Abstract
Rationale: Prior approaches to measuring healthcare capacity strain have been constrained by using individual care units, limited metrics of strain, or general, rather than disease-specific, populations.
Objectives: We sought to develop a novel composite strain index and measure its association with intensive care unit (ICU) admission decisions and hospital outcomes.
Methods: Using more than 9.2 million acute care encounters from 27 Kaiser Permanente Northern California and Penn Medicine hospitals from 2013 to 2018, we deployed multivariable ridge logistic regression to develop a composite strain index based on hourly measurements of 22 capacity-strain metrics across emergency departments, wards, step-down units, and ICUs. We measured the association of this strain index with ICU admission and clinical outcomes using multivariable logistic and quantile regression.
Results: Among high-acuity patients with sepsis (n = 90,150) and acute respiratory failure (ARF; n = 45,339) not requiring mechanical ventilation or vasopressors, strain at the time of emergency department disposition decision was inversely associated with the probability of ICU admission (sepsis: adjusted probability ranging from 29.0% [95% confidence interval, 28.0–30.0%] at the lowest strain index decile to 9.3% [8.7–9.9%] at the highest strain index decile; ARF: adjusted probability ranging from 47.2% [45.6–48.9%] at the lowest strain index decile to 12.1% [11.0–13.2%] at the highest strain index decile; P < 0.001 at all deciles). Among subgroups of patients who almost always or never went to the ICU, strain was not associated with hospital length of stay, mortality, or discharge disposition (all P ≥ 0.13). Strain was also not meaningfully associated with patient characteristics.
Conclusions: Hospital strain, measured by a novel composite strain index, is strongly associated with ICU admission among patients with sepsis and/or ARF. This strain index fulfills the assumptions of a strong within-hospital instrumental variable for quantifying the net benefit of admission to the ICU for patients with sepsis and/or ARF.
Keywords: sepsis, acute respiratory failure, capacity strain, triage, intensive care unit
Healthcare capacity strain is an operations concept that can be defined as approaching or exceeding the limits of a care team’s, hospital’s, or health system’s ability to provide high-quality care for all patients who may need it at a given time (1, 2). Considerable research has shown that capacity-strain metrics in the intensive care unit (ICU) (3–12), ward (13, 14), and emergency department (15–17) are associated with variability in the processes of care—including disposition decisions and timing, limitations in life-sustaining therapy, physician rounding time, and adherence to evidence-based clinical practices—and certain adverse clinical outcomes.
Although prior studies by us and others have identified associations between hospital capacity strain and key processes of care and clinical outcomes, they have been limited to specific hospital treatment locations (e.g., the ICU or the emergency department), individual or narrowly focused strain characteristics, or data measured at nongranular time intervals (3–6, 12, 13). Although strain is increasingly understood as a system-level construct, no robust hospital-wide index of capacity strain exists. Such a tool could prove useful for clinical care and research and in work to improve patient safety and hospital operations.
We therefore sought to develop a composite, multivariable strain index based on granular measurements of multiple capacity strain metrics across emergency departments, wards, step-down units, and ICUs in two diverse health systems and to examine its association with emergency department disposition decisions (i.e., ICU vs. ward admission) and clinical outcomes among patients admitted with sepsis and/or acute respiratory failure (ARF). Furthermore, we sought to validate this strain index as a within-hospital instrumental variable for use in determining the net benefit of ICU admission for patients with sepsis and/or ARF, conditions that are common, morbid, and expensive (18–23), and for patients for whom optimal ICU admission practices are unknown (4, 12, 24–26).
Methods
The study protocol was approved with a waiver of informed consent by the institutional review boards of Kaiser Permanente Northern California and the University of Pennsylvania.
Study Overview, Sites, and Data Sources
This study has two components: 1) creation of the strain index and 2) measurement of the association of the strain index with ICU admission and clinical outcomes among patients with sepsis and/or ARF. We used the electronic health record data of patients treated at 22 Kaiser Permanente Northern California hospitals and 5 Penn Medicine hospitals between 2013 and 2018 to create two distinct patient populations. First, the strain population, whose data contributed to construction of the strain index, included all patients who spent any time in any medical or medical–surgical acute care location during the study period. Second, the clinical cohorts were defined as adult patients (age ≥ 18 yr) with sepsis and/or ARF who were admitted from the emergency department to a medical or medical–surgical ward, step-down unit, or ICU.
Strain-Index Components
We developed the composite strain index by including hospital data from adult medical or medical–surgical inpatient locations (i.e., general wards, step-down units, and ICUs) and from all patients in the emergency departments (including pediatric and obstetric patients). We sought candidate strain metrics that captured the direct workload of ICU care teams, perceived strain on ICU care teams, and strain in non-ICU locations (including emergency departments, wards, and step-down units) to account for patients competing for ICU beds or strain that might impact ICU throughput. Final capacity strain metrics included measures of patient volume, measures of illness acuity, and use of certain life-support therapies determined within prespecified time intervals (Table 1). We assessed patient occupancy on an hourly basis in all locations. As additional measures of acuity and bedside workload, and to capture patients who might be competing for ICU beds, we measured counts of patients requiring vasopressors, mechanical ventilation, bilevel positive airway pressure, and other respiratory support (a composite of non-rebreather mask, high-flow nasal cannula, or fraction of inspired oxygen [FiO2] ≥ 60%) in all locations using sliding 8-hour windows. To capture ICU-specific strain with greater detail, we measured daily census illness acuity using the mean Laboratory-based Acute Physiology Score version 2 (LAPS2), a severity score calibrated for all hospitalized adults with 24 variable inputs, including vital signs, neurologic assessment, laboratory values, and demographics (possible range, 0–414) (27, 28). We also measured ICU turnover (number of newly admitted patients in the prior 24 h) and discharges (number of discharged patients in the prior 24 hours, excluding within-hospital transfers) hourly. All count measures were standardized to the bed capacity of the units in which they were measured (6, 9), and then all measures were standardized by the absolute difference from their local median on the basis of the unit, hospital, and calendar year. (See Appendices 1–3 in the online supplement for additional details regarding capacity strain metrics.)
Table 1.
Capacity-strain metrics, locations, and time intervals
| Strain Metric | Measurement Location |
Measurement Interval | |||
|---|---|---|---|---|---|
| ICU | Ward | SDU | Emergency Department | ||
| Occupancy | Measured | Measured | Measured | Measured | Hourly |
| Census acuity | Measured | Not measured | Not measured | Not measured | Daily at 7 a.m. |
| Turnover | Measured | Not measured | Not measured | Not measured | Hourly |
| Discharges | Measured | Not measured | Not measured | Not measured | Hourly |
| Vasopressors | Measured | Measured | Measured | Measured | 8-h windows (7 a.m. to 3 p.m., 3 p.m. to 11 p.m., 11 p.m. to 7 a.m.) |
| Mechanical ventilation | Measured | Measured | Measured | Measured | |
| BiPAP | Measured | Measured | Measured | Measured | |
| Other respiratory support* | Measured | Measured | Measured | Not measured | |
Definition of abbreviations: BiPAP = bilevel positive airway pressure; ICU = intensive care unit; SDU = step-down unit.
Composite of non-rebreather mask, high-flow nasal cannula, or fraction of inspired oxygen ≥ 60%.
Sepsis and ARF Clinical Cohort Development
We defined our clinical cohorts on the basis of clinical definitions for sepsis or ARF occurring during the emergency department stay. We defined the emergency department stay as the interval of time each patient was physically located in the emergency department, including any emergency department boarding time (i.e., the period after an admission order was placed but before physical transfer to a non–emergency department location).
We defined sepsis on the basis of an adaptation of the Sepsis-3 criteria (29, 30) requiring suspected or confirmed infection and at least one physiologic criterion indicative of organ failure during the emergency department stay. Suspected or confirmed infection was defined as at least one antimicrobial order and at least one microbiologic culture order. Physiologic criteria included 1) Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score ≥ 2 (29, 30), 2) quick SOFA score ≥ 2 (4, 29, 30), 3) serum lactate ≥ 4 mmol/L, 4) a single oxygen saturation measurement by pulse oximetry ≤ 85% during receipt of any supplemental oxygen, 5) receipt of FiO2 ≥ 60% or via a non-rebreather mask for at least two measurements at least 2 hours apart, or 6) receipt of any noninvasive ventilation (including bilevel positive airway pressure and continuous positive airway pressure) or high-flow nasal cannula. For all scores, we calculated maximum values on the basis of the sum of the most abnormal subscores recorded during the emergency department stay.
Because ARF is a clinical syndrome with multiple potential etiologies that lacks a single standardized clinical or research definition (22), we defined ARF for this study on the basis of indicators of hypoxemic or hypercarbic respiratory failure at any time during the emergency department stay, including 1) a single oxygen saturation measurement by pulse oximetry ≤ 85% during receipt of any supplemental oxygen, 2) receipt of supplemental oxygen ≥ 6 L/min or FiO2 ≥ 40% for at least two measurements at least 2 hours apart; 3) arterial carbon dioxide partial pressure > 45 mm Hg or mixed venous carbon dioxide partial pressure > 50 mm Hg and respiratory rate ≥ 22 breaths/min, 4) arterial carbon dioxide partial pressure > 60 mm Hg or mixed venous carbon dioxide partial pressure > 65 mm Hg and pH ≤ 7.3 on a single blood gas, or 5) receipt of any noninvasive ventilation.
Because our focus was on patients who could potentially be admitted to either the ward or the ICU, patients receiving either invasive mechanical ventilation or vasopressors in the emergency department were excluded from the primary clinical cohorts. We also excluded patients who were on comfort measures only or who had hospice status in the emergency department, but we included patients with simple do-not-resuscitate or do-not-intubate status (4). Patients could be included in one or both of the sepsis and ARF cohorts. We considered multiple hospital admissions by the same patient as unique events for all analyses (4). (See Table E1 in the online supplement for additional details regarding the clinical cohorts).
Assignment of Strain Metrics to Clinical Cohorts
We defined emergency department disposition time—when clinical cohort patients were being evaluated in the emergency department and a disposition decision (i.e., ICU vs. ward admission) was likely being made—as a 4-hour window starting from 1 hour before to 3 hours after the first collection time of routine emergency department laboratory results (i.e., complete blood count, basic metabolic profile, lactate, or venous or arterial blood gas). For each patient in the clinical cohorts, we calculated the mean of each strain metric at that patient’s hospital across the five on-the-hour values included within their emergency department disposition time window. (See Appendix 4 in the online supplement for additional details regarding assignment of strain metrics.) These values represented the average hospital-wide strain experienced by each patient during the time of their emergency department disposition decision-making.
Composite Strain-Index Development
We first used L2 penalized (ridge) logistic regression to measure the association of the capacity strain metrics at the time of the emergency department disposition decision with admission to the ICU versus the ward, adjusted for patient characteristics. Ridge regression is used for analyzing regression data with a large number of variables that systematically penalizes outlier variable coefficients by moving them closer to the null (31). We included a priori–designated patient-level covariates: age, sex, race, ethnicity, insurance status, LAPS2, and Comorbidity Point Score version 2 (COPS2), a comorbidity burden score based on the 12 months of preceding International Classification of Diseases diagnosis coding (possible range, 0–1,014) (27, 28, 32). From this “coefficient creation model,” we extracted β-coefficients for each strain metric; for each clinical cohort patient, we then calculated their strain index as a sum of the products of each strain metric β-coefficient and each standardized strain metric value at that patient’s hospital (33–36).
Final Strain-Index Model
Because our study included two clinical cohorts and hospitals with distinct characteristics (e.g., teaching status, urban vs. rural, small vs. large, with and without step-down units, etc.), we evaluated how the composite strain index differed when 1) using clinical cohort–specific (i.e., sepsis or ARF) strain β-coefficients for each metric, 2) using health system–specific or hospital-specific strain β-coefficients, 3) stratifying by the presence of a step-down unit, and 4) restricting to higher-acuity patients alone (i.e., LAPS2 ≥ 100). To balance discrimination with parsimony, we selected a final composite strain index that 1) used disease- and hospital-specific β-coefficients by stratifying the regression-coefficient creation model by clinical cohort and hospital, 2) did not stratify by the presence of a step-down unit but imputed step-down–unit strain metric values = 0 for hospitals without step-down units (equivalent to no deviation from standardized baseline), and 3) included only high-acuity patients with a LAPS2 ≥ 100 (because lower-acuity patients were infrequently admitted to the ICU). The single-value composite strain index therefore takes into account strain metric values standardized to hospital, clinical cohort, bed capacity, timing of emergency department disposition decision, and the unit-, hospital-, or year-specific median and is adjusted for, but does not include, patient-level covariates. Put differently, we created 54 hospital-specific sets of strain metric β-coefficients, one for each of the 27 hospitals in each of the sepsis and ARF clinical cohorts, such that the β-coefficients varied in strength of contribution both within and among hospital-specific sets. (See Appendix 5 in the online supplement for additional details regarding development of the composite capacity strain index; see Table E2 in the online supplement for an example set of strain metric β-coefficients).
Statistical Analysis
We sought to demonstrate that a novel composite strain index fulfills the assumptions of a strong within-hospital instrumental variable for use in determining the net benefit of admission to the ICU for patients with sepsis and/or ARF by validating the necessary assumptions that it is 1) highly associated with ICU versus ward admission (the exposure of interest); 2) not associated with clinical outcomes of interest, other than through its association with the exposure of interest; and 3) not meaningfully associated with potentially confounding patient-level characteristics (26).
Association of the strain index with ICU admission
To test our hypothesis that the strain index would be inversely associated with ICU admission, we used multivariable logistic regression, stratified by clinical cohort and adjusted for patient-level covariates with hospital as a fixed effect, with a primary outcome of ICU admission from the emergency department. Because hospital-level analyses were superior to health system–level analyses during strain-index development, we opted to adjust for hospital rather than health system in all analyses. On the basis of prior work, we classified step-down–unit admissions as ward admissions for this outcome assessment (4).
To determine whether the composite, multivariable strain index improved the discrimination between ICU and ward admission compared with a simpler measure of strain, we repeated the above analyses using the ICU occupancy strain metric alone as an exposure; in prior work, we have shown its association with ICU admission (4).
Association of the strain index with clinical outcomes
Determining whether the strain index is associated with clinical outcomes independently of its potential association with emergency department disposition decision required that we evaluate cohorts whose emergency department disposition decision was not sensitive to strain. To do so, we identified separate sepsis and ARF subcohorts whose emergency department disposition decision was minimally impacted by the strain index: patients almost always admitted to the ward and patients almost always admitted to the ICU. We defined the “usually-ward” subcohorts as 1) sepsis patients without end-organ dysfunction (i.e., SOFA = 0) and 2) ARF patients with a LAPS2 ≤ 50, on the basis of low observed ICU admission rates. In both cases, we excluded patients who required mechanical ventilation or vasopressors in the emergency department. We defined “usually-ICU” sepsis and ARF subcohorts as those in whom both invasive mechanical ventilation and vasopressors were started in the emergency department.
The primary clinical outcome was hospital length of stay (LOS), defined as the time from inpatient admission to hospital discharge, using a “placement-of-death” approach. To account for in-hospital deaths, defined as death at discharge or a transition to hospice, deaths were ranked as equivalent to undesirable hospital LOSs, including the 95th or 99th percentile of hospital LOS, or to the longest observed hospital LOS (37, 38). To analyze the ranked LOS outcome, we performed multivariable median quantile regression (39) to measure the association between the strain index and median hospital LOS, adjusted for patient-level covariates with hospital as a fixed effect. Quantile regression estimates were calculated with bootstrapped 95% confidence intervals (95% CIs) using 5,000 runs. Quantile regression estimates can be interpreted to represent the predicted change in median hospital LOS with a 1-unit change in the strain index at the specified quantile. Secondary outcomes of hospital mortality and hospital discharge disposition (i.e., discharge home vs. not home) were analyzed using multivariable logistic regression.
Association of the strain index with patient-level characteristics
Finally, to determine whether the strain index was associated with patient characteristics, we used analysis of variance (ANOVA) testing. Given the large sample size, for any statistically significant relationships in the ANOVA results, we visually inspected the associations with the strain index by using box and scatter plots and, for continuous variables, by using the Pearson correlation coefficient (R).
Exposure, covariate, and outcome variables were missing at low rates (<1%), allowing for complete case analysis. P values < 0.05 were considered statistically significant. Analyses were conducted using Stata (StataCorp), R language for statistical computing (R Foundation), and SAS computer programming language (SAS Institute).
Results
Clinical Cohorts and the Strain Index
The clinical cohorts included 90,150 patients who met criteria for sepsis and 45,339 patients who met criteria for ARF (26,404 patients were included in both clinical cohorts). The 86.1% and 83.4% of patients with sepsis and ARF, respectively, came from Kaiser Permanente Northern California, consistent with it providing 22 of the 27 hospitals (81.5%) in the sample. Among patients with sepsis and ARF with a LAPS2 ≥ 100, mean age was 73.6 and 72.9 years, 60.7% and 61.1% were of white race, 20.5% and 29.5% were admitted to the ICU, median hospital LOS was 3.9 and 3.9 days, and observed in-hospital mortality was 17.2% and 20.2%, respectively (Table 2). We used 9,278,610 acute care encounters to develop the strain index, which had a range of −6.19 to 7.97. Figures E1 and E2 display the strain-index variability among and within hospitals over time.
Table 2.
Clinical cohort patient characteristics
| Characteristics | Sepsis Cohort (n = 90,150) | ARF Cohort (n = 45,339) |
|---|---|---|
| Age, mean yr (SD) | 73.6 (15.1) | 72.9 (14.7) |
| Male, n (%) | 46,994 (52.1) | 22,232 (49.0) |
| Race, n (%) | ||
| White | 54,732 (60.7) | 27,715 (61.1) |
| Black | 11,171 (12.4) | 6,807 (15.0) |
| Asian | 9,073 (10.1) | 4,169 (9.2) |
| Other* | 15,174 (16.8) | 6,648 (14.7) |
| Hispanic ethnicity, n (%) | 8,869 (9.8) | 3,662 (8.1) |
| Insurance, n (%) | ||
| Private | 70,939 (78.7) | 33,348 (73.6) |
| Medicare/Medicaid | 12,252 (13.6) | 7,563 (16.7) |
| Other/unknown | 6,959 (7.7) | 4,428 (9.8) |
| LAPS2,† mean (SD) | 130.2 (24.3) | 132.3 (25.7) |
| COPS2,‡ mean (SD) | 109.9 (61.4) | 114.8 (60.2) |
| Admitted to the ICU, n (%) | 18,456 (20.5) | 13,370 (29.5) |
| Hospital LOS, d, median (IQR) | 3.9 (2.4–6.7) | 3.9 (2.2–5.8) |
| Discharge home, n (%) | 51,880 (57.6) | 26,240 (57.9) |
| Hospital mortality,§n (%) | 15,465 (17.2) | 9,146 (20.2) |
Definition of abbreviations: ARF = acute respiratory failure; COPS2 = Comorbidity Point Score version 2; ICU = intensive care unit; IQR = interquartile range; LAPS2 = Laboratory-based Acute Physiology Score version 2; LOS = length of stay; SD = standard deviation.
Includes Hawaiian or Pacific Islander, American Indian or Native American, self-reported race as “multiple” or “other,” or unknown.
LAPS2 possible range of 0–414 and univariate relationship with hospital mortality (before applying inclusion and exclusion criteria): 0–49, 0.7%; 50–99, 16.9%; ≥100, 82.5%. Inclusion criteria were restricted to patients without mechanical ventilation or vasopressors and with a LAPS2 ≥ 100 in the emergency department.
COPS2 possible range of 0–1,014 and univariate relationship with hospital mortality (before application of inclusion and exclusion criteria): 0–64, 17.1%; ≥65, 82.9%.
Defined as death or transition to hospice.
Association of the Strain Index with ICU Admission
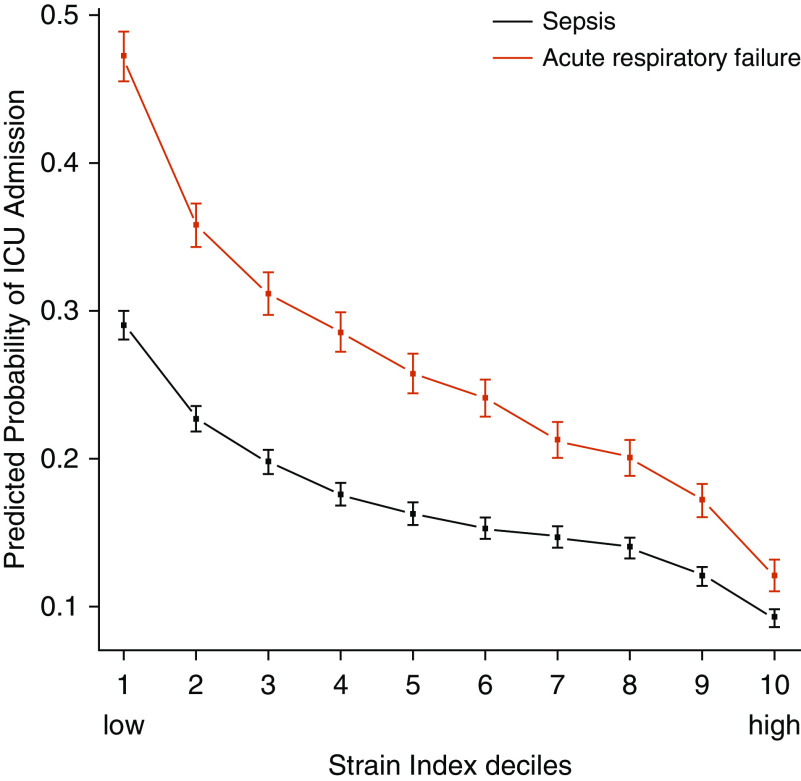
Among patients with sepsis and/or ARF who did not require mechanical ventilation or vasopressors but had high acuity (LAPS2 ≥ 100) in the emergency department, higher strain at the time of the emergency department disposition decision was associated with decreased probability of ICU admission (Figure 1 and Table E3). Among patients with sepsis, the adjusted predicted probability of ICU admission decreased from 29.0% (95% CI, 28.0–30.0%) when emergency department disposition occurred during the lowest strain index decile to 9.3% (95% CI, 8.7–9.9%) during the highest strain index decile. Compared with the lowest (first) strain index decile, the odds ratios (ORs) for ICU admission decreased monotonically from the second strain index decile (OR, 0.72; 95% CI, 0.67–0.77) to the highest (10th) strain index decile (OR, 0.25; 95% CI, 0.23–0.27) (all P < 0.001). Among patients with ARF, the adjusted predicted probability of ICU admission decreased from 47.2% (95% CI, 45.6–48.9%) during the lowest strain index decile to 12.1% (95% CI, 11.0–13.2%) during the highest strain index decile. Similar to the sepsis ORs, the ARF ORs for ICU admission decreased from the second strain index decile (OR, 0.62; 95% CI, 0.57–0.68) to the highest strain index decile (OR, 0.15; 95% CI, 0.14–0.17) (all P < 0.001).
Figure 1.
Association of the strain index with intensive care unit (ICU) admission among patients with sepsis and acute respiratory failure (ARF). As the strain index increases by decile (x-axis), the adjusted predicted probability of ICU admission decreases for patients with sepsis and ARF (y-axis). Note that the strain-index deciles were calculated separately for the sepsis and ARF cohorts. Squares and whiskers display point estimates and 95% confidence intervals for the adjusted predicted probability of ICU admission.
When the analyses were repeated using ICU occupancy alone as the strain exposure, rather than using the composite strain index, ranges of adjusted predicted probabilities of ICU admission between the lowest and highest strain deciles were narrower and, in many cases, did not decrease monotonically across deciles (Figure E3). Among patients with sepsis, the adjusted predicted probability of ICU admission decreased from 20.0% (95% CI, 19.1–20.9%) in the lowest ICU occupancy decile to 14.3% (95% CI, 13.6–15.1%) in the highest. Among patients with ARF, the adjusted predicted probability of ICU admission decreased from 30.3% (95% CI, 28.9–31.8%) in the lowest ICU occupancy decile to 20.1% (95% CI, 18.9–21.3%) in the highest (Table E4).
Independent Association of the Strain Index with Hospital LOS, Hospital Mortality, and Hospital Discharge Disposition
Usually-ward subgroup
Patients who met inclusion criteria for sepsis but who had a SOFA score = 0 and did not require mechanical ventilation or vasopressors in the emergency department (n = 6,562) were admitted to the ward 88.9% of the time. Patients who met inclusion criteria for ARF but who had a LAPS2 ≤ 50 and did not require mechanical ventilation or vasopressors in the emergency department (n = 3,571) were admitted to the ward 88.5% of the time. Among these lower-acuity patients with sepsis or ARF, the strain index was not associated with hospital LOS (Table 3), hospital mortality (Table E5), or discharge home (Table E5) (all P ≥ 0.33).
Table 3.
Association of strain index with hospital LOS in “usually-ward” and “usually-ICU” subgroups of patients with sepsis and ARF
| Cohort | Quantile Regression Estimate (Bootstrap 95% CI)*; P Value |
|
|---|---|---|
| Usually Ward† | Usually ICU‡ | |
| Death set as 95th-percentile LOS§ | ||
| Sepsis‖ | −0.07 (−0.06 to 0.46); 0.58 | −0.35 (−1.06 to 2.67); 0.70 |
| ARF¶ | −0.06 (−0.23 to 0.17); 0.58 | −0.07 (−1.01 to 0.77); 0.89 |
| Death set as 99th-percentile LOS** | ||
| Sepsis | −0.07 (−0.02 to 0.50); 0.55 | −0.55 (−2.46 to 10.41); 0.84 |
| ARF | −0.06 (−0.24 to 0.19); 0.55 | −0.19 (−4.35 to 2.33); 0.91 |
| Death set as longest observed LOS†† | ||
| Sepsis | −0.07 (−0.03 to 0.49); 0.56 | 10.36 (−24.87 to 70.25); 0.64‡‡ |
| ARF | −0.06 (−0.24 to 0.20); 0.54 | 1.27 (−35.27 to 20.43); 0.92‡‡ |
Definition of abbreviations: 95% CI = 95% confidence interval; ARF = acute respiratory failure; ICU = intensive care unit; LOS = length of stay.
Models were adjusted for patient-level covariates of age, sex, ethnicity, race, insurance, Laboratory-based Acute Physiology Score version 2, Comorbidity Point Score version 2, and hospital.
Based on 5,000 runs.
Sepsis patients with Sequential (Sepsis-related) Organ Failure Assessment = 0 and no mechanical ventilation or vasopressors in the emergency department (88.9% admitted to the ward). ARF patients with Laboratory-based Acute Physiology Score version 2 ≤ 50 and no mechanical ventilation or vasopressors in the emergency department (88.5% admitted to the ward).
Mechanical ventilation and vasopressors in the emergency department (99.5% of patients with sepsis and 99.2% of patients with ARF admitted to the ICU).
For the usually-ward group, the 95th-percentile LOS = 12.7 days for sepsis and 10.8 days for ARF; for the usually-ICU group, the 95th-percentile LOS = 31.2 days for sepsis and 29.7 days for ARF.
n = 6,562 for the usually-ward group, and n = 2,169 for the usually-ICU group.
n = 3,571 for the usually-ward group, and n = 3,150 for the usually-ICU group.
For the usually-ward group, the 99th-percentile LOS = 26.5 days for sepsis and 25.1 days for ARF; for the usually-ICU group, the 99th-percentile LOS = 58.5 days for sepsis and 56.6 days for ARF.
For the usually-ward group, the longest observed LOS = 288.9 days for sepsis and 83.7 days for ARF; for the usually-ICU group, the longest observed LOS = 341.0 days for sepsis and 341.0 days for ARF.
Divergent point estimates in these analyses are due to the presence of outlier patients with extremely long hospital LOS in the usually-ICU subgroup that then serve as the LOS assignment for deaths in the “placement-of-death” approach.
Usually-ICU subgroup
Patients with sepsis who required mechanical ventilation and vasopressors in the emergency department (n = 2,169) were admitted to the ICU 99.5% of the time. Patients with ARF who required mechanical ventilation and vasopressors in the emergency department (n = 3,150) were admitted to the ICU 99.2% of the time. Among these higher-acuity patients with sepsis or ARF, the strain index was not associated with hospital LOS (Table 3), hospital mortality (Table E5), or discharge home (Table E6) (all P > 0.13).
Association of the Strain Index with Patient Characteristics
Among patients with sepsis who did not require mechanical ventilation or vasopressors but had high acuity (LAPS2 ≥ 100) in the emergency department, the strain index demonstrated statistically significant associations with age, race, LAPS2, COPS2, and insurance (all ANOVA P < 0.001). Among patients with ARF who did not require mechanical ventilation or vasopressors but had high acuity (LAPS2 ≥ 100) in the emergency department, the strain index demonstrated statistically significant associations with race, LAPS2, COPS2, and insurance (all ANOVA P < 0.001). Although these associations were statistically significant, box and scatter plots revealed minute effect sizes and, for continuous variables, very small correlations (R = −0.026 to 0.015) (Table E7 and Figures E4–E17).
Discussion
This study rectifies limitations among prior examinations of capacity strain, including those by our own group, by creating a hospital-wide composite strain index that 1) is based on temporally granular measurements of multiple capacity strain metrics across emergency departments, wards, step-down units, and ICUs; 2) was measured across many hospitals in two diverse health systems; and 3) is optimized for selected diagnoses and hospitals. Our first key finding is that this composite strain index, as a measure of hospital strain, was highly associated with emergency department disposition to the ICU versus the ward for patients with sepsis and/or ARF who did not require mechanical ventilation or vasopressors but had high acuity in the emergency department. Between the lowest and highest deciles of strain, there was at least a threefold difference in admissions to the ICU. This trend persisted in a consistent dose–response manner across the entire spectrum of strain (i.e., not just comparing the lowest with the highest strain deciles), among and within different hospitals and health systems, and across clinical cohorts. This finding suggests that although patient triage would ideally be dictated by convictions regarding where patients receive optimal care, the capacity strain of the system plays a major role in influencing these critical choices. This tremendous variability highlights the subjectivity of ICU admission decisions that could potentially be improved with evidence-based guidelines.
The second important finding of our study is that this novel composite strain index fulfills the requirements to be used as a within-hospital instrumental variable for future studies of which patients may benefit from ICU versus ward admission. Obtaining such unbiased measurements is methodologically challenging. Traditional approaches to risk adjustment in retrospective data are unlikely to account for confounding-by-indication between ICU and ward cohorts. Prospective randomization, although possible (40), carries significant logistical and ethical hurdles. Finally, prior efforts at using instrumental variables (26, 41) have examined those that vary among hospitals rather than within hospitals, making them unable to address the clinically relevant decision between whether to admit a patient to the ICU or the ward within the same hospital. Our study demonstrates that the composite strain index fulfills the assumptions of a strong within-hospital instrumental variable as it is 1) highly associated with ICU versus ward admission (the exposure of interest); 2) not associated with hospital LOS, hospital mortality, or hospital discharge disposition (outcomes of interest) when the exposure of interest is held constant; and 3) not meaningfully associated with potentially confounding patient-level characteristics (26). This is important because a promising approach for enhancing the cost-effectiveness of acute care delivery is to only admit patients to the ICU who truly benefit from such resource-intensive care (18).
Third, although the magnitude of the data and the analytic complexity of the strain index construction would require substantial analytic resources to replicate in other health systems, we have shown that such an investment would provide benefits beyond those obtainable with simpler, and more commonly used, approaches to gauging strain. Specifically, the discrimination between ICU and ward admission generated by the novel strain index is considerably greater than that produced by simpler measures of ICU occupancy, and these simpler approaches may not discriminate at all in the middles of their distributions. These findings suggest that many factors within and outside of ICUs influence triage.
Limitations
The results of this study should be interpreted in the context of a number of limitations. First, in this large, clinical retrospective analysis, the most likely potential source of data error is in accurate counts of interventions that are routinely turned on and off over time (e.g., vasopressors and noninvasive respiratory support). Use of these interventions may not be documented with perfect fidelity, and documentation may vary across hospitals and health systems on the basis of electronic health record software, local implementation, and clinical workflows. Undercounts, however, would bias toward decreased strain index discrimination rather than toward type I error. Second, we rely on bed capacity as a standardizing denominator. Although we took into account known bed-capacity changes during the study period—such as the opening of a new ICU or the phasing-out of a ward—we were not able to take into account changes in functional bed capacity such as temporary maintenance closures, staffing-associated closures, double-bedding, and hallway beds, among others. The impact of these uncaptured bed-capacity changes is partially, but not fully, accounted for by standardizing to hospital- and unit-specific yearly medians. Finally, although this is, to our knowledge, the most extensive hospital-wide measure of capacity strain to date, there are certainly additional strain metrics not included in our index, in particular dynamic measures of bedside nursing workload, such as those that required higher nurse-to-patient ratios (13).
Conclusions
In summary, we find that although patient disposition ought to be predicated on estimates of optimal care for a given patient, for high-acuity patients with sepsis and ARF in the emergency department, it is in fact often influenced by strain across the hospital. Furthermore, such hospital strain is strongly associated with ICU admission, with as much as a threefold reduction in the likelihood of ICU admission, and is not associated with hospital outcomes or patient characteristics, thereby fulfilling the assumptions of a strong within-hospital instrumental variable. This provides a potential method for producing unbiased estimates of the net benefit of ICU admission for patients with sepsis and/or ARF. Future work will estimate these benefits, explore whether they can be generalized for other diagnoses and hospitals, and examine their potential for enhancing the understanding of organizational and care-delivery characteristics that may account for outcome differences.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health grants R01HL136719 (S.D.H.), R35GM128672 (V.X.L.), and K23HL146894 (R.K.); the Permanente Medical Group (G.J.E.); and Agency for Healthcare Research and Quality grant K12HS026372 (G.L.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of any funders, including the Agency for Healthcare Research and Quality.
Author Contributions: Conception and design of study: G.L.A., D.S.S., M.K.D., R.K., B.B., E.D., G.J.E., S.D.H., and V.X.L. Data acquisition: G.L.A., M.C., B.B., E.D., V.X.L. Analysis and data interpretation: G.L.A., M.C., D.S.S., M.K.D., R.K., B.B., W.W., G.J.E., S.D.H., and V.X.L. Drafting and revision of the manuscript: G.L.A., M.C., D.S.S., M.K.D., R.K., B.B., W.W., E.D., G.J.E., S.D.H., and V.X.L. All authors approve of the final version to be published. G.L.A. takes final responsibility for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 2.Kerlin MP, Harhay MO, Vranas KC, Cooney E, Ratcliffe SJ, Halpern SD. Objective factors associated with physicians’ and nurses’ perceptions of intensive care unit capacity strain. Ann Am Thorac Soc. 2014;11:167–172. doi: 10.1513/AnnalsATS.201306-141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anesi GL, Gabler NB, Allorto NL, Cairns C, Weissman GE, Kohn R, et al. Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: a 2-hospital study in South Africa. J Intensive Care Med. 2018;35:1104–1111. doi: 10.1177/0885066618815804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anesi GL, Liu VX, Gabler NB, Delgado MK, Kohn R, Weissman GE, et al. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc. 2018;15:1328–1335. doi: 10.1513/AnnalsATS.201804-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SE, Rey MM, Pardo D, Weinreb S, Ratcliffe SJ, Gabler NB, et al. The allocation of intensivists’ rounding time under conditions of intensive care unit capacity strain. Am J Respir Crit Care Med. 2014;190:831–834. doi: 10.1164/rccm.201406-1127LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabler NB, Ratcliffe SJ, Wagner J, Asch DA, Rubenfeld GD, Angus DC, et al. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188:800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua M, Halpern SD, Gabler NB, Wunsch H. Effect of ICU strain on timing of limitations in life-sustaining therapy and on death. Intensive Care Med. 2016;42:987–994. doi: 10.1007/s00134-016-4240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenzie MS, Auriemma CL, Olenik J, Cooney E, Gabler NB, Halpern SD. An observational study of decision making by medical intensivists. Crit Care Med. 2015;43:1660–1668. doi: 10.1097/CCM.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159:447–455. doi: 10.7326/0003-4819-159-7-201310010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissman GE, Gabler NB, Brown SE, Halpern SD. Intensive care unit capacity strain and adherence to prophylaxis guidelines. J Crit Care. 2015;30:1303–1309. doi: 10.1016/j.jcrc.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41:2712–2719. doi: 10.1097/CCM.0b013e318298a139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews KS, Durst MS, Vargas-Torres C, Olson AD, Mazumdar M, Richardson LD. Effect of emergency department and ICU occupancy on admission decisions and outcomes for critically ill patients. Crit Care Med. 2018;46:720–727. doi: 10.1097/CCM.0000000000002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohn R, Harhay MO, Weissman GE, Anesi GL, Bayes B, Greysen SR, et al. Ward capacity strain: a novel predictor of delays in intensive care unit survivor throughput. Ann Am Thorac Soc. 2019;16:387–390. doi: 10.1513/AnnalsATS.201809-621RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohn R, Harhay MO, Weissman GE, Anesi GL, Bayes B, Song H, et al. The association of geographic dispersion with outcomes among hospitalized pulmonary service patients. Ann Am Thorac Soc. 2020;17:249–252. doi: 10.1513/AnnalsATS.201906-471RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein SL, Aronsky D, Duseja R, Epstein S, Handel D, Hwang U, et al. Society for Academic Emergency Medicine, Emergency Department Crowding Task Force. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1–10. doi: 10.1111/j.1553-2712.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- 16.Salehi L, Phalpher P, Valani R, Meaney C, Amin Q, Ferrari K, et al. Emergency department boarding: a descriptive analysis and measurement of impact on outcomes. CJEM. 2018;20:929–937. doi: 10.1017/cem.2018.18. [DOI] [PubMed] [Google Scholar]
- 17.Sun BC, Hsia RY, Weiss RE, Zingmond D, Liang L-J, Han W, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61:605–611, e6. doi: 10.1016/j.annemergmed.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anesi GL, Admon AJ, Halpern SD, Kerlin MP. Understanding irresponsible use of intensive care unit resources in the USA. Lancet Respir Med. 2019;7:605–612. doi: 10.1016/S2213-2600(19)30088-8. [DOI] [PubMed] [Google Scholar]
- 19.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 20.Halpern NA, Pastores SM. Critical care medicine beds, use, occupancy, and costs in the United States: a methodological review. Crit Care Med. 2015;43:2452–2459. doi: 10.1097/CCM.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46:1889–1897. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Steingrub JS, Lagu T, et al. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8:76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med. 2017;45:1443–1449. doi: 10.1097/CCM.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Admon AJ, Seymour CW, Gershengorn HB, Wunsch H, Cooke CR. Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest. 2014;146:1452–1461. doi: 10.1378/chest.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127:923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA. 2015;314:1272–1279. doi: 10.1001/jama.2015.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 28.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 29.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freidman J, Hastie T, Tibshirani R, Simon N, Narasimhan B, Qian J. R Glmnet: lasso and elastic-net regularized generalized linear models. Package version 2.0-18. Vienna, Austria: R Foundation for Statistical Computing; 2020 [updated 2020 Jun 16; accessed 2020 Sep 24]. Available from: https://cran.r-project.org/web/packages/glmnet/index.html. [Google Scholar]
- 32.Liu V, Kipnis P, Gould MK, Escobar GJ. Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care. 2010;48:739–744. doi: 10.1097/MLR.0b013e3181e359f3. [DOI] [PubMed] [Google Scholar]
- 33.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26:2333–2355. doi: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol. 2013;42:1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies NM, von Hinke Kessler Scholder S, Farbmacher H, Burgess S, Windmeijer F, Smith GD. The many weak instruments problem and Mendelian randomization. Stat Med. 2015;34:454–468. doi: 10.1002/sim.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small DS, Rosenbaum PR. War and wages: the strength of instrumental variables and their sensitivity to unobserved biases. J Am Stat Assoc. 2008;103:924–933. [Google Scholar]
- 37.Lin W, Halpern SD, Prasad Kerlin M, Small DSA. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2017;26:292–311. doi: 10.1177/0962280214545121. [DOI] [PubMed] [Google Scholar]
- 38.Ranganathan P, Pramesh CS. Censoring in survival analysis: potential for bias. Perspect Clin Res. 2012;3:40. doi: 10.4103/2229-3485.92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenker R, Portnoy S, Ng PT, Zeileis A, Grosjean P, Ripley BD. Quantreg: quantile regression. Package version 5.42. Vienna, Austria: R Foundation for Statistical Computing; 2020 [updated 2020 Sep 9; accessed 2020 Sep 24]. Available from: https://cran.r-project.org/web/packages/quantreg/index.html.
- 40.Guidet B, Leblanc G, Simon T, Woimant M, Quenot JP, Ganansia O, et al. ICE-CUB 2 Study Network. Effect of systematic intensive care unit triage on long-term mortality among critically ill elderly patients in France: a randomized clinical trial. JAMA. 2017;318:1450–1459. doi: 10.1001/jama.2017.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Intensive care unit admission and survival among older patients with chronic obstructive pulmonary disease, heart failure, or myocardial infarction. Ann Am Thorac Soc. 2017;14:943–951. doi: 10.1513/AnnalsATS.201611-847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.