Abstract
Rationale: Many clinical studies have focused on the epidemiological and clinical characteristics of inpatients with coronavirus disease (COVID-19). However, there are few reports about the clinical follow-up of discharged patients.
Objectives: To describe the follow-up of patients with COVID-19 in Wenzhou City, Zhejiang, China.
Methods: We retrospectively reviewed 4-week follow-ups in patients with COVID-19, including computed tomographic (CT) chest scanning, blood testing, and oropharyngeal-swab testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ribonucleic acid. The chest CT scans and blood tests were performed on the last day before discharge and 2 weeks and 4 weeks after discharge. The oropharyngeal-swab tests were performed at both 1 week and 2 weeks after discharge. Fifty-one patients with common COVID-19 were enrolled in the study. All the CT and clinical data were collected between January 23 and March 28, 2020.
Results: Compared with the abnormalities found on the the last CT scans before discharge, the abnormalities in the lungs at the first and second follow-ups after discharge had been gradually absorbed. The cases with focal ground-glass opacity were reduced from 17.7% to 9.8% of cases. The cases with multiple ground-glass opacities decreased from 80.4% to 23.5%. The cases with consolidation were reduced from 49.0% to 2.0%. The cases with interlobular septal thickening were reduced from 80.4% to 35.3%. The cases with subpleural lines were reduced from 29.4% to 7.8%. The cases with irregular lines were reduced from 41.2% to 15.7%. The lung lesions of 25.5% patients were shown to be fully absorbed on the first CT scans after discharge, and the rate of lung recovery increased to 64.7% after the second follow-up. Nucleic-acid test results became recurrently positive in 17.6% of discharged patients, of whom only 33.3% complained of clinical symptoms. There were no differences in the characteristics of the last CT scans before discharge between the patients with recurrently positive test results and the patients with negative test results. The lung damage was fully absorbed in 55.6% of discharged patients with recurrence of positive test results for SARS-CoV-2 ribonucleic acid.
Conclusions: The lung damage due to COVID-19 could be reversible for patients with common COVID-19. A few cases showed recurring positive results of nucleic-acid tests after discharge.
Keywords: computed tomographic imaging, follow-up, SARS-CoV-2, COVID-19, recurrently positive
Since December 2019, a cluster of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been identified in Wuhan, China (1, 2). The pneumonia caused by the virus was named coronavirus disease (COVID-19) by the World Health Organization. The virus has led to worldwide panic because of its significant infectivity and virulence (3, 4).
Many clinical studies have focused on the epidemiological and clinical characteristics of inpatients with COVID-19, such as the onset of symptoms and blood-test results (1, 2). However, there are few reports about the clinical follow-up of discharged patients with COVID-19 (5). According to the criteria of discharge released by the China National Health and Fitness Commission, the nucleic-acid tests for SARS-CoV-2 ribonucleic acid (RNA) were repeated after discharge, and the results of a few patients were positive (6, 7). Therefore, it is necessary to keep the follow-up visits of patients with COVID-19. In addition, follow-up chest computed tomography of discharged patients could help with better understanding the effects on the lungs caused by COVID-19.
In our hospital, chest computed tomographic (CT) scans, blood examinations, and nucleic-acid tests for SARS-CoV-2 RNA were widely used in the follow-up evaluations of discharged patients with COVID-19. The purpose of this study is to describe the clinical follow-up of patients who had been discharged from our hospital for 4 weeks.
Methods
Patients
Our study was approved by the ethics committees of the Ruian People’s Hospital and the Ruian Hospital of Traditional Chinese Medicine. The requirement for informed consent was waived. There were 51 patients infected with SARS-CoV-2 enrolled in this study. All the patients were in the group with common COVID-19 (Table 1) who were treated in the Ruian People’s Hospital. According to the “Pneumonia Diagnosis and Treatment Program for Novel Coronavirus Infection (Trial Version 5)” issued by the National Health Commission of the People’s Republic of China (Table 1), patients with common COVID-19 had fever, some respiratory-infection symptoms, and pneumonia on radiographic images (8). The patients were discharged from the Ruian People’s Hospital after two negative nucleic-acid test results, with tests being taken at least 24 hours apart, and after displaying the indications of clinical recovery according to the China National Health and Fitness Commission criteria (see Table E1 in the online supplement).
Table 1.
The clinical classification of pneumonitis using the “Pneumonia Diagnosis and Treatment Program for Novel Coronavirus Infection (Trial Version 5)”
| Mild | Clinical symptoms are mild, and there is no pneumonia on radiographic images |
| Common | Fever with some respiratory-infection symptoms; pneumonia is present on radiographic images |
| Severe | Meeting any of the following criteria: |
| a. Respiratory distress, RR ≥ 30 breaths/min | |
| b. At rest, finger-clip oxygen saturation ≤ 93% | |
| c. PaO2/FiO2 ≤ 300 mm Hg (1 mm Hg = 0.133 kPa) | |
| Critical | Meeting any of the following criteria: |
| a. Respiratory failure, requiring mechanical ventilation | |
| b. Shock | |
| c. Other organ failures requiring ICU monitoring |
Definition of abbreviations: FiO2 = fraction of inspired oxygen; ICU = intensive care unit; PaO2 = arterial oxygen tension/pressure; RR = respiration rate.
The patients with mild and common coronavirus disease (COVID-19) were regarded as the nonsevere group, and the patients with severe and critical COVID-19 were regarded as the severe group. Reprinted by permission from Reference 8.
Data Collection
The clinical data, including blood-test results, chest CT-scan findings, and oropharyngeal-swab test results, were retrospectively collected in our study. Blood examination included blood routine testing and testing for alanine aminotransferase, aspartate transaminase, lactate dehydrogenase, blood urea nitrogen, and creatinine. Clinical data were collected between January 23 and March 28, 2020. For all of the patients with COVID-19, the blood examinations and CT scans were performed on the last day before discharge, around 2 weeks after discharge, and about 4 weeks after discharge. In addition, oropharyngeal-swab testing was performed 1 and 2 weeks after discharge. The frequency of follow-up tests and chest CT imaging were suggested by the National Health Commission of the People’s Republic of China.
The CT chest scans were performed using one of two multislice spiral scanners: the uCT 528 scanner (United Imaging Healthcare) or the Siemens SOMATOM Perspective CT Scanner (Siemens Medical Solutions). The pixel spacing of CT images was 0.72 mm and 0.85 mm for the uCT and Siemens scanners, respectively. The slice thickness was 5 mm for both scanners. All chest-scan acquisitions were performed in the spiral mode at a pitch of 1.125, with a peak tube voltage of 100 kilovolts and an adaptive tube current. The scan covered the entire chest and provided a detailed look from the thoracic inlet to the costophrenic angle. The oropharyngeal swab was tested using the COVID-19 (ORF lab/N gene) nucleic-acid detection kit (Shanghai BioGerm Medical Biotechnology Co., Ltd.). The detection kit was officially approved by the National Medical Products Administration and received European Union European Conformity certification. The detection limit of the kit was 968 copies/ml (9).
Data Analysis
All of the CT scans were analyzed on a radiologic picture-archiving-and-communication-system workstation (Greenlander version 6.0; Mindray Healthcare). Two or more senior general radiologists with over 15 years of clinical experience reviewed and evaluated the CT imaging features, came to a consensus, and eventually reported the CT findings in the electronic information system. All CT findings were taken from the original clinical reports. The CT imaging features were evaluated, including focal ground-glass opacities (GGOs), multiple GGOs (Figures 1A and 1B), diffuse GGOs, consolidation (Figures 1C and 1D), interlobular septal thickening (Figure 1E), subpleural lines (Figure 1E), irregular lines (Figure 1E), bronchiectasis (Figure 2), and reticular patterns (Figure 1F). In our study, focal GGO indicated that multiple or single ground-glass attenuations were concentrated in one lung lobe. Multiple GGOs meant that multiple ground-glass attenuations were present in at least two lung lobes. Diffuse GGO referred to large areas with increased density in both lungs on chest CT scans. Consolidation was defined as having higher density than GGO and showing blurred margins for pulmonary blood vessels and bronchial tubes. A reticular pattern was defined as a collection of innumerable, small linear opacities.
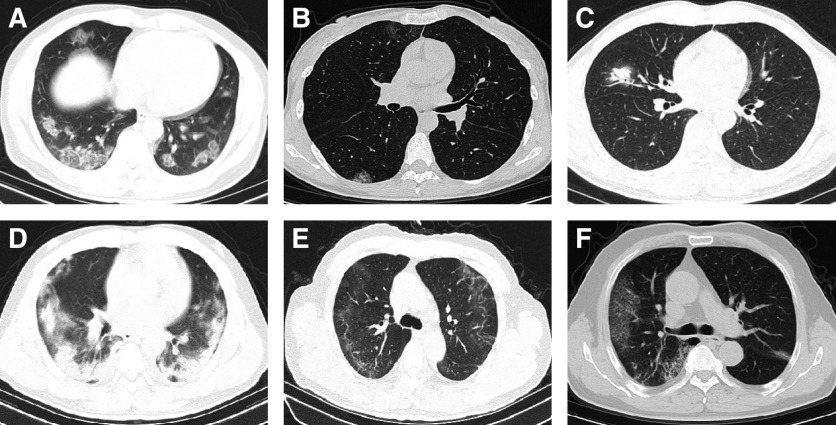
Figure 1.
Chest computed tomographic (CT) features of patients with COVID-19. (A) Chest CT scan of a 48-year-old male patient. Multiple ground-glass opacities (GGOs) are shown in the lower lobes. (B) Chest CT scan of a 34-year-old female patient after 3 days of treatment. Multiple patchy GGOs are shown in the lower lobes. (C) Chest CT scan of a 51-year-old male patient at admission. Consolidation in the right middle lobe and air bronchogram signs are shown. (D) Chest CT scan of a 36-year-old male patient after 8 days of treatment. Multiple consolidations are shown in both lungs. (E) The last chest CT scan before discharge of a 65-year-old male patient. Subpleural lines and interlobular septal thickening are shown in the right lung, and irregular lines were present in left lung. (F) Chest CT scan of a 70-year-old male patient after 3 days of treatment. Multiple reticular patterns are shown in the right lung, and small consolidations are shown in the left lung.
Figure 2.
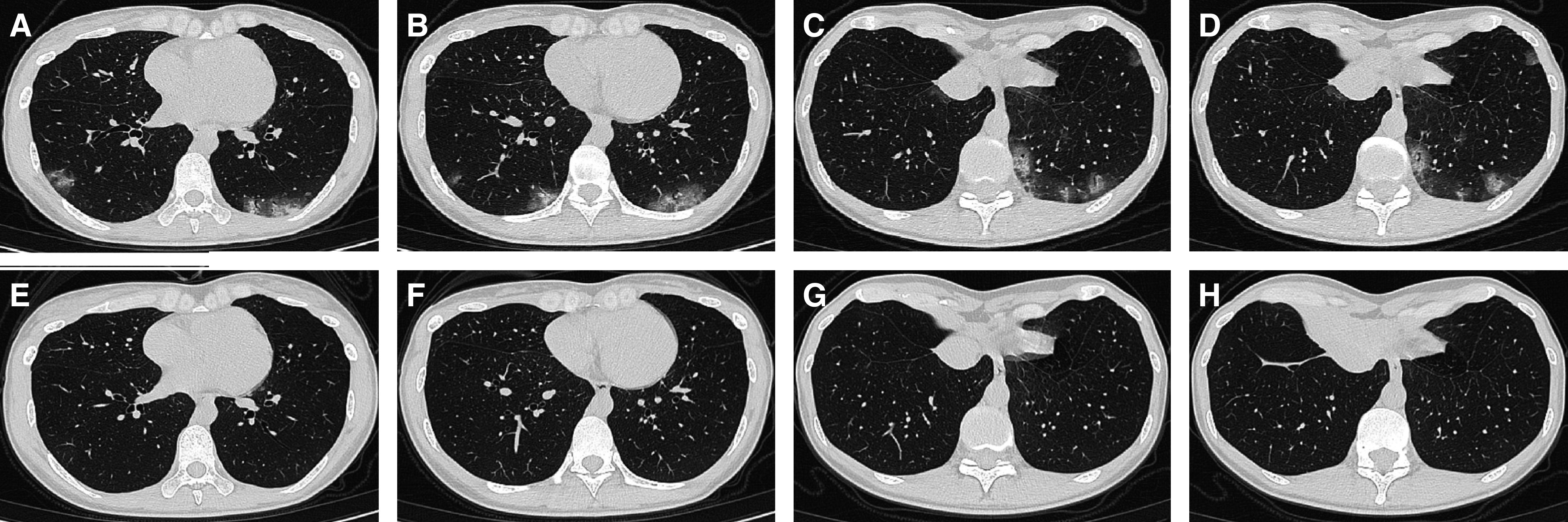
The chest computed tomographic (CT) scans from a 34-year-old female patient with coronavirus disease (COVID-19). (A–D) The chest CT scans at admission. (E–H) The chest CT scans 4 weeks after discharge show near recovery from multiple patchy ground-glass opacities in the lower lobes, consolidations, and bronchiectasis.
Results
Clinical Characteristics
Fifty-one patients with common COVID-19 completed the clinical follow-up for 4 weeks. The clinical characteristics of these patients are presented in Table 2. The clinical characteristics of male and female patients are shown in Tables E3 and E4, respectively.
Table 2.
Clinical characteristics of patients
| Age, yr | 46.6 ± 13.9 (14–70) |
| Male/female | 21/30 |
| Smoking | 3 (5.9) |
| Comorbidity | 8 (15.9) |
| Diabetes | 4 (7.8) |
| Hypertension | 7 (13.7) |
| Coronary heart disease | 1 (2) |
| Pregnancy | 1 (2) |
| Symptoms 4 wk after discharge | |
| Cough | 8 (15.7) |
| Sputum | 2 (3.9) |
| Throat discomfort | 3 (5.9) |
Data are mean ± standard deviation (range), ratio, or n (%).
Follow-Up Chest CT Scans
Three CT examinations were performed on 51 patients. The first CT scans were captured on the last day before discharge. The median interval between discharge and the first follow-up CT scan was 10 days, with a range of 7–16 days. The median interval between the first and second follow-up CT scans was 31 days, with a range of 20–37 days.
Compared with abnormalities on the last CT scan before discharge, the abnormalities in the lungs at the first and second follow-ups after discharge had gradually resolved (Table 3). Each of the numbers mentioned in this section represents the number of subjects with the specific finding, not the total number of such findings. The number of subjects with focal GGOs was reduced from nine (17.7%) to five (9.8%) at the first follow-up CT scan after discharge and remained at five (9.8%) at the second follow-up CT scan after discharge. The reduction in GGO cases indicated that the GGO lesions were completely resolved in these cases. The number of subjects with multiple GGOs decreased from 41 (80.4%) to 32 (62.8%) and then to 12 (23.5%). The number of subjects with diffuse GGOs remained at one (2.0%) at the first follow-up CT scan after discharge and was reduced to zero at the second follow-up CT scan. The number of subjects with consolidations was reduced from 25 (49.0%) to 4 (7.8%) and then to 1 (2.0%). The number of subjects with interlobular septal thickening was reduced from 41 (80.4%) to 25 (49.0%) and then to 18 (35.3%). The number of subjects with subpleural lines was reduced from 15 (29.4%) to 11 (21.6%) and then to 4 (7.8%). The number of subjects with irregular lines was reduced from 21 (41.2%) to 16 (31.4%) and then to 8 (15.7%). The number of subjects with bronchiectasis was reduced from 17 (33.3%) to 6 (11.8%) and then to 2 (3.9%). The number of subjects with a reticular pattern was reduced from two (3.9%) to one (2.0%) and then to zero. The lung lesions of 13 patients (25.5%) were fully absorbed as shown on the first CT scans after discharge, and the rate of lung recovery increased to 64.7% after the second follow-up CT scan (Figure 3). At the first follow-up CT scan, 84% (21 of 25) of the consolidation cases were resolved, which was the quickest resolution among the different types of lesions. Between the first and second CT follow-ups, 75% (3 of 4) of the subjects with consolidation had recovered, which was the quickest recovery compared with subjects with other types of lesions (excluding those with diffuse GGO and a reticular pattern because of the small number of cases). During the 4-week follow-up, the recovery from consolidation (96%; 24 of 25) was quicker than the recovery from other types of lesions.
Table 3.
Comparison of chest CT scans before discharge and at the first and second CT follow-ups after discharge
| Last CT Scan before Discharge [n (%)] | First Follow-Up CT Scan after Discharge [n (%)] | Second Follow-Up CT Scan after Discharge [n (%)] | |
|---|---|---|---|
| GGO | 9 (17.7) | 5 (9.8) | 5 (9.8) |
| Multiple GGOs | 41 (80.4) | 32 (62.8) | 12 (23.5) |
| Diffuse GGO | 1 (2.0) | 1 (2.0) | 0 (0) |
| Consolidation | 25 (49.0) | 4 (7.8) | 1 (2.0) |
| Interlobular septal thickening | 41 (80.4) | 25 (49.0) | 18 (35.3) |
| Subpleural lines | 15 (29.4) | 11 (21.6) | 4 (7.8) |
| Irregular lines | 21 (41.2) | 16 (31.4) | 8 (15.7) |
| Bronchiectasis | 17 (33.3) | 6 (11.8) | 2 (3.9) |
| Reticular pattern | 2 (3.9) | 1 (2.0) | 0 (0) |
Definition of abbreviations: CT = computed tomographic; GGO = ground-glass opacity.
Figure 3.
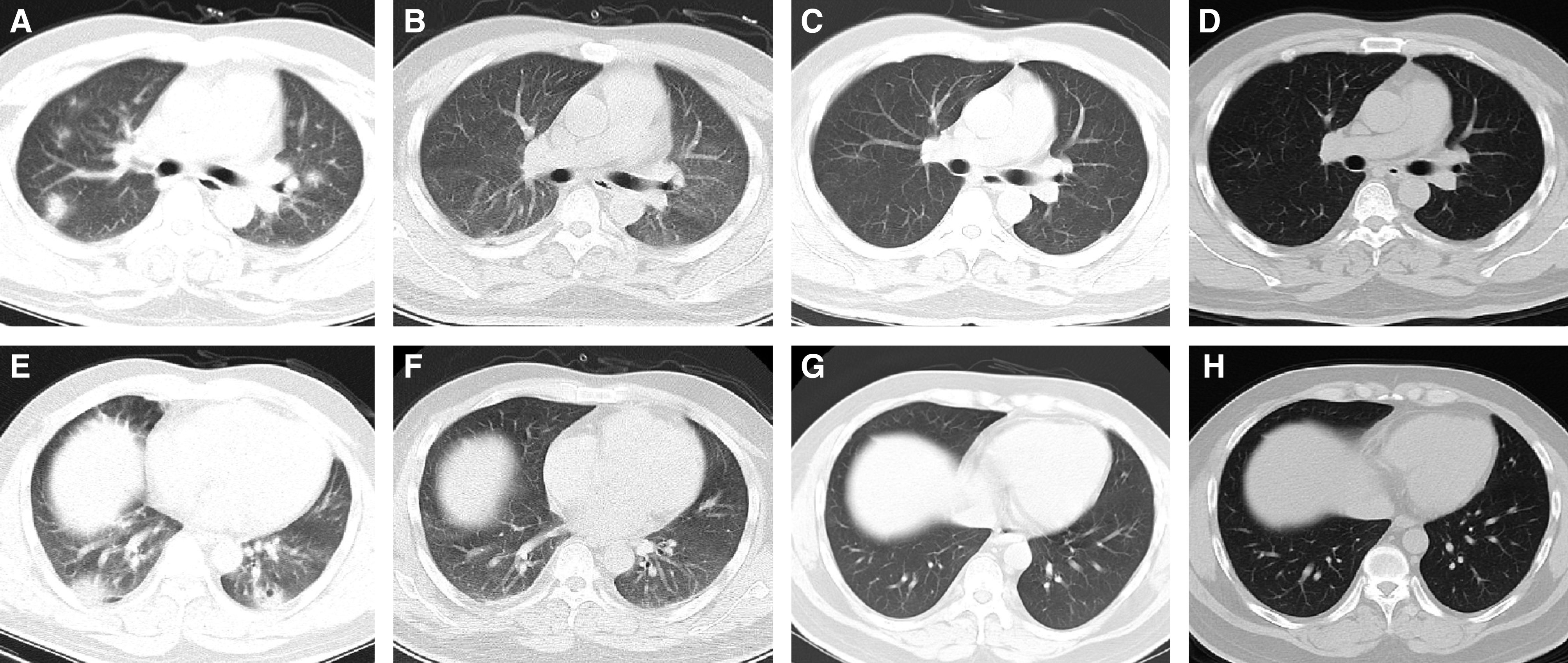
The chest computed tomographic (CT) scans from a 35-year-old male patient with coronavirus disease (COVID-19). (A and E) The chest CT scans at admission. Chest CT scans demonstrated multiple consolidation in the upper and lower lobes, ground-glass opacity (GGO) in the peripheral lung, and a small cavity in the lower lobe of the left lung. (B and F) The last chest CT scans before discharge. The consolidations are mostly absorbed and have transformed into small, patchy GGOs. (C and G) The chest CT scans 2 weeks after discharge. The GGO was absorbed completely. (D and H) The chest CT scans 4 weeks after discharge shows no recurrence.
Follow-Up Blood Tests
The blood laboratory tests were performed on the same day as the follow-up CT scans. The patients were regrouped on the basis of their recovery from lung damage into partially absorbed (Figure 4) and fully absorbed (Figure 3) groups. However, there was no significant difference between these two groups at the 2-week follow-up CT scan and at the 4-week follow-up (Tables E4 and E5).
Figure 4.
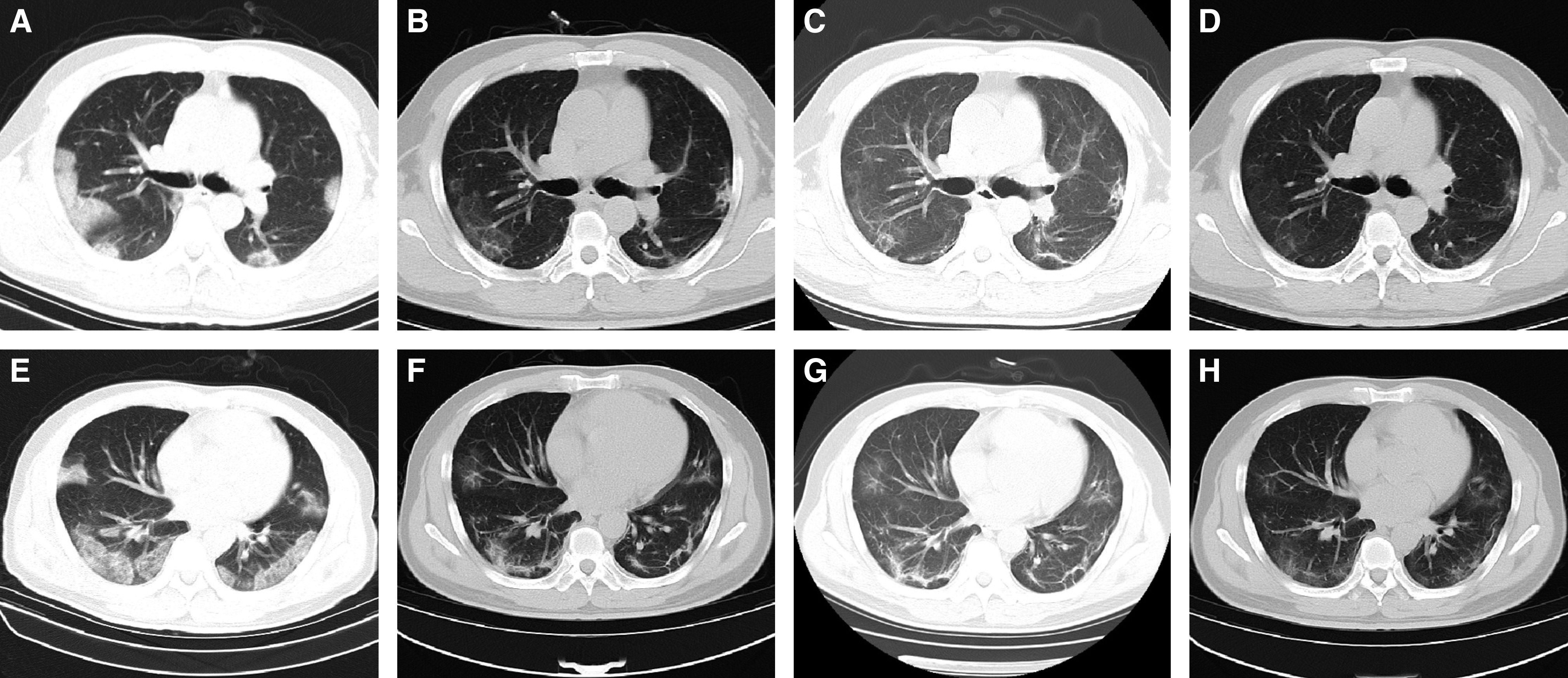
The chest computed tomographic (CT) scans from a 48-year-old male patient with coronavirus disease (COVID-19). (A and E) The chest CT scans at admission. Multiple patchy ground-glass opacities (GGOs), consolidation, and interlobular septal thickening were shown in the lower lobes. (B and F) The last chest CT scans before discharge. Most of the subpleural consolidations were absorbed. Small patchy consolidation in the lower right lung was present. GGOs transformed into irregular lines and subpleural lines. The density of the interlobular septal thickening decreased. (C and G) The chest CT scans 2 weeks after discharge. The consolidation in the right lower lobe transformed into GGO. The density of subpleural GGO in both lungs was gradually absorbed. (D and H) The chest CT scans 4 weeks after discharge show that the damage was partly absorbed. The consolidation in the left upper lobe transformed into GGO, and the subpleural GGO was further absorbed.
Discharged Patients with Recurrent Positive SARS-CoV-2 RNA Test Results
All 51 patients had two negative nucleic-acid test results before discharge, with tests being taken at least 24 hours apart. However, oropharyngeal-swab tests for SARS-CoV-2 RNA were performed for all these patients at 1 and 2 weeks after discharge, respectively. Five patients had weakly positive recurrence at 1 week after discharge. Two weeks after discharge, test results for four of the patients went from being positive to being negative, and in one case, results remained weakly positive. However, oropharyngeal-swab test results for SARS-CoV-2 RNA turned weakly positive recurrently in four other patients, whose nucleic-acid test results were negative in the first week. In total, results for 9 of 51 patients (17.6%) were recurrently weakly positive. Among them, three patients complained of dry cough, one patient complained of cough with sputum, and the other six patients showed no clinical symptoms. Out of the 9 patients, one was pregnant, one had diabetes, and another had hypertension.
We compared the last CT scans before discharge of patients with recurrently positive nucleic-acid test results with those of patients with negative test results (Table 4). There were no differences in the characteristics of CT scans between the two subgroups. The subsequent analysis of the blood-test results before discharge between these two subgroups showed no significant differences either (Table 5). Considering the symptoms at 4 weeks after discharge, there was no significant difference in the clinical characteristics between the patients with recurring positive results and the patients with negative results (Table E6). In addition, there was no significant difference (P = 0.25) in length of the hospital stay between patients with recurring positive results and patients with negative results (Table E7).
Table 4.
Comparison of the last CT features before discharge in patients with recurring positive results versus patients with negative results
| Recurring Positive Results (n = 9) [n (%)] | Negative Results (n = 42) [n (%)] | P Value | |
|---|---|---|---|
| Focal GGO | 0 (0) | 8 (19.1) | 0.17 |
| Multiple GGOs | 2 (22.2) | 32 (76.2) | 0.11 |
| Diffuse GGO | 0 (0) | 1 (2.4) | 0.62 |
| Consolidation | 6 (66.7) | 20 (47.6) | 0.57 |
| Interlobular septal thickening | 8 (88.9) | 32 (76.2) | 0.78 |
| Subpleural lines | 3 (33.3) | 13 (30.1) | 0.92 |
| Irregular lines | 3 (33.3) | 19 (45.2) | 0.67 |
| Bronchiectasis | 6 (66.7) | 12 (28.6) | 0.17 |
| Reticular pattern | 0 (0) | 3 (7.1) | 0.39 |
Definition of abbreviations: CT = computed tomographic; GGO = ground-glass opacity.
P values were obtained using the chi-square test.
Table 5.
Comparison of the last laboratory test results before discharge in patients with recurring positive results versus patients with negative results
| Recurring Positive Results (n = 9) (Mean ± Standard Deviation) | Negative Results (n = 42) (Mean ± Standard Deviation) | P Value | |
|---|---|---|---|
| Leu, ×109/L | 5.04 ± 1.58 | 5.62 ± 1.68 | 0.37 |
| Lym, ×109/L | 1.45 ± 0.47 | 1.52 ± 0.48 | 0.71 |
| Neu, ×109/L | 3.04 ± 1.06 | 3.48 ± 1.38 | 0.39 |
| PLT, ×109/L | 308.0 ± 98.66 | 279.9 ± 104.8 | 0.34 |
| ALT, IU/L | 25.88 ± 28.29 | 29.5 ± 22.36 | 0.41 |
| AST, IU/L | 23.75 ± 15.69 | 25.07 ± 12.85 | 0.28 |
| LDH, U/dl | 212.2 ± 71.22 | 215.1 ± 62.22 | 0.93 |
| BUN, mmol/L | 4.12 ± 1.36 | 3.86 ± 0.87 | 0.54 |
| Cr, μmol/L | 64.33 ± 5.24 | 67.14 ± 10.02 | 0.51 |
| Na+, mmol/L | 139.4 ± 1.77 | 138.4 ± 2.47 | 0.30 |
| K+, mmol/L | 3.92 ± 0.34 | 3.69 ± 0.37 | 0.11 |
Definition of abbreviations: ALT = alanine aminotransferase; AST = aspartate transaminase; BUN = blood urea nitrogen; Cr = creatinine; LDH = lactate dehydrogenase; Leu = leukocytes; Lym = lymphocytes; Neu = neutrophils; PLT = platelets.
P values were obtained using the Mann-Whitney U test.
In the first follow-up CT scan after discharge, the lung lesions were fully absorbed in one case of recurrently positive test results. The lung damage of four patients was fully absorbed at the second follow-up CT scan. By the second follow-up CT scan, the abnormalities in lungs of five patients with recurrently positive nucleic-acid test results were fully absorbed, and the lung damage of four other patients was partly absorbed in comparison with before. Moreover, none of the patients further infected other people.
Discussion
COVID-19 has been raging in China for over 3 months. As of March 31, 2020, China has cured over 76,000 patients with COVID-19. However, the SARS-CoV-2 outbreak is a global pandemic. The number of patients with COVID-19 in America and Europe has been increasing rapidly. Follow-up observation of chest computed tomography and clinical features could assist with COVID-19 diagnosis and treatment. In our study, 51 patients with common COVID-19 who met the discharge criteria (Table E1) were evaluated during the 4 weeks after discharge. These patients are returnees from Wuhan or close contacts of returnees. Local disease control centers and hospitals conducted screening, diagnosis, and treatment in the early stage. The prevention and treatment work were relatively well done.
Compared with the CT features before discharge, the lesions were significantly reduced and the lesion density was decreased. Furthermore, the lung lesions of 64.7% of discharged patients were fully absorbed after the 4-week follow-up. This indicated that the damage to lung tissue by COVID-19 could be reversible for patients with common COVID-19. It also suggested that the prognosis of nonsevere patients (Table 1) is favorable, and clinical intervention should be conducted in time to prevent patients with common COVID-19 from worsening and developing severe COVID-19.
We noticed that 2 and 4 weeks after discharge, patients with lung consolidations due to common COVID-19 recovered more quickly than patients with other types of lesions. As far as we know, the dynamic change of chest CT imaging in patients with COVID-19 was as follows. In the early stage, chest CT findings of patients with COVID-19 mainly manifest as GGO and interstitial changes. As the disease progresses, consolidations may occur in some patients. After active treatment, the consolidations are absorbed gradually, so recovery from consolidations could be relatively fast compared with recovery from other types of lesions. The lung lesions were absorbed gradually, as shown by chest CT imaging.
Our follow-up observations showed that nucleic-acid tests results became recurrently positive in 17.6% of patients during the 2 weeks after discharge. On the premise of a normalized sampling operation, the false-negative results from oropharyngeal-swab tests at the time of discharge are presumed to be the reason for later positive results from nucleic-acid tests. Angiotensin-converting enzyme-2 was identified as the cell-entry receptor of SARS-CoV-2, which was highly expressed in the lungs rather than in the upper respiratory tract (10, 11). Therefore, we speculate that the viral load of SARS-CoV-2 in the lungs is higher than it is in the upper respiratory tract. As a patient’s condition improved, the viral load, especially in the upper respiratory tract, decreased significantly. At this period, there could be a false-negative result from the oropharyngeal- or nasopharyngeal-swab test. Thus, combining sputum, blood, and other specimens may be more clinically meaningful for the comprehensive judgment of whether patients have become virus-negative. Besides, a positive nucleic-acid test result does not mean that the virus still survives. In nine patients with recurrently positive nucleic-acid test results, only one-third complained about clinical symptoms. By the second follow-up CT scan, the abnormalities in the lungs of five patients were fully absorbed, and the lung damage of four other patients were partly absorbed compared with before. All the contacts of each patient were identified and traced until the fourth week after discharge. None of the discharged patients further infected other people. Viral culture is required to determine whether these patients are still contagious. However, a recent study has shown that SARS-CoV-2 could not be isolated 8 days after the onset of symptoms, although a high viral load could be detected in the throat swab and sputum culture samples of patients (12). According to the results for our patient cohort, there may be viral nucleic-acid fragments detected in the samples, which are not necessarily infectious. In addition, there were no differences in the characteristics of the last CT scans or in the blood-test results before discharge between the patients with recurrently positive test results and the patients with negative test results. Therefore, we cannot use these indicators to predict whether the results of a SARS-CoV-2 RNA test would be recurrently positive.
Limitations
The limitation of our study is that only patients with common COVID-19 were enrolled. The follow-up visit was too short. Long-term CT follow-up is required to determine whether the reticular patterns evolve into irreversible fibrosis. For the patients with positive nucleic-acid results in the second week after discharge, no further follow-up nucleic-acid testing was performed.
Conclusions
The lung damage due to COVID-19 could be reversible for patients with common COVID-19. A few cases showed recurring positive results from nucleic-acid tests after discharge, which were probably related to the false negativity of the nucleic-acid test results at the time of discharge.
Supplementary Material
Footnotes
Supported by the Ruian Science and Technology Bureau (MS2020023, MS2020025) and the National Key Research and Development Program of China (2016YFC1304000, 2016YFC1304002).
Author Contributions: C.L. performed experiments, analyzed data, and co-wrote the paper. L.Y. performed experiments and co-wrote the paper. R.X., X.Z., C.Y., and Z.W. collected the data. R.L., D.S., Y.G., J.Y., and Q.S. analyzed the data. X.W. and M.J. designed experiments and revised the paper. All authors provided critical feedback and helped shape the research and analysis as well as the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health: the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing Y, Mo P, Xiao Y, Zhao O, Zhang Y, Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 2020;25:2000191. doi: 10.2807/1560-7917.ES.2020.25.10.2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J-F, Yan K, Ye H-H, Lin J, Zheng J-J, Cai T. SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standards for discharge. Int J Infect Dis. 2020;97:212–214. doi: 10.1016/j.ijid.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D, Xu W, Lei Z, Huang Z, Liu J, Gao Z, et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health and Health Commission of the People’s Republic of China. Pneumonia Diagnosis and Treatment Program for Novel Coronavirus Infection (Trial Version 5). Feb 8, 2020 [accessed 2020 Aug 26]. http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml.
- 9.Wang X, Yao H, Xu X, Zhang P, Zhang M, Shao J, et al. Limits of detection of 6 approved RT-PCR kits for the novel SARS-coronavirus-2 (SARS-CoV-2) Clin Chem. 2020;66:977–979. doi: 10.1093/clinchem/hvaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.