Abstract
Insulclock® is an electronic device designed to improve treatment adherence and insulin injection tracking. This randomized, single-center, pilot study assessed the clinical impact of Insulclock on glycemic control and variability, treatment adherence, and satisfaction in patients with uncontrolled type 1 diabetes mellitus (T1DM). We also compared these outcomes between the Active and Masked groups (with or without receiving reminders and app alerts). Sixteen patients completed the study: 10 in the Active group and 6 in the Masked group. Insulclock use was associated with a decrease in mean glucose (−27.0 mg/dL [1.5 mmol/L]; P = 0.013), glucose standard deviation (−14.4 mg/dL [0.8 mmol/L]; P = 0.003), and time above range (−12.5%; P = 0.0026), and an increase in time in range (TIR) (+7%; P = 0.038) in the overall population. The use of app information and alerts in the Active group was associated with an increase in TIR (+8%; P = 0.026). We observed a −3.9 (P = 0.1352) and −5.4 (P = 0.032) reduction per month in the number of missed and mistimed insulin doses in the overall population, respectively. Most of the items of the Insulin Treatment Satisfaction Questionnaire (ITSQ) improved after 4 weeks of Insulclock use. This pilot study points out an improvement in glycemic levels, adherence, and satisfaction in T1DM patients, supporting the development of clinical trials powered to confirm these effects.
Keywords: Insulclock, Self-management, Glucose variability, Adherence, Treatment satisfaction
Introduction
Despite the recognized potential of intensive insulin therapy for controlling glycemic levels and delaying the onset of diabetes-associated complications,1 many people with type 1 diabetes mellitus (T1DM) do not meet nor maintain glycemic targets.2 Treatment compliance with the prescribed regimen and incorrect injection techniques are among the leading barriers to achieving optimal glycemic levels.3–6
Mobile Health (mHealth) technology is a tool with increasing popularity aiming to facilitate the self-management of chronic diseases including diabetes, comprising recent advancements such as insulin pens with memory function or electronic pen caps.7–9 Alongside with these interventions, health applications (apps) have been developed for data log and review or educational purposes.10,11 However, to date, these devices and apps have been mainly intended to monitor disease outcomes without targeting the optimal performance of insulin injections.
Insulclock® is a small electronic device developed to facilitate the optimal administration of insulin. This device works as an add-on module of commercially available insulin pens and monitors the date, time and dose of injections, the type of insulin injected, the duration of injections and insulin temperature. The Insulclock app allows automatic data logging, report generation and reminder setting, among other functions. We recently described the main capabilities and tests carried out to optimize Insulclock performance.12 In this pilot study, we present the clinical impact of Insulclock on glycemic indices, treatment compliance, and treatment satisfaction in patients with persistent poorly controlled T1DM.
Methods
Study design
This single-center, randomized, prospective, open-label, pilot study was conducted at the General Hospital of Segovia, after classification by the Spanish Agency for Medicines and Health Products (AEMPS) and ethical approval by the Ethics Review Committee of the General Hospital of Segovia. The study was conducted in compliance with the ethical principles of the Declaration of Helsinki. Each participant provided written informed consent before inclusion in the study.
The study was scheduled across five visits. At Visit 1 (screening, week 1), patients started with masked Insulclock for a 1-week run-in period. A patient diary was provided to record study variables. At Visit 2 (baseline, week 0), demographic and clinical information were collected, concomitant medications were registered, and patients completed the Insulin Treatment Satisfaction Questionnaire (ITSQ). Participants were randomized 1:1 to the Active Insulclock group or the Masked Insulclock group. At Visit 3 (week 1), the masked FreeStyle Libre Pro™ CGM device (professional use) was applied by a health care professional in the upper arm to all the participants. At Visit 4 (week 3), the FreeStyle Libre Pro was removed. At Visit 5 (end of study, week 4) participants completed the ITSQ (Supplementary Fig. S1).
Study population
We included patients aged 18–80 years with uncontrolled T1DM, defined as glycated hemoglobin (HbA1C) levels ≥8% for at least 1 year, and/or variations ≥1% in HbA1C within the previous 2 years and attending regular (≥4 per year) follow-up visits at the Endocrinology department. We excluded pregnant or breastfeeding women, individuals with a history of or current alcohol or drug abuse, acute infection, cognitive decline or dementia, or any medical condition that may compromise the use of the device or study participation.
Study outcomes
The primary aim of the study was to assess the effect of Insulclock on glycemic control and variability, treatment adherence, and treatment satisfaction. The secondary objective was to compare these outcomes between patients in the Active and Masked Insulclock groups.
Glycemic variability indices were monitored according to international recommendations13,14 with the FreeStyle Libre Pro and included glucose standard deviation (SD), time in range (TIR), time above range (TAR), and time below range (TBR).
A late meal bolus (mistimed) was considered when Insulclock detected the insulin injection ≥30 min after a glucose rise in the continuous glucose monitoring (CGM) and a missed dose when no injection was detected ≥2 h after a glucose rise in the CGM. We used the GRID (Glucose Rate Increase Detector) algorithm to identify meal glucose excursions.15
Participants completed the ITSQ, which comprises 22 items scored on a 7-point Likert scale ranging from 1 (extremely satisfied) to 7 (extremely dissatisfied).16
Study devices
Participants self-administered rapid insulin with Humalog Kwikpen® pens coupled with Insulclock and according to routine clinical practice. By means of acoustic and visual alarms, participants received information for a correct injection technique or to prevent stacking insulin.
In the Active group, participants received acoustic and visual alarms and had access to the Insulclock app with integrated information on insulin doses, glucose levels and injection time reminders. In the Masked group, participants knew the dose, time, and duration of injections, but they did not receive any reminder and were masked to the Insulclock app.
Participants wore the masked Freestyle Libre Pro (Abbott Diabetes Care, Witney, Oxon, United Kingdom) for 14 days, which automatically records glucose data every 15 min.
Statistical analyses
Continuous variables were described by the mean and SD, median, interquartile range, and extremes (Min, Max). Categorical variables were described by number and percentage. Comparisons between two independent groups were performed using the Student's t-test for unpaired data for continuous variables or the Chi-square test for categorical variables. The level of statistical significance was set at P < 0.05. All statistical analyses were performed using the SAS software for Windows, version 9.2 (SAS Institute, Cary, SC).
Results
Among the 21 participants included, 1 dropped out in the Active group and 4 in the Masked group: 1 because of mobile phone incompatibility with the device and 4 because of patients' decision. Sixteen patients were analyzed: 10 in the Active Insulclock group and 6 in the Masked Insulclock group. Mean age (SD) in the overall population was 40.1 (13.9) years, and 56.3% were men (Table 1).
Table 1.
Demographic and Clinic Characteristics of Patients at Baseline
| Total | Active Insulclock® | Masked Insulclock | P | |
|---|---|---|---|---|
| Age, years | 40.1 (13.9) | 43.1 (13.8) | 35.2 (13.8) | 0.285 |
| Sex, male, n (%) | 9 (56.3) | 5 (50.0) | 4 (66.7) | 0.515 |
| Duration of diabetes, years | 20.4 (11.9) | 20.9 (12.5) | 19.5 (11.9) | 0.828 |
| Weight, kg | 69.4 (10.6) | 65.2 (9.5) | 75.1 (10.1) | 0.085 |
| BMI, kg/m2 | 24.8 (3.9) | 23.2 (3.0) | 27.0 (4.2) | 0.070 |
| Microvascular complications, n (%) | ||||
| Retinopathy | 6 (37.5) | 4 (40.0) | 2 (33.3) | |
| Nephropathy | 2 (12.5) | 2 (20.0) | 0 (0) | |
| Neuropathy | 5 (31.3) | 3 (30.0) | 2 (33.3) | |
| SBP, mmHg | 121.9 (18.4) | 118.0 (8.3) | 126.8 (27.5) | 0.515 |
| DBP, mmHg | 75.0 (10.3) | 72.4 (9.7) | 78.3 (11.5) | 0.434 |
| Insulin dose, IU/kg | ||||
| Long-acting | 0.39 (0.21) | 0.31 (0.09) | 0.50 (0.27) | |
| Rapid-acting | 0.41 (0.22) | 0.34 (0.13) | 0.50 (0.27) | |
Data are expressed as mean (SD), except for sex and microvascular complications (%).
Statistical significance between groups was determined using either the Student's t-test or the chi-square test for continuous or categorical variables, respectively.
BMI, body mass index; BPM, beats per minute; DBP, diastolic blood pressure; IU, international units; SBP, systolic blood pressure; SD, standard deviation.
Glycemic control and treatment adherence
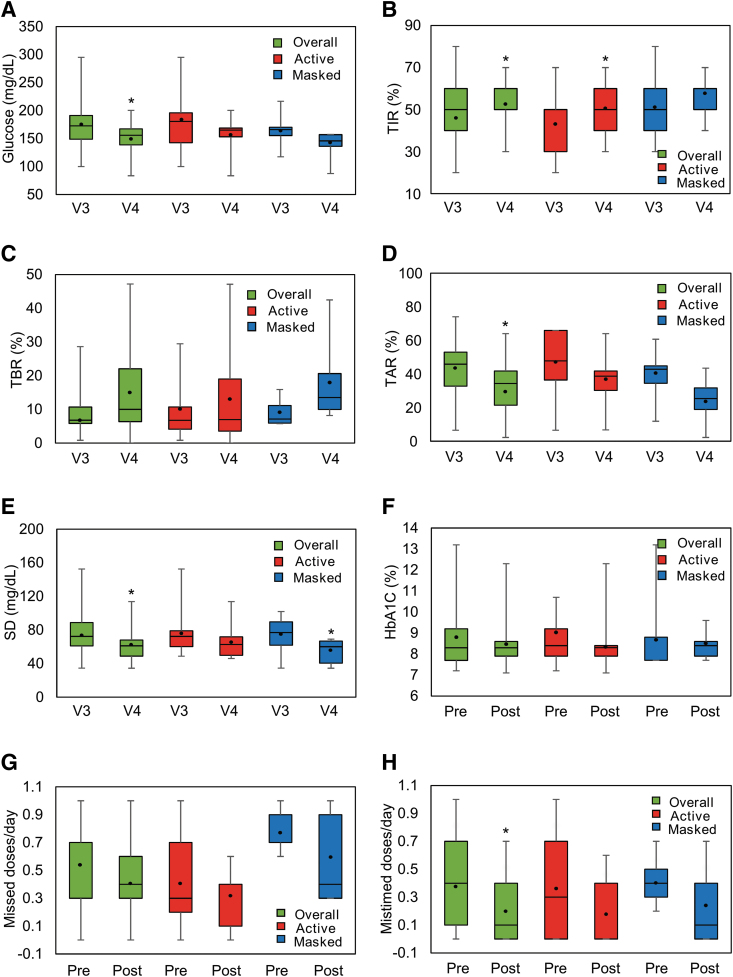
Mean glucose concentration decreased in the overall population, and in Active and Masked groups, with significant differences in the overall population (P = 0.013) (Fig. 1A).
FIG. 1.
Effect of Insulclock® on glycemic control, variability, and treatment adherence. The box-plots show median, Q1, Q3, Min, and Max values for (A) glucose, (B) TIR, (C) TBR, (D) TAR, and (E) SD at Visit 3 (V3) and Visit 4 (V4), and (F) HbA1C, (G) missed and (H) mistimed insulin doses at baseline (pre) and Visit 5 (post). Black dots indicate the mean.*P < 0.05 from baseline (Student's t-test). HbA1C, glycated hemoglobin; SD, standard deviation; TAR, time above range; TBR, time below range; TIR, time in range. Color graphics are available online.
TIR increased in both groups after 14 days, with significant differences in the overall population (+7%; P = 0.038) and in the Active Insulclock group (+8%; P = 0.026) (Fig. 1B). We observed a numerical increase in TBR in the overall population (+5.3%; P = 0.0826) (Fig. 1C). Significant differences were observed in the overall population for TAR (−12.5%; P = 0.0026) (Fig. 1D).
Mean SD significantly decreased in the overall population (−14.4 mg/dL [0.8 mmol/L]; P = 0.003) and in the Masked group (−18.5 mg/dL [1.0 mmol/L]; P = 0.037) (Fig. 1E).
No significant differences were observed in the reduction of mean HbA1C for any comparison (Fig. 1F).
The number of missed and mistimed insulin doses decreased per month in the overall population by −3.9 (P = 0.135) and −5.4 (P = 0.032), respectively (Fig. 1G, H).
Individual trends are depicted in Supplementary Figure S2.
Treatment satisfaction
Most of the items of the ITSQ improved after 4 weeks of Insulclock use. Significant differences were observed in the perception of insulin treatment interference in work/school activities and in the item assessing the potential of current insulin treatment for avoiding severe hypoglycemic episodes (Supplementary Fig. S3).
Discussion
This prospective, pilot study showed that the Insulclock device contributes to improving mean glucose and variability indices, adherence to insulin treatment, and treatment satisfaction in poorly controlled T1DM patients.
The use of Insulclock was associated with an overall improvement in glycemic control, although only some variables reached statistical significance. This could be expected in the context of a pilot study with a limited sample size, which makes it difficult to establish reliable conclusions. In our case, it could be also related to the chosen target population with significant adherence problems since, as previously shown, engagement with mHealth technologies is more difficult in nonadherent patients.17 Despite this, the significant improvement in TIR (+8%) in the Active group, and the decrease in mean glucose (−27.0 mg/dL), glucose variability (SD −14.4 mg/dL) and TAR (−12.5%) and the increase in TIR (+7%) in the overall population are of remarkable importance. Although glycemic indices were still not optimal at Visit 414,18 and differences did not reach statistical significance for some variables, they trended in the right direction with the use of Insulclock.
Previous studies assessing the effectiveness of mHealth technology have provided conflicting results.19,20 The authors hypothesized that telemedicine might not be suitable for all patient populations, particularly for those with persistent noncompliance issues.21 Therefore, the improvements observed in our study in patients with persistent poorly controlled glycemic levels seem promising.
In line with the improvement observed in glycemic indices, the mean number of missed and mistimed doses was reduced by −3.9 and −5.4 per month, respectively. This reduction is particularly important, since a previous study showed that two missed mealtime injections per week correlated with a 0.5% increase in HbA1C levels.22 Although omissions and late injections were still high at Visit 4, the reduction observed may represent an important achievement with a positive impact on glycemic control in this complex population. These results agree with recent data showing a decrease from 25% to 14% in missed bolus dose injections after 6 months of treatment with a smart insulin pen.23
Individual trends revealed dramatic improvements in specific patients in the Active Insulclock group. Understanding what motivated these patients to change compliance habits would require further investigation. It is worth mentioning that the patient with the greatest glucose improvement during the study period was the only one using a tutor from the Insulclock app. Although we cannot obtain reliable conclusions from a single patient, it would be interesting to study the effect of reinforcing this role.
In agreement with the results observed for glycemic variability, ITSQ items related to hypoglycemia control showed the most considerable improvements. However, only two items reached statistical significance, which could indicate that achieving substantial changes in quality of life requires longer time of follow-up and more pronounced clinical improvements.
This is the first clinical study assessing the effect of an insulin pen cap on clinical outcomes, treatment adherence and satisfaction. One of the main strengths is that it targeted patients with persistent uncontrolled glycemic levels, a particularly vulnerable population with a high risk for complications.
The main limitations of this study are the reduced sample size and follow-up time, as usual in pilot studies. In addition, we observed a considerable drop-out rate (23.8%), which could be explained considering the particularly challenging population analyzed.
In conclusion, this pilot study points out an improvement in glycemic levels, adherence, and quality of life in T1DM patients, supporting the development of future clinical trials to confirm such clinical benefit.
Supplementary Material
Acknowledgments
We thank Carla Granados from Trialance SCCL for providing medical writing assistance. We thank the European Commission who funded this project through grant number 739148, and Insulcloud, S.L. for providing the devices and telemedicine system under test. Insulcloud, S.L. did not contribute to study design or data analysis.
Author Disclosure Statement
F.G.-P. has taken part in advisory panels for Sanofi and Novo Nordisk; has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals and Lilly; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Co. and Lilly. C.A. has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals and Lilly and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, and Bristol-Myers Squibb Co. Sara Gómez-Rodriguez, Margarita Cruz-Bravo, C.M.-S. and G.P. have nothing to disclose. L.R.-V. is an Insulcloud S.L. employee.
Funding Information
Medical writing, statistical assistance, and sensors for continuous glucose monitoring were funded by the European Union Horizon 2020, Project ID: 674505. Insulcloud S.L. provided the Insulclock devices. The conception and design, acquisition, analysis, and interpretation of data were conducted thanks to the unconditional effort of participating investigators.
Supplementary Material
References
- 1. The Diabetes Control And Complications Trial: The effect of intensive treatment of diabetes on development and progression of long term complications in insulin dependent diabetes melitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Gomez-Peralta F, Menéndez-Torre E, Barreiro SC, et al. : Clinical characteristics and management of patients with type 1 diabetes—a National Population-Based Spanish Study (SED1). Diabetes 2019;68:2459 [Google Scholar]
- 3. Grassi G, Scuntero P, Trepiccioni R, et al. : Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol 2014;1:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Juarez DT, Tan C, Davis J, et al. : Factors affecting sustained medication adherence and its impact on healthcare utilization in patients with diabetes. J Pharm Health Serv Res 2013;4:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, et al. : Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention—a review. J Diabetes Complications 2016;30:167–177 [DOI] [PubMed] [Google Scholar]
- 6. Cramer JA: A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218–1224 [DOI] [PubMed] [Google Scholar]
- 7. Basatneh R, Najafi B, Armstrong DG: Health sensors, smart home devices, and the internet of medical things: an opportunity for dramatic improvement in care for the lower extremity complications of diabetes. J Diabetes Sci Technol 2018;12:577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hou C, Xu Q, Diao S, et al. : Mobile phone applications and self-management of diabetes: a systematic review with meta-analysis, meta-regression of 21 randomized trials and GRADE. Diabetes Obes Metab 2018;20:2009–2013 [DOI] [PubMed] [Google Scholar]
- 9. Arsand E, Muzny M, Bradway M, et al. : Performance of the first combined smartwatch and smartphone diabetes diary application study. J Diabetes Sci Technol 2015;9:556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou C, Carter B, Hewitt J, et al. : Do mobile phone applications improve glycemic control (HbA 1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016;39:2089–2095 [DOI] [PubMed] [Google Scholar]
- 11. Wu IXY, Kee JCY, Threapleton DE, et al. : Effectiveness of smartphone technologies on glycaemic control in patients with type 2 diabetes: systematic review with meta-analysis of 17 trials. Obes Rev 2018;19:825–838 [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, et al. : Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther 2019;21:209–214 [DOI] [PubMed] [Google Scholar]
- 13. Wright LA-C, Hirsch IB: Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther 2017;19:S16–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harvey RA, Dassau E, Zisser H, et al. : Design of the glucose rate increase detector: a meal detection module for the health monitoring system. J Diabetes Sci Technol 2014;8:307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson RT, Skovlund SE, Marrero D, et al. : Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther 2004;26:565–578 [DOI] [PubMed] [Google Scholar]
- 17. Adu MD, Malabu UH, Malau-Aduli AEO, et al. : Users' preferences and design recommendations to promote engagements with mobile apps for diabetes self-management: multi-national perspectives. PLoS One 2018;13:e0208942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monnier L, Colette C, Wojtusciszyn A, et al. : Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017;40:832–838 [DOI] [PubMed] [Google Scholar]
- 19. Montori VM, Helgemoe PK, Guyatt GH, et al. : Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care 2004;27:1088–1094 [DOI] [PubMed] [Google Scholar]
- 20. Chase HP, Pearson JA, Wightman C, et al. : Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care 2003;26:1475–1479 [DOI] [PubMed] [Google Scholar]
- 21. Yaron M, Sher B, Sorek D, et al. : A randomized controlled trial comparing a telemedicine therapeutic intervention with routine care in adults with type 1 diabetes mellitus treated by insulin pumps. Acta Diabetol 2019;56:667–673 [DOI] [PubMed] [Google Scholar]
- 22. Burdick J, Chase HP, Slover RH, et al. : Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics 2004;113(Pt 1):e221–e224 [DOI] [PubMed] [Google Scholar]
- 23. Adolfsson P, Hartvig NV, Kaas A, et al. : Improved insulin adherence after introduction of a smart connected insulin pen. Diabetes 2019;68:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.