Abstract
Tissue repair is critical for animal survival. The skin epidermis is particularly exposed to injuries which necessitates rapid repair. The coordinated action of distinct epidermal stem cells recruited from various skin regions together with other cell types including fibroblasts and immune cells is required to ensure efficient and harmonious wound healing. A complex crosstalk ensures the activation, migration and plasticity of these cells during tissue repair.
Introduction
The skin is the first barrier protecting animals against UV radiation and pathogens from the external environment. It is composed of an epithelial layer, the epidermis, and the underlying dermis, which are separated by a basement membrane1. The epidermis contains pilo-sebaceous units that include a hair follicle and sebaceous glands, and are connected with the interfollicular epidermis (IFE) through the infundibulum1. The skin epidermis also contains other appendages, such as sweat glands, which regulate the body temperature through perspiration1. The dermis is composed of an upper (papillary) and a lower layer (reticular) of fibroblasts, blood vessels, immune cells and extracellular matrix (ECM)2, 3. Specialized fibroblasts form the dermal papilla, which regulates hair follicle growth and the erector pili muscle, responsible for pilo-erection. Partially integrated into the reticular dermis is a layer of dermal adipocytes that form the dermal white adipose tissue (DWAT)4. Underneath the dermis, the hypodermis (or subcutaneous adipose tissue) is composed of adipocytes, blood vessels and inflammatory cells4. This layer is important for thermoregulation and mechanical protection2, 4 (Fig. 1a).
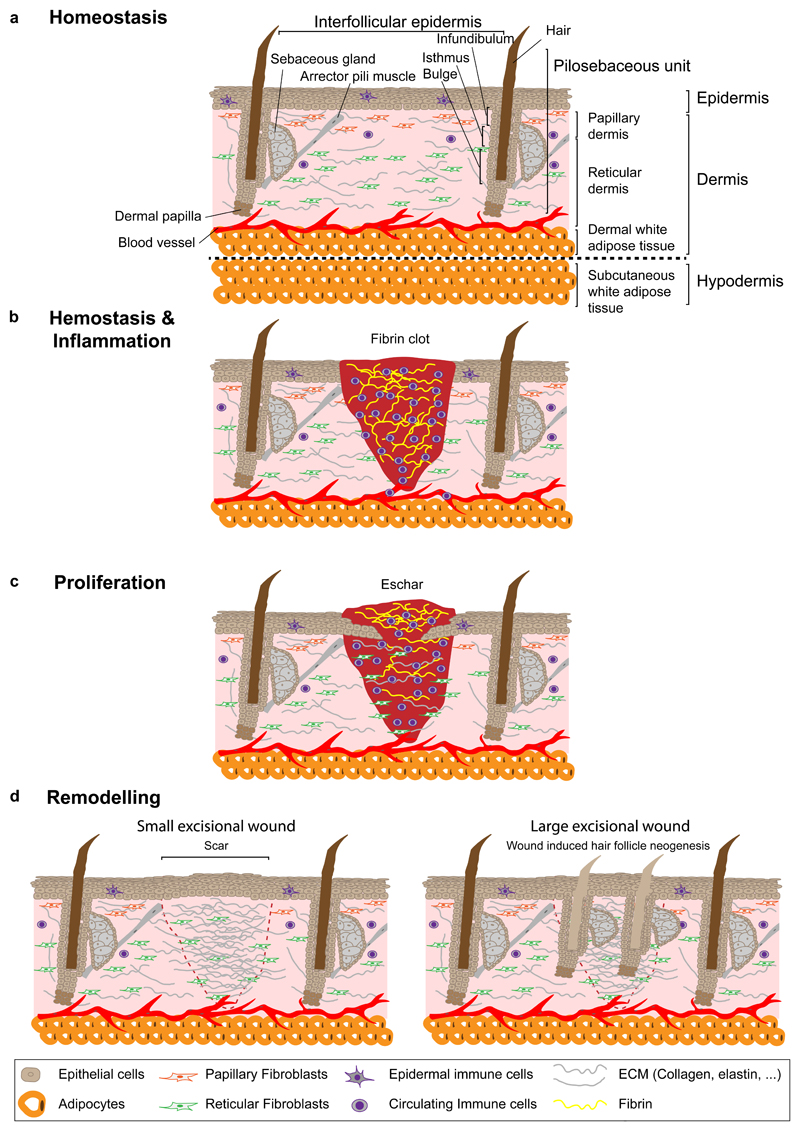
Figure 1. Overview of skin homeostasis and wound healing phases.
a, The skin is composed of dermis and epidermis. In the epidermis, epithelial cells are organized into a pilo-sebaceous unit, the hair follicle and its associated sebaceous glands, and the surrounding tissue, the IFE. The dermis consists of a papillary and a reticular layer located in the upper and lower part, respectively. Dermal papilla controls the hair follicle cycle and arrector pili muscle ensures its movement. The dermis includes fibroblasts, blood vessels, immune cells, sensory nerves and in its lower portion, the DWAT, which contains adipocytes. Below the skin lies the hypodermis or SWAT. b, Haemostasis and inflammation start immediately after wounding. The fibrin clot prevents further blood loss and provides a scaffold for the migration of immune, dermal and epidermal cells. c, During the proliferation phase, keratinocytes, fibroblasts and endothelial cells proliferate and migrate to the wound site and reform the ECM. d, During the remodelling phase the collagen in the dermis is remodelled and cells from earlier stages are removed. In small excisional wounds in mice, hair follicles are not regenerated and dermal scar tissue compensates for skin loss (left panel). In large excisional injuries, WIHN can be observed after complete re-epithelialization (right panel). DWAT, dermal white adipose tissue; ECM, extracellular matrix; SWAT, subcutaneous white adipose tissue; WIHN, wound induced hair follicle neogenesis.
Upon tissue damage, the skin has to be repaired as quickly as possible to prevent excessive blood loss and infection. Wound healing occurs through distinct overlapping phases: haemostasis, inflammation, proliferation and remodelling5. Haemostasis occurs immediately after tissue damage and results in the formation of a blood clot, which stops the haemorrhage and triggers the recruitment of different immune cells, including neutrophils, macrophages and lymphocytes, to prevent infection and further activate the inflammatory response5, 6 (Fig. 1b). The proliferation phase coordinates epidermal re-epithelialization and dermal repair5 (Fig. 1c). The remodelling phase removes cells that are no longer necessary and induces ECM remodelling6. In small excisional wounds (<1cm diameter in mice), hair follicles are not re-formed and dermal scar tissue compensates for skin loss7 (Fig. 1d). However, in large wounds (>1cm diameter), regeneration of hair follicles (wound-induced hair follicle neogenesis, WIHN) can be observed after re-epithelialization during the remodelling phase, resembling hair follicle embryonic development and a regeneration phase, typically 13-14 days after injury in mice 7, 8 (Fig. 1d).
Although the key steps of wound healing are well-described at the tissue level, a more in-depth characterization of the behaviour of individual cells at the clonal level and their fate transitions has only yet begun. The emergence of lineage tracing and intravital microscopy, coupled with transcriptional and epigenetic profiling, provide important insights about cellular and molecular mechanisms responsible for wound healing9–12. In this Perspective article, we describe recent advances with an emphasis on skin epithelial stem cell populations, their heterogeneity, clonal dynamics and remarkable plasticity during wound healing. Finally, we discuss the role of fibroblast populations and immune cells during repair and regeneration.
Skin epithelial stem cells during homeostasis
The skin epithelium renews throughout life in a continuous turnover ensured by stem and progenitor cells that balance proliferation and differentiation to replace dead and terminally differentiated cells1, 13, 14. Epithelial stem cells reside in a specific microenvironment called niche that is composed of a variety of cell types. Niche cells influence stem cell behaviour directly by cell contact or indirectly via ECM components and growth factors15. Although skin stem cells are able to regenerate the entire repertoire of skin epithelial lineages upon transplantation, lineage tracing has demonstrated that during physiological conditions, epidermal compartments are sustained by their own pool of resident stem cells14–16 (Fig. 2a).
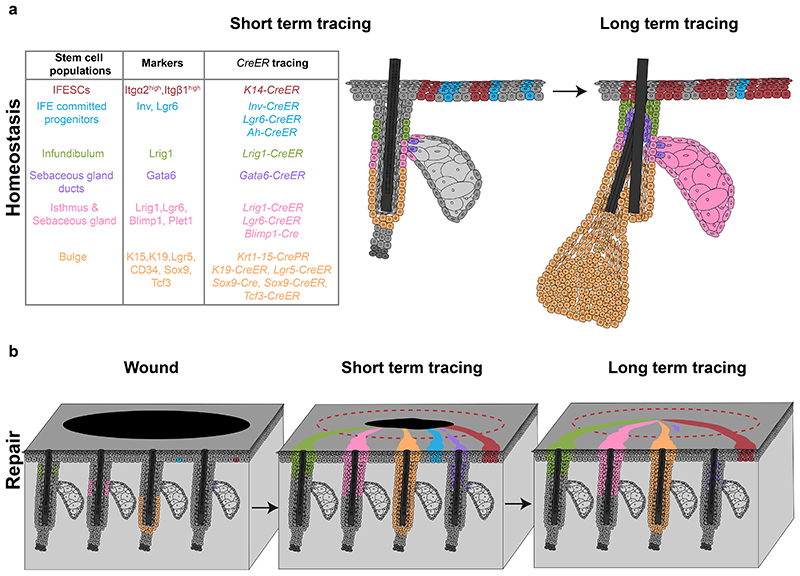
Figure 2. Skin epithelial stem cell populations during homeostasis and repair.
a, Skin epithelial stem cells express specific markers and can be lineage-traced with CreER mouse strains (left table). IFE stem cells and committed progenitors are located in the basal layer of the IFE and give rise to suprabasal, differentiated cells. Stem cells and committed progenitors can be traced using a K14-CreER or Inv-CreER mouse strains induced at low dose respectively. IFE committed progenitors also express Lgr6. Infundibulum stem cells are located in the upper part of the isthmus and express Lrig1. A population of sebaceous gland duct stem cells expressing Gata6 are located at the entrance of the gland but only maintains the junctional zone. Isthmus and sebaceous gland stem cells are basal cells located at the junction between the hair follicle and the gland, express Lrig1, Lgr6 and Blimp1 and give rise to the entire sebaceous gland and the isthmus. Bulge stem cells are located in the permanent lowest portion of the hair follicle, express K15, K19, Lgr5, CD34, Sox9 and Tcf3 and give rise to the entire hair follicle. b, Upon wounding, both IFESCs and committed progenitors are recruited and contribute to tissue repair. Only IFESCs will reside in the newly formed IFE long-term. Isthmus, sebaceous gland and infundibulum stem cells are recruited, contribute to IFE repair and remain long-term. Bulge stem cells are recruited to the IFE and a small proportion can remain long-term as IFESCs. Gata6+ sebaceous gland duct stem cells are recruited to the IFE, migrate suprabasally, de-differentiate and are re-established as IFESCs in the long-term. IFE, interfollicular epidermis; IFESCs, interfollicular epidermis stem cells.
During adult homeostasis, hair follicles undergo cycles of growth (anagen) and degeneration (catagen), followed by a resting stage (telogen)1. The hair follicle stem cells (HFSC) responsible for cyclic regeneration are located in the permanent non-cyclic follicle portion called bulge17–20. HFSCs were first identified based on their slow-cycling properties19, 21, 22. They have higher clonogenicity in vitro and give rise to IFE, hair follicle and sebaceous gland lineages upon transplantation17, 18, 20, 23, 24. Slow-cycling HFSCs were first isolated and characterised using K5-rtTA/TRE-H2BGFP and Krt15-EGFP transgenic mice25, 26, revealing expression of specific markers, such as Cd3418, 23, Krt1527, 28, Krt1929, Lgr530, Sox931, 32 and Tcf333. In sharp contrast with transplantation experiments, lineage tracing using Krt15-CrePR34, Shh-Cre35 Lgr5-CreER30, K19-CreER29, Sox9-Cre32 and Tcf3-CreER33 mouse strains established that HFSCs only contribute to hair follicle regeneration during physiological conditions, and do not maintain the sebaceous gland, infundibulum or IFE (Fig. 2a).
The IFE is composed of a single layer of proliferative basal cells and several layers of differentiated non-proliferative cells1. Basal cells replenish the suprabasal cells that are lost as terminally differentiated squames. In mice, it takes about a week for a basal cell to transit to the surface of the skin and about a month to replenish the whole IFE36. Early proliferation kinetic experiments reported maintenance of the IFE by small proliferative units that contain stem cells and progenitors37. However, lineage-tracing at clonal density later demonstrated that these units do not have a fixed size or predictable proliferation kinetics38, 39. Instead, in these studies IFE homeostasis was sustained by a single population of committed progenitors that balance renewal and differentiation in a stochastic manner38, 40–43. However, further studies provided evidence that basal epidermal cells are heterogeneous and some cells, depending on the skin regions, exhibit stem cell characteristics44, 45. These cells were more quiescent, persisted longer, and could give rise to more rapidly cycling committed progenitors with a shorter life-span44, 45 (Fig. 2a). Profiling of murine stem and progenitor cells showed that the two populations are molecularly different and that stem cells express higher level of basal integrins, as do human epidermal stem cells, whereas committed progenitors are primed toward differentiation44, 46.
The isthmus, a region located between bulge and sebaceous gland, contains its own pool of resident stem cells that express Blimp147, Lgr648, Lrig149, Gata650 or Plet151. These multipotent cells give rise to all epidermal lineages upon transplantation48–50, 52. Lineage tracing using Blimp1-cre47, Lgr6-CreER48, 53 and Lrig1-CreER54 has confirmed that these cells maintain the isthmus and sebaceous gland during homeostasis (Fig. 2a). In addition, Lrig1-expressing cells also give rise to cells of the infundibulum54 (Fig. 2a), whereas Gata6-expressing stem cells only contribute to the maintenance of the sebaceous gland ducts but not the gland itself during homeostasis50 (Fig. 2a). Altogether, these data show that during physiological conditions skin stem cells are confined to restricted compartments. Presently, the molecular mechanisms that restrain the movement of these cells across different territories remain unclear. As in the intestine55, cell-specific expression of different guidance molecules might confine cell types in specific territories.
Skin epithelial stem cells during wound healing
During wound healing stem cells are activated and recruited from different skin regions. Interestingly, lineage restriction and spatial confinement of resident skin stem cells are transiently lost during repair, allowing contribution of multiple epidermal stem cells15, 34, 54, 56, 57 (Fig. 2b).
The involvement of HFSCs in wound healing was already proposed 40 years ago after dermabrasion experiments in mice58 and further confirmed by more recent analyses of proliferation kinetics22. Lineage tracing targeting label retaining cells26 or using Krt15-CrePR34, Shh-Cre57, K19-CreER54 and Lgr5- CreER54 reporter strains showed that HFSCs rapidly migrate from the bulge to the wound and contribute to epidermal repair (Fig. 2b). These data demonstrate that HFSCs are highly plastic during wound healing, similarly to their expanded fate potential upon transplantation17, 20, 30, 49, 52.
Clonal analysis of IFE stem cells (IFESCs) and committed progenitor cells following injury of mouse tail skin showed that IFESCs are recruited to the wound, contribute to epidermal repair and persist up to 35 days44 (Fig. 2b). By contrast, committed progenitors are initially recruited but their progeny do not remain in the wound long-term44. Additional lineage tracing with Dlx1-CreER and Slc1a3-CreER reporters, which mark slow and rapidly cycling stem cells from different micro-domains of tail and back-skin IFE, demonstrated that upon wounding, both stem cell populations repopulate the two IFE regions59. However, in the long-term both cell populations only persist in their region of origin and not the region they migrate to during wound healing59. These observations suggest that during repair all basal cells present some degree of plasticity, a change in behaviour and functional contribution, but the wound does not reset the clock completely and cells keep a memory of their original location and hierarchy.
Similarly to HFSCs, Lrig1 and Lgr6-expressing stem cells from the upper isthmus are mobilized following wounding and possibly activated even more rapidly than HFSCs54. HFSCs have been assumed to be quickly lost during regeneration and to only serve as a transient bandage that allows other stem cells from the IFE and upper isthmus/infundibulum to sustain long-term repair34. However, a more recent study showed that the proportion of hair follicle and Lrig1-derived cells located in the epidermis drops dramatically 3 weeks after an injury, whereas remaining cells can persist up to one year thereafter54 (Fig. 2b). The persistence of these cells in the re-epithelialized IFE is proportional to the amount of stem cells labelled in the beginning and suggests a stochastic competition between equipotent stem cells rather than a hard-wired process54. Importantly, glabrous skin, such as the ventral (or palmar) part of the paw, heals correctly with slower kinetics compared to human skin, showing that HFSCs are dispensable for wound healing60. Moreover, similar to the contributions of hair follicles and infundibulum in skin with hair, sweat gland duct progenitors help to regenerate the injured epidermis in mouse paws61. Altogether, these studies suggest that the vacant niche created by an injury activates a broad range of stem cells to assume characteristics that differ from their homeostatic roles. Additional studies will be required to better understand the signals that activate distant stem cells, respective timelines and the mechanisms that disrupt and re-establish the boundaries between skin compartments. It further remains unclear how differentiation programmes get rewired during wound healing and how the balance between proliferation, migration and differentiation is achieved.
Migration, proliferation and compartmentalization
Epidermal injury is typically followed by increased keratinocyte proliferation5. Interestingly, proliferation is not observed at the wound edge but rather at a distance of 0.5 to 1.5 mm away from the edge9, 10, 62, in a proliferative zone that surrounds the wound. At the leading edge, keratinocytes do not proliferate but migrate as cellular sheet9, 10 (Fig. 3).
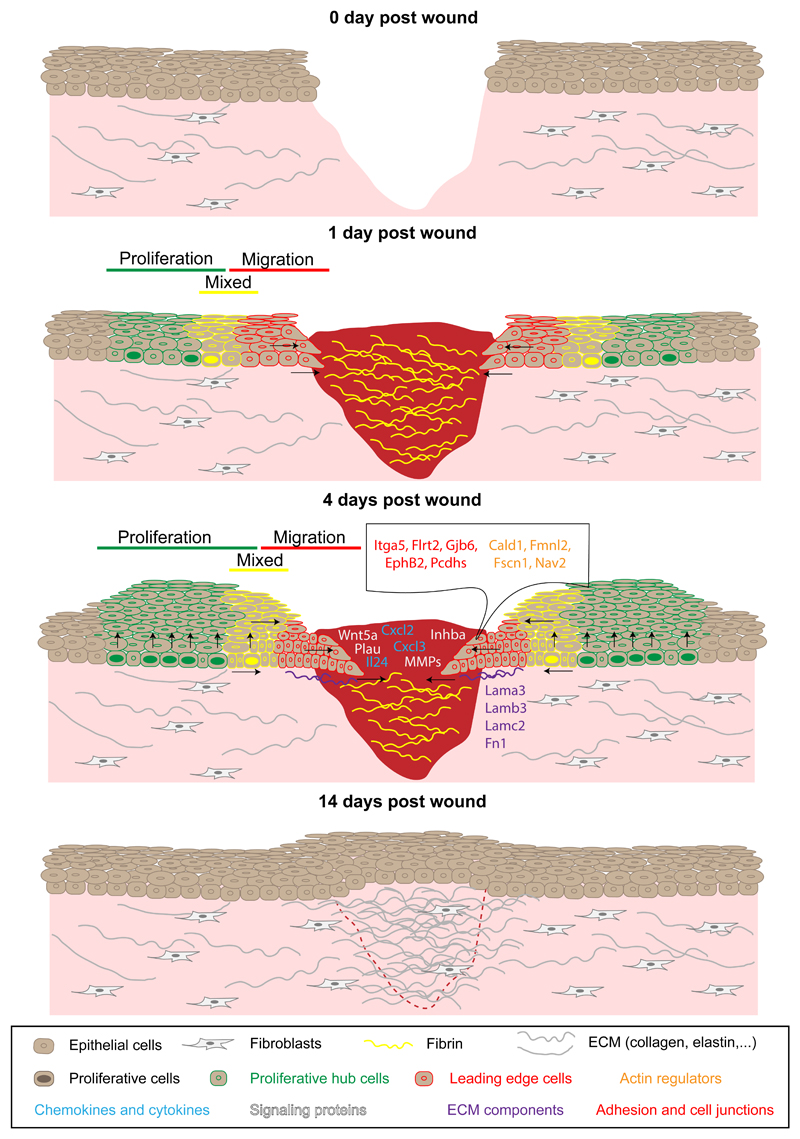
Figure 3. Epidermal migration, proliferation and compartmentalization during wound healing.
Epithelial cells start to migrate into the wound bed within 12 hours after injury. The day after wounding, IFE cells located close to the wound show an elongated shape toward the direction of the wound and are quiescent, whereas cells located at a distance start to proliferate, which leads to the establishment of a proliferative and a migrating leading edge compartment. Between these two zones, a mixed region is observed containing both migrating and proliferative cells. Four days after wounding, leading edge cells are compressed and upregulate the expression of specific genes that promote inflammation and regeneration. This gene signature is transient and disappears when the IFE is healed. IFE, interfollicular epidermis; ECM, extracellular matrix.
Intravital microscopy during wound healing demonstrated that both basal and suprabasal layers migrate during wound healing10. The speed of migration is greatest closer to the leading edge and decreases thereafter10. At a distance of 0.5 mm from the edge both migration and proliferation co-occur10. In this mixed region, basal cells are elongated toward the wound and orient their division in this direction10. In tail epidermis, cells present at the leading edge are initially elongated parallel to the direction of the wound, suggesting active migration, but assume perpendicular orientation 2 to 4 days after wounding, possibly because they are pushed and compressed by cells behind9. Whether proliferation is necessary for cell migration remains unclear. Pharmacological inhibition of cell proliferation prevents wound closure and cell compression at the leading edge in tail skin9. By contrast, proliferation is dispensable for wound closure in mouse ear epidermis10. However, cells also display a more elongated shape in wounds with inhibited proliferation, suggesting a compensatory effect 10. Differences in wound size and region-specific dermal populations could explain the discrepancies observed between ear and tail IFE. However, in Rac1 knockout mice with perturbed cell migration and elongation, a defect in the orientation of cell division is evident, suggesting its control by migration of the leading edge10. Altogether, these observations imply that cell migration at the leading edge comes first after wounding and that the displacement of cells in this region triggers orientated cell division of the cells following behind. Increased proliferation can itself generate a surplus of migrating cells that later push the leading edge toward the wound centre.
Transcriptional profiling of cells from migration and proliferation zones indicated two molecularly distinct and transient regions9. Cells at the leading edge expressed transiently higher levels of matrix metalloproteinases, pro-inflammatory molecules, genes controlling the cytoskeleton, microtubule and actin remodelling, ECM ligands and cell adhesion molecules such as integrin α59 (Fig 3). The constant size of the leading edge and its independence of wound size or skin area suggest that the signals controlling marker expression are local and potentially propagated from cell to cell within the epidermis. The leading edge might act as a transient scaffold enabling harmonious wound healing. By secreting higher lever of proteins that control ECM remodelling and blood clot dissolution, the leading edge might promote the progression of tissue regeneration toward the wound centre and protect stem cells and their progeny from tissue remodelling.
In addition, the acquired migratory phenotype of keratinocytes displays some features of epithelial-to-mesenchymal transition (EMT) including downregulation of cell adhesion molecules, increased motility and upregulation of EMT markers, such as Slug63, 64. Whether EMT is required for efficient wound healing or leading edge migration will require further analysis.
Stem cell population dynamics
During homeostasis, IFESCs and committed progenitors divide asymmetrically at the population level to maintain a constant number of epidermal cells65. However, during wound healing, cell numbers need to increase to compensate for lost cells until re-epithelialization is completed. Excess of renewal over differentiation can be achieved by increasing symmetric renewal or decreasing the proportion of cells that undergo differentiation, as during oesophageal wound repair66 or in vitro culture of keratinocytes67. Upon tail injury, clonal analysis of K14-CreER (IFESCs and progenitors) and Lrig1-CreER (infundibulum stem cells) mouse strains demonstrated streaks of labelled cells arising from single IFE or infundibulum cells, both basal and suprabasal, that project toward the wound centre9. IFE-derived clones decreased by more than 90% during the first week, due to rapid terminal differentiation of committed progenitors, but overall cell ratios demonstrated that committed progenitors continued to divide mostly asymmetrically at the population level9. The proportion of basal and suprabasal cells from Lrig1-CreER-derived clones was similar to IFE-derived clones, although the size of the infundibulum-derived clones was slightly bigger9. Clonal persistence, clone size and basal/suprabasal cell ratio were consistent with a hierarchical model in which rare stem cells reside at the top of the hierarchy, dividing asymmetrically at a much more rapid pace compared to homeostasis and give rise to progenitors, so that the equilibrium between proliferation and differentiation remains balanced. Irrespective of their initial locations, wounding seems to induce the activation of a minor stem cell population, whereas lineage hierarchy and balance between self-renewal and differentiation of committed progenitors remain unchanged from homeostasis9.
Recently, clonal analysis of human skin epidermis was performed after grafting in vitro reconstructed, genetically engineered skin into a patient with a severe form of the genetic skin disorder Epidermolysis Bullosa68. Sequencing of the integration site of the retrovirus used to replace the mutated gene revealed that viral integration in keratinocyte colonies originating from progenitors rapidly decreased over time68. By contrast, the integration sites of the most clonogenic colonies derived from stem cells increased, supporting the notion that only human skin stem-cell-derived colonies are able to renew and expand long-term in vivo, whereas progenitor cells possess limited potential to self-renew and revert back into a stem cell like state68.
Stem cell plasticity
When stem cells from the hair follicle and infundibulum are recruited to the IFE upon injury, they progressively lose their initial identity and are reprogrammed to an IFE fate34. The molecular mechanisms responsible for this plasticity are still incompletely understood. In a comparison of chromatin landscapes of injured IFE and homeostatic HFSCs and IFESCs, the wounded IFE exhibited a hybrid signature between HFSCs and IFESCs, in which the open chromatin regions were enriched for both IFESC (Klf5) and HFSC (Sox9) transcription factors12. This hybrid stage called ‘lineage infidelity’ seems to ensure proper re-establishment of the epidermal barrier12. Although this hybrid state is transient during repair, it persists in skin cancer12, 69.
Differentiated suprabasal epidermal cells are able to revert back to a stem cell state upon wounding70, 71, a phenomenon also observed in airway epithelium after lineage ablation of basal stem cells72. However, lineage tracing and photolabelling of suprabasal IFE cells demonstrated that these cells cannot adopt a basal state again under wound healing conditions9,10. Contrastingly, a population of Gata6-expressing cells residing in the isthmus, which during homeostatic conditions give rise to the sebaceous duct, can be mobilized during wound healing to migrate toward the injured IFE and revert from a differentiated to a basal stem cell fate50. This reversion does not occur immediately after injury, as the suprabasal cells require a few days to access the basal layer and undergo stem cell reprograming50. This intriguing observation raises the question of whether other differentiated epidermal cells are also able to revert back to a stem cell state or whether this is a unique property of the Gata6-expressing population. It is possible that the timing of reversion is important and that experiments performed on the tail and ear epidermis induced the labelling of the suprabasal cells too early to observe the reversion9, 10. Further experiments will be necessary to identify the mechanisms underlying this cellular plasticity and reprogramming of differentiated cells during wound healing. Other cases of dedifferentiation have been previously described in the hair follicle73, 74. After depilation or laser ablation to induce the loss of bulge HFSCs, hair germ cells73 as well as infundibulum or sebaceous gland cells74 are able to repopulate the stem cell niche and establish functional HFSCs. Similarly to skin, cells in the gut epithelium that are committed to terminal differentiation can revert back to a progenitor-like state and contribute to tissue repair following injury75–77. However, intestinal stem cells are required to ensure tissue repair following ionizing radiation78, demonstrating that despite the ability of committed cells to re-assume stemness, regular tissue resident stem cells are essential for repair.
The degree of damage can also influence cellular plasticity. In relatively small wounds, re-epithelialization occurs without reforming hair follicles, whereas de novo hair follicle formation is apparent in large wounds8. Lineage tracing confirmed that these de novo hair follicles do not originate from HFSCs but IFE cells8. Analogous to hair follicle morphogenesis during embryonic development79–81, WIHN depends on Wnt signalling as overexpression of Dickkopf Wnt signalling pathway inhibitor 1 (Dkk1), or β-catenin deletion in IFE basal cells prevents de novo hair follicle regeneration8. Epidermal deletion of Wntless, a gene required for the secretion of Wnt ligands, suppresses WIHN, suggesting that keratinocyte-derived Wnt is essential82. Different mouse strains have different susceptibilities to WIHN83, 84. Toll like receptor 3 (Tlr3) is increased in mouse strains with enhanced WIHN and dsRNA is a key signal that triggers skin regeneration through Tlr384. Msh homeobox 2 (Msx2) is also crucial for WIHN85. Fibroblast growth factor 9 (Fgf9), secreted by γδ T cells, triggers Wnt expression by wound-induced fibroblasts, further amplifying the Wnt signal required for WIHN. These data illustrate the importance of a crosstalk between immune cells, fibroblasts and keratinocytes to enable successful WIHN86.
Plasticity upon wound healing is also observed in other skin lineages. In the dermis, myofibroblasts, located close to de novo formed hair follicles, are converted into adipocytes in large wounds87. This cell fate conversion depends on BMP signalling originating from newly formed hair follicles that activate the expression of Zfp423, a transcription factor regulating adipocyte development87. Cell plasticity has also been described in other epithelia, such as the mammary gland, lung and intestinal epithelium88. It will be important to define whether generic mechanisms conserved across different tissues and species control cellular plasticity after lineage ablation and during tissue repair, inflammation or tumorigenesis.
Crosstalk between stem cells and the niche
During wound repair, fibroblasts are responsible for ECM synthesis in the dermis and for the fibrotic response leading to scar formation5. However, during embryonic development and in certain body locations, such as the oral cavity, wounds heal without forming scars89. The dermis of mouse back skin is composed of fibroblasts of different developmental origin90, 91. At least two fibroblastic lineages give rise to the upper (papillary fibroblasts, dermal papillae, and erector pili muscle) and lower dermis (reticular fibroblasts, preadipocytes, hypodermal adipocytes), respectively90. Upon wounding, fibroblasts of the lower dermis are recruited first, followed by fibroblasts of the upper dermis90 (Fig. 1). Reticular fibroblasts secrete the collagens responsible for scar tissue formation and are unable to regenerate the hair follicle after transplantation90. This observation may potentially explain why de novo hair follicle formation rarely occurs during wound healing. The upper papillary dermis is the only fibroblast lineage competent to regenerate hair follicles after transplantation, but it is recruited late during repair90. Interestingly, activation of Wnt/β-catenin signalling in epidermal cells increases the recruitment of papillary fibroblasts and also the number of hair follicles regenerated in the wound bed90. In sharp contrast, inhibition of β-catenin in fibroblasts promotes hair follicle regeneration during wound healing and correlates with a decrease of reticular fibroblasts and an increase of papillary fibroblasts92. In addition to different locations, two distinct fibroblastic lineages can also be identified on the basis of embryonic expression of Engrailed-1 (En-1)91. The En-1 positive lineage of fibroblasts (EPFs) produces ECM composed of collagen type I and III, becomes more abundant postnatally and is responsible for fibrotic scarring after injury91. By contrast, the En-1 negative lineage becomes less abundant after birth but has a higher regenerative capacity and their dominance during embryonic development might explain why embryos are able to regenerate scarless skin91, 93. Of note, the preferential location of EPFs in the reticular dermis after birth suggests they encompass the reticular populations previously described90, 91. Altogether, these data support the importance of surrounding dermal fibroblasts in skin regeneration.
A crosstalk between epidermal and immune cells also plays a major role during wound healing. Interestingly, after epidermal stem cells have been challenged with an inflammatory stimulus, the skin retains a memory of past inflammation and heals faster upon wounding. This memory is associated with chromatin remodelling that primes stem cells to respond more rapidly upon injury, in particular by promoting cell migration, which is partly mediated by the Aim2 inflammasome11. This remarkable observation is reminiscent of an ‘alert state’, as described in muscle stem cells, in which stem cells contralateral to the injured muscle undergo accelerated cell cycle entry upon tissue damage94. By contrast, ageing is associated with a defect in wound healing, linked to a decrease in epidermal migration and impaired signalling between epidermal and dendritic epidermal T cells95. Aged epidermal cells demonstrate a defect in Stat3 activation following wounding, which in turn prevents the activation of SKINTS genes that code for immunoglobulins and activate dendritic epidermal T cells in the wound bed95.
Upon wounding, skin stem cell populations are also activated by Activin A, which is overexpressed in the dermis and the epidermis9, 96. Overexpression of Activin A enhances wound healing96, 97, promotes keratinocyte migration and the formation of granulation tissue by dermal cells98, 99. Moreover, Activin A recruits Foxp3-expressing regulatory T cells, which further support accelerated healing97. These data illustrate the importance of the crosstalk between immune cells, dermal cells and keratinocytes during wound healing97.
Conclusions and outlook
Taken together, this body of work provides previously unappreciated insights into the cellular and molecular mechanisms regulating wound healing and the importance of a crosstalk between different skin cell populations. However, many open questions remain regarding the signals that stimulate stem cells to migrate to the wound centre. We also know little about the control of skin compartmentalization during homeostasis and repair. How rapidly after wounding are these boundaries re-established? Mechanistically, it will be interesting to further elucidate how cells move between and within basal and suprabasal layers and learn more about the role of the leading edge. Factors controlling chromatin remodelling and lineage infidelity in chronic wounds and cancer also remain to be discovered.
Lineage ablation experiments at the leading edge should aid in defining the role and function of involved compartments during wound repair. Moreover, single-cell RNA-sequencing and ATAC-sequencing of stem cell populations isolated during different regeneration stages should provide insights into the chromatin and transcriptional landscape associated with cellular heterogeneity. These studies might also elucidate further mechanisms that mediate the reprogramming of cells from the hair follicle and infundibulum as they are recruited to the IFE. Finally, understanding the role of the niche in the reprogramming of differentiated cells toward a stem cell fate will be an important challenge for the future.
Footnotes
Competing interests
The authors declare no competing interests.
Acknowledgements
We apologize to all authors whose work could not be cited due to space constraints.
C.B. is an investigator of WELBIO. S.D. is supported by a fellowship of the FNRS/TELEVIE.
This work was supported by a consolidator grant of the European Research Council.
References
- 1.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annual review of cell and developmental biology. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature medicine. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch MD, Watt FM. Fibroblast heterogeneity: implications for human disease. The Journal of clinical investigation. 2018;128:26–35. doi: 10.1172/JCI93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Experimental dermatology. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science (New York, NY) 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 6.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, et al. Principles and mechanisms of regeneration in the mouse model for wound-induced hair follicle neogenesis. Regeneration (Oxford, England) 2015;2:169–181. doi: 10.1002/reg2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 9.Aragona M, et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nature communications. 2017;8:14684. doi: 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nature cell biology. 2017;19:155–163. doi: 10.1038/ncb3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naik S, et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 2017;550:475–480. doi: 10.1038/nature24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Y, et al. Stem Cell Lineage Infidelity Drives Wound Repair and Cancer. Cell. 2017;169:636–650.:e614. doi: 10.1016/j.cell.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature reviews Molecular cell biology. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belokhvostova D, et al. Homeostasis, regeneration and tumour formation in the mammalian epidermis. The International journal of developmental biology. 2018;62:571–582. doi: 10.1387/ijdb.170341fw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales KAU, Fuchs E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Developmental cell. 2017;43:387–401. doi: 10.1016/j.devcel.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kretzschmar K, Watt FM. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harbor perspectives in medicine. 2014;4 doi: 10.1101/cshperspect.a013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 18.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 20.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 21.Braun KM, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development (Cambridge, England) 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 22.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 23.Trempus CS, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. The Journal of investigative dermatology. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 24.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nature biotechnology. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 26.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science (New York, NY) 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyle S, et al. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. Journal of cell science. 1998;111(Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. The Journal of investigative dermatology. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- 29.Youssef KK, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nature cell biology. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 30.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 31.Vidal VP, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Current biology : CB. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 32.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell stem cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard JM, Nuguid JM, Ngole D, Nguyen H. Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development (Cambridge, England) 2014;141:3143–3152. doi: 10.1242/dev.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature medicine. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 35.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Developmental cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Potten CS. Epidermal cell production rates. The Journal of investigative dermatology. 1975;65:488–500. doi: 10.1111/1523-1747.ep12610194. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS. The epidermal proliferative unit: the possible role of the central basal cell. Cell and tissue kinetics. 1974;7:77–88. doi: 10.1111/j.1365-2184.1974.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 38.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 39.Ro S, Rannala B. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO reports. 2004;5:914–920. doi: 10.1038/sj.embor.7400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy E, et al. Bimodal behaviour of interfollicular epidermal progenitors regulated by hair follicle position and cycling. The EMBO journal. 2016;35:2658–2670. doi: 10.15252/embj.201693806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim X, et al. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science (New York, NY) 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Developmental cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Rompolas P, et al. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science (New York, NY) 2016;352:1471–1474. doi: 10.1126/science.aaf7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascre G, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Danes A, et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature. 2016;536:298–303. doi: 10.1038/nature19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 47.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snippert HJ, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science (New York, NY) 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 49.Jensen KB, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell stem cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E. Wounding induces dedifferentiation of epidermal Gata6(+) cells and acquisition of stem cell properties. Nature cell biology. 2017;19:603–613. doi: 10.1038/ncb3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nijhof JG, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development (Cambridge, England) 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- 52.Jensen UB, et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. Journal of cell science. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fullgrabe A, et al. Dynamics of Lgr6(+) Progenitor Cells in the Hair Follicle, Sebaceous Gland, and Interfollicular Epidermis. Stem cell reports. 2015;5:843–855. doi: 10.1016/j.stemcr.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell stem cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batlle E, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 56.Jaks V, Kasper M, Toftgard R. The hair follicle-a stem cell zoo. Experimental cell research. 2010;316:1422–1428. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 58.Argyris T. Kinetics of epidermal production during epidermal regeneration following abrasion in mice. The American journal of pathology. 1976;83:329–340. [PMC free article] [PubMed] [Google Scholar]
- 59.Sada A, Jacob F, Leung E, Wang S, White BS. Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nature cell biology. 2016;18:619–631. doi: 10.1038/ncb3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? The Journal of investigative dermatology. 2008;128:1059–1061. doi: 10.1038/jid.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu CP, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. The Journal of investigative dermatology. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 63.Savagner P, et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. Journal of cellular physiology. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 64.Haensel D, Dai X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Developmental Dynamics. 2018;247:473–480. doi: 10.1002/dvdy.24561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nature reviews Molecular cell biology. 2013;14:489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- 66.Doupe DP, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science (New York, NY) 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roshan A, et al. Human keratinocytes have two interconvertible modes of proliferation. Nature cell biology. 2016;18:145–156. doi: 10.1038/ncb3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirsch T, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Latil M, et al. Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell stem cell. 2017;20:191–204.:e195. doi: 10.1016/j.stem.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu X, Sun X, Li X, Sheng Z. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet (London, England) 2001;358:1067–1068. doi: 10.1016/S0140-6736(01)06202-X. [DOI] [PubMed] [Google Scholar]
- 71.Mannik J, Alzayady K, Ghazizadeh S. Regeneration of multilineage skin epithelia by differentiated keratinocytes. The Journal of investigative dermatology. 2010;130:388–397. doi: 10.1038/jid.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tata PR, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 74.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Es JH, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature cell biology. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buczacki SJ, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 77.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell stem cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Developmental cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 81.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Developmental cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 82.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. The Journal of investigative dermatology. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson AM, et al. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. The Journal of investigative dermatology. 2013;133:881–889. doi: 10.1038/jid.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson AM, et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell stem cell. 2015;17:139–151. doi: 10.1016/j.stem.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hughes MW, et al. Msx2 Supports Epidermal Competency during Wound-Induced Hair Follicle Neogenesis. The Journal of investigative dermatology. 2018;138:2041–2050. doi: 10.1016/j.jid.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gay D, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nature medicine. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plikus MV, Guerrero-Juarez CF. Regeneration of fat cells from myofibroblasts during wound healing. Science (New York, NY) 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science (New York, NY) 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. Journal of dental research. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 90.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rinkevich Y, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science (New York, NY) 2015;348:aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rognoni E, et al. Inhibition of beta-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development (Cambridge, England) 2016;143:2522–2535. doi: 10.1242/dev.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang D, et al. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nature cell biology. 2018;20:422–431. doi: 10.1038/s41556-018-0073-8. [DOI] [PubMed] [Google Scholar]
- 94.Rodgers JT, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keyes BE, et al. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Nature cell biology. 2016;167:1323–1338.:e1314. doi: 10.1016/j.cell.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munz B, et al. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. European journal of immunology. 1999;18:5205–5215. doi: 10.1093/emboj/18.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haertel E, Joshi N, Hiebert P, Kopf M, Werner S. Regulatory T cells are required for normal and activin-promoted wound repair in mice. European journal of immunology. 2018;48:1001–1013. doi: 10.1002/eji.201747395. [DOI] [PubMed] [Google Scholar]
- 98.Wankell M, et al. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. The EMBO journal. 2001;20:5361–5372. doi: 10.1093/emboj/20.19.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bamberger C, et al. Activin controls skin morphogenesis and wound repair predominantly via stromal cells and in a concentration-dependent manner via keratinocytes. The American journal of pathology. 2005;167:733–747. doi: 10.1016/S0002-9440(10)62047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]