Abstract
Objectives
Children with perinatally-acquired HIV (PHIV) and taking antiretroviral therapy (ART) have a high prevalence of subclinical cardiac disease. We hypothesised that cardiac disease may be a consequence of dysregulated systemic immune activation driven by HIV infection. We examined cardiovascular and proinflammatory biomarkers and their association with echocardiographic abnormalities in children with PHIV.
Design
Cross-sectional analysis of soluble biomarkers from a prospective cohort of children aged 6-16 years with PHIV and age-matched HIV-uninfected comparison group.
Methods
Cryopreserved plasma samples were used to measure seven soluble biomarkers using multiplex bead assay (Luminex). Multivariable logistic regression assessed how biomarker levels related to cardiac abnormalities.
Results
A total of 406 children participated in this study (195 PHIV and 211 HIV-uninfected). Mean (standard deviation (SD)) ages of PHIV and HIV-uninfected participants were 10.7 (2.6) and 10.8 (2.8) years, respectively. Plasma levels of CRP, TNF-α, ST2, VCAM-1 and GDF-15 were significantly higher in the PHIV group compared to uninfected control (p<0.001). Among children with PHIV, with one-unit representing one SD in biomarker level, a one-unit increase in CRP and GDF-15, was associated with increased odds of having left ventricular (LV) diastolic dysfunction [adjusted odds ratio (aOR), 1.49 (1.02–2.18; P<0.040)] and [aOR 1.71 (1.18–2.53; P=0.006)] respectively. Each one unit increase in GDF-15 was associated with increased odds of LV hypertrophy [aOR 1.84 (95% CI 1.10-3.10; p<0.021)].
Conclusion
Children with PHIV had higher levels of proinflammatory and cardiovascular biomarkers compared to HIV-uninfected children. Increased CRP and GDF-15 were associated with cardiac abnormalities in children with PHIV.
Keywords: biomarkers, perinatal HIV, ART, children, echocardiographic abnormalities
Introduction
Prior to widespread adoption of antiretroviral therapy (ART), cardiac disease in children was an indicator of advanced HIV infection and typically manifested as dilated cardiomyopathy and congestive heart failure [1–4]. This has become rare since the scale up of ART. However, several studies in the ART era report a high burden of subclinical cardiac disease, typified by left ventricular (LV) diastolic dysfunction [5, 6], LV hypertrophy and RV dilatation [7–10].
The underlying mechanism of cardiac disease in individuals with HIV is poorly understood. HIV infection is associated with dysregulated systemic immune activation, and in adults several studies have shown an association of non-AIDS defining illnesses, including cardiovascular disease, with markers of chronic inflammation [11]. Pro-inflammatory biomarkers including interleukin 6 (IL-6), tumour necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) are reportedly elevated in adults with HIV and associated with several cardiovascular conditions [12].
Cardiac biomarkers, including N-terminal pro B-type Natriuretic Peptide (NT-proBNP) and cardiac troponin I (cTnI), are released in response to cardiac damage and stress and have a major diagnostic and prognostic role across a wide spectrum of cardiac diseases [13–18]. More recently, growth differentiating factor-15 (GDF-15) and suppression of tumorigenicity 2 (ST2) have been found to predict cardiovascular events in both the general population and in adults with HIV [14, 19, 20]. These biomarkers are released by cardiomyocytes and upregulated in response to mechanical strain [14]. Adhesion molecules including intercellular and vascular adhesion molecules 1 (ICAM-1 and (VCAM-1, respectively) are associated with endothelial dysfunction and strongly linked to a higher risk of cardiovascular disease in the general population [21].
Children with PHIV have higher markers of immune activation and inflammation compared to HIV-exposed but uninfected controls [13] and more recently, these markers were found to be associated with HIV-related comorbidity, chronic lung disease in PHIV [22]. However, little is known about the effect of HIV on specific cardiovascular and pro-inflammatory biomarkers and their association with cardiac abnormalities among children in the contemporary ART era. The main objectives of this study were to examine cardiovascular and proinflammatory biomarkers and to determine their association with cardiac structural and functional abnormalities in children with PHIV.
Methods
Study design
The INHALE (Investigation of heart and lung disease) was a prospective cohort study, conducted between August 2014 and December 2017, that investigated heart and lung diseases in older children and adolescents aged between 6-16 years, on ART for at least 6 months with no acute clinical symptoms.
The study enrolled 201 children with PHIV and 282 HIV-uninfected controls. Children with PHIV were followed up at 18 months. Details of the inclusion/exclusion criteria as well as findings on lung and cardiac diseases of INHALE study have been published elsewhere [7, 23–26]. Ethical approval was obtained from the Medical Research Council of Zimbabwe, Biomedical Research and Training Institute (BRTI) and London School of Hygiene & Tropical Medicine.
Transthoracic echocardiograms were performed at baseline and 18-month follow-up in children with PHIV only [7, 23], according to American Society of Echocardiography (ASE) recommendations [27]. Briefly, the following cardiac measures were performed: left ventricular (LV), right ventricular (RV), left (LA) and right atrial (RA) dimensions and function. Cardiac dimensions were converted to z-scores using local references [25], to provide LV and RV diameter in diastole z-scores (LVddz) and (RVddz) respectively, interventricular septum in diastole z-scores (IVSdz), LV posterior wall in diastole z-scores (LVPWdz), LA and RA z-scores and finally tricuspid annular plane systolic excursion (TAPSE) z-scores.
Measurement of soluble biomarkers
Cryopreserved baseline plasma samples from the INHALE study participants were used to investigate the levels of circulating biomarkers. Seven biomarkers associated with cardiovascular disease in individuals without HIV were selected. Plasma levels of the following soluble biomarkers: CRP, GDF-15, ICAM-1, IL-6, ST2, TNF-α and VCAM-1 were measured using the Luminex multiplex bead assay on a MagPix instrument. On the day of the assay, cryopreserved plasma samples were thawed and equilibrated to room temperature. Samples were then spun at 16,000 xg for 4 minutes immediately prior to dilution. Assays were then run as per manufacturer’s protocol (Luminex technology, Hertogenbosch, Netherlands). Each sample and the standards used to create each standard curve were run in duplicate. The means of the technical replicates were taken, and the values from the blank fluorescence intensity were subtracted from each reading. Standard curves for each analyte were plotted using five parameter logistic curve fitting. Where samples were diluted, the concentration read from the standard curve were multiplied by the dilution factor to generate a final reading. Where samples had measurements falling outside the standard curve, they were repeated at an appropriate dilution. After repeats, biomarkers with detectable levels falling below the standard curve were assigned half the lower limit of quantification. All panels were run at the Biomedical Research and Training Institute laboratory in Harare.
Statistical Analysis
Data were analysed using STATA 15 (StataCorp, Texas, USA) and R Studio (Version 1.1.383). Baseline demographic and clinical characteristics from the INHALE study are presented as means (standard deviation (SD)) or median ± interquartile ranges (IQR) or proportions with corresponding percentages (n, %), as appropriate. Comparison of clinical characteristics between participants with and without HIV was done using chi-squared or independent t-tests, as appropriate. Differences in biomarker levels between groups, either those with and without HIV or (among those with HIV) those virally suppressed (HIV viral load <400 copies/ml) or not were assessed using Wilcoxon rank sum test. Comparisons of biomarker levels between virally suppressed children with PHIV and those without HIV were done as a sensitivity analysis.
A correlation matrix between the biomarkers under study was constructed using the ggcorplot package in R. Spearman rank correlation coefficients were calculated for each pair of biomarkers in the HIV-positive and HIV-negative group. We explored relationships between biomarkers and associations between biomarkers and echocardiographic Z-scores (for RV and LV diameter and wall thickness, RV function and LA diameter) for those with HIV. Biomarker levels were first transformed into standardised log10 levels. The association between log10-transformed biomarkers and echocardiographic Z-scores was explored using linear regression in univariable and multivariable analysis adjusting for continuous age, sex, body surface area and log10 viral load. Multivariable logistic regression was used to determine the odds of having specific cardiac abnormalities, including LV diastolic dysfunction, LV hypertrophy, RV and LA dilatation in relation to continuous transformed biomarker levels, with a one-unit change in biomarker signifying a one standard deviation increase. A p-value of 0.05 or less was considered significant.
Results
A total of 406 participants with available stored plasma samples were included in the analysis (n=195 with HIV, 98 (48%) female and n=211 without HIV, 109 (52%) female). Those excluded did not have stored baseline plasma samples (n= 77). Mean ages were similar in HIV and HIV-uninfected participants. Participants with HIV were more likely to be stunted (p<0.001). Among participants with HIV, median (IQR) age at ART initiation was 6 (3 – 8) years and duration on ART was 4.8 (2.7 – 6.4) years; 152 (78%) were virally suppressed (Table 1).
Table 1. Clinical characteristics of the participants with and without HIV.
| Characteristics | HIV+ N= 195 Mean (SD) | HIV- N=211 Mean (SD) | P-value |
|---|---|---|---|
| Female, n (%) a | 94 (48.0) | 109 (52.0) | 0.487 |
| Age, y | 10.7 (2.6) | 10.8 (2.8) | 0.671 |
| Height-for-age z-score b, | -1.27 (1.1) | -0.27 (1.1) | <0.001 |
| Stunted, N (%) a, c, | 48 (25.0) | 15 (7.0) | <0.001 |
| Weight-for age z-score b, d | -1.11 (1.3) | -0.23 (1.1) | <0.001 |
| Wasted, N (%) a, c, d | 44 (23.0) | 8 (4.0) | <0.001 |
| Body surface area, m2 | 1.05 (0.2) | 1.18 (0.3) | <0.001 |
| Viral load | |||
| Viral load, copies/ml, median (IQR) | 19 (19 – 208) | ||
| Viral suppression, (<400 copies/ml) n (%) | 152 (78) | ||
| CD4 count, cells/μl, median (IQR) d | 710 (473 – 899) | ||
| CD4 count<200, (cells/mm3), N (%) | 9 (5) | ||
| Age at HIV diagnosis, y, median (IQR) d | 5 (3 – 7) | ||
| Age at ART start, y, median (IQR) d | 6 (3 – 8) | ||
| Duration on ART, y, median (IQR) d | 4.8 (2.7 – 6.4) | ||
| ART regimen | |||
| 2 NRTI + PI, n (%) | 24 (21) | ||
| 2 NRTI + NNRTI, n (%) | 88 (75) | ||
| Unknown, n (%) | 5 (4) |
chi-squared test
Weight-for-age and height-for-age z-scores were calculated using British 1990 Growth Reference [53].
Z-score <-2.
Missing values: Weight-for age z-score- n=1 HIV+, n=1 HIV-; Body surface area- n=2 HIV+, n=1 HIV-: CD4 count- n=3; Age at ART start- n=3; Age at HIV diagnosis- n=1; Duration on ART- n=2
y, years; SD, standard deviation; IQR, interquartile range; BMI, body mass index; BP, blood pressure; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase.
Echocardiographic data were available for 191/195 (98%) PHIV at baseline. Of these, 80 (42%) had cardiac abnormalities (LV dilatation 8 (4%); LV hypertrophy 21 (11%); LV diastolic dysfunction 43 (23%) and systolic dysfunction 3 (2%); LA dilatation 16 (8%); dilated cardiomyopathy 1(1%); RV dilatation 12 (6%) and systolic dysfunction 4 (2%) [7].
Comparison of biomarkers between children with PHIV and children without HIV
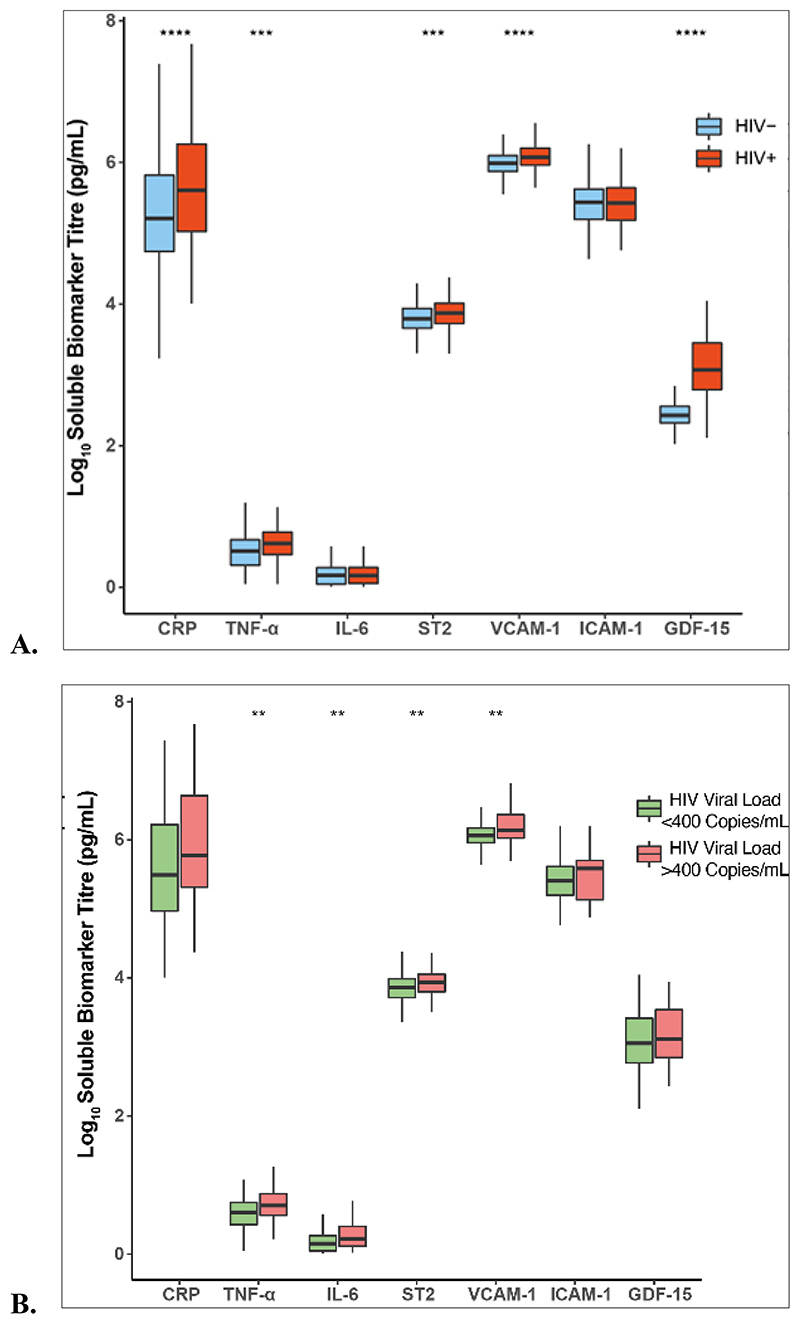
The distribution of circulating biomarkers by HIV status and viral suppression is shown in Figure 1. Inflammatory biomarker levels including CRP and TNF-α, were higher among PHIV compared to uninfected controls, (p<0.001). Similarly, levels of GDF-15, VCAM-1 and ST2 were elevated in PHIV participants compared to children without HIV (p<0.001). There was no difference in IL-6 and ICAM-1 levels by HIV status. Among participants with HIV, those who were virally suppressed had lower TNF-α, IL-6, ST2 and VCAM-1 (p=0.01). Virally suppressed children had higher levels of CRP, GDF-15, and VCAM-1 (p<0.001), TNF-α and ST2 (p<0.01) compared to uninfected controls.
Figure 1. Comparison of biomarkers by HIV status and Viral suppression.
A) shows the comparison of log10 biomarker levels between HIV+ and HIV- groups and B) is a comparison of log10 biomarker levels by viral suppression within the HIV+ group using Wilcoxon rank sum test. Stars represent levels of significance, * 0.05, ** 0.01, ***0.001, **** 0.0001
Relationship between biomarkers and echocardiographic findings in participants with PHIV
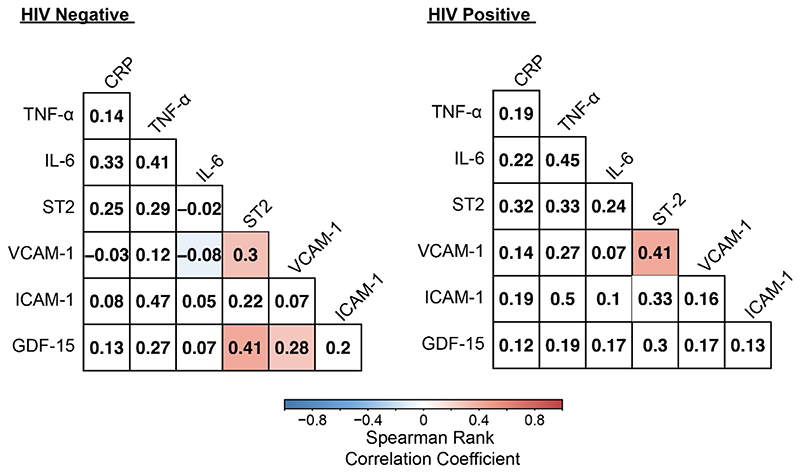
Biomarkers were moderately correlated for ST2 and VCAM-1 in PHIV while GDF-15, VCAM-1 and ST2 were correlated in children without HIV (p<0.05) (Figure 2). GDF-15 and VCAM-1 were significantly associated with higher interventricular septum z-scores (IVSdz) (adjusted β, 0.317 ; p<0.001) and (0.177 ; p=0.029), respectively. GDF-15 and IL-6 were associated with increased LV posterior wall z-scores (LVPWdz), while only GDF-15 was associated with lower RV diameter z-scores (RVDdz) (Table 2).
Figure 2. Spearman rank correlations between biomarkers among those without and with HIV.
Number within square indicates Spearman rank correlation coefficient between biomarkers. Correlations are significant at (p <0.05).
Table 2. Multiple Linear Regression of Biomarkers and Echocardiographic z-scores in the HIV group.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Univariate β ± SE | P-value | Adjusted β ± SE | P-value | |
| LVdd z-scores | ||||
| Log10 CRP | 0.179 ± 0.095 | 0.063 | 0.185± 0.095 | 0.053 |
| Log10 TNF-alpha | 0.150 ± 0.089 | 0.093 | 0.137 ± 0.096 | 0.154 |
| Log10 IL-6 | 0.082 ± 0.112 | 0.460 | 0.169 ± 0.122 | 0.167 |
| Log10 ST2/IL-33R | 0.152 ± 0.094 | 0.108 | 0.162 ± 0.092 | 0.081 |
| Log10 VCAM-1 | 0.090 ± 0.075 | 0.231 | 0.083 ± 0.076 | 0.279 |
| Log10 ICAM-1 | 0.052 ± 0.062 | 0.409 | 0.058 ± 0.059 | 0.329 |
| Log10 GDF-15 | 0.227 ± 0.106 | 0.034 | 0.174 ± 0.113 | 0.128 |
| IVSd z-scores | ||||
| Log10 CRP | 0.029 ± 0.07 | 0.680 | 0.021 ± 0.07 | 0.774 |
| Log10 TNF-alpha | 0.137 ± 0.079 | 0.086 | 0.145± 0.079 | 0.068 |
| Log10 IL-6 | 0.032 ± 0.075 | 0.062 | 0.027 ± 0.081 | 0.740 |
| Log10 ST2/IL-33R | 0.025 ± 0.079 | 0.752 | 0.020 ± 0.08 | 0.805 |
| Log10 VCAM-1 | 0.144 ± 0.079 | 0.069 | 0.177 ± 0.08 | 0.029 |
| Log10 ICAM-1 | 0.098 ± 0.077 | 0.206 | 0.083± 0.077 | 0.283 |
| Log10 GDF-15 | 0.288 ± 0.078 | <0.001 | 0.317 ± 0.083 | <0.001 |
| LVPWd z-scores | ||||
| Log10 CRP | 0.129 ± 0.078 | 0.098 | 0.142 ± 0.079 | 0.074 |
| Log10 TNF-alpha | 0.013 ± 0.822 | 0.872 | 0.051± 0.087 | 0.557 |
| Log10 IL-6 | 0.171 ± 0.090 | 0.058 | 0.249 ± 0.097 | 0.011 |
| Log10 ST2/IL-33R | 0.075± 0.080 | 0.354 | 0.113 ± 0.085 | 0.185 |
| Log10 VCAM-1 | 0.032 ± 0.072 | 0.657 | 0.089 ± 0.078 | 0.257 |
| Log10 ICAM-1 | 0.088± 0.087 | 0.313 | 0.073 ± 0.088 | 0.404 |
| Log10 GDF-15 | 0.242 ± 0.089 | 0.008 | 0.327 ± 0.100 | 0.001 |
| LA z-scores | ||||
| Log10 CRP | 0.0.191 ± 0.115 | 0.099 | 0.198 ± 0.109 | 0.073 |
| Log10 TNF-alpha | 0.154 ± 0.097 | 0.114 | 0.172 ± 0.105 | 0.102 |
| Log10 IL-6 | 0.069 ± 0.153 | 0.653 | 0.136 ± 0.152 | 0.372 |
| Log10 ST2/IL-33R | 0.169 ± 0.101 | 0.099 | 0.196 ± 0.102 | 0.057 |
| Log10 VCAM-1 | 0.045 ± 0.076 | 0.553 | 0.102 ± 0.087 | 0.247 |
| Log10 ICAM-1 | 0.041 ± 0.069 | 0.550 | 0.009± 0.071 | 0.891 |
| Log10 GDF-15 | 0.064 ± 0.126 | 0.611 | -0.012 ± 0.136 | 0.928 |
| RVdd z-scores | ||||
| Log10 CRP | -0.052 ± 0.115 | 0.652 | -0.065 ± 0.106 | 0.541 |
| Log10 TNF-alpha | -0.027 ± 0.106 | 0.793 | -0.029 ± 0.094 | 0.753 |
| Log10 IL-6 | -0.030 ± 0.122 | 0.807 | 0.015 ± 0.118 | 0.899 |
| Log10 ST2/IL-33R | -0.075 ± 0.097 | 0.442 | -0.087 ± 0.098 | 0.377 |
| Log10 VCAM-1 | -0.029 ± 0.077 | 0.708 | 0.063 ± 0.081 | 0.438 |
| Log10 ICAM-1 | -0.013 ± 0.078 | 0.861 | -0.092 ± 0.076 | 0.228 |
| Log10 GDF-15 | -0.028 ± 0.125 | 0.818 | -0.250 ± 0.247 | 0.037 |
| TAPSE z-scores | ||||
| Log10 CRP | 0.022 ± 0.076 | 0.766 | 0.020 ± 0.076 | 0.794 |
| Log10 TNF-alpha | -0.033 ± 0.075 | 0.654 | -0.050 ± 0.082 | 0.544 |
| Log10 IL-6 | -0.045 ± 0.080 | 0.573 | -0.051 ± 0.094 | 0.586 |
| Log10 ST2/IL-33R | -0.105 ± 0.069 | 0.131 | -0.119 ± 0.076 | 0.123 |
| Log10 VCAM-1 | 0.012 ± 0.060 | 0.839 | 0.023 ± 0.067 | 0.730 |
| Log10 ICAM-1 | -0.066 ± 0.067 | 0.327 | -0.077 ± 0.069 | 0.266 |
| Log10 GDF-15 | -0.006 ± 0.085 | 0.938 | -0.049 ± 0.095 | 0.602 |
Multivariable model adjusted for age, sex, and body surface area and log10 viral load. Each row of the table represents a separate linear regression model. P-values were not corrected for multiple testing.
Abbreviations: SE, standard error; IL-6, interleukin 6, TNF-α, tumour necrosis factor-alpha; CRP, C-reactive protein; GDF-15, growth differentiating factor-15, ST2, suppression of tumorigenicity 2; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecules 1.
A one-unit increase in CRP and GDF-15 was associated with increased odds of having a cardiac abnormality increased, 1.50 (95% confidence interval (CI) 1.10 – 2.03; p=0.010)and 1.53 (95% CI 1.03– 2.27; p=0.035) respectively, after adjusting for age category, sex, body surface area and viral suppression (Table 3). Each one-unit increase in CRP and GDF-15 was associated with increased odds of LV diastolic dysfunction, 1.49 (95% CI 1.02–2.18; P=0.040) and 1.71 (95% CI 1.17–2.52; p=0.006), with the odds of LV hypertrophy increased by 1.84 (95% CI 1.10–3.10; p<0.021) for a one-unit change in GDF-15. There was an associated decreased risk of LV diastolic dysfunction with every one unit increase of ICAM-1, 0.73 (95% CI 0.56 – 0.96; p=0.023)
Table 3. Logistic Regression of Individual Biomarkers and cardiac abnormalities in the HIV group.
| Any cardiac abnormality | LV diastolic dysfunction | LV Hypertrophy | LA dilatation | RV dilatation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Odds Ratio | Adjusted Odds Ratio | Adjusted Odds Ratio | Adjusted Odds Ratio | Adjusted Odds Ratio | ||||||
| aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Log10 CRP | 1.50 (1.10 – 2.03) | 0.010 | 1.49 (1.02 – 2.18) | 0.040 | 1.31 0.82 – 2.09) | 0.253 | 1.16 (0.68 – 1.96) | 0.585 | 1.16 (0.66- 2.06) | 0.605 |
| Log10 TNF-alpha | 0.94 (0.69 – 1.30) | 0.729 | 0.84 (0.60 – 1.18) | 0.324 | 0.88 (0.55 – 1.40) | 0.578 | 1.12 (0.59 – 2.17) | 0.715 | 1.17 (0.83- 1.64) | 0.379 |
| Log10 IL-6 | 1.16 (0.84 – 1.61) | 0.364 | 1.08 (0.75 – 1.55) | 0.674 | 1.28 (0.78 – 2.10) | 0.335 | 1.33 (0.79 – 2.27) | 0.285 | 0.87 (0.19-3.98) | 0.859 |
| Log10 ST2/IL-33R | 1.35 (0.99 – 1.85) | 0.061 | 1.28 (0.88 – 1.84) | 0.195 | 1.21 (0.75 – 1.93) | 0.438 | 0.92 (0.52 – 1.62) | 0.764 | 1.18 (0.74- 1.89) | 0.479 |
| Log10 VCAM-1 | 1.18 (0.89 – 1.57) | 0.243 | 1.13 (0.84– 1.53) | 0.424 | 1.20 (0.81 – 1.78) | 0.374 | 0.92 (0.53 – 1.58) | 0.753 | 1.01 (0.71- 1.44) | 0.947 |
| Log10 ICAM-1 | 0.86 (0.66 – 1.11) | 0.248 | 0.73 (0.56 – 0.96) | 0.023 | 0.99 (0.60 – 1.66) | 0.987 | 1.13 (0.57 – 2.22) | 0.725 | 1.03 (0.53- 1.99) | 0.939 |
| Log10 GDF-15 | 1.53 (1.03 – 2.27) | 0.035 | 1.71 (1.17 – 2.52) | 0.006 | 1.84 (1.10 – 3.10) | 0.021 | 1.03 (0.42 – 2.54) | 0.942 | 0.88 (0.36- 2.13) | 0.774 |
Models were adjusted for age-category, sex, body surface area and viral suppression. Odds ratios represent the odds of having an abnormality for a one-unit change in biomarker level with one-unit representing one standard deviation
Discussion
In this study, we explored cardiovascular and proinflammatory biomarkers and their association with echocardiographic measures of structure and function in children with PHIV. Overall, children with PHIV had elevated levels of CRP and TNF-α compared to those without HIV and similarly these biomarkers were also higher in virally suppressed children compared to uninfected controls, suggesting persistent systemic inflammation despite ART. CRP was associated with having a cardiac abnormality and, more specifically, LV diastolic dysfunction. Signs of inflammation have been previously observed in the myocardium of adults with HIV using cardiac magnetic resonance imaging [28]. Our findings support the hypothesis that systemic inflammation may play a role in the pathogenesis of HIV-related cardiac disease. While ART reduces immune activation, it does not completely normalise, and levels of proinflammatory biomarkers may remain elevated among those with HIV [29]. Chronic inflammation may cause cardiomyopathy through promoting apoptosis, fibrosis and inducing hypertrophy through alterations in the extracellular matrix [30, 31]. In particular, TNF-α exerts negative inotropic effects which result in contractile dysfunction [32]. Therefore, it is plausible that persistent inflammation and suboptimal immune recovery underlie the altered cardiac structure and function observed in children with PHIV.
Circulating biomarkers are dynamic and may be influenced by the complex interaction between metabolic, inflammation and viral load status in HIV. Notably, we found that viral suppression was associated with reduced TNF-α and IL-6. It is likely that in this cohort of children with PHIV, inflammatory damage may have been cumulative given the delayed initiation of ART: the median age at ART initiation in our cohort was 6 years.
GDF-15 was more likely to be elevated in children with PHIV, including those with LV diastolic dysfunction and hypertrophy. The release of GDF-15 increases in response to cardiomyocyte tissue injury such as in the context of LV hypertrophy and dilated cardiomyopathy [33]. In a group of elderly individuals, GDF-15 was associated with lower ejection fraction, concentric LV remodelling and hypertrophy. Of note, elevated GDF-15 level was associated with interventricular septum and LV posterior wall z-scores, a finding previously reported among hypertensive patients with LV hypertrophy [34]. The findings in the present study suggest that elevated GDF-15 in children with PHIV may be an important indicator of myocardial remodelling. Several reports have shown an association of GDF-15 with all-cause mortality, subclinical cardiovascular disease, endothelial dysfunction, and LV hypertrophy independent of conventional risk factors. This suggests that GDF-15 may provide additional information on the risk of cardiovascular disease over and above traditional risk factors [35].
Although the level of ST2 was significantly higher in children with PHIV, it was not associated with any cardiac abnormalities. In contrast, Secemsky et al found that ST2 was associated with LV diastolic dysfunction among adults with HIV [14]. More recently, elevated ST2 levels were associated with LV diastolic dysfunction among adults with HIV from Tanzania [36]. Our findings are different to published data in adults, possibly because the disease phenotypes may be at an earlier stage in children (but in time may develop into the adult phenotype). ST2 is a marker for myocardial fibrosis and several studies have reported an association with adverse cardiac outcomes and all-cause mortality [37–39].
ICAM-1 and VCAM-1 are markers of vascular injury. Impaired endothelial function promotes atherogenesis and hypertension, both of which are important risk factors for cardiac remodelling resulting in heart failure [40]. Both markers are associated with subclinical atherosclerosis and all-cause mortality in the general population [41] and are elevated in HIV infection [42]. Miller et al found that children with HIV had elevated ICAM-1 and VCAM-1 which was related to HIV disease severity (low CD4 counts and higher viral load) [43]. In this study, only VCAM-1 was elevated in children with PHIV and was associated with increased interventricular septum z-scores. VCAM-1 has previously been linked to LV wall thickness or LV mass indexes in patients with hypertension [44, 45]. This biomarker could potentially be used as an early marker for increased wall thickness in children with HIV. Unexpectedly, we found an associated decreased risk of LV diastolic dysfunction with every one unit increase of ICAM-1. This could be due to multiple testing.
It is notable that we reported a high prevalence (42%) of echocardiographic abnormalities in this cohort. There is evidence of a general burden of cardiac abnormalities in children with HIV from Sub-Saharan Africa and elsewhere, with estimates ranging between 14-89% [9, 10, 46–48]. Similarly, in a large contemporary cohort of adults with HIV, Mondy et al reported a high prevalence of subclinical cardiac abnormalities including LV diastolic dysfunction (26%), LV systolic dysfunction (18%) and pulmonary hypertension in 57% [49]. Other studies have also corroborated this high burden in adults with HIV in this ART era [50, 51].
The panel of biomarkers investigated in this study illustrate different processes in the development of HIV-related cardiomyopathy, including myocardial insult, inflammation, and cardiac remodelling [18]. GDF-15 and ST2 are emerging biomarkers, which may have the same potential as natriuretic peptides to impact the way cardiac disease is evaluated and managed [18]. We propose that measuring circulating biomarkers will be important in young people with HIV to enable early detection of cardiac complications and provide pathophysiological insights. This facilitates prompt interventions to reduce cardiovascular morbidity during the transition to adulthood. Furthermore, these biomarkers might be able to replace more expensive and difficult to access tests including echocardiography, particularly in resource limited settings, although further prospective studies are needed to confirm this. Our findings also provide evidence of a link between inflammatory biomarkers and PHIV comorbidities as previously reported [22].
The primary limitation of this study was its cross-sectional design. Serial measurements of biomarkers may have provided incremental prognostic evidence and reflected changes in myocardial remodelling over time. However, single-time measurements of biomarkers have been shown to be predictive of adverse cardiovascular outcomes [52]. This study had limited statistical power to model infrequent outcomes. We did not have data on metabolic parameters including lipids and insulin resistance, which are important risk factors for cardiovascular disease. Data on echocardiographic abnormalities in children without HIV was lacking and, therefore, it is unknown if these associations exist among children without HIV. Cardiac MRI data may have provided additional insights into myocardial inflammation. Longitudinal follow-up of children with PHIV, together with integration of metabolic data, will be necessary to understand whether our findings translate to cardiovascular events.
In conclusion, CRP, TNF-α, ST2, VCAM-1 and GDF-15 were elevated in children with PHIV and established on ART. CRP and GDF-15 were associated with echocardiographic abnormalities in PHIV and provide insights into possible role of inflammation in the comorbidities of children with PHIV.
Acknowledgements
We would like to acknowledge all the INHALE participants and families for contributing to the study.
Funding
This study is funded by Fogarty International Center (Fogarty D43TW009539) and TRENT Program. RAF is funded by the Wellcome Trust (206316_Z_17_Z). JPK is supported by a Medical Research Council Clinical Academic Research Partnership (MRC CARP) award (MR/T024062/1). EDM is funded by EDCTP2 programme supported by European Union and Novartis Global Health Basel Switzerland (grant TMA2019CDF-2776).
Footnotes
Authors Contribution
EDM led and coordinated the study. LMY and JM tested for the biomarkers. AMR and DHB performed the statistical analysis. EDM drafted the manuscript. JPK, LMY, HAM, SLRJ and RAF critically revised the manuscript for important intellectual content. All authors contributed to the manuscript and approved final version of the article.
References
- 1.Al-Attar, Orav JE, Exil V, Vlach SA, Lipshultz S. Predictors of Cardiac Morbidity and Related Mortality in Children With Acquired Immunodeficiency Syndrome. Journal of the American College of Cardiology. 2003;41(9):1598–605. doi: 10.1016/s0735-1097(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 2.Herskowitz A. Cardiomyopathy and other symptomatic heart diseases associated with HIV infection. Current opinion in cardiology. 1996;11(3):325–31. doi: 10.1097/00001573-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Luginbuhl LM, Orav E, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with hiv infection. Jama. 1993;269(22):2869–75. [PubMed] [Google Scholar]
- 4.Stewart JM, Kaul A, Gromisch DS, Reyes E, Woolf PK, Gowitz MH. Symptomatic cardiac dysfunction in children with human immunodeficiency virus infection. American heart journal. 1989;117(1):140–4. doi: 10.1016/0002-8703(89)90668-6. [DOI] [PubMed] [Google Scholar]
- 5.Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34(19):1432–6. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 6.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circulation Heart failure. 2010;3(1):132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majonga ED, Rehman AM, Simms V, Mchugh G, Mujuru HA, Nathoo K, et al. High prevalence of echocardiographic abnormalities in older HIV-infected children taking antiretroviral therapy. AIDS (London, England) 2018;32(18):2739. doi: 10.1097/QAD.0000000000002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idris NS, Cheung MMH, Grobbee DE, Burgner D, Kurniati N, Uiterwaal CSPM. Cardiac Effects of Antiretroviral-Naïve versus Antiretroviral-Exposed HIV Infection in Children. PloS one. 2016;11(1):e0146753. doi: 10.1371/journal.pone.0146753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chelo D, Wawo E, Siaha V, Anakeu A, Ndongo FA, Ndombo POK, et al. Cardiac anomalies in a group of HIV-infected children in a pediatric hospital: an echocardiographic study in Yaounde, Cameroon. Cardiovascular Diagnosis and Therapy. 2015;5(6):444–53. doi: 10.3978/j.issn.2223-3652.2015.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namuyonga J, Lubega S, Musiime V, Lwabi P, Lubega I. Cardiac Dysfunction Among Ugandan HIV-Infected Children on Antiretroviral Therapy. The Pediatric infectious disease journal. 2016 doi: 10.1097/INF.0000000000000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu DC, Sereti I, Ananworanich J. Serious Non-AIDS events: Immunopathogenesis and interventional strategies. AIDS Research and Therapy. 2013;10(1):29. doi: 10.1186/1742-6405-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker JV, Duprez D. Biomarkers and HIV-Associated Cardiovascular Disease. Current opinion in HIV and AIDS. 2010;5(6):511–6. doi: 10.1097/COH.0b013e32833ed7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson JD, Williams PL, Yu W, Colan SD, Mendez A, Zachariah JP, et al. Cardiac and inflammatory biomarkers in perinatally HIV-infected and HIV-exposed uninfected children. Aids. 2018;32(10):1267–77. doi: 10.1097/QAD.0000000000001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Secemsky EA, Scherzer R, Nitta E, Wu AH, Lange DC, Deeks SG, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. JACC: Heart Failure. 2015;3(8):591–9. doi: 10.1016/j.jchf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusconi PG, Ludwig DA, Ratnasamy C, Mas R, Harmon WG, Colan SD, et al. Serial Measurements of Serum NT-proBNP as Markers of Left Ventricular Systolic Function and Remodeling in Children with Heart Failure. American heart journal. 2010;160(4):776–83. doi: 10.1016/j.ahj.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangat J, Carter C, Riley G, Foo Y, Burch M. The clinical utility of brain natriuretic peptide in paediatric left ventricular failure. European journal of heart failure. 2009;11(1):48–52. doi: 10.1093/eurjhf/hfn001. [DOI] [PubMed] [Google Scholar]
- 17.Wells SM, Sleeper M. Cardiac troponins. Journal of Veterinary Emergency and Critical Care. 2008;18(3):235–45. [Google Scholar]
- 18.Gaggin HK, Januzzi JL., Jr Biomarkers and diagnostics in heart failure. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2013;1832(12):2442–50. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126(13):1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherzer R, Shah SJ, Secemsky E, Butler J, Grunfeld C, Shlipak MG, et al. Association of Biomarker Clusters With Cardiac Phenotypes and Mortality in Patients With HIV Infection. Circulation: Heart Failure. 2018;11(4):e004312. doi: 10.1161/CIRCHEARTFAILURE.117.004312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waheed HJ, Aref IS, Majid MA, Fadhil NK. Impact of Adhesion Molecules as a Predictive Marker for Cardiovascular Disease [Google Scholar]
- 22.Hameiri-Bowen D, Sovershaeva E, Flaegstad T, Gutteberg TJ, Ngwira LG, Simms V, et al. Soluble biomarkers associated with chronic lung disease in older children and adolescents with perinatal HIV infection. Aids. 2021;35(11):1743–51. doi: 10.1097/QAD.0000000000002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majonga ED, Rehman AM, McHugh G, Mujuru HA, Nathoo K, Odland JO, et al. Incidence and Progression of Echocardiographic Abnormalities in Older Children with Human Immunodeficiency Virus and Adolescents Taking Antiretroviral Therapy: A Prospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020;70(7):1372–8. doi: 10.1093/cid/ciz373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai SR, Nair A, Rylance J, Mujuru H, Nathoo K, McHugh G, et al. Human Immunodeficiency Virus-Associated Chronic Lung Disease in Children and Adolescents in Zimbabwe: Chest Radiographic and High-Resolution Computed Tomographic Findings. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(2):274–81. doi: 10.1093/cid/cix778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majonga ED, Rehman AM, McHugh G, Mujuru HA, Nathoo K, Patel MS, et al. Echocardiographic reference ranges in older children and adolescents in sub-Saharan Africa. International Journal of Cardiology. 2017;248(Supplement C):409–13. doi: 10.1016/j.ijcard.2017.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rylance J, McHugh G, Metcalfe J, Mujuru H, Nathoo K, Wilmore S, et al. Chronic lung disease in HIV-infected children established on antiretroviral therapy. Aids. 2016;30(18):2795–803. doi: 10.1097/QAD.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23(5):465–95. doi: 10.1016/j.echo.2010.03.019. 576-7. [DOI] [PubMed] [Google Scholar]
- 28.Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, et al. HIV-1-Related Cardiovascular Disease Is Associated With Chronic Inflammation, Frequent Pericardial Effusions, and Probable Myocardial Edema-Clinical Perspective. Circulation: Cardiovascular Imaging. 2016;9(3):e004430. doi: 10.1161/CIRCIMAGING.115.004430. [DOI] [PubMed] [Google Scholar]
- 29.Eric N, Janet L, Steven KG. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS (London, England) 2016;30(10):1495. doi: 10.1097/QAD.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91(11):988–98. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 31.Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, Visner MS. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest. 1992;90(2):389–98. doi: 10.1172/JCI115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhu SD. Cytokine-Induced Modulation of Cardiac Function. Circulation Research. 2004;95(12):1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 Functions As a Protective and Antihypertrophic Factor Released From the Myocardium in Association With SMAD Protein Activation. Circulation Research. 2006;98(3):342–50. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 34.Wesseling M, de Poel JHC, de Jager SCA. Growth differentiation factor 15 in adverse cardiac remodelling: from biomarker to causal player. ESC Heart Fail. 2020;7(4):1488–501. doi: 10.1002/ehf2.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wollert KC, Kempf T, Wallentin L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clinical chemistry. 2017;63(1):140–51. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 36.Kingery JR, Goyal P, Hosalli R, Lee MH, Desderius B, Kalokola F, et al. HIV-Associated Myocardial Diastolic Dysfunction and Soluble ST2 Concentration in Tanzanian Adults: A Cross-Sectional Study. The Journal of infectious diseases. 2020 doi: 10.1093/infdis/jiaa328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. American heart journal. 2010;160(4):721–8. doi: 10.1016/j.ahj.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Rehman SU, Mueller T, Januzzi JL. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. Journal of the American College of Cardiology. 2008;52(18):1458–65. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 39.Choi H, Dey AK, Sharma G, Bhoite R, Burkholder G, Fedson S, et al. Etiology and pathophysiology of heart failure in people with HIV. Heart failure reviews. 2021;26(3):497–505. doi: 10.1007/s10741-020-10048-8. [DOI] [PubMed] [Google Scholar]
- 40.Premer C, Kanelidis AJ, Hare JM, Schulman IH. Rethinking Endothelial Dysfunction as a Crucial Target in Fighting Heart Failure. Mayo Clinic Proceedings: Innovations, Quality Outcomes. 2019;3(1):1–13. doi: 10.1016/j.mayocpiqo.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–42. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 42.Melendez MM, McNurlan MA, Mynarcik DC, Khan S, Gelato MC. Endothelial adhesion molecules are associated with inflammation in subjects with HIV disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(5):775–80. doi: 10.1086/527563. [DOI] [PubMed] [Google Scholar]
- 43.Miller TL, Somarriba G, Orav EJ, Mendez AJ, Neri D, Schaefer N, et al. Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus-1. Journal of acquired immune deficiency syndromes. 2010;55(2):182–8. doi: 10.1097/QAI.0b013e3181e222c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malmqvist K, Wallén HN, Held C, Kahan T. Soluble cell adhesion molecules in hypertensive concentric left ventricular hypertrophy. J Hypertens. 2002;20(8):1563–9. doi: 10.1097/00004872-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Kuroda YT, Komamura K, Tatsumi R, Mori K, Yoneda K, Katayama Y, et al. Vascular cell adhesion molecule-1 as a biochemical marker of left ventricular mass in the patients with hypertension*. American Journal of Hypertension. 2001;14(9):868–72. doi: 10.1016/s0895-7061(01)02139-2. [DOI] [PubMed] [Google Scholar]
- 46.Animasahun BA, Diaku-Akinwumi IN, Ubuan PO, Ibitoye E. Cardiac size and systolic function of HIV-infected Lagos children accessing routine care: a pilot study. Journal of Xiangya Medicine. 2018;3(4) [Google Scholar]
- 47.Singh P, Hemal A, Agarwal S, Kumar D. Cardiac Manifestations in HIV Infected Children. Indian Journal of Pediatr. 2014 doi: 10.1007/s12098-014-1481-9. [DOI] [PubMed] [Google Scholar]
- 48.Miller RF, Kaski JP, Hakim J, Matenga J, Nathoo K, Munyati S, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(4):576–82. doi: 10.1093/cid/cis911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(3):378–86. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 50.Okeke NL, Alenezi F, Bloomfield GS, Dunning A, Clement ME, Shah SH, et al. Determinants of Left Ventricular Hypertrophy and Diastolic Dysfunction in an HIV Clinical Cohort. Journal of cardiac failure. 2018;24(8):496–503. doi: 10.1016/j.cardfail.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovascular journal of Africa. 2012;23(2):90–7. doi: 10.5830/CVJA-2011-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaggin HK, Szymonifka J, Bhardwaj A, Belcher A, De Berardinis B, Motiwala S, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC: Heart Failure. 2014;2(1):65–72. doi: 10.1016/j.jchf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17(4):407–29. [PubMed] [Google Scholar]