Abstract
Mutually orthogonal aminoacyl-tRNA synthetase (aaRS)/tRNA pairs provide a foundation for encoding non-canonical amino acids (ncAAs) into proteins, and encoded non-canonical polymer and macrocycle synthesis. Here we discover quintuply orthogonal pyrrolysyl-tRNA synthetase (PylRS)/ tRNAPyl pairs. We discover empirical sequence identity thresholds for mutual orthogonality, and use these for agglomerative clustering of PylRS and tRNAPyl sequences; this defines numerous sequence clusters, spanning five classes of PylRS/ tRNAPyl pairs (existing classes: +N, A, B, and newly defined classes: C, S). Most PylRS clusters belong to classes that are unexplored for orthogonal pair generation. By testing pairs from distinct clusters and classes, and pyl tRNAs with unusual structures, we resolve 80% of the pairwise specificities required to make quintuply orthogonal PylRS/tRNAPyl pairs; we control the remaining specificities by engineering and directed evolution. Overall, we create 924 mutually orthogonal PylRS/tRNAPyl pairs, 1324 triply orthogonal pairs, 128 quadruply orthogonal pairs, and 8 quintuply orthogonal pairs. These advances may provide a key foundation for encoded polymer synthesis.
Introduction
The genetic code of living cells has been reprogrammed to enable the site-specific incorporation of non-canonical amino acids (ncAAs) and hydroxy acids into proteins, and the encoded synthesis of non-canonical polymers and macrocyclic peptides and depsipeptides.1–4 These advances are underpinned by the discovery of aminoacyl-tRNA synthetases (aaRSs) and tRNAs that are orthogonal – in their aminoacylation specificity – with respect to the synthetases and tRNAs of the host organism, and mutually orthogonal with respect to each other. Several of these pairs,5–16 have been altered to recognise distinct amino acids (Supplementary Note 1). While initial work incorporated ncAAs in response to the amber codon, recent work has taken advantage of other codons including additional stop codons,17 quadruplet codons,18–21 codons containing non-canonical bases22–24 and sense codons in organisms with genomic code compression and tRNA deletion.3,25,26 Mutually orthogonal pairs provide a foundation for incorporating combinations of ncAAs and encoded cellular polymer synthesis and, despite recent progress, the discovery of such pairs remains an outstanding challenge.1,2,5,6,17,18,27–30
The pyrrolysyl-tRNA synthetase PylRS/tRNAPyl pairs are the most widely used systems for genetic code expansion.2 These pairs enable the site-specific incorporation of ncAAs in all domains of life;31 the anticodon of the pyl tRNAs tested can be mutated to decode diverse codons,6,19,21,32,33 as it is not a recognition element for PylRS enzymes;34 and the PylRS active site does not recognise canonical amino acids and can accept, or be evolved to accept, diverse ncAAs and hydroxy acids.4,10,35–40
Most genetic code expansion work with pyrrolysyl systems has focussed on the Methanosarcina mazei (Mm)PylRS/MmtRNAPylCUA pair and the closely related Methanosarcina barkeri (Mb)PylRS/MbtRNAPylCUA pair.31 The PylRS enzymes of these pairs are composed of two domains: an amino (N)-terminal domain and a carboxy (C)-terminal domain. The C-terminal domain binds the amino acid substrate and catalyses the aminoacylation of the cognate tRNAPyl, and the N-terminal domain contacts the variable and T loops of the tRNAPyl to enhance binding affinity and specificity.34,41 Both domains are required to create a functional MmPylRS/MmtRNAPyl pair in E. coli, and it was widely thought that all PylRS systems required both domains for activity.42,43 We demonstrated that a newly defined group of PylRS enzymes12 – ΔN PylRS, lacking an N-terminal domain (in the same polypeptide or in trans) – are active and orthogonal.7 These pairs, and their engineered derivatives, were combined with pairs from the canonical +N group to enable the creation of mutually orthogonal pyl systems. We further showed that PylRS and tRNAPyl sequences in the ΔN group clustered into two classes, A and B, on the basis of their sequence identity, and we created triply orthogonal pairs composed of a pair derived from the +N group, a class A pair, and a class B pair.6 The discovery of new mutually orthogonal pyl systems has been combined with strategies for providing codons with which to encode non-canonical monomers, and this has enabled the incorporation of several distinct non-canonical amino acids into a protein and the encoded cellular synthesis of non-canonical polymers and macrocycles.3,7,21,28,44–48 Despite these advances there were no criteria with which to effectively search genomic data for mutually orthogonal pyl systems and we hypothesized that many orthogonal and mutually orthogonal systems remained to be discovered.
Here we leverage experimental PylRS/tRNAPyl cross reactivity data to empirically define sequence identity thresholds for mutually orthogonal PylRS enzymes and pyl tRNAs. We then perform agglomerative clustering on 351 PylRS sequences, to define clusters of sequences that pass the empirical thresholds; 84% of the resulting clusters belong to PylRS classes that have not been explored in the search for orthogonal pairs. We identify and cluster tRNAPyl sequences from the same organisms for members of 95% of the PylRS sequence clusters. Using both the empirical orthogonality thresholds and the presence of exotic structural features that may confer orthogonality, we select a set of pyl tRNAs which, along with PylRS enzymes from the same organism, form the starting point of an experimental search for mutually orthogonal pairs.
We identify two new classes of PylRS and tRNAPyl sequences, which we name class C and class S, and we show that the majority of our PylRS enzymes and pyl tRNAs are active and orthogonal in E. coli. We explore the specificity of class S and class C systems with respect to each other and with respect to previously characterized class N, A and B PylRS systems. Strikingly our sequence-based approach allows us to control 20 of the 25 aminoacyl-tRNA synthetase/tRNA pairwise specificities required to make a set of quintuply orthogonal PylRS/tRNAPyl pairs without additional engineering; we control the remaining five specificities by tRNAPyl engineering and directed evolution. Overall, we create 924 mutually orthogonal PylRS/tRNAPyl pairs, 1324 triply orthogonal pairs, 128 quadruply orthogonal pairs, and 8 quintuply orthogonal pairs.
Results
Cross-reactivity and sequence identity of pairs
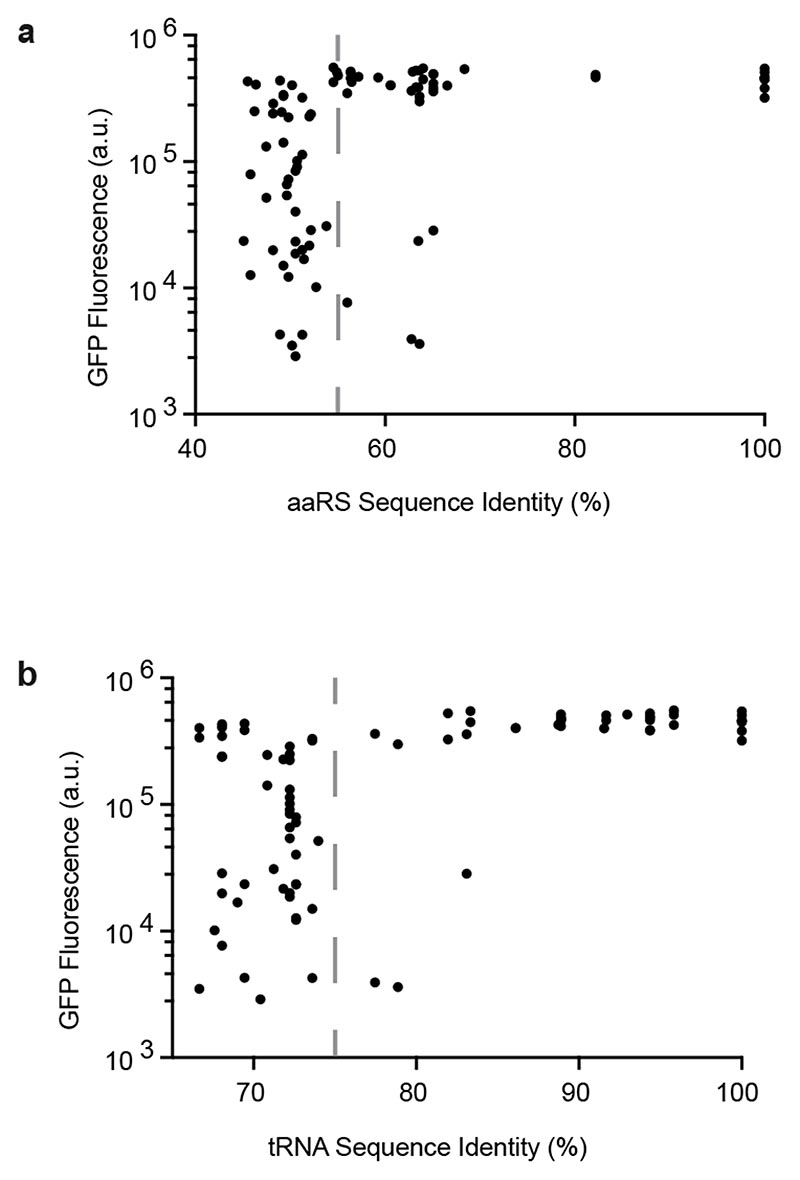
We previously defined the cross-reactivity profiles of PylRS/tRNAPyl pairs belonging to three distinct classes (N, A, and B); we showed that certain non-cognate pairs, drawn from the ΔN PylRS classes A and B, exhibit a surprising degree of natural orthogonality with respect to one another. We postulated that this mutual orthogonality might be related to the sequence identity between the pairs, and we therefore decided to draw on ΔN PylRS/tRNAPyl activity data6 to quantify this relationship.
Strikingly, we found that if two ΔN PylRS enzymes had a sequence identity of over 55%, then one ΔN PylRS enzyme would show high activity with the tRNAPyl that naturally pairs with the other ΔN PylRS enzyme (or vice-versa) in approximately 90% of cases (Fig. 1a and Supplementary Fig. 1). Below 55% sequence identity, ΔN PylRS enzymes exhibited a range of activities with the pyl tRNAs of other ΔN PylRS enzymes. Similarly, if two tRNAPyl genes shared a sequence identity of over 75%, then one tRNAPyl would show high activity with the synthetase of the other tRNAPyl (or vice-versa) in approximately 90% of cases (Fig. 1b and Supplementary Fig. 1). Below 75% sequence identity, pyl tRNAs exhibited a range of activities with the ΔN PylRS enzymes of other pyl tRNAs.
Fig. 1. Relationship between sequence identity and cross-reactivity in previously characterized ΔN PylRSs and pyl tRNAs.
a. Activity of each combination of ΔN PylRSi and ΔN tRNAPylj, measured by production of GFP(150AllocK)His6 from cells bearing a GFP(150TAG)His6 gene in the presence of 4 mM AllocK 1, plotted against the sequence identity between ΔN PylRSi and ΔN PylRSj, where ΔN PylRSj is the synthetase from the same organism as ΔN tRNAPylj. ΔN PylRS proteins with greater than 55% sequence identity (dashed grey line) are predominantly active with each other’s pyl tRNAs (88% of cases). ΔN PylRS proteins with less than 55% sequence identity may or may not be active with each other’s pyl tRNAs. b. Activity of each combination ΔN PylRS with greater thani and ΔN tRNAPylj, plotted against the sequence identity between ΔN tRNAPyli and ΔN tRNAPylj, where ΔN tRNAPyli is the tRNAPyl from the same organism as ΔN PylRSi. ΔN pyl tRNAs with greater than 75% sequence identity (dashed grey line) are predominantly active with each other’s synthetases (93% of cases). ΔN pyl tRNAs with less than 75% sequence identity may or may not be active with each other’s synthetases. Dots represent the mean of three biological replicates, error bars are shown in Supplementary Figure 1. All numerical values are provided in Supplementary Table 2.
This analysis suggested that the development of new multiply orthogonal pairs should focus on PylRS/tRNAPyl pairs whose synthetase and tRNA sequence identities are less than 55% and 75%, respectively.
Identification and clustering of PylRS sequences
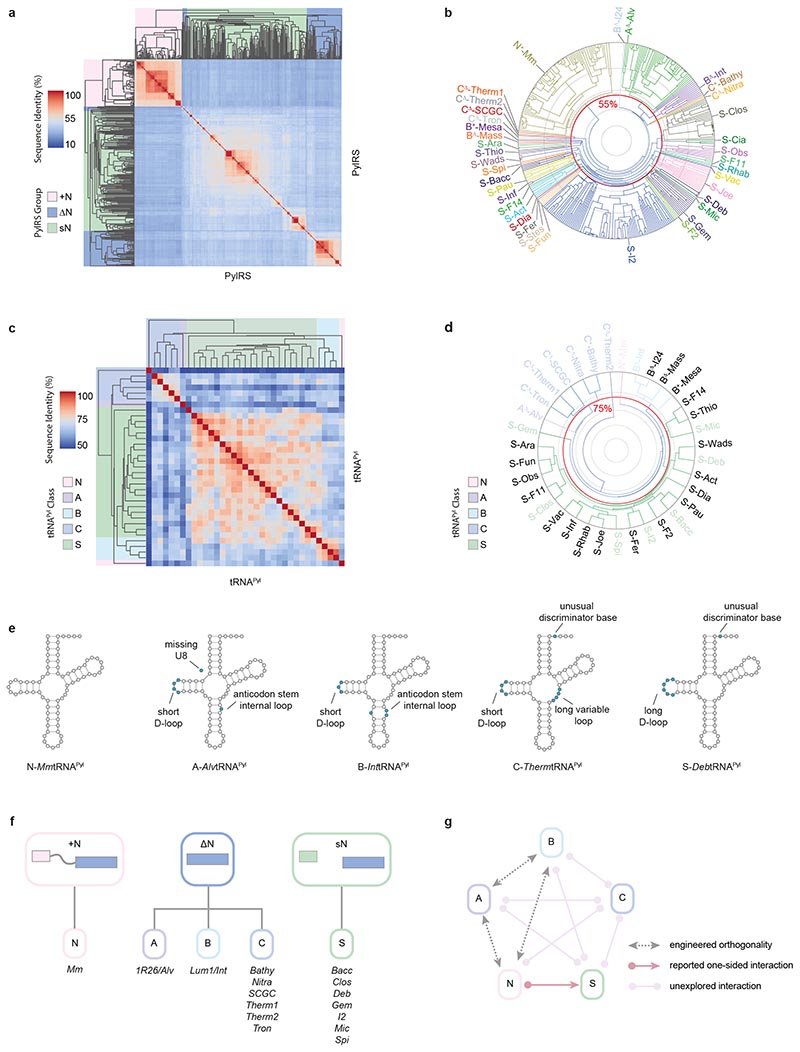
We assembled a database of PylRS sequences by performing a BLAST search for sequence similarity to the Candidatus Methanomethylophilus alvus (Alv) ΔN PylRS sequence (class A; henceforth referred to as AΔ-AlvPylRS). We retrieved 351 PylRS protein sequences (Supplementary Fig. 2 and Supplementary Table 1); of these, 79 belonged to the archaeal +N group, 66 belonged to the archaeal ΔN group, and 204 belonged to the bacterial (sN) group. In addition, two PylRS genes, despite being classified as archaeal, possessed a separately encoded N-terminal domain – we termed these the archaeal sN group.
We performed an agglomerative hierarchical clustering to visualise the sequence diversity among PylRS catalytic domains (Fig. 2a and Supplementary Table 1). We observed two major groups: a dense cluster consisting of the class N PylRS sequences, and a loose cluster consisting of the bacterial and other archaeal PylRS sequences. The latter cluster itself contained several denser sub-clusters, including those corresponding to the known archaeal class A and B sequences. To discover mutually orthogonal systems, we focussed on identifying PylRS sequences with pairwise sequence identities of less than 55% (Fig. 1a). To achieve this, we set a linkage distance threshold for the agglomerative clustering such that two clusters would be merged if, and only if, the average of the percentage identities of each PylRS in the two clusters was greater than 55%. This led to 37 clusters (Fig. 2b, Supplementary Fig. 3, Supplementary Table 1). Three clusters represented the known PylRS classes N, A, and B. By contrast, there were 25 bacterial sN-group clusters and 9 further archaeal clusters (seven ΔN-group and two archaeal sN-group). This analysis demonstrated that substantial sequence diversity among Pyl systems remains to be explored.
Fig. 2. Selection of candidate PylRS and tRNAPylCUA sequences and partitioning of the pyl system into five distinct sequence-defined classes.
a. Clustergram of 351 PylRS C-terminal domain amino acid sequences retrieved. Three groups: +N (red), ΔN (blue), and sN (green) are shown over the dendrogram. Using a clustering threshold of 55%, 37 clusters were obtained. The heatmaps display percentage sequence identity scores. b. Dendrogram of the 37 clusters generated from agglomerative hierarchical clustering of the 351 PylRS C-terminal domain amino acid sequences. The 37 PylRS representative sequences are labelled. The radial coordinate represents percentage sequence identity (log scale). Grey contours 20% intervals, red contour 55% sequence identity, the clustering threshold value. c. Clustergram of 35 tRNAPyl sequences from the same organism as a representative PylRS from each cluster. The five pyl system classes are indicated over the dendrograms: N (red), A (purple), B (light blue), C (dark blue), and S (green). d. Dendrogram showing the eight clusters generated from agglomerative hierarchical clustering of the 35 identified tRNAPyl sequences. Coloured labels correspond to the 16 tRNAPyl sequences chosen for experimental characterization along with their cognate PylRS enzymes. The radial coordinate represents percentage sequence identity (log scale). Grey contours 20% intervals, red contour 75% sequence identity, the clustering threshold value. e. A representative tRNAPyl from each class is shown; notable structural differences with respect to the canonical N-Mm tRNAPyl in blue. f. Schematic of the three Pyl system groups and their division into five classes. Names of the Pyl systems chosen for characterisation are annotated below each class. For classes A and B, the AΔ-1R26PylRS/A-AlvtRNAPyl and BΔ-Lum1PylRS/B-InttRNAPyl pairs were used. For all other classes, PylRS/tRNAPyl pairs were derived from the same organism. g. Schematic of the interaction network between all five classes. Classes N and S are known to interact (red arrow); interactions between classes N, A and B can be abolished by tRNA engineering (grey arrows). All other interactions between classes are unexplored (pink arrows).
Identification and clustering of tRNAPyl sequences
For a representative PylRS enzyme from 35 of the 37 clusters we identified the corresponding pyl tRNA gene from the same organism49 (Supplementary Table 1).
We performed an agglomerative hierarchical clustering of the 35 tRNAPyl sequences (Fig. 2c). We observed tighter grouping than with the PylRS sequences. Of the nine identified archaeal pyl tRNAs, three grouped with class B and six formed a clear albeit more loosely related grouping, which we termed class C. Meanwhile, bacterial sN pyl tRNAs grouped together strongly; we assigned these to a new class S. Curiously, the pyl tRNAs of the two archaeal sN PylRS enzymes are fairly weakly related and fall into classes B and C, indicating they may not have a common origin.
By setting a linkage distance threshold for agglomerative clustering to 75% sequence identity (Fig. 1b) we generated eight clusters of tRNAPyl genes (Fig. 2d, Supplementary Table 1). Of these clusters, three represented the known PylRS classes N, A, and B. There were two bacterial clusters, one of which only contained a single tRNAPyl. The three remaining clusters were from class C, in line with the looser interrelatedness of the members of this class.
We selected 16 pyl tRNAs – including at least one member of each tRNA cluster and pyl tRNAs with exotic structural features that are not observed in canonical class N tRNAPyl (Fig. 2e, Supplementary Note 2) – for further investigation (Fig. 2f). These 16 tRNAs included 13 pyl tRNAs which were uncharacterised in E. coli (six archaeal class C, and seven bacterial class S), along with three previously characterised pyl tRNAs from classes N, A and B.
To finalise our representative set of PylRS/tRNAPyl pairs for experimental characterisation, we combined each chosen tRNAPyl with the synthetase from the same organism – with the exception of A-AlvtRNAPyl and B-Candidatus Methanomassiliicoccus intestinalis (Int)tRNAPyl, which form highly active heterologous cognate pairs with the previously reported PylRS enzymes AΔ- Candidatus Methanomethylophilus sp.1R26 (1R26)Py1RS and BΔ-Methanomassiliicoccus luminyensis 1 (Lum1)PylRS, respectively (Fig. 2f).6 We note that, of the ten inter-class relationships, only four have been even partially characterised in E. coli (Fig. 2g): N+-MbPylRS is known to interact with S-Desulfitobacterium hafniense (Dh)-tRNAPyl,42 while sets of engineered pairs from classes N, A, and B (such as N+-MmPylRS/N-Methanosarcina spelaei (Spe)tRNAPyl, AΔ-1R26PylRS/A-AlvtRNAPyl-8, and BΔ-Lum1PylRS/B-InttRNAPyl-17C10), are known to be triply orthogonal to one another.6,7
In preparing the systems for characterisation, we observed that multiple class S PylRS genes were recalcitrant to cloning, and that even those that could be successfully cloned resulted in reduced growth when expressed in E. coli cells. We hypothesized that these issues might be related to their separately expressed N-terminal domain protein (PylSn), and prepared variants of each class S PylRS system with the PylSn gene removed, which abrogated the toxicity effects. These variants (which we term SΔ) were characterised alongside (or in place of) the wild-type enzymes (which we term S+).
Active PylRS enzymes and pyl tRNAs
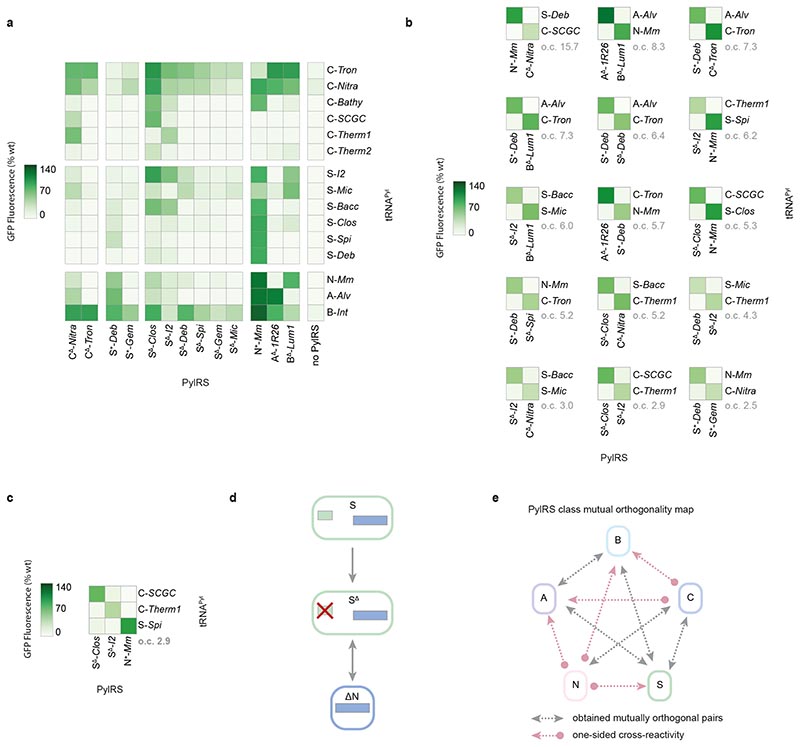
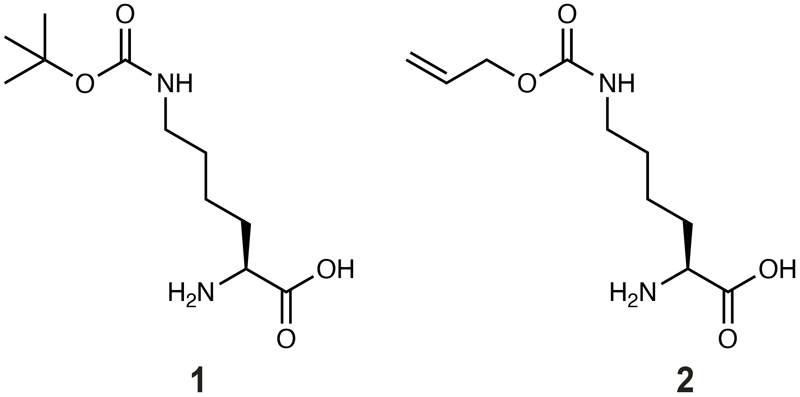
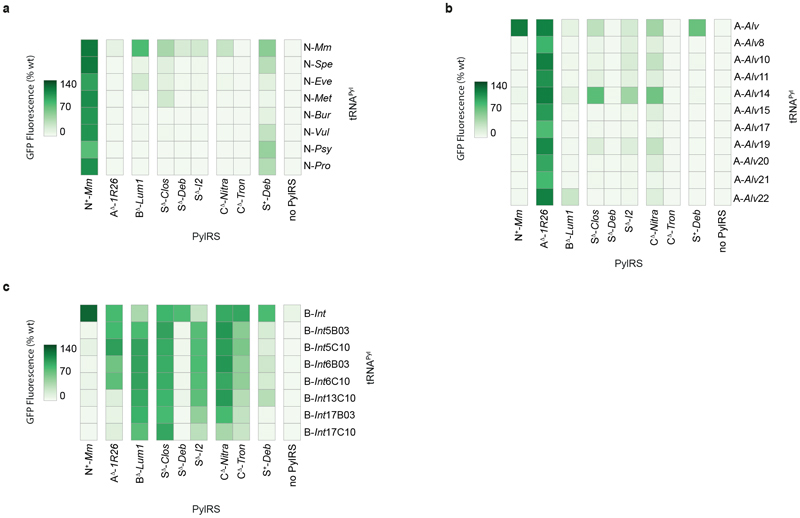
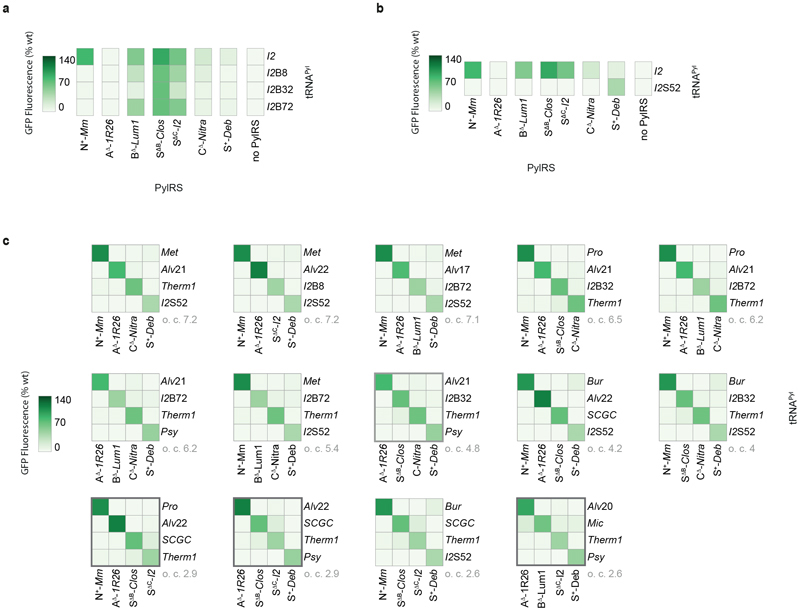
We measured the activities of each chosen PylRS enzyme with each chosen tRNAPyl via the production of green fluorescent protein (GFP) from a gene coding for GFP containing an amber codon at position 150, in the presence of the non-canonical amino acid N6-((allyloxy)carbonyl)-L-lysine (AllocK 1, Extended Data Fig. 1) – a known substrate of previously characterised PylRS enzymes (Fig. 3a, Supplementary Table 2).50 15 out of 16 pyl tRNAs gave rise to PylRS-dependent GFP production (at a level at least 30% of that produced from a control GFP gene without an amber stop codon, ‘wtGFP control’) in the presence of at least one PylRS enzyme (Fig. 3a). This included all class C pyl tRNAs and all but one class S tRNAPyl. In addition, 13 out of 20 PylRS enzymes led to GFP production at a level at least 30% of the wtGFP control, in the presence of at least one tRNAPyl (Supplementary Note 3). This demonstrated that most PylRS enzymes and pyl tRNAs were expressed and active in E. coli.
Fig. 3. Activity mapping of candidate PylRS enzymes and pyl tRNAs, and discovery of new triply orthogonal, PylRS/tRNAPyl pairs.
a. Heatmap displaying the activity of combinations of the selected pyl tRNAs and PylRS enzymes, measured by production of GFP(150AllocK)His6 from cells bearing a GFP(150TAG)His6 gene in the presence of 4 mM AllocK 1. Values are the percentage of wild-type GFP. Only PylRS enzymes and pyl tRNAs that have greater than 30% activity with at least one tRNAPyl or PylRS enzyme, respectively, are shown. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). b. Activity heatmaps of representative sets from each family of doubly orthogonal PylRS/tRNAPyl pairs obtained from the activity screen. Orthogonality coefficient (o.c) is shown in grey; the set with the highest o.c. in each family is displayed. Distinct members of a family share the same set of PylRS enzymes but use different pyl tRNAs. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). c. Activity heatmap of the set with the highest o.c. from the family of triply orthogonal PylRS/tRNAPyl pairs obtained from the activity screen. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). d. The generation of SΔ PylRS variants by deletion of the N-terminal domain from class S PylRS enzymes. We considered SΔ PylRS variants as engineered members of the ΔN group; their activity profiles are too diverse to be considered as a distinct class. e. The mutual interaction network between all five PylRS classes based on the activity between the characterised PylRS enzymes and pyl tRNAs. Mutually orthogonal pairs can be found using PylRS enzymes from: classes A and B; classes A and S; classes B and S; classes C and N; and classes C and S (double-headed grey arrows). Therefore, five out of ten possible mutually orthogonal combinations were discovered; the other five each showed one undesired cross reactivity (single-headed red arrows). These orthogonal interactions were identified without engineering to tailor PylRS:tRNAPyl interactions. For no two PylRS classes did all combinations of pairs possess two-sided cross-reactivity.
Mutually orthogonal PylRS/tRNAPyl pairs
Next, we used our activity measurements (Fig. 3a) to determine whether any of the pyrrolysine systems we had discovered formed naturally mutually orthogonal sets (Fig. 3b,c). We first defined the criteria for mutually orthogonal pairs by reference to the interactions between them. The network of interactions between multiple aaRS/tRNA pairs may be represented as a matrix where each element xi,j is the activity of the aaRS protein of column j with the tRNA of row i (measured in this case by GFP(150AllocK)His6 production in the presence of aaRSj and tRNAi). When aaRSi, denotes the cognate aaRS of tRNAi all diagonal elements xi,i represent the paired activities we wish to maximise. All off-diagonal elements represent the cross-reactivity between a non-cognate aaRS and tRNA, which should be minimised. A diagonal interaction matrix of order N therefore represents a perfectly orthogonal set of N pairs.
In order to exclude sets of pairs with unacceptably low activity, or unacceptably high cross-reactivity, we deemed that the activity of a cognate pair should be greater than 40% of wild-type GFP production, but that each cross-reactivity (between a tRNAPyl and a PylRS enzyme belonging to different pairs) should be less than 20% of wild-type GFP production. Since pairs composed of a PylRS and tRNAPyl from different organisms can have activity equal to, or exceeding, that of the corresponding homologous pairs, we included heterologous PylRS/tRNAPyl combinations as possible cognate pairs in our search. In addition, we defined a metric, henceforth known as the ‘orthogonality coefficient’ (o.c.), as the quotient of the lowest intra-pair activity over the highest inter-pair cross-reactivity. This metric provides a quantitative measure of mutual orthogonality between a set of aaRS/tRNA pairs. Previously characterised triply orthogonal pairs (N+-MmPy1RS/N-SpetRNAPyl, AΔ-1R26PylRS/A-AlvtRNAPyl-8, BΔ-Lum1PylRS/B-InttRNAPvl-l7C10) used for the incorporation of three distinct non-canonical amino acids have an o.c. of approximately 5.0 (Supplementary Table 2),6 however, we reasoned that since mutual orthogonality could be improved by further engineering, a lower cut-off (o.c. > 2.5) would be more useful in initial screens.
In our initial search we considered an interaction matrix to be sufficiently orthogonal if: (i) all diagonal elements were greater than 40% of the wtGFP control, (ii) all off-diagonal elements were less than 20% of the wtGFP control, and (iii) the quotient of the smallest diagonal element over the largest off-diagonal element was greater than 2.5.
We uncovered 46 doubly orthogonal pairs (henceforth referred to as ‘doublets’); the highest doublet o.c. is 15.7. Since many doublets involve the same two PylRS enzymes, and differ only in the pyl tRNAs used, we grouped these doublets into families; members of a family share the same set of PylRS enzymes but use different pyl tRNAs. We thus obtained fifteen doublet families (Fig. 3b and Supplementary Table 2); all but one family contains a class C or class S PylRS enzyme. Similarly, we obtained two triply orthogonal pairs (or ‘triplets’), both from the same family; the highest triplet o.c. is 2.9 (Fig. 3c and Supplementary Table 2).
These families shed important insights on the PylRS/tRNAPyl activity profiles. The highly orthogonal class C pyl tRNAs – C-Candidatus Methanohalarchaeum thermophilum 1 (Therm1)tRNAPyl and C-Candidate division MSBL1 archaeon SCGC-AAA382A20 (SCGC)tRNAPyl – appear in doublets when either tRNAPyl is paired with CΔ-Nitrososphaeria archaeon (Nitra)PylRS. However, these pyl tRNAs also form a surprising inter-class doublet family when paired respectively with SΔ-Desulfosporosinus sp. I2 (I2)PylRS and SΔ-Clostridiales bacterium (Clos)PylRS; this doublet forms part of a triplet family with an N+-MmPylRS pair (e.g. N+-MmPylRS/S-Spirochaetales bacterium (Spi)tRNAPyl). Further doublet families involving S+ or SΔ PylRS enzymes (e.g. with S+-Deltaproteobacteria bacterium (Deb)PylRS and SΔ-DebPylRS, or SΔ-DebPylRS and SΔ-I2PylRS) illustrate not only divergence between S+ PylRS enzymes and their SΔ variants, but between different SΔ PylRS variants. As such, we do not consider SΔ PylRS enzymes as a distinct class, but rather as synthetically derived PylRS variants that expand the ΔN group (Fig. 3d).
The relationship between the five PylRS classes may itself be described on the basis of the doubly orthogonal pairs formed by representative PylRS enzymes from each class and the appropriate pyl tRNAs (which may or may not belong to the same classes). For five of these ten inter-class relationships, we obtained mutually orthogonal representative PylRS/RNAPyl pairs (Fig. 3e). For the remaining five inter-class relationships, no characterized PylRS/tRNAPyl pairs met our criteria for mutual orthogonality. Two cases for lack of mutual orthogonality between general PylRS classes R1 and R2 can be defined: (1) ‘two-sided cross-reactivity’ – for any two pairs of the form R1-PylRS/Ti-tRNAPyl and R2-PylRS/Tj-tRNAPyl (where Ti and Tj are arbitrary tRNA classes), both cross-reactivities R1-PylRS/Tj-tRNAPyl and R2-PylRS/Ti-tRNAPyl are too high (i.e. off-diagonal elements in the interaction matrix are greater than 20% wtGFP control or result in o.c. < 2.5); (2) ‘one-sided cross-reactivity’ – there exist pairs R1-PylRS/Ti -tRNAPyl and R2-PylRS/Tj-tRNAPyl such that only one cross-reactivity R1-PylRS/Tj-tRNAPyl or R2-PylRS/Ti-tRNAPyl is too high (i.e. only one off-diagonal element in the interaction matrix is greater than 20% wtGFP control or results in o.c. < 2.5). Strikingly, all five non-orthogonal inter-class relationships fall into the second class. Therefore, of the 25 pairwise specificities required to make a set of quintuply orthogonal pairs (using one PylRS from each of the five classes), our computational approach was able to resolve 20 of these – all five cognate interactions and 15 out of 20 non-cognate interactions.
Eliminating inter-class cross-reactivities
As a starting point for generating a quintuply orthogonal pair, we examined the inter-class interaction matrix for five specific PylRS/tRNAPyl pairs (Fig. 4a-b). For classes N, A, and B, we used pairs N+-MmPylRS/N-MmtRNAPyl, AΔ-lR26PylRS/A-AlvtRNAPyl, and BΔ-Lum1PylRS/B-InttRNAPyl, since these were the starting point for a previously reported triplet.6 For class C, a natural starting point is CΔ-NitraPylRS/C-Therm1tRNAPyl, the most active class C pair for which the tRNAPyl is orthogonal to all other PylRS classes. For class S, we simply chose the most active pairing of a wild-type class S PylRS enzyme, S+-DebRS/S-SpitRNAPyl. Of the twenty possible inter-class synthetase/tRNA interactions in the matrix (off-diagonal elements), only nine are sufficiently low to meet our initial criterion for cross-reactivity (less than 20% of wild-type GFP production levels). In order to bring the other eleven interactions under this threshold, we sought to replace the tRNAs involved in undesired cross-reactions (off diagonal interactions) with more orthogonal variants, i.e. substitute the rows of the interaction matrix such that the off-diagonal elements are progressively eliminated.
Fig. 4. Screening of engineered pyl tRNAs permits the control of 18 out of 20 cross-reactivities between specific members of the five Pyl classes.
a. Schematic of the interactions between five specific PylRS/tRNAPyl pairs (one from each class) that represent a logical starting point for the development of quintuply orthogonal pairs through a tRNAPyl engineering strategy. For classes N, A, and B, we chose active pairs for which the inter-class cross-reactivities (arrows highlighted in blue) have previously been controlled by tRNAPyl engineering. For class C, we chose the pair CΔ-NitraPylRS/C-Therm1tRNAPyl pair, for which the tRNAPyl is naturally orthogonal to all other PylRS classes. Finally, for class S, we chose the most active intraclass PylRS/tRNAPyl pair. b. Activity heatmap of the set of PylRS enzymes and pyl tRNAs chosen as a basis for developing quintuply orthogonal pairs. The pyl tRNAs that require engineering or replacement to control unwanted cross-reactions are labelled in red, while the pyl tRNAs that already satisfy all necessary orthogonality requirements are labelled in green. Green box: the natural orthogonality of C-Therm1 tRNAPyl. Blue box: class interactions that have previously orthogonalized by tRNAPyl engineering and screening. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). c. Activity heatmap from b, updated based on the results of the class N tRNAPyl screen. N-MettRNAPyl, the most orthogonal tRNA from the class N tRNAPyl screen with respect to the chosen PylRS enzymes, is paired with N+-MmPylRS. N-MettRNAPyl satisfies all orthogonality requirements (green box). Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). d. Activity heatmap from c, updated based on the results of the A-AlvtRNAPyl screen. A-AlvtRNAPyl-21, the most orthogonal tRNAPyl from the class A tRNAPyl screen, is paired with AΔ-1R26PylRS. A-AlvtRNAPyl-21 satisfies all orthogonality requirements (green box). Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). e. Activity heatmap from d, updated based on the results of the class B tRNAPyl screen. B-InttRNAPyl-17C10, the most orthogonal tRNAPyl from the class B tRNAPyl screen, does not satisfy all necessary orthogonality requirements due only to cross-reactivity with CΔ-NitraPylRS (red box). Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). f. Schematic summarizing the key results of the N, A, and B tRNAPyl screens. By screening of natural and engineered pyl tRNAs, 18 out of 20 interactions – which need to be orthogonalised to generate quintuply orthogonal PylRS/tRNAPyl pairs –controlled (left diagram, arrows highlighted in blue show interactions that were successfully controlled). Three fully orthogonal pyl tRNAs (labelled in green with their cognate PylRS enzymes) were identified and for each of the remaining two pyl tRNAs (in red) only one cross reaction remains to be controlled.
To find a class N tRNAPyl with orthogonality to all other classes, we screened seven pyl tRNAs from homologous class N Pyl systems (Fig. 4c and Extended Data Fig. 2a).6 N-Methanococcoides methylutens (Met)tRNAPyl and N-Methanococcoides burtonii (Bur)tRNAPyl gave rise to around 10% or less activity with class A, B, C, and S PylRS enzymes while retaining over 85% of the activity of N-MmtRNAPyl with N+-MmPylRS.
To find a class A tRNAPyl with orthogonality to all other classes, we screened ten engineered variants of A-AlvtRNAPyl (Fig. 4d and Extended Data Fig. 2b).7 Two pyl tRNAs (A-AlvtRNAPyl-17 and A-Alvt RNAPyl-21) gave rise to less than 10% of wild-type GFP levels in the presence of class N, B, C, and S PylRS enzymes while retaining over 70% of activity with AΔ-1R26PylRS.
To find a class B tRNAPyl with orthogonality to all other classes, we screened seven previously reported B-InttRNAPyl variants (Fig. 4e and Extended Data Fig. 2c).6 However, although we obtained tRNAs with orthogonality to class N, A, and S PylRS proteins, all tested pyl tRNAs gave rise to significant levels of GFP production (over 40%) in the presence of CΔ-NitraPylRS. Since the B-InttRNAPyl variants carried a range of mutations with respect to their parent tRNAPyl, in both the acceptor stem and variable loop, we hypothesised that CΔ-NitraPylRS may recognise multiple identity elements in B-InttRNAPyl; this suggested that the non-cognate interaction between CΔ-NitraPylRS and B-InttRNAPyl cannot easily be abrogated without also destroying recognition of B-InttRNAPyl by BΔ-Lum1PylRS.
Overall, the screen led to the abrogation of a further nine inter-class PylRS/tRNAPyl interactions (Fig. 4f); this left only two undesired interactions – between the class C PylRS and class B tRNAPyl, and between the class N PylRS and class S tRNAPyl. Notably, three out of five tRNAs now fulfilled all orthogonality requirements. Our results demonstrate the substantial extent to which the evolutionary divergence of PylRS sequences can be exploited to generate orthogonal interactions.
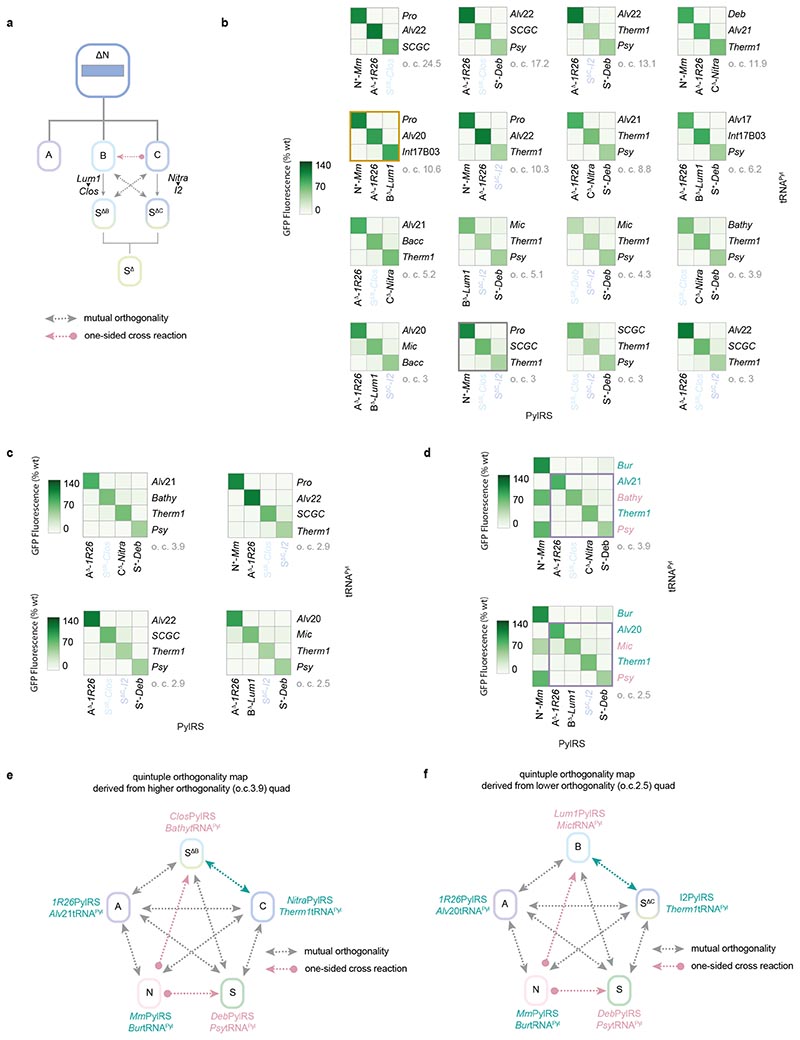
Quadruply orthogonal PylRS/tRNAPyl pairs
We investigated additional approaches to eliminate inter-class (off-diagonal) interactions for a fourth PylRS/tRNAPyl pair, and form a mutually orthogonal quadruplet.
As noted above, the engineered SΔPylRS variants belong to the expanded ΔN group (Fig. 3d). The activities of different SΔ variants resemble the activities of different ΔN classes; for instance SΔ-I2PylRS is active with C-Therm1tRNAPyl (Fig. 3a; much like CΔ-NitraPylRS), and SΔ-ClosPylRS is active with engineered B-InttRNAPyl variants (Extended Data Fig. 2c; much like BΔ-Lum1PylRS). We therefore speculated that substitution of the class B or C pair with a pair containing a SΔ PylRS supplying a desired B-like or C-like activity might resolve the issue of cross-reactivity between class C PylRS enzymes and class B pyl tRNAs (Fig. 5a). We refer to these substituting PylRS variants as SΔB or SΔC, respectively.
Fig. 5. Unique activity patterns of SΔ PylRS enzymes enable the development of quadruply orthogonal pairs.
a. Schematic of the strategy of replacing a class B or C PylRS enzyme with a SΔ PylRS variant to resolve the undesired cross-reactivity between class C PylRS and class B tRNAPyl. The diverse activities of SΔ PylRS variants mean that a different variant can be found to substitute for either class B (e.g. SΔ-ClosPylRS for BΔ-Lum1PylRS, as labelled) or class C (e.g. SΔ-I2PylRS for CΔ-NitraPylRS, as labelled) PylRS enzymes. b. Activity heatmaps of the highest o.c. sets from each family of triply orthogonal PylRS/tRNAPyl pairs obtained following the results of the N, A, and B tRNAPyl screens. The substitution of a B or C class PylRS with different SΔ PylRS variants (labelled in two shades of blue) allows the generation of many of the new families. Gold box: representative set from the previously reported triply orthogonal N+-MmPylRS, AΔ-1R26PylRS, BΔ-Lum1PylRS family. Silver box: representative set from the only triply orthogonal family found prior to the N, A, and B tRNAPyl screens. Orthogonality coefficient, o.c. is shown in grey. Distinct members of a family share the same set of PylRS enzymes but use different pyl tRNAs. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). c. Activity heatmaps of the highest o.c. sets from each family of quadruply orthogonal PylRS/tRNAPyl pairs obtained following the results of the class N, A, and B tRNAPyl screens. The substitution of a class B or C PylRS with different SΔ PylRS variants (labelled in two shades of blue) allows the generation of all such families. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). d. Activity heatmaps of the two quadruplet families with a single SΔ PylRS variant substituting for a class B or class C PylRS, shown along with the most orthogonal fifth pair from the final class (class N). The pyl tRNAs that require engineering or replacement to minimize unwanted cross-reactions are labelled in red, while the pyl tRNAs that already satisfy all necessary orthogonality requirements are labelled in green. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). e. Schematic of the interactions between the quadruply orthogonal set with the highest o.c. (class B PylRS substituted by SΔB, o.c. 3.9) and the most orthogonal fifth pair from the final class (class N). f. Schematic of the interactions between the quadruply orthogonal set with the lowest o.c. (class C PylRS substituted by SΔC, o.c. 2.5) and the most orthogonal fifth pair from the final class (class N).
By allowing the replacement of class B or C PylRS enzymes with SΔ PylRS variants, we obtained a total of 946 doublets in 25 families, 1425 triplets in 16 families, and – crucially – 96 quadruplets in four families (Fig. 5b-c and Supplementary Table 2). Notably, the highest triplet o.c. is 24.5 – approximately five times higher than a previously reported triplet used for the incorporation of three distinct non-canonical amino acids (N+-MmPylRS/N-SpetRNAPyl, AΔ-1R26PylRS/A-AlvtRNAPyl-8, BΔ-Lum1PylRS/B-InttRNAPyl-17C10, Supplementary Table 2), and approximately two times higher than the highest o.c. triplet from the previously reported N+-MmPylRS, AΔ-1R26PylRS, BΔ-Lum1PylRS family.6 Most triplet and all quadruplet families involve substitution of the class B and/or class C pair with pairs containing SΔ PylRS variants.
To understand how far the SΔ PylRS substitution strategy had advanced the development of quintuply orthogonal pairs, we considered the quadruplets in the context of our inter-class interaction network (Fig. 5d-f and Extended Data Fig. 3). For the [A, S ΔB, C, S] (o.c. 3.9) and [A, B, SΔC, S] (o.c. 2.5) quadruplet families formed with a single SΔ PylRS variant substituting for a class B or class C PylRS respectively, the cross-reactivity between classes B and C (Fig. 4e) had effectively been replaced by cross-reactivity between class N and SΔB or B (Fig. 5d-f). This results in the generation of a diagonal submatrix of order 4 in the overall interaction matrix, and thus the quadruplets. However, including cross-reactivity between classes N (N+-MmPylRS) and S (any tRNAPyl paired with S+-DebPylRS), two cross-reactivities still remained to be eliminated. Meanwhile, for the [N, A, SΔB, SΔC] and [A, SΔB, SΔC, S] quadruplets (both o.c. 2.9) formed via substitution of both class B and class C PylRS enzymes with SΔ PylRS variants, one main cross-reactivity (between N+-MmPylRS and any tRNAPyl paired with S+-DebPylRS) persisted (Extended Data Fig. 3). However, there also remained some residual cross-reaction between the class SΔB and SΔC pairs which would restrict the o.c. of any potential quintuplets. We hypothesised that a general solution to all of these cross-reactivity problems would be further engineering of the pyl tRNAs paired with class B/SΔB and class S PylRS enzymes.
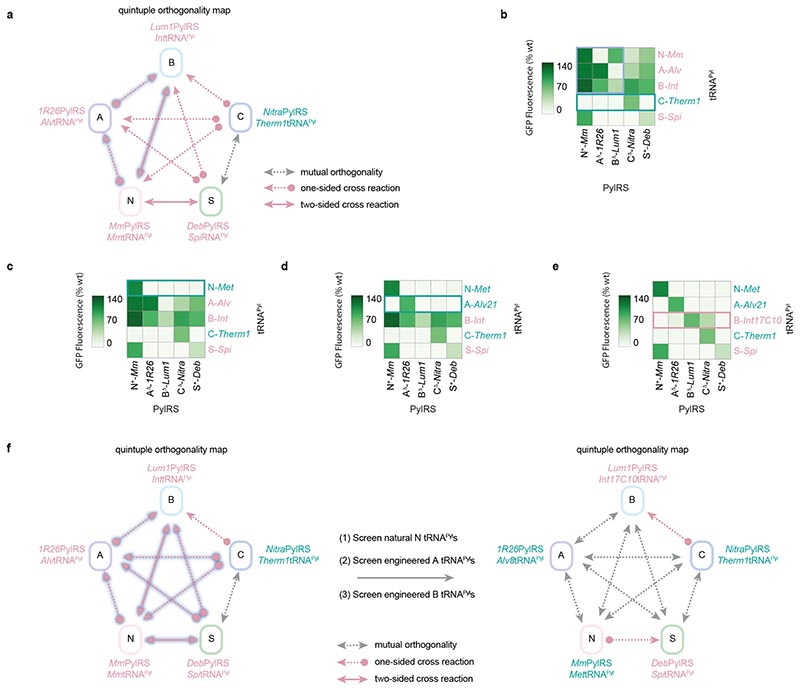
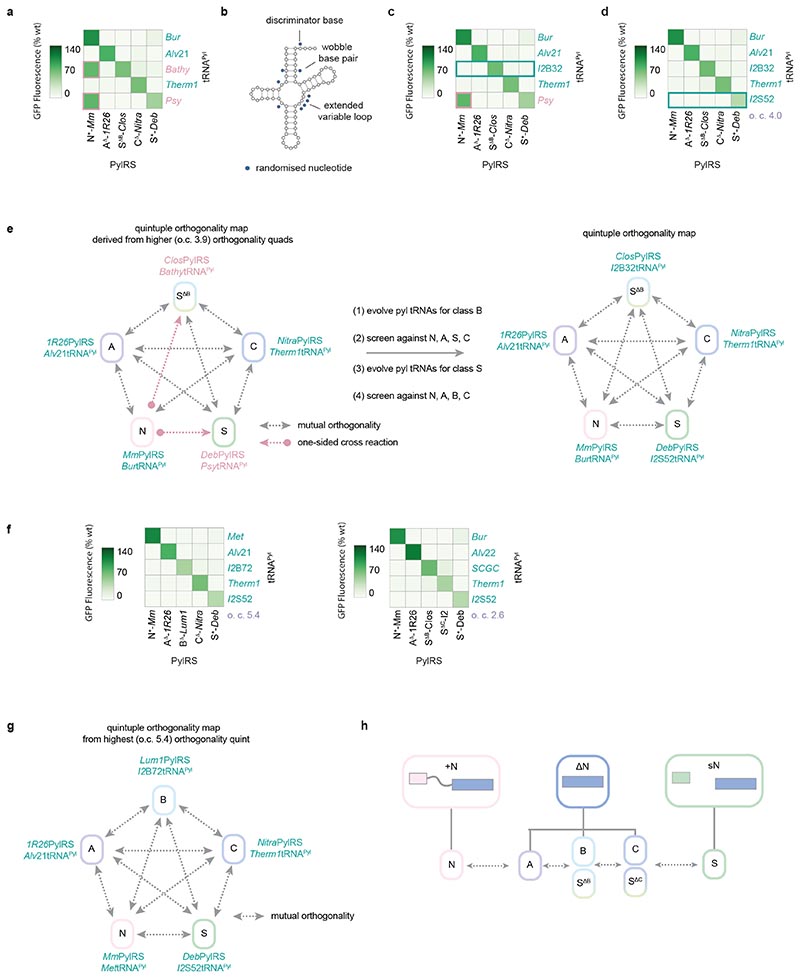
Quintuply orthogonal PylRS/tRNAPyl pairs
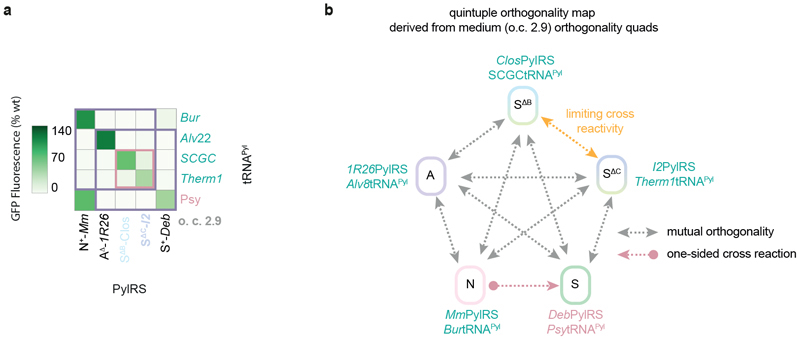
We aimed to: (1) discover a tRNAPyl that functions with a class B PylRS enzyme (or SΔB PylRS variant) but is orthogonal to all other PylRS classes, (2) discover a tRNAPyl that functions with a class S PylRS but is orthogonal to all other PylRS classes, and (3) replace the pyl tRNAs that pair with SΔB-ClosPylRS and S+-DebPylRS with our new pyl tRNAs in the highest o.c. quadruplet. We anticipated that this would mitigate undesired cross-reactivity with a fifth pair and thereby enable the generation of a quintuply orthogonal set of pairs (Fig. 6a).
Fig. 6. Quintuply orthogonal PylRS/tRNAPyl pairs via directed evolution.
a. Activity heatmap combining the quadruplet with the highest o.c. and the most orthogonal fifth pair from the final class. Cross-reactivities are boxed in red; pyl tRNAs to be replaced with quintuply orthogonal evolved variants are labelled in red. b. The library used for evolution of quintuply orthogonal pyl tRNAs from the S-I2tRNAPyl scaffold. The cloverleaf structure of S-I2tRNAPyl is shown. c. Activity heatmap from a, updated based on the results of the directed evolution of pyl tRNAs specific to class B (or SΔB). S-I2tRNAPyl-B32, the most orthogonal tRNA from the directed evolution, satisfies all necessary orthogonality requirements (green box). As a result, the quintuply orthogonal SΔB-ClosPylRS/S-I2tRNAPyl-B32 pair substitutes effectively for class B and requires no further engineering. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). d. Activity heatmap from c, updated based on the results of the directed evolution of a pyl tRNA specific to class S. S-I2tRNAPyl-S52 satisfies all necessary orthogonality requirements (green box). As a result, the S+-DebPylRS/S-I2tRNAPyl-S52 pair requires no further engineering, and completes a quintuply orthogonal set of pairs (o.c. 4.0). Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). e. Schematic of the overall tRNAPyl evolution strategy and resulting pairs. The cross-reactions between class N and class B (or its SΔ equivalent), and between class N and class S are successively destroyed to yield a set of five pairs where all twenty cross-reactions are minimized. f. Activity heatmaps from two families of quintuply orthogonal pairs that incorporate the evolved pyl tRNAs; the quintuplets with the highest o.c.. Data represents the average of three biological replicates. All numerical values and bar charts including error bars showing s.d. are provided (Supplementary Table 2). g. Schematic of the interactions within the quintuply orthogonal set of pairs with the highest o.c. (5.4), which is formed with one PylRS from each class. h. Schematic highlighting the successful division of pyrrolysine systems into five mutually orthogonal functional classes: N, A, B or SΔB, C or SΔC, and S.
We noted that engineered B-tRNAPyl variants were active with CΔ-NitraPylRS (Extended Data Fig. 2c), and postulated that a different parent tRNAPyl, such as one from a bacterial class S system, might provide a better starting point for the discovery of a class B- or SΔB-specific tRNAPyl variant with orthogonality towards CΔ-NitraPylRS (and the other PylRS enzymes, as necessary), via directed evolution.
We chose S-I2tRNAPyl, a bacterial tRNA, as a starting point for directed evolution; this tRNA has exceptionally high activity with SΔB-ClosPylRS (giving rise to 93% of wild-type GFP levels), yet fairly modest cross-reactivity with CΔ-NitraPylRS (23% of wild-type GFP levels). In fact, S-I2tRNAPyl is most cross-reactive with N+-MmPylRS (68% of wild-type GFP levels); accordingly, we focussed our efforts on abrogating S-I2tRNAPyl recognition by N+-MmPylRS.
Previous work demonstrated that N+-MmPylRS can be much more sensitive to expansions in the short variable loop of tRNAPyl than ΔN PylRS enzymes from classes A7 and B6 (Supplementary Table 2). Since SΔB-ClosPylRS is an artificial ΔN class PylRS, we hypothesised that it may tolerate insertions into the variable loop of S-I2tRNAPyl, and synthesised a library of S-I2tRNAPyl mutants in which the variable loop was expanded to four nucleotides (Fig. 6b). Positions 8 and 22, which could make important tertiary contacts with the variable loop, were also randomised. In addition, to widen the diversity of activity profiles within the library, three bases thought to be generally important to the recognition of S-I2tRNAPyl were also randomised; namely, position 69 (the discriminator base, an important identity element for PylRS), and positions 5 and 64. The latter two form a wobble base pair in the S-I2tRNAPyl acceptor stem; such pairs are known to distort RNA helices and thereby dictate aminoacyl-tRNA synthetase recognition.51
We selected S-I2tRNAPyl mutants that allowed cells also expressing SΔB-ClosPylRS to grow on 100 μg mL-1 chloramphenicol in the presence of AllocK 1, by enabling production of protein from a gene coding for chloramphenicol acetyl transferase containing an amber codon at position 111. We then performed successive screens on the selected S-I2tRNAPyl variants to identify pyl tRNAs that have minimal cross-reactivity with N+-MmPylRS, AΔ-1R26PylRS, CΔ-NitraPylRS, and S+-DebPylRS. Cells harbouring GFP(150TAG)His6, an S-I2tRNAPyl variant, and one of N+-MmPylRS, AΔ-1R26PylRS, CΔ-NitraPylRS, or S+-DebPylRS were provided with AllocK and screened for the absence of GFP expression. These screens revealed three library members, S-I2tRNAPyl-B8, S-I2tRNAPyl-B32, and S-I2tRNAPyl-B72, which remain highly active with SΔB-ClosPylRS but have little cross-reactivity with N+-MmPylRS, AΔ-1R26PylRS, CΔ-NitraPylRS, and S+-DebPylRS (Fig. 6c and Extended Data Fig. 4a). In particular, S-I2tRNAPyl-B32 and S-I2tRNAPyl-B72 both retain over 75% of the activity of their parent S-I2tRNAPyl with SΔB-ClosPylRS but have over 20-fold lower activity than their parent tRNA with N+-MmPylRS and around three-fold lower activity than their parent with CΔ-NitraPylRS.
While performing screens on S-I2tRNAPyl mutants, we observed that – despite their expanded variable loops – some S-I2tRNAPyl mutants have increased activity with S+-DebPylRS. We hypothesised that the N-terminal domain protein in the split S+-DebPylRS enzyme may have different recognition of variable loop nucleotides from N+ PylRS. Therefore, to discover a tRNAPyl that pairs with a class S PylRS that is orthogonal to all other PylRS classes used in a quintuplet, we selected S-I2tRNAPyl expanded variable loop mutant library members that are selectively aminoacylated by S+-DebPylRS.
We performed a positive selection for S-I2tRNAPyl mutants that are active with S+-DebPylRS, followed by screening to minimize cross-reactivity with N+-MmPylRS, AΔ-1R26PylRS, SΔB-ClosPylRS, and CΔ-NitraPylRS. These screens identified one library member, S-I2tRNAPyl-S52, which is active with S+-DebPylRS (43% of wild-type GFP production) but possesses very low activity with SΔ-ClosPylRS, N+-MmPylRS, AΔ-1R26PylRS, and CΔ-NitraPylRS (all less than 2% of wild-type GFP production) (Fig. 6d and Extended Data Fig. 4b). When compared with the wild-type (S-I2tRNAPyl), S-I2tRNAPyl-S52 is over three-fold more active with S+-DebPylRS and 120-fold less active with SΔB-ClosPylRS.
By substituting our S-I2tRNAPyl mutants into the quadruplet with the highest o.c. (from the [A, SΔB, C, S] family), we minimized cross-reactivities with N+-MmPylRS. This enabled us to combine the updated quadruplets with a fifth orthogonal pair from class N (for example N+-MmPylRS/N-BurtRNAPyl) to generate a family of mutually orthogonal quintuplets with o.c. values of up to 4.0 (Fig. 6d). Thus, by mutation, selection and screening from a single tRNA scaffold we eliminated the two final cross-reactivities (Fig. 6e). Despite their very different activities, S-I2tRNAPyl-B32 and S-I2tRNAPyl-S52 differ by only four nucleotides; this demonstrates the power of synthetically engineering identity elements to control tRNA recognition by aminoacyl-tRNA synthetases.
Because S-I2tRNAPyl-S52 is also orthogonal to both BΔ-Lum1PylRS and SΔC-I2PylRS, we obtained two additional quintuplet families, by substitution of SΔB-ClosPylRS with BΔ-Lum1PylRS, or CΔ-NitraPylRS with SΔC-I2PylRS. Overall, using our original o.c. threshold of 2.5, we obtained 1136 doublets in 27 families (highest o.c. 72.0), 2359 triplets in 26 families (highest o.c. 39.6), 919 quadruplets in 14 families (highest o.c. 7.2), and 90 quintuplets in 3 families (highest o.c. 5.4). Upon increasing the o.c. threshold to 5.0 – comparable to the o.c. value for PylRS/tRNAPyl pairs previously used for incorporating three distinct non-canonical amino acids – we obtained 924 doublets in 22 families, 1324 triplets in 18 families, 128 quadruplets in 7 families, and 8 quintuplets in 1 family (Fig. 6f, Extended Data Fig. 4c, Supplementary Fig. 4, Supplementary Table 2). The quintuplets with the highest o.c. values have the following composition: N+-MmPylRS with a (non-cognate) natural class N tRNAPyl; AΔ-1R26PylRS with an engineered A-AlvtRNAPyl variant; BΔ-Lum1PylRS with an engineered S-I2tRNAPyl variant; CΔ-NitraPylRS with a (non-cognate) natural class C tRNAPyl; and S+-DebPylRS with an engineered S-I2tRNAPyl variant (Fig. 6g). We characterized the amber suppression efficiency and accuracy of each quintuply orthogonal PylRS/tRNAPyl pair in the most orthogonal quintuplet by producing GFP150AllocKHis6 and Ub11AllocKHis6 from GFP150TAGHis6 or Ub11TAGHis6 respectively, and measuring protein titres as well as MS spectra (Supplementary Fig. 5 to 10 and Supplementary Table 4 and 5). Our results demonstrate the successful and unprecedented division of homologous PylRS/tRNAPyl systems into five classes that are mutually orthogonal in their aminoacylation specificity (Fig. 6h).
Discussion
We have defined sequence identity threshold criteria to effectively search genomic data for PylRS and tRNAPyl orthogonality. By applying these thresholds to generate sequence clusters we have computationally searched PylRS sequences for multiply orthogonal systems. Using this approach we have identified orthogonal systems from hundreds of PylRS systems spanning all PylRS groups. By combining our computational approach with directed evolution and engineering we have generated the first quadruply and quintuply orthogonal PylRS systems. These advances, along with strategies for generating codons that can be used to encode non-canonical monomers, may facilitate the synthesis of proteins containing an increasing number of ncAAs, and the encoded cellular synthesis of more diverse polymers and macrocycles.2,3,18,19,21–26 Moreover, mining the data generated through our approach may provide further insight into the sequence requirements for mutually orthogonal systems and enable the creation of more refined rules for predicting orthogonality.
Methods
Identification of PylRS sequences
We identified PylRS sequences by performing a BLAST search against the NCBI non-redundant protein sequence database using the AΔ-AlvPylRS protein as the query sequence, filtering for expected values below 1 x 10-30. Partial protein sequences and sequences from synthetic constructs were removed by manual inspection, and we ultimately obtained 351 PylRS sequences. We collected these sequences into a database, where each PylRS sequence was assigned a unique identifier based on the organism name and the NCBI Accession ID. We aligned the obtained PylRS sequences using Clustal Omega52 and extracted sequences corresponding to the C terminal domain (CTD) of the PylRS enzymes, with reference to the known annotation of the CTD in N+-MmPylRS6.
Identification of tRNAPyl sequences
Using the NCBI Nucleotide database, we obtained all available nucleotide sequences of the host genome or metagenomic read containing each of our identified PylRS sequences. We ran the tRNA detection program ARAGORN49(version 1.2.38) on each nucleotide sequence, allowing for introns of up to 100 nucleobases and scoring thresholds at 90% of default levels. Discovered pyl tRNAs were added to the PylRS sequence database. For the PylRS sequence with identifier Marc.6481 (isolated from candidate division MSBL1 archaeon SCGC-AAA382A20, subsequently referred to as CΔ-SCGCPylRS), no corresponding tRNA could be found by ARAGORN, but a putative sequence had been previously reported53 and was therefore added to the database. Meanwhile, PylRS sequences with identifiers Mthe.7552 and Mthe.9096 were found to originate from the same organism (Candidatus Methanohalarchaeum thermophilum), but ARAGORN only found a corresponding tRNAPyl proximate to one of the PylRS sequences (Mthe.7552, subsequently referred to as CΔ-Therm1PylRS). Manual searching of the nucleotide sequence revealed a second tRNAPyl proximate to the other PylRS sequence (Mthe.9096, subsequently referred to as CΔ-Therm2PylRS); this was also added to the database. During preparation of this manuscript these two pyl tRNAs were independently reported.46 After manual curation to remove pseudogenes, we obtained pyl tRNAs for 284 PylRS genes.
Analysis of previously characterised PylRS/tRNAPyl pairs
From our database of PylRS and tRNAPyl sequences, we obtained the sequences of class A and B PylRS and tRNAPyl sequences that we had previously characterized experimentally.6 A matrix of pairwise sequence percentage identities for all pairs of PylRS sequences and all pairs of tRNAPyl was then calculated using python (version 3.9.7).54 For PylRS sequences, percentage identities were calculated from the multiple sequence alignment of C terminal domains. For tRNAPyl sequences, percentage identities were calculated from a manually performed multiple sequence alignment that was based on secondary structure predictions from ARAGORN and RNAfold .55,56
We considered the experimental activity data previously reported for these sequences, namely the level of GFP production from a GFP gene containing an in-frame amber codon at position 150 (GFP150TAGHis6) obtained in the presence of each combination of PylRS enzyme, and tRNAPylCUA, as well as 8 mM Nε-Boc-L-lysine (subtracted by the level of GFP production in the presence of only GFP gene and tRNAPylCUA). For each combination of PylRS enzyme and tRNAPylCUA, we plotted this activity against the percentage sequence identity of the PylRS sequence with the sequence of the PylRS from the same organism as the tRNAPylCUA. Similarly, we plotted the activity for each combination of PylRS enzyme and tRNAPylCUA against the percentage identity of the tRNAPylCUA with the sequence of the tRNAPylCUA from the same organism as the PylRS enzyme.
Clustering of PylRS C terminal domain sequences
Using python (version 3.9.7), we calculated a matrix of percentage identities from the multiple sequence alignment of C terminal domains for all pairs of PylRS sequences in our database. We then used this matrix to perform unweighted average linkage agglomerative hierarchical clustering (UPGMA) of aligned PylRS CTD sequences with a cluster merging threshold of 55% sequence identity, using the biopython (version 1.79) and the scikit-learn (version 1.0.1) python libraries.54,57
Alignment and clustering of tRNAPyl sequences
For each PylRS cluster, we chose a representative PylRS sequence for which a corresponding tRNAPyl sequence could be found. For two of the 37 PylRS clusters, no tRNAPyl sequence was found; these were excluded from further analysis. For the 35 obtained pyl tRNAs, we performed a manual multiple sequence alignment that was based on secondary structure predictions from ARAGORN and RNAfold. This multiple sequence alignment was then used to calculate a matrix of percentage identities for all pairs of chosen tRNAPyl, using the biopython (version 1.79) and scikit-learn (version 1.0.1) python libraries. We then used this matrix to perform unweighted average linkage agglomerative hierarchical clustering (UPGMA) of aligned chosen tRNAPyl sequences with a cluster merging threshold of 75% sequence identity.
DNA constructs
PylRS and tRNAPyl genes were synthesized by IDT as gBlock double-stranded DNA fragments. We cloned all new pyl tRNAs into a minimal pMB1 backbone under an lpp promoter. Previously reported pyl tRNAs were used in the same format. Certain tRNAs differed from the canonical sequence at the anticodon loop; these positions were mutated to the consensus bases found in E. coli to improve the efficiency of the tRNAs in E. coli translation as has been previously described.6 PylRS sequences were cloned into a p15A backbone under a glnS promoter. For each new PylRS sequence, a 5’ untranslated region was generated using the online tool De Novo DNA58–63 predicted to maximise translation initiation efficiency (see Supplementary Table 3) and inserted between the +1 site of the glnS promoter and the start codon of the gene. For class S PylRS enzymes, polycistronic operons consisting of the separately expressed N- and C-terminal domains were constructed using intergenic regions predicted by De Novo DNA. The optimal arrangement of the two domains was chosen by maximizing predicted translation initiation rates. N+MmPylRS, AΔ-AlvPylRS, and B Δ-Lum1PylRS were used in similar p15A constructs containing C-terminal tags as previously described.6 The p15A vectors also encoded a chloramphenicol acetyltransferase (CAT) gene with an amber codon at position 111 under a constitutive cat promoter and a GFP gene with an amber codon at position 150 under an L-arabinose inducible pBAD promoter.
Measuring the activity and specificity of PylRS/tRNPylCUA pairs
To measure the activity of the PylRS/tRNAPylCUA pairs we transformed 0.4 μL of pMB1 plasmid encoding a tRNAPylCUA gene into 4-10 μL E.coli DH10B chemically competent cells bearing a p15A plasmid encoding a PylRS gene, a CAT111TAG gene, as well as a GFP150TAGHis6 gene. We recovered the transformed cells for approximately 1h at 37°C and 75Or.p.m. in 180 μL of SOC medium (Super optimal broth with catabolite repression) in a 96 well Costar microtitre plate format. We then used 40 μL of the rescued cells to inoculate 760 μL of selective 2xYT-st (2xYT medium containing 75 μg mL-1 spectinomycin and 12.5 μg mL-1 tetracycline) medium in a 1.2 mL 96 well plate format and the cultures were grown overnight at 37°C and 750 r.p.m. After a minimum of 16 h, 40 μL of the overnight cultures were used to inoculate 760 μL of 2xYT-st medium, containing 0.05% L-arabinose and 4 mM Nε-Alloc-L-lysine (AllocK), in a 1.2 mL 96 well plate format. Cells were grown for 18-24 h at 37°C and 750 r.p.m. Ultimately, 100 μL of each culture was transferred into 96 well flat bottom Costar plates and fluorescence and optical density (OD) were measured using a PHERAstar FS plate reader. Measured GFP/OD600 values were normalised by the GFP/OD600 value of cells expressing GFP from a GFP150AsnHis6 gene (referred to as ‘wtGFP control’).
Identification of sets of mutually orthogonal PylRS/tRNAPyl pairs
Using python (version 3.9.7), we identified sets of mutually orthogonal PylRS/tRNAPyl pairs based on the GFP activity data. For any given set of PylRS/tRNAPyl pairs, the quotient of the lowest intra-pair activity over the highest inter-pair cross reactivity was defined as the orthogonality coefficient, o.c.. Sets of pairs were considered mutually orthogonal if the lowest intra-pair activity was greater than 40% of the wtGFP control, the highest inter-pair cross-reactivity was less than 20% of the wtGFP control, and the o.c. was higher than 2.5. We grouped mutually orthogonal sets together into families if they involved the same PylRS enzymes.
S-I2tRNAPylCUA library generation
The library of S-I2tRNAPylCUA with randomized nucleotides was constructed by Golden Gate cloning into a pMB1 vector using PCR primers as listed in Supplementary Table 3, a Q5 DNA polymerase, a Bbs1-HF restriction enzyme, and a T4 DNA ligase (all enzymes were purchased from New England Biolabs (NEB)). The library was transformed into electrocompetent E.coli DH10B cells with a transformation efficiency of more than 1x108 colony forming units.
Selection and screening to identify orthogonal S-I2tRNAPylCUA hits
The S-I2tRNAPylCUA library was transformed into electrocompetent E.coli DH10B cells bearing a p15A plasmid encoding CAT(111TAG), GFP(150TAG)His6 and either SΔ-ClosPylRS or S+-DebPylRS. Cells were recovered for one hour in 1 mL SOC at 37°C 220 r.p.m. supplemented with AllocK and plated onto LB agar plates containing 4 mM AllocK, 75 μgmL-1 spectinomycin, 12.5 μgmL-1 tetracycline and 100 μgmL-1 chloramphenicol. The plates were incubated at 37°C for 18-24 h. After incubation a combined total of 576 colonies from either selection (colonies grown in presence of SΔ-Clos PylRS or S+-DebPylRS, respectively) were picked into 500 μL 2xYT-st and the colonies were grown over night at 750 r.p.m. and 37°C. 40 μL of the overnight culture were then given into 760 μL 2xYT-st containing 0.05% L-arabinose in presence and absence of 4 mM AllocK. Plasmids from clones which were selectively fluorescent in presence of AllocK were extracted (DNA miniprep from Qiagen), digested with NcoI restriction enzyme and T5 exonuclease (both from NEB) and retransformed into chemically competent cells bearing a p15A plasmid encoding CAT(111TAG), GFP(150TAG)His6 and one of the following PylRS genes – N+-MmPylRS, SΔ-ClosPylRS, CΔ-NitraPylRS, AΔ-1R26PylRS, or S+-DebPylRS. Plasmids of clones which fulfilled the orthogonality requirements were isolated and sequenced.
Quantifying GFP150AllocKHis6 and Ub11AllocKHis6 protein production yields with quintuply orthogonal PylRS/tRNAPylCUA pairs
To measure the protein yield for single ncAA incorporations with the quintuply orthogonal PylRS/tRNAPylCUA pairs from the set with the highest o.c., we co-transformed the pMB1 plasmid (encoding a tRNAPylCUA gene) and the p15A plasmid (encoding a PylRS gene, a CAT111TAG gene, as well as a GFP150TAGHis6, or Ub11TAGHis6gene) into competent E.coli DH10B by electroporation. As controls we also co-transformed GFPHis or Ub11TCAHis6 together with AlvtRNAPyl-21CUA and MmPylRS in the same plasmid set-up.
We recovered the transformed cells for approximately 1 h at 37°C and 220 r.p.m. in 600 μL of SOC medium (Super optimal broth with catabolite repression). We used 160 μL of the rescued cells to inoculate 5 mL of selective 2xYT-st (2xYT medium containing 75 μg mL-1 spectinomycin and 12.5 μg mL-1 tetracycline) medium in a 50 mL glass tubes and the cultures were grown overnight at 37°C and 220 r.p.m in a shaking incubator. After a minimum of 16 h, 140 μL of the overnight cultures were used to inoculate 5 mL of 2xYT-st medium, containing 0.05% L -arabinose and 4 mM N-Alloc-L-lysine (AllocK), in a 50 mL glass tube. Cells were grown for 16-18 h at 37°C and 220 r.p.m. Cells were spun down, aspirated and the cell pellets were frozen at -20 °C for a minimum of 1 h.
The pellets were resuspended in 800 μL BugBuster® Protein Extraction Reagent containing cOmplete™ protease inhibitor and lysed for one hour with head-over-tail rotation. Lysed cells were spun down and the supernatant incubated for 1-16 h at 4 °C with 160 μL NiNTA agarose beads. The beads were washed five times with 800 μL 25 mM imidazole in PBS at pH 8.5 and the proteins were eluted five times with 160 μL 250 mM imidazole in PBS pH 8.5 (for GFP samples), or five times with 100 μL 250 mM imidazole in PBS pH 8.5 (for Ub samples). Protein concentrations for GFP were measured by quantifying the absorption at 280 nm. Protein concentrations for ubiquitin were measured using Pierce™ BCA Protein Assay Kit from Thermo Fisher following the manufacturers protocol.
Electrospray ionization mass spectrometry
Denatured protein samples (~10 μM) were subjected to liquid chromatography-mass spectrometry analysis. Briefly, proteins were separated on a C4 BEH 1.7 μm, 1.0 × 100 mm ultraperformance liquid chromatography column (Waters) using a modified nanoAcquity (Waters) to deliver a flow of approximately 50 μl min-1. The column was developed over 20 min with a gradient of acetonitrile (2–80% v/v) in 0.1% v/v formic acid. The analytic column outlet was directly interfaced via an electrospray ionization source, with a hybrid quadrupole time-of-flight mass spectrometer (Xevo G2, Waters). Data were acquired over a m/z range of 300-2,000, in positive-ion mode with a cone voltage of 30 V. Scans were summed together manually and deconvoluted using MaxEnt1 (Masslynx, Waters). The theoretical molecular weights of proteins with ncAAs was calculated by first computing the theoretical molecular weight of wild-type protein using an online tool (http://web.expasy.org/protparam/) and then manually correcting for the theoretical molecular weight of ncAAs.
Extended Data
Supplementary Material
Acknowledgements
This work was supported by the Medical Research Council (MRC), UK (MC_U105181009 and MC_UP_A024_1008) and an ERC Advanced Grant SGCR, all to J.W.C.. For the purpose of Open Access, the MRC Laboratory of Molecular Biology has applied a CC BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission. D.L.D. was supported by the Boehringer Ingelheim Fonds and Magdalene College, Cambridge.
Footnotes
Author contributions
D.L.D., A.T.B. and J.W.C. designed the project. A.T.B. and D.L.D. performed the experiments. A.T.B. generated the computational discovery pipeline with inputs from D.L.D.. A.T.B., D.L.D and J.W.C. wrote the paper.
Competing interests
The authors declare the following competing financial interest: J.W.C. is a founder of the company Constructive Bio.
Data availability
All materials generated or analysed in this study are available from the corresponding author upon reasonable request. All generated data sets are provided in the supplementary information. Protein and nucleotide sequences were obtained from the NCBI Protein and NCBI Nucleotide databases, respectively.
Code availability
The code for PylRS and tRNAPyl clustering and mutually orthogonal PylRS/tRNAPyl pair identification is available at https://github.com/JWChin-Lab/Quint-Pyl.
References
- 1.Chin JW. Expanding and reprogramming the genetic code. Nature. 2017;550:53. doi: 10.1038/nature24031. [DOI] [PubMed] [Google Scholar]
- 2.De La Torre D, Chin JW. Reprogramming the genetic code. Nature Reviews Genetics. 2021;22:169–184. doi: 10.1038/s41576-020-00307-7. [DOI] [PubMed] [Google Scholar]
- 3.Robertson WE, et al. Sense codon reassignment enables viral resistance and encoded polymer synthesis. Science. 2021;372:1057–1062. doi: 10.1126/science.abg3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinck M, et al. Genetically programmed cell-based synthesis of non-natural peptide and depsipeptide macrocycles. Nature Chemistry. 2023;15:61–69. doi: 10.1038/s41557-022-01082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervettini D, et al. Rapid discovery and evolution of orthogonal aminoacyl-tRNA synthetase–tRNA pairs. Nat Biotechnol. 2020:1–11. doi: 10.1038/s41587-020-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunkelmann DL, Willis JCW, Beattie AT, Chin JW. Engineered triply orthogonal pyrrolysyl–tRNA synthetase/tRNA pairs enable the genetic encoding of three distinct non-canonical amino acids. Nature Chemistry. 2020;12:535–544. doi: 10.1038/s41557-020-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis JCW, Chin JW. Mutually orthogonal pyrrolysyl-tRNA synthetase/tRNA pairs. Nature Chemistry. 2018;10:831–837. doi: 10.1038/s41557-018-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan G, James CM, Krzycki JA. Pyrrolysine Encoded by UAG in Archaea: Charging of a UAG-Decoding Specialized tRNA. Science. 2002;296:1459–1462. doi: 10.1126/science.1069588. [DOI] [PubMed] [Google Scholar]
- 9.Krzycki JA. The direct genetic encoding of pyrrolysine. Curr Opin Microbiol. 2005;8:706–712. doi: 10.1016/j.mib.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Neumann H, Peak-Chew SY, Chin JW. Genetically encoding Nε-acetyllysine in recombinant proteins. Nat Chem Biol. 2008;4:232. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Brock A, Herberich B, Schultz PG. Expanding the Genetic Code of Escherichia coli. Science. 2001;292:498–500. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 12.Borrel G, et al. Unique Characteristics of the Pyrrolysine System in the 7th Order of Methanogens: Implications for the Evolution of a Genetic Code Expansion Cassette. Archaea. 2014;2014:11. doi: 10.1155/2014/374146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park H-S, et al. Expanding the Genetic Code of Escherichia coli with Phosphoserine. Science. 2011;333:1151–1154. doi: 10.1126/science.1207203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogerson DT, et al. Efficient genetic encoding of phosphoserine and its nonhydrolyzable analog. Nat Chem Biol. 2015;11:496. doi: 10.1038/nchembio.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes RA, Ellington AD. Rational design of an orthogonal tryptophanyl nonsense suppressor tRNA. Nucleic Acids Research. 2010;38:6813–6830. doi: 10.1093/nar/gkq521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee A, Sun SB, Furman JL, Xiao H, Schultz PG. A Versatile Platform for Single- and Multiple-Unnatural Amino Acid Mutagenesis in Escherichia coli. American Chemical Society. 2013 doi: 10.1021/bi4000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Italia JS, et al. Mutually Orthogonal Nonsense-Suppression Systems and Conjugation Chemistries for Precise Protein Labeling at up to Three Distinct Sites. J Am Chem Soc. 2019 doi: 10.1021/jacs.8b12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann H, Wang K, Davis L, Garcia-Alai M, Chin JW. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature. 2010;464:441. doi: 10.1038/nature08817. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, et al. Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double-labelling and FRET. Nat Chem. 2014;6:393. doi: 10.1038/nchem.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JC, et al. An expanded genetic code with a functional quadruplet codon. Proceedings of the National Academy of Sciences. 2004;101:7566–7571. doi: 10.1073/pnas.0401517101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunkelmann DL, Oehm SB, Beattie AT, Chin JW. A 68-codon genetic code to incorporate four distinct non-canonical amino acids enabled by automated orthogonal mRNA design. Nature Chemistry. 2021;13:1110–1117. doi: 10.1038/s41557-021-00764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malyshev DA, et al. A semi-synthetic organism with an expanded genetic alphabet. Nature. 2014;509:385–388. doi: 10.1038/nature13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer EC, et al. New codons for efficient production of unnatural proteins in a semisynthetic organism. Nature Chemical Biology. 2020;16:570–576. doi: 10.1038/s41589-020-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. A semi-synthetic organism that stores and retrieves increased genetic information. Nature. 2017;551:644. doi: 10.1038/nature24659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fredens J, et al. Total synthesis of Escherichia coli with a recoded genome. Nature. 2019;569:514–518. doi: 10.1038/s41586-019-1192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, et al. Defining synonymous codon compression schemes by genome recoding. Nature. 2016;539:59. doi: 10.1038/nature20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann H, Slusarczyk AL, Chin JW. De Novo Generation of Mutually Orthogonal Aminoacyl-tRNA Synthetase/tRNA Pairs. American Chemical Society. 2010 doi: 10.1021/ja9068722. [DOI] [PubMed] [Google Scholar]
- 28.Beranek V, Willis JCW, Chin JW. An Evolved Methanomethylophilus alvus Pyrrolysyl-tRNA Synthetase/tRNA Pair Is Highly Active and Orthogonal in Mammalian Cells. Biochemistry. 2019;58:387–390. doi: 10.1021/acs.biochem.8b00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee A, Xiao H, Schultz PG. Evolution of multiple, mutually orthogonal prolyl-tRNA synthetase/tRNA pairs for unnatural amino acid mutagenesis in Escherichia coli . Proc Natl Acad Sci USA. 2012;109:14841–14846. doi: 10.1073/pnas.1212454109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Italia JS, et al. An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes. Nature Chemical Biology. 2017;13:446–450. doi: 10.1038/nchembio.2312. [DOI] [PubMed] [Google Scholar]
- 31.Chin JW. Expanding and Reprogramming the Genetic Code of Cells and Animals. Annu Rev Biochem. 2014;83:379–408. doi: 10.1146/annurev-biochem-060713-035737. [DOI] [PubMed] [Google Scholar]
- 32.Ambrogelly A, et al. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc Natl Acad Sci USA. 2007;104:3141–3146. doi: 10.1073/pnas.0611634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott TS, et al. Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nat Biotechnol. 2014;32:465. doi: 10.1038/nbt.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, et al. Crystal structures reveal an elusive functional domain of pyrrolysyl-tRNA synthetase. Nat Chem Biol. 2017;13:1261. doi: 10.1038/nchembio.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi T, Yanagisawa T, Sakamoto K, Yokoyama S. Recognition of Non-α-amino Substrates by Pyrrolysyl-tRNA Synthetase. J Mol Biol. 2009;385:1352–1360. doi: 10.1016/j.jmb.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 36.Polycarpo CR, et al. Pyrrolysine analogues as substrates for pyrrolysyl-tRNA synthetase. FEBS Letters. 2006;580:6695–6700. doi: 10.1016/j.febslet.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bindman NA, Bobeica SC, Liu WR, Van Der Donk WA. Facile Removal of Leader Peptides from Lanthipeptides by Incorporation of a Hydroxy Acid. J Am Chem Soc. 2015;137:6975–6978. doi: 10.1021/jacs.5b04681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y-M, et al. Ligation of Expressed Protein α-Hydrazides via Genetic Incorporation of an α-Hydroxy Acid. ACS Chemical Biology. 2012;7:1015–1022. doi: 10.1021/cb300020s. [DOI] [PubMed] [Google Scholar]
- 39.Ohtake K, et al. Engineering an Automaturing Transglutaminase with Enhanced Thermostability by Genetic Code Expansion with Two Codon Reassignments. ACS Synthetic Biology. 2018;7:2170–2176. doi: 10.1021/acssynbio.8b00157. [DOI] [PubMed] [Google Scholar]
- 40.Polycarpo C, et al. An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc Natl Acad Sci U S A. 2004;101:12450–12454. doi: 10.1073/pnas.0405362101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozawa K, et al. Pyrrolysyl-tRNA synthetase–tRNAPyl structure reveals the molecular basis of orthogonality. Nature. 2008;457:1163. doi: 10.1038/nature07611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herring S, et al. The amino-terminal domain of pyrrolysyl-tRNA synthetase is dispensable in vitro but required for in vivo activity. FEBS Lett. 2007;581:3197–3203. doi: 10.1016/j.febslet.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang R, Krzycki JA. PylSn and the homologous N-terminal domain of pyrrolysyl-tRNA synthetase bind the tRNA that is essential for the genetic encoding of pyrrolysine. J Biol Chem. 2012:jbc.M112.396754. doi: 10.1074/jbc.M112.396754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meineke B, Heimgärtner J, Eirich J, Landreh M, Elsässer SJ. Site-Specific Incorporation of Two ncAAs for Two-Color Bioorthogonal Labeling and Crosslinking of Proteins on Live Mammalian Cells. Cell Reports. 2020;31:107811. doi: 10.1016/j.celrep.2020.107811. [DOI] [PubMed] [Google Scholar]
- 45.Meineke B, Heimgärtner J, Lafranchi L, Elsässer SJ. Methanomethylophilus alvus Mx1201 Provides Basis for Mutual Orthogonal Pyrrolysyl tRNA/Aminoacyl-tRNA Synthetase Pairs in Mammalian Cells. ACS Chemical Biology. 2018;13:3087–3096. doi: 10.1021/acschembio.8b00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, et al. The tRNA discriminator base defines the mutual orthogonality of two distinct pyrrolysyl-tRNA synthetase/tRNAPyl pairs in the same organism. Nucleic Acids Research. 2022;50:4601–4615. doi: 10.1093/nar/gkac271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer JT, Söll D, Tharp JM. Directed Evolution of Methanomethylophilus alvus Pyrrolysyl-tRNA Synthetase Generates a Hyperactive and Highly Selective Variant. Front Mol Biosci. 2022 doi: 10.3389/fmolb.2022.850613. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tharp JM, Vargas-Rodriguez O, Schepartz A, Söll D. Genetic Encoding of Three Distinct Noncanonical Amino Acids Using Reprogrammed Initiator and Nonsense Codons. ACS Chemical Biology. 2021;16:766–774. doi: 10.1021/acschembio.1c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laslett D. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Research. 2004;32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katayama H, Nozawa K, Nureki O, Nakahara Y, Hojo H. Pyrrolysine Analogs as Substrates for Bacterial Pyrrolysyl-tRNA Synthetase in Vitro and in Vivo. Bioscience, Biotechnology, and Biochemistry. 2012;76:205–208. doi: 10.1271/bbb.110653. [DOI] [PubMed] [Google Scholar]
- 51.Varani G, McClain WH. The G·U wobble base pair. EMBO reports. 2000;1:18–23. doi: 10.1093/embo-reports/kvd001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guan Y, Haroon MF, Alam I, Ferry JG, Stingl U. Single-cell genomics reveals pyrrolysine-encoding potential in members of uncultivated archaeal candidate division MSBL1. Environmental Microbiology Reports. 2017;9:404–410. doi: 10.1111/1758-2229.12545. [DOI] [PubMed] [Google Scholar]
- 54.Cock PJA, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruber AR, Lorenz R, Bernhart SH, Neubock R, Hofacker IL. The Vienna RNA Websuite. Nucleic Acids Research. 2008;36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenz R, et al. ViennaRNA Package 2.0. Algorithms for Molecular Biology. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedregosa F, et al. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research. 2011;12:2825–2830. [Google Scholar]
- 58.Reis AC, Salis HM. An Automated Model Test System for Systematic Development and Improvement of Gene Expression Models. ACS Synthetic Biology. 2020;9:3145–3156. doi: 10.1021/acssynbio.0c00394. [DOI] [PubMed] [Google Scholar]
- 59.Cetnar DP, Salis HM. Systematic Quantification of Sequence and Structural Determinants Controlling mRNA stability in Bacterial Operons. ACS Synthetic Biology. 2021;10:318–332. doi: 10.1021/acssynbio.0c00471. [DOI] [PubMed] [Google Scholar]
- 60.Espah Borujeni A, et al. Precise quantification of translation inhibition by mRNA structures that overlap with the ribosomal footprint in N-terminal coding sequences. Nucleic Acids Res. 2017;45:5437–5448. doi: 10.1093/nar/gkx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espah Borujeni A, Salis HM. Translation Initiation is Controlled by RNA Folding Kinetics via a Ribosome Drafting Mechanism. J Am Chem Soc. 2016;138:7016–7023. doi: 10.1021/jacs.6b01453. [DOI] [PubMed] [Google Scholar]
- 62.Espah Borujeni A, Channarasappa AS, Salis HM. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res. 2013;42:2646–2659. doi: 10.1093/nar/gkt1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salis HM, Mirsky EA, Voigt CA. Automated design of synthetic ribosome bindingsites to control protein expression. Nat Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All materials generated or analysed in this study are available from the corresponding author upon reasonable request. All generated data sets are provided in the supplementary information. Protein and nucleotide sequences were obtained from the NCBI Protein and NCBI Nucleotide databases, respectively.
The code for PylRS and tRNAPyl clustering and mutually orthogonal PylRS/tRNAPyl pair identification is available at https://github.com/JWChin-Lab/Quint-Pyl.