Abstract
Methods to acquire and process synaptic-resolution electron-microscopy datasets have progressed very rapidly, allowing production and annotation of larger, more complete connectomes. More accurate neuronal matching techniques are enriching cell type data with gene expression, neuron activity, behaviour and developmental information, providing ways to test hypotheses of circuit function. In a variety of behaviours such as learned and innate olfaction, navigation and sexual behaviour, connectomics has already revealed interconnected modules with a hierarchical structure, recurrence and integration of sensory streams. Comparing individual connectomes to determine which circuit features are robust and which are variable is one key research area; new work in comparative connectomics across development, experience, sex and species will establish strong links between neuronal connectivity and brain function.
Rapid progress in connectomics
Connectomics has developed extremely rapidly in the last five years and some of the most impactful developments have been in insect brains [1]. Increased electron-microscopy (EM) imaging speed and improvements in automated segmentation mean that complete synaptic-resolution wiring diagrams of the size of a whole fruit fly brain are now feasible. Although many insect species are now potential targets for EM connectomics, progress so far has mostly been in Drosophila melanogaster, which is therefore the focus of this review.
Improvements to sample preparation, and serial-section transmission [••2] and focused ion beam scanning EM imaging [••3,4] have made pipelines more robust. This, together with optimised image alignment, has improved automated segmentation methods for neurons [••3,5,6] and synapses [••3,7,8]. A newly generated EM volume can now be densely populated with connectivity and neuronal morphologies, many of which may be immediately identifiable.
Despite this progress in automation, manual proofreading to remove false merges between neurons and find missed branches remains essential. The proofreading effort for the central brain of Drosophila (∼40 000 neurons) has been reduced from order 2000 to ∼30 person-years. But this is still a major effort, depending on organised proofreading projects, which range from completely in-house to community-distributed. Compared with the first similar effort [9], the speed-up offered by automated segmentation allows research groups to contribute weeks to months of effort while deriving significant insights. Nevertheless, so far, only dedicated and highly coordinated proofreading teams have delivered complete connectomes of tens of thousands of neurons, typical of the adult fly brain and ventral nerve cord (VNC) [••3].
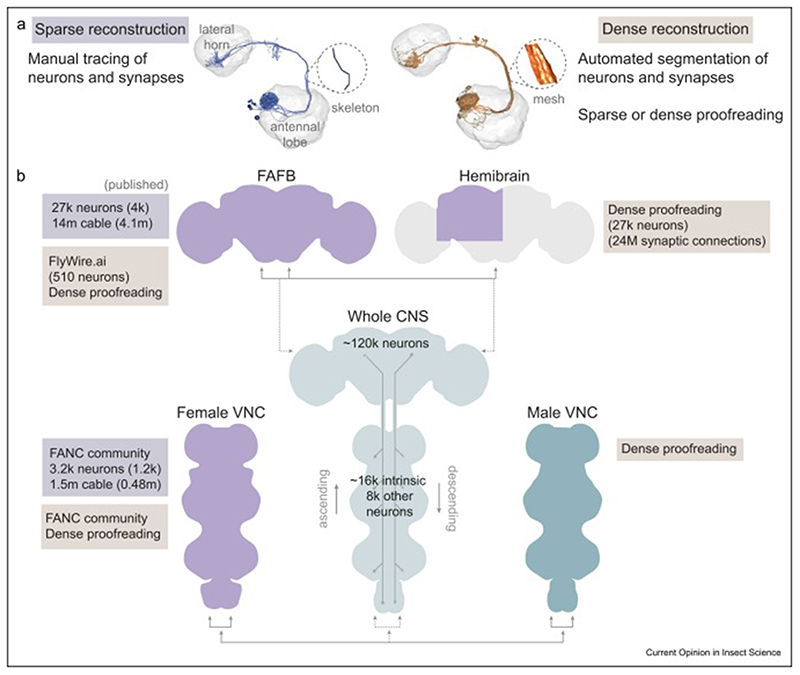
The pioneer connectomics volume in Drosophila was the larval first instar (L1) central nervous system (CNS) [9]. It sparked analysis in several areas from learning and memory [10,•11] to developmental mechanisms [12,•13], often linking the observed connectivity with behaviour (reviewed in Ref. [14]). Seven subvolumes comprising an adult olfactory glomerulus allowed some initial subcellular analysis and morphological diversity discovery [15]. The first adult whole-brain volume evolved from sparse manual tracing [16] to dense automated reconstruction followed by sparse proofreading [••5] (Figure 1). The release of the dense connectome for a partial adult female brain [••3] was the first resource allowing researchers instant access to a finished insect connectome. Together, both volumes allow comparisons between individuals and across three hemispheres [17]. Since then, a sparse female VNC reconstruction has been published [••2] and a dense autosegmentation will be released soon (personal communication, W. Allen Lee and J. Phelps). A densely reconstructed male VNC is also expected shortly (personal communication, S. Berg, G. Rubin, G. Card and G. Jefferis).
Figure 1. Adult Drosophila melanogaster EM connectomics volumes.
(a) Comparison between sparse and dense EM reconstruction and proofreading. The reconstructed olfactory-projection neurons DA1 lPNs are shown as an example for sparse (purple, data from https://fafb.catmaid.virtualflybrain.org/) [79] and dense reconstruction (orange, data from https://neuprint.janelia.org/) [••3,•17]. The neuron format (skeleton or volumetric mesh) is independent of the reconstruction being sparse or dense. The examples shown are constrained by the publicly available data. (b) Adult Drosophila melanogaster EM datasets available and future ones (whole central nervous system (CNS)). Comparisons possible within (across hemispheres) and between them (across individuals) are indicated by arrows. Dashed lines indicate future comparisons. For each project, the number of reconstructed neurons, total reconstructed cable length or synaptic connections is shown. Numbers in brackets are for published data. For the full adult fly brain (FAFB) and the female VNC sparse reconstructions, the number of unpublished neurons captures any skeleton with more than 100 nodes. The total reconstructed cable includes any skeleton with more than two nodes.
Reconstructing whole volumes provides immediate benefits beyond the analytical power. Proofreading and neuron identification are significantly accelerated by cross-comparisons. Although generating a connectome is still resource-intensive, current progress [•18] means that central nervous system (CNS) volumes for both sexes should become available within 2–3 years. Mapping dimorphic neurons and connections between male and female central nervous system (CNS) volumes could explain observed differences in behaviour (Box 1).
Box 1 Resources and challenges.
Datasets:
L1 larval central nervous system (CNS) [9]: published sparse connectome available via Virtual Fly Brain (https://l1em.catmaid.virtualflybrain.org/).
Whole female brain, full adult fly brain (FAFB): sparse (https://temca2data.org/) and dense reconstruction communities (FlyWire, https://flywire.ai/) [••5,16]. Published sparse connectome data available via Virtual Fly Brain (https://fafb.catmaid.virtualflybrain.org/).
Partial female brain, hemibrain: connectome data, including segmentation, image data and queries available via neuPrint (https://neuprint.janelia.org/) [••3,74].
Female VNC, FANC: sparse connectome available via Virtual Fly Brain (https://fanc.catmaid.virtualflybrain.org/). Dense proofreading community (https://www.lee.hms.harvard.edu/phelps-hildebrand-graham-et-al-2021)
Male VNC, MANC: connectome data will be available via neuPrint [74].
Tools for analysis or visualisation:
Neuroglancer (https://github.com/google/neuroglancer): WebGL-based viewer for volumetric data, often used for EM connectomics projects.
NeuronBridge (https://neuronbridge.janelia.org/) [27,•30]: resource allowing similarity matching between MultiColour Flp-out LM images and EM morphologies.
natverse (https://natverse.org/) [37]: collection of R packages for neuroanatomical analysis, including light microscopy (LM) and EM connectomics data.
NAVis and friends (https://navis.readthedocs.io/en/latest/source/other_libraries.html#) [37]: collection of Python packages for neuroanatomical analysis, with some overlap in functionality to natverse.
Virtual Fly Brain (http://www.virtualflybrain.org/): Drosophila melanogaster neuroscience resource integrating published data with a rich vocabulary for cell types.
Insect Brain Database (https://insectbraindb.org/) [75]: neuroscience resource for species, neuroanatomy and functional information for a wide range of non-Drosophila melanogaster insects.
Fruit Fly Brain Observatory (https://www.fruitflybrain.org/) [76]: ecosystem of tools to explore Drosophila neuroanatomical data, including morphology, connectivity and physiology, with the aim of generating networks and models.
Brain Circuits (https://braincircuits.io/): website listing available EM connectomics and light microscopy (LM) libraries and atlases for vertebrates and invertebrates, together with available tools. Also provides own analysis tools for EM datasets.
Challenges:
Proofreading: a) ensuring a significant and consistent neuron, cell type or region completeness. This is affected by the proofreading strategy (distributed community or centralised), resources available, quality of image data and awareness of issues that impinge on morphology and synapse-segmentation outputs. Furthermore, the quality of the segmentation will depend on how representative the ground-truth data are with regard to the heterogeneity of image features almost always observed in bigger volumes. b) Storage and handling of larger EM volumes and segmentations, together with significant amounts of concurrent edits requires a robust but user-friendly proofreading and annotation system.
Neuron and cell type identification: obtaining whole-neuron morphologies is crucial for this step. Prior knowledge, leveraging other EM volumes and the ability to use automated morphological and connectivity-similarity methods [••3,•17,36] to compare new morphologies to previously identified single neurons or cell types, together with expert assessment, drives this process. The output quality and comprehensiveness of this process is significantly affected by the quality of the data being mapped and what it is being mapped to. On the one hand, there is the inherently sparse nature of light microscopy (LM) data for cell types and the incomplete descriptions in publications. On the other, the truncated and large number of new EM morphologies, the difficulty in assigning cell types and the lack of comprehensive inter- and intraindividual comparisons for both sexes. Common to both is the experience-dependent or -independent morphological and connectivity variability [•17,31,77]. This process of mapping cell types also leads to the identification of driver lines [••28,•29] that can be used for behavioural assays and the increase in the availability of sparsely labelled lines [78] is facilitating this process. However, similar but distinct cell types morphologically or connectivity-wise in EM are often not distinguishable in light microscopy (LM).
Mapping other types of data: features extracted from EM image data, such as neurotransmitter predictions, can be directly linked to a morphological entity, often classified at the cell type level (Figure 2). However, the small number of neurons known to express nonfast-acting neurotransmitters makes it difficult to collect enough ground truth data to generate confident predictions for a comprehensive set of neurotransmitters, currently identified by immunostaining methods. Linking EM to transcriptomics data is dependent on having expression markers for cell types as identified by light microscopy (LM) methods, thus only available for a small subset of types. The recent Fly Cell Atlas effort identified 80 clusters of neurons or glia types in the adult head, though the majority of neuron types in the central brain were unannotated [•21].
Stereotypy and its impact on the interpretation of the wiring diagram: the observed natural variability is also a factor on how we interpret circuit connectivity, and what behavioural changes it might cause, if any. Establishing robust ways of testing the links between observed connectivity and behavioural output, for a range of paradigms, contexts, internal states and so on, will help to clearly assess the impact of stereotypy.
Wiring diagrams can generate and test behavioural hypotheses or help interpret experimental results. This approach is becoming more widespread and we showcase some examples below and in Figure 2. These insights are enriched by augmenting the connectome with other types of data. Machine learning approaches can predict neurotransmitter identity from EM data with high accuracy [•19]. Further work could broaden the applicable neurotransmitters and compare across datasets. Nevertheless, this signal context already aids in interpreting connectivity features and supporting functional observations [•20]. Single-cell transcriptomic approaches are increasingly prevalent and more comprehensive with whole adult data now available [•21–24]. The next steps could fully map these data in the central nervous system (CNS) at the resolution of connectomics cell types. Integrating developmental information onto the static connectome can also uncover valuable insights. In the larva, developmental patterns were shown to affect synaptic specificity. The interaction of spatial, hemilineage, and temporal identity rules drives the observed connectivity patterns, with related neurons more likely to share connections and partners [•13,25]. These refinement rules are apparent in the proprioceptive motor circuit, where first-, second- and third-order types downstream of the sensory input can each be assigned to a specific temporal-hemilineage identity that impinges on neuronal identity and connectivity [•13]. Furthermore, circuit structure is also impacted, with neuronal outputs born earlier than inputs [25].
Figure 2.
What can be done with a reconstructed neuron? Centre: Visualisation of the hemibrain surface and a single aSP-g2 neuron (hemibrain bodyid=641278400). From top left, going clockwise: · Find genetic driver lines using NeuronBridge [••27,•30]. Use colour-depth search or PatchPerPixMatch 29 to find driver lines expressing the neuron of interest. · Group neurons based on morphology (using NBLAST 36). This is used to define types. · Explore identity and weights of inputs and outputs. When exploring connectivity, it is useful to collapse by type and check not only the number of synapses but also the proportion of synapses from or to connecting neurons. · Explore connectivity motifs (examples shown). · Compare across databases — how stereotypic is the neuron? Are parts missing between datasets? Here, the hemibrain aSP-g2 neuron (black) and the homologous full adult fly brain (FAFB) aSP-g2 neuron (red) are both plotted in full adult fly brain (FAFB) coordinates. Notice the hemibrain neuron is truncated. Morphological comparisons can also be made against neurons from light microscopy (LM) datasets, for example, FlyCircuit (www.flycircuit.tw) [80]. · Find other neurons within that type, either manually identifying neurons travelling together within a tract, or by comparing similarity within a set of known neurons (using NBLAST 36). Many neurons on the hemibrain dataset were curated by type, so are easily found. Visualisation of all aSP-g neurons within the hemibrain dataset. · Computational modelling of network function using connectivity data. In this example, a model of fan-shaped body (FB) circuit using actual connectivity information showed a strong correlation between bump position and travel direction (left panel). This correlation is lost when specific connections are eliminated from the model (right panel, PNFv neurons omitted) (image kindly provided by R. I. Wilson, author of 20. · Check distribution of input synapses on the dendritic tree (green) and axon (orange). It is useful to plot neurons of interest and their partners to examine whether input connectivity is axo-dendritic feedforward, axo-axonic or other. Strong inputs to a hemibrain aSP-g neuron (black) in each subcompartment are plotted: olfactory projection neuron DA1 lPN synapses on dendrites (blue), and local neuron synapses on axons (purple). Dotted line shows a zoomed-in section in inset. Additionally, the spatial distribution of synapses along the dendritic tree might affect the functional connectivity strength, as was suggested for MBONs [39] and EPG neurons [61]. · Synapse placement — split a neuron into axon and dendrite. Flow-centrality analysis with natverse [79] produced an axon and dendrite split, showing pre- (red) and postsynaptic (blue) connections. · Relate structure to function, for example, compartmentalisation. In this Amacrine cell, thin, loopy branches provide electrotonic separation between compartments. This morphology suggests the neuron performs local computations, which was confirmed functionally [81] (image used with permission from publisher). · Get neurotransmitter prediction [•19].
Intersecting gene expression, developmental rules, neurotransmitter and connectivity information at the cell type level will undoubtedly strengthen the interpretation of neuronal function [26].
Integrative connectomics
Establishing direct links between the structural connectivity information of connectomics with circuit physiology and behaviour remains a challenge. The first step requires matching morphologies between EM and lower-resolution light-level microscopy (LM) data. Methods to semi-automate this process have been developed, taking advantage of extensive light microscopy (LM) driver lines and MultiColour FlpOut libraries [••27–•30]. The success of this process strongly correlates with the amount of data available per morphology, as the range of natural variability in both EM and light microscopy (LM data), and potential developmental abnormalities need to be considered, not only to map individual neurons but to define cell types [•17,31] — the reproducible and recognisable unit of neuronal morphology and connectivity. Collapsing information by cell type is important as it can overcome inherent variability in biological datasets. In the hemibrain, over 5200 morphological cell types were identified, with some further broken down by connectivity. Only 20% of those were previously described [••3]. We expect that a subset of these morphological and connectivity types will be further refined or consolidated as more cross-comparisons are possible.
Additional uncertainties include the relationship between connectomics and physiological connection strengths and how neuronal firing might be impacted by variations in synapse location, these issues can only be conclusively addressed by detailed physiological measurements from many neuron types [•32]. Understanding the relationship between connectivity and activity will be of major importance in the next few years, eventually allowing us to interpret connection strengths in the context of whole circuits and behaviour, and provide a deeper understanding of natural variability. Other factors such as the influence of gap junctions, neuromodulators and glia are recognised unknowns, however harder to address with current datasets. Staining protocols, as well as automated segmentation methods are usually optimised to recognise chemical synapses and neurons, thus those other features are either not discernible in the image data or not segmented correctly, precluding a comprehensive analysis without very significant effort [33]. Focused approaches to identify these factors would necessitate changes to staining methods, resegmentation of existing datasets after algorithm optimisation or mapping markers identified by light microscopy (LM) to EM neurons [34]. Certainly, the availability of additional EM volumes will allow a more detailed analysis of stereotypy per cell type, for morphology and connectivity and further comparisons to other data types (Figure 2).
Importantly, releasing datasets and annotated connectomes freely and quickly to the community enables a shared framework to link to literature and support communication across labs. This aids the understanding of the multiple functions and contexts in which a given circuit might be acting.
From connectomics to behaviour
Initial studies relating connectomics to behaviour
The earliest mapping between connectome and function in Drosophila was in the larva, dissecting the circuit underpinning rolling escape behaviour [9]. Second-order neurons were identified in EM, that integrate combinations of nociceptive and mechanosensory signals triggering rolling, depending on their input. Further analysis of deeper circuit levels revealed that convergence of multimodal information occurred at multiple layers, in both the brain and VNC.
In the adult, EM connectivity was crucial to interpret and corroborate light microscopy (LM) and behaviour data in the olfactory system, demonstrating the interaction between innate and learned circuits for aversive memory retrieval [•35]. This work discovered key neurons in the lateral horn, a brain region implicated in sensory processing for innate behaviour, downstream of a mushroom body output neuron (MBON). Comprehensive EM reconstruction followed by quantification of morphological similarity to light microscopy (LM) data [36], allowed the unequivocal matching between connectome and genetically defined cell types [•35]. The availability of good bridging registrations between light microscopy (LM) and EM brain templates was crucial for this in silico mapping [37].
Memory circuits of the mushroom body
Olfactory memories are formed through a plastic, compartmentalised and recurrent network; coincidence between odour and reinforcing signals carried by valencespecific dopaminergic neurons (DANs) changes the connection strength between mushroom body (MB) Kenyon Cells and their downstream partners, MBONs. After training with punishment or reward, flies will avoid or approach the associated odour in subsequent exposures.
EM reconstruction of larval MB circuitry unveiled dense recurrent connections between Kenyon Cells, and reciprocal connectivity between DANs and both Kenyon Cells and MBONs [10]. Extending this map revealed that punishment is indirectly conveyed to DANs from nociceptive and mechanosensory neurons, while previous experience is fed back onto many DANs by neurons downstream of MBONs [•11]. This feedback enables adaptive regulation of DANs based on experience and could serve as the basis for encoding prediction errors and expectation. Further work identified substantial convergence between MBONs and lateral horn neurons, providing an initial architecture and mechanism for integrating learned and innate behaviour [38], similar to the adult [•35].
Repeated exposure to a learned odour without expected reinforcement leads to memory extinction. Recent work in adults found that extinction is an active mechanism, in which a newly formed memory trace competes with the old one [39]. The new memory is formed by recurrent feedback from MBONs to opposite-valence DANs, whose activity serves as a new ‘reinforcement’ signal. The overall effect of the competing pathways — avoidance- and approach-promoting — is integrated and neutralised, leading to behavioural extinction. Analysing synapse location showed that inhibitory synapses from an approach-promoting MBON targeted the dendritic root of an avoidance-promoting MBON, and could therefore shunt activity from entire dendrites [39]. Following aversive learning, release of such strong inhibition would bias the MBON network towards odour avoidance. Indeed, inhibition between MBONs serves to integrate opposing memory traces after extinction. Additionally, recurrent inhibition and disinhibition between MBONs and DANs enables specific gain control with subsequent odour exposure, thereby adapting learning rules by expectation [39,••40].
A new study describes a feedforward circuit from a reward-representing MBON to DANs in other MB compartments, required for second-order learning [41]. In second-order learning, animals first learn to associate a stimulus with reinforcement and then to associate a new stimulus with the first one. The authors used hemibrain connectivity and neurotransmitter predictions to identify cholinergic interneurons downstream of reward-representing MBONs. These interneurons are activated in the initial learning phase, and excite DANs in other MB compartments. This plasticity enables a novel stimulus to acquire a similar valence to the initially learned one.
Sexually dimorphic circuits
Two genes coding for transcription factors, fruitless and doublesex, shape sexually dimorphic features of neural circuitry in flies, and regulate dimorphic patterns of social behaviour. Catalogues of fruitless and doublesex-expressing sexually dimorphic neurons [42–45] serve as the foundation to study the role of circuit components in sexual behaviour. Recent work compared a doublesex+ neuronal cluster (termed aDN) between sexes [•46]. Using light microscopy (LM) images in males, they predicted that aDN neurons receive direct input from LC10 visual neurons that are required for orienting and tracking female movement during courtship. In females, connectomic analysis showed that aDN neurons instead receive olfactory inputs, and are required for selecting communal egg-laying sites. Whereas the male light microscopy (LM) analysis confirmed specific hypotheses, the female analysis enabled a comprehensive mapping of the network, discovering novel components, and generating unexpected behavioural hypotheses. In the near future, comparing male and female connectomes will link many known morphological changes to actual wiring differences between the sexes.
The female connectome has guided recent studies of circuits regulating female-specific behaviours: sexual receptivity, egg-laying and female aggression. Wang et al. [•47] and Wang et al. [•48] identified the descending neurons controlling male-acceptance and egglaying, and their upstream circuitry, linking mating status with egg-laying, and demonstrating multisensory integration such as egg-laying substrate and the male courtship song. fruitless−doublesex+ pC1 neurons regulate both sex-specific female behaviours: they promote virgin receptivity by directly activating vaginal-plate-opening descending neurons, and block virgin egg-laying by indirectly inhibiting oviposition-descending neurons. Mating status is incorporated into the circuit via abdominal ganglia neurons, indirectly inhibited by male sex-peptide transmitted during copulation. These neurons activate the pC1a subtype, suggesting pC1 neurons contribute to a switch between an internal state of virgin female receptivity and mated egg-laying.
The circuitry for female aggression was also studied using the connectome [•49,•50]. aIPg neurons bidirectionally control female aggression and their reconstruction in the brain [••3,16] found reciprocal connections with pC1d, a pC1 subtype that promotes persistent aggression. Strong pC1 subtype interconnectivity might shift a female’s decision between aggression and receptivity, promoting an internal state according to social context and needs.
Our group used the female connectome to dissect circuits processing the male pheromone [•51]. We identified a novel olfactory projection neuron population responsible for the previously described effects of male pheromone on female sexual behaviour. A single olfactory channel diverges into two ‘labelled lines’ in the second neuronal layer, each with unique responses to a male and sex-specific behavioural effects. The third-order circuitry then fans out to tens of cell types, some of them specifically responding to distinct aspects of the signal, such as a speed-sensitive sensor for male approach, and a multimodal sensor integrating male smell and taste. This architecture produces detectors for specific social contexts, each with a dedicated pathway to promote an appropriate behavioural response.
Navigation and the central complex
The brain’s navigation system has been a highly influential area of neuroscience for decades. Recent studies in insects have finally revealed detailed circuit mechanisms [52–54], inaccessible in vertebrates, and connectomics has played a key role.
The fly’s sense of direction emerges from integrating sensory and motor signals in the ellipsoid body (EB), generating an interconnected network, operating as a ring attractor. This circuit relies on combining both internal self-motion cues (angular velocity) [55,56] and external cues (polarised light, visual landmarks, mechanosensory wind cues or others), carried by ring neurons. Ring neurons form all-to-all connections with sensory-channel specificity, and connect to compass neurons (termed EPG [56]). Plastic connectivity from ring to EPG neurons allows the compass to learn and maintain heading in response to a stable sensory scene, and to quickly remap new scenes [57,58]. Connectomic analysis confirmed the basic architecture and identified additional ring neuron subtypes that provide diverse sensory input, supporting the emerging view of EB as multimodal cue integrator [••3,59–••61]. How are sensory cues prioritised? Connectomic analysis provided multiple insights. First, relative synaptic weights differ between various ring neurons onto EPG neurons. Second, there is a consistent hierarchical organisation of inputs along EPG dendrites, such that self-motion inputs are closest to the root and are probably the most influential, followed by mechanosensory, visual and finally sleep-related ring neurons. Third, the authors found a hierarchy in the structure of mutual inhibition across ring neuron types, which could privilege salient sensory stimuli among others [••61]. Altogether, these results suggest that prioritising sensory cues for navigation could be hard-wired, reflecting cue reliability and the species’ evolutionary needs.
Flies are able to integrate their own turns along a path to compute the travelled journey and estimate a straight vector back to the starting point. This process, called path integration, requires a constantly updating vector computation of movement direction and speed, relative to a target location. While path integration is best known for central place foragers such as bees and ants [62], it was also confirmed in Drosophila [63], inviting connectomics circuit dissection. Path integration is suggested to be computed in another region of the central complex, downstream of the EB, the fan-shaped body (FB). Analysis of the hemibrain revealed a densely recurrent architecture of FB interneurons, tiling either horizontal layers or vertical columns, suggesting that columnar FB neurons are phase-shifted in their anatomical heading angle relative to neighbouring cells. These repeated connectivity motifs and phase shifts, combined with self-motion and head-direction inputs, could support vector-based navigational computation in the FB, ideal to perform coordinate transformations necessary for path integration [••61].
Emerging experimental work strengthens the anatomically based models [••61,62]: flies indeed perform path integration when foraging [63], remember target location after it disappeared and when faced with two distinct vanished targets, flies centre their search at a location between them [64]. These results suggest that flies accumulate experience while foraging to develop an internal sense of the target centre — a coordinate system centred on the world, rather than the self. Two papers recently provided evidence that the FB network indeed encodes a travel vector, by combining heading information from EB compass neurons with velocity pre-motor signals to perform vector computations [•20,•65]. Downstream, this body-centred velocity vector is transformed to a world-centred velocity vector, which can serve as the basis for remembering a target location during navigation.
Connectomic analysis described additional FB input from multiple regions, for example linking the FB to the MB [••61], supporting the idea of the FB as a centre for context-dependent navigational control, enabling flexible regulation of behaviour [66]. Additionally, recent work described FB input from olfactory neurons, and demonstrated that olfactory context modulates turning behaviour in response to wind [67].
Other perspectives
The trend in generating larger and denser connectomes will enable comparative connectomics in D. melanogaster to directly link plasticity during an animal’s lifetime to behavioural diversity and circuit architecture. Similarly, comparing connectomes across sex and developmental stages will teach us about processes, leading to network maturation and specification [68,69].
Despite the extreme morphological variability of insect species and their rich behavioural repertoires, insect brains have evolved from a common ground plan dating back 500 million years [70]. Recent connectomes of the central complex of bumble bees [71] and butterfly lamina [72] highlighted the usefulness of comparisons to D. melanogaster, demonstrating both conserved core circuitry and species-specific modifications. Even in vertebrates, the recent description of a zebrafish ring-attractor network with similarities to the fly [73], suggests great value in comparisons beyond insects. We expect that inter-species comparisons will directly identify neuroanatomical changes and conservation correlated with species differentiation and behavioural evolution.
Acknowledgements
We thank Gwyneth Card, Stuart Berg and Gerry Rubin, and also Jasper Maniates-Selvin and Wei-Chung Allen Lee, for personal communications regarding the male and female VNC. For statistics on published and unpublished connectomics data, we would like to thank the full adult fly brain (FAFB) CATMAID community for access to the unpublished quantification, Sven Dorkenwald from FlyWire for providing the published numbers, Jasper Phelps and Wei-Chung Allen Lee for access to the unpublished FANC community data and to Virtual Fly Brain for maintaining the CATMAID instances for published full adult fly brain (FAFB) and FANC data. We thank Kimberly Meechan and Irene Varela for CATMAID tracing of neurons, used in Figure 2. We are also grateful to István Taisz and Stanley Heinze for providing comments on the paper.
This work was supported by Marie Curie Individual (H2020-IF-748478) and European Molecular Biology Organisation (EMBO) long-term (ALTF 164-2016) fellowships to DSG, a Wellcome Trust Collaborative Award (220343/Z/20/Z), an MRC/NSF (National Science Foundation) NeuroNex2 Award (MC_EX_MR/T046279/1), an National Institutes of Health (NIH) BRAIN Initiative Award (1RF1MH120679-01), and core support from the Medical Research Council (MRC) (MC-U105188491) to GSXEJ.
Footnotes
Conflict of interest statement
None declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Schlegel P, Costa M, Jefferis GS. Learning from connectomics on the fly. Curr Opin Insect Sci. 2017;24:96–105. doi: 10.1016/j.cois.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Phelps JS, Hildebrand DGC, Graham BJ, Kuan AT, Thomas LA, Nguyen TM, Buhmann J, Azevedo AW, Sustar A, Agrawal S, et al. Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy. Cell. 2021;184:759–774.:e18. doi: 10.1016/j.cell.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper described the acquisition of the adult female VNC EM dataset by transmission EM (TEM) using a new method, GridTape, to automate the collection and imaging of serial-sections, enabling fully automated, and high throughput TEM imaging. The study also describes the proofreading of 1000 sensory and motor neurons and the alignment to an LM template, facilitating EM to LM matching
- 3.Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura S-Y, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. A connectome and analysis of the adult central brain. eLife. 2020;9:e57443. doi: 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper presents the hemibrain dataset, a partial adult Drosophila female brain acquired via focused ion beam scanning EM and the first densely reconstructed Drosophila volume. It consists of approximately 27 000 neurons and around 24 million synaptic connections
- 4.Xu CS, Pang S, Hayworth KJ, Hess HF. Enabling FIB-SEM systems for large volume connectomics and cell biology. bioRxiv. 2019 doi: 10.1101/852863. [DOI] [Google Scholar]
- 5.Dorkenwald S, McKellar CE, Macrina T, Kemnitz N, Lee K, Lu R, Wu J, Popovych S, Mitchell E, Nehoran B, et al. FlyWire: online community for whole-brain connectomics. Nat Methods. 2022;19:119–128. doi: 10.1038/s41592-021-01330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes FlyWire, the automated segmentation of FAFB, the framework supporting it both for data handling, visualisation and proofreading, and the establishment of an open community. It demonstrates that the segmentation quality is of good enough quality to significantly accelerate proofreading tasks
- 6.Li PH, Lindsey LF, Januszewski M, Zheng Z, Bates AS, Taisz I, Mike T, Nichols M, Li F, Perlman E, et al. Automated reconstruction of a serial-section EM drosophila brain with flood-filling networks and local realignment. bioRxiv. 2020 doi: 10.1101/605634. [DOI] [Google Scholar]
- 7.Buhmann J, Sheridan A, Malin-Mayor C, Schlegel P, Gerhard S, Kazimiers T, Krause R, Nguyen TM, Heinrich L, Lee W-CA, et al. Automatic detection of synaptic partners in a whole-brain Drosophila electron microscopy data set. Nat Methods. 2021;18:771–774. doi: 10.1038/s41592-021-01183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich L, Funke J, Pape C, Nunez-Iglesias J, Saalfeld S. In: Medical Image Computing and Computer Assisted Intervention — MICCAI 2018. Frangi AF, Schnabel JA, Davatzikos C, Alberola-López C, Fichtinger G, editors. Springer International Publishing; 2018. Synaptic cleft segmentation in non-isotropic volume electron microscopy of the complete Drosophila brain; pp. 317–325. [Google Scholar]
- 9.Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- 10.Eichler K, Li F, Litwin-Kumar A, Park Y, Andrade I, Schneider-Mizell CM, Saumweber T, Huser A, Eschbach C, Gerber B, et al. The complete connectome of a learning and memory centre in an insect brain. Nature. 2017;548:175–182. doi: 10.1038/nature23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eschbach C, Fushiki A, Winding M, Schneider-Mizell CM, Shao M, Arruda R, Eichler K, Valdes-Aleman J, Ohyama T, Thum AS, et al. Recurrent architecture for adaptive regulation of learning in the insect brain. Nat Neurosci. 2020;23:544–555. doi: 10.1038/s41593-020-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper extended the larva MB connectome, found sensory pathways feeding into MB modulatory neurons representing reinforcement, and provided functional evidence to the significance of feedback circuitry between MBONs and MB modulatory neurons
- 12.Valdes-Aleman J, Fetter RD, Sales EC, Heckman EL, Venkatasubramanian L, Doe CQ, Landgraf M, Cardona A, Zlatic M. Comparative connectomics reveals how partner identity, location, and activity specify synaptic connectivity in Drosophila. Neuron. 2021;109:105–122.:e7. doi: 10.1016/j.neuron.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mark B, Lai S-L, Zarin AA, Manning L, Pollington HQ, Litwin-Kumar A, Cardona A, Truman JW, Doe CQ. A developmental frameworklinking neurogenesis and circuit formation in the CNS . eLife. 2021;10:e67510. doi: 10.7554/eLife.67510. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper analyses a set of 160 interneurons in the larval L1 connectome, showing that developmental and temporal identity are reflected in anatomical features and higher shared connectivity between related neurons. These rules also seem impinge on circuit formation and function
- 14.Eschbach C, Zlatic M. Useful road maps: studying Drosophila larva’s central nervous system with the help of connectomics. Curr Opin Neurobiol. 2020;65:129–137. doi: 10.1016/j.conb.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber L, Rybak J, Hansson BS, Cantera R. Synaptic spinules inthe olfactory circuit ofDrosophila melanogaster. Front Cell Neurosci. 2018;12(1-7):86. doi: 10.3389/fncel.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell. 2018;174:730–743.:e22. doi: 10.1016/j.cell.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlegel P, Bates AS, Stürner T, Jagannathan SR, Drummond N, Hsu J, Serratosa Capdevila L, Javier A, Marin EC, Barth-Maron A, et al. Information flow, cell types and stereotypy in a full olfactory connectome. eLife. 2021;10:e66018. doi: 10.7554/eLife.66018. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Connectomics study on olfactory pathways in the adult using the FAFB and hemibrain datasets. Presents a comprehensive assessment of stereotypy within- and across individuals
- 18.Hayworth KJ, Peale D, Januszewski M, Knott GW, Lu Z, Xu CS, Hess HF. Gas cluster ion beam SEM for imaging of large tissue samples with 10 nm isotropic resolution. Nat Methods. 2020;17:68–71. doi: 10.1038/s41592-019-0641-2. [DOI] [PubMed] [Google Scholar]; • This paper describes a new gas cluster ion beam scanning EM technique that would allow imaging at & 10 nm isotropic resolution with a wider milling area. The thicker and more reliable sectioning results in a faster acquisition, even for large volumes
- 19.Eckstein N, Bates AS, Du M, Hartenstein V, Jefferis GSXE, Funke J. Neurotransmitter classification from electron microscopy images at synaptic sites in drosophila. bioRxiv. 2020 doi: 10.1101/2020.06.12.148775. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study develops a machine learning approach to identify neurotransmitter identity by analysing the image context surrounding synaptic sites. Applied to the FAFB dataset for six types of neurotransmitters
- 20.Lu J, Behbahani AH, Hamburg L, Westeinde EA, Dawson PM, Lyu C, Maimon G, Dickinson MH, Druckmann S, Wilson RI. Transforming representations of movement from body-to world-centric space. Nature. 2022;601:98–104. doi: 10.1038/s41586-021-04191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Together with Lyu et al. (2021), both papers provided evidence that the FB network encodes a travel vector, by combining heading information from EB compass neurons with velocity pre-motor signals. Downstream in the pathway, they describe the anatomy, physiology, and behavioural effects of a mechanism for a reference-frame transformation of this velocity vector, from a body-centred to a world-centred reference
- 21.Li H, Janssens J, De Waegeneer M, Kolluru SS, Davie K, Gardeux V, Saelens W, David FPA, Brbić M, Spanier K, et al. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science. 2022;375:eabk2432. doi: 10.1126/science.abk2432. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First single-cell RNAseq dataset of the entire adult Drosophila for both sexes, in addition to 17 dissected tissues. A number of neuron types and glia encompassing peripheral and central nervous system ones were identified
- 22.Croset V, Treiber CD, Waddell S. Cellular diversity in the midbrain revealed by single-cell transcriptomics. Elife. 2018;7:e34550. doi: 10.7554/eLife.34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davie K, Janssens J, Koldere D, De Waegeneer M, Pech U, Kreft Ł, Aibar S, Makhzami S, Christiaens V, Bravo González-Blas C, et al. A single-cell transcriptome atlas of the aging Drosophila brain. Cell. 2018;174:982–998.:e20. doi: 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen AM, Neville MC, Birtles S, Croset V, Treiber CD, Waddell S, Goodwin SF. A single-cell transcriptomic atlas of the adult ventral nerve cord. Elife. 2020;9:e54074. doi: 10.7554/eLife.54074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y-W, Wreden CC, Levy M, Marshall ZD, MacLean JN, Heckscher ES. Sequential addition of neuronal stem cell temporal cohorts generates a feed-forward circuit in the Drosophila larval nerve cord. bioRxiv. 2022 doi: 10.1101/2022.04.05.487221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates AS, Janssens J, Jefferis GS, Aerts S. Neuronal cell types in the fly: single-cell anatomy meets single-cell genomics. Curr Opin Neurobiol. 2019;56:125–134. doi: 10.1016/j.conb.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Meissner GW, Dorman Z, Nern A, Forster K, Gibney T, Jeter J, Johnson L, He Y, Lee K, Melton B, et al. An image resource of subdivided Drosophila GAL4-driver expression patterns for neuron-level searches. bioRxiv. 2020 doi: 10.1101/2020.05.29.080473. [DOI] [Google Scholar]; •• This work describes a dataset of 27k MultiColour Flp-out (MCFO) images obtained from GAL4 drivers, allowing the visualisation of single-neuron morphologies in sparse samples. Furthermore, it goes on to describe the development of NeuronBridge, an open search tool allowing the matching of these MCFO images to EM morphologies, via the use of two automated similarity methods, Color MIPs and PatchPerPixMatch
- 28.Otsuna H, Ito M, Kawase T. Color depth MIP mask search: a new tool to expedite Split-GAL4 creation. bioRxiv. 2018 doi: 10.1101/318006. [DOI] [Google Scholar]; •• This work describes a new method of using colour maximum intensity projections (MIPs) to identify neurons that overlap with LM data for GAL4 driver lines. These results are integrated into NeuronBridge. Furthermore, the authors share a new open access Fiji plugin and MIP-preprocessed data for two large libraries (over 13k lines)
- 29.Mais L, Hirsch P, Managan C, Wang K, Rokicki K, Svirskas RR, Dickson BJ, Korff W, Rubin GM, Ihrke G, et al. PatchPerPixMatch for automated 3d search of neuronal morphologies in light microscopy. bioRxiv. 2021 doi: 10.1101/2021.07.23.453511. [DOI] [Google Scholar]; • This work describes a neuron similarity tool, PatchPerPixMatch, that performs full 3D automated searches of neuronal morphologies against MCFO data of GAL4 driver lines. The results are integrated in NeuronBridge
- 30.Clements J, Goina C, Hubbard PM, Kawase T, Olbris DJ, Otsuna H, Svirskas R, Rokicki NeuronBridge: an intuitive web application for neuronal morphology search across large data sets. bioRxiv. 2022 doi: 10.1101/2022.07.20.500311. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This work describes NeuronBridge, a web application that facilitates matching of neurons between EM and LM
- 31.Scaplen KM, Talay M, Fisher JD, Cohn R, Sorkaç A, Aso Y, Barnea G, Kaun KR. Transsynaptic mapping of Drosophila mushroom body output neurons. eLife. 2021;10:e63379. doi: 10.7554/eLife.63379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu TX, Davoudian PA, Lizbinski KM, Jeanne JM. Connectomic o features underlying diverse synaptic connection strengths and subcellular computation. Curr Biol. 2022;32:559–569.:e5. doi: 10.1016/j.cub.2021.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study relates hemibrain connectivity data and electrophysiological recordings for pairs of connected neurons to show that anatomy can predict many of the physiological properties. Activity is affected by the subcellular electrical compartments defined by morphology, while synaptic location within each arbour does not influence it
- 33.Park A, Croset V, Otto N, Devika A, Treiber C, Meschi E, Sims D, Waddell S. Gliotransmission of D-serine promotes thirst-directed behaviors in Drosophila. Current Biology. 2022;32(18):3952–3970.:e8. doi: 10.1101/2022.03.07.483255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammer G, Vieira RM, Fendl S, Borst A. Anatomical distribution and functional roles of electrical synapses in Drosophila. Curr Biol. 2022;32:2022–2036.:e4. doi: 10.1016/j.cub.2022.03.040. [DOI] [PubMed] [Google Scholar]
- 35.Dolan M-J, Belliart-Guérin G, Bates AS, Frechter S, Lampin-Saint-Amaux A, Aso Y, Roberts RJV, Schlegel P, Wong A, Hammad A, et al. Communication from learned to innate olfactory processing centers is required for memory retrieval in drosophila. Neuron. 2018;100:651–668.:e8. doi: 10.1016/j.neuron.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Paper describing a new interaction between the adult olfactory innate and memory pathways that is required for the retrieval of aversive memories. It integrated connectomics and behavioural data, exemplifying the ability to map new cell types across LM and EM, and the value added from the latter
- 36.Costa M, Manton JD, Ostrovsky AD, Prohaska S. Jefferis GSXE: NBLAST: rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron. 2016;91:293–311. doi: 10.1016/j.neuron.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bates AS, Manton JD, Jagannathan SR, Costa M, Schlegel P, Rohlfing T, Jefferis GS. The natverse, a versatile toolbox for combining and analysing neuroanatomical data. eLife. 2020;9:e53350. doi: 10.7554/eLife.53350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eschbach C, Fushiki A, Winding M, Afonso B, Andrade IV, Cocanougher BT, Eichler K, Gepner R, Si G, Valdes-Aleman J, et al. Circuits for integrating learned and innate valences in the insect brain. eLife. 2021;10:e62567. doi: 10.7554/eLife.62567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felsenberg J, Jacob PF, Walker T, Barnstedt O, Edmondson-Stait AJ, Pleijzier MW, Otto N, Schlegel P, Sharifi N, Perisse E, et al. Integration of parallel opposing memories underlies memory extinction. Cell. 2018;175:709–722.:e15. doi: 10.1016/j.cell.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Lindsey JW, Marin EC, Otto N, Dreher M, Dempsey G, Stark I, Bates AS, Pleijzier MW, Schlegel P, et al. The connectome of the adult Drosophila mushroom body provides insights into function. eLife. 2020;9:e62576. doi: 10.7554/eLife.62576. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper fully reconstructed the connectome of the adult Drosophila MB from the hemibrain volume, and provided insights into structural and connectivity motifs
- 41.Yamada D, Bushey D, Feng L, Hibbard K, Sammons M, Funke J, Litwin-Kumar A, Hige T, Aso Y. Hierarchical architecture of dopaminergic circuits enables second-order conditioning in Drosophila. bioRxiv. 2022 doi: 10.1101/2022.03.30.486484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou C, Pan Y, Robinett CC, Meissner GW, Baker BS. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron. 2014;83:149–163. doi: 10.1016/j.neuron.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 43.Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nojima T, Rings A, Allen AM, Otto N, Verschut TA, Billeter J-C, Neville MC, Goodwin SF. A sex-specific switch between visual and olfactory inputs underlies adaptive sex differences in behavior. Curr Biol. 2021;31:1175–1191.:e6. doi: 10.1016/j.cub.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper describes the sexually dimorphic aDN neurons, having distinct morphology, upstream connectivity and behavioural roles between sexes. Male aDNs were required for visual tracking of female movement and orienting during courtship, whereas female aDNs do not receive visual but rather olfactory inputs, and were required for communal egg laying site selection
- 47.Wang K, Wang F, Forknall N, Yang T, Patrick C, Parekh R, Dickson BJ. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature. 2021;589:577–581. doi: 10.1038/s41586-020-2972-7. [DOI] [PubMed] [Google Scholar]; • This paper identified the circuitry controlling female vaginal plate opening, a behaviour that promotes mating. They identified vpoDNs, descending neurons responsible for this behaviour, and how auditory information about the male courtship song is integrated with the female mating state, to control the mating decision
- 48.Wang F, Wang K, Forknall N, Patrick C, Yang T, Parekh R, Bock D, Dickson BJ. Neural circuitry linking mating and egg laying in Drosophila females. Nature. 2020;579:101–105. doi: 10.1038/s41586-020-2055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper identified the neural circuitry linking egg laying to mating status. Female oviDNs, descending neurons controlling egg laying, get indirectly disinhibited by sex peptide-to-pC1 pathway, to enable egg laying after mating
- 49.Deutsch D, Pacheco D, Encarnacion-Rivera L, Pereira T, Fathy R, Clemens J, Girardin C, Calhoun A, Ireland E, Burke A, et al. The neural basis for a persistent internal state in females. eLife. 2020;9:e59502. doi: 10.7554/eLife.59502. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper showed that activating female pC1d/e neurons induced persistent behavioural changes in presence of a male, and long-lasting neuronal activity in regions downstream to pC1 neurons. They also reconstructed the wiring map of pC1d and e and described reciprocal connections with aIPg neurons
- 50.Schretter CE, Aso Y, Robie AA, Dreher M, Dolan M-J, Chen N, Ito M, Yang T, Parekh R, Branson KM, et al. Cell types and neuronal circuitry underlying female aggression in. eLife. 2020;9:e58942. doi: 10.7554/eLife.58942. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper described sexually dimorphic aIPg neurons controlling female aggression, and their reciprocal interactions with pC1d neurons
- 51.Taisz I, Donà E, Münch D, Bailey SN, Morris WJ, Meechan KI, Stevens KM, Varela I, Gkantia M, Schlegel P, et al. Generating parallel representations of position and identity in the olfactory system. bioRxiv. 2022 doi: 10.1101/2022.05.13.491877. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper analysed the circuit processing a male pheromone, cVA, and found that it is relayed onto parallel processing streams, generating separate representations of (‘where’) and identity (‘what’). Distinct temporal response properties (phasic versus tonic) and cell type specific multimodal integration motifs refine the represented stimulus features. Mechanistically, increasing bilateral odour contrast by contralateral inhibition produces olfactory spatial receptive fields that can encode angular direction of an odour source
- 52.Kim SS. Plasticity between visual input pathways and the head direction system. Curr Opin Neurobiol. 2021;71:60–68. doi: 10.1016/j.conb.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Fisher YE. Flexible navigational computations in the Drosophila central complex. Curr Opin Neurobiol. 2022;73:102514. doi: 10.1016/j.conb.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Seelig JD, Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521:186–191. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green J, Adachi A, Shah KK, Hirokawa JD, Magani PS, Maimon G. A neural circuit architecture for angular integration in Drosophila. Nature. 2017;546:101–106. doi: 10.1038/nature22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner-Evans D, Wegener S, Rouault H, Franconville R, Wolff T, Seelig JD, Druckmann S, Jayaraman V. Angular velocity integration in a fly heading circuit. eLife. 2017;6:e23496. doi: 10.7554/eLife.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SS, Hermundstad AM, Romani S, Abbott LF, Jayaraman V. Generation of stable heading representations in diverse visual scenes. Nature. 2019;576:126–131. doi: 10.1038/s41586-019-1767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher YE, Lu J, D’Alessandro I, Wilson RI. Sensorimotor experience remaps visual input to a heading-direction network. Nature. 2019;576:121–125. doi: 10.1038/s41586-019-1772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okubo TS, Patella P, D’Alessandro I, Wilson RI. A neural network for wind-guided compass navigation. Neuron. 2020;107:924–940.:e18. doi: 10.1016/j.neuron.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardcastle BJ, Omoto JJ, Kandimalla P, Nguyen B-CM, Keleş MF, Boyd NK, Hartenstein V, Frye MA. A visual pathway for skylight polarization processing in Drosophila. eLife. 2021;10:e63225. doi: 10.7554/eLife.63225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hulse BK, Haberkern H, Franconville R, Turner-Evans DB, Takemura S-Y, Wolff T, Noorman M, Dreher M, Dan C, Parekh R, et al. A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and contextdependent action selection. eLife. 2021;10:e66039. doi: 10.7554/eLife.66039. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper fully reconstructed the connectome of the adult Drosophila central complex from the hemibrain volume, and provided insights into structural and connectivity motifs
- 62.Stone T, Webb B, Adden A, Weddig NB, Honkanen A, Templin R, Wcislo W, Scimeca L, Warrant E, Heinze S. An anatomically constrained model for path integration in the bee brain. Curr Biol. 2017;27:3069–3085.:e11. doi: 10.1016/j.cub.2017.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim IS, Dickinson MH. Idiothetic path integration in the fruit fly Drosophila melanogaster. Curr Biol. 2017;27:2227–2238.:e3. doi: 10.1016/j.cub.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 64.Behbahani AH, Palmer EH, Corfas RA, Dickinson MH. Drosophila re-zero their path integrator at the center of a fictive food patch. Curr Biol. 2021;31:4534–4546.:e5. doi: 10.1016/j.cub.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyu C, Abbott LF, Maimon G. Building an allocentric travelling o direction signal via vector computation. Nature. 2021;601:92–97. doi: 10.1038/s41586-021-04067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Together with Lu et al. (2022), both papers provided evidence that the FB network encodes a travel vector, by combining heading information from EB compass neurons with velocity pre-motor signals. Downstream in the pathway, they describe the anatomy, physiology and behavioural effects of a mechanism for a reference-frame transformation of this velocity vector, from a body-centred to a world-centred reference
- 66.Shiozaki HM, Ohta K, Kazama H. A multi-regional network encoding heading and steering maneuvers in Drosophila. Neuron. 2020;106:126–141.:e5. doi: 10.1016/j.neuron.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Matheson AMM, Lanz AJ, Licata AM, Currier TA, Syed MH, Nagel KI. Organization of central circuits for wind-guided olfactory navigation. bioRxiv. 2021 doi: 10.1101/2021.04.21.440842. [DOI] [Google Scholar]
- 68.Witvliet D, Mulcahy B, Mitchell JK, Meirovitch Y, Berger DR, Wu Y, Liu Y, Koh WX, Parvathala R, Holmyard D, et al. Connectomes across development reveal principles of brain maturation. Nature. 2021;596:257–261. doi: 10.1038/s41586-021-03778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, Nguyen KCQ, Tang LT-H, Bayer EA, Duerr JS, et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571:63–71. doi: 10.1038/s41586-019-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X, Edgecombe GD, Hou X, Goral T, Strausfeld NJ. Preservational pathways of corresponding brains of a Cambrian Euarthropod. Curr Biol. 2015;25:2969–2975. doi: 10.1016/j.cub.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 71.Sayre ME, Templin R, Chavez J, Kempenaers J, Heinze S. A projectome of the bumblebee central complex. Elife. 2021;10:e68911. doi: 10.7554/eLife.68911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsushita A, Stewart F, Ilić M, Chen P-J, Wakita D, Miyazaki N, Murata K, Kinoshita M, Belušič G, Arikawa K. Connectome of the lamina reveals the circuit for early color processing in the visual pathway of a butterfly. Curr Biol. 2022;32:2291–2299.:e3. doi: 10.1016/j.cub.2022.03.066. [DOI] [PubMed] [Google Scholar]
- 73.Petrucco L, Lavian H, Wu YK, Svara F, Štih V, Portugues R. Neural dynamics and architecture of the heading direction circuit in a vertebrate brain. bioRxiv. 2022 doi: 10.1101/2022.04.27.489672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clements J, Goina C, Hubbard PM, Kawase T, Olbris DJ, Otsuna H, Svirskas R, Rokicki neuPrint: analysis tools for EM connectomics. bioRxiv. 2022 doi: 10.1101/2022.07.20.500311. [DOI] [Google Scholar]
- 75.Heinze S, El Jundi B, Berg BG, Homberg U, Menzel R, Pfeiffer K, Hensgen R, Zittrell F, Dacke M, Warrant E, et al. A unified platform to manage, share, and archive morphological and functional data in insect neuroscience. eLife. 2021;10:e65376. doi: 10.7554/eLife.65376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lazar AA, Liu T, Turkcan MK, Zhou Y. Accelerating with FlyBrainLab the discovery of the functional logic of the brain in the connectomic and synaptomic era. eLife. 2021;10:e62362. doi: 10.7554/eLife.62362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chou Y-H, Yang C-J, Huang H-W, Liou N-F, Panganiban MR, Luginbuhl D, Yin Y, Taisz I, Liang L, Jefferis GSXE, et al. Mating-driven variability in olfactory local interneuron wiring. Sci Adv. 2022;8:eabm7723. doi: 10.1126/sciadv.abm7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bates AS, Schlegel P, Roberts RJV, Drummond N, Tamimi IFM, Turnbull R, Zhao X, Marin EC, Popovici PD, Dhawan S, et al. Complete connectomic reconstruction of olfactory projection neurons in the fly brain. Curr Biol. 2020;30:3183–3199.:e6. doi: 10.1016/j.cub.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiang A-S, Lin C-Y, Chuang C-C, Chang H-M, Hsieh C-H, Yeh C-W, Shih C-T, Wu J-J, Wang G-T, Chen Y-C, et al. Threedimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol. 2011;21:1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 81.Meier M, Borst A. Extreme compartmentalization in a Drosophila amacrine cell. Curr Biol. 2019;29:1545–1550.:e2. doi: 10.1016/j.cub.2019.03.070. [DOI] [PubMed] [Google Scholar]