Abstract
Obesity has become a burgeoning epidemic in India, even though the country is still dealing with undernutrition. As a significant determinant of the Metabolic Syndrome (MetS) and non-communicable diseases (NCDs) such as type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD), understanding the Indian context of the problem and learning how to deal with the obesity epidemic in this country has gained paramount importance.
This narrative review points to the unique features of the obesity epidemic in India and its associated contributing factors, including the evolving nature of the Indian diet, the peculiarity of the increased adiposity at lower BMIs, unique obesity-associated genetic variants in Indians, the contribution of the gut microbiome, the impact of chronic inflammation and the role of ambient air pollution, and the contribution of decreased physical activity levels concerning the rapid urbanisation and the built environment. We believe that disseminating our insights into these unique features influencing the development of obesity in India will help increase global awareness and pave the way for better control and management of this obesity epidemic.
Keywords: obesity, India, epidemic, body mass index
1. Introduction
Obesity is defined as excessive fat accumulation leading to impairment in the health of an individual(1). The threat of obesity comes from the severity of its associated risk of noncommunicable diseases such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), stroke, musculoskeletal disorder, fatty liver disease and cancer, thereby contributing to disability for life, decrease in life expectancy (2) and economic disruption.
The imbalance between calories consumed and expended is the primary determinant of positive energy balance leading to obesity. Factors such as increased sedentary lifestyle, decreased physical activity, and increased intake of high-calorie food rich in fat and sugars contribute to its increasing prevalence. As India is the second-most populous country in the world and going through a rapid economic transition, it is easy to understand the magnitude of the health burden risk posed by the increasing prevalence of obesity. A few recent reviews on obesity in India have focused on the prevalence of obesity in India, forecasting prevalence in the near future, with specific emphasis on socioeconomic factors and the epidemiology of childhood obesity(3–7). However, to develop a comprehensive strategy with a chance to control/mitigate the epidemic of obesity in India, it is essential to understand the contribution of and connection between diverse factors, such as the population, geographical condition, lifestyle, socioeconomic state (SES), dietary patterns and biology of obesity.
2. Trends in the prevalence of obesity in India
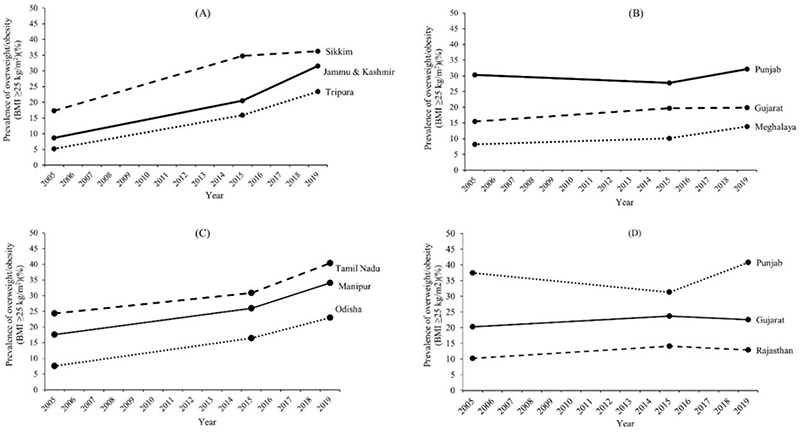
The Indian National Family Health Surveys (NFHS)(8–11) provide combined prevalence data for overweight and obesity (BMI ≥25 kg/m2). Time/trends of overweight and obesity (BMI ≥25 kg/m2) prevalence in Indian adults from the NFHS surveys(8–11) indicate a countrywide increase over time. On the whole, the prevalence of overweight and obesity (BMI ≥25 kg/m2) in India has increased from 12% to 23% in men; and from 15% to 24% in women in the same period (2005-2019)(8–10). Of relevance, these changes in prevalence are not homogeneous in the whole territory, exhibiting major variability ranges amongst the Indian states. States like Jammu & Kashmir and Manipur have witnessed skyrocketing increases in prevalence among men and women, respectively, between 2005-2006 (NFHS 3) and 2019-2021 (NFHS 5) (Figure 1, Table 1). In contrast, states like Punjab and Gujarat have witnessed minimal change, but at higher and lower prevalence levels, respectively, likely due to ethnic differences.
Figure 1. Prevalence of overweight and obesity (BMI ≥25 kg/m2) in select Indian states.
Prevalence of overweight and obesity (BMI ≥25 kg/m2) in select Indian states with high or low change in prevalence (% per year) between 2005 and 2019 (National Family Health Survey NFHS 3 – 5)(8–11). Jammu & Kashmir, Sikkim and Tripura had the highest (A) and Punjab, Gujarat and Meghalaya had the lowest change in the rate of prevalence (B) amongst men. Manipur, Tamil Nadu and Odisha had the highest (C) and Gujarat, Rajasthan and Punjab had the lowest change in the rate of prevalence (D) amongst women.
Table 1.
Rate of change (% change per year) of prevalence of overweight and obesity (BMI ≥25 kg/m2) between [National Family Health Survey (NFHS) 3 (2005-2006)](8) and NFHS 5 (2019-2021)(10, 11). The prevalence for states/union territories that were not included in either of the data sets are represented as '-’ and have been excluded in the analysis. *Data from Dadra and Nagar Haveli and Daman and Diu was merged during the NFHS 5 survey, and the cumulative data is represented here.
| State/Union Territory | Men | Women | ||||
|---|---|---|---|---|---|---|
| NFHS 3 (2005-2006) NFHS 5 (2019-2021) | NFHS 3 (2005-2006) NFHS 5 (2019-2021) | |||||
| Prevalence of overweight and obesity (BMI ≥25 kg/m2) | Prevalence of overweight and obesity (BMI ≥25 kg/m2) | Rate of change of prevalence (% change per year) | Prevalence of overweight and obesity (BMI ≥25 kg/m2) | Prevalence of overweight and obesity (BMI ≥25 kg/m2) | Rate of change of prevalence (% change per year) | |
| Andaman & Nicobar Islands | - | 45.3 | - | - | 31.8 | - |
| Andhra Pradesh | 17.6 | 31.1 | 0.96 | 17.7 | 36.3 | 1.33 |
| Arunachal Pradesh | 10.6 | 27.6 | 1.21 | 10.5 | 23.9 | 0.96 |
| Assam | 6.7 | 16.2 | 0.68 | 9.0 | 15.2 | 0.44 |
| Bihar | 8.5 | 14.7 | 0.44 | 5.3 | 15.9 | 0.76 |
| Chandigarh | - | 34.4 | - | - | 44.0 | - |
| Chhattisgarh | 6.5 | 14.9 | 0.60 | 6.7 | 14.1 | 0.53 |
| Dadra & Nagar Havelli | - | 21.4 | - | - | 26.8 | - |
| Daman & Diu | - | - | - | - | ||
| Goa | 20.8 | 32.6 | 0.84 | 27.0 | 36.1 | 0.65 |
| Gujarat | 15.5 | 19.9 | 0.31 | 20.3 | 22.6 | 0.16 |
| Haryana | 14.4 | 28.3 | 0.99 | 21.0 | 33.1 | 0.86 |
| Himachal Pradesh | 16.0 | 30.6 | 1.04 | 17.3 | 30.4 | 0.94 |
| Jammu & Kashmir | 8.7 | 31.6 | 1.64 | 22.7 | 29.3 | 0.47 |
| Jharkhand | 5.3 | 15.1 | 0.70 | 5.9 | 11.9 | 0.43 |
| Karnataka | 14.0 | 30.9 | 1.21 | 18.1 | 30.1 | 0.86 |
| Kerala | 24.3 | 36.4 | 0.86 | 34.0 | 38.1 | 0.29 |
| Lakshadweep | - | 41.3 | - | - | 33.5 | - |
| Madhya Pradesh | 5.4 | 15.6 | 0.73 | 8.6 | 16.6 | 0.57 |
| Maharashtra | 15.9 | 24.7 | 0.63 | 17.1 | 23.4 | 0.45 |
| Manipur | 13.4 | 30.3 | 1.21 | 17.6 | 34.1 | 1.18 |
| Meghalaya | 8.2 | 13.9 | 0.41 | 7.1 | 11.5 | 0.31 |
| Mizoram | 16.9 | 31.9 | 1.07 | 12.0 | 24.2 | 0.87 |
| Nagaland | 8.4 | 23.9 | 1.11 | 8.9 | 14.4 | 0.39 |
| NCT of Delhi | 24.0 | 38.0 | 1.00 | 32.9 | 41.3 | 0.60 |
| Puducherry | - | 43.3 | - | - | 46.2 | - |
| Punjab | 30.3 | 32.2 | 0.14 | 37.5 | 40.8 | 0.24 |
| Rajasthan | 8.4 | 15.0 | 0.47 | 10.2 | 12.9 | 0.19 |
| Sikkim | 17.3 | 36.3 | 1.36 | 19.5 | 34.7 | 1.09 |
| Tamil Nadu | 19.8 | 37.0 | 1.23 | 24.4 | 40.4 | 1.14 |
| Telangana | - | 32.3 | - | - | 30.1 | - |
| Tripura | 5.2 | 23.4 | 1.30 | 7.8 | 21.5 | 0.98 |
| Uttar Pradesh | 9.9 | 18.5 | 0.61 | 11.1 | 21.3 | 0.73 |
| Uttarakhand | 11.4 | 27.1 | 1.12 | 16.0 | 29.7 | 0.98 |
| West Bengal | 6.1 | 16.2 | 0.72 | 12.5 | 22.7 | 0.73 |
| Odisha | 6.9 | 22.2 | 1.09 | 7.6 | 23.0 | 1.10 |
These findings, heterogeneity and cultural influence, are in line with those by the NCD Risk Factor Collaboration, where 2416 population-based studies were pooled to track worldwide time trends in the prevalence of overweight (BMI 25–30 kg/m2) and obesity (BMI >30 kg/m2) between 1975 to 2016(12). Their findings showed that obesity in India has ballooned up much faster than in the rest of the world. Among men, the prevalence of overweight and obesity has increased by about four-fold (from 4.45% to 15.66%) and sixteen fold (from 0.17% to 2.87%) respectively in India compared to two fold (from 17.75% to 28.37%) a four-fold (from 3.05% to 11.55%) worldwide. Among women who were overweight and obese, these increases were three fold (from 6.03% to 17.10%) and nine fold (from 0.56% to 5.31%) respectively, higher in India compared to 1.5 fold (from 17.06% to 24.77%) and 2.4 fold (from 6.56% to 15.70%) worldwide(12).
The complexity of tackling the problem of overweight and obesity in India is exacerbated because its prevalence varies between states, rural and urban populations, men and women, geographical conditions, lifestyle, SES and dietary patterns(13). Moreover, although obesity is lower in rural populations, the upward trend is clear, and ~20% of rural Indian adults have been predicted to become overweight or obese by 2030(14). In contrast with other nations, the National Family Health Survey (NFHS) survey conducted in 2015-16 reported a higher incidence of obesity in those with higher socioeconomic status, linked to their sedentary lifestyle and high-calorie intake(9).
In terms of regional variations, Mohan et al. in 2008 reported clear regional differences in the prevalence of both obesity and abdominal obesity (prevalence high to low: South India > North India > East India > West India)(15). This regional disparity continues more than a decade later, as evident from NFHS-5 data released in 2019-21(10, 11), and is likely linked to regional disparities in prevailing economic conditions. The Southern states have consistently fared better in terms of both Gross State Domestic Product (Constant prices) as well as Per Capita Net State Domestic Product (Constant prices) compared to the Northern or Eastern states(16, 17). However, the Western states stand as an anomaly, usually faring the highest in terms of both Gross State Domestic Product (Constant prices) as well as Per Capita Net State Domestic Product (Constant prices)(16, 17) but the lowest in obesity prevalence(10, 11). Historically, these states were the major conduit for all the major invasions into the country; therefore, the likely mixing of local with non-local gene pools could be a reason for this observed disparity(18).
3. Relevance of understanding obesity in a traditionally undernourished country
India is in a transitional state of nutrition, facing a dual paradoxical burden where undernutrition and obesity coexist(19). The prevalence of undernutrition is consistently decreasing in parallel with an increase in obesity. Based on India's National Family Health Surveys (NFHS) data collected in 1998-1999, 2005-2006, 2015-2016 and 2019-2021, the prevalence of underweight has consistently reduced from 36% (in 1998-1999) to 19% (in 2019-2021) of Indian women (age 15-49 years) and from 28% (in 2005-2006) to 16% (in 2019-2021) of Indian men (age 15-49 years)(8–11). Conversely, the prevalence of overweight and obesity (BMI ≥25 kg/m2) in the same age group (age 15-49 years) has steadily gone up from 11% (in 1998-1999) to 24% (in 2019-2021) amongst Indian women and from 12% (in 2005-2006) to 23% (in 2019-2021) amongst Indian men(8–10, 20). Young et al. attribute the reduction in the prevalence of underweight (from 33% to 19%) and simultaneous increase in the prevalence of overweight and obesity (BMI ≥25 kg/m2) (from 15% to 24%) in Indian women (age 20-49 years) to improved SES, progressive urbanisation, improved diet diversity, latrine use, and higher education levels and decision-making and ownership of money(21).
Obesity is a significant risk factor for noncommunicable diseases (NCDs) such as CVD, T2DM, and hypertension. These comorbidities increase the burden of death and disability in India(22). According to the World Health Organization's (WHO) NCD progress monitor of 2015, it estimated that 61% of the total deaths in India were attributed to NCDs, and about 23% of the total population was at risk of premature death from NCDs(23). The shift in principal causes of premature mortality and morbidity from communicable, maternal, and neonatal conditions to NCDs has been observed rapidly in India since the 1990s(24). In India, ischemic heart disease - the most common form of cardiovascular disease – was ranked sixth in 1990 as the leading cause of disability-adjusted life-years (DALYs), and has been ranked second in 2019(24). T2DM was the ninth leading cause of DALYs in 2019(24). Non-alcoholic fatty liver disease (NAFLD) is another NCD associated with obesity, gaining prominence fast. NAFLD, a medical condition in which excess fat deposits in the liver, is a leading cause of chronic liver diseases globally. NAFLD affects about 25% of the global adult population ranging from 13.5% in Africa to 31.8% in the Middle East(25). In India, a recent systematic review reported an estimated pooled prevalence of 38.6% among adults and 35.4% among children(26). Moreover, major risk factors for NCDs, including high systolic blood pressure, high fasting plasma glucose, high total cholesterol, and high body-mass index, have consistently worsened in India between 1990 to 2016(27).
In terms of the economic impact of obesity and its associated complications, Okunogbe et al. recently utilised a cost-of-illness approach to calculate and compare the cost of obesity per capita in 2019 in eight countries representing diverse economic and geographical contexts(28). Their model included direct and indirect costs but could not include a few indirect cost components such as early retirement costs or long-term disability costs due to the feasibility of data availability across the eight countries. Though the cost of obesity per capita was lowest in India (United State Dollars (USD) 17), compared to USD 940 in Australia (highest) in 2019, the authors of the study projected a 17 times increase in these costs (USD 290) for India versus a three-fold increase (USD 2956) for Australia in 2060. In a 2015 study with data from 325 (15-49-year-old) women, the authors noted a significant increase in the individual average health expenditure per month amongst women, and this increase was correlated to the BMI. Mean expenditure increased steadily, from normal weight (BMI 18.5 – 24.9 kg/m2) – Indian Rupees (INR) 68 (±133.4 SD), overweight (BMI 25-30 kg/m2) – INR 132.7 (±234.4 SD), obese (BMI >30 kg/m2) – INR 143.7 (±204.9 SD), to morbidly obese (BMI >35 kg/m2) – INR 224.8 (±370.9 SD)(29). In their analysis of the economic burden of overweight and obesity on the public health system in the South Indian state of Andhra Pradesh conducted in 2017, Panda et al. concluded that the estimated total cost in one year due to all diseases related to overweight and obesity was USD 55 million, of which the lion’s share (~18%) was due to cardiovascular disease(30).
4. Causes and possible mechanisms contributing to obesity and comorbidities in India
4.1. The Evolving Indian Diet
To revise the Recommended Daily Allowance (RDA) values of nutrients for Indians, the Indian Council of Medical Research (ICMR)–National Institute of Nutrition (NIN) Expert Committee on RDA (2020) has defined the normal BMI reference for Indian adult man and woman of 19-39 years as weighing 65 and 55 kg respectively in contrasts with the 60 and 50 kg, respectively, considered by the earlier committees of 1989 and 2010(31). This change in the Indian reference was based on the evolving anthropometry and, therefore, the dietary needs of Indians. Further, as a nod toward the recognition of obesity as an increasingly significant problem for India, the ICMR-NIN Expert Committee on RDA (2020) reduced the Estimated Average Requirement (EAR) for energy for a sedentary Indian man by 9.1% [2320 kcal/day to 2110 kcal/day] and by 12.6% [1900 kcal/day to 1660 kcal/day] for a sedentary Indian non-pregnant, non-lactating woman, compared to the recommendations provided in 2010(32).
A study using data obtained from the Consumption Expenditure Survey (CES) compared the average daily calorie consumption in India with the EAT-Lancet recommendations and found that the calories gained are predominantly through the consumption of cereals than through the consumption of proteins, fruits, vegetables and legumes, pointing to the nutritional reasons for deficiency of protein and micronutrients(33). Similarly, another cross-sectional study on Asian Indian adolescents and youth reported a disproportionate consumption of micro and macronutrients(34). The consumption of fruits, vegetables, tubers, roots, milk products, sweets and oils was more than 9.0% (210 kcal/day) of the recommended daily allowance, and the daily energy percentage obtained from protein consumption was markedly less compared to the energy obtained from carbohydrates and fat. Also, fat consumption was way higher than the daily recommended allowance(34).
To identify components of the Indian diet likely contributing to the increasing prevalence of obesity, we utilised the TATA-NIN dashboard(35), a centralised data repository with harmonised datasets about crop production, nutrient intake and health outcomes. Using this dashboard, we analysed correlations between state-level intakes of foods and the prevalence of overweight and obesity (BMI ≥25 kg/m2) for all food types for which both intake and high BMI prevalence data were available for >10 states (Table 2).
Table 2.
Pearson's correlation (r) between state-wise mean monthly intake of foods and prevalence of overweight and obesity (BMI ≥25 kg/m2) in men and women(35).
| Food type | Men | Women | ||||
|---|---|---|---|---|---|---|
| n | r | P | n | r | P | |
| Oil (g) | 34 | 0.444 | 0.008 | 34 | 0.418 | 0.014 |
| Cereals (g) | 36 | -0.571 | <0.001 | 36 | -0.583 | <0.001 |
| Spices (g) | 32 | 0.672 | <0.001 | 32 | 0.633 | <0.001 |
| Meat and egg (g) | 23 | 0.440 | 0.036 | 23 | 0.414 | 0.049 |
| Pulses (g) | 33 | 0.413 | 0.017 | 34 | 0.454 | 0.007 |
| Sugar (g) | 33 | 0.183 | 0.309 | 35 | 0.383 | 0.023 |
| Milk (g) | 23 | 0.176 | 0.421 | 23 | 0.300 | 0.164 |
| Vegetable (g) | 32 | -0.131 | 0.473 | 33 | -0.230 | 0.199 |
| Fruits (g) | 32 | 0.083 | 0.652 | 33 | 0.004 | 0.982 |
| Tobacco and ganja (g) | 10 | 0.007 | 0.985 | 12 | 0.068 | 0.834 |
| Packaged processed food (g) | 22 | 0.348 | 0.112 | 22 | 0.409 | 0.059 |
| Salt (g) | 33 | -0.027 | 0.880 | 33 | -0.100 | 0.578 |
n = number of states for which monthly intake data is available.
P < 0.05 are shown in bold
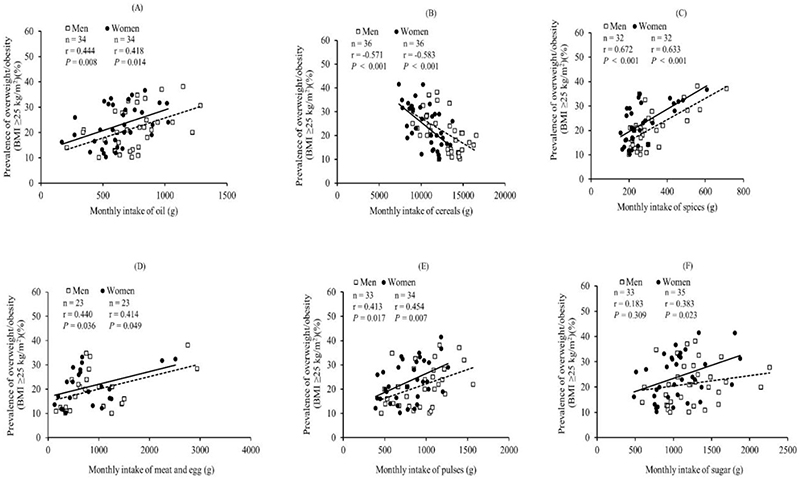
As expected, intake of 'all oils' (includes data on mustard oil, groundnut/peanut oil, refined oil – sunflower, soybean, safflower, hydrogenated fats, and all other edible oils) was positively associated with the prevalence of high BMI in both men and women (Figure 2A). Intake of 'cereals' (includes all rice/rice products, wheat/wheat products, millets, and all other cereals and bread) was negatively associated with high BMI in both men and women (Figure 2B). There can be at least two reasons for this observation. One, 'cereals' primarily reflect whole grain cereals, not refined grains, as intake of refined grains has been documented under 'packaged foods'. Whole grain cereals benefit body weight regulation by providing more dietary fibre, vitamins, and minerals than refined versions(36, 37). Two, expenditure on non-cereal foods has consistently increased in rural and urban India, at the expense of expenditure on cereal-based foods, primarily due to an increase in income levels across the board(38, 39). As such, levels of cereal intake could act as a proxy for the SES of the population, with higher SES having a higher prevalence of high BMI. The positive association between intake of 'spices' and the prevalence of high BMI (Figure 2C) can similarly be explained by the intake of spices acting as a proxy for the purchasing power and, therefore, SES of the population(40).
Figure 2. Scatter plots of correlations between state-wise mean monthly intakes and prevalence of overweight and obesity (BMI ≥25 kg/m2).
Scatter plots of correlations between state-wise mean monthly intakes of (A) oil, (B) cereals, (C) spices, (D) meat and egg, (E) pulses and (F) sugar and prevalence of overweight and obesity (BMI ≥25 kg/m2) in men (open squares) and women (filled circles)(35). Monthly intake values labelled as imprecise in the TATA-NIN dashboard(35) were removed from this analysis.
For both intakes of 'all meat and egg' (includes eggs, fish/prawns and meats: chicken, beef, pork and all other kinds of meat consumed) (Figure 2D) and ‘pulses’ (includes all pulses/legumes and legume products consumed) (Figure 2E), we observed positive association of intakes with the prevalence of high BMI. This was expected as protein foods (meats and eggs represent non-vegetarian sources and legumes represent vegetarian sources) account for the largest share of the food budget(41, 42), and higher intake levels are likely due to higher SES.
Our analysis also unexpectedly identified a female-specific positive association between intake of 'sugar' (includes sugar distributed through the public distribution system, honey, candy, jaggery and rock sugar) with the prevalence of high BMI (Figure 2F). Of note, this was seen despite a similar mean monthly sugar intake for men (range of mean monthly intake: 591-2262 g) and women (range of mean monthly intake: 483-1874 g). This could be based on findings of Chukijrungroat et al.(43) in rats due to a higher likelihood of women being prone to hepatic steatosis via a higher capacity for de novo lipogenesis from sugar (sucrose, broken down into glucose and fructose in our body). Female rats exhibited significantly higher levels of hepatic steatosis but less hepatic inflammation than male rats after consuming a high-fat, high-fructose diet for 12 weeks(43). Despite the strong evidence of whole-body metabolism being sexually dimorphic, studies on human metabolism are usually done on subjects from only one sex, primarily males(44, 45). Therefore, further in-depth exploration of these findings, using study designs that compare the metabolic effects of various foods on body weight regulation between men and women, will be needed for understanding the underlying causes and appropriate remedial measures.
Beyond the kind of foods consumed, the level of processing of foods plays a significant role in overall caloric intake and health outcomes. In mice, body mass increased on the consumption of cooked meat compared to uncooked meat, and this increase was not attributable to differences in activity or intake levels(46). Similarly, in an inpatient, randomised controlled trial on 20 weight stable adults, Hall et al. reported exposure to an ultra-processed diet for 14 days resulted in increased ad libitum energy intake and weight gain when compared to exposure to an unprocessed diet for an equal period, even after matching the two diets in calories, fat, sugar, fibre sodium and macronutrients(46). A recent systematic review and meta-analysis concluded that consumption of ultra-processed foods and health status were associated with a worse cardiometabolic profile (including significantly increased risk of obesity) and a higher risk of CVD and all-cause mortality(47). Pandav et al. have recently highlighted the accelerating growth of the market for packaged junk foods and soft drinks in India; the retail value of these products went up by 42 times between 2006 and 2019(48). Singh et al. reported in 2021 that amongst 13,274 Indian children aged 9-14 years, 53% consumed packed foods or packaged sweetened beverages at least once a day(49).
4.2. Adiposity
Beyond body weight, BMI is a robust and easy measure of adiposity(50, 51). However, adiposity measured as BMI is susceptible to misinterpretations in individuals with high physical activity levels or when generalised between ethnic groups(52, 53). Regional fat content and distribution influence metabolic health more significantly (54). Central adiposity or centralised fat distribution, as measured by waist circumference, waist-to-hip ratio or waist-to-height ratio, are better predictors of CVD risk than BMI in populations as diverse as original from Europe, the USA, Thailand and Peru(55–59).
The latest NFHS survey (NFHS – 5, 2019-2021) reported the prevalence of adults with a high-risk waist-to-hip ratio (women ≥0.85 and men ≥0.90) for the first time(11). More than half the women (56.7%) and nearly half of the men (47.7%) surveyed had a high-risk waist-to-hip ratio.
These numbers emphasise the potential contribution and need for a better understanding of the role of central obesity in metabolic health amongst Indians.
Indians have lower muscle mass and higher body fat content than other ethnicities(60). This results in BMI being a relatively insensitive cardiometabolic risk predictor of overweight in Indians. Two decades back, Dudeja et al. (61) reported 15% of males and 27% of females as overweight and obese when using BMI ≥25kg/m2 as a cut-off, which leapt up to 35% of males (2.3 times) and 89% of females (3.3 times) as overweight when they used percentage body fat as cut-off (25% for males and 30% for females)(62, 63). Based on these observations, recommendations were made to change the BMI cut-offs for Indians to BMI ≥23 kg/m2 for overweight and >30 kg/m2 for obese, respectively(64). However, these modified BMI cut-offs for Indians are yet to be widely adopted, likely either due to a lack of awareness or to maintain uniformity when collecting data, to be able to compare anthropometric and clinical characteristics of Indians with those of other ethnicities.
Though in absolute terms, Indian neonates with birth weights appropriate for gestational age (AGA) do not have a higher fat mass than those from other populations, both AGA neonates and those with birth weights small for gestational age (SGA) are much better at preserving fat mass, but not fat-free mass(65, 66). In Indian children, the progressive increase in adiposity was related to the nutritional status of the mothers during pregnancy, parental health and genetics(67, 68).
Comorbidities of obesities, such as insulin resistance(69, 70) and CVD risk(71, 72), appear at lower BMI in Indians than in other ethnic groups. Migration to developed countries with a western obesogenic environment further exacerbates these problems(73), with multiple existing reports of higher levels of obesity and associated coronary risk factors in Indians living in those countries, compared to those in India(74, 75). Lifestyle factors leaning toward positive energy balance, either through the reduction in physical activity levels or an increase in calorie consumption in Indians, therefore, can easily tip the balance towards increased risk of metabolic syndrome and associated NCDs.
4.3. Genetics
The genetic component of obesity, as measured by heritability estimates (proportion of phenotypic variation attributed to genetic variation), is substantial. It ranges from 40 to 70%, with the highest estimates from monozygotic twin studies(76). The genetic basis of monogenic forms of obesity, both syndromic and non-syndromic, has been successfully identified(77). However, the ever-increasing number of obesity-susceptibility loci [~1000 Single nucleotide polymorphisms (SNPs)] can still explain only ~10% of the heritability of obesity, underlying the need for further technological advances and larger sample sizes to identify the large remaining heritability of obesity(60).
Heritability estimates of obesity have shown minimal ethnicity-based differences(76). Reports from large-scale genome-wide association studies (GWAS) in Indians are only now becoming available, which could shed light on any India-specific loci contributing to obesity. Though genetic loci reported being associated with obesity specifically in the Indian population are not widespread, a few novel ones have recently been reported: rs1526538, an intronic SNP of THSD7A(78), rs6913677 in BAI3, rs2078267 SLC22A11 and rs8100011 in ZNF45(79).
Compounded with obesity, obesity-associated complications and comorbidities are also heritable and have ethnicity-specific components. Indian- or South Asian-specific novel genetic loci associated with multiple comorbidities of obesity, such as T2DM (five intronic SNPs: rs9552911 in SGCG(80), rs998451 in TMEM163(81), rs16861329 in ST6GAL1, rs7178572 in HMG20A and rs4812829 in HNF4A(82); rs3923113 located near GRB14 and rs1802295 located in the exonic region of VPS26A(82)), glycaemic control (a missense variant in G6PD and a non-coding variant near PIEZ01 associated with HbA1c levels(83)) and heart failure (a deletion of 25 bp in MYBPC3(84)) have been reported. Whole-exome sequencing in individuals with low BMI but NAFLD and controls revealed variants in Phosphatidylethanolamine N-methyltransferase (PEMT) and Oxysterol-binding protein-related protein 10 (OSBPL10), which have a role in dietary choline and cholesterol homeostasis, respectively. They could potentially be used to identify the exacerbated risk of NAFLD(85). Few obesity traits were mapped to the intronic region of Alpha-Ketoglutarate Dependent Dioxygenase (FTO) (rs9939609) and Melanocortin 4 receptor (MC4R) (rs17782313) by Vasan et al.(86). Also, among the 32 susceptible genetic variants to predict T2DM and related comorbidities, Janipalli et al. found that MC4R showed the most association with BMI(87). Genetic variants in intronic regions of the genes Rhomboid domain containing 1 (RHBDD1) (rs2177596), Mitogen-activated protein kinase 1 (MAPK1) (rs17759796), FTO (rs1121980) and MC4R (rs6567160) were associated with BMI and Alzheimer's disease(88). These trends might indicate that whereas the Indian population might not be more genetically susceptible to obesity than other populations, they might have genetic trains that might make them more susceptible to cardiometabolic complications. Future large-scale GWAS studies incorporating in-depth phenotyping of the subjects will shed further light on India-specific obesity-susceptible loci and their role in the aetiology of obesity and its complications in Indians.
4.4. The gut microbiome
Animal model studies have shown that the obesity-associated gut microbiome has a higher capacity for energy harvest from food and that the colonisation of germ-free mice can transmit this trait with 'obese microbiota'(89). An obese-type gut microbial profile associated with altered bacterial genes and metabolic pathways and reduced bacterial diversity has also been reported for humans (89). However, considering the large inter-individual variability of human gut microbial profiles and multiple confounding factors, such as diet, antibiotic intake, stool consistency, and many others, consistency has been lacking amongst reports of a human 'obese-type' gut microbial profile(90).
The Indian gut microbiome has its peculiarities, being characterised by a community rich in genera Prevotella, Bacteroides, Roseburia and Megasphaera(90–93). This flora reflects the predominantly plant-based, whole-grain carbohydrate and fibre-rich habitual diets of Indians(94). As far as gut microbial profiles of obesity amongst Indians is concerned, very few studies have been reported. Patil et al. reported prominence of genus Bacteroides and high archaeal density with elevated faecal short-chain fatty acids (SCFA) levels among individuals with obesity(95).
Levels of faecal Faecalibacterium prausnitzii were higher in 11-14-year-old Indian children with obesity than in the non-obese children(96).
Harnessing the power of gut microbiome modulation for managing and controlling obesity is still in its infancy and will need many future studies focusing on identifying and controlling the confounders of obesity-gut microbiome associations(94). Stool consistency and stool transit time explain the lion's share of interindividual(94) and temporal intraindividual variation in human gut microbial profiles(94). These factors are heavily influenced by dietary composition, especially the soluble and insoluble fibre content of food(97), which varies considerably in different parts of India(92). In-depth studies linking geographically regular diets, associated gut microbial profiles and the prevalence of obesity in other parts of India are needed to understand the link between diet, the gut microbiome and obesity in Indians.
4.5. Chronic systemic inflammation: Role of pollution
Obesity and its associated metabolic disorders, T2D and CVD, are now well known to be associated with chronic systemic inflammation (CSI)(97, 98). Multiple studies have reported the existence of CSI in overweight Indians, with or without T2DM(99–102). Though pro-inflammatory cytokines are known to increase energy expenditure, evident in exercise-induced inflammation or cancer-associated inflammation leading to cachexia, obesity is considered a state of ‘inflammation resistance’ whereby the increased energy expenditure response to pro-inflammatory cytokines is blunted(103). As such, CSI might be linked to body composition, preferential expansion of intraabdominal adipose tissue depots, harmful effects of ectopic lipotoxicity, or severe pro-inflammatory responses induced by nutritional overload.
Another factor of great relevance to India is ambient air pollution. The localised inflammatory response in the lungs driven by exposure to fine particulate matter (PM2.5) causes adipose tissue dysfunction in animal models (loss of brown adipose tissue and their transition toward a white adipose phenotype; increased inflammation, insulin resistance and lipolysis in white adipose tissue), thereby leading to vascular inflammation and endothelial cell insulin resistance(104). A few studies have reported associations between either gases or particulate matter and markers of CSI in Indians(105, 106), underlining the role of air quality on CSI and, in turn, on cardiometabolic health. Pollution is a matter of grave concern as Indian cities, such as New Delhi, are routinely featured among the most polluted cities in the world(107). Factors apart from air pollutants contributing to CSI in Indians need to be systematically delineated to add to the arsenal of preventing measures in the fight against obesity and related disorders.
4.6. The built environment and physical activity
Among South Asian countries, India had the highest (34%) prevalence of insufficient physical activity in 2016(108). The ICMR-INDIAB study conducted in 2014 reported that more than half of Indians are inactive, and inactivity is more prevalent in urban areas and among females(109). Against this backdrop, it is a matter of grave concern that the rapid economic transition in India has brought about unprecedented levels of rural to urban migration(110, 111).
The Indian cities have seen rapid expansions in the last couple of decades due to the migration of people into cities searching for better opportunities(112, 113). According to the World Urbanization Prospects 2018 published by the United Nations, >50% of the Indian population will live in urban areas by 2046(114). The infrastructure of these cities was not built to accommodate and withstand such a rapid onslaught(113). This is leading to the accelerated shrinking of urban green spaces, including public parks, walkable roads, and sidewalks(115, 116). Access to urban green spaces is a well-appreciated factor in increasing physical activity levels, reducing the risk of obesity and CVD, and improving the overall health of the residents(117–119).
Such rapid expansions have also contributed to longer commute times, further eating into the availability of time to increase physical activity levels(120). Additionally, a mismatch between rapid urbanisation and a slower pace of development of public transport systems is leading to increasing use of personal doorstep transport options(121), and increased usage of public transport has been reported to be associated with a decrease in the prevalence of obesity(122). A combination of these factors is likely to have an ever-worsening effect on the metabolic health of Indians unless remedial measures are sought, planned, and implemented at least at the same rate at which urbanisation is happening in this country.
5. Conclusion
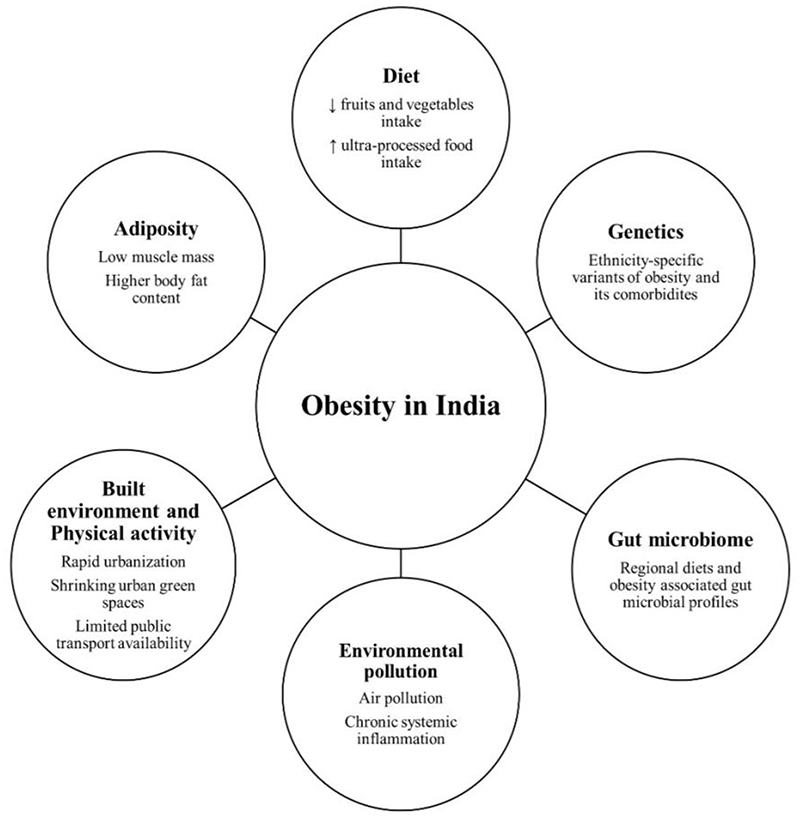
The second-most populous country in the world is becoming fat, and fast. This review has delineated this country's unique facets of obesity (Figure 3). India and many other developing countries are witnessing rapid economic and associated nutrition and health transition(123). Jaacks et al. have proposed a four-stage model of obesity, and India is in stage 1 of that model. India’s obesity is characterised by a higher prevalence of obesity in women than men, in those with higher SES than in lower SES, and in adults compared to children(124). Therefore, the worst seems yet to be as far as the epidemic of obesity in India is concerned. Progress to a high prevalence of obesity and other NCDs as part of economic progress is not predetermined(125). As outlined for CVDs, one of the NCDs associated with obesity, a comprehensive public health approach can stem this progress(126).
Figure 3. Framework of obesity in India.
Overall framework of obesity in India and its risk factors.
The next obvious question is what needs to be done to contain the escalating obesity epidemic in India. At a micro level, large-scale funding needs to be made available for in-depth research into the molecular characterisation of the peculiarities of obesity, adiposity and increased susceptibility to comorbidities in Indians and its correlation with human whole-body physiology and epidemiological studies. Support systems, including improved public transport and infrastructure and urban green spaces, must be available at a macro-social level. Awareness programs must be planned and conducted in schools, workplaces, and communities. Further, insights into the effectiveness of these programs through implementation research also need to be encouraged. Only such a multi-pronged approach can succeed in stemming this tide of the rapid increase in obesity amongst Indians.
Acknowledgement
No specific funding was received for this work. AM is supported by the Wellcome Trust/DBT India Alliance Fellowship [grant number IA/CPHI/19/1/504593].
Abbreviations
- T2DM
Type 2 Diabetes Mellitus
- CVD
Cardiovascular Disease
- SES
Socio-Economic Status
- BMI
Body Mass Index
- NCD
Non-Communicable Disease
- WHO
World Health Organization
- DALY
Disability-Adjusted Life - Year
- NAFLD
Non-Alcoholic Fatty Liver Disease
- GDP
Gross Domestic Product
- NFHS
National Family Health Survey
- RDA
Recommended Daily Allowance
- ICMR
Indian Council of Medical Research
- NIN
National Institute of Nutrition
- EAR
Estimated Average Requirement
- CES
Consumption Expenditure Survey
- AGA
Appropriate for Gestational Age
- SGA
Small for Gestational Age
- SNP
Single Nucleotide Polymorphism
- GWAS
Genome-Wide Association Study
- SCFA
Short-Chain Fatty Acid
- CSI
Chronic Systemic Inflammation
Footnotes
Declarations of interest
The authors declare no conflict of interest.
Author contributions
The authors' contributions to the manuscript are as follows – AM and AV-P: conceptualised the idea for this review; NN, SA and PCN: wrote the manuscript; NN and SA: analysed data and prepared the tables and figures. All authors critically reviewed the manuscript, and read and approved the final version.
References
- 1.World Health Organization. Obesity and overweight. 2022. [WWW document]. URL https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.World Health Organization. Global status report on noncommunicable diseases 2014. doi: 10.1161/STROKEAHA.115.008097. [WWW document] URL https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf. [DOI] [PubMed]
- 3.Luhar S, Timæus IM, Jones R, et al. Forecasting the prevalence of overweight and obesity in India to 2040 Joe W (ed.) Joe W, editor. PLoS One. 2020;15:e0229438. doi: 10.1371/journal.pone.0229438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corsi DJ, Subramanian SV. Socioeconomic Gradients and Distribution of Diabetes, Hypertension, and Obesity in India. JAMA Netw Open. 2019;2:e190411. doi: 10.1001/jamanetworkopen.2019.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjani H, Mehreen T, Pradeepa R, et al. Epidemiology of childhood overweight &obesity in India: A systematic review. Indian J Med Res. 2016;143:160. doi: 10.4103/0971-5916.180203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahirwar R, Mondal PR. Prevalence of obesity in India: A systematic review. Diabetes Metab Syndr Clin Res Rev. 2019;13:318–321. doi: 10.1016/j.dsx.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Kalra S, Unnikrishnan A. Obesity in India: The weight of the nation. J Med Nutr Nutraceuticals. 2012;1:37. [Google Scholar]
- 8.International Institute for Population Sciences [IIPS], ICF. National Family Health Survey (NFSH-3), 2005-06. Mumbai: IIPS; [WWW document]. URL http://rchiips.org/nfhs/NFHS-3Data/VOL-2/Report-Volume-II(1632K).pdf. [Google Scholar]
- 9.International Institute for Population Sciences [IIPS], ICF. National Family Health Survey (NFHS-4), 2015-16. Mumbai: IIPS; [WWW document]. URL http://rchiips.org/nfhs/NFHS-4Reports/India.pdf. [Google Scholar]
- 10.International Institute for Population Sciences [IIPS], ICF. National Family Health Survey (NFHS-5), 2019-20. Key indicators 22 States/UTs from Phase-1. [WWW document]. URL https://main.mohfw.gov.in/sites/default/files/NFHS-5_Phase-I.pdf.
- 11.International Institute for Population Sciences [IIPS], ICF. National Family Health Survey (NFHS-5), 2019-21. Key indicators, India and 14 States/UTs (Phase-2) [WWW document]. URL https://main.mohfw.gov.in/sites/default/files/NFHS-5_Phase-II_0.pdf.
- 12.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016:a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie H, Roser M. Obesity. Our World Data. 2017 [Google Scholar]
- 14.Swain S, Chowdhury S. Trends of nutritional status among rural adults in six states of India: findings from national survey data. Clin Epidemiol Glob Heal. 2018;6:181–187. [Google Scholar]
- 15.Mohan V, Mathur P, Deepa R, et al. Urban rural differences in prevalence of self-reported diabetes in India-The WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80:159–168. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh KK, Sivakumar I, Saravanakumar N, Sathishkumar R. Regional disparities and Indian states: A macro level study. J Crit Rev. 2020;7 [Google Scholar]
- 17.Reserve Bank of India. Handbook of Statistics on Indian States 2020-21. [WWW document]. URL https://rbidocs.rbi.org.in/rdocs/Publications/PDFs/0HSIS241121FL7A6B5C0ECBC64B0ABF0A097B1AD40C83.PDF.
- 18.Singh G, Talwar I, Sharma R, Matharoo K, Bhanwer AJS. Genetic differentiation and population structure of five ethnic groups of Punjab (North-West India) Mol Genet Genomics. 2016;291:2055–2063. doi: 10.1007/s00438-016-1239-3. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Bhurtyal A, Wei J, Akhtar P, Wang L, Wang Y. Double Burden of Malnutrition and Nutrition Transition in Asia: A Case Study of 4 Selected Countries with Different Socioeconomic Development. Adv Nutr. 2020;11:1663–1670. doi: 10.1093/advances/nmaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Institute for Population Sciences [IIPS], ORC Macro. National Family Health Survey (NFHS-2), 1998-99. Mumbai: IIPS; 2000. [Google Scholar]
- 21.Young MF, Nguyen P, Tran LM, Avula R, Menon P. A double edged sword? Improvements in economic conditions over a decade in India led to declines in undernutrition as well as increases in overweight among adolescents and women. J Nutr. 2020;150:364–372. doi: 10.1093/jn/nxz251. [DOI] [PubMed] [Google Scholar]
- 22.Patel V, Chatterji S, Chisholm D, et al. Chronic diseases and injuries in India. Lancet. 2011;377:413–428. doi: 10.1016/S0140-6736(10)61188-9. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Noncommunicable Diseases Progress Monitor 2015. 2015 [Google Scholar]
- 24.World Health Organization. WHO methods and data sources for global burden of disease estimates 2000-2011. [WWW document]. URL https://www.academia.edu/8015723/Global_Health_Estimates_Technical_Paper_WHO_HIS_HSI_GHE_2013_4_Acknowledgments.
- 25.Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019;103:22–27. doi: 10.1097/TP.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 26.Shalimar Elhence A, Bansal B, et al. Prevalence of Non-alcoholic Fatty Liver Disease in India: A Systematic Review and Meta-analysis. J Clin Exp Hepatol. 2022;12:818–829. doi: 10.1016/j.jceh.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dandona L, Dandona R, Kumar GA, et al. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet. 2017;390:2437–2460. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okunogbe A, Nugent R, Spencer G, Ralston J, Wilding J. Economic impacts of overweight and obesity: current and future estimates for eight countries. BMJ Glob Heal. 2021;6 doi: 10.1136/bmjgh-2021-006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal P, Agrawal S. Health care expenditure associated with overweight/obesity: a study among urban married women in Delhi, India. Int J Community Med Public Heal. 2015:308–317. doi: 10.18203/2394-6040.ijcmph20150488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panda P, Ayyanar R, Boyanagari VK. Burden and cost of overweight and obesity in south India public health system. Obes Med. 2019;13:55–58. [Google Scholar]
- 31.ICMR. Nutrient Requirements for Indians, Recommended Daily Allowances (RDA) and Estimated Average Requirements (EAR). A Report of the Expert Group of the National Insitute of Nutrition (ICMR) [WWW document]. URL https://www.nin.res.in/RDA_short_Report_2020.html.
- 32.Indian Counsil of Medical Research (ICMR) Nutrient requirements and recommended dietary allowances for Indians. A report of the expert group of the ICMR. [WWW document]. URL https://www.nin.res.in/downloads/DietaryGuidelinesforNINwebsite.pdf.
- 33.Sharma M, Kishore A, Roy D, Joshi K. A comparison of the Indian diet with the EAT- Lancet reference diet. BMC Public Health. 2020;20:1–13. doi: 10.1186/s12889-020-08951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta N, Shah P, Goel K, et al. Imbalanced dietary profile, anthropometry, and lipids in urban asian indian adolescents and young adults. J Am Coll Nutr. 2010;29:81–91. doi: 10.1080/07315724.2010.10719820. [DOI] [PubMed] [Google Scholar]
- 35.Tata-NIN Centre of Excellence in Public Health Nutrition. The state of food and nutrition in India. 2022. [WWW document]. URL http://www.tatanin.org.
- 36.Choi S-W, Claycombe KJ, Martinez JA, Friso S, Schalinske KL. Nutritional epigenomics: a portal to disease prevention. Adv Nutr. 2013;4:530–532. doi: 10.3945/an.113.004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith CE, Tucker KL. Health benefits of cereal fibre: a review of clinical trials. Nutr Res Rev. 2011;24:118–131. doi: 10.1017/S0954422411000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sendhil R, Kiran Kumara TM, Ramasundaram P, Sinha M, Kharkwal S. Nutrition status in India: Dynamics and determinants. Glob Food Sec. 2020;26:100455 [Google Scholar]
- 39.Tak M, Shankar B, Kadiyala S. Dietary Transition in India: Temporal and Regional Trends, 1993 to 2012. Food Nutr Bull. 2019;40:254–270. doi: 10.1177/0379572119833856. [DOI] [PubMed] [Google Scholar]
- 40.Bhathal SK, Kaur H, Bains K, Mahal AK. Assessing intake and consumption level of spices among urban and rural households of Ludhiana district of Punjab, India. Nutr J. 2020;19:121. doi: 10.1186/s12937-020-00639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hess JM, Cifelli CJ, Agarwal S, Fulgoni VL. Comparing the cost of essential nutrients from different food sources in the American diet using NHANES 2011–2014. Nutr J. 2019;18:68. doi: 10.1186/s12937-019-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirvonen K, Bai Y, Headey D, Masters WA. Affordability of the EAT–Lancet reference diet: a global analysis. Lancet Glob Heal. 2020;8:e59–e66. doi: 10.1016/S2214-109X(19)30447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chukijrungroat N, Khamphaya T, Weerachayaphorn J, Songserm T, Saengsirisuwan V. Hepatic FGF21 mediates sex differences in high-fat high-fructose diet-induced fatty liver. Am J Physiol Metab. 2017;313:E203–E212. doi: 10.1152/ajpendo.00076.2017. [DOI] [PubMed] [Google Scholar]
- 44.Geidl-Flueck B, Hochuli M, Németh Á, et al. Fructose- and sucrose- but not glucose- sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J Hepatol. 2021;75:46–54. doi: 10.1016/j.jhep.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Faeh D, Minehira K, Schwarz J-M, Periasamy R, Park S, Tappy L. Effect of Fructose Overfeeding and Fish Oil Administration on Hepatic De Novo Lipogenesis and Insulin Sensitivity in Healthy Men. Diabetes. 2005;54:1907–1913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 46.Yengo L, Sidorenko J, Kemper KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125:308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandav C, Smith Taillie L, Miles DR, Hollingsworth BA, Popkin BM. The WHO South-East Asia Region Nutrient Profile Model Is Quite Appropriate for India: An Exploration of 31,516 Food Products. Nutrients. 2021;13:2799. doi: 10.3390/nu13082799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh SA, Dhanasekaran D, Ganamurali NLP, Sabarathinam S. Junk food-induced obesity-a growing threat to youngsters during the pandemic. Obes Med. 2021;26:100364. doi: 10.1016/j.obmed.2021.100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eknoyan G. Adolphe Quetelet (1796-1874)--the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- 51.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. Int J Epidemiol. 2014;43:655–65. doi: 10.1093/ije/dyu058. [DOI] [PubMed] [Google Scholar]
- 52.Rahman M, Berenson AB. Accuracy of current body mass index obesity classification for white, black, and Hispanic reproductive-age women. Obstet Gynecol. 2010;115:982–988. doi: 10.1097/AOG.0b013e3181da9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrido-Chamorro RP, Sirvent-Belando JE, Gonzalez-Lorenzo M, Martin-Carratala ML, Roche E. Correlation between body mass index and body composition in elite athletes. J Sports Med Phys Fitness. 2009;49:278–84. [PubMed] [Google Scholar]
- 54.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 55.van Dijk SB, Takken T, Prinsen EC, Wittink H. Different anthropometric adiposity measures and their association with cardiovascular disease risk factors: a meta-analysis. Netherlands Hear J. 2012;20:208–218. doi: 10.1007/s12471-011-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paniagua L, Lohsoonthorn V, Lertmaharit S, Jiamjarasrangsi W, Williams MA. Comparison of waist circumference, body mass index, percent body fat and other measure of adiposity in identifying cardiovascular disease risks among Thai adults. Obes Res Clin Pract. 2008;2:215–223. doi: 10.1016/j.orcp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menke A, Muntner P, Wildman RP, Reynolds K, He J. Measures of adiposity and cardiovascular disease risk factors. Obesity (Silver Spring) 2007;15:785–95. doi: 10.1038/oby.2007.593. [DOI] [PubMed] [Google Scholar]
- 58.Wai WS, Dhami RS, Gelaye B, et al. Comparison of Measures of Adiposity in Identifying Cardiovascular Disease Risk Among Ethiopian Adults. Obesity. 2012;20:1887–1895. doi: 10.1038/oby.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knowles KM, Paiva LL, Sanchez SE, et al. Waist Circumference, Body Mass Index, and Other Measures of Adiposity in Predicting Cardiovascular Disease Risk Factors among Peruvian Adults. Int J Hypertens. 2011;2011:1–10. doi: 10.4061/2011/931402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102:632. doi: 10.1017/S0007114508207221. [DOI] [PubMed] [Google Scholar]
- 61.Dudeja V, Misra A, Pandey RM, Devina G, Kumar G, Vikram NK. BMI does not accurately predict overweight in Asian Indians in northern India. Br J Nutr. 2001;86:105–112. doi: 10.1079/bjn2001382. [DOI] [PubMed] [Google Scholar]
- 62.Hortobágyi T, Israel RG, O’Brien KF. Sensitivity and specificity of the Quetelet index to assess obesity in men and women. Eur J Clin Nutr. 1994;48:369–75. [PubMed] [Google Scholar]
- 63.Pollock M, Wilmore J. Exercise in health and disease; Evaluation and Prescription for Prevention and Rehabilitation. 2nd Editio. Philadelphia: WB Saunders Company; 1990. [Google Scholar]
- 64.Misra A, Chowbey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 65.Wells CJ. Reconsidering the “Thin-Fat” Indian Neonate. J Nutr. 2020;150:658–660. doi: 10.1093/jn/nxaa026. [DOI] [PubMed] [Google Scholar]
- 66.Kuriyan R, Naqvi S, Bhat KG, et al. The Thin But Fat Phenotype is Uncommon at Birth in Indian Babies. J Nutr. 2020;150:826–832. doi: 10.1093/jn/nxz305. [DOI] [PubMed] [Google Scholar]
- 67.Yajnik CS, Deshmukh US. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev Endocr Metab Disord. 2008;9:203. doi: 10.1007/s11154-008-9087-z. [DOI] [PubMed] [Google Scholar]
- 68.Yajnik CS. Early Life Origins of Insulin Resistance and Type 2 Diabetes in India and Other Asian Countries. J Nutr. 2004;134:205–210. doi: 10.1093/jn/134.1.205. [DOI] [PubMed] [Google Scholar]
- 69.Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:2329–35. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 70.Raji A, Seely EW, Arky RA, Simonson DC. Body Fat Distribution and Insulin Resistance in Healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 71.Patel SA, Deepa M, Shivashankar R, et al. Comparison of multiple obesity indices for cardiovascular disease risk classification in South Asian adults: The CARRS Study. Kiechl S, editor. PLoS One. 2017;12:e0174251. doi: 10.1371/journal.pone.0174251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pais P, Pogue J, Gerstein H, et al. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet. 1996;348:358–363. doi: 10.1016/s0140-6736(96)02507-x. [DOI] [PubMed] [Google Scholar]
- 73.Misra A, Ganda OP. Migration and its impact on adiposity and type 2 diabetes. Nutrition. 2007;23:696–708. doi: 10.1016/j.nut.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Patel DJ, Winterbotham M, Britt RP, et al. Coronary risk factors in people from the Indian subcontinent living in West London and their siblings in India. Lancet. 1995;345:405–409. doi: 10.1016/s0140-6736(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 75.Patel JV, Vyas A, Cruickshank JK, et al. Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis. 2006;185:297–306. doi: 10.1016/j.atherosclerosis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 76.Stryjecki C, Alyass A, Meyre D. Ethnic and population differences in the genetic predisposition to human obesity. Obes Rev. 2018;19:62–80. doi: 10.1111/obr.12604. [DOI] [PubMed] [Google Scholar]
- 77.Fairbrother U, Kidd E, Malagamuwa T, Walley A. Genetics of Severe Obesity. Curr Diab Rep. 2018;18:85. doi: 10.1007/s11892-018-1053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nizamuddin S, Govindaraj P, Saxena S, et al. A novel gene THSD7A is associated with obesity. Int J Obes. 2015;39:1662–1665. doi: 10.1038/ijo.2015.144. [DOI] [PubMed] [Google Scholar]
- 79.Giri AK, Prasad G, Bandesh K, et al. Multifaceted genome-wide study identifies novel regulatory loci in SLC22A11 and ZNF45 for body mass index in Indians. Mol Genet Genomics. 2020;295:1013–1026. doi: 10.1007/s00438-020-01678-6. [DOI] [PubMed] [Google Scholar]
- 80.Saxena R, Saleheen D, Been LF, et al. Genome-Wide Association Study Identifies a Novel Locus Contributing to Type 2 Diabetes Susceptibility in Sikhs of Punjabi Origin From India. Diabetes. 2013;62:1746–1755. doi: 10.2337/db12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabassum R, Chauhan G, Dwivedi OP, et al. Genome-Wide Association Study for Type 2 Diabetes in Indians Identifies a New Susceptibility Locus at 2q21. Diabetes. 2013;62:977–986. doi: 10.2337/db12-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kooner JS, Saleheen D, Sim X, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Q, Graff M, Rowland B, et al. Analyses of biomarker traits in diverse UK biobank participants identify associations missed by European-centric analysis strategies. J Hum Genet. 2022;67:87–93. doi: 10.1038/s10038-021-00968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dhandapany PS, Sadayappan S, Xue Y, et al. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41:187–191. doi: 10.1038/ng.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bale G, Vishnubhotla RV, Mitnala S, et al. Whole-Exome Sequencing Identifies a Variant in Phosphatidylethanolamine N-Methyltransferase Gene to be Associated With Lean-Nonalcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2019;9:561–568. doi: 10.1016/j.jceh.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vasan SK, Fall T, Neville MJ, et al. Associations of Variants in FTO and Near MC4R With Obesity Traits in South Asian Indians. Obesity. 2012;20:2268–2277. doi: 10.1038/oby.2012.64. [DOI] [PubMed] [Google Scholar]
- 87.Janipalli CS, Kumar MVK, Vinay DG, et al. Analysis of 32 common susceptibility genetic variants and their combined effect in predicting risk of Type 2 diabetes and related traits in Indians. Diabet Med. 2012;29:121–127. doi: 10.1111/j.1464-5491.2011.03438.x. [DOI] [PubMed] [Google Scholar]
- 88.D’Silva S, Chakraborty S, Kahali B. Concurrent outcomes from multiple approaches of epistasis analysis for human body mass index associated loci provide insights into obesity biology. Sci Rep. 2022;12:7306. doi: 10.1038/s41598-022-11270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 90.Castaner O, Goday A, Park Y-M, et al. The Gut Microbiome Profile in Obesity: A Systematic Review. Int J Endocrinol. 2018;2018:1–9. doi: 10.1155/2018/4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jain A, Li XH, Chen WN. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express. 2018;8:104. doi: 10.1186/s13568-018-0632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhakan DB, Maji A, Sharma AK, et al. The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. Gigascience. 2019;8 doi: 10.1093/gigascience/giz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tandon D, Haque MMRS, et al. A snapshot of gut microbiota of an adult urban population from Western region of India. Arora PK, editor. PLoS One. 2018;13:e0195643. doi: 10.1371/journal.pone.0195643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulkarni AS, Kumbhare SV, Dhotre DP, Shouche YS. Mining the Core Gut Microbiome from a Sample Indian Population. Indian J Microbiol. 2019;59:90–95. doi: 10.1007/s12088-018-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patil DP, Dhotre DP, Chavan SG, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–657. doi: 10.1007/s12038-012-9244-0. [DOI] [PubMed] [Google Scholar]
- 96.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AMS, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010;103:335–338. doi: 10.1017/S0007114509992182. [DOI] [PubMed] [Google Scholar]
- 97.Burkitt D, Walker AR, Painter N. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;300:1408–1411. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 98.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asegaonkar SB, Marathe A, Tekade ML, et al. High-sensitivity C-reactive protein: a novel cardiovascular risk predictor in type 2 diabetics with normal lipid profile. J Diabetes Complications. 2011;25:368–370. doi: 10.1016/j.jdiacomp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 100.Bandyopadhyay R, Paul R, Basu AK, Chakraborty PP, Mitra S. Study of C Reactive Protein in Type 2 Diabetes and its Relation with Various Complications from Eastern India. J Appl Pharm Sci. 3:156–159. 1AD. [Google Scholar]
- 101.Vikram N. Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis. 2003;168:305–313. doi: 10.1016/s0021-9150(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 102.Wasir JS, Misra A, Vikram NK, Pandey RM, Luthra K. C-reactive protein, obesity, and insulin resistance in postmenopausal women in urban slums of North India. Diabetes Metab Syndr Clin Res Rev. 2007;1:83–89. [Google Scholar]
- 103.Wang H, Ye J. Regulation of energy balance by inflammation: Common theme in physiology and pathology. Rev Endocr Metab Disord. 2015;16:47–54. doi: 10.1007/s11154-014-9306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Della Guardia L, Shin AC. The role of adipose tissue dysfunction in PM 2.5 -induced vascular pathology. Am J Physiol Circ Physiol. 2022;322:H971–H972. doi: 10.1152/ajpheart.00156.2022. [DOI] [PubMed] [Google Scholar]
- 105.Khafaie MA, Salvi SS, Ojha A, Khafaie B, Gore SS, Yajnik CS. Systemic inflammation (C-reactive protein) in type 2 diabetic patients is associated with ambient air pollution in Pune City, India. Diabetes Care. 2013;36:625–630. doi: 10.2337/dc12-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elango N, Kasi V, Vembhu B, Poornima JG. Chronic exposure to emissions from photocopiers in copy shops causes oxidative stress and systematic inflammation among photocopier operators in India. Environ Heal. 2013;12(1):1–12. doi: 10.1186/1476-069X-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.IQ Air. IQ Air - World’s most polluted cities (historical data 2017-2021) 2022. [WWW document]. URL https://www.iqair.com/in-en/world-most-polluted-cities.
- 108.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Heal. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 109.Anjana RM, Pradeepa R, Das AK, et al. Physical activity and inactivity patterns in India - results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5] Int J Behav Nutr Phys Act. 2014;11 doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitra A, Murayama M. Rural to Urban Migration: A District-Level Analysis for India. Int J Migr Heal Soc Care. 2009;5:35–52. [Google Scholar]
- 111.Imbert C, Papp J. Costs and benefits of rural-urban migration: Evidence from India. J Dev Econ. 2020;146:102473 [Google Scholar]
- 112.Sultana S, Satyanarayana ANV. Urban heat island intensity during winter over metropolitan cities of India using remote-sensing techniques: impact of urbanization. Int J Remote Sens. 2018;39:6692–6730. [Google Scholar]
- 113.Sun L, Chen J, Li Q, Huang D. Dramatic uneven urbanization of large cities throughout the world in recent decades. Nat Commun. 2020;11:5366. doi: 10.1038/s41467-020-19158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.United Nations. United Nations, Department of Economic and Social Affairs, Population Division (2018). World urbanization prospects: The 2018 revision. New York: United Nations, Department of Economic and Social Affairs (DESA), Population Division, Population Estimates; 2022. [WWW document]. URL https://population.un.org/wup/Country-Profiles/ [Google Scholar]
- 115.Ramaiah M, Avtar R. Urban Green Spaces and Their Need in Cities of Rapidly Urbanizing India: A Review. Urban Sci. 2019;3:94. [Google Scholar]
- 116.Rigolon A, Browning M, Lee K, Shin S. Access to Urban Green Space in Cities of the Global South: A Systematic Literature Review. Urban Sci. 2018;2:67. [Google Scholar]
- 117.Kondo M, Fluehr J, McKeon T, Branas C. Urban Green Space and Its Impact on Human Health. Int J Environ Res Public Health. 2018;15:445. doi: 10.3390/ijerph15030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seo S, Choi S, Kim K, Kim SM, Park SM. Association between urban green space and the risk of cardiovascular disease: A longitudinal study in seven Korean metropolitan areas. Environ Int. 2019;125:51–57. doi: 10.1016/j.envint.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 119.Wang H, Dai X, Wu J, Wu X, Nie X. Influence of urban green open space on residents’ physical activity in China. BMC Public Health. 2019;19:1093. doi: 10.1186/s12889-019-7416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pucher J, Peng Z, Mittal N, Zhu Y, Korattyswaroopam N. Urban Transport Trends and Policies in China and India: Impacts of Rapid Economic Growth. Transp Rev. 2007;27:379–410. [Google Scholar]
- 121.Singh SK. Urban Transport in India: Issues, Challenges, and the Way Forward. 2012 [Google Scholar]
- 122.She Z, King DM, Jacobson SH. Analyzing the impact of public transit usage on obesity. Prev Med (Baltim) 2017;99:264–268. doi: 10.1016/j.ypmed.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 123.Bishwajit G. Nutrition transition in South Asia: the emergence of non-communicable chronic diseases. F1000Research. 2015;4:8. doi: 10.12688/f1000research.5732.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Popkin BM, Ng SW. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev. 2022;23 doi: 10.1111/obr.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–237. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]