Abstract
A role for vitamin D in immune modulation and in cancer has been suggested. Here, we report that mice with increased availability of vitamin D display greater immune-dependent resistance to transplantable cancers and augmented responses to checkpoint blockade immunotherapies. Similarly, in humans, vitamin D-induced genes correlate with improved responses to immune checkpoint inhibitor treatment, as well as with immunity to cancer and increased overall survival. In mice, resistance is attributable to the activity of vitamin D on intestinal epithelial cells, which alters microbiome composition, favoring Bacteroides fragilis that positively regulate cancer immunity. Our findings indicate a previously unappreciated connection between vitamin D, microbial commensal communities and immune responses to cancer. Collectively, they highlight vitamin D levels as a potential determinant of cancer immunity and immunotherapy success.
The micronutrient vitamin D has an important role in immune modulation and in shaping commensal microbial communities (1–6). Vitamin D has also been studied for its potential role in cancer, with studies showing it can decrease cancer cell proliferation, promote apoptosis, reduce angiogenesis (7–9), and dampen the pro-tumorigenic activity of cancer-associated fibroblasts (10, 11). In some but not all studies, higher blood levels or increased dietary intake of vitamin D have been correlated with a lower incidence of colorectal, breast, prostate and pancreatic tumors and/or decreased cancer mortality (12–21). However, to what extent the activity of vitamin D impacts cancer development, and whether this involves the immune system and/or the microbiome, remains unclear.
Vitamin D (calciferol) is a term that includes both vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) forms of the vitamin. Vitamin D3 is derived from animal-sourced foods or is produced by skin in response to ultraviolet radiation whereas vitamin D2 is derived from plants and fungi (22). Irrespective of source, both vitamin D2 and D3 are converted in the liver and other tissues to 25-hydroxyvitamin D [25-OHD], the main circulating form of vitamin D (22). 25-OHD is then converted primarily in kidney to 1,25-dihydroxy-vitamin D [1,25-(OH)2D], which can bind to vitamin D receptor (VDR) to regulate expression of vitamin D-responsive genes (22). Notably, vitamin D and its 25-OHD and 1,25-(OH)2D metabolites (collectively called VitD henceforth) are bound by the blood carrier protein “group-specific component” (Gc) globulin, also known as vitamin D binding protein. Gc possesses a domain at its N-terminus with high affinity for 25-OHD and lower affinity for its precursor calciferol and for 1,25-(OH)2D (23, 24). Gc binding sequesters VitD, principally 25-OHD, away from tissues, acting as a blood reservoir (24, 25). Despite the prominent role of VitD in calcium homeostasis, Gc-/- mice (and a rare human patient displaying bi-allelic GC loss) do not display bone abnormalities (e.g., rickets or osteomalacia) associated with VitD deficiency (24, 26). Rather, animals lacking Gc globulin display low levels of VitD in blood, which results in more rapid and profound tissue responses to VitD at the expense of low buffering capacity (24).
Cross-presentation of tumor antigens by type 1 conventional dendritic cells (cDC1) is critical for generating anti-cancer CD8+ T cells (27, 28). In mice and humans, cDC1 express DNGR-1 (a.k.a. CLEC9A), a receptor that binds to F-actin exposed by dying cells and promotes cross-presentation of antigens within the corpses (29, 30). Previously, we showed that secreted gelsolin (sGSN), an extracellular protein that circulates in plasma and is secreted by tumor cells, severs F-actin and blocks DNGR-1 ligand binding, dampening anti-cancer immunity and the efficacy of immunogenic anti-cancer therapies (31, 32). Interestingly, Gc globulin possesses a C-terminal actin-binding domain and functions as an actin scavenging protein in partnership with sGSN, a role that is independent of VitD buffering (33). We therefore set out to test whether, like sGSN, Gc acts as a barrier to anti-cancer CD8+ T cell responses. Here, we show that this is indeed the case but that it is not attributable to actin scavenging but to Gc regulation of VitD availability. We uncover a complex interplay whereby increased VitD levels promote responses from intestinal epithelial cells that modulate the gut microbiome, which in turn acts to potentiate anti-cancer immunity. Remarkably, the effect of increased VitD availability on immune-mediated resistance to cancer can be transferred in dominant fashion to microbiota-replete mice by transplantation of fecal matter or oral inoculation with the bacterium Bacteroides fragilis provided dietary vitamin D intake is maintained. In humans, we show that vitamin D levels correlate with lower cancer incidence and that hallmarks of VDR activity are associated with better disease outcomes in cancer patients and improved responses to checkpoint blockade immunotherapy. Overall, our data suggest that VitD can regulate the microbiome and anti-cancer immunity, with possible clinical and public health applications.
Gc-deficient mice display immune-dependent transmissible tumor resistance
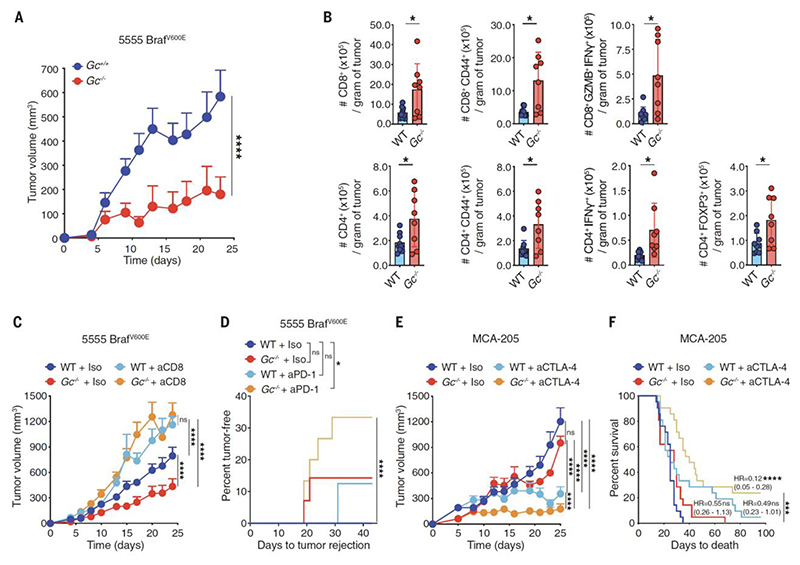
We set out to test whether Gc, like sGSN, acts as a barrier to anti-cancer immunity. We used the transplantable 5555 BrafV600E melanoma cell line, the growth of which is greatly attenuated in sGsn-/- mice (31) and examined its ability to grow in Gc-/- mice (24) vs. Gc +/+ littermate controls that were separated at weaning and housed in different cages. Gc-deficient mice (fully backcrossed to the C57BL/6J background) controlled the 5555 BrafV600E melanoma cell line significantly better than Gc-sufficient littermate controls (Fig. 1A) and displayed greater intra-tumoral accumulation of total and activated CD4+ and CD8+ T cells (Fig. 1B). The relative resistance of Gc-/- mice to 5555 BrafV600E melanoma was abrogated by antibody-mediated CD8+ T cell depletion (Fig. 1C). Additionally, Gc-/- mice bearing 5555 BrafV600E melanoma or MCA-205 fibrosarcoma tumors displayed greater responses to anti-PD1 and anti-CTLA-4 checkpoint blockade immunotherapies than C57BL6/J wild type (WT) mice (Fig. 1D-F). Thus, like sGsn-/-, Gc-/- mice exhibit enhanced CD8+ T cell-dependent resistance to transplantable tumors and superior responsiveness to checkpoint blockade immunotherapies.
Fig. 1. Loss of Gc increases CD8+ T cell dependent tumor control and augments response to immunotherapy.
(A) Growth profile of 0.2 x 106 5555 BrafV600E cancer cells implanted in separately housed groups of Gc-/- mice (n=8) and Gc+/+ littermate control mice (n=11). (B) Quantification of the indicated intratumoral immune cell populations in separately housed groups of WT C57BL/6J (n=9) or Gc-/- (n=8) mice at day 15 post-inoculation with 5555 BrafV600E cancer cells. Data are presented as number of cells per gram of tumor from two independent experiments. (C) As in (A) but mice received anti-CD8 antibody or isotype-matched control (300μg intraperitoneally (i.p.) on days -3, 1, 4, 7, 10, 13, 16, 19, 22). WT C57BL/6J + isotype (n=12), WT C57BL/6J + anti-CD8 (n=12), Gc-/- + isotype (n=14) and Gc-/- + anti-CD8 (n=13). (D) Percent of 5555 BrafV600E tumor rejection from two independent experiments in separately housed WT C57BL/6J or Gc-/- groups of mice that received anti-PD-1 monoclonal antibody or isotype-matched control (200μg i.p. every 3 days from day 3 to day 18). WT + isotype (n=15), WT + anti-PD-1 (n=16), Gc-/- + isotype (n=14), Gc-/- + anti-PD-1 (n=15). (E-F) Separately housed WT C57BL/6J or Gc-/- groups of mice implanted with 0.5 x 106 MCA-205 and given isotype-matched control or anti-CTLA-4 (50μg injected i.p. on days 6, 9 and 12). (E) Growth profile (n=10 mice per group). (F) Survival (Kaplan-Meier) curves from two independent experiments (n=21 mice per group). Data in (A, C and E) are presented as tumor volume (mm3) ± SEM and are representative of two independent experiments. Tumor growth profiles (A, C and E) were compared using Bonferroni-corrected two-way ANOVA. Groups in (B) were compared using two-tailed unpaired t test with Welch’s correction. Incidence of tumor rejection and survival (Kaplan-Meier) curves in (D and F) were compared using Log-rank (Mantel-Cox) test for comparison of each group with WT C57BL/6J + isotype and Log-rank for trend for comparison of all groups. In (F) hazard ratios (HR) with 95% confidence interval are shown in brackets, calculated as a ratio of each group / WT + isotype. *p<0.05, ***p< 0.001, ****p< 0.0001; ns, not significant.
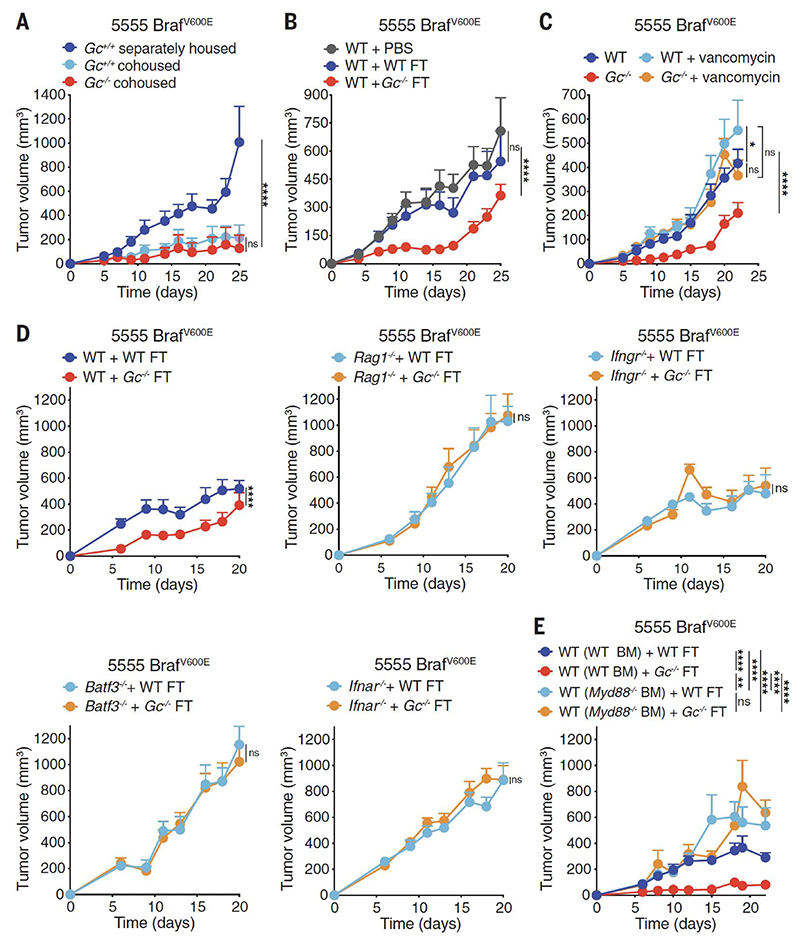
To control for possible differences in microbiota between Gc-/- mice and Gc +/+ controls separated at weaning, we repeated the experiments in Gc-/- and Gc +/+ littermates kept in the same cages. Intriguingly, co-housed Gc +/+ mice acquired the tumor resistance phenotype of their Gc-deficient littermates (Fig. 2A). Similarly, C57BL6/J WT mice (bred as an independent line) became more resistant to tumor challenge when co-housed with Gc-/- mice (Supplementary Fig. 1A). This transmissible tumor resistance was reversible as Gc +/+ littermate controls co-housed since birth with Gc-/- mice were less able to control tumors when separated for at least a month before tumor challenge (Fig. 1A and 2A). These data suggest that: a) Gc-/- and Gc +/+ mice exhibit genotype-driven divergence in microbiota composition, which dictates their differential ability to control tumors; b) the Gc-/--associated component of the microbiota that mediates tumor resistance can be transmitted in a dominant fashion to co-housed mice by coprophagy. Consistent with the latter, fecal transplant (FT) from Gc-/- donors into microbiota-replete C57BL/6 WT mice led to enhanced tumor control (Fig. 2B). Further, single administration of certain antibiotics (vancomycin, metronidazole or neomycin) inhibited or decreased the ability of Gc-/- mice to control transplantable tumors (Fig. 2C and Supplementary Fig. 1B).
Fig. 2. Fecal transplants from Gc-/- mice increase anti-cancer immunity.
(A-E) Growth profile of 0.2 x 106 5555 BrafV600E cancer cells implanted into: (A) Separately housed Gc+/+ (n=12) and co-housed Gc+/+ (n=7) and Gc-/- (n=6) groups of mice. (B) Separately housed groups of WT C57BL/6J mice (n=10 per group) that received orally PBS or fecal transplant (FT) from WT or Gc-/- donors twice (days -14 and - 12) before tumor inoculation (day 0). (C) Separately housed groups of WT C57BL/6J or Gc-/- mice that received or not vancomycin (0.5 g/L) in the drinking water starting from 2 weeks prior to tumor inoculation. WT (n=11), WT + vancomycin (n=10), Gc-/- (n=11), Gc-/- + vancomycin (n=10). (D-E) the indicated separately housed groups of mice that received oral FT from WT C57BL/6J or Gc-/- donors twice (days -14 and -12) prior to tumor inoculation (day 0). (D) WT (n=11 per group), Rag1-/- (n=9 per group), Ifngr1-/- (n=10 per group), Batf3-/- (n=10) and Ifnar-/- (n=10 per group) mice, (E) Irradiated CD45.1 WT mice reconstituted using bone marrow (BM) from CD45.2 WT or Myd88-/- donors. WT (WT BM) + WT FT (n=11), WT (WT BM) + Gc-/- FT (n=12), WT (Myd88-/- BM) + WT FT (n=10), WT (Myd88-/- BM) Gc-/- FT (n=10). Data in (A-E) are presented as tumor volume (mm3) ± SEM and are representative of two independent experiments. Tumor growth profiles (A-E) were compared using Bonferroni-corrected two-way ANOVA. *p<0.05, **p<0.01, ***p< 0.001, ****p< 0.0001; ns, not significant.
The anti-tumor effect of the intestinal microbiome of Gc-/- mice was not accompanied by obvious signs of gut inflammation or histological changes to the intestinal barrier (Supplementary Fig. 1C). Extent of gut-associated lymphoid tissue, gut permeability, total leukocyte numbers, and immune cell composition of intestinal lamina propria were all grossly similar between WT and Gc-/-, except for a decrease in the frequency of IL-17-producing CD4+ T cells in the small intestine and of total CD4+ T cells and Tregs in the colon of Gc-deficient hosts (Supplementary Fig. 1D-I). Moreover, FT of Gc-/- fecal matter into WT mice did not increase the severity of dextran sodium sulphate (DSS)-induced colitis (Supplementary Fig. 2A-D). Collectively, these data suggest that the commensal organisms present in the intestine of Gc-deficient mice do not markedly alter barrier function or mucosal immunity, either at steady state or after induction of intestinal inflammation.
To confirm that the transmissible resistance to transplantable tumors was immune-dependent and to dissect the pathways involved, we tested different immune-deficient strains (Fig. 2D and Supplementary Fig. 3A). FT from Gc-/- donors into mice deficient in T and B cells (Rag1-/-) or IFN-γ receptor (Ifngr-/-) did not confer enhanced protection to subsequent tumor challenge (Fig. 2D). Similarly, mice deficient in CD8+ T cells and MHC class I presentation (Tap1-/-) or cDC1 (Batf3-/-) did not display enhanced control of transplantable tumors when given Gc-/- fecal matter (Fig. 2D and Supplementary Fig. 3A). Global deletion of type I IFN receptor (IFNAR) or MyD88 (an adaptor molecule that operates downstream of IL-1 receptor and Toll-like receptors) also diminished tumor resistance conferred by Gc-/- FT (Fig. 2D and Supplementary Fig. 3B). Using bone marrow radiation chimeras, MyD88 expression in the hematopoietic compartment was found to be necessary and sufficient for enhanced tumor control (Fig. 2E and Supplementary Fig. 3C). In contrast, the DNA sensor cGAS and the TLR adaptor molecule TRIF were dispensable for increased tumor resistance following Gc-/- FT administration (Supplementary Fig. 3B). Collectively, these data indicate a key role for innate and adaptive immunity in the enhanced tumor resistance conferred by Gc-/- microbiota.
Vitamin D availability determines transmissible tumor resistance in mice
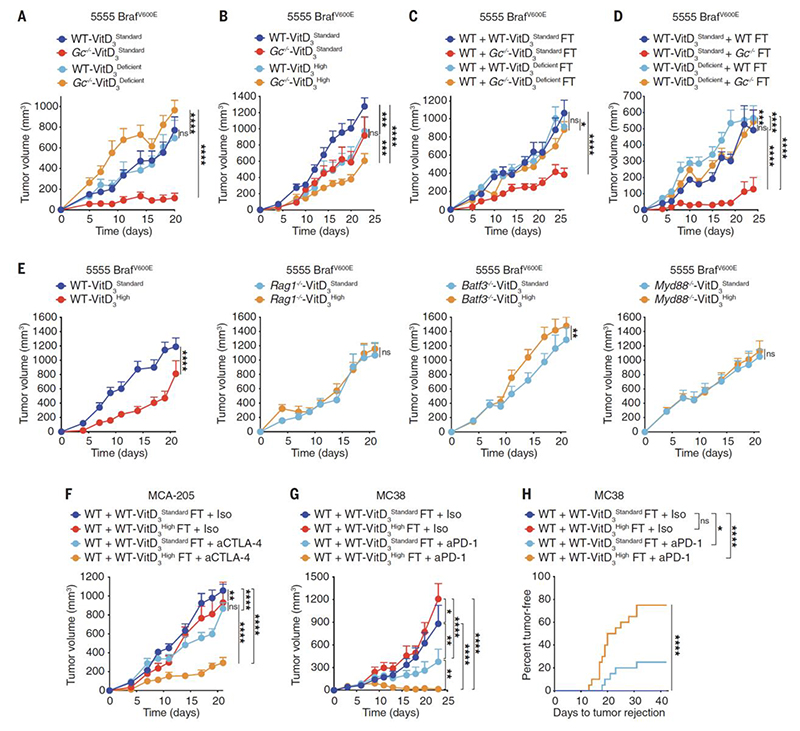
Because mice deficient in sGSN do not transfer tumor resistance to co-housed WT mice (31), we hypothesized that a deficiency in actin scavenging was not responsible for the enhanced tumor resistance in Gc-/- mice. As expected (24), Gc-/- mice displayed lower levels of vitamin D3 and 25-OHD3 in plasma, indicative of VitD redistribution to tissues (Supplementary Fig. 4A). The main vitamin D in mouse chow is cholecalciferol (VitD3). To test whether Gc deficiency enhances tumor resistance in a VitD-dependent manner, WT and Gc-/- mice were put on a VitD3-deficient diet for approximately 4 weeks to deplete their VitD reservoirs (Supplementary Fig. 4A). Remarkably, this completely abrogated the enhanced ability of Gc-/- mice to resist tumors (Fig. 3A). In the converse experiment, increased dietary VitD3 supplementation led to elevated total VitD serum levels (Supplementary Fig. 4A) and decreased tumor growth in WT mice to the point that they became comparable to Gc-deficient animals fed with standard VitD3 chow (Fig. 3B). The latter strain displayed even greater tumor resistance when placed on a VitD3 high diet (Fig. 3B). Collectively, these data suggest that enhanced VitD availability, induced by loss of Gc and/or by dietary VitD3 supplementation, promotes increased resistance to transplantable tumors in mice.
Fig. 3. Loss of Gc increases VitD-dependent anti-cancer immunity by altering the gut microbiome.
(A-E) Growth profile of 0.2 x 106 5555 BrafV600E cancer cells implanted into: (A-B) WT C57BL/6J or Gc-/- mice that were fed a VitD3 standard (2 IU/g), deficient (0 IU/g) or high (10 IU/g) diet starting from 3.5 weeks before tumor inoculation. (A) WT + VitD3Standard (n=8), WT + VitD3Deficient (n=9), Gc-/- + VitD3Standard (n=8), Gc-/- + VitD3Deficient (n=9). (B) WT + VitD3Standard (n=12), WT + VitD3High (n=13), Gc-/- + VitD3Standard (n=12), Gc-/- + VitD3High (n=13). (C) WT C57BL/6J (n=10 per group) that received (on days -14 and -12 prior to tumor inoculation) FT from WT C57BL/6J or Gc-/- donors that had been fed with VitD3 standard or deficient diet. (D) WT C57BL/6J mice that were fed a VitD3 standard or deficient diet starting 3.5 weeks before FT (on days -14 and -12 prior to tumor inoculation) with fecal matter from WT C57BL/6J or Gc-/- donors. WT-VitD3Standard + WT FT (n=7), WT-VitD3Standard + WT Gc-/- (n=10), WT-VitD3Deficient + WT FT (n=10), WT-VitD3Deficient + WT Gc-/- FT (n=10). (E) WT C57BL/6J, Rag1-/-, Batf3-/- or Myd88-/- mice (n=10 per group) that were fed with VitD3 standard or high diet starting from 3.5 weeks before tumor inoculation. (F) Growth profile of 0.5 x 106 MCA-205 cancer cells implanted into WT C57BL/6J mice (n=10 per group) that received (on days -14 and -12 prior tumor inoculation) FT from WT C57BL/6J donors that were fed with VitD3 standard or high diet. Mice were treated i.p. with 50μg of isotype-matched control or anti-CTLA-4 antibody on days 6, 9 and 12. (G-H) Separately housed groups of WT C57BL/6J mice implanted with 0.5 x 106 MC38 that received (on days -14 and -12 prior to tumor inoculation) FT from WT C57BL/6J donors that were fed with VitD3 standard or high diet. Mice were treated i.p. with 200μg of isotype-matched control or anti-PD-1 monoclonal antibody every 3 days from day 3 to day 12. (G) Growth profile (n=10 mice per group). (H) Percent tumor rejection from two independent experiments (n=20 mice per group). Data in (A-G) are presented as tumor volume (mm3) ± SEM and are representative of two independent experiments. Tumor growth profiles (A-G) were compared using Bonferroni-corrected two-way ANOVA. Incidence of tumor rejection in (H) were compared using Log-rank (Mantel-Cox) test for comparison of each group with WT C57BL/6J + isotype and Log-rank for trend for comparison of all groups. *p<0.05, **p<0.01, ***p< 0.001, ****p< 0.0001; ns, not significant.
We next assessed if, as for Gc deficiency, dietary VitD3 supplementation increases tumor resistance via the microbiota. Consistent with that notion, a VitD3 high diet did not increase the ability of germ-free mice to resist tumors (Supplementary Fig. 4B). Further, the capacity to transmit increased tumor resistance to WT mice was abrogated when fecal material was derived from Gc-/- mice that had been placed on a VitD3-deficient diet (Fig. 3C). Conversely, increasing dietary VitD3 in WT mice conferred their fecal matter the ability to transmit tumor control, which was prevented by treatment with vancomycin (Supplementary Fig. 4C and D). Importantly, FT from WT mice that were fed with VitD3 high diet transferred tumor resistance to C576BL/6 mice from three different sources that were imported and housed in geographically-distinct animal units (Supplementary Fig. 4E). Finally, we established that VitD availability in the recipient mice, was also necessary for the beneficial anti-tumor effects of FT from Gc-/- donors. Indeed, enhanced resistance to tumors was prevented if the recipients were placed on a VitD3 deficient diet (Fig. 3D).
In parallel, we tested whether manipulation of dietary VitD3 impacted tumor growth by modulating cancer immunity. Like Gc deficient hosts (Fig. 1C) or WT mice gavaged with Gc-/- fecal matter (Fig. 2D-E and Supplementary Fig. 3A and B), mice fed with a VitD3 high diet did not exhibit increased tumor resistance if rendered deficient in T and B cells, cDC1 or MyD88 (Fig. 3E). Further resembling Gc-/- mice, fecal transplants from WT mice that were fed with VitD3 high diet increased the therapeutic efficacy of anti-CTLA-4 and anti-PD-1 immune checkpoint blockade inhibitors in transplantable cancer models other than 5555 BrafV600E melanoma such as MCA-205 and MC38 (Fig. 3F, G, H). Collectively, these results establish that 1) high VitD levels favor a mouse microbiome that augments anti-cancer immunity; and 2) the favorable effect can be transferred by FT as long as VitD remains available to the recipient mice.
Increased vitamin D levels in mice favor a microbiome that potentiates cancer immunity
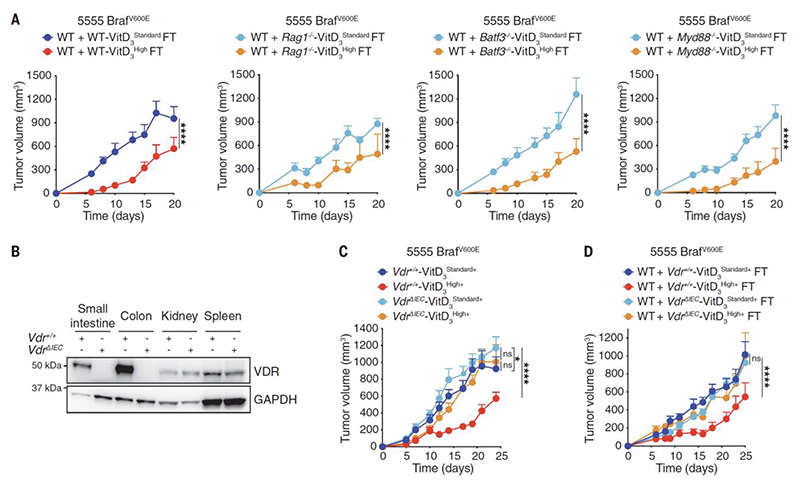
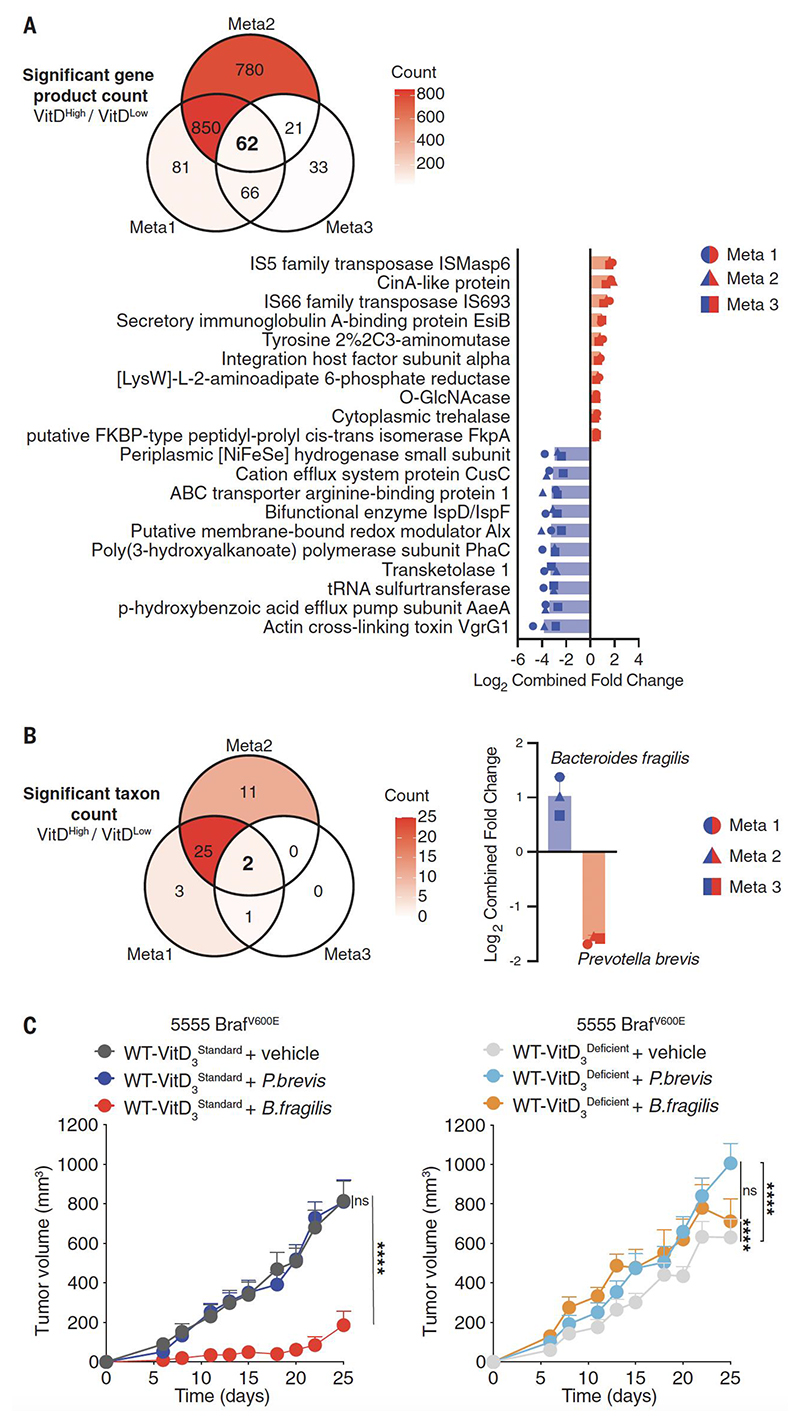
The fact that Rag1-/-, Batf3-/- or Myd88-/- mice did not display VitD-driven increased immune resistance to cancer (Fig. 3E) was not because immune defects in those mice compromised the ability of VitD3 high diet to promote the favorable alterations in microbiota. Indeed, fecal matter from all the immunodeficient mice given VitD3 high diets was able to induce greater tumor resistance upon FT into WT mice (Fig. 4A). These data suggest that the ability of high VitD availability to alter the microbiome is largely independent of the immune system. To look for a non-immune component, we turned our attention to the possible effects of VitD on intestinal epithelial cells (IECs). Although it did not alter gut permeability (Supplementary Fig. 1E), a VitD3 high diet induced profound changes in gene expression in colonic tissue of WT mice (Supplementary Fig. 5A). Gene expression analysis did not reveal marked compositional differences in specific immune cell populations, as predicted, but alterations in cellular signaling, cell junction organization, as well as in defense from microbes (Supplementary Fig. 5B-D). This is consistent with the ability of VitD, acting via VDR, to directly regulate the expression of multiple genes that impact host physiology (22, 34). To directly assess the importance of VDR in intestinal epithelial cells (IECs), we bred Vdrfl/fl mice to VillinCre mice to generate a VdrΔIEC strain that lacks VDR expression in IECs (Fig. 4B). Upon weaning, VDRΔIEC mice were maintained on diets complemented with calcium, phosphorus and lactose to mitigate the osteomalacia-like effects of abrogating VitD responsiveness in gut epithelium (35). This altered diet did not prevent the ability of VitD3 supplementation to increase tumor resistance in control WT mice [Fig. 4C, VitD3 high+ diet (where the + symbol denotes calcium/phosphorus/lactose complementation)]. However, VitD3 high+ diet failed to increase tumor resistance in littermate VDRΔIEC mice (Fig. 4C). Furthermore, the fecal matter of VDRΔIEC mice on VitD3 high+ diet was no longer able to transmit tumor resistance, unlike that of control WT littermates (Fig. 4D). These data indicate that VitD acts via IECs to favor a gut microbiome that increases immune-mediated cancer control.To look for VitD-associated alterations in the microbiome, we carried out shotgun metagenomic analyses of fecal samples from mice in which we altered VitD levels by manipulating diet and/or genotype. We found that bacterial species alpha diversity was largely similar across all samples while beta diversity and taxonomic profiles showed major differences across genotype but not diet (Supplementary Fig. 6A-D, 7A-B, 8A-B). To gain further insight into To gain further insight into bacterial species modulated by VitD availability, we combined 3 meta-analyses of different comparisons across experiments: Meta1, differences driven by genotype (WT vs. Gc-/-) in a VitD3Standard condition; Meta2, differences driven by genotype (WT vs. Gc-/-) in the presence of varying levels of dietary VitD (VitD3Standard and VitD3High); Meta3, differences driven by genotype (WT vs. Gc-/-) consistent with those driven by increased dietary VitD in WT (VitD3Standard vs. VitD3High). This approach allowed us to identify 62 gene products and 2 taxa that were consistently regulated by VitD availability across conditions (Fig. 5A-B, Supplementary Fig. 9A-C). Higher VitD availability increased the abundance of Bacteroides fragilis at the expense of Prevotella brevis (Fig. 5B. Supplementary Fig. 9A-C, 10A-B). Because the ability of Gc-/- mice to transmit tumour resistance through microbiota depends on the presence of dietary VitD, we removed background differences driven by genotype by contrasting Gc-/- and WT in mice fed VitD3Deficient and VitD3Standard diets and focused on taxonomic differences observed exclusively in the presence of VitD (VitD3Standard). This analysis further confirmed the VitD-dependent increase in Bacteroides fragilis and reduction of P. brevis (Supplementary Fig. 10C). Therefore, we assessed whether either bacterium could impact tumor resistance in a VitD-dependent manner. Remarkably, three rounds of oral gavage with Bacteroides fragilis was sufficient to induce increased resistance to subsequent tumor transplantation across WT C576BL/6 mice procured from different sources and housed in two different animal units (Fig. 5C, left panel, Supplementary Fig. 10D). However, and in line with our earlier data using FT (Fig. 3D), tumor resistance induced by Bacteroides fragilis was prevented if the recipient mice were placed on a diet deficient in VitD3 (Fig. 5C, right panel). Thus, VitD availability is necessary to maintain a niche in which Bacteroides fragilis can thrive. Consistent with that notion, gavage with the bacterium led to slightly lower levels of the organism in the intestine of mice placed on a VitD3-deficient diet compared to those on a VitD3-standard diet (Supplementary Fig. 10E). In contrast to Bacteroides fragilis, gavage with Prevotella brevis did not increase tumor resistance (Fig. 5C) and, in fact, decreased it slightly in mice placed on a VitD3-deficient diet (Fig. 5C, right panel and Supplementary Fig. 10F).
Fig. 4. VitD acts via VDR in the gut epithelium to alter gut microbiome and permit tumor control.
(A, C-D) Growth profile of 0.2 x 106 5555 BrafV600E cancer cells implanted into: (A) WT C57BL/6J mice that received (on days -14 and -12 prior to tumor inoculation) FT from WT C57BL/6J, Rag1-/-, Batf3-/- or Myd88-/- donors that had been fed for 3.5 weeks on a VitD3 standard or VitD3 high diet. WT + WT-VitD3Standard FT (n=10), WT + WT-VitD3High FT (n=10), WT + Rag1-/-- VitD3Standard FT (n=10), WT + Rag1-/--VitD3High FT (n=9), WT + Batf3-/--VitD3Standard FT (n=11), WT + Batf3-/--VitD3High FT (n=11), WT + Myd88-/--VitD3Standard FT (n=9), WT + Myd88-/--VitD3High FT (n=9). (B) Lysates from the indicated mouse tissues of Vdr+/+ and VdrΔ IEC mice immunoblotted for VDR and GAPDH. (C) Vdr+/+ or VdrΔIEC mice kept on a VitD3 standard+ (2 IU/g) diet complemented with 2% calcium, 1.25% phosphorus and 20% lactose were then maintained on the same diet or switched to a VitD3 high+ (10 IU/g) diet (similarly complemented with 2% calcium, 1.25% phosphorus and 20% lactose) from 3.5 weeks before tumor inoculation. Vdr+/+-VitD3Standard+ (n=12), Vdr+/+-VitD3High+ (n=11), VdrΔIEC -VitD3Standard+ (n=15), VdrΔIEC - VitD3High+ (n=15) (D) WT C57BL/6J mice (n=10 per group) received (on days -14 and -12 prior to tumor inoculation) FT from the groups in (C), i.e., Vdr+/+ or VdrΔIEC donors that were fed with VitD3 standard+ or VitD3 high+ diet. Data in (A, C and D) are presented as tumor volume (mm3) ± SEM and are representative of two independent experiments. Tumor growth profiles (A, C and D) were compared using Bonferroni-corrected two-way ANOVA. *p<0.05, ****p< 0.0001; ns, not significant.
Fig. 5. B.fragilis promotes tumor resistance in a VitD-dependent manner.
(A-B) Meta-analysis of metagenomic data to determine (A) common features in microbial gene products (top 20/62 features in each direction shown, 20/62) and (B) last known taxon associated with differences in VitD availability. Fecal samples were sequenced from WT or Gc-/- mice that had been fed with VitD3 standard (2 IU/g), deficient (0 IU/g) or high (10 IU/g) diet for 3.5 weeks. Comparison is of mice with high VitD availability [WT + VitD3High (n=13), Gc-/- + VitD3Standard (n=20), Gc-/- + VitD3High (n=13) vs. mice with normal or low VitD availability [WT + VitD3Standard (n=22), WT + VitD3Deficient (n=10), Gc-/- + VitD3Deficient (n=10)]. In (A, B), count of significant features indicated in the Venn diagram and shown by color scale (top) and ranked bar plots (bottom) show common features across 3 meta-analyses as indicated. (C) Growth profile of 0.2 x 106 5555 BrafV600E cancer cells implanted into separately housed WT C57BL/6 groups of mice (n=10 per group) fed with VitD3 standard (left graph) or deficient diet (right graph), starting 3.5 weeks before receiving B. fragilis, P. brevis or vehicle. Mice received 109 B. fragilis or P. brevis by oral gavage on days -14, -12 and -10 prior to tumor inoculation. Data in (A, B) are presented as average log2 median fold change from three meta-analyses of data from two independent experiments. Data in (C) are presented as tumor volume (mm3) ± SEM and are representative of two independent experiments for P. brevis and 3 independent experiments for B. fragilis. In (A, B), p values were calculated using the Mann–Whitney–Wilcoxon U test on parts per million (PPM) relative abundances for that feature in samples within each group for pairwise comparisons. The combined p value (cp) for meta-analysis of within-group comparisons was calculated using Fishers P value. For each feature type, the cut-offs for the meta-analysis were: p< 0.2, cp< 0.1, false discovery rate (FDR)<0.15. Tumor growth profiles (A, C and D) were compared using Bonferroni-corrected two-way ANOVA. ****p< 0.0001; ns, not significant.
Vitamin D levels in humans correlate with cancer resistance
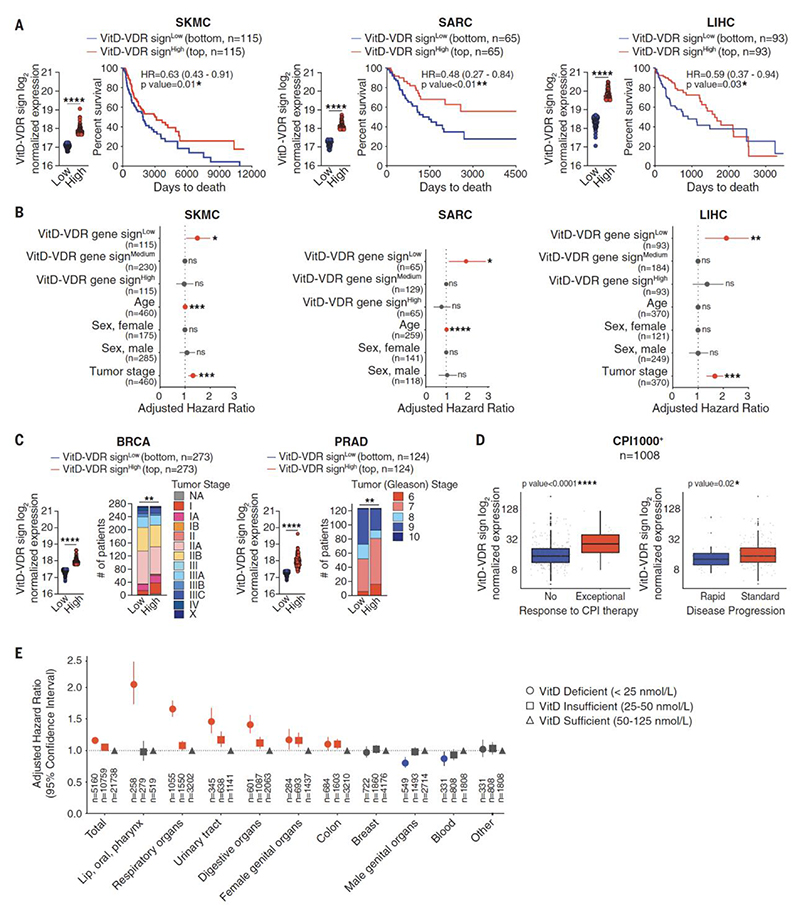
Polymorphisms in genes that encode proteins that participate in 1,25-(OH)2D biosynthesis (CYP2R1, CYP27A1, CYP27B1), that restrict VitD availability (GC) or mediate VitD biological functions (VDR) have been variously correlated with cancer risk, alterations in microbiota and/or changes in immune parameters in health and disease (36–40) (https://www.ebi.ac.uk/gwas/; Supplementary Fig. 11A and Supplemental Table 1). VDR is an ubiquitously expressed (Supplementary Fig. 11B) nuclear receptor that functions as a ligand-activated transcription factor. We therefore hypothesized that the expression of VDR target genes in any tissue, healthy or malignant, may act as a surrogate measurement of VitD availability in that tissue (24, 41). We assembled a gene signature (VitD-VDR sign) consisting of 237 VDR target genes from several human cell types identified using ChIP-sequencing datasets (Supplemental Table 2; 11, 42–46). We confined our analysis to ChIPseq data to increase resolution and ensure that we analyzed only primary VDR targets even if this might exclude other relevant VitD-inducible genes. We examined the expression of the VitD-VDR sign in different cancers using data from The Cancer Genome Atlas (TCGA) collection (Supplemental Table 2). Analysis of skin cancer (n=460), sarcoma (n=259), liver hepatocellular carcinoma (n=370), breast cancer (n=1092) and prostate adenocarcinoma (n=497) revealed that lower expression of the VitD-VDR signature correlated with poorer survival or more advanced disease (Fig. 6A-C). In the same cancers, the VDR transcript did not correlate with patient survival, highlighting a specific association of VDR target genes, but not necessarily VDR expression with cancer progression (Supplementary Fig. 11C and D). Comparison of human tumors with high versus low VitD-VDR sign revealed that VitD-VDR signhigh cancers displayed specific enrichment for genes and gene signatures of the same immune elements that we found to be required to restrict growth of mouse tumors following increased VitD availability (Supplementary Fig. 11E). This correlation between high VitD-VDR signature and gene signatures of anti-tumor immunity prompted us to further test the value of VitD-VDR sign in predicting responses to immunotherapy. We analyzed >1000 patients treated with immune checkpoint inhibitors (CPI1000+ cohort) across seven cancer types using bioinformatic pipelines and standardized clinical criteria, as reported (47). Low expression of VitD-VDR sign and, to a lesser extent, of VDR, was associated with resistance to immune checkpoint inhibitors and more rapid disease progression (Fig. 6D, Supplementary Fig. 11F). Overall, these data suggest that, in humans as in mice, lower VitD tissue availability is associated with lower overall immune-mediated control and worse cancer outcome.
Fig. 6. VitD correlates with lower risk of cancer and increased patient survival.
(A) Prognostic value of VitD-VDR gene signature levels for overall survival and hazard ratio comparing samples with the lowest (VitD-VDR signLow) versus highest (VitD-VDR signHigh) expression in the indicated TCGA datasets. Skin cutaneous melanoma (SKMC, n=460), sarcoma (SARC, n=259), liver hepatocellular carcinoma (LIHC, n=370), bottom and top 25% of patient cohort. (B) Hazard ratio, adjusted for age, sex and tumor stage, comparing samples with the lowest (VitD-VDR signLow) or highest (VitD-VDR signHigh) versus medium (VitD-VDR signMedium) expression in the indicated TCGA datasets as in (A). (C) Prognostic value of VitD-VDR signature levels for tumor stage comparing samples with the lowest (VitD-VDR signLow) versus highest (VitD-VDR = signHigh) expression in the indicated TCGA datasets. Breast cancer (BRCA, n=1092), prostate adenocarcinoma (PRAD, n=497), bottom and top 25% of patient cohort. (D) VitD-VDR signature levels in samples with no response vs. exceptional response (left) and rapid vs. standard disease progression (right) of patients (n=1008) treated with checkpoint inhibitors (CPI1000+ cohort). (E) Estimated hazard ratio, adjusted for sex, age and Charlson’s comorbidity index, in the VitD deficient (<25 nmol/L) or insufficient (25-50 nmol/L) group versus the VitD sufficient (50-125 nmol/L) group of individuals (n=1,496,766) that were living in Denmark between 2008-2017. In (A) data are presented as mean of log2 normalized expression ± SEM. In (C) data are represented as number of patients that are subdivided based on the tumour stage. In (D) data are presented as log2 normalized expression box-and-whisker plot with median, 25th and 75th percentiles represented by the box and min/max by the whiskers. Survival (Kaplan-Meier) curves in (A) were compared using Log-rank (Mantel-Cox) test. In (A, B and E) hazard ratios (HR) with 95% confidence interval showed. In (A and C) gene signature levels between groups were compared using two-tailed unpaired t test with Welch’s correction. In (C) frequency of tumour stage was compared between groups using Chi-squared test. In (D) expression of gene signature between the groups was compared using Wilcoxon signed-rank test. *p<0.05, **p<0.01, ***p< 0.001, ****p< 0.0001; ns, not significant.
Several human epidemiological studies have associated high total (bound and unbound to Gc) and free VitD serum levels with decreased cancer onset and extended patient survival (12–21). However, these studies are inconclusive and limited by relatively small sample sizes. Therefore, we analyzed combined data from the Danish Central Person Registry, the Cancer Registry and the Register of Laboratory Results for Research to include clinical information from a very large cohort of participants (1,496,766 individuals) that lived in Denmark and had at least one vitamin D (25-OHD) serum measurement registered between 2008-2017 (48, 49) (Fig. 6E). Time elapsed since one year following first 25-OHD serum measurement until first diagnosis of cancer was analyzed by a Cox regression model using age as the underlying time scale and adjusting for sex, sample collection time and Charlson’s comorbidity index calculated on the five years before the sample was taken, as described before (50). Skin pigmentation, which can impact VitD3 production in response to sun exposure, was not available as a variable but the analysis is unlikely to be affected by differences in ethnicity as the Danish population is highly homogeneous (86% of Danish descent). Further, the relatively northerly latitude of Denmark means that most of the year is “vitamin D winter”; i.e. the period during which cutaneous synthesis of vitamin D3 does not occur. Skin cancer was excluded from the study because sun exposure is a major confounder as it contributes to both VitD3 synthesis and skin carcinogenesis. (In the previous analysis of cancer outcomes (Fig. 6A, B and D), this confounder is not relevant as we correlated VitD-induced transcripts with outcome of patients that already developed skin cancer.) Notably, and consistent with our preclinical mouse models, we found that a low serum measurement of 25-OHD, indicative of vitamin D deficiency at the time the sample was taken, is associated with increased cancer risk in 6/10 individual cancer cohorts over the following decade. This analysis highlights that low vitamin D serum levels can be a prospective risk factor for cancer development in humans (Fig. 6E).
Discussion
The interplay between diet, microbiome and the immune system is increasingly recognized as an important component of immunity, including to cancer (51–53). Studies in mice and humans have shown gut commensals to influence anti-cancer immune responses and impact the efficacy of immune checkpoint blockade therapy (54–60). The host factors that allow gut-resident microbes to modulate anti-cancer immune responses remain elusive. Here, we show that increased VitD availability upon genetic deletion of Gc or following vitamin D dietary supplementation alters the gut microbiome to enhance cancer immunity (graphical summary in Supplementary Fig. 12A, B). Specifically, VitD levels appear to regulate the abundance and/or metabolic properties of Bacteroides fragilis, an anaerobic Gram-negative bacterium that is part of the normal microbiome of humans and mice. Remarkably, gavage of WT mice with fecal matter from Gc-/- mice or a non-enterotoxic clinical isolate of Bacteroides fragilis was sufficient to confer increased immune-mediated tumor resistance. This did not require antibiotic-mediated conditioning of the recipient mice but necessitated continued availability of dietary vitamin D, demonstrating the dependence of the Bacteroides fragilis “niche” on the micronutrient. Our data further indicate that this niche requires the activity of VitD on IECs but further work will be required to understand which VDR-dependent IEC-derived factors are involved and whether they allow for Bacteroides fragilis expansion or alter its immunomodulatory activity. With regards to the latter, we do not presently know how Bacteroides fragilis acts to boost cancer immunity although our findings suggest that MyD88-dependent receptor signaling and type I IFN production are necessary, as are cDC1-dependent T cell responses. Interestingly, Bacteroides fragilis has been previously associated with favorable anti-tumor immune responses following treatment of patients with anti-CTLA-4 whereas gut-resident Prevotella species had the opposite effect (55, 61). Further, vitamin D supplementation in healthy human volunteers is associated with a significant increase in intestinal Bacteroides species and in the Bacteroides / Prevotella ratio (62, 63) and abundance of Bacteroides fragilis in human infant fecal samples shows a positive correlation with maternal plasma 25-(OH)D levels (64). Thus, our data suggest a model in which VitD levels in humans, as in mice, modulate the ability of intestinal cells to produce mediators that select for an altered microbiome that includes organisms such as Bacteroides fragilis, which potentiate cancer immunity (graphical summary in Supplementary Fig. 12C). Whether this comes at the risk of adverse effects, especially given the ability of Bacteroides fragilis to become pathogenic (65), will require further assessment. However, in mice, we do not see evidence for Bacteroides fragilis-associated exacerbation of gut inflammation and the bacterium is also reported to protect gut integrity and reduce colorectal cancer induction (66, 67).
In some but not all studies, higher blood levels or increased dietary intake of vitamin D have been correlated with a lower risk of colorectal, breast, prostate and pancreatic tumors (12–21). Our data from nearly 1.5 million individuals, the largest ever such cohort, confirms that a low VitD measurement correlates with increased subsequent risk of cancer incidence. Notably, this may be an underestimate of the true effect of VitD in cancer protection as those individuals who were found to be VitD deficient may have subsequently redressed it with dietary supplements, a factor that is not considered in our analysis. Interestingly, VitD levels at diagnosis of melanoma have been reported to positively correlate with both thinner tumors and better survival (68). As it is exceedingly difficult to control for diet and sunlight exposure, and because a single measurement of VitD may not reflect actual vitamin D availability, we derived a VitD-VDR gene signature as a surrogate of tissue VitD activity. We show that this VitD-VDR gene signature correlates with cancer patient survival, consistent with studies showing that VitD can decrease cancer cell proliferation, promote apoptosis, reduce angiogenesis (7–9), and dampen the pro-tumorigenic activity of cancer-associated fibroblasts (10, 11). Importantly, we further show that the VitD-VDR gene signature correlates with signatures of anti-cancer immunity and with patient responses to immunotherapy. Similarly, VDR expression in melanoma correlates with immune score and increased patient survival, possibly because VDR signals help counteract immunosuppressive Wnt signaling (69). Notably, a recent study reports that greater VitD levels at baseline or after dietary correction correlate with higher responsiveness to immune checkpoint blockade therapy in a cohort of advanced melanoma patients (70). Thus, in humans as in mice, VitD activity appears to potentiate immune responses to cancer.
In sum, here we report that disrupted vitamin D signalling in IECs alters the intestinal microbiome, which in turns impacts immunity to cancer in mice. Further, we show that the vitamin D status of human patients and VitD-VDR signatures within tumors impacts cancer incidence, survival and/or the response to immunotherapy. Further work will be necessary to assess to what extent of overlap between these two findings. Longitudinal studies in humans will help to disentangle the interaction between VitD availability with the microbiome and immunity to cancer, as well as to better assess the effects of vitamin D dietary supplementation.
Supplementary Material
Acknowledgements
We thank the members of the Immunobiology Laboratory for helpful discussions and suggestions. We thank Gitta Stockinger for reading an original draft of the manuscript and valuable input. We thank the Crick Science Technology Platforms including Biological Research Facility, Flow Cytometry, Metabolomics and Experimental Histopathology as well as CRUK Manchester Institute Biological Resource Unit and the Frederick National Laboratory Gnotobiotics Facility (NCI, NIH) for their support throughout this project.
Funding
This work was supported by The Francis Crick Institute, which receives core funding from Cancer Research UK (CC2090), the UK Medical Research Council (CC2090), and the Wellcome Trust (CC2090); an ERC Advanced Investigator grant (AdG 268670); a Wellcome Investigator Award (106973/Z/15/Z); and a prize from the Louis-Jeantet Foundation. E.G. is currently supported by Cancer Research UK Institute Award (C5759/A27412) and a Royal Society Research Grant (RG\R2\232348). M.P.C was supported by Boehringer Ingelheim Fonds. K.H.J.L. was supported by a Wellcome Imperial 4i Clinical Research Fellowship (216327/Z/19/Z). A.B. was supported by the Human Frontier Science Program postdoctoral fellowship (LT0061/2022), EMBO non-stipendiary long-term fellowship (ALTF 662-2021). M.A.K. and S.Z. were supported by a Cancer Research UK Institute Award (DRCSGL-2023/100001). This research was funded in part by the Wellcome Trust (grants CC2090, 106973/Z/15/Z, and 216327/Z/19/Z). K.C.L., R.R.R., and R.S.G. are supported by the Intramural Research Program of the National Institutes of Health (CCR-NCI). K.R.P. and S.B. were supported by ERC (grant no. 866028) and UK Medical Research Council (project no. MC_UU_00025/11). J.C.L. is a Lister prize fellow. T.J and G.J.P were supported by the Danish National Research Foundation (DNRF148).
Footnotes
Author contributions: E.G. conducted experiments and analyzed data with assistance from M.P.C., K.C.L., K.H.J.L., A.C., C.P., S.P., A.B., T.C.D., M.D.B., M.A.K., P.W. and A.V. S.L. provided technical support. S.Z. oversaw the research activity planning and execution that was performed by M.A.K.. K.C.L. performed experiments on germ free mice. T.J. and K.C.L provided access to data and metadata analysis pipeline for bioinformatic analysis. P.C., K.C.L., R.R.R., G.J.P., B.S. and E.G. carried out bioinformatic analyses. J.C.L. and R.S.G. helped with analysis and experimental design. C.M.M., E.C., C.P., A.I. helped with the harvesting and processing of tissue and tumor samples. E.N., E.G. and B.G. process and cut tissue for histological analysis. A.C., A.S.B. and S.L.P. conducted histopathology assessments blindly. S.B. and K.R.P. cultured B.fragilis and P.brevis. E.G., A.C. and S.O. performed fecal microbiota transplantations as well as B.fragilis and P.brevis oral administration. S.A.P.D and J.I.M. measured the vitamin D concentration in mouse serum. R.G. performed the RNA sequencing of mouse colon. N.C.R. managed mouse colonies. E.G. and C.R.S. designed the study, interpreted data and wrote the manuscript. All authors reviewed and edited the manuscript.
Competing interests: C.R.S. is a founder of Adendra Therapeutics and owns stock options and/or is a paid consultant/advisory board member for Adendra Therapeutics, Bicara Therapeutics, Montis Biosciences and Bicycle Therapeutics, all unrelated to this work. C.R.S. has an additional appointment as a Visiting Professor in the Faculty of Medicine at Imperial College London and holds honorary professorships at University College London and King’s College London.
Data and materials availability
All data are available in the manuscript or the supplementary materials. Materials and reagents described in this study are either commercially available or available on request from the corresponding author. Shotgun metagenomics data are available through the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) under BioProject ID PRJNA1077927. Colon RNA sequencing data have been deposited in the Gene Expression Omnibus (GEO) database under the accession number GSE219214.
References and notes
- 1.Yamamoto EA, Jørgensen TN. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol. 2020;10:3141. doi: 10.3389/fimmu.2019.03141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int J Mol Sci. 2018;19:2663. doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas RM, Gorman S, Geldenhuys S, Hart PH. Vitamin D and immunity. F1000prime Reports. 2014;6:118. doi: 10.12703/P6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark A, Mach N. Role of Vitamin D in the Hygiene Hypothesis : The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front Immunol. 2016;7:1–12. doi: 10.3389/fimmu.2016.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, Neale RE. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. 2019;58:2895–2910. doi: 10.1007/s00394-018-1842-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen F-A, Rühlemann MC, Szymczak S, Holm K, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396–1406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 8.Jeon S-M, Shin E-A. Exploring vitamin D metabolism and function in cancer. Experimental & Molecular Medicine. 2018;50:20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlberg C, Muñoz A. An update on vitamin D signaling and cancer. Seminars in Cancer Biology. 2020:1–14. doi: 10.1016/j.semcancer.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer-Mayorga G, Gómez-López G, Barbáchano A, Fernández-Barral A, Peña C, Pisano DG, Cantero R, Rojo F, Muñoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, Margolis KL, O’Sullivan MJ, Ockene JK, Phillips L, Pottern L, Prentice RL, et al. Investigators, Calcium plus Vitamin D Supplementation and the Risk of Colorectal Cancer. New Engl J Medicine. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 13.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJB, Norat T, Pischon T, Jansen EHJM, Slimani N, Byrnes G, Rinaldi S, Tjønneland A, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolcott CG, Wilkens LR, Nomura AMY, Horst RL, Goodman MT, Murphy SP, Henderson BE, Kolonel LN, Marchand LL. Plasma 25-Hydroxyvitamin D Levels and the Risk of Colorectal Cancer: The Multiethnic Cohort Study. Cancer Epidemiology Prev Biomarkers. 2010;19:130–134. doi: 10.1158/1055-9965.EPI-09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association Between Vitamin D and Risk of Colorectal Cancer: A Systematic Review of Prospective Studies. J Clin Oncol. 2011;29:3775–3782. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 17.Smedt JD, Kelst SV, Boecxstaens V, Stas M, Bogaerts K, Vanderschueren D, Aura C, Vandenberghe K, Lambrechts D, Wolter P, Bechter O, et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): a randomized controlled trial. BMC Cancer. 2017;17:562. doi: 10.1186/s12885-017-3538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Chen H, Zhao M, Peng P. Prognostic value of circulating vitamin D binding protein, total, free and bioavailable 25-hydroxy vitamin D in patients with colorectal cancer. Oncotarget. 2017;8:40214–40221. doi: 10.18632/oncotarget.16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, Friedenberg G, Ridge C, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. New Engl J Med. 2018;380:33–44. [Google Scholar]
- 20.Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, Rubinson DA, Schrag D, Miksad R, Bullock AJ, Allen J, et al. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321:1370–1379. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urashima M, Ohdaira H, Akutsu T, Okada S, Yoshida M, Kitajima M, Suzuki Y. Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients With Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA. 2019;321:1361. doi: 10.1001/jama.2019.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikle DD. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddad JG, Hu YZ, Kowalski MA, Laramore C, Ray K, Robzyk P, Cooke NE. Identification of the sterol- and actin-binding domains of plasma vitamin D binding protein (Gc-globulin) Biochemistry-us. 1992;31:7174–81. doi: 10.1021/bi00146a021. [DOI] [PubMed] [Google Scholar]
- 24.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duchow EG, Cooke NE, Seeman J, Plum LA, DeLuca HF. Vitamin D binding protein is required to utilize skin-generated vitamin D. Proc National Acad Sci. 2019;116:24527–24532. doi: 10.1073/pnas.1915442116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson CM, Fink SL, Bassyouni H, Argiropoulos B, Brown L, Laha TJ, Jackson KJ, Lewkonia R, Ferreira P, Hoofnagle AN, Marcadier JL. Vitamin D–Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N Engl J Med. 2019;380:1150–1157. doi: 10.1056/NEJMoa1807841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böttcher JP, Reis e Sousa C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends in Cancer. 2018;4:784–792. doi: 10.1016/j.trecan.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nature Reviews Immunology. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 29.Canton J, Blees H, Henry CM, Buck MD, Schulz O, Rogers NC, Childs E, Zelenay S, Rhys H, Domart M-C, Collinson L, et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat Immunol. 2021;22:140–153. doi: 10.1038/s41590-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry CM, Castellanos CA, Reis e Sousa C. DNGR-1-mediated cross-presentation of dead cell-associated antigens. Semin Immunol. 2023;66:101726. doi: 10.1016/j.smim.2023.101726. [DOI] [PubMed] [Google Scholar]
- 31.Giampazolias E, Schulz O, Lim KHJ, Rogers NC, Chakravarty P, Srinivasan N, Gordon O, Cardoso A, Buck MD, Poirier EZ, Canton J, et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell. 2021;184:4016–4031.:e22. doi: 10.1016/j.cell.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim KHJ, Giampazolias E, Schulz O, Rogers NC, Wilkins A, Sahai E, Strid J, Reis e Sousa C. Loss of secreted gelsolin enhances response to anticancer therapies. J Immunother Cancer. 2022;10:e005245. doi: 10.1136/jitc-2022-005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee WM, Galbraith RM. The Extracellular Actin-Scavenger System and Actin Toxicity. New Engl J Medicine. 1992;326:1335–1341. doi: 10.1056/NEJM199205143262006. [DOI] [PubMed] [Google Scholar]
- 34.Koivisto O, Hanel A, Carlberg C. Key Vitamin D Target Genes with Functions in the Immune System. Nutrients. 2020;12:1140. doi: 10.3390/nu12041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–7. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 36.Mondul AM, Shui IM, Yu K, Travis RC, Stevens VL, Campa D, Schumacher FR, Ziegler RG, Bueno-de-Mesquita HB, Berndt S, Crawford ED, et al. Genetic Variation in the Vitamin D Pathway in Relation to Risk of Prostate Cancer— Results from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiology Prev Biomarkers. 2013;22:688–696. doi: 10.1158/1055-9965.EPI-13-0007-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, Zhang X, Chen X, Liu L, Lu C, Tang X, Shi J, Li M, Zhou M, Zhang Z, Xiao L, et al. GC Glu416Asp and Thr420Lys polymorphisms contribute to gastrointestinal cancer susceptibility in a Chinese population. Int J Clin Exp Med. 2011;5:72–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Karami S, Andreotti G, Koutros S, Barry KH, Moore LE, Han S, Hoppin JA, Sandler DP, Lubin JH, Burdette LA, Yuenger J, et al. Pesticide Exposure and Inherited Variants in Vitamin D Pathway Genes in Relation to Prostate Cancer. Cancer Epidemiology Prev Biomarkers. 2013;22:1557–1566. doi: 10.1158/1055-9965.EPI-12-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña-Chilet M, Ibarrola-Villava M, Feito M, Gomez-Fernandez C, Planelles D, Carretero G, Lluch A, Nagore E, Ribas G. rs12512631 on the group specific complement (vitamin D-binding protein GC) implicated in melanoma susceptibility. Plos One. 2012;8:e59607. doi: 10.1371/journal.pone.0059607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson LN, Cotterchio M, Cole DEC, Knight JA. Vitamin D-Related Genetic Variants, Interactions with Vitamin D Exposure, and Breast Cancer Risk among Caucasian Women in Ontario. Cancer Epidemiology Prev Biomarkers. 2011;20:1708–1717. doi: 10.1158/1055-9965.EPI-11-0300. [DOI] [PubMed] [Google Scholar]
- 41.Chun RF. New perspectives on the vitamin D binding protein: NEW PERSPECTIVES ON VITAMIN D BINDING PROTEIN. Cell Biochem Funct. 2012;30:445–456. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- 42.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, Watson CT, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Research. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, Lau SL, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nurminen V, Seuter S, Carlberg C. Primary Vitamin D Target Genes of Human Monocytes. Frontiers in Physiology. 2019;10 doi: 10.3389/fphys.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawai M, Kinoshita S, Yamazaki M, Yamamoto K, Rosen CJ, Shimba S, Ozono K, Michigami T. Intestinal clock system regulates skeletal homeostasis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanel A, Neme A, Malinen M, Hämäläinen E, Malmberg H-R, Etheve S, Tuomainen T-P, Virtanen JK, Bendik I, Carlberg C. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Scientific Reports. 2020;10 doi: 10.1038/s41598-020-78288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Litchfield K, Reading JL, Puttick C, Thakkar K, Abbosh C, Bentham R, Watkins TBK, Rosenthal R, Biswas D, Rowan A, Lim E, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184:596–614.:e14. doi: 10.1016/j.cell.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Healt. 2011;39:42–5. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 49.Arendt JFH, Hansen AT, Ladefoged SA, Sørensen HT, Pedersen L, Adelborg K. Existing Data Sources in Clinical Epidemiology: Laboratory Information System Databases in Denmark. Clin Epidemiology. 2020;12:469–475. doi: 10.2147/CLEP.S245060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 51.Zhou C-B, Zhou Y-L, Fang J-Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends in Cancer. 2021;7:647–660. doi: 10.1016/j.trecan.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Villemin C, Six A, Neville BA, Lawley TD, Robinson MJ, Bakdash G. The heightened importance of the microbiome in cancer immunotherapy. Trends Immunol. 2023;44:44–59. doi: 10.1016/j.it.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Alexander M, Turnbaugh PJ. Deconstructing Mechanisms of Diet-Microbiome-Immune Interactions. Immunity. 2020;53:264–276. doi: 10.1016/j.immuni.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre M-L, Chang EB, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, Poirier-Colame V, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Routy B, Chatelier EL, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 57.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 58.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, Lewis IA, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 60.Lam KC, Araya RE, Huang A, Chen Q, Modica MD, Rodrigues RR, Lopès A, Johnson SB, Schwarz B, Bohrnsen E, Cogdill AP, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338–5356.:e21. doi: 10.1016/j.cell.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo C-H, Wu D-C, Jao S-W, Wu C-C, Lin C-Y, Chuang C-H, Lin Y-B, Chen C-H, Chen Y-T, Chen J-H, Hsiao K-H, et al. Enrichment of Prevotella intermedia in human colorectal cancer and its additive effects with Fusobacterium nucleatum on the malignant transformation of colorectal adenomas. J Biomed Sci. 2022;29:88. doi: 10.1186/s12929-022-00869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020;40:551–556. doi: 10.21873/anticanres.13984. [DOI] [PubMed] [Google Scholar]
- 63.Singh P, Rawat A, Alwakeel M, Sharif E, Khodor SA. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep. 2020;10:21641. doi: 10.1038/s41598-020-77806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talsness CE, Penders J, Jansen EHJM, Damoiseaux J, Thijs C, Mommers M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS ONE. 2017;12:e0188011. doi: 10.1371/journal.pone.0188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patrick S. A tale of two habitats: Bacteroides fragilis, a lethal pathogen and resident in the human gastrointestinal microbiome. Microbiol (Read, Engl) 2022;168 doi: 10.1099/mic.0.001156. [DOI] [PubMed] [Google Scholar]
- 66.Sofi MH, Wu Y, Ticer T, Schutt S, Bastian D, Choi H-J, Tian L, Mealer C, Liu C, Westwater C, Armeson KE, et al. A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight. 2021;6:e136841. doi: 10.1172/jci.insight.136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YK, Mehrabian P, Boyajian S, Wu W-L, Selicha J, Vonderfecht S, Mazmanian SK. The Protective Role of Bacteroides fragilis in a Murine Model of Colitis-Associated Colorectal Cancer. mSphere. 2018;3:e00587–18. doi: 10.1128/mSphere.00587-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang Y-M, Affleck P, Elliott F, Chan M, Leake S, Karpavicius B, Haynes S, Kukalizch K, et al. Serum 25-Hydroxyvitamin D3 Levels Are Associated With Breslow Thickness at Presentation and Survival From Melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muralidhar S, Filia A, Nsengimana J, Poźniak J, O’Shea SJ, Diaz JM, Harland M, Randerson-Moor JA, Reichrath J, Laye JP, van der Weyden L, et al. Vitamin D–VDR Signaling Inhibits Wnt/β-Catenin–Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019;79:5986–5998. doi: 10.1158/0008-5472.CAN-18-3927. [DOI] [PubMed] [Google Scholar]
- 70.Galus Ł, Michalak M, Lorenz M, Stoińska-Swiniarek R, Małecka DT, Galus A, Kolenda T, Leporowska E, Mackiewicz J. Vitamin D supplementation increases objective response rate and prolongs progression-free time in patients with advanced melanoma undergoing anti–PD-1 therapy. Cancer. 2023;129:2047–2055. doi: 10.1002/cncr.34718. [DOI] [PubMed] [Google Scholar]
- 71.Odamaki T, Xiao J-Z, Sakamoto M, Kondo S, Yaeshima T, Iwatsuki K, Togashi H, Enomoto T, Benno Y. Distribution of Different Species of the Bacteroides fragilis Group in Individuals with Japanese Cedar Pollinosis. Appl Environ Microbiol. 2008;74:6814–6817. doi: 10.1128/AEM.01106-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric Salmonellosis Disrupts the Microbial Ecology of the Murine Gastrointestinal Tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG, Colaço B, Pires MJ, Colaço J, Ferreira R, Ginja M. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Animal. 2013;42:217–224. doi: 10.1038/laban.254. [DOI] [PubMed] [Google Scholar]
- 75.Cardoso A, Castro AG, Martins AC, Carriche GM, Murigneux V, Castro I, Cumano A, Vieira P, Saraiva M. The Dynamics of Interleukin-10-Afforded Protection during Dextran Sulfate Sodium-Induced Colitis. Front Immunol. 2018;9:400. doi: 10.3389/fimmu.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCulloch JA, Badger JH, Cannon N, Rodrigues RR, Valencia M, Barb JJ, Fernandes MR, Balaji A, Crowson L, O’hUigin C, Dzutsev A, et al. JAMS - A framework for the taxonomic and functional exploration of microbiological genomic data. bioRxiv. 2023:2023.03.03.531026 [Google Scholar]
- 77.Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, Wong MC, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM, Fernandes MR, Costa RGF, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28:545–556. doi: 10.1038/s41591-022-01698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc National Acad Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript or the supplementary materials. Materials and reagents described in this study are either commercially available or available on request from the corresponding author. Shotgun metagenomics data are available through the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) under BioProject ID PRJNA1077927. Colon RNA sequencing data have been deposited in the Gene Expression Omnibus (GEO) database under the accession number GSE219214.