Abstract
The public health burden of Alzheimer’s disease (AD) is related not only to cognitive symptoms, but also to neuropsychiatric symptoms, including apathy. Apathy is defined as a quantitative reduction of goal-directed activity in comparison to a previous level of functioning and affects 30%–70% of persons with AD. Previous attempts to treat apathy in AD—both nonpharmacologically and pharmacologically—have been wanting. Catecholaminergic treatment with methylphenidate has shown encouraging results in initial trials of apathy in AD. Understanding the neuronal circuits underlying motivated behavior and their reliance on catecholamine actions helps provide a rationale for methylphenidate actions in the treatment of apathy in patients with AD. Anatomical, physiological, and behavioral studies have identified parallel, cortical-basal ganglia circuits that govern action, cognition, and emotion and play key roles in motivated behavior. Understanding the distinct contributions to motivated behavior of subregions of the prefrontal cortex—dorsolateral, orbital-ventromedial, and dorsomedial—helps to explain why degeneration of these areas in AD results in apathetic behaviors. We propose that the degeneration of the prefrontal cortex in AD produces symptoms of apathy. We further propose that methylphenidate treatment may ameliorate those symptoms by boosting norepinephrine and dopamine actions in prefrontal-striatal-thalamocortical circuits.
Keywords: Alzheimer’s disease, apathy, methylphenidate, prefrontal cortex, catecholamines, norepinephrine, dopamine
INTRODUCTION
The public health burden of Alzheimer’s disease (AD) is related not only to cognitive symptoms, but also to neuropsychiatric symptoms. Most patients with AD will develop at least one such symptom over the course of the disease.1 Common neuropsychiatric symptoms in AD include agitation, depression, and apathy.2 Apathy is defined as a quantitative reduction of goal-directed activity in comparison to a previous level of functioning3 and affects 30%-70% of persons with AD.2,4 Even in the prodromal condition of mild cognitive impairment, apathy has an estimated prevalence of 15%-18%4,5 and is associated with a significantly increased risk of incident dementia.6 Numerous studies have shown that apathy is among the neuropsychiatric symptoms most associated with caregiver time and distress.7-9 Thus, apathy adds to the public health burden of AD, particularly for caregivers, and is an important target for treatment.
Previous efforts to treat apathy in AD have left a large unmet need. Nonpharmacologic interventions would be preferred as first-line interventions if effective, because they are lower risk than pharmacologic interventions. Interventions to engage the patient in recreational and social activities have been studied in long-term care settings.10-12 However, the evidence base for effectiveness of these interventions is slim, and the interventions themselves are neither well systematized nor widely used in clinical practice. A recent consensus paper on nonpharmacologic strategies for treating apathy in AD addresses the interaction of one-on-one interventions with Information and Communication Technologies.13
Several pharmacologic options for the treatment of apathy in AD have also been explored. For cholinesterase inhibitors, several randomized clinical trials have demonstrated modest improvements in apathy.14-18 However, about half of the patients showed no significant relief of apathetic symptoms following treatment.16 Antidepressant medications have not been found to improve apathy in AD19 and may in fact worsen symptoms.20 Another pharmacologic treatment that has failed in the treatment of apathy in AD is modafinil,21 a drug of unclear mechanism. While modafinil may bind weakly to the dopamine and norepinephrine transporters, the histaminergic and orexinergic systems are also thought to be responsible for its pharmacologic effects.22 Clearly, better pharmacologic options are needed for apathy in AD.
Based on clinical anecdotal reports, methylphenidate is under consideration for the treatment of apathy in AD and has shown encouraging results in initial trials.23,24 Understanding the neuronal circuits underlying motivated behavior and their reliance on catecholamine actions helps provide a rationale for methylphenidate actions in the treatment of apathy in patients with mild to moderate AD.
NEUROBIOLOGY AND PHARMACOLOGY OF APATHY
Lesions to Prefrontal Cortical-Basal Ganglia Circuits Induce Apathy
Anatomical, physiological, and behavioral studies25,26 have identified parallel, cortical-basal ganglia circuits that govern action, cognition, and emotion and play key roles in motivated behavior (Fig. 1A). Basal ganglia circuits are especially important for mediating habits—often unconscious, complex emotional, cognitive, and motor responses formed from repetitive experience.27,28
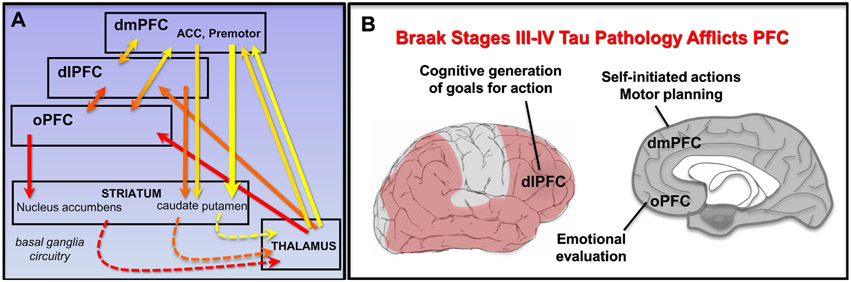
FIGURE 1.
Prefrontal cortical brain regions that mediate motivated behavior affected in Alzheimer’s disease. (A) Prefrontal cortical-basal ganglia circuits that play key roles in motivated behavior. Dashed lines represent indirect connections. (B) Tau pathology afflicts prefrontal cortical circuits, mediating motivated behaviors beginning in Braak Stage III/IV. dmPFC: dorsomedial prefrontal cortex; dlPFC: dorsolateral prefrontal cortex; oPFC: orbital prefrontal cortex; ACC: anterior cingulate cortex. Premotor: premotor cortex.
Traditional research in rodents has focused on the role of dopamine inputs to the nucleus accumbens in driving motivation. For example, rats normally choose to climb a tall barrier for a small reward, unless they have dopamine depletion from the nucleus accumbens.29 However, similar motivational deficits occur with insults to the medial prefrontal cortex (PFC).30 The PFC expands exponentially in primate brains31 and is essential for the complex and elaborated aspects of motivated behavior in humans. As described below, the degeneration of PFC, as occurs in AD, produces symptoms of apathy. Thus, understanding the distinct contributions of PFC subregions to motivated behavior, for example, as reviewed by Levy or Knight,32-34 helps to explain why degeneration of these areas results in apathetic behaviors (Fig. 1B). The relationship of PFC subregions to dimensions of the apathy syndrome is briefly summarized here:
Dorsolateral PFC:
The dorsolateral PFC is able to generate top-down goals for future actions by creating internally generated representations that are independent of sensory stimulation.35,36 Thus, lesions to the dorsolateral PFC in humans are typified by the loss of a cognitive plan or goal for actions. This is described sometimes as a “pseudodepressive” syndrome that is characterized by reduced initiative and motivation, flattened affect, reduced verbalizations, and behavioral slowness arising from an inability to plan and maintain sequences of goals and actions.34
Orbital-ventromedial PFC:
Lesions to these ventral aspects of the PFC cause a loss of emotional evaluation and an inability to link emotional-affective signals to behaviors, consistent with their connections with limbic regions such as the amygdala. A key feature of orbital-ventromedial PFC dysfunction is emotional blunting.37,38 Emotion and affect may reveal the motivational value of a given behavior and orient decision-making.32 In the setting of orbital or ventromedial PFC lesions, a diminished reactivity to emotion and reward may produce a decision-making deficit—that is, an inability to evaluate the emotional consequences of one’s own choices—and a decrease in goal-directed behavior.32,39
Dorsomedial PFC:
Lesions to the dorsal and medial aspects of PFC often include damage to the anterior cingulate and premotor/supplementary motor areas that are important for the organization and initiation of self-generated behaviors. Thus, these lesions can produce a profound loss of self-initiated action and spontaneous behaviors, which can be temporarily reversed by external stimulation. At its most extreme, lesions to the region of the anterior cingulate can produce akinetic mutism, where patients lose the will to move or talk, even though they are capable of both.34
All of these PFC subregions interconnect,40 and coalesce in “hubs” such as the rostral anterior cingulate.41 They also contribute to cortical-cortical networks involved in additional aspects of motivated behavior. For example, altered PFC connectivity with areas such as the insular cortex has been implicated in symptoms of apathy.42 As the present review is focused on methylphenidate as a treatment for apathy in AD, we will concentrate on PFC subregions where catecholamine and methylphenidate actions have been extensively studied.
Degeneration of Prefrontal Cortical Circuits in Alzheimer’s Disease and Its Relationship to Symptoms of Apathy
The PFC circuits needed for motivated, appropriate behavior are disrupted by AD tau and amyloid pathology beginning in Braak Stage III. Cortical tau pathology and subsequent neuronal loss lead to widespread degeneration of the PFC (Fig. 1B), and tau pathology correlates with cognitive impairment.43 In PFC, tau pathology concentrates in layer III and V glutamatergic pyramidal cells, as tangles build inside of neurons and kill them. However, these neurons likely lose function long before they fully degenerate, for example, due to loss of synapses.44 The PFC is also a focus of amyloid pathology.45 In later Braak stages, the entire PFC expresses significant buildup of both tau and amyloid AD degeneration.46
Neuroimaging studies have confirmed that the symptoms of apathy in AD correlate in vivo with pathology in the PFC. These studies have compared AD patients with extensive symptoms of apathy to those at similar stages of disease with minimal apathy. They have found that apathy is correlated with alterations in the PFC, especially in the anterior cingulate, dorsomedial PFC, and orbital PFC regions. Early SPECT imaging studies demonstrated an association between apathy and reduced regional cerebral blood flow in the orbitofrontal cortex44 and anterior cingulate.47,48 Similarly, a structural imaging study showed a correlation between apathy and gray matter loss in medial PFC (including anterior cingulate).49
The advent of PET radioligands for amyloid and tau has enabled the study of apathy in association with AD-specific neuropathology. PET amyloid imaging with [11C]PiB has shown a correlation between apathy and the accumulation of fibrillar amyloid in the PFC, including the orbital, ventromedial, and polar PFC subregions, as well as the anterior cingulate.50 Most recently, tau PET imaging has been used to link apathy with tau pathology in the orbital PFC using [11C]PBB351 or the right anterior cingulate and dorsolateral PFC using [18F]flortaucipir.52 Thus, the neuroimaging findings in AD are consonant with earlier data from lesion studies, and corroborate a major role for PFC insults in apathy.
Strategy for Treatment: Boosting Actions of Norepinephrine and Dopamine in Prefrontal-Striatal Circuits With Methylphenidate
Researchers have wondered whether they could lessen symptoms of apathy by administering methyl-phenidate, a treatment for Attention Deficit Hyperactivity Disorder (ADHD) that is known to improve motivation in non-AD patients.53 Methylphenidate is thought to have many of its beneficial catecholamine actions in ADHD by strengthening PFC function,53 and it may boost remaining PFC circuits in AD patients as well. Methylphenidate may also enhance motivation by enhancing dopamine actions in striatum, a region more resilient in AD.
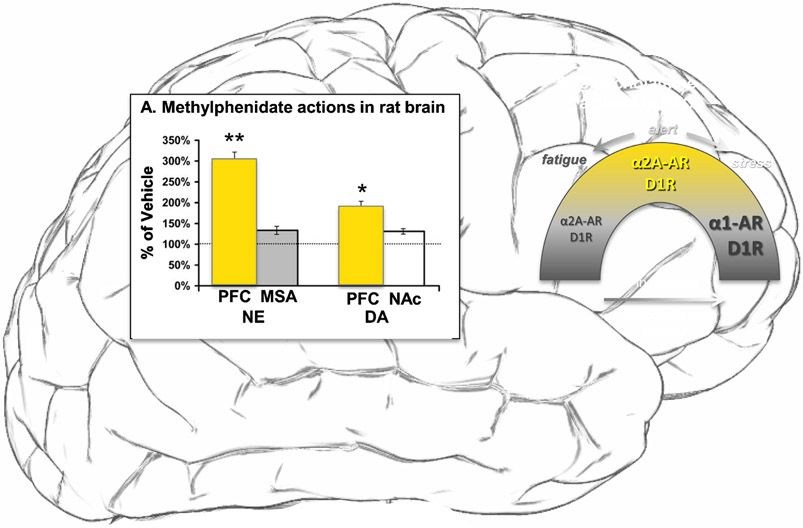
The catecholamines norepinephrine and dopamine are synthesized by neurons in brainstem that project to forebrain, with dense projections to subcortical structures such as the thalamus and striatum, respectively, and a more delicate projection to the cortex, including the PFC.54,55 Methylphenidate blocks dopamine transporters and increases extracellular dopamine concentrations in the striatum56 and in the medial PFC.57 A comprehensive examination of catecholamine changes (Fig. 2A) made the surprising discovery that methylphenidate produces an even greater increase in norepinephrine than dopamine in rat medial PFC.58 The low, clinically relevant doses used had greater effects on PFC than those seen subcortically in nucleus accumbens or septum.58 Thus, methylphenidate effects on PFC catecholamine actions may be particularly important to understanding the clinical benefits of this compound, as described below.
FIGURE 2.
Actions of methylphenidate via catecholamines in prefrontal cortex. (A) Methylphenidate has greater effects on catecholamine levels in prefrontal cortex than in subcortical structures in rat brain. (B) Catecholamines have an inverted U dose response on prefrontal top-down control, where either too little or too much is detrimental to function. The beneficial effects of moderate norepinephrine levels are through high affinity, postsynaptic alpha-2A-AR, while the detrimental actions at high levels of norepinephrine release are through low affinity alpha-1-AR. In contrast, dopamine has both beneficial and detrimental actions through increasing engagement of D1R. PFC: prefrontal cortex, MSA: medial septal area, NAc: nucleus accumbens. Adapted from Berridge and Arnsten, 2013.53
Catecholamine Actions in Striatum and Thalamus
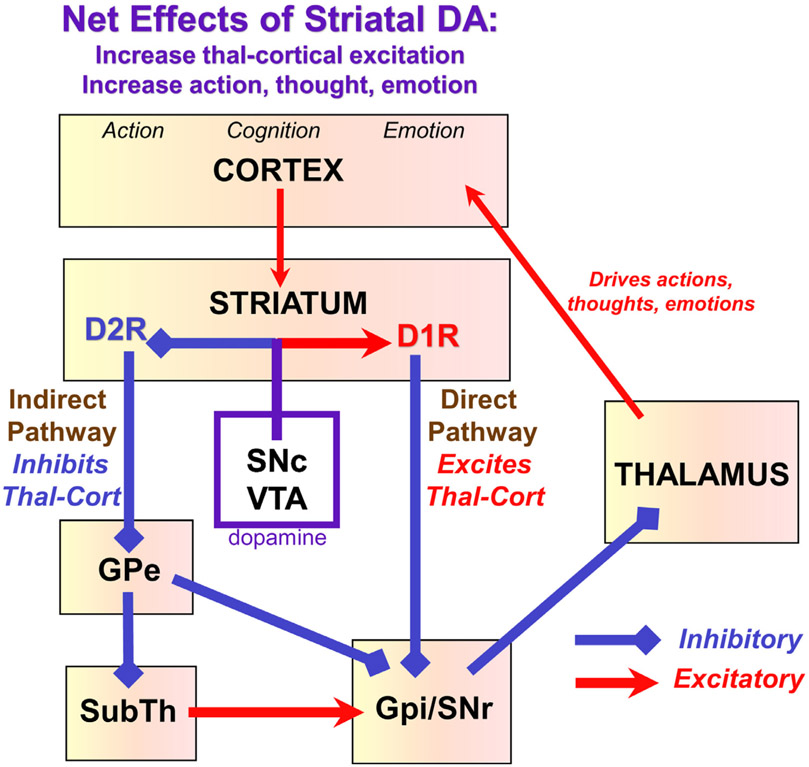
The effects of dopamine on striatal physiology and function have been studied extensively.26 The parallel pathways through the basal ganglia governing motor, cognitive and affective responses25 all involve the interplay of the Direct and Indirect circuits within the basal ganglia (Fig. 3). The Direct Pathway excites movements, thoughts, and emotions, and is potentiated by DA stimulation of D1R.59 In contrast, the Indirect Pathway inhibits movements, thoughts and emotions, but is suppressed by DA stimulation of D2R, resulting in the disinhibition of action.59 Thus, dopamine has an overall excitatory effect in striatum through both D1R and D2R mechanisms. Catecholamine actions in the thalamus have received much less attention than dopamine actions in the striatum. However, recordings from the thalamus in rats indicate that moderate increases in locus ceruleus activity enhance sensory processing through the thalamus.60,61 Of relevance to the current review, systemic administration of methylphenidate enhanced visual processing in lateral geniculate nucleus.62 Thus, some of methylphenidate’s beneficial actions may occur by enhancing thalamic functions.
FIGURE 3.
Dopamine effects on basal ganglia circuitry. The basal ganglia have parallel circuits for the control of movement, cognition, and emotion. The basal ganglia regulate the output of the thalamus, and its ability to excite the cortex. There are two major pathways emanating from the striatum: a Direct pathway that overall excites thalamocortical projections (by inhibiting the inhibitory effects of Gpi/SNr on thalamus), and an Indirect pathway that overall inhibits thalamocortical projections (by a still more complex series of connections). Dopamine facilitates movements, thoughts, and emotions by exciting the Direct pathway via D1R, and inhibiting the Indirect pathway via D2R. DA: dopamine; Thal-Cort: thalamo-cortical; GPe: globus pallidus external segment, GPi: globus pallidus internal segment; SubTh: subthalamic nucleus; SNc: substantia nigra pars compacta; SNr: substantia nigra pars reticulate; VTA: ventral tegmental area.
Catecholamines have an Inverted U Dose/Response in PFC
Both norepinephrine and dopamine have an inverted U dose/response on PFC function,58 where either insufficient or excessive levels of catecholamines impair PFC function, while moderate levels are essential for optimal function (Fig. 2B). The level of catecholamine release increases with increasing arousal (Fig. 2B), and the amount of catecholamine release can engage different receptors, for example, where moderate levels of norepinephrine during alert waking engage high affinity α2A-AR, while very high levels of norepinephrine release during stress can engage lower affinity receptors, such as α1A-AR.63 Thus, the level of catecholamine release can serve as a “switch” by engaging differing receptors.
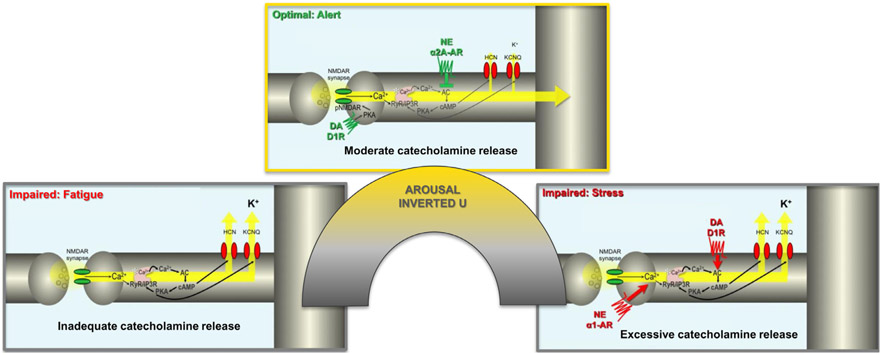
The dorsolateral PFC is able to create goals for action through recurrent, excitatory N-methyl-D-aspartate (NMDA) receptor circuits that can maintain information without sensory stimulation, the foundation of abstract thought and goal-directed behavior.36,64 As summarized in Figure 4, the connectivity of these circuits is powerfully controlled by catecholamines, with moderate levels strengthening and refining inputs, and high-level weakening connections.65 Thus, while low doses of methylphenidate may boost PFC function, these findings caution that excessive catecholamine release in PFC, for example, via doses of methylphenidate that are too high, can worsen PFC function and may be counterproductive to therapeutic strategies. An inverted U dose response on PFC physiology and function has been seen with methylphenidate administration in both rats and monkeys.66-68
FIGURE 4.
Catecholamine effects on prefrontal cortical connectivity. Catecholamines have powerful effects on the connectivity of prefrontal cortical recurrent excitatory circuits needed to generate top-down goals for action. These newly evolved synapses rely on N-methyl-D-aspartate (NMDA) receptors for neurotransmission. They also contain the molecular machinery to rapidly weaken connections through feedforward calcium-cAMP signaling, opening nearby potassium (K+) channels to functionally disconnect the circuit. Moderate levels of catecholamine release strengthen connectivity, through alpha-2A-AR inhibiting cAMP opening of K+ channels. D1R within the synapse may also enhance firing by phosphorylating NMDA receptors to maintain them within the synapse. In contrast, excessive catecholamine release weakens connectivity by driving calcium-cAMP opening of K+ channels through alpha-1AR and D1R actions at locations away from the synapse. For more detailed discussion of this topic, see the following video on how stress and fatigue can alter prefrontal function: https://www.youtube.com/watch?v=vdDvChLuQsA&t=6s.
Methylphenidate Effects in Human Subjects
In contrast to animal studies where within-subjects dose-response studies are common, understanding the methylphenidate dose-response on PFC executive function requires between study comparisons, and can reflect differing results between individuals with ADHD and those with healthier PFC abilities. For example, a meta-analysis of therapeutic doses of methylphenidate in ADHD patients shows improved executive functioning and enhancement of PFC activity measured with fMRI.69 However, studies of cognition in humans rarely administer high doses, and thus a true dose/response curve cannot easily be assessed. A meta-review of single-dose studies of methylphenidate in adults indicates that it is the lower doses (e.g., 10-20 mg) that are more optimal for working memory and paired associates learning, while higher doses (e.g., 40-60 mg) can actually impair performance70 and that the optimal dose for impulse control may differ from that for working memory.71
Positron emission tomography (PET) imaging has enabled investigators to relate doses of methylphenidate to catecholamine transporter occupancy.72,73 The estimated dose of oral methylphenidate required to block 50% of dopamine transporters in striatum (ED50) corresponded to 0.25 mg/kg,72 and of norepinephrine transporters in thalamus and other norepinephrine transporter-rich areas to 0.14 mg/kg.73 Therefore, the average efficacious maintenance doses of methylphenidate in ADHD (0.35-0.55 mg/kg) occupy 70%-80% of norepinephrine transporters but only 60%-70% of dopamine transporters. Those findings are consistent with the higher in vitro affinity of methylphenidate for norepinephrine transporters than dopamine transporters74 and suggest the potential relevance of norepinephrine transporter inhibition in the therapeutic effects of methylphenidate.
PET studies have also permitted the effect of methylphenidate on extracellular levels of dopamine to be translated from animals to human brain. Volkow et al.75 showed that oral methylphenidate (average dose 0.8 mg/kg) significantly increased extracellular dopamine in the brains of healthy control subjects, as evidenced by a significant 20% reduction in D2 receptor availability in striatum. A subsequent study by Clatworthy et al.71 reported that methylphenidate effects on dopamine release ([11C]-raclopride receptor availability) in differing striatal subregions of young healthy subjects correlated with performance on a working memory versus reversal task. These results provide direct evidence that oral methylphenidate at doses within the therapeutic range significantly increases extracellular dopamine in human brain. Unfortunately, data on increases in extracellular norepinephrine in human brain by therapeutic doses of methylphenidate are not available, and PET imaging to detect a catecholamine receptor signal is limited to subcortical brain regions.
Caveats and Limitations
Several cautionary notes must be kept in mind when exploring methylphenidate as a therapeutic for apathy in AD. First, the dose of methylphenidate must be optimized, as elderly patients may have changes in bioavailability and drug metabolism. As described above, high doses can worsen PFC function, as well as having worrisome side effects such as tachycardia. Previous and current studies of apathy in AD have not attempted a thorough exploration of the safety and efficacy of methylphenidate at doses greater than 20 mg/day.23,24 However, these doses are based on longstanding clinical experience in older adults,76 and not all participants with apathy in AD are able to tolerate a dose of 20 mg/day.23 Therefore, higher doses may not be feasible in this population. Second, the degenerative course of the disease will likely limit the beneficial actions of methylphenidate, due to neurofibrillary tangle accumulation in locus ceruleus neurons,46 reduced catecholamine concentrations in the aging cortex as compensatory mechanisms become inadequate,77-79 and the eventual degeneration of the PFC pyramidal cell circuits that guide motivated behavior.46,80 Under these decorticate conditions, increasing dopamine actions in the basal ganglia may remain, but may produce agitation and perseveration rather than enhanced motivation. Third, this review considers methylphenidate for apathy as a syndrome in isolation, whereas it frequently co-occurs with other neuropsychiatric symptoms, particularly depression.81 Since we excluded current diagnosis of major depressive episode in our previous23 and current trials, they will not address, for example, how methylphenidate may interact with traditional antidepressants or may enhance motivation to participate in nonpharmacologic interventions for depression in AD. Future studies will need to address these and other issues for patients with AD and multiple neuropsychiatric symptoms.
CONCLUSION
In this review, we have attempted to establish the public health and clinical significance of the apathy syndrome in AD, as well as the unmet need for better treatment of the symptoms of apathy. The catecholaminergic treatment methylphenidate is currently under consideration for the treatment of apathy in AD. Understanding the neuronal circuits subserving motivated behavior and their reliance on catecholamine actions provides a rationale for methylphenidate in the treatment of apathy in patients with AD. Anatomical, physiological, and behavioral studies have identified parallel, cortical-basal ganglia-thalamic circuits that govern action, cognition, and emotion and play key roles in motivated behavior. Understanding the distinct contributions to motivated behavior of subregions of the PFC—dorsolateral, orbital-ventromedial, dorsomedial—helps to explain why degeneration of these areas results in apathetic behaviors. We propose that the degeneration of the PFC in AD produces symptoms of apathy. We further propose that methylphenidate treatment may ameliorate those symptoms by boosting norepinephrine and dopamine actions in prefrontal-striatal-thalamocortical circuits.
Initial trials of methylphenidate for the treatment of apathy in AD have yielded encouraging results. In a Phase II trial (ADMET), methylphenidate (up to 10 mg orally twice daily) showed significant improvement in symptoms of apathy compared to placebo.24 More recently, Padala et al. reported that in male veterans with mild AD and apathy, this same dose of methylphenidate treatment significantly improved apathy, as well as cognition, functional status, caregiver burden, depression, and clinical global impression of change after 12 weeks of treatment.23 Both of these preliminary studies found methylphenidate at these doses to be safe and well tolerated in this population. The primary aim of the ADMET 2 study is to determine whether methylphenidate is effective in improving clinically significant apathy in a larger sample of 200 participants with AD, over a longer 6-month treatment period.
Directions for Future Research
ADMET 2 will still leave unanswered several important questions about the neurobiology of apathy and the neurobiology of methylphenidate treatment. A blood-based biomarkers administrative supplement for the ADMET 2 grant provides for collection of blood specimens in a portion of the participants to generate preliminary data about a number of potential biomarkers of apathy and its treatment in AD. The objectives are to understand biomarker correlates of apathy and of change in apathy over time, and to identify possible predictors of treatment response. In the original ADMET trial, a distinct group of approximately one-third of the sample had a dramatic response to treatment, but another third showed minimal improvement, and the final third had no benefit from treatment.24 Importantly, no significant clinical differences were observed between responders and nonresponders, suggesting that biomarker information may be necessary for prediction of treatment response. In order to elucidate this heterogeneity of response, the biomarker substudy of ADMET 2 will investigate a number of specific blood-based biomarkers, including microRNA (focused on dopamine and norepinephrine transporters), lipidomics, and markers of oxidative stress, inflammation (cytokines), and neuronal loss (S 100 calciumbinding protein B, neurofilament light chain protein). This substudy will generate preliminary data about promising blood-based biomarkers that may be further evaluated in a larger sample.
Apart from blood-based biomarkers, PET studies may yet elucidate the relative contribution of dopaminergic versus noradrenergic contributions to apathy and to methylphenidate treatment response. Longitudinal PET studies could be undertaken to compare brain dopamine and norepinephrine transporter binding in the same AD participants—to determine their relative associations with baseline apathy and longitudinal change in apathy, and to determine the predictive value of these PET measures for methylphenidate treatment response.
Acknowledgments
This work was supported by grants R01-AG046543 and P50-AG047270 from the National Institute on Aging..
Dr. van Dyck reports consulting fees from Roche, Eisai, and Kyowa Kirin; and grants for clinical trials from Biogen, Roche, Eisai, Eli Lilly, Genentech, Janssen, Novartis, Biohaven, Merck, Toyama, TauRx, and Forum. Dr. Arnsten reports consulting fees from Lundbeck; and royalties from Shire/Takeda. Dr. Porsteinsson reports DSMB membership fees from Acadia, Functional Neuromodulation, Neurim, and Tetra Discovery Partners; consulting fees from Avanir, BioXcel, Eisai, Grifols, Lundbeck, Merck, Pfizer, and Toyama; and grants for clinical trials from AstraZeneca, Avanir, Biogen, Biohaven, Eisai, Eli Lilly, Genentech/Roche, Janssen, Merck, Novartis, and Toyama. Dr. Levey reports consulting fees from Karuna and GENUV; and grants from Abbvie, Biogen, Cognito, Eisai, Genentech, and Novartis. Dr. Lanctôt reports consulting fees from Otsuka/Lundbeck, Kondor, and ICG Pharma; unpaid consulting for Highmark Interactive; and grants for clinical trials from AbbVie Canada.
Contributor Information
Christopher H. van Dyck, Yale School of Medicine, New Haven, CT.
Amy F.T. Arnsten, Yale School of Medicine, New Haven, CT.
Prasad R. Padala, University of Arkansas for Medical Sciences, Central Arkansas Veterans Healthcare System, Little Rock, AR
Olga Brawman-Mintzer, Medical University of South Carolina and Ralph H. Johnson Veterans Administration Medical Center, Charleston, SC
Alan J. Lerner, University Hospitals - Case Western Reserve University, Cleveland, OH
Anton P. Porsteinsson, University of Rochester, Rochester, NY
Roberta W. Scherer, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD
Allan I. Levey, Emory University, Atlanta, GA
Nathan Herrmann, Sunnybrook Research Institute, Toronto, ON, Canada
Nimra Jamil, Johns Hopkins University School of Medicine, Baltimore, MD.
Jacobo E. Mintzer, Medical University of South Carolina and Ralph H. Johnson Veterans Administration Medical Center, Charleston, SC
Krista L. Lanctôt, Sunnybrook Research Institute, Toronto, ON, Canada
Paul B. Rosenberg, Johns Hopkins University School of Medicine, Baltimore, MD.
REFERENCES
- 1.Steinberg M, Shao H, Zandi P, et al. : Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 2008; 23:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Steinberg M, Tschanz JT, et al. : Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000; 157:708–714 [DOI] [PubMed] [Google Scholar]
- 3.Robert P, Lanctôt KL, Agüera-Ortiz L, et al. : Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry 2018; 54:71–76 [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Lopez O, Jones B, et al. : Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288:1475–1483 [DOI] [PubMed] [Google Scholar]
- 5.Geda YE, Roberts RO, Knopman DS, et al. : Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65:1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pink A, Stokin GB, Bartley MM, et al. : Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: a population-based study. Neurology 2015; 84:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Guarino PD, Dysken MW, et al. : Neuropsychiatric symptoms and caregiver burden in individuals with Alzheimer’s disease: the TEAM-AD VA Cooperative Study. J Geriatr Psychiatry Neurol 2018; 31:177–185 [DOI] [PubMed] [Google Scholar]
- 8.Dauphinot V, Delphin-Combe F, Mouchoux C, et al. : Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: a cross-sectional study. J Alzheimers Dis 2015; 44:907–916 [DOI] [PubMed] [Google Scholar]
- 9.Truzzi A, Valente L, Engelhardt E, et al. : The association between caregiver distress and individual neuropsychiatric symptoms of dementia. Dement Neuropsychol 2013; 7:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero-Arias J, Goni-Imizcoz M, Gonzalez-Bernal J, et al. : The efficacy of nonpharmacological treatment for dementia-related apathy. Alzheimer Dis Assoc Disord 2011; 25:213–219 [DOI] [PubMed] [Google Scholar]
- 11.Lam LC, Lui VW, Luk DN, et al. : Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia—a randomized control trial. Int J Geriatr Psychiatry 2010; 25:133–141 [DOI] [PubMed] [Google Scholar]
- 12.Leone E, Deudon A, Bauchet M, et al. : Management of apathy in nursing homes using a teaching program for care staff: the STIM-EHPAD study. Int J Geriatr Psychiatry 2013; 28:383–392 [DOI] [PubMed] [Google Scholar]
- 13.Manera V, Abrahams S, Aguera-Ortiz L, et al. : Recommendations for the nonpharmacological treatment of apathy in brain disorders. Am J Geriatr Psychiatry 2020; 28:410–420 [DOI] [PubMed] [Google Scholar]
- 14.Tariot PN, Solomon PR, Morris JC, et al. : A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 2000; 54:2269–2276 [DOI] [PubMed] [Google Scholar]
- 15.Tariot PN, Cummings JL, Katz IR, et al. : A randomized, doubleblind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J Am Geriatr Soc 2001; 49:1590–1599 [PubMed] [Google Scholar]
- 16.Cummings JL, Nadel A, Masterman D, et al. : Efficacy of metrifo-nate in improving the psychiatric and behavioral disturbances of patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol 2001; 14:101–108 [DOI] [PubMed] [Google Scholar]
- 17.Feldman H, Gauthier S, Hecker J, et al. : A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology 2001; 57:613–620 [DOI] [PubMed] [Google Scholar]
- 18.Cummings JL, Koumaras B, Chen M, et al. : Effects of rivastigmine treatment on the neuropsychiatric and behavioral disturbances of nursing home residents with moderate to severe probable Alzheimer’s disease: a 26-week, multicenter, open-label study. Am J Geriatr Pharmacother 2005; 3:137–148 [DOI] [PubMed] [Google Scholar]
- 19.Berman K, Brodaty H, Withall A, et al. : Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry 2012; 20:104–122 [DOI] [PubMed] [Google Scholar]
- 20.Leontjevas R, Teerenstra S, Smalbrugge M, et al. : More insight into the concept of apathy: a multidisciplinary depression management program has different effects on depressive symptoms and apathy in nursing homes. Int Psychogeriatr 2013; 25:1941–1952 [DOI] [PubMed] [Google Scholar]
- 21.Frakey LL, Salloway S, Buelow M, et al. : A randomized, doubleblind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer’s disease. J Clin Psychiatry 2012; 73:796–801 [DOI] [PubMed] [Google Scholar]
- 22.Ishizuka T, Murotani T, Yamatodani A: Action of modafinil through histaminergic and orexinergic neurons. Vitam Horm 2012; 89:259–278 [DOI] [PubMed] [Google Scholar]
- 23.Padala PR, Padala KP, Lensing SY, et al. : Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer’s disease: a double-blind, randomized, placebo-controlled trial. Am J Psychiatry 2018; 175:159–168 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg PB, Lanctôt KL, Drye LT, et al. : Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry 2013; 74:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 1986; 9:357–381 [DOI] [PubMed] [Google Scholar]
- 26.Kreitzer AC: Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 2009; 32:127–147 [DOI] [PubMed] [Google Scholar]
- 27.Everitt BJ, Robbins TW: Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005; 8:1481–1489 [DOI] [PubMed] [Google Scholar]
- 28.Graybiel AM: Habits, rituals, and the evaluative brain. Annu Rev Neurosci 2008; 31:359–387 [DOI] [PubMed] [Google Scholar]
- 29.Cousins MS, Atherton A, Turner L, et al. : Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res 1996; 74:189–197 [DOI] [PubMed] [Google Scholar]
- 30.Basso JC, Morrell JI: The medial prefrontal cortex and nucleus accumbens mediate the motivation for voluntary wheel running in the rat. Behav Neurosci 2015; 129:457–472 [DOI] [PubMed] [Google Scholar]
- 31.Elston GN, Benavides-Piccione R, Elston A, et al. : Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol 2006; 288:26–35 [DOI] [PubMed] [Google Scholar]
- 32.Levy R, Dubois B: Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006; 16:916–928 [DOI] [PubMed] [Google Scholar]
- 33.Levy R: Apathy: a pathology of goal-directed behaviour: a new concept of the clinic and pathophysiology of apathy. Rev Neurol (Paris) 2012; 168:585–597 [DOI] [PubMed] [Google Scholar]
- 34.Szczepanski SM, Knight RT: Insights into human behavior from lesions to the prefrontal cortex. Neuron 2014; 83:1002–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuster JM: The prefrontal cortex, mediator of cross-temporal contingencies. Hum Neurobiol 1985; 4:169–179 [PubMed] [Google Scholar]
- 36.Goldman-Rakic PS: Cellular basis of working memory. Neuron 1995; 14:477–485 [DOI] [PubMed] [Google Scholar]
- 37.Rosen HJ, Hartikainen KM, Jagust W, et al. : Utility of clinical criteria in differentiating frontotemporal lobar degeneration (FTLD) from AD. Neurology 2002; 58:1608–1615 [DOI] [PubMed] [Google Scholar]
- 38.Boone KB, Miller BL, Swartz R, et al. : Relationship between positive and negative symptoms and neuropsychological scores in frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2003; 9:698–709 [DOI] [PubMed] [Google Scholar]
- 39.Bechara A, Damasio H, Damasio AR: Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 2000; 10:295–307 [DOI] [PubMed] [Google Scholar]
- 40.Barbas H, Pandya DN: Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 1989; 286:353–375 [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Jbabdi S, Zhu Z, et al. : A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. eLife 2019; 8:e43761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones SA, De Marco M, Manca R, et al. : Altered frontal and insular functional connectivity as pivotal mechanisms for apathy in Alzheimer’s disease. Cortex 2019; 119:100–110 [DOI] [PubMed] [Google Scholar]
- 43.Giannakopoulos P, Herrmann FR, Bussière T, et al. : Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003; 60:1495–1500 [DOI] [PubMed] [Google Scholar]
- 44.DeKosky ST, Scheff SW: Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990; 27:457–464 [DOI] [PubMed] [Google Scholar]
- 45.Devanand DP, Mikhno A, Pelton GH, et al. : Pittsburgh compound B (11C-PIB) and fluorodeoxyglucose (18 F-FDG) PET in patients with Alzheimer disease, mild cognitive impairment, and healthy controls. J Geriatr Psychiatry Neurol 2010; 23:185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braak H, Thal DR, Ghebremedhin E, et al. : Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70:960–969 [DOI] [PubMed] [Google Scholar]
- 47.Migneco O, Benoit M, Koulibaly PM, et al. : Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: a study in Alzheimer’s disease and non-demented patients. Neuroimage 2001; 13:896–902 [DOI] [PubMed] [Google Scholar]
- 48.Lanctot KL, Moosa S, Herrmann N, et al. : A SPECT study of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007; 24:65–72 [DOI] [PubMed] [Google Scholar]
- 49.Apostolova LG, Akopyan GG, Partiali N, et al. : Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007; 24:91–97 [DOI] [PubMed] [Google Scholar]
- 50.Mori T, Shimada H, Shinotoh H, et al. : Apathy correlates with prefrontal amyloid β deposition in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2014; 85:449–455 [DOI] [PubMed] [Google Scholar]
- 51.Kitamura S, Shimada H, Niwa F, et al. : Tau-induced focal neurotoxicity and network disruption related to apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2018; 89:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marshall GA, Gatchel JR, Donovan NJ, et al. : Regional tau correlates of instrumental activities of daily living and apathy in mild cognitive impairment and Alzheimer’s disease dementia. J Alzheimers Dis 2019; 67:757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berridge CW, Arnsten AF: Psychostimulants and motivated behavior: arousal and cognition. Neurosci Biobehav Rev 2013; 37:1976–1984 [DOI] [PubMed] [Google Scholar]
- 54.Berger B, Gaspar P, Verney C: Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci 1991; 14:21–27 [DOI] [PubMed] [Google Scholar]
- 55.Morrison JH, Foote SL: Noradrenergic and serotonergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol 1986; 243:117–138 [DOI] [PubMed] [Google Scholar]
- 56.Kuczenski R, Segal DS: Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem 1997; 68:2032–2037 [DOI] [PubMed] [Google Scholar]
- 57.Kodama T, Kojima T, Honda Y, et al. : Oral administration of meth ylphenidate (Ritalin) affects dopamine release differentially between the prefrontal cortex and striatum: a microdialysis study in the monkey. J Neurosci 2017; 37:2387–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berridge CW, Devilbiss DM, Andrzejewski ME, et al. : Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 2006; 60:1111–1120 [DOI] [PubMed] [Google Scholar]
- 59.Kravitz AV, Freeze BS, Parker PR, et al. : Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010; 466:622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moxon KA, Devilbiss DM, Chapin JK, et al. : Influence of norepinephrine on somatosensory neuronal responses in the rat thalamus: a combined modeling and in vivo multi-channel, w. Brain Res 2007; 1147:105–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogawski MA, Aghajanian GK: Activation of lateral geniculate neurons by norepinephrine: mediation by an alpha-adrenergic receptor. Brain Res 1980; 182:345–359 [DOI] [PubMed] [Google Scholar]
- 62.Navarra RL, Clark BD, Zitnik GA, et al. : Methylphenidate and atomoxetine enhance sensory-evoked neuronal activity in the visual thalamus of male rats. Exp Clin Psychopharmacol 2013; 21:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnsten AFT: Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plast 2000; 7:133–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang M, Yang Y, Wang CJ, et al. : NMDA receptors subserve working memory persistent neuronal firing In dorsolateral prefrontal cortex. Neuron 2013; 77:736–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnsten AFT, Wang M, Paspalas CD: Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 2012; 76:223–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devilbiss DM, Berridge CW: Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry 2008; 64:626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gamo NJ, Wang M, Arnsten AFT: Methylphenidate and atomoxetine improve prefrontal cortical function via noradrenergic alpha-2 and dopaminergic D1 receptor stimulation. J Amer Acad Child Adol Psychiatry 2010; 49:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berridge CW, Arnsten AF: Catecholamine mechanisms in the prefrontal cortex: proven strategies for enhancing higher cognitive function. Curr Opin Behav Sci 2015; 4:33–40 [Google Scholar]
- 69.Rubia K, Alegria AA, Cubillo AI, et al. : Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry 2014; 76:616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linssen AM, Sambeth A, Vuurman EF, et al. : Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int J Neuropsychopharmacol 2014; 17:961–977 [DOI] [PubMed] [Google Scholar]
- 71.Clatworthy PL, Lewis SJ, Brichard L, et al. : Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci 2009; 29:4690–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volkow ND, Wang GJ, Fowler JS, et al. : Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 1998; 155:1325–1331 [DOI] [PubMed] [Google Scholar]
- 73.Hannestad J, Gallezot JD, Planeta-Wilson B, et al. : Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry 2010; 68:854–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eshleman AJ, Carmolli M, Cumbay M, et al. : Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther 1999; 289:877–885 [PubMed] [Google Scholar]
- 75.Volkow ND, Wang G, Fowler JS, et al. : Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001; 21:Rc121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franzen JD, Padala PR, Wetzel MW, et al. : Psychostimulants for older adults. Curr Psychiatry 2012; 11:23–32 [Google Scholar]
- 77.Goldman-Rakic PS, Brown RM: Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience 1981; 6:177–187 [DOI] [PubMed] [Google Scholar]
- 78.Hoogendijk WJ, Feenstra MG, Botterblom MH, et al. : Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann Neurol 1999; 45:82–91 [DOI] [PubMed] [Google Scholar]
- 79.Wenk GL, Pierce DJ, Struble RG, et al. : Age-related changes in multiple neurotransmitter systems in the monkey brain. Neuro-biol Aging 1989; 10:11–19 [DOI] [PubMed] [Google Scholar]
- 80.Bussière T, Giannakopoulos P, Bouras C, et al. : Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol 2003; 463:281–302 [DOI] [PubMed] [Google Scholar]
- 81.Lyketsos CG, Sheppard JM, Steinberg M, et al. : Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry 2001; 16:1043–1053 [DOI] [PubMed] [Google Scholar]