Abstract
Methamphetamine (METH) administration alters gene expression in the nucleus accumbens (NAc). Previously, we showed that an acute injection of METH produced prolonged increases in the expression of immediate early genes (IEGs) in the NAc of HDAC2-deficent mice, suggesting that HDAC2 might be an important regulator of gene expression in the rodent brain. Here we tested the possibility that HDAC2 deletion might also impact METH-induced changes in the expression of various classes of HDACs in the NAc. Wild-type (WT) and HDAC2 Knockout (KO) mice were given a METH (20 mg/kg) injection and NAc tissue was collected at 1hr, 2hrs, and 8hrs post treatment. We found that METH caused decreased HDAC3, HDAC4, HDAC7, HDAC8, and HDAC11 but increased HDAC6 mRNA levels in the NAc of WT mice. In contrast, METH caused increases in HDAC3, HDAC4, HDAC7, HDAC8, and HDAC11 mRNA levels in HDAC2KO mice. These observations suggest that METH-induced large scale transcriptional changes in the NAc that might be secondary to suppression of several HDACs, in part, via HDAC2-regulated mechanisms. Our findings further implicate HDACs as potential novel therapeutic targets for substance use disorders.
Introduction
Methamphetamine (METH) use disorders are serious international health problems with an estimated 25 million METH users worldwide (Chomchai and Chomchai, 2015). METH causes a sense of euphoria, increased alertness, and hypersexuality in humans (Cruickshank and Dyer, 2009). These clinical effects last several hours because of the long elimination half-life (10-12 hours) of METH (Segal and Kuczenski, 2006). In rodents, METH increases locomotor activity and produces stereotypic behaviors (Jing et al., 2014). In addition, an acute METH injection alters gene expression in various brain regions (Cadet et al., 2001; Cadet et al., 2010) including the nucleus accumbens (NAc), an important structure involved in the acute and chronic effects of psychostimulants (Kalivas and Volkow, 2005). Moreover, METH-induced molecular alterations are accompanied by time-dependent post-translational histone modifications (Godino et al., 2015; Jayanthi et al., 2014; Martin et al., 2012).
Gene expression is regulated, in part, by dynamic interactions between chromatin, post-translational histone modifications, and transcription factors (TFs) (Bannister and Kouzarides 2011). In eukaryotic cells, DNA exists in chromatin whose basic subunits are called nucleosomes (Erdel et al., 2011). Each nucleosome is composed of 4 core histones including histones H2A, H2B, H3, and H4 that form an octomeric structure (2 of each core histone) (Morrison et al., 2007). Histones have N-terminal tails that contain lysine residues that can be reversibly acetylated by histone acetyltransferases (HATs) or deacetylated by histone deacetylases (HDACs) (Zhang et al., 2008). HDACs are divided into four known classes based on sequence similarities (Morrison et al., 2007). These include Class I (HDAC1, HDAC2, HDAC3, and HDAC8), Class IIA (HDAC4, HDAC5, HDAC7, and HDAC9), Class IIB (HDAC6 and HDAC10), Class III (Sirtuin1-7), and Class IV (HDAC11) (Gregoretti et al., 2004). Acetylation of histones allows for increased accessibility of TFs onto gene promoters with secondary increases in transcriptional activity (Verdone et al., 2006). In contrast, deacetylation of histones by repressor complexes containing HDACs results in condensed chromatin states and suppressed transcriptional activity (Kelly and Cowley, 2013).
Of interest is the fact that HDACs appear to be involved in psychostimulant-induced behaviors (Kalda and Zharkovsky, 2015; Nestler et al., 2012). For example, administration of the non-specific HDAC inhibitors, butyric acid or valproic acid, potentiates amphetamine-induced behavioral sensitization in mice (Kalda et al., 2007). An acute injection of METH produced time-dependent HDAC2 protein accumulation in the rat NAc (Martin et al., 2012). Moreover, decreases in the striatal expression of glutamate receptors produced by chronic METH administration were mediated, in part, by increased HDAC1 and HDAC2 protein abundance on the promoters of these genes (Jayanthi et al., 2014). More recently, our laboratory reported that binge toxic METH injections impacted the mRNA expression of several HDACs (Omonijo et al., 2014). In the present study, we used transgenic mice with a conditional deletion of HDAC2 to test the potential role of this protein in the regulation of METH-induced changes in the expression of several HDACs. Here we report that a single injection of METH produced decreased HDAC3, HDAC4, HDAC7, HDAC8, and HDAC11 mRNA levels in the NAc of wild-type mice. In the absence of HDAC2, however, the METH injection increased their expression. These results support the notion that HDAC2 may be an important regulator of gene expression after acute METH treatment.
Materials and Methods
Animals and Drug treatment
We used male HDAC2flox/flox Camk2a Cre+ mice (HDAC2KO) and age-matched HDAC2flox/flox Camk2a Cre- (WT) littermates weighing 30-40 g (N = 48) in this study. HDAC2KO mice were generated by cross-breeding HDAC2loxP/loxP transgenic mice (Montgomery, et al., 2007) containing loxP sites between exon 2 and exon 4 of the mouse HDAC2 gene with the C57BL/6 Camk2a-Cre transgenic mouse line (B6.Cg-Tg(Camk2a-cre)T29-1Stl/J) purchased from The Jackson Laboratory (Bar Harbor, ME). Mouse breeding was conducted by the National Institute of Drug Abuse (NIDA) Biomedical Research Center (BRC) Breeding Facility Core in Baltimore, MD. Genotyping for the specific deletion of HDAC2 was conducted by the Charles River’s Laboratory Testing Management® (LTM) division. All mice were housed in a temperature-controlled (22.2+0.2°C) vivarium with ad libitum access to food and water. After 14 weeks of age, mice of both genotypes received a single injection (i.p.) of METH (20 mg/kg) and were subsequently euthanized at three different time points (1hr, 2hrs, or 8hrs post injection).
This dose of METH produces robust changes in gene expression and increased HDAC2 protein levels within the NAc (Martin et al. 2012). The time course of tissue collection was chosen based on our previous experiments showing HDAC2 involvement in the regulation of METH-induced IEG expression (Torres et al., 2015). Control mice were euthanized at the 1hr and 8hr time points after a single saline injection. All animal use procedures were conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the NIDA Intramural Research Program (IRP) Animal Care and Use Committee (ASP #12-CNRB-50).
RNA isolation
Nucleus accumbens tissue was rapidly dissected, immediately placed on dry ice and stored at -80°C. Total RNA was isolated using Qiagen RNeasy® Mini kits and genomic DNA contamination was eliminated using Qiagen RNase-free DNase (Qiagen, Valencia, CA) following the manufacturer's protocol. Analysis of total RNA integrity was conducted using an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA) and showed no degradation based on RIN values. Quantification of individual RNA obtained from six mice per group was then assessed using a Nanodrop 2000 spectrophotometer system (Thermo Scientific, Waltham, MA).
Quantitative Real-Time Reverse Transcription-PCR Analysis
Un-pooled total RNA (0.5μg) for each mouse was reverse-transcribed to cDNA using oligo dT primers from the Advantage RT for PCR kit (Clontech, Mountain View, CA). Specific gene primers for members of the HDAC family and PCR amplification were designed using the LightCycler Probe Design software (Roche, Indianapolis, IN). Primer sequences are list on Table 1. Each custom designed primer was then manufactured and HPLC-purified by the Synthesis and Sequencing Facility of the Johns Hopkins University (Baltimore, MD). The expression levels of each amplicon were examined by qRT-PCR using a LightCycler 480 II system (Roche, Indianapolis, IN) with iQ SYBR Green Supermix (BioRad, Hercules, CA). Purity of each qRT-PCR product was verified by separate melting curve analyses. Quantitative PCR values were normalized to clathrin (Cltc) based on the observation that this reference gene had the most stable expression across both genotypes at various time points after the acute METH injection. Results are reported as fold changes calculated as the normalized gene expression value of each group relative to controls.
Table 1.
List of qRT-PCR primers.
| Gene Name | Forward | Reverse |
|---|---|---|
| HDAC1 | GCC CTT CCA ATA TGA CTA AC | GAG CAG ATG GAA ATT CGT |
| HDAC3 | ACC AGA TCC GCC AGA CAA T | TTC AGC GTC GGC CTC GT |
| HDAC4 | ACC AGG CCT TGC TGG ATG | AGT GGC CTC CGC TTC TT |
| HDAC5 | CTG GGC AAG ATC CTT AC | CTC CTC CTC TTC TAG GT |
| HDCA6 | CAG CGC ATC TTA CGC ATC A | TGC GGA GAT GCT CCA CG |
| HDAC7 | CAG CCG CCT CAA ACT GGA T | TGC AGC TCG TTC CAG ATG GT |
| HDAC8 | CAA TCC GAA GGC AGT GGT T | GCC AGC TGC CAC TGT AGG |
| HDAC9 | GCT GTG AGT CGC TGG GAA | CTT GCC AGG AGG GAT TGA AG |
| HDAC10 | CTC CCA CTG GCC TTC GAG | CTG CAG CAG CTG TGT GAG A |
| HDAC11 | AGG TGC CCA AGG GTC TCT G | TGT GGC TGG AGC TAA GCTGT |
| Cltc | GGG GTT AAA GTC ACA CAG | AAG TAT CCG TAA GTG GAG |
Statistical Analyses
Quantitative PCR data were analyzed using two-way analysis of variance (ANOVA) followed by Fisher’s LSD where appropriate (SPSS 20, IBM, Armonk, NY). All data are presented as means ± SEM and considered statistically significant when p < 0.05.
Results
To test the potential role of HDAC2 in the regulation of METH-induced changes in Class I HDACs, we measured HDAC1, HDAC2, HDAC3, and HDAC8 mRNA levels in the NAc of WT and HDAC2KO mice. The METH injection did not cause significant alterations in HDAC1 [F(3,40) = 1.51, p = 0.22] (Figure 1A) nor in HDAC2 [F(3,20) = 3.04, p = 0.05] (Figure 1B) mRNA levels in WT or HDAC2KO mice. However, there were main effects of genotype [F(1, 40) = 25.83, p < 0.001] and significant genotype*METH interactions [F(3,40) = 4.58, p < 0.01] in HDAC3 mRNA expression (Figure 1C). HDAC3 mRNA levels were decreased at the 1hr and 8hr time points after the METH injection in WT mice relative to saline controls (Figure 1C). In contrast, HDAC3 mRNA levels were increased at the three time points in HDAC2KO mice compared to WT littermates (Figure 1C). There were significant main effects of genotype [F(1, 38) = 10.9, p < 0.001] and genotype*METH interactions [F(3,38) = 3.19, p < 0.05] in HDAC8 mRNA levels (Figure 1D). HDAC8 expression was comparable in both genotypes at the 1hr time point, but had significantly increased (2.4-fold) in HDAC2KO at the 2hr time point relative to saline controls and WT littermates (Figure 1D). HDAC8 levels returned to normal values at the 8hr time point in HDAC2KO mice but were decreased in WT littermates.
Figure 1:
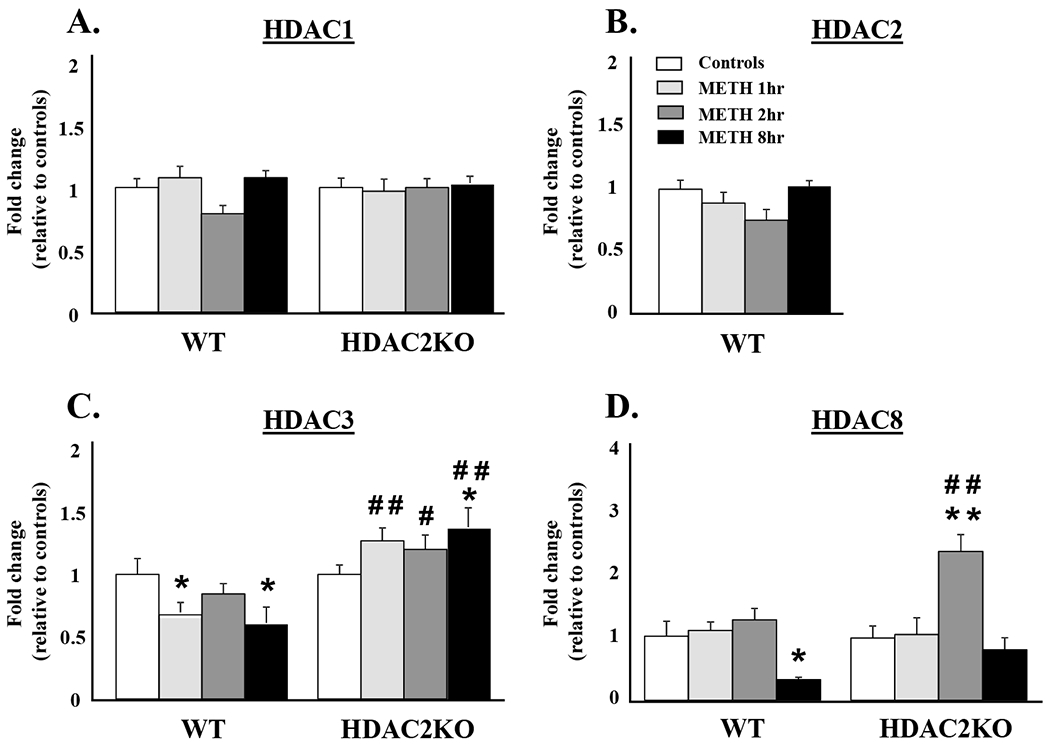
METH-induced transcriptional responses in members of the Class I HDAC family: (A) HDAC1, (B) HDAC2, (C) HDAC3, and (D) HDAC8. WT and HDAC2KO mice were injected with a single dose of METH (20 mg/kg) and euthanized 1hr, 2hrs, and 8hrs later. Total RNA was extracted from the NAc and used in qRT-PCR assays. The relative amounts of transcripts were normalized to Cltc. Statistical significance was determined by ANOVAs followed by post-hoc tests were appropriate. Data are presented as means ± SEM (n = 6 animals per genotype per time point) and were examined using two-way ANOVAs. Key to statistics: * = p < 0.05, ** = p < 0.01, in comparison to respective saline treated controls; # = p < 0.05, ## = p < 0.01, in comparison to respective METH treated WT mice.
The effects of METH on Class IIA HDACs are shown in Figure 2. The METH injection caused significant main effects of genotype [F(1,40) = 29.67, p < 0.001] and significant genotype*METH interactions [F(3,40) = 16.17, p < 0.01] on HDAC4 mRNA levels (Figure 2A). Decreased levels were observed at the 1hr time point in both WT and HDAC2KO mice relative to saline controls. HDAC4 mRNA levels remained decreased in WT mice, at both the 2hr and 8hr time points, whereas they significantly increased in HDAC2KO mice (Figure 2A).
Figure 2:

Differential effects of acute METH treatment on class IIA HDAC mRNA levels: (A) HDAC4, (B) HDAC5, (C) HDAC7, and (D) HDAC9. The graphs show results from qRT-PCR analyses. Data are presented as means ± SEM (n = 6 animals per genotype per time point) and were examined using two-way ANOVAs. Key to statistics: * = p < 0.05, ** = p < 0.01, in comparison to respective saline treated controls; ## = p < 0.01, ### = p < 0.001, in comparison to respective METH treated WT mice.
METH treatment did not alter HDAC5 mRNA levels in the NAc of either WT or HDAC2KO mice [F (3,40) = 1.27, p = 0.29] (Figure 2B). Figure 2C shows significant main effects of genotype [F(1,40) = 13.83, p < 0.005] and genotype*METH interactions [F(3,40) = 7.63, p < 0.01] in HDAC7 mRNA levels. HDAC7 mRNA levels decreased at the 1hr and 2hr, but not at the 8hr, (normalized) time points after the METH injection in WT mice compared to saline controls. In contrast, HDAC7 mRNA levels increased at the 1hr time point in HDAC2KO mice compared to saline-treated controls and to WT litter mates. HDAC7 mRNA levels increased at the 8hr time point in HDAC2KO mice in comparison to WT mice. There were no significant changes in HDAC9 mRNA expression at any time point after the METH injection in WT or HDAC2KO mice [F(3,39) = 1.48, p = .23] (Figure 2D).
Figure 3 illustrates the effects of METH on Class IIB HDACs. We observed significant main effects of genotype [F(1, 40) = 17.25, p < 0.001] and genotype*METH interactions [F(3,40) = 3.51, p < 0.05] on HDAC6 mRNA expression (Figure 3A). In contrast to the observations with the other HDACs, there were significant increases in HDAC6 mRNA levels at the three time points in WT mice (Figure 3A). However, significant increases in HDAC6 mRNA levels were only observed at the 8hr time point in HDAC2KO mice. There were no significant METH-induced changes in HDAC10 mRNA levels in either WT or HDAC2KO mice [F(3,39) = 0.70, p = 0.55] (Figure 3B). Finally, METH caused significant main effects of genotype [F(1,38 ) = 24.58, p < 0.001] and significant genotype*METH interactions [F(3,38) = 5.19, p < 0.01] on HDAC11 mRNA expression (Figure 3C). METH caused significant decreases in HDAC11 mRNA levels at the 3 time points post treatment in WT mice compared to saline controls. HDAC11 mRNA levels showed increased levels in HDAC2KO mice compared to WT littermates at all time points (Figure 3C).
Figure 3:
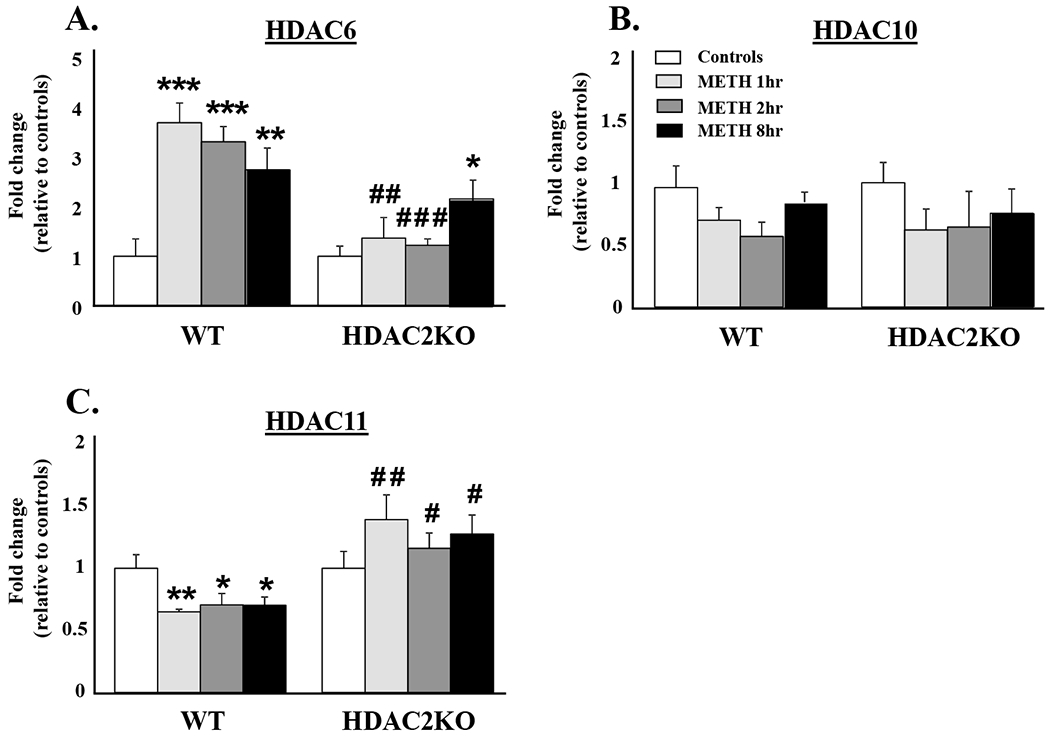
Effects of acute METH on the expression of Class IIB and Class IV members of the HDAC family: (A) HDAC6, (B) HDAC10, and (C) HDAC11. RNA extraction, qRT-PCR, and statistical analyses are as described in the methods section. Data are presented as means ± SEM (n = 6 animals per genotype per time point) and were examined using two-way ANOVAs. Key to statistics:* = p < 0.05, ** = p < 0.01, *** = p < 0.001, in comparison to respective saline treated controls; # = p < 0.05, ## = p < 0.01, ### = p < 0.001, in comparison to respective METH treated genotype.
Discussion
In the present study, we assessed the potential role of HDAC2 in mediating METH-induced transcriptional changes in members of the HDAC family by using conditional HDAC2KO mice. The main findings of this report are that: (1) acute METH caused time-dependent decreases in HDAC3, HDAC4, HDAC7, HDAC8, and HDAC11 mRNA in the NAc of WT mice; (2) there were increased HDAC6 mRNA levels after acute METH and (3) relative to WT mice, HDAC2KO mice showed increased HDAC3, HDAC4, HDAC7, HDAC8, and HDAC11 mRNA levels after a single METH injection. These findings suggest that acute METH causes down-regulation of HDACs in the NAc, and implicate HDAC2 as a repressor of various HDACs following METH treatment.
Previous work from our laboratory had shown that binge METH injections did not cause significant changes in HDAC1 or HDAC2 mRNA levels at 16 hours after these injections (Omonijo et al., 2014). In the present study, we also show that acute METH exposure does not alter HDAC1 or HDAC2 mRNA levels in the NAc of WT mice. Similarly, there were no changes in HDAC1 mRNA levels in HDAC2KO mice following a single METH injection. These lacks of changes may not be surprising given that the enzymatic activity of HDAC1 and HDAC2 is mostly redundant (Montgomery et al., 2007). Indeed, among members of the class I HDACs, HDAC1 and HDAC2 are the most comparable with an estimated 83% amino acid identity (Brunmeir et al., 2009). Given that deletion of HDAC2 does not alter HDAC1 expression in mouse embryonic stem cell lines (Winter et al., 2013), together these findings suggest that HDAC2 might not be intimately involved in the regulation of HDAC1 mRNA levels.
In contrast, acute METH decreased HDAC3 mRNA levels in WT mice. The cloning and characterization of HDAC3 was published in 1998 (Emilliani et al., 1998) and is known to be a negative regulator of transcription through the formation of SMRT and NCoR protein complexes (Karagianni and Wong, 2007). Considering the regulatory role of HDAC3 on gene expression, the down-regulation of HDAC3 mRNA after acute METH exposure might be, in part, a trigger for METH-induced transcriptional activation through increased histone acetylation. This notion is consistent with findings showing that cocaine exposure produces decreased HDAC3 enrichment on the promoters of c-fos and Nr4a2 (Rogge et al., 2013), two genes known to be up-regulated following acute cocaine (Hope et al., 1992) and METH treatment (Akiyama et al., 2008). We also show that HDAC2KO mice display increased HDAC3 mRNA levels after a single METH injection relative to WT mice. Because HDAC3 is essential for drug-induced memory consolidation (Malvaez et al., 2013), it is possible that HDAC3 might be involved in the improved cognition observed after the loss of HDAC2 (Morris et al., 2013). Another Class I HDAC affected by the acute METH injection was HDAC8. The characterization of HDAC8 was published by two researcher groups (Buggy et al., 2000; Hu et al., 2000). HDAC8 was shown to mediate transcriptional repression independent of protein complexes (Haider et al., 2011). Here, we show that HDAC8 mRNA expression was decreased at the 8hr time point after acute METH treatment in the NAc of WT mice. These findings are similar to our previous report (Omonijo et al., 2014) and suggest that METH indeed impacts HDAC8 transcription. We also showed that, in HDAC2KO mice, HDAC8 mRNA levels were up-regulated at the 2hr time point and returned to baseline at the 8hr time point following acute METH treatment. Because overexpression of HDAC8 decreases pCREB activity (Gao et al., 2009), and METH prolongs expression of pCREB-responsive IEGs in the absence of HDAC2 (Torres et al., 2015), the transient up-regulation of HDAC8 might serve as a compensatory mechanism to repress pCREB-dependent transcription activation in HDAC2KO mice. Nevertheless, further studies are needed to fully explore this possibility.
The METH injection also impacted HDAC4 and HDAC7 mRNA expression. Unlike the Class I HDACs, members of the Class IIA subgroup are unique in that they shuttle between the nucleus and cell cytoplasm (Chawla et al., 2003). Herein, we observed that acute METH decreased HDAC4 mRNA levels in the NAc of WT mice. These findings are similar to our observations for HDAC3 and suggests that METH might down-regulate the gene expression of HDAC4 by similar molecular mechanisms because HDAC4 is enzymatically inactive when not associated with HDAC3 (Guenther et al., 2001). We also observed that, relative to WT mice, HDAC2KO mice displayed increased HDAC4 mRNA levels at the 2hr and 8hr time points after the acute METH injection. These observations suggest that HDAC2 is indeed involved in regulation HDAC4 transcription. METH also caused decreased HDAC7 mRNA levels in WT mice. The METH-induced down-regulation of HDAC7 is interesting given that HDAC7 inhibits NR4A1 (Dequiedt et al., 2005), an orphan nuclear receptor known to be overexpressed after METH treatment (McCoy et al., 2011). Thus, decreases in HDAC7 mRNA might account for the METH-induced transcriptional activation of members of this nuclear family that play a key role in cell survival (Akiyama et al., 2008). METH also increased HDAC7 mRNA in HDAC2KO mice. Because HDAC7 also combines with protein complexes containing HDAC3 (Fischle et al., 2001), it is tempting to speculate whether HDAC2 might co-regulate these two enzymes to exert better control of their epigenetic effects.
Interestingly, HDAC6 mRNA was up-regulated in WT mice following the acute METH injection. HDAC6 is unique among other HDACs in that it has a zinc finger ubiquitin-binding domain able to identify misfolded proteins for lysosome degradation (Lee et al., 2010; Ouyang et al., 2012). HDAC6 also possesses tubulin deacetylase activity (Haggarty et al., 2003). Acetylation of microtubules plays a critical role in cell stability whereas deacetylation results in cytoskeleton disarrangement (Li et al., 2007). Because METH is known to increase blood brain barrier permeability (Ramirez et al., 2009), the up-regulation of HDAC6 mRNA might be involved in METH-induced disruption of cytoskeletal viability. This idea is consistent with the fact that endothelial cells treated with METH show α-tubulin degradation secondary to HDAC6 activation (Fernandes et al., 2015). The acute METH injection also caused down-regulation of HDAC11 mRNA in WT mice. Given that HDAC11 also suppresses transcriptional activity (Sahakian et al., 2015; Gao et al., 2002), decreases in this enzyme, in addition to the decreased expression of some of the other HDACs, might serve to potentiate METH-induced global changes in gene expression. We also found that, similar to the other HDACs, HDAC2 deletion resulted in overexpression of HDAC11 mRNA at the 3 time points after the METH injections. Because overexpression of HDAC11 decreases the acetylation of H3K9 and H3K14 (Liu et al., 2009), METH-induced HDAC11 overexpression may also decrease the acetylation status of these histones in HDAC2KO mice.
In summary, we report that acute METH treatment represses the mRNA expression of several members of the HDAC family. In addition, we report that conditional deletion of HDAC2 causes METH-induced up-regulation in HDAC3, HDAC4, HDAC8, HDAC7 and HDAC11 mRNA levels in NAc. In contrast, acute METH treatment did not affect HDAC1, HDAC2, HDAC5 HDAC9, or HDAC10 mRNA levels in the NAc of WT or HDAC2KO mice. These findings supports the notion that HDAC2 might act as a negative regulator of METH-induced transcriptional changes in the brain. Moreover, our work shows that HDAC2 is an important repression of some, but not all, HDAC family members after METH exposure. Future studies will focus on investigating a direct association between METH-induced repression of HDAC family members and global increase in gene expression after METH exposure.
Acknowledgements
The authors wish to thank Ezekiell Mouzon at Mount Sinai School of Medicine for the gracious initial donation of HDAC2 floxed mice.
This research was supported by funds of the Intramural Research Program of the DHHS/NIH/NIDA.
List of nonstandard abbreviations:
- METH
methamphetamine
- HDAC
histone deacetylase
- KO
knock-out
- CamK2a
Calcium/Calmodulin-Dependent Protein Kinase II Alpha
References
- Akiyama K, Isao T, Ide S, Ishikawa M, Saito A (2008) mRNA expression of the Nurr1 and NGFI-B nuclear receptor families following acute and chronic administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 32(8):1957–1966. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, and Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21(3): 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunmeir R, Lagger S, Seiser C (2009) Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53(2-3):275–289. [DOI] [PubMed] [Google Scholar]
- Buggy JJ, Sideris ML, Mak P, Lorimer DD, McIntosh B, Clark JM (2000) Cloning and characterization of a novel human histone deacetylase, HDAC8. Biochem J 350 Pt 1:199–205. [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord Drug Targets 9(5):526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B (2001) Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: evidence from cDNA array. Synapse 41(1):40–48. [DOI] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H (2003) Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem 85(1):151–159. [DOI] [PubMed] [Google Scholar]
- Chomchai C and Chomchai S (2015) Global patterns of methamphetamine use. Curr Opin Psychiatry 28(4):269–274. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC and Dyer KR (2009) A review of the clinical pharmacology of methamphetamine. Addiction 104(7):1085–1099. [DOI] [PubMed] [Google Scholar]
- Darcy MJ, Calvin K, Cavnar K, Ouimet CC (2010) Regional and subcellular distribution of HDAC4 in mouse brain. J. Comp. Neurol 518, 722–740. [DOI] [PubMed] [Google Scholar]
- Dequiedt F, Van Lint J, Lecomte E, Van Duppen V, Seufferlein T, Vandenheede JR, Wattiez R, Kettmann R (2005) Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J Exp Med 201(5):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emiliani S, Fischle W, Van Lint C, Al-Abed Y, Verdin E (1998) Characterization of a human RPD3 ortholog, HDAC3. Proc Natl Acad Sci U S A 95(6):2795–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdel F, Krug J, Längst G, Rippe K (2011) Targeting chromatin remodelers: signals and search mechanisms. Biochim Biophys Acta 1809(9):497–508. [DOI] [PubMed] [Google Scholar]
- Fernandes S, Salta S, Summavielle T (2015) Methamphetamine promotes α-tubulin deacetylation in endothelial cells: the protective role of acetyl-l-carnitine. Toxicol Lett 234(2):131–138. [DOI] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Fillion M, Hendzel MJ, Voelter W, Verdin E (2001) Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo. J Biol Chem 276(38):35826–35835. [DOI] [PubMed] [Google Scholar]
- Gao L, Cueto MA, Asselbergs F, Atadja P (2002) Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem 277(28):25748–25755. [DOI] [PubMed] [Google Scholar]
- Godino A, Jayanthi S, Cadet JL (2015). Epigenetic landscape of amphetamine and methamphetamine addiction in rodents. Epigenetics 10(7):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoretti IV, Lee YM, Goodson HV (2004) Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol 338(1):17–31. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459(7243):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Barak O, Lazar MA (2001) The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21(18):6091–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. (2003). Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A 100(8):4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Joseph CG, Neidle S, Fierke CA, Fuchter MJ (2011) On the function of the internal cavity of histone deacetylase protein 8: R37 is a crucial residue for catalysis. Bioorg Med Chem Lett 21(7):2129–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. (1992). Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A 89(13):5764–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Chen Z, Fredrickson T, Zhu Y, Kirkpatrick R, Zhang GF, Johanson K, Sung CM, Liu R, Winkler J (2000) Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. J Biol Chem 275(20):15254–15264. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL (2014) Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry 76(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Zhang M, Li JX, Huang P, Liu Q, Li YL, Liang H, Liang JH (2014) Comparison of single versus repeated methamphetamine injection induced behavioral sensitization in mice. Neurosci Lett 560:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalda A, Heidmets LT, Shen HY, Zharkovsky A, Chen JF (2007) Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav Brain Res 181(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalda A, and Zharkovsky A (2015) Epigenetic Mechanisms of Psychostimulant-Induced Addiction. Int Rev Neurobiol 120:85–105. [DOI] [PubMed] [Google Scholar]
- Kalivas PW and Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162(8):1403–1413 [DOI] [PubMed] [Google Scholar]
- Karagianni P and Wong J (2007) HDAC3: taking the SMRT-N-CoRrect road to repression. Oncogene 26(37):5439–5449. [DOI] [PubMed] [Google Scholar]
- Kelly RD and Cowley SM (2013) The physiological roles of histone deacetylase (HDAC) 1 and 2: complex co-stars with multiple leading parts. Biochem Soc Trans 41(3):741–749. [DOI] [PubMed] [Google Scholar]
- Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao TP (2010) HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J 29(5):969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Jiang H, Chang M, Xie H, Hu L (2007) HDAC6 α-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases. J Neurol Sci 304(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Q, D'ercole AJ, Ye P (2009) Histone deacetylase 11 regulates oligodendrocyte-specific gene expression and cell development in OL-1 oligodendroglia cells. Glia 57(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA (2013) HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A 110(7):2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, Cadet JL (2012) Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLoS One 7(3):e34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MT, Jayanthi S, Wulu JA, Beauvais G, Ladenheim B, Martin TA, Krasnova IN, Hodges AB, Cadet JL (2011) Chronic methamphetamine exposure suppresses the striatal expression of members of multiple families of immediate early genes (IEGs) in the rat: normalization by an acute methamphetamine injection. Psychopharmacology (Berl) 215(2):353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. (2007). Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21(14):1790–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM (2013) Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci 33(15):6401–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BE, Majdzadeh N, D'Mello SR (2007) Histone deacetylases: focus on the nervous system. Cell Mol Life Sci 64(17):2258–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2012) Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci 10(3):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonijo O, Wongprayoon P, Ladenheim B, McCoy MT, Govitrapong P, Jayanthi S, Cadet JL. (2014). Differential effects of binge methamphetamine injections on the mRNA expression of histone deacetylases (HDACs) in the rat striatum. Neurotoxicology 45:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Ali YO, Ravichandran M, Dong A, Qiu W, MacKenzie F, Dhe-Paganon S, Arrowsmith CH, Zhai RG (2012) Protein aggregates are recruited to aggresome by histone deacetylase 6 via unanchored ubiquitin C termini. J Biol Chem 287(4):2317–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, Dykstra H, Weksler BB, Romero IA, Couraud PO, Persidsky Y (2009) Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab 29(12):1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge GA, Singh H, Dang R, Wood MA (2013) HDAC3 is a negative regulator of cocaine-context-associated memory formation. J Neurosci 33(15):6623–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian E, Powers JJ, Chen J, Deng SL, Cheng F, Distler A, Woods DM, Rock-Klotz J, Sodre AL, Youn JI, Woan KV, Villagra A, Gabrilovich D, Sotomayor EM, Pinilla-Ibarz J (2015) Histone deacetylase 11: A novel epigenetic regulator of myeloid derived suppressor cell expansion and function. Mol Immunol 63(2):579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R (2006) Human methamphetamine pharmacokinetics simulated in the rat: single daily intravenous administration reveals elements of sensitization and tolerance. Neuropsychopharmacology 31(5):941–955. [DOI] [PubMed] [Google Scholar]
- Torres OV, McCoy MT, Ladenheim B, Jayanthi S, Brannock C, Tulloch I, Krasnova IN, Cadet JL (2015) CAMKII-conditional deletion of histone deacetylase 2 potentiates acute methamphetamine-induced expression of immediate early genes in the mouse nucleus accumbens. Sci Rep 5:13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone L, Agricola E, Caserta M, Di Mauro E (2006) Review Histone acetylation in gene regulation. Brief Funct Genomic Proteomic 5(3):209–221. [DOI] [PubMed] [Google Scholar]
- Winter M, Moser MA, Meunier D, Fischer C, Machat G, Mattes K, Lichtenberger BM, Brunmeir R, Weissmann S, Murko C, Humer C, Meischel T, Brosch G, Matthias P, Sibilia M, Seiser C (2013) Divergent roles of HDAC1 and HDAC2 in the regulation of epidermal development and tumorigenesis. EMBO J 32(24):3176–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fang H, Jiao J, Xu W (2008) The structure and function of histone deacetylases: the target for anti-cancer therapy. Curr Med Chem 15(27):2840–2849. [DOI] [PubMed] [Google Scholar]


