Abstract
Background
Previous observational studies have indicated a protective effect of drinking milk on asthma and allergy. In Mendelian Randomization, one or more genetic variants are used as unbiased markers of exposure to examine causal effects. We examined the causal effect of milk intake on hay fever, asthma, forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) by using the lactase rs4988235 genotype associated with milk intake.
Methods
We performed a Mendelian Randomization study including 363961 participants from the UK Biobank.
Results
Observational analyses showed that self-reported milk-drinkers vs. non-milk drinkers had an increased risk of hay fever: odds ratio (OR)=1.36 (95% CI: 1.32, 1.40, p<0.001), asthma: OR=1.33 (95% CI: 1.38, 1.29, p<0.001), yet a higher FEV1: β=0.022 (SE=0.004, p<0.001) and FVC: β=0.026 (SE=0.005, p<0.001). In contrast, genetically determined milk-drinking vs. not drinking milk was associated with a lower risk of hay fever: OR=0.791 (95% CI: 0.636, 0.982, p=0.033), and asthma: OR=0.587 (95% CI: 0.442, 0.779, p=0.001), and lower FEV1: β=-0.154 (standard error, SE=0.034, p<0.001) liter, and FVC: β=-0.223 (SE=0.034, p<0.001) liter in univariable MR analyses. These results were supported by multivariable Mendelian randomization analyses although not statistically significant.
Conclusions
As opposed to observational results, genetic association findings indicate that drinking milk has a protective effect on hay fever and asthma but may also have a negative effect on lung function. The results should be confirmed in other studies before any recommendations can be made.
Keywords: allergic disease, asthma, hay fever, milk, lung function
Introduction
The increase in allergic disease and asthma in the past decades has been attributed to changes in lifestyle and/or environmental factors (1). There has been a decline in the consumption of milk in this period (1) and thus there is a great interest in determining the impact of drinking or avoiding milk, including raw farm milk, breastfeeding, and intake of milk during pregnancy, on the development of allergic disease and asthma (2–17). Previous observational studies have suggested a possible protective effect of drinking milk on asthma (12, 14). However, the apparent protection of milk against asthma may result from avoidance of milk among parents of asthmatic children, rather than an actual prophylactic effect (13). Furthermore, milk consumption has been suspected to increase mucus and asthma symptoms (18).
Lactose is the primary carbohydrate in milk. It is metabolized by the lactase enzyme on the enterocytes. Most Northern Europeans can digest lactose their whole life, but in some individuals, lactase activity is reduced after they are accustomed to normal food. The single nucleotide polymorphism LCT-13910 C/T rs4988235 genotype exhibits complete correlation with lactase persistence in Northern Europeans. It is located 13910 base pairs upstream of the lactase gene on chromosome 2q21-22 within intron 13 of the adjacent MCM6 gene. The C and T alleles are the lactase non-persistent and lactase-persistent alleles, respectively. The rs4988235 genotype is autosomal recessively inherited. Homozygotes for the C allele cannot digest lactose, whereas heterozygotes and homozygotes for the T allele are able to digest lactose. Milk consumption may cause lactase non-persistent individuals symptoms of lactose intolerance, e.g., diarrhea and flatulence. They tend to consume less milk compared with lactase persistent individuals (19).
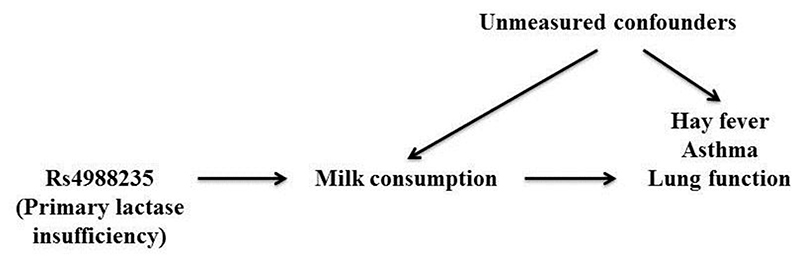
Causal inference from conventional epidemiologic studies between milk intake and allergic respiratory disease is difficult due to the potential confounding and reverse causation. Mendelian randomization examines causality by using one or more genetic variants, typically single nucleotide polymorphism (SNP), as instruments for exposures. It is based on the random allocation of genes from parents to offspring, which will not be associated with the confounding factors inherent in conventional observational studies. The validity of the instrument is dependent on three assumptions, referred to as ‘relevance’, ‘independence’, and ‘exclusion’. Here we used the rs4988235 genotype as an instrument for milk intake to test the causality of the association of milk consumption with hay fever, asthma, and lung function (illustrated in Fig. 1), in a Mendelian randomization meta-analysis of 363961 participants from the UK Biobank study.
Figure 1. Directed acyclic graph of an instrumental variable.
The instrument (the rs4988235 genotype) must be associated with the exposure (milk consumption), it must be independent of unmeasured confounders, and it must be independent of the outcome (hay fever/asthma/lung function) given the exposure (milk consumption) and the unmeasured confounders.
Methods
Study population
The UK Biobank is a large prospective study with more than 500000 participants (409694 of genetically European ancestry) from across the United Kingdom and aged 40–69 years at recruitment/initial assessment in 2006–2010 (20). We included 363961 participants with data on the rs4988235 genotype (including 83976 cases of hay fever and 40364 cases of asthma) of genetically determined European ancestry.
Genotypes
The lactase SNP was directly genotyped and on the opposite strand as the LCT-13910 C/T rs4988235 genotype which means that the G and A alleles are the lactase non-persistent and lactase-persistent alleles, respectively. Approximately 450000 of the participants have been genotyped using the UK Biobank Axiom array from Affymetrix. We had data on the rs4988235 genotype from this array. Minor allele frequency and Hardy Weinberg Equilibrium p-value for the rs4988235 were 0.25 and 0.600, respectively. We analyzed the genetic variant in two ways (1) an additive model with a per allele milk-increasing effect, (2) major homozygotes vs. heterozygotes and minor homozygotes combined.
In additional analyses, we used the asthma-associated SNPs 1) rs36045143, rs1837253, rs12413578, rs10876864, rs1059513, rs301805, rs1342326, rs744910, rs3894194, and rs2284033 in a simple genetic risk score for asthma (21, 22), 2) the rs10838738, rs10938397, rs10968576, rs11847697, rs12444979, rs13107325, rs1424233, rs1514175, rs1555543, rs17782313, rs1805081, rs206936, rs2112347, rs2241423, rs2287019, rs2568958, rs29941, rs3810291, rs4929949, rs543874, rs713586, rs7647305, rs9939609, rs10146997, rs1121980, and rs7138803 associated with body mass index (BMI) in a weighted genetic risk score (23), 3) and the rs16969968 associated with smoking (24).
Milk intake
We defined milk drinking status according to the answer to the question: “What type of milk do you mainly use?” (N= 363961). If the participants answered “Full cream”, “Semi-skimmed”, or “Skimmed”, they were categorized as milk-drinkers (Nmilk-drinkers= 335107). Otherwise (including the answers “Soya”, “Other type of milk”, “Never/rarely have milk”, “Do not know”, or “Prefer not to answer”), they were categorized as no-milk drinkers (Nno-milk drinkers=28854). In additional analyses, we restricted the no-milk drinkers to those answering “Never/rarely have milk”.
The amount of milk consumed (in glasses) was defined among those who answered the following question at the first assessment: “How many glasses/carton/ 250ml of milk (excluding milkshakes) did you consume yesterday?”. The options were half a glass, one, two, three, four, five or six glasses of milk (N of responders to this question=2298).
Hay fever and asthma
Hay fever was defined as a positive answer to the question: “Has a doctor ever told you that you have had any of the following conditions? Hay fever, allergic rhinitis, or eczema” (N= 363961, Ncases= 83976). Asthma was defined as a positive answer to the question: “Has a doctor ever told you that you have had any of the following conditions? Asthma” (N= 363961, Ncases=40364).
Due to the non-specific nature of the hay fever variable (i.e. including eczema), we assessed two other definitions of hay fever in additional analyses using information from the initial assessment visit 2006–2010: 1) We defined hay fever status according to self-reported use of medication for hay fever. From the complete list of >6000 recorded types of medication, we manually excluded the medication that <100 persons were taking or which we knew was not used in the treatment of hay fever. For each of the remaining medications, we assessed the general indication and excluded those not related to or too non-specific to treatment of hay fever. We ended up with 31 entries of medication as previously published (23). Persons who reported taking one or more of the medications on the list were defined as having hay fever according to this definition, and persons who did not were defined as not having hay fever. According to this definition, 7110 participants out of 363961 were defined as having hay fewer. 2) We defined hay fever status according to doctor diagnosis. The participants stated in the UK Biobank touch screen questionnaire whether they had been told by a doctor that they have other serious illnesses or disabilities. If they answered “Yes” they were later asked by an interviewer: “In the touch screen you selected that you have been told by a doctor that you have other serious illnesses or disabilities, could you now tell me what they are?” If the participant answered “hay fever” we defined the participant as having hay fever. Otherwise, we defined the participant as not having hay fever. According to this definition, 14135 participants out of 363691 were defined as having hay fever.
Lung function
Forced expiratory volume in 1-second (FEV1) and forced vital capacity (FVC) were measured at baseline by spirometry using a Vitalograph Pneumotrac 6800 (Vitalograph, UK) operated via a PC for data capture and in order to visualize flow graphs from each test. The spirometer was calibrated in the beginning of each day by the Spirotrac software supplied with the Pneumotrac 6800. FEV1 and FVC were measured in 327803 of the included individuals and expressed in liters.
Other covariates
All the 363961 individuals included had data on age, sex, and 40 genetic principal components. Other covariates had the following number of missing values: smoking status categorized as never, former or current smokers (Nmissing=1427), smoking quantity was expressed in cigarettes per day, alcohol status categorized as drinkers and non-drinkers (Nmissing=258), and body mass index (BMI) expressed in kg/m2 (Nmissing=1247).
Statistical analyses
Statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), STATA, version 12 (StataCorp, College Station, TX, USA), and the statistical software R, version 3.3.3 (http://www.r-project.org/). The p-values are two-tailed, and statistical significance was defined as p<0.05. Participants with missing value in one or more variables were excluded in the regression models. The univariable genetic association analyses of the milk-associated SNP and milk intake status, hay fever, asthma and lung function were assessed using linear and logistic regression and performed unadjusted; adjusted for age, gender, and genetic principal components (PCs); and further adjusted for smoking, alcohol, and BMI (Table 3–4). The conventional observational analyses of the associations of milk intake status with hay fever, asthma and lung function were assessed by logistic and linear regression analyses and were performed unadjusted; adjusted for age, and gender; and further adjusted for smoking status, alcohol status and BMI (Table 2 and 5). Third, for the model adjusted for age, gender, and genetic PCs, instrumental variable (IV) analyses were performed by the “MendelianRandomization” package in R using the inverse variance-weighted method (“mr_ivw”), similar to the ratio method for a single IV. This model is considered the most correct: it is most likely to account for factors unaccounted for by the genetic instruments (such as the principal components) and least likely to infer additional bias by breaking the ‘randomization’ (by adjusting for, e.g., BMI that could infer bias if associated with an unmeasured confounder).
Table 3. Associations of genetically determined levels of milk intake with lung function (N=325737).
| Unadjusted | Adjusted for age, sex, and PCs | Further adjusted for smoking, alcohol and BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | Beta | SE | P | |
| FEV1, liter | |||||||||
| Lactase genotype | |||||||||
| GG (non-persistence) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| GA (persistence) | -0.017 | 0.006 | 0.004 | -0.011 | 0.004 | 0.008 | -0.009 | 0.004 | 0.023 |
| AA (persistence) | 0.017 | 0.003 | <0.001 | 0.010 | 0.002 | <0.001 | 0.009 | 0.002 | <0.001 |
| AA vs. GA/GG | 0.016 | 0.003 | <0.001 | 0.010 | 0.002 | <0.001 | 0.008 | 0.002 | <0.001 |
| Per milk-increasing allele | -0.014 | 0.002 | <0.001 | -0.009 | 0.002 | <0.001 | -0.008 | 0.002 | <0.001 |
| Genotype × milk* | 0.201 | 0.536 | 0.775 | ||||||
| Drinking vs. not drinking milk (genetically determined) | -0.154 | 0.034 | <0.001 | ||||||
| Per glass of genetically determined higher milk intake** | -0.138 | 0.031 | <0.001 | ||||||
| FVC, liter | |||||||||
| Lactase genotype | |||||||||
| GG (non-persistence) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| GA (persistence) | -0.027 | 0.008 | <0.001 | -0.020 | 0.006 | <0.001 | -0.017 | 0.006 | 0.003 |
| AA (persistence) | -0.044 | 0.008 | <0.001 | -0.031 | 0.006 | <0.001 | -0.026 | 0.006 | <0.001 |
| AA vs. GA/GG | 0.020 | 0.004 | <0.001 | 0.014 | 0.003 | <0.001 | 0.011 | 0.003 | <0.001 |
| Per milk-increasing allele | -0.019 | 0.003 | <0.001 | -0.013 | 0.002 | <0.001 | -0.011 | 0.002 | <0.001 |
| Genotype × milk* | 0.247 | 0.623 | 0.933 | ||||||
| Drinking vs. not drinking milk (genetically determined) | -0.223 | 0.034 | <0.001 | ||||||
| Per glass of genetically determined higher milk intake** | -0.200 | 0.031 | <0.001 | ||||||
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; principal component, SE, standard error.
rs4988235 genotype (per allele) × drinking milk (no/yes) interaction test.
SNP-milk intake estimates, N=2298. SNP-outcome estimates: N=325737.
Table 4. Associations of genetically determined milk intake with hay fever and asthma (N=361302).
| unadjusted | Adjusted for age, sex, and PCs | +smoking, alcohol, and BMI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | LCI | UCI | P | OR | SE**** | LCI | UCI | P | OR | LCI | UCI | p | |
| Hay fever | |||||||||||||
| Lactase genotype | |||||||||||||
| GG (non-persistence) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| GA (persistence) | 0.989 | 0.956 | 1.022 | 0.514 | 1.001 | 0.968 | 1.036 | 0.939 | 1.000 | 0.967 | 1.035 | 0.967 | |
| AA (persistence) | 0.956 | 0.925 | 0.988 | 0.007 | 0.983 | 0.951 | 1.016 | 0.304 | 0.983 | 0.951 | 1.016 | 0.305 | |
| AA vs. GA/GG | 0.965 | 0.951 | 0.981 | <0.001 | 0.982 | 0.966 | 0.997 | 0.022 | 0.982 | 0.967 | 0.998 | 0.026 | |
| Per milk-increasing allele | 0.972 | 0.960 | 0.985 | <0.001 | 0.986 | 0.006 | 0.974 | 0.999 | 0.036 | 0.987 | 0.974 | 0.999 | 0.041 |
| Genotype × milk* | 0.047 | 0.044 | 0.048 | ||||||||||
| Drinking vs. not drinking milk (genetically determined) | 0.791 | 0.636 | 0.982 | 0.033 | |||||||||
| Per glass of genetically determined higher milk intake***** | 0.810 | 0.667 | 0.983 | 0.033 | |||||||||
| Hay fever, no-milk drinkers** | |||||||||||||
| Lactase genotype | |||||||||||||
| GG (non-persistence) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| GA (persistence) | 1.030 | 0.929 | 1.145 | 0.568 | 1.033 | 0.930 | 1.149 | 0.535 | 1.039 | 0.934 | 1.155 | 0.479 | |
| AA (persistence) | 1.033 | 0.933 | 1.143 | 0.529 | 1.062 | 0.958 | 1.177 | 0.253 | 1.069 | 0.963 | 1.185 | 0.210 | |
| AA vs. GA/GG | 1.007 | 0.957 | 1.060 | 0.785 | 1.032 | 0.980 | 1.088 | 0.229 | 1.035 | 0.982 | 1.090 | 0.203 | |
| Per milk-increasing allele | 1.010 | 0.970 | 1.051 | 0.635 | 1.029 | 0.022 | 0.987 | 1.072 | 0.177 | 1.031 | 0.989 | 1.075 | 0.148 |
| Hay fever, milk drinkers*** | |||||||||||||
| Lactase genotype | |||||||||||||
| GG (non-persistence) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| GA (persistence) | 0.989 | 0.955 | 1.025 | 0.549 | 1.002 | 0.967 | 1.038 | 0.911 | 1.001 | 0.966 | 1.037 | 0.965 | |
| AA (persistence) | 0.954 | 0.921 | 0.987 | 0.007 | 0.980 | 0.946 | 1.014 | 0.246 | 0.979 | 0.945 | 1.014 | 0.232 | |
| AA vs. GA/GG | 0.963 | 0.947 | 0.979 | <0.001 | 0.978 | 0.962 | 0.994 | 0.008 | 0.978 | 0.962 | 0.995 | 0.009 | |
| Per milk-increasing allele | 0.970 | 0.957 | 0.983 | <0.001 | 0.984 | 0.007 | 0.970 | 0.997 | 0.016 | 0.984 | 0.970 | 0.997 | 0.017 |
| Per glass of genetically determined higher milk intake***** | 0.775 | 0.632 | 0.949 | 0.014 | |||||||||
| Asthma | |||||||||||||
| Lactase genotype | |||||||||||||
| GG (non-persistence) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| GA (persistence) | 0.976 | 0.933 | 1.021 | 0.286 | 0.976 | 0.934 | 1.021 | 0.295 | 0.971 | 0.928 | 1.05 | 0.198 | |
| AA (persistence) | 0.944 | 0.904 | 0.986 | 0.009 | 0.944 | 0.904 | 0.987 | 0.010 | 0.937 | 0.896 | 0.979 | 0.004 | |
| AA vs. GA/GG | 0.964 | 0.944 | 0.984 | <0.001 | 0.964 | 0.944 | 0.984 | <0.001 | 0.960 | 0.941 | 0.981 | <0.001 | |
| Per milk-increasing allele | 0.969 | 0.953 | 0.986 | <0.001 | 0.969 | 0.008 | 0.953 | 0.986 | <0.001 | 0.966 | 0.950 | 0.982 | <0.001 |
| Genotype × milk* | 0.615 | 0.594 | 0.495 | ||||||||||
| Drinking vs. not drinking milk (genetically determined) | 0.587 | 0.442 | 0.779 | 0.001 | |||||||||
| Per glass of genetically determined higher milk intake***** | 0.620 | 0.481 | 0.799 | 0.001 | |||||||||
Note: ‘Genetically determined’ refers to (1) an additive model with a per allele (A) milk-increasing effect, and (2) major homozygotes (AA) vs. heterozygotes (GA) and minor homozygotes combined (GG). AA: N=205376, GA: N=133909, GG: N=22017.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OR, odds ratio; LCI, lower confidence interval; UCI, upper confidence interval; PC, principal component; SE, standard error.
rs4988235 genotype × drinking milk (no/yes) interaction test.
N=28854.
N=335107.
Used in IV-analyses.
SNP-milk intake estimates, N=2298. SNP-outcome estimates: Nhay fever =361302. Nhay fever, milk drinkers=335107. Nasthma=361302.
Table 2. Associations of self-reported milk intake with hay fever and asthma.
| N | Unadjusted | Adjusted for age and sex | + smoking, alcohol and BMI OR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | LCI | UCI | P | OR | LCI | UCI | P | OR | LCI | UCI | P | ||
| Hay fever | |||||||||||||
| Non-milk drinkers | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Milk-drinkers | 363961 | 1.387 | 1.350 | 1.425 | <0.001 | 1.358 | 1.322 | 1.395 | <0.001 | 1.363 | 1.326 | 1.401 | <0.001 |
| Per glass of milk yesterday* | 2298 | 0.968 | 0.852 | 1.101 | 0.629 | 0.972 | 0.853 | 1.107 | 0.679 | 0.978 | 0.858 | 1.116 | 0.749 |
| Asthma | |||||||||||||
| Non-milk drinkers | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Milk-drinkers | 363961 | 1.326 | 1.281 | 1.374 | <0.001 | 1.303 | 1.258 | 1.349 | <0.001 | 1.334 | 1.287 | 1.382 | <0.001 |
| Per glass of milk yesterday* | 2298 | 1.095 | 0.902 | 1.328 | 0.355 | 1.099 | 0.903 | 1.337 | 0.345 | 1.148 | 0.940 | 1.401 | 0.175 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OR, odds ratio; LCI, lower confidence interval; UCI, upper confidence interval.
Among those who reported to have a milk intake yesterday.
Table 5. Associations of self-reported milk intake with lung function.
| N | Unadjusted | Adjusted for age and sex | +smoking, alcohol and BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | P | Beta | SE | P | Beta | SE | P | ||
| FEV1 | ||||||||||
| Non-milk drinkers | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| Milk-drinkers | 327803 | 0.109 | 0.005 | <0.001 | 0.016 | 0.004 | <0.001 | 0.022 | 0.004 | <0.001 |
| Per glass of milk yesterday* | 2068 | 0.135 | 0.024 | <0.001 | 0.019 | 0.017 | 0.283 | 0.027 | 0.017 | 0.122 |
| FVC | ||||||||||
| Non-milk drinkers | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| Milk-drinkers | 327803 | 0.139 | 0.007 | <0.001 | 0.011 | 0.005 | 0.023 | 0.026 | 0.005 | <0.001 |
| Per glass of milk yesterday* | 2068 | 0.184 | 0.031 | <0.001 | 0.040 | 0.022 | 0.063 | 0.057 | 0.021 | 0.008 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SE, standard error.
Among those who reported to have a milk intake yesterday.
In additional analyses, we performed multivariable MR analyses incl. genetically predicted milk consumption, smoking, and BMI in a single model using the ‘ivreg2’ and ‘bootstrap’ in Stata, adjusted for age, gender, and the first 10 PCs. We also examined the association between a genetic risk score for asthma and drinking milk, adjusted for age, gender, and the first 10 PCs.
Results
Descriptive statistics of the participants are shown in Table 1. The rs4988235 genotype was significantly associated with smoking status, BMI, and drinking milk in crude analyses. When restricting the no-milk drinkers to those answering “Never/rarely have milk”, the results were similar.
Table 1. Characteristics of the UK Biobank study population (N=363961).
| rs4988235 genotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | P-value (Kruskal Wallis’ or Chi2 test) | ||||||||
| Characteristics | N | % or mean (SD) | N | % or mean (SD) | N | % or mean (SD) | Genotype | Hay fever | Asthma | FEV1 | FVC |
| All | 22088 | 6.1 | 134908 | 37.1 | 206965 | 56.9 | NA | NA | NA | NA | NA |
| Sex | |||||||||||
| Women | 11993 | 54.3 | 73602 | 54.6 | 112959 | 54.6 | 0.7251 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Men | 10095 | 45.7 | 61306 | 45.4 | 94006 | 45.4 | |||||
| Age, years | 22088 | 56.9 (8.0) | 134908 | 56.9 (8.0) | 206965 | 56.9 (8.0) | 0.8053 | <0.0001 | <0.0001 | NA | NA |
| Smoking status | |||||||||||
| Never | 12210 | 55.5 | 74345 | 55.3 | 113124 | 54.9 | 0.0013 | <0.0001 | <0.0001 | 0.0006 | <0.0001 |
| Former | 7884 | 35.8 | 47941 | 35.7 | 73838 | 35.8 | |||||
| Current | 1908 | 8.7 | 12106 | 9.0 | 19178 | 9.3 | |||||
| Drinking alcohol | |||||||||||
| No | 660 | 3.0 | 4244 | 3.1 | 6397 | 3.1 | 0.3865 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Yes | 21417 | 97.0 | 130567 | 96.9 | 200418 | 96.9 | |||||
| Alcohol intake, drinks/week BMI group | 15990 | 8.5 (8.8) | 97021 | 8.5 (8.8) | 148872 | 8.6 (8.6) | 0.2590 | <0.0001 | 0.0051 | NA | NA |
| <18.5 kg/m2 | 139 | 0.6 | 711 | 0.5 | 976 | 0.5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 18.5–25 kg/m2 | 7566 | 34.4 | 44793 | 33.3 | 67054 | 32.5 | |||||
| >25–30 kg/m2 | 9342 | 42.4 | 57465 | 42.7 | 88492 | 42.9 | |||||
| >30 kg/m2 | 4972 | 22.6 | 31481 | 23.4 | 49723 | 24.1 | |||||
| BMI, kg/m2 | 22019 | 27.2 (4.7) | 134450 | 27.3 (4.7) | 206245 | 27.4 (4.7) | <0.0001 | <0.0001 | <0.0001 | NA | NA |
| Drinking milk | |||||||||||
| No | 2074 | 9.4 | 10723 | 7.9 | 16057 | 7.8 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Yes | 20014 | 90.6 | 124185 | 92.1 | 190908 | 92.2 | |||||
| Milk yesterday, glasses* | 137 | 0.97 (0.47) | 880 | 1.11 (0.69) | 1281 | 1.15 (0.75) | 0.1371 | 0.6246 | 0.7065 | NA | NA |
| Fizzy drinks yesterday, glasses Orange juice | 216 | 1.25 (0.82) | 1263 | 1.17 (0.74) | 1740 | 1.21 (0.74) | 0.1135 | 0.0731 | 0.0793 | NA | NA |
| yesterday, glasses | 647 | 0.98 (0.52) | 3741 | 0.97 (0.51) | 5437 | 0.96 (0.49) | 0.7292 | 0.1040 | 0.0084 | NA | NA |
| Energy intake yesterday**, kJ | 2381 | 8900 (3212) | 13921 | 8863 (3186) | 20180 | 8835 (3080) | 0.7856 | 0.0001 | 0.2708 | NA | NA |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
Among those who reported to have a milk intake yesterday.
Calculated.
Observational analyses
Self-reported milk-drinkers vs. non-milk drinkers had an OR=1.36 (95% CI: 1.32, 1.40, p<0.001) and OR=1.33 (95% CI: 1.29, 1.38, p<0.001) of hay fever and asthma, respectively (Table 2, adjusted for age, sex, smoking and alcohol status, and BMI). Milk intake quantity was not significantly associated with hay fever or asthma in the smaller sample with available data on quantity of milk intake (n=2298).
Self-reported milk-drinkers had higher FEV1: β=0.022 (SE=0.004, p<0.001) and FVC: β=0.026 (SE=0.005, p<0.001) than non-milk drinkers (Table 5, adjusted for age, sex, smoking and alcohol status, and BMI). Milk intake quantity (per glass of milk yesterday) was significantly associated with higher FVC: β=0.057 (SE=0.021, p=0.008) but not with FEV1: β=0.027 (SE=0.017, p=0.122).
Mendelian randomization analyses
The rs4988235 variant was associated with 1.06-fold increased odds of drinking milk per each lactose persistence allele (95% CI: 1.04, 1.08, SE=0.011, p<0.001, N=363961) (adjusted for age, sex and 40 PCs). Among milk drinkers, each lactose persistence allele increased milk intake by 0.065 liters (95% CI: 0.017, 0.113, SE=0.025, p=0.008, N=2298) (adjusted for age, sex and 40 PCs).
Genetically determined higher milk intake was associated with a lower risk of hay fever: OR=0.986 (95% CI: 0.974, 0.999, p=0.036) per milk-increasing allele (Table 4, adjusted for age, sex and PCs). Genetically determined drinking vs. not drinking milk was associated with a lower risk of hay fever: OR=0.791 (95% CI: 0.636, 0.982, p=0.033). The odds ratio of hay fever was OR=0.810 (95% CI: 0.667, 0.983, p=0.003) per glass of genetically determined higher milk intake.
There was a statistically significant interaction between genotype and milk intake status. In milk-drinkers, the associations were strengthened with an odds ratio of hay fever: OR=0.984 (95% CI: 0.970, 0.997, p=0.016) per milk-increasing allele. In non-milk drinkers the association was absent with an odds ratio of hay fever: OR=1.029 (95% CI: 0.987, 1.072) per milk-increasing allele (Table 4, adjusted for age, sex, and PCs).
Genetically determined higher milk intake was associated with a lower risk of asthma: OR=0.969 (95% CI: 0.953, 0.986, p<0.001) per milk-increasing allele (Table 4, adjusted for age, sex and PCs). Genetically determined drinking vs. not drinking milk was associated with a lower risk of asthma: OR=0.587 (95% CI: 0.442, 0.779, p=0.001). The odds ratio of asthma was OR=0.620 (95% CI: 0.481, 0.799, p=0.001) per glass of genetically determined higher milk intake.
Genetically determined higher milk intake was associated with lower FEV1: β=-0.009 (SE=0.002, p<0.001) liter per milk-increasing allele (Table 3, adjusted for age, sex, and PCs). Genetically determined drinking vs. not drinking milk was associated with lower FEV1: β=-0.154 (SE=0.034, p<0.001) liter. FEV1 was β=-0.138 (SE=0.031, p<0.001) liter per glass of genetically determined higher milk intake.
Genetically determined higher milk intake was also associated with lower FVC: β=-0.013 (SE=0.002, p<0.001) liter per milk-increasing allele (Table 3, adjusted for age, sex, and PCs).
Genetically determined drinking vs. not drinking milk was associated with lower FVC: β=-0.223 (SE=0.034, p<0.001) liter. FVC was β=-0.200 (SE=0.031, p<0.001) liter per glass of genetically determined higher milk intake.
The primary definition of hay fever showed among milk-drinkers an OR=0.984 (95% CI: 0.970, 0.997, p=0.016, N=335107, Ncases=75664) per milk increasing allele. Using self-reported hay fever medication as alternative definition of hay fever yielded an OR=0.949 (95% CI: 0.911, 0.988, p=0.011, N=335107, Ncases=6415) per milk increasing allele. Using self-reported hay fever classified as serious illness as alternative definitions of hay fever indicated an OR=0.997 (95% CI: 0.968, 1.026, p=0.820, N=335107, Ncases=12688) per milk increasing allele.
The weighted genetic BMI-score was significantly associated with BMI with an estimate=0.92 (95% CI: 0.85, 0.99, p<0.001, N=84946) kg/m2 higher BMI per BMI-increasing allele. The smoking associated SNP was associated with an estimate=0.96 (95% CI: 0.61, 1.32, p<0.001, N=5006) cigarettes per day per smoking-increasing allele among current smokers and an estimate=0.11 (95% CI: 0.07, 0.15, p<0.001, N=81853) cigarettes per day per smoking-increasing allele among never, former, and current smokers.
Multivariable MR analyses of genetically determined milk drinking, BMI and smoking quantity and adjusted for age, sex, and 10 principal components, showed non-significant inverse effects of genetically determined milk drinking on asthma with OR=0.66 (95% CI: 0.17, 2.64, p=0.555, N=81564, Fmilk=4.7, FBMI=235, Fsmoking=11.1) for having asthma, OR=0.72 (95% CI: 0.17, 3.00, N=81564, p=0.650, Fmilk=4.7, FBMI=235, Fsmoking=11.1) for having hay fever, FEV1 with estimate=-0.70 (95% CI: -24.3, 22.9, p=0.954, N=73525, Fmilk= 4.1, FBMI=220, Fsmoking=5.5) liter higher FEV1, and estimate=-1.3 (95% CI: -38.8, 36.1, N=73525, p=0.944, Fmilk=4.1, FBMI=220, Fsmoking=5.5) liter higher FVC per milk-increasing allele, respectively. When restricting the no-milk drinkers to those answering “Never/rarely have milk”, the results were similar.
The genetic risk score for asthma was significantly associated with a higher odds ratio of asthma: OR=1.08 (95% CI: 1.08, 1.09, p<0.001, N=353576) per asthma-increasing allele, adjusted for age, sex, and 10 principal components. The genetic risk-score for asthma was positively associated with drinking milk with OR=1.006 (95% CI: 1.000, 1.012, p=0.0498, N=353576) of drinking milk per asthma-increasing allele, adjusted for age, sex, and 10 principal components. Genetically determined higher risk of asthma was not associated with amount of milk intake among milk drinkers: estimate=0.008 (95% CI: -0.007, 0.023, p=0.315, N=2219) glasses of milk per asthma-increasing allele, adjusted for age, sex, and 10 principal components.
Discussion
While observational studies showed higher risk of asthma and hay fever among milk drinkers and higher lung function, univariable Mendelian randomization analyses showed lower risk of hay fever and asthma, but lower FEV1 and FVC, for genetically determined higher milk intake and for genetically determined milk-drinkers compared to non-milk drinkers. These results were supported by multivariable Mendelian randomization analyses although these analyses lacked power and were not statistically significant.
Unlike our observational results, most previous traditional observational studies on the association of milk consumption on allergies and asthma show a protective effect of milk on hay fever and asthma. Mai and colleagues found that asthma was significantly associated with infrequent milk consumption among girls, but not boys (12). Another study found that milk consumption did not protect against childhood asthma and concluded that previously observed protection of milk against asthma could be due to parents avoiding milk for their asthmatic children (13). A third study found that the prevalence of recent asthma at age three was lower in children who at age two consumed full cream milk daily than in those who did not. Likewise, the prevalence of recent wheeze was lower in children who consumed milk products daily compared to those who did not (14). Even intake of milk during pregnancy has been subject to investigating regarding a possible protective effect on asthma and allergy in the offspring. Thus, Bunyavanich and colleagues found that a higher maternal intake of, for example, milk in early pregnancy was associated with lower risk of allergy and asthma in mid-childhood (11).
Observational studies of drinking milk and lung volume are few. Thiara describes how consumption of milk has been believed to increase mucus secretion and worsen asthma (18). Haas and colleagues found that milk lipids may disturb gas exchange in 11 asthmatic patients, but not in 10 healthy controls (15). Woods and colleagues concluded that dairy products are unlikely to be a bronchoconstrictor in most asthmatic patients (16). Yusoff and colleagues found an eight weeks period of egg- and milk-free diet to reduce allergic symptoms and improve lung function in asthmatic children (17). However, the study was only single-blinded and not randomized; the parents were given the option to choose for their children either the intervention group avoiding eggs and milk, or the control group consuming their ordinary diet. The available studies are quite small and inconclusive.
Although a genetic marker of milk consumption is less likely to be associated with common confounding factors and is unaffected by reverse causation, violation of the main principles of Mendelian randomization may hamper causal inference. According to the relevance assumption, the instrument must be associated with the exposure, i.e. the rs4988235 genotype needs to be associated with milk consumption. The risk of violating this assumption is reduced by using a genotype biologically associated with the digestion of lactose (63). In accordance with previous studies, we found that the rs4988235 was strongly associated with milk intake status supporting that rs4988235 is well-suited as a genetic instrument of milk intake. The exclusion assumption says that the rs4988235 genotype needs to be independent of the hay fever/asthma/lung function given the milk consumption and unmeasured confounders, i.e. the rs4988235 genotype can only affect hay fever/asthma/lung function through milk consumption. This assumption is strengthened by the fact that the genotype codes for a lactose digesting enzyme rather than a complex trait such as BMI. To avoid confounding by population stratification, we adjusted for population structure using 40 principal components. The use of a single SNP prevents the use of pleiotropy robust methods such as MR Egger and MRMix (25). However, our study included multivariable MR-analyses to account for the possible pleiotropic effect of the lactase SNP through smoking and BMI. In addition, we examined the association between a genetic asthma-score and drinking milk to investigate any reverse causation, e.g., if asthmatics stop drinking milk after they develop asthma.
A limitation of the study is the use of self-reported hay fever and asthma rather than clinical diagnoses or measurements. Non-differential misclassification of a binary outcome is likely to attenuate associations towards the null. We also defined milk intake status from self-report. Milk intake quantity was assessed in a very small number of individuals which makes the quantity measures in liter somewhat unreliable. The primary hay fever variable included eczema. Therefore, we assessed another two hay fever variables, one of which yielded a similar results and the other a non-significant result. The main weaknesses of the additional hay fever variables were the fact that one was based on self-reported medication rather than doctor-diagnosed hay fever, and the other only counted hay fever cases that were classified as a serious illness by the participants. It is likely that those drinking (more) milk have other dietary habits as well, e.g., substituting fizzy drinks or juice, or having a different total energy intake. However, the rs4988235 genotype was not associated with any of these. Although different dietary habits might still be an issue in the observational analyses, it is not likely to be in the Mendelian Randomization analyses.
In conclusion, as opposed to our traditional observational results that found higher risk of asthma and hay fever among milk drinkers and higher lung function but are likely to suffer from confounding and reverse causation, our genetic results indicate that drinking milk infers a lower risk of hay fever and asthma, but lower FEV1 and FVC. The results should be confirmed in other studies before any recommendations can be made.
Ethics
Each participant has given informed consent. An independent Ethics and Governance Council oversees adherence to the Ethics and Governance Framework (20).
Funding and acknowledgements
Tea Skaaby was supported by grants from the Lundbeck Foundation (Grant number R165-2013-15410 and R219-2016-471), the Harboe Foundation (Grant number 16152), the A.P. Møller Foundation for the Advancement of Medical Science (Grant number 15-363), Aase and Einar Danielsen’s Foundation (Grant number 10-001490) and the Weimann’s grant. This research has been conducted using the UK Biobank Resource (Application number: 17765). The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk).
Footnotes
Conflicts of interest
None
References
- 1.Aadahl M, Linneberg A, Moller TC, Rosenorn S, Dunstan DW, Witte DR, et al. Motivational counseling to reduce sitting time: a community-based randomized controlled trial in adults. AmJPrevMed. 2014;47:576–586. doi: 10.1016/j.amepre.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Brick T, Schober Y, Booking C, Pekkanen J, Genuneit J, Loss G, et al. omega-3 fatty acids contribute to the asthma-protective effect of unprocessed cow’s milk. The Journal of allergy and clinical immunology. 2016;137:1699–1706.:e1613. doi: 10.1016/j.jaci.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. The Journal of allergy and clinical immunology. 2014;133:551–559. doi: 10.1016/j.jaci.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Sozanska B, Pearce N, Dudek K, Cullinan P. Consumption of unpasteurized milk and its effects on atopy and asthma in children and adult inhabitants in rural Poland. Allergy. 2013;68:644–650. doi: 10.1111/all.12147. [DOI] [PubMed] [Google Scholar]
- 5.Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. The Journal of allergy and clinical immunology. 2011;128:766–773.:e764. doi: 10.1016/j.jaci.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 6.Waser M, Michels KB, Bieli C, Floistrup H, Pershagen G, von Mutius E, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37:661–670. doi: 10.1111/j.1365-2222.2006.02640.x. [DOI] [PubMed] [Google Scholar]
- 7.van Elten TM, van Rossem L, Wijga AH, Brunekreef B, de Jongste JC, Koppelman GH, et al. Breast milk fatty acid composition has a long-term effect on the risk of asthma, eczema, and sensitization. Allergy. 2015;70:1468–1476. doi: 10.1111/all.12703. [DOI] [PubMed] [Google Scholar]
- 8.Soto-Ramirez N, Karmaus W, Zhang H, Liu J, Billings D, Gangur V, et al. Fatty acids in breast milk associated with asthma-like symptoms and atopy in infancy: a longitudinal study. The Journal of asthma : official journal of the Association for the Care of Asthma. 2012;49:926–934. doi: 10.3109/02770903.2012.719251. [DOI] [PubMed] [Google Scholar]
- 9.Rothenbacher D, Weyermann M, Beermann C, Brenner H. Breastfeeding, soluble CD14 concentration in breast milk and risk of atopic dermatitis and asthma in early childhood: birth cohort study. Clin Exp Allergy. 2005;35:1014–1021. doi: 10.1111/j.1365-2222.2005.02298.x. [DOI] [PubMed] [Google Scholar]
- 10.Wijga AH, van Houwelingen AC, Kerkhof M, Tabak C, de Jongste JC, Gerritsen J, et al. Breast milk fatty acids and allergic disease in preschool children: the Prevention and Incidence of Asthma and Mite Allergy birth cohort study. The Journal of allergy and clinical immunology. 2006;117:440–447. doi: 10.1016/j.jaci.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA, Jr, et al. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. The Journal of allergy and clinical immunology. 2014;133:1373–1382. doi: 10.1016/j.jaci.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mai XM, Becker AB, Sellers EA, Liem JJ, Kozyrskyj AL. Infrequent milk consumption plus being overweight may have great risk for asthma in girls. Allergy. 2007;62:1295–1301. doi: 10.1111/j.1398-9995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 13.Fussman C, Todem D, Forster J, Arshad H, Urbanek R, Karmaus W. Cow’s milk exposure and asthma in a newborn cohort: repeated ascertainment indicates reverse causation. The Journal of asthma : official journal of the Association for the Care of Asthma. 2007;44:99–105. doi: 10.1080/02770900601180669. [DOI] [PubMed] [Google Scholar]
- 14.Wijga AH, Smit HA, Kerkhof M, de Jongste JC, Gerritsen J, Neijens HJ, et al. Association of consumption of products containing milk fat with reduced asthma risk in pre-school children: the PIAMA birth cohort study. Thorax. 2003;58:567–572. doi: 10.1136/thorax.58.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas F, Bishop Mc, Fau, Salazar-Schicchi J, Fau, Axen KV, Fau, Lieberman D, Fau, Axen K. Effect of milk ingestion on pulmonary function in healthy and asthmatic subjects. doi: 10.3109/02770909109089462. [DOI] [PubMed] [Google Scholar]
- 16.Woods RK, Weiner Jm, Fau, Abramson M, Fau, Thien F, Fau, Walters EH. Do dairy products induce bronchoconstriction in adults with asthma? doi: 10.1016/S0091-6749(98)70192-7. [DOI] [PubMed] [Google Scholar]
- 17.Yusoff NA, Hampton Sm, Fau, Dickerson JW, Fau, Morgan JB. The effects of exclusion of dietary egg and milk in the management of asthmatic children: a pilot study. doi: 10.1177/146642400412400211. [DOI] [PubMed] [Google Scholar]
- 18.Thiara G, Goldman RD. Milk consumption and mucus production in children with asthma. Canadian family physician Medecin de famille canadien. 2012;58:165–166. [PMC free article] [PubMed] [Google Scholar]
- 19.Bergholdt HK, Larsen MK, Varbo A, Nordestgaard BG, Ellervik C. Lactase persistence, milk intake, hip fracture, and bone mineral density: A study of 97,811 Danish individuals and a meta-analysis. Journal of internal medicine. 2018 doi: 10.1111/joim.12753. [DOI] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoSMed. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Lee PH, Chaffin MD, Chung W, Loh PR, Lu Q, et al. A genome-wide cross-trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nature genetics. 2018;50:857–864. doi: 10.1038/s41588-018-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skaaby T, Taylor AE, Thuesen BH, Jacobsen RK, Friedrich N, Mollehave LT, et al. Estimating the causal effect of body mass index on hay fever, asthma and lung function using Mendelian randomization. Allergy. 2017 doi: 10.1111/all.13242. [DOI] [PubMed] [Google Scholar]
- 24.Skaaby T, Taylor AE, Jacobsen RK, Paternoster L, Thuesen BH, Ahluwalia TS, et al. Investigating the causal effect of smoking on hay fever and asthma: a Mendelian randomization meta-analysis in the CARTA consortium. SciRep. 2017;7:2224. doi: 10.1038/s41598-017-01977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi G, Chatterjee N. Mendelian randomization analysis using mixture models for robust and efficient estimation of causal effects. Nat Commun. 2019;10:1941. doi: 10.1038/s41467-019-09432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]