Abstract
The exact relationship between cognitive functioning, cortical excitability, and synaptic plasticity in dementia is not completely understood. Vascular cognitive impairment (VCI) is deemed to be the most common cognitive disorder in the elderly since it encompasses any degree of vascular-based cognitive decline. In different cognitive disorders, including VCI, transcranial magnetic stimulation (TMS) can be exploited as a noninvasive tool able to evaluate in vivo the cortical excitability, the propension to undergo neural plastic phenomena, and the underlying transmission pathways. Overall, TMS in VCI revealed enhanced cortical excitability and synaptic plasticity that seem to correlate with the disease process and progression. In some patients, such plasticity may be considered as an adaptive response to disease progression, thus allowing the preservation of motor programming and execution. Recent findings also point out the possibility to employ TMS to predict cognitive deterioration in the so-called “brains at risk” for dementia, which may be those patients who benefit more of disease-modifying drugs and rehabilitative or neuromodulatory approaches, such as those based on repetitive TMS (rTMS). Finally, TMS can be exploited to select the responders to specific drugs in the attempt to maximize the response and to restore maladaptive plasticity. While no single TMS index owns enough specificity, a panel of TMS-derived measures can support VCI diagnosis and identify early markers of progression into dementia. This work reviews all TMS and rTMS studies on VCI. The aim is to evaluate how cortical excitability, plasticity, and connectivity interact in the pathophysiology of the impairment and to provide a translational perspective towards novel treatments of these patients. Current pitfalls and limitations of both studies and techniques are also discussed, together with possible solutions and future research agenda.
1. Introduction
1.1. Vascular Cognitive Impairment
Globally, vascular cognitive impairment (VCI) is defined as a decline in cognition due to cerebrovascular injury. It is currently viewed as an “umbrella term” encompassing mild VCI, vascular dementia (VaD), and mixed dementia [1–3]. Mild VCI is a decline in cognition not fully satisfying the diagnostic criteria for dementia [4, 5]. VaD identifies cognitively impaired patients who have lost their functional independence due to vascular lesions and includes different subtypes, such as poststroke dementia, multi-infarct dementia, strategic infarct dementia, and the subcortical ischemic VaD. Finally, mixed dementia is the result of both vascular and degenerative pathophysiology, most commonly of Alzheimer's disease- (AD-) type [6]. Hence, VCI is deemed to be the most common cognitive disorder in the elderly, with a growing impact on patients' quality of life (QoL) and on social and healthcare system [2]. Moreover, vascular-derived impairment has a great prevalence in all types of cognitive decline, where its contribution to the deficits is considerable. Of note, this is the only contribution that can be, at least in part, treatable and preventable [7, 8].
In addition to the affected cognitive domains, which typically are attention, processing speed, and executive functioning [9], VCI can impact also on several neuropsychiatric aspects, such as behavioral and mood disturbances, making this disorder extremely heterogeneous [10–12]. Apathy, irritability, disinhibition, and psychomotor retardation are common examples of the behavioral changes found in VCI patients, while depression is the most reported mood disorder. Behavioral and mood changes correlate with the worsening of cognitive and functional status and significantly reduce the QoL of patients and caregivers [13–15].
The pathophysiology accounting for cognitive and behavioral-mood dysfunction in VCI is still not completely defined. The so-called “disconnection hypothesis,” based on the analysis of brain images of large samples [16, 17], points to the result of a “disruption” of cortical and/or subcortical loops implicated in cognition and mood-affect regulation, due to acute of chronic cerebrovascular lesions [18–20]. In magnetic resonance imaging (MRI) of stroke or cerebral small vessel disease, ischemic white matter lesions (WMLs) are clinically associated with cognitive impairment [21]. In large community-based populations [16, 22, 23], WMLs are also associated with nonmotor sequelae, and cognitive and mood-behavior impairment was especially linked with the ischemic disruption of the prefrontal cortical-subcortical circuits [24]. In stroke survivors, the atrophy of the medial temporal lobe predicts early cognitive dysfunction [25]. Even subcortical ischemic vascular disease, including silent lacunar infarcts and WMLs, is associated with executive dysfunction and late-life depression, which is a clinical and neuroimaging condition now referred as “vascular depression” [20]. Taken together, it has been clearly established that cognitive limitations and depressive disorders are tightly intertwined in patients with both acute and chronic cerebrovascular diseases, such as stroke and small vessel disease, respectively [9, 11, 13, 26].
VCI diagnosis must capitalize from clinical and neuropsychological evaluations, as well as from structural and functional neuroimaging [11]. However, the search for novel hallmarks of disease process and progression, such as serological, cerebrospinal fluid (CSF), and instrumental markers, is needed to allow an early, tailored, and accurate screening of VCI patients. This will also pave the way to innovative therapeutic strategies and to the identification of predictors of drug response [27, 28]. Moreover, the noninvasively investigation of cortical circuitry in VCI patients has produced intriguing findings on abnormal cortical connectivity [29] and plasticity [30, 31].
Overall, neural plasticity refers to the brain's ability, particularly of the cerebral cortex, of reorganizing and adapting to different constantly changing environmental stimuli. This takes place through phenomena of modification of synaptic connection strength (like long-term potentiation (LTP) and long-term depression (LTD)), modification of the representation pattern and neuronal activity, modulation of gene induction and expression, changes in cerebral blood flow, and neurotrophin release [32]. Neural plasticity is an essential substrate for learning and memory [33], and its involvement in dementia (such as AD), movement disorders (such as Parkinson's disease), and neuropsychiatric disorders (such as major depression) [34] is well documented. Although abnormalities in brain plasticity and its components have been widely demonstrated in dementia, their role in the pathophysiology of VCI and in the counteraction against disease progression is still not understood. In this scenario, the contribution of noninvasive and translational brain stimulation techniques, namely, transcranial magnetic stimulation (TMS), is becoming of pivotal importance.
1.2. Transcranial Magnetic Stimulation
From the pioneering application of TMS to assess the primary motor cortex (M1) and the cortical-spinal conductivity [35], scientists boost the potentialities of this technique, which is employed today to study cortical excitability, to map connectivity, and to probe the propensity to undergo plastic phenomena [36]. This gives novel insights into the pathophysiology underlying several neurological and neuropsychiatric diseases [37, 38].
A functional assessment of global cortical excitability and cortico-spinal conduction results from the application of single magnetic pulses at adequate stimulator intensity over the M1 that elicits a motor evoked potential (MEP) recordable on the contralateral target muscle [39, 40]. The MEP amplitude is an aggregate measure of the excitation state of M1's output cells, nerve roots, and the conduction along the peripheral motor nerves till the muscles [41]. The resting motor threshold (rMT), i.e., the minimum intensity of stimulation needed to evoke a MEP, is a basic index of M1 excitability, as it is a compound measure of the membrane excitability of cortical neurons, the neural inputs into pyramidal cells within the cortex, and the excitability of spinal motor neurons, neuromuscular junctions, and muscles [42].
During a tonic contraction of the contralateral muscles, the result of a suprathreshold TMS pulse applied to the M1 is a few hundred milliseconds suppression of the electromyographic (EMG) activity of those muscles [43]. This phenomenon, called contralateral cortical silent period (cSP), is exploited as a functional measure of intracortical inhibitory circuits [44, 45], mainly mediated by the gamma-aminobutyric acid- (GABA-) B transmission [46]. Conversely, activation of the muscle and stimulation of the hemisphere of the same side evoke the ipsilateral silent period (iSP), which it is thought to receive some modulated effects from transcallosal output neurons that project to contralateral GABAergic interneurons [47, 48].
Paired-pulse TMS paradigm allows the assessment of the short-interval intracortical inhibition (SICI) and the intracortical facilitation (ICF) of the motor response [49, 50]. The activity of GABA-A interneurons is the most likely mediator of SICI [51, 52], whereas the neurophysiology of ICF is more complex. It probably relates to the activation of a cortical circuit projecting upon cortico-spinal cells different from that preferentially activated by single-pulse TMS. ICF seems dependent to a great extent on the activity of glutamatergic excitatory interneurons, although other mediators are known to contribute [53, 54].
Researchers have also the possibility of investigating the sensory-motor interactions in the cerebral cortex by using specific TMS protocols. One of these allows for the investigation of the short-latency afferent inhibition (SAI), which mainly reflects the central cholinergic circuits' integrity [55]. Indeed, while the muscarinic antagonistic scopolamine in healthy subjects reduces or abolishes SAI [56], acetylcholine positively modulates it [57]. It has been suggested that SAI may depend on the integrity of circuits linking sensory input and motor output [58, 59], thus providing valuable diagnostic information in a variety of cognitive and movement disorders [60, 61]. Finally, TMS also allows the study of synaptic plasticity through different paradigms of paired-associative stimulation (PAS), e.g., by applying a magnetic stimulus after a brief period of exercise or by using repetitive low-frequency median nerve stimulation combined with TMS over the contralateral M1 [62]. PAS has shown to lead to LTP-/LTD-like changes within the sensory-motor pathways [63].
Figure 1 schematically illustrates the technical aspects and the neurophysiological correlate of SICI, ICF, SAI, PAS, and repetitive TMS (rTMS).
Figure 1.
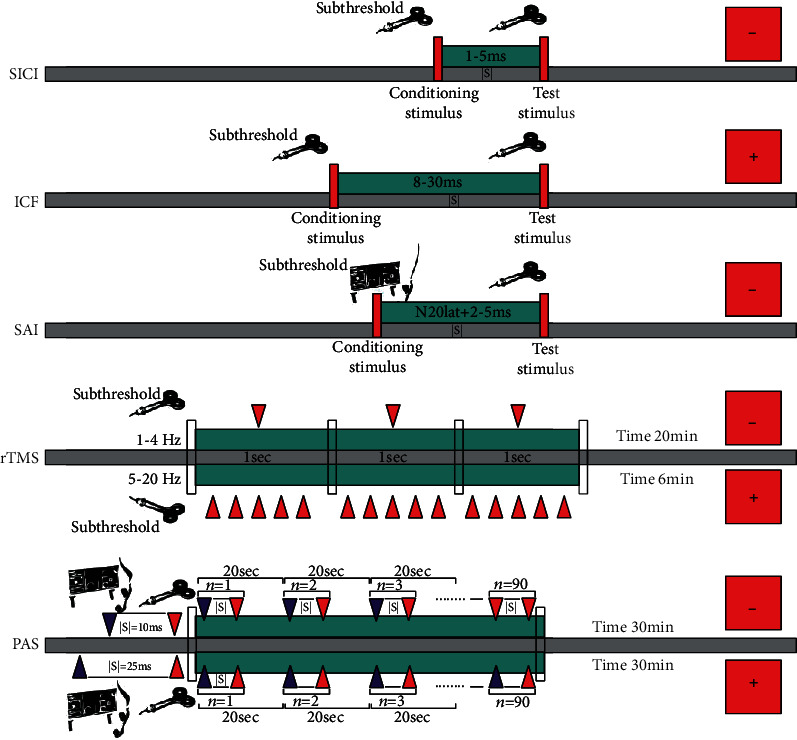
Schematic representation of some TMS measures and protocols of stimulation. Legend (in alphabetic order): ICF: intracortical facilitation; ISI: interstimulus interval; SAI: short-latency afferent inhibition; PAS: paired-associative stimulation; rTMS: repetitive transcranial magnetic stimulation; SICI: short-interval intracortical inhibition; +: facilitatory/excitatory effect; -: inhibitory/suppressive effect.
1.3. Repetitive TMS
Repetitive TMS (rTMS) over the same cortical target induces a transient modification of the cortex excitability, which decreases by using low frequencies (≤1 Hz) and increases by using high frequencies (5-20 Hz) [64]. The neurobiology of rTMS seems to share many features with LTD and LTP's induction by tetanic stimulation in cortical slices [65], such as the dependence from N-methyl-D-aspartate- (NMDA-) receptor activity [66], the sensibility to prior synaptic activation [67], and the strict link with stimulation frequency [68]. The short-term changes in synaptic efficacy and the rapid downregulation of GABA-related inhibitory circuits are key processes of calcium- and sodium channel-dependent LTP plasticity [69, 70]. Conversely, by inducing LTD-like responses, rTMS decreases the synaptic efficacy [71, 72].
The effects of repeated sessions of rTMS persist in time and act by enhancing plasticity when needed but also by downregulating it when plasticity becomes inappropriate or even maladaptive [73]. For all those reasons, the translational therapeutic and rehabilitative applications of rTMS may cover a wide range of neurological and psychiatric disorders [74, 75]. Accordingly, in October 2008, the Food and Drug Administration (FDA) approved rTMS as an add-on treatment of adult drug-resistant major depressive disorder (MDD). Besides, specific rTMS paradigms, like the theta-burst stimulation [76] or the quadripulse stimulation [77], may help in a better comprehension of synaptic plasticity phenomena or even more complex responses, such as the metaplasticity (i.e., “plasticity of synaptic plasticity”) [78–80].
Overall, rTMS is safe and well tolerated. A discomfort caused by scalp or facial muscle twitching and transient headache are the most commonly reported side effects [81], while the induction of seizures is a very rare but serious adverse effect, although not common even employing supratherapeutic stimulations [82]. Nevertheless, epileptic patients or those with risk factors of epilepsy should be managed with extreme caution.
1.4. Aim e Rationale
To date, the exact relationship between cognitive functioning, motor cortical excitability, and synaptic plasticity in VCI is not completely unveiled. In this work, we review all the TMS and rTMS studies related to VCI to provide a timely translational perspective on how cortical excitability and network connectivity interact to determine the pathophysiology and plastic changes in VCI and its subtypes, and how these findings may be exploited by experimental treatments. Current pitfalls and limitations of both studies and techniques are also discussed, together with possible solutions and future research agenda.
2. Methods
A literature search was carried out to find all the relevant studies of TMS and rTMS in VCI. A PubMed-based literature review was performed by using the following search queries:
(“transcranial magnetic stimulation” [MeSH Terms] OR (“transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “transcranial magnetic stimulation” [All Fields] OR (“repetitive” [All Fields] AND “transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “repetitive transcranial magnetic stimulation” [All Fields]) AND “vascular” [All Fields] AND (“cognitive dysfunction” [MeSH Terms] OR (“cognitive” [All Fields] AND “dysfunction” [All Fields]) OR “cognitive dysfunction” [All Fields] OR (“cognitive” [All Fields] AND “impairment” [All Fields]) OR “cognitive impairment” [All Fields])
(“transcranial magnetic stimulation” [MeSH Terms] OR (“transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “transcranial magnetic stimulation” [All Fields] OR (“repetitive” [All Fields] AND “transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “repetitive transcranial magnetic stimulation” [All Fields]) AND (“dementia, vascular” [MeSH Terms] OR (“dementia” [All Fields] AND “vascular” [All Fields]) OR “vascular dementia” [All Fields])
(“transcranial magnetic stimulation” [MeSH Terms] OR (“transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “transcranial magnetic stimulation” [All Fields] OR (“repetitive” [All Fields] AND “transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “repetitive transcranial magnetic stimulation” [All Fields]) AND “vascular” [All Fields] AND (“depressive disorder” [MeSH Terms] OR (“depressive” [All Fields] AND “disorder” [All Fields]) OR “depressive disorder” [All Fields] OR “depression” [All Fields] OR “depression” [MeSH Terms])
(“transcranial magnetic stimulation” [MeSH Terms] OR (“transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “transcranial magnetic stimulation” [All Fields] OR (“repetitive” [All Fields] AND “transcranial” [All Fields] AND “magnetic” [All Fields] AND “stimulation” [All Fields]) OR “repetitive transcranial magnetic stimulation” [All Fields]) AND (“cadasil” [MeSH Terms] OR “cadasil” [All Fields])
Two independent authors (FF and MP) screened titles and abstracts of all retrieved publications, and disagreements were solved by the consensus of a third author (RB). Duplicated entries, retracted publications, studies on other diseases different from VCI or its subtypes, works on animals or in vitro, studies without statistical analysis, non-English written papers, publications that are not research studies (i.e., commentaries, letters, editorials, and reviews), and any other article that did not fit with the scope of this review were excluded. Articles listed in the references were also reviewed in search of more data. We considered studies indexed from the database inception to April 2020.
3. Results and Discussion
A total of 77 results were originally retrieved. Of these, 20 peer-reviewed publications were selected according to the above inclusion and exclusion criteria. The examination of the references from relevant papers detected 5 additional studies fitting the purpose of this review. Eventually, a total of 25 papers were included (Figure 2), and their main findings were analyzed clustering within two groups, one on TMS studies (summarized in Table 1) and the other on rTMS studies (summarized in Table 2). More in details, we included in the TMS group 6 studies on mild VCI [30, 31, 83–86], 6 on VaD [87–92], 3 on vascular depression [93–95], and 4 on cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) [96–99], while the rTMS studies group consisted of 2 articles in mild VCI [100, 101] and 4 in vascular depression [102–105].
Figure 2.

Flow diagram showing the search strategy, the number of records identified, and the number of included/excluded studies [106]. This figure is reproduced from Moher, David et al. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535 (under the Creative Commons Attribution License/public domain).
Table 1.
TMS studies in patients with vascular cognitive impairment.
| VCI subtype | Study, year | Study design | Patients n | Main findings |
|---|---|---|---|---|
| Mild VCI | Bella et al., 2011 [30] | Cross-sectional | 10 | ↑ Intracortical excitatory neuronal circuits |
| Bella et al., 2013 [31] | Case-control | 9 | ↑ Excitability during the progression of VCI | |
| Lanza et al., 2013 [83] | Cross-sectional | 15 | = Transcallosal inhibitory functioning, unlike AD and mild cognitive impairment | |
| List et al., 2013 [84] | Cross-sectional | 20 | ↑ Cortical plasticity as a compensatory mechanism | |
| List et al., 2014 [85] | Cross-sectional | 12 | ↓ LTP-like plasticity in the affected hemisphere = Motor learning between hemispheres, maybe due to a GABA-B effect in the affected side |
|
| Bella et al., 2016 [86] | Cross-sectional | 25 | Central cholinergic pathway not clearly affected | |
|
| ||||
| Vascular dementia | Alagona et al., 2004 [87] | Cross-sectional | 20 AD 20 SIVD 20 HC |
↓ Motor threshold in SIVD compared to AD and HC |
| Di Lazzaro et al., 2008 [88] | Cross-sectional | 12 VaD 12 AD 12 HC |
= Short-latency afferent inhibition in VaD patients and significantly reduced in AD | |
| Nardone et al., 2008 [89] | Cross-sectional | 20 SIVD 25 HC |
↓ Mean short-latency afferent inhibition in patients | |
| Pennisi et al., 2011 [90] | Cross-sectional | 20 VaD 20 mild VCI |
↑ Cortical excitability in demented patients only | |
| Nardone et al., 2011 [91] | Cross-sectional | 28 | Microbleeds on cholinergic function are independent of white matter lesion extent and ischemic stroke | |
| Guerra et al., 2015 [92] | Cross-sectional | 7 VCI 9 AD |
↑ Excitability and plasticity in AD and VaD Hyperexcitability promoted plasticity |
|
|
| ||||
| Vascular depression | Bella et al., 2011 [93] | Cross-sectional | 15 MDD 10 non-depressed |
Neurophysiology of vascular depression differs from MDD, and it is similar to that of subcortical ischemic vascular disease |
| Concerto et al., 2013 [94] | Cross-sectional | 11 depressed 11 MDD |
Different patterns of cortical excitability between late-onset vascular depression and early-onset nonvascular MDD | |
| Pennisi et al., 2016 [95] | Case-control | 16 MDD 11 nondepressed |
↑ Risk of dementia in vascular depression, probably due to subcortical vascular burden or to the lack of compensatory functional cortical changes | |
|
| ||||
| CADASIL | Manganelli et al., 2008 [96] | Cross-sectional | 10 CADASIL 10 HC |
↓ Short-latency afferent inhibition in patients ↓ Resting motor threshold significantly reduced in patients |
| List et al., 2011 [97] | Cross-sectional | 12 CADASIL 10 HC |
↑ Cortical plasticity in patients compared to HC | |
| Palomar et al., 2013 [98] | Cross-sectional | 10 | Acetylcholine and glutamate were involved Abnormal sensory-motor plasticity correlated with cognition |
|
| Nardone et al., 2014 [99] | Cross-sectional | 8 CADASIL 8 AD |
↓ Cholinergic functioning, with restoration by L-3,4-dihydroxyphenylalanine in AD group only | |
Legend (in alphabetical order): AD: Alzheimer's disease; CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; GABA: gamma-aminobutyric acid; HC: healthy controls; LTP: long-term potentiation; MDD: major depressive disorder; n: patients' number; SIVD: subcortical ischemic vascular disease; VaD: vascular dementia; VCI: vascular cognitive impairment; ↑: increase/enhancement; ↓: decrease/reduction; =: no significant change/modification.
Table 2.
Repetitive TMS studies in patients with vascular cognitive impairment.
| VCI subtype | Study, year | Study characteristics | Main findings |
|---|---|---|---|
| Mild VCI | Rektorova et al., 2005 [100] | Type of study: randomized, controlled, blinded, crossover Subjects: 7 Type of coil: figure-of-eight coil (7 cm diameter) Stimulation site: left DLPFC (active), left M1 (control) Stimulation frequency: 10 Hz Intensity: 100% rMT Length: 3 rTMS blocks, separated by a 10 min interval; in each block, fifteen 10-pulse trains, each of 1 s duration, delivered with an intertrain interval of 10 s Duration: 1 session Total number of pulses delivered: 450 |
Significant positive effect of active stimulation on the Stroop color-word interference test |
| Sedlackova et al., 2008 [101] | Type of study: randomized, controlled, blinded, crossover Subjects: 7 Type of coil: figure-of-eight coil (7 cm diameter) Stimulation site: left DLPFC (active), left M1 (control) Stimulation frequency: 1 Hz; 10 Hz Intensity: 100% rMT Length: for the 10 Hz stimulation: 3 rTMS blocks, separated by a 10-minute interval; in each block, fifteen 10-pulse trains, each of 1 s duration, delivered with an intertrain interval of 10 s; for the 1 Hz stimulation: continuous Duration: 4 sessions (two at 1 Hz and two at 10 Hz) Total number of pulses delivered: 450 at 10 Hz; 1,800 at 1 Hz |
Significant improvement in the Stroop color-word interference test after the stimulation of DLPFC but not M1; improvement in the digit symbol subtest of the Wechsler Adult Intelligence Scale-revised after rTMS, regardless of the stimulation site. No measurable effect in any other neuropsychological test | |
|
| |||
| Vascular depression | Fabre et al., 2004 [102] | Type of study: open trial Subjects: 11 Type of coil: figure-of-eight coil Stimulation site: left prefrontal cortex Stimulation frequency: 10 Hz Intensity: 100% rMT Length: twenty 8 s trains, with 52 s intertrain intervals Duration: 10 sessions over two weeks |
Five out of 11 patients responded to rTMS in terms of clinically meaningful improvement in HDRS scores, with a decrease by at least 25% from baseline; improvement of verbal fluency, visuospatial memory, and delayed recall |
| Jorge et al., 2008 [103] | Type of study: prospective, randomized, sham-controlled Subjects: 92, randomized in active (48) and sham (44) groups; experiment 1: two groups of 15 patients each; experiment 2: 33 “real” patients and 29 sham patients Type of coil: figure-of-eight coil (7 cm diameter) Stimulation site: left DLPFC Stimulation frequency: 10 Hz Intensity: 110% rMT Length: 30 minutes Duration: 10 sessions (experiment 1), 15 sessions (experiment 2), 6 s period of stimulation, with a total of 20 trains separated by 1 min pauses, over 10 days Total number of pulses delivered: 12,000 (experiment 1); 18,000 (experiment 2) |
Experiment 1: significant decrease in HDRS scores for real stimulation compared to sham; experiment 2: significant decrease in HDRS scores, increase in response rates, and remission rates for real stimulation compared to sham. Response rates to rTMS negatively correlated with age and positively correlated with higher frontal gray matter volume | |
| Robinson et al., 2009 [104] | Same patients and protocol of the experiment 2 of the study by Jorge and colleagues (2008) [103]. After rTMS or sham treatment, all responders were given citalopram for 9 weeks | Among the 33 “real” patients, 13 responded (>50% decrease in HDRS score); among them, 9 patients continued to be responders whereas the reaming 4 had a relapse of depression during the course of citalopram treatment | |
| Narushima et al., 2010 [105] | Type of study: prospective, randomized, sham-controlled Subjects: 43, randomized in “real” (32 patients) and “sham” (11 patients) groups Type of coil: figure-of-eight coil (7 cm diameter) Stimulation site: left DLPFC Stimulation frequency: 10 Hz Intensity: 110% rMT Duration: 10 sessions, 6 s period of stimulation, with a total of 20 trains separated by 1 min pauses, performed over 10 days Total number of pulses delivered: 12,000 (12 patients)–18,000 (31 patients) |
Significant difference in the response and remission rate of the HDRS scores between active and sham groups, in favor of the “real” stimulation group; increased low-theta power in the subgenual cingulate predicted the response to rTMS | |
Legend (in alphabetical order): DLPFC: dorsolateral prefrontal cortex; HDRS: Hamilton depression rating scale; M1: primary motor cortex; rMT: resting motor threshold; rTMS: repetitive transcranial magnetic stimulation; VCI: vascular cognitive impairment.
3.1. Mild Vascular Cognitive Impairment
The identification of mild VCI subjects at risk for clinical progression into VaD or mixed dementia is a crucial challenge for both clinicians and researchers because it may raise the chances to early diagnose and to delay the disease progression.
A previous study on nondemented elderly patients with subcortical ischemic vascular disease and clinical-cognitive profile of mild VCI [30] found that prefrontal subcortical loops lesioned by the ischemic interruption due to WMLs or lacunar infarcts lead to functional changes of the intracortical excitatory neuronal circuits (i.e., increased ICF). In this patient class, a further study has also shown that iSP is spared [83], unlike neurodegenerative disorders, such as AD and mild cognitive impairment (MCI), that show abnormal iSP since the early stages. This suggests a functional integrity of the transcallosal inhibitory connections in VCI, at least in the early phase [107].
A TMS study carried out on the same participants after a 2-year follow-up [31] found that, compared to the baseline, patients exhibited an increased global cortical excitability (reduction of the median rMT) and a significant worsening of the score of neuropsychological tests evaluating the frontal lobe abilities. The researchers interpreted the findings as indicative of plastic compensatory mechanisms in response to cortical disconnection [31, 108]. In particular, the study hypothesizes that rMT might become abnormal when VCI progresses to VaD and that its value can be used as a “neurophysiological cut-off” segregating patients who will progress from those who will remain cognitively stable. It is known, indeed, that higher motor cortex facilitation marks higher risk to convert from normal aging brain to cognitive impairment up to an overt dementia [31]. These findings are in line with the observation of enhanced cortical plasticity and reorganization, probably as compensatory mechanisms due to impaired cerebral autoregulation, in nondemented patients with severe ischemic small vessel disease [84, 85].
Notably, mild VCI individuals do not show impaired cholinergic activity compared to age-matched controls [86], which might suggest a distinctive cholinergic profile characterizing the early stages of VaD and differentiating it from the “cholinergic” forms of cognitive decline, such as MCI and AD [109]. However, cholinergic involvement in VaD is still under debate, and the few available TMS data show conflicting results [88, 89, 91]. The high heterogeneity in the location and severity of subcortical infarcts, leading to variations in the resultant distribution and magnitude of the cholinergic denervation, may be a reasonable explanation [91]. Finally, SAI might be useful in the identification of responders to the acetylcholinesterase inhibitors and, indirectly, in the differentiation between “cholinergic” and “non-cholinergic” forms of dementia [86].
3.2. Vascular Dementia
A common feature of AD and VaD patients is the increase of M1 excitability, (i.e., reduction of rMT), which differentiate them from normal brain aging [87]. Different studies converge on the hypothesis that an enhanced excitability and plasticity seems to have a role in counteracting cognitive decline in the elderly [110] as a compensatory response to neuronal loss and vascular injury [111]. However, this likely hypothesis warrants future experimental investigations on longitudinal studies and further clinical-pathological correlations. In AD patients, a reorganization of cortical functions has been reported since the early stages, likely due to the occurrence of a frontal and medial shift of the “center of gravity” of the TMS-based cortical motor map representations [112, 113]. A similar pattern has been shown also in subcortical ischemic VaD, which identifies a homogenous subtype of patients characterized by insidious onset, gradual course, and relatively slow progression, which make them hard to differentiate them from AD patients [114].
Although much less is known, plastic phenomena have been also reported to take place also in VaD. While exploring the relationship between excitability and plasticity in subcortical ischemic VaD, a cross-sectional study found that M1 had enhanced excitability in both AD and subcortical ischemic VaD patients, and more interestingly, M1 was plastically rearranged in both groups [92]. The results demonstrated indeed a functional cortical reorganization of all patients, with a slightly smaller frontal shift in the center of gravity for subcortical ischemic VaD compared to AD. A direct correlation between parameters of cortical excitability and those associated with the topographic shift of cortical maps was also noted [92]. Authors hypothesized that partially overlapping electrophysiological mechanisms probably act in the same manner in both VaD and AD, although they may differ both in location (subcortical vs. cortical) and origin (vascular vs. degenerative). Therefore, these disorders might share a common neurophysiological platform represented by a progressive neuronal loss in the motor areas in AD and a vascular disconnection in the white matter in subcortical ischemic VaD [115]. Eventually, these alterations will promote a functional brain rearrangement allowing to preserve motor programming and execution [84, 85].
Neurochemically, the reduction of rMT in both VaD and AD might represent a marker of impaired glutamatergic transmission, with an imbalance between non-NMDA and NMDA activity [116, 117]. Coherently, enhanced cortical excitability has been observed after the administration of an NMDA antagonist [118]. However, the facilitation of cortico-spinal outputs might also be caused by reduced intracortical inhibition [33]. Indeed, an increased GABA release may be a response to an overactivation of glutamate as part of the neuronal defense mechanisms leading to the compensation for excitotoxicity [119]. However, the studies here reviewed did not find significant changes of the TMS-related measures of inhibition, such as cSP, iSP, and SICI, while a significant SAI reduction was found in subcortical ischemic VaD [89]. In a different study, however, the reduction of SAI was noted in AD but not in VaD, apart from 25% of VaD patients that probably had a mixed dementia [88]. Even microbleeds in subcortical ischemic VaD might to have an impact on SAI-related cholinergic pathways, which was independent of the WMLs extent and ischemic stroke [91].
3.3. Vascular Depression
TMS studies are in line with the other findings in classifying vascular depression as a distinct nosologic entity, different from early-onset MDD [94]. In vascular depression, depressive symptoms, rather than signs of a primary disease status, are part of the wide spectrum of clinical presentations of the subcortical cerebrovascular disease [93]. Another difference between geriatric vascular depression and early-onset MDD is the enhancement of ICF observed only in former [93, 94]. According to the vascular depression hypothesis, this finding may imply that the disruption of the frontal-striatal circuits caused by vascular lesions may predispose, precipitate, or perpetuate a late-life depression [120].
However, from a neurophysiological perspective, very little is known on plasticity preserving cognitive functions in geriatric depression. By investigating the evolution of neurophysiological parameters in nondepressed patients with mild VCI and those with vascular depression, it has been shown that only nondepressed patients had a high level of ICF at the initial TMS evaluation [95]. At follow-up, a glutamate-related enhanced plasticity may have taken place in nondepressed patients that might be protective against cognitive deterioration, giving also cues on the possible role played by the late-life depression in the progression of VCI. Further, reduced rMT in both patient groups at follow-up points to the glutamatergic neurotransmission involvement. However, no specific change of neurophysiological parameter correlated with cognitive decline in depressed patients, suggesting that cognitive deterioration in vascular depression might be related to the subcortical lesion load or to the lack of compensatory cortical inputs [95].
3.4. CADASIL
The mutations in the Notch3 gene on chromosome 19 causes CADASIL, that manifests with progressive cognitive decline till dementia, migraine, psychiatric disorders, and cerebral ischemic events. For this reason, it represents a genetic model of VaD that is interesting to study from a neuropsychologic and electrophysiological point of view [121].
In the first TMS study, a reduction in rMT and SAI [96] was found and attributed to the disruption of different cortical-subcortical circuits caused by vascular lesions and locations [122], such those affecting the external capsule [123]. Regarding SAI, the significant reduction observed in both AD and CADASIL may be due to the involvement of different pathways, in that the L-3,4-dihydroxyphenylalanine (L-DOPA) administration was able to restore SAI only in AD [99], thus also providing therapeutic insights.
CADASIL patients also present an impaired sensory-motor plasticity induced by PAS [97]. Further, an association between WMLs load and lowered fractional anisotropy, along with an abnormal enhancement of LTP-like plasticity induced by PAS, has been observed particularly in the frontal commissural fibers. The authors' suggestion was that the increase in cortical plasticity might compensate the deterioration of cognitive and motor functions [97]. However, older patients with impaired cognition manifested opposite results, with a lower PAS-induced cortical plasticity, as well as a reduction of SAI and ICF [98]. In this study, a lower LTP-like plasticity in a stage of overt cognitive disorder may have failed in the compensatory mechanisms [98].
3.5. Repetitive TMS in Vascular Cognitive Impairment
Several rTMS studies, although methodologically heterogeneous, have shown that specific paradigms of stimulation might improve cognitive performance and have been proposed as a possible alternative to conventional neuroleptic therapy to behavioral symptoms of dementia. This is of particular interest because current pharmacological treatments suffer of significant limitations, such as nonspecific effects, insufficient tailoring to the individual, and moderate-to-severe adverse effects [124]. In this context, the target for an ideal rTMS treatment would be: (i) modulation of activity specifically in the targeted cortex, (ii) modulation of activity in a dysfunctional network, (iii) restoration of adaptive balance in a disrupted network, (iv) guiding plasticity for best outcome, and (v) suppression of maladaptive changes for functional advantage.
In a randomized controlled pilot study on patients with subcortical ischemic vascular disease and a clinical diagnosis of mild VCI, high-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC) induced a long-lasting improvement of the executive performance, likely due to an indirect activation of the midbrain monoaminergic neurons (dopamine) and/or of the brainstem (noradrenaline and serotonine) and their cortical and subcortical targets [100]. In the same patients, rTMS was able to alleviate depressive symptoms, suggesting a potential application even in individuals with vascular depression, although WMLs and global vascular risk factors were predictors of poor response [125].
Few years later, a randomized, controlled, crossover study on 7 mild VCI patients [101] stimulated the left DLPFC and the left M1 both at low- and high-frequency rTMS for 4 sessions (two at 1 Hz and two at 10 Hz). The authors found a significant improvement in the Stroop color-word interference test after the stimulation of the DLPFC but not the M1. An improvement was also noted in the digit symbol subtest of the revised Wechsler Adult Intelligence Scale after rTMS, regardless of the stimulation site [101].
3.6. Repetitive TMS in Vascular Depression
Based on the FDA approval for the treatment of drug-resistant MDD in adults [126] and according to the view that depressed patients exhibit a significant interhemispheric asymmetry in motor cortex excitability (i.e., lower excitability of the left hemisphere) [34], two main rTMS protocols, i.e., high-frequency rTMS (5-20 Hz) over the left DLPFC and low-frequency rTMS (1 Hz) on the right DLPFC [127], have been evaluated. The protocol using the high-frequency rTMS [128] reached a remission rate up of 15% in the “real” (treated) stimulation group with respect to 5% of the “sham” (simulated) stimulation group [129].
Globally, rTMS seems to be less effective in late-onset patients with geriatric depression [130, 131], probably due to the brain atrophy (especially in the frontal lobes) and ischemic WMLs (especially in the prefrontal areas) characterizing this age group, both disrupting the connections between DLPFC and subcortical areas underlying mood and affect control [132]. Nevertheless, an earlier analysis [133] did not find age as a significant predictor of response, whereas positive predictors were a shorter duration of the current depressive episode (<2 years) and the degree of treatment resistance (≤1 treatment failure vs. >1).
In the attempt of exploiting rTMS as a therapeutic option for vascular depression, a small open trial showed that 10 sessions of high-frequency rTMS applied over the left DLPFC improved not only verbal fluency, visuospatial memory, and delayed recall but also depressive symptoms [102]. In a larger prospective randomized sham-controlled study, high-frequency rTMS over the same cortical region successfully treated depressive symptoms and increased both response and remission rates [103]. These results suggested that rTMS may modulate both cognitive ability and depressive symptoms, probably by activating different but closely spaced neural networks. Preliminary findings were confirmed by two subsequent randomized trials, one that combined rTMS with citalopram treatment [104] and one by using electroencephalography (EEG) in the follow-up period [105]. The studies showed significant differences in response and remission rates of depressive symptoms between active and sham groups, favoring the “real” stimulation. The second study also found that the increased “low-theta” band power in the subgenual cingulate cortex predicted the response to rTMS [105].
Finally, low-frequency rTMS over the right DLPFC was tested in a patient with drug-resistant depression and cerebral amyloid angiopathy, which is a chronic neurovascular disorder characterized by a progressive amyloid-β fibril deposition within the wall of cerebral blood vessels, eventually leading to hemorrhagic events and dementia. Stimulation intensity was set to 110% of the rMT, and rTMS was applied at 1 Hz for 1,600 pulses per day for 3 weeks. A long-lasting decrease in depression rating scales was noted, thus opening the way for the treatment of depression in other forms of cerebrovascular and degenerative diseases [134].
3.7. Translational Considerations
To date, the prediction of dementia onset and progression is beyond the possibilities of conventional tools. However, differently from AD and other degenerative disorders, VaD can be slowed, delayed, or even avoided through a careful prevention and control of vascular risk factors [135]. Besides the prevention of vascular accidents, maintaining the functional status in the elderly is a further key factor in the prevention and management of VCI.
Because of the VCI's heterogeneous construct, the selection of appropriate outcome measures to employ in pharmacological trials is of particular importance. In this context, the early discovery of new therapeutic targets would lead to a better prevention and treatment of VaD, and accordingly with the reviewed literature, TMS can be of help [33]. An enhancement of cortical plasticity might be induced to counteract cognitive decline, and the evaluation of where and how much this happens in different patients' subpopulations may shed light on the pathophysiological bases of decline or preservation of cognition [115].
Although a single TMS measure cannot be used to diagnose VCI, collectively the parameters of interest may act as footprints of VCI pathophysiology. Moreover, TMS can help to identify different profiles of cortical excitability for VCI subtypes and for the prediction of the “brain at risk” to convert into an overt VaD [28, 31, 95]. These findings will also support the study design of trials to test new drugs and novel nonpharmacological approaches. Finally, clinicians can exploit TMS in patients with overt dementia for the selection of the response to specific drugs [110], and the efficacy of treatment can be maximized by selecting the patients on the basis of putative neurophysiological markers.
Neurotrophins have an important role in the response to vascular damage and in stroke recovery [136, 137], and their release and modulation may also be behind the mechanisms of action of noninvasive brain stimulation in dementia. Several murine models of VaD have been used for testing rTMS [138], showing that low-frequency rTMS positively impact on cognitive deficit by upregulating the release of the hippocampal brain-derived neurotrophic factor (BDNF) and the expression of the NMDA glutamate receptor [139]. A different study found that increased expression in the Bcl-2 gene and a decrease in the Bax gene led low-frequency rTMS to be effective in learning and memory, as well as in the protection of pyramidal cells from apoptosis and in the promotion of hippocampal synaptic plasticity [140]. Moreover, rTMS significantly improved learning and memory and increased acetylcholinesterase and choline acetyltransferase activity, the density of cholinergic neurons, and the number of BDNF-immunoreactive cells at the level of hippocampal CA1 region [141]. Finally, in VaD rats, synaptic plasticity showed to be synergic with mesenchymal stem cells transplantation and with the promotion of autophagy [142]. However, the effectiveness of rTMS as VCI disease-modifying therapy in humans deserves further translational considerations, larger samples size, and well-controlled investigations [143].
Similarly, the clinical efficacy of rTMS on the cognitive aspects of vascular depression is still a matter of debate. It cannot be excluded, indeed, that cognitive improvement might be the consequence of an indirect effect on depressive symptoms rather than an improvement of cognition per se. In this context, while findings on rTMS in vascular depression are still limited and a conclusive evidence is yet to be reached, rTMS data in MDD (which is often associated with cognitive changes, especially executive dysfunction) are much more robust [144]. In MDD, the treatment-induced response did not seem to be directly related to a relief from depression or other treatment variables, thus suggesting that improvement of cognition and mood may follow different mechanisms [145]. Based on earlier controlled studies [146–148], improvement in both verbal fluency and visuospatial memory suggests that rTMS may enhance specific aspects of cognition independently from positive mood changes through a general alerting effect or a learning facilitation [102]. Moreover, since previous investigations did not find significant correlations between cognitive functioning and depression scores [149–151], it has been proposed that rTMS might independently modulate cognitive abilities and depression symptoms by activating different neural pathways and brain regions. In addition, in a pilot study on treatment-resistant depressed patients [151], left frontal high-frequency rTMS was associated with better performance of tests evaluating frontal lobe abilities and reduction in depression severity. The authors hypothesized that the cognitive improvement could be due to a direct or indirect (i.e., transsynaptic) modulation of the DLPFC [151], probably secondary to the activation of monoaminergic neurons in the midbrain (dopamine) or in the brainstem (noradrenaline and serotonin) and their cortical and subcortical targets [152, 153].
Lastly, it was demonstrated that rTMS not only improved executive dysfunction in MDD patients but also restored the interhemispheric asymmetry of rMT and ICF, thus implying that specific electrocortical changes may correlate to executive functions, both before and after treatment [154]. Although the pattern of motor cortex excitability in vascular depression differs from that previously reported in MDD and is similar to that of patients with subcortical vascular disease [28], the clinical presentations of these patients are similar, i.e., psychomotor retardation, difficulties at work, apathy, lack of insight, and executive dysfunction. This may suggest that, in vascular depressed patients, the enhancement of ICF could play a compensatory glutamate-mediated role in response to vascular damage of the frontal cortical-subcortical circuits implicated in mood-affect regulation and cognition [94]. Nevertheless, as mentioned above, the effects of rTMS on cognitive functioning can depend on additional factors (e.g., the paradigms used and the parameters studied), and it is not always observed [155], thus warranting further evidence in both MDD and vascular depression.
At this stage, it is not certain that the findings of the studies we reviewed have a direct impact in the daily decision-making algorithm of VCI patients' care, although they suggest that TMS can help to screen populations at risk. Once the population at risk is identified, a careful prevention and vascular risk factors control can be achieved more easily. Further longitudinal studies combining TMS with other neurophysiological techniques, such as high-density EEG and multimodal evoked potentials, as well as with advanced structural and functional neuroimaging (such as diffusion tensor imaging and functional MRI) and serum or CSF analysis will clarify the impact of cognitive and mood deficits on VCI plasticity.
Figure 3 illustrates the TMS findings in VCI, proposes a diagnostic algorithm, and summarizes the main rTMS effects.
Figure 3.
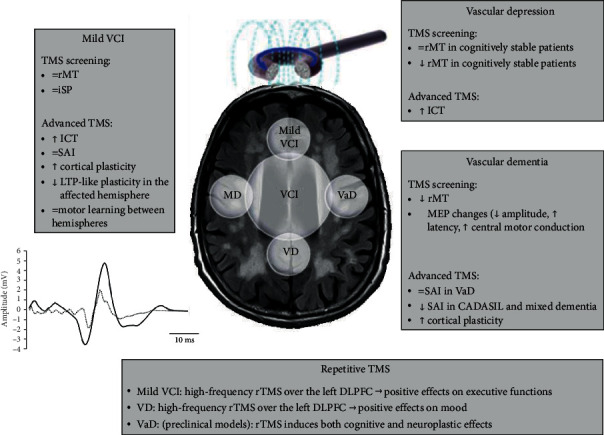
TMS findings, proposed diagnostic algorithm, and main rTMS effects in VCI. Legend (in alphabetic order): CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; DLPFC: dorsolateral prefrontal cortex; ICF: intracortical facilitation; iSP: ipsilateral silent period; LTP: long-term potentiation; MD: mixed dementia; MEP: motor evoked potential; rMT: resting motor threshold; rTMS: repetitive transcranial magnetic stimulation; SAI: short-latency afferent inhibition; TMS: transcranial magnetic stimulation; VaD: vascular dementia; VCI: vascular cognitive impairment; VD: vascular depression.
3.8. Critical Aspects, Possible Solutions, and Future Research
A major limitation in the implementation of the studies employing noninvasive brain stimulation in VCI is the relatively small sample sizes that make the generalization of these results to large populations troublesome. The same holds for the difficulty to recruit enough elderly healthy controls without neuroimaging evidence of cerebrovascular disease.
Second, the relatively low spatial resolution of TMS often determines the lack of systematic correlation between the pattern of cortical excitability and the anatomical distribution and severity of vascular lesions. Combining TMS with advanced imaging, neuronavigational systems, and other electrophysiological techniques may overcome this issue.
Third, although the TMS-related measures of cortical excitability are sensitive to the “global weight” of many neurotransmitters, so far we do not have more detailed information linking TMS findings with specific cognitive or behavioral changes [54, 111]. In this context, hypothesizing the presence a specific “signature” characteristic of VCI patients could be risky given the paucity of previous data and the difficulty that similar approaches are encountered in other dementing conditions, such as the non-AD dementias [33]. Additionally, even in the absence of evident motor deficit, vascular lesions significantly contribute to degenerative dementias and their progression. Therefore, it cannot be excluded that some of the enrolled patients had a mixed dementia rather than a pure VaD. In other words, TMS profile alone is not currently capable of distinguishing VaD from AD [115].
It should also be noted that antithrombotic agents, oral antidiabetic therapy, antihypertensive drugs, and statins, commonly prescribed to elders, might affect the measures of cortical excitability and their response to rTMS treatments [156, 157]. Thus, both TMS and rTMS studies need to consider this possible confounding factor.
Finally, for an adequate definition of sensitivity and specificity, the individual TMS measures in all patients and controls would be necessary. Besides, the estimation of the number of false positives would require an independent follow-up allowing the assessment of the cognitive status. Those requirements have been met only by a few studies, so that the next applications of TMS in VCI need methodological improvements and higher standardization levels.
Regarding rTMS, it is relatively expensive and requires technical expertise. Moreover, the magnetic coil must to be held still, and sham stimulation and operator blindness are often difficult. The majority of reported investigations are open-label or uncontrolled, and the treatment response could be affected by changes in brain morphology (e.g., cortical atrophy or CSF distribution). Moreover, determining the most appropriate target for stimulation is often challenging, and inferring to what extent cortical response characteristics of the motor system are representative of other brain areas is often speculative. Finally, there is a wide range of TMS parameters and rTMS settings that need to be considered in these applications.
Possible solutions may consist of [158]: (i) fully report of the results all rTMS trials, including negative findings; (ii) more studies in healthy individuals or in those with mild disease, thus allowing finessing of stimulation parameters and establishing the tolerability of protocols; (iii) further studies on the etiological models of dementia, including preclinical ones, thus aiding the choice of stimulation site and other technical set up; (iv) optimization of the treatment efficacy through methods of stratification, where patients are selected on the basis, for instance, of neuropsychological, electrophysiological, or genetic markers; and (v) use of novel methodological factors that can increase the stimulation efficacy, as well as the combination of rTMS with objective outcome measures (e.g., those derived from EEG, CSF, or MRI).
4. Conclusions
Overall, there is a mounting interest towards new diagnostic and therapeutic tools for cognitive assessment and rehabilitation in dementia, including VCI. Current data, although obtained from heterogeneous studies, have revealed that TMS and rTMS can provide, respectively, valuable diagnostic clues and induce beneficial effects on some cognitive domains and neuropsychiatric manifestations. Challenges still exist in terms of appropriate patient selection and optimization of the stimulation protocols. Recent findings from animal models are exciting, but their clinical significance needs to be validated. Together with the clinical exam, psychocognitive assessment, and neuroimaging, a systematic TMS evaluation of VCI patients can aid the diagnostic process, enhance the therapeutic arsenal, and predict the prognosis.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Mariagiovanna Cantone and Giuseppe Lanza contributed equally to this work.
References
- 1.Román G. C., Sachdev P., Royall D. R., et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. Journal of the Neurological Sciences. 2004;226(1–2):81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Moorhouse P., Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. The Lancet. Neurology. 2008;7(3):246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 3.van der Flier W. M., Skoog I., Schneider J. A., et al. Vascular cognitive impairment. Nature Reviews. Disease Primers. 2018;4(1):p. 18003. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien J. T. Vascular cognitive impairment. The American Journal of Geriatric Psychiatry. 2006;14(9):724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 5.Skrobot O. A., Black S. E., Chen C., et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s & Dementia. 2018;14(3):280–292. doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Emrani S., Lamar M., Price C. C., et al. Alzheimer’s/vascular spectrum dementia: classification in addition to diagnosis. Journal of Alzheimer’s disease. 2020;73(1):63–71. doi: 10.3233/JAD-190654. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick P. B., Counts S. E., Nyenhuis D. Vascular cognitive impairment and dementia. Biochimica Et Biophysica Acta. 2016;1862(5):860–868. doi: 10.1016/j.bbadis.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azarpazhooh M. R., Hachinski V. Vascular cognitive impairment: a preventable component of dementia. Handbook of Clinical Neurology. 2019;167:377–391. doi: 10.1016/B978-0-12-804766-8.00020-0. [DOI] [PubMed] [Google Scholar]
- 9.Vasquez B. P., Zakzanis K. K. The neuropsychological profile of vascular cognitive impairment not demented: a meta-analysis. Journal of Neuropsychology. 2015;9(1):109–136. doi: 10.1111/jnp.12039. [DOI] [PubMed] [Google Scholar]
- 10.Aizenstein H. J., Baskys A., Boldrini M., et al. Vascular depression consensus report – a critical update. BMC Medicine. 2016;14(1):p. 161. doi: 10.1186/s12916-016-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalaria R. N., Akinyemi R., Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016;1862(5):915–925. doi: 10.1016/j.bbadis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulos G. S., Meyers B. S., Young R. C., Campbell S., Silbersweig D., Charlson M. Vascular depression’ hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 13.Gupta M., Dasgupta A., Khwaja G. A., Chowdhury D., Patidar Y., Batra A. Behavioural and psychological symptoms in poststroke vascular cognitive impairment. Behavioural Neurology. 2014;2014:5. doi: 10.1155/2014/430128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiel C., Sudo F. K., Alves G. S., et al. Neuropsychiatric symptoms in vascular cognitive impairment: a systematic review. Dementia & Neuropsychologia. 2015;9(3):230–236. doi: 10.1590/1980-57642015dn93000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith E. E. Clinical presentations and epidemiology of vascular dementia. Clinical Science. 2017;131(11):1059–1068. doi: 10.1042/CS20160607. [DOI] [PubMed] [Google Scholar]
- 16.Inzitari D., Pracucci G., Poggesi A., et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339(jul06 1):p. b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Carlo A., Baldereschi M., Lamassa M., et al. Daily function as predictor of dementia in cognitive impairment, no dementia (CIND) and mild cognitive impairment (MCI): an 8-year follow-up in the ILSA study. Journal of Alzheimer’s disease. 2016;53(2):505–515. doi: 10.3233/JAD-160087. [DOI] [PubMed] [Google Scholar]
- 18.de Groot J. C., de Leeuw F.-E., Oudkerk M., Hofman A., Jolles J., Breteler M. M. B. Cerebral white matter lesions and depressive symptoms in elderly adults. Archives of General Psychiatry. 2000;57(11):1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 19.Vernooij M. W., Ikram M. A., Vrooman H. A., et al. White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry. 2009;66(5):545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 20.Prins N. D., van Dijk E. J., den Heijer T., et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain: A Journal of Neurology. 2005;128(9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien J. T., Erkinjuntti T., Reisberg B., et al. Vascular cognitive impairment. The Lancet. Neurology. 2003;2(2):89–98. doi: 10.1016/S1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 22.Steffens D. C., Helms M. J., Krishnan K. R., Burke G. L. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30(10):2159–2166. doi: 10.1161/01.STR.30.10.2159. [DOI] [PubMed] [Google Scholar]
- 23.Teodorczuk A., O’Brien J. T., Firbank M. J., et al. White matter changes and late-life depressive symptoms: longitudinal study. The British Journal of Psychiatry. 2007;191(3):212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 24.Bonelli R. M., Cummings J. L. Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinyemi R. O., Firbank M., Ogbole G. I., et al. Medial temporal lobe atrophy, white matter hyperintensities and cognitive impairment among Nigerian African stroke survivors. BMC Research Notes. 2015;8(1) doi: 10.1186/s13104-015-1552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballard C., Rowan E., Stephens S., Kalaria R., Kenny R. A. Prospective follow-up study between 3 and 15 months after stroke. Stroke. 2003;34(10):2440–2444. doi: 10.1161/01.STR.0000089923.29724.CE. [DOI] [PubMed] [Google Scholar]
- 27.Carnevale L., Lembo G. Innovative MRI techniques in neuroimaging approaches for cerebrovascular diseases and vascular cognitive impairment. International Journal of Molecular Sciences. 2019;20(11):p. 2656. doi: 10.3390/ijms20112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinciguerra L., Lanza G., Puglisi V., et al. Update on the neurobiology of vascular cognitive impairment: from lab to clinic. International Journal of Molecular Sciences. 2020;21(8):p. 2977. doi: 10.3390/ijms21082977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallett M., di Iorio R., Rossini P. M., et al. Contribution of transcranial magnetic stimulation to assessment of brain connectivity and networks. Clinical Neurophysiology. 2017;128(11):2125–2139. doi: 10.1016/j.clinph.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bella R., Ferri R., Pennisi M., et al. Enhanced motor cortex facilitation in patients with vascular cognitive impairment-no dementia. Neuroscience Letters. 2011;503(3):171–175. doi: 10.1016/j.neulet.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Bella R., Ferri R., Lanza G., et al. TMS follow-up study in patients with vascular cognitive impairment-no dementia. Neuroscience Letters. 2013;534:155–159. doi: 10.1016/j.neulet.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Gonçalves-Ribeiro J., Pina C. C., Sebastião A. M., Vaz S. H. Glutamate transporters in hippocampal LTD/LTP: not just prevention of excitotoxicity. Frontiers in Cellular Neuroscience. 2019;13:p. 357. doi: 10.3389/fncel.2019.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantone M., di Pino G., Capone F., et al. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clinical Neurophysiology. 2014;125(8):1509–1532. doi: 10.1016/j.clinph.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Cantone M., Bramanti A., Lanza G., et al. Cortical plasticity in depression. ASN Neuro. 2017;9(3) doi: 10.1177/1759091417711512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker A. T., Jalinous R., Freeston I. L. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell J. Transcranial brain stimulation: past and future. Brain and Neuroscience Advances. 2018;2, article 2398212818818070 doi: 10.1177/2398212818818070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomes-Osman J., Indahlastari A., Fried P. J., et al. Non-invasive brain stimulation: probing intracortical circuits and improving cognition in the aging brain. Frontiers in Aging Neuroscience. 2018;10:p. 177. doi: 10.3389/fnagi.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Pino G., Pellegrino G., Assenza G., et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nature Reviews. Neurology. 2014;10(10):597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- 39.Hoogendam J. M., Ramakers G. M. J., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimulation. 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Lazzaro V., Oliviero A., Mazzone P., et al. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Experimental Brain Research. 2001;141(1):121–127. doi: 10.1007/s002210100863. [DOI] [PubMed] [Google Scholar]
- 41.Rossini P. M., Burke D., Chen R., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossini P. M., Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68(7):484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- 43.Chen R., Lozano A. M., Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Experimental Brain Research. 1999;128(4):539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- 44.Cantello R., Gianelli M., Civardi C., Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42(10):1951–1959. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- 45.Werhahn K. J., Kunesch E., Noachtar S., Benecke R., Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. The Journal of Physiology. 1999;517(2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siebner H. R., Dressnandt J., Auer C., Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle & Nerve. 1998;21(9):1209–1212. doi: 10.1002/(SICI)1097-4598(199809)21:9<1209::AID-MUS15>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 47.Chen R., Cros D., Curra A., et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clinical Neurophysiology. 2008;119(3):504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi M., Pascual-Leone A. Transcranial magnetic stimulation in neurology. The Lancet. Neurology. 2003;2(3):145–156. doi: 10.1016/S1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 49.Kujirai T., Caramia M. D., Rothwell J. C., et al. Corticocortical inhibition in human motor cortex. The Journal of Physiology. 1993;471(1):501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziemann U., Rothwell J. C., Ridding M. C. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology. 1996;496(3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Lazzaro V., Pilato F., Dileone M., et al. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. The Journal of Physiology. 2006;575(3):721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreri F., Pasqualetti P., Määttä S., et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. NeuroImage. 2011;54(1):90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 53.Di Lazzaro V., Pilato F., Oliviero A., et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. Journal of Neurophysiology. 2006;96(4):1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- 54.Ziemann U. TMS and drugs. Clinical Neurophysiology. 2004;115(8):1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Tokimura H., di Lazzaro V., Tokimura Y., et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. The Journal of Physiology. 2000;523(2):503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.di Lazzaro V., Oliviero A., Profice P., et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Experimental Brain Research. 2000;135(4):455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- 57.di Lazzaro V., Oliviero A., Pilato F., et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(8):1064–1069. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sailer A., Molnar G. F., Paradiso G., Gunraj C. A., Lang A. E., Chen R. Short and long latency afferent inhibition in Parkinson’s disease. Brain. 2003;126(8):1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- 59.Martorana A., Mori F., Esposito Z., et al. Dopamine modulates cholinergic cortical excitability in Alzheimer’s disease patients. Neuropsychopharmacology. 2009;34(10):2323–2328. doi: 10.1038/npp.2009.60. [DOI] [PubMed] [Google Scholar]
- 60.Di Lazzaro V., Pilato F., Dileone M., et al. In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology. 2006;66(7):1111–1113. doi: 10.1212/01.wnl.0000204183.26231.23. [DOI] [PubMed] [Google Scholar]
- 61.Di Lazzaro V., Pilato F., Dileone M., et al. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clinical Neurophysiology. 2007;118(10):2207–2214. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Carson R. G., Kennedy N. C. Modulation of human corticospinal excitability by paired associative stimulation. Frontiers in Human Neuroscience. 2013;7:p. 823. doi: 10.3389/fnhum.2013.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caramia M. D., Scalise A., Gordon R., Michalewski H. J., Starr A. Delayed facilitation of motor cortical excitability following repetitive finger movements. Clinical Neurophysiology. 2000;111(9):1654–1660. doi: 10.1016/S1388-2457(00)00356-4. [DOI] [PubMed] [Google Scholar]
- 64.Chen R. Guideline on therapeutic use of repetitive transcranial magnetic stimulation: useful but know the methods and limitations. Clinical Neurophysiology. 2020;131(2):461–462. doi: 10.1016/j.clinph.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 65.Wilson M. T., Fulcher B. D., Fung P. K., Robinson P. A., Fornito A., Rogasch N. C. Biophysical modeling of neural plasticity induced by transcranial magnetic stimulation. Clinical Neurophysiology. 2018;129(6):1230–1241. doi: 10.1016/j.clinph.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Cheeran B., Koch G., Stagg C. J., Baig F., Teo J. Transcranial magnetic stimulation: from neurophysiology to pharmacology, molecular biology and genomics. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2010;16(3):210–221. doi: 10.1177/1073858409349901. [DOI] [PubMed] [Google Scholar]
- 67.Jung P., Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. The Journal of Neuroscience. 2009;29(17):5597–5604. doi: 10.1523/JNEUROSCI.0222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Lazzaro V., Profice P., Pilato F., Dileone M., Oliviero A., Ziemann U. The effects of motor cortex rTMS on corticospinal descending activity. Clinical Neurophysiology. 2010;121(4):464–473. doi: 10.1016/j.clinph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Ziemann U., Chen R., Cohen L. G., Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51(5):1320–1324. doi: 10.1212/WNL.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 70.Ziemann U., Tergau F., Wischer S., Hildebrandt J., Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalography and Clinical Neurophysiology. 1998;109(4):321–330. doi: 10.1016/S0924-980X(98)00023-X. [DOI] [PubMed] [Google Scholar]
- 71.Cirillo G., di Pino G., Capone F., et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimulation. 2017;10(1):1–18. doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Gladding C. M., Fitzjohn S. M., Molnár E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacological Reviews. 2009;61(4):395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lefaucheur J.-P. Transcranial magnetic stimulation. Handbook of Clinical Neurology. 2019;160:559–580. doi: 10.1016/B978-0-444-64032-1.00037-0. [DOI] [PubMed] [Google Scholar]
- 74.Lefaucheur J.-P., Aleman A., Baeken C., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018) Clinical Neurophysiology. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Fisicaro F., Lanza G., Grasso A. A., et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Therapeutic Advances in Neurological Disorders. 2019;12, article 175628641987831 doi: 10.1177/1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C.-T., Huang Y.-Z., Bai Y.-M., Tsai S.-J., Su T.-P., Cheng C.-M. Critical role of glutamatergic and GABAergic neurotransmission in the central mechanisms of theta-burst stimulation. Human Brain Mapping. 2018;40(6):2001–2009. doi: 10.1002/hbm.24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumoto H., Ugawa Y. Quadripulse stimulation (QPS) Experimental Brain Research. 2020;238(7-8):1619–1625. doi: 10.1007/s00221-020-05788-w. [DOI] [PubMed] [Google Scholar]
- 78.Yger P., Gilson M. Models of metaplasticity: a review of concepts. Frontiers in Computational Neuroscience. 2015;9:p. 138. doi: 10.3389/fncom.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He W., Fong P.-Y., Leung T. W. H., Huang Y.-Z. Protocols of non-invasive brain stimulation for neuroplasticity induction. Neuroscience Letters. 2020;719:p. 133437. doi: 10.1016/j.neulet.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 80.Di Pino G., Pellegrino G., Capone F., Di Lazzaro V. Human cerebral cortex metaplasticity and stroke recovery. Austin Journal of Cerebrovascular Disease & Stroke. 2014;1(2) [Google Scholar]
- 81.Burt T., Lisanby S. H., Sackeim H. A. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. The International Journal of Neuropsychopharmacology. 2002;5(1):73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 82.Anderson B., Mishory A., Nahas Z., et al. Tolerability and safety of high daily doses of repetitive transcranial magnetic stimulation in healthy young men. The journal of ECT. 2006;22(1):49–53. doi: 10.1097/00124509-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 83.Lanza G., Bella R., Giuffrida S., et al. Preserved transcallosal inhibition to transcranial magnetic stimulation in nondemented elderly patients with leukoaraiosis. BioMed Research International. 2013;2013:5. doi: 10.1155/2013/351680.351680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.List J., Duning T., Kürten J., Deppe M., Wilbers E., Flöel A. Cortical plasticity is preserved in nondemented older individuals with severe ischemic small vessel disease. Human Brain Mapping. 2013;34(6):1464–1476. doi: 10.1002/hbm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.List J., Hertel-Zens S., Kübke J. C., Lesemann A., Schreiber S. J., Flöel A. Cortical reorganization due to impaired cerebral autoregulation in individuals with occlusive processes of the internal carotid artery. Brain Stimulation. 2014;7(3):381–387. doi: 10.1016/j.brs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Bella R., Cantone M., Lanza G., et al. Cholinergic circuitry functioning in patients with vascular cognitive impairment--no dementia. Brain Stimulation. 2016;9(2):225–233. doi: 10.1016/j.brs.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 87.Alagona G., Ferri R., Pennisi G., et al. Motor cortex excitability in Alzheimer’s disease and in subcortical ischemic vascular dementia. Neuroscience Letters. 2004;362(2):95–98. doi: 10.1016/j.neulet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Di Lazzaro V., Pilato F., Dileone M., et al. In vivo functional evaluation of central cholinergic circuits in vascular dementia. Clinical Neurophysiology. 2008;119(11):2494–2500. doi: 10.1016/j.clinph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 89.Nardone R., Bergmann J., Tezzon F., Ladurner G., Golaszewski S. Cholinergic dysfunction in subcortical ischaemic vascular dementia: a transcranial magnetic stimulation study. Journal of Neural Transmission. 2008;115(5):737–743. doi: 10.1007/s00702-007-0003-6. [DOI] [PubMed] [Google Scholar]
- 90.Pennisi G., Ferri R., Alagona G., et al. Motor cortex hyperexcitability in subcortical ischemic vascular dementia. Archives of Gerontology and Geriatrics. 2011;53(2):e111–e113. doi: 10.1016/j.archger.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Nardone R., De Blasi P., Seidl M., et al. Cognitive function and cholinergic transmission in patients with subcortical vascular dementia and microbleeds: a TMS study. Journal of Neural Transmission. 2011;118(9):1349–1358. doi: 10.1007/s00702-011-0650-5. [DOI] [PubMed] [Google Scholar]
- 92.Guerra A., Petrichella S., Vollero L., et al. Neurophysiological features of motor cortex excitability and plasticity in subcortical ischemic vascular dementia: a TMS mapping study. Clinical Neurophysiology. 2015;126(5):906–913. doi: 10.1016/j.clinph.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 93.Bella R., Ferri R., Cantone M., et al. Motor cortex excitability in vascular depression. International Journal of Psychophysiology. 2011;82(3):248–253. doi: 10.1016/j.ijpsycho.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Concerto C., Lanza G., Cantone M., et al. Different patterns of cortical excitability in major depression and vascular depression: a transcranial magnetic stimulation study. BMC psychiatry. 2013;13(1):p. 300. doi: 10.1186/1471-244X-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pennisi M., Lanza G., Cantone M., et al. Correlation between motor cortex excitability changes and cognitive impairment in vascular depression: pathophysiological insights from a longitudinal TMS study. Neural Plasticity. 2016;2016:10. doi: 10.1155/2016/8154969.8154969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manganelli F., Ragno M., Cacchiò G., et al. Motor cortex cholinergic dysfunction in CADASIL: a transcranial magnetic demonstration. Clinical Neurophysiology. 2008;119(2):351–355. doi: 10.1016/j.clinph.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 97.List J., Duning T., Meinzer M., et al. Enhanced rapid-onset cortical plasticity in CADASIL as a possible mechanism of preserved cognition. Cerebral Cortex. 2011;21(12):2774–2787. doi: 10.1093/cercor/bhr071. [DOI] [PubMed] [Google Scholar]
- 98.Palomar F. J., Suárez A., Franco E., Carrillo F., Gil-Néciga E., Mir P. Abnormal sensorimotor plasticity in CADASIL correlates with neuropsychological impairment. Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84(3):329–336. doi: 10.1136/jnnp-2012-303960. [DOI] [PubMed] [Google Scholar]
- 99.Nardone R., Höller Y., Thomschewski A., et al. Dopamine differently modulates central cholinergic circuits in patients with Alzheimer disease and CADASIL. Journal of Neural Transmission. 2014;121(10):1313–1320. doi: 10.1007/s00702-014-1195-1. [DOI] [PubMed] [Google Scholar]
- 100.Rektorova I., Megova S., Bares M., Rektor I. Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. Journal of the Neurological Sciences. 2005;229–230:157–161. doi: 10.1016/j.jns.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 101.Sedlackova S., Rektorova I., Fanfrdlova Z., Rektor I. Neurocognitive effects of repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia. Journal of Psychophysiology. 2008;22(1):14–19. doi: 10.1027/0269-8803.22.1.14. [DOI] [Google Scholar]
- 102.Fabre I., Galinowski A., Oppenheim C., et al. Antidepressant efficacy and cognitive effects of repetitive transcranial magnetic stimulation in vascular depression: an open trial. International Journal of Geriatric Psychiatry. 2004;19(9):833–842. doi: 10.1002/gps.1172. [DOI] [PubMed] [Google Scholar]
- 103.Jorge R. E., Moser D. J., Acion L., Robinson R. G. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Archives of General Psychiatry. 2008;65(3):268–276. doi: 10.1001/archgenpsychiatry.2007.45. [DOI] [PubMed] [Google Scholar]
- 104.Robinson R. G., Tenev V., Jorge R. E. Citalopram for continuation therapy after repetitive transcranial magnetic stimulation in vascular depression. The American Journal of Geriatric Psychiatry. 2009;17(8):682–687. doi: 10.1097/JGP.0b013e3181a88423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Narushima K., McCormick L. M., Yamada T., Thatcher R. W., Robinson R. G. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(1):75–84. doi: 10.1176/jnp.2010.22.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):p. e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoeppner J., Wegrzyn M., Thome J., et al. Intra- and inter-cortical motor excitability in Alzheimer’s disease. Journal of Neural Transmission. 2012;119(5):605–612. doi: 10.1007/s00702-011-0738-y. [DOI] [PubMed] [Google Scholar]
- 108.Delvendahl I., Jung N. H., Kuhnke N. G., Ziemann U., Mall V. Plasticity of motor threshold and motor-evoked potential amplitude – a model of intrinsic and synaptic plasticity in human motor cortex? Brain Stimulation. 2012;5(4):586–593. doi: 10.1016/j.brs.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Nardone R., Bergmann J., Christova M., et al. Short latency afferent inhibition differs among the subtypes of mild cognitive impairment. Journal of Neural Transmission. 2012;119(4):463–471. doi: 10.1007/s00702-011-0725-3. [DOI] [PubMed] [Google Scholar]
- 110.Lanza G., Bramanti P., Cantone M., Pennisi M., Pennisi G., Bella R. Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behavioural Neurology. 2017;2017:16. doi: 10.1155/2017/1421326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rossini P. M., Rossi S., Babiloni C., Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Progress in Neurobiology. 2007;83(6):375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 112.Ferreri F., Pauri F., Pasqualetti P., Fini R., Dal Forno G., Rossini P. M. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation study. Annals of Neurology. 2003;53(1):102–108. doi: 10.1002/ana.10416. [DOI] [PubMed] [Google Scholar]
- 113.Ferreri F., Pasqualetti P., Määttä S., et al. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation follow-up study. Neuroscience Letters. 2011;492(2):94–98. doi: 10.1016/j.neulet.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 114.Skrobot O. A., O'Brien J., Black S., et al. The vascular impairment of cognition classification consensus study. Alzheimer’s & Dementia. 2017;13(6):624–633. doi: 10.1016/j.jalz.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 115.Pennisi G., Bella R., Lanza G. Motor cortex plasticity in subcortical ischemic vascular dementia: what can TMS say? Clinical Neurophysiology. 2015;126(5):851–852. doi: 10.1016/j.clinph.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 116.Di Lazzaro V., Oliviero A., Pilato F., Saturno E., Dileone M., Tonali P. A. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease: evidence of impaired glutamatergic neurotransmission? Annals of Neurology. 2003;53(6):824–824; author reply 825. doi: 10.1002/ana.10600. [DOI] [PubMed] [Google Scholar]
- 117.Di Lazzaro V., Oliviero A., Pilato F., et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75(4):555–559. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lazzaro V. D., Oliviero A., Profice P., et al. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. The Journal of Physiology. 2003;547(2):485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paula-Lima A. C., Brito-Moreira J., Ferreira S. T. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. Journal of Neurochemistry. 2013;126(2):191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 120.Bella R., Pennisi G., Cantone M., et al. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology. 2010;56(3):298–302. doi: 10.1159/000272003. [DOI] [PubMed] [Google Scholar]
- 121.Di Donato I., Bianchi S., De Stefano N., et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC medicine. 2017;15(1):p. 41. doi: 10.1186/s12916-017-0778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swartz R. H., Sahlas D. J., Black S. E. Strategic involvement of cholinergic pathways and executive dysfunction: does location of white matter signal hyperintensities matter? Journal of Stroke and Cerebrovascular Diseases. 2003;12(1):29–36. doi: 10.1053/jscd.2003.5. [DOI] [PubMed] [Google Scholar]
- 123.O’Sullivan M., Jarosz J. M., Martin R. J., Deasy N., Powell J. F., Markus H. S. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628–634. doi: 10.1212/wnl.56.5.628. [DOI] [PubMed] [Google Scholar]
- 124.Elder G. J., Taylor J.-P. Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimer’s Research & Therapy. 2014;6(9):p. 74. doi: 10.1186/s13195-014-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brunoni A. R., Benseñor I. M., Alves T. C. T. F. Therapeutic interventions for vascular depression: a systematic review. Revista Brasileira De Psiquiatria. 2011;33(4):400–409. doi: 10.1590/S1516-44462011000400015. [DOI] [PubMed] [Google Scholar]
- 126.Horvath J. C., Mathews J., Demitrack M. A., Pascual-Leone A. The NeuroStar TMS device: conducting the FDA approved protocol for treatment of depression. Journal of Visualized Experiments. 2010;45 doi: 10.3791/2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fregni F., Pascual-Leone A. Transcranial magnetic stimulation for the treatment of depression in neurologic disorders. Current Psychiatry Reports. 2005;7(5):381–390. doi: 10.1007/s11920-005-0041-4. [DOI] [PubMed] [Google Scholar]
- 128.O’Reardon J. P., Solvason H. B., Janicak P. G., et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biological Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 129.George M. S., Lisanby S. H., Avery D., et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives of General Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 130.Figiel G. S., Epstein C., McDonald W. M., et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. The Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(1):20–25. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- 131.Manes F., Jorge R., Morcuende M., Yamada T., Paradiso S., Robinson R. G. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. International Psychogeriatrics. 2001;13(2):225–231. doi: 10.1017/S1041610201007608. [DOI] [PubMed] [Google Scholar]
- 132.Jalenques I., Legrand G., Vaille-Perret E., Tourtauchaux R., Galland F. Therapeutic efficacy and safety of repetitive transcranial magnetic stimulation in depressions of the elderly: a review. L’Encephale. 2010;36(Suppl 2):D105–D118. doi: 10.1016/j.encep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 133.Lisanby S. H., Husain M. M., Rosenquist P. B., et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 134.Gray Z. A., Greenberg S. M., Press D. Z. rTMS for treatment of depression in a patient with cerebral amyloid angiopathy: a case report on safety and efficacy. Brain stimulation. 2014;7(3):495–497. doi: 10.1016/j.brs.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hakim A. M. Vascular disease: the tsunami of health care. Stroke. 2007;38(12):3296–3301. doi: 10.1161/STROKEAHA.107.492793. [DOI] [PubMed] [Google Scholar]
- 136.Di Lazzaro V. Val66Met BDNF gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimulation. 2015;8(1):92–96. doi: 10.1016/j.brs.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Di Pino G., Pellegrino G., Capone F., et al. Val66Met BDNF polymorphism implies a different way to recover from stroke rather than a worse overall recoverability. Neurorehabilitation and Neural Repair. 2016;30(1):3–8. doi: 10.1177/1545968315583721. [DOI] [PubMed] [Google Scholar]
- 138.Yang H., Shi O., Jin Y., et al. Functional protection of learning and memory abilities in rats with vascular dementia. Restorative Neurology and Neuroscience. 2014;32(5):689–700. doi: 10.3233/RNN-140409. [DOI] [PubMed] [Google Scholar]
- 139.Yukimasa T., Yoshimura R., Tamagawa A., et al. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39(2):52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- 140.Yang H.-Y., Liu Y., Xie J.-C., Liu N.-N., Tian X. Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behavioural Brain Research. 2015;281:149–155. doi: 10.1016/j.bbr.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 141.Zhang X.-Q., Li L., Huo J.-T., Cheng M., Li L.-H. Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regeneration Research. 2018;13(8):1384–1389. doi: 10.4103/1673-5374.235251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang F., Zhang C., Hou S., Geng X. Synergistic effects of mesenchymal stem cell transplantation and repetitive transcranial magnetic stimulation on promoting autophagy and synaptic plasticity in vascular dementia. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2019;74(9):1341–1350. doi: 10.1093/gerona/gly221. [DOI] [PubMed] [Google Scholar]
- 143.Bordet R., Ihl R., Korczyn A. D., et al. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Medicine. 2017;15(1):p. 107. doi: 10.1186/s12916-017-0869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Concerto C., Lanza G., Cantone M., et al. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. International Journal of Psychiatry in Clinical Practice. 2014;19(4):252–258. doi: 10.3109/13651501.2015.1084329. [DOI] [PubMed] [Google Scholar]
- 145.Nadeau S. E., Bowers D., Jones T. L., Wu S. S., Triggs W. J., Heilman K. M. Cognitive effects of treatment of depression with repetitive transcranial magnetic stimulation. Cognitive and Behavioral Neurology. 2014;27(2):77–87. doi: 10.1097/WNN.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 146.Loo C., Mitchell P., Sachdev P., McDarmont B., Parker G., Gandevia S. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. The American Journal of Psychiatry. 1999;156(6):946–948. doi: 10.1176/ajp.156.6.946. [DOI] [PubMed] [Google Scholar]
- 147.Triggs W. J., McCoy K. J. M., Greer R., et al. Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biological Psychiatry. 1999;45(11):1440–1446. doi: 10.1016/S0006-3223(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 148.Loo C., Sachdev P., Elsayed H., et al. Effects of a 2- to 4-week course of repetitive transcranial magnetic stimulation (rTMS) on neuropsychologic functioning, electroencephalogram, and auditory threshold in depressed patients. Biological Psychiatry. 2001;49(7):615–623. doi: 10.1016/S0006-3223(00)00996-3. [DOI] [PubMed] [Google Scholar]
- 149.Martis B., Alam D., Dowd S. M., et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clinical Neurophysiology. 2003;114(6):1125–1132. doi: 10.1016/S1388-2457(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 150.Hausmann A., Pascual-Leone A., Kemmler G., et al. No deterioration of cognitive performance in an aggressive unilateral and bilateral antidepressant rTMS add-on trial. The Journal of Clinical Psychiatry. 2004;65(6):772–782. doi: 10.4088/JCP.v65n0608. [DOI] [PubMed] [Google Scholar]
- 151.Kedzior K. K., Rajput V., Price G., Lee J., Martin-Iverson M. Cognitive correlates of repetitive transcranial magnetic stimulation (rTMS) in treatment-resistant depression- a pilot study. BMC psychiatry. 2012;12(1):p. 163. doi: 10.1186/1471-244X-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paus T., Castro-Alamancos M. A., Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. The European Journal of Neuroscience. 2001;14(8):1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 153.Strafella A. P., Paus T., Barrett J., Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. The Journal of Neuroscience. 2001;21(15, article RC157) doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spampinato C., Aguglia E., Concerto C., et al. Transcranial magnetic stimulation in the assessment of motor cortex excitability and treatment of drug-resistant major depression. IEEE transactions on neural systems and rehabilitation engineering. 2013;21(3):391–403. doi: 10.1109/TNSRE.2013.2256432. [DOI] [PubMed] [Google Scholar]
- 155.George M. S., Lisanby S. H., Sackeim H. A. Transcranial magnetic stimulation: applications in neuropsychiatry. Archives of General Psychiatry. 1999;56(4):300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- 156.Paulus W., Classen J., Cohen L. G., et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulation. 2008;1(3):151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 157.Ziemann U., Reis J., Schwenkreis P., et al. TMS and drugs revisited 2014. Clinical Neurophysiology. 2015;126(10):1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 158.Pennisi G., Bella R., Lanza G., Di Lazzaro V. The contribution of non-invasive brain stimulation techniques in the experimental treatment of cognitive and neuropsychiatric symptoms in vascular dementia; Monduzzi Editore International Proceedings Division; 2016. pp. 13–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


