Abstract
The premise of cancer immunotherapy is that cancers are specifically visible to an immune system tolerised to healthy self. The promise of cancer immunotherapy is that immune effector mechanisms and immunological memory can jointly eradicate cancers and inoperable metastases and de facto vaccinate against recurrence. For some patients with hitherto incurable diseases, including metastatic melanoma, this promise is being realised by game-changing immunotherapies based on αβ T cells. Today’s challenges are to bring benefit to greater numbers of patients of diverse ethnicities, targeting more cancer types, and achieving cure while incurring fewer adverse events. In meeting those challenges, unique benefits may be offered by γδ T cells which compose a second T cell lineage with distinct recognition capabilities and functional traits that bridge innate and adaptive immunity. γδ T cell-based clinical trials, including “off-the-shelf” adoptive cell therapy (ACT) and agonist antibodies are yielding promising results, although identifiable problems remain. In addressing those problems, we advocate that immunotherapies be guided by the distinctive biology of γδ T cells as elucidated by ongoing research.
Immunotherapies based on Adaptive Immunity
The specific recognition of human cancers and the potential to vaccinate is rooted in adaptive immunity, wherein massively diverse reactivities of cell-type defining antigen receptors, B cell receptors (BCRs / immunoglobulins [Ig]) and T cell receptors (TCRs) derive from the quasi-random somatic recombination of “V-(D)-J” gene segments that encode them, and additionally from somatic mutation for Ig genes(1, 2).
Adaptive immunity in most jawed vertebrates comprises B cells, αβ T cells, and γδ T cells. Contemporary cancer immunotherapy originated in B cell biology, specifically immunoglobulins (a.k.a. antibodies) used to target molecules including EGFR isoforms and CD20 commonly over-expressed by carcinomas and lymphomas, respectively. Such immunoglobulins work in part by ADCC (antibody-dependent cellular cytotoxicity), in which their “Fc” region engages Natural Killer (NK) cells or macrophages via “Fc Receptors” (FcR), provoking killing of target cells bound by the antigen-specific Variable (V)-regions of the antibodies. This bridging of adaptive (B cell) and innate (NK cell / macrophage) immunity has been extremely successful, but at least two major limitations exist: intra-tumoral ADCC-competent cells can be scarce(3) and the number of antibody targets discriminating cancer cells from healthy counterparts is de facto limited.
These limitations were addressed by using cytolytic T cells which recognise major histocompatibility complex (MHC) proteins (HLA in humans) presenting neoantigenic peptides derived from proteins somatically mutated in cancer cells owing to genome instability(4). However, chronically stimulated, neoantigen-specific, tumour-infiltrating T lymphocytes (TILs) frequently upregulate inhibitory “checkpoint” receptors including PD-1, CTLA-4, TIM3, and LAG3, and become functionally exhausted. Limiting exhaustion by antibody-meditated immune checkpoint blockade (ICB) has enjoyed game-changing success, becoming first-line treatment for multiple solid tumours(5). Nonetheless, many tumours have few mutations and hence few neoantigens, and they can also suppress β2-microglobulin which is required for the expression of Class I MHC-peptide complexes, as well as for both CD1 which presents lipid antigens to TCRαβ+ NKT cells, and MR1 which presents metabolite antigens to TCRαβ+ MAIT cells(6). Thus, tumours become invisible to a panoply of αβ T cells. Furthermore, ICB-driven αβ T cell derepression en masse may induce uncontrolled autoreactivity causing permanent and severe adverse events (AEs)(7).
So-called CAR-T immunotherapy is based on combining B and T cell biology. Specifically, gene segments encoding tumour antigen-specific IgV-regions are fused to gene segments encoding T cell signalling mediators, whereupon the resultant chimaeric antigen receptors (CARs) are introduced ex vivo into a patient’s T cells that are expanded and then reinfused(8). By combining antibody specificity with cytolytic T lymphocyte (CTL) capabilities, CAR-T cells deliver ADCC to antigen-expressing cancer cells, invulnerable to MHC loss or low neoantigen load.
Nonetheless, while transformative efficacy has been seen in several haematologic cancers, practical challenges exist(9), including: limited cancer-specific antigenic targets; the fragility of T cells in advanced cancer patients exposed to radiation and/or chemotherapeutics; time-consuming logistics of T cell expansion and transduction; uncertainty that transduction will target CTLs; the likelihood of tumour immune-evasion via CAR-specific antigen loss; and the unpredictability of transduced CAR-T cells reaching and thriving within a hostile solid tumour microenvironment (TME), notwithstanding efforts to overcome this by vaccination-based CAR-T boosting(10,11). Additionally, necessary precautions to constrain severe acute AEs limit the numbers of accredited CAR-T treatment centres, each of which is limited in the numbers of treatments it can perform. This raises serious questions about the accessibility and inclusivity of such an expensive treatment.
In sum, the transformative potentials of therapies rooted in adaptive immunity are qualified by the limited spectrum of tumours that they reach, by collateral damage, and by logistics. As will now be considered, γδ-based therapies may be much less affected by such limitations (Table 1).
Table 1. γδ T cells offer escape from issues limiting αβ T cell-based immunotherapies.
| Phenotype relevant to immunotherapies | αβ T cells | γδ T cells |
|---|---|---|
| MHC-restriction limits unrestricted clinical application | Yes, for most αβ T cells | No obligate MHC restriction for γδ T cells |
| Resistant to cancer cell loss of β2-microglobulin | No | Yes (function may be enhanced by this status) Yes: no evidence for widespread GVHD |
| Function as allogeneic therapy off-the-shelf (a) | No: drives graft-versus-host disease (GVDH) | |
| Function as allogeneic therapy off-the-shelf (b) | No: requires "cloaking" to avoid rejection | No: requires "cloaking" to avoid rejection; may also veto rejection |
| Home to and function within extralymphoid tissues | Some subsets (e.g., TRM) adapt to tissues | Many subsets naturally localize to and function within tissues |
| Readily recognise tumors with low neoantigen load | Only unconventional subsets (NKT, MAIT) may do this |
Yes |
| Recognise a potentially vast diversity of cancer surface antigens | No | Yes |
| Responds to ICB | Yes | Yes, with PD-1+ non-Vδ2 cells showing less exhaustion than PD1+ CD8+ αβ T cells |
| Mostly cytolytic | No | Yes |
| Adverse events | Potentially high because of CRS and cross-reactivity to normal self | Limited CRS because small fraction of CD3+ cells, and because of natural therapeutic window |
| Establishing cure via immunological memory | Yes, directly | Yes, with capacity to orchestrate CD8+ αβ T cell memory |
| ADCC | No, unless CAR-T engineered | Yes, naturally |
| Capacity to cross-present peptide antigens to αβ T cells | No | Yes for Vγ9Vδ2 T cells |
γδ cells: Nature’s CAR-T cells
The suitability of γδ T cells for immunotherapy is suggested by their natural CAR-T cell biology. First, γδTCRs function like antibodies in recognising native antigens(12,13), but because TCRδ V-D-J gene-segment recombination may achieve unmatched diversity(12), the range of tumour targets may be substantially broader, including autologous surface antigens expressed at high levels and/or in altered conformations by many different cancers (Table 2). Parenthetically, evolutionary data highlight overlaps of B and γδ T cell biology, with lizards lacking γδ T cells showing B cell amplification, whereas the B cell compartment may be more limited in marsupials harbouring an extra chain, TCRμ, that amplifies γδ TCR diversity(14).
Table 2. Ligands of human γδ TCRs for which direct binding is documented.
| Ligand | TCRγδ V-usage | Origin of γδ T cells, context | Affinity (KD) | Structure of the TCR/ligand complex |
References |
|---|---|---|---|---|---|
| HLA or HLA-like | |||||
| CD1a | Vδ1/Vγ4 |
PBMC-sorted
γδ T cells using CD1a tetramers |
15-24 μM | resolved |
Wegrecki, Nat Commun.
2022, PMID: 35790773 |
| CD1b | Vδ1 |
PBMC-sorted γδ T cells using
CD1b tetramers loaded with microbial lipids |
9 μM | not reported |
Reijneveld, PNAS. 2020
PMID: 32868441 |
| CD1c | Vδ1 |
PBMC-sorted γδ T cells using
CD1c tetramers loaded with microbial lipids |
23-30 μM | Not reported | Roy, J Immunol 2016, PMID: 26755823 |
|
CD1d |
V81 |
PBMC-sorted γδ T cells using CD1d tetramers |
16-33 μM |
resolved |
Luoma, Immunity, 2013
PMID: 24239091 Uldrich, Nat Immunol, 2013 PMID: 24076636 |
| EPCR | Vδ5/Vγ4 |
Clonally expanded γδ T cells in
the context of CMV-infection |
90 μM | Not reported |
Willcox, Nat Immunol,
2012 PMID: 22885985 |
| MR1 | Vδ3/Vγδ Vδ1/Vγ9 | PBMC-sorted γδ T cells using MR1 tetramers | 0.6-13 μM | resolved |
Le Nours, Science, 2019, PMID: 31857486 Rice, PNAS, 2021, PMID:
34845016 |
| HLA-A2/MART-1 | Vδ1/Vγδ |
γδ T cells differentiated in vitro
from HSPC |
3-71 μM | resolved |
Benveniste, Sci Immunol, 2018 PMID:
30552102 |
| HLA-DR | Vδ1/Vγ3 |
Clonally expanded γδ T cells in
the context of CMV-infection |
3-32 μM | Not reported |
Deseke, J Exp Med,
2022 PMID: 35852466 |
| Others | |||||
| Annexin A2 | Vδ3/Vγ8 |
PBMC γδ T cells reactive
against lymphoma B cells in vitro |
3 μM | Not reported |
Marlin, PNAS, 2017
PMID: 28270598 |
| BTNL3 | Vγ4 |
Intestinal γδ T cells
CMV expanded Vγ4Vδ1 T cells |
20 μM | Not reported |
Melandri, Nat Immunol, 2018, PMID: 30420626
Willcox, Immunity, 2019, PMID: 31628053 |
| BTN2A1 | Vδ2/Vγ9 Vδ1/Vγ9 | Blood Vγ9Vδ2 T cells, CMV-expanded or BTN2A1 tetramer-sorted Vγ9Vδ1 T cells | 40-50 μM | Not reported |
Rigau, Science, 2020,
PMID: 31919129 Karunakaran, Immunity, 2020, PMID: 32155411 |
Second, γδ T cells combine antibody-like recognition with: high expression of granzymes and perforin that facilitate target cell lysis(12); some capacity to present antigen to αβ T cells(15); and expression of activating natural killer cell receptors (NKRs) including natural cytotoxicity receptors(16) and Fc-receptors(17, 18) that can supplement TCRγδ-mediated cancer cell recognition. Following nonclonal NKR engagement, γδ T cells can phenocopy innate immune cells in responding rapidly, delivering effector function without prior clonal expansion, and orchestrating adaptive immunity by antigen presentation and by promoting an immunogenic cytokine milieu(19). Likewise, many human γδ T cells are naturally tissue-tropic(12). Nonetheless, human peripheral blood and tissue-associated γδ T cells also phenocopy adaptive immunity in displaying durable clonotypic, TCR-mediated responses to various challenges(20-22). We advocate that this capacity to straddle innate and adaptive immunity endows γδ T cell-based immunotherapies with unique advantages.
Off-the-shelf therapies
Because γδ T cells are not MHC-restricted, they can be transfused as allografts with little danger of graft-versus-host disease (GVHD) that confounds allogeneic αβ T cell therapies. Hence, in relation to logistics, γδ ACT could be prepared in advance from healthy donors and administered “off-the-shelf”, meeting stringent timeframes for patient treatment, permitting rigorous pre-infusion characterisation and refinement of the product, and permitting the patient to be informed that the identical product has shown demonstrable efficacy in other recipients.
Nonetheless, allogeneic grafts risk rejection by histo-incompatible hosts(23). Several “cloaking” approaches have been developed to limit this, most often by reducing MHC expression, which is particularly facile for γδ ACT derived from inducible pluripotent stem cells(24). Alternatively, CAR-T cells can be transduced with an alloimmune defense receptor (ADR) comprising part of the ligand for 4-1BB linked to a CD3 signalling motif(23). Since alloreactive T and NK cells disproportionately upregulate 4-1BB, they can be specifically targeted by ADR-expressing γδ CAR-T cells that thereby escape deletion. Additionally, a “veto” effect exists whereby adoptively transferred NK cells target graft-reactive CD8 T cells(25): given their parallels with NK cell biology, γδ-based ACT might be optimised to veto their rejection.
Natural cancer cell recognition by blood Vγ9Vδ2 T cells
γδ-based immunotherapies have primarily focussed on Vγ9Vδ2 T cells, the predominant blood γδ T cell subset, and Vδ1 T cells that are commonly enriched in tissues. Being easier to obtain, Vγ9Vδ2 T cells were first into the clinic(26). Vγ9Vδ2 T cells rapidly respond to myriad microbial infections, commonly reflecting polyclonal TCRVγ9Vδ2 reactivity to “phosphoantigens” (pAgs), hydroxymethy-but-2-enyl pyrophosphate (HMBPP) and isopentenyl pyrophosphate (IPP). HMBPP is an intermediate in the microbial MEP (methylerythritol phosphate) pathway that generates cholesterol and sterol derivatives, whereas IPP is an intermediate common to the MEP pathway and its host cell counterpart, the mevalonate pathway(27). Virus-infected and cancer cells often upregulate IPP, e.g., by hydroxy methyl glutaryl co-enzyme reductase upregulation. pAgs bind the intracellular B30.2 domain of Butyrophilin 3A1(BTN3A1)(28), cementing association with BTN2A1 which directly binds TCRVγ9(29–32). BTN and BTN-like (BTNL) proteins are understudied Ig-domain-containing members of the B7-superfamily of lymphocyte regulators(33). Hence, rather than detecting pathogen-specific or cancer cell-specific antigens, Vγ9Vδ2 T cells recognise altered surface expression of BTN2A1/3A1 as immediate consequences of infection or cell transformation(12) and of AMPK sensing of ATP levels during metabolic crisis in cancer cells(34).
BTN2A1 binds to germline-encoded residues of TCRVγ9, eliciting nonclonal responses that are defining hallmarks of innate immunity(35,36). Nonetheless, CDR3δ sequences are also important, implying that additional TCR contacts are made, possibly with BTN3A1 and/or its relatives, BTN3A2 and BTN3A3, which are required for optimal pAg responses(37–39). Vγ9Vδ2 TCRs are not conserved in rodents which has inevitably limited the preclinical models available to support the development of Vγ9Vδ2 T cell-based immunotherapeutics(40).
Immunotherapeutic Vγ9Vδ2 T cells
Natural and synthetic pAgs, e.g., the drug BrHPP (Phosphostim®), can support Vγ9Vδ2 T cell expansion in vitro as a preliminary to ACT, but they display poor pharmacokinetics in vivo. Instead, intracellular IPP levels can be elevated by amino bisphosphonates (ABPs) which inhibit farnesyl pyrophosphate synthase that catalyses geranyl pyrophosphate catabolism downstream of IPP in the MEP and mevalonate pathways(40,41). Because ABPs, e.g., zoledronate and pamidronate, were clinically approved for treating osteoporosis and /or osteolytic cancer metastases, it was easier to obtain regulatory approval for them as Vγ9Vδ2 cell activators in cancer settings. While largely safe, these approaches showed limited clinical efficacy, commonly attributed to Vγ9Vδ2 cell exhaustion caused by chronic stimulation.
These disappointments notwithstanding, Vγ9Vδ2 T cell-based immunotherapeutics remain attractive for many reasons considered above and listed in Table 1. Moreover, ICB combination-therapy has the potential to derepress Vγ9Vδ2 T cells(42). In a Phase I trial of allogeneic Vγ9Vδ2 T cells at Fuda Cancer Hospital, China, safety was confirmed and 18 patients with advanced liver or lung cancer receiving five or more infusions showed greatly prolonged survival (Table 3). In8Bio (Birmingham, USA) has likewise developed Vγ9Vδ2 cells for allogeneic treatment of leukaemia following haematopoietic stem cell transplantation (HSCT) (Table 3). The logic is based on many years’ findings that when risk of relapse was high post-HSCT, long term clinical remission positively correlated with robust and durable γδ T cell reconstitution(43, 44). Preliminary results appear promising, including no current reports of GVHD, durable Vγ9Vδ2 T cell reconstitution probably attributable to highly effective lymphodepletion pre-infusion, and disease stabilisation.
Table 3. Examples of ongoing γδ T cell-based clinical trials in cancer.
| Approach | Clinical trial(s) | Institution/ Company | Therapeutic (product) | Cancer indications | ||
|---|---|---|---|---|---|---|
| NCT04165941, NCT05664243 | U. Alabama, IN8Bio | Chemotherapy-resistant allogeneic or autologous expanded γδ T cells (DeltEX) | Glioblastoma | |||
| NCT03533816 | U. Kansas, IN8Bio | Allogeneic expanded γδ T cells (EAGD) post-HSCT | Leukemias and myelodysplastic syndromes | |||
| NCT05886491 | Takeda | Allogeneic expanded Vδ1 T cells (TAK012) | Relapsed /refractory (r/r) AML | |||
| Unmodified ACT | NCT05358808 | TC Biopharm | Allogeneic expanded γδ T cells (TCB-008) | r/r AML | ||
| NCT05015426 | Lee Moffit Cancer Center | Allogeneic expanded γδ T cells (AAPC) | AML | |||
| NCT05400603 | Emory University | Allogeneic expanded γδ T cells | r/r Neuroblastoma | |||
| NCT03183206, NCT03183219, NCT03183232, NCT03180437 | Fuda Cancer Hospital affiliated with Jinan University (Guangzhou) | Allogeneic expanded Vγ9Vδ2 T cells | late-stage lung and liver cancer | |||
| NCT04696705 | Beijing GD Initiative Cell Therapy Technology | Allogeneic expanded γδ T cells | Non-Hodgkin lymphoma, peripheral T cell lymphoma | |||
| NCT04764513 | Chinese PLA General Hospital | Allogeneic expanded γδ T cells | AML, ALL, myelodysplastic syndromes and lymphoma | |||
| NCT04765462 | Chinese PLA General Hospital | Expanded allogeneic γδ T cells | Various solid tumours | |||
| NCT06069570 | Kiromic BioPharma | Allogeneic expanded γδ T cells combined with radiotherapy | Metastatic Non-Small Cell Lung Cancer | |||
| CAR-ACT | NCT05546723 | Luminary | BAFF-transduced Vδ1+Vδ2 T cells (LMY-920) | r/r Multiple myeloma | ||
| NCT04735471, NCT04911478 | Adicet | CD20-specific CAR-transduced Vδ1 T cells (ADI-001) | B cell lymphomas | |||
| NCT05302037 | Cytomed | NKG2D ligand-specific CAR-transduced Vγ9Vδ2 T cells (CTM-N2D) | Solid and hematological tumors | |||
| NCT06193486 | Lee Moffitt Cancer Center | PSCA-specific CAR-transduced γδ T cells | Metastatic Castration Resistant (mcr) Prostate Cancer | |||
| NCT06150885 | Ever Supreme Biotech | HLA-G-specific CAR-transduced γδ T cells | r/r solid tumours | |||
| Conjugated ACT | NCT05653271 | Acepodia | Anti-CD20 conjugated Vδ2 T cells (ACE1831) | Non-Hodgkin’s Lymphoma | ||
| NCT06415487 | Acepodia | Ant-EGFR conjugated Vδ2 cells (ACE2016) | Solid tumours | |||
| γδTCR-ACT | NCT04688853 | Gadeta | aβ T cells transduced with Vγ9Vδ2 TCR (TEG-002) | r /r multiple myeloma | ||
| NCT04014894 | Eureka | CD19-specific antibody/ γδTCR-transduced T cells (ET019003) | B cell lymphoma | |||
| NCT04864054 | Eureka | GPC3-specific antibody/ γδTCR transduced T cells (ECT204) | Liver cancers | |||
| NCT04502082; NCT04634357 | Eureka | Alpha-fetoprotein-specific antibody/ γδTCR transduced T cells (ET140203) | Liver cancers | |||
| Engagers | NCT04243499, NCT05307874 | ImCheck | BTN3A agonist (ICT01) | Hematological and solid tumors | ||
| NCT05369000 | Lava | PSMA-targeting bispecific γδ T Cell engager (LAVA-1207) | mcr Prostate Cancer | |||
In8Bio has also developed an innovative protocol termed drug resistant immunotherapy (DRI) that focusses the innate responsiveness of Vγ9Vδ2 T cells toward chemotherapy-treated tumours in situ. Specifically, Vγ9Vδ2 cells from glioblastoma (GBM) patients are expanded using zoledronate + IL2, whereupon the cells are transduced with a methylguanine DNA methyltransferase (MGMT) gene that confers resistance to temozolomide (TMZ), a chemotherapeutic standard-of-care for GBM, that by inducting genome damage may induce TCR and NKR antigens for Vγ9Vδ2 cells. The MGMT-transduced Vγ9Vδ2 cells are then administered proximal to the residual tumour site via a Rickham catheter used for TMZ delivery. Improved methods for Vγ9Vδ2 cell preparation and maintenance offer opportunities to repeatedly and locally administer fresh, non-exhausted cells. DRI was successfully applied to four human/mouse xenograft models of primary and refractory GBM(45) and is being delivered clinically (Table 3).
In parallel to ACT, antibody-based engagers are being developed to activate and expand Vγ9Vδ2 cells in vivo, thereby countering the cells’ presumed exhaustion in the TME. Because their numbers are limited, Vγ9Vδ2 activation en masse is less likely than αβ T cell agonism to cause AEs. Based on the role of BTN3A molecules in Vγ9Vδ2 T cell activation, ImCheck Therapeutics (Marseille, France) has developed BTN3A-specific agonist antibodies that substitute for pAgs in driving TCR acitvation. With evidence for tumour suppression in xenograft models reconstituted with Vγ9Vδ2 T cells(46), an antibody (ICT0) has been in a phase 1/2a clinical trial in haematological and solid cancers (Table 3), also with promising results.
Likewise, Lava Therapeutics (Utrecht, The Netherlands) has developed bispecific antibodies, so-called “gammabodies” combining tumour-targeting specificities with a TCR Vγ9 binding domain(47). Those reagents activated Vγ9Vδ2-dependent cytotoxicity against tumour cells in vitro and based on encouraging pre-clinical data, clinical trials commenced. Whereas Lava discontinued (albeit not for safety reasons) a Phase I trial in haematological cancers of LAVA-051 that co-jointly targeted Vγ9 and CD1d, there is a Phase I trial in metastatic castration-resistant prostate cancer, using LAVA-1207 that co-jointly targets Vγ9 and PMSA (Table 3). Other targets include CD123 and CD40 for blood cancers, EGFR for solid tumours, and undisclosed targets in partnership with Janssen (Pennsylvania, USA). γδ-engagers may be insufficient to fully overcome γδ T cell suppression by the TME, but might be effectively combined with ICB modalities targeting checkpoints most relevant for Vγ9Vδ2 cells.
Combining ACT and engagers, Acepodia (California, USA; Taipei, Taiwan) has used innovative chemistry to conjugate allogeneic Vδ2+ T cells to anti-CD20 (ACE1831), for treatment of non-Hodgkin’s lymphoma (Table 3). Encouraging safety and efficacy data reported in May 2024 have added momentum to an analogous approach (ACE2016) targeting EGFR-expressing solid tumors.
There have been multiple uses of Vγ9Vδ2 cells as CAR-T cells. For example, those targeting MUC1-Tn showed similar or superior potency to CAR-αβ T cells in vitro(48), and could be sustained in vivo with human cytokines, displaying IL-2-dependent activity against a metastatic gastric cancer cell line. The attractiveness of CAR-Vγ9Vδ2 T cells should be enhanced by culture methods that improve cell yields and cytolytic potentials, as reported by Leucid (London, U.K.)(49). Another attractiveness is their overcoming off-target activity and exhaustion attributable to high background phosphoprotein levels induced in αβ T cells by CD3ζ-based CARs, a phenotype not observed in Vγ9Vδ2 T cells transduced with chimaeric co-stimulatory receptors that signal via DAP10 and PI3-kinase thereby synergising with CD3ζ-signals induced via the natural Vγ9Vδ2 TCR(50). Nevertheless, it will be important to ascertain that potent CAR-driven signalling does not disrupt signature, innate-like, γδ T cell responses to cytokines and NKR ligands.
Avoiding such concerns, and because γδ T cell numbers can be very limiting, Gadeta (Utrecht, Netherlands) combined Vγ9Vδ2-mediated cancer cell recognition with proven CAR-T cell modalities, by transducing primary αβ T cells with Vγ9Vδ2 TCRs selected for relatively high affinity and strong tumour cell killing(51–53). The resulting TEG (T Cells Engineered to Express a Defined Gamma Delta TCR) have been applied in phase I as ACT targeting multiple myeloma (Table 3). Likewise, Immunocore (Oxfordshire, UK) is developing soluble “ImmTAC” constructs, combining Vγ9Vδ2 ectodomains with an anti-CD3 domain, thereby eliciting substantial T cell responses toward cells recognised by TCRVγ9Vδ2. Lacking MHC-restriction, Vγ9Vδ2-ImmTACs may be efficacious in greater numbers of patients than MHC-restricted TCRαβ-based ImmTacs in clinical use(54), but the potential for AEs will need scrutiny.
Natural cancer cell recognition by non-Vγ9Vδ2 γδ T cells
As well as Vγ9Vδ2 T cells, human blood contains Vδ1+, Vδ3+, and Vδ5+ T cells that are usually strikingly enriched in tissues(55–58). Vδ1+ cells are the most abundant and have therefore received most attention, but because their biology seems largely applicable to Vδ3+ and Vδ5+, it is common to refer to these cells collectively as “non-Vγ9Vδ2” or “Vδ2neg” γδ T cells. Of note, repertoire deep-sequencing from blood and tissues has often revealed large, durable, clonal expansions of Vδ2neg cells which are rarely shared across individuals(20–22), implying some form of immunological memory that is a hallmark of adaptive immunity highly describable in immunotherapy. However, the molecular basis of such “γδ memory” remains unelucidated.
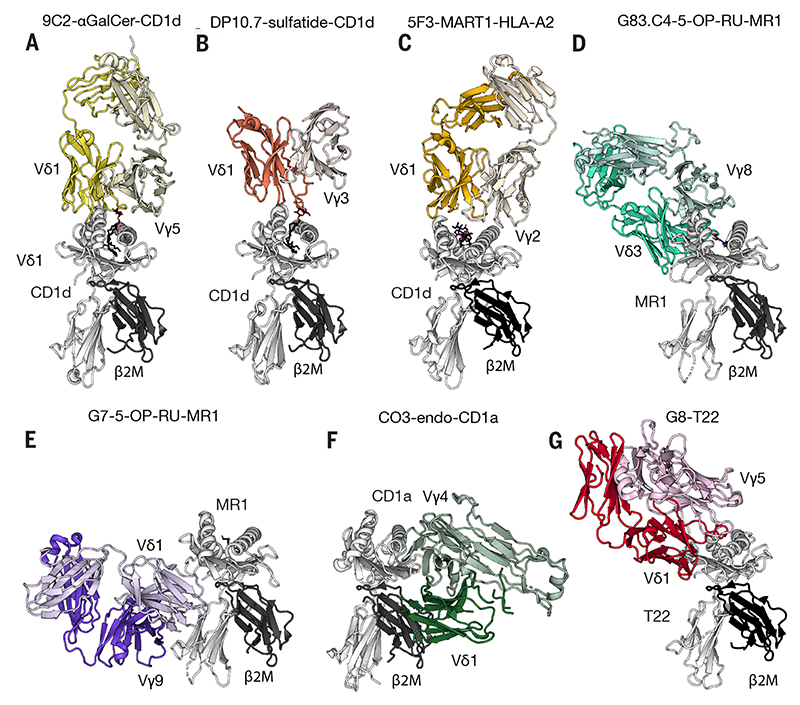
Candidate ligand approaches have identified some overlaps of Vδ1 and αβ T cell reactivities (Table 2), including NKT-like reactivities toward CD1d(59–61). Interestingly, CD1d-restricted Vδ1 T cells can be found within human hepatosplenic T cell lymphomas that can be very aggressive(62). There are also Vδ1 and Vδ3 TCR reactivities toward other CD1 molecules(63–66), which can be over-expressed on haematological malignancies, and towards MR1 (Fig. 1)(67), and Vδ1 T cells recognizing melanoma-associated antigens presented by MHC could be generated in vitro from haematopoietic progenitors(68).
Fig. 1. Gallery of human γδTCR structures solved in complex with their ligands highlighting diverse docking modes.
(a) Vγ5Vδ1 TCR-CD1d-α-GalCer (b) Vγ3Vδ1 TCR-CD1d-sulfatide (c) Vγ2Vδ1 TCR-HLA-A2-MART1 (d) VγδVδ3 TCR-MR1-5-OP-RU (e) Vγ9Vδ1 TCR-MR1-5-OP-RU (f) Vγ4Vδ1 TCR-CD1a-endo (g) Vγ5Vδ1 TCR-T22. MHC and MHC-like molecules coloured in grey; distinct coloring for the γδTCRs.
Nonetheless, γδTCR and αβTCR reactivities are distinct. Thus, while some lipids (e.g., sulfatides) can increase Vδ1+ cell recognition of CD1d(59), they are not mandatory(69). Likewise, whereas αβTCRs bind MHC, CD1 and MR1 ligands in ‘end-to-end’ orientations, the antibody-like nature of γδTCRs is evident in ‘down under’ and ‘sideways’ recognition modes(66,67) (Fig. 1). Some such γδTCRs display reasonable affinities for ligands but nevertheless signal poorly, conceivably attributable to unusual docking modes akin to how the TCRαβ-pMHC docking orientation affects CD8+ T cell signalling(70). This underscores how constraining it may be to view γδ T cells simply as αβ T cells with unconventional TCRs: rather, the cells’ optimal clinical exploitation will rely on elucidating exactly how TCRγδ-ligand engagement transduces signals, particularly when integrated with other inputs, e.g., NKRs and cytokines, thereby dictating consequent cell expansion, homing, effector function, and durability.
In this regard, unbiased methods have identified novel tumour antigens recognized by non-Vγ9Vδ2 T cells (Table 2). For example, clonally expanded Vδ1, Vδ3 and Vδ5 T cells from immunosuppressed patients showing CMV reactivation, displayed dual reactivity toward CMV-infected and tumour cells, and by immunizing mice with the target tumour cells, antibodies were obtained that blocked clonotypic tumour cell killing. This identified relevant TCR ligands including the tyrosine receptor EphA2, a membrane translocated form of Annexin A2, and Endothelial Protein C Receptor (EPCR) which is a CD1d homolog(71–73). Each was recognized in native conformation, and each was over-expressed on several cancer cell types and on cells dysregulated by AMPK activation or oxidative stress.
In another approach, CRISPR/Cas9 deployment identified HLA-DR as a ligand of a γδTCR expanded in the context of CMV infection and showing reactivity toward B cell lymphomas(74). Likewise, there are data for TCRγδ recognition of unusual MHC conformations not commonly found on healthy cells (J.D.-M., unpublished). It is intriguing that despite the potential for diversity in TCRδ, many ligands show core structural relatedness to MHC, but with no evidence of tumour-specific antigenic cargoes. Provocatively, Kaufman speculated that hereditary Vγ/Vδ selection may have been based on MHC-like “W” genes, since been lost from most vertebrates(75,76). Additionally, many nonpolymorphic MHC molecules lacking ascribed functions might have fundamental roles as “stress antigens” underpinning γδ T cell-dependent, neoantigen-independent immunosurveillance(77).
Going forward, unbiased TCRγδ antigen identification approaches, including use of TCRγδ multimers, will be coupled with spatial “-omics” to evaluate antigen expression in tumours and assess correlations with clinical outcomes. These direct, practicable methods can complement complex yet evolving approaches to predict HLA-dependent cancer neoantigens for αβ TILs(4). Of note, the unprocessed nature of γδ tumour antigens makes them relatively easy to target via “binders” comprising either γδ TCRs or monoclonal antibodies blanketing epitopes on the same targets. Additionally, the overlap of TCRγδ ligand expression in cancers and infections, e.g., CMV(20,78) or toxoplasmosis(79), facilitates studies of tumour-reactive γδ T cells in patients without the complexities of immuno-toxic cancer treatment regimens.
Immunotherapeutic non-Vγ9Vδ2 T cells
The application of Vδ1+ cells to cancer immunotherapy (Table 3) builds on strong associations between the numbers and activation state of Vδ1+ cells and progression-free and overall survival in haematological and solid cancers, including breast, lung, and colorectal carcinoma (CRC)(80–82). In CRC liver metastases, Vδ1 T cells constituted the largest TIL subset, showed potent Type 1 effector functions, and correlated with lower metastasis numbers and improved overall survival(83). Likewise, associations of γδ T cell reconstitution and survival post HSCT (above) were stronger for blood Vδ1+ cells than for Vδ2+ cells(43).
Vδ1 ACT was enabled by clinical-grade protocols for robust cell expansion from tissues or from blood(84). In particular, the “Delta One T (DOT)” protocol achieves >1,000-fold expansions in 2-3 weeks, while also inducing upregulation of NKRs contributing to tumour cell targeting(84, 85). DOT cells showed marked efficacy in various patient-derived xenograft models of AML(85, 86), an indication for which the cells have been granted orphan drug designation for allogeneic application by Takeda Pharmaceuticals (Boston, USA). In an alternative approach, Onechain Therapeutics (Barcelona, Spain) uses Notch-activated CD34+ stem cells as its source of allogeneic Vδ1 ACT.
To date however, there has been little exploitation of the cells’ adaptive biology, leaving scope for therapeutic optimisation, e.g., by elucidating the basis of durable clonotypic responses to cancer-associated antigens considered above. The limited polymorphisms of such antigens, e.g., EPCR, suggests a potentially broad-ranging utility, for instance by coupling cancer-targeting Vδ1 TCRs to cytotoxic drugs, evoking antibody-drug conjugates (ADC) that are showing immunotherapeutic successes(87). Currently there are no clinical data from the use of engagers to specifically activate non-Vγ9Vδ2 T cells.
By contrast, cells expanded by the DOT or similar approaches have proved amenable to CAR engineering, e.g., targeting CD123(86), CD20(88), or glypican-3(89). Independently conducted studies demonstrated that CAR-Vδ1 T cells were extremely potent in vitro and in vivo, while also revealing the importance of human IL-15 for persistence in immunodeficient murine hosts(87, 89).
Of note, allogeneic CD20 CAR-Vδ1 T cells developed by Adicet Bio (Boston, USA) showed favourable interim results in a phase 1 trial in B cell malignancies, including those failing αβ-CAR-T therapy (Table 3), although long-term efficacy will likely depend upon solving “the durability problem”, i.e., to sustain donor cells post-infusion. Recently, Adicet was granted Phase I trial approval for ADI-270 comprising allogeneic Vδ1 cells expressing both a signalling CD27 CAR that binds CD70 overexpressed on renal cell carcinoma, and a dominant negative TGFβ receptor to limit immunosuppression by the TME.
Hedging their bets, Luminary Therapeutics (Minneapolis, USA) use allogeneic mixtures of Vδ1+ and Vδ2+ T cells as substrates for non-viral delivery of large genetic payloads that cloak the cells (see above) and that targets multiple solid tumor antigens, e.g., BAFF receptors overexpressed on B cell malignancies (Table 3), and CD70 and CSPG4 overexpressed in head-and-neck cancers.
Immune checkpoint blockade, γδ competence, and tissue normality-sensing
The expanding armamentarium of γδ-based therapeutics needs to be viewed in context. Indeed, whereas ICB is largely viewed as derepressing αβ T cells in the TME, Vδ1 T cells were recently invoked to explain the efficacy of anti-PD-1 in MHCI-deficient CRC(90, 91). Likewise, positive outcomes of ICB in melanomas with low mutational burden (LMB) were significantly associated with high Vδ1 transcript levels(92). Such data offer frontline evidence that γδ T cells can broaden the range of tumours targeted by αβ T cells and extend the reach of ICB, which is important given that early applications of γδ-based immunotherapies in solid tumours will likely need to be in combination with standard-of-care ICB. Vδ1 T cell efficacy in MHClow CRC and LMB melanoma, together with studies of Vδ2neg T cells in kidney cancer(93) seem consistent with evidence that PD-1+ Vδ1 cells do not comply with the gene expression signature of exhausted αβ T cells, and very rapidly generate potent effector responses upon derepression(92).
Conceivably PD-1 contributes to controlling tissue-resident γδ T cell activation at steady-state. Indeed, healthy human colonocytes are not ignored by TCRγδ+ intestinal intraepithelial lymphocytes (IEL) but are engaged via TCR binding to BTNL3+BTNL8 dimers(58). BTNL3 binds germline-encoded Vγ4 sequences within or abutting CDR2 and Hypervariable region (HV)4. This innate modality, which contrasts with CDR3 motifs that confer clonotypic adaptive specificities(64, 94), was subsequently shown for Vγ9-BTN2A1 binding(31,38) (above), and probably underscores Vγ7-BTNL6 engagement in the mouse small intestine where healthy enterocytes express BTNL6-BTNL1 dimers(64). Neither Btnl1-deficient mice nor humans hypomorphic for BTNL3 develop normal TCRγδ+ IEL repertoires (58,95), and likewise skin TCRVγ5+ IEL fail to mature in mice lacking SKINT1 or SKINT2, two BTNL-related proteins expressed by foetal thymic epithelial cells and keratinocytes(96).
Importantly, however, steady-state TCR-BTNL interactions have profound impacts beyond γδTCR repertoire selection. Thus, in mice transiently exposed to blocking anti-SKINT1 antibodies, the differentiation programme and viability of keratinocytes were compromised resulting in impaired epidermal barrier function. Additionally, intraepidermal γδ cells lost competence to respond to local challenges including ultraviolet irradiation, with consequent accumulation of mutagenic cyclobutane pyrimidine dimers and local inflammatory lesions(97).
The active engagement of healthy tissues by local γδ T cells is termed “normality sensing”(97), and has distinct implications for cancer therapy. First, signal transduction from innate TCRγδ engagement evidently promotes effector functions distinct from cytolysis and inflammatory cytokines induced by adaptive ligands. Second, TCRγδ agonists mimicking innate engagement might sustain γδ T cell competence, potentially solving “the durability problem” (above). This might likewise be achieved by an appropriately designed TCRγδ-based CAR. Indeed, normality sensing conferred competence to respond via the 4-1BB (TNFRSF9) co-stimulatory receptor(97), the signalling domain of which is commonly included in latter generation CAR constructs. Conversely, over-active CAR-T signalling might promote exhaustion by overriding innate TCR signals that maintain the cells’ competence. Third, normality sensing γδ T cells can contribute to tissue integrity and to limiting inflammation probably related to the cells’ capacity for wound healing (98,99). This might usefully promote cancer lesion resolution at distinct stages of treatment, e.g., post-surgery adjuvant settings. Improved models to test this hypothesis seem merited given that cancers are described as “wounds that do not heal”(100). Fourth, by actively discriminating normal cells from cancer cells by use of innate and adaptive modalities, γδTCRs naturally create a therapeutic window limiting attacks on healthy tissues.
Therapeutic windows and rethinking activation thresholds
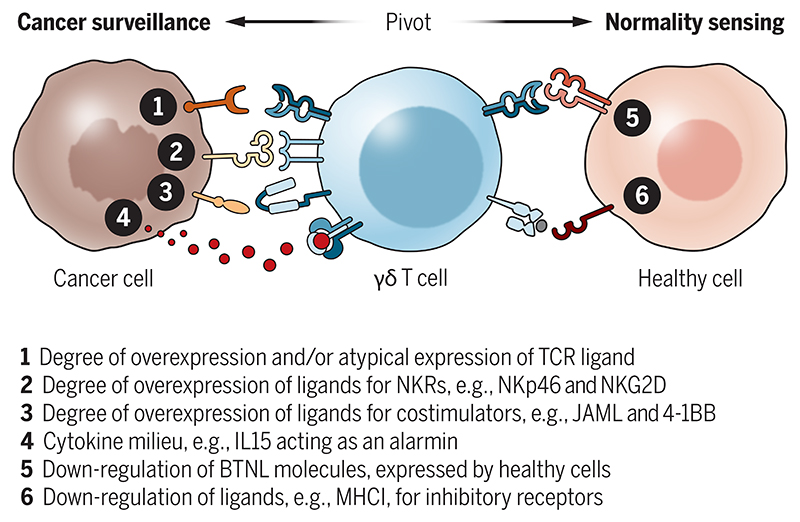
For immunotherapies, the therapeutic window equates to tolerance of normal self. This would seem particularly germane to γδ T cells given their focus on self-antigens. Biochemical and genetic data are consistent with deletion of developing γδ T cells carrying high affinity TCRs, or with their phenotypic skewing away from IL-17(12). Additionally, tolerance may be imposed peripherally by several thresholds limiting γδ T cell activation (12) (Fig. 2). The first would be high ligand density, which contrasts with the capacity of TCRαβ to initiate responses to very low pMHC densities(101), facilitated in part by TCR-ligand catch-bonds that TCRγδ cannot form(102). Indeed, the αβ T cell immunotherapy paradigm of “higher affinity is better” may not apply to γδ T cells if enhanced affinity γδTCRs adversely target normal cells expressing low levels of cognate ligands.
Fig. 2. A multipartite-avidity model to maintain peripheral tolerance to self, thereby creating a therapeutic window.
A γδ T cell can pivot from normality sensing to cancer cell surveillance by loss of healthy cell markers and acquisition of TCR and innate receptor ligands, a combination of which sets the threshold for full cell activation.
The next class of thresholds (Fig. 2; numbers 2-4) would be overexpression by cancer cells of ligands for different classes of innate receptors including: (i) co-stimulators not commonly implicated in αβ T cell activation, e.g., JAML, which binds the coxsackie-adenovirus entry receptor(103), and CD100, which binds plexin B2(104); (ii) cytokine receptors, e.g., IL-15R that can detect high levels of IL-15 as an alarmin; (iii) scavenger receptors among which the most thoroughly explored γδ T cell activator is WC1 expressed by Artiodactyla(12,105); and (iv) NKRs, e.g., NKp46 which recognises ecto-calreticulin induced by endoplasmic reticulum stress that is common in cancer cells(106), and NKG2D, which binds self-encoded ligands induced by DNA damage, excessive growth factor receptor signalling, osmotic shock, and other perturbations(16). Applying this knowledge, Vγ9Vδ2 cells have been transduced with a CAR with the ectodomain of NKG2D, promoting their targeting of solid tumours expressing NKG2D ligands(107). Based on positive pre-clinical results in a xenograft ovarian cancer model, this approach has reached clinical testing in relapsed or refractory solid cancers (Table 3). Similarly, NKp46 was a defining trait of anti-tumour human Vδ1 T cells in colorectal cancer(82), and likewise of BTNL3-selected colonic γδ IEL that seemingly limit IBD severity(95), a predisposing condition for CRC.
Further thresholds would be downregulation of ligands, including BTNLs that sustain γδ T cells rather than fully activate them (above), and MHCI that could suppress γδ T cells by signalling from killer inhibitory receptors (KIRs)(108) (Fig. 2). Which of these (and possibly other) thresholds underpins natural target cell discrimination by γδ T cells in different cancer types needs to be determined if γδ T cell-based therapies are to be optimised in a timely fashion. Importantly, the clear phenotypic distinction of competent versus functionally differentiated versus exhausted versus stem-like progenitor γδ T cells in normal tissues and tumours should provide invaluable prognostic biomarkers for the success or otherwise of γδ T cell immunotherapies. Spatial “-omics” will be essential, as will a better understanding of how γδ T cell signalling integrates innate and clonotypic inputs to promote different phenotypic outcomes.
Achieving appropriate immunotherapeutic phenotypes
In addition to maintaining appropriate therapeutic windows, the community needs to guard against potentially adverse γδ phenotypes. γδ T cell deficient mice are significantly more susceptible in multiple solid tumour models(109), and mice expressing a Vγ1Cγ4 TCR transgene showed increased resistance to T cell lymphomas(110). Added to this are several aforementioned associations of γδ T cells with favourable outcomes in human cancer(111). Nonetheless, cancer promotion has been ascribed to innate IL-17-producing γδ cells, commonly found in subepithelial tissues of lung, skin, reproductive organs and other sites, where they are readily activated by IL-1β and IL-23(109). Moreover, there have been some associations of γδ T cell activation and worse clinical outcomes, including in pancreatic cancer(111, 112). Toward resolving these paradoxes, elegant mouse molecular genetic experiments demonstrated that TCRγδ+ IEL, including BTNL-selected NKp46+ cells protected against early stages of CRC, whereas if such control was evaded, invasive CRC growth was enhanced by IL-17-producing γδ cells(113). Thus, it has become commonplace to screen against IL-17 production in evaluating clinical protocols for γδ T cell activation in ACT or via engagers. Nonetheless, human IL-17-producing γδ T cells have been extremely challenging to identify or induce(95) and a large-scale study showed negligible IL-17 RNA expression by human γδ T cells in CRC(114).
Interestingly, whereas the cytolytic activity of TCRαβ+ CD8 T cells is a lynchpin of cancer immunotherapies, there are murine tumour-promoting (“T-pro”) CD8 T cells that combine IL-17 production with other traits, including amphiregulin production(115). Similarly, amphiregulin-producing Vδ1 T cells identified in CRC were distinct from CRC-associated cytolytic Vδ1 cells(116). This has fuelled γδ T cell culture protocols skewed against amphiregulin production. Nonetheless, amphiregulin-producing murine γδ T cells are strongly associated with tissue repair(12), again raising the possibility that amphiregulin-producing human cells might be beneficial in driving wound resolution at appropriate treatment junctures.
Toward cancer remissions; durable immune orchestration
There has been much debate over whether the most critical trait of CAR-T cells is their initial impact on a tumour or their long-term sustainability that permits durable immune surveillance. As has been considered, γδ T cells can satisfy both demands by combining rapid, nonclonal delivery of effector functions with durable clonotypic expansions. Thus, a major practical goal is for γδ–based therapeutics to deliver this combination, with one intriguing possibility being to directly gene-edit endogenous γδ T cells in vivo.
Of note, γδ T cell immunotherapies may achieve sustainable cancer remissions via innate orchestration of true adaptive immunity. For example, γδ T cells are prominent and beneficial in early responses of cattle to vaccination(12), and human γδ T cell representation strongly correlated with malaria sporozoite vaccination immunogenicity(12,117,118), as may also be true in emerging cancer vaccine settings. Indeed, establishment of high quality CD8+ TCRαβ+ memory cells was γδ T cell-dependent in settings as diverse as West Nile virus infection(119) and contact hypersensitivity(120), and may also be so in cancer. Thus, γδ-based immunotherapies should not be viewed merely as filling gaps where αβ T cells are ineffective, but as adjuvants that promote αβ T cell efficacy. This re-emphasises that off-the-shelf, allogeneic γδ T cell-based immunotherapies should most likely retain innate competences that swiftly reboot host adaptive immunity, prior to γδ ACT graft rejection.
Concluding remarks
The distinctive capacity of γδ T cells to bridge innate and adaptive immunity makes them highly attractive candidates for tackling cancer. γδ T cell immunotherapeutics are in clinical trials, reflecting practical advances in expanding and engineering cells for ACT and in developing γδ T cell-specific engagers. Safety profiles seem good, and there are promising read-outs of efficacy. Furthermore, γδ-based immunotherapies are predicted to combine well with ICB in solid tumours, emphasising the need for reliable markers of γδ T cell status, particularly within tumors.
Good science underlies good drugs, and the refinement of first generation γδ-based therapies will benefit from instilling “γδ-unique” traits that in turn require advances in better understanding γδ T cell biology. Ideally, this will integrate efforts in basic research, experimental medicine, pharmaceutical science, and trial design. The goals are cheaper, off-the-shelf treatments that benefit increased numbers of patients of diverse ethnicities with a wider range of cancers; fewer AEs; and reliable prognostic biomarkers of treatment success. As we move beyond some of the constraints of αβ T cells, we should learn how factors in the TME specifically suppress γδ T cells, thereby identifying additional therapeutic targets. Current research efforts are intensive and there is good reason for optimism.
Acknowledgements
AH is funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK (CRUK) (FC001003), the UK Medical Research Council (FC001003), and the Wellcome Trust (FC001003). JDM is funded by Agence National pour la Recherche (ANR-19-CElS-0024-02) and Institut National du Cancer (2021-199). JR is supported by an NHMRC investigator grant; and BSS is funded by Fundação para a Ciência e Tecnologia (PTDC/MED-ONC/6829/2020). The authors thank B. Gully for Fig. 1; J. Brock for the illustration in the in-print summary; S. Mensurado and L. Barros for assistance with the manuscript; and L. Lamb, A. Banerjee, N. Sobhana, and Y. Wu for discussions. We apologise profusely to those whose work could not be considered given space limitations.
Footnotes
Conflicts of interest
AH and BSS were co-founders of GammaDelta Therapeutics and Lymphact SA, respectively, and currently hold sponsored research agreements with Takeda Development Center Americas, Inc.; JR previously collaborated with Lava Therapeutics, and currently has a collaboration with Gadeta B.V. All listed companies focus on γδ T cell therapeutics but none was at any point consulted regarding the content of this article and had no direct influence over it.
References
- 1.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–81. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Davis M, Chien Y. Paul’s Fundamental Immunology. 8th ed. Wolters Kluwer; 2022. T Cell Antigen Receptors and Antigen Recognition. [Google Scholar]
- 3.Wong JKM, Dolcetti R, Rhee H, Simpson F, Souza-Fonseca-Guimaraes F. Weaponizing natural killer cells for solid cancer immunotherapy. Trends Cancer. 2023;9:111–121. doi: 10.1016/j.trecan.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher TN, Scheper W, Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, Liu J, Subudhi SK, Poon C, Gant KL, Herbrich SM, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186:1652–1669. doi: 10.1016/j.cell.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity. 2018;48:453–473. doi: 10.1016/j.immuni.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Hassel JC, Heinzerling L, Aberle J, Bähr O, Eigentler TK, Grimm MO, Grünwald V, Leipe J, Reinmuth N, Tietze JK, Trojan J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. doi: 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Baker DJ, Arany Z, Baur JA, Epstein JA, June CH. CAR T therapy beyond cancer: the evolution of a living drug. Nature. 2023;619:707–715. doi: 10.1038/s41586-023-06243-w. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Rezvani K, Rafei H. Next-generation chimeric antigen receptors for T- and natural killer-cell therapies against cancer. Immunol Rev. 2023 doi: 10.1111/imr.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, Fichter M, Wang C, Liang S, Silva M, Kumari S, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, Christ E, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 12.Hayday A. Paul’s Fundamental Immunology. 8th ed. Wolters Kluwer, publ; 2022. Gamma Delta T Cells. [Google Scholar]
- 13.Willcox BE, Willcox CR. γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. 2019;20:121–128. doi: 10.1038/s41590-018-0304-y. [DOI] [PubMed] [Google Scholar]
- 14.Morrissey KA, Wegrecki M, Praveena T, Hansen VL, Bu L, Sivaraman KK, Darko S, Douek DC, Rossjohn J, Miller RD, Le Nours J. The molecular assembly of the marsupial γμ T cell receptor defines a third T cell lineage. Science. 2021;371:1383–1388. doi: 10.1126/science.abe7070. [DOI] [PubMed] [Google Scholar]
- 15.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Santos B, Strid J. Working in “NK Mode”: Natural Killer Group 2 Member D and Natural Cytotoxicity Receptors in Stress-Surveillance by γδ T Cells. Front Immunol. 2018;9:851. doi: 10.3389/fimmu.2018.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, Poccia F, Fournié J-J, Battistini L. FcgammaRIII discriminates between 2 subsets of Vgamma9Vdelta2 effector cells with different responses and activation pathways. Blood. 2004;104:1801–7. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- 18.Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, Merville P, Moreau J-F, Déchanet-Merville J. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa) Blood. 2012;119:1418–27. doi: 10.1182/blood-2011-06-363655. [DOI] [PubMed] [Google Scholar]
- 19.Hayday AC. γδ T Cell Update: Adaptate Orchestrators of Immune Surveillance. J Immunol. 2019;203:311–320. doi: 10.4049/jimmunol.1800934. [DOI] [PubMed] [Google Scholar]
- 20.Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdörfer L, Reinhardt A, Ravens I, Beck M, Geffers R, von Kaisenberg C, et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol. 2017;18:393–401. doi: 10.1038/ni.3686. [DOI] [PubMed] [Google Scholar]
- 21.Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, Salim M, Mohammed F, Price DA, Chudakov DM, et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat Commun. 2017;8:14760. doi: 10.1038/ncomms14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, Oo YH, Willcox BE. Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J Hepatol. 2018;69:654–665. doi: 10.1016/j.jhep.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo F, Mamonkin M, Brenner MK, Heslop HE. Taking T-Cell Oncotherapy Off-the-Shelf. Trends Immunol. 2021;42:261–272. doi: 10.1016/j.it.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueda T, Shiina S, Iriguchi S, Terakura S, Kawai Y, Kabai R, Sakamoto S, Watanabe A, Ohara K, Wang B, Xu H, et al. Optimization of the proliferation and persistency of CAR T cells derived from human induced pluripotent stem cells. Nat Biomed Eng. 2023;7:24–37. doi: 10.1038/s41551-022-00969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller RG. The veto phenomenon and T-cell regulation. Immunol Today. 1986;7:112–4. doi: 10.1016/0167-5699(86)90151-9. [DOI] [PubMed] [Google Scholar]
- 26.Fournié J-J, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, Ysebaert L, Laurent G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10:35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riganti C, Massaia M, Davey MS, Eberl M. Human γδ T-cell responses in infection and immunotherapy: common mechanisms, common mediators? Eur J Immunol. 2012;42:1668–76. doi: 10.1002/eji.201242492. [DOI] [PubMed] [Google Scholar]
- 28.Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, Breathnach R, Bonneville M, Scotet E, Adams EJ. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan L, Ma X, Yang Y, Qu Y, Li X, Zhu X, Ma W, Duan J, Xue J, Yang H, Huang J-W, et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature. 2023 doi: 10.1038/s41586-023-06525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, McWilliam HEG, Hudson C, Tutuka C, Wheatley AK, Kent SJ, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. 2020;367 doi: 10.1126/science.aay5516. [DOI] [PubMed] [Google Scholar]
- 31.Karunakaran MM, Willcox CR, Salim M, Paletta D, Fichtner AS, Noll A, Starick L, Nöhren A, Begley CR, Berwick KA, Chaleil RAG, et al. Butyrophilin-2A1 Directly Binds Germline-Encoded Regions of the Vγ9Vδ2 TCR and Is Essential for Phosphoantigen Sensing. Immunity. 2020;52:487–498. doi: 10.1016/j.immuni.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao C-HC, Nguyen K, Jin Y, Vinogradova O, Wiemer AJ. Ligand-induced interactions between butyrophilin 2A1 and 3A1 internal domains in the HMBPP receptor complex. Cell Chem Biol. 2022;29:985–995. doi: 10.1016/j.chembiol.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afrache H, Gouret P, Ainouche S, Pontarotti P, Olive D. The butyrophilin (BTN) gene family: from milk fat to the regulation of the immune response. Immunogenetics. 2012;64:781–94. doi: 10.1007/s00251-012-0619-z. [DOI] [PubMed] [Google Scholar]
- 34.Mamedov MR, Vedova S, Freimer JW, Das Sahu A, Ramesh A, Arce MM, Meringa AD, Ota M, Chen PA, Hanspers K, Nguyen VQ, et al. CRISPR screens decode cancer cell pathways that trigger γδ T cell detection. Nature. 2023;621:188–195. doi: 10.1038/s41586-023-06482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 36.Hayday A, Vantourout P. The Innate Biologies of Adaptive Antigen Receptors. Annu Rev Immunol. 2020;38:487–510. doi: 10.1146/annurev-immunol-102819-023144. [DOI] [PubMed] [Google Scholar]
- 37.Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW, Snijders AP, Malim MH, Hayday AC. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc Natl Acad Sci U S A. 2018;115:1039–1044. doi: 10.1073/pnas.1701237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willcox CR, Salim M, Begley CR, Karunakaran MM, Easton EJ, von Klopotek C, Berwick KA, Herrmann T, Mohammed F, Jeeves M, Willcox BE. Phosphoantigen sensing combines TCR-dependent recognition of the BTN3A IgV domain and germline interaction with BTN2A1. Cell Rep. 2023;42:112321. doi: 10.1016/j.celrep.2023.112321. [DOI] [PubMed] [Google Scholar]
- 39.Fulford T, Soliman C, Castle RG, Rigau M, Ruan Z, Dolezal O, Seneviratna R, Brown HG, Hanssen E, Hammet A, Li S, et al. Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers. BioRxiv. doi: 10.1038/s41590-024-01892-z. [DOI] [PubMed] [Google Scholar]
- 40.Beck BH, Kim H-G, Kim H, Samuel S, Liu Z, Shrestha R, Haines H, Zinn K, Lopez RD. Adoptively transferred ex vivo expanded gamma delta-T cells mediate in vivo antitumor activity in preclinical mouse models of breast cancer. Breast Cancer Res Treat. 2010;122:135–44. doi: 10.1007/s10549-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gundermann S, Klinker E, Kimmel B, Flierl U, Wilhelm M, Einsele H, Kunzmann V. A comprehensive analysis of primary acute myeloid leukemia identifies biomarkers predicting susceptibility to human allogeneic Vγ9Vδ2 T cells. J Immunother. 2014;37:321–30. doi: 10.1097/CJI.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y. Cancer immunotherapy harnessing γδ T cells and programmed death-1. Immunol Rev. 2020;298:237–253. doi: 10.1111/imr.12917. [DOI] [PubMed] [Google Scholar]
- 43.Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang K-Y, Abhyankar S, Lamb LS. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39:751–7. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]
- 44.Minculescu L, Marquart HV, Ryder LP, Andersen NS, Schjoedt I, Friis LS, Kornblit BT, Petersen SL, Haastrup E, Fischer-Nielsen A, Reekie J, et al. Improved Overall Survival, Relapse-Free-Survival, and Less Graft-vs.-Host-Disease in Patients With High Immune Reconstitution of TCR Gamma Delta Cells 2 Months After Allogeneic Stem Cell Transplantation. Front Immunol. 2019;10:1997. doi: 10.3389/fimmu.2019.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamb LS, Pereboeva L, Youngblood S, Gillespie GY, Nabors LB, Markert JM, Dasgupta A, Langford C, Spencer HT. A combined treatment regimen of MGMT-modified γδ T cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci Rep. 2021;11:21133. doi: 10.1038/s41598-021-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Gassart A, Le K-S, Brune P, Agaugué S, Sims J, Goubard A, Castellano R, Joalland N, Scotet E, Collette Y, Valentin E, et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vγ9Vδ2 T cell-mediated antitumor immune response. Sci Transl Med. 2021;13:eabj0835. doi: 10.1126/scitranslmed.abj0835. [DOI] [PubMed] [Google Scholar]
- 47.King LA, Toffoli EC, Veth M, Iglesias-Guimarais V, Slot MC, Amsen D, van de Ven R, Derks S, Fransen MF, Tuynman JB, Riedl T, et al. A Bispecific γδ T-cell Engager Targeting EGFR Activates a Potent Vγ9Vδ2 T cell-Mediated Immune Response against EGFR-Expressing Tumors. Cancer Immunol Res. 2023;11:1237–1252. doi: 10.1158/2326-6066.CIR-23-0189. [DOI] [PubMed] [Google Scholar]
- 48.Zhai X, You F, Xiang S, Jiang L, Chen D, Li Y, Fan S, Han Z, Zhang T, An G, Zhang B, et al. MUC1-Tn-targeting chimeric antigen receptor-modified Vγ9Vδ2 T cells with enhanced antigen-specific anti-tumor activity. Am J Cancer Res. 2021;11:79–91. [PMC free article] [PubMed] [Google Scholar]
- 49.Beatson RE, Parente-Pereira AC, Halim L, Cozzetto D, Hull C, Whilding LM, Martinez O, Taylor CA, Obajdin J, Luu Hoang KN, Draper B, et al. TGF-β1 potentiates Vγ9Vδ2 T cell adoptive immunotherapy of cancer. Cell Rep Med. 2021;2:100473. doi: 10.1016/j.xcrm.2021.100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher J, Don DW, Barisa M, Hurtado MO, Abramowski P, Porter L, Day W, Borea R, Inglott S, Anderson J, Pe'er D. Engineering γδ T cells limits tonic signaling associated with chimeric antigen receptors. Sci Signal. 2019;12(598):eaax1872. doi: 10.1126/scisignal.aax1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcu-Malina V, Heijhuurs S, van Buuren M, Hartkamp L, Strand S, Sebestyen Z, Scholten K, Martens A, Kuball J. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood. 2011;118:50–9. doi: 10.1182/blood-2010-12-325993. [DOI] [PubMed] [Google Scholar]
- 52.Straetemans T, Kierkels GJJ, Doorn R, Jansen K, Heijhuurs S, Dos Santos JM, van Muyden ADD, Vie H, Clemenceau B, Raymakers R, de Witte M, et al. GMP-Grade Manufacturing of T Cells Engineered to Express a Defined γδTCR. Front Immunol. 2018;9:1062. doi: 10.3389/fimmu.2018.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johanna I, Straetemans T, Heijhuurs S, Aarts-Riemens T, Norell H, Bongiovanni L, de Bruin A, Sebestyen Z, Kuball J. Evaluating in vivo efficacy - toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J Immunother Cancer. 2019;7:69. doi: 10.1186/s40425-019-0558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson IB, Mulvaney R, Dieckmann N, Vantellini A, Canestraro M, Amicarella F, O'Dwyer R, Cole DK, Harper S, Dushek O, Kirk P. Tuning the potency and selectivity of ImmTAC molecules by affinity modulation. Clin Exp Immunol. 2023:uxad120. doi: 10.1093/cei/uxad120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowers Y, Holtmeier W, Harwood J, Morzycka-Wroblewska E, Kagnoff MF. The V delta 1 T cell receptor repertoire in human small intestine and colon. J Exp Med. 1994;180:183–90. doi: 10.1084/jem.180.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenna T, Golden-Mason L, Norris S, Hegarty JE, O'Farrelly C, Doherty DG. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin Immunol. 2004;113:56–63. doi: 10.1016/j.clim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–50. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML, Laing A, et al. A. Hayday, Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific γδ T Cell Compartments. Cell. 2016;167:203–218.:e17. doi: 10.1016/j.cell.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, Bendelac A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur J Immunol. 2012;42:2505–10. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, Savage PB, et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity. 2013;39:1032–42. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, Godfrey DI. CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol. 2013;14:1137–45. doi: 10.1038/ni.2713. [DOI] [PubMed] [Google Scholar]
- 62.Bachy E, Urb M, Chandra S, Robinot R, Bricard G, de Bernard S, Traverse-Glehen A, Gazzo S, Blond O, Khurana A, Baseggio L, et al. CD1d-restricted peripheral T cell lymphoma in mice and humans. J Exp Med. 2016;213:841–57. doi: 10.1084/jem.20150794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy S, Ly D, Castro CD, Li N-S, Hawk AJ, Altman JD, Meredith SC, Piccirilli JA, Moody D, Adams EJ. Molecular Analysis of Lipid-Reactive Vδ1 γδ T Cells Identified by CD1c Tetramers. J Immunol. 2016;196:1933–42. doi: 10.4049/jimmunol.1502202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D, Irving PM, et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol. 2018;19:1352–1365. doi: 10.1038/s41590-018-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reijneveld JF, Ocampo TA, Shahine A, Gully BS, Vantourout P, Hayday AC, Rossjohn J, Moody DB, Van Rhijn I. Human γδ T cells recognize CD1b by two distinct mechanisms. Proc Natl Acad Sci U S A. 2020;117:22944–22952. doi: 10.1073/pnas.2010545117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wegrecki M, Ocampo TA, Gunasinghe SD, von Borstel A, Tin SY, Reijneveld JF, Cao T-P, Gully BS, Le Nours J, Moody DB, Van Rhijn I, et al. Atypical sideways recognition of CD1a by autoreactive γδ T cell receptors. Nat Commun. 2022;13:3872. doi: 10.1038/s41467-022-31443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Nours J, Gherardin NA, Ramarathinam SH, Awad W, Wiede F, Gully BS, Khandokar Y, Praveena T, Wubben JM, Sandow JJ, Webb AI, et al. A class of γδ T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science. 2019;366:1522–1527. doi: 10.1126/science.aav3900. [DOI] [PubMed] [Google Scholar]
- 68.Benveniste PM, Roy S, Nakatsugawa M, Chen ELY, Nguyen L, Millar DG, Ohashi PS, Hirano N, Adams EJ, Zúñiga-Pflücker JC. Generation and molecular recognition of melanoma-associated antigen-specific human γδ T cells. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aav4036. [DOI] [PubMed] [Google Scholar]
- 69.Hayday A, Vantourout P. A long-playing CD about the γδ TCR repertoire. Immunity. 2013;39:994–6. doi: 10.1016/j.immuni.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Zareie P, Szeto C, Farenc C, Gunasinghe SD, Kolawole EM, Nguyen A, Blyth C, Sng XYX, Li J, Jones CM, Fulcher AJ, et al. Canonical T cell receptor docking on peptide-MHC is essential for T cell signaling. Science. 2021;372 doi: 10.1126/science.abe9124. [DOI] [PubMed] [Google Scholar]
- 71.Harly C, Joyce SP, Domblides C, Bachelet T, Pitard V, Mannat C, Pappalardo A, Couzi L, Netzer S, Massara L, Obre E, et al. Human γδ T cell sensing of AMPK-dependent metabolic tumor reprogramming through TCR recognition of EphA2. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.aba9010. [DOI] [PubMed] [Google Scholar]
- 72.Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, Khairallah C, Lomenech A-M, Harly C, Bonneville M, Moreau J-F, et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc Natl Acad Sci U S A. 2017;114:3163–3168. doi: 10.1073/pnas.1621052114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau J-F, Hayday AC, Willcox BE, Déchanet-Merville J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–9. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 74.Deseke M, Rampoldi F, Sandrock I, Borst E, Böning H, Ssebyatika GL, Jürgens C, Plückebaum N, Beck M, Hassan A, Tan L, et al. A CMV-induced adaptive human Vδ1+ γδ T cell clone recognizes HLA-DR. J Exp Med. 2022;219 doi: 10.1084/jem.20212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaufman J. The new W family reconstructs the evolution of MHC genes. Proc Natl Acad Sci U S A. 2022;119(5):e2122079119. doi: 10.1073/pnas.2122079119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okamura K, Dijkstra JM, Tsukamoto K, Grimholt U, Wiegertjes GF, Kondow A, Yamaguchi H, Hashimoto K. Discovery of an ancient MHC category with both class I and class II features. Proc Natl Acad Sci U S A. 2021;118(51):e2108104118. doi: 10.1073/pnas.2108104118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janeway CA, Jr, Jones B, Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988;9:73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 78.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, Michelson S, Méric C, Hallet MM, Kourilsky P, Potaux L, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma L, Papadopoulou M, Taton M, Genco F, Marchant A, Meroni V, Vermijlen D. Effector Vγ9Vδ2 T cell response to congenital Toxoplasma gondii infection. JCI Insight. 2021;6 doi: 10.1172/jci.insight.138066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y, Kyle-Cezar F, Woolf RT, Naceur-Lombardelli C, Owen J, Biswas D, Lorenc A, Vantourout P, Gazinska P, Grigoriadis A, Tutt A, et al. An innate-like Vδ1+ γδ T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aax9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y, Biswas D, Usaite I, Angelova M, Boeing S, Karasaki T, Veeriah S, Czyzewska-Khan J, Morton C, Joseph M, Hessey S, et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat Cancer. 2022;3:696–709. doi: 10.1038/s43018-022-00376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mikulak J, Oriolo F, Bruni E, Roberto A, Colombo FS, Villa A, Bosticardo M, Bortolomai I, Lo Presti E, Meraviglia S, Dieli F, et al. NKp46-expressing human gut-resident intraepithelial Vδ1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruni E, Cimino MM, Donadon M, Carriero R, Terzoli S, Piazza R, Ravens S, Prinz I, Cazzetta V, Marzano P, Kunderfranco P, et al. Intrahepatic CD69+Vδ1 T cells re-circulate in the blood of patients with metastatic colorectal cancer and limit tumor progression. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Almeida AR, V Correia D, Fernandes-Platzgummer A, da Silva CL, da Silva MG, Anjos DR, Silva-Santos B. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clin Cancer Res. 2016;22:5795–5804. doi: 10.1158/1078-0432.CCR-16-0597. [DOI] [PubMed] [Google Scholar]
- 85.Di Lorenzo B, Simões AE, Caiado F, Tieppo P, V Correia D, Carvalho T, da Silva MG, Déchanet-Merville J, Schumacher TN, Prinz I, Norell H, et al. Broad Cytotoxic Targeting of Acute Myeloid Leukemia by Polyclonal Delta One T Cells. Cancer Immunol Res. 2019;7:552–558. doi: 10.1158/2326-6066.CIR-18-0647. [DOI] [PubMed] [Google Scholar]
- 86.Sánchez Martínez D, Tirado N, Mensurado S, Martínez-Moreno A, Romecín P, Gutiérrez Agüera F, V Correia D, Silva-Santos B, Menéndez P. Generation and proof-of-concept for allogeneic CD123 CAR-Delta One T (DOT) cells in acute myeloid leukemia. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koganemaru S, Kuboki Y, Koga Y, Kojima T, Yamauchi M, Maeda N, Kagari T, Hirotani K, Yasunaga M, Matsumura Y, Doi T. U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer. Mol Cancer Ther. 2019;18:2043–2050. doi: 10.1158/1535-7163.MCT-19-0452. [DOI] [PubMed] [Google Scholar]
- 88.Nishimoto KP, Barca T, Azameera A, Makkouk A, Romero JM, Bai L, Brodey MM, Kennedy-Wilde J, Shao H, Papaioannou S, Doan A, et al. Allogeneic CD20-targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin Transl Immunology. 2022;11:e1373. doi: 10.1002/cti2.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, Nishimoto KP, Brodey MM, Tabrizizad M, Gundurao SRY, Bai L, et al. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Vries NL, van de Haar J, Veninga V, Chalabi M, Ijsselsteijn ME, van der Ploeg M, van den Bulk J, Ruano D, van den Berg JG, Haanen JB, Zeverijn LJ, et al. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature. 2023;613:743–750. doi: 10.1038/s41586-022-05593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silva-Santos B, Mensurado S. γδ T cells maintain sensitivity to immunotherapy in MHC-I-deficient tumors. Nat Immunol. 2023;24:387–388. doi: 10.1038/s41590-023-01429-w. [DOI] [PubMed] [Google Scholar]
- 92.Davies D, Kamdar S, Woolf R, Zlatareva I, Iannitto ML, Morton C, Haque Y, Martin H, Biswas D, Ndagire S, Munonyara M, et al. PD-1 defines a distinct, functional, tissue-adapted state in Vδ1+ T cells with implications for cancer immunotherapy. Nat Cancer. 2024 doi: 10.1038/s43018-023-00690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rancan C, Arias-Badia M, Dogra P, Chen B, Aran D, Yang H, Luong D, Ilano A, Li J, Chang H, Kwek SS, et al. Exhausted intratumoral Vδ2-γδ T cells in human kidney cancer retain effector function. Nat Immunol. 2023;24:612–624. doi: 10.1038/s41590-023-01448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willcox CR, Vantourout P, Salim M, Zlatareva I, Melandri D, Zanardo L, George R, Kjaer S, Jeeves M, Mohammed F, Hayday AC, et al. Butyrophilin-like 3 Directly Binds a Human Vγ4+ T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity. 2019;51:813–825.:e4. doi: 10.1016/j.immuni.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dart RJ, Zlatareva I, Vantourout P, Theodoridis E, Amar A, Kannambath S, East P, Recaldin T, Mansfield JC, Lamb CA, Parkes M, et al. Conserved γδ T cell selection by BTNL proteins limits progression of human inflammatory bowel disease. Science. 2023;381(6663):eadh0301. doi: 10.1126/science.adh0301. Epub 2023 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jandke A, Melandri D, Monin L, Ushakov DS, Laing AG, Vantourout P, East P, Nitta T, Narita T, Takayanagi H, Feederle R, et al. Butyrophilin-like proteins display combinatorial diversity in selecting and maintaining signature intraepithelial γδ T cell compartments. Nat Commun. 2020;11:3769. doi: 10.1038/s41467-020-17557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McKenzie DR, Hart R, Bah N, Ushakov DS, Muñoz-Ruiz M, Feederle R, Hayday AC. Normality sensing licenses local T cells for innate-like tissue surveillance. Nat Immunol. 2022;23:411–422. doi: 10.1038/s41590-021-01124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–9. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 99.Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 101.Huang J, Brameshuber M, Zeng X, Xie J, Li Q, Chien Y, Valitutti S, Davis MM. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013;39:846–57. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mallis RJ, Duke-Cohan JS, Das DK, Akitsu A, Luoma AM, Banik D, Stephens HM, Tetteh PW, Castro CD, Krahnke S, Hussey RE, et al. Molecular design of the γδ T cell receptor ectodomain encodes biologically fit ligand recognition in the absence of mechanosensing. Proc Natl Acad Sci U S A. 2021;118:e2023050118. doi: 10.1073/pnas.2023050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGraw JM, Thelen F, Hampton EN, Bruno NE, Young TS, Havran WL, Witherden DA. JAML promotes CD8 and γδ T cell antitumor immunity and is a novel target for cancer immunotherapy. J Exp Med. 2021;218 doi: 10.1084/jem.20202644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Witherden DA, Watanabe M, Garijo O, Rieder SE, Sarkisyan G, Cronin SJ, Verdino P, Wilson IA, Kumanogoh A, Kikutani H, Teyton L, et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity. 2012;37:314–25. doi: 10.1016/j.immuni.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baldwin CL, Damani-Yokota P, Yirsaw A, Loonie K, Teixeira AF, Gillespie A. Special features of γδ T cells in ruminants. Mol Immunol. 2021;134:161–169. doi: 10.1016/j.molimm.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 106.Sen Santara S, Lee D-J, Crespo Â, Hu JJ, Walker C, Ma X, Zhang Y, Chowdhury S, Meza-Sosa KF, Lewandrowski M, Zhang H, et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature. 2023;616:348–356. doi: 10.1038/s41586-023-05912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ang WX, Ng YY, Xiao L, Chen C, Li Z, Chi Z, Tay JC-K, Tan WK, Zeng J, Toh HC, Wang S. Electroporation of NKG2D RNA CAR Improves Vγ9Vδ2 T Cell Responses against Human Solid Tumor Xenografts. Mol Ther Oncolytics. 2020;17:421–430. doi: 10.1016/j.omto.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lafarge X, Pitard V, Ravet S, Roumanes D, Halary F, Dromer C, Vivier E, Paul P, Moreau J-F, Déchanet-Merville J. Expression of MHC class I receptors confers functional intraclonal heterogeneity to a reactive expansion of gammadelta T cells. Eur J Immunol. 2005;35:1896–905. doi: 10.1002/eji.200425837. [DOI] [PubMed] [Google Scholar]
- 109.Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392–404. doi: 10.1038/s41568-019-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Penninger JM, Wen T, Timms E, Potter J, Wallace VA, Matsuyama T, Ferrick D, Sydora B, Kronenberg M, Mak TW. Spontaneous resistance to acute T-cell leukaemias in TCRV gamma 1.1J gamma 4C gamma 4 transgenic mice. Nature. 1995;375:241–4. doi: 10.1038/375241a0. [DOI] [PubMed] [Google Scholar]
- 111.Mensurado S, Blanco-Domínguez R, Silva-Santos B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023;20:178–191. doi: 10.1038/s41571-022-00722-1. [DOI] [PubMed] [Google Scholar]
- 112.Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK, Avanzi A, et al. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell. 2016;166:1485–1499. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reis BS, Darcy PW, Khan IZ, Moon CS, Kornberg AE, Schneider VS, Alvarez Y, Eleso O, Zhu C, Schernthanner M, Lockhart A, et al. TCR-Vγδ usage distinguishes protumor from antitumor intestinal γδ T cell subsets. Science. 2022;377:276–284. doi: 10.1126/science.abj8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ran R, Trapecar M, Brubaker DK. Systematic Analysis of Human Colorectal Cancer scRNA-seq Revealed Limited Pro-tumoral IL-17 Production Potential in Gamma Delta T Cells. bioRxiv. 2024 doi: 10.1016/j.neo.2024.101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kwong BY, Roberts SJ, Silberzahn T, Filler RB, Neustadter JH, Galan A, Reddy S, Lin WM, Ellis PD, Langford CF, Hayday AC, et al. Molecular analysis of tumor-promoting CD8+ T cells in two-stage cutaneous chemical carcinogenesis. J Invest Dermatol. 2010;130:1726–36. doi: 10.1038/jid.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harmon C, Zaborowski A, Moore H, St Louis P, Slattery K, Duquette D, Scanlan J, Kane H, Kunkemoeller B, McIntyre CL, Scannail AN, et al. γδ T cell dichotomy with opposing cytotoxic and wound healing functions in human solid tumors. Nat Cancer. 2023;4:1122–1137. doi: 10.1038/s43018-023-00589-w. [DOI] [PubMed] [Google Scholar]
- 117.Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, Samake Y, Sissoko K, Mwakingwe-Omari A, Lane J, Imeru A, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis. 2022;22:377–389. doi: 10.1016/S1473-3099(21)00332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang L-J, Sarwar UN, Zephir KL, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016;22:614–23. doi: 10.1038/nm.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang T, Gao Y, Scully E, Davis CT, Anderson JF, Welte T, Ledizet M, Koski R, Madri JA, Barrett A, Yin Z, et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177:1825–32. doi: 10.4049/jimmunol.177.3.1825. [DOI] [PubMed] [Google Scholar]
- 120.Muñoz-Ruiz M, Llorian M, D'Antuono R, Pavlova A, Mavrigiannaki AM, McKenzie D, García-Cassani B, Iannitto ML, Wu Y, Dart R, Davies D, et al. IFN-γ-dependent interactions between tissue-intrinsic γδ T cells and tissue-infiltrating CD8 T cells limit allergic contact dermatitis. J Allergy Clin Immunol. 2023;152:1520–1540. doi: 10.1016/j.jaci.2023.07.015. [DOI] [PubMed] [Google Scholar]