Abstract
Type-2 immunity helps protect the host from infection, but also plays key roles in tissue homeostasis, metabolism and repair. Unfortunately, inappropriate type-2 immune reactions may lead to allergy and asthma. Group-2 innate lymphoid cells (ILC2s) in the lungs respond rapidly to local environmental cues, such as the release of epithelium-derived type-2 initiator cytokines/alarmins, producing type-2 effector cytokines such as IL-4, IL-5 and IL-13 in response to tissue damage and infection. ILC2s are associated with the severity of allergic asthma and experimental models of lung inflammation have shown how they act as playmakers, receiving signals variously from stromal and immune cells as well as the nervous system, and then disseminating cytokine cues to elicit effector functions and potentiate CD4+ T helper cell activation that characterise the pathology of allergic asthma. Recent breakthroughs identifying stromal and neuronal-derived microenvironmental cues that regulate ILC2s, along with studies recognizing the potential plasticity of ILC2s, have improved our understanding of the immunoregulation of asthma and opened new avenues for drug discovery.
Keywords: asthma, allergy, innate lymphoid cells, alarmin, type-2 immunity, neuroimmunity
1. Introduction
Asthma is a heterogeneous respiratory disease characterised by chronic lower airway inflammation, airway hyperresponsiveness and airway remodelling. According to World Health Organization (WHO) statistics, approximately 235 million people suffer from asthma, globally (1), resulting in a high health and economic cost. Asthma-associated immune phenotypes (endotypes) can vary widely. For example, allergen-induced type-2 immune reactions characterised by cytokines, including IL-4, IL-5 and IL-13, derived predominantly from innate group 2 innate lymphoid cells (ILC2) and adaptive T helper 2 (Th2) cells, and eosinophilia are associated with the type-2 high asthma endotype (2). Indeed, blood eosinophil levels are a strong determinant of patient responsiveness to type-2 cytokine targeting antibody treatments. By contrast, the type-2 low asthma endotype, associated with a marked increase in IL-17A and neutrophilic or paucigranulocytic airway inflammation, is frequently linked with exposure to irritants or obesity (2). However, these features are not always mutually exclusive and can be strongly influenced by co-morbidities such as allergic rhinitis, respiratory viral infection, chronic rhinosinusitis, nasal polyps, atopic dermatitis, and obesity. Although asthma is a common human respiratory illness, many of our mechanistic insights into asthma are derived from animal models. This has included the discovery of lymphocyte specialisation with the characterisation of polarised Th cell subsets within the adaptive arm of the immune system (3) and, more recently, novel innate lymphoid cell (ILC) populations, which have been shown to play central roles in asthma-associated immune reactions (4, 5).
Key amongst the roles of ILC2s is their ability to integrate signals from the epithelium, stromal cells, nervous system and other immune cells and to subsequently orchestrate downstream effector functions. In this review, we will discuss how the discovery of ILC2s in mice and humans has added greater complexity, but also a greater appreciation of the immune mechanisms that underlie asthma, and how breakthroughs in understanding ILC2 biology may offer new pathways for potential therapeutic intervention.
1.1. Type-2 lymphocytes in asthma
Traditionally, allergic asthma was considered a Th2 cell-driven pathology. These cells are characterised by their expression of the cytokines IL-4, IL-5, IL-9 and IL-13, which stimulate so many of the critical mediators of allergic asthma, including Th2 cell polarisation, eosinophilia, mastocytosis, goblet cell hyperplasia, muscle contraction, IgE switching and mucus hypersecretion (6–11). However, research in mouse models led to the discovery of ILC2s as new protagonists in protective type-2 immunity and allergic asthma (4, 12). In contrast to T cells, ILCs lack somatically-recombined antigen-specific receptors and respond to microenvironmental cues, such as cytokines, rather than directly to antigen stimulation. ILCs are divided into five categories - natural killer cells (NK), ILC1s, ILC2s, ILC3s and lymphoid tissue inducer cells (LTi). NK cells are innate cytotoxic cells, comparable to cytotoxic T cells, whereas ILC1s, ILC2s and ILC3s are analogous to Th1, Th2 and Th17 helper cells, respectively. LTi cells are essential for secondary lymphoid organ development (13).
ILC2s are potent cytokine producers, expressing predominantly IL-5, IL-13 but also IL-4 and IL-9 amongst others (Figure 1) (12–15). Like T cells and B cells, ILC2s are derived from common lymphoid progenitor (CLP) cells in the bone marrow via an ILCP intermediate that can further differentiate into ILC1, ILC2, ILC3 and NK cells (16). ILC2 commitment requires the transcription factors GATA3, RAR-related orphan receptor α (RORα) and B cell lymphoma leukemia 11b protein (BCL11b) (16–18). Both foetal-liver-derived and bone marrow (BM) progenitors can differentiate into ILC2s and, during the first couple of weeks after birth, ILC2 numbers expand greatly in the lung. This has been attributed to their stimulation by IL-33 released by airway epithelial cells due to mechanical stress induced by the onset of breathing (19–21). Indeed, in adult mice, the majority of tissue ILC2s are of early postnatal source rather than foetal or adult origin (22)
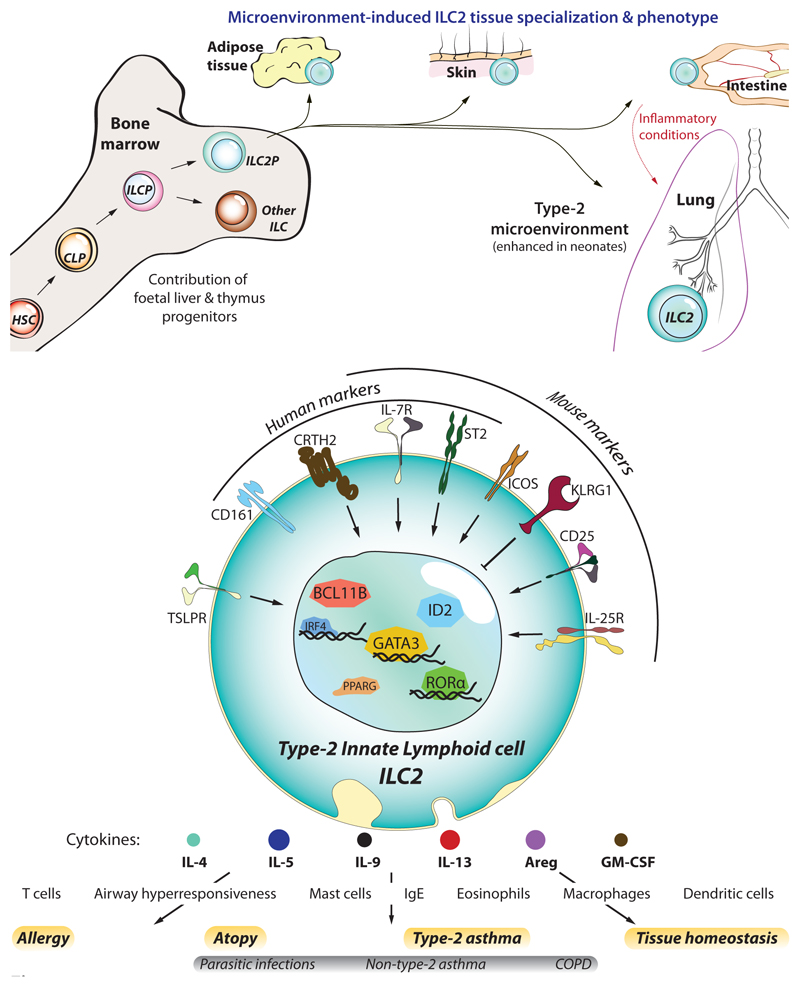
Figure 1. ILC2 development and identification.
(a) Like other haematopoietic cells, ILC2s derive from haematopoietic stem cells (HSC) that differentiate into common lymphoid progenitors (CLP), and further commit towards the innate lymphocyte lineage as ILC progenitors (ILCP), and ILC2 progenitors (ILC2P). Though ILC2s first develop in foetal tissues, a significant fraction of them arise during the neonatal period whereupon they colonise most peripheral tissues (skin, intestine, lung, and visceral adipose tissue – VAT). Here they becom resident lymphocytes and adapt to the tissue microenvironment by acquiring a specialized genetic program tailored to each tissue. In particular, at least half of the ILC2s residing in the lung are from neonatal origin, a period where lungs display a strong type-2-biased microenvironment and allergic sensitisation likely occurs. However, under inflammatory conditions such as parasite infection or allergic reactions, the lung receives a transient ingress of ILC2s from the small intestine, which are characterized by increased sensitivity to IL-25 and higher expression of KLRG1, that contribute to lung inflammation. (b) ILC2s can be identified by their lack of lineage specific markers (CD3, CD4, CD8a, CD11b, CD11c, CD19, FcεRI, Ly6C, Ly6G, NK1.1/CD56, TCRαβ, TCRγδ, and Ter119), and expression of IL-7Rα (CD127) and high levels of the transcription factor GATA3. In addition, other molecules can serve to identify them in humans (i.e. CD161, CRTH2, and ST2) and mice (ST2, CD25, KLRG1, ICOS). Despite also providing contact-dependent signals, the foremost contribution of ILC2s to allergy and asthma is their overwhelming capacity to rapidly produce type-2 cytokines upon stimulation by cytokines/alarmins (IL-25, IL33, TSLP). In turn, ILC2-derived cytokines orchestrate the spectrum of immune effector cells that mediate the immunopathology of asthma, such as eosinophils and mast cells.
During homeostasis, ILC2s are primarily located in mucosal barrier sites such as the lung and intestine, but are also found in many other tissues (13), where they take on tissue-specific characteristics dictated by microenvironmental cues (23, 24). Gene expression profiles have helped to differentiate ILC subsets (25, 26), and even subdivide the major subsets based on differential gene expression (27–31). One such subdivision defined natural ILC2s (nILC2 - characterized by IL-5, Arginase 1 and IL-33R (ST2) expression), and inflammatory ILC2s (iILC2 - expressing IL-25R (IL-17BR) and elevated IL-13) (27–29, 32). However, ever-increasing resolution of single cell-omics, such as mass cytometry and single cell RNA sequencing, has indicated that ILC2s represent a continuum of cells which adapt to microenvironmental cues and become specialised to the tissues where they reside (23, 24, 30, 33). In this way, skin-resident ILC2s respond to IL-18 by expressing IL-18Rα, while gut-resident ILC2s express the IL-25R, making them particularly sensitive to IL-25, and stomach and lung ILC2s display higher levels of ST2 (24, 34). However, despite being primarily tissue resident cells (35), ILC2s can migrate to distant tissues upon immune challenges and acquire the phenotype characteristic of the target tissue (23, 27, 28).
Although ILC2s play a pivotal role in helminth parasite clearance and post-clearance tissue repair (12, 36), ILC2 dysregulation is implicated in various allergic diseases such as asthma, rhinitis and atopic dermatitis. Strikingly, numerous ILC2-related genes have been identified by genome-wide association studies (GWAS) of asthma, with single nucleotide polymorphisms located in the genes encoding IL-13, RORα, ST2 and thymic stromal lymphopoietin (TSLP) (37–40). Early mouse studies also implicated innate mechanisms in lung inflammation, with Rag2-deficient mice, which lack T and B cells, continuing to display airway inflammation and asthma-like symptoms during allergen challenge (41). This non-B, non-T cell compartment has been defined as ILC2 (4, 42) and their roles in human asthma are the subject of extensive investigation.
1.2. Human asthma
Nasal polyps associated with chronic rhinosinusitis were one of the first human tissues found to harbour ILC2s (43). Since then, ILC2 have been shown to be consistently expanded in atopy/asthma patients within the peripheral blood and lung (44, 45) and their frequencies rise during allergy season, significantly correlating with symptoms (46). In humans, upon allergen challenge, the numbers of CD161+CRTH2+ILC2s decrease among peripheral blood mononuclear cells (PBMC) and accumulate in the lung (as measured in bronchoalveolar lavage, BAL) (47). In addition, BAL ILC2s increase their expression of genes encoding the transcription factors AHR, GATA3, BATF, PPARG and IRF4, that promote ILC2 activity, and the cytokines and cytokine receptors that drive ILC2 function (including IL-2RA, IL-4Rα, ST2 (IL1RL1, IL-33R), IFNGR1, IL-25R (IL-17RB), IL-13 and AREG) (47).
Asthmatic patients have increased frequencies of ILC2s in blood, sputum and BAL, which is associated with elevated IL-5 and IL-13 expression (48–55). However, the correlation between asthma severity and ILC2 levels has varied between studies. Smith et al. reported higher blood and sputum ILC2 numbers in severe asthma patients compared to mild asthmatics (50); whilst Yu et al. published that mild asthmatics display higher frequencies of ILC2s than moderate and severe asthmatics (51). Such differences may be due to multiple factors, e.g. the changing frequencies of eosinophil and CD4+ T cells, differences in disease classification criteria and the varied treatment histories in the two studies. Notably, successful asthma treatment significantly reduces blood ILC2 levels (52), whilst allergen rechallenge increases type-2 cytokine expressing ILC2s in sputum (53). Pediatric severe therapy-resistant asthma (STRA) patients show significantly higher levels of ILC2 in blood and sputum as compared to difficult asthma patients and controls (56, 57). STRA patients also showed increased frequencies of IL-13-positive ILC2s. Notably, systemic steroid treatment of STRA patients reduced ILC2 levels which was accompanied by improvements in the disease. Asthmatic patients also show elevated levels of ILC2 initiator cytokines like IL-33 (54) and TSLP (55), in the BAL as compared to controls. Collectively, these studies demonstrate associations between ILC2s and human asthma.
Human ILC2s have been commonly defined as CD45+Lin-IL-7Rα+CRTH2+ cells in human blood. However, a recent report has suggested that human ILC2 populations are more heterogeneous than previously appreciated (45). Indeed, IL-7Rα+CRTH2+, IL-7Rα+CRTH2- and IL-7Rα-CRTH2-ILC2s expressing IL-5 were identified in blood and BAL. These ILC2 subpopulations show differential expression of ILC2 markers such as GATA3, IL-25R, KLRG1, ICOS and CD161, but all three subpopulations caused airway hyperresponsiveness when transferred to Rag2-/-γc-/- mice. Further studies are required to determine whether these three populations are bonafide subpopulations of ILC2 or occur at different stages of differentiation/activation/plasticity and how they may impact disease.
Viral infections generally provoke a type-1 immune profile. However, the respiratory viruses rhinovirus (58) and influenza virus infections (59) promote ILC2 lung infiltration, and exacerbate lung inflammation and airway hyperreactivity to secondary infections and allergen challenges in an ILC2 dependent-manner (60). Asthma patients also present increased IL-25 production upon rhinovirus infection and mouse models indicate that IL-25R blockade is sufficient to suppress rhinovirus-induced IL-33 and TSLP expression and subsequent ILC2 and eosinophilic lung infiltration.
2. Stimulation of lung ILC2s by epithelium and stromal cell-derived factors
An intact airway epithelium is critical for lung homeostasis and dysregulation of this physical barrier leads to the release of cytokines or cytokine-like alarmin molecules that activate juxtaposed immune cell sentinels. ILC2s express and display a spectrum of cytokine receptors that allow them to sense initiator alarmins/cytokines that stimulate their proliferation and promote the secretion of type-2 effector cytokines (Figure 2). Inhaled allergens, infectious agents or airborne pollutants can all induce epithelial cell stress or cell death. For example, various plant-derived, insect-derived or fungal allergens contain proteases that can directly damage epithelial cell tight junctions or activate airway epithelial cells via protease-activated receptors (PAR) (61–63). Three major activators of ILC2s are IL-33, TSLP and IL-25. These may be produced at homeostatic levels or released and/or upregulated upon infection or tissue damage.
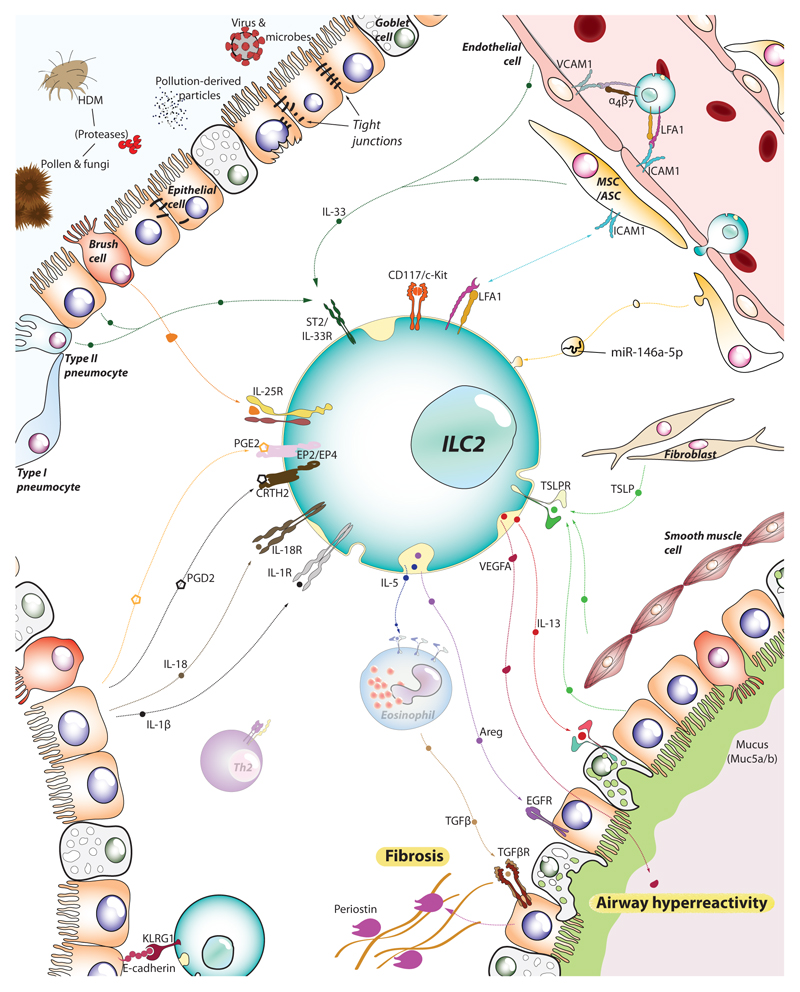
Figure 2.
ILC2s sense the tissue microenvironment and kick-off the type-2 immune response. As tissue resident lymphocytes, ILC2s are strategically positioned in the mucosal barrier sites, poised to monitor the release of alarmins by epithelial, endothelial and stromal cells. Epithelial cells, brush cells, multipotent and adventitial stromal cells (MSC/ASC) and pneumocytes (together with immune cells such as alveolar macrophages and dendritic cells) can detect allergens and irritants and, in turn, release IL-33, IL-25 and TSLP. Together with IL-1β and IL-18 liberated by dying cells, these alarmins stimulate ILC2s to secrete IL-5, IL-13, amphiregulin (Areg), and vascular endothelial factor A (VEGFA), that engage their respective receptors in epithelial cells, goblet cells and smooth muscle cells amongst others. The activation of these cells leads to airway hyperresponsiveness, mucus production and fibrosis, cardinal pathophysiological features of asthma and allergy. Endothelial cells also respond to the local inflammation and support further recruitment of ILC2s and other immune effector cells from the blood and distal tissues (e.g. intestine), boosting the inflammatory process.
2.1. IL-33
IL-33 is a nuclear protein that is released as an alarmin. In humans, airway bronchial epithelial cells and endothelial cells are major sources of IL-33 (64–67). In mice, type II alveolar pneumocytes (68) and adventitial stromal cells (ASCs) (69) constitutively produce IL-33 while endothelial cells only produce IL-33 under chronic inflammation (70–72). Transgenic expression of IL-33 causes severe inflammation in multiple organs (73). The mechanism of IL-33 release into the extracellular space remains elusive, though epithelial necrosis or active secretion have been suggested (71, 74–76). Once released, mast cell and neutrophil-derived extracellular proteases are proposed to process IL-33 into its more active form (74, 77), which binds to IL-1 receptor-like 1 (IL-1RL1, IL-33R, ST2) (78) expressed on ILC2s in the lungs. In the absence of processing, IL-33 is quickly oxidized and its activity neutralised (79).
The importance of IL-33 in allergic lung inflammation has been shown in multiple models (54, 80–83). Subsequent studies demonstrated that asthmatic patients display higher levels of IL-33 in their BAL, sputum and serum and human bronchial epithelial cells (HBEC) as compared to healthy controls (54, 65, 84). IL-33 potently activates ILC2s, and both ST2 or IL-33 deficient animals display reduced AHR, ILC2 and IL-5/IL-13 levels in the BAL and lung as compared to controls (42, 85). ILC2-derived type-2 cytokines can subsequently recruit eosinophils and other immune cells to the lung. ILC2 expression of IL-4 and IL-13 may further compromise epithelial tight junctions by altering their structure (86, 87). Indeed, ILC2s co-cultured with HBEC in the presence of IL-33 produced IL-13 that disrupted epithelial cell tight junctions, an effect prevented by inclusion of anti-IL-13 neutralizing antibody (88). In-vivo, intranasal administration of IL-33 increased airway epithelial permeability in Rag2–/– mice, but not in Rag2–/–Il2g–/– or ILC2-deficient Rorasg/sg mice (88). Allergen-induced uric acid production in the airways of mice has been shown to enhance IL-33 release by airway epithelium and thereby promote a type-2 response (76), and a similar mechanism involving IL-33 and TSLP has been reported to induce lung ILC2 following RSV infection (89).
2.2. TSLP
TSLP is produced by various non-immune and immune cells, but the major sources of TSLP in the lung are epithelium, fibroblasts and smooth muscle cells (90, 91), which produce TSLP in response to allergen, infection or trauma (5, 90, 92, 93). TSLP signalling occurs via a heteromeric receptor composed of TSLP receptor (TSLPR) and IL-7Rα (94) expressed on ILC2. Significantly, TSLP levels are increased in the serum, BAL and sputum of asthmatic patients as compared to healthy controls (95–97). Overexpression of TSLP in mice caused spontaneous development of airway inflammation, whilst TSLPR-deficient mice displayed decreased airway inflammation, lymphocyte infiltration and AHR (98). TSLPR-deficient mice also have fewer lung ILC2 and reduced IL-5 and IL-13 secretion in response to Alternaria alternata-induced acute lung inflammation and respiratory syncytial virus infection (99, 100). However, in vitro, TSLP alone does not induce ILC2 proliferation and type-2 cytokine secretion but instead acts synergistically with IL-33 (5). Indeed, these cytokines also appear to reciprocally regulate the expression of the others receptor on ILC2s (99). Notably, TSLP in the BAL fluids of patients with severe refractory asthma correlated with steroid resistance (55, 101), and airway ILC2s (but not blood ILC2s) from these patients showed dexamethasone resistance. Dexamethasone treatment increased IL-7Rα expression on ILC2s and thereby increased TLSP mediated pSTAT5 and MEK signalling. Inhibition of MEK and STAT5 reversed ILC2 steroid resistance. Therefore, combining anti-TSLP neutralizing antibody with conventional therapy could potentially help treat severe refractory asthma (55, 101).
2.3. IL-25
In mice, IL-25 overexpression or exogenous IL-25 administration induces type-2 immune responses including an increase in type-2 cytokine secretion, airway inflammation, lymphocyte infiltration, AHR and fibrosis (4, 27, 42). The IL-25 receptor, which consists of IL-17RB and IL-17RA subunits, is differentially expressed on ILC2s dependent on tissue location (24). Asthmatic patients display higher serum IL-25 levels than healthy controls (102) and IL-25 neutralization during allergen-induced experimental asthma reduces all hallmarks of asthma (103). Though, in mice, IL-33 is a more potent activator of lung ILC2s and AHR (42). The cellular source of IL-25 remained elusive for a long time. However, in 2016 multiple groups independently discovered intestinal tuft cells as the source of IL-25(104–106) which, in a feed-forward mechanism, stimulates IL-13 expression from ILC2s that can lead to further tuft cell differentiation from progenitor cells. The source of IL-25 in the lungs is somewhat more ambiguous. von Moltke et al. reported that mouse DCLK1+ lung tuft cells (known as brush cells in the trachea) also express IL-25 (104) and this was supported by further studies in which A. alternata and house dust mite (HDM)-induced allergic lung inflammation promoted the proliferation of IL-25-positive brush cells (107). However, it has been proposed that DCLK1+ solitary chemosensory cells (SCC) in the human upper airways are the source of human IL-25 in patients with nasal polyposis and that this correlated with ILC2 levels (108, 109).
The cytokine TGFβ is another epithelial cell-derived activator of ILC2s. Lung ILC2s express TGFβRII and in vitro treatment with TGFβ enhances ILC2 chemokinesis (110). Furthermore, deficiency of airway epithelial-derived TGFβ leads to reduced BAL lymphocyte infiltration, eosinophil recruitment and diminished AHR as compared to control animals, in response to HDM allergen. TGFβ-deficiency specifically impaired ILC2 accumulation without affecting T lymphocytes (110). Therefore, the beneficial effects of TGFβ neutralization (111, 112) in asthma might, at least partially, be ILC2-mediated.
3. ILC2 effector functions
The cardinal type-2 cytokines IL-4, IL-5, IL-9 and IL-13 are all produced by ILC2s (representing up to 50 – 80% of all IL-5 and IL-13 producers) and have been shown to contribute to the induction of goblet cell hyperplasia, mucus hypersecretion, eosinophilia, airway hyperresponsiveness and fibrosis in the lung (4, 6, 9, 13, 113, 114).
Mucus hypersecretion is a major cause of airway obstruction, and is accompanied by goblet cell hyperplasia. Epidemiological studies have demonstrated a strong association between early life rhinovirus infection with asthma in later life (115). In neonatal mice, rhinovirus infection led to an increase in lung IL-25, IL-33 and TSLP secretion, with airway epithelium-derived IL-25 inducing ILC2-mediated IL-13 production which subsequently promoted mucus hyperproduction and goblet cell hyperplasia (116–118). Adoptive transfer of ILC2s from infected neonatal mice to healthy neonatal recipients was sufficient to cause mucosal cell metaplasia (118). In vivo allergen challenge also promotes murine ILC2s to produce amphiregulin which further contributes to epithelial cells mucus overproduction. In an HDM mouse model, ILC2-derived amphiregulin can activate the EGFR signalling pathway, which ultimately causes mucus hypersecretion by epithelial cells.
IL-4 and IL-13 can enhance histamine-induced airway hyperresponsiveness in human bronchi (119), though the role of ILC2-derived IL-4 and IL-13 in this process is unknown. However, in contrast to wildtype mice, both individual IL-25 or IL-33 receptor-deficient mice and combined knock-out mice, which cannot elicit ILC2 responses, have impaired airway hyperresponsiveness in allergen-induced experimental asthma models (42). Shen et al. have recently demonstrated that ILC2-derived vascular endothelial factor A (VEGFA) is crucial for airway hyperresponsiveness (120). In IL-33 or A. alternata-induced airway allergy models, adoptive transfer of ILC2s to Rag2–/–Il2rg–/– mice was sufficient to induce airway hyperresponsiveness. In this model, inhibition of VEGFA signalling by SU1498 abolished airway hyperresponsiveness, whilst neutralization of IL-13 was only partially effective. Notably, VEGFA also regulated IL-13 secretion by ILC2s in an autocrine manner (120).
Recently ILC2 have been implicated in lung fibrosis. In an HDM model with overexpression of smad5, IL-25 neutralization reduced bronchiolar collagen deposition and airway remodelling (121), whilst ILC2-deficient mice displayed diminished collagen deposition and pulmonary fibrosis following IL-25-induced acute lung inflammation (113). Adoptive transfer of ILC2s also exacerbated bleomycin-induced pulmonary fibrosis (122). Experimental lung fibrosis is also reduced in ST2-deficient mice (122). The relative importance of specific ILC2-derived cytokines in fibrosis is unclear. In an allergen-induced experimental asthma model, ILC2-derived IL-5 recruited eosinophils to the lung and eosinophil-derived TGFβ contributed to lung fibrosis, with IL-5-deficient mice exhibiting reduced airway remodelling (123, 124). Furthermore, both IL-4-deficient and IL-13-deficient mice display reduced subepithelial fibrosis and periostin deposition. However, this reduction is also accompanied by a reduction in TGFβ1 level, making it difficult to delineate the direct and indirect effects of IL-4 and IL13 (125, 126). Indeed, the differential contribution of IL-4 and IL-13 derived from ILC2s, Th2 cells, mast cells or basophils to subepithelial fibrosis, is yet to be determined.
4. ILC2 migration/homing/integrins
Although ILC2 are often referred to as tissue resident, they are also recruited from the blood circulation and other tissues during inflammation (28, 35). Indeed, many studies using human ILC2s harvest them from peripheral blood. During asthma, lung endothelial cells upregulate the expression of the cellular adhesion molecules ICAM1 and VCAM1(127) and recent studies have implicated both of these molecules in ILC2 recruitment to the lung (128, 129). Karta et al. demonstrated, using bone marrow chimaeras, that the increase in lung ILC2 levels following A. alternata challenge is due to both local proliferation and migration from bone marrow and circulation (128). This migration from the blood circulation to the airways required αLβ2 (LFA1) integrin expression (common to both human and mouse ILC2) and blocking either β2 or αL inhibited allergen-induced ILC2 recruitment to the lung (128). The authors went on to propose that ICAM1, expressed by lung endothelial cells, and a known ligand of αLβ2, as a potential partner for ILC2-expressed αLβ2, supporting a role for endothelial cells in ILC2 recruitment to the airways (128). The contribution of endothelial cell-mediated ILC2 recruitment was further supported by the finding that allergen-induced IL-33 secretion promotes RAGE-mediated VCAM1 expression by lung endothelial cells and that antibodies that inhibit VCAM1 binding to α4β7 on ILC2 repress lung ILC2 recruitment in response to HDM or IL-33-induced airway inflammation (129).
Recruitment of ILC2s into the lungs requires CCR4 and/or CCR8 in mice (130, 131), with IL-33 inducing CCR8 (and CCR1) but not of CCR4 (130). CCR8 inhibition reduced ILC2 recruitment and type-2 inflammation (130, 131). Importantly, human peripheral blood ILC2s also express CCR8, raising the potential for therapeutic benefit in asthma.
Although ILC2s display tissue residence and adaptation in human and mice (22, 23, 33, 35), they can also participate and/or coordinate immunity in distal organs (23, 28, 132, 133). For example, during murine allergen challenges, gut IL-25R+KLRG1hi ILC2s migrate from the lamina propria to the lung in an S1P-dependent manner, substantially contributing to allergic inflammation (23, 28, 32). Reciprocally, lung resident ILC2s also migrate towards the intestine to contribute to the antiparasitic response (23). Additionally, intestinal ILC2s are able to detect IL-33 released from keratinocytes upon mechanical skin injury. In turn these ILC2 activate intestinal mast cells via IL-4 and IL-13, which mediate allergen-induced anaphylaxis (133). This invites the question of whether a similar mechanism could potentiate asthma in atopic patients?
5. ILC2 immune networking
Complex diseases, like asthma, involve an interplay of numerous immune and non-immune cells. ILC2s represent a pivotal hub in the crosstalk between innate immune cells (e.g. macrophages, dendritic cells and granulocytes) and adaptive lymphocytes (B and T cells) (Figure 3). ILC2s modulate the immune response by sending and receiving multiple ligands and soluble factors that induce immune cell activation as well as stromal cell reactions.
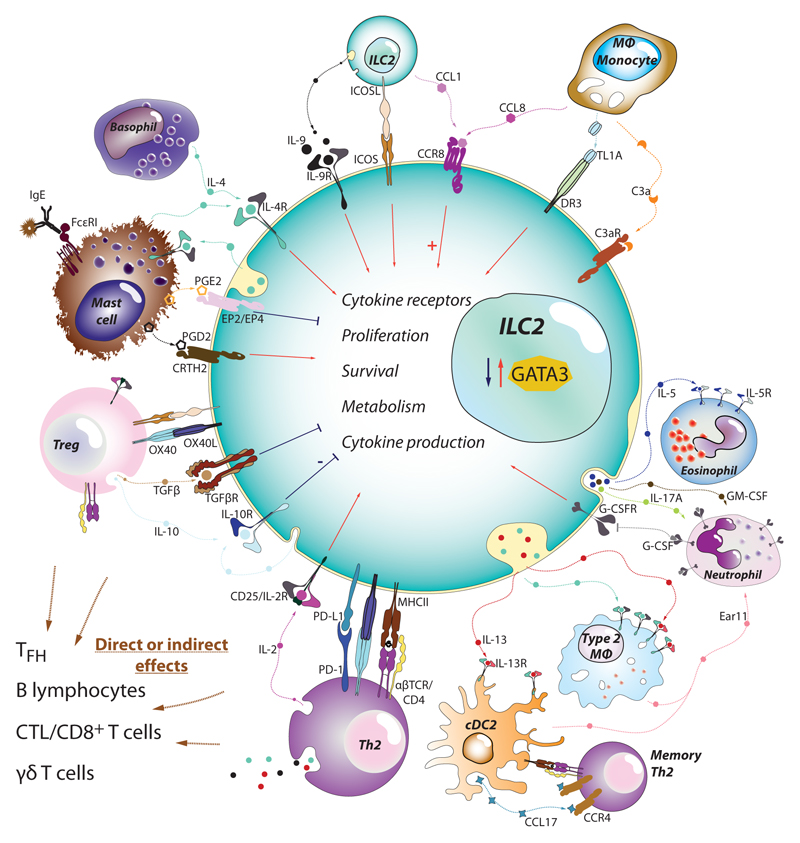
Figure 3.
ILC2s as central playmakers within the immune cell network in asthma. Since their discovery, ILC2s have been found to directly interact with most of the immune cells participating in asthma and allergy. ILC2s integrate the information provided by alarmins with the signals derived from other innate leukocytes such as basophils, neutrophils, macrophages and mast cells. Cytokines (IL-2, IL-4, IL-9, IL-10…), chemokines (CCL1, CCL8), ligand-receptor interactions (ICOS-ICOSL), lipid mediators (PGD2, PGE2, PGI2, LTD4…) and other soluble factors will stimulate or suppress ILC2 expression of alarmin receptors, proliferation, survival, transcriptional factor levels (e.g. GATA3), and cytokine production. The net effect is the capacity of ILC2s to activate and positively modulate T cells, macrophages, dendritic cells into becoming type-2 immune cells (Th2, M2 or cDC2 respectively). In parallel, ILC2s also provide support for mast cells, eosinophils and neutrophils, effectors that will contribute extensively to the pathology associated with asthma
5.1. ILC2 interactions stromal cells
Lung adventitial stromal cells (ASCs) have been reported to support ILC2 proliferation and cytokine production (134). In vitro, ASCs activate ILC2 principally via TSLP production. In vivo, ASC depletion reduces lung ILC2 numbers at least partly through the loss of ASC-derived IL-33. Reciprocally, depletion of ILC2s during helminth infection also reduces IL-33 expressing ASC expansion, suggesting a feedback circuit (69). However, further studies are required to uncover the signals involved in ILC2 recruitment to the adventitial cuffs and to understand the effect of ASC and ILC2 interactions in asthma. Of note, a recent study in adipose tissue has suggested that multipotent stromal cells (MSCs) in the adipose tissue can potentiate ILC2 proliferation and type-2 cytokine production that was attributable to integrin-mediated signalling via ICAM-1 on stromal cells binding to LFA-1 on ILC2s (135). Consequently, ILC2-derived IL-4 and IL-13 fed back to induce eotaxin secretion from MSCs supporting eosinophil recruitment. With similar stromal cells present in the lungs, it is possible that a parallel circuit may occur in the airways alongside the ASC:TSLP pathway.
Interestingly, small extracellular vesicles (SEV) derived from human mesenchymal stromal cells (MSC) have been reported to ameliorate ILC2-dependent airway hyperresponsiveness following IL-33 administration. This inhibitory effect was microRNA(miR)-146-5p-mediated, with SEVs isolated from MSCs able to inhibit IL-5 and IL-13 secretion by ILC2s isolated from allergic rhinitis patients (136). However, the in vivo consequences of this interaction and the molecular mechanisms involved remain to be determined.
5.2. ILC2 interactions with T cells
Significantly, in addition to providing a potent innate source of type-2 cytokines, ILC2s can also help potentiate adaptive Th2 cell responses, either directly or indirectly through third-party cells (6, 137–139). A proportion of mouse ILC2s express MHC class II molecules together with variable levels of CD80 and CD86, giving them the capacity to present antigens to T cells (12, 138) to induce T cell proliferation (though not with the same efficiency as professional antigen-presenting cells). In turn Th2 cells produce IL-2, which stimulates ILC2 expansion and cytokine production (138). The antigen-presenting function of ILC2s has also been reported to be at least partially dependent on ILC2-intrinsic C3ar1 expression (140). In humans, ILC2s are also able to uptake, process and present antigens on MHC class II molecules and this required stimulation with IL-1β and IL-18 (138, 141). Furthermore, by rapidly producing IL-13, ILC2s promote the migration of DCs to the lymph nodes to prime T cells at the initiation of the adaptive immune response (6).
ILC2s can provide additional contact-dependent pro-type-2 signals to T cells. Besides expressing PD-1, ILC2s also display PD-L1 on their surface. Surprisingly, and in contrast to its role in promoting immune exhaustion, PD-L1 on ILC2s has been reported to promote Th2 differentiation and expansion in a Th2-intrinsic PD-1-dependent manner (142). Lymphocyte-specific PD-L1-deficiency resulted in reduced numbers of ILC2s and DCs, and diminished type-2 immunity (142). The co-stimulatory molecule OX40L, expressed by ILC2s in the lungs, also contributes to allergic airway disease by inducing the expansion of OX40-expressing Th2 cells and regulatory T cells upon IL-33 and allergen stimulation (139). Of note, engagement of OX40 on T lymphocytes has been shown to heighten expression of IL-9 by initiating chromatin reorganization characteristic of Th2/Th9 cell (143), and it would be interesting to know if such Th-derived IL-9 feeds back to stimulate ILC2s to promote a Th9 immune bias. Though ILC2s also express ICOSL, this molecule seems not to have a role in directly activating Th2 cells (144). In contrast, interaction in-trans between ICOS and ICOSL on neighbouring ILC2s supports the survival and expansion of ILC2s.
ILC2s were found to mediate their effects beyond the initiation of primary T cell responses by enhancing memory T cell migration to sites of secondary allergen challenge (137). ILC2-derived IL-13 was critical for eliciting the secretion of the Th2 cell-attracting chemokine CCL17 by IRF4+CD11b+CD103– DCs. Thus, ILC2s can promote initial Th2 differentiation and co-operate with DCs to support memory Th2 cell responses to allergens.
5.3. ILC2 interactions with Tregs
Regulatory T cells (Tregs), are a subset of T lymphocytes that mediate immune tolerance, e.g. by competing with other lymphocytes for IL-2 and by producing the immunosuppressive cytokines IL-10 and TGFβ (145, 146). Consequently, it is important to understand how Tregs interact with ILC2 (139, 147, 148). Normally ILC2s will promote Treg fitness and function. Both ICOSL (147) and OX40L (139) expressed by ILC2s promote proliferation of Tregs expressing their cognate ligands(139, 147). In addition, autocrine IL-9 expression by ILC2 induces GITRL, which promotes Treg function to resolve inflammation in arthritis models (148). Presumably, such pathways are ineffective in the lungs of asthmatics. Indeed, in food allergies ILC2s bypass the immunoregulation induced by Tregs (145). Notably, a fraction of ILC2s also expresses IL-10 (12, 149, 150), constituting 5-10% in inflammatory airway disease. Interestingly, an increase in IL-10-producing ILC2s was associated with a reduction in eosinophilic infiltration (151), suggesting that skewing ILC2s towards IL-10 production may reduce type-2 inflammation.
5.4. Innate Th2 cells and memory ILC2s
The discovery of Th2 cells that appear to behave as innate lymphocytes, particularly after being primed by antigens from either allergens or parasites (152, 153), suggests that these cells may complement the innate functions of ILC2s, in type-2 immunity. These ‘innately licensed’ Th2 cells produce predominantly IL-13, and antigen-dependent stimulation is still required to induce their expression of IL-4. Although they could compensate to some extent in the absence of ILC2s, they were unable to protect from parasite infection (152). Conversely, it has been proposed that innate leukocytes can acquire a long-lasting memory-like state termed “trained” immunity, that can epigenetically resemble that of their adaptive counterparts (154, 155). “Memory-like” ILC2s have been reported to arise following allergen sensitisation and last for several months making individuals more susceptible to subsequent challenges, and more sensitive to unrelated allergens (156, 157).
5.5. ILC2s regulate, and are in turn regulated by mast cells, eosinophils, neutrophils and basophils
Eosinophils are the most prominent population of effector cells in type-2 immunity-induced asthma, despite non-type-2 asthma indicating that they are not completely required for all cases of asthma. Indeed, mice lacking eosinophils either do not develop, or develop milder forms of, many of the features of allergic asthma (158). Asthma preclinical models indicate that ILC2s contribute to eosinophil infiltration in lungs during allergen-driven type-2 immune responses (4–6). In patients with allergic rhinitis, allergen challenge induces a significant increase in ILC2 numbers in nasal tissue, strongly correlating with eosinophil infiltration (159).
Together with eosinophils, mast cells are pivotal in atopy/asthma (160). Intriguingly, both ILC2s and mast cells are activated by stem cell factor (SCF), which is elevated in serum of asthmatic patients (89). While ILC2s promote mast cell activation (161), the feedback provided by mast cells to ILC2s may have inflammatory or anti-inflammatory consequences. When activated by IgE binding to the FcεRI, mast cells produce IL-4 that can induce ILC2 proliferation and IL-13 production thereby contributing to histamine/serotonin-induced anaphylaxis (162). Mast cells are also major producers of eicosanoids including leukotrienes and prostaglandins (163–166). Both mouse and human lung ILC2 express leukotriene receptor CysLT1R which binds leukotriene D4 (LTD4) to induce ILC2 proliferation and type-2 cytokine production (167). Similarly, ILC2s respond to autocrine/paracrine (168) and mast cell-derived prostaglandin D2 (PGD2) by inducing IL-13 production and upregulating ST2 and IL-17RA expression, making them more responsive to IL-33 and IL-25 activation (169–171). On the other hand, the lipid mediators PGE2(172), PGI2 (173) and lipoxin A4 (174) exhibit inhibitory effects on ILC2s. Similarly, IL-33-activated mast cells produce IL-2, which has been shown in murine papain-induced asthma to promote Tregs that curb ILC2 inflammatory effects through IL-10 (175).
The association between ILC2s and basophils in the context of asthma has not been extensively studied. However, in atopic dermatitis models, IL-33 directly stimulates basophils, which in turn produce IL-4 that synergises with IL-33 to directly enhance ILC2 activation (176). By contrast, basophil inhibition reduces ILC2 accumulation in inflamed skin (177). Likewise, in papain-induced lung inflammation, basophil-derived IL-4 was required for ILC2 activation, IL-5 and IL-13 production and induction of AHR (178). Given the increased numbers of basophils in the sputum of asthmatic patients (179–181) and that, in humans, they respond to allergen by upregulating IL-4 (182), it is certainly possible that an equivalent mechanism for promoting ILC2 proliferation exists in humans.
Neutrophilic infiltration is commonly associated with COPD or non-type-2 asthma (183, 184) but even in asthmatic patients with a type-2 asthma endotype allergen challenge promotes lung neutrophilic infiltration (47). Usually they represent a minor population and were often considered to be the escapees from the suppressive effects of IL-4 and the type-2 environment (185). However, recent studies have identified signals that can drive neutrophil recruitment during type-2 immunity, including the M2 macrophage-derived molecule Ym1 (encoded by Chil3) (186, 187). ILC2 also contribute to neutrophil recruitment in the type-2 microenvironment (188). ILC2s promote the induction of myeloid-derived Ear11, an RNase-like protein which recruits neutrophils upon A. alternata and ragweed challenges.
The modulation between ILC2s and neutrophils seems to be mutual, as it has been recently described that neutrophils cap or curtail ILC2 responses (189). Neutrophils consume granulocyte-colony stimulating factor (G-CSF) and an excess of G-CSF upon neutrophil depletion has a dual effect: there is an increase in ILC2s with higher production of IL-4 and IL-13 (both in lungs and BAL). Both human and murine ILC2s upregulate the expression of the G-CSF receptor (G-CSFR) upon IL-33 stimulation, and in turn, the neutrophil depletion-related excess of G-CSF enhances the expression of GATA3, IL-5 and IL-13. Interestingly, this did not intensify AHR, suggesting that exacerbation of type-2 immunity without further abnormalities is not always sufficient to induce asthma.
In the mucosal barrier sites, activated myeloid cells and endothelial cells are also sources of the cytokine, TL1A (Tnfsf15) that can activate both mouse and human ILC2 via death receptor 3 (DR3) (190–192). In response to ovalbumin or intranasal papain challenge, DR3–/– mice showed reduced accumulation of ILC2 in BAL and lung compared to controls (190, 191). However, TL1A signalling is not necessary for ILC2 differentiation, and IL-33 and IL-25 signalling remain functional in DR3–/– ILC2s (190). Interestingly, TL1A neutralizing antibody ameliorates lymphocyte and eosinophil accumulation in the BAL of rats undergoing ovalbumin-induced experimental lung inflammation (193). Thus, TL1A signalling might be a potential therapeutic target in asthma, though the contribution of ILC2 remains to be identified.
6. Neuroimmune modulation of ILC2 and asthma
Many neuropeptides, biogenic amines and neurotransmitters promote communication between the nervous system and immune cells. The lungs are innervated by a complex network of sensory, sympathetic and parasympathetic neurons (194, 195) with ILC2s residing in close proximity (Figure 4) (29, 196, 197). Notably, allergen-induced airway hyperreactivity in mice is abrogated when the vagal lung-innervating neurons responsible for the detection of noxious stimuli (nociceptive Trpv1-expressing neurons) are inhibited or deleted (198). Originally attributed to a direct effect of neurons on airway muscle contraction, it has become apparent that both depleting or blocking Trpv1+ lung afferents modulates lung ILC2s, prompting the concept that ILC2s are regulated by neuronal cues (199). Recently, Drake and colleagues found that patients with persistent asthma have denser innervation and higher substance P expression in lung biopsies than patients with intermittent asthma or healthy individuals, and this was associated with a failure to respond to bronchodilation treatment. In mice, they found that IL-5-induced eosinophil infiltration promoted airway remodelling, expansion of lung innervation, and airway hyperreactivity (200). Although they did not examine the source of IL-5, it is tempting to speculate whether ILC2-derived IL-5 may underlie this phenotype, with ILC2s and afferent neurons creating a positive feedback loop (via eosinophils) that may contribute to the development of asthma.
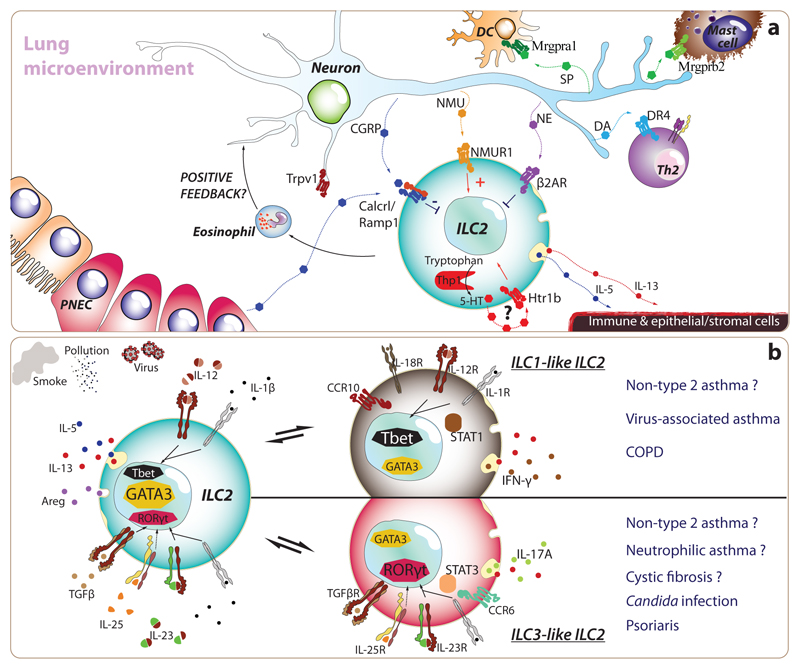
Figure 4. Environmental and microenvironmental cues leading to typical and atypical ILC2s in asthma.
(a) Recently, researchers have discovered that ILC2s express many receptors for neurotransmitters and neuropeptides, as well as exceptionally high levels of certain enzymes implicated in the synthesis of neurotransmitters. Both sympathetic and vagal sensory neurons project their afferent fibres within lung parenchyma. In particular, transient-receptor-potential-cation channel-subfamily-V-member-1-expressing nociceptive neurons (Trpv1+), which specialize in the detection of noxious stimuli from the environment (e.g. irritants, extreme temperatures, tissue damage), appear to play key roles in promoting airway hyperresponsiveness. Besides activating type-2 classical dendritic cells (cDC2) and mast cells through the production of Substance P (SP), and promoting type-2 T helper cell differentiation through the release of dopamine, lung afferent neurons release many peptides and biogenic amines which directly target ILC2s, which reside in close proximity of neural fibres. Neuromedin U (NMU) promotes ILC2 expansion and Th2 cytokine production while norepinephrine (NE) and calcitonin gene related peptide (CGRP) curb the activation of ILC2s. Furthermore, unusually for an immune cell, ILC2s can secrete CGRP and express the enzymatic machinery necessary to produce serotonin (5-TH). Murine models have shown that inhibition or deletion of nociceptive neurons prevents or decreases the severity of allergic immune responses and asthma, suggesting that modulation of neuro-immune units can represent a targetable treatment approach. (b) ILC2s are characterized by the expression of cardinal type-2 cytokines (i.e. IL-4, IL-5, IL-9, IL-13 and Areg) and the transcription factor, GATA3. Nonetheless, ILC2s exhibit a remarkable ability to adapt their biology to match that of their environment. This reversible plasticity allows ILC2s to acquire ILC1- and ILC3-like features such as specific expression of Tbet and RORγt and IFN-γ and IL-17A, respectively. Indeed, the changes induced by IL-1β and IL-12, in the case of ILC1-like ILC2s, or by IL-1β, IL-23 and TGFβ for ILC3-like ILC2s suggest a profound modulation in the transcriptional signature. Both human and murine ILC2s present this capacity in vitro, but we are only starting to understand the prevalence of this process in vivo. Interestingly, the plasticity of ILC2s provides a plausible hypothesis to understand how ILC2s may participate and promote COPD and non-type-2 asthma that are characterised by type-1 and type-3 immunity respectively.
ILC2s are enriched in receptors for neuronal cues (196, 201, 202), including the neuropeptides neuromedin U (NMU), calcitonin gene-related peptide (CGRP) and β2 adrenergic receptor agonists (β2AR). In the lungs of mice NMU receptor (NMUR1)-positive ILC2s respond to NMU and IL-25 to promote eosinophilia and AHR (29). Furthermore, NMUR1-deficient mice fail to mount productive type-2 immune responses against helminths or HDM challenge (197, 202). However, NMU-deficient mice do not present gross alterations in response to HDM (29), and the extent of NMU involvement in allergen-induced airway inflammation and lung eosinophilia has not been reported in Nmur1-deficient mice (29). The consequence of deleting NMU in specific neurons, and restricting NMUR1-deficiency to ILC2 will help further delineate the specific neural regulation of ILC2s by the NMU pathway. While NMU boosts ILC2 activity, the effects of CGRP on ILC2s are more heterogeneous. Clusters of rare epithelial pulmonary neuroendocrine cells (PNEC) secrete CGRP promoting ILC2 expansion, IL-5 and IL-13 production and infiltration of Th2 lymphocytes and eosinophils in an OVA-challenge model of lung inflammation (203). Furthermore, asthmatic patients show increased PNEC levels as compared to healthy controls, suggesting a role in human asthma (203), though the mechanism by which allergens stimulate PNEC still requires further investigation. Indeed, by contrast, recent publications have reported that CGRP curbs ILC2-induced inflammation in response to either IL-25 or IL-33 (201, 204, 205). By affecting chromatin regulatory elements, CGRP reduced expression of pro-inflammatory genes such as Il4, Il5, Il9, Il13, Il17a or Il17f, and promote an immunoregulatory gene signature in ILC2s (e.g. Areg and Il10ra expression), even in response to IL-33 and NMU (201, 204, 205). Mice challenged with intranasal IL-33 experienced a reduction ILC2-dependent eosinophilia, type-2 cytokines and AHR when CGRP was co-administered, and CGRP-deficient mice displayed an increase in ILC2 proliferation upon papain challenge (204). In both mice and humans, the β2AR is highly expressed by ILC2s from the lungs, and β2AR agonists impair ILC2 responses, whilst β2AR-deficiency enhances ILC2 activation (196).
ILC2s also express tryptophan hydroxylase (Tph1) one of the enzymes required to produce serotonin (5-TH), and an intrinsic lack of Tph1 expression precludes activation of ILC2s during parasitic infection (206). Though this study did not evaluate the role of ILC2-specific serotonin or Tph1 expression in murine allergy models, 5-TH has been implicated in asthma (207) and Tph1 is positively regulated by GATA3 in ILC2s (208), making it a potential modulator during allergic/asthmatic type-2 responses.
Although ILC2s appear to represent a hub for signals from the nervous system, other immune cells may also complement the neuro-immune modulatory axis during type-2 inflammation. Indeed, lung T cells express the dopamine receptor DR4, which synergises with STAT5 signalling to induce T cell proliferation and Th2 programming upon allergen challenge (209). Interestingly, dopaminergic fibres are more abundant in the lungs of infants/children in both mice and humans, and have the potential to contribute to the polarization of the type-2 lymphocytes that prevail in lungs early after birth (19, 209). In addition, substance P-producing nociceptive neurons are able to detect cysteine protease activity derived from HDM, and activate MRGPRB2+ mast cells and MRGPRA1+ cDC2 to initiate the inflammatory response (210, 211).
Thus, ILC2s sit within a complex neuro-immune network in which their production of, or response to, neurotransmitters can help maintain tissue homeostasis or contribute to the pathophysiology of lung disease. The development and analysis of more refined experimental models will allow a greater understanding of neuro-ILC2 interactions.
6.1. Sex hormone modulation of ILC2s
Male and female sex hormones differentially impact ILC2s, with females having higher numbers of ILC2s as compared to males, which mirrors the sex bias in asthma, where women display greater susceptibility and severity of asthma than men (212). In mice, A. alternata or HDM-induced models of airway inflammation resulted in fewer ILC2s and less inflammation in males as compared to females. Moreover, ILC2s isolated from females produced higher levels of IL-5 and IL-13 (213). Orchiectomy in male mice exacerbated airway inflammation in response to A. alternata, and adoptive bone marrow transfer from the androgen receptor (AR)-deficient mice into wildtype mice demonstrated that androgen influences ILC2 function in a cell-intrinsic manner (213). In female mice, the KLRG1– ILC2 subset predominates, but can be diminished by an increase in androgen levels (214). In addition, AR signalling in ILC2p suppresses their differentiation to ILC2s (214). AR is also expressed by lung epithelium, and testosterone suppresses IL-33 and TSLP secretion further suppressing ILC2 proliferation in a cell-extrinsic manner (213).
7. Environmental factors
Circadian rhythms have been associated with asthma (215), and it is notable that ILC2s (at least in the gut) show marked oscillations in circadian regulator genes (216). This is also in line with ILC2-mediated circadian regulation of eosinophil homeostasis (217). Such changes are probably linked to feeding behaviour, which has been shown to promote IL-5 and IL-13 production by ILC2s (217). Upon activation, ILC2s upregulate the glycolytic pathway, a process that requires arginase 1 (218, 219). Supporting this, fasting or a ketogenic diet, which fail to provide fuel for glycolysis, reduces IL-13 production (217) and result in less severe allergic responses (219). Furthermore, fibre-rich diets also downmodulate airway inflammatory disease in mice, due to intestinal microbiota-derived short chain fatty acids (SCFA) metabolites acting to curtail ILC2 metabolism and inhibit histone deacetylases, thereby suppressing GATA3 expression, ILC2 proliferation and cytokine production (220, 221).
Although ILC2s require glucose during their initial activation (219), they then rely on autophagy (221) and the acquisition of microenvironmental lipids (stored as lipid droplets), for anabolic processes such as proliferation and cytokine production (219, 222). As a consequence, ILC2s are particularly fit to thrive in environments characterised by inflammation-associated tissue damage where Pla2g5-expressing macrophages engulf dead cells and provide ILC2s with free fatty acids (223). Taken together, it is possible that dietary intervention may prove beneficial in modifying type-2 responses generally, and specifically ILC2s, but large carefully-controlled trials are required (224).
Diet also affects the microbiome, which has also been linked to asthma susceptibility, but little is known about the possible role of ILC2s in this context (for an extensive discussion see (225–227). Several lines of evidence indicate that allergy and asthma sensitisation occur early in life (226, 228). Coincidentally, ILC2s accumulate in the lung during this period (postnatal days 10-14 in mice, 2-3 years in humans), and promote a type-2 immune microenvironment (19, 217) responsible for enhanced responses against HDM (21). This suggests that tuning of ILC2s during this period may prevent or promote asthma later in life. Indeed, averting microbial colonization during this time exacerbated IL-4, IL-5 and IL-13 production in subsequent allergic responses (229).
A recent study found that in mice, pre-pregnancy and prenatal maternal exposure to diesel exhaust particles (DEP) enhanced allergic responses in the offspring (230). This process was dependent on IL-25R+ ILC2s and type-2 NK cells, which upon allergen-induced activation secreted granzyme B and IL-13 promoting further IL-25 production from bronchoalveolar epithelia. Furthermore, traffic-related particulate matter, such as DEP, have been found to boost IL-4, IL-5, IL-13 and IL-17A expression in murine allergy models, with an active contribution of ILC2s to the related immunopathology (231, 232).
8. ILC2 plasticity
Although, typical type-2-secreting ILC2s are associated with allergic asthma, changes in the local airway cytokine environment can instruct ILC2s to acquire type 1 (Th1) or type 3 (Th17) phenotypes (Figure 4) (24). Consequently, these re-educated ILC2s may contribute to non-allergic asthma and even COPD. IL-1β is a key player in ILC2 plasticity and is produced by airway epithelial cells (233), resulting in higher levels of IL-1β in the sputum of asthmatic patients as compared to healthy controls (234). IL-1α/β induces IL-5 and IL-13 production from ILC2s and, when IL-1β synergises with IL-33, this potentiates the expression of ST2, IL17RB, and TSLPR on ILC2s, making them more responsive to these cytokines. Paradoxically, IL-1β also induces Tbet and IL-12 receptor expression by ILC2s. This fosters mixed ILC1/ILC2 gene signatures in ILC2s (235) and conversion to an ILC1-like in the presence of IL-12 (236, 237). Indeed, several preclinical studies have also confirmed that triggers of COPD, such as cigarette smoke or respiratory infection with bacteria or viruses, promote an IL-12-dependent switch in ILC2s from GATA3+ST2+ cells towards a GATA3loTBET+IL18Rα+ phenotype (71, 237). The plasticity of ILC2s has recently been linked to asthma (238). The authors report that severe asthma patients have increased frequencies of a subset of CCR10+ ILC2s that express IFNγ and very little type-2 cytokine. This is mirrored by an increase in T-bet expression and a reduction in GATA3 expression. Strikingly, in mice, inhibition of CCR10+ ILC2 recruitment to the lungs exacerbated allergen-induced asthma, suggesting that repolarisation to IFNγ suppresses the type-2 response.
Murine ILC2s have also been reported to be able to switch to an ILC2-ILC3 hybrid phenotype upon IL-23 (239) or IL-25-driven inflammation (27, 28). In these settings ILC2s acquired Rorgt and IL-17A expression, while downregulating IL-5 and GATA3. These cells still co-expressed IL-13, and were associated with the development of psoriasis-like skin pathology (239). Likewise, human ILC2s develop a RORgt+CCR6+ ILC3-like reversible phenotype in skin lesions of patients with psoriasis (240). Additionally, in cystic fibrosis, a proportion of ILC2s appear to reversibly transdifferentiate into ILC3-like cells, as a consequence of IL-1β, TGFβ and IL-23 (241). When ILC2s from nasal polyps of patients with cystic fibrosis were compared to ILC2s infiltrating nasal polyps of allergic patients, both groups were still strong IL-13-producers, but in cystic fibrosis they were associated with neutrophilic infiltration. Thus, type-3 exILC2s may contribute to neutrophilia observed in non-type-2 asthma.
9. Clinical relevance and therapeutic potential
Asthma treatment strategies are based on asthma endotype and biomarkers. Here, we will only discuss therapeutics in the context of ILC2 function. The most common treatment involves inhaled β2 agonists, which may be combined with corticosteroids, to induce airway smooth muscle relaxation. However, many immune cells, including mouse and human lung ILC2s, also express β2AR. Notably, deletion of β2AR from ILC2s increased their proliferation and cytokine expression, whilst β2 agonist treatment attenuated ILC2 functions (196). Thus, the therapeutic effects of β2 agonist might be due to cumulative effects on both smooth muscle and ILC2s.
In most patients, inhaled corticosteroids reduce inflammation in the lung. Although the majority of asthma patients respond to treatment, 3 - 10 % of patients are corticosteroid resistant and represent the majority of asthma-related hospitalizations. Although corticosteroids can attenuate both human and mouse ILC2 viability and type-2 cytokine secretion (55, 101), TSLP-mediated corticosteroid resistance is observed in human and mouse lung ILC2s (101, 242). Notably, the anti-TSLP monoclonal antibody, tezepelumab (AMG-157) has shown compelling benefit in asthmatic patients, decreasing annual exacerbation rates by up to 70% in a glucocorticoid non-responsive patient cohort (243). Of the other ILC2-stimulating cytokines, IL-33 signalling inhibitors are also in phase II clinical trials, with anti-ST2 mAb (GSK3772847) assessed for asthma treatment and anti-IL-33 antibodies (RG6149/AMG282 and ANB020) trialled in atopic dermatitis, where ANB020 has shown promising results. However, in spite of promising preclinical results, there has been very little progress in targeting IL-25 signalling in human asthma (58, 103, 244).
IL-5 neutralizing antibodies (mepolizumab and reslizumab) and IL-5R blocking antibodies (benralizumab that depletes IL-5Rα-expressing cells by antibody-dependent cellular cytotoxicity) (245) effectively deplete eosinophil numbers and are approved for treating severe eosinophilic asthma (246–248). By contrast, anti-IL-13 antibody (lebrikizumab and tralokinumab) clinical trials for asthma have disappointed in phase III trials for asthma, despite early promise (249–251). The failure of anti-IL-13 antibodies may be attributable to the functional redundancy of IL-4 and IL-13 which both signal via IL-4Rα. Indeed, dupilumab, which binds to IL-4Rα and abrogates IL-4 and IL-13 signalling, reduces asthma exacerbations and is approved for clinical use in moderate to severe uncontrolled asthma (252). Dupilumab has also shown effectiveness in allergic rhinitis-induced co-morbid asthma, nasal polyposis and atopic eczema (253–255). Interestingly, unlike IL-5 inhibition, the patient blood eosinophil counts did not play any determinant role in dupilumab efficacy (256). Therefore, targeting multiple facets of ILC2 function might be more effective in controlling asthma than targeting any single cytokine.
Beyond antibody treatment options, prostaglandin and leukotriene signalling are additional targets for inhibiting ILC2 function. Human ILC2s express prostaglandin D2 receptor (CRTH2) and its ligand PGD2 activates ILC2s (168). Although, fevipiprant, a CRTH2 antagonist, exhibited promising results in a phase II trial, it recently failed to meet primary end-points in phase III LUSTER trials. Although other CRTH2 antagonists remain in development e.g. timapiprant, and positive effects continue to be reported in small studies, the LUSTER results have raised doubts as to whether such inhibitors will be effective in severe uncontrolled asthma (257).
Conclusions
Whilst studies in experimental mouse models have highlighted the potential contribution of ILC2s in allergic lung diseases, their roles in human asthma are still being unveiled. In this regard, ILC2s appear amongst a field of players whose complex interplay builds momentum, but ultimately leads to an immunological own goal. By responding to epithelium-derived cytokines (IL-33, IL-25, TSLP) ILC2s respond rapidly to these first signals by helping to kick-off the type-2 immune response. Furthermore, due to their expression of receptors for neuropeptides (NMU) and neurotransmitters they also receive signals from the nervous system allowing ILC2s to sense the wider field. These stimuli allow ILC2s to become the playmakers sending out cytokine passes (IL-4, IL-5, IL-9, IL-13) to bring eosinophils, mast cells, goblet cells and push their adaptive Th2 cell relatives forwards in the type-2 inflammation game. However, ILC2s are not content with a one-dimensional attack and can react flexibly (with plasticity) if they receive fresh team orders to play in a different formation by switching to a new cytokine pattern that can elicit type-1 or type-3 (IL-17) immunity. We are continuing to peel back the pages of the asthma playbook, and in so doing we are identifying new ways to defend from the resulting pathology and develop novel therapeutics.
Footnotes
Disclosure Statement
A.N.J.M. works for the MRC who have licenced anti-IL-25R antibodies. A.N.J.M. has grant funding from UK Medical Research Council (U105178805) and Wellcome Trust (100963/Z/13/Z). N.R.R has grant funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 896454.
Contributor Information
Noe Rodriguez-Rodriguez, Email: noer@mrc-lmb.cam.ac.uk.
Mayuri Gogoi, Email: mgogoi@mrc-lmb.cam.ac.uk.
Acknowledgements
We are grateful to the Sophie Kitching for her help with the article.
Literature Cited
- 1.Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol. 2014;795:17–29. doi: 10.1007/978-1-4614-8603-9_2. [DOI] [PubMed] [Google Scholar]
- 2.Koczulla AR, Vogelmeier CF, Garn H, Renz H. New concepts in asthma: clinical phenotypes and pathophysiological mechanisms. Drug Discov Today. 2017;22:388–96. doi: 10.1016/j.drudis.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 4.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–8.:e1-4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie ANJ, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Ye CJ, Marson A, Erle DJ, Locksley RM, et al. A tissue checkpoint regulates type 2 immunity. Nature Immunology. 2016;17:1381–7. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunology. 2016;9:275–86. doi: 10.1038/mi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature Immunology. 2011;12:1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake LY, Iijima K, Bartemes K, Kita H. Group 2 Innate Lymphoid Cells Promote an Early Antibody Response to a Respiratory Antigen in Mice. The Journal of Immunology. 2016;197:1335–42. doi: 10.4049/jimmunol.1502669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie ANJ. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. Journal of Allergy and Clinical Immunology. 2012;129 doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, McKenzie ANJ, Mebius RE, Powrie F, Spits H, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054–66. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 15.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker JA, Clark PA, Crisp A, Barlow JL, Szeto A, Ferreira ACF, Rana BMJ, Rodriguez-Jolin HE, Rodriguez N, Sivasubramaniam M, Pannell R, et al. Polychromic Reporter Mice Reveal Unappreciated Innate Lymphoid Cell Progenitor Heterogeneity and Elusive ILC3 Progenitors in Bone Marrow. Immunity. 2019;51:104–18.:e7. doi: 10.1016/j.immuni.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherrier DE, Serafini N, Di Santo JP. Innate Lymphoid Cell Development: A T Cell Perspective. Immunity. 2018;48:1091–103. doi: 10.1016/j.immuni.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Cherrier DE, Chea S, Vosshenrich C, Serafini N, Petit M, Liu P, Golub R, Di Santo JP. An Id2(RFP)-Reporter Mouse Redefines Innate Lymphoid Cell Precursor Potentials. Immunity. 2019;50:1054–68.:e3. doi: 10.1016/j.immuni.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saluzzo S, Gorki AD, Rana BMJ, Martins R, Scanlon S, Starkl P, Lakovits K, Hladik A, Sharif O, Warszawska JM, Jolin H, et al. First-Breath-Induced Type 2 Pathways Shape the Lung Immune Environment. Cell Rep. 2017;18:1893–905. doi: 10.1016/j.celrep.2017.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga S, Hozumi K, Hirano KI, Yazawa M, Terooatea T, Minoda A, Nagasawa T, Koyasu S, Moro K. Peripheral PDGFRalpha(+)gp38(+) mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med. 2018;215:1609–26. doi: 10.1084/jem.20172310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kleer IM, Kool M, de Bruijn MJ, Willart M, van Moorleghem J, Schuijs MJ, Beyaert R, Hams E, Fallon PG, Hammad H, Hendriks RW, et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity. 2016;45:1285–98. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Schneider C, Lee J, Koga S, Ricardo-Gonzalez RR, Nussbaum JC, Smith LK, Villeda SA, Liang HE, Locksley RM. Tissue-Resident Group 2 Innate Lymphoid Cells Differentiate by Layered Ontogeny and In Situ Perinatal Priming. Immunity. 2019;50:1425–38.:e5. doi: 10.1016/j.immuni.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricardo-Gonzalez RR, Schneider C, Liao C, Lee J, Liang HE, Locksley RM. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. The Journal of experimental medicine. 2020;217 doi: 10.1084/jem.20191172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, Locksley RM. Tissue signals imprint ILC2 identity with anticipatory function. Nature Immunology. 2018;19:1093–9. doi: 10.1038/s41590-018-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Björklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, Sandberg R, Mjösberg J. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nature Immunology. 2016;17:451–60. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 26.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M, Immunological Genome C. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–17. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Jr, Paul WE. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–9. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, Usher N, Zhu J, Urban JF, Paul WE, Germain RN. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359:114–9. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R-EEE, Nyman J, Dionne D, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–6. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E, Shapiro H, et al. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166:1231–46.:e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, Wang G, Tian Y, Ying J, Fan Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020 doi: 10.1038/s41422-020-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MM, Patel PS, Bao K, Danhorn T, O’Connor BP, Reinhardt RL. BATF acts as an essential regulator of IL-25-responsive migratory ILC2 cell fate and function. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.aay3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Fergusson JR, Tang CL, Kam MH, Dennis K, et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2017;46:148–61. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh-Takayama N, Kato T, Motomura Y, Kageyama T, Taguchi-Atarashi N, Kinoshita-Daitoku R, Kuroda E, Di Santo JP, Mimuro H, Moro K, Ohno H. Bacteria-Induced Group 2 Innate Lymphoid Cells in the Stomach Provide Immune Protection through Induction of IgA. Immunity. 2020;52:635–49.:e4. doi: 10.1016/j.immuni.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–5. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015;112:10762–7. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson W, Consortium G. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, Belgrave D, den Dekker HT, Husby A, Sevelsted A, Faura-Tellez G, Mortensen LJ, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–5. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 39.He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, Zamar D, Bosse Y, Kozyrskyj AL, James A, Laprise C, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124:222–9. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, Miyagawa T, Doi S, Kameda M, Fujita K, Miyatake A, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44:787–93. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–46. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–41. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Mjösberg JM, Trifari S, Crellin NK, Peters CP, Van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25-and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunology. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 44.Yu QN, Guo YB, Li X, Li CL, Tan WP, Fan XL, Qin ZL, Chen D, Wen WP, Zheng SG, Fu QL. ILC2 frequency and activity are inhibited by glucocorticoid treatment via STAT pathway in patients with asthma. Allergy. 2018;73:1860–70. doi: 10.1111/all.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Sirohi K, Verma M, McKay J, Michalec L, Sripada A, Danhorn T, Rollins D, Gorska MM, Martin RJ, Alam R. Optimal identification of human conventional and nonconventional (CRTH2(-)IL7Ralpha(-)) ILC2s using additional surface markers. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. Journal of Allergy and Clinical Immunology. 2014;134:1193–5.:e4. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Winkler C, Hochdörfer T, Israelsson E, Hasselberg A, Cavallin A, Thörn K, Muthas D, Shojaee S, Lüer K, Müller M, Mjösberg J, et al. Activation of group 2 innate lymphoid cells after allergen challenge in asthmatic patients. Journal of Allergy and Clinical Immunology. 2019;144:61–9.:e7. doi: 10.1016/j.jaci.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 48.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–8.:e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, Wu J, Zhao J, Wang J, Zhang Y, Liu L, Cao L, Liu Y, Dong L. Type 2 innate lymphoid cells: A novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med. 2015;109:1391–6. doi: 10.1016/j.rmed.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, Gauvreau GM, Lemiere C, Martin J, Nair P, Sehmi R, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.:e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 51.Yu QN, Tan WP, Fan XL, Guo YB, Qin ZL, Li CL, Chen D, Lin ZB, Wen W, Fu QL. Increased Group 2 Innate Lymphoid Cells Are Correlated with Eosinophilic Granulocytes in Patients with Allergic Airway Inflammation. Int Arch Allergy Immunol. 2018;176:124–32. doi: 10.1159/000488050. [DOI] [PubMed] [Google Scholar]
- 52.Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, Wang X, Hu M, Tang R, Chen Z. IL-13(+) Type 2 Innate Lymphoid Cells Correlate with Asthma Control Status and Treatment Response. Am J Respir Cell Mol Biol. 2016;55:675–83. doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 53.Chen R, Smith SG, Salter B, El-Gammal A, Oliveria JP, Obminski C, Watson R, O’Byrne PM, Gauvreau GM, Sehmi R. Allergen-induced Increases in Sputum Levels of Group 2 Innate Lymphoid Cells in Subjects with Asthma. Am J Respir Crit Care Med. 2017;196:700–12. doi: 10.1164/rccm.201612-2427OC. [DOI] [PubMed] [Google Scholar]
- 54.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, Gorentla B, Gorska MM, Chu H, Martin RJ, Alam R, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136:59–68.:e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Verma M, Michalec L, Liu W, Sripada A, Rollins D, Good J, Ito Y, Chu H, Martin RJ, Alam R, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141:257–68.:e6. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagakumar P, Puttur F, Gregory LG, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Pulmonary type-2 innate lymphoid cells in paediatric severe asthma: phenotype and response to steroids. Eur Respir J. 2019;54 doi: 10.1183/13993003.01809-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624–6.:e6. doi: 10.1016/j.jaci.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 58.Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, Zhu J, Man Ching Y, Shamji B, Edwards M, Westwick J, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li BWS, de Bruijn MJW, Lukkes M, van Nimwegen M, Bergen IM, KleinJan A, GeurtsvanKessel CH, Andeweg A, Rimmelzwaan GF, Hendriks RW. T cells and ILC2s are major effector cells in influenza-induced exacerbation of allergic airway inflammation in mice. European Journal of Immunology. 2019;49:144–56. doi: 10.1002/eji.201747421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajput C, Han M, Ishikawa T, Lei J, Jazaeri S, Bentley JK, Hershenson MB. Early life heterologous rhinovirus infections induce an exaggerated asthma-like phenotype. Journal of Allergy and Clinical Immunology. 2020 doi: 10.1016/j.jaci.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–34. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–92.:e6. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Namvar S, Warn P, Farnell E, Bromley M, Fraczek M, Bowyer P, Herrick S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin Exp Allergy. 2015;45:982–93. doi: 10.1111/cea.12426. [DOI] [PubMed] [Google Scholar]
- 64.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 66.Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–50. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 68.Hardman CS, Panova V, McKenzie ANJ. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. European Journal of Immunology. 2013;43:488–98. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahlgren MW, Jones SW, Cautivo KM, Dubinin A, Ortiz-Carpena JF, Farhat S, Yu KS, Lee K, Wang C, Molofsky AVAB, Tward AD, et al. Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches. Immunity. 2019;50:707–22.:e6. doi: 10.1016/j.immuni.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–95. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 71.Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, Mori M, Pham TH, Ward CK, Criner GJ, Marchetti N, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity. 2015;42:566–79. doi: 10.1016/j.immuni.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Shinoda K, Hirahara K, Iinuma T, Ichikawa T, Suzuki AS, Sugaya K, Tumes DJ, Hara T, Tani-Ichi S, Ikuta K, Okamoto Y, et al. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc Natl Acad Sci U S A. 2016;113:E2842–51. doi: 10.1073/pnas.1512600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, Cote-Sierra J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun. 2014;55:33–41. doi: 10.1016/j.jaut.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–8. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, Kephart GM, Kurabayashi M, Kita H. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. 2014;192:4032–42. doi: 10.4049/jimmunol.1400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502–7. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Qin J, Li X, Gorman DM, Bazan JF, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 79.Cohen ES, Scott IC, Majithiya JB, Rapley L, Kemp BP, England E, Rees DG, Overed-Sayer CL, Woods J, Bond NJ, Veyssier CS, et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Commun. 2015;6:8327. doi: 10.1038/ncomms9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–81. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, Kita H. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193:1549–59. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009;386:181–5. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013;50:803–9. doi: 10.3109/02770903.2013.816317. [DOI] [PubMed] [Google Scholar]
- 85.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saatian B, Rezaee F, Desando S, Emo J, Chapman T, Knowlden S, Georas SN. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1:e24333. doi: 10.4161/tisb.24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029-38. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- 88.Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, Kubo T, Wawrzyniak P, Ruckert B, Sudo K, Nakae S, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018;141:300–10.:e11. doi: 10.1016/j.jaci.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 89.Fonseca W, Malinczak CA, Schuler CF, Best SKK, Rasky AJ, Morris SB, Cui TX, Popova AP, Lukacs NW. Uric acid pathway activation during respiratory virus infection promotes Th2 immune response via innate cytokine production and ILC2 accumulation. Mucosal Immunol. 2020 doi: 10.1038/s41385-020-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L375–82. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- 92.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–9. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calven J, Yudina Y, Hallgren O, Westergren-Thorsson G, Davies DE, Brandelius A, Uller L. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases. J Innate Immun. 2012;4:86–99. doi: 10.1159/000329131. [DOI] [PubMed] [Google Scholar]
- 94.Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, Pauwels K, Tavernier J, Lambrecht BN, Hammad H, De Winter H, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun. 2017;8:14937. doi: 10.1038/ncomms14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, Corrigan CJ, Ying S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J Immunol. 2018;200:2253–62. doi: 10.4049/jimmunol.1701455. [DOI] [PubMed] [Google Scholar]
- 96.Gluck J, Rymarczyk B, Kasprzak M, Rogala B. Increased Levels of Interleukin-33 and Thymic Stromal Lymphopoietin in Exhaled Breath Condensate in Chronic Bronchial Asthma. Int Arch Allergy Immunol. 2016;169:51–6. doi: 10.1159/000444017. [DOI] [PubMed] [Google Scholar]
- 97.Berraies A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Increased expression of thymic stromal lymphopoietin in induced sputum from asthmatic children. Immunol Lett. 2016;178:85–91. doi: 10.1016/j.imlet.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 99.Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, Kita H, Boyd KL, Peebles RS., Jr TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020 doi: 10.1111/all.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML, Hartert TV, Zhou B, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol. 2016;138:814–24.:e11. doi: 10.1016/j.jaci.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, Betsuyaku T, Koyasu S, Asano K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 102.Tang W, Smith SG, Beaudin S, Dua B, Howie K, Gauvreau G, O’Byrne PM. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int Arch Allergy Immunol. 2014;163:5–10. doi: 10.1159/000355331. [DOI] [PubMed] [Google Scholar]
- 103.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 104.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–5. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–30. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–33. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bankova LG, Dwyer DF, Yoshimoto E, Ualiyeva S, McGinty JW, Raff H, von Moltke J, Kanaoka Y, Frank Austen K, Barrett NA. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25-producing airway brush cells leading to type 2 inflammation. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, Blasetti M, Doghramji L, Kennedy DW, Adappa ND, Palmer JN, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;142:460–9.:e7. doi: 10.1016/j.jaci.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel NN, Kohanski MA, Maina IW, Triantafillou V, Workman AD, Tong CCL, Kuan EC, Bosso JV, Adappa ND, Palmer JN, Herbert DR, et al. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2018 doi: 10.1002/alr.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Denney L, Byrne AJ, Shea TJ, Buckley JS, Pease JE, Herledan GM, Walker SA, Gregory LG, Lloyd CM. Pulmonary Epithelial Cell-Derived Cytokine TGF-beta1 Is a Critical Cofactor for Enhanced Innate Lymphoid Cell Function. Immunity. 2015;43:945–58. doi: 10.1016/j.immuni.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC, Jr, Lonning S, Gauldie J, Jordana M. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med. 2008;177:593–603. doi: 10.1164/rccm.200706-958OC. [DOI] [PubMed] [Google Scholar]
- 112.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–80. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 113.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, Voehringer D, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111:367–72. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal Chitin from Asthma-Associated Home Environments Induces Eosinophilic Lung Infiltration. The Journal of Immunology. 2011;187:2261–7. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Meel ER, den Dekker HT, Elbert NJ, Jansen PW, Moll HA, Reiss IK, de Jongste JC, Jaddoe VWV, Duijts L. A population-based prospective cohort study examining the influence of early-life respiratory tract infections on school-age lung function and asthma. Thorax. 2018;73:167–73. doi: 10.1136/thoraxjnl-2017-210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, Sajjan US, Hershenson MB. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134:429–39. doi: 10.1016/j.jaci.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han M, Rajput C, Hong JY, Lei J, Hinde JL, Wu Q, Bentley JK, Hershenson MB. The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J Immunol. 2017;199:1308–18. doi: 10.4049/jimmunol.1700216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajput C, Cui T, Han M, Lei J, Hinde JL, Wu Q, Bentley JK, Hershenson MB. RORalpha-dependent type 2 innate lymphoid cells are required and sufficient for mucous metaplasia in immature mice. Am J Physiol Lung Cell Mol Physiol. 2017;312:L983–L93. doi: 10.1152/ajplung.00368.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manson ML, Safholm J, James A, Johnsson AK, Bergman P, Al-Ameri M, Karrman-Orre AC, Mardh C, Dahlen SE, Adner M. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J Allergy Clin Immunol. 2020;145:808–17.:e2. doi: 10.1016/j.jaci.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 120.Shen X, Pasha MA, Hidde K, Khan A, Liang M, Guan W, Ding Y, Haczku A, Yang Q. Group 2 innate lymphoid cells promote airway hyperresponsiveness through production of VEGFA. J Allergy Clin Immunol. 2018;141:1929–31.:e4. doi: 10.1016/j.jaci.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KH, Campbell GA, McKenzie AN, Lloyd CM. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013;68:82–90. doi: 10.1136/thoraxjnl-2012-202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, Kurowska-Graham GJ, Stolarska M, Liew FY, McSharry C, Xu D. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134:1422–32.:e11. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–60. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 126.Agrawal S, Townley RG. Role of periostin, FENO, IL-13, lebrikzumab, other IL-13 antagonist and dual IL-4/IL-13 antagonist in asthma. Expert Opin Biol Ther. 2014;14:165–81. doi: 10.1517/14712598.2014.859673. [DOI] [PubMed] [Google Scholar]
- 127.Gosset P, Tillie-Leblond I, Janin A, Marquette CH, Copin MC, Wallaert B, Tonnel AB. Expression of E-selectin, ICAM-1 and VCAM-1 on bronchial biopsies from allergic and non-allergic asthmatic patients. Int Arch Allergy Immunol. 1995;106:69–77. doi: 10.1159/000236892. [DOI] [PubMed] [Google Scholar]
- 128.Karta MR, Rosenthal PS, Beppu A, Vuong CY, Miller M, Das S, Kurten RC, Doherty TA, Broide DH. beta2 integrins rather than beta1 integrins mediate Alternaria-induced group 2 innate lymphoid cell trafficking to the lung. J Allergy Clin Immunol. 2018;141:329–38.:e12. doi: 10.1016/j.jaci.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perkins TN, Oczypok EA, Milutinovic PS, Dutz RE, Oury TD. RAGE-dependent VCAM-1 expression in the lung endothelium mediates IL-33-induced allergic airway inflammation. Allergy. 2019;74:89–99. doi: 10.1111/all.13500. [DOI] [PubMed] [Google Scholar]
- 130.Puttur F, Denney L, Gregory LG, Vuononvirta J, Oliver R, Entwistle LJ, Walker SA, Headley MB, McGhee EJ, Pease JE, Krummel MF, et al. Pulmonary environmental cues drive group 2 innate lymphoid cell dynamics in mice and humans. Science Immunology. 2019;4 doi: 10.1126/sciimmunol.aav7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knipfer L, Schulz-Kuhnt A, Kindermann M, Greif V, Symowski C, Voehringer D, Neurath MF, Atreya I, Wirtz S. A CCL1/CCR8-dependent feed-forward mechanism drives ILC2 functions in type 2-mediated inflammation. Journal of Experimental Medicine. 2019;216:2763–77. doi: 10.1084/jem.20182111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Campbell L, Hepworth MR, Whittingham-Dowd J, Thompson S, Bancroft AJ, Hayes KS, Shaw TN, Dickey BF, Flamar AL, Artis D, Schwartz DA, et al. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. Journal of Experimental Medicine. 2019;216:2714–23. doi: 10.1084/jem.20180610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leyva-Castillo JM, Galand C, Kam C, Burton O, Gurish M, Musser MA, Goldsmith JD, Hait E, Nurko S, Brombacher F, Dong C, et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity. 2019;50:1262–75.:e4. doi: 10.1016/j.immuni.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dahlgren MW, Jones SW, Cautivo KM, Dubinin A, Ortiz-Carpena JF, Farhat S, Yu KS, Lee K, Wang C, Molofsky AV, Tward AD, et al. Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches. Immunity. 2019;50:707–22.:e6. doi: 10.1016/j.immuni.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rana BMJ, Jou E, Barlow JL, Rodriguez-Rodriguez N, Walker JA, Knox C, Jolin HE, Hardman CS, Sivasubramaniam M, Szeto A, Cohen ES, et al. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. The Journal of experimental medicine. 2019;216:1999–2009. doi: 10.1084/jem.20190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, Peng YQ, Xu ZB, Fan XL, Wu ZJ, Chen D, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles. 2020;9:1723260. doi: 10.1080/20013078.2020.1723260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Halim TYF, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, McKenzie ANJ. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nature Immunology. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–95. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, Haim-Serrao EM, Vilmovsky L, Teichmann SA, Rodewald HR, Botto M, et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity. 2018;48:1195–207.:e6. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gour N, Smole U, Yong HM, Lewkowich IP, Yao N, Singh A, Gabrielson E, Wills-Karp M, Lajoie S. C3a is required for ILC2 function in allergic airway inflammation. Mucosal Immunology. 2018;11:1653–62. doi: 10.1038/s41385-018-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rao A, Strauss O, Kokkinou E, Bruchard M, Tripathi KP, Schlums H, Carrasco A, Mazzurana L, Konya V, Villablanca EJ, Björkström NK, et al. Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs. Nature Communications. 2020;11:2049. doi: 10.1038/s41467-020-15695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwartz C, Khan AR, Floudas A, Saunders SP, Hams E, Rodewald HR, McKenzie ANJ, Fallon PG. ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. Journal of Experimental Medicine. 2017;214:2507–21. doi: 10.1084/jem.20170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiao X, Fan Y, Li J, Zhang X, Lou X, Dou Y, Shi X, Lan P, Xiao Y, Minze L, Li XC. Guidance of super-enhancers in regulation of IL-9 induction and airway inflammation. Journal of Experimental Medicine. 2018;215:559–74. doi: 10.1084/jem.20170928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, Akbari O. ICOS: ICOS-Ligand Interaction Is Required for Type 2 Innate Lymphoid Cell Function, Homeostasis, and Induction of Airway Hyperreactivity. Immunity. 2015;42:538–51. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. Journal of Allergy and Clinical Immunology. 2016;138:801–11.:e9. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, Galle-Treger L, Maazi H, Lo R, Freeman GJ, Sharpe AH, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator–inducible T-cell costimulator ligand interaction. Journal of Allergy and Clinical Immunology. 2017;139:1468–77.:e2. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. InterleuKin-33 And Interferon-Γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation During Immune Perturbation. Immunity. 2015;43:161–74. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Herrmann M, Kaplan MH, Weigmann B, et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nature Medicine. 2017;23:938–44. doi: 10.1038/nm.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bando JK, Gilfillan S, Di Luccia B, Fachi JL, Sécca C, Cella M, Colonna M. ILC2s are the predominant source of intestinal ILC-derived IL-10. The Journal of experimental medicine. 2020;217 doi: 10.1084/jem.20191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morita H, Kubo T, Rückert B, Ravindran A, Soyka MB, Rinaldi AO, Sugita K, Wawrzyniak M, Wawrzyniak P, Motomura K, Tamari M, et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. Journal of Allergy and Clinical Immunology. 2019;143:2190–201.:e9. doi: 10.1016/j.jaci.2018.12.1018. [DOI] [PubMed] [Google Scholar]
- 151.Seehus CR, Kadavallore A, Torre BDL, Yeckes AR, Wang Y, Tang J, Kaye J. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nature Communications. 2017;8:1–13. doi: 10.1038/s41467-017-02023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Paul WE. Innate immunological function of T H2 cells in vivo. Nature Immunology. 2015;16:1051–9. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Minutti CM, Drube S, Blair N, Schwartz C, McCrae JC, McKenzie AN, Kamradt T, Coffer PJ, Sibilia M, Sijts AJ, Fallon PG, et al. Epidermal Growth Factor Receptor Expression Licenses Type-2 Helper T Cells to Function in a T Cell ReceptorIndependent Fashion. Immunity. 2017;47:710–22.:e6. doi: 10.1016/j.immuni.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lau CM, Adams NM, Geary CD, Weizman OE, Rapp M, Pritykin Y, Leslie CS, Sun JC. Epigenetic control of innate and adaptive immune memory. Nature Immunology. 2018;19:963–72. doi: 10.1038/s41590-018-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Netea MG, Joosten LAB. Trained Immunity and Local Innate Immune Memory in the Lung. Cell Press; 2018. pp. 1463–5. [DOI] [PubMed] [Google Scholar]
- 156.Martinez-Gonzalez I, Mathä L, Steer CA, Ghaedi M, Poon GFT, Takei F. Allergen-Experienced Group 2 Innate Lymphoid Cells Acquire Memory-like Properties and Enhance Allergic Lung Inflammation. Immunity. 2016;45:198–208. doi: 10.1016/j.immuni.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 157.Martinez-Gonzalez I, Mathä L, Steer CA, Takei F. Immunological Memory of Group 2 Innate Lymphoid Cells. Elsevier Ltd; 2017. pp. 423–31. [DOI] [PubMed] [Google Scholar]
- 158.Lee JJ, Dimina D, Macias MMP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 159.Dhariwal J, Cameron A, Trujillo-Torralbo MB, Del Rosario A, Bakhsoliani E, Jackson DJ, Edwards MR, Rana BMJ, Cousins DJ, Hansel TT, Johnston SL, et al. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergens. American Journal of Respiratory and Critical Care Medicine. 2017;195:1586–96. doi: 10.1164/rccm.201609-1846OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. Journal of Clinical Investigation. 2006;116:1633–41. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leyva-Castillo JM, Galand C, Mashiko S, Bissonnette R, McGurk A, Ziegler SF, Dong C, McKenzie ANJ, Sarfati M, Geha RS. ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation. Journal of Allergy and Clinical Immunology. 2020 doi: 10.1016/j.jaci.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burton OT, Medina Tamayo J, Stranks AJ, Miller S, Koleoglou KJ, Weinberg EO, Oettgen HC. IgE promotes type 2 innate lymphoid cells in murine food allergy. Clinical & Experimental Allergy. 2018;48:288–96. doi: 10.1111/cea.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866–98. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 164.Liu D, Ji L, Wang Y, Zheng L. Cyclooxygenase-2 expression, prostacyclin production and endothelial protection of high-density lipoprotein. Cardiovasc Hematol Disord Drug Targets. 2012;12:98–105. doi: 10.2174/1871529x11202020098. [DOI] [PubMed] [Google Scholar]
- 165.Schmidt LM, Belvisi MG, Bode KA, Bauer J, Schmidt C, Suchy MT, Tsikas D, Scheuerer J, Lasitschka F, Grone HJ, Dalpke AH. Bronchial epithelial cell-derived prostaglandin E2 dampens the reactivity of dendritic cells. J Immunol. 2011;186:2095–105. doi: 10.4049/jimmunol.1002414. [DOI] [PubMed] [Google Scholar]
- 166.Hallstrand TS, Lai Y, Henderson WR, Jr, Altemeier WA, Gelb MH. Epithelial regulation of eicosanoid production in asthma. Pulm Pharmacol Ther. 2012;25:432–7. doi: 10.1016/j.pupt.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maric J, Ravindran A, Mazzurana L, Van Acker A, Rao A, Kokkinou E, Ekoff M, Fauland A, Nilsson G, Wheelock CE, et al. Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation. J Allergy Clin Immunol. 2019;143:2202–14.:e5. doi: 10.1016/j.jaci.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 169.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, Klenerman P, Ogg G. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901.:e3. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wojno ED, Monticelli LA, Tran SV, Alenghat T, Osborne LC, Thome JJ, Willis C, Budelsky A, Farber DL, Artis D. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8:1313–23. doi: 10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maric J, Ravindran A, Mazzurana L, Bjorklund AK, Van Acker A, Rao A, Friberg D, Dahlen SE, Heinemann A, Konya V, Mjosberg J. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J Allergy Clin Immunol. 2018;141:1761–73.:e6. doi: 10.1016/j.jaci.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou W, Toki S, Zhang J, Goleniewksa K, Newcomb DC, Cephus JY, Dulek DE, Bloodworth MH, Stier MT, Polosuhkin V, Gangula RD, et al. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. Am J Respir Crit Care Med. 2016;193:31–42. doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, Oboki K, Ohno T, Matsuda A, Yamaguchi S, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation By Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–86. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Imai Y, Yasuda K, Nagai M, Kusakabe M, Kubo M, Nakanishi K, Yamanishi K. IL-33– Induced Atopic Dermatitis–Like Inflammation in Mice Is Mediated by Group 2 Innate Lymphoid Cells in Concert with Basophils. Journal of Investigative Dermatology. 2019;139:2185–94.:e3. doi: 10.1016/j.jid.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 177.Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M, Artis D. Basophils Promote Innate Lymphoid Cell Responses in Inflamed Skin. The Journal of Immunology. 2014;193:3717–25. doi: 10.4049/jimmunol.1401307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S, Kubo M. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–71. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 179.Suzuki Y, Wakahara K, Nishio T, Ito S, Hasegawa Y. Airway basophils are increased and activated in eosinophilic asthma. Allergy: European Journal of Allergy and Clinical Immunology. 2017;72:1532–9. doi: 10.1111/all.13197. [DOI] [PubMed] [Google Scholar]
- 180.Boita M, Heffler E, Omedè P, Bellocchia M, Bussolino C, Solidoro P, Giorgis V, Guerrera F, Riva G, Brussino L, Bucca C, et al. Basophil Membrane Expression of Epithelial Cytokine Receptors in Patients with Severe Asthma. International Archives of Allergy and Immunology. 2018;175:171–6. doi: 10.1159/000486314. [DOI] [PubMed] [Google Scholar]
- 181.Salter BM, Oliveria JP, Nusca G, Smith SG, Tworek D, Mitchell PD, Watson RM, Sehmi R, Gauvreau GM. IL-25 and IL-33 induce Type 2 inflammation in basophils from subjects with allergic asthma. Respiratory Research. 2016;17 doi: 10.1186/s12931-016-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salter BM, Oliveria JP, Nusca G, Smith SG, Watson RM, Comeau M, Sehmi R, Gauvreau GM. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. Journal of Allergy and Clinical Immunology. 2015;136:1636–44. doi: 10.1016/j.jaci.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 183.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. Massachussetts Medical Society; 2017. pp. 965–76. [DOI] [PubMed] [Google Scholar]
- 184.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. Massachussetts Medical Society; 2015. pp. 1241–9. [DOI] [PubMed] [Google Scholar]
- 185.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Wynn TA, Gause WC. An essential role for T H 2-type responses in limiting acute tissue damage during experimental helminth infection. Nature Medicine. 2012;18:260–6. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sutherland TE, Logan N, Rückerl D, Humbles AA, Allan SM, Papayannopoulos V, Stockinger B, Maizels RM, Allen JE. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nature Immunology. 2014;15:1116–25. doi: 10.1038/ni.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.He CH, Lee CG, DelaCruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, Nour AM, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Reports. 2013;4:830–41. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Panova V, Gogoi M, Rodriguez-Rodriguez N, Sivasubramaniam M, Jolin HE, Walker JA, Rana BMJ, Drynan LF, Hodskinson M, Pannell R, King G, et al. Group-2 innate lymphoid cell-dependent regulation of tissue neutrophil migration by alternatively activated macrophage-secreted Ear11. Mucosal Immunology. 2020:1–12. doi: 10.1038/s41385-020-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patel DF, Peiró T, Bruno N, Vuononvirta J, Akthar S, Puttur F, Pyle CJ, Suveizdyte K, Walker SA, Singanayagam A, Carlin LM, et al. Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte-dendritic cell antigen presentation. Science Immunology. 2019;4 doi: 10.1126/sciimmunol.aax7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, Sciume G, Richard AC, Hayes ET, Gomez-Rodriguez J, Chen X, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7:958–68. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, Yan D, Xu M, Lee WP, Grogan JL. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–40. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Machida K, Aw M, Salter BM, Ju X, Mukherjee M, Gauvreau GM, O’Byrne PM, Nair P, Sehmi R. Role of TL1A/DR3 Axis in the Activation of ILC2s in Eosinophilic Asthmatics. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.201909-1722OC. [DOI] [PubMed] [Google Scholar]
- 193.Clarke AW, Poulton L, Shim D, Mabon D, Butt D, Pollard M, Pande V, Husten J, Lyons J, Tian C, Doyle AG. An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease. MAbs. 2018;10:664–77. doi: 10.1080/19420862.2018.1440164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Godinho-Silva C, Cardoso F, Veiga-Fernandes H. Neuro–Immune Cell Units: A New Paradigm in Physiology. Annual Review of Immunology. 2019;37:19–46. doi: 10.1146/annurev-immunol-042718-041812. [DOI] [PubMed] [Google Scholar]
- 195.Lee L-Y, Yu J. Sensory Nerves in Lung and Airways. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2014. pp. 287–324. [DOI] [PubMed] [Google Scholar]
- 196.Moriyama S, Brestoff JR, Flamar AL, Moeller JB, Klose CSN, Rankin LC, Yudanin NA, Monticelli LA, Putzel GG, Rodewald HR, Artis D. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–61. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 197.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Veiga-Fernandes H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–81. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111:11515–20. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, Laedermann C, Tran JV, Lai N, Chiu IM, Ghasemlou N, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87:341–54. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Drake MG, Scott GD, Blum ED, Lebold KM, Nie Z, Lee JJ, Fryer AD, Costello RW, Jacoby DB. Eosinophils increase airway sensory nerve density in mice and in human asthma. Science Translational Medicine. 2018;10 doi: 10.1126/scitranslmed.aar8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nagashima H, Mahlakõiv T, Shih HY, Davis FP, Meylan F, Huang Y, Harrison OJ, Yao C, Mikami Y, Urban JF, Caron KM, et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity. 2019;51:682–95.:e6. doi: 10.1016/j.immuni.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549:282–6. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360 doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wallrapp A, Burkett PR, Riesenfeld SJ, Kim SJ, Christian E, Abdulnour REE, Thakore PI, Schnell A, Lambden C, Herbst RH, Khan P, et al. Calcitonin Gene-Related Peptide Negatively Regulates Alarmin-Driven Type 2 Innate Lymphoid Cell Responses. Immunity. 2019;51:709–23.:e6. doi: 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu H, Ding J, Porter CBM, Wallrapp A, Tabaka M, Ma S, Fu S, Guo X, Riesenfeld SJ, Dionne D, Nguyen LT, et al. Transcriptional Atlas of Intestinal Immune Cells Reveals that Neuropeptide alpha-CGRP Modulates Group 2 Innate Lymphoid Cell Responses. Immunity. 2019;51:696–708.:e9. doi: 10.1016/j.immuni.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Flamar AL, Klose CSN, Moeller JB, Mahlakõiv T, Bessman NJ, Zhang W, Stokic-Moriyama S, Trtica V, Rankin LC, Putzel GG, Rodewald HR, et al. Interleukin-33 Induces the Enzyme Tryptophan Hydroxylase 1 to Promote Inflammatory Group 2 Innate Lymphoid Cell-Mediated Immunity. Immunity. 2020;52:606–19.:e6. doi: 10.1016/j.immuni.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Flanagan TW, Sebastian MN, Battaglia DM, Foster TP, Cormier SA, Nichols CD. 5-HT2 receptor activation alleviates airway inflammation and structural remodeling in a chronic mouse asthma model. Life Sciences. 2019;236 doi: 10.1016/j.lfs.2019.116790. [DOI] [PubMed] [Google Scholar]
- 208.Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, Hu G, Barron L, Nakayama T, Belkaid Y, Zhao K, et al. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity. 2014;40:378–88. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang W, Cohen JA, Wallrapp A, Trieu KG, Barrios J, Shao F, Krishnamoorthy N, Kuchroo VK, Jones MR, Fine A, Bai Y, et al. Age-Related Dopaminergic Innervation Augments T Helper 2-Type Allergic Inflammation in the Postnatal Lung. Immunity. 2019;51:1102–18.:e7. doi: 10.1016/j.immuni.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, Reber LL, Marichal T, Cenac N, Dong X, Tsai M, et al. House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation. Nature Immunology. 2019;20:1435–43. doi: 10.1038/s41590-019-0493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aderhold PA, Dewan ZNA, Perner C, Flayer CH, Zhu X, Voisin T, Camire RB, Chiu IM, Chow OA, Sokol CL. Allergen-induced dendritic cell migration is controlled through Substance P release by sensory neurons. bioRxiv. 2020:2020.06.05.136929. doi: 10.1016/j.immuni.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Laffont S, Blanquart E, Guery JC. Sex Differences in Asthma: A Key Role of Androgen-Signaling in Group 2 Innate Lymphoid Cells. Front Immunol. 2017;8:1069. doi: 10.3389/fimmu.2017.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, Hamilton RG, et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep. 2017;21:2487–99. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, Kovats S. A Major Population of Functional KLRG1(-) ILC2s in Female Lungs Contributes to a Sex Bias in ILC2 Numbers. Immunohorizons. 2018;2:74–86. doi: 10.4049/immunohorizons.1800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Krakowiak K, Durrington HJ. The Role of the Body Clock in Asthma and COPD: Implication for Treatment. Pulmonary Therapy. 2018;4:29–43. doi: 10.1007/s41030-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Godinho-Silva C, Domingues RG, Rendas M, Raposo B, Ribeiro H, da Silva JA, Vieira A, Costa RM, Barbosa-Morais NL, Carvalho T, Veiga-Fernandes H. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature. 2019;574:254–8. doi: 10.1038/s41586-019-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nussbaum JC, Van Dyken SJ, Von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Monticelli LA, Buck MD, Flamar AL, Saenz SA, Wojno EDT, Yudanin NA, Osborne LC, Hepworth MR, Tran SV, Rodewald HR, Shah H, et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nature Immunology. 2016;17:656–65. doi: 10.1038/ni.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Karagiannis F, Masouleh SK, Wunderling K, Surendar J, Schmitt V, Kazakov A, Michla M, Hölzel M, Thiele C, Wilhelm C. Lipid-Droplet Formation Drives Pathogenic Group 2 Innate Lymphoid Cells in Airway Inflammation. Immunity. 2020;52:620–34.:e6. doi: 10.1016/j.immuni.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 220.Thio CLP, Chi PY, Lai ACY, Chang YJ. Regulation of type 2 innate lymphoid cell–dependent airway hyperreactivity by butyrate. Journal of Allergy and Clinical Immunology. 2018;142:1867–83.:e12. doi: 10.1016/j.jaci.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 221.Lewis G, Wang B, Shafiei Jahani P, Hurrell BP, Banie H, Aleman Muench GR, Maazi H, Helou DG, Howard E, Galle-Treger L, Lo R, et al. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Frontiers in Immunology. 2019;10 doi: 10.3389/fimmu.2019.02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wilhelm C, Harrison OJ, Schmitt V, Pelletier M, Spencer SP, Urban JF, Ploch M, Ramalingam TR, Siegel RM, Belkaid Y. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. Journal of Experimental Medicine. 2016;213:1409–18. doi: 10.1084/jem.20151448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yamaguchi M, Samuchiwal SK, Quehenberger O, Boyce JA, Balestrieri B. Macrophages regulate lung ILC2 activation via Pla2g5-dependent mechanisms. Mucosal Immunology. 2018;11:615–26. doi: 10.1038/mi.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Cabo R, Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med. 2019;381:2541–51. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 225.Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Cell Press; 2020. pp. 241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: Early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017;140:24–40. doi: 10.1016/j.jaci.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 227.Miller RL, Peden DB. Environmental effects on immune responses in patients with atopy and asthma. J Allergy Clin Immunol. 2014;134:1001–8. doi: 10.1016/j.jaci.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lodge CJ, Lowe AJ, Gurrin LC, Hill DJ, Hosking CS, Khalafzai RU, Hopper JL, Matheson MC, Abramson MJ, Allen KJ, Dharmage SC. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. Journal of Allergy and Clinical Immunology. 2011;128:782–8.:e9. doi: 10.1016/j.jaci.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 229.Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Déjardin F, Sparwasser T, Bérard M, Cerf-Bensussan N, Eberl G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity. 2019;50:1276–88.:e5. doi: 10.1016/j.immuni.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 230.Qian Q, Paul Chowdhury B, Sun Z, Lenberg J, Alam R, Vivier E, Gorska MM. Maternal diesel particle exposure promotes offspring asthma through NK cell-derived granzyme B. Journal of Clinical Investigation. 2020 doi: 10.1172/JCI130324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, Chatila ZK, Daher N, Sioutas C, Chatila TA. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. Journal of Allergy and Clinical Immunology. 2015;136:441–53. doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.De Grove KC, Provoost S, Hendriks RW, McKenzie ANJ, Seys LJM, Kumar S, Maes T, Brusselle GG, Joos GF. Dysregulation of type 2 innate lymphoid cells and TH2 cells impairs pollutant-induced allergic airway responses. Journal of Allergy and Clinical Immunology. 2017;139:246–57.:e4. doi: 10.1016/j.jaci.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shi L, Manthei DM, Guadarrama AG, Lenertz LY, Denlinger LC. Rhinovirus-induced IL-1beta release from bronchial epithelial cells is independent of functional P2X7. Am J Respir Cell Mol Biol. 2012;47:363–71. doi: 10.1165/rcmb.2011-0267OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Busse PJ, Birmingham JM, Calatroni A, Manzi J, Goryachokovsky A, Fontela G, Federman AD, Wisnivesky JP. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol. 2017;139:1808–18.:e6. doi: 10.1016/j.jaci.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ohne Y, Silver JS, Thompson-Snipes LA, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, Liu YJ. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nature Immunology. 2016;17:646–55. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 236.Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, Casanova JL, Yssel H, Di Santo JP. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. Journal of Experimental Medicine. 2016;213:569–83. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, Pritchard GH, Berlin AA, Hunter CA, Bowler R, Erjefalt JS, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nature Immunology. 2016;17:626–35. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Beuraud C, Lombardi V, Luce S, Horiot S, Naline E, Neukirch C, Airouche S, Perchet T, Golub R, Devillier P, Chollet-Martin S, et al. CCR10(+) ILC2s with ILC1-like properties exhibit a protective function in severe allergic asthma. Allergy. 2019;74:933–43. doi: 10.1111/all.13679. [DOI] [PubMed] [Google Scholar]
- 239.Bielecki P, Riesenfeld SJ, Kowalczyk MS, Vesely MCA, Kroehling L, Yaghoubi P, Dionne D, Jarret A, Steach HR, McGee HM, Porter CBM, et al. Skin inflammation driven by differentiation of quiescent tissue-resident ILCs into a spectrum of pathogenic effectors. Cold Spring Harbor Laboratory; 2018. [Google Scholar]
- 240.Bernink JH, Ohne Y, Teunissen MBM, Wang J, Wu J, Krabbendam L, Guntermann C, Volckmann R, Koster J, van Tol S, Ramirez I, et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat Immunol. 2019;20:992–1003. doi: 10.1038/s41590-019-0423-0. [DOI] [PubMed] [Google Scholar]
- 241.Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, Kradolfer CMA, Shikhagaie MM, Seys S, Hellings PW, van Drunen CM, et al. IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nature Communications. 2019;10:1–15. doi: 10.1038/s41467-019-09883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu S, Verma M, Michalec L, Liu W, Sripada A, Rollins D, Good J, Ito Y, Chu HW, Gorska MM, Martin RJ, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. Journal of Allergy and Clinical Immunology. 2018;141:257–68.:e6. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, van der Merwe R. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med. 2017;377:936–46. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 244.Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie ANJ. Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity. Clinical and Experimental Allergy. 2011;41:1447–55. doi: 10.1111/j.1365-2222.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- 245.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, Reed JL, Woods R, Dall’acqua WW, Stephens GL, Erjefalt JS, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–53.:e2. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 246.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M, Investigators ZT. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med. 2017;376:2448–58. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 247.Chipps BE, Hirsch I, Trudo F, Alacqua M, Zangrilli JG. Benralizumab efficacy for patients with fixed airflow obstruction and severe, uncontrolled eosinophilic asthma. Ann Allergy Asthma Immunol. 2020;124:79–86. doi: 10.1016/j.anai.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 248.Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, Ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 249.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 250.Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, Yokoyama A, Olsson J, Gray S, Holweg CT, Eisner M, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–96. doi: 10.1016/S2213-2600(16)30265-X. [DOI] [PubMed] [Google Scholar]
- 251.Panettieri RA, Jr, Sjobring U, Peterffy A, Wessman P, Bowen K, Piper E, Colice G, Brightling CE. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med. 2018;6:511–25. doi: 10.1016/S2213-2600(18)30184-X. [DOI] [PubMed] [Google Scholar]
- 252.Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, Olsson RF, Martin UJ, Goldman M, investigators Bs Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7:46–59. doi: 10.1016/S2213-2600(18)30406-5. [DOI] [PubMed] [Google Scholar]
- 253.Weinstein SF, Katial R, Jayawardena S, Pirozzi G, Staudinger H, Eckert L, Joish VN, Amin N, Maroni J, Rowe P, Graham NMH, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018;142:171–7.:e1. doi: 10.1016/j.jaci.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 254.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, Mullol J, Greos LS, Bosso JV, Laidlaw TM, Cervin AU, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–50. doi: 10.1016/S0140-6736(19)31881-1. [DOI] [PubMed] [Google Scholar]
- 255.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, Silverberg JI, Deleuran M, Kataoka Y, Lacour JP, Kingo K, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375:2335–48. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 256.Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, Pavord ID, Zhang B, Staudinger H, Pirozzi G, Amin N, et al. Liberty Asthma QUEST: Phase 3 Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate Dupilumab Efficacy/Safety in Patients with Uncontrolled, Moderate-to-Severe Asthma. Adv Ther. 2018;35:737–48. doi: 10.1007/s12325-018-0702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shrimanker R, Borg K, Connolly C, Thulborn S, Cane J, Xue L, Hynes G, Hinks T, Kots M, Girardello L, Petruzzelli S, et al. Late Breaking Abstract - Effect of timapiprant, a DP2 antagonist, on airway inflammation in severe eosinophilic asthma. European Respiratory Journal. 2019;54:RCT3784 [Google Scholar]