Abstract
Background
Survivors of teenage and young adult (TYA) cancer are at risk of cerebrovascular events, but the magnitude of and extent to which this risk varies by cancer type, decade of diagnosis, age at diagnosis and attained age remains uncertain. This is the largest ever cohort study to evaluate the risks of hospitalisation for a cerebrovascular event among long-term survivors of TYA cancer.
Methods
The population-based Teenage and Young Adult Cancer Survivor Study (N=178,962) was linked to Hospital Episode Statistics data for England to investigate the risks of hospitalisation for a cerebrovascular event among 5-year survivors of cancer diagnosed when aged 15-39 years. Observed numbers of first hospitalisations for cerebrovascular events were compared to that expected from the general population using standardised hospitalisation ratios (SHR) and absolute excess risks (AER) per 10,000 person-years. Cumulative incidence was calculated with death considered a competing risk.
Results
Overall, 2,782 cancer survivors were hospitalised for a cerebrovascular event—40% higher than expected (SHR=1.4, 95% confidence interval [CI]=1.3-1.4). Survivors of central nervous system (CNS) tumours (SHR=4.6, CI=4.3-5.0), head & neck tumours (SHR=2.6, CI=2.2-3.1) and leukaemia (SHR=2.5, CI=1.9-3.1) were at greatest risk. Males had a significantly higher AER than females (AER=7 versus 3), especially among head & neck tumour survivors (AER=30 versus 11). By age 60, 9%, 6% and 5% of CNS tumour, head & neck tumour, and leukaemia survivors, respectively, had been hospitalised for a cerebrovascular event. Beyond age 60, every year 0.4% of CNS tumour survivors were hospitalised for a cerebral infarction (versus 0.1% expected. Whereas at any age, every year 0.2% of head & neck tumour survivors were hospitalised for a cerebral infarction 7 (versus 0.06% expected).
Conclusions
Survivors of a CNS tumour, head & neck tumour, and leukaemia are particularly at risk of hospitalisation for a cerebrovascular event. The excess risk of cerebral-infarction among CNS tumour survivors increases with attained age. For head & neck tumour survivors this excess risk remains high across all ages. These groups of survivors, and in particular males, should be considered for surveillance of cerebrovascular risk factors and potential pharmacological interventions for cerebral infarction prevention.
Keywords: teenage and young adult cancer, cerebrovascular disease, strokes
Introduction
Improvements in survival over the last few decades mean that more than 80% of teenagers and young adults (TYA, age 15-39 years) diagnosed with cancer can expect to survive at least 5-years from diagnosis 1, 2. This has resulted in a large population of cancer survivors, who may be at increased risk of treatment related long-term adverse health effects3, 4. Notwithstanding this, TYA cancer survivors are an understudied population and little is known about the risks of long-term adverse health outcomes in this group5, 6.
Previous studies of cancer survivors have observed an increased risk of cerebrovascular events after irradiation of the head, neck and mediastinum7–13. In addition to treatment, local recurrence and brain metastases are also potential causes of cerebrovascular events in cancer populations14. Development of a cerebrovascular event after treatment for cancer is a serious, and potentially life-threatening adverse event15, yet only one other large-scale population-based study has explored the risk of hospitalisation for a cerebrovascular event in individuals diagnosed with cancer, between the ages of 15 and 39 years (n=43,154 1-year survivors) 16. In this Danish study, there was a 30% increased risk of hospitalisation for a cerebrovascular event for all survivors combined compared to the general population, however, the risk varied by type of cerebrovascular event and cancer type. The magnitude of the effect of attained age, age at cancer diagnosis and decade of cancer diagnosis on the risk of specific types of cerebrovascular events (e.g. cerebral infarction, cerebral haemorrhage) has not previously been investigated and has often been encompassed within studies of all types of cardiovascular events combined9, 16, 17.
Within the UK, the risk of having a cerebrovascular event doubles every decade after the age of 55 18. Currently it is unknown what the risk of cerebrovascular events is in TYA cancer survivors and whether this effect doubles beyond the age of 55. If this holds true for survivors of TYA cancer survivors this could lead to a large population of cancer survivors developing a cerebrovascular event in older age.
To our knowledge, this is the largest study to investigate the risk of hospitalisation for specific cerebrovascular events in survivors of all and specific types of cancer diagnosed in individuals aged 15-39 years. In addition, this is the first study to investigate whether the risk of hospitalisation for a cerebrovascular event varies with attained age, age at diagnosis and decade of diagnosis.
Methods
Teenage and Young Adult Cancer Survivor Study
The Teenage and Young Adult Cancer Survivor Study (TYACSS) is a large population-based cohort consisting of 200,945 5-year survivors of cancer diagnosed aged 15-39 years between 1971 and 2006 in England and Wales. The cohort was ascertained from cancer registrations recorded by the Office for National Statistics and the Welsh Cancer Registry. Cancer registrations were coded according to the relevant revisions of the International Classification of Diseases (ICD) (topography) and International Classification of Diseases for Oncology (ICDO) (morphology). We mapped all ICD-topography and ICDO-morphology codes into 15 main cancer types based on the classification of cancers for TYAs 19, 20. We refined the classification slightly to allow for inclusion of finer groupings of carcinomas (see Online Table 1). Ethical approval for the TYACSS was obtained from the National Research Ethics Service (ref: 16/LO/0895) and legal permission to process identifiable information without individual patient consent from the Confidentiality Advisory Group (ref: 3-03(c)2010).
Ascertainment of Cerebrovascular Events from Hospital Episodes Statistics
The inpatient Hospital Episode Statistics (HES) database is a national electronic database of routinely collected data on individual hospital admissions to National Health Service (NHS) and private (if care was commissioned by the NHS) hospitals in England. For this study HES inpatient data were available from April 1997 to December 2012. Prior to linking the survivor cohort by NHS number, date of birth, postcode and sex to the inpatient HES database, we excluded all Welsh survivors (N= 11,099 patients) due to HES being restricted to England only. Individuals were also excluded if they had died or emigrated before the start of the follow-up period (1st April 1997) (N=10,128 patients) (see Online Figure 1 for flow-diagram of exclusions). It was not possible to determine the actual linkage rate of the survivor cohort to the inpatient HES database as not all survivors would have been admitted to hospital within the HES years 1997-2012. For the vast majority of survivors the key linkage identifiers—NHS number, date of birth, postcode and sex—were available and it is thus more likely that such survivors would not have been admitted to hospital rather than an actual failure to link the survivor to the correct HES record(s). Of the 179,718 survivors eligible to be linked with HES, 84% (150,942) had at least one inpatient admission record in HES.
Each inpatient admission initiates a record in the HES database and contains information on the date of admission, methods of admission and discharge, patient demographics, and up to 20 diagnosis variables containing information on the primary condition, conditions diagnosed during hospital admission, and any pre-existing co-morbidities. All diagnosis variables are coded using the International Classification of Disease version 10 (ICD-10) and are coded at the time of hospital discharge by trained clinical coders or clinicians using all available clinical notes21. Cerebrovascular events (ICD-10: I60-I68) were identified from all 20 diagnosis variables recorded in HES and categorised into the following subgroups: subarachnoid haemorrhage (ICD-10: I60), cerebral haemorrhage (ICD-10: I61-I62), cerebral infarction/occlusion/stenosis (ICD-10: I63, I65-I66), and ‘other cerebrovascular event’ (ICD-10: I64, I67-I68) (stroke not specified as haemorrhage or infarction (ICD-10: I64), other cerebrovascular events (ICD-10: I67), cerebrovascular disorders in diseases elsewhere classified (ICD-10:I68)). If an individual developed the same type of cerebrovascular event more than once, only the first event was retained for the analysis to ensure that any cerebrovascular event was not counted more than once due to potential duplicate recordings. Individuals who developed a cerebrovascular event within the first 5-years from cancer diagnosis were excluded as time at risk started at 5-year survival. Individuals with a first event recorded as sequelae of cerebrovascular disease (ICD-10: I69), but no prior recorded cerebrovascular event in HES were also excluded, as such survivors would most likely have had a prior cerebrovascular event before April 1997. After these exclusions, a cohort of 178,962 5-year survivors remained (see Online Figure 1 for flow-diagram of exclusions).
Statistical Analysis
Individuals were followed-up for cerebrovascular events from 5-year survival or 1st April 1997, whichever date was most recent. Follow-up ended at the first occurrence of death, emigration, hospitalisation for a cerebrovascular event or study end date (31st December 2012). Cerebrovascular related hospitalisation rates for the general population were derived from the entire (anonymised) HES dataset for England (N=13,476,762) by dividing the number of individuals with a hospitalisation by the mid-year general population estimates 22 for each age (1-year bands), sex and calendar-year (1-year bands). The accumulated person-years within each corresponding age, sex and calendar year stratum in the survivor cohort were multiplied by the general population rates to obtain the expected number of cerebrovascular hospitalisations.
Analyses were conducted to investigate the risk of a hospitalisation for all cerebrovascular events combined and for specific types of cerebrovascular events. The observed number of hospitalisations for a cerebrovascular event was divided by the number expected from the general population (referred to as a standardised hospitalisation ratio (SHR))17. The absolute excess risk (AER) is the additional number of hospitalisation for a cerebrovascular event compared to that expected based on general population hospitalisation rates, it is calculated as the observed minus the expected number of hospitalisation divided by the person-years at risk. The AER has been expressed per 10,000 person-years throughout the manuscript 23. For each cerebrovascular event subtype, SHRs and AERs were stratified by the following factors: cancer diagnosis, sex, age at cancer diagnosis (15-19/20-24/25-29/30-34/35-39 years), decade of cancer diagnosis (1971-1979/1980-1989/1990-1999/2000-2006) and attained age (20-44/45-49/50-54/55-59/≥60years). To investigate the simultaneous effect of these variables on the risk of hospitalisation for a cerebrovascular event, multivariable Poisson regression was conducted to derive relative risks (RR) and excess hospitalisation ratios (EHR). RRs can be interpreted as the ratio of the SHRs adjusted for potential confounders. EHRs can be interpreted as the ratio of the AERs adjusted for potential confounders24. Negative binomial regression was preferred to Poisson when the data showed signs of over-dispersion (see Online Table 2 for dispersion parameters and regression methods used). A likelihood ratio test was used to test for linear trend of a factor by comparing the log-likelihood of a model including the factor variable of interest (e.g. attained age (20-44/45-49/50-54/55-59/≥60 years)), which was coded such that it had the median value of the variable at each level, with the log-likelihood of a model without the factor variable of interest. Cumulative incidence with attained age as the time scale was calculated treating death as a competing risk. A p-value of <0.05 (2-sided test) was taken as statistically significant. All statistical analyses were conducted in Stata statistical software (version 14.1, Stata Corp., College Station, TX).
From here on “hospitalisation for a cerebrovascular event” and “cerebral infarction/occlusion/stenosis” will be referred to as “cerebrovascular event” and “cerebral infarction”, respectively.
Results
Cohort Characteristics
A total of 178,962 5-year survivors of TYA cancer were included in the analysis, contributing 1,837,996 person-years of follow-up. Characteristics of the cohort can be found in Online Table 3. The median follow-up was 11.3 years (range: 0-15.8 years) with 36% of individuals followed for at least 15 years. In total, 2,782 (1.6%) individuals were hospitalised for at least one cerebrovascular event. With respect to specific types of cerebrovascular events; 618 (0.35% (0.18% expected)) individuals were hospitalised for a cerebral haemorrhage, 1,296 (0.72% (0.48% expected)) for a cerebral infarction, 262 (0.15% (0.15%expected)) for a subarachnoid haemorrhage and 1,114 (0.62% (0.49% expected)) for ‘other cerebrovascular events’.
Risk of any cerebrovascular event
Survivors experienced a 40% significantly increased risk of developing any cerebrovascular event compared to that expected from the general population (SHR=1.4, 95% confidence interval [CI]=1.3-1.4); corresponding to four excess events per 10,000 person-years (PY) (Table 1). Female survivors experienced significantly fewer excess cerebrovascular events than male survivors (AER=3 and AER=7, respectively; pheterogeneity<0.001). In terms of age at cancer diagnosis, SHRs for a cerebrovascular event were highest among individuals diagnosed with a cancer aged 15-19 years (SHR=3.6, CI=3.0-4.2; AER=9) and there was a significant trend for the SHR to decrease with increasing age at diagnosis (ptrend <0.001). The SHR declined significantly with attained age (ptrend<0.001), although the p-value for trend for the RR just fell short of significance when evaluated in the multivariable model (ptrend =0.086) (Table 2). The AERs remained elevated at all ages, however; the multivariable analysis showed a significant increasing trend in the number of excess cerebrovascular events with increasing attained age—survivors older than 60 years had 2-fold the number of excess cerebrovascular events than survivors aged 20-44 years (EHR=2.4, CI=1.5-4.1;ptrend<0.001) (Table 2).
Table 1.
Standardised Hospitalisation Ratios and Absolute Excess Risks of any cerebrovascular event, Cerebral Haemorrhage (ICD10: I61-I62), Cerebral infarction (ICD10: I63, I65-66), Other Cerebrovascular Event (ICD10:I64, I67-I68) by TYA cancer diagnosis, sex, age at diagnosis and decade of diagnosis.
| Any CV Event (ICD10: I60-I68) | Cerebral Haemorrhage (ICD10: I61-I62) | Cerebral infarction (ICD10: I63, I65-I66) | Other CV Event (ICD10: I64, I67-I68)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | SHR (95% CI) | AER (95% CI) | 1 O | SHR (95% CI) | AER (95% CI) | 1 O | SHR (95% CI) | AER (95% CI) | 1 O | SHR (95% CI) | AER (95% CI) | |
| All Survivors FPN | 2782 | 1.4 (1.3,1.4) | 4.2 (3.6,4.8) | 618 | 2.0 (1.8,2.1) | 1.6 (1.4,1.9) | 1296 | 1.5 (1.4,1.6) | 2.4 (2.0,2.7) | 1114 | 1.4 (1.3,1.5) | 1.7 (1.3,2.0) |
| Breast | 288 | 0.8 (0.7,0.9) | -2.0 (-3.1,-0.8) | 48 | 1.0 (0.7,1.3) | -0.0 (-0.5,0.4) | 127 | 0.9 (0.8,1.1) | -0.4 (-1.1,0.4) | 117 | 0.8 (0.7,1.0) | -1.0 (-1.8,-0.3) |
| Testicular | 278 | 1.0 (0.9,1.1) | 0.1 (-1.3,1.4) | 61 | 1.3 (1.0,1.7) | 0.6 (-0.1,1.2) | 128 | 1.0 (0.8,1.2) | -0.1 (-1.0,0.8) | 97 | 0.9 (0.8,1.2) | -0.2 (-1.0,0.6) |
| Cervix | 317 | 1.2 (1.1,1.4) | 2.4 (0.9,3.8) | 47 | 1.2 (0.9,1.6) | 0.4 (-0.2,0.9) | 137 | 1.4 (1.1,1.6) | 1.5 (0.5,2.4) | 149 | 1.4 (1.2,1.6) | 1.7 (0.7,2.7) |
| Melanoma | 155 | 0.8 (0.7,0.9) | -2.0 (-3.2,-0.8) | 61 | 2.0 (1.5,2.6) | 1.5 (0.7,2.3) | 47 | 0.6 (0.4,0.8) | -1.7 (-2.4,-1.0) | 47 | 0.6 (0.4,0.8) | -1.5 (-2.1,-0.8) |
| CNS tumour | 616 | 4.6 (4.3,5.0) | 33.9 (30.5,37.4) | 183 | 8.3 (7.2,9.6) | 11.2 (9.3,13.0) | 256 | 4.4 (3.9,5.0) | 13.8 (11.6,16.0) | 268 | 5.1 (4.5,5.8) | 15.1 (12.8,17.3) |
| Hodgkin Lymphoma | 228 | 1.6 (1.4,1.8) | 4.8 (3.1,6.6) | 43 | 1.7 (1.3,2.4) | 1.1 (0.3,1.9) | 135 | 2.1 (1.8,2.5) | 4.2 (2.9,5.6) | 76 | 1.4 (1.1,1.7) | 1.2 (0.2,2.2) |
| NHL | 139 | 1.6 (1.4,1.9) | 6.7 (3.8,9.5) | 33 | 2.4 (1.6,3.3) | 2.3 (1.0,3.7) | 70 | 1.8 (1.4,2.3) | 3.9 (1.9,6.0) | 47 | 1.4 (1.0,1.9) | 1.7 (0.1,3.4) |
| Thyroid | 65 | 0.9 (0.7,1.2) | -0.6 (-2.7,1.5) | 9 | 0.8 (0.4,1.6) | -0.2 (-1.0,0.6) | 34 | 1.2 (0.8,1.7) | 0.7 (-0.8,2.3) | 27 | 1.0 (0.6,1.4) | -0.1 (-1.5,1.2) |
| Gastrointestinal | 88 | 0.9 (0.7,1.2) | -1.0 (-3.9,1.9) | 19 | 1.3 (0.8,2.1) | 0.7 (-0.6,2.1) | 43 | 1.0 (0.7,1.4) | 0.1 (-2.0,2.1) | 43 | 1.1 (0.8,1.5) | 0.7 (-1.4,2.7) |
| STS | 84 | 1.3 (1.0,1.6) | 3.4 (0.4,6.5) | 22 | 2.2 (1.4,3.3) | 2.0 (0.5,3.6) | 42 | 1.5 (1.1,2.0) | 2.4 (0.2,4.5) | 36 | 1.4 (1.0,2.0) | 1.8 (-0.2,3.8) |
| Leukaemia | 70 | 2.5 (1.9,3.1) | 10.2 (6.2,14.2) | 26 | 5.2 (3.4,7.6) | 5.1 (2.7,7.6) | 28 | 2.3 (1.5,3.3) | 3.9 (1.3,6.4) | 27 | 2.6 (1.7,3.8) | 4.1 (1.6,6.6) |
| GU (other) | 93 | 1.6 (1.3,1.9) | 8.3 (3.7,12.8) | 22 | 2.5 (1.6,3.8) | 3.2 (1.0,5.4) | 46 | 1.8 (1.3,2.4) | 4.9 (1.7,8.1) | 33 | 1.3 (0.9,1.9) | 2.0 (-0.7,4.7) |
| Ovary | 52 | 1.0 (0.7,1.3) | -0.3 (-3.1,2.5) | 7 | 0.9 (0.4,1.9) | -0.1 (-1.2,0.9) | 23 | 1.1 (0.7,1.6) | 0.4 (-1.5,2.3) | 20 | 0.9 (0.5,1.4) | -0.5 (-2.2,1.2) |
| Bladder | 83 | 1.0 (0.8,1.2) | -0.3 (-4.1,3.5) | 10 | 0.8 (0.4,1.4) | -0.6 (-1.9,0.7) | 47 | 1.2 (0.9,1.6) | 1.5 (-1.4,4.3) | 32 | 0.9 (0.6,1.3) | -0.6 (-2.9,1.8) |
| Head & Neck | 123 | 2.6 (2.2,3.1) | 21.5 (15.4,27.7) | 13 | 1.7 (0.9,3.0) | 1.5 (-0.4,3.5) | 75 | 3.5 (2.8,4.4) | 15.0 (10.2,19.8) | 51 | 2.7 (2.0,3.5) | 8.9 (5.0,12.8) |
| Other | 103 | 1.5 (1.2,1.9) | 5.8 (2.6,9.1) | 14 | 1.3 (0.7,2.2) | 0.6 (-0.6,1.7) | 58 | 2.0 (1.5,2.5) | 4.7 (2.2,7.1) | 44 | 1.6 (1.2,2.2) | 2.8 (0.6,4.9) |
| P-heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Male | 1322 | 1.5 (1.5,1.6) | 6.6 (5.6,7.6) | 322 | 2.2 (2.0,2.5) | 2.5 (2.0,3.0) | 659 | 1.6 (1.5,1.7) | 3.5 (2.8,4.2) | 505 | 1.6 (1.4,1.7) | 2.5 (1.9,3.2) |
| Female | 1460 | 1.3 (1.2,1.3) | 2.7 (2.1,3.4) | 296 | 1.8 (1.6,2.0) | 1.1 (0.8,1.4) | 637 | 1.4 (1.3,1.5) | 1.6 (1.2,2.1) | 609 | 1.3 (1.2,1.4) | 1.1 (0.7,1.6) |
| P-heterogeneity | <0.001 | <0.001 | 0.004 | <0.001 | 0.016 | <0.001 | 0.001 | 0.006 | ||||
| Age at FPN Diagnosis(years) | ||||||||||||
| 15-19 | 148 | 3.6 (3.0,4.2) | 8.9 (6.9,10.9) | 44 | 5.3 (3.8,7.1) | 3.0 (1.9,4.1) | 67 | 4.3 (3.3,5.4) | 4.3 (2.9,5.6) | 56 | 4.1 (3.1,5.4) | 3.5 (2.3,4.8) |
| 20-24 | 219 | 2.0 (1.8,2.3) | 5.2 (3.9,6.6) | 53 | 2.7 (2.0,3.5) | 1.6 (0.9,2.2) | 101 | 2.3 (1.9,2.8) | 2.7 (1.8,3.7) | 81 | 2.2 (1.7,2.7) | 2.1 (1.2,2.9) |
| 25-29 | 407 | 1.6 (1.4,1.7) | 4.2 (3.1,5.4) | 91 | 2.1 (1.7,2.5) | 1.3 (0.8,1.9) | 192 | 1.8 (1.5,2.1) | 2.4 (1.7,3.2) | 164 | 1.7 (1.5,2.0) | 2.0 (1.2,2.7) |
| 30-34 | 722 | 1.3 (1.2,1.4) | 3.6 (2.5,4.6) | 176 | 2.1 (1.8,2.4) | 1.8 (1.3,2.3) | 318 | 1.4 (1.2,1.5) | 1.7 (1.0,2.4) | 255 | 1.2 (1.1,1.4) | 0.8 (0.2,1.5) |
| 35-39 | 1286 | 1.2 (1.2,1.3) | 3.5 (2.4,4.6) | 254 | 1.6 (1.4,1.8) | 1.5 (1.0,2.0) | 618 | 1.3 (1.2,1.4) | 2.3 (1.6,3.1) | 558 | 1.3 (1.2,1.4) | 1.7 (1.0,2.4) |
| P-trend | <0.001 | <0.001 | <0.001 | 0.079 | <0.001 | 0.007 | <0.001 | 0.002 | ||||
| Decade of FPN Diagnosis | ||||||||||||
| 1971-1979 | 809 | 1.1 (1.1,1.2) | 3.9 (1.8,6.0) | 118 | 1.2 (1.0,1.4) | 0.8 (-0.0,1.6) | 404 | 1.2 (1.1,1.4) | 3.0 (1.5,4.5) | 376 | 1.2 (1.1,1.3) | 2.3 (0.8,3.7) |
| 1980-1989 | 1097 | 1.5 (1.4,1.6) | 5.6 (4.6,6.7) | 235 | 2.0 (1.7,2.3) | 1.9 (1.4,2.3) | 519 | 1.6 (1.5,1.8) | 3.2 (2.5,4.0) | 448 | 1.5 (1.4,1.7) | 2.5 (1.8,3.2) |
| 1990-1999 | 700 | 1.6 (1.4,1.7) | 3.4 (2.7,4.2) | 195 | 2.5 (2.1,2.8) | 1.6 (1.2,2.0) | 309 | 1.8 (1.6,2.0) | 1.8 (1.4,2.3) | 239 | 1.5 (1.3,1.7) | 1.1 (0.7,1.5) |
| 2000-2006 | 176 | 1.7 (1.4,1.9) | 3.1 (1.9,4.2) | 70 | 3.7 (2.9,4.7) | 2.2 (1.5,3.0) | 64 | 1.5 (1.1,1.9) | 0.9 (0.2,1.6) | 51 | 1.4 (1.0,1.8) | 0.6 (0.0,1.2) |
| P-trend | <0.001 | <0.001 | <0.001 | 0.089 | <0.001 | <0.001 | 0.007 | <0.001 | ||||
Abbreviations: CV=cerebrovascular; ICD= International classification of disease; O= observed; SHR=standardised hospitalisation ratio; AER=absolute excess risk; CI=confidence interval; FPN=first primary neoplasms; CNS=central nervous system; NHL=non-hodgkin lymphoma; STS=soft-tissue sarcoma; GU=genitourinary.
‘Constituting ICD10: I64-Stroke not specified as haemorrhage or infarction (n=454); I67.0 – Dissection of cerebral arteries, non-ruptured (n=10); I67.1 – Cerebral aneurysm, non-ruptured (n=98); I67.2 – Cerebral atherosclerosis (n=38); I67.4- Hypertensive encephalopathy (n=12); I67.5-I67.8 – Other specified cerebrovascular disease (n=399); I67.9- Cerebrovascular disease, unspecified (n=244); I68 – Cerebrovascular disorders in diseases elsewhere classified (n=3).
Table 2. Standardised Hospitalisation Ratio, Relative Risk, Absolute Excess Risk and Excess Hospitalisation Ratio of any and specific cerebrovascular events according to attained age.
| Cerebrovascular Event | Attained Age (years) | O | SHR (95% CI) | RR (95% CI)* | AER (95% CI) | EHR (95% CI)* |
|---|---|---|---|---|---|---|
| Any CV Event (ICD10: I60-I68) | 20-44 | 549 | 2.0 (1.9,2.2) | 1.0 Ref | 3.7 (3.1,4.3) | 1.0 Ref |
| 45-49 | 433 | 1.5 (1.3,1.6) | 0.9 (0.7,1.0) | 3.7 (2.6,4.7) | 1.6 (1.2,2.2) | |
| 50-54 | 512 | 1.5 (1.4,1.7) | 0.9 (0.8,1.1) | 6.1 (4.5,7.6) | 2.1 (1.4,2.9) | |
| 54-59 | 455 | 1.3 (1.2,1.4) | 0.9 (0.7,1.0) | 5.4 (3.3,7.4) | 2.4 (1.5,3.6) | |
| 60+ | 833 | 1.1 (1.0,1.2) | 0.8 (0.7,1.0) | 3.5 (0.9,6.2) | 2.4 (1.5,4.1) | |
| P-trend | <0.001 | 0.086 | 0.122 | <0.001 | ||
| Cerebral Haemorrhage(ICD10: I61-I62) | 20-44 | 193 | 3.6 (3.1,4.2) | 1.0 Ref | 1.8 (1.5,2.2) | 1.0 Ref |
| 45-49 | 110 | 2.2 (1.8,2.6) | 0.7 (0.6,0.9) | 1.6 (1.0,2.1) | 1.1 (0.7,1.6) | |
| 50-54 | 106 | 1.9 (1.6,2.3) | 0.7 (0.5,1.0) | 1.7 (1.0,2.4) | 1.2 (0.7,2.0) | |
| 54-59 | 92 | 1.7 (1.4,2.1) | 0.7 (0.5,1.0) | 1.9 (1.0,2.8) | 1.1 (0.6,2.2) | |
| 60+ | 117 | 1.2 (1.0,1.4) | 0.6 (0.4,0.8) | 0.7 (-0.2,1.7) | 1.0 (0.4,2.3) | |
| P-trend | <0.001 | 0.016 | 0.137 | 0.610 | ||
| Cerebral infarction (ICD10: I63, I65-66) | 20-44 | 197 | 2.2 (1.9,2.5) | 1.0 Ref | 1.4 (1.0,1.8) | 1.0 Ref |
| 45-49 | 191 | 1.8 (1.5,2.0) | 0.9 (0.7,1.1) | 2.2 (1.5,2.9) | 2.7 (1.8,4.1) | |
| 50-54 | 242 | 1.8 (1.6,2.0) | 0.9 (0.7,1.2) | 3.7 (2.7,4.8) | 3.7 (2.3,6.0) | |
| 54-59 | 238 | 1.6 (1.4,1.8) | 0.8 (0.6,1.1) | 4.2 (2.7,5.7) | 5.1 (2.9,8.8) | |
| 60+ | 428 | 1.1 (1.0,1.3) | 0.7 (0.5,0.9) | 2.5 (0.6,4.4) | 5.6 (2.9,10.8) | |
| P-trend | <0.001 | 0.018 | <0.001 | <0.001 | ||
| Other CV event (ICD10: I64, I67-I68) | 20-44 | 161 | 1.8 (1.6,2.1) | 1.0 Ref | 1.0 (0.6,1.3) | 1.0 Ref |
| 45-49 | 160 | 1.6 (1.3,1.8) | 1.0 (0.8,1.3) | 1.5 (0.8,2.2) | 3.0 (1.9,4.9) | |
| 50-54 | 207 | 1.6 (1.4,1.9) | 1.1 (0.9,1.4) | 2.8 (1.8,3.8) | 4.4 (2.6,7.4) | |
| 54-59 | 184 | 1.3 (1.2,1.6) | 1.0 (0.7,1.3) | 2.3 (1.0,3.6) | 4.6 (2.5,8.7) | |
| 60+ | 402 | 1.1 (1.0,1.3) | 0.9 (0.7,1.3) | 2.3 (0.5,4.2) | 6.4 (3.1,12.9) | |
| P-trend | <0.001 | 0.605 | <0.001 | <0.001 |
Abbreviations: CV=cerebrovascular; ICD= International classification of disease; O= observed; SHR=standardised hospitalisation ratio; AER=absolute excess risk; CI=confidence interval; RR=relative risk; EHR=excess hospitalisation ratio; ref= reference level
Multivariable models and p-values are adjusted for cancer diagnosis, sex, age at cancer diagnosis, decade of cancer diagnosis and attained age
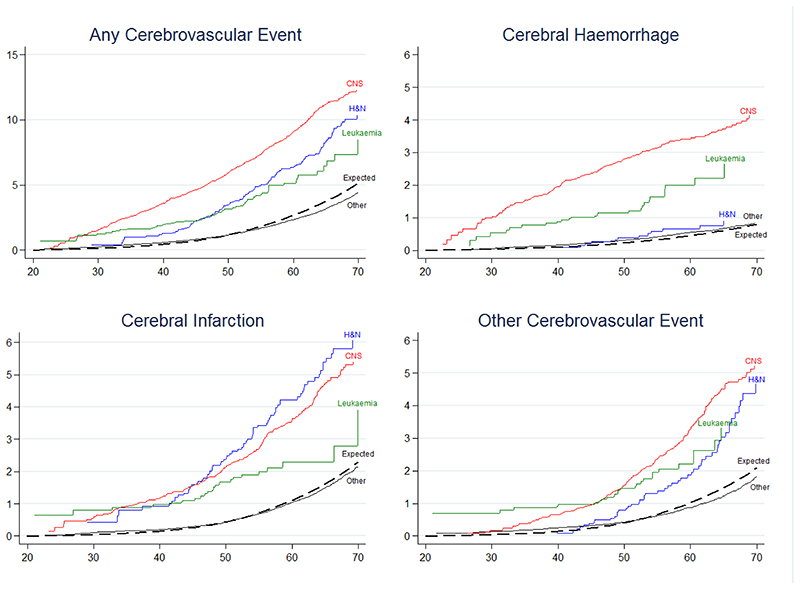
With regards to TYA cancer type, survivors at the highest risk of developing a cerebrovascular event were: central nervous system (CNS) tumour survivors (SHR=4.6, CI=4.3-5.0; AER=34); head & neck tumour survivors (SHR=2.6, CI=2.2-3.1; AER=22) and leukaemia survivors (SHR=2.5. CI=1.9-3.1; AER=10). The cumulative incidence of developing a cerebrovascular event by age 60 was 9.0%, 6.4% and 5.1% among CNS tumour, head & neck tumour and leukaemia survivors, respectively, whereas 2.3% was expected (Figure 1a). Due to having substantially increased risk, further analyses were conducted for CNS tumour, head & neck tumour and leukaemia survivors.
Figure 1. Cumulative Incidence for all and specific types of cerebrovascular events, by attained age.
Abbreviations: H&N=head and neck, CNS= central nervous system. Other = all TYA cancers excluding CNS, H&N and leukaemia.
CNS tumour survivors
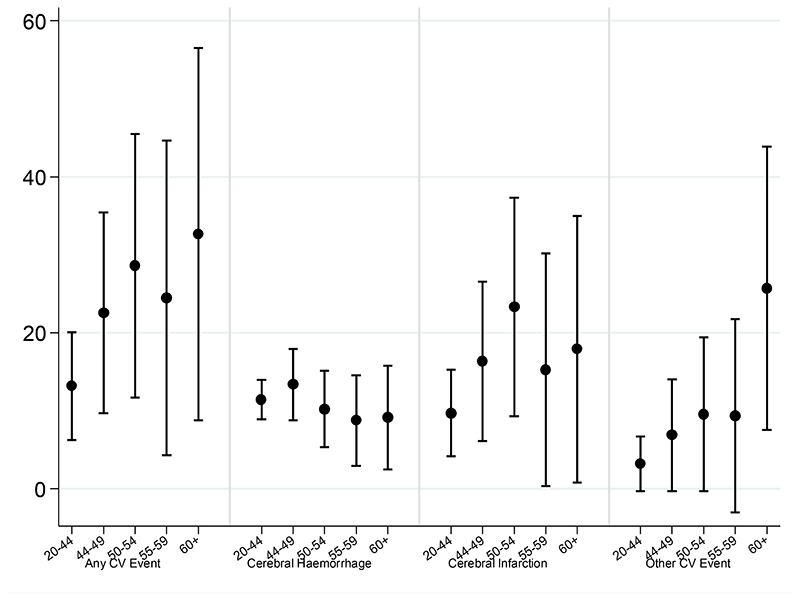
Survivors of all CNS tumour types were at increased risk of developing a cerebrovascular event, particularly, survivors of embryonal tumours (SHR=12.9, CI=8.0-19.7; AER=78) and glial tumours (SHR=10.8, CI=9.5-12.3; AER=72) (Table 3). The SHR was highest among survivors diagnosed when aged 15-19 years (SHR=12.7, CI=10.1-15.9); however survivors aged 35-39 years at diagnosis were still at 3.5-fold increased risk (SHR=3.5, CI=3.0-4.0). The SHR declined with increasing attained age, however remained elevated among all ages (ptrend<0.001). In contrast, the AER increased with attained age (ptrend<0.001) reaching 55 additional hospitalisations per 10,000 PY within individuals aged ≥60years (Figure 2, Table 3).
Table 3.
SHRs and AERs of any cerebrovascular event, cerebral haemorrhage (ICD10: I61-I62), Cerebral infarction (ICD10:I63, I65-66) or other cerebrovascular event (ICD10:I64, I67-I68) among CNS tumours survivors by tumour diagnosis, sex, age at diagnosis, decade of diagnosis and attained age
| Any CV Event (ICD10: I60-I68) | Cerebral Haemorrhage (ICD10: I61-I62) | Cerebral infarction (ICD10: I63, I66-67) | Other CV Event (ICD10: I64, I67-I68) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | SHR (95% CI) | AER (95% CI) | 1 O | SHR (95% CI) | AER (95% CI) | 1 O | SHR (95% CI) | AER (95% CI) | O | SHR (95% CI) | AER (95% CI) | |
| All CNS tumours | 616 | 4.6 (4.3,5.0) | 33.9 (30.5,37.4) | 183 | 8.3 (7.2,9.6) | 11.2 (9.3,13.0) | 256 | 4.4 (3.9,5.0) | 13.8 (11.6,16.0) | 268 | 5.1 (4.5,5.8) | 15.1 (12.8,17.3) |
| CNS tumour type | ||||||||||||
| Glial Tumour | 254 | 10.8 (9.5,12.3) | 72.3 (62.5,82.1) | 108 | 26.2 (21.5,31.6) | 32.1 (25.8,38.3) | 94 | 9.3 (7.5,11.4) | 25.9 (20.1,31.8) | 89 | 10.1 (8.1,12.4) | 24.8 (19.1,30.5) |
| Embryonal Tumour | 21 | 12.9 (8.0,19.7) | 78.0 (41.9,114.2) | * | * | * | 13 | 18.8 (10.0,32.1) | 49.1 (20.9,77.3) | 12 | 20.2 (10.4,35.3) | 45.6 (18.5,72.7) |
| Craniopharyngioma | 24 | 6.0 (3.8,8.9) | 43.9 (22.8,65.0) | 5 | 7.3 (2.4,17.0) | 9.3 (-0.2,18.7) | 12 | 6.8 (3.5,11.9) | 22.1 (7.5,36.8) | 10 | 6.5 (3.1,12.0) | 18.5 (5.0,32.0) |
| Other Pituitary Tumours | 115 | 3.4 (2.8,4.0) | 21.7 (16.1,27.4) | 12 | 2.1 (1.1,3.7) | 1.7 (-0.1,3.5) | 55 | 3.8 (2.8,4.9) | 10.8 (6.9,14.7) | 61 | 4.6 (3.5,5.9) | 12.8 (8.7,16.9) |
| Meningioma | 52 | 2.1 (1.5,2.7) | 12.9 (6.1,19.6) | 13 | 3.4 (1.8,5.7) | 4.3 (1.0,7.7) | 24 | 2.2 (1.4,3.3) | 6.2 (1.7,10.8) | 20 | 1.9 (1.2,3.0) | 4.6 (0.4,8.7) |
| Ependymoma | 30 | 4.9 (3.3,7.0) | 35.5 (19.5,51.5) | 6 | 5.7 (2.1,12.4) | 7.3 (0.2,14.3) | 17 | 6.1 (3.6,9.8) | 21.0 (9.1,32.9) | 13 | 5.5 (3.0,9.5) | 15.7 (5.3,26.2) |
| Germ Cell Intracranial | 9 | 8.1 (3.7,15.3) | 40.5 (10.3,70.8) | * | * | * | 5 | 10.5 (3.4,24.6) | 23.1 (0.7,45.5) | 5 | 12.6 (4.1,29.5) | 23.4 (1.1,45.7) |
| Other specified | 56 | 2.0 (1.5,2.6) | 10.3 (4.8,15.7) | 22 | 4.8 (3.0,7.3) | 6.5 (3.1,9.9) | 19 | 1.5 (0.9,2.4) | 2.4 (-0.8,5.6) | 26 | 2.3 (1.5,3.4) | 5.5 (1.8,9.2) |
| Other Unspecified | 55 | 5.9 (4.4,7.7) | 47.2 (32.2,62.2) | 14 | 9.0 (4.9,15.0) | 12.6 (5.2,20.1) | 17 | 4.1 (2.4,6.5) | 13.0 (4.8,21.2) | 32 | 8.7 (5.9,12.2) | 28.9 (17.6,40.3) |
| P-heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Sex Male | 339 | 4.8 (4.3,5.3) | 40.8 (35.3,46.3) | 105 | 8.5 (6.9,10.2) | 13.9 (10.9,16.9) | 140 | 4.2 (3.5,4.9) | 16.0 (12.5,19.5) | 153 | 5.8 (4.9,6.8) | 19.1 (15.4,22.7) |
| Female | 277 | 4.4 (3.9,4.9) | 28.0 (23.8,32.3) | 78 | 8.1 (6.4,10.2) | 8.9 (6.6,11.1) | 116 | 4.8 (3.9,5.7) | 11.9 (9.2,14.7) | 115 | 4.4 (3.7,5.3) | 11.6 (8.9,14.3) |
| P-heterogeneity | 0.272 | <0.001 | 0.805 | 0.006 | 0.280 | 0.066 | 0.028 | 0.001 | ||||
| Age at FPN Diagnosis (years) | ||||||||||||
| 15-19 | 79 | 12.7 (10.1,15.9) | 37.7 (28.7,46.8) | 28 | 21.8 (14.5,31.5) | 13.7 (8.4,19.0) | 32 | 13.6 (9.3,19.2) | 15.2 (9.5,20.9) | 30 | 14.8 (10.0,21.1) | 14.4 (8.9,19.9) |
| 20-24 | 67 | 6.3 (4.9,8.0) | 25.5 (18.3,32.8) | 17 | 8.5 (5.0,13.7) | 6.7 (3.1,10.4) | 28 | 6.7 (4.4,9.6) | 10.7 (6.1,15.4) | 28 | 7.5 (5.0,10.9) | 10.9 (6.3,15.6) |
| 25-29 | 113 | 5.7 (4.7,6.8) | 33.1 (25.7,40.5) | 34 | 9.7 (6.7,13.6) | 10.7 (6.7,14.8) | 48 | 5.8 (4.3,7.7) | 14.0 (9.2,18.8) | 49 | 6.7 (5.0,8.9) | 14.7 (9.9,19.5) |
| 30-34 | 146 | 4.1 (3.4,4.8) | 32.5 (25.5,39.5) | 53 | 9.1 (6.8,11.9) | 13.8 (9.6,17.9) | 52 | 3.3 (2.5,4.4) | 10.7 (6.5,14.8) | 56 | 4.0 (3.1,5.2) | 12.3 (8.1,16.6) |
| 35-39 | 211 | 3.5 (3.0,4.0) | 38.7 (31.3,46.0) | 51 | 5.4 (4.0,7.1) | 10.5 (7.0,14.1) | 96 | 3.5 (2.8,4.2) | 17.4 (12.5,22.3) | 105 | 4.1 (3.4,5.0) | 20.3 (15.2,25.4) |
| P-trend | <0.001 | 0.393 | <0.001 | 0.791 | <0.001 | 0.555 | <0.001 | 0.111 | ||||
| Decade of FPN Diagnosis | ||||||||||||
| 1971-1979 | 142 | 3.3 (2.8,3.9) | 53.4 (40.9,66.0) | 26 | 4.2 (2.7,6.1) | 10.4 (5.1,15.6) | 59 | 3.0 (2.3,3.8) | 20.6 (12.6,28.5) | 83 | 4.5 (3.6,5.5) | 34.2 (24.7,43.7) |
| 1980-1989 | 234 | 4.6 (4.1,5.3) | 38.5 (32.2,44.8) | 49 | 5.8 (4.3,7.7) | 8.4 (5.6,11.2) | 107 | 4.9 (4.0,5.9) | 17.7 (13.5,21.9) | 115 | 5.9 (4.9,7.1) | 19.8 (15.5,24.2) |
| 1990-1999 | 179 | 5.4 (4.6,6.2) | 24.8 (20.3,29.2) | 72 | 11.9 (9.3,15.0) | 11.1 (8.3,13.9) | 71 | 5.4 (4.2,6.8) | 9.8 (7.0,12.6) | 57 | 4.9 (3.7,6.3) | 7.7 (5.2,10.2) |
| 2000-2006 | 61 | 8.5 (6.5,10.9) | 31.5 (22.5,40.5) | 36 | 26.5 (18.6,36.7) | 20.3 (13.4,27.1) | 19 | 6.3 (3.8,9.9) | 9.3 (4.4,14.3) | 13 | 5.4 (2.9,9.2) | 6.2 (2.1,10.3) |
| P-trend | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | 0.451 | <0.001 | ||||
| Attained Age (years) | ||||||||||||
| 20-44 | 200 | 8.4 (7.3,9.7) | 24.6 (20.7,28.5) | 87 | 17.9 (14.4,22.1) | 11.4 (8.9,14.0) | 65 | 8.2 (6.3,10.4) | 7.9 (5.7,10.1) | 50 | 6.6 (4.9,8.7) | 5.9 (4.0,7.8) |
| 44-49 | 118 | 5.5 (4.6,6.6) | 36.0 (28.1,43.9) | 40 | 10.5 (7.5,14.4) | 13.4 (8.8,17.9) | 52 | 6.4 (4.8,8.5) | 16.2 (11.0,21.5) | 54 | 7.2 (5.4,9.5) | 17.2 (11.9,22.5) |
| 50-54 | 104 | 4.4 (3.6,5.4) | 41.8 (31.4,52.1) | 24 | 6.1 (3.9,9.1) | 10.2 (5.3,15.1) | 40 | 4.1 (2.9,5.5) | 15.5 (9.1,21.8) | 58 | 6.6 (5.0,8.5) | 25.2 (17.6,32.9) |
| 54-59 | 88 | 3.9 (3.1,4.8) | 51.3 (36.8,65.7) | 15 | 4.2 (2.4,7.0) | 8.8 (3.0,14.6) | 46 | 4.4 (3.2,5.9) | 27.5 (17.2,37.8) | 44 | 4.9 (3.6,6.6) | 27.1 (17.1,37.2) |
| 60+ | 106 | 2.5 (2.1,3.0) | 54.6 (37.3,71.9) | 17 | 2.9 (1.7,4.6) | 9.1 (2.5,15.8) | 53 | 2.4 (1.8,3.2) | 26.0 (14.1,37.9) | 62 | 3.2 (2.4,4.1) | 35.7 (22.7,48.6) |
| P-trend | <0.001 | <0.001 | <0.001 | 0.441 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
Abbreviations: CV=cerebrovascular; ICD= International classification of disease; O= observed; SHR=standardised hospitalisation ratio; AER=absolute excess risk; CI=confidence interval; FPN=first primary neoplasms; CNS=central nervous system; NHL=non-Hodgkin lymphoma; STS=soft-tissue sarcoma; GU=genitourinary.
Omitted from table due to few events (less than 5 observed).
Figure 2. Absolute Excess Risk (95% CI) for any and specific types of cerebrovascular event (CV) according to attained age among survivors of CNS tumours.
Head & neck tumour survivors
Male survivors of a head & neck tumour had a significantly greater AER for a cerebrovascular event than female survivors (ptrend=0.002), with an excess of 30 events compared to 11 per 10,000 PY, respectively (Table 4). The AER remained significantly increased for all ages; however the multivariable analysis showed that the AER did not increase significantly with attained age (ptrend=0.071) (Figure 3, Online Table 6).
Table 4.
SHRs and AERs of any cerebrovascular event, Cerebral infarction (ICD10: I63, I65-66) or Other Cerebrovascular Event (ICD10: I67-I68) among survivors of head and neck tumours by sex, age at diagnosis, decade of diagnosis and attained age
| Any CV Event (ICD10: I60-I68) | Cerebral infarction (ICD10: I63, I65-66) | Other CV Event (ICD10: I64, I67-I68) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O | SHR (95%CI) | AER (95%CI) | O | SHR (95%CI) | AER (95%CI) | O | SHR (95%CI) | AER (95%CI) | |
| All HN tumours | 123 | 2.6 (2.2,3.1) | 21.5 (15.4,27.7) | 75 | 3.5 (2.8,4.4) | 15.0 (10.2,19.8) | 51 | 2.7 (2.0,3.5) | 8.9 (5.0,12.8) |
| Sex | |||||||||
| Male | 91 | 2.9 (2.3,3.6) | 30.4 (20.9,39.9) | 58 | 3.8 (2.9,4.9) | 21.5 (14.0,29.0) | 36 | 2.9 (2.0,4.0) | 11.7 (5.8,17.5) |
| Female | 32 | 2.1 (1.4,2.9) | 10.5 (3.5,17.6) | 17 | 2.8 (1.6,4.4) | 6.9 (1.8,12.0) | 15 | 2.3 (1.3,3.8) | 5.4 (0.6,10.2) |
| p-heterogeneity | 0.098 | 0.002 | 0.258 | 0.001 | 0.494 | 0.027 | |||
| Age at FPN Diagnosis (years) | |||||||||
| 15-24 | 12 | 4.6 (2.4,8.0) | 16.0 (4.4,27.6) | 8 | 7.8 (3.3,15.3) | 11.9 (2.4,21.3) | * | * | * |
| 25-29 | 14 | 2.6 (1.4,4.3) | 13.5 (1.9,25.2) | 8 | 3.3 (1.4,6.5) | 8.8 (0.1,17.5) | 8 | 3.9 (1.7,7.6) | 9.4 (0.6,18.1) |
| 30-34 | 31 | 2.5 (1.7,3.6) | 20.4 (8.6,32.3) | 18 | 3.2 (1.9,5.1) | 13.5 (4.5,22.4) | 13 | 2.7 (1.4,4.5) | 8.7 (1.1,16.3) |
| 35-39 | 66 | 2.5 (1.9,3.2) | 28.1 (16.8,39.4) | 41 | 3.3 (2.4,4.5) | 20.1 (11.3,28.9) | 27 | 2.4 (1.6,3.5) | 11.0 (3.9,18.1) |
| p-trend | 0.152 | 0.053 | 0.116 | 0.106 | 0.288 | 0.096 | |||
| Decade of FPN Diagnosis | |||||||||
| 1971-1979 | 35 | 1.8 (1.3,2.5) | 25.5 (6.8,44.2) | 19 | 2.0 (1.2,3.2) | 15.2 (1.7,28.8) | 19 | 2.2 (1.3,3.4) | 16.1 (2.7,29.6) |
| 1980-1989 | 40 | 2.5 (1.8,3.4) | 21.0 (10.1,31.9) | 25 | 3.4 (2.2,5.1) | 15.5 (6.9,24.0) | 18 | 2.8 (1.7,4.5) | 10.1 (2.9,17.3) |
| 1990-2006 | 48 | 4.2 (3.1,5.6) | 20.5 (12.9,28.1) | 31 | 6.5 (4.4,9.2) | 14.6 (8.5,20.7) | 14 | 3.5 (1.9,5.9) | 5.6 (1.5,9.7) |
| p-trend | <0.001 | 0.799 | <0.001 | 0.991 | 0.158 | 0.119 | |||
| Attained Age (years) | |||||||||
| 20-44 | 23 | 4.6 (2.9,6.8) | 13.2 (6.3,20.1) | 15 | 8.6 (4.8,14.2) | 9.7 (4.2,15.3) | 6 | 3.7(1.4,8.1) | 3.2 (-0.3,6.7) |
| 44-49 | 22 | 3.8 (2.4,5.7) | 22.6 (9.7,35.4) | 14 | 6.2 (3.4,10.4) | 16.3 (6.1,26.5) | 7 | 3.4(1.4,7.1) | 6.9 (-0.3,14.0) |
| 50-54 | 23 | 3.2 (2.1,4.9) | 28.6 (11.7,45.5) | 16 | 5.4 (3.1,8.7) | 23.3 (9.3,37.3) | 8 | 3.0 (1.3,6.0) | 9.5 (-0.3,19.4) |
| 54-59 | 18 | 2.3 (1.3,3.6) | 24.5 (4.3,44.7) | 10 | 2.7 (1.3,5.0) | 15.3 (0.4,30.2) | 7 | 2.3 (0.9,4.7) | 9.3 (-3.0,21.7) |
| 60+ | 37 | 1.8 (1.3,2.5) | 32.7 (8.8,56.5) | 20 | 1.8 (1.1,2.9) | 17.9 (0.8,35.0) | 23 | 2.4 (1.5,3.6) | 25.7 (7.5,43.9) |
| p-trend | <0.001 | 0.029 | <0.001 | 0.159 | 0.215 | 0.002 | |||
Abbreviations: CV=cerebrovascular; ICD= International classification of disease; O= observed; SHR=standardised hospitalisation ratio; AER=absolute excess risk; CI=confidence interval; FPN=first primary neoplasms; CNS=central nervous system; NHL=non-Hodgkin lymphoma; STS=soft-tissue sarcoma; GU=genitourinary.
Omitted from table due to few events (less than 5 observed).
Figure 3. Absolute Excess Risk (95% CI) for any and specific types of cerebrovascular (CV) event according to attained age among survivors of head & neck tumours.
Leukaemia survivors
Survivors of all types of leukaemia were at an increased risk of developing a cerebrovascular event and risk did not depend on type of leukaemia (pheterogeneity=0.130) (Table 5). Leukaemia survivors diagnosed aged 15-19 years had the greatest SHR (SHR=5.1, CI=2.8-8.6) and the SHR decreased significantly with older age at diagnosis (ptrend=0.007). The AER increased significantly with attained age until age 55 years (ptrend=0.014) reaching 20 additional hospitalisations per 10,000 PY within individuals aged 50-54 years.
Table 5. SHR, AER, RR and EHR of any cerebrovascular event among survivors of leukaemia by , type of leukaemia, sex, age at diagnosis, decade of diagnosis and attained age.
| O | SHR (95%CI) | RR (95%CI) | AER (95%CI) | EHR (95%CI) | |
|---|---|---|---|---|---|
| All Leukaemia | 70 | 2.5 (1.9,3.1) | 10.2 (6.2,14.2) | ||
| Type of Leukaemia | |||||
| Acute Lymphoblastic | 18 | 3.7 (2.2,5.9) | 1.0 Ref | 12.4 (4.6,20.3) | 1.0 Ref |
| Acute Myeloid | 16 | 1.7 (1.0,2.8) | 0.6 (0.3,1.1) | 4.5 (-0.8,9.7) | 0.3 (0.1,1.7) |
| Chronic Myeloid | 13 | 2.9 (1.6,5.0) | 0.9 (0.4,2.0) | 12.3 (2.1,22.5) | 1.1 (0.3,3.8) |
| Other | 23 | 2.3 (1.5,3.5) | 0.9 (0.4,1.9) | 15.9 (4.5,27.3) | 1.0 (0.3,3.5) |
| p-heterogeneity | 0.13 | 0.297 | 0.15 | 0.322 | |
| Sex | |||||
| Male | 47 | 2.7 (2.0,3.6) | 1.0 Ref | 13.0 (7.1,18.9) | 1.0 Ref |
| Female | 23 | 2.1 (1.3,3.1) | 0.8 (0.5,1.4) | 6.6 (1.4,11.9) | 0.5 (0.2,1.3) |
| p-heterogeneity | 0.29 | 0.409 | 0.262 | 0.258 | |
| Age at FPN Diagnosis (years) | |||||
| 15-19 | 14 | 5.1 (2.8,8.6) | 1.0 Ref | 12.0 (4.2,19.9) | 1.0 Ref |
| 20-24 | 6 | 2.2 (0.8,4.7) | 0.4 (0.2,1.1) | 4.7 (-2.3,11.8) | 0.4 (0.1,1.8) |
| 25-29 | 10 | 2.4 (1.2,4.4) | 0.4 (0.2,1.0) | 8.1 (-0.5,16.8) | 0.4 (0.1,1.8) |
| 30-34 | 22 | 3.0 (1.9,4.5) | 0.5 (0.2,1.2) | 17.4 (6.4,28.4) | 0.6 (0.1,2.7) |
| 35-39 | 18 | 1.6 (0.9,2.5) | 0.2 (0.1,0.7) | 7.3 (-2.0,16.6) | 0.2 (0.0,1.3) |
| p-trend | 0.007 | 0.017 | 0.696 | 0.15 | |
| Decade of FPN Diagnosis | |||||
| 1971-1979 | 13 | 2.3 (1.2,3.9) | 1.0 Ref | 21.7 (0.5,42.9) | 1.0 Ref |
| 1980-1989 | 23 | 2.3 (1.4,3.4) | 1.2 (0.5,2.9) | 11.0 (3.0,19.0) | 1.0 (0.2,4.5) |
| 1990-2006 | 34 | 2.7 (1.9,3.7) | 1.7 (0.6,4.7) | 8.3 (3.9,12.8) | 1.1 (0.2,5.9) |
| p-trend | 0.527 | 0.162 | 0.116 | 0.775 | |
| Attained Age (years) | |||||
| 20-44 | 22 | 2.7 (1.7,4.2) | 1.0 Ref | 5.5 (1.9,9.1) | 1.0 Ref |
| 44-49 | 19 | 3.1 (1.9,4.9) | 1.6 (0.8,3.3) | 17.5 (5.9,29.1) | 5.0 (1.3,18.9) |
| 50-54 | 14 | 2.6 (1.4,4.4) | 1.6 (0.7,3.5) | 20.4 (3.1,37.8) | 6.0 (1.2,30.8) |
| 55+ | 15 | 1.6 (0.9,2.7) | 1.3 (0.5,3.6) | 16.3 (-4.7,37.4) | 7.9 (1.1,55.7) |
| p-trend | 0.142 | 0.429 | 0.014 | 0.01 |
Abbreviations: O= observed; SHR=standardised hospitalisation ratio; AER=absolute excess risk; CI=confidence interval; RR=relative risk; EHR=excess hospitalisation ratio.
Risk of a cerebral haemorrhage
The SHR for a cerebral haemorrhage was twice that expected (SHR=2.0, CI=1.8-2.1). The risk of developing a cerebral haemorrhage among survivors diagnosed more recently (1990-2006), was increased nearly 4-fold relative to that expected from the general population (SHR=3.7, CI=2.9-4.7); whereas it was only 20% increased among survivors diagnosed earlier (1971-1979) (SHR= 1.2, CI=1.0-1.4) (Table 1). This increase in risk for the recent treatment decade was further confirmed by the multivariable analysis (Ptrend=0.005) (Online Table 4). In terms of attained age, the SHR was highest for survivors younger than 45 years (SHR=3.6, CI=3.1-4.2) and declined with increasing attained age, however remained significantly elevated for all ages (SHR at age 60+years = 1.2, CI=1.0-1.4) (Table 2).
With regards to TYA cancer type, the SHR was highest among survivors of a CNS tumour and leukaemia, with an 8-fold (SHR=8.3, CI=7.2-9.6) and 5-fold (SHR=5.2, CI=3.4-7.6) increased risk, respectively (Table 1). The cumulative incidence of developing a cerebral haemorrhage by age 60 was 3.4% and 2.0% among CNS tumour and leukaemia survivors, respectively, whereas 0.4% was expected (Figure 1b). Notably, survivors of melanoma were at increased risk of developing a cerebral haemorrhage (SHR=2.0 CI=1.5-2.6); but were not at significantly increased risk of any other cerebrovascular event.
CNS tumour survivors
The risk of developing a cerebral haemorrhage was significantly elevated among all CNS tumour survivors. Survivors of glial tumours were at greatest risk (SHR=26.2, CI=21.5-31.6)(Table 3). The SHR and AER of developing a cerebral haemorrhage among CNS survivors diagnosed more recently (1990-2006), was 27-fold increased relative to that expected from the general population (SHR=26.5, CI=18.6-36.7; AER=20); whereas it was only 4-fold increased among survivors diagnosed earlier (1971-1979) (SHR= 4.2 , CI=2.7-6.1; AER=10). This increase in the SHRs and AERs by more recent treatment decade was-confirmed by the multivariable analysis (ptrend<0.001 and 0.005, respectively) (Online Table 5).
Risk of cerebral infarction
Overall, survivors had a 50% significantly increased risk of a cerebral infarction compared to that expected (SHR=1.5; CI=1.4-1.6; AER=2). In terms of age at cancer diagnosis, the SHR and AER for a cerebral infarction was highest among survivors diagnosed aged 15-19 years (SHR=4.3, CI=3.3-5.4; AER=4) (Table 1); who had twice the risk of a cerebral infarction than survivors diagnosed aged 35-39 years (RR=0.5, CI=0.4-0.7) (Online Table 4). The AER increased with increasing attained age (ptrend<0.001) (Table 2); after adjusting for potential confounders, survivors aged over 60 years had 6-fold the number of excess cerebral infarction than survivors aged less than 45 years (EHR=5.6, CI=2.9-10.8).
With regards to TYA cancer type, the SHR was highest among survivors of CNS tumours (SHR=4.4, CI=3.9-5.0), head & neck tumours (SHR=3.5, CI=2.8-4.4), leukaemia (SHR=2.3, CI=1.5-3.3), and Hodgkin lymphoma (SHR=2.1, CI=1.8-2.5) (Table 1). The cumulative incidence of developing a cerebral infarction before age 60 was 4.2%, 3.6%, 2.3% and 2.0% among head & neck tumour, CNS tumour, leukaemia and Hodgkin lymphoma survivors, respectively, whereas 0.9% was expected (Figure 1c).
CNS tumour survivors
The risk of developing a cerebral infarction was significantly elevated among all CNS tumour survivors, with the exception of ‘other specified’ CNS tumour group. Survivors of embryonal tumours (SHR=18.8, CI=10.0-32.1) and germ cell tumours (SHR=10.5, CI=3.4-24.6)were at greatest risk (Table 3). The AER was substantially elevated among survivors of embryonal tumours, with 49 excess cerebral infarctions per 10,000PY (Table 3). AERs increased sharply with attained age reaching 26 excess cerebral infarctions per 10,000 PY in survivors aged 60 years or older (ptrend<0.001) (Figure 2, Table 3). In terms of absolute risk, every year approximately 0.4% of CNS tumour survivors over age 60 years were hospitalised for a cerebral infarction compared to approximately 0.2% in the general population.
Head & neck tumour survivors
Among head & neck tumour survivors, the AER for a cerebral infarction was higher among males than females (AER=22 and AER=7, respectively; Ptrend<0.001) (Table 4). The SHR decreased with attained age (ptrend<0.001), however remained elevated for all ages. The AER was high for all ages (AER=10-23); however did not vary significantly with attained age (ptrend=0.159) (Figure 3). In terms of absolute risk, every year approximately 0.2% of head & neck tumour survivors were hospitalised for a cerebral infarction compared to approximately 0.06% in the general population.
Risk of subarachnoid haemorrhage
The risk of being hospitalised for a subarachnoid haemorrhage among TYA cancer survivors did not differ significantly from that observed in the general population (SHR=1.0, CI=0.9-1.1; AER=0) (Results not shown).
Risk of ‘other cerebrovascular events’
Survivors had a 40% significantly increased risk of any ‘other cerebrovascular events’ compared to that expected (SHR=1.4, CI=1.3-1.5; AER=2) (Table 1). The SHR and AER was highest among survivors diagnosed aged 15-19 years (SHR=4.1, CI=3.1-5.4; AER=3) (Table 1); survivors diagnosed aged 15-19 years had over twice the risk of developing ‘other cerebrovascular events’ than survivors aged 35-39 years at diagnosis (Online Table 4). The AER increased with increasing attained age reaching 2 per 10,000 person-years after age 60 years, which was confirmed by the multivariable analysis, where the EHR increased substantially with attained age (ptrend<0.001) (Table 2).
With regards to TYA cancer type, the risk was highest among survivors of: a CNS tumour (SHR=5.1, CI=4.5-5.8; AER=15), head & neck tumours (SHR=2.7, CI=2.0-3.5;AER=9) and leukaemia (SHR=2.6, CI=1.7-3.8; AER=4) (Table 1). The cumulative incidence of developing an ‘other cerebrovascular event’ by age 60 was 3.3%, 2.2% and 1.9% among CNS tumour, leukaemia and head & neck tumour survivors, respectively, whereas 0.8% was expected (Figure 1d).
CNS tumour survivors
Among CNS tumour survivors, the SHR and AER for ‘other cerebrovascular events’ was highest among survivors of embryonal tumours (SHR=20.2, CI=10.4-35.3 AER=46) and germ cell intracranial tumours (SHR=12.6, CI=4.1-29.5; AER=23) (Table 3). AERs increased substantially with attained age reaching 36 excess ‘other cerebrovascular events’ per 10,000 PY in CNS tumour survivors aged 60 years or older (ptrend<0.001) (Figure 2, Table 3).
Head & neck tumour survivors
Among head & neck tumour survivors, the AER increased significantly with attained age reaching 26 excess ‘other cerebrovascular events’ per 10,000PY in survivors aged 60 years or older (ptrend=0.002) (Figure 3, Table 4).
Discussion
Main Findings
In this largest ever study of TYA cancer survivors, we report a 40% increased risk of hospitalisation for a cerebrovascular event compared to that expected in the general population. TYA cancer survivors are at 2-fold, 1.5-fold and 1.4-fold increased risk of a cerebral haemorrhage, cerebral infarction and `other cerebrovascular eventˋ, respectively. By age 60, 9%, 6% and 5% of CNS tumour, head & neck tumour, and leukaemia survivors, respectively, had been hospitalised for a cerebrovascular event. Male TYA cancer survivors had a significantly higher number of excess cerebrovascular events compared to female survivors, especially cerebral infarction among head and neck tumour survivors. We found that the excess risk of developing a cerebral infarction among CNS tumour survivors increases significantly with attained age. Beyond age 60, every year approximately 0.4% of CNS tumour survivors can expect to be hospitalised for a cerebral infarction. The excess risk of developing a cerebral infarction among head & neck tumour survivors did not vary with attained age but was consistently high. Every year approximately 0.2% of head & neck tumour survivors can expect to be hospitalised for a cerebral infarction.
Previous studies
The only other large-scale cohort investigating hospitalisation for cerebrovascular disease—a Danish cohort study of 43,153 1-year survivors of TYA cancer (age 15-39 years)—found a significant 1.3-fold increased risk of hospitalisation for any cerebrovascular disease for all survivors combined, which is comparable to the 1.4-fold increased risk observed in our study of 178,964 5-year survivors of TYA cancer 16. In a smaller scale study; van Laar et al. did not observe a significantly increased risk of cerebrovascular disease among 1,880 5-year survivors of TYA cancer diagnosed between 15 and 29 years in Yorkshire, England; however the study included only 7 cerebrovascular events compared to 3,201 in the current study17.
The Danish study by Rugbjerg et al. demonstrated that CNS tumour survivors had a substantially increased risk of being hospitalised for a cerebrovascular event (SHR=3.9, CI: 3.4-4.5), particularly for a cerebral haemorrhage (SHR=7.6, CI=5.7-10.2) and other undefined cerebrovascular disease (SHR=4.5, CI=3.6-5.5) 16; these findings are generally consistent with the risk estimates observed in our study. In contrast, this study showed that CNS tumour survivors had a 4.4-fold (CI=3.9-5.0) increased risk of developing a cerebral infarction—significantly higher than the 2.9-fold (CI=2.2-3.8) risk observed by Rugbjerg et al. One explanation for this difference could be the more recent diagnosis period of our cohort (individuals diagnosed 1971-2006) compared to the Danish cohort (individuals diagnosed 1943-2009); hence the background risk of developing a cerebral infarction in our study was lower. To our knowledge, we provide for the first time, risk estimates for cerebrovascular disease by type of CNS tumour among TYA cancer survivors. Risk estimates for developing cerebrovascular disease for other tumour types, such as testicular, Hodgkin lymphoma, non-Hodgkin lymphoma, ovary, cervical, melanoma, thyroid and leukaemia were remarkably similar to those found by Rugbjerg et al16.
Potential Radiation-Induced Cerebrovascular Disease
Although the mechanisms of radiation-induced cerebrovascular disease are unknown, cranial irradiation has been implicated in causing direct damage to endothelial cells of the cerebral arteries resulting in weakened vessels, accelerated atherosclerosis and vascular insufficiency secondary to stenosis or occlusion12, 13, 25. Previous studies among childhood CNS tumour survivors have reported a strong linear dose-response relationship between the amount of cranial irradiation exposure and risk of developing stroke7, 8, 10, 11. Prophylactic cranial irradiation for childhood leukaemia has also been shown to increase the risk of stroke in a dose-dependent manner11. Given these observations among childhood cancer survivors, the substantially increased risks of cerebrovascular disease observed here among TYA survivors of CNS tumour and leukaemia are probably, to at least some extent, related to cranial irradiation as well. Nonetheless, some cerebrovascular events—particularly cerebral haemorrhage—may be caused by compression or damage of intracranial vessels from local recurrence of a CNS tumour or brain metastasis from other tumours (e.g. melanoma or head & neck cancer)14. This could explain the increase in cerebral haemorrhage among CNS tumour survivors treated most recently—a higher proportion of high grade glial tumours (which are likely to recur) were observed in 1990-2006 compared to previous decades. We cannot rule out the possibility that the increase in cerebral haemorrhage among CNS tumour survivors diagnosed more recently may be an artefact of greater frequency of CNS imaging or improved diagnosis of haemorrhage in more recent years. Melanoma survivors were at increased risk of a cerebral haemorrhage, but not any other type of cerebrovascular event. Melanoma metastases are commonly haemorrhagic 26 , therefore this increase could in fact be due to metastases to the brain from recurrent melanoma subsequent to 5-year survival.
This study observed a substantially increased risk of cerebral infarction among head and neck tumour survivors. Several previous studies—mainly among head and neck cancer patients diagnosed after age 40 years—have observed an increased risk of cerebral infarction after radiation to the head and neck 12, 13, 27, 28; this is likely due to radiation-induced damage of the carotid artery 25. The exact mechanism underlying the radiation-induced damage of the carotid artery remains elusive; however it may be due to direct damage to endothelial cells which are very radiosensitive, injury to the microvasculature network or accelerated atherosclerosis 25, 29–32. Radiation-induced carotid artery disease can lead to higher risk of cerebral infarction due to stenosis of the carotid artery itself or by embolism of a dislodged thrombus blocking a cerebral artery 25. However, there is the possibility that both head and neck cancer and cerebrovascular disease have a shared aetiology, thus smoking and alcohol may also be implicated in the increased risk of cerebrovascular disease. Nonetheless, all head and neck tumour survivors in the TYA cohort were diagnosed under age 40 years, and thus, exposure to smoking and alcohol is likely to play a lesser role in the aetiology of head and neck cancer compared to patients diagnosed after age 40 years33. In addition to carotid artery irradiation, an increased risk of cerebral infarction after mediastinal irradiation has also been observed among a population of Hodgkin lymphoma survivors, likely due to cardioemboli9. This, in addition to carotid artery irradiation could explain the 2-fold increased risk of cerebral infarction observed among Hodgkin lymphoma survivors in our study.
In this study, the risk of cerebral haemorrhage was greatest among CNS tumour survivors diagnosed most recently (2000-2006). The increasing trend observed within this diagnosis period was significant for glial tumour survivors only (results not shown). Among survivors, the proportion of gliomas considered high grade increased from 9.5% in 1971-1979 to 17.5% in 2000-2006. High grade tumours are more difficult to treat successfully and are likely to recur and it is thus not inconceivable that, although a greater proportion of these individuals are becoming 5-year survivors, more aggressive treatment and/or recurrence of the tumour could increase the risk of cerebral haemorrhage.
Recommendations for prevention of cerebrovascular disease
To our knowledge, there are no specific guidelines for stroke prevention among cancer survivors. The American Heart Association/American Stroke Association (AHA/ASA) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines recommend that individuals who are considered to be at high risk of stroke in the general population (e.g. history of coronary heart disease, previous stroke, high blood pressure, and diabetes mellitus) undergo regular blood pressure checks, implement lifestyle modifications (stop smoking and reduce alcohol consumption) and are considered for pharmacological intervention (e.g. antihypertensive medication and statins) to reduce the risk of stroke 34, 35. In this study, the absolute excess risk of cerebral infarction among CNS tumour survivors and ‘Other Cerebrovascular Event’ among head & neck tumour survivors increased with attained age, resulting in substantial numbers of excess cerebral infarctions and ‘Other Cerebrovascular Event’ among older survivors. There is evidence suggesting that stroke risk is increased in cancer survivors treated with cranial irradiation in the absence of atherosclerotic risk factors 8, 12. As previously mentioned, cranial irradiation has been implicated in causing accelerated atherosclerosis, therefore it could be argued that cranial irradiation itself is an atherosclerotic risk factor and patients treated with cranial irradiation should be considered at high risk such as patients with hypertension and diabetes. It may therefore not be unreasonable to suggest that TYA CNS tumour and head & neck tumour survivors (likely treated with head & neck irradiation) should be considered for pharmacological interventions even in the absence of other risk factors. A randomised intervention study may be required to ascertain if, and to the extent to which, these survivors would benefit from such treatments.
Study strengths and limitations
Most previous studies used questionnaires to ascertain cerebrovascular events which rely on self-report and may suffer from non-response or recall bias. A strength of the current study—in addition to the large cohort size—was that the ascertainment of cerebrovascular events was entirely population-based through linkage of the TYACSS cohort with the population-based HES dataset, thereby eliminating potential non-response or recall bias. A limitation of our study is the lack of detailed treatment information, particularly the lack of detailed information on treatment with cranial radiotherapy (e.g. availability of cumulative radiation dose to the cranium). However, it would not be practically feasible to collect detailed information on treatment for the entire cohort of 178,962 survivors due the destruction of older medical notes in addition to cost and time restrictions. Nested case-control studies would be required to investigate the risks of developing cerebrovascular disease in relation to elements of treatment.
Modifiable lifestyle factors such as hypertension, diabetes, obesity and smoking may contribute to the elevated risk of cerebrovascular disease among cancer survivors treated with radiotherapy to the head, neck and mediastinum 9, 12, 27,however, we cannot confirm this as collecting information on lifestyle factors was beyond the scope of this study.
We acknowledge that survivors in this cohort were treated between 1971 and 2006, thus the findings presented may not be translatable to individuals treated with newer therapies in recent years e.g. proton beam therapy. Future prospective studies would be needed to investigate risks of cerebrovascular events after newer treatment protocols.
Conclusions
In this large scale study investigating cerebrovascular events in teenage and young adult cancer survivors, we found that survivors of a CNS tumour, head & neck tumour, and leukaemia are particularly at risk of hospitalisation for a cerebrovascular event. The excess risk of developing a cerebral infarction among CNS tumour survivors increases with attained age.For head & neck tumour survivors this excess risk remains high across all ages. These groups of survivors, and in particular males, should be considered for surveillance of cerebrovascular risk factors and potential pharmacological interventions for cerebral infarction prevention.
Supplementary Material
Clinical Perspective.
What is new? Teenage and young adult cancer survivors are widely acknowledged to be an understudied population and little is known about the risks of long-term adverse health outcomes in this group of survivors. This is the largest ever study to investigate the risk of hospitalisation for specific cerebrovascular events among survivors of all and specific types of cancer diagnosed in individuals aged 15-39 years. In addition, this is the first study to investigate whether the risk of hospitalisation for a cerebrovascular event varies with attained age, age at diagnosis and decade of diagnosis.
What are the clinical implications? This study found that survivors of a CNS tumour, head & neck tumour, and leukaemia are particularly at risk of hospitalisation for a cerebrovascular event. The absolute excess risk of developing a cerebral infarction among CNS tumour survivors’ increases with attained age and that for head & neck tumour survivors this excess risk remained high across all ages. These groups of survivors should be considered for surveillance of cerebrovascular risk factors and potential pharmacological interventions for cerebral infarction prevention.
Acknowledgements
The Teenage and Young Adult Cancer Survivor Study acknowledges and thanks its data providers: Office for National Statistics, Welsh Cancer Registry and NHS-Digital.
Study collaborators include: Professor Sarah Darby, University of Oxford; Dr Angela Edgar, Royal Hospital for Sick Children, Edinburgh; Dr Richard Feltbower, University of Leeds; Dr Lorna Anne Fern, University College London; Dr Diana Greenfield, University of Sheffield; Dr Tony Moran, North West Cancer Intelligence Service; Professor John Radford, University of Manchester; Dr Peter Rose, University of Oxford; Dr Helen Alexandra Spoudeas, University College London Hospitals NHS Foundation Trust; Dr William Hamish Wallace, Royal Hospital for Sick Children, Edinburgh; Professor Jeremy Whelan, University College London Hospitals NHS (National Health Service) Foundation Trust London.
Finally, we are thankful to the National Cancer Research Institute - Teenage and Young Adult -Clinical Studies Group.
Sources of Funding
This study would not have been possible without funding from Cancer Research UK (grant number C386/A11709. Other funding was through a post-doctoral fellowship to Dr Raoul Reulen from the National Institute for Health Research (PDF-2012-05-280).
Footnotes
Disclosures
All authors declare that they have no conflicts of interest in relation to this work. This report is independent research and the views expressed in this publication are those of the author(s) and not necessarily those of the NHS-Digital, Cancer Research UK, the National Institute for Health Research, the Office of National Statistics or the Welsh Cancer Registry.
References
- 1.Cancer Research UK. CancerStats: Teenage and Young Adult Cancer-Survival. 2013.
- 2.Trama A, Botta L, Foschi R, Ferrari A, Stiller C, Desandes E, Maule MM, Merletti F, Gatta G. Survival of European adolescents and young adults diagnosed with cancer in 2000-07: population-based data from EUROCARE-5. The Lancet Oncology. 2016 doi: 10.1016/S1470-2045(16)00162-5. [DOI] [PubMed] [Google Scholar]
- 3.Woodward E, Jessop M, Glaser A, Stark D. Late effects in survivors of teenage and young adult cancer: does age matter? Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2011;22:2561–8. doi: 10.1093/annonc/mdr044. [DOI] [PubMed] [Google Scholar]
- 4.Coccia PF, Altman J, Bhatia S, Borinstein SC, Flynn J, George S, Goldsby R, Hayashi R, Huang MS, Johnson RH, Beaupin LK, et al. Adolescent and Young Adult Oncology. Journal of the National Comprehensive Cancer Network. 2012;10:1112–1150. doi: 10.6004/jnccn.2012.0117. [DOI] [PubMed] [Google Scholar]
- 5.The Adolescent and Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. 2006.
- 6.Barr RD, Ferrari A, Ries L, Whelan J, Bleyer WA. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016;170:495–501. doi: 10.1001/jamapediatrics.2015.4689. [DOI] [PubMed] [Google Scholar]
- 7.Mueller S, Sear K, Hills NK, Chettout N, Afghani S, Gastelum E, Haas-Kogan D, Fullerton HJ. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. International journal of radiation oncology, biology, physics. 2013;86:643–8. doi: 10.1016/j.ijrobp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller S, Fullerton HJ, Stratton K, Leisenring W, Weathers RE, Stovall M, Armstrong GT, Goldsby RE, Packer RJ, Sklar CA, Bowers DC, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. International journal of radiation oncology, biology, physics. 2013;86:649–55. doi: 10.1016/j.ijrobp.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bruin ML, Dorresteijn LD, van't Veer MB, Krol AD, van der Pal HJ, Kappelle AC, Boogerd W, Aleman BM, van Leeuwen FE. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. Journal of the National Cancer Institute. 2009;101:928–37. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 10.Haddy N, Mousannif A, Tukenova M, Guibout C, Grill J, Dhermain F, Pacquement H, Oberlin O, El-Fayech C, Rubino C, Thomas-Teinturier C, et al. Relationship between the brain radiation dose for the treatment of childhood cancer and the risk of long-term cerebrovascular mortality. Brain : a journal of neurology. 2011;134:1362–72. doi: 10.1093/brain/awr071. [DOI] [PubMed] [Google Scholar]
- 11.Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, Robison LL, Packer RJ, Oeffinger KC. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5277–82. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 12.Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, van Leeuwen FE, Bartelink H. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:282–8. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 13.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke; a journal of cerebral circulation. 2011;42:2410–8. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 14.Dearborn JL, Urrutia VC, Zeiler SR. Stroke and Cancer-A Complicated Relationship. Journal of neurology translational neuroscience. 2014;2:1039. [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad PK, Signorello LB, Friedman DL, Boice JD, Jr, Pukkala E. Long-term non-cancer mortality in pediatric and young adult cancer survivors in Finland. Pediatr Blood Cancer. 2012;58:421–7. doi: 10.1002/pbc.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rugbjerg K, Mellemkjaer L, Boice JD, Kober L, Ewertz M, Olsen JH. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943-2009. Journal of the National Cancer Institute. 2014;106:dju110. doi: 10.1093/jnci/dju110. [DOI] [PubMed] [Google Scholar]
- 17.van Laar M, Feltbower RG, Gale CP, Bowen DT, Oliver SE, Glaser A. Cardiovascular sequelae in long-term survivors of young peoples’ cancer: a linked cohort study. British journal of cancer. 2014;110:1338–41. doi: 10.1038/bjc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroke Association. State of the Nation-Stroke statistics. 2016.
- 19.Birch JM, Alston RD, Kelsey AM, Quinn MJ, Babb P, McNally RJ. Classification and incidence of cancers in adolescents and young adults in England 1979-1997. British journal of cancer. 2002;87:1267–74. doi: 10.1038/sj.bjc.6600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106:1425–30. doi: 10.1002/cncr.21773. [DOI] [PubMed] [Google Scholar]
- 21.Spencer A. Hospital Episode Statistics (HES): Improving the quality and value of hospital data. 2011.
- 22.Office for National Statistics. Population Estimates for UK, England and Wales, Scotland and Northern Ireland. 2016 [Google Scholar]
- 23.Breslow NE, Day NE. Statistical Methods in Cancer Research: Volume II, The Design and Analysis of Cohort Studies. IARC Scientific Publications; Lyon, France: 1987. [PubMed] [Google Scholar]
- 24.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Statistics in medicine. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Cao Y. Radiation-induced carotid artery stenosis: a comprehensive review of the literature. Interv Neurol. 2014;2:183–92. doi: 10.1159/000363068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo H, Jung E, Gwak HS, Shin SH, Lee SH. Surgical outcomes of hemorrhagic metastatic brain tumors. Cancer Res Treat. 2011;43:102–7. doi: 10.4143/crt.2011.43.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu CN, Chen SW, Bai LY, Mou CH, Hsu CY, Sung FC. Increase in stroke risk in patients with head and neck cancer: a retrospective cohort study. British journal of cancer. 2011;105:1419–23. doi: 10.1038/bjc.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang YS, Lee CC, Chang TS, Ho HC, Su YC, Hung SK, Lee MS, Chou P, Chang YH, Lee CC. Increased risk of stroke in young head and neck cancer patients treated with radiotherapy or chemotherapy. Oral Oncol. 2011;47:1092–7. doi: 10.1016/j.oraloncology.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Dorresteijn LDA, Kappelle AC, Scholz NMJ, Munneke M, Scholma JT, Balm AJM, Bartelink H, Boogerd W. Increased carotid wall thickening after radiotherapy on the neck. European Journal of Cancer. 2005;41:1026–1030. doi: 10.1016/j.ejca.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Cheng SK, Ting AW, Lam L, Wei WI. CArotid stenosis after radiotherapy for nasopharyngeal carcinoma. Archives of Otolaryngology-Head Neck Surgery. 2000;126:517–521. doi: 10.1001/archotol.126.4.517. [DOI] [PubMed] [Google Scholar]
- 31.Bashar K, Healy D, Clarke-Moloney M, Burke P, Kavanagh E, Walsh SR. Effects of neck radiation therapy on extra-cranial carotid arteries atherosclerosis disease prevalence: systematic review and a meta-analysis. PLoS One. 2014;9:e110389. doi: 10.1371/journal.pone.0110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gujral DM, Chahal N, Senior R, Harrington KJ, Nutting CM. Radiation-induced carotid artery atherosclerosis. Radiother Oncol. 2014;110:31–8. doi: 10.1016/j.radonc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S. An analysis of risk factors for oral cancer in young people: a case-control study. Oral Oncology. 2004;40:304–313. doi: 10.1016/j.oraloncology.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2014;45:3754–832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scottish Intercollegiate Guidelines Network. Risk estimation and the prevention of cardiovascular disease: a national clinical guideline. 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.