Abstract
Purpose
To compare antigen-specific intraocular immune responses between different clinical phenotypes of tuberculin skin test (TST)–positive and TST-negative uveitis.
Design
Single center, retrospective cross-sectional study.
Methods
Patients requiring diagnostic or therapeutic vitrectomy for the management of intraocular inflammation were divided into 3 groups based on Standardization of Uveitis Nomenclature (SUN) classification criteria for tubercular uveitis. Group 1 included patients with ocular tuberculosis (OTB; n = 23) who were TST-positive patients, met the SUN criteria, and/or had a polymerase chain reaction (PCR)–positive test for TB. Group 2 included patients with uveitis of unknown origin (UNK; n = 24) who were undifferentiated TST-positive patients who had not met SUN criteria. Group 3 included non-TB uveitis patients (n = 24) who were TST-negative either with or without a well-defined non-TB diagnosis. Total vitreous cells were activated with Mycobacterium tuberculosis–specific Early Secreted Antigenic Target-6 (ESAT-6) or the retinal autoantigen, interphotoreceptor retinoid-binding protein peptide (pIRBP 1-20), stained for intracellular interferon gamma (IFNγ), tumor necrosis factor-alfa (TNFα), and interleukin 17 (IL-17), and analyzed by flow cytometry. Antigen-specific single and dual (polyfunctional) cytokine responses to ESAT-6 and IRBP were compared between the 3 groups.
Results
All cytokine responses to ESAT-6 were higher in the UNK group compared with the non-TB control subjects, while all except IL-17 were comparable between the OTB and non-TB groups. Polyfunctional responses—IFNγ /IL-17 (P = .002), TNFα/IL-17 (P = .02), and TNFα/IFNγ (P = .01)—were significantly greater for UNK than the OTB group. Polyfunctional cells also produced more cytokine per cell than respective monofunctional cells. IRBP cytokine responses were comparable between different groups and were not affected by the clinical phenotype or duration of disease.
Conclusion
The intraocular polyfunctional cytokine response is stronger in undifferentiated TST-positive uveitis than in OTB patients, likely representing an exaggerated anti-TB immune response rather than active infection.
Ocular tuberculosis (OTB) is one of the more common causes of infectious uveitis in both TB-endemic and non–TB-endemic countries.1 This condition has diverse clinical manifestations that are also shared by other infectious and noninfectious uveitis entities.2 The Standardization of Uveitis Nomenclature (SUN) Working Group recently reported classification criteria for the diagnosis of OTB.3 Together with appropriate clinical signs, these criteria include evidence of current or previous TB infection (histologically or microbiologically proven TB, or positive interferon-gamma release assay [IGRA] or positive tuberculin skin test [TST]), and negative tests for syphilis and sarcoidosis. Histologic and/or microbiologic evidence of TB are rarely found in ocular samples. Tests for immunoreactivity to TB antigens (TST and/or IGRA) therefore become critical to the etiologic diagnosis of TB, provided that the other conditions are fulfilled. In addition, given the limitations of currently available molecular diagnostic tests (such as polymerase chain reaction [PCR]),4 much reliance is placed on the TST/IGRA for diagnosis.
TB immunoreactivity denotes the memory T-cell response (antigen recall) to TB antigens that can be tested in vivo (as in TST) or in vitro (as in IGRA). Such T cell memory responses can be detected during active disease, latent infection, or even after the infection has been cleared from the body.5 The inability of TST or IGRA to distinguish active TB disease from inactive TB infection represents a formidable challenge in the diagnosis of OTB. This has therapeutic implications because it may lead to over-diagnosis of OTB and the initiation of potentially toxic anti-TB therapy (ATT), especially if appropriate clinical signs are absent and non-TB diagnoses are not ruled out. Conversely, especially in TB-endemic countries, the significance of a positive IGRA or TST may be downplayed by the treating ophthalmologist, thus denying ATT to the patient and prolonging intraocular inflammation.
The significance of this problem is best highlighted by the high prevalence of TB immunoreactivity among patients with uveitis compared with the general population.6–9 This pattern has been recognized in successive studies in TB-endemic and non–TB-endemic countries. For example, in Thailand and Indonesia (both TB-endemic countries), the IGRA positivity in a cohort of consecutive uveitis patients was 36% and 61%, respectively, much higher than their respective countrywide prevalence.6, 7 In similar cohorts of patients in the United States and the Netherlands (non–TB-endemic countries), IGRA positivity was found to be 14.4% and 13%, respectively, among patients with uveitis, again much higher than the countrywide prevalence (5% and 0.01%, respectively).8, 9 In all of the above studies, only a minority of patients with IGRA-positive uveitis were classified as active OTB (though based on variable diagnostic criteria), while the majority (~60% and above) were reported as uveitis of unknown origin that did not match either OTB or any known non-TB entity.
The above data show that there exists a large subset of patients with undifferentiated uveitis and positive TB immunoreactivity, in both TB-endemic and non–TB-endemic countries. The current classification criteria do not recognize these patients as having OTB,3 and the need for initiating ATT in such patients remains unclear. Furthermore, the impact of systemic TB immunoreactivity on the intraocular immune response of this large cohort of patients with uveitis remains largely unknown. Our earlier study had demonstrated that the intraocular T cells in patients with clinically diagnosed OTB are reactive to both mycobacterial and retinal autoantigens, with the autoreactive T cells being more proinflammatory and resistant to activation-induced cell death.10 While the demonstration of an autoreactive immune response in eyes with infectious uveitis was significant, the OTB diagnostic criteria used in our earlier study did not strictly match the recently introduced SUN classification criteria. Hence, it is possible that the OTB cohort in that study was comprised of patients with “true” OTB as well as undifferentiated uveitis with TB immunoreactivity. Any difference in the intraocular immune response between the above two clinical phenotypes remained unrecognized.
In the current study, we have investigated the intraocular antigen-specific immune response in different clinical phenotypes of TB-immunoreactive uveitis. Here, we have applied the SUN classification criteria for OTB, and/or positive TB PCR to strictly differentiate true OTB from TB-immunoreactive uveitis of unknown origin (labeled as the UNK group). Our study revealed a clear separation between these two subgroups in the cytokine response to TB-specific antigen. Furthermore, a proinflammatory cytokine response to the retinal autoantigen—interphotoreceptor retinoidbinding protein (IRBP)—was noted in both the subgroups of TB-immunoreactive uveitis as well as patients with non–TB-immunoreactive uveitis. However, the IRBP-specific response was not influenced by the TB immunoreactivity, clinical phenotype, inflammatory score, or the duration of disease.
Methods
Study Design
Our single-center, retrospective cross-sectional study aimed to compare the antigen-specific cytokine responses between different subgroups of patients with TB-immunoreactive or non–TB-immunoreactive uveitis. TB immunoreactivity was defined by a positive TST, with or without a positive IGRA result. The patients included in our study were divided into 3 groups. Group 1 (OTB) was restricted to patients who fulfilled the SUN classification criteria for OTB and/or tested positive for TB-PCR from the vitreous sample. Notably, the diagnostic accuracy for OTB with the SUN criteria was 98.2% (95% confidence interval 96.5–99.1), and the misclassification rate was as low as 3.6% during validation.3 Group 2 included the remaining patients with a positive TST who did not fulfill the SUN criteria and who had a negative TB-PCR study, classified as uveitis of unknown origin (UNK). Group 3 was comprised of TST-negative patients, with or without a well-defined non-TB uveitis entity, who were classified as non-TB control subjects.
Vitreous samples were collected from all patients by pars plana vitrectomy. The entire cellular infiltrate was isolated from the sample and activated with either the TB-specific antigen, early secreted antigenic target 6-kDa (ESAT-6) peptide, or the retinal autoantigenic peptide IRBP (1-20). Intracellular cytokine response and surface markers (where applicable) were measured with multicolor flow cytometry.
Inclusion And Exclusion Criteria
Patients requiring diagnostic or therapeutic vitrectomy for the management of intraocular inflammation between October 1, 2020 and November 30, 2021 were included in the study. All patients had vitreous haze of ≥2+ (National Institutes of Health photographic scale) at the time of surgery.11 All patients included in the study received a TST (5 tuberculin units; Span Diagnostics, India), wherein ≥10 mm of induration at the end of 48 hours was considered a positive reaction. IGRAs were not performed in all patients because these tests are not recommended in the guidelines for management of extrapulmonary TB in India.12 The study was approved by the LV Prasad Eye Institute Ethics Committee (study code 2019-133-IM-26) and adhered to the tenets of Declaration of Helsinki. Written informed consent was obtained from each patient before their inclusion in the study.
For data analysis, the diagnosis of OTB was restricted to patients fulfilling the SUN classification criteria (published online April 2, 2021),3 and/or positive TB-PCR from vitreous samples. The SUN criteria included 4 clinical signs in the posterior segment: serpiginous-like choroiditis, occlusive retinal vasculitis, choroidal tuberculoma, and multifocal choroiditis (in the presence of active systemic TB). The OTB classification was applied when clinical signs were accompanied by evidence of Mycobacterium tuberculosis infection (histologic/microbiologic, positive IGRA, or positive TST) and negative tests for sarcoidosis and syphilis. Patients with positive TST with or without IGRA but having undifferentiated uveitis not fulfilling SUN classification criteria for OTB and having negative TB-PCR were categorized as UNK. Patients with negative TST with or without a negative IGRA and having negative TB-PCR were categorized as non-TB control subjects. Finally, those with a positive TST with or without IGRA but presenting with a well-characterized non-TB entity were excluded from the study.
Indications For Pars Plana Vitrectomy In The Study
Indications for pars plana vitrectomy included: 1) clearing of inflammatory debris in patients with nonresolving or recurrent inflammation after corticosteroid or non-steroidal immunosuppressive therapy; 2) diagnostic vitrectomy to sample for PCR assay; and 3) management of complications of uveitis, such as rhegmatogenous or tractional retinal detachment, in the presence of active inflammation. However, eyes with vitreous haemorrhage were excluded.
Vitreous Sample Collection
Vitreous samples were collected by 25-G microincision pars plana vitrectomy in all patients. Undiluted vitreous (0.5 mL) was collected for TB-PCR, while the remaining vitrectomy was performed under Ringer lactate infusion. The entire aspirate was collected into a 20-cc syringe by slow manual aspiration. Most eyes had complete posterior vitreous detachment (PVD) or had an incomplete PVD with a stump attached to the disc that could be easily trimmed with the cutter. In rare cases, PVD had to be induced through active suction from the vitrectomy machine, and the remaining surgery completed with slow manual aspiration. Vitreous base excision was not performed for any of the patients.
Vitreous Cell Isolation
Vitreous fluid samples were immediately placed on ice and processed within 2 hours of surgery. The samples were diluted with an approximately equal volume of sterile phosphate-buffered saline and filtered through a cell strainer (pore size 40 μm) to make single-cell suspensions. The cells were then centrifuged at 500 g for 20 minutes and the pellet was carefully washed once with RPMI 1640. The cell pellet was resuspended in 0.32 mL complete RPMI medium (RPMI medium 1640 with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptavidin, and 2 mM L-glutamine [GIBCO/Invitrogen]). Fifteen microliters of this cell suspension was mixed with an equal volume of dilute trypan blue for viability checking as well as counting of cells with a hemacytometer using a compound microscope (Nikon Corporation). Antigen-specific stimulation experiments were carried out on the same day without any freeze-down to investigate the cytokine responses in as close-to–in vivo conditions as possible.
Antigen-Specific Stimulation
M tuberculosis ESAT-6 peptide 1 (NR-34824; BEI Resources) and IRBP1 peptide (IRBP [1-20], human, GenScript, RP20269) were used for antigen-specific stimulation of the total cell isolate. Briefly, cells were plated in 96 well U-shaped culture plates (approximately 0.75 × 105/mL) and stimulated with 10 μg/mL of ESAT-6 or 10 μg/mL of IRBP, along with 2 μg/mL of anti-CD28 antibody (Invitrogen, 16-0281-38). The control samples were activated with anti-CD28 alone. These were then incubated at 37°C and 5% CO2 for approximately 14 hours with 10 μg/mL Brefeldin A and 2 μmol/mL monensin added during the last 8 hours of incubation. The final peptide-stimulated cytokine response (in percent) was calculated by subtracting the control response from the total peptide-stimulated response.
Flow Cytometry
Intracellular cytokine staining was carried out as described previously,10 with minor modifications. Briefly, after stimulation with the antigenic peptide, cells were harvested and stained with LIVE/DEAD fixable blue dead cell stain kit (Invitrogen, L34962) for 15 minutes. After washing, they were fixed for 1 hour at 4°C with fixation buffer (Invitrogen, 00-5123-43) and stained with antibodies (1:100) in permeabilization buffer (Invitrogen, 00-8333-56) for 45 minutes. Antibodies used were as follows: interferon-gamma (IFNγ) monoclonal antibody (4S.B3), PerCP-Cyanine5.5, eBioscience (Invitrogen, 45-7319-42), interleukin 17 (IL-17) monoclonal antibody (eBio64DEC17), APC-eFluor 780, eBioscience (Invitrogen, 47-7179-42), and tumor necrosis factor-alfa (TNFα) monoclonal antibody (MAb11), eFluor 450, eBioscience (Invitrogen, 48-7349-42). Cells were then washed and resuspended in 2% fetal bovine serum (Thermo Fisher Scientific) for flow cytometry. Cells were acquired in CytoFLEX S N2-V3-B5-R3 (Beckman Coulter) and analyzed by CytExpert (Beckman Coulter) and SPICE 6 software (https://niaid.github.io/spice). For intracellular cytokine gating, we have followed the guidelines suggested by International Multiconsortia Proficiency Panel conducted by the Cancer Immunotherapy Consortium.13, 14 We also analyzed T cell surface markers (CD3, CD4, and CD8) and the monocyte marker CD14 in select samples where sufficient cells were isolated. However, the small number of samples where T cell immunophenotyping could be performed did not allow meaningful comparison of intracellular cytokines gated to specific T cell markers.
Statistical Analyses
We performed the nonparametric Kruskal–Wallis test to compare ≥3 independent experimental groups. The Wilcoxon matched-pairs signed-rank test was used to compare the median from 2 paired groups. Statistical tests were performed using Prism software (version 9.0; GraphPad) and SPICE 6 (ImmunoTechnology Section, VRC/NIAID/NIH) software.15 Results were expressed as median with interquartile range. P < .05 was considered significant.
Results
Patient Profiles
The patient profiles of each sub-group are listed in the Table 1. The numbers of patients included in each group were similar. Only 1 eye was included for each patient. The median age was lower for the OTB group, but no statistical difference was noted between any of the groups (Supplemental Figure 1). The clinical signs in the OTB group (n = 23) comprised occlusive retinal vasculitis (n = 8), serpiginous-like choroiditis (SLC, n = 6), and multifocal choroiditis (MFC, n = 5), following the SUN criteria.3 Four patients with MFC, 2 with panuveitis, and 1 with intermediate uveitis were included in the OTB group after testing positive for TB-PCR from vitreous samples. One patient with MFC had active pulmonary TB. Three patients with retinal vasculitis and 1 patient with serpiginous-like choroiditis also tested positive for TB-PCR. Thus, nearly half (11/21) of patients with OTB tested positive on PCR. One-third of patients (8/21) had chest radiographic signs that included healed/active pulmonary TB or mediastinal lymphadenopathy.
Table 1. Clinical Categorization of Patients Undergoing Pars Plana Vitrectomy.
| SN | Distinguishing Features | OTB (n = 23) | UNK (n = 24) | Non-TB Control Subjects (n = 24) |
|---|---|---|---|---|
| 1 | Clinical phenotypes | Retinal vasculitis, SLC, MFC (as per SUN criteria, 21/23); intermediate or panuveitis with positive TB-PCR (2/23) |
Intermediate or panuveitis (all cases); not matching any well-de?ned non-TB diagnosis |
Intermediate or panuveitis (23/24); MFC (1/24) |
| 2 | Tuberculin skin test | Positive | Positive | Negative |
| 3 | TB-PCR | Positive or negative | Negative | Negative |
MFC = multifocal choroiditis; OTB = ocular tuberculosis; PCR = polymerase chain reaction; SLC = serpiginous-like choroiditis; SUN = Standardization of Uveitis Nomenclature; TB = tuberculosis; UNK = uveitis of unknown origin.
In the UNK and non-TB groups, almost all patients were diagnosed with either panuveitis or intermediate uveitis, with only 1 case of MFC in the non-TB group. More than half (13/24) of patients in the UNK group had chest radiographic signs, all of which were nonspecific and unrelated to healed or active TB. Approximately two-thirds of the patients in each of the 3 groups had duration of ocular disease >3 months. All patients with OTB began treatment with ATT postvitrectomy. Four patients in the UNK group also received ATT based on the decision of the treating ophthalmologist.
Specific Monofunctional And Polyfunctional Cytokine Responses To Esat-6 Distinguish Different Tb-Immunoreactive Phenotypes
Total cell yields from the vitreous samples varied from the low thousands to >1.5 × 105 cells but the range of cell yields was similar for all 3 groups (Supplemental Figure 1). This impacted the number of flow cytometric analyses which were possible for each sample, but sufficient cell yields were available to perform intracellular cytokine studies on all samples. The median proportion of CD3+ T cells (CD3+) monocyte/myeloid cells (CD14+) in the samples for which there were sufficient cells was 2.16 (Supplemental Figure 2, A). In the CD3+ T cell population, there was a predominance of CD4+ over CD8+ T cells (Supplemental Figure 2, B through D).
We compared the intracellular single and dual cytokine responses from vitreous cellular infiltrates of each subgroup of patients, following activation with the M tuberculosis–specific protein ESAT-6. Since the total number of cells isolated from the vitreous samples was variably low (Supplemental Figure 1), the gating strategy was centered on the presumed lymphocyte population in the initial forward scatter/side scatter gate of the total cell population (Supplemental Figure 3). It was assumed that the cytokine data in the experiments below were derived from the predominant CD4+ T cell population, for the following reasons. First, the antigen-specific activation (ESAT-6 or IRBP) and anti-CD28 costimulation (in the absence of innate immune triggers) used in these experiments limits the cytokine response to T cells. Secondly, myeloid cells present in the vitreous sample facilitate antigen presentation to the CD4+ T cells and follows a similar strategy routinely used for antigen-specific activation of T cells via peripheral blood mononuclear cells.16 Finally, the expression of multiple proinflammatory cytokines (TNFα, IFNγ, and IL-17) from single cells (polyfunctional response) is also characteristic of CD4+ T cell responses.17
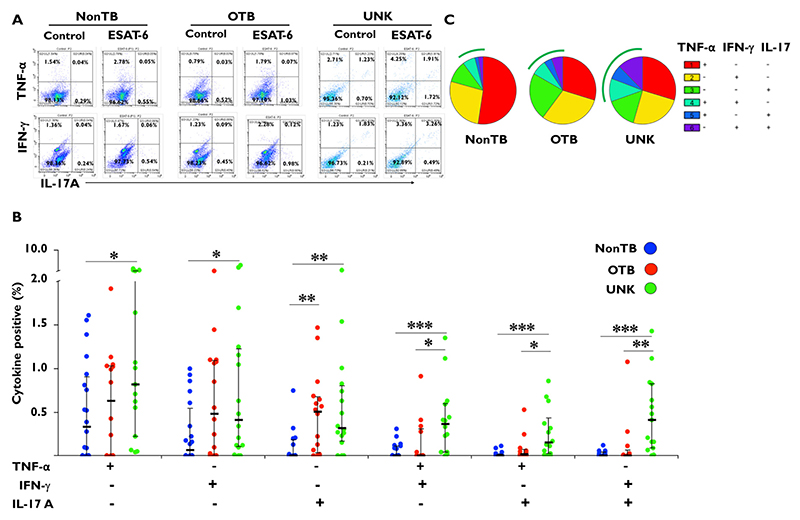
We initially compared data between patients with OTB and patients with non-TB uveitis. Cytokine responses to ESAT-6 from vitreous cells were similar in both groups apart from the IL-17 response (P = .007) that was higher in the OTB group (Figure 1). We then compared responses from the UNK patients to the patients with non-TB uveitis. All monofunctional and polyfunctional cytokine responses in the UNK group were significantly higher than the non-TB group. We finally compared responses between the OTB group and the UNK group. While the monofunctional cytokine responses were comparable between these 2 groups, all 3 polyfunctional cytokine responses—IFNγ /IL-17 (P = .002), TNFα/IL-17 (P = .02), and TNFα/IFNγ (P = .01), were significantly greater for UNK than the OTB group.
Figure 1.
Specific monofunctional and polyfunctional cytokine responses to early secreted antigenic target 6-kDa (ESAT-6) distinguish different tuberculosis (TB)-immunoreactive phenotypes. Cells from vitrectomy samples of patients with uveitis were stimulated with 10 μg/mL of ESAT-6 peptide along with anti-CD28 antibody (2 μg/mL) for approximately 14 hours. In the last 8 hours,10 μg/mL Brefeldin A and/or 2 μmol/mL monensin were added. Cells were fixed, stained, and analyzed for tumor necrosis factor-alfa (TNFα), interleukin 17 (IL-17), and interferon-gamma (IFNγ) by flow cytometry. (A) Representative dot plot from 1 patient sample for each of the 3 groups: non-TB control subjects, patients with ocular TB (OTB), and patients with uveitis of unknown origin (UNK). (B) Bar figure representing TNFα, IFNγ, and IL-17 mono- and dual-cytokine responses in each group. (C) Pie chart representing the proportion of TNFα, IFNγ, and IL-17 mono- and dual-cytokine responses in each group. The arcs represent the total polyfunctional component of the antigen-specific response in each group. ESAT-6–specific cytokine percentages were subtracted from paired unstimulated samples and the resulting positive cytokines percentages from different groups were compared using the Wilcoxon rank sum test. Data are shown as median ± interquartile ratio. P < 0.5 was considered statistically significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (Non-TB group, n = 24; OTB group, n = 23; and UNK group, n = 24.)
Irbp-Specific Cytokine Responses Are Comparable Between Tb-Immunoreactive And Nonreactive Phenotypes
A role for autoimmunity has been proposed in the immunopathogenesis of most cases of noninfectious uveitis18 and recently in infectious uveitis.10 Our earlier study demonstrated antigen-specific cytokine responses to both ESAT-6 and retinal crude extract from vitreous samples of patients with clinically diagnosed ocular TB. However, tissue crude extracts lack specificity, and so cross-reactivity between ESAT-6 and peptide fragments in the crude extract may have been possible. In the current study, we compared responses to the uveitogenic peptide IRBP 1-2019 with ESAT-6 where there is no sequence homology as shown by a basic local alignment search tool alignment (data not shown).
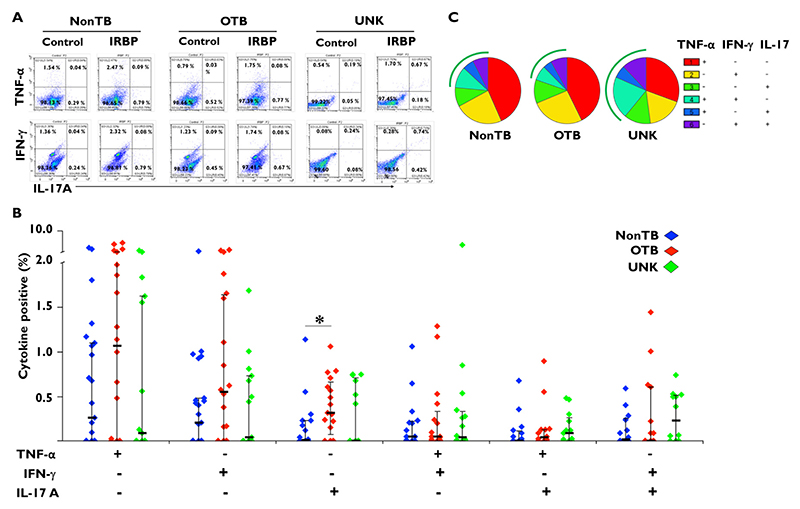
Our data show that proinflammatory cytokine responses were induced by stimulation with IRBP 1-20 in vitreous samples from all 3 patient groups in the study (Figure 2). However, apart from a greater IL-17 response in the OTB group than the non-TB uveitis group (P = .02), we did not find a significant difference in either the monofunctional or polyfunctional cytokine responses between any of the groups in the study.
Figure 2.
Interphotoreceptor retinoid-binding protein (IRBP)-specific cytokine responses are comparable between tuberculosis (TB)-immunoreactive and TB-nonreactive phenotypes. Vitreous infiltrated cells from patients with uveitis were stimulated with 10 μg/mL of IRBP1 peptide (IRBP [1-20]) along with anti-CD28 antibody (2 μg/mL) for approximately 14 hours. In the last 8 hours,10 μg/mL Brefeldin A and/or 2 μmol/mL monensin were added. Cells were fixed, stained, and analyzed for tumor necrosis factor-alfa (TNFα), interleukin 17 (IL-17), and interferon-gamma (IFNγ) by flow cytometry. (A) Representative dot plot from 1 patient sample for each of the 3 groups: non-TB control subjects, patients with ocular TB (OTB), and patients with uveitis of unknown origin (UNK). (B) Bar figure representing TNFα, IFNγ, and IL-17 mono- and dual-cytokine responses in each group. (C) Pie chart representing the proportion of TNFα, IFNγ, and IL-17 mono- and dual-cytokine responses in each group. The arcs represent the total polyfunctional component of the antigen-specific response in each group. IRBP1-specific cytokine percentages were subtracted from paired unstimulated samples and the resulting positive cytokines percentages from different groups were compared using the Wilcoxon rank sum test. Data are shown as median ± IQR. P < 0.5 was considered statistically significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (Non-TB group, n = 24; OTB group, n = 23; and UNK group, n = 24.)
Esat-6 Responsive Polyfunctional Cells Are Phenotypically Different Compared To Respective Monofunctional Cells
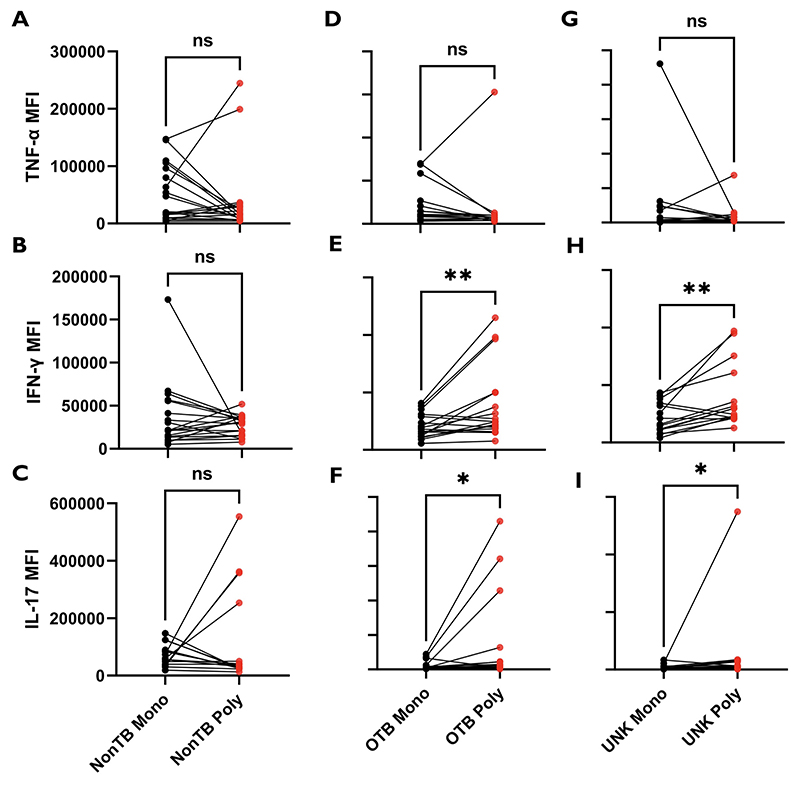
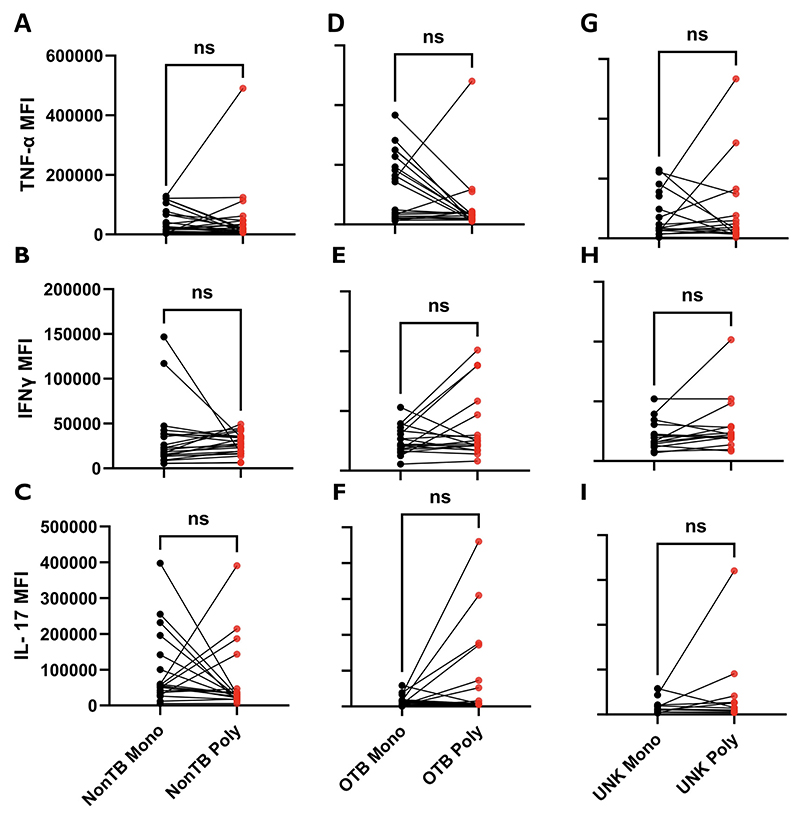
Polyfunctional or multifunctional cells are single cells expressing ≥2 cytokines.20 Since polyfunctional cytokine responses to ESAT-6 were the primary differentiating factor between the UNK and OTB groups, we sought to further characterize the polyfunctional phenotype to ESAT-6. We used the median fluorescent intensity (MFI) to measure the total cytokine produced per cell and compared the MFI for a specific cytokine in the polyfunctional cells with the MFI for the same cytokine from monofunctional cells of the same sample (Figure 3). We noted that the ESAT-6–responsive polyfunctional cells produced significantly more IFNγ (P < .01) and IL-17 (P < .05) per cell than sample-matched monofunctional cells in both the OTB and the UNK groups but not in the non-TB group. We also attempted to compare the activation and proliferation profiles of polyfunctional and monofunctional cells with the surface markers HLA-DR and Ki-67 respectively. However, the expression levels for these markers were too low for a meaningful analysis. We also investigated whether similar differences in cytokine secretion exist between the monofunctional and polyfunctional cells responsive to IRBP. However, no such difference was found for any of the 3 proinflammatory cytokines (Figure 4). Furthermore, there was no difference in the mono- or dual-cytokine secretion between ESAT-6 and IRBP responses in paired samples of the same patient (Supplemental Figure 4).
Figure 3.
Early secreted antigenic target 6-kDa (ESAT-6)-specific expression of cytokines in polyfunctional T cells as compared with paired monofunctional cells in different groups. After performing intracellular cytokine assay after treatment with 10 μg/mL of ESAT-6, the magnitude of expression of cytokines (median fluorescent intensity [MFI]) in polyfunctional T cells was compared with their paired monofunctional counterpart. (A through C) Monofunctional vs polyfunctional MFI for tumor necrosis factor-alfa (TNFα), interferon-gamma (IFNγ), and interleukin 17 (IL-17), respectively, in the non-TB group. (D through F) Monofunctional versuss polyfunctional MFI for TNFα, IFNγ, and IL-17, respectively, in the ocular TB (OTB) group. (G through I) Monofunctional vs polyfunctional MFI for TNFα, IFNγ, and IL-17, respectively, in the uveitis of unknown origin (UNK) group. Median fluorescent intensities of different groups were compared using the Wilcoxon matched-pairs signed-rank test. P < 0.5 was considered statistically significant. ns = P > .05; *P ≤ .05; **P ≤ .01.
Figure 4.
Interphotoreceptor retinoid-binding protein (IRBP)-specific expression of cytokines in polyfunctional T cells compared with paired monofunctional cells in different groups. After performing intracellular cytokine assay after treatment with 10 μg/mL of IRBP peptide, the magnitude of expression of cytokines (median fluorescent intensity [MFI]) in polyfunctional T cells was compared with their paired monofunctional counterpart. (A through C) Monofunctional vs polyfunctional MFI for tumor necrosis factor-alfa (TNFα), interferon-gamma (IFNγ), and interleukin 17 (IL-17), respectively, in the non-TB group. (D through F) Monofunctional vs polyfunctional MFI for TNFα, IFNγ, and IL-17, respectively, in the ocular TB (OTB) group. (G through I) Monofunctional vs polyfunctional MFI for TNFα, IFNγ, and IL-17, respectively, in the uveitis of unknown origin (UNK) group. Median fluorescent intensities of different groups were compared using the Wilcoxon matched-pairs signed-rank test. P < 0.5 was considered statistically significant. ns = P > .05.
Irbp Response Is Not Influenced By Clinical Phenotype Or Duration Of Disease
While several factors such as microbial pathogens and autoinflammation may trigger the onset of uveitis, autoimmunity to retinal antigens has been proposed to be the driving force for the persistence of inflammation in different forms of uveitis.21 Our earlier study in clinically diagnosed tubercular uveitis had revealed that autoreactive T cells in vitreous samples were resistant to activation-induced cell death and could potentially prolong the inflammatory response in the eye. Hence, we asked if the autoimmune/ IRBP-specific cytokine responses were influenced by any patient or disease related factor. On multivariate linear regression analysis, we found that the clinical subgroup (OTB, UNK, or non-TB control), phenotype (see patient profiles; Table 1), or the duration of disease (<3 or ≥3 months) did not have any influence on the IRBP responses in the vitreous samples.
Discussion
Tests for TB immunoreactivity (TST and IGRA) are central to the current diagnostic strategy for OTB. Their role in OTB diagnosis has been reinforced by the publication of the machine learning–based SUN classification criteria.3 However, the utility of these tests is undermined by the high prevalence of TB immunoreactivity in the general uveitis population with a majority of such patients being classified as UNK.6–9 Our study revealed fundamental differences in the intraocular immune response between undifferentiated TST-positive patients with uveitis (UNK group) and patients classified as OTB based on the SUN criteria and/or positive TB-PCR. Contrary to our expectations, we noted that the cytokine responses to the M tuberculosis antigen ESAT-6 were significantly higher in the UNK group not only compared with patients with non-TB uveitis but also to the OTB group. Specifically, the UNK and OTB groups differed in the polyfunctional (dual-cytokine) responses to ESAT-6, and the polyfunctional cells expressed higher levels of proinflammatory cytokines than the corresponding monofunctional cells. In addition, we also demonstrated that retinal autoantigen IRBP-specific intraocular cytokine responses occurred in all cases of posterior segment uveitis, regardless of their clinical phenotype, chronicity, or TB immunoreactive status.
Several studies have documented higher frequencies of polyfunctional cytokine responses in peripheral blood samples from patients with latent or successfully treated pulmonary TB compared with active TB.22–24 Polyfunctional responses have also been reported from local sites of inflammation, such as from bronchoalveolar lavage, in individuals with latent TB.25 Pathogen-specific polyfunctionality of T cells has been proposed to be a marker of T cell efficacy and immune protection, not only in TB infections but in other infections.20, 26 Considering that the OTB phenotype in our study was based on the SUN criteria that has high diagnostic accuracy and a low misclassification rate,3 we assume that this group more likely correlates with active TB infection in the eye. Notably, all patients in the OTB group were treated with ATT in our study. Conversely, the UNK group, only 4 (16.7%) of whom were considered for ATT, could be representative of latent infection. However, contradictory results such as a predominant polyfunctional response in active TB have also been reported in other studies.27, 28 Alternatively, high CD4+ T cell TNFα single-cytokine response in active TB has been used to discriminate it from latent TB.29
Regardless of its ability to distinguish between active and latent infection, the main strength of our data lies in demonstrating a robust demarcation in the intraocular TB antigen–specific cytokine response between two different clinical phenotypes of TB-immunoreactive uveitis.One of these phenotypes (OTB) requires mandatory treatment with ATT, while in the other larger group, namely undifferentiated TST+ uveitis (UNK), the indications for ATT are not well-defined. Our data offer mechanistic support to the SUN classification criteria for tubercular uveitis. They provide a concrete immunologic basis for future studies on diagnostic tests, biomarkers, and treatment outcomes in OTB, to follow the SUN criteria for patient inclusion. For patients who are TST+ who cannot be classified as OTB based on the SUN criteria (UNK group in the current study), the data suggest that judicious anti-inflammatory therapy without the need for adjunctive ATT may be sufficient to control inflammation and prevent immunemediated tissue damage. The protective host immune response, consisting of antigen-specific polyfunctional cells, can be expected to control any reactivation of infection, within or outside the eye, though it may require careful monitoring to avoid a shift in phenotype from undifferentiated UNK to overt OTB.
Our study also documented the antigen-specific intraocular response to the retinal antigen IRBP, a known uveitogenic autoantigen in experimental models.30 Immune responses to retinal antigens have been observed in the peripheral blood in patients with uveitis31–33 but may also be present in normal individuals.32 We, and others, have also reported retinal antigen (crude extract)-specific response in tubercular uveitis, and birdshot chorioretinopathy, respectively.10, 34 To the best of our knowledge, this is the first report of an IRBP-specific response from intraocular T cells in patients with uveitis. That we did not find any influence of the clinical phenotype, or any difference between infectious and noninfectious etiology, raises the question whether this autoreactivity is a cause or a consequence of intraocular inflammation. One possibility is that the IRBP response is an epiphenomenon resulting from the tissue damage and release of retinal antigens in a proinflammatory context to the periphery.35 Nevertheless, considering that the retinal autoreactive cells are highly proinflammatory and resistant to activation-induced cell death,10 it is possible that these cells might have a contributory pathologic role in intraocular inflammation.
Our study was limited by the use of single peptides for eliciting both the antimycobacterial and retinal antigen–specific responses. Thus, T cell responses to other immunodominant peptides (mycobacterial and retinal) are not covered in our data. However, our attempt to activate vitreous T cells with a peptide pool comprised of multiple ESAT-6 peptides and those from culture filtrate protein 10-kDa consistently yielded lower cytokine responses than the single ESAT-6 peptide used in this study (data not shown). This could be attributed to the dilution of immunodominant peptides within the peptide pool. We also did not specifically identify the source of cellular cytokine in order to minimize cell losses from a relatively low starting yield from vitreous samples. However, for reasons described above, we believe that the cytokine response noted in our study was derived predominantly from CD4+ and CD8+ T cells. Further studies using single cell RNA sequencing technology are planned.
In summary, our study demonstrates that TST-positive undifferentiated uveitis generates a stronger monofunctional and polyfunctional intraocular cytokine response than active OTB, suggesting that the anti-TB immune response in TST-positive undifferentiated uveitis is more effective in protecting from pathogen-based tissue damage. An additional autoreactive anti-IRBP response was characteristic of both TB- and non-TB–associated intraocular inflammation.
Supplementary Material
Supplemental Material available at AJO.com.
Table 2. Clinical Characteristics of Patients Assigned to Each of the 3 Groups: Ocular Tuberculosis (OTB), Uveitis of Unknown Origin (UNK) and Nontuberculosis Control Subjects.
| OTB | UNK | Non-TB | |
|---|---|---|---|
| Total patients, n | 23 | 24 | 24 |
| Gender (M:F) | 17:6 | 12:12 | 9:15 |
| Median age, years (IQR) | 29 (27-45) | 42 (28.5-49.8) | 41 (24-47) |
| Clinical phenotypes | PU = 2; IU = 1; MFC = 5; RV = 8; SLC = 6 | PU = 12; IU = 12 | PU = 6; IU = 17; MFC = 1 |
| Duration, ≤3 months:>3 months | 8:15 | 8:16 | 6:18 |
| Abnormal chest radiography, n (%) | 8 (34.8) | 13 (54.2) | 2 (8.3) |
| M tuberculosis PCR, n (%) | 11 (47.8) | 0 | 0 |
| Anti-TB therapy, n (%) | 23 (100) | 4 (16.7) | NA |
F = female; IQR = interquartile range; IU = intermediate uveitis; M = male; MFC = multifocal choroiditis; NA = not applicable; OTB = ocular tuberculosis; PCR = polymerase chain reaction; PU = panuveitis; RV = retinal vasculitis; SLC = serpiginous-like choroiditis; TB = tuberculosis; UNK = uveitis of unknown origin.
Funding/Support
S.B. was funded by the DBT Wellcome Trust India Alliance Fellowship in Clinical and Public Health Research (IA/CPHI/18/1/503975) and the Hyderabad Eye Research Foundation. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest.
Financial Disclosures: The authors indicate no financial support or conflicts of interest. All authors attest that they meet the current ICMJE criteria for authorship.
References
- 1.Agrawal R, Gunasekeran DV, Raje D, et al. Global variations and challenges with tubercular uveitis in the Collaborative Ocular Tuberculosis Study. Invest Ophthalmol Vis Sci. 2018;59(10):4162–4171. doi: 10.1167/iovs.18-24102. [DOI] [PubMed] [Google Scholar]
- 2.Gupta V, Shoughy SS, Mahajan S, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):14–24. doi: 10.3109/09273948.2014.986582. [DOI] [PubMed] [Google Scholar]
- 3.Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for tubercular uveitis. Am J Ophthalmol. 2021;228:142–151. doi: 10.1016/j.ajo.2021.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Agrawal R, Gunasekaran DV, et al. The Collaborative Ocular Tuberculosis Study (COTS)-1 report 3: polymerase chain reaction in the diagnosis and management of tubercular uveitis: global trends. Ocul Immunol Inflamm. 2019;27(3):465–473. doi: 10.1080/09273948.2017.1406529. [DOI] [PubMed] [Google Scholar]
- 5.Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. doi: 10.1136/bmj.k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathanapitoon K, Kunavisarut P, Sirirungsi W, Rothova A. Looking for ocular tuberculosis: prevalence and clinical manifestations of patients with uveitis and positive QuantiFERON®-TB Gold test. Ocul Immunol Inflamm. 2018;26(6):819–826. doi: 10.1080/09273948.2016.1245760. [DOI] [PubMed] [Google Scholar]
- 7.La Distia Nora R, Sitompul R, Bakker M, et al. Tuberculosis and other causes of uveitis in Indonesia. Eye. 2018;32(3):546–554. doi: 10.1038/eye.2017.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakin M, Kesav N, Cheng SK, Caplash S, Gangaputra S, Sen HN. The association between QuantiFERON-TB Gold test and clinical manifestations of uveitis in the United States. Am J Ophthalmol. 2021;230:181–187. doi: 10.1016/j.ajo.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groen-Hakan F, van Laar JAM, Bakker M, van Hagen PM, Hardjosantoso H, Rothova A. Prevalence of positive QuantiFERON-TB Gold in-tube test in uveitis and its clinical implications in a country nonendemic for tuberculosis. Am J Ophthalmol. 2020;211:151–158. doi: 10.1016/j.ajo.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Tagirasa R, Parmar S, Barik MR, Devadas S, Basu S. Autoreactive T cells in immunopathogenesis of TB-associated uveitis. Invest Ophthalmol Vis Sci. 2017;58(13):5682–5691. doi: 10.1167/iovs.17-22462. [DOI] [PubMed] [Google Scholar]
- 11.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SK, Ryan H, Khaparde S, et al. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. 2017;145(4):448–463. doi: 10.4103/ijmr.IJMR_1950_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeil LK, Price L, Britten CM, et al. A harmonized approach to intracellular cytokine staining gating: results from an international multiconsortia proficiency panel conducted by the Cancer Immunotherapy Consortium (CIC/CRI) Cytometry A. 2013;83(8):728–738. doi: 10.1002/cyto.a.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price LS, Adamow M, Attig S, et al. Gating harmonization guidelines for intracellular cytokine staining validated in second international multiconsortia proficiency panel conducted by Cancer Immunotherapy Consortium (CIC/CRI) Cytometry A. 2021;99(1):107–116. doi: 10.1002/cyto.a.24244. [DOI] [PubMed] [Google Scholar]
- 15.Roederer M, Nozzi JL, Nason MX. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79A:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5(48):eabd2071. doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 18.Lee RW, Nicholson LB, Sen HN, et al. Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol. 2014;36:581–594. doi: 10.1007/s00281-014-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avichezer D, Silver PB, Chan CC, Wiggert B, Caspi RR. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest Ophthalmol Vis Sci. 2000;41(1):127–131. [PubMed] [Google Scholar]
- 20.Boyd A, Almeida JR, Darrah PA, et al. Pathogen-specific T cell polyfunctionality is a correlate of T cell efficacy and immune protection. PLoS One. 2015;10(6):e0128714. doi: 10.1371/journal.pone.0128714. Erratum in: PLoS One. 2015;10(9):e0138395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day CL, Abrahams DA, Lerumo L, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187(5):2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenum S, Grewal HM, Hokey DA, et al. The frequencies of IFNγ +IL2+TNFα+ PPD-specific CD4+CD45RO+ T-cells correlate with the magnitude of the QuantiFERON® Gold in-tube response in a prospective study of healthy Indian adolescents. PLoS One. 2014;9(7):e101224. doi: 10.1371/journal.pone.0101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrère-Kremer S, Rubbo PA, Pisoni A, et al. High IFN-γ release and impaired capacity of multi-cytokine secretion in IGRA supernatants are associated with active tuberculosis. PLoS One. 2016;11(9):e0162137. doi: 10.1371/journal.pone.0162137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvela J, Moyer M, Leahy P, et al. Mycobacterium tuberculosis-induced bronchoalveolar lavage gene expression signature in latent tuberculosis infection is dominated by pleiotropic effects of CD4+ T cell-dependent IFN-γ production despite the presence of polyfunctional T cells within the airways. J Immunol. 2019;203(8):2194–2209. doi: 10.4049/jimmunol.1900230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burel JG, Apte SH, Groves PL, McCarthy JS, Doolan DL. Polyfunctional and IFN-γ monofunctional human CD4+ T cell populations are molecularly distinct. JCI Insight. 2017;2(3):e87499. doi: 10.1172/jci.insight.87499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caccamo N, Guggino G, Joosten SA, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40(8):2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 28.Prezzemolo T, Guggino G, La Manna MP, et al. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol. 2014;5:180. doi: 10.3389/fimmu.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harari A, Rozot V, Bellutti Enders F, et al. Dominant TNF-α+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17(3):372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Qian H, Horai R, Chan CC, Caspi RR. Mouse models of experimental autoimmune uveitis: comparative analysis of adjuvant-induced vs spontaneous models of uveitis. Curr Mol Med. 2015;15(6):550–557. doi: 10.2174/1566524015666150731100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Smet MD, Yamamoto JH, Mochizuki M, et al. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am J Ophthalmol. 1990;110(2):135–142. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi M, Usui Y, Okunuki Y, et al. Immune responses to interphotoreceptor retinoid-binding protein and S-antigen in Behçet’s patients with uveitis. Invest Ophthalmol Vis Sci. 2010;51(6):3067–3075. doi: 10.1167/iovs.09-4313. [DOI] [PubMed] [Google Scholar]
- 33.Kijlstra A, Hoekzema R, vd Lelij A, Doekes G, Rothova A. Humoral and cellular immune reactions against retinal antigens in clinical disease. Curr Eye Res. 1990;9(suppl):85–89. doi: 10.3109/02713689008999425. [DOI] [PubMed] [Google Scholar]
- 34.Kuiper JJ, Rothova A, Schellekens PA, et al. Detection of choroid- and retina-antigen reactive CD8(+) and CD4(+) T lymphocytes in the vitreous fluid of patients with birdshot chorioretinopathy. Hum Immunol. 2014;75(6):570–577. doi: 10.1016/j.humimm.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Adamus G. Are anti-retinal autoantibodies a cause or a consequence of retinal degeneration in autoimmune retinopathies? Front Immunol. 2018;9:765. doi: 10.3389/fimmu.2018.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.