Conspectus
Heteroatom-doped porous carbon membranes (HPCMMs) with a tailor-made pore architecture, chemical composition, atomic structural order, and surface state represent an exciting family of porous carbon materials for diverse potential applications in catalysis, water treatment, biofiltration, energy conversion/storage, and so forth. Conventional porous carbon membranes possess intrinsic structural integrity, interconnectivity, and chemical purity across the atomic-to-macro world and have been popularly incorporated into devices as separators or chemically inert conductive supports, circumventing otherwise the inevitable complicated processing and structure weakness of their fine powderous counterpart. Motivated by the distinguished heteroatom-doping effect that revolutionizes the chemical and physical nature of carbon materials, the HPCMM research surges very recently, and focuses not only on the eminent conductive supports or separators but also on electro(co)catalysts in energy devices. Synergy of the porous nature, incorporation of heteroatoms, and the membrane state creates a vivid profile pattern and new task-specific usage. It is also noteworthy that the inherent structural merits of HPCMMs plus a high electron conductivity imbue them as a reliable binder-free model electrode to derive the intrinsic structure–property relationship of porous carbons in electrochemical environments, excluding the complex and adverse factors in association with polymer binders in carbon powder-based electrodes. HPCMMs are of both intense academic interest and practical value because of their well-defined properties endowed by controllable structure and porosity at both atomic and macroscopic scales in a membrane form. The sole aim of this article is to bring this group of porous carbon materials to the forefront so their comprehensive properties and functions can be better understood to serve the carbon community to address pressing materials challenges in our society.
In this Account, we highlight the latest discovery and proceedings of HPCMMs, particularly the advancements in how to tailor structures and properties of HPCMMs by rational structure design of porous polymer membranes as sacrificial template built up especially from heteroatom-rich poly(ionic liquid)s (PILs). We will also stress the carbonization craft and the state-of-the-art electrochemical applications for HPCMMs. Key factors and thoughts in heteroatom doping and porous systems in HPCMMs are discussed. A future perspective of the challenges and promising potential of HPCMMs is cast on the basis of these achievements.
1. Introduction
Heteroatom-doped porous carbon membranes (HPCMMs) are a unique group of carbon materials carrying an interconnected porous network in a membrane state, in which some of carbon atoms are selectively replaced by heteroatom such as nitrogen (N), sulfur (S), phosphorus (P), boron (B), and selenium (Se). Oxygen due to its wide presence in the majority of carbonaceous materials is not discussed here. In stark contrast to easy-to-make porous carbon powders that dominate the current research of porous carbons, HPCMMs are synthetically more demanding. This is due to their hierarchical pores in the entire micro-, meso- to macropore size range, and the covalent doping of heteroatoms, plus a macroscopically visible membrane shape that can be directly used in devices without complicated processing procedures. Such structural features across an unusually wide dimension range are highly preferable to their powder counterparts. Mechanical rolling of thermally expanded graphite flakes, chemical vapor deposition, vacuum filtration-assisted deposition, pyrolysis of thermosetting polymer precursors or biotemplates, though technologically mature for fabrication of porous carbon membranes,1−8 are restrictedly disadvantageous in terms of the fine control of their pore sizes, in particular in the micropore regime, the complex pore architectures, and/or easiness in scalable production.
Equally important to pores, heteroatom-doping can impart desirable properties to carbons that are otherwise absent or rather weak in nondoped ones. The electronegativity, charge density, and size of heteroatoms are all decisive factors to reshape the property profile of carbons, including their conductivity, band configuration, and surface activity. It is not only about the type of heteroatoms, but also the same heteroatom in a different bond configuration with carbons matters to the doping effect, not to mention that the mechanism of heteroatom effect in graphitic carbons is still under debate. Bear in mind that heteroatom doping may not always bring in favorable features. For example, carbons are functionalized by heteroatoms usually at the price of a limited degree of graphitization (thus limited structural order), as heteroatoms are in nature “defects” in a carbon framework. Such a trade-off also exists between the evolution of micropores and crystallinity of HPCMMs. Therefore, the design and synthesis of HPCMMs need to take consideration of the target use in systems and devices. For instance, a high graphitization degree may be irrelevant to its gas separation function but favors electrocatalytic reactions. Fairly speaking, there exists no best structure but always the most suitable structure of HPCMMs for a system.
To achieve desirable HPCMMs, a key issue is how to prepare a suitable porous polymer membrane (PPM) as a template. Fueled by substantial advance in the past two decades in macromolecular design and engineering, especially those containing thermally robust aromatic, cyano or dicyanamide groups, a rich library of PPMs has been made and offered great opportunities to template HPCMMs by one-step pyrolysis. In comparison to biomass-derived membranes, synthetic PPMs have a larger freedom in engineering pores and heteroatoms. As for PPMs from biomass (e.g., silk and cotton), their renewable aspect is obviously a profound advantage.9−12 Nevertheless, activation reagents, porogens or additive salts, are usually required to achieve HPCMMs with hierarchical porous structures or high surface areas. For heteroatoms, N, S, and sometimes P are the most common ones present in bio(macro)molecules, which may dope carbons upon carbonization. Other heteroatoms, including B, Se, or more, are rarely found in bio(macro)molecules but can be easily positioned into synthetic polymers on demand.
Another issue is the carbonization craft for creating HPCMMs, in which the temperature program, carbonization environment, and inner oncotic pressure of PPMs during pyrolysis have to be aligned with the final usage of HPCMMs. For example, preheating of macromolecules containing cyano groups at 300 °C could form a thermally more robust polytriazine network than ones without cyano groups. It can increase the carbonization yield and preserve the morphology at the stage of cross-linking. In this regard, the porous structure, the chemical composition, and structural integrity of HPCMMs are largely, if not entirely, dictated by that of the corresponding PPMs and the employed carbonization craft. Unusually, design of HPCMMs needs to attentively consider the polymer chain characteristics, including their molecular weight, charges, constitutional atoms beyond C/H/O, and chain topology, because they jointly regulate the structure of PPMs at the molecular and macroscopic level that ends up largely in the final HPCMMs.
Currently, porous carbons are profoundly involved in fabrication of electrochemical energy devices.13−15 Without insulating polymer binders, HPCMM itself is directly utilized as electrode, thus avoiding binder-related dilemmas of pulverous carbons in electrochemical applications. Therefore, recent applications of HPCMMs are dominated by electrochemical conversion and storage, where the high conductivity and heteroatoms are necessary for achieving high performance. For the above reasons, the HPCMM topic is intrinsically interdisciplinary and spans from polymer science, carbon materials, and electrochemistry. This article will present the latest advance in template synthesis, structural control, and electrochemical applications of HPCMMs.
2. PIL Structures as Templates
Template synthesis of carbons carrying rich micro/meso-/macropores or their combination is a classic approach and utilizes historically either hard or soft templates. To template HPCMMs by inorganic molds with both tiny pores and a macroscopic membrane shape is rather difficult, leaving the soft template the benchmark approach. Frequently the soft template is unnecessary to remove after carbonization and serves as precursors of carbon and heteroatom, i.e., a sacrificial template that is transferred into the final HPCMM by carbonization.
2.1. Porous PIL Membrane as Precursor
Morphology-maintaining carbonization of homologous porous polymer membranes (PPMs) is a common, conceptually straightforward method. The primary larger pores, i.e., the macropores, in HPCMMs come from that of PPMs, and the secondary smaller pores, usually the micro/mesopores, from the pyrolytic decomposition or chemical activation of the polymer entity. To note, the chemical composition of HPCMMs is determined by the temperature-dependent pyrolytic product of PPMs. A sufficiently high temperature (>800 °C) improves the graphitization degree and conductivity and are beneficial for electrochemical energy application. As mature fabrication techniques of PPMs, such as (co)polymer assembly and electrospinning,16,17 have been well-established to date, the remaining issue to address is the nature of polymers in PPMs, as not all polymers are suitable as both membrane materials and carbon precursors.
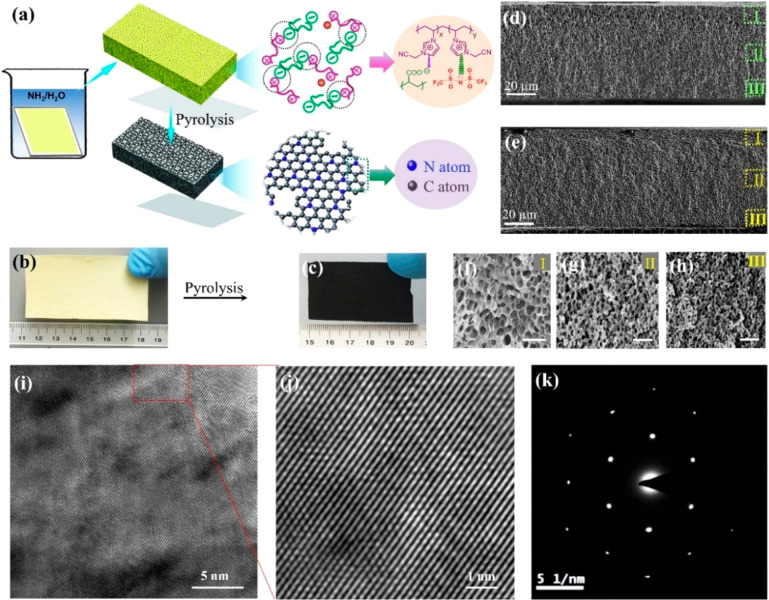
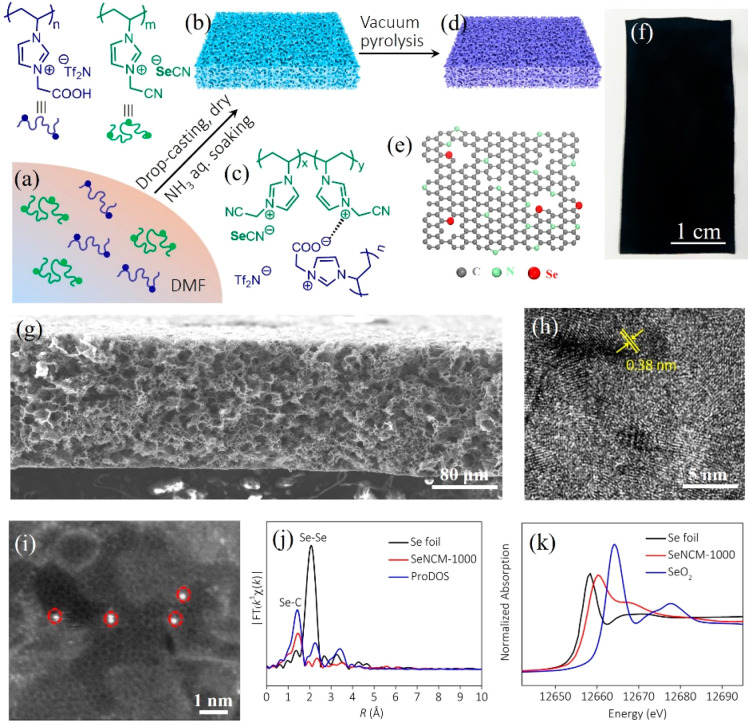
Poly(ionic liquid) (PIL) produced via polymerization of ionic liquids (ILs), emerges as a family of heteroatom-rich polyelectrolytes. Due to superior thermal stability of IL species as the building units, PILs are thermally more stable than most of the common neutral polymers; at the same pyrolysis temperature PILs may form carbons in a higher yield due to thermally enabled cross-linking reactions, such as the cyclization of cyano groups. Therefore, PILs are recognized as distinguished carbon precursors. PILs were first used in the presence of FeCl3 to produce mesoporous carbons in 2010 by us;18 thereafter, we found pyrolysis of a hydrophobic PIL poly(1-cyanomethyl-3-vinylimidazolium bis(trifluoromethanesulfonyl)imide) (termed PCMVImTf2N, chemical structures of PILs are all shown in Figure S1) alone at 1000 °C produced N-coped micro/mesoporous carbons with a Brunauer–Emmett–Teller specific surface area (SBET) of up to 520 m2/g.19 In 2013, we assembled PIL-derived PPMs with hierarchical and gradient pores along their cross-section via electrostatic complexation of poly(acrylic acid) (termed PAA) and PCMVImTf2N (this membrane is termed PCMVImTf2N-PAA PPM).20 The multifaceted roles of PCMVImTf2N in these studies, i.e., as carbon precursor and membrane materials, ignited our passion in the synthesis of HPCMMs directly out of the PCMVImTf2N-PAA PPM. Conventional carbonization protocols by pyrolysis of this PPM under inert gases, i.e., N2 and Ar, were tested to fail, leaving behind only carbon cracks rather than an intact membrane. To our delight, we discovered that carbonization under a narrow window of vacuum (1–10 mbar) was decisive to preserve the overall membrane morphology of PCMVImTf2N-PAA PPM (Figure 1a–c).21 In hindsight, it is spotted that vacuum carbonization can promptly pump out the in situ-pyrolytic volatile fragments to avoid their condensation back into carbon membranes, thus sufficiently decreasing the inner oncotic pressure of the PPM during pyrolysis to hold its integrity.
Figure 1.
(a) Schematic illustration of synthesis of HPCMMs from PCMVImTf2N-PAA PPMs. (b, c) Photographs of a PPM and the derived HPCMM. (d, e) Cross-sectional SEM images of HPCMMs derived from PCMVImTf2N-PAA PPMs with PAA of MW ∼ 100 kDa and 250 kDa at 1000 °C, respectively. (f, g) High-magnification cross-sectional SEM images of the HPCMM in (e). Scale bar is 200 nm. (i, j) Representative HRTEM images at different magnifications of the HPCMM prepared at 1000 °C. (k) Selected-area electron diffraction (SAED) pattern that is indicative of a single-crystal-like characteristic. Reproduced with permission from ref (21). Copyright 2017 Springer Nature.
This breakthrough enables morphology-maintaining carbonization of PPMs into HPCMMs. Next, by exerting precise control over the pore size in PCMVImTf2N-PAA PPMs through the molecular weight (MW) of the constitutional polymers, a series of graphitic HPCMMs with a gradient porous architecture along the cross-section were obtained. The reason MW of polymers matters to HPCMM is because it can modulate the pore size in PPMs that largely ends up in the final carbon membrane. The inextricable relationship between polymer MW and carbon membranes was investigated in detail by varying the MW of PAA while keeping other experimental parameters unchanged. Pyrolysis of PCMVImTf2N-PAA PPMs with PAA of MW ∼ 100k Da at 1000 °C produced an asymmetric, three-dimensionally interconnected porous architecture with the macropore size visible in SEM images and dropping along the cross-section gradually from 1.5 μm to 900 and 550 nm in zones I to II and III (Figure 1b), respectively, while with PAA MW ∼ 250k Da, from 250 to 75 and 32 nm (Figure 1c). Apparently, the pore size of HPCMMs is correlated with the MW of PAA. To our surprise, the pore wall appears single-crystal-like across the entire membrane (Figure 1j), which equips HPCMMs with an unusually high apparent conductivity of 200 S cm–1 at 25 °C despite a high pore volume of 0.79 cm3 g–1 and a large SBET of 907 m2/g. In addition to the inherent advantage brought by PIL as precursor, vacuum pyrolysis is believed to accelerate graphitization to a higher degree than that under inert gas, and in our opinion is appealing for carbon industry to attain highly graphitic carbons.
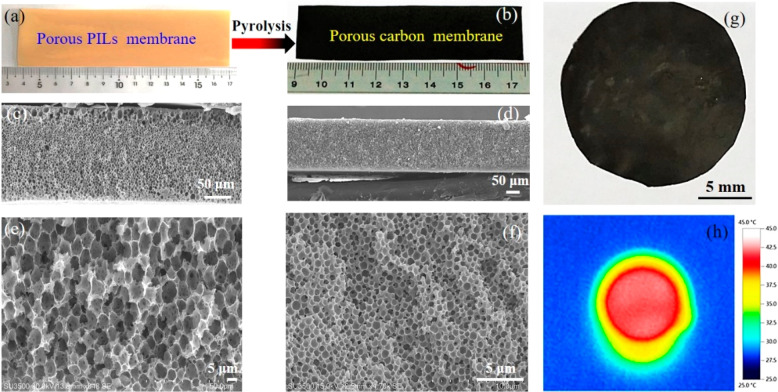
Beside MW, the porous system of HPCMMs is modulatable by the hydrophobic nature of the membrane polymers, here exemplified by the PIL due to its anion-adaptive hydrophobicity. A series of PILs, PCMVImX (X represents counter-anions in the hydrophobicity sequence of Br < PF6 < Tf2N), and a COOH-bearing PIL, poly(1-carboxymethyl-3-vinylimidazolium Tf2N) (termed PCAVImTf2N) were synthesized and applied as an ionic complexation pair to fabricate three all-PIL PPMs.22 It was found that the PCMVImBr-PCAVImTf2N PPM exhibited irregular pores of size >20 μm. For PCMVImPF6-PCAVImTf2N PPM, an asymmetric porous structure was identified along its cross-section from the top (micron/submicron-sized random pores) to the bottom (pores of an average size of 5 μm). When using the most hydrophobic PIL PCMVImTf2N, the PCMVImTf2N-PCAVImTf2N PPM contains only uniform pores of an average size of 0.6 μm. Vacuum carbonization of these three PPMs produced integrated HPCMMs with visible macropores in SEM images (Figure 2) similar to their precursor PPMs. N2 sorption isotherms confirmed the presence of micro- and mesopores; thus all three HPCMMs are composed of hierarchical pores. The micropores in HPCMMs most likely arise from volatilization of Tf2N anion clusters at high temperature, and the small mesopores from fusion of micropores at even higher temperatures. Via measurements by X-ray photoelectron spectroscopy (XPS) and elemental mapping, heteroatoms such as N and P existing in PPMs were found in the carbon matrix. An eminent highlight of such a bottom-up template method is the flexibility in the shape design, e.g., a rectangular one of 2.7 × 8.4 mm2 or a circular one with a diameter of 8 mm (Figure 2b,g). The variable shape provides an excellent engineering platform for microminiaturization of current carbon-based energy devices.
Figure 2.
(a, b) Photographs of a PCMVImPF6-PCAVImTf2N PPM and its HPCMM. (c, d and e, f) Low- and high-magnification cross-sectional SEM images of HPCMMs derived from the PCMVImPF6-PCAVImTf2N and PCMVImTf2N-PCAVImTf2N PPMs, respectively. (g, h) Digital photograph of a circular HPCMM and its infrared radiation image taken by an IR camera under 1 kW m–2 irradiation for 1 h, respectively. Reproduced with permission from ref (22). Copyright 2018 American Chemical Society.
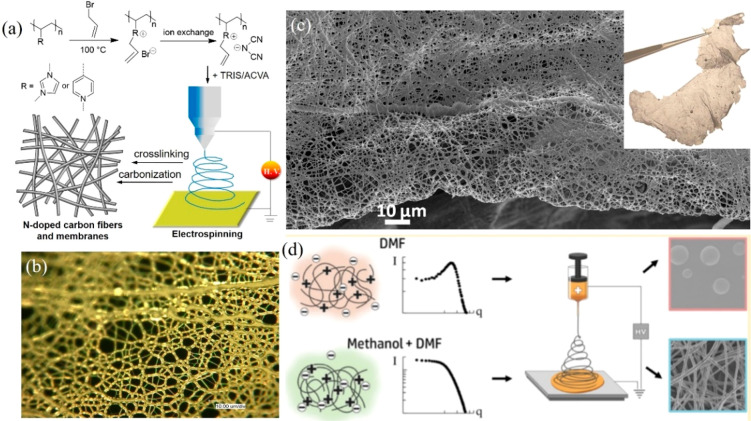
Direct carbonization of electrospun polymer fibers is an approved commercial method to manufacture carbon membranes. Producing HPCMMs by this method is, however, impeded by the limited choice of polymer fibers, which will upon carbonization be converted to carbon fibers and membranes. Our study found that thermally cross-linkable PIL fibers are admirable precursors to N-doped carbon fibers and membranes.23 In the study, two PILs, poly(3-allyl-1-vinylimidazolium dicyanamide) (PAVIm-DCA) and poly(1-allyl-4-vinylpyridinium dicyanamide) (PAVP-DCA) were employed (Figure 3a). The incorporated DCA anions are crucial as they thermally cyclize into triazine rings at ca. 300 °C to covalently fix the fiber morphology in PPMs (Figure 3a,b). The subsequent carbonization at 1000 °C under N2 converted PIL mats into carbon membranes with a N content of 8.0% and a satisfactory conductivity of 200 S cm–1. Such HPCMMs contain only micron-sized pores, and the surface area is very limited (<5 m2/g) (Figure 3c).
Figure 3.
(a) Synthetic scheme of a HPCMM from electrospun PILs. (b) Optical microscopy image of a PAVP-DCA PPM before cross-linking. (c) Representative SEM images of a HPCMM. The inset is its photograph. Reproduced with permission from ref (23). Copyright 2011 Royal Society of Chemistry. (d) Production scheme of electrospun PCMVImTf2N fibers. Reproduced with permission from ref (24). Copyright 2019 American Chemical Society.
Generally speaking, electrospinning of PIL into fibers is a challenge owing to their ionic nature-induced jet instability. Molecular understanding of PILs in solution casts a deep insight for a rational design of electrospun PIL fibers. Small-angle X-ray scattering (SAXS) analysis by Josef et al. explained why PCMVImTf2N fibers could not be electrospun from its N,N-dimethylformamide (DMF) solution. Due to strong ion-solvation power of DMF molecules, PCMVImTf2N in DMF acted as a conventional cationic polyelectrolyte with disassociated counter-anions, which is unfavorable for jet formation because of improve conductivity of the spinning solution (Figure 3d).24 By contrast, addition of methanol to the DMF solution shifted the PIL solution structure stepwise from a dissociation form to an ion-association form, in favor of formation of fiber, as PILs in a DMF/methanol mixture solution will assume more a conventional neutral polymer-like state. With this knowledge, PCMVImTf2N fibers were readily electrospun from its solution in a DMF/methanol mixture. After treatment with benzenepentacarboxylic acid for stabilization, these fibers were carbonized into fibers/membranes with a SBET of 287 m2/g.25 To note, the morphology-maintaining carbonization of PIL-based PPMs allows for a homogeneous incorporation of heteroatoms into the entire carbon matrix in a controlled fashion. HPCMMs derived from non-PIL-based electrospun fibers have also been reported. Ding et al. electrospun poly(tetrafluoroethylene) and poly(vinyl alcohol) cross-linked by boric acid, and after pyrolysis produced B–F–N codoped porous carbon membranes with hierarchical pores and a SBET of ∼750 m2 g–1.26
2.2. PIL as Additive to Membrane Templates
PILs can serve as carbonization additives (≤10 wt %) to better the carbonization yield, incorporate heteroatoms, induce micropores, and maintain the integrated membrane morphology of other polymer membrane templates. Traditionally, water stream and CO2 are activation agents for carbon,27 but chemicals such as phosphoric acid, zinc chloride, and alkaline hydroxides are preferred in many cases of porous carbon production at 600–800 °C. In such cases, a labor/energy consumptive purification step is required to remove residue chemicals. In stark contrast, a task-specific PIL can act as a soft “activation agent” to prepare HPCMMs from biotemplates without postsynthetic steps, as it ends up as a part of HPCMMs. In our study, PCMVImTf2N was used to activate commercial cellulose filter papers into HPCMMs at only 400 °C.28 Such HPCMMs were insulators but exhibited improved mechanical strength and flexibility, suitable surface area (∼400 m2/g) and fire-retardancy. To be stressed, the Tf2N anions in PCMVImTf2N can create micro/mesopores in the biosubstrate due to their catalytic reaction with oligo-/polysaccharides,18 and simultaneously deposit heteroatoms. This method can be expanded to prepare HPCMMs with metallic species. For instance, by strong complexation between Fe3+ and PCMVImTf2N, a hybrid FeCl3/PCMVImTf2N activation system was applied to commercial cellulose filter papers to produce flexible, FeN4 site-anchored HPCMMs with a SBET of 696 m2/g at 1000 °C (Figure 4).29 These studies support that low-cost HPCMMs could be accessed from biotemplates by employing the right PIL-based “activation agent”.
Figure 4.
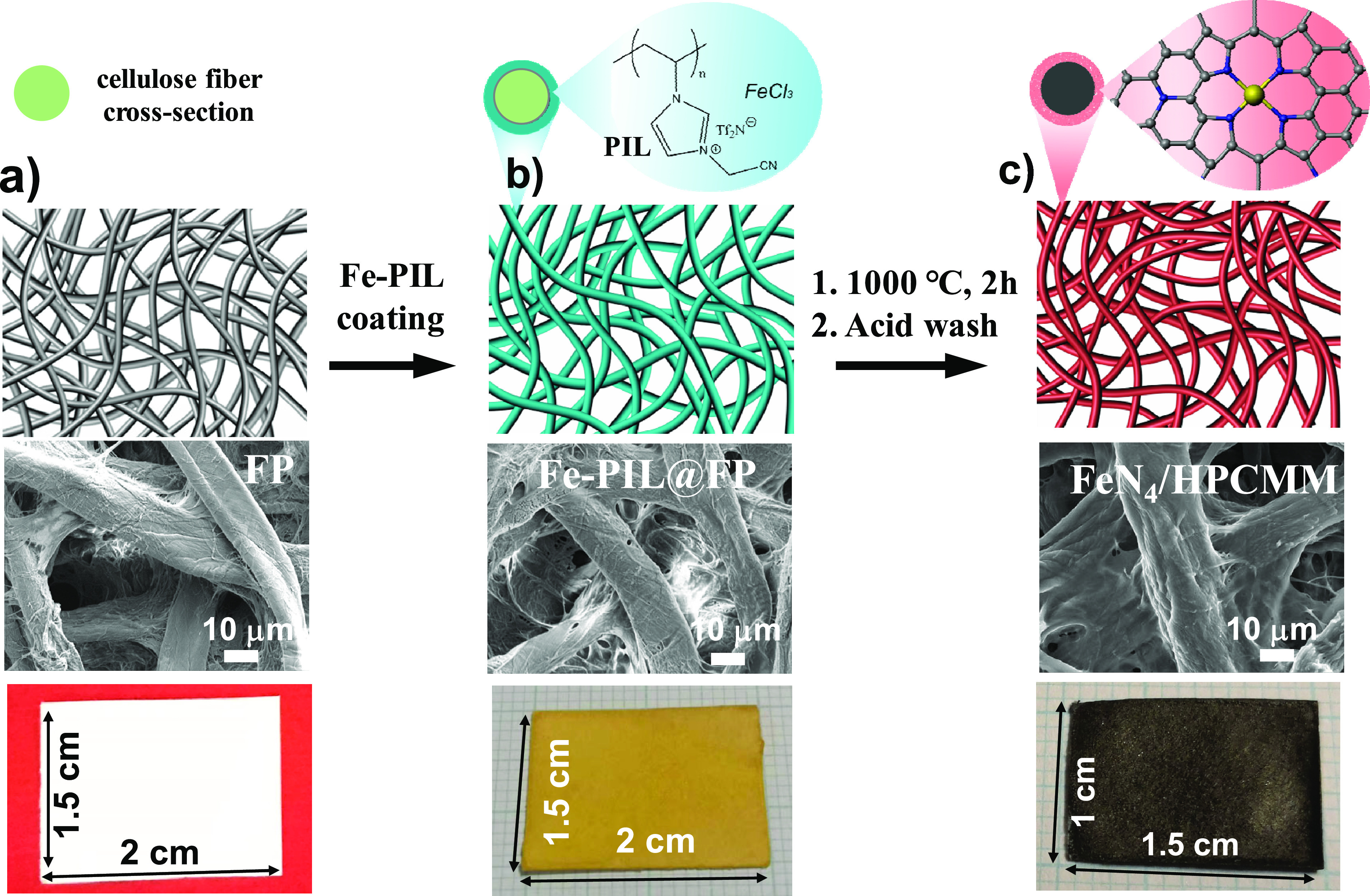
(a)–(c) Schematic synthetic route from cellulose filter paper toward FeN4 site-functionalized HPCMMs via using FeCl3/PCMVImTf2N as a hybrid activation system. Reproduced with permission from ref (29). Copyright 2020 Wiley-VCH.
3. Control of Heteroatom Doping and Porosity
3.1. Heteroatom Doping
The heteroatoms discussed here refer to nonmetals, e.g., N, P, B, S, and Se, that bond covalently with carbon; metal doping in the form of single atoms or nanoparticles is skipped here unless they are the electrocatalysts supported by HPCMMs. In graphitic carbons produced at a sufficiently high temperature (>800 °C), the survival heteroatom(s) solely or jointly incorporated into carbons can disturb the sp2-hybridized graphitic network, induce defects, bend individual graphitic planes, enlarge interlayer spacing, and interfere charge distribution. Heteroatom-doping expands the property window of carbons to enable “intelligent” materials for sensing, sorption, support, catalysis and energy conversion/storage technologies. N-doping is in the core of heteroatom-doping, being the most extensively studied to date,30 due to its broad presence in carbon precursors. In most cases, cations such as imidazolium, pyridinium, and dicyanamide ions are common structural units in PILs, making them a favorable N source.
N-doped carbon materials are useful building blocks for a variety of electronic devices. For example, by taking the advantage of the N-doping effect, electron-deficient water-dispersible carbon nanobubbles (CNBs) could “glue” to and thus stabilize multiwall carbon nanotubes (MWCNTs) in solution via the so-called donor–acceptor interaction between both (Figure 5a,b).31 Electron transfer between CNBs and MWCNTs enables CNB/MWCNT composites dispersible in water. This phenomenon can be utilized to prepare conductive carbon membranes, all-carbon inks, and solders, as well as new carbon p–n junctions to improve charge separation.
Figure 5.
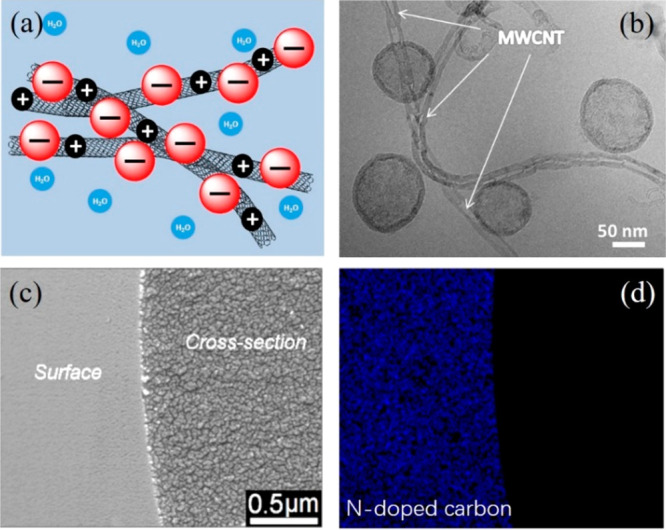
(a) Schematic formation mechanism of N-doped CNB (in red)/MWCNT (in gray) heterojunction in water. (b) Cryo-TEM image of the N-doped CNB/MWCNT composite in water. CNBs are found to attach to MWCNTs. Reproduced with permission from ref (31). Copyright 2014 Wiley-VCH. (c, d) SEM image and the corresponding N mapping of N-doped microporous carbon fibers derived from PCMVImTf2N-coated commercial microporous carbon fibers. Reproduced with permission from ref (32). Copyright 2017 Wiley-VCH.
The concept of “N-doped carbon/carbon heterojunction” can be expanded to fabricate ultrasensitive CO2 fiber sensors. PILs are well-known for strong surface interactions with various substrates to form a thin coating by a variety of noncovalent interactions. Taking this advantage, PCMVImTf2N-coated microporous carbon fibers were made and carbonized, so the doped N atoms and micropores will be simultaneously introduced into the skin of parent carbon fibers (Figure 5c,d), changing the original “inert” heteroatom-free carbon fibers into a high-sensitive CO2 detector.32 This PIL-based surface-coating strategy is simple, scalable, and technically relevant to fabrication of advanced carbon heterostructures.
To note, different N configurations in a carbon framework display varied functions. Graphitic N is sp2 hybridized and bonded to three C atoms to give one electron pair into the aromatic π-system, thus displaying a positive charged characteristicn as evidenced by XPS analysis. By contrast, pyridinic N in an sp2 hybridization is bonded to two C atoms, contributes only one p-electron to the π system and localizes its lone electron pair in a sp2 orbital; thus it displays a strong electron donating character. In this context, pyridinic N is negatively polarized, while the surrounding C atoms are positively charged. The valence band as such moves to a more positive direction. These positively charged carbon atoms due to their electrophilic feature are responsible for various catalytic reactions, e.g., electrocatalytic CO2 conversion. The positive charge also makes N-doped carbons tend to oxidize other compounds instead of being oxidized and combusted. We have proven that HPCMMs can be well-preserved even when burnt in a butane flame (∼1300 °C) in air for at least 60 s.22 Therefore, when designing HPCMMs, it is important to know that the electronic structure of carbons is sensitive toward the doping pattern.
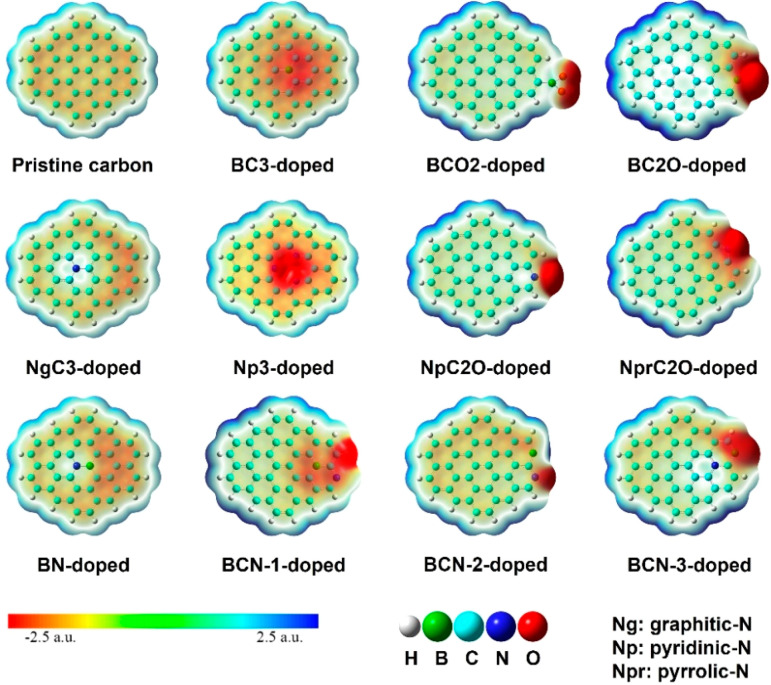
There has arisen considerable interest in improving carbon materials by codoping of multiple heteroatoms. It was reported that B/N codoped graphitic carbons could shift the Fermi level to the valence band and improve the wettability of pore interfaces, which facilitates charge storage and transfer. In addition, the density functional theory (DFT) calculation shows synergy of the N/B codoping to enhance adsorption energies on HPCMM surfaces (Figure 6). These structural metrics of B/N codoped carbons are prerequisites for achieving high-performance in supercapacitor. On the basis of our PPM fabrication technique, by blending H3BO3 into the PCMVImTf2N-PAA PPM system, a N/B codoped porous carbon membrane with an ultrahigh SBET of 1501 m2 g–1 was fabricated.33 All-solid-state symmetric supercapacitors built up from two identical B/N codoped porous carbon membranes showed an excellent areal capacitance of 1.0 F cm–2, high energy and power densities of 0.14 mW h cm–2 and 29 mW cm–2, respectively, and satisfactory recyclability (over 15 000 cycles). The concept of “blend-into” synthesis of multiple heteroatoms codoped carbons holds great potential in exploring new porous carbons.
Figure 6.
Electrostatic potential mappings of a B/N codoped porous carbon membrane surface model calculated by DFT. Reproduced with permission from ref (33). Copyright 2019 American Chemical Society.
It is well-known that counter-anions in PILs can be inorganic, such as BF4, PF6, and SeCN. Some heteroatoms in anions could be inserted into carbon frameworks after appropriate pyrolysis of corresponding PILs. Very recently, by morphology-maintaining carbonization, a N/Se codoped porous carbon membrane with a SBET of 450 m2/g was made by direct pyrolysis of a PCMVImSeCN-PCAVImTf2N PPM (Figure 7a–h). It is noteworthy that the semimetal, i.e., Se, doped carbons have been rarely studied so far in the carbon community. To deeply explore the structure of N/Se codoped porous carbon membrane at an atomic level, a suite of advanced characterization techniques has been employed. Atomic-resolution scanning transmission electron microscopy (STEM) revealed that most of Se atoms are located at the edge of graphitic HPCMM (Figure 7i). The extended X-ray absorption fine structure (EXAFS) spectrum of N/Se codoped membrane revealed that Se atoms were indeed exclusively bonded to C atoms (Figure 7j), i.e., in a single atom state. Moreover, the atomic configurations of Se atoms were determined as the edge-atoms of graphitic planes. The X-ray absorption near edge structure (XANES) spectra also displayed the adsorption edge position to be located between the Se foil and SeO2, indicating the positively charged character of Se atoms (Figure 7k).34 The enlarged interlayer spacing due to large-sized Se atoms, positively charged Se atoms, and the integrated membrane shape endowed the N/Se codoped porous carbon membrane electrode with surprisingly high performance in hydrazine fuel cells. Compared to the state-of-the-art studies of carbons that are doped by light elements such as N, P, B, S, and F, semimetal doping can be of particular interest for future studies.
Figure 7.
(a)–(e) Synthetic scheme of the N/Se codoped porous carbon membrane. (f) Photograph of the N/Se codoped porous carbon membrane. (g, h) SEM and HRTEM images of N/Se codoped porous carbon membrane, respectively. (i) Representative STEM-HAADF image of N/Se codoped carbon. Se atoms are highlighted by red circles. (j) Se K-edge Fourier transformed EXAFS spectrum in the R space of the N/Se codoped porous carbon membrane compared with the other two references. (k) Se K-edge XANES spectra of the N/Se codoped carbon membrane, Se foil and SeO2 as references. Reproduced with permission from ref (34). Copyright 2019 Wiley-VCH.
3.2. Porosity
As discussed above, heteroatoms, and porogen may coexist in a single PIL chain. By judicious choice of PIL chemical structures, one can obtain tailor-made HPCMMs carrying desirable heteroatoms and a controlled porosity. The commonly used PIL anions, e.g., Tf2N and bis(perfluoroethylsulfonyl)imide (Beti, (termed PCMVImTf2N, chemical structure shown in Figure S2), are apparently a micropore-forming agent.22 It has been demonstrated that when pairing the same IL cation with Tf2N or Beti anions, carbon products exhibited a micropore-dominant feature with a high SBET of 640.4 and 662.7 m2/g, respectively.35 Meanwhile, the cations of ILs also play a pivotal role in determining the porosity of resultant carbons. With 1-(cyanomethyl)-3-methylimidazolium Tf2N (MCNImTf2N, carrying one cyano group) and 1,3-bis(cyanomethyl)imidazolium Tf2N (BCNImTf2N, carrying two cyano groups) as examples (chemical structures shown in Figure S3), the resultant carbon produced at 800 °C from the former exhibits significant mesoporosity, while the latter with strictly microporosity. It is assumed that during carbonization, the cyano groups on the cation start to rearrange into a stable polytriazine network at T > 200 °C. Then the large Tf2N anions are clustered and trapped in the network and volatilized at T > 400 °C to form microporosity. Mesopores come from the fusion of preformed micropores at 800 °C due to insufficient cross-linking density, as MCNImTf2N has only one cross-linkable cyano group. Replacing Tf2N by chloride without changing the cation results in complete loss of micro/mesoporosity, demonstrating the important porogen role of Tf2N anions. In addition, vacuum carbonization is a powerful morphology-maintaining carbonization technique, which could largely keep the original macropore structures of PPMs. Thus, by precise design of PIL chemical structures plus the employment of vacuum carbonization technique, tailored porous systems could be readily obtained. Learning from ILs, PIL chemical structures carrying cations and anions can tune not only heteroatoms but also porosity. For example, the SBET of HPCMMs derived from vacuum carbonization of PCMVImX/PCAVImTf2N PPMs (X = PF6– and Tf2N–) are 158 and 499 m2 g–1, respectively, highlighting the formidable micropore-making ability of Tf2N anion.22
4. Applications in Electrochemical Devices
Porous carbon membranes as commercialized products,36 have a long application history in separation techniques, particularly when equipped with a micropore-enabled molecular sieving function for gas separation.37−39 Dopant in carbon membranes is sometimes needed to improve affinity toward one or more of permeating gases.8 As motivated by the prosperous activities in the fabrication of porous carbons for electrochemical energy storage,40−42 recent progress in HPCMMs is dominated by electrochemical applications, where a high electron conductivity obtained at a carbonization temperature of around 1000 °C or above is a prerequisite. The unique merits of such HPCMMs as either electroactive species or functional supports for electrochemical devices are self-evident. Their structural integrity, connectivity, and excellent conductivity make them ideal binder-free electrode candidates, avoiding binder-related adverse effects, i.e., deteriorating the overall conductivity of electrode and the stability of devices, as observed often in those prepared from powderous carbons. Note that doping of graphitic carbons by selected heteroatoms may make porous carbon membranes electrochemically active, so HPCMMs themselves have been directly used as metal-free electrodes showing satisfied performance and long-term operational stability. Frankly speaking, despite significant promise, the exploration of electrochemical applications of HPCMMs is still in their burgeoning phase, as limited by the synthetic ddilemma.
4.1. Electrocatalysts in Fuel Cells and Electrolyzers
Electrode materials determine, to a great extent, the overall performance of electrochemical devices, thereby pursuing active and safe electrocatalysts is at the heart of current electrochemical energy devices, such as fuel cells and electrolyzers. Ideal electrocatalysts should possess catalytic sites of high activity and density, fast mass transfer, and satisfactory conductivity. In this regard, HPCMMs are favorable in terms of customized pore architectures, heteroatom doping, and superior conductivity not interfered by binders.
Electrochemical conversion of gaseous carbon dioxide (CO2) into value-added products is a subject of global research because of the presence of excess CO2 in atmosphere and its geo-independent distribution. Exploration of efficient electrocatalysts with low overpotential is indispensable for affordable large-scale CO2 conversion at a minimum electricity consumption.
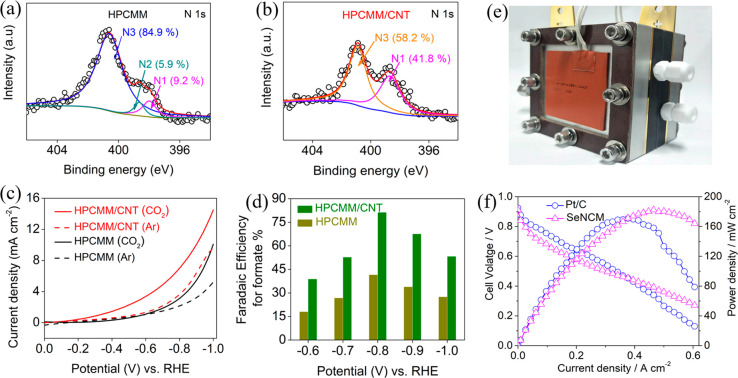
Recently, we found that pyrolysis of multiwalled carbon nanotube (MWCNT)-hybridized PCMVImTf2N-PAA PPMs at 900 °C could produce MWCNT-functionalized HPCMMs (HPCMM/CNTs).43 These HPCMM/CNTs were excellent metal-free electrocatalysts for conversion of CO2 to formate with a Faradaic efficiency (FE) of 81%, much higher than that of pristine HPCMMs (FE of 40%). XPS characterization disclosed that the relative content of pyridinic N in HPCMM/CNTs is 41.9%, which is 4.6 times higher than that in pristine HPCMMs (Figure 8a–d). This change stems from a unique templating effect of MWCNTs that the cation−π interactions between PIL and MWCNT favor the growth of pyridinic N over graphic N on the surface of MWCNTs. Considering that other parameters such as SBET and conductivity between HPCMM/CNTs and pristine HPCMMs are similar, and in combination with previous reports,44 we attributed the high-performance of HPCMM/CNTs in CO2 reduction reaction (CO2RR) to the increased amount of pyridinic N in the membrane. The mechanism of CO2RR by HPCMM/CNTs is proposed that the CO2 molecule is first adsorbed to the basic carbon atom adjacent to pyridinic N. Subsequently, the adsorbed CO2 molecule is reduced to a COO* radical. The stabilized COO* is then protonated most likely by HCO3– since its pKa value (10.33) is smaller than that of H2O (15.7). This step is followed by a second and rapid electron-transfer reduction reaction to release formate as the product. This work unequivocally implies that N configuration should be particularly designed for N-doped metal-free carbocatalyst because different states of N atoms in carbon frameworks display varied functions.
Figure 8.
(a, b) High resolution N1 XPS spectra of pristine HPCMM and HPCMM/CNT, respectively. (c) Cathodic linear sweep voltammetry scan at 5 mV s–1 in a CO2-saturated or argon-saturated aqueous 0.1 M KHCO3 solution. (d) Faradaic efficiencies for formate production vs applied potential at the HPCMM and HPCMM/CNT electrodes. Reproduced with permission from ref (43). Copyright 2017 Wiley-VCH. (e) Digital photograph of practical hydrazine fuel cell device. (f) Polarization and power density curves for direct hydrazine fuel cells using SeNCM-1000 and Pt/C as electrocalalyst at 80 °C. Test conditions: the anolyte is 6.0 M KOH + 0.5 M hydrazine hydrate with a flow rate of 1 mL min–1; the cathode is oxygen with a flow rate of 0.2 slpm. Reproduced with permission from ref (34). Copyright 2019 Wiley-VCH.
Although pyridinic N-rich HPCMM/CNTs are active in electrocatalytic CO2RR, their activity toward electrocatalytic hydrazine oxidation reaction (HzOR) is very limited. Very recently, we found that introduction of large-sized semimetallic Se atoms in N-doped porous carbon membrane could remarkably improve the electrode performance in HzOR (Figure 8e,f).34 The EXAFS spectrum of N/Se codoped carbon membranes revealed that Se atoms were in a positive state at the edge of graphitic planes, being attributed to “structure defects”. These structural features render superior HzOR activities with a low onset potential of 0.34 V (vs RHE) and a large peak current density of 30.8 mA cm–2. When applied as an electrode in a practical hydrazine fuel cell, the device exhibited a high peak power density of 182 mW cm–2 and long-term operational stability at 80 °C. On the basis of these achievements, we envision that by a judicious choice of types of heteroatoms and their configurations, functional HPCMMs can be well-suited for task-specific practical application.
4.2. Functional Support for Electrocatalysts in Fuel Cells and Electrolyzers
Aside from programmable atomic structure and porosity of HPCMMs by molecular structure of PILs, the hallmark of polyelectrolyte-derived complex membranes is their high affinity toward metal ions and nanoparticles. During pyrolysis, the strong binding force between heteroatoms such as N, and metals could restrict the movement of metal species, so to evenly distribute metal species during carbonization and finally into nanoparticle-anchored HPCMMs. These features make HPCMMs exciting supports for some electrochemically active metal nanoparticles. Electronically, these HPCMMs are classified as “non-innocent” supports, suggesting that they could change the electron flow and participate in the catalytic reactions of metallic nanoparticles (MNPs). Taking N-doped carbons as example, the introduction of N lifts up the work function of carbons to be more positive than those of nondoped ones. Thus, when metal nanoparticles are in close contact with N-doped carbons, the electron density is inevitably shifted toward carbons, causing metal nanoparticles to be more positive (or reactive) for various reactions. In solid state physics, it is referred to as the Mott–Schottky effect, and the mechanism of charge transfer is detailed in ref (45).
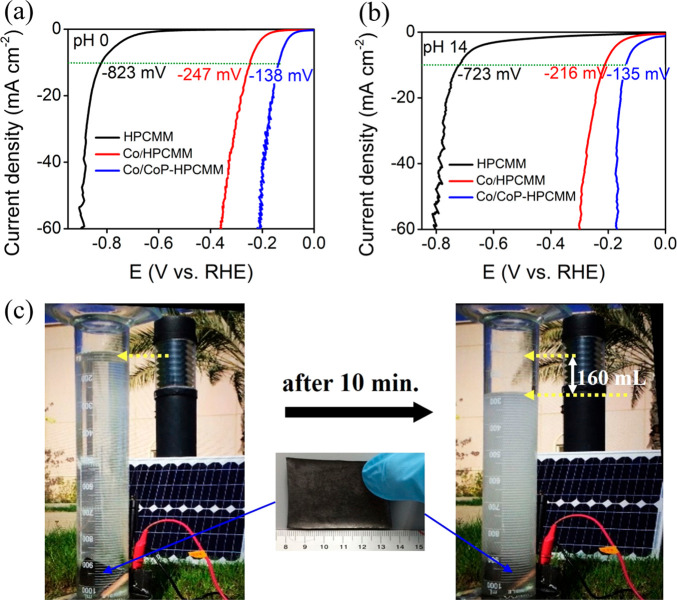
In this context, by employing HPCMMs as functional supports, a series of high-performance electrodes have been reported for the H2 evolution reaction (HER), the oxygen evolution reaction (OER), the CO2 reduction reaction (CO2RR), the N2 reduction reaction (NRR), and so on.21,43,46,47 In our study, cobalt nanoparticle-loaded HPCMMs (termed Co/HPCMMs) were fabricated by direct pyrolysis of PCMVImTf2N-PAA PPMs with PAA of MW ∼ 10 000 in the presence of Co2+, which were active bifunctional electrocatalysts for overall water splitting in alkaline media.46 Under optimized conditions, polarization curves obtained from linear sweep voltammetry (LSV) measurements showed that Co/HPCMMs exhibited a high HER activity with a current density of 10 mA cm–2 at an overpotential of 158 mV after internal resistance (IR) correction, while a low overpotential of 199 mV was required to reach a current density of 10 mA cm–2 for OER.
In order to raise the water splitting performance of the HPCMM-based electrode, we prepared Co/CoP Janus metal nanoparticle-functionalized HPCMMs (Co/CoP-HPCMMs) by phosphatization treatment of Co/HPCMMs at 350 °C using a tubular furnace with two temperature zones. These Co/CoP-HPCMMs exhibited superior HER activity with a low overpotential of 135 and 138 mV at 10 mA cm–2 in acidic and alkaline conditions, respectively (Figure 9a,b). Such extraordinary performance could be attributed to a rectifying effect between the Janus-type metal catalytic species (Co and CoP) and N-doped carbon. An important feature of the HPCMM synthesis via a single-step vacuum carbonization is that our synthetic route toward freestanding Co/CoP-HPCMM electrodes can be easily scaled up. As a proof-of-concept demonstration for the solar driven electrolysis of water, a large piece of Co/CoP-HPCMM of 5.6 × 4 cm2 in size was prepared and driven by a solar panel (up to 20 V) for HER in 1 M KOH. After 10 min HER operation, 160 mL of H2 was collected (Figure 9c), indicating the practicality of Co/CoP-HPCMM electrode in simple, decentral H2 production in an environment-friendly manner.
Figure 9.
(a, b) HER performance of HPCMM, Co/HPCMM, and Co/CoP-HPCMM in 0.5 M H2SO4 (pH 0) and 1 M KOH (pH 14), respectively. (c) Solar driven HER employing Co/CoP-HPCMM of 5.6 × 4 cm 2 in size as the working electrode in 0.1 M KOH. Reproduced with permission from ref (46). Copyright 2017 American Chemical Society.
4.3. Electrodes in Other Electrochemical Devices
HPCMMs as binder-free electrode are also promising for battery design. In conventional powderous electrodes, polymeric binders, being mostly insulators, are commonly used to integrate the electroactive species together. However, the pore volume, porosity, and accessibility of the resultant electrodes are affected by insulating polymer binders, resulting in undesirable side effects, such as structural inhomogeneity, polymer-related side reactions at high voltage, deteriorative electronic conductivity and cycling durability. In this regard, it would be advantageous to develop binder-free HPCMMs as electrode. For instance, Shen et al. prepared a Fe3C-doped asymmetric porous carbon membrane as a binder-free integral anode in lithium-ion battery without additional collector, polymer binder, or conductive agents.48 Such an electrode possessed a high SBET and simultaneously a high electronic conductivity, enabling a fast electron transfer and ion diffusion, leading to excellent rate performance and cycle stability. The rate capability reached 605.5 mAh g–1 at a current density of 0.1 A g–1, and 601.0 mAh g–1 was retained when the current density returned to 0.1 A g–1. Similarly, Xu et al. demonstrated a successful application of an ordered mesoporous carbon membrane with continuous microporous channels as a freestanding air cathode for Li–O2 battery, showing a discharge capacity of 35.6 μAh cm–2 at 0.38 mA cm–2.49 Owing to their tunable conductivity, large SBET, and abundant surface sites, HPCMMs also show great potential in supercapacitors, as discussed previously.33
5. Conclusions and Outlook
It is beyond question that HPCMMs are a type of challenging carbon materials in view of their demanding synthesis and a full control of structure parameters in an unusually wide range of dimension and chemical composition. Template synthesis of HPCMMs by PIL-derived porous polymer membranes is the state of the art and a key progress achieved recently that offers a scalable route to high-quality HPCMMs of tailor-made porous structures and rich heteroatoms. Distinctively different from other types of carbon (nano)materials, the structural connectivity in a 3D manner is maintained entirely from the nano- to macroscopic scale. This unique fundamental property makes HPCMMs highly conductive and useful in electrochemical devices as electrocatalysts or their active support, where long-term high conductivity is of paramount importance. Preparation of HPCMMs from PILs should have a thorough consideration of porosity, heteroatoms, and carbonization yield, because these factors jointly determine the property, application spectrum, performance, and potential scalability of carbon membranes. It is well-known that large counter-anions such as Tf2N and Bf2N are micropore makers (and mesopore makers when ≥800 °C) in carbons. Meanwhile, the ionic cross-linking density of PPMs is related to the molecular weight of the polymers. Therefore, by tuning the molecular weight of polymers, macropores of PPMs can be constructed in a controllable manner that will end up in large part in the final carbon products. To dope carbons with N atoms, imidazolium-, pyridinium-, and triazolium-based IL species are excellent choices of precursors, because the N and C atoms in these units are in an sp2 hybridization state to favor a high carbonization yield and a high N retention rate during carbonization. In addition, counteranions such as PF6–, N(CN)2–, BF4–, and SeCN– and unlimited heteroatom-containing side groups are feasible sources of heteroatoms. Considering the enormously large library of PIL chemical structures, new HPCMMs containing many different heteroatoms and their combinations are to be discovered.
One of the major challenges in the research area of HPCMMs currently lies in the limited synthetic approaches, particularly the scalable, low-cost ones that can meanwhile balance the pore structure, heteroatoms, and simultaneously the required properties. Biotemplates are one of the most promising methods, and their limitations in the type and content of heteroatoms can be circumvented by pre- or postcarbonization functionalization. In addition, how to cost-effectively convert existent heteroatom-free porous carbon membranes into HPCMMs is a crucial alternative.
Commercially available carbon papers (i.e., Toray) are derived from carbonization of electrospun polymer fibers, which are widely utilized as conductive support and gas diffusion membrane in various devices. Fair to say that these carbon papers lack micropores or small mesopores and are of low-to-no electroactivity due to the lack of heteroatoms. How to upgrade them to HPCMMs with hierarchical pores in a cost-effective way is an urgent question to be tackled. Only when the synthetic obstacle is fully addressed can the field gather a sufficiently large number of researchers of diverse backgrounds. Overall membrane materials are investigated both as a fundament research topic and as an important technical form of products in industry. Such a dual role is true for HPCMMs. Similar to carbon fibers that have been long commercialized using polymers as precursors, HPCMMs produced from a polymeric template are a promising step toward future real-life use. Equally important is the more in-depth investigations of the physicochemical properties of HPCMMs, where the interplay of heteroatom doping effects and their electrochemical behavior is not yet fully understood. As for expanded applications, HPCMMs were previously used as gas separation barrier and conductive supports, and are currently used dominantly in electrocatalysis, yet HPCMM-based electro-assisted separation techniques, e.g., electrodialysis, have not been demonstrated but are highly possible. In addition, the superior fire-retardant property of HPCMMs may enable them as possible aerobic high-temperature reactors.
Further development in HPCMMs requires an unusually broad range of knowledge needed to investigate and understand this system, particularly the expertise in polymer science, solid-state materials science, membrane science, and electrochemistry. The different voices in these fields have to be joined together to go deep enough to address the true challenges in the HPCMM topic. This unique knowledge pattern in a wide scope is not often seen and it is one of the purposes of this article to strengthen the research tie between these fields.
Acknowledgments
J. Yuan is grateful for financial support from European Research Council (ERC) Starting Grant NAPOLI-639720, Swedish Research Council Grant 2018-05351, Dozentenpreis 15126 from Verband der Chemischen Industrie e.V. (VCI) in Germany, the Wallenberg Academy Fellow program (Grant KAW 2017.0166) in Sweden, and the Stockholm University Strategic Fund SU FV-2.1.1-005. H. Wang acknowledges the financial support from Nankai University, the National Science Foundation of China (No. 21875119), the Natural Science Foundation of Tianjin (19JCYBJC17500) and the Fundamental Research Funds for the Central Universities”, Nankai University (63201043).
Biographies
Yucheng Wang, born on April 21, 1993, in Hubei, China, received his B.S. degree from Hubei University in 2014 and a Ph.D. degree from Xiamen University in 2019. From 2018 to 2019, he stayed as an international visitor at Institute National de la Recherche Scientifique-Énergie Matériaux et Télécommunications (INRS-EMT, Canada). He is currently a postdoctoral researcher at Stockholm University (Sweden). His research focuses on fuel cells.
Yue Shao was born in 1993 in Ningbo, China. He received his B.S. (Honours) at the University of Prince Edward Island, Canada, in 2016. He then worked with Prof. Douglas Stephan at the University of Toronto in frustrated Lewis pairs chemistry. Later, he continued his research with Prof. Hong Wang at Nankai University, China, on poly(ionic liquid)-derived functional materials. He is currently a master student at Tsinghua University and studies soft Janus nanoparticles and their self-organized nanostructures.
Hong Wang, born on November 4, 1982, in Huining Gansu province, China, received his Ph.D. degree from the Lab of Advanced Materials, Fudan University, in 2013. After multiple research stays at the Hong Kong University of Science and Technology (Hong Kong), King Abdullah University of Science and Technology (Kingdom of Saudi Arabia), Max Planck Institute of Colloids and interfaces (Germany), and University of Toronto (Canada), he joined Nankai University as a professor in China (2017). His current research is exclusively in the area of poly(ionic liquid)-based functional materials science.
Jiayin Yuan, born on November 4, 1979, in Hefei, China, received his Ph.D. from Universität Bayreuth, Germany, in 2009. He joined the Max Planck Institute of Colloids and Interfaces in Potsdam as a research group leader and completed his Habilitation in 2015. After a one-year Associate Professor appointment at Clarkson University, USA, in 2017, he is now a Full Professor at Stockholm University (Sweden). He received the European Research Council (ERC) Starting Grant in 2014 and a Wallenberg Academy Fellowship from the Knut and Alice Wallenberg Foundation in 2017. His research focuses on functional polymers and carbons.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/accountsmr.0c00010.
Chemical structures and their abbreviations of ionic liquids and poly(ionic liquid)s mentioned in the main text (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Dowell M. B.; Howard R. A. Tensile and compressive properties of flexible graphite foils. Carbon 1986, 24 (3), 311–323. 10.1016/0008-6223(86)90232-0. [DOI] [Google Scholar]
- Li D.; Müller M. B.; Gilje S.; Kaner R. B.; Wallace G. G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3 (2), 101–105. 10.1038/nnano.2007.451. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Chen Z.; Du X.; Logan J. M.; Sippel J.; Nikolou M.; Kamaras K.; Reynolds J. R.; Tanner D. B.; Hebard A. F.; Rinzler A. G. Transparent, Conductive Carbon Nanotube Films. Science 2004, 305 (5688), 1273. 10.1126/science.1101243. [DOI] [PubMed] [Google Scholar]
- Dikin D. A.; Stankovich S.; Zimney E. J.; Piner R. D.; Dommett G. H. B.; Evmenenko G.; Nguyen S. T.; Ruoff R. S. Preparation and characterization of graphene oxide paper. Nature 2007, 448 (7152), 457–460. 10.1038/nature06016. [DOI] [PubMed] [Google Scholar]
- Kim K. S.; Zhao Y.; Jang H.; Lee S. Y.; Kim J. M.; Kim K. S.; Ahn J.-H.; Kim P.; Choi J.-Y.; Hong B. H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457 (7230), 706–710. 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- Liu J.; Min S.; Wang F.; Zhang Z. Biomass-derived three-dimensional porous carbon membrane electrode for high-performance aqueous supercapacitors: An alternative of powdery carbon materials. J. Power Sources 2020, 466, 228347. 10.1016/j.jpowsour.2020.228347. [DOI] [Google Scholar]
- Yeh C.-N.; Raidongia K.; Shao J.; Yang Q.-H.; Huang J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7 (2), 166–170. 10.1038/nchem.2145. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Tian C.; Chai S.; Nelson K.; Han K. S.; Hagaman E. W.; Veith G. M.; Mahurin S. M.; Liu H.; Dai S. New Tricks for Old Molecules: Development and Application of Porous N-doped, Carbonaceous Membranes for CO2 Separation. Adv. Mater. 2013, 25 (30), 4152–4158. 10.1002/adma.201300793. [DOI] [PubMed] [Google Scholar]
- Wang C.; Chen W.; Xia K.; Xie N.; Wang H.; Zhang Y. Silk-Derived 2D Porous Carbon Nanosheets with Atomically-Dispersed Fe-Nx-C Sites for Highly Efficient Oxygen Reaction Catalysts. Small 2019, 15 (7), 1804966. 10.1002/smll.201804966. [DOI] [PubMed] [Google Scholar]
- Wang C.; Xie N.-H.; Zhang Y.; Huang Z.; Xia K.; Wang H.; Guo S.; Xu B.-Q.; Zhang Y. Silk-Derived Highly Active Oxygen Electrocatalysts for Flexible and Rechargeable Zn–Air Batteries. Chem. Mater. 2019, 31 (3), 1023–1029. 10.1021/acs.chemmater.8b04572. [DOI] [Google Scholar]
- Zhou B.; Zhang M.; He W.; Wang H.; Jian M.; Zhang Y. Blue rose-inspired approach towards highly graphitic carbons for efficient electrocatalytic water splitting. Carbon 2019, 150, 21–26. 10.1016/j.carbon.2019.05.009. [DOI] [Google Scholar]
- Zhu Y.; Sun W.; Luo J.; Chen W.; Cao T.; Zheng L.; Dong J.; Zhang J.; Zhang M.; Han Y.; Chen C.; Peng Q.; Wang D.; Li Y. A cocoon silk chemistry strategy to ultrathin N-doped carbon nanosheet with metal single-site catalysts. Nat. Commun. 2018, 9 (1), 3861. 10.1038/s41467-018-06296-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.; Lin Y.; Connell J. W.; Cheng H.-M.; Gogotsi Y.; Titirici M.-M.; Dai L. Carbon-Based Metal-Free Catalysts for Energy Storage and Environmental Remediation. Adv. Mater. 2019, 31 (13), 1806128. 10.1002/adma.201806128. [DOI] [PubMed] [Google Scholar]
- Borchardt L.; Oschatz M.; Kaskel S. Carbon Materials for Lithium Sulfur Batteries—Ten Critical Questions. Chem. - Eur. J. 2016, 22 (22), 7324–7351. 10.1002/chem.201600040. [DOI] [PubMed] [Google Scholar]
- Fan L. Z.; Hu Y. S.; Maier J.; Adelhelm P.; Smarsly B.; Antonietti M. High Electroactivity of Polyaniline in Supercapacitors by Using a Hierarchically Porous Carbon Monolith as a Support. Adv. Funct. Mater. 2007, 17 (16), 3083–3087. 10.1002/adfm.200700518. [DOI] [Google Scholar]
- Radjabian M.; Abetz V. Advanced porous polymer membranes from self-assembling block copolymers. Prog. Polym. Sci. 2020, 102, 101219. 10.1016/j.progpolymsci.2020.101219. [DOI] [Google Scholar]
- Liu T.; Serrano J.; Elliott J.; Yang X.; Cathcart W.; Wang Z.; He Z.; Liu G. Exceptional capacitive deionization rate and capacity by block copolymer–based porous carbon fibers. Science Advances 2020, 6 (16), eaaz0906 10.1126/sciadv.aaz0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.; Giordano C.; Antonietti M. Ionic Liquid Monomers and Polymers as Precursors of Highly Conductive, Mesoporous, Graphitic Carbon Nanostructures. Chem. Mater. 2010, 22 (17), 5003–5012. 10.1021/cm1012729. [DOI] [Google Scholar]
- Zhao Q.; Fellinger T.-P.; Antonietti M.; Yuan J. A novel polymeric precursor for micro/mesoporous nitrogen-doped carbons. J. Mater. Chem. A 2013, 1 (16), 5113. 10.1039/c3ta10291b. [DOI] [Google Scholar]
- Zhao Q.; Yin M.; Zhang A. P.; Prescher S.; Antonietti M.; Yuan J. Hierarchically Structured Nanoporous Poly(Ionic Liquid) Membranes: Facile Preparation and Application in Fiber-Optic pH Sensing. J. Am. Chem. Soc. 2013, 135 (15), 5549–5552. 10.1021/ja402100r. [DOI] [PubMed] [Google Scholar]
- Wang H.; Min S.; Ma C.; Liu Z.; Zhang W.; Wang Q.; Li D.; Li Y.; Turner S.; Han Y.; Zhu H.; Abou-hamad E.; Hedhili M. N.; Pan J.; Yu W.; Huang K.-W.; Li L.-J.; Yuan J.; Antonietti M.; Wu T. Synthesis of single-crystal-like nanoporous carbon membranes and their application in overall water splitting. Nat. Commun. 2017, 8 (1), 13592. 10.1038/ncomms13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y.; Jiang Z.; Zhang Y.; Wang T.; Zhao P.; Zhang Z.; Yuan J.; Wang H. All-Poly(ionic liquid) Membrane-Derived Porous Carbon Membranes: Scalable Synthesis and Application for Photothermal Conversion in Seawater Desalination. ACS Nano 2018, 12 (11), 11704–11710. 10.1021/acsnano.8b07526. [DOI] [PubMed] [Google Scholar]
- Yuan J.; Márquez A. G.; Reinacher J.; Giordano C.; Janek J.; Antonietti M. Nitrogen-doped carbon fibers and membranes by carbonization of electrospun poly(ionic liquid)s. Polym. Chem. 2011, 2 (8), 1654. 10.1039/c1py00196e. [DOI] [Google Scholar]
- Josef E.; Guterman R. Designing Solutions for Electrospinning of Poly(ionic liquid)s. Macromolecules 2019, 52 (14), 5223–5230. 10.1021/acs.macromol.9b00691. [DOI] [Google Scholar]
- Josef E.; Yan R.; Guterman R.; Oschatz M. Electrospun Carbon Fibers Replace Metals as a Current Collector in Supercapacitors. ACS Applied Energy Materials 2019, 2 (8), 5724–5733. 10.1021/acsaem.9b00854. [DOI] [Google Scholar]
- Yan J.; Dong K.; Zhang Y.; Wang X.; Aboalhassan A. A.; Yu J.; Ding B. Multifunctional flexible membranes from sponge-like porous carbon nanofibers with high conductivity. Nat. Commun. 2019, 10 (1), 5584. 10.1038/s41467-019-13430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T. H.; Huang Y.-K.; Tan A. T. L.; Dravid V. P.; Huang J. Steam Etched Porous Graphene Oxide Network for Chemical Sensing. J. Am. Chem. Soc. 2011, 133 (39), 15264–15267. 10.1021/ja205693t. [DOI] [PubMed] [Google Scholar]
- Men Y.; Siebenbürger M.; Qiu X.; Antonietti M.; Yuan J. Low fractions of ionic liquid or poly(ionic liquid) can activate polysaccharide biomass into shaped, flexible and fire-retardant porous carbons. J. Mater. Chem. A 2013, 1 (38), 11887. 10.1039/c3ta12302b. [DOI] [Google Scholar]
- Wang Y.-C.; Wan L.-Y.; Cui P.-X.; Tong L.; Ke Y.-Q.; Sheng T.; Zhang M.; Sun S.-H.; Liang H.-W.; Wang Y.-S.; Zaghib K.; Wang H.; Zhou Z.-Y.; Yuan J. Porous Carbon Membrane-Supported Atomically Dispersed Pyrrole-Type Fe–N4 as Active Sites for Electrochemical Hydrazine Oxidation Reaction. Small 2020, 16 (31), 2002203. 10.1002/smll.202002203. [DOI] [PubMed] [Google Scholar]
- Gong K.; Du F.; Xia Z.; Durstock M.; Dai L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323 (5915), 760–764. 10.1126/science.1168049. [DOI] [PubMed] [Google Scholar]
- Kuzmicz D.; Prescher S.; Polzer F.; Soll S.; Seitz C.; Antonietti M.; Yuan J. The Colloidal Stabilization of Carbon with Carbon: Carbon Nanobubbles as both Dispersant and Glue for Carbon Nanotubes. Angew. Chem., Int. Ed. 2014, 53 (4), 1062–1066. 10.1002/anie.201307459. [DOI] [PubMed] [Google Scholar]
- Gong J.; Antonietti M.; Yuan J. Poly(Ionic Liquid)-Derived Carbon with Site-Specific N-Doping and Biphasic Heterojunction for Enhanced CO2 Capture and Sensing. Angew. Chem., Int. Ed. 2017, 56 (26), 7557–7563. 10.1002/anie.201702453. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Wei S.; Wu Y.; Wang Y.-L.; Zhang M.; Roy D.; Wang H.; Yuan J.; Zhao Q. Poly(Ionic Liquid)-Derived Graphitic Nanoporous Carbon Membrane Enables Superior Supercapacitive Energy Storage. ACS Nano 2019, 13 (9), 10261–10271. 10.1021/acsnano.9b03514. [DOI] [PubMed] [Google Scholar]
- Wang T.; Wang Q.; Wang Y.; Da Y.; Zhou W.; Shao Y.; Li D.; Zhan S.; Yuan J.; Wang H. Atomically Dispersed Semimetallic Selenium on Porous Carbon Membrane as an Electrode for Hydrazine Fuel Cells. Angew. Chem., Int. Ed. 2019, 58 (38), 13466–13471. 10.1002/anie.201907752. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Wang X.; Luo H.; Baker G. A.; Dai S. Facile Ionothermal Synthesis of Microporous and Mesoporous Carbons from Task Specific Ionic Liquids. J. Am. Chem. Soc. 2009, 131 (13), 4596–4597. 10.1021/ja900686d. [DOI] [PubMed] [Google Scholar]
- Wen W.; Huang T.; Guan S.; Zhao Y.; Chen A. Self-Assembly of Single Chain Janus Nanoparticles with Tunable Liquid Crystalline Properties from Stilbene-Containing Block Copolymers. Macromolecules 2019, 52 (8), 2956–2964. 10.1021/acs.macromol.9b00154. [DOI] [Google Scholar]
- Hägg M.-B., Carbon membrane. In Encyclopedia of Membranes, Enrico Drioli L. G., Ed.; Springer, 2015. [Google Scholar]
- Lei L.; Bai L.; Lindbråthen A.; Pan F.; Zhang X.; He X. Carbon membranes for CO2 removal: Status and perspectives from materials to processes. Chem. Eng. J. 2020, 401, 126084. 10.1016/j.cej.2020.126084. [DOI] [Google Scholar]
- Dipak Rana A. F. I.; Matsuura T.; Foley H.. Carbon-based Membranes for Separation Processes; Springer: New York, NY, 2011. [Google Scholar]
- He Y.; Zhuang X.; Lei C.; Lei L.; Hou Y.; Mai Y.; Feng X. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. 10.1016/j.nantod.2018.12.004. [DOI] [Google Scholar]
- Wu J.; Sharifi T.; Gao Y.; Zhang T.; Ajayan P. M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31 (13), 1804257. 10.1002/adma.201804257. [DOI] [PubMed] [Google Scholar]
- Chen S.; Qiu L.; Cheng H.-M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120 (5), 2811–2878. 10.1021/acs.chemrev.9b00466. [DOI] [PubMed] [Google Scholar]
- Wang H.; Jia J.; Song P.; Wang Q.; Li D.; Min S.; Qian C.; Wang L.; Li Y. F.; Ma C.; Wu T.; Yuan J.; Antonietti M.; Ozin G. A. Efficient Electrocatalytic Reduction of CO2 by Nitrogen-Doped Nanoporous Carbon/Carbon Nanotube Membranes: A Step Towards the Electrochemical CO2 Refinery. Angew. Chem., Int. Ed. 2017, 56 (27), 7847–7852. 10.1002/anie.201703720. [DOI] [PubMed] [Google Scholar]
- Guo D.; Shibuya R.; Akiba C.; Saji S.; Kondo T.; Nakamura J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351 (6271), 361–5. 10.1126/science.aad0832. [DOI] [PubMed] [Google Scholar]
- Li X.-H.; Antonietti M. Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: functional Mott–Schottky heterojunctions for catalysis. Chem. Soc. Rev. 2013, 42 (16), 6593–6604. 10.1039/c3cs60067j. [DOI] [PubMed] [Google Scholar]
- Wang H.; Min S.; Wang Q.; Li D.; Casillas G.; Ma C.; Li Y.; Liu Z.; Li L.-J.; Yuan J.; Antonietti M.; Wu T. Nitrogen-Doped Nanoporous Carbon Membranes with Co/CoP Janus-Type Nanocrystals as Hydrogen Evolution Electrode in Both Acidic and Alkaline Environments. ACS Nano 2017, 11 (4), 4358–4364. 10.1021/acsnano.7b01946. [DOI] [PubMed] [Google Scholar]
- Wang H.; Wang L.; Wang Q.; Ye S.; Sun W.; Shao Y.; Jiang Z.; Qiao Q.; Zhu Y.; Song P.; Li D.; He L.; Zhang X.; Yuan J.; Wu T.; Ozin G. A. Ambient Electrosynthesis of Ammonia: Electrode Porosity and Composition Engineering. Angew. Chem., Int. Ed. 2018, 57 (38), 12360–12364. 10.1002/anie.201805514. [DOI] [PubMed] [Google Scholar]
- Shen W.; Kou W.; Liu Y.; Dai Y.; Zheng W.; He G.; Wang S.; Zhang Y.; Wu X.; Fan S.; Li X. Fe3C-doped asymmetric porous carbon membrane binder-free integrated materials as high performance anodes of lithium-ion batteries. Chem. Eng. J. 2019, 368, 310–320. 10.1016/j.cej.2019.02.199. [DOI] [Google Scholar]
- Xu S.-M.; Liang X.; Ren Z.-C.; Wang K.-X.; Chen J.-S. Free-Standing Air Cathodes Based on 3D Hierarchically Porous Carbon Membranes: Kinetic Overpotential of Continuous Macropores in Li-O2 Batteries. Angew. Chem., Int. Ed. 2018, 57 (23), 6825–6829. 10.1002/anie.201801399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.