Abstract
Podocyte injury in focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) results from the imbalance between adaptive responses that maintain homeostasis and cellular dysfunction that can culminate in cell death. Therefore, an in situ analysis was performed to detect morphological changes related to cell death and autophagy in renal biopsies from adult patients with podocytopathies. Forty-nine renal biopsies from patients with FSGS (n = 22) and MCD (n = 27) were selected. In situ expression of Wilms Tumor 1 protein (WT1), light chain microtubule 1-associated protein (LC3) and caspase-3 protein were evaluated by immunohistochemistry. The foot process effacement and morphological alterations related to podocyte cell death and autophagy were analyzed with transmission electronic microscopy. Reduction in the density of WT1-labeled podocytes was observed for FSGS and MCD cases as compared to controls. Foot process width (FPW) in control group was lower than in cases of podocytopathies. In FSGS group, FPW was significantly higher than in MCD group and correlated with proteinuria. A density of LC3-labeled podocytes and the number of autophagosomes in podocytes/ pedicels were higher in the MCD group than in the FSGS group. The number of autophagosomes correlated positively with the estimated glomerular filtration rate in cases of MCD. The density of caspase-3-labeled podocytes in FSGS and MCD was higher than control group, and a higher number of podocytes with an evidence of necrosis was detected in FSGS cases than in MCD and control cases. Podocytes from patients diagnosed with FSGS showed more morphological and functional alterations resulting from a larger number of lesions and reduced cell adaptation.
Introduction
Focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) are among the main causes of idiopathic nephrotic syndrome, termed as podocytopathies. These conditions show diffused foot processes effacement under electron microscopy [1].
Podocytes (visceral epithelial cells) are terminally differentiated cells lining the outer surface of glomerular capillaries, and their differentiation coincides with the progressive expression of maturity markers, including the Wilms tumor suppressor gene 1 (WT1) [2]. These cells appear to have an intrinsic system that could withstand tension but they may be undergo damage when the stresses exceed this capacity. Thus, podocyte injury is a result of the imbalance between the adaptive responses that maintain homeostasis and cellular dysfunction, leading to cell death [3].
To maintain homeostasis, podocytes have a high rate of autophagy, which is related to the formation of autophagosomes [4]. Proteins that are important for the formation of the autophagosome include light chain microtubule 1-associated protein (LC3), a member of the autophagy-related protein family 8 encoded by the MAP1LC3 gene, which is required for the elongation and maturation of the autophagosome [5].
So, considering the research line of our group that has been looking for possible biomarkers, morphological alterations and mechanisms of lesions that may allow differentiation of the diagnosis of MCD from that of FSGS when sclerosis lesions are not sampled in renal biopsies [6–8], we propose to analyze in situ morphological and functional alterations related to apoptosis, necrosis and autophagy in renal biopsies of adult patients with FSGS and MCD.
Materials and methods
This study was approved by the Ethics and Research Committee of the Federal University of Triângulo Mineiro with the number 2.949.713. All samples were archived and cases were identified by codes with letters and numbers to ensure that individuals were anonymized. Because it is a retrospective study, ethics committee waived the requirement for informed consent.
Patients
A total of 49 cases of idiopathic podocytopathies in adults, including patients with FSGS (n = 22) and MCD (n = 27), were selected from the Nephropathology Service of the General Pathology Discipline of the Federal University of Triângulo Mineiro Uberaba, Minas Gerais, Brazil. FSGS group was defined by the presence of segmental and focal sclerosis (mesangial matrix increase) in light microscopy (LM) and foot process effacement in transmission electron microscopy (TEM). MCD group was defined by the absence of lesions in LM and foot process effacement as the only alteration in TEM analysis. All cases that presented morphological alterations compatible with other entities were excluded from this study. For analyzes by light microscopy, we used autopsy kidney fragments from patients whose death was not related to renal or infectious diseases to compose the control group (n = 18). Cases of non-glomerular proteinuria and isolated hematuria were selected to compose the control group (n = 12) for analyses by transmission electron microscopy.
Renal histopathology
The kidney samples were evaluated by LM, immunofluorescence, TEM, and immunohistochemistry. For LM, the paraformaldehyde-fixed fragment was dehydrated in graduated alcohols, diaphanized in xylol, embedded in paraffin and submitted to serial cuts of 2μm thickness. The paraffin material slides were stained with hematoxylin and eosin, Sirius Red, periodic acid silver methenamine stain and Masson's trichrome. For direct immunofluorescence, the fragment was frozen in liquid nitrogen and sectioned with 2μm thickness sections. The frozen material slides were screened for IgA, IgG and IgM immunoglobulins, kappa and lambda light chains, C1q and C3 complement fractions, and fibrinogen by fluorescein isothiocyanate conjugated antibodies (Dako, Copenhagen, Denmark). For TEM, the sample was fixed with Karnovsky 2.5% + ruthenium red 0.2% and post-fixed in osmium tetroxide 2%, then dehydrated in graded alcohols and acetone solutions and embedded in Epon 812 resin. Ultrathin 60-nm-thick sections were cut and placed onto nickel grids and were subsequently contrasted with lead citrate 1% and uranyl acetate 3% and examined under an EM-900 transmission electron microscope (Zeiss, Germany) [8, 9].
Immunohistochemistry for WT1, LC3, and caspase 3
Sequential histological sections of 2 μm of paraffin-embedded fragments were subjected to immunohistochemistry (Table 1) which was performed manually using the Novolink non-biotinylated polymer system (Novolink Polymer Detection System Kit, BL, UK).
Table 1. Immunohistochemistry specifications.
| Primary antibody | Supplier | Clone | Antigen retrieval buffer | Antibody dilutions | Catalog numbers |
|---|---|---|---|---|---|
| Monoclonal mouse anti-human Wilms’ tumor 1 | Dako | 6F-H2 | Citrate pH 6.0 | 1:500 | M3561 |
| Monoclonal mouse anti-human LC3 | BIORBYT | 166AT1234 | Citrate pH 6.0 | 1:1200 | orb97657 |
| Monoclonal rabbit anti-human Caspase 3 | NSJBIO | RM250 | Citrate pH 6.0 | 1:1000 | R20270 |
Immunostaining quantification
All glomeruli without sclerosis of each sample were analyzed. Digital images of each glomerulus were captured using the AxionCam ICc5 (Zeiss, Germany) digital camera attached to the light microscope on the 40x objective lens. Immunostained cells showing an intense brownish staining and located outside glomerular loop were defined as podocytes. For each case, all immunostained brownish-colored podocytes were quantified in each glomerulus and the area of each glomerulus was measured. The result was expressed in cell density per glomerular area according to Venkatareddy et al. [10].
Transmission electron microscopy
In TEM were evaluated the foot process width, the number of autophagosomes in podocytes / pedicels and evidence of necrosis in podocytes. Two glomeruli from each patient were evaluated and the images of all viable glomerular loops and all available podocytes were captured at an amplification of 7000X and the image analysis was performed with ImageJ 1.5 software.
To determine the foot process width (FPW), the length of the glomerular basement membrane (GBM) was measured and the number of foot process was counted similar to the Deegens et al. technique [11]. Each foot process was defined as a secondary epithelial segment connected to the GBM between two slit diaphragms. Thus, the FPW was obtained by dividing the number of foot process counted by the length of GBM analyzed [11] corrected by the correction factor π / 4 to exclude the assumed random variation in the section angle in relation to the long axis of the podocyte [12]. For each patient, the average foot process width was expressed in nanometers.
To evaluate podocyte number, all available podocytes were captured at an amplification of 7000X and their nucleus were observed in each image, without repeating fields. So, for each case the average number of podocytes was obtained.
The number of autophagosomes in podocytes/foot process and the number of podocytes with evidence of necrosis were counted in each image without repeating fields and the results were expressed as the average number of each variable analyzed for each case. Autophagosomes were identified as vesicles containing undigested cytoplasmic material [13]. Evidence of necrosis in podocytes were oncosis (increased cell volume), nuclear edema (increased nuclear volume with decreased electrodensity) and cell lysis (cytoplasmic membrane rupture and loss of intracytoplasmic content) [14].
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software version 7.0. The normality in the frequentist statistics was tested by the Shapiro Wilk test. Comparison between cases with normal distribution and similar variations, the ANOVA (F) test was used, followed by the Tukey post-test or Student's t test (t). The Kruskal-Wallis tests (H), followed by the Dunn post-test or Mann-Whitney test (U) were used to compare cases with non-normal distribution. For the analysis of associations, the chi-square test (χ2) was used. Correlations were assessed with the Pearson (r) and Spearman (rS) tests. Differences were considered significant when p <0.05.
Results
Epidemiological and clinical-laboratory analysis of patients
Of the 49 cases of podocytopathies studied in adults, the majority were male (53.06%) and white (71.43%), with a mean age of 35.4 ± 13.0 years. Among patients with FSGS, 13 (59.1%) were male and 15 (68.2%) were white, and the mean age was 35.1 ± 2.5 years. Of all patients with MCD, 13 (63.6%) were male and 20 (74.1%) were white, while their mean age was 35.6 ± 13.4 years. In the control group, 9 (50.0%) patients were males and 9 (50.0%) were females, and the mean age was 42.8 ± 13.2 years.
Patients from both groups had nephrotic proteinuria, with a mean value of 6.3 ± 4.9 g/24 h in FSGS group and 4.4 ± 4.2 g/24 h in MCD group. The mean serum albumin level was 2.6 ± 0.9 mg/dL in patients with FSGS and 2.7 ± 0.8 mg/dL in those with MCD. The mean serum creatinine level was 1.5 ± 1.0 mg/dL and 1.0 ± 0.5 mg/dL and the average estimated glomerular filtration rate (eGFR) was 72.9 ± 39.9 mL/min/1.73m2 and 88.4 ± 28.4 mL/min/1.73m2 in FSGS and MCD groups, respectively. Hematuria was reported in 6 (27.3%) patients with FSGS group and 10 (37.0%) patients with MCD, and systemic arterial hypertension was present in 16 (72.7%) patients in the FSGS group and in 7 (25.9%) patients in the MCD group.
Morphological analysis of patient biopsies
At least 10 glomeruli per patient were evaluated in LM and in TEM analysis, at least two glomeruli were evaluated per patient and an average of 12.8 ± 5.4 podocytes and 15.8 ± 5.6 glomerular loops were observed with a length of 12552.6 ± 1314.8 nm (Table 2).
Table 2. Morphological analysis.
| Control (n = 18) | FSGS (n = 22) | MCD (n = 27) | |
| Number of WT1-labeled podocytes | |||
| Mean ± SD | 28.3±4.7 | 18.0±7.8 | 18.6±6.4 |
| Median (Min-Max) | 28.0 (19.0–38.0) | 17.0 (7.0–37.0) | 17.5 (9.0–34.0) |
| Control (n = 12) | FSGS (n = 22) | MCD (n = 27) | |
| Number of evaluated podocytes in TEM | |||
| Mean ± SD | 11.0±5.5 | 11.1±4.5 | 13.6±5.7 |
| Median (Min-Max) | 9.5 (4.0–21.0) | 10.5 (5.0–19.0) | 12.0 (4.0–25.0) |
| Number of glomerular loops in TEM | |||
| Mean ± SD | 15.5±6.3 | 15.0±5.2 | 16.2±5.9 |
| Median (Min-Max) | 15.0 (7.0–28.0) | 15.0 (6.0–23.0) | 16.0 (5.0–25.0) |
| Length of glomerular loops in TEM (nm) | |||
| Mean ± SD | 11962.4±1370.9 | 12944.4±998.4 | 12280.0±1458.3 |
| Median (Min-Max) | 11570.6 (10149.3–15151.0) | 13000.0 (11000.0–14000.0) | 12000.0 (11000.0–18000.0) |
| Foot process widht (nm) | |||
| Mean ± SD | 471.0±101.9 | 2503.0±1506.5 | 1488.9±793.4 |
| Median (Min-Max) | 452.1 (395.2-777-3) | 2739.2 (493.0–5550.3) | 1327.6 (521.2–3011.8) |
FSGS, Focal segmental glomerulosclerosis; n, Number of cases; WT1, Antibody anti-human Wilms’tumor 1 protein; SD, Standard deviation; Min, Minimum; Max, Maximum; TEM, Transmission electronic microscopy; nm, Nanometer
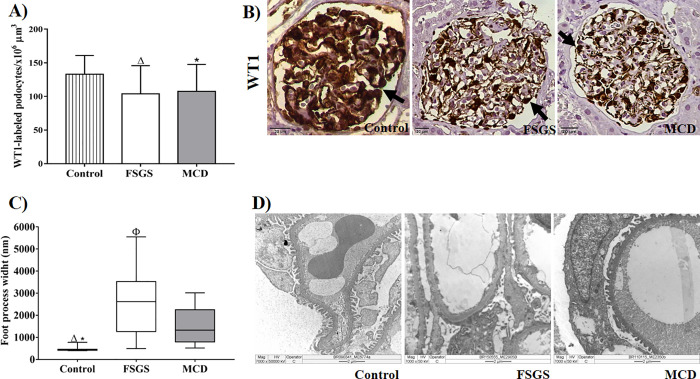
Previous results from our group have shown that podocytes have increased expression of urokinase plasminogen activator receptor (uPAR) in situ in biopsies from adult [8] and pediatric [9] patients with morphological lesions compatible with FSGS. From these findings, we evaluated the possible causes of cellular injury in podocytopathies. As these diseases progress with changes in podocytes, we evaluated the number of podocytes using the expression of WT1 and observed a significant reduction in the density of podocytes both in FSGS and MCD cases as compared to controls (Fig 1A and 1B, p = 0.0430, F = 0.1014, Table 2). We also evaluated the functionality of podocytes by measuring their width. We observed that FPW was higher in the cases of FSGS and MCD than in control group. Among podocytopathies, FPW was greater in FSGS group than in MCD [(Fig 1C and 1D, p<0.0001, H = 29.09), Table 3].
Fig 1. Morphological evaluation of podocytes in renal biopsies of patients with FSGS and MCD.
(A) Density of WT1-labeled podocytes in three groups. ANOVA test followed by Tukey’s multiple comparison test. The bars represent the mean and the vertical lines represent the standard error of the mean. (B) WT1-labeled podocytes in the control, FSGS, and MCD groups (arrows). (C) Difference in the foot process width between control, FSGS and MCD groups showing the extensive foot process effacement in FSGS group. Kruskal-Wallis test followed by Dunn's multiple comparisons test. The horizontal lines represent the medians, bars represent the 25–75% percentiles, and the vertical lines represent the 10–90% percentiles. (D) TEM showing more foot process effacement in a case of FSGS and more foot process preserved in a case of MCD and in a case of control group. Δ: Significant difference between FSGS and control group. *: Significant difference between MCD and control group. Φ: Significant difference between FSGS and MCD group.
Table 3. Podocytes lesions and adaptations analysis in renal biopsies of patients with FSGS and MCD.
| Control (n = 18) | FSGS (n = 22) | MCD (n = 27) | |
| Autophagy evidences | |||
| Number of LC3-labeled podocytes | |||
| Mean ± SD | 25.6±12.4 | 14.5±4.0 | 15.8±5.5 |
| Median (Min-Max) | 20.0 (15.0–52.0) | 14.0 (10.0–24.0) | 16.0 (9.0–27.0) |
| Control (n = 12) | FSGS (n = 22) | MCD (n = 27) | |
| Number of autophagosomes in TEM | |||
| Mean ± SD | 139.2±60.9 | 54.0±25.6 | 79.2±40.3 |
| Median (Min-Max) | 134.0 (47.0–235.0) | 55.0 (10.0–92.0) | 80.0 (20.0–150.0) |
| Number of autophagic vesicles in TEM | |||
| Mean ± SD | 4.0±2.4 | 2.9±2.7 | 4.4±3.4 |
| Median (Min-Max) | 4.0 (0.0–8.0) | 2.0 (0.0–10.0) | 4.0 (0.0–11.0) |
| Apoptosis evidences | |||
| Number of Caspase 3-labeled podocytes | |||
| Mean ± SD | 2.4±0.9 | 7.6±2.9 | 6.9±1.4 |
| Median (Min-Max) | 2.0 (1.0–4.0) | 8.0 (3.0–12.0) | 7.0 (5.0–10.0) |
| Necrosis evidences | |||
| Number of podocytes with oncosis in TEM | |||
| Mean ± SD | 0.8±1.2 | 0.3±0.6 | 0.6±1.0 |
| Median (Min-Max) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) | 0.0 (0.0–4.0) |
| Number of podocytes with nuclear edema in TEM | |||
| Mean ± SD | 0.0±0.0 | 0.3±0.8 | 0.1±0.3 |
| Median (Min-Max) | 0.0 (0.0–0.0) | 0.0 (0.0–3.0) | 0.0 (0.0–1.0) |
| Number of podocytes with cell lysis in TEM | |||
| Mean ± SD | 0.0±0.0 | 0.3±1.0 | 0.0±0.0 |
| Median (Min-Max) | 0.0 (0.0–0.0) | 0.0 (0.0–4.0) | 0.0 (0.0–0.0) |
FSGS, Focal segmental glomerulosclerosis; MCD, Minimal change disease; n, Number of cases; LC3, Antibody anti-human LC3; SD, Standard deviation; Min, Minimum; Max, Maximum; TEM, Transmission electronic microscopy; Caspase 3, Antibody anti-human Caspase 3
Podocytes of FSGS patients show more lesions and less cellular adaptation than those of MCD patients
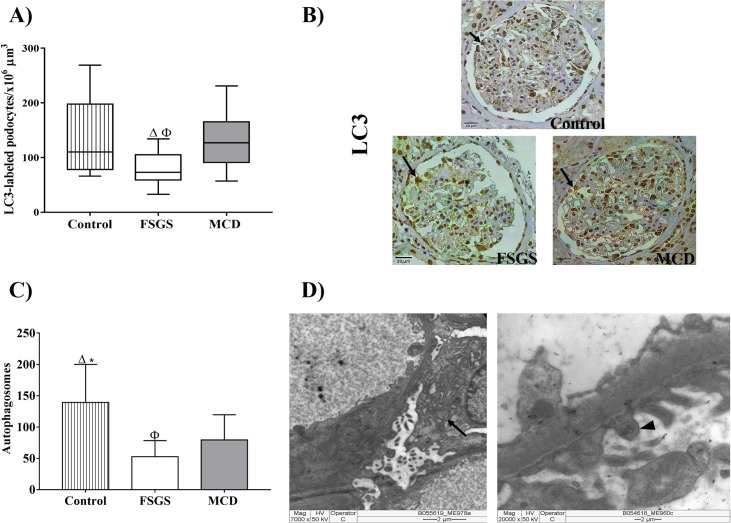
As FSGS group showed a functional reduction in podocytes, we used LM and TEM to find evidences of lesions and cellular adaptations in podocytes. Glomeruli of FSGS patients showed lower density of LC3-labeled podocytes, evidence of autophagy when compared to the control group and the MCD group (Fig 2A and 2B, p = 0.0162, H = 8.243, Table 3). A greater number of autophagosomes was observed in podocytes and pedicels of patients from control group compared with FSGS and MCD groups. Besides, podocytes and pedicels of patients with MCD showed great number of autophagosomes than in those with FSGS (Fig 2C and 2D, p<0.0001, F = 16.15, Table 3), showing that autophagy is less prevalent in podocytes of FSGS cases.
Fig 2. Evidence of podocyte autophagy in renal biopsy of patients with FSGS and MCD.
(A) Density of LC3-labeled podocytes in three groups. ANOVA test followed by Tukey’s multiple comparison test. The bars represent the mean and the vertical lines represent the standard error of the mean. (B) LC3-labeled podocytes in the control, FSGS, and MCD groups (arrows). (C) Difference in the number of autophagosomes between control, FSGS and MCD groups implies higher autophagy in MCD and control group. ANOVA test followed by Tukey’s multiple comparison test. Bars represent the mean and vertical lines represent standard error of the mean. (D) TEM showing autophagosomes characterized as a multivesicle bodies containing undigested cytoplasmic material in podocytes (arrows) and pedicels (arrow head). Δ: Significant difference between FSGS and control group. Φ: Significant difference between FSGS and MCD group.
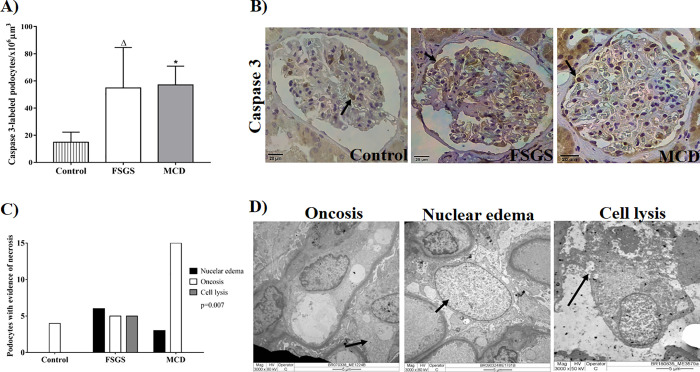
After the analysis of podocyte viability through autophagy, the possible mechanisms underlying cell death in podocytes were evaluated. A higher density of caspase-3-labeled podocytes were observed in glomeruli of patients from the FSGS group and from the MCD group compared to the control group (Fig 3A and 3B, p < 0.0001, F = 23.69, Table 3). Thus, apoptosis-mediated death of podocytes occurs in both podocytopathies. However, a higher number of podocytes with evidence of necrosis such as nuclear edema and cell lysis was observed in FSGS cases than in MCD and control cases (Fig 3C and 3D, p = 0.007, χ2 = 14.09, Table 3). Cell lysis, a morphological characteristic of necrosis, was absent in all MCD and control cases.
Fig 3. Evidence of cell death in renal biopsy of patients with FSGS and MCD.
(A) Density of podocytes labeled with caspase-3 in three groups. ANOVA test followed by Tukey’s multiple comparison test. The bars represent the mean and the vertical lines represent the standard error of the mean. (B) Caspase 3-labeled podocytes in the control, FSGS, and MCD groups (arrows). (C) Morphological evidence of necrosis in podocytes from control, FSGS and MCD group, indicating a higher number of podocytes with nuclear edema and cell lysis in FSGS cases. Chi-square test. (D) TEM illustrating podocytes (arrows) with morphological alterations compatible with necrosis. Δ: Significant difference between FSGS and control group. *: Significant difference between MCD and control group.
Evaluation of impaired podocyte function
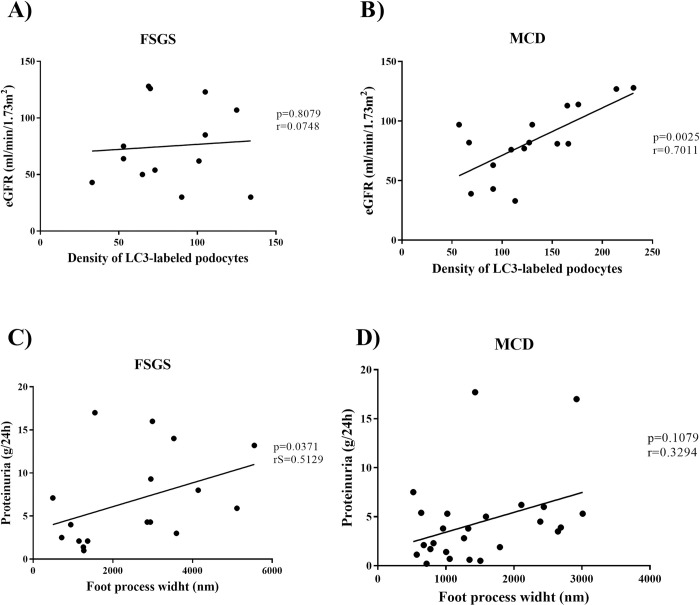
As podocytes from FSGS group showed morphological changes and those from MCD group exhibited better cell adaptation properties, we evaluated the correlation between morphological alterations and relevant clinical data in patients with podocytopathies. A significant positive correlation was detected between eGFR and density of LC3-labeled podocytes in MCD cases (Fig 4B, p = 0.0025, r = 0.7011), which was not observed in FSGS cases (Fig 4A, p = 0.8079, r = 0.0748). Patients with FSGS showed positive and significant correlation between proteinuria levels and FPW (Fig 4C, p = 0.0371, rS = 0.5129) that was absent in patients from MCD group (Fig 4C, p = 0.1079, r = 0.3294).
Fig 4. Evaluation of impaired podocyte function.
Correlation between the estimated glomerular filtration rate and density of LC3-labeled podocytes in (A) FSGS and (B) MCD group. Correlation between proteinuria and foot process width in (C) FSGS and (D) MCD group. r: Pearson's correlation coefficient. rS: Spearman’s correlation coefficient.
This result indicates that proteinuria exhibits a direct relationship with the extension of foot process effacement in FSGS group.
To complement and validate our results, we performed a comparative analysis between sclerotic and non-sclerotic glomeruli. Analyzing all glomeruli available in each sample (with and without sclerosis), a significant reduction in the density of WT1-labeled podocytes both in FSGS and MCD cases was observed compared to control group (p = 0.0390; F = 3.44). Glomeruli of FSGS patients showed lower density of LC3-labeled podocytes compared to the control group and MCD group (p = 0.0297; F = 3.801). A higher density of caspase-3-labeled podocytes were observed in glomeruli of patients from FSGS group and from MCD group compared to control group (p < 0.0001, F = 23.75). Thus, the comparison between sclerotic and non-sclerotic glomeruli showed results similar to those previously reported in the study.
Discussion
The podocytopathies FSGS and MCD are glomerular diseases that are among the main causes of nephrotic syndrome. A podocyte is a primarily damaged cell and the extent of damage may vary [1].
One way to evaluate podocyte loss is through the analysis of specific podocyte markers such as WT1, which is related to the maintenance of podocyte differentiation [2]. We investigated whether there exists any differences in the density of podocytes in renal biopsies of patients with FSGS and MCD through immunostaining for WT1. As a result, we found a significant reduction in the density of WT1-labeled podocytes both in FSGS and MCD cases as compared to controls, but there was no difference between the two podocytopathies. Animal model studies have demonstrated the relationship between reduction in WT1-labeled podocytes and glomerulosclerosis [15]. However, we have previously shown that there is no difference in the density of WT1-labeled podocytes in biopsies of pediatric patients with FSGS and MCD [9]. Unlike other studies, we excluded glomeruli that presented sclerosis. We believe a possible explanation would be that mechanisms of podocyte injury may cause phenotypic podocyte modifications, resulting in reduced expression of markers in situ, such as WT1, in both podocytopathies [16]. In addition, another mechanism would be that parietal epithelial cells (PECs), which are progenitors of podocytes, invade glomerular tuff and replace podocytes. This way, PECs can play a protective role in glomerular diseases, such as FSGS, responding to damage to podocytes with proliferation [17, 18]. Another possibility is that podocytes detach from the glomerular loop in response to hypertrophic stress analogous to the “mitotic catastrophe” model suggested by Lasagni et al. [19] and similar to what is observed in aging human glomeruli [20] which causes a reduction in WT1 immunostaining. Although we have not been able to identify detached podocytes in Bowman's space or tubular lumens by histology, and the number of podocytes per glomerulus was not measurable in association with FSGS development, it is possible that the methods used were not sensitive enough to detect these events in a subset of glomeruli.
The common ultrastructural findings between FSGS and MCD is the foot process effacement resulting from change in the binding complex between GBM and the pedicel, leading to a disorder in actin cytoskeleton [21]. To measure podocyte lesion, the extension of foot process effacement was assessed by the evaluation of mean FPW which was higher in FSGS cases than in MCD cases. Thus, a more diffused foot process effacement was noted in patients with FSGS. This observation has been demonstrated in a quantitative study, wherein the mean of FPW was higher in FSGS cases than in MCD cases [11].
The difference in foot process effacement between FSGS and MCD cases, suggests that podocyte damage in these diseases occurs owing to different mechanisms, which is reinforced by the difference in the expression of some podocyte proteins [6, 7]. It has been reported that the expression of dystroglycan, an adhesion molecule between the podocyte and GBM, is significantly lower in biopsies of patients with MCD than in those of patients with FSGS [22]. Another protein, uPAR, is upregulated in podocytes from cases as compared to those from MCD cases [8, 9] and is related to the activation of β3 integrin, leading to the foot process effacement in FSGS [23]. In this study, the mean FPW showed a positive and significant correlation with the levels of proteinuria in FSGS cases. The mean FPW also positively correlated with proteinuria in cases of proteinuric diseases, including FSGS [24] and IgA nephropathy [25]. However, the relation between foot process effacement and proteinuria is still controversial. Some morphometric studies that evaluated the FPW and its correlation with proteinuria failed to show this relationship, suggesting that proteinuria mainly depends on the nature of the underlying disease and not on the effacement [11, 26]. In experimental models with puromycin aminonucleoside nephrosis, effacement precedes proteinuria [27]. In addition, it has been shown that effacement is associated with narrowing of glomerular filtration slits and development of tight junctions between foot processes [28]. These data suggest that, after foot process effacement by some specific cause, this leads to proteinuria. Therefore, we suggest in our study that, possibly, proteinuria may be associated with impaired function of podocytes, as this clinical sign may reflect the impairment of the selective permeability function of podocytes that favors protein leakage to the urinary space, indicating decreased glomerular function. So, in agreement with another study, we believe that once effacement is initiated, the degree of proteinuria correlates with the degree of effacement [25].
In general, podocyte lesion evolves with better prognosis in MCD but progresses more easily to renal disease at terminal stages in FSGS [29]. Accordingly, we assessed whether podocytes from patients with MCD could better adapt to injury than those from patients with FSGS. We investigated the mechanism underlying podocyte autophagy, a process that maintains cellular homeostasis [30]. We observed that MCD cases showed higher density of LC3-labeled podocytes and higher of autophagosomes than FSGS cases, indicating that podocytes from MCD could adapt better than those from patients with FSGS. Furthermore, we observed a positive and significant correlation between the density of LC3-labeled podocytes and eGFR. Ogawa-Akiyama el al, demonstrated positive areas of LC3 co-located with positive areas of WT1 in immunofluorescence, suggesting the occurrence of autophagy in podocytes of patients with MCD [31]. A study with renal biopsies of patients with MCD and with FSGS showed that podocytes from MCD patients had higher levels of Beclin-mediated autophagic activity1 than podocytes of FSGS patients. In addition, repeated renal biopsies from patients with MCD made it possible to track autophagic activity of podocytes and confirmed patients that maintained high autophagic activity of podocytes maintained the status of MCD, while patients with podocytes with decreased autophagic activity, evolved to FSGS, demonstrating that the autophagic activity of podocytes plays a critical protective role in kidney injury in cases of podocytopathies [32]. So, our findings allow the suggestion that autophagy may be playing a protective role against podocyte lesions in cases of MCD.
Podocyte dysfunction may lead to death due to apoptosis and necrosis [33]. Morphological manifestations of cell death have historically been employed to classify cell death in three different forms. Type I cell death corresponds to apoptosis and is characterized with little or no ultra-structural modifications in cytoplasmic organelles, formation of apoptotic blebs and bodies, maintenance of the integrity of the plasmatic membrane until the final stage, and phagocytosis by resident phagocytes. Type II cell death is defined as an autophagy-dependent cell death with the formation of large-scale autophagic vacuolization, which may cause cell death under specific circumstances; however, autophagy may promote cell survival. Type III cell death or necrosis is morphologically characterized by increased cell volume (oncosis), organelle swelling, rupture of the plasma membrane, and subsequent loss of intracellular content [14].
Podocyte apoptosis has been proposed as a mechanism of podocyte loss and glomerulosclerosis in a transforming growth factor -β1 transgenic mouse model [34], a knockout for CD2AP [35], a puromycin-induced lesion model [15], and mouse and human glomerular epithelial cell cultures [36]. In human samples, in situ apoptosis was observed only in cases of lupus nephritis [37] and IgA nephropathy [38]. In the present study, no difference was observed in the analysis of apoptosis with caspase-3 immunostaining, and this finding differs from the literature that reports the existence of a relationship between apoptosis and glomerulosclerosis. However, apoptosis reported in the literature was observed at a higher frequency in cultured podocytes than in podocytes in vivo which demonstrates a technical difficulty in analyzing apoptosis in situ. Besides caspase 3, caspase 6 and 7 are also apoptotic effector caspases. Thus, another hypothesis that did not find difference in apoptosis among the studied podocytopathies is that this form of cell death may occur through a caspase-3-independent pathway [39, 40].
On the other hand, higher number of podocytes with evidence of necrosis was reported in FSGS cases, proving that these cases, besides presenting fewer cell adaptations, have more lesions. Necrosis as a mechanism of podocyte cell death is less studied, probably owing to technical limitations and difficulties in detecting necrotic structures in vivo. An example of podocyte necrosis was demonstrated in a transplanted kidney biopsy sample from a patient with severe acute renal failure secondary to antibody-mediated rejection, wherein a podocyte with oncosis and cell lysis was observed [41]. Most necrotic podocytes probably detach from the GBM early in the process and are lost in the tubular system [33].
In our study, podocytes from patients with FSGS showed more evidence of cell death and less autophagy than podocytes from patients with MCD, suggesting that in FSGS cases podocytes present more lesions and fewer cell adaptations.
Conclusions
Podocytes of patients with FSGS present fewer cellular adaptations and more morphological and functional changes and that these changes may be related to the mechanisms of cell death by necrosis and caspase 3-independent apoptosis.
Acknowledgments
The authors appreciate the help of the following employees from Federal University of Triangulo Mineiro, General Pathology Discipline and Nephropatology service: Alberto Borba, Edson Santos, João Noberto, Laura Penna, Lívia Alves and Vandair Gonçalves.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors appreciate the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), and Fundação de Ensino e Pesquisa de Uberaba (FUNEPU).
References
- 1.Büscher AK, Weber S. Educational paper: the podocytopathies. Eur J Pediatr. 2012;171(8):1151–60. 10.1007/s00431-011-1668-2 . [DOI] [PubMed] [Google Scholar]
- 2.Mundel P, Reiser J, Kriz W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol. 1997;8(5):697–705. . [DOI] [PubMed] [Google Scholar]
- 3.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89(6):1221–30. Epub 2016/03/19. 10.1016/j.kint.2016.01.012 . [DOI] [PubMed] [Google Scholar]
- 4.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542–52. 10.1038/sj.cdd.4401765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. 10.1007/978-1-59745-157-4_4 . [DOI] [PubMed] [Google Scholar]
- 6.Machado JR, Rocha LP, Neves PD, Cobô EeC, Silva MV, Castellano LR, et al. An overview of molecular mechanism of nephrotic syndrome. Int J Nephrol. 2012;2012:937623 10.1155/2012/937623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aparecida da Silva C, Molinar Mauad Cintra M, de Castro Côbo E, Vinícius da Silva M, Bichuette Custódio F, Rosa Miranda Corrêa R, et al. Renal biopsy: use of biomarkers as a tool for the diagnosis of focal segmental glomerulosclerosis. Dis Markers. 2014;2014:192836 10.1155/2014/192836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva CA, Araújo LS, Dos Reis Monteiro MLG, de Morais Pereira LH, da Silva MV, Castellano LRC, et al. Evaluation of the Diagnostic Potential of uPAR as a Biomarker in Renal Biopsies of Patients with FSGS. Dis Markers. 2019;2019:1070495 Epub 2019/05/02. 10.1155/2019/1070495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira LHM, da Silva CA, Monteiro MLGD, Araújo LS, Rocha LP, Reis MBDR, et al. Podocin and uPAR are good biomarkers in cases of Focal and segmental glomerulosclerosis in pediatric renal biopsies. PLoS One. 2019;14(6):e0217569 Epub 2019/06/12. 10.1371/journal.pone.0217569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, et al. Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol. 2014;25(5):1118–29. Epub 2013/12/19. 10.1681/ASN.2013080859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int. 2008;74(12):1568–76. Epub 2008/08/27. 10.1038/ki.2008.413 . [DOI] [PubMed] [Google Scholar]
- 12.Gundersen HJ, Seefeldt T, Osterby R. Glomerular epithelial foot processes in normal man and rats. Distribution of true width and its intra- and inter-individual variation. Cell Tissue Res. 1980;205(1):147–55. 10.1007/bf00234450 . [DOI] [PubMed] [Google Scholar]
- 13.Liang S, Jin J, Gong J, Lin B, Li Y, He Q. How many podocyte autophagosomes are there in immunoglobulin A nephropathy and idiopathic membranous nephropathy? Int Urol Nephrol. 2016;48(12):2109–14. Epub 2016/08/31. 10.1007/s11255-016-1398-5 . [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. Epub 2008/10/10. 10.1038/cdd.2008.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60(3):957–68. 10.1046/j.1523-1755.2001.060003957.x . [DOI] [PubMed] [Google Scholar]
- 16.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16(4):1013–23. 10.1681/ASN.2004080720 . [DOI] [PubMed] [Google Scholar]
- 17.Shankland SJ, Anders HJ, Romagnani P. Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens. 2013;22(3):302–9. 10.1097/MNH.0b013e32835fefd4 . [DOI] [PubMed] [Google Scholar]
- 18.Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int. 2015;88(5):999–1012. Epub 2015/05/20. 10.1038/ki.2015.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P. Podocyte mitosis—a catastrophe. Curr Mol Med. 2013;13(1):13–23. 10.2174/1566524011307010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O'Connor C, et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol. 2015;26(12):3162–78. Epub 2015/06/02. 10.1681/ASN.2014080752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennon R, Randles MJ, Humphries MJ. The importance of podocyte adhesion for a healthy glomerulus. Front Endocrinol (Lausanne). 2014;5:160 10.3389/fendo.2014.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannico G, Yang H, Neilson EG, Fogo AB. Dystroglycan in the diagnosis of FSGS. Clin J Am Soc Nephrol. 2009;4(11):1747–53. Epub 2009/09/24. 10.2215/CJN.01510209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. 10.1038/nm1696 . [DOI] [PubMed] [Google Scholar]
- 24.Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, et al. Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol. 2003;14(8):2063–71. 10.1097/01.asn.0000078803.53165.c9 . [DOI] [PubMed] [Google Scholar]
- 25.Tewari R, Nada R, Rayat CS, Boruah D, Dudeja P, Joshi K, et al. Correlation of proteinuria with podocyte foot process effacement in IgA nephropathy: an ultrastructural study. Ultrastruct Pathol. 2015;39(2):147–51. Epub 2014/09/30. 10.3109/01913123.2014.960543 . [DOI] [PubMed] [Google Scholar]
- 26.van den Berg JG, van den Bergh Weerman MA, Assmann KJ, Weening JJ, Florquin S. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int. 2004;66(5):1901–6. 10.1111/j.1523-1755.2004.00964.x . [DOI] [PubMed] [Google Scholar]
- 27.Inokuchi S, Shirato I, Kobayashi N, Koide H, Tomino Y, Sakai T. Re-evaluation of foot process effacement in acute puromycin aminonucleoside nephrosis. Kidney Int. 1996;50(4):1278–87. 10.1038/ki.1996.439 . [DOI] [PubMed] [Google Scholar]
- 28.Shirato I. Podocyte process effacement in vivo. Microsc Res Tech. 2002;57(4):241–6. 10.1002/jemt.10082 . [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg AZ, Kopp JB. Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–17. Epub 2017/02/27. 10.2215/CJN.05960616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000;69:303–42. 10.1146/annurev.biochem.69.1.303 . [DOI] [PubMed] [Google Scholar]
- 31.Ogawa-Akiyama A, Sugiyama H, Kitagawa M, Tanaka K, Kano Y, Mise K, et al. Podocyte autophagy is associated with foot process effacement and proteinuria in patients with minimal change nephrotic syndrome. PLoS One. 2020;15(1):e0228337 Epub 2020/01/24. 10.1371/journal.pone.0228337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong Y, et al. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J Pathol. 2014;234(2):203–13. Epub 2014/08/04. 10.1002/path.4382 . [DOI] [PubMed] [Google Scholar]
- 33.Braun F, Becker JU, Brinkkoetter PT. Live or Let Die: Is There any Cell Death in Podocytes? Semin Nephrol. 2016;36(3):208–19. 10.1016/j.semnephrol.2016.03.008 . [DOI] [PubMed] [Google Scholar]
- 34.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108(6):807–16. 10.1172/JCI12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiffer M, Mundel P, Shaw AS, Böttinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem. 2004;279(35):37004–12. 10.1074/jbc.M403534200 . [DOI] [PubMed] [Google Scholar]
- 36.Sanwal V, Pandya M, Bhaskaran M, Franki N, Reddy K, Ding G, et al. Puromycin aminonucleoside induces glomerular epithelial cell apoptosis. Exp Mol Pathol. 2001;70(1):54–64. 10.1006/exmp.2000.2345 . [DOI] [PubMed] [Google Scholar]
- 37.Cui JH, Qiao Q, Guo Y, Zhang YQ, Cheng H, He FR, et al. Increased apoptosis and expression of FasL, Bax and caspase-3 in human lupus nephritis class II and IV. J Nephrol. 2012;25(2):255–61. 10.5301/JN.2011.8451 . [DOI] [PubMed] [Google Scholar]
- 38.Tashiro K, Kodera S, Takahashi Y, Horikoshi S, Shirato I, Tomino Y. Detection of apoptotic cells in glomeruli of patients with IgA nephropathy. Nephron. 1998;79(1):21–7. 10.1159/000044986 . [DOI] [PubMed] [Google Scholar]
- 39.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol. 2005;16(9):2615–25. 10.1681/ASN.2005020142 . [DOI] [PubMed] [Google Scholar]
- 40.Mohr S, McCormick TS, Lapetina EG. Macrophages resistant to endogenously generated nitric oxide-mediated apoptosis are hypersensitive to exogenously added nitric oxide donors: dichotomous apoptotic response independent of caspase 3 and reversal by the mitogen-activated protein kinase kinase (MEK) inhibitor PD 098059. Proc Natl Acad Sci U S A. 1998;95(9):5045–50. 10.1073/pnas.95.9.5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liapis H, Romagnani P, Anders HJ. New insights into the pathology of podocyte loss: mitotic catastrophe. Am J Pathol. 2013;183(5):1364–74. Epub 2013/09/03. 10.1016/j.ajpath.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.