0.28, 1.17 | 0.126 | 0.105 |
| UKBB | Aortic valve stenosis | 3528 | 364 033 | 0 | 1.12 | 0.82, 1.53 | 0.482 | 1.32 | 0.89, 1.94 | 0.166 | 0.226 |
| GWAS meta-analysis (Nielsen et al.) | Atrial fibrillation | 60 620 | 970 216 | 0 | 1.07 | 0.97, 1.17 | 0.161 | 1.08 | 0.93, 1.26 | 0.307 | 0.860 |
| FinnGen | Atrial fibrillation | 17 325 | 97 214 | 29 | 1.11 | 0.87, 1.41 | 0.413 | 1.13 | 0.76, 1.67 | 0.540 | 0.784 |
| CARDIoGRAMplusC4D + UKBB | Coronary artery disease | 122 733 | 424 528 | 30 | 1.00 | 0.91, 1.10 | 0.982 | 0.99 | 0.86, 1.14 | 0.932 | 0.954 |
| FinnGen | Coronary artery disease | 16 631 | 160 268 | 0 | 0.95 | 0.77, 1.18 | 0.647 | 0.81 | 0.61, 1.07 | 0.135 | 0.025 |
| HERMES | Heart failure | 47 309 | 930 014 | 0 | 1.02 | 0.92, 1.13 | 0.771 | 1.07 | 0.94, 1.23 | 0.308 | 0.101 |
| FinnGen | Heart failure | 9576 | 159 286 | 11 | 0.90 | 0.71, 1.15 | 0.415 | 0.82 | 0.58, 1.14 | 0.233 | 0.242 |
| MEGASTROKE | Stroke | 40 585 | 406 111 | 5 | 0.99 | 0.88, 1.10 | 0.810 | 0.94 | 0.81, 1.09 | 0.397 | 0.265 |
| UKBB | Stroke | 12 036 | 355 525 | 9 | 0.90 | 0.75, 1.07 | 0.241 | 0.89 | 0.71, 1.12 | 0.331 | 0.543 |
| FinnGen | Stroke | 14 171 | 133 027 | 0 | 1.15 | 0.92, 1.42 | 0.217 | 1.11 | 0.83, 1.47 | 0.487 | 0.913 |
| ISGC | Intracerebral hemorrhage | 3223 | 3725 | 47 | 0.96 | 0.46, 2.04 | 0.925 | 0.69 | 0.19, 2.43 | 0.560 | 0.318 |
| UKBB | Intracerebral hemorrhage | 1504 | 366 057 | 0 | 0.75 | 0.46, 1.21 | 0.237 | 0.83 | 0.46, 1.49 | 0.528 | 0.887 |
| FinnGen | Intracerebral hemorrhage | 1224 | 163 533 | 0 | 2.08 | 1.10, 3.92 | 0.024 | 2.08 | 0.94, 4.61 | 0.071 | 0.202 |
| UKBB | Subarachnoid hemorrhage | 1292 | 366 269 | 26 | 0.76 | 0.46, 1.28 | 0.309 | 0.83 | 0.38, 1.81 | 0.634 | 0.828 |
| FinnGen | Subarachnoid hemorrhage | 1019 | 163 508 | 56 | 1.00 | 0.47, 2.14 | 0.999 | 2.01 | 0.59, 6.86 | 0.265 | 0.137 |
| MEGASTROKE | Ischaemic stroke | 34 217 | NA | 0 | 0.96 | 0.83, 1.11 | 0.553 | 0.90 | 0.75, 1.08 | 0.270 | 0.477 |
| UKBB | Ischaemic stroke | 6566 | 360 995 | 0 | 0.87 | 0.68, 1.10 | 0.248 | 1.00 | 0.75, 1.33 | 0.991 | 0.239 |
| FinnGen | Ischaemic stroke | 8046 | 164 286 | 41 | 1.05 | 0.81, 1.37 | 0.712 | 1.15 | 0.75, 1.77 | 0.523 | 0.428 |
| MEGASTROKE | Large artery stroke | 3373 | 406 111 | 28 | 0.95 | 0.65, 1.38 | 0.770 | 0.91 | 0.49, 1.66 | 0.748 | 0.896 |
| MEGASTROKE | Small vessel stroke | 5386 | 406 111 | 34 | 0.77 | 0.55, 1.07 | 0.114 | 0.67 | 0.42, 1.07 | 0.093 | 0.093 |
| MEGASTROKE | Cardioembolic stroke | 7193 | 406 111 | 0 | 0.83 | 0.63, 1.10 | 0.191 | 0.81 | 0.56, 1.18 | 0.269 | 0.883 |
| UKBB | Transient ischaemic attack | 4813 | 362 748 | 0 | 0.79 | 0.60, 1.03 | 0.076 | 0.78 | 0.56, 1.08 | 0.136 | 0.727 |
| FinnGen | Transient ischaemic attack | 6729 | 164 286 | 0 | 1.15 | 0.88, 1.50 | 0.313 | 1.09 | 0.76, 1.57 | 0.648 | 0.684 |
| UKBB | Venous thromboembolism | 16 412 | 351 149 | 46 | 1.01 | 0.87, 1.17 | 0.903 | 1.02 | 0.79, 1.32 | 0.900 | 0.504 |
| FinnGen | Venous thromboembolism | 6913 | 169 986 | 46 | 1.02 | 0.78, 1.34 | 0.875 | 0.93 | 0.57, 1.52 | 0.784 | 0.471 |
| UKBB | Peripheral arterial disease | 4593 | 362 968 | 25 | 0.80 | 0.61, 1.05 | 0.112 | 0.85 | 0.56, 1.29 | 0.445 | 0.936 |
| FinnGen | Peripheral arterial disease | 5323 | 167 843 | 38 | 0.71 | 0.52, 0.97 | 0.030 | 0.63 | 0.38, 1.03 | 0.063 | 0.178 |
CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; CI, confidence interval; CVD, cardiovascular disease; HERMES; Heart Failure Molecular Epidemiology for Therapeutic Targets; ISGC, International Stroke Genetic Consortium; OR, odds ratio; SNP, single-nucleotide polymorphism; UKBB, UK Biobank. The I2 statistic was used to present the heterogeneity among estimates for each SNP in one analysis. The P-value for the intercept in the MR-Egger regression was used present the pleiotropy (P < 0.05). The UK Biobank was included in GWAS meta-analysis (Nielsen et al.), HERMES consortium, and ISGC.
Discussion
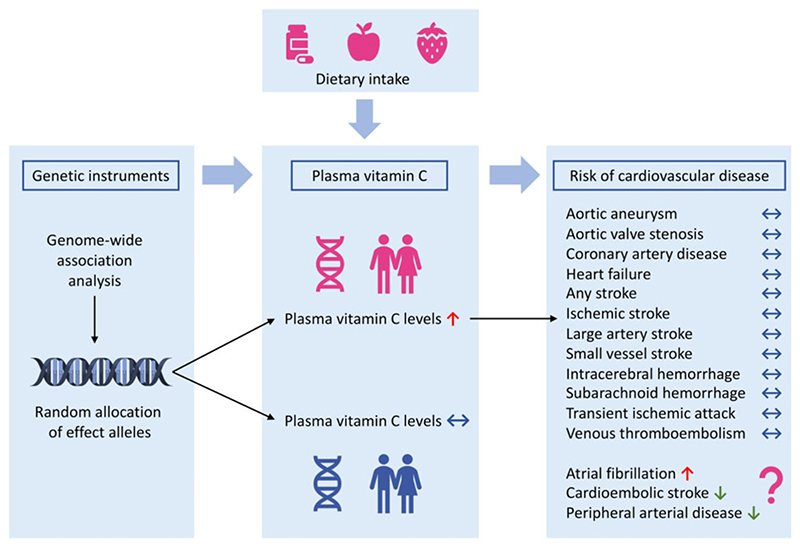
This MR study found no clear pattern of associations between genetically predicted vitamin C levels and risk of CVDs (Figure 2). However, genetically predicted vitamin C levels showed suggestive inverse associations with cardioembolic stroke and peripheral artery disease but a suggestive positive association with atrial fibrillation (Figure 2).
Figure 2. Summary of associations of genetically predicted plasma vitamin C levels with risk of cardiovascular disease.
The overall lack of support for a protective association between vitamin C and CVDs in the present MR study was consistent with most RCTs3,10,11 and some27,28 but not all4–7 observational studies. A recent review article concluded that findings differed between RCTs for vitamin C supplementation with null findings and observational studies on dietary vitamin C intake suggesting a protective association.8 Several signalling pathways were highlighted using the network pharmacology approach,9 whereas no evidence was found to support that vitamin C supplementation reduced the risk of CVD in healthy participants in a systematic review of RCTs.10 This discrepancy may be caused by residual confounding by other cardio-protective nutrients, such as magnesium,29,30 from foods rich in vitamin C (e.g. fruit and vegetables), or healthy lifestyle behaviours among individuals with a vitamin C-rich diet.31 Even though the present MR study did not support cardiovascular benefits of increasing circulating vitamin C levels, our findings did not hint any information on possible health benefits from a diet abundant in vitamin C, suggested by previous evidence.4,32,33 Instead, the present study did not justify vitamin C supplementation as a primary prevention strategy for CVD.
Higher plasma vitamin C levels were associated with a reduced risk of total stroke in cohort studies.34 Nevertheless, a daily supplementation of 500 mg of vitamin C together with 400 IU of vitamin E did not decrease the risk of total stroke in an RCT involving 14 641 US male physicians followed-up of for a mean of 8 years.35 Data on cardioembolic stroke are sparse. Likewise, few studies have investigated whether vitamin C status was associated with incident peripheral artery disease, although a clinical study revealed that acute vitamin C administration might restore peripheral endothelial function in patients with coronary artery disease.36 Plasma vitamin C was inversely associated with risk of atrial fibrillation in middle-aged women with low baseline intake but not in men37 and might prevent post-operative atrial fibrillation albeit with heterogeneous findings.38 Our study, on the contrary, found a possible positive association of genetically predicted circulating vitamin C levels with atrial fibrillation, a finding that needs to be verified in other studies.
A previous MR study found no association between plasma vitamin C proxied by rs33972313 in the SLC23A1 gene region and ischaemic heart disease,20 which is consistent with our findings. In addition, genetically proxied plasma vitamin C was not associated with certain cardiovascular risk factors, such as type 2 diabetes19 and urate.39
The major strength of the present study is the MR design, which diminished residual confounding and other biases, thereby strengthening the causal inference. In addition, we examined the association of genetically predicted vitamin C levels with CVDs using several data sources and the consistency of results the consistency of results supports the robustness of our findings. Along with the use of multiple independent SNPs as instrumental variables for plasma vitamin C, combining results for one outcome from different data sources increased the statistical power to detect weak associations even though we might have overlooked associations for certain outcomes with small number of cases. We confined the analyses to individuals of European ancestry, with the exception for the analysis for coronary artery disease based on consortium data where >80% of participants are individual of European descent. Thus, our findings were less likely to be influenced by population structure bias. Nonetheless, the population confinement limited the generalizability of our findings.
A potential limitation in MR studies is horizontal pleiotropy. We excluded an SNP (rs174547) with pleiotropic effects in the analysis to minimize bias from horizontal pleiotropy. Results were broadly consistent across sensitivity analyses and no evidence of horizontal pleiotropy was detected by MR-Egger regression. We also examined the association of plasma vitamin C with CVD using rs3397231320 in the SLC23A1 gene, which encodes the sodium-dependent vitamin C transporter 1, as instrumental variable and found consistent results. No evidence of bias from horizontal pleiotropy was detected. Another limitation is that we could not assess potential interaction effects of vitamin C with other antioxidants (e.g. vitamin E and β-carotene) on CVD. Our findings were based on generally healthy population and therefore cannot be generalized to populations with special features, such as individuals with vitamin C deficiency, patients with diabetes or kidney disease, and heavy smokers. There was small sample overlap in certain analyses. This overlap might have caused minor bias in the estimates towards the observational associations. Potential non-linear associations could not be examined in this MR study based on summary-level data.
In conclusion, this MR study suggests that elevating circulating vitamin C levels may not benefit the primary prevention of most CVDs. Whether increased vitamin C levels may decrease the risk of cardioembolic stroke and peripheral artery disease needs confirmation.
Acknowledgments
Genetic association estimates for CVDs were obtained from a GWAS meta-analysis of atrial fibrillation (Nielsen et al.), CARDIoGRAMplusC4D (Coronary ARtery DIsease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics), HERMES (Heart Failure Molecular Epidemiology for Therapeutic Targets) consortium, MEGASTROKE, ISGC (International Stroke Genetic Consortium), the UK Biobank study, and the FinnGen consortium. The authors thank all investigators for sharing these data. The MEGASTROKE project received funding from sources specified at http://www.megastroke.org/acknowledgments.html. The author list of MEGASTROKE is listed in https://www.megastroke.org/authors.html. Analyses of UK Biobank data were performed under application 29202.
Funding
A.M.M. is supported by EC-Innovative Medicines Initiative (BigData@Heart). S.B. is supported by Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z). J.S.Z. is supported by the National Natural Science Foundation of China (82073529, 81903316). S.C.L. is supported by research grants from the Karolinska Institutet’s Research Foundation Grants (Grant number 2020-01842), the Swedish Research Council for Health, Working Life and Welfare (Forte; grant no. 2018-00123), the Swedish Research Council (Vetenskapsrådet; grant no. 2016-01042 and 2019-00977), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden; grant no. 20190247).
Footnotes
Ethical approval
All studies included in cited genome-wide association studies had been approved by a relevant review board. The present MR analyses were approved by the Swedish Ethical Review Authority (2019-02793).
Conflict of interest:
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Role of the funder
Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Data availability
All data analysed in this study are available OSF data respiratory (https://osf.io/6qd8f/).
References
- 1.Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137:2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 2.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease. Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 3.Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17:1328. doi: 10.3390/ijms17081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khaw K-T, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 5.Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Hu FB, Manson JE, Willett WC. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol. 2003;42:246–252. doi: 10.1016/s0735-1097(03)00575-8. [DOI] [PubMed] [Google Scholar]
- 6.Pfister R, Sharp SJ, Luben R, Wareham NJ, Khaw K-T. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition-Norfolk prospective study. Am Heart J. 2011;162:246–253. doi: 10.1016/j.ahj.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Simon JA, Hudes ES, Tice JA. Relation of serum ascorbic acid to mortality among US adults. J Am Coll Nutr. 2001;20:255–263. doi: 10.1080/07315724.2001.10719040. [DOI] [PubMed] [Google Scholar]
- 8.Morelli MB, Gambardella J, Castellanos V, Trimarco V, Santulli G. Vitamin C and cardiovascular disease: an update. Antioxidants (Basel) 2020;9:1227. doi: 10.3390/antiox9121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu N, Huang B, Jiang W. Targets of vitamin C with therapeutic potential for cardiovascular disease and underlying mechanisms: a study of network pharmacology. Front Pharmacol. 2020;11:591337. doi: 10.3389/fphar.2020.591337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Khudairy L, Flowers N, Wheelhouse R, Ghannam O, Hartley L, Stranges S, Rees K. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;3:Cd011114. doi: 10.1002/14651858.CD011114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myung S-K, Ju W, Cho B, Oh S-W, Park SM, Koo B-K, Park B-J, Korean Meta-Analysis Study Group Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234–1239. doi: 10.1038/s41588-018-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122:433–443. doi: 10.1161/CIRCRESAHA.117.312086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman ÅK, Wilk JB, Morley MP, Chaffin MD, Helgadottir A, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11:163. doi: 10.1038/s41467-019-13690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese A-K, van der Laan SW, Gretarsdottir S, Anderson CD, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo D, Falcone GJ, Devan WJ, Brown WM, Biffi A, Howard TD, Anderson CD, Brouwers HB, Valant V, Battey TWK, Radmanesh F, et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. 2014;94:511–521. doi: 10.1016/j.ajhg.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consortium F. FinnGen documentation of R4 release. 2020. https://finngen.gitbook.io/documentation/ (20 December 2020)
- 19.Zheng J-S, Luan J, Sofianopoulou E, Imamura F, Stewart ID, Day FR, Pietzner M, Wheeler E, Lotta LA, Gundersen TE, Amiano P, et al. Plasma vitamin C and type 2 diabetes: genome-wide association study and Mendelian randomization analysis in European populations. Diabetes Care. 2021;44:98–106. doi: 10.2337/dc20-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobylecki CJ, Afzal S, Davey Smith G, Nordestgaard BG. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am J Clin Nutr. 2015;101:1135–1143. doi: 10.3945/ajcn.114.104497. [DOI] [PubMed] [Google Scholar]
- 21.Yuan S, Bäck M, Bruzelius M, Mason AM, Burgess S, Larsson S. Plasma phospholipid fatty acids, FADS1 and risk of 15 cardiovascular diseases: a Mendelian randomisation study. Nutrients. 2019;11:3001. doi: 10.3390/nu11123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28:30–42. doi: 10.1097/EDE.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Spiller W, Davies NM, Palmer TM. Software application profile: mrrobust—a tool for performing two-sample summary Mendelian randomization analyses. Int J Epidemiol. 2019;48:684–690. [Google Scholar]
- 27.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 28.Ascherio A, Rimm EB, Hernan MA, Giovannucci E, Kawachi I, Stampfer MJ, Willett WC. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med. 1999;130:963–970. doi: 10.7326/0003-4819-130-12-199906150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Larsson SC, Burgess S, Michaëlsson K. Serum magnesium levels and risk of coronary artery disease: Mendelian randomisation study. BMC Med. 2018;16:68. doi: 10.1186/s12916-018-1065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson SC, Traylor M, Burgess S, Boncoraglio GB, Jern C, Michaëlsson K, Markus HS, MEGASTROKE project of the International Stroke Genetics Consortium Serum magnesium and calcium levels in relation to ischemic stroke: Mendelian randomization study. Neurology. 2019;92:e944–e950. doi: 10.1212/WNL.0000000000007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson J, Pullar J, Wilson R, Spittlehouse J, Vissers M, Skidmore P, Willis J, Cameron V, Carr A. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: findings of the CHALICE cohort study. Nutrients. 2017;9:831. doi: 10.3390/nu9080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum NNa, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw K-T. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr. 2008;87:64–69. doi: 10.1093/ajcn/87.1.64. [DOI] [PubMed] [Google Scholar]
- 35.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erbs S, Gielen S, Linke A, Möbius-Winkler S, Adams V, Baither Y, Schuler G, Hambrecht R. Improvement of peripheral endothelial dysfunction by acute vitamin C application: different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am Heart J. 2003;146:280–285. doi: 10.1016/S0002-8703(03)00184-4. [DOI] [PubMed] [Google Scholar]
- 37.Pfister R, Michels G, Brägelmann J, Sharp SJ, Luben R, Wareham NJ, Khaw K-T. Plasma vitamin C and risk of hospitalisation with diagnosis of atrial fibrillation in men and women in EPIC-Norfolk prospective study. Int J Cardiol. 2014;177:830–835. doi: 10.1016/j.ijcard.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Hemilä H, Suonsyrjä T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17:49. doi: 10.1186/s12872-017-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobylecki CJ, Afzal S, Nordestgaard BG. Genetically high plasma vitamin C and urate: a Mendelian randomization study in 106 147 individuals from the general population. Rheumatology (Oxford) 2018;57:1769–1776. doi: 10.1093/rheumatology/key171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analysed in this study are available OSF data respiratory (https://osf.io/6qd8f/).