Abstract
Xenopus tadpoles have emerged as a powerful in vivo model system to study mucociliary epithelia such as those found in the human airways. The tadpole skin has mucin-secreting cells, motile multi-ciliated cells, ionocytes (control local ionic homeostasis) and basal stem cells. This cellular architecture is very similar to the large airways of the human lungs and represents an easily accessible and experimentally tractable model system to explore the molecular details of mucociliary epithelia. Each of the cell types in the tadpole skin has a human equivalent and a conserved network of genes and signalling pathways for their differentiation has been discovered. Great insight into the function of each of the cell types has been achieved using the Xenopus model and this has enhanced our understanding of airway disease. This simple model has already had a profound impact on the field but, as molecular technologies (e.g. gene editing and live imaging) continue to develop apace, its use for understanding individual cell types and their interactions will likely increase. For example, its small size and genetic tractability make it an ideal model for live imaging of a mucociliary surface especially during environmental challenges such as infection. Further potential exists for the mimicking of human genetic mutations that directly cause airway disease and for the pre-screening of drugs against novel therapeutic targets.
Introduction
As we breathe, we inhale particles, toxins and pathogens with the potential to compromise health. The mucociliary epithelium in the airways of the lung provides a robust first line of defence. Mucus provides a physical barrier to entry, whilst beating cilia transport the mucus (and trapped entities) away from the lungs in a process called mucociliary clearance. Breakdown of mucociliary clearance is a feature of airway diseases such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and asthma[1]. A comprehensive understanding of mucociliary biology and clearance mechanisms is needed to develop more effective therapeutics for these diseases. Mammalian model organisms are commonly used to study the lung mucociliary epithelium because of their anatomical similarity to humans. However, in vivo studies are challenging because the airways are not readily accessible and require invasive procedures that remove them from their native environment. Here, I introduce a complementary, easily accessible, non-mammalian model system to study mucociliary biology − the skin of the Xenopus tadpole[2]. Rather than an exhaustive account of the literature, this minireview aims to equip the reader with an overall understanding of the similarities between the tadpole skin and the human airways. The common cell types are discussed, with emphasis placed on the key findings in the tadpole that have proven to be directly relevant to human airways and disease. The review concludes with a look to the future, highlighting where the tadpole model can be of most use in the quest to understand mucociliary biology and for the treatment of airway disease.
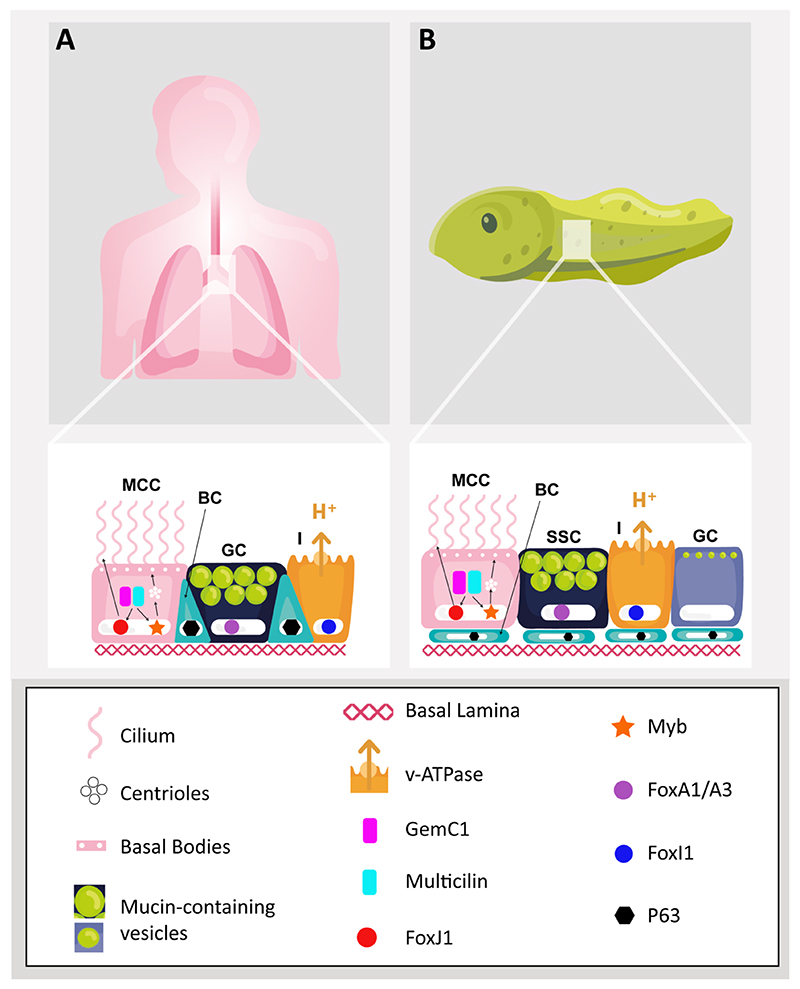
Xenopus embryos have been used for many years to research developmental processes because they are produced externally in large numbers, rapidly develop and can be genetically manipulated using simple tools[3]. Two species of Xenopus, X. laevis and X. tropicalis, are used in biomedical research, often interchangeably, since their developmental features and pathways are conserved. By the time the embryo has become a free-swimming tadpole, its skin is a mucociliary epithelium with motile multi-ciliated cells (MCCs), mucin secreting cells and ionocytes, the latter regulating the local ionic environment[4]. These cell types all reside above a population of basal stem cells [5]. A basal lamina containing fibronectin and laminin supports the epithelium [6,7]. This architecture is remarkably similar to the epithelium in the large airways of the human lung, where mucin secreting goblet cells, MCCs and ionocytes form a pseudostratified epithelium with basally residing stem cells[8,9]. Moreover, these cell types are highly similar to their human airway counterparts in development, function and gene expression. A comparison of the large airways and the tadpole skin epithelia is given in Figure 1.
Figure 1. Comparison of human large airway epithelium and tadpole skin.
(A) A mucociliary epithelium extends from the human nasal cavity to the trachea and bronchioles. Details of the tracheobronchial epithelial cell types are shown. It is a pseudostratified epithelium with basal stem cells (BC; turquoise) residing slightly below and in between multiciliated cells (MCC; pink), mucin-secreting goblet cells (GC; dark blue) and ionocytes (I; orange). A fibronectin-rich basal lamina is also shown (B) The tadpole skin is a mucociliary epithelium with multiciliated cells (MCC; pink), small secretory cells (SSC; dark blue), goblet cells (GC; purple) and ionocytes (I; orange), residing above basal stem cells (BC; turquoise) and a fibronectin-rich basal lamina. Key features that are conserved in the human epithelium and the tadpole skin are highlighted. For the ciliated cells, the conserved gene regulatory network is shown and arrows indicate how GemC1/Multicilin drive centriole biogenesis and basal body formation through Myb, whilst ciliogenesis is driven through FoxJ1.
The Xenopus model has some unique advantages over other animal and human cell culture models to study mucociliary epithelia. As it is a developing system, the embryonic skin can be used to investigate the dynamic interaction and movement of the different cell types and also how they are initially patterned. The tadpole develops fast − several hours - compared to weeks for human tracheobronchial epithelial cultures. Furthermore, gaining access to the skin mucociliary epithelium is very simple compared to the cumbersome air-liquid interface that needs to be generated for human cultures. In addition, using the embryonic form of Xenopus instead of mature animal models (e.g. mice) has an animal welfare benefit. Xenopus produce thousands of embryos from a single fertilization, whereas many adult mammals must be sacrificed for similar experiments. Easy access to the skin of Xenopus tadpoles provides unique imaging opportunities, whilst rearing the embryos is straightforward and inexpensive, requiring only simple salt solutions.
Motile, multi-ciliated cells (MCCs)
The ciliated cells are the most striking feature of the tadpole skin (Fig. 2A), as noted over half a century ago in seminal electron microscopy studies[10,11]. Many cilia project out from each MCC and beat co-ordinately to generate directional fluid flow, just like in the human airways. Although a large number of studies have been conducted into the structure and function of MCCs, here the focus is on a few selected studies that best demonstrate the similarities between the tadpole and human airway MCCs, but also show how the tadpole model has provided new insight into cilia biology and human disease.
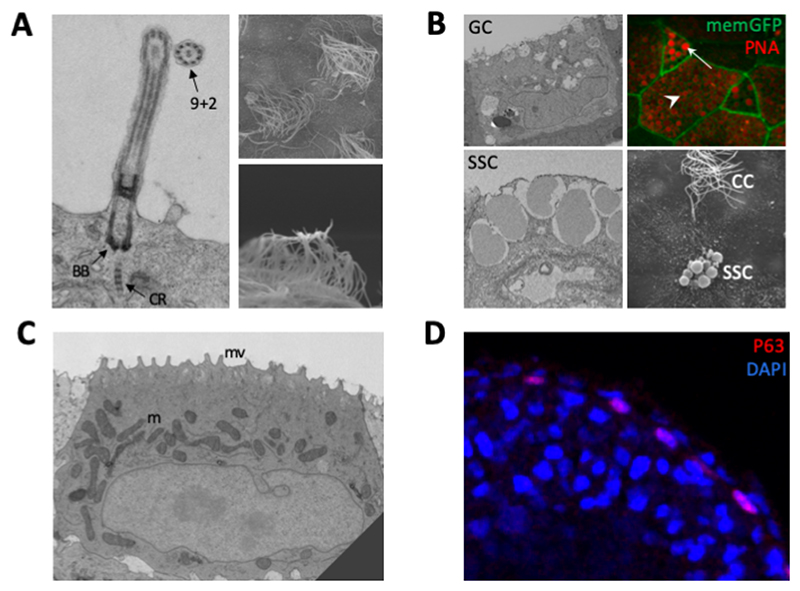
Figure 2. The tadpole skin cell types.
(A) Multi-ciliated cells. Transmission electron micrograph (left) shows a single cilium with key features of ciliary rootlet (CR), Basal Body (BB) and ‘9+2’ arrangement of microtubules highlighted. Scanning electron micrographs (right) show multiple cilia. (B) Secretory cells. Goblet cells (GC) and small secretory cells (SSC) shown in transmission electron micrographs of skin sections. Apical vesicles containing mucins are evident. Immunofluorescence shows peanut agglutinin (PNA) binding to mucins in SSCs (arrow) and goblet cells (arrowhead). Membrane-GFP marks the plasma membrane. Scanning electron micrograph of tadpole skin shows an SSC near to a ciliated cell (CC). (C) Transmission electron micrograph of an ionocyte, which is mitochondria-rich (m) and has apical microvilli (mv). (D) Immunofluorescence of a section of tadpole skin showing P63 (a marker of stem cells) staining. DAPI marks the nuclei. P63 positive basal cells are found in the inner layer of the skin. Images in (B) and (C) reproduced from Dubaissi et al (2011), Fig. 3[4]
MCC differentiation: An evolutionarily conserved gene regulatory network
As ciliated cells differentiate, cilia are generated in a process called multi-ciliogenesis. The transcription factor, FOXJ1, was one of the earliest identified markers of MCCs in the human airways[12] and a knockout mouse model showed that this transcription factor is essential for ciliogenesis[13]. FoxJ1 was subsequently identified in MCCs in the Xenopus tadpole skin and knockdown of FOXJ1 function mimicked the defects observed in mammalian ciliogenesis[14]. This study also identified the genetic targets of FoxJ1 for the first time, showing that the model can be used, not just to confirm, but to extend, our understanding of this cell type. The targets of FoxJ1 were mostly genes encoding the protein machinery required for ciliogenesis and cilia motility, but the study also found that FoxJ1 was insufficient to generate multiple cilia[14]. Further research led to the identification, in Xenopus, of Multicilin, which is required for centriole amplification to generate the multiple basal bodies that each act as a template for formation of a single cilium[15]. The transcription factor, Myb, was found to be downstream of Multicilin in both Xenopus and mice and is integral to the process of centriole amplification[16]. A protein related to Multicilin, GemC1, was later identified as the key factor at the top of the gene regulatory network simultaneously controlling the pathways of centriole biogenesis, via Multicilin, and ciliogenesis, via FoxJ1[17]. Many of these factors, originally identified in Xenopus, were subsequently found to be defective in human disease. For example, mutations in MCIDAS (the gene encoding Multicilin) and cyclin O (CCNO − needed for centriole amplification), a downstream target of Myb, led to a reduced number of cilia and defective mucociliary clearance[18,19]. Indeed, in the case of CCNO, knockdown of the gene in the tadpole skin showed a reduction of cilia in the MCCs, supporting the clinical phenotype as well as demonstrating the translational potential of the model.
The polarization of cilia
Polarity is needed both within an individual MCC (rotational polarity), so that the cilia all move in the same direction, and across the plane of an epithelium (tissue-level polarity), to ensure fluid flow. Research using the tadpole model has led the field in understanding how planar cell polarity (PCP) proteins direct this process. For example, the core PCP protein, Dishevelled (Dvl), localizes to one side of the basal body, which has downstream effects on the organisation of actin and microtubules [20,21]. This aligns all the basal bodies in the cell in one direction. When Dvl was inhibited, the basal bodies became randomly orientated[20]. Tissue-level polarity was also shown to involve core PCP proteins, first in Xenopus, and then in mouse airways[22,23]. The core PCP proteins, Vangl2 and Frizzled, appear to direct this tissue-level polarity, given that depletion of these proteins in cells adjacent to MCCs affects cilia orientation within MCCs [22]. In the study of MCCs in mouse trachea, asymmetric distribution (i.e. to opposite membranes) of membrane-bound PCP proteins directs the polarity of cilia across the tissue through specific microtubule interactions extending from one pole of the cell and connecting with basal bodies[23]. The importance of PCP is exemplified in some chronic human inflammatory airway diseases, where core PCP proteins are mislocalized, overproduced or absent [24–26]. Moreover, some genetic diseases cause defects in cilia polarity that impair mucociliary clearance. For example, the gene, growth-arrest specific protein-2-like-2 (GAS2L2), has been found to be mutated in some individuals and knockdown of this gene in Xenopus tadpole skin led to polarity defects in ciliated cells [27].
Ciliary motility
Axonemal dynein motor proteins form outer and inner dynein arms and generate the force to slide adjacent microtubules with respect to each other, effecting the movement of cilia[28]. Indeed, motile ciliopathies such as primary ciliary dyskinesias (PCD), which cause defective mucociliary clearance in the human airways, are frequently caused by mutations in genes that encode axonemal dyneins[29]. Genes such as DNAH5 (commonly mutated in PCD) were identified as downstream targets of FoxJ1 and associated transcription factors in Xenopus MCCs[14,30]. A recent study in Xenopus described a liquid-like organelle (dynein axonemal particles or DynAPs) containing axonemal dyneins, dynein axonemal assembly factors (DNAAFs) and chaperone proteins[31]. Significantly, depletion of individual DNAAFs that have been implicated in PCD[32], caused disruption of the whole DynAP complex and dynein arms were not localized to the cilia, resulting in immotile cilia[31]. The authors hypothesised that the DynAPs exist to facilitate fast assembly and movement of dyneins to ciliary axonemes. Meanwhile, human mutations in another DNAAF, ZMYND10, causes PCD due to loss of axonemal dynein arms[33]. The identification of DynAPs provides a plausible explanation for this phenomenon. As for many of the studies highlighted, the ZMYND10 study used the tadpole model to further understand the clinical phenotype − immotile cilia - of mutations in this protein.
Secretory cells
The tadpole skin has two types of secretory cells that structurally resemble the human airway mucin-secreting goblet cells (Fig. 2B). Indeed, the first secretory cells to be identified in the tadpole skin were named goblet cells by analogy to the human cell type[34]. However, the other secretory cell type − the small secretory cells (SSCs) − actually bear more resemblance to human goblet cells, with large, apically-localized electron-dense vesicles[35]. Irrespective of their names, both cell types secrete the same gel-forming mucin, MucXS, with properties reminiscent of the human airway mucins, MUC5AC and MUC5B [36,37]. These large, polymeric heavily O-glycosylated proteins underpin the characteristic viscoelastic properties of mucus[38]. Mucin polymers form through disulphide bonding between specific domains (VWF and cysteine knot domains) and have central (mucin) domains rich in threonine and serine residues that become O-glycosylated[39]. In both the human airways and the tadpole skin, mucins are condensed inside vesicles until secretion, when they decondense and rapidly expand in size to provide the structural framework of the protective mucus barrier [40]. Just as the airway mucins provide protection against infection in the airways[41], knock down of MucXS in the tadpole skin led to a loss of the mucus barrier and increased susceptibility to infection[36].
It remains unclear why the tadpole skin requires two mucin-secreting cell types, but in fact airway mucins can also be secreted by other cell types in addition to goblet cells[42]. Very little else is known about the tadpole skin goblet cells, other than that they secrete a lectin called Intelectin-2[43]. Intelectin-2 is secreted by human intestinal goblet cells and has also been identified in the airways[44]. In the tadpole, Intelectin-2 is believed to bind and agglutinate bacteria[43].
The SSCs appear to be most similar to the human goblet cells and this extends to their key markers. The SSCs express the forkhead box A transcription factor, FoxA1, and knockdown of FoxA1 led to their depletion[35] which caused an increased susceptibility to infection, similar to when MucXS was depleted[36]. The related transcription factor, FOXA3, promotes gene expression in human and mouse airway goblet cells[45]. FoxA1 is also an important transcription factor driving the gene expression of Muc2 in mouse intestinal goblet cells[46].
As demonstrated for the ciliated cells, as more becomes known about the tadpole skin secretory cells, this has the potential to enhance our understanding of human airway goblet cells. This includes aspects such as the gene regulatory network that stimulates their differentiation, mucin synthesis and secretion and the factors involved in regulating these processes. Ultimately, this can facilitate the development of new therapeutics since mucin hypersecretion and goblet cell hyperplasia are common features of airway diseases such asthma, COPD and CF[47].
Ionocytes
Ionocytes were originally identified in the developing Xenopus embryonic skin, appearing shortly after the ciliated cells[4,48]. These cells are rich in mitochondria and often have apical microvilli (Fig. 2C). The genes identified as markers of this cell type include ion channels, proton pumps and enzymes such as carbonic anhydrases[4]. Two subtypes of ionocytes have been identified in the tadpole skin[48]. The v-ATPase proton pump is expressed in both, but shows different subcellular localization[4]. In some cells, v-ATPase localizes apically, whilst in others it localizes basolaterally. Apical localization of v-ATPase corresponds to expression of the anion exchanger, AE1, whilst basolateral expression of v-ATPase corresponds with the expression of the chloride-bicarbonate exchanger, Pendrin, and the transmembrane enzyme carbonic anhydrase 12[4,48]. It is thought that the Xenopus tadpole, which is found in freshwater environments, requires v-ATPase to pump out protons, in order to take in sodium ions (against the concentration gradient), by generating a local electrochemical gradient [49]. The transcription factor Foxi1 was found to be an early marker of the tadpole ionocytes[48]. Knockdown of Foxi1 led to loss of ionocytes, whilst its overexpression led to increased numbers of ionocytes[4]. Depletion of ionocytes also had a corresponding non-cell autonomous effect on neighbouring MCCs, which still developed, but had fewer cilia and numerous basal bodies were found within the cytoplasm rather than docked to the apical membrane[4].
It is known that regulation of ionic balance is needed in the human airways in order to maintain hydration of the mucus. In CF, mutations in CFTR leads to defective chloride/ bicarbonate transport and airway surface dehydration[50]. This results in thick and sticky mucus that cannot be cleared effectively by cilia[51]. Until recently, CFTR was believed to be predominantly expressed in ciliated cells, with other ion channels found in secretory cells[52]. However, two independent single cell RNAseq studies of mouse and human airways revealed the additional presence of pulmonary ionocytes, named after those identified in the tadpole skin[8,9]. Like the tadpole ionocytes, the pulmonary ionocytes express genes for v-ATPase and the transcription factor, Foxi1. Knockout of Foxi1 led to loss of pulmonary ionocytes, leading to increased viscosity of airway mucus (like in CF) [9]. Intriguingly, more than 50% of the CFTR transcripts in the airway cells were found clustered with the pulmonary ionocytes, indicating that this cell type could be a crucial target for treatment of CF airway disease[9]. Whilst it is unclear if the tadpole skin ionocytes express CFTR and no subtypes of ionocytes have yet been identified in the human airways, it seems certain that this cell type will receive more attention in the coming years. Greater understanding of the tadpole ionocytes could provide insight of importance to human airway physiology and ultimately to the treatment of disease.
Basal stem cells
The tadpole skin is a bilayered epithelium (Fig. 1B). Precursors of MCCs, ionocytes and SSCs originally reside in the inner layer and then intercalate into the outer layer to fully differentiate alongside the goblet cells[4,35,53]. However, the inner layer of the epithelium remains and contains undifferentiated basal cells. These cells strongly express the transcription factor tumour-related protein 63 (P63; Fig. 2D), which is also expressed in human airway basal stem cells[5,54]. P63 is essential for the maintenance and survival of airway basal cells[55]. Knockout of P63 in mouse airways resulted in a highly ciliated airway with loss of basal cells[55], whilst P63 overexpression in the tadpole led to a depletion in the number of MCCs, SSCs and ionocytes[56]. Injury models in the mouse airways have shown that the basal cells can become both secretory cells and ciliated cells, and they can also self-renew to maintain the basal cell population[57]. The pulmonary ionocytes were also shown to derive from the basal stem cells[9]. Interestingly, isolated human airway basal stem cells can differentiate into a mucociliary epithelium at an air-liquid interface, demonstrating that the basal cells are bona fide stem cells[58]. It is unclear if the tadpole basal cells are also capable of converting into the other cell types, but a recent study of isolated basal cells (which are likely the P63+ stem cells) shows that they are capable of regenerating a mucociliary epithelium [59]. A similar transcriptional profile between airway basal cells and the tadpole skin basal cells has been described, indicating that they have a conserved function[56]. Airway basal cells are altered in disease and are a target for gene therapy. For example, in COPD, basal cells are depleted[60], whilst a proof-of-principle study showed correction of CFTR mutations in basal cells isolated from CF patients[61]. Understanding the tadpole basal cells in greater detail could facilitate further development of therapeutics to generate lasting changes in airway basal stem cells to treat disease.
Conserved signalling pathways in the development and repair of mucociliary epithelia: Notch, Wnt and BMP signalling
The key signalling pathways required for the differentiation of the tadpole skin and the human airway mucociliary epithelium are also conserved[62]. Notch signalling controls the balance of secretory and ciliated cell fate in the development of the airways and the tadpole skin[53,63,64]. In the tadpole skin, SSCs increase in response to Notch activation[64], whilst ciliated cells and ionocytes decrease[48,53]. Moreover, Notch activation in the developing mouse airways causes an increase in secretory cells at the expense of ciliated cells[65], whilst pulmonary ionocytes have also been shown to express downstream targets of Notch[8]. It is not clear whether Notch signalling is required for the initial formation of basal stem cells in the developing airways, but in the tadpole skin, Notch activation increases the numbers of P63-positive basal cells[6]. However, after development, adult airway basal stem cells do require Notch signalling to differentiate into secretory or ciliated cells in the steady-state and upon injury[66].
A side-by-side comparison of the developing tadpole skin and regenerating human airway epithelial cells showed similar responses to BMP activation and inhibition[67]. BMP inhibition is required for the specification of ciliated cells, ionocytes and SSCs in the tadpole skin and for differentiation of goblet cells and ciliated cells in human airway epithelia. It is hypothesised that, in Xenopus, attenuation of BMP signalling is needed prior to downstream Notch signalling to stimulate differentiation of the different cell types. Addition of exogenous BMP4 ligand to Xenopus embryos led to a reduction in ciliated cells, ionocytes and SSCs, but also to P63-positive basal cells[67]. In mouse tracheal basal cells, addition of BMP4 inhibited the proliferation and differentiation of P63-positive cells[68]. Meanwhile, in vivo injury to the mouse trachea led to upregulation of BMP antagonists indicating that attenuation of BMP signalling is required for proliferation and subsequent regeneration of the tracheal epithelium[68]. Defects in BMP signalling have been described in mild asthma and in COPD induced by cigarette smoking[69,70].
Canonical Wnt signalling, via activation of b-catenin-induced gene expression, is also important in the development of both the airways and tadpole skin. Wnt-β catenin has been implicated in expression of FoxJ1 and ciliogenesis in ciliated cells of the tadpole skin[71]. The same study also showed that Wnt signalling regulates expression of FoxA1 in SSCs and inhibition leads to loss of secretory vesicles. Meanwhile, activating β-catenin in mouse airways led to goblet cell hyperplasia with enhanced expression of the mucin, Muc5ac[72]. Meanwhile, P63 is directly regulated by β-catenin at its promoter in both mammalian cells and Xenopus[73,74]. Indeed, Wnt/ β-catenin has recently been shown to upregulate P63 in basal cells of the tadpole and isolated human airway epithelial cells, preventing differentiation to other cell types[56]. b-catenin knockdown led to an increase in ciliated cells at the expense of basal cells, whilst chronic Wnt activation caused basal cell hyperplasia[56]. As for the other signalling pathways, several airway diseases have been associated with defective Wnt signalling. For example, asthmatic airway remodelling has been shown to stem from aberrant activation of Wnt/ β-catenin signalling[75], which may also drive cigarette-induced COPD[76]. Since the major signalling pathways in the tadpole skin and the airways are conserved, it may be possible to test out drug targets of these pathways to treat airway disease using the tadpole model before moving into mammalian models and ultimately for the treatment of human patients.
Conclusions and Future outlook
The research findings indicate that the tadpole skin is similar in composition and function to the mucociliary epithelium of the large airways in humans. Its small size, genetic tractability and accessibility makes it a powerful in vivo model to understand this epithelium in health and disease. This has been most clearly demonstrated for the ciliated cells, where many studies have used Xenopus to understand the function of genes or signalling pathways; findings which are mostly conserved in human airway ciliated cells. The other cell types in the tadpole skin also have equivalents in the human airways, but these are at an earlier stage of research. As we look to the future, understanding more about the role of ionocytes in the tadpole skin will provide great insight into the function of these cells in the human airways, including their role in diseases such as CF. Likewise, the SSCs appear to be highly similar to human airway goblet cells so a thorough understanding of their function is warranted. As single cell technologies continue to develop rapidly, the specific functions of genes in each cell type will need to be understood. The ability to quickly identify, knockdown and overexpress genes in the Xenopus model make it an ideal candidate for this task.
New genome-editing technologies such as CRISPR-Cas9 have proven to be successful in Xenopus[77] and open up a pathway towards mimicking human genetic mutations in airway diseases, as well as endogenous tagging of target genes with fluorophores. The fact that the tadpole is small and its skin is accessible means that live in vivo imaging is feasible, unlike for mammalian airways. Fluorescently labelling mucins and cilia would enable study of processes such as mucin packaging into secretory granules and their subsequent secretion, and cilia interactions with mucus during clearance. The ability to manipulate the local environment of the tadpole together with the capability to generate large amounts of material lends this system to rapid high-throughput drug screening programmes to initially identify novel drugs affecting mucin secretion or cilia beating. In addition, the introduction of pathogens to the media can enable studies of host-pathogen interactions at a mucociliary surface. In fact, the tadpole model has already been employed to screen muco-active agents and study the impact of particulate matter from diesel engines[78,79]. Other areas where the tadpole model could be used in future include studies of drug delivery across mucus barriers, which hinders the bioavailability of drugs and testing of gene therapy approaches following mimicking of human genetic mutations[38].
I envisage that this model will be increasingly used alongside and, in some cases, as a replacement for, other models of mucociliary epithelia. It is ideally suited as a tool to understand the function of particular genes, to mimic genetic mutations and for the pre-screening of drugs. It could be used for hypothesis generation and testing, with key findings validated in mammalian models. Furthermore, as the tadpole skin is an accessible larval model it does not require some of the invasive techniques required for mammalian in vivo studies of the airways, making it an ideal candidate for the reduction, refinement and replacement of animals in research.
Perspectives
The Xenopus tadpole has a mucociliary skin surface with cell types similar to the human airways; including motile multiciliated cells, mucin-secreting cells, ionocytes and basal stem cells. The Xenopus model is ideally suited to researching mucociliary epithelia and their role in airway disease.
Each cell type has conserved gene regulatory networks and functions, as well as signalling pathways that control their differentiation. The Xenopus model has been used to further our understanding of these aspects as well as to understand the clinical phenotypes of airway disease, particularly for ciliated cell dysfunction.
The unique combination of conserved cell types, genetic tractability and ease of accessibility makes the tadpole skin model ideally suited for live imaging of mucociliary epithelia. By employing the latest genome editing technologies to mimic disease and label proteins, in combination with environmental challenge, there is great potential to employ the tadpole model in future, perhaps even in place of some more established mammalian models.
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- COPD
chronic obstructive pulmonary disease
- MCC
multi-ciliated cell
- PCP
planar cell polarity
- Dvl
Dishevelled
- PCD
primary ciliary dyskinesia
- DNAAF
dynein axonemal assembly factor
- DynAP
dynein axonemal particle
- SSC
small secretory cell
- BMP
bone morphogenetic protein
Acknowledgements
I thank David Thornton, Richard Grencis and Ian Roberts for critical review of the manuscript, general advice and support. I thank Emily Whitaker-Bethel for graphical illustrations in Figure 1.
Funding
E.D. is supported by a project grant (NC/S001034/1) from the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs).
Footnotes
Author Contributions
E.D. conceived, wrote and edited the manuscript.
Competing Interests
I declare that I have no competing interests associated with the manuscript.
References
- [1].De Rose V, Molloy K, Gohy S, Pilette C, Greene CM. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediators Inflamm. 2018 doi: 10.1155/2018/1309746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Walentek P, Quigley IK. What we can learn from a tadpole about ciliopathies and airway diseases: Using systems biology in Xenopus to study cilia and mucociliary epithelia. Genesis. 2017;55:e23001. doi: 10.1002/dvg.23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ishibashi S, Saldanha F, Amaya E. Basic Science Methods for Clinical Researchers. Academic Press; 2017. Xenopus as a Model Organism for Biomedical Research; pp. 263–290. [Google Scholar]
- [4].Dubaissi E, Papalopulu N. Embryonic frog epidermis: a model for the study of cell-cell interactions in the development of mucociliary disease. Disease Models Mechanisms. 2011;4:179–192. doi: 10.1242/dmm.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lu P, Barad M, Vize PD. Xenopus p63 expression in early ectoderm and neurectoderm. Mech Dev. 2001;102:275–278. doi: 10.1016/s0925-4773(01)00315-x. [DOI] [PubMed] [Google Scholar]
- [6].Sirour C, Hidalgo M, Bello V, Buisson N, Darribère T, Moreau N. Dystroglycan is involved in skin morphogenesis downstream of the Notch signaling pathway. Mol Biol Cell. 2011;22:2957–2969. doi: 10.1091/mbc.E11-01-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fey J, Hausen P. Appearance and distribution of laminin during development of Xenopus laevis. Differentiation. 1990;42:144–152. doi: 10.1111/j.1432-0436.1990.tb00755.x. [DOI] [PubMed] [Google Scholar]
- [8].Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].S F, Billett RPG. Fine structural changes in the differentiating epidermis of Xenopus laevis embryos. J Anat. 1971;108:465. [PMC free article] [PubMed] [Google Scholar]
- [11].Steinman RM. An electron microscopic study of ciliogenesis in developing epidermis and trachea in the embryo of Xenopus laevis. Developmental Dynamics. 1968;122:19–55. doi: 10.1002/aja.1001220103. [DOI] [PubMed] [Google Scholar]
- [12].Pelletier GJ, Brody SL, Liapis H, White RA, Hackett BP. A human forkhead/winged-helix transcription factor expressed in developing pulmonary and renal epithelium. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1998;274(3):L351–359. doi: 10.1152/ajplung.1998.274.3.L351. [DOI] [PubMed] [Google Scholar]
- [13].Brody SL, Yan XH, Wuerffel MK, Song S-K, Shapiro SD. Ciliogenesis and Left-Right Axis Defects in Forkhead Factor HFH-4-Null Mice. American Journal of Respiratory Cell and Molecular Biology. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- [14].Stubbs JL, Oishi I, Izpisúa Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol. 2012;14:140–147. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tan FE, Vladar EK, Ma L, Fuentealba LC, Hoh R, Espinoza FH, et al. Myb promotes centriole amplification and later steps of the multiciliogenesis program. Development. 2013;140:4277–4286. doi: 10.1242/dev.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou F, Narasimhan V, Shboul M, Ching YL, Reversade B, Roy S. Gmnc Is a Master Regulator of the Multiciliated Cell Differentiation Program. Current Biology. 2015;25:3267–3273. doi: 10.1016/j.cub.2015.10.062. [DOI] [PubMed] [Google Scholar]
- [18].Boon M, Wallmeier J, Ma L, Loges NT, Jaspers M, Olbrich H, et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Commun. 2014;5:1–8. doi: 10.1038/ncomms5418. [DOI] [PubMed] [Google Scholar]
- [19].Wallmeier J, Al-Mutairi DA, Chen C-T, Loges NT, Pennekamp P, Menchen T, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014;46:646–651. doi: 10.1038/ng.2961. [DOI] [PubMed] [Google Scholar]
- [20].Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, Mitchell BJ. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. The Journal of Cell Biology. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP Pathway Instructs the Planar Orientation of Ciliated Cells in the Xenopus Larval Skin. Current Biology. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vladar EK, Bayly RD, Sangoram A, Scott MP, Axelrod JD. Microtubules Enable the Planar Cell Polarity of Airway Cilia. Current Biology. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vladar EK, Nayak JV, Milla CE, Axelrod JD. Airway epithelial homeostasis and planar cell polarity signaling depend on multiciliated cell differentiation. JCI Insight. 2016;1:183. doi: 10.1172/jci.insight.88027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, et al. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight. 2018;3:20S. doi: 10.1172/jci.insight.98738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luis AB, Corrales A, Herrera MA, Colino CG, Cumplido J, Tadeo JM, et al. A pathway-based association study reveals variants from Wnt signalling genes contributing to asthma susceptibility. Clin Exp Allergy. 2017;47:618–626. doi: 10.1111/cea.12883. [DOI] [PubMed] [Google Scholar]
- [27].Bustamante-Marin XM, Wei-Ning Y, Sears PR, Werner ME, Brotslaw EJ, Mitchell BJ, et al. Lack of GAS2L2 causes PCD by impairing cilia orientation and mucociliary clearance. Am J Hum Genet. 2019;104(2):229–245. doi: 10.1016/j.ajhg.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brooks ER, Wallingford JB. In vivo investigation of cilia structure and function using Xenopus. Methods Cell Biol. 2015;127:131–159. doi: 10.1016/bs.mcb.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kurkowiak M, Ziętkiewicz E, Witt M. Recent advances in primary ciliary dyskinesia genetics. Journal of Medical Genetics. 2015;52:1–9. doi: 10.1136/jmedgenet-2014-102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chung M-I, Kwon T, Tu F, Brooks ER, Gupta R, Meyer M, et al. Coordinated genomic control of ciliogenesis and cell movement by RFX2. elifesciences. 2014 doi: 10.7554/eLife.01439.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huizar RL, Lee C, Boulgakov AA, Horani A, Tu F, Marcotte EM, et al. A liquid-like organelle at the root of motile ciliopathy. elifesciences. 2018 doi: 10.7554/eLife.38497.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Horani A, Druly TE, Zariwala MA, Patel AC, Levinson BT, Van Arendonk LG, et al. Whole-Exome Capture and Sequencing Identifies HEATR2 Mutation as a Cause of Primary Ciliary Dyskinesia. Am J Hum Genet. 2012;91:685–693. doi: 10.1016/j.ajhg.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zariwala MA, Gee HY, Kurkowiak M, Al-Mutairi DA, Leigh MW, Hurd TW, et al. ZMYND10 Is Mutated in Primary Ciliary Dyskinesia and Interacts with LRRC6. Am. J Hum Genet. 2013;93:336–345. doi: 10.1016/j.ajhg.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hayes JM, Kim SK, Abitua PB, Park TJ, Herrington ER, Kitayama A, et al. Identification of novel ciliogenesis factors using a new in vivo model for mucociliary epithelial development. Developmental Biology. 2007;312:115–130. doi: 10.1016/j.ydbio.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dubaissi E, Rousseau K, Lea R, Soto X, Nardeosingh S, Schweickert A, et al. A secretory cell type develops alongside multiciliated cells, ionocytes and goblet cells, and provides a protective, anti-infective function in the frog embryonic mucociliary epidermis. Development. 2014;141:1514–1525. doi: 10.1242/dev.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dubaissi E, Rousseau K, Hughes GW, Ridley C, Grencis RK, Roberts IS, et al. Functional characterization of the mucus barrier on the Xenopus tropicalis skin surface. Proc Natl Acad Sci USA. 2018;115:726–731. doi: 10.1073/pnas.1713539115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ridley C, Thornton DJ. Mucins: the frontline defence of the lung. Biochem Soc Trans. 2018;45(5):1099–1106. doi: 10.1042/BST20170402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. International Journal of Pharmaceutics. 2017;532:555–572. doi: 10.1016/j.ijpharm.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thornton DJ, Sharpe C, Ridley C. Intracellular Processing of Human Secreted Polymeric Airway Mucins. Ann Am Thorac Soc. 2018;15(3):S154–158. doi: 10.1513/AnnalsATS.201802-143AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2010;298(1):L15–22. doi: 10.1152/ajplung.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2013;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am J Respir Crit Care Med. 2019;199(6):715–727. doi: 10.1164/rccm.201804-0734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nagata S. Isolation, characterization, and extra-embryonic secretion of the Xenopus laevis embryonic epidermal lectin, XEEL. Glycobiology. 2005;15:281–290. doi: 10.1093/glycob/cwi010. [DOI] [PubMed] [Google Scholar]
- [44].Gu N, Kang G, Jin C, Xu Y, Zhang Z, Erle DJ, et al. Intelectin is required for IL-13-induced monocyte chemotactic protein-1 and-3 expression in lung epithelial cells and promotes allergic airway inflammation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2010;298(3):L290–296. doi: 10.1152/ajplung.90612.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, et al. Foxa3 Induces Goblet Cell Metaplasia and Inhibits Innate Antiviral Immunity. Am J Respir Crit Care Med. 2014;189(3):301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ye DZ, Kaestner KH. Foxa1 and Foxa2 Control the Differentiation of Goblet and Enteroendocrine L-and D-Cells in Mice. Gastroenterology. 2009;137:2052–2062. doi: 10.1053/j.gastro.2009.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shaykhiev R. Emerging biology of persistent mucous cell hyperplasia in COPD. Thorax. 2019;74:4–6. doi: 10.1136/thoraxjnl-2018-212271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Quigley IK, Stubbs JL, Kintner C. Specification of ion transport cells in the Xenopus larval skin. Development. 2011;138:705–714. doi: 10.1242/dev.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Harvey BJ. Energization of sodium absorption by the H(+)-ATPase pump in mitochondria-rich cells of frog skin. Journal of Experimental Biology. 1992;172:289–309. doi: 10.1242/jeb.172.1.289. [DOI] [PubMed] [Google Scholar]
- [50].Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci. 2017;74:93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Morrison CB, Markovetz MR, Ehre C. Mucus, mucins, and cystic fibrosis. Pediatric Pulmonology. 2019;54:S84–S96. doi: 10.1002/ppul.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, et al. Characterization of Wild-Type and ΔF508 Cystic Fibrosis Transmembrane Regulator in Human Respiratory Epithelia. Mol Biol Cell. 2005;16(5):2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Deblandre GA, Wettstein DA, Koyano-Nakagawa N, Kintner C. A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development. 1999;126:4715–4728. doi: 10.1242/dev.126.21.4715. [DOI] [PubMed] [Google Scholar]
- [54].Warner SMB, Hackett T-L, Shaheen F, Hallstrand TS, Kicic A, Stick SM, et al. Transcription Factor p63 Regulates Key Genes and Wound Repair in Human Airway Epithelial Basal Cells. American Journal of Respiratory Cell and Molecular Biology. 2013;49(6):978–988. doi: 10.1165/rcmb.2012-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. American Journal of Physiology-Cell Physiology. 2004;287:C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- [56].Haas M, Vazquez JLG, Sun DI, Tran HT, Brislinger M, Tasca A, et al. ΔN-Tp63 Mediates Wnt/β-Catenin-Induced Inhibition of Differentiation in Basal Stem Cells of Mucociliary Epithelia. Cell Rep. 2019;28:3338–3352.:e6. doi: 10.1016/j.celrep.2019.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. PNAS. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Levardon H, Yonker LM, Hurley BP, Mou H. Expansion of Airway Basal Cells and Generation of Polarized Epithelium. Bio-protocol. 2018;8:e2877. doi: 10.21769/BioProtoc.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kim HY, Jackson TR, Stuckenholz C, Davidson LA. Tissue mechanics drives regeneration of a mucociliated epidermis on the surface of Xenopus embryonic aggregates. Nat Commun. 2020;11:1–10. doi: 10.1038/s41467-020-14385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ghosh M, Miller YE, Nakachi I, Kwon JB, Barón AE, Brantley AE, et al. Exhaustion of Airway Basal Progenitor Cells in Early and Established Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;197(7):885–896. doi: 10.1164/rccm.201704-0667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vaidyanathan S, Salahudeen AA, Sellers ZM, Bravo DT, Choi SS, Batish A, et al. High-Efficiency, Selection-free Gene Repair in Airway Stem Cells from Cystic Fibrosis Patients Rescues CFTR Function in Differentiated Epithelia. Cell Stem Cell. 2020;26:161–171.:e4. doi: 10.1016/j.stem.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Morrisey EE, Rustgi AK. The Lung and Esophagus: Developmental and Regenerative Overlap. Trends in Cell Biology. 2018;28:738–748. doi: 10.1016/j.tcb.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tsao P-N, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kurrle Y, Kunesch K, Bogusch S, Schweickert A. Serotonin and MucXS release by small secretory cells depend on Xpod, a SSC specific marker gene. Genesis. 2020;58:4715. doi: 10.1002/dvg.23344. [DOI] [PubMed] [Google Scholar]
- [65].Guseh JS, Bores SA, Stanger Ben Z, Zhou Q, Anderson WJ, Melton DA, et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rock JR, Gao X, Xue Y, Randell SH, Kong Y-Y, Hogan BLM. Notch-Dependent Differentiation of Adult Airway Basal Stem Cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cibois M, Luxardi G, Chevalier B, Thomé V, Mercey O, Zaragosi L-E, et al. BMP signalling controls the construction of vertebrate mucociliary epithelia. Development. 2015;142:2352–2363. doi: 10.1242/dev.118679. [DOI] [PubMed] [Google Scholar]
- [68].Tadokoro T, Gao X, Hong CC, Hotten D, Hogan BLM. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development. 2016;143:764–773. doi: 10.1242/dev.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kariyawasam HH, Xanthou G, Barkans J, Aizen M, Kay AB, Robinson DS. Basal Expression of Bone Morphogenetic Protein Receptor Is Reduced in Mild Asthma. Am J Respir Crit Care Med. 2012;177(10):1074–1081. doi: 10.1164/rccm.200709-1376OC. [DOI] [PubMed] [Google Scholar]
- [70].Zuo W-L, Yang J, Strulovici-Barel Y, Salit J, Rostami M, Mezey JG, et al. Exaggerated BMP4 signalling alters human airway basal progenitor cell differentiation to cigarette smoking-related phenotypes. Eur Respir J. 2019;53:1702553. doi: 10.1183/13993003.02553-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Walentek P, Beyer T, Hagenlocher C, Müller C, Feistel K, Schweickert A, et al. ATP4a is required for development and function of the Xenopus mucociliary epidermis-a potential model to study proton pump inhibitor-associated pneumonia. Developmental Biology. 2015;408:292–304. doi: 10.1016/j.ydbio.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, et al. β-Catenin regulates differentiation of respiratory epithelial cells in vivo. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2005;289(6):L971–979. doi: 10.1152/ajplung.00172.2005. [DOI] [PubMed] [Google Scholar]
- [73].Ruptier C, De Gaspéris A, Ansieau S, Granjon A, Tanière P, Lafosse I, et al. TP63 P2 promoter functional analysis identifies β-catenin as a key regulator of ΔNp63 expression. Oncogene. 2011;30:4656–4665. doi: 10.1038/onc.2011.171. [DOI] [PubMed] [Google Scholar]
- [74].Kjolby RAS, Harland RM. Genome-wide identification of Wnt/β-catenin transcriptional targets during Xenopus gastrulation. Developmental Biology. 2017;426:165–175. doi: 10.1016/j.ydbio.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kwak HJ, Park DW, Seo J-Y, Moon J-Y, Kim TH, Sohn JW, et al. The Wnt/β-catenin signaling pathway regulates the development of airway remodeling in patients with asthma. Exp Mol Med. 2015;47:e198. doi: 10.1038/emm.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Heijink IH, de Bruin HG, van den Berge M, Bennink LJC, Brandenburg SM, Gosens R, et al. Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax. 2013;68:709–716. doi: 10.1136/thoraxjnl-2012-201667. [DOI] [PubMed] [Google Scholar]
- [77].Nakayama T, Grainger RM, Cha SW. Simple embryo injection of long single-stranded donor templates with the CRISPR/Cas9 system leads to homology-directed repair in Xenopus tropicalis and Xenopus laevis. Genesis. 2020;58:15. doi: 10.1002/dvg.23366. [DOI] [PubMed] [Google Scholar]
- [78].Yang H-S, Sim HJ, Cho H, Bang WY, Kim HE, Kwon TK, et al. Alpha-tocopherol exerts protective function against the mucotoxicity of particulate matter in amphibian and human goblet cells. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-63085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sim HJ, Kim S-H, Myung K-J, Kwon T, Lee H-S, Park TJ. Xenopus: An alternative model system for identifying muco-active agents. PLOS ONE. 2018;13:e0193310. doi: 10.1371/journal.pone.0193310. [DOI] [PMC free article] [PubMed] [Google Scholar]