Abstract
The role of the left angular gyrus (AG) in language processing remains unclear. In this study, we used transcranial magnetic stimulation (TMS) to test the hypothesis that the left AG causally supports the processes necessary for context-dependent integration and encoding of information during language processing. We applied online TMS over the left AG to disrupt the online context-dependent integration during a language reading task, specifically while human participants integrated information between two sequentially-presented paragraphs of text (“context” and “target” paragraphs). We assessed the effect of TMS on the left AG by asking participants to retrieve integrated contextual information when given the target condition as cue in a successive memory task. Results from the memory task showed that TMS applied over the left AG during reading impaired the formation of integrated context-target representation. These results provide the first evidence of a causal link between the left AG function, online information integration and associative encoding during language processing.
Keywords: semantic, context, narrative, TMS, angular gyrus
Introduction
The left angular gyrus (AG) is implicated in a wide range of cognitive activities including memory retrieval, language and semantic processing, numerical processing, spatial cognition, attention and theory of mind (Bonnici, Cheke, Green, FitzGerald, & Simons, 2018; Bonnici, Richter, Yazar, & Simons, 2016; Ciaramelli, Rosenbaum, Solcz, Levine, & Moscovitch, 2010; Hartwigsen, Golombek, & Obleser, 2015; Humphreys & Lambon Ralph, 2015, 2017; Seghier, 2013). Amongst these activities, its specific role during semantic and language tasks is still not well understood. Recent neuroimaging evidence suggests that this region may support the integration of contextual information during language processing (Bonnici et al., 2016; Branzi, Humphreys, Hoffman, & Lambon Ralph, 2020; Humphreys & Lambon Ralph, 2015, 2017; Ramanan, Piguet, & Irish, 2018; van der Linden, Berkers, Morris, & Fernandez, 2017). For instance, the left AG, differently from other regions within the semantic network such as the anterior temporal lobes (ATLs), is engaged in language and semantic tasks but only when information can be integrated into a contextual support (e.g., Branzi et al., 2020). Furthermore, the left AG responds more to stimuli with strong rather than weak contextual or thematic associations (Bar, Aminoff, & Schacter, 2008; Davey et al., 2015), and finally, it is involved in reactivation and recombination of information presented in a given context (Bonnici et al., 2016; Jonker, Dimsdale-Zucker, Ritchey, Clarke, & Ranganath, 2018; Ramanan et al., 2018; Shimamura, 2011; I. C. Wagner et al., 2015).
Whilst these functional magnetic resonance imaging (fMRI) investigations have advanced our understanding of the left AG’s role in different aspects of cognition, to date no study has provided evidence for a causal link between processes supporting online contextual integration during naturalistic language processing and the functioning of the left AG. Some previous studies have assessed the causal role of left AG for processing two-word combinations (e.g., student–pupil) (Koen, Thakral, & Rugg, 2018; Sliwinska, James, & Devlin, 2015). Going beyond two-word combinations is critically important because two-word combinations do not engage the left AG to the same extent as language tasks involving multi-item context integration (e.g., sentence processing) (see Humphreys, Hoffman, Visser, Binney, & Lambon Ralph, 2015; Humphreys & Lambon Ralph, 2015; Humphries, Binder, Medler, & Liebenthal, 2007), suggesting that this brain region may be particularly important for multi-item and time-extended integration of information (e.g., Humphreys & Lambon Ralph, 2015; Ramanan et al., 2018).
In the present study, we evaluated the causal role of the AG for context-dependent integration of information using transcranial magnetic stimulation (TMS) during naturalistic reading. Online TMS was applied over the left AG to temporarily interrupt the AG integration function while participants read narratives composed of two consecutive passages (“context” and “target” conditions). We hypothesised that if the left AG is necessary for continuous integration of information, AG TMS applied between context and target presentation should affect the encoding of an integrated context-target representation. We also used this new TMS exploration to test a more specific hypothesis that the AG is particularly engaged when integration involves ongoing coherently-related content (Humphreys & Lambon Ralph, 2015, 2017; van der Linden et al., 2017; Branzi et al., 2020). For instance, recent neuroimaging evidence has shown that AG activity is positively engaged during information processing and is predictive of better memory retention, only when this information fits with a knowledge-based schema or semantic context (van der Linden et al., 2017). Thus, in the current experiment an AG TMS effect should be observed only when context and target passages are semantically coherent (i.e., same schema or semantic context), but not when the semantic context changes between the passages (and thus there is need to reset or update the current schema). To test this hypothesis, AG TMS was applied during two narrative conditions in which the same target paragraph was preceded by different types of context: (i) a highly congruent context (HC) which maximised the information contained in a single coherent story assimilated across both passages; versus (ii) a low−congruent (LC) paragraph with a divergent meaning, thus requiring updating the semantic context (see Figure 1A). In the subsequent memory task, participants were presented with the target condition (both HC and LC) as a cue, and asked to retrieve context-related information that required access to an integrated representation (“Cue Target” trials) (see Figure 1B). We expected AG TMS to impair behavioural performance in HC conditions, but to have no effect on the retrieval of LC conditions.
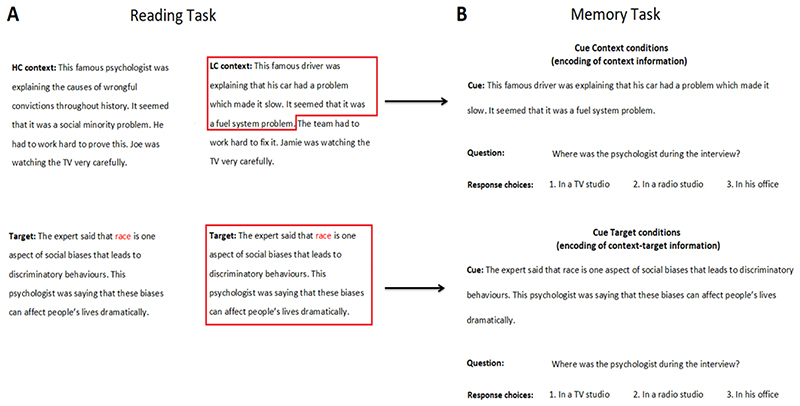
Figure 1.
(A) Examples of stimuli for HC and LC conditions (context and target paragraphs). Homonym word is coloured in red. For a complete list of the stimuli, see Branzi et al., (2020). (B) Examples of stimuli for Cue Context (LC only in this example) and Cue Target conditions, assessing the encoding of context and context-target information, respectively. In Cue Context conditions, the cues for LC and HC conditions are identical to the LC and HC contexts, except for some information that was removed because it contained the answer to the questions in the memory task. For instance, the cues for LC and HC conditions in Figure 1B would correspond to the LC and HC contexts presented in Figure 1A, except for the final sentences “The team had to work hard to fix it. Jamie was watching the TV very carefully.” and “He had to work hard to prove this. Joe was watching the TV very carefully.”, which were removed from the Cue Context conditions because they contained the answer to the question (i.e., location of the interview). Questions for Cue Target and Cue Context conditions were the same across participants. However, for each narrative item, a given participant was asked different questions for Cue Target and Cue Context conditions. For instance, if in the Cue Context condition we were asking the question about the location of the interview (as in Figure 1B), in the Cue Target condition we would have asked the question about who was listening to the interview (and vice versa).
Furthermore, the literature suggests that memory representations that include elements following and preceding the update of information are harder to retrieve than representations that require retrieval of associations within the same event (Speer & Zacks, 2005; Swallow, Zacks, & Abrams, 2009). Accordingly, we expected to find increased response times (RTs) and error rates for low over high context-to-target congruency under standard conditions (i.e., no AG TMS), and that AG TMS would diminish this effect given the AG’s role in contextual integration. To rule out the possibility that TMS was affecting encoding in general, rather than the encoding of context-target integrated representations specifically (i.e., associative memory), we employed a control condition in the memory task. In “Cue Context” trials, participants were required to retrieve the same context-related information as in the Cue Target conditions (Figure 1B). However, they were provided with some contextual information as cue.
Materials and Methods
Participants
Participants were selected from an existing database of volunteers that have been screened for contraindications to TMS. Specifically, participants were excluded if they had a current or previous neuropsychiatric or neurological illness, were taking any psychoactive medications, had a prior head injury that required hospitalization/surgery, if they had cardiac pace maker and/or cochlear implants fitted, had any joint replacements or metal implants in any part of their body including the head, had a prior experience of a seizure, had a diagnosis or family history of epilepsy, and finally, if they might be pregnant. Eighteen volunteers took part in the study (average age = 22, standard deviation (SD) = 3; N female = 12). All participants were right-handed (Oldfield, 1971), native English speakers with no history of neurological or psychiatric disorders and normal or corrected−to−normal vision. The participants included in the study were also screened for any developmental or acquired language impairments. Written informed consent was obtained from all participants. The experiment was approved by the local ethics committee.
Stimuli
Reading task
The experimental stimuli used in the reading task were the same as in a previous study (Branzi et al., 2020). Thus, a total of 40 narrative paragraph pairs were employed in the reading task. For each narrative pair, the same second paragraph (target) was preceded by different first paragraphs (contexts) that could be either high−congruent (HC) or low−congruent (LC) with the target in terms of meaning. Both HC and LC context paragraphs could be integrated with the target paragraphs, though a reworking of the evolving semantic context was required after LC contexts (see Figure 1A for an example of the stimuli; for the full list of the stimuli used in the reading task see Branzi et al., 2020). Homonym words (e.g., race, bank, etc.), presented at the beginning of the target paragraph, were employed to determine the exact point in the paragraph in which the shift in the semantic context should have been experienced. Finally, the “No Context” (NC) condition, where the target (the same as in HC and LC conditions) was preceded by a string of numbers, was employed as a control condition. The NC condition allowed us to verify that any TMS effect in the HC and LC conditions was due to integration processes, and not to bottom-up attention triggered by the presentation of the target paragraph.
Memory task
75 experimental stimuli were employed. In the memory task, the presentation of a cue displayed on the screen was followed by a question about the context of the narrative along with three possible response choices (see Figure 1B). Importantly, in the memory task there were different types of conditions. These conditions did not differ with respect to the type of information that participants had to retrieve (the same contextual information), rather they differed in the type of cue (see below).
Cue Target conditions
In this condition, the cues contained the information presented in the target paragraph for both HC and LC narratives [i.e., HC Cue Target conditions (n = 25) and LC Cue Target conditions (n = 25)]. This condition tested participants’ ability to retrieve target-context integrated representations.
Cue Context conditions
In this condition (n = 25), the cues contained only a part of the context paragraph (see an example in Figure 1B). Thus, participants were presented with this information as a cue, and then had to retrieve the “missing” contextual information. Therefore, unlike the Cue Target condition, this condition did not require participants to retrieve the integrated representation of the two sequential paragraphs, but only tested participants’ ability to retrieve the context-paragraph representation. In both conditions, the type of contextual information to be retrieved during the memory task covered a variety of episodic details (e.g., who, where, when) (Figure 1B).
Task procedures
Structure of the experimental sessions
Before starting the experimental study, all participants signed an informed consent and were given written and oral instructions. A PC running ePrime (Psychology Software Tools, Inc., Pittsburgh, PA) was used to present the stimuli and record participants’ behavioural responses. The participants completed three sessions on different days. During two out of three sessions, participants received TMS (TMS over the left AG and TMS over the vertex). In one session, TMS was not used. The sessions were at least 1 week apart (average days between sessions = 12, SD = 3), and the order of sessions was counterbalanced across participants.
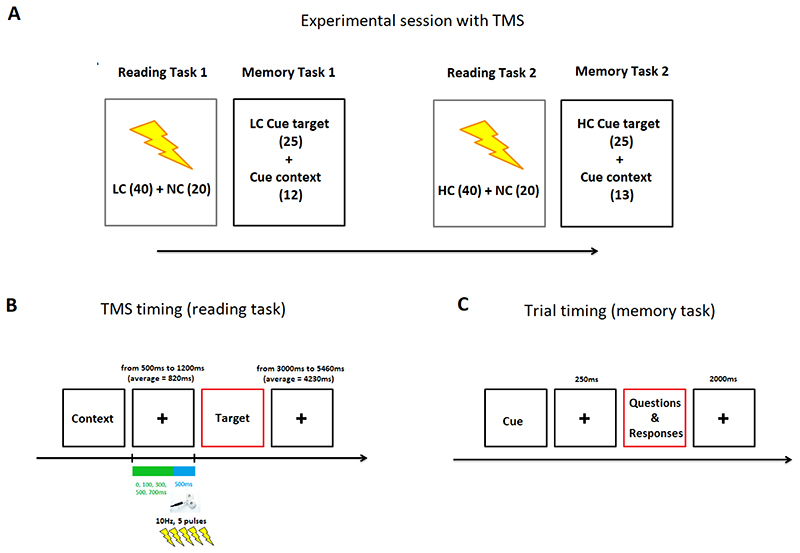
In each session, participants performed two different tasks: a reading task and a memory task. Both reading and memory tasks were divided into two blocks (i.e., “Reading Task 1” and “Reading Task 2”; and “Memory Task 1” and “Memory Task 2”), which were presented in an interleaved fashion (see Figure 2A). Each session started with Reading Task 1 (see Figure 2A), in which participants read half of the narrative stimuli. This was followed by a memory task (i.e., Memory Task 1), comprised of questions about the narratives presented in Reading Task 1. After a short break (10 minutes), the remaining narratives were presented (Reading Task 2, see Figure 2A), which was followed by Memory Task 2, including questions relating to the narratives presented in Reading Task 2.
Figure 2.
(A) An example of the structure of a session in which TMS was used. (B) Trial timing in the reading task. (C) Trial timing in the memory task.
During the TMS sessions, online TMS was delivered during each trial in the reading tasks (for detail see Figure 2B and Stimulation parameters and stimulation sites). TMS was not applied during the memory task. We did not apply TMS during the memory task since the goal of the present study was to measure the contribution of the left AG to context integration during language comprehension. Since the left AG is implicated not only in language processing, but also in memory retrieval (Humphreys & Lambon Ralph, 2015; Rugg & King, 2018; Rugg & Vilberg, 2013), avoiding TMS during the memory task ensured that any observed TMS effect reflected the contribution of the AG for integrating information during the reading task. At the end of each TMS session, participants completed a questionnaire in which they reported the extent to which TMS was perceived as uncomfortable and distracting (scales from 1 = not very, to 7 = very).
Reading task
As noted above, the reading task was divided in two blocks (Reading Task 1 and Reading Task 2). In both, participants were presented with 40 narrative items per condition (HC, LC and NC). As in our previous study (Branzi et al., 2020), each trial consisted of two text paragraphs (context and target) that participants had to read silently (verbal material and numbers). Contexts and targets were displayed on the screen until participants pressed a button to indicate that they had finished reading the paragraph (for both contexts and targets). The instruction emphasized speed, given the limited amount of time for reading (max. duration for context was 12 seconds (s) and for targets was 7.5s), but also the need to understand and encode the information presented in the narratives. We informed participants that at the end of each reading task they would perform a memory task, requiring them to answer questions on the content of the narratives. We also specified that, in order to perform the task, it would be necessary to integrate the information presented in context and target paragraphs. Rest time was varied between context and targets (range between 500 milliseconds (ms) and 1200ms, average time = 820ms) and between trials (range between 3000ms and 5460ms, average time = 4230ms) during which a black fixation cross was presented.
Importantly, HC and LC narrative stimuli were presented separately (see Figure 2A). This was because HC and LC conditions terminated with the same target paragraph, and therefore, mixing them during the reading task would have created confusion during the memory task. Thus, in each session, Reading Task 1 included either HC trials (40) or LC trials (40), and half of the NC trials (20) (see Figure 2A). Reading Task 2 included the remaining narratives (either LC trials or HC trials, and the remaining 20 NC trials). The order of LC and HC conditions (i.e., whether they were assigned to Reading Task 1 or 2) was counterbalanced across participants and sessions. The stimuli presented in the reading task were the same across all three sessions.
Memory task
The memory task was divided in two blocks (Memory Task 1 and Memory Task 2). In both, participants were presented with 25 items per condition (Cue Context, LC Cue Target and HC Cue Target). Memory was probed using a three alternative-forced-choice task. Each trial started with the presentation of a cue displayed on the screen until participants made a button response. This was followed by the presentation of the questions and response choices, which were displayed until the participants made their selection by button response up to a time limit of 8.5s (see Figure 2C). The instruction emphasized speed, given the limited amount of time for responding. Rest time (black fixation cross) was presented between cue and questions/response choices and between trials (fixed time intervals: 250ms and 2000ms, respectively).
Importantly, Cue Context, LC Cue Target and HC Cue Target were presented in the following way: In Memory Task 1, LC Cue Target trials (25) were presented with half of the Cue Context trials (12 or 13), always after reading the LC narratives. In Memory Task 2, The HC Cue Target trials (25) were presented with the remaining Cue Context trials (13 or 12), always after the HC narratives (see Figure 2A). The order of presentation of LC and HC conditions (i.e., whether they were assigned to Memory Task 1 or 2) was counterbalanced across participants and sessions. Note that, for each participant, HC Cue Target trials (25) and LC Cue Target trials (25) referred to different narratives. Although the question stimuli for Cue Target and Cue Context trials were the same across participants, for the same narrative item, each participant was asked different questions in Cue Target (HC or LC) and Cue Context conditions (see Figure 1B). Finally, the stimuli presented during the memory task were always different across the three sessions.
Stimulation parameters and stimulation sites
TMS was delivered using a Magstim Rapid2 stimulator (Magstim Co., Whitland, UK) and a figure-of-eight coil with a diameter of 70 mm. Stimulation was performed at 120% of the individual’s motor threshold, measured before the start of the first session (mean stimulation intensity = 71, SD = 8, range = 54 - 84). The resting motor threshold of the relaxed contralateral abductor pollicis brevis muscle was measured as the lowest stimulation intensity able to cause a visible twitch in the muscle 5 out of 10 times (Sandrini, Umilta, & Rusconi, 2011).
For each trial, one train of five pulses (10Hz for 500ms) was delivered. This stimulation protocol has already been used over the inferior parietal cortex to induce inhibitory effects during language (Capotosto et al., 2017; Hartwigsen et al., 2010; Sliwinska et al., 2015) and non-linguistic processing (Riddle, Scimeca, Cellier, Dhanani, & D’Esposito, 2020), which is consistent with the inhibitory theory related to alpha oscillations in parietal-occipital areas (Klimesch, 2012). The first pulse was administered before the presentation of the target to avoid disrupting reading (TMS to the AG can induce eye twitches) (see Figure 2B). A pilot study confirmed that this procedure was successful for obtaining the expected (inhibitory) TMS effect on behaviour. The rest time between the context and target phases was variable and randomized (see Figure 2). Thus, participants were not able to anticipate when they would receive TMS. Finally, the TMS frequency, intensity and duration were well within established international safety limits (Rossini et al., 2015).
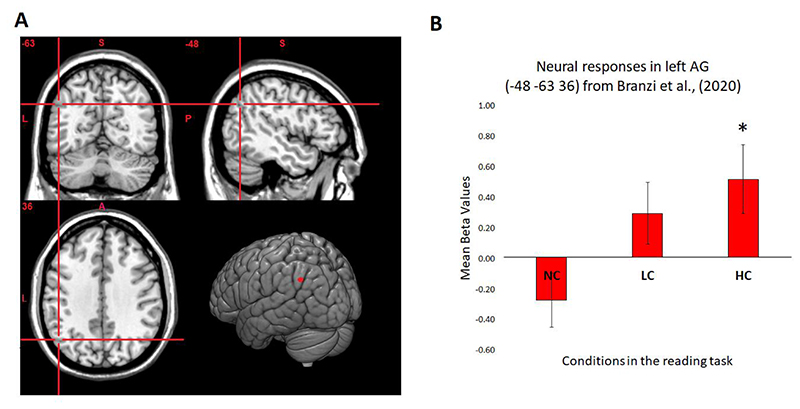
The stimulation site for the left AG region corresponded to the MNI coordinates (x = −48, y = −63, z = 36) derived from our previous fMRI study, in which the same experimental material was used, and where, in accord with other studies (for a review see Ramanan et al., 2018), the left AG activity was modulated by context integration and was positively engaged (against rest) for coherent conditions only, i.e., for HC conditions (Branzi et al., 2020) (see Figure 3B). Anatomically, this peak falls within an area between posterior PGa and PGp, two subregions of the AG, as defined by cytoarchitectonic parcellation (Caspers et al., 2008; Caspers et al., 2006).
Figure 3.
(A) The left AG stimulation site pinpointed on the MNI cortical template. (B) The neural data results are derived from Branzi et al. (2020), where the same stimuli and task were employed. The neural responses measured at the left AG stimulation site (sphere of 10mm radius) reflect neural activity of left AG against rest during context integration, i.e., measured at the onset of the target paragraph. Error bars correspond to standard errors (SEs). The neural activity measured at the stimulation site shows a context dependent effect [main effect of condition: F (2, 42) = 15.784, p < 0.001, ηp2 = 0.429, post-hoc t-test comparisons Bonferroni-corrected indicate that HC > NC and LC > NC (p < 0.001 and p = 0.012, respectively)] and that neural responses against the baseline are positively enhanced for HC conditions only [one-sample t-test for HC conditions: t (21) = 2.249, p = 0.035; one-sample t-test for LC conditions: t (21) = 1.427, p = 0.168].
During the AG TMS testing session, a Polaris Vicra infrared camera (Northern Digital, Waterloo, ON, Canada) was used in conjunction with the Brainsight frameless stereotaxy system (Rogue Research, Montreal, QC, Canada) to register the participant’s head to their own MRI scan to accurately target stimulation throughout the experiment. As in many previous studies that investigated the role of the AG in cognition, we selected the vertex as the control site (Bonnici et al., 2018; Davey et al., 2015; Koen et al., 2018; Thakral, Madore, Kalinowski, & Schacter, 2020; Thakral, Madore, & Schacter, 2017; Yazar, Bergstrom, & Simons, 2014, 2017). The vertex has also been used in many other TMS studies (Jung, Bungert, Bowtell, & Jackson, 2016; Silvanto, Cattaneo, Battelli, & Pascual-Leone, 2008) and is a suitable control site for the AG, since behavioural RT side-effects induced by TMS over AG or vertex do not differ (Meteyard & Holmes, 2018). The location of the vertex was established for each participant by using the international 10-20 system (Steinmetz, Furst, & Meyer, 1989). The halfway intersection of the two lines was marked using a skin marker.
Behavioural and data analysis
Discomfort/distractibility scores and reading times
To rule out the possibility that AG TMS effects measured in the memory task could be due to general disruption of reading processing induced by TMS, i.e., non-specific TMS effects, we obtained discomfort and distractibility self-report measures during each TMS session, and assessed the impact of TMS on the reading task performance. At the end of each TMS session participants completed a questionnaire in which they reported the extent to which TMS was perceived as uncomfortable and distracting (scales from 1 = not very, to 7 = very). Thus, we conducted separate t-tests for each measure (discomfort and distractibility), comparing ratings between sites (AG versus vertex).
Behavioural analyses were also performed on reading times to quantify the impact of discomfort and distractibility induced by TMS on the reading task performance (as measured by reading times). Trials exceeding three SDs above or below a given participant’s mean reading time were excluded from the analyses, causing a loss of 0.2% of data, across all task conditions and type of sessions. Then, the TMS effect was assessed by conducting a 3 × 3 within-subject ANOVA with the repeated-measures factors Condition (NC, LC and HC) and Session-type (No TMS, TMS to left AG, and TMS to vertex). Bonferroni correction for multiple comparisons was applied on post-hoc pairwise contrasts. Finally, correction for non-sphericity (Greenhouse-Geisser procedure) was applied to the degrees of freedom and p-values associated with factors having more than two levels (i.e., Condition and Session-type).
Memory Task
Behavioural analyses were performed on RTs and accuracy measures. RT analysis was conducted only for correct trials. Having eliminated error trials (see Table 1), trials exceeding three SDs above or below a given participant’s mean were excluded from the RT analyses, causing a loss of 2% of data, across all task conditions and type of sessions. Thus, for each Session-type (No TMS, AG TMS and vertex TMS) the remaining set of data on which we performed the RT analyses consisted, on average, of 16 trials per condition (SD = 2.7, 2.1 and 2.7 for No TMS, AG TMS and Vertex TMS, respectively). We conducted two separate 3 × 3 within-subject ANOVAs (one for RTs and one for accuracy measures) with the repeated-measures factors Condition (Cue Context, LC Cue Target and HC Cue Target) and Session-type (No TMS, TMS to left AG, and TMS to vertex). Correction for multiple comparisons was applied on the planned pairwise comparisons, according to our hypotheses. In detail, (1) LC Cue Target conditions were expected to be slower than HC Target conditions in absence of AG TMS (i.e., during vertex TMS and No TMS sessions only); and (2) for HC Cue Target Conditions, we expected AG TMS to induce slower RTs than vertex TMS and No TMS. Finally, correction for non-sphericity (Greenhouse-Geisser procedure) was applied to the degrees of freedom and p-values associated with factors having more than two levels (i.e., Condition and Session-type).
Table 1. Memory task.
Descriptive statistics for RTs and Accuracy measures.
| Descriptive Statistics RTs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NO TMS | AG | VERTEX | |||||||
| Cue Context | LC Cue Target | HC Cue Target | Cue Context | LC Cue Target | HC Cue Target | Cue Context | LC Cue Target | HC Cue Target | |
| Mean | 3134 | 3239 | 2950 | 2978 | 3288 | 3267 | 3144 | 3391 | 2951 |
| Std. Error | 119 | 122 | 73 | 138 | 181 | 139 | 201 | 167 | 120 |
| Std. Deviation | 503 | 520 | 308 | 586 | 768 | 588 | 851 | 707 | 508 |
| Descriptive Statistics Accuracy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NO TMS | AG | VERTEX | ||||||||
| Cue Context | LC Cue Target | HC Cue Target | Cue Context | LC Cue Target | HC Cue Target | Cue Context | LC Cue Target | HC Cue Target | ||
| Mean | 0.67 | 0.64 | 0.63 | 0.67 | 0.64 | 0.64 | 0.64 | 0.63 | 0.62 | |
| Std. Error | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 | 0.02 | |
| Std. Deviation | 0.13 | 0.15 | 0.12 | 0.10 | 0.12 | 0.15 | 0.15 | 0.17 | 0.10 | 35 |
Results
Discomfort/distractibility scores and reading times
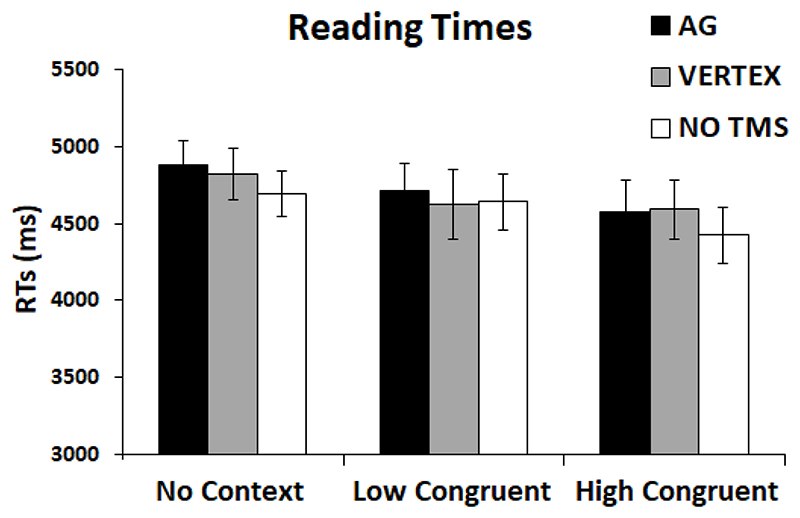
The scores obtained were moderate (AG distracting: average = 3.8, SD = 1.3; AG uncomfortable: average = 3.9, SD = 1.3; vertex distracting: average = 3.3, SD = 1.2; vertex uncomfortable: average = 3, SD = 1.4). TMS over AG or vertex showed similar distractibility scores [t (17) = 1.22, p = 0.238]. TMS over the AG, however, obtained higher scores on the discomfort scale as compared to TMS over the vertex [t (17) = 2.12, p = 0.049]. Interestingly, reading times for the target passage showed that there was no significant effect of Session-type [F (1.919, 32.618) = 0.551, p = 0.581, ηp2 = 0.031] or significant Condition × Session-type interaction [F (1.893, 32.181) = 0.231, p = 0.920, ηp2 = 0.013]. Thus, despite TMS over the AG obtained higher scores of discomfort as compared to TMS over the vertex, applying TMS over the left AG did not disrupt reading performance more than TMS over the vertex (Figure 4).
Figure 4. Reading task.
Results for reading times for NC, LC and HC conditions. Error bars correspond to SEs.
Memory task
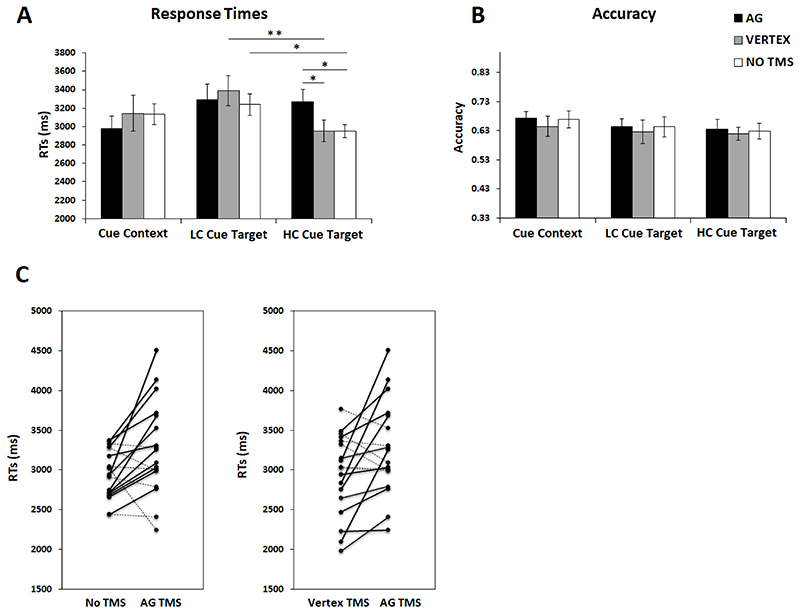
The results for the memory task are summarised in Figure 5 and Table 2. RTs showed a main effect of Condition [F (1.998, 33.968) = 9.443, p = 0.001, ηp2 = 0.357], suggesting that speed for LC Cue Target condition was slower as compared HC Cue Target (p = 0.003) and Cue Context conditions (p = 0.009). There was no significant difference in speed between the HC Cue Target and Cue Context conditions (p > 0.999). The main effect of Session-type was not significant [F (1.966, 33.42) = 0.188, p = 0.826, ηp2 = 0.011], suggesting that overall performance was not particularly affected by TMS. RT results revealed the expected significant Condition × Session-type interaction [F (3.235, 54.996) = 3.446, p = 0.02, ηp2 = 0.169]. Importantly, to ensure that this significant interaction was not solely driven by the Cue Context conditions, we further conducted a 2 × 3 within-subject ANOVA for RTs with the repeated-measures factors Condition (LC Cue Target and HC Cue Target) and Session-type (no TMS, TMS to left AG, and TMS to vertex). Again we found a significant Condition × Session-type interaction [F (1.979, 33.646) = 4.037, p = 0.027, ηp2 = 0.192]. This was assessed via planned pairwise comparisons. For all pairwise comparisons, we provide an effect size (Cohen’s d) and a Bayes factor (BF10 > 3 suggests substantial evidence for a difference between the pairs, and BF10 < 0.3 suggests substantial evidence for a null effect, see Jeffreys, 1961). Reporting Bayes factors is useful for hypothesis testing because they provide a coherent approach to determining whether non-significant results support a null hypothesis over a theory, or whether the data are just insensitive.
Figure 5. Memory task.
Results for (A) RTs and (B) Accuracy (proportion of correct responses) for Cue Context, LC Cue Target and HC Cue Target conditions. Error bars correspond to SEs. (C) Individual stimulation RT effects during the HC Cue Target condition in the memory task. Continuous black lines indicate slower performance for AG TMS as compared to No TMS or Vertex TMS, whereas dashed black lines reflect faster performance for AG TMS as compared to No TMS or Vertex TMS.
Table 2. Memory task.
RT results relative to the planned comparisons.
| RT results relative to planned comparisons | ||||
|---|---|---|---|---|
| Planned comparisons | Mean and Std. Error | T-test statistics | Effect size (d) | Bayes Factor (BF10) |
| No TMS: LC Cue Target > HC Cue Target | 289 (111) | t (17) = 2.613, p = 0.027 | 0.610 | 6.380 |
| Vertex TMS: LC Cue Target > HC Cue Target | 440 (116) | t (17) = 3.779, p = 0.0015 | 0.890 | 52.687 |
| AG TMS: LC Cue Target > HC Cue Target | 21 (91) | t (17) = 0.227, p > 0.999 | 0.053 | 0.290 |
| HC Cue Target: AG TMS > Vertex TMS | 316 (128) | t (17) = 2.463, p = 0.024 | 0.580 | 4.958 |
| HC Cue Target: AG TMS > No TMS | 317 (123) | t (17) = 2.576, p = 0.02 | 0.607 | 5.991 |
As mentioned above, our planned t-tests focussed on one-sided procedure for hypothesis testing because our hypotheses dictated a specific direction of the effects: we expected (1) LC Cue Target conditions to be slower than HC Target conditions in absence of AG TMS (i.e., during vertex TMS and No TMS sessions only); and that (2) for HC Cue Target Conditions, AG TMS should induce slower RTs than in the other two sessions (vertex TMS and No TMS).
In keeping with (1), we found that LC Cue Target conditions were significantly slower than HC Cue Target conditions when TMS was applied to the vertex (control site) [t (17) = 3.779, p = 0.0015, Cohen’s d = 0.89; 95% confidence interval CI: 0.332 - 1.431; BF10 = 52.687] or when it was not applied at all [t (17) = 2.613, p = 0.027, Cohen’s d = 0.61, CI: 0.103 - 1.114; BF10 = 6.380]. Interestingly, the same effect was not observed when TMS was applied to the left AG [t (17) = 0.227, p > 0.999, Cohen’s d = 0.053; CI: -0.410 – 0.515; BF10 = 0.29], suggesting that AG TMS may have affected HC Cue Target conditions specifically. Accordingly, and in line with (2) HC Cue Target conditions became slower when TMS was applied to the left AG as compared to when it was delivered to the vertex [t (17) = 2.463, p = 0.024, Cohen’s d = 0.58, CI: 0.072 – 1.074; BF10 = 4.958] or when it was not delivered at all [t (17) = 2.576, p = 0.02, Cohen’s d = 0.607, CI: 0.095 – 1.104; BF10 = 5.991].
In light of the results observed for the discomfort scale, we conducted a regression analysis to ensure that the observed TMS effect (AG TMS > vertex TMS) for the HC Cue Target condition was not driven by differences in discomfort scores (see Holmes & Meteyard, 2018). Our results showed that AG TMS > vertex TMS differences in discomfort measures were not predictive of AG TMS > vertex TMS differences in RTs for the HC Cue Target condition (Beta = 0.042, p = 0.868; The overall model fit was R^2 = 0.002), leading to the conclusion that perceived discomfort did not play any influence on the observed TMS effect (AG TMS > Vertex TMS) for the HC Cue Target condition. This conclusion was further corroborated by a further linear mixed-effects models analysis (Baayen, Davidson, & Bates, 2008). The model was fitted using RT in the memory task as the dependent variable, and Session-type (AG TMS and vertex TMS) and Condition (LC Cue Target and HC Cue Target) as two fixed effect factors at the level of individual trials. Importantly, we included the discomfort scores as a covariate of the fixed effect terms. Furthermore, by-item and by-subject random intercepts were included in the model to account for variability in the RT responses at the level of individual participants and individual items, in addition to the variability of individual trials already modelled by the fixed effect terms (Type of session and Condition). The model parameters were estimated using the restricted maximum likelihood (REML) approach. In line with the regression analysis outcome, results showed that, whilst the Type of session × Condition interaction was significant (F=3.659, p=0.026), the Type of session × discomfort scores (F=1.915, p=0.167) and Condition × discomfort scores interactions (F=1.122, p=0.326) were not significant.
Finally, TMS to the left AG did not impair RT performance for LC Cue Target conditions [AG TMS versus vertex TMS: t (17) = -0.549, p > 0.999, Cohen’s d = -0.129, CI: -0.591 – 0.337; BF10 = 0.17; AG TMS versus No TMS: t (17) = 0.281, p > 0.782, Cohen’s d = 0.066, CI: -0.397 – 0.528; BF10 = 0.30] or Cue Context conditions [AG TMS versus vertex TMS: t (17) = -1.109, p > 0.999, Cohen’s d = -0.261, CI: -0.728 – 0.213; BF10 = 0.128; AG TMS versus No TMS: t (17) = -1.303, p > 0.999, Cohen’s d = -0.307, CI: -0.776 – 0.170; BF10 = 0.118].
Note that RTs for error responses were not included in the main analyses because the hypothesis of AG TMS impairing the integration and encoding of a context-target integrated representation can be assessed only by examining RTs for correct trials (and/or accuracy measures). In fact, one possibility is that AG TMS prevents the formation of an association between the information presented in context and target paragraphs, leading to a decrease in accuracy measures. Another possibility is that AG TMS does not prevent the formation of context-target associations, but it makes them weaker and therefore harder to retrieve. This should result in slower RTs for correct trials only. Consistent with this latter alternative, RT results for error responses did not show any significant TMS effect or interaction (main effect of Session-type: [F (1.665, 28.310) = 1.803, p = 0.187, ηp2 = 0.096]; main effect of Condition: F (1.919, 32.626) = 1.351, p = 0.273, ηp2 = 0.074]; Condition × Session-type interaction [F (2.417, 41.088) = 1.282, p = 0.292, ηp2 = 0.07]).
Like previous studies assessing associative memory performance with three or more response choices (Clouter, Shapiro, & Hanslmayr, 2017; Cooper, Greve, & Henson, 2019; Wang, Clouter, Chen, Shapiro, & Hanslmayr, 2018; Yazar et al., 2017) accuracy was not high (on average 64%). Nevertheless, participants were engaged in the task (chance level is 33%). The accuracy data did not reveal any significant effect (main effect of Condition: [F (1.894, 32.2) = 2.087, p = 0.143, ηp2 = 0.109]; main effect of Session-type: [F (1.946, 33.084) = 0.515, p = 0.598, ηp2 = 0.029]; Condition × Session-type interaction: [F (3.166, 53.83) = 0.030, p = 0.994, ηp2 = 0.002]).
In summary, we found substantial evidence (BF10 > 3; Cohen’s d >= 0.58) that TMS over the left AG selectively impaired encoding of context-target integrated representation for highly coherent narratives (HC Cue Target conditions), but not for the other conditions (BF10 < 0.3). The medium-large effect sizes reported here for AG TMS accord with the evidence that a small number of trials per condition does not necessarily represent an issue when it comes to power (Rounder & Haff, 2018). Note that it is unlikely that the TMS effects observed in the present study reflect disruption of retrieval processes (i.e., long-lasting after-effects of TMS) rather than encoding processes during the reading task. In fact, EEG-TMS combined studies show that the type of stimulation used in the present study induces online and short-lasting effects only (e.g., (Thut et al., 2011). Thus the short trains of stimulation delivered in the present study, interleaved with long intervals (>16s), probably caused direct and measurable condition-specific interference with patterns of ongoing neuronal discharge at the time of stimulation (Valero-Cabre, Amengual, Stengel, Pascual-Leone, & Coubard, 2017). Furthermore, if the AG TMS had induced general disruption of retrieval processes, this effect should also have been observed for the other conditions, and especially for the Cue Target condition that showed similar RTs in the baseline conditions (No TMS: Cue Context versus HC Cue Target: t(17) = 1.738, p=0.1; vertex TMS: Cue Context versus HC Cue Target: t(17) = 1.417, p=0.175). However, no such effect was observed.
Discussion
In the present study, we sought evidence for the hypothesis that the left AG is critical for integration of context-dependent information during language processing (Branzi et al., 2020; Humphreys & Lambon Ralph, 2015, 2017). This hypothesis was tested by asking participants to read short narratives consisting of two sequential paragraphs (context and target), and by delivering TMS pulses over the left AG between the context and target paragraphs, to disrupt online integration and therefore encoding of the narrative content (context and target integrated representation).
In a memory task, we measured RTs and proportion of correct responses to test the hypothesis that TMS-induced temporary disruption of AG activity during reading would have had an effect on encoding, and therefore recall of integrated memory representation (context-target). We hypothesised that this effect would have been observed for HC conditions specifically, that is, when incoming information (target) matches the current knowledge-based schema (Humphreys & Lambon Ralph, 2015, 2017; Speer & Zacks, 2005; Swallow et al., 2009). In line with this hypothesis, we found that TMS delivered over the left AG made participants slower in retrieving context-related information during the memory task, only for coherent narratives (HC Cue Target conditions). This result also accords with the left AG profile of activation found in our previous fMRI study where, during reading, neural responses for HC conditions were the most enhanced as compared to the rest baseline (see Figure 3B). Importantly, this AG TMS effect (observed only for the HC Cue Target condition) cannot be explained by non-specific stimulation side effects (e.g., auditory noise or discomfort). Discomfort measures were not predictive of the observed AG TMS effect nor were reading times affected by AG TMS.
Our results align with previous TMS evidence establishing the causal role of the left AG for the retrieval of highly congruent thematic associations (Davey et al., 2015). In this study, the authors tested the contribution of the left AG in semantic retrieval using a thematic matching task. As with our study, this type of task involves some context-dependent processing of information. In fact, matching the picture-probe with the target-word requires linking conceptual representations of objects from different semantic categories that are nevertheless found or used together in similar contexts. Our findings also accord with previous work showing that TMS over the AG can influence the encoding of novel associations into memory (Hermiller, VanHaerents, Raij, & Voss, 2019; Tambini, Nee, & D’Esposito, 2018). Nevertheless, the results from the present study are distinct from previous work, as they establish a critical role of the left AG in continuous online integration of linguistic information.
In contrast to our findings, a recent TMS study failed to reveal a link between left AG functioning during encoding and subsequent associative memory performance (Koen et al., 2018). Differences in the type of task and stimuli might explain contrasting outcomes. In Koen et al (2018), participants were presented with word-pairs and were required to perform a size judgment task on them. Therefore, unlike our and other studies (Davey et al., 2015; van der Linden et al., 2017), integration was unlikely to be loading on automatic and context-dependent integration of information: the comparative size judgement task requires comparative working memory for two study items, rather than integration of rich and detailed contextual information. The importance of the left AG for the latter process has been demonstrated in a recent study, where activity of the left AG (1) increased as the presentation of coherent items (pictures of thematically-related objects) unfolded over encoding, and (2) predicted later memory performance (van der Linden et al., 2017). In accordance with our findings, these results suggest that the left AG activity is crucial for encoding and re-activation of long-term schema-consistent associative memories. In short, if the left AG supports context-dependent integration of rich and highly relational content, we should not necessarily expect its disruption to impair memory for arbitrary word-pairs (see also Bonnici et al., 2016 for results consistent with this view).
Despite some TMS and patient studies indicating a role of the AG for language comprehension (Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Hartwigsen et al., 2015), our results did not reveal any AG TMS effect in reading times. Rather, our findings align with previous evidence showing that TMS effects in speech comprehension are more likely to be observed under perceptually challenging conditions (Hartwigsen et al., 2015).
Taken together, our results are consistent with the proposal that the AG supports context-dependent processing of information (Branzi et al., 2020; Humphreys & Lambon Ralph, 2015, 2017). However, what is the nature of the processes reflected by AG activity during language tasks? Some researchers have proposed that the left AG (more specifically the core, posterior AG area associated with the default mode network and targeted in the present study) might play a key role in semantic representation or, more specifically, event semantics (Binder & Desai, 2011; Binder, Desai, Graves, & Conant, 2009; Humphries, Binder, Medler, & Liebenthal, 2007). Others have argued that left AG may support online, automatic information buffering processes on content that is perceived or represented elsewhere in the brain (Humphreys & Lambon Ralph, 2015, 2017; Vilberg & Rugg, 2012). According to both proposals, this portion of the AG is positively engaged in semantic tasks, but only when these require some context-dependent processing (Baldassano et al., 2017; Branzi et al., 2020; Lerner, Honey, Silbert, & Hasson, 2011). However, the very same parietal region is commonly deactivated during semantic tasks that do not require such processes (Humphreys et al., 2015; Visser & Lambon Ralph, 2011), which is inconsistent with proposals that the AG underpins semantic representation or a more general role in semantic processing.
In considering the AG’s function beyond semantic cognition alone, it is important to note that the left AG is positively activated by tasks requiring episodic processing (Bonnici et al., 2016; Ramanan et al., 2018; Rugg & King, 2018; Tibon, Fuhrmann, Levy, Simons, & Henson, 2019). One common aspect shared by the semantic context integration tasks (such as that probed in the present study) and episodic memory tasks is that they both require online manipulation, retrieval and integration of information (e.g., episodic details such as the contextual who, what, when, where, and why knowledge). Thus, it is possible to relate the left AG’s core function to at least two mnemonic mechanisms. One possibility is that the left AG is directly involved in the formation and representations of episodes (Bonnici et al., 2016; Ramanan et al., 2018; Shimamura, 2011). This hypothesis fits with findings showing that the left AG’s activity is prominently observed at the end of an event, i.e., when all the information has been already presented (e.g., Humphries et al., 2007). Alternatively, the left AG may buffer the combination of past information (episodically retrieved) with newly presented information to update the growing contextual meaning, as time unfolds. This hypothesis is consistent with both previous neuroimaging evidence (Branzi et al., 2020; Humphreys & Lambon Ralph, 2015, 2017; Ramanan et al., 2018; van der Linden et al., 2017; Vilberg & Rugg, 2008; Wagner, Shannon, Kahn, & Buckner, 2005), and various neuropsychological findings (for a review see Ramanan et al., 2018). In fact, patients with left AG lesions are not amnesic like patients with hippocampal lesions on standard source and associative memory paradigms. Rather, damage to the left AG seems mainly to diminish the confidence in recollection and the efficient online control of information (Simons et al., 2008; Simons, Peers, Mazuz, Berryhill, & Olson, 2010). This neuropsychological observation mirrors our finding that TMS on the left AG affects RTs, but not accuracy measures. Together these results may suggest that disrupting the normal functioning of the left AG does not cause the key information to be lost (as per classical amnesia), but rather the formation of a less vivid and rich representation of a given episode, therefore making it less easy to retrieve (see also Yazar et al., 2014). Thus, our results seem to be more in line with the online buffering hypothesis, rather than with possibility that AG reflects the coding and integration of this information per se (i.e., episodic and semantic representations might be coded in other regions, e.g., hippocampal regions and ATL, respectively). Of course, the left AG’s role in online buffering of internal and external information sources would be a necessary precursor to the generation and the updating of context meaning, processing of the information in an episode and so on.
Finally, in the memory task we also observed slower RTs for LC Cue Target conditions as compared to HC Cue Target conditions. This result is consistent with previous studies showing that memory performance is affected by “event boundaries” (Swallow et al., 2009). In these studies event boundaries were identified as changes in temporal, spatial and personal dimensions in the course of information processing. To our knowledge, this is the first study to demonstrate that the same is true for “semantic boundaries” (i.e., a change of semantic context).
To conclude, our findings provide the first evidence of a causal role of the left AG in context-dependent integration of information and associative encoding during online naturalistic language processing. This result suggests that the AG role observed in previous studies (e.g., Baldassano et al., 2017; Branzi et al., 2020; Humphries et al., 2007) may be causally related to such processes. Future studies can investigate whether the context-dependent processes supported by left AG during language tasks reflect domain-general buffering processes (Humphreys & Lambon Ralph, 2015, 2017), or formation of (semantic or/and episodic) representations per se.
Acknowledgements
This research was supported by a Postdoctoral Fellowship from the European Union’s Horizon 2020 research and innovation programme, under the Marie Sklodowska-Curie grant agreement No 658341 to FMB; an ERC Advanced Grant (GAP: 670428 - BRAIN2MIND_NEUROCOMP) and MRC Programme Grant (MR/R023883/1) to MALR and a Beacon Anne McLaren Research Fellowship (University of Nottingham) to JJ. We also acknowledge the Medical Research intramural funding (MC_UU_00005/18).
Footnotes
Competing interests: The authors declare that no competing interests exist.
References
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. Journal of memory and language. 2008;59(4):390–412. [Google Scholar]
- Baldassano C, Chen J, Zadbood A, Pillow JW, Hasson U, Norman KA. Discovering Event Structure in Continuous Narrative Perception and Memory. Neuron. 2017;95(3):709–721.:e705. doi: 10.1016/j.neuron.2017.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008;28(34):8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Cheke LG, Green DAE, FitzGerald THMB, Simons JS. Specifying a Causal Role for Angular Gyrus in Autobiographical Memory. Journal of Neuroscience. 2018;38(49):10438–10443. doi: 10.1523/JNEUROSCI.1239-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Richter FR, Yazar Y, Simons JS. Multimodal Feature Integration in the Angular Gyrus during Episodic and Semantic Retrieval. J Neurosci. 2016;36(20):5462–5471. doi: 10.1523/JNEUROSCI.4310-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzi FM, Humphreys GF, Hoffman P, Lambon Ralph MA. Revealing the neural networks that extract conceptual gestalts from continuously evolving or changing semantic contexts. Neuroimage. 2020:116802. doi: 10.1016/j.neuroimage.2020.116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Baldassarre A, Sestieri C, Spadone S, Romani GL, Corbetta M. Task and Regions Specific Top-Down Modulation of Alpha Rhythms in Parietal Cortex. Cerebral Cortex. 2017;27(10):4815–4822. doi: 10.1093/cercor/bhw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, et al. The human inferior parietal lobule in stereotaxic space. Brain Structure & Function. 2008;212(6):481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Rosenbaum RS, Solcz S, Levine B, Moscovitch M. Mental Space Travel: Damage to Posterior Parietal Cortex Prevents Egocentric Navigation and Reexperiencing of Remote Spatial Memories. Journal of Experimental Psychology-Learning Memory and Cognition. 2010;36(3):619–634. doi: 10.1037/a0019181. [DOI] [PubMed] [Google Scholar]
- Clouter A, Shapiro KL, Hanslmayr S. Theta Phase Synchronization Is the Glue that Binds Human Associative Memory. Curr Biol. 2017;27(20):3143–3148.:e3146. doi: 10.1016/j.cub.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Cooper E, Greve A, Henson RN. Investigating Fast Mapping Task Components: No Evidence for the Role of Semantic Referent nor Semantic Inference in Healthy Adults. Front Psychol. 2019;10:394. doi: 10.3389/fpsyg.2019.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, et al. Automatic and Controlled Semantic Retrieval: TMS Reveals Distinct Contributions of Posterior Middle Temporal Gyrus and Angular Gyrus. Journal of Neuroscience. 2015;35(46):15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1-2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR. Phonological decisions require both the left and right supramarginal gyri. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(38):16494–16499. doi: 10.1073/pnas.1008121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Golombek T, Obleser J. Repetitive transcranial magnetic stimulation over left angular gyrus modulates the predictability gain in degraded speech comprehension. Cortex. 2015;68:100–110. doi: 10.1016/j.cortex.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Hermiller MS, VanHaerents S, Raij T, Voss JL. Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity. Hippocampus. 2019;29(7):595–609. doi: 10.1002/hipo.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes NP, Meteyard L. Subjective Discomfort of TMS Predicts Reaction Times Differences in Published Studies. Front Psychol. 2018;9:1989. doi: 10.3389/fpsyg.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Hoffman P, Visser M, Binney RJ, Lambon Ralph MA. Establishing task- and modality-dependent dissociations between the semantic and default mode networks. Proc Natl Acad Sci U S A. 2015;112(25):7857–7862. doi: 10.1073/pnas.1422760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Fusion and Fission of Cognitive Functions in the Human Parietal Cortex. Cereb Cortex. 2015;25(10):3547–3560. doi: 10.1093/cercor/bhu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Mapping Domain-Selective and Counterpointed Domain-General Higher Cognitive Functions in theLateral Parietal Cortex: Evidence from fMRI Comparisons of Difficulty-Varying Semantic Versus Visuo-SpatialTasks, and Functional Connectivity Analyses. Cereb Cortex. 2017;27(8):4199–4212. doi: 10.1093/cercor/bhx107. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. Neuroimage. 2007;36(3):924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys H. The Theory of Probability. Oxford Univ. Press; 1961. [Google Scholar]
- Jonker TR, Dimsdale-Zucker H, Ritchey M, Clarke A, Ranganath C. Neural reactivation in parietal cortex enhances memory for episodically linked information. Proc Natl Acad Sci U S A. 2018;115(43):11084–11089. doi: 10.1073/pnas.1800006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Bungert A, Bowtell R, Jackson SR. Vertex Stimulation as a Control Site for Transcranial Magnetic Stimulation: A Concurrent TMS/fMRI Study. Brain Stimul. 2016;9(1):58–64. doi: 10.1016/j.brs.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Thakral PP, Rugg MD. Transcranial magnetic stimulation of the left angular gyrus during encoding does not impair associative memory performance. Cognitive Neuroscience. 2018;9(3-4):127–138. doi: 10.1080/17588928.2018.1484723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci. 2011;31(8):2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteyard L, Holmes NP. TMS SMART - Scalp mapping of annoyance ratings and twitches caused by Transcranial Magnetic Stimulation. J Neurosci Methods. 2018;299:34–44. doi: 10.1016/j.jneumeth.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Piguet O, Irish M. Rethinking the Role of the Angular Gyrus in Remembering the Past and Imagining the Future: The Contextual Integration Model. Neuroscientist. 2018;24(4):342–352. doi: 10.1177/1073858417735514. [DOI] [PubMed] [Google Scholar]
- Riddle J, Scimeca JM, Cellier D, Dhanani S, D’Esposito M. Causal Evidence for a Role of Theta and Alpha Oscillations in the Control of Working Memory. Current Biology. 2020;30(9):1748. doi: 10.1016/j.cub.2020.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Haaf JM. Power, dominance, and constraint: A note on the appeal of different design traditions. Advances in Methods and Practices in Psychological Science. 2018;1(1):19–26. [Google Scholar]
- Rugg MD, King DR. Ventral lateral parietal cortex and episodic memory retrieval. Cortex. 2018;107:238–250. doi: 10.1016/j.cortex.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Umilta C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neuroscience and Biobehavioral Reviews. 2011;35(3):516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11(3):277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J Neurophysiol. 2008;99(5):2725–2730. doi: 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans. Neuropsychologia. 2008;46(4):1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation Between Memory Accuracy and Memory Confidence Following Bilateral Parietal Lesions. Cerebral Cortex. 2010;20(2):479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska MW, James A, Devlin JT. Inferior parietal lobule contributions to visual word recognition. J Cogn Neurosci. 2015;27(3):593–604. doi: 10.1162/jocn_a_00721. [DOI] [PubMed] [Google Scholar]
- Speer NK, Zacks JA. Temporal changes as event boundaries: Processing and memory consequences of narrative time shifts. Journal of Memory and Language. 2005;5(1):125–140. [Google Scholar]
- Steinmetz H, Furst G, Meyer BU. Craniocerebral topography within the international 10-20 system. Electroencephalogr Clin Neurophysiol. 1989;72(6):499–506. doi: 10.1016/0013-4694(89)90227-7. [DOI] [PubMed] [Google Scholar]
- Swallow KM, Zacks JM, Abrams RA. Event Boundaries in Perception Affect Memory Encoding and Updating. Journal of Experimental Psychology-General. 2009;138(2):236–257. doi: 10.1037/a0015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Nee DE, D’Esposito M. Hippocampal-targeted Theta-burst Stimulation Enhances Associative Memory Formation. Journal of Cognitive Neuroscience. 2018;30(10):1452–1472. doi: 10.1162/jocn_a_01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, Kalinowski SE, Schacter DL. Modulation of hippocampal brain networks produces changes in episodic simulation and divergent thinking. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(23):12729–12740. doi: 10.1073/pnas.2003535117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, Schacter DL. A Role for the Left Angular Gyrus in Episodic Simulation and Memory. Journal of Neuroscience. 2017;37(34):8142–8149. doi: 10.1523/JNEUROSCI.1319-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS Causes Local Entrainment of Natural Oscillatory Signatures. Current Biology. 2011;21(14):1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibon R, Fuhrmann D, Levy DA, Simons JS, Henson RN. Multimodal Integration and Vividness in the Angular Gyrus During Episodic Encoding and Retrieval. Journal of Neuroscience. 2019;39(22):4365–4374. doi: 10.1523/JNEUROSCI.2102-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabre A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neuroscience and Biobehavioral Reviews. 2017;83:381–404. doi: 10.1016/j.neubiorev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- van der Linden M, Berkers R, Morris RGM, Fernandez G. Angular Gyrus Involvement at Encoding and Retrieval Is Associated with Durable But Less Specific Memories. J Neurosci. 2017;37(39):9474–9485. doi: 10.1523/JNEUROSCI.3603-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46(7):1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. The neural correlates of recollection: transient versus sustained FMRI effects. J Neurosci. 2012;32(45):15679–15687. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Lambon Ralph MA. Differential Contributions of Bilateral Ventral Anterior Temporal Lobe and Left Anterior Superior Temporal Gyrus to Semantic Processes. Journal of Cognitive Neuroscience. 2011;23(10):3121–3131. doi: 10.1162/jocn_a_00007. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wagner IC, van Buren M, Kroes MCW, Gutteling TP, van der Linden M, Morris RG, et al. Schematic memory components converge within angular gyrus during retrieval. Elife. 2015;4 doi: 10.7554/eLife.09668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Clouter A, Chen QY, Shapiro KL, Hanslmayr S. Single-Trial Phase Entrainment of Theta Oscillations in Sensory Regions Predicts Human Associative Memory Performance. Journal of Neuroscience. 2018;38(28):6299–6309. doi: 10.1523/JNEUROSCI.0349-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar Y, Bergstrom ZM, Simons JS. Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One. 2014;9(10):e110414. doi: 10.1371/journal.pone.0110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar Y, Bergstrom ZM, Simons JS. Reduced multimodal integration of memory features following continuous theta burst stimulation of angular gyrus. Brain Stimul. 2017;10(3):624–629. doi: 10.1016/j.brs.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]