Abstract
Disturbed interoception (i.e., the sensing, awareness, and regulation of internal body signals) has been found across several mental disorders, leading to the development of interoception-based interventions (IBIs). Searching PubMed and PsycINFO, we conducted the first systematic review of randomized-controlled trials (RCTs) investigating the efficacy of behavioral IBIs at improving interoception and target symptoms of mental disorders in comparison to a non-interoception-based control condition [CRD42021297993]. Thirty-one RCTs fulfilled inclusion criteria. Across all studies, a pattern emerged with 20 (64.5%) RCTs demonstrating IBIs to be more efficacious at improving interoception compared to control conditions. The most promising results were found for post-traumatic stress disorder, irritable bowel syndrome, fibromyalgia and substance use disorders. Regarding symptom improvement, the evidence was inconclusive. The IBIs were heterogenous in their approach to improving interoception. The quality of RCTs was moderate to good. In conclusion, IBIs are potentially efficacious at improving interoception for some mental disorders. In terms of symptom reduction, the evidence is less promising. Future research on the efficacy of IBIs is needed.
Keywords: body awareness, interoception, interoception-based interventions, mental disorders, randomized-controlled trial
The concept of interoception has brought the body, and particularly its psychophysiological states, into the spotlight of mental health research and practice.1,2 The term interoception describes the sensing, awareness and interpretation of the physiological signals arising within the body, involving major, visceral systems such as the gastrointestinal, respiratory, and cardiovascular systems, and in some definitions also certain cutaneous sensations such as affective touch, pain and temperature.3,4 Interoception has been associated with the awareness and regulation of emotional states at a behavioral5,6 and neural level,3,7,8 building on preceding, peripheral theories of emotions. These theories highlighted the role of physiological processes at the core of emotional awareness.5,8–10 More recently, new technologies that can capture simultaneously both peripheral and central signals have led to the burgeoning of research on the multifaceted ways in which body–brain couplings influence cognition and emotion.11 The emerging perspectives on emotion reaffirm the influence of higher-order factors such as memory, appraisals or beliefs, yet highlight the bi-directional pathways by which peripheral signals interact with central mechanisms to give rise to our emotions. This bi-directional influence of peripheral and central processing in emotion has been found to be particularly relevant in the etiology and treatment of mental health disorders.
Indeed, fast accumulating evidence points to interoceptive disturbances in mental health conditions,12 including depression,13 eating disorders,14–16 somatic symptom disorders,17 addiction disorders,18–20 depersonalization/derealization disorder,21 borderline personality disorder,22 suicidality,23 obsessive-compulsive disorder,24 post-traumatic stress disorder (PTSD),25 schizophrenia26 and anxiety disorders.27–29 In addition to such diagnosis-specific findings, it has been suggested that interoceptive deficits, including related neural disruptions in a network centered around the mid-dorsal insula, might instead represent ‘transdiagnostic’ mechanisms conferring a common vulnerability across multiple mental health disorders.12,30,31
Interoceptive processing is multidimensional, ranging from peripheral mechanisms of body physiology to high-order processing of interoceptive signals.1,2 Establishing the level, mechanism, and modality of interoceptive disruption is key to understanding and treating mental health disorders. However, this has proven far from straightforward and methodologically challenging.12,32 Ongoing measurement and terminology debates notwithstanding, several useful schemes have been proposed to classify the dimensions of interoception and suggest appropriate measures for each level, starting with a tripartite classification.33 This scheme distinguished between1 interoceptive accuracy referring to the ability of detecting physiological signals like one’s heart rate correctly during behavioral tasks2; interoceptive self-report or sensibility, referring to subjective, retrospective, typically questionnaire-based, accounts of one’s own everyday interoceptive abilities, and3 interoceptive insight, metacognition, or awareness, operationalized as confidence–accuracy correspondence in behavioral tasks, or as the (mis)alignment of interoceptive beliefs with veridical body states, as frequently noted in functional, somatic symptom and eating disorders.34 Several extensions with clinical relevance have been proposed since,35–37 highlighting preconscious levels of interoceptive functioning, such as the finding that fear memories may be heightened by cardiovascular signals particularly in anxious individuals38; interoceptive attention, as for example, excessive attention to bodily changes in certain anxiety disorders and interoceptive attribution, as for instance catastrophizing about bodily signals during a panic attack.28
A range of current mental health treatments appear to alter interoception via effects on body physiology or on the cognitive processing of such signals across the aforementioned dimensions.35 Indeed, interoception can be manipulated at cellular, or, systems level, including for example via neural stimulation (e.g., vagus nerve stimulation) and pharmacological interventions (e.g., on interoceptive immune or appetite pathways).39,40 Moreover, an increasing number of mental health interventions target interoceptive disturbances at the psychological, or behavioral level.41,42 These psychological or behavioral, non-invasive Interoception-Based Interventions (IBIs) are defined as interventions that include “first-person reflection upon or cultivation of specific modes of experience, and practices that explicitly involve interoceptive awareness”.43 This definition stems from an interdisciplinary consideration of the various clinical and scientific traditions that have separately emphasized the importance of interoception for physical and psychological health and wellbeing.43 For example, Buddhist and contemplative practices have long attempted to cultivate healthy interoceptive habits and attentional stances towards bodily signals, with some of these insights reaching Western, secular psychological treatments in the form of mindfulness treatment or Acceptance and Commitment Therapy.44 Separately from these traditions, increased scientific understanding of the bidirectional influences between physiology and psychology, and their effects on mental health, has led to the inclusion of interoceptive procedures, such as interoceptive exposure, and the development of novel therapeutic targets such as the training of interoceptive accuracy in many other psychological therapies. As a result of these different traditions, there are important differences in how these fields define and target interoception and which levels and facets of interoceptive processing they consider key for mental health and wellbeing. These differences, as well as some similarities, have been considered extensively elsewhere41,43 and a similar analysis escapes the scope of the present systematic review. However, we rely on these previous analyses that pointed towards the collective significance of these therapeutic endeavors to define IBIs as involving both first person reflections, experiences and practices that promote interoception, broadly defined.41 In particular, while certain scientific and clinical traditions would narrowly define and therapeutically target interoception as the cognitive ability to perceive one’s bodily signals accurately, broader definitions and alternative therapeutic targets focus on people’s attention tendencies and attitudes towards their bodily signals and their regulation.45,46 While the various interventions covered in this review are not identical and they may involve elements that go beyond targeting any strict definition of interoception, they share an emphasis in targeting interoception broadly defined, and as we outline below, they also need to include measures of interoception. Moreover, given these different traditions and as we explain below, we tried to systematically present the variability in the multiple dimensions, modalities, and terms of interoception used by the existing RCTs, using also a common scheme that categorizes as far as possible the therapeutic focus on each intervention.
Interoceptive exposure (IE) is one of the most common IBIs of recent years,41,42 considered particularly effective for panic disorder.47 IE is typically applied as a part of cognitive behavioral therapy, involving the safe exposure to enhanced interoceptive sensations (e.g. hyperventilation following exercise) to facilitate reappraisal and better regulation of such sensations.48 Typically, interoceptive accuracy or perceptual metacognition is not measured in such interventions. By contrast, other IBIs may attempt to train interoceptive accuracy, or perceptual metacognition per se with beneficial effects on anxiety symptoms,49,50 while practices such as mindfulness, stemming out of contemplative traditions, target more attentional and attributive aspects of interoception, and appear to be effective in improving mental health symptoms in depression51 and subclinical eating disorders.52 However, observed changes of interventions such as interoceptive exposure or mindfulness practices that target interoception have been largely qualitative and limited, with few validated measures quantifying the mechanism of change. More generally, beyond a handful of non-systematic narrative reviews and opinion papers,35,41,42 the literature on these psychological IBIs has not been systematically reviewed. Hence, the quality, efficacy, and mechanisms of action of these interventions in improving interoception and reducing mental health symptoms has not been systematically assessed. It is also unknown whether the different interoceptive dimensions and modalities targeted by these interventions have differential effects across or within different diagnostic categories. This lack of a systematic evaluation of the literature thus hinders the understanding and improvement of this potential, non-invasive therapeutic path from interoception to mental health.
Accordingly, we preregistered and conducted the first systematic review of randomized controlled trials (RCTs) investigating the immediate and long-term efficacy of IBIs at improving interoception and target symptoms compared to a non-IBI control condition in patients diagnosed with mental health disorders. Although a meta-analysis of the literature would offer additional insights in the future, the currently available number of RCTs and clinical and methodological diversity of the used IBI’s, including their characteristics and measures, would render quantitative comparisons potentially misleading.53 Therefore, we decided that a systematic review and qualitative evaluation of the literature would be most appropriate at this stage. The main objective of this systematic review was to provide a comprehensive and reliable literature overview of the characteristics and efficacy of these IBIs in1 improving interoception (primary aim) and2 reducing target symptom severity (secondary aim) in mental health disorders. Additionally, we aimed to systematically assess and present the methodological quality, assessment instruments and intervention focus of the available RCTs across and within modalities and domains of interoception and the targeted mental health disorders. We chose to perform a systematic review as this method allows the exploration and narrative synthesis of the efficacy of heterogenous data (e.g., varied by type of intervention, disorders, outcomes, control conditions) and may help to identify relevant data for future meta-analyses. Accordingly, we considered as outcomes and systematically presented the variability in the multiple modalities and dimensions of interoception utilized, including physiological measures, validated behavioral tasks, or self-report.35 Given that the identified interventions did not always use the terms described in the experimental and physiological literature on interoception, to distinguish between the various levels of interoception targeted, we used the following scheme to facilitate the reader; we presented the efficacy of IBIs regarding improvements in interoception and target symptoms, based on their therapeutic focus on1 physiological signals,2 the appraisal of these signals, or3 a combination of both.
Methods
This systematic review was conducted according to the PRISMA guidelines54 and was preregistered at PROSPERO (registration number: CRD42021297993) and OSF (https://osf.io/fxucj).
Search strategy
A comprehensive literature search was independently conducted by NH and MB, using the PubMed and PsycINFO databases to identify eligible studies published up to December 1, 2021. Two distinct search strategies were selected: firstly, we replicated and updated the search of a previous (non-systematic) review of IBIs conducted by Khoury and colleagues (2018),41 and secondly, we expanded their method to potentially identify additional papers using broader search terms. The first search strategy used the following search terms: “interoception” OR “interoceptive” OR “body awareness” AND “affective disorders” OR “depression” OR “anxiety” OR “eating disorders” OR “anorexia nervosa” OR “bulimia nervosa” OR “psychosomatic” OR “addiction” OR “addictive disorders”. The second search strategy used the following search terms: “body awareness” OR “interocept*”. For identification of RCTs, both strategies added the terms “randomized”, “RCT” or “random*” to be found in the title/abstract sections. Search strategy URLs are available in supplementary materials (Table S1). Additionally, references from relevant studies and reviews were manually checked for further eligible RCTs, not yet identified through database searching.
Inclusion and exclusion criteria
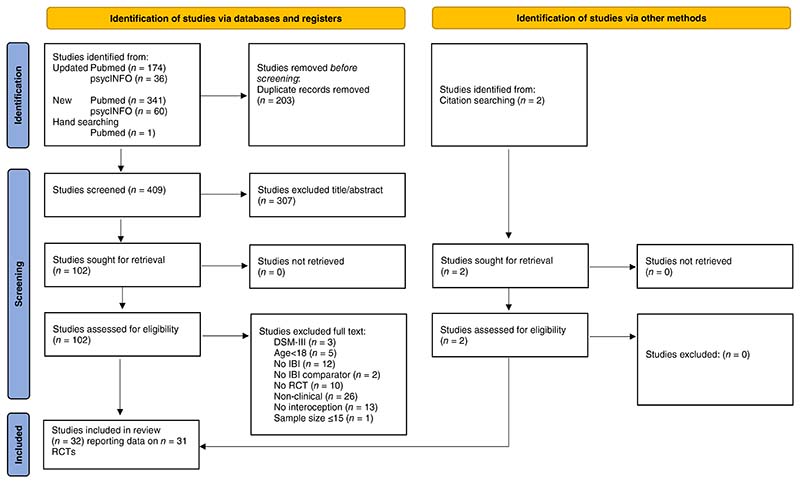
The following inclusion criteria were used to select studies1: randomized controlled trial (RCT) design, with participants randomly assigned to two or more trial arms,2 adult (≥18 year old) clinical population with a mental health disorder diagnosis, which had to be obtained from a clinician, trained researcher, or validated questionnaire according to DSM-5,55 DSM-IV56 or ICD-10 criteria,57,3 study published in English,4 comparing an IBI (see Introduction for definition) against a non-interoceptive control condition (e.g., active control, treatment as usual, waitlist) and5 using both at least one validated measure assessing interoception and mental health target symptoms (e.g., depressive symptoms in depression). Lastly,6 although not pre-registered we also excluded studies when no relevant data was reported to interpret the direction of the IBI based on statistical analyses, i.e., no between-group effects were reported or could be calculated based on mean scores and standard deviation, because the samples were too small. This led to the exclusion of one study with a total sample size n = 10 participants58 (Fig. 1).
Fig. 1. PRISMA flowchart of study selection.
Screening and selection of studies
Screening and selection of eligible studies was conducted independently by two authors (NH and MB). Title and abstract screening were performed according to the eligibility criteria and all articles that met initial inclusion criteria were retrieved as full text. Study selection was performed independently by NH and MB, with disagreements resolved through discussion or including consultation with the other authors. Figure 1 details the flowchart of study selection. If no test statistics or effect sizes of between-group comparisons were reported, they were calculated manually based on post-treatment mean scores and standard deviations. Negative effect sizes indicating symptom reduction were changed to positive values, and vice versa positive effect sizes indicating deterioration to negative values, so that positive effect sizes indicate favorable effects for the IBI.
Data extraction
Data extraction was performed independently by NH and MB. Extracted data include first author, year of publication, sample size, mental health diagnosis, type of intervention and comparator. The following indices were extracted from intention to treat analysis, when available, for both the IBI and the comparator: duration of treatment, total number of sessions, length of sessions, frequency of sessions. Furthermore, measure of interoception, target symptom measure, post intervention between group comparison (including P-value and effect sizes in standardized mean difference, SMD) and follow-up between-group comparison (including follow-up time, P-value, and effect size).
Studies using at least one measure of interoception were included, including a wide range of interoceptive dimensions that were used as therapeutic targets and measured with different means. For example, measures ranged from interoceptive accuracy and interoceptive insight, assessed through experimental tasks to self-report measures of interoceptive sensibility, or mindful body awareness, or measures capturing symptoms that coincide with interoception, such as pain or irritable bowel symptoms. This variability reflects the aforementioned multidimensionality and debates regarding the concept of interoception. Highlighting and systematically presenting this variability in therapeutic targets was considered an important function of the present review.
Data synthesis
Results were reported in tables, figures and narratively summarized. For this, significant between-group differences at improving interoception and symptoms were pre-registered as primary and secondary outcomes, respectively. For illustrative purposes, RCTs were labeled as demonstrating significant between-group differences in favor of the IBI (↑Table 1), no statistical differences between groups (—Table 1) or significant between-group effects in favor of the control condition (↓Table 1). For this, we used between-group comparisons of total scores of interoception and target symptom measures when available. Considering the multi-dimensionality of interoception mentioned above, in the case that a measure consisted only of subscales, we labeled the overall direction of the effect if at least one subscale showed significant between-group effects. This was the case, for example, for the Multidimensional Assessment of Interoceptive Awareness (MAIA),46 which has no total score and consists of eight subscales that might each represent clinically relevant dimensions of interoception. Most studies did not apply corrections for multiple comparisons in this case, yet as we report in the results section, no study had significant findings that relies only on a single subscale and in fact, in most studies results were consistent across multiple subscales.
Table 1. Interoception immediate and long-term outcomes.
| Post – between-group | Follow-up – between-group | Post – between-group | Follow-up – between-group | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Disorder | N | Intervention | Duration(weeks) | Focus of IBI | Comparator | Interoception measure | P-value** | ES in SMD | Direction | P-value** | ES in SMD | Direction | Symptom measure | P-value* | ES in SMD | Direction | P-value* | ES in SMD | Direction |
| Majore-Dusele et al. | 2021 | CP | 29 | MBDMT+TAU | 5 | ISM | TAU | FFMQ | 0.21 | — | — | 0.43 | — | — | NRS | 0.02 | — | ↑MBDMT | 0.04 | — | ↑MBDMT |
| Van der Maas et al. | 2015 | CP | 114 | PMT+TAU | 12 | ISM | TAU | SBC | <0.05 | 0.44 | ↑PMT | <0.05 | 0.73 | ↑PMT | PDI | >0.05 | −0.28 | — | >0.05 | 0.003 | — |
| Roberts et al. | 2022 | CP | 95 | MORE | 8 | ISM | AC | MAIA* | 0.001–0.008 | — | ↑MORE | N.A. | N.A. | N.A. | BPI | 0.03 | 0.54 | ↑MORE | N.A. | N.A. | N.A. |
| Seferiadis et al. | 2016 | CP | 113 | BBAT | 10 | ISM | AC | SF-36* | 0.110, 0.120 | −0.30, −0.31 | — | 0.044, 0.688 | −0.4, −0.08 | ↑BBAT | NDI | 0.07 | 0.37 | — | 0.368 | 0.18 | — |
| Mehling et al. | 2005 | CP | 36 | Breathing therapy | 6–8 | IS | AC | SF-36* | 0.45–0.32 | — | — | 0.27–0.45 | — | — | VAS | 0.74 | — | — | 0.56 | — | — |
| Ahmadi et al. | 2020 | CP | 60 | Feldenkrais method | 5 | IS | AC | MAIA | <0.001 | 1.73 | ↑Feldenkrais method | N.A. | N.A. | N.A. | MPQ | 0.166 | 0.13 | — | N.A. | N.A. | N.A. |
| Paolucci et al. | 2017 | CP | 53 | Feldenkrais method | 5 | IS | AC | MAIA | >0.05 | — | — | >0.05 | — | — | VAS | >0.05 | — | — | 0.005 | — | ↑Feldenkrais method |
| Nicholas et al | 2014 | CP | 140 | CBT + IE | 3 | ISM | AC | PSEQ | >0.05 | 0.22 | — | 0.57 | −0.01 | — | MPI | >0.05 | 0.06 | — | 0.82 | 0.04 | — |
| Zangrando et al. | 2017 | CP | 51 | SMG | 3 | IS | AC | MAIA | 0.024 | −0.6 | ↑SMG | 0.097 | −0.47 | — | VAS | 0.005 | 0.95 | ↑SMG | 0.098 | 0.58 | — |
| De Jong et al. | 2016 | CP & MDD | 40 | MBCT+TAU | 8 | ISM | Waitlist | MAIA* | 0.023–0.863 | 0.13–0.48 | ↑MBCT | N.A. | N.A. | N.A. | PCS | 0.294 | 0.03 | — | N.A. | N.A. | N.A. |
| Fissler et al. | 2016 | MDD | 74 | MBCT | 3 | ISM | AC | MAIA* | 0.001–>0.05 | 0.05–0.86 | ↑MBCT | N.A. | N.A. | N.A. | BDI-II | 0.001 | 1.3 | ↑MBCT | N.A. | N.A. | N.A. |
| Danielsson et al. | 2014 | MDD | 62 | BBAT | 10 | ISM | AC | SBC | >0.05 | — | — | N.A. | N.A. | N.A. | MADRS | >0.05 | — | — | N.A. | N.A. | N.A. |
| AC(exercise) | >0.05 | — | — | N.A. | N.A. | N.A. | >0.05 | — | — | N.A. | N.A. | N.A. | |||||||||
| Feinstein et al. | 2018 | MDD & Anxiety | 31 | Floatation-REST | 0 | IS | AC | MAIA* | <0.001 | — | ↑flotation-REST | N.A. | N.A. | N.A. | STAI | 0.001 | — | ↑flotation-REST | N.A. | N.A. | N.A. |
| Kendall et al. | 2000 | FM | 20 | BBAT | 21 | ISM | AC | CSQ | <0.05 | — | #BBAT | N.A. | N.A. | N.A. | FIQ | <0.05 | — | ↓BBAT | >0.05 | — | — |
| ASES | <0.05–>0.05 | — | ↓BBAT | 0.005–0.016 | — | ↓BBAT | |||||||||||||||
| Bravo et al | 2019 | FM | 41 | BBAT+TAU | 5 | ISM | TAU | BARS | <0.05 | — | ↑BBAT | 0.072 | — | — | VAS | 0.037 | — | ↑BBAT | 0.593 | — | — |
| Mannerkorpi & Arndorw | 2004 | FM | 36 | BBAT | 12 | ISM | Waitlist | BARS | 0.025 | — | ↑BBAT | N.A. | N.A. | N.A. | FIQ | >0.05 | — | — | N.A. | N.A. | N.A. |
| Gaylord et al. | 2011 | IBS | 75 | MBSR | 8 | ISM | AC | FFMQ | 0.03 | — | ↑MBSR | 0.04 | — | ↑MBSR | IBS-SS | 0.006 | — | ↑ MBSR | 0.001 | — | ↑MBSR |
| Henrich et al. | 2020 | IBS | 67 | MBCT-IBS | 6 | ISM | Waitlist | FFMQ | <0.01 | 0.37 | ↑MBCT-IBS | <0.001 | 0.61 | ↑MBCT-IBS | GSRS-IBS | <0.01 | 0.33 | ↑MBCT-IBS | 0.003 | 0.86 | ↑MBCT-IBS |
| Craske et al. | 2011 | IBS | 110 | CBT + IE | 10 | ISM | AC | PVAQ | <0.05 | 0.64 | ↑CBT + IE | <0.05 | 0.82 | ↑IE | BSS | >0.05 | 0.43 | — | >0.05 | 0.7 | — |
| AC (SM) | >0.24 | 0.29 | — | 0.06 | 0.56 | >0.05 | 0.44 | — | >0.05 | 0.33 | — | ||||||||||
| Quadt et al. | 2021 | ASD | 121 | ADIE | 8 | IS | AC | MAIA* | 0.095–0.573 | 0–0.3 | — | N.A. | N.A. | N.A. | STAI-T | >0.05 | 0.13 | — | 0.004 | 0.3 | ↑ADIE |
| BPQ | N.A. | N.A. | N.A. | 0.001 | 0.4 | ↑ADIE | |||||||||||||||
| HTT:IAccuracy | >0.001 | 0.5 | ↑ADIE | >0.001 | 0.51 | ↑ADIE | |||||||||||||||
| HDT:IAccuracy | 0.001 | 1.1 | ↑ADIE | N.A. | N.A. | N.A. | |||||||||||||||
| HDT:IAwareness | 0.383 | 0.26 | — | N.A. | N.A. | N.A. | |||||||||||||||
| Black et al. | 2015 | Sleep disturbance | 49 | MAPs | 6 | ISM | AC | FFMQ | 0.008 | 0.76 | ↑MAPs | N.A. | N.A. | N.A. | PSQI | 0.002 | 0.89 | ↑MAPs | N.A. | N.A. | N.A. |
| Nakamura et al. | 2011 | PTSD | 63 | MBB | 2 | ISM | AC | FFMQ | 0.052 | 0.46 | ↑MBB | N.A. | N.A. | N.A. | MOS-SS | 0.012 | 1.89 | ↑MBB | N.A. | N.A. | N.A. |
| Fetzner & Asmundson | 2015 | PTSD | 33 | Exercise & IP | 2 | IS | AC (CD) | ASI-3* | <0.01 | — | ↓IP | N.A. | N.A. | N.A. | PCL | >0.05 | — | — | N.A. | N.A. | N.A. |
| AC (EO) | 0.03 | — | ↓IP | N.A. | N.A. | N.A. | >0.05 | — | — | N.A. | N.A. | N.A. | |||||||||
| Classen et al. | 2021 | PTSD | 37 | SP | 20 | ISM | Waitlist | SBC* | 0.007–0.65 | 0.14–0.91 | ↑SP | N.A. | N.A. | N.A. | PCL | 0.71 | 0.12 | — | N.A. | N.A. | N.A. |
| Mehling et al. | 2018 | PTSD | 47 | IExercise | 12 | ISM | Waitlist | MAIA* | 0.000–0.958 | −1.05 | ↑IE | N.A. | N.A. | N.A. | CAPS | 0.038 | 0.9 | ↑IE | N.A. | N.A. | N.A. |
| Nordbrandt et al. | 2020 | PTSD | 338 | BBAT+TAU | 20 | ISM | TAU | MAIA | >0.05 | — | — | N.A. | N.A. | N.A. | HTQ | >0.05 | — | — | N.A. | N.A. | N.A. |
| AC+ TAU | >0.05 | — | — | N.A. | N.A. | N.A. | >0.05 | — | — | N.A. | N.A. | N.A. | |||||||||
| Price et al. | 2019 & 2019 | SUD | 217 | MABT+TAU | 8–10 | ISM | TAU | MAIA* | <0.001–0.19 | 0.00–0.75 | ↑MABT | >0.05 | — | — | TLFB | 0.01 | — | ↑MABT | <0.05 | 0.32 | ↑MABT |
| AC+TAU | — | <0.001–0.19 | 0.00–0.66 | ↑MABT | >0.05 | — | — | — | — | — | >0.05 | — | — | ||||||||
| Worden et al. | 2017 | SUD | 41 | AS+TAU | 3 | ISM | TAU | ASI-3* | 0.002 | 1.01 | ↑AS | 0.74 | −0.23 | — | TLFB | >0.05 | 0.22 | — | 0.66 | 0.27 | — |
| Price et al. | 2012 | SUD | 46 | MABT+TAU | 8 | ISM | TAU | SBC* | 0.750.88 | — | — | 0.010.59 | — | ↑MABT | TLFB | <0.02 | — | ↑MABT | 0.1 | — | — |
| Carrard et al. | 2011 | ED | 74 | CBT-IG | 24 | IM | Waitlist | EDI-2* | 0.024 | 0.52 | ↑CBT-IG | >0.05 | 0.23 | — | EDEQ | <0.001 | 0.38 | ↑CBT-IG | >0.05 | 0.3 | — |
| Catalan-Matamoros et al. | 2011 | ED | 28 | BBAT+TAU | 7 | ISM | TAU | EDI-2* | 0.31 | — | — | N.A. | N.A. | N.A. | EAT-40 | 0.039 | — | ↑BBAT | N.A. | N.A. | N.A. |
Note:
in favor of the interoception-based intervention,
no significant differences,
in favor of the control condition,
interoception measures with subscales only, see Table S4 for subscale P-values.
P-values reported here in inconsistent ways, included decimal points because we are necessarily following the reporting system of each paper.
Single session interventions or lasting less than one week anything will be marked 0.
Disorder: ASD, autism spectrum disorder, CP, Chronic Pain, ED, Eating Disorder, FM, Fibromyalgia, IBS, irritable bowel syndrome, MDD, Major Depressive Disorder; SUD, Substance Use Disorder.
Intervention: ADIE, Aligning Dimensions of Interoceptive Experience, BBAT, Basic Body Awareness Therapy, CBT-IE, Cognitive Behavioral Therapy with Interoceptive Exposure, Floatation-REST, Reduced Environmental Stimulation Therapy, IExercise, Integrative Exercise, IG, Internet Group, MABT, Mindful Awareness in Body-oriented Therapy, MAPs, Mindful Awareness Practices, MBB, Mind–Body Bridging, MBCT, Mindfulness-Based Cognitive Therapy, MBDMT, Mindful-Based Dance Movement Therapy, MBSR, Mindfulness-Based Stress Reduction, MORE, Mindfulness-Oriented Recovery Enhancement, PMT, Psychomotor Therapy; SP, Sensorimotor Psychotherapy.
Comparator: AC, Active Control, CD, cognitive distraction, EO, exercise only, SM, stress management; TAU, treatment as usual.
Interoception measure: ASES, Arthritis Self–Efficacy Scale, ASI-3, Anxiety Sensitivity-3, BARS, Body Awareness Rating Scale, BDI-II, Beck Depression Inventory-II, BPI, Brief Pain Inventory, BPQ, Body Perception Questionnaire, BSS, Bowel Symptom Severity Index, CAPS, Clinician-Administered PTSD Scale, CSQ, Coping Strategies Questionnaire, EAT-40, Eating Attitudes Test, EDEQ, Eating Disorder Examination Questionnaire, EDI-2, Eating Disorder Inventory-2, FFMQ, Five-Facet Mindfulness Questionnaire, FIQ, Fibromyalgia Impact Questionnaire, GSRS-IBS, Gastrointestinal Symptom Rating Scale-IBS, HDT, Heartbeat Discrimination task (Katkin et al., 1983); in which participants state if a presented external stimulus is synchronous or asynchronous to their own heartbeat, HTT, Heartbeat Tracking task (Schandry et al., 1981); in which participants silently attend to their heart beats for a specific time frame and state how many heartbeats they felt, HTQ, Harvard Trauma Questionnaire, IAccuracy, Interoceptive Accuracy IAweress, Interoceptive Awareness, IBS-SS, Irritable Bowel Syndrome-Scoring System, IP, interoceptive prompts, MADRS, Montgomery-Asberg Depression Rating Scale, MAIA, Multidimensional Assessment of Interoceptive Awareness, MOS-SS, Medical Outcomes Study-Sleep Scale, MPI, Multidimensional Pain Inventory, MPQ, McGill Pain Questionnaire, NDI, Neck Disability Index, NRS, Numeric Rating Scale, PCL,
Although not preregistered, in order to give readers a more integrated overview of central findings we also presented post-intervention results along an available, tripartite classification scheme, distinguishing between observed “indicative”, “preliminary” and “inconclusive” evidence for a given mental health disorder. The existence of ≥2 RCTs with >50% demonstrating superior efficacy was defined as “indicative evidence” of efficacy, while the existence of only one RCT for a given disorder demonstrating efficacy in the absence of conflicting evidence was defined as “preliminary evidence” of efficacy. Lastly, results were labeled as “inconclusive evidence” of efficacy when there were ≥1 RCTs of which ≥50% did not find the IBI to be superior.
Quality assessment
The quality of RCTs was assessed using a modified, enhanced version of the Jadad scale59 and presented visually in one, comprehensive figure (Fig. S1). The original scale comprises three scoring criteria: (i) randomization; (ii) double-blinding; and (iii) withdrawals/dropouts. One point each was assigned if the following criteria were fulfilled: (i) randomization; (ii) blinding and (iii) description of dropouts.59 One additional point was assigned to criterion (i) if the randomization method was described appropriately. Jadad scale criterion (ii) was adapted as double blinding cannot be realized in trials of psychological interventions.60 Thus, criterion (ii) was rated as fulfilled if researchers were blinded to treatment allocation. In total, a maximum score of 4 points could be achieved, with higher scores indicating better study quality.
Results
Study Characteristics
A total of 32 studies reporting data on 31 RCTs published between 2000 and 2021 were included in the systematic review as shown in Figure 1. Mental health disorders studied included patients with chronic pain (n = 9), chronic pain and depression (n = 1), depression (n = 2), anxiety and depression (n = 1), PTSD (n = 5), autism spectrum disorders (n = 1), eating disorders (n = 2), irritable bowel syndrome (n = 3), sleep disturbance (n = 1), fibromyalgia (n = 3) and substance use disorders (n = 3) (Table 1). The sample sizes ranged between 20 and 338 participants with a mean number of 75.5 participants (SD = 64.1) across all studies (Table 1). A total of 16 out of 31 (51.6%) RCTs reported follow-up data ranging between.
Quality of studies
The quality of included studies was moderate to good with an average score of 3.2 out of a maximum of 4 (Fig. S1). All studies reported randomization (100%) with 77.4% describing appropriate randomization procedures, e.g., using sealed envelopes or a randomization algorithm. Except for one study,61 all RCTs documented number and reasons for dropouts (96.8%). Regarding blinding of researchers to treatment allocation, only 41.9% described appropriate masking, while the rest showed increased risk of bias. Lastly, 54.8% (n = 17/31) of RCTs reported the pre-registration of a study protocol.
Interoception-based interventions
The applied IBIs varied in their approach and intensity (frequency, and duration) (Table S2). The average number of intervention sessions across all studies was 11.5 (SD = 8.4, range 1–46) with a mean duration of 8.5 weeks (SD = 5.9, range single-session – 24 weeks). The reviewed IBIs also varied in the level of interoception they targeted. Given that these interventions do not always use the terms described in the experimental and physiological literature on interoception to distinguish between the various levels of interoception targeted we used the following scheme to help readers understand the central findings in this regard. Lastly, the types of interoception each intervention is targeting could not be meaningfully distinguished to accuracy, awareness, and sensibility, as the terms used by each therapeutic intervention do not always directly correspond to these dimensions as categorized in the experimental and psychophysics traditions (we now address this point directly in the text in our introduction, please see also above), and furthermore (as now outlined in the discussion) in terms of measures, almost all studies investigated interoception only by self-report limiting almost all results to a focus on the dimensions of interoceptive awareness and sensibility. IBIs were distinguished into three categories:
Interventions focusing solely on enhancing the bottom-up processing of, or the attention to physiological signals, such as breathing or exercise or veridical perception (IAccuracy) interventions without any components of reflection or appraisal of the experience in conversation with the therapist.
Interventions focusing on the (metacognitive) appraisal of or reflection on these signals and their meaning without any concomitant instructions to attend to bodily signals, improve or modify them (via exercise or similar).
Interventions involving both elements such as mindfulness based cognitive therapy, incorporating attention to bodily signals and reflection upon them with the therapist (Table S2).
The majority of RCTs (n = 23/31, 74.2%) used IBIs that both targeted processing and attention to the physiological signal itself, but also the metacognitive appraisal of the signal, including “mindfulness-based cognitive therapy” (MBCT) (n = 3). The most frequently applied IBI was “basic body awareness therapy” (BBAT) (n = 7), consisting of movement and massage therapy, followed by reflections about participants’ experiences during the sessions (Table S2).
Control conditions
IBIs were mainly compared against active controls (AC, n = 17/31, 54.8%) followed by treatment as usual (TAU, n = 8/31, 25.8%) and waitlist (WL, n = 6/31, 19.4%) (Table 1). A total of five RCTs applied a three-arm design, including two RCTs comparing an IBI plus TAU, against TAU plus an AC condition and TAU alone, accounting for dosage effects. Active controls encompassed an equally intensive treatment as the IBI-condition, however, without any interoceptive components (e.g., focus on exteroceptive cues). In the waitlist conditions participants did not receive any kind of treatment for the duration of the RCT. Overall, TAU varied markedly regarding intensity and type of treatment ranging from pharmacotherapy only to group therapy with 36 sessions (Table S3).
Interoception measures
The most frequent used measure for interoception was the Multi-dimensional Assessment of Interoceptive Awareness (MAIA) (n = 11/31, 35.5%) followed by the Five-Facet Mindfulness Questionnaire (FFMQ) (n = 5/31, 16.1%) and Scale of Body Connection (SBC) (n = 4/31, 12.9%) (Table 1). Although interoception can be assessed by experimental tasks and questionnaires, almost all RCTs used exclusively self-report measures (n = 30/31, 96.8%). In fact, only one study applied both questionnaires and validated tasks, measuring interoceptive accuracy (i.e., heartbeat tracking and heartbeat discrimination).49 As aforementioned, when verbal report measures have multiple subscales without aggregate scoring, we considered the overall direction of the effect if at least one subscale showed significant between-group effects.
Efficacy of interoception-based interventions on interoception
Across all studies, a pattern emerged, with most, but not all IBIs being more efficacious at improving interoception in mental health disorders compared to control conditions (Tables 1 and 2). In total, 20 out of 31 RCTs (64.5%) demonstrated statistically superior between-group effects of IBIs at improving interoception. A total of nine studies (29%) found no differences and two RCTs (6.5%) found the control condition to be significantly superior at improving interoception. Between-group effect sizes post-treatment ranged from d = −0.30–1.73 and were available for 18 RCTs (58%). Of the 16 studies reporting follow-up data, eight RCTs (50.0%) reported a significant prolonged improvement in interoception in favor of the IBI at follow-up (Table 1).
Table 2. Evaluation of efficacy for improving interoception and symptoms per mental disorder according to a scheme.
| RCTs per disorder | Interoception | Symptoms | ||||||
|---|---|---|---|---|---|---|---|---|
| Superior to control (n, %) | No differences (n, %) | Inferior to control (n, %) | Evidence of efficacy | Superior to control (n, %) | No differences (n, %) | Inferior to control (n, %) | Evidence of efficacy | |
| All disorders (n = 31) | 20 (64.5%) | 9 (29%) | 2 (6.5%) | 15 (48.4%) | 15 (48.4%) | 1 (3.2%) | ||
| Chronic pain (n = 9) | 4 (44.4%) | 5 (55.6%) | 0 | Inconclusive | 3 (33.3%) | 6 (66.7%) | 0 | Inconclusive |
| Chronic pain and depression (n = 1) | 1 (100%) | 0 | 0 | Preliminary | 0 | 1 (100%) | 0 | Inconclusive |
| Depression (n = 2) | 1 (50%) | 1 (50%) | 0 | Inconclusive | 1 (50%) | 1 (50%) | 0 | Inconclusive |
| Anxiety and depression (n = 1) | 1 (100%) | 0 | 0 | Preliminary | 1 (100%) | 0 | 0 | Preliminary |
| PTSD (n = 5) | 3 (60%) | 1 (20%) | 1 (20%) | Indicative | 2 (40%) | 3 (60%) | 0 | Inconclusive |
| Autism spectrum disorders (n = 1) | 1 (100%) | 0 | 0 | Preliminary | 0 | 1 (100%) | 0 | Inconclusive |
| Eating disorders (n = 2) | 1 (50%) | 1 (50%) | 0 | Inconclusive | 2 (100%) | 0 | 0 | Indicative |
| Irritable bowel syndrome (n = 3) | 3 (100%) | 0 | 0 | Indicative | 2 (66.7%) | 1 (33.3%) | 0 | Indicative |
| Sleep disturbance (n = 1) | 1 (100%) | 0 | 0 | Preliminary | 1 (100%) | 0 | 0 | Preliminary |
| Fibromyalgia (n = 3) | 2 (66.7%) | 0 | 1 (33.3%) | Indicative | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | Inconclusive |
| Substance use disorders (n = 3) | 2 (66.7%) | 1 (33.3%) | 0 | Indicative | 2 (66.7%) | 1 (33.3%) | 0 | Indicative |
Note: Indicative evidence for efficacy ≥2 RCTs with >50% demonstrating superior efficacy; inconclusive evidence of efficacy ≥1 RCTs of which ≥50% did not find the IBI to be superior; inferior to control = statistical between-effects in favor of the control condition; no difference = no significant differences; preliminary evidence of efficacy = one available RCT demonstrating efficacy; superior to control group = statistical between-effects in favor of the IBI.
Indicative evidence for the superiority of IBIs at improving interoception was found in participants with irritable bowel syndrome with all three RCTs (100%) demonstrating significant better between-group effects on interoception and effect sizes ranging from 0.29 to 0.64 (Table 2). Furthermore, indicative evidence for substance-use disorder (SMDs ranging from −0.10 to 0.75) having two out of three RCTs (66.7%) demonstrating superiority at improving interoception. The studies for irritable bowel syndrome (n = 110,75,67; quality = ø3.7 out of max. 4 on the Jadad scale) and substance use disorder (n = 217,41,46; quality = ø3.0 on the Jadad scale) had good to very good quality and sample sizes were sufficient. Lastly, indicative evidence was also identified for fibromyalgia (no SMDs available) with two studies demonstrating superiority and one inferiority, and in PTSD (SMDs ranging from −0.01 to 1.05) with three out of five demonstrating superiority (60%) and two out of five inferiority (20%) (Table 2). For fibromyalgia sample sizes of the RCTs were small (n = 41,36,20) and quality was moderate to good (ø2.7 on the Jadad scale). For PTSD, the largest trial with 338 participants found no significant between-group effects at improving interoception and the three trials with positive results in favor of the IBI had small to medium sample sizes (n = 63,37,47) and moderate to good quality (ø2.7 on the Jadad scale). Preliminary evidence for IBIs at improving interception was found in chronic pain with depression, anxiety and depression, autism spectrum disorder and sleep disturbances (one RCT available per disorder showing positive effects) (Table 2). Inconclusive evidence was found in patients diagnosed with chronic pain, depression and eating disorders (Table 2).
Considering the differing sample sizes, a total of six RCTs49,62–66 had at least 50 participants in each arm, enabling a more confident detection of a medium effect (d = 0.5) with a power of 0.8 at alpha = 0.05 using a one-sided test.67 Of those, three (50.0%)49,63,66 reported superior effects in changing interoception for the IBI, which is somewhat smaller than the percentage over all studies (61.3%). Of those six RCTs, two reported between-group effect sizes ranging from d = −0.30 to 1.10 in comparison to active controls (Table 1).
No clear differences in efficacy of improving interoception were found based on the type of IBI (Table 1). Out of the seven trials focusing on enhancing awareness of interoceptive signals, four (57.1%) demonstrated superior effects49,68–70 in favor of the IBI, and one (14.3%) inferior effects.71 Out of the 23 studies focusing on both interoceptive signal awareness and metacognitive appraisal of interoceptive signals, 15 studies (65.2%) found the IBI to be significantly more efficacious at improving interoceptive sensibility and one study (4.3%) significantly less efficacious72 (Table 1).
However, differences in efficacy at improving interoceptive sensibility were found according to the type of comparator condition (Table 1). Of the 19 RCTs comparing an IBI against an AC, with or without treatment as usual, 10 (52.6%) detected significant superior effects at improving interoceptive sensibility in favor of the IBI condition and two (10.5%) detected inferior effects.71,72 Six studies compared an IBI against TAU alone, of which three (50.0%) found significant superior effects for the IBI (Table 1). Lastly, of the six studies comparing IBIs to waitlist condition, all (100%) found significant positive results in improving interoceptive sensibility for the IBI.
Efficacy of interoception-based interventions on mental health symptoms
Regarding differences in symptom improvements, 15/31 RCTs (48.4%) demonstrated significant between-group effects in symptoms in favor of the IBI against a comparator condition, while 15 out of 31 (48.4%) RCTs found no significant differences and one RCT found the comparator condition significantly better regarding symptom improvement (3.2%) (Table 1&2). Between-group effect sizes post-treatment ranged from d = −0.28–1.89 and were available for 17 RCTs (54.84%). A total of 16 RCTs (51.6%) reported follow-up data on mental health symptoms of which six (37.5%) reported a significant prolonged improvement in favor of the IBI at follow-up (Table 1).
Indicative evidence for significant greater efficacy at improving symptoms in favor of the IBI was found for eating disorders (n = 2/2, 100.0%) with one effect size of d = 0.38 and substance use disorder (n = 2/3, 66.7%) with effect sizes ranging from 0.22–0.38 and irritable bowel syndrome (n = 2/3, 66.7%) with effect sizes ranging from 0.33 to 0.44. (Table 2). For all other mental health disorders, evidence was inconclusive and preliminary. Notably, indicative evidence for IBIs were identified as improving interoception in fibromyalgia and PTSD, did not correspond to any significant improvements in disorder symptoms (Table 2). Furthermore, in larger trials with sufficient power (n ≥ 50 per arm), to detect a medium effect (d = 0.5) with a power of 0.8 at alpha = 0.05 using a one-sided test,67 only one out of six RCTs (16.7%) found the IBI to improve symptoms significantly better than the control group.66 However, these six trials compared an IBI either against TAU or an active control where smaller effects are expected. Regarding the type of IBI, from the seven interventions focusing on interoceptive signal awareness, two (28.6%) identified significant symptom improvement in favor of the IBI, whereas 11 (47.8%) trials focusing on interoceptive signals, and the appraisal of these signals revealed greater improvement on participants’ symptoms. The results also differed with respect to type of comparator (Table 1). Whereas two-thirds of the trials comparing against TAU (4/6, 66.7%) found the IBI to achieve superior symptom improvements, this was only the case in 42.1% (8/19) in comparison to active control and 50.00% (3/6) using waitlist (Table 1).
Discussion
This systematic review is the first to investigate the efficacy of IBIs at improving interoception and reducing target symptoms in mental health disorders. Across the included RCTs (n = 31), a pattern emerged with most, but not all IBIs (20/31, 64.5%) being significantly more efficacious at improving interoception in mental health disorders compared to control conditions. Indicative evidence of efficacy was found for PTSD, irritable bowel syndrome, fibromyalgia, and substance use disorder. Preliminary evidence of efficacy was found for chronic pain with depression, anxiety and depression, autism spectrum disorder and sleep disturbances. Inconclusive evidence of efficacy was found for chronic pain, depression, and eating disorders.
The secondary aim was to identify significant between-group differences of IBIs at improving mental health symptoms. The emerging pattern of results was different in this case, with indicative evidence of efficacy observed in eating disorders, irritable bowel syndrome and substance use disorder. For all other disorders the evidence was inconclusive with less than half of the examined RCTs exhibiting reliable evidence of symptom improvement. Eating and substance use disorders lie at the interface between physical and mental health and are characterized not only with interoception abnormalities,73,74 but also with difficulties of insight regarding their eating, or substance use behaviors and their effects on the body. However, given the variability in both the methods and the quality of the available RCTs (see below for further discussion), it remains unclear whether IBIs are particularly suited to certain disorders at the interface between physical and mental health (e.g., disorders that affect appetitive brain–body systems), or whether better interventions stand to improve symptoms across a wider pool of mental health conditions. Importantly, given that symptom improvement was more common among the RCTs that targeted both interoceptive processing and interoceptive metacognition (see also below), further research in this area is warranted.
The included IBIs were heterogenous in their approach to improving interoception, i.e., the interoceptive dimension targeted. IBIs were grouped in three categories: (i) IBIs focusing solely on sensing of interoceptive signals; (ii) IBIs focusing solely on the appraisal of interoceptive signals; and (iii) IBIs targeting both. The most frequently applied type of IBI targeted both improving the perception of physiological signals and the metacognitive appraisal of the signals. In terms of improving interoception no differences in efficacy were found between these groups. However, IBIs focusing on both, more often yielded significant symptom improvement compared to control conditions. This outcome may be attributed to the dual mechanism of action of IBIs reinforcing the experience of interoceptive signals and their metacognitive appraisal, which are both components of interoceptive processing. This might also indicate that in addition to improving interoceptive accuracy other interoceptive dimensions must be targeted to improve symptoms.
In terms of type of control conditions, the efficacy of IBIs at improving interoception differed depending on the comparator used, in that RCTs comparing against waitlist more often found favorable effects. However, there was no such finding regarding symptom improvements. This outcome is notable as comparisons against waitlist usually tend to find larger effect sizes than comparisons against TAU or active controls.75–78 Additionally, the outcome could be explained as those trials comparing against waitlist condition were mostly underpowered and thus might have missed small or moderate differences (Table 1). Furthermore, all trials comparing an IBI with TAU tested the IBI in addition to TAU vs. TAU alone. Thus, ‘dosage’ in all TAU-trials was not balanced except for two three-armed RCTs that included TAU alone and TAU with an AC in comparison to an IBI. Hence, it is unclear whether differential effects of TAU-comparisons are due to the interoception component or merely a higher therapeutic ‘dose’. Moreover, despite the higher dose, few RCTs comparing an IBI with TAU against TAU alone found positive results in favor of the IBI, which can also signify the efficacy of TAU practice, in some cases. Lastly, TAU varied greatly regarding intensity and type of treatment ranging from pharmacotherapy only to group therapy with 36 sessions and thus no comprehensive conclusions can be drawn regarding treatment dosage. As the intensity of TAU has been shown to significantly affect between-group comparisons,79 future meta-analytic studies should take TAU intensity into account as a potential moderator.
Across all RCTs, the power was low with an average of 75.5 participants (SD = 64.1) per RCT. More than half of the trials (17/31, 54.86%) had n ≤ 60 participants. In those RCTs with sufficient power to detect medium to large effect sizes (n ≥ 50 per arm) 50% found significant effects of the IBI in improving interoception, which is somewhat smaller than the average across all studies (64.5%). In terms of symptom reduction, the results differed markedly depending on power. While across all studies 45.2% of the trials found the IBI to be significantly better in improving mental symptoms, 16.7% of the RCTs with at least 50 participants per arm found the IBI to be significantly better. Hence the results regarding the efficacy of IBIs at improving mental symptoms must be considered with utmost caution and require further investigation. Future RCTs should consider a priori power calculations. In addition, only about half of the reviewed studies (16/31 of RCTs) had follow-up data so the current results are based on post-intervention differences and hence conclusions about the long-term effects of IBIs are not warranted based on this evidence.
The quality of the included RCTs was overall sufficient with most trials reporting appropriate randomization procedures and reasons for withdrawal. However, risk of bias persisted in terms of researcher blinding of treatment allocation and lack of pre-registration of study protocols, increasing researcher’s freedom and risk of publication bias. In addition, most RCTs used exclusively self-report to measure intervention effects on interoception and in 11 RCTs the scales used consisted of subscales only with no total scores. In most of these cases, statistical comparisons were performed without correction for multiple analyses, thus potentially leading to an over-estimation of significant effects on interoception. However, it should be noted that despite the multidimensionality of interoception, in the majority of studies without aggregate measures or omnibus analyses (9 out of 11), 50% or more of the subscales used showed consistent results. Thus, future studies could optimize the interoception measures used to focus on meaningful aggregate self-report measures, as well as physiological and behavioral measures that can improve the quality of measurement.
Strikingly, indicative evidence for IBIs in significantly improving interoception in PTSD and fibromyalgia does not correspond with significant improvements in symptomatic change. More generally, as summarized above, the current systematic review found that IBIs can improve interoception across many disorders, while primary mental health symptoms remain unaffected by the same IBIs with the exception of substance use disorders and irritable bowel syndrome. These findings suggest that symptom improvement may be harder to treat, either because targeting interoceptive deficits by behavioral interventions is not sufficient, or because the existing IBIs are not targeting interoception in a clinically efficacious manner. Interestingly, most of the reviewed studies do not statistically analyze the relationship between these two variables, however they discuss the following possibilities: some studies attributed this discrepancy mainly to small sample sizes with low power.80–84 Other explanations given were that the exposure to the applied IBI was not sufficient69,84; attention to bodily signals or the IBI itself through exercise may have increased chronic pain63,83; interoception might not be a mechanism of change63; the control condition may have been too strong, or that self-report measures may have impacted outcome81; the lack of difference may be attributed to a ceiling effect of the symptom outcome measure, or the concomitant pharmacological treatment, there is the possibility that IBIs may only be efficacious for specific subgroups.84
Therefore, these possibilities call for a better characterization of the comorbidity between mental health symptoms and interoceptive deficits. For instance, disturbed interoception can be only one of many predisposing factors for the gradual development of mental health symptoms. In addition, the trajectory of symptom improvement compared to improvement of interoception, can be very distinct and therefore further research should target and explore both predisposing and maintenance mechanisms, as well as compare and contrast the effects of targeting different domains and levels of interoception on clinical outcomes. Future studies should empirically investigate in greater specificity whether interoception is a mediator for change in symptom improvement. Indeed, even among the few available studies on invasive, neurostimulation or pharmacological treatments targeting interoception and mental health, it has become clear that specific interoceptive biomarkers for mental health need to be established before interventions can have meaningful clinical results.31,35,42,85–87 However, such insights have not for the most part been taken up by the available RCTs on behavioral IBIs. For example, there is evidence for dysfunctions in both cardiac and gastrointestinal interoception in eating disorders and the potential to alter them by vagus nerve stimulation, interoceptive exposure or reduced environmental stimulation (i.e. floatation therapy; 89). Yet the existing RCTs in eating disorders have targeted only conscious levels of body awareness and interoception, such as the feeling of relaxation following slow breathing or the pleasure of eating, with interoception interventions specifically targeting and measuring cardiac or gastrointestinal interoception, being limited to a handful of case or pilot studies.88–90 In summary, with the exception of substance use disorders and irritable bowel syndrome, this systematic review of RCTs testing the efficacy of behavioral interoceptive interventions did not find evidence to support the hypothesis that improving disturbed interoception is accompanied by reductions of mental health symptoms.
This systematic review has limitations. As research into IBIs is a novel field and no previous systematic investigation was conducted, this study aimed not only to review the evidence on the efficacy of IBIs, but also summarize the different approaches, quality of the studies, comparators, or measures used. However, future meta-analytic investigations are required to calculate overall effects of IBIs compared to different controls and regarding different outcomes. Although power was considered in interpreting the data, systematic reviews can only do so in a limited manner and meta-analytic evaluation is required, taking into consideration the sample size of the trial. Furthermore, meta-analyses can include RCTs with very small sample sizes, whereas we had to exclude one RCT as no metric test results were reported or could be calculated. Although this is a single small-scale study and therefore the effects of bias are unlikely to be significant, excluding articles with small sample sizes may result in publishing bias. In addition, as IBIs have not been sufficiently defined, we included all interventions aiming to improve interoception. As the field progresses and more RCTs become available, future reviews and meta-analytic evaluations might narrow their analyses to specific types of IBIs to increase homogeneity and internal validity or focus on specific measures of interoception.
Furthermore, some limitations of the present review depend to a large extent on the quality of the included RCTs. The same applies to the assessment of interoception. As aforementioned, with the exception of one study, all RCTs used self-report measures to assess interoception and future RCTs should apply also additional, experimental, and physiological measures, which can provide additional information regarding effects on interoceptive sensing, accuracy, or metacognition, and can be less biased by beliefs of one’s ability to sense interoceptive signals and other cognitive biases.
In conclusion, interoception-based interventions are potentially efficacious at improving interoception in mental health disorders. We found indicative and preliminary evidence for improved interoception in several mental health disorders, including irritable bowel syndrome, fibromyalgia, PTSD, substance use disorder, autism spectrum disorder and sleep disturbances. To date, there is no consistent evidence for the potential efficacy of IBIs in improving interoception in chronic pain, depression, or eating disorders. In terms of improving symptoms, indicative evidence of efficacy was found for eating disorders, irritable bowel syndrome and substance use disorders. The applied IBIs varied markedly in the heir approach to improving interoception with those focusing on both the perception and appraisal of bodily signals achieving better improvements in symptoms than those focusing on improving the sensing of interoceptive signals only. For many mental health disorders more RCTs with sufficient power are required to confidently determine the efficacy of IBIs.
Supplementary Material
Acknowledgments
This project was supported by the EU Horizon 2020 research and innovation program under grant agreement No.818070, for the Consolidator Award METABODY (to AF).
Footnotes
Author contributions
NH and MB contributed equally to the screening, extraction, synthesis of data and writing up of the review. MT and PJ revised the draft and senior authors AF and CS revised the draft and supervised the overall research process.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- 1.Suksasilp C, Garfinkel SN. Towards a comprehensive assessment of interoception in a multi-dimensional framework. Biol Psychol. 2022;168:108262. doi: 10.1016/j.biopsycho.2022.108262. [DOI] [PubMed] [Google Scholar]
- 2.Berntson GG, Khalsa SS. Neural circuits of Interoception. Trends Neurosci. 2021;44:17–28. doi: 10.1016/j.tins.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig AD. Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 4.von Mohr M, Fotopoulou A. The Cutaneous Borders of Interoception: Active and Social Inference of Pain and Pleasure on the Skin. The Interoceptive Mind: From Homeostasis to Awareness. Oxford University Press; New York, NY, US: 2019. pp. 102–120. [Google Scholar]
- 5.Füstös J, Gramann K, Herbert BM, Pollatos O. On the embodiment of emotion regulation: Interoceptive awareness facilitates reappraisal. Soc Cogn Affect Neurosci. 2013;8:911–917. doi: 10.1093/scan/nss089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 7.Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig AD. Human feelings: Why are some more aware than others? Trends Cogn Sci. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Damasio AR. Descartes’ error and the future of human life. Sci Am. 1994;271:144. doi: 10.1038/scientificamerican1094-144. [DOI] [PubMed] [Google Scholar]
- 10.James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- 11.Azzalini D, Rebollo I, Tallon-Baudry C. Visceral signals shape brain dynamics and cognition. Trends Cogn Sci. 2019;23:488–509. doi: 10.1016/j.tics.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Brewer R, Murphy J, Bird G. Atypical interoception as a common risk factor for psychopathology: A review. Neurosci Biobehav Rev. 2021;130:470–508. doi: 10.1016/j.neubiorev.2021.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons WK, Burrows K, Avery JA, et al. Depression-related increases and decreases in appetite: Dissociable patterns of aberrant activity in reward and interoceptive Neurocircuitry. Am J Psychiatry. 2016;173:418–428. doi: 10.1176/appi.ajp.2015.15020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalsa SS, Craske MG, Li W, Vangala S, Strober M, Feusner JD. Altered interoceptive awareness in anorexia nervosa: Effects of meal anticipation, consumption and bodily arousal. Int J Eating Disord. 2015;48:889–897. doi: 10.1002/eat.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berner LA, Simmons AN, Wierenga CE, et al. Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychol Med. 2018;48:142–154. doi: 10.1017/S0033291717001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral Interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology. 2016;41:521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16:266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. You are the danger: Attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 2014;142:110–119. doi: 10.1016/j.drugalcdep.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedeño L, Couto B, Melloni M, et al. How do you feel when you Can’t feel your body? Interoception, functional connectivity and emotional processing in depersonalization-Derealization disorder. PloS One. 2014;9:e98769. doi: 10.1371/journal.pone.0098769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Back SN, Bertsch K. Interoceptive processing in borderline personality pathology: A review on neurophysiological mechanisms. Curr Behav Neurosci Rep. 2020;7:232–238. [Google Scholar]
- 23.DeVille DC, Khalsa SS, Lapidus RC, White E, Paulus MP, Aupperle RL. A Transdiagnostic multilevel examination of interoceptive processing in individuals with a remote history of suicidal behavior. Behav Ther. 2021;52:1080–1092. doi: 10.1016/j.beth.2021.01.006. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultchen D, Zaudig M, Krauseneck T, Berberich G, Pollatos O. Interoceptive deficits in patients with obsessive-compulsive disorder in the time course of cognitive-behavioral therapy. PloS One. 2019;14:e0217237. doi: 10.1371/journal.pone.0217237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhardt KM, Zerubavel N, Young AS, et al. A multi-method assessment of interoception among sexual trauma survivors. Physiol Behav. 2020;226:113108. doi: 10.1016/j.physbeh.2020.113108. [DOI] [PubMed] [Google Scholar]
- 26.Critchley HD, Ewing DL, van Praag CG, Habash-Bailey H, Eccles JA, Meeten F, et al. Transdiagnostic expression of interoceptive abnormalities in psychiatric conditions. medRxiv. 2019:19012393 [Google Scholar]
- 27.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein DJ, Craske MG, Rothbaum BO, et al. The clinical characterization of the adult patient with an anxiety or related disorder aimed at personalization of management. World Psychiatry. 2021;20:336–356. doi: 10.1002/wps.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA. Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: Evidence for a central fear mechanism. Arch Gen Psychiatry. 2001;58:125–131. doi: 10.1001/archpsyc.58.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during Interoception across psychiatric disorders. Am J Psychiatry. 2021;178:761–770. doi: 10.1176/appi.ajp.2020.20091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalsa SS, Adolphs R, Cameron OG, et al. Interoception and mental health: A roadmap. Biol Psychiatry: Cognit Neurosci Neuroimaging. 2018;3:501–513. doi: 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonaz B, Lane RD, Oshinsky ML, et al. Diseases, disorders, and comorbidities of Interoception. Trends Neurosci. 2021;44:39–51. doi: 10.1016/j.tins.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Saramandi A, Crucianelli L, Koukoutsakis A, Nisticò V, Baiza A, Goeta D, et al. Belief Updating about Interoception and Body Size Estimation in Anorexia Nervosa. PsyArXiv. 2022 [Google Scholar]
- 35.Nord CL, Garfinkel SN. Interoceptive pathways to understand and treat mental health conditions. Trends Cogn Sci. 2022;26:499–513. doi: 10.1016/j.tics.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Murphy J, Catmur C, Bird G. Alexithymia is associated with a multi-domain, multidimensional failure of interoception: Evidence from novel tests. J Exp Psychol Gen. 2018;147:398–408. doi: 10.1037/xge0000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy J, Catmur C, Bird G. Classifying individual differences in interoception: Implications for the measurement of interoceptive awareness. Psychon Bull Rev. 2019;26:1467–1471. doi: 10.3758/s13423-019-01632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garfinkel SN, Gould van Praag CD, Engels M, et al. Interoceptive cardiac signals selectively enhance fear memories. J Exp Psychol Gen. 2021;150:1165–1176. doi: 10.1037/xge0000967. [DOI] [PubMed] [Google Scholar]
- 39.Wittenberg GM, Stylianou A, Zhang Y, et al. Effects of immunomodulatory drugs on depressive symptoms: A mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25:1275–1285. doi: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris LS, Voon V, Leggio L. Stress, motivation, and the gut-brain Axis: A focus on the ghrelin system and alcohol use disorder. Alcohol Clin Exp Res. 2018;42:1378–1389. doi: 10.1111/acer.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoury NM, Lutz J, Schuman-Olivier Z. Interoception in psychiatric disorders: A review of randomized, controlled trials with Interoception-based interventions. Harv Rev Psychiatry. 2018;26:250–263. doi: 10.1097/HRP.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weng HY, Feldman JL, Leggio L, Napadow V, Park J, Price CJ. Interventions and manipulations of Interoception. Trends Neurosci. 2021;44:52–62. doi: 10.1016/j.tins.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farb N, Daubenmier J, Price CJ, et al. Interoception, contemplative practice, and health. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes SC, Levin ME, Plumb-Vilardaga J, Villatte JL, Pistorello J. Acceptance and commitment therapy and contextual behavioral science: Examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180–198. doi: 10.1016/j.beth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, Stewart A. Body awareness: Construct and self-report measures. PloS One. 2009;4:e5614. doi: 10.1371/journal.pone.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The multidimensional assessment of interoceptive awareness (MAIA) PloS One. 2012;7:e48230. doi: 10.1371/journal.pone.0048230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown TA, Barlow DH. Long-term outcome in cognitive-behavioral treatment of panic disorder: Clinical predictors and alternative strategies for assessment. J Consult Clin Psychol. 1995;63:754–765. doi: 10.1037//0022-006x.63.5.754. [DOI] [PubMed] [Google Scholar]
- 48.Deacon BJ, Lickel JJ, Farrell NR, Kemp JJ, Hipol LJ. Therapist perceptions and delivery of interoceptive exposure for panic disorder. J Anxiety Disord. 2013;27:259–264. doi: 10.1016/j.janxdis.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Quadt L, Garfinkel SN, Mulcahy JS, et al. Interoceptive training to target anxiety in autistic adults (ADIE): A single-center, superiority randomized controlled trial. eClinicalMedicine. 2021;39:39. doi: 10.1016/j.eclinm.2021.101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bornemann B, Herbert BM, Mehling WE, Singer T. Differential changes in self-reported aspects of interoceptive awareness through 3 months of contemplative training. Front Psychol. 2015;5 doi: 10.3389/fpsyg.2014.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JM, Crane C, Barnhofer T, et al. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: A randomized dismantling trial. J Consult Clin Psychol. 2014;82:275–286. doi: 10.1037/a0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daubenmier J, Kristeller J, Hecht FM, et al. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. J Obes. 2011;2011:651936. doi: 10.1155/2011/651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cochrane Handbook for Systematic Reviews of Interventions. 3rd edn. Cochrane: John Wiley & Sons; 2022. [Google Scholar]
- 54.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 56.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 57.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Word Health Organization; Geneve, Switzerland: 1993. [Google Scholar]
- 58.Price CJ, Merrill JO, McCarty RL, Pike KC, Tsui JI. A pilot study of mindful body awareness training as an adjunct to office-based medication treatment of opioid use disorder. J Subst Abuse Treat. 2020;108:123–128. doi: 10.1016/j.jsat.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 60.Donker T, Griffiths KM, Cuijpers P, Christensen H. Psychoeducation for depression, anxiety and psychological distress: A meta-analysis. BMC Med. 2009;7:79. doi: 10.1186/1741-7015-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrard I, Crépin C, Rouget P, Lam T, Golay A, Van der Linden M. Randomised controlled trial of a guided self-help treatment on the internet for binge eating disorder. Behav Res Ther. 2011;49:482–491. doi: 10.1016/j.brat.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Seferiadis A, Ohlin P, Billhult A, Gunnarsson R. Basic body awareness therapy or exercise therapy for the treatment of chronic whiplash associated disorders: A randomized comparative clinical trial. Disabil Rehabil. 2016;38:442–451. doi: 10.3109/09638288.2015.1044036. [DOI] [PubMed] [Google Scholar]
- 63.Van der Maas LC, Köke A, Pont M, et al. Improving the multi-disciplinary treatment of chronic pain by stimulating body awareness: A cluster-randomized trial. Clin J Pain. 2015;31:660–669. doi: 10.1097/AJP.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 64.Nordbrandt MS, Sonne C, Mortensen EL, Carlsson J. Trauma-affected refugees treated with basic body awareness therapy or mixed physical activity as augmentation to treatment as usual-a pragmatic randomised controlled trial. PloS One. 2020;15:e0230300. doi: 10.1371/journal.pone.0230300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicholas MK, Asghari AA, Sharpe LA, et al. Cognitive exposure versus avoidance in patients with chronic pain: Adherence matters. Eur J Pain. 2014;18:424–437. doi: 10.1002/j.1532-2149.2013.00383.x. [DOI] [PubMed] [Google Scholar]
- 66.Price CJ, Thompson EA, Crowell SE, et al. Immediate effects of interoceptive awareness training through mindful awareness in body-oriented therapy (MABT) for women in substance use disorder treatment. Subst Abus. 2019;40:102–115. doi: 10.1080/08897077.2018.1488335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Lawrence Erlbaum Associates, Publishers; Hillsdale, NJ: 1988. [Google Scholar]
- 68.Zangrando F, Piccinini G, Tagliolini C, et al. The efficacy of a preparatory phase of a touch-based approach in treating chronic low back pain: A randomized controlled trial. J Pain Res. 2017;10:941–949. doi: 10.2147/JPR.S129313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmadi H, Adib H, Selk-Ghaffari M, et al. Comparison of the effects of the Feldenkrais method versus core stability exercise in the management of chronic low back pain: A randomised control trial. Clin Rehabil. 2020;34:1449–1457. doi: 10.1177/0269215520947069. [DOI] [PubMed] [Google Scholar]
- 70.Feinstein JS, Khalsa SS, Yeh H, et al. The elicitation of relaxation and interoceptive awareness using floatation therapy in individuals with high anxiety sensitivity. Biol Psychiatry: Cognit Neurosci Neuroimaging. 2018;3:555–562. doi: 10.1016/j.bpsc.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fetzner MG, Asmundson GJ. Aerobic exercise reduces symptoms of posttraumatic stress disorder: A randomized controlled trial. Cogn Behav Ther. 2015;44:301–313. doi: 10.1080/16506073.2014.916745. [DOI] [PubMed] [Google Scholar]
- 72.Kendall SA, Brolin-Magnusson K, Sören B, Gerdle B, Henriksson KG. A pilot study of body awareness programs in the treatment of fibromyalgia syndrome. Arthritis Care Res. 2000;13:304–311. doi: 10.1002/1529-0131(200010)13:5<304::aid-anr10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 73.Konstantakopoulos G, Tchanturia K, Surguladze SA, David AS. Insight in eating disorders: Clinical and cognitive correlates. Psychol Med. 2011;41:1951–1961. doi: 10.1017/S0033291710002539. [DOI] [PubMed] [Google Scholar]
- 74.Raftery D, Kelly PJ, Deane FP, et al. Insight in substance use disorder: A systematic review of the literature. Addict Behav. 2020;111:106549. doi: 10.1016/j.addbeh.2020.106549. [DOI] [PubMed] [Google Scholar]
- 75.Cuijpers P, Cristea IA. How to prove that your therapy is effective, even when it is not: A guideline. Epidemiol Psychiatr Sci. 2016;25:428–435. doi: 10.1017/S2045796015000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohr DC, Spring B, Freedland KE, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78:275–284. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- 77.Mohr DC, Ho J, Hart TL, et al. Control condition design and implementation features in controlled trials: A meta-analysis of trials evaluating psychotherapy for depression. Transl Behav Med. 2014;4:407–423. doi: 10.1007/s13142-014-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barth J, Munder T, Gerger H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: A network meta-analysis. PLoS Med. 2013;10:e1001454. doi: 10.1371/journal.pmed.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munder T, Geisshüsler A, Krieger T, et al. Intensity of treatment as usual and its impact on the effects of face-to-face and internet-based psychotherapy for depression: A preregistered meta-analysis of randomized controlled trials. Psychother Psychosom. 2022;91:200–209. doi: 10.1159/000521951. [DOI] [PubMed] [Google Scholar]
- 80.Classen CC, Hughes L, Clark C, Hill Mohammed B, Woods P, Beckett B. A pilot RCT of a body-oriented group therapy for complex trauma survivors: An adaptation of sensorimotor psychotherapy. J Trauma Dissociation. 2021;22:52–68. doi: 10.1080/15299732.2020.1760173. [DOI] [PubMed] [Google Scholar]
- 81.Craske MG, Wolitzky-Taylor KB, Labus J, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther. 2011;49:413–421. doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Jong M, Lazar SW, Hug K, et al. Effects of mindfulness-based cognitive therapy on body awareness in patients with chronic pain and comorbid depression. Front Psychol. 2016;7:967. doi: 10.3389/fpsyg.2016.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mannerkorpi K, Arndorw M. Efficacy and feasibility of a combination of body awareness therapy and qigong in patients with fibromyalgia: A pilot study. J Rehabil Med. 2004;36:279–281. doi: 10.1080/16501970410031912. [DOI] [PubMed] [Google Scholar]
- 84.Worden BL, Genova M, Tolin DF. Randomized pilot of an anxiety sensitivity-based intervention for individuals in a substance use day program. J Psychoactive Drugs. 2017;49:333–343. doi: 10.1080/02791072.2017.1329570. [DOI] [PubMed] [Google Scholar]
- 85.Schoeller F, Haar AJH, Jain A, Maes P. Enhancing human emotions with interoceptive technologies. Phys Life Rev. 2019;31:310–319. doi: 10.1016/j.plrev.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Livermore JJA, Holmes CL, Moga G, et al. A single oral dose of citalopram increases interoceptive insight in healthy volunteers. Psychopharmacology (Berl) 2022;239:2289–2298. doi: 10.1007/s00213-022-06115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paulus M, Stewart J, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Frontiers Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boswell JF, Anderson LM, Oswald JM, Reilly EE, Gorrell S, Anderson DA. A preliminary naturalistic clinical case series study of the feasibility and impact of interoceptive exposure for eating disorders. Behav Res Ther. 2019;117:54–64. doi: 10.1016/j.brat.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plasencia M, Sysko R, Fink K, Hildebrandt T. Applying the disgust conditioning model of food avoidance: A case study of acceptance-based interoceptive exposure. Int J Eating Disord. 2019;52:473–477. doi: 10.1002/eat.23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zucker NL, LaVia MC, Craske MG, et al. Feeling and body investigators (FBI): ARFID division—An acceptance-based interoceptive exposure treatment for children with ARFID. Int J Eating Disord. 2019;52:466–472. doi: 10.1002/eat.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.