Abstract
Background:
Anti PD-1/PD-L1 antibody therapy is a standard treatment for advanced non-small cell lung cancer (NSCLC), and PD-L1 immunohistochemistry is used as a predictive biomarker for therapeutic response. However, because not all NSCLC patients with a high PD-L1 respond, and some patients with low PD-L1 expression show durable benefit, more accurate, predictive biomarkers are needed. Circulating miRNA and miRNA packaged in extracellular vesicles (EVs) are considered to play a role in intercellular communication among immune cells and between immune cells and tumor cells and may represent a good source of mechanism-related biomarkers.
Methods:
Pretreatment plasma of advanced NSCLC patients treated with single agent anti PD-1 or PD-L1 antibody was used in this study. Plasma EVs were isolated using size-exclusion chromatography. Whole plasma and EV containing RNAs were extracted. The miRNA profile was analyzed with a next generation sequencing platform.
Results:
Samples from 14 responders (patients who showed PR or SD ≥ 6 months) and 15 non-responders (patients who showed PD in RECIST) were analyzed. In total, 32 miRNAs (p=0.0030 – 0.0495) from whole plasma and 7 EV-associated miRNAs (p=0.041 – 0.0457) showed significant concentration differences between responders and non-responders. The results of some of these circulating miRNAs were validated in a separated cohort with 8 responders and 13 non-responders. The tumor PD-L1 level was also assessed using immunohistochemistry for patients involved in both cohorts.
Conclusions:
Specific circulating miRNAs in whole plasma and plasma EVs are differentially expressed between responders and non-responders and have potential as predictive biomarkers for anti PD-1/PD-L1 treatment response.
Keywords: microRNA, extracellular vesicle, anti-PD-1, anti-PD-L1, lung cancer
1. Introduction:
Approximately 20 – 25% of unselected NSCLC patients show good responses with checkpoint immunotherapy1. The level of PD-L1 protein expression on the tumor cell surface is a reasonable, mechanism-based predictive marker for anti-PD-1 therapeutic response. However, it is still an inadequate biomarker, as many studies show only a relatively weak predictive value of PD-L1 expression toward the therapeutic efficacy of anti-PD-1 antibodies2. This is in undoubtedly due to the complexity of the immune response and the interaction of multiple factors including tumor mutation burden, tumor microenvironment, and local and systemic immune responses3. To optimize the use of these agents, avoid unnecessary cost and potentially serious side effects for patients receiving no benefit, the development of effective biomarkers is critical to optimize the therapeutic efficacy of these treatments.
Small RNAs in circulation, such as microRNAs (miRNAs) have been suggested as biomarkers for various diseases and health conditions including NSCLC5. However, most of the studies have not considered Extracellular vesicle (EV)-encapsulated miRNAs, but rather analyze miRNAs in whole biofluids like serum or plasma, which contain many non-EV RNAs complexed with lipoproteins and RNA-binding proteins6,7. EVs are small membrane-encapsulated particles (including exosomes and microvesicles) released from a wide variety of cell types (specifically from both immune and cancer cells) and have been implicated to be involved in many biological processes, including modulating the immune response4. EVs contain a wide variety of biomolecules including proteins, metabolites and RNAs. There have not been studies on either EVs or whole biofluids to investigate the possibility of using miRNA to predict patients’ response to any type of immunotherapy.
To address this, we investigated whether the miRNA patterns from whole plasma and EVs might be used as biomarkers to predict the efficacy of anti-PD-1/PD-L1 immunotherapy for NSCLC patients. From a set of 29 pre-treatment plasma samples obtained from NSCLC patients undergoing PD-1/PD-L1 immunotherapy (14 responders and 15 non-responders, cohort 1), 32 miRNAs in whole plasma, and 7 in EVs were identified that exhibited concentration differences between responders and non-responders using a next generation sequencing (NGS) platform. These immunotherapy response associated miRNAs appear to be involved in biological processes and pathways related to cancer and immune response. Furthermore, some of these miRNAs can also be used to predict patients’ response toward anti-PD-1/PD-L1 immunotherapy. Some of these immunotherapy response associated circulating miRNAs were validated in second cohort containing 8 responders and 13 non-responders.
2. Methods and Materials:
Study design and patient selection
Plasma samples from metastatic NSCLC patients were collected before PD-1/PD-L1 monotherapy as part of Total Cancer Care Protocol (TCCP) at The Ohio State University. Based on previously established surrogate endpoints for survival8, we classified patients who showed PR (partial response) or SD (stable disease) lasting more than 6 months as “responders”, and the patients who showed PD (progressive disease) as “non-responders”. The cohorts used in the study included a total of 29 NSCLC subjects: 14 responders and 15 non-responders in cohort 1, and a total of 21 NSCLC subjects: 8 responders and 13 non-responders in cohort 2. PD-L1 immunohistochemistry (IHC) was performed with PD-L1 IHC 28–8 pharmDx (Dako North America, Inc, CA, USA) or PD-L1 IHC 22C3 pharmDx (Dako North America, Inc). Percent of tumor cells positive for PD-L1 equals to or more than 1% was assigned as positive for PD-L1 IHC.
EV isolation and miRNA characterization
The plasma samples were spun at 10,000 X g at 4°C for 10 minutes to remove cellular debris prior to EV and RNA isolation. EVs were isolated from 200 μL of plasma using qEV size-exclusion column (Izon Science, Cambridge, MA). Eluate fractions (~500 μL/per fraction) were collected individually and the particle count was determined using the qNano (Izon Science, Cambridge, MA) for each fraction. The fractions containing EV particles were then pooled and concentrated to ~200 μL using Amicon 10K centrifugation filters (EMD Millipore, Billerica, MA). RNA was isolated from either 200 μL of concentrated EVs, or 200 μL of whole plasma using the miRNeasy kit (QIAGEN, Germantown MD) according to the manufacturer’s instructions. To characterize the miRNA spectrum in EVs and whole plasma, a modified small-RNAseq (sRNAseq) library construction protocol was used9.
Data analysis
Sequence files were processed with sRNAnalyzer, a small RNA data analysis pipeline developed in-house10. After removing short (less than 15 nucleotides) and low quality reads, the remaining processed reads, were mapped against various sequence databases. For miRNA, miRBase V21 (www.mirbase.org) was used with 0 mismatches allowed. The miRNA mapping results were normalized using read count per million (RPM) of processed reads. The detectable miRNAs were selected based on having more than 10 mapped reads in at least 70% of the samples. The detectable miRNAs that had a ≥ ± 1.5 fold concentration difference and p-value < 0.05 (Wilcoxon rank-sum test) were selected as miRNA associated with the efficacy of anti-PD-1 immunotherapy. In addition, several invariant miRNAs, such as miR-30a-5p, were identified based on its low coefficient of variance across samples. The miRTarBase database (mirtarbase.mbc.nctu.edu.tw/)11 was used to identify miRNA targets for functional analysis. Only targets that were identified by at least two different experimental techniques, were selected. The functional enrichment analysis was performed with DAVID (Database for Annotation, Visualization and Integrated Discovery, https://david.ncifcrf.gov/)12.
qRT-PCR
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) based on TaqMan Advanced miRNA assay (Thermo Fisher, Waltham, MA) was used to verify the concentration changes of miRNAs. The invariant miRNA, miR-30a-5p, was used to normalize the PCR data. Ct values greater than 35 were excluded from analysis.
Classification performance
The area under the curve (AUC) value from a receiver operating characteristic (ROC) curve was calculated using the best performing miRNA panels with either two or three miRNAs from the five miRNAs (miR-199a-3p, miR-200c-3p, miR-21–5p, miR-28–5p and miR-30e-3p) validated in cohort 2 and PD-L1 IHC in cohort 1 and 2. Positive likelihood ratios were calculated based on the on the classification rates.
3. Results and Discussion
This is the first study to investigate differences in the circulating miRNA profiles in pre-treatment whole plasma and EVs between responders and non-responders receiving single agent anti-PD-1 immunotherapy. The study involved two patient cohorts and the characteristics of the two cohorts, cohort 1: 29 advanced NSCLC patients (14 responders and 15 non-responders), and cohort 2: 21 advanced NSCLC patients (8 responders and 13 non-responders), are listed in Table 1. The cohort 2 tended to have more patients with performance status 2, and more responders for pembrolizumab treatment. PD-L1 IHC data was available for 16 out of the 29 patients of cohort 1, and 11 out of the 21 patients of cohort 2. The small RNAs in whole plasma and plasma EVs were characterized using sRNAseq. General sequencing statistics in whole plasma and EVs of responder and non-responder samples are listed in Table 2. On average, about 4 million reads mapped to about 1,000 different miRNAs from whole plasma and around 650,000 reads mapped to about 650 different miRNAs in purified EVs. Compared to whole plasma, the EVs have much less miRNA, which is in agreement with published reports13. When looking at the overall difference between responders and non-responders, there are significantly more miRNAs in non-responder whole plasma samples.
Table 1:
Patient characteristics.
| Cohort 1 | Cohort 2 | ||||
|---|---|---|---|---|---|
| Non-responder | Responder | Non-responder | Responder | ||
| n=15 | n=14 | n=13 | n=8 | ||
| Age | median | 68 | 62.5 | 64.5 | 63.5 |
| Range | 50–83 | 45–86 | 44–77 | 55–75 | |
| Gender | male | 6 | 8 | 10 | 5 |
| Female | 9 | 6 | 3 | 3 | |
| Race | Caucasian | 15 | 12 | 11 | 7 |
| African American | 0 | 2 | 2 | 1 | |
| Smoking history | current | 3 | 4 | 4 | 3 |
| former | 11 | 10 | 9 | 5 | |
| never | 1 | 0 | 0 | 0 | |
| pack year, average | 37.9 | 50.8 | 38.9 | 40.0 | |
| Performance status | 0 | 3 | 2 | 0 | 2 |
| 1 | 10 | 11 | 10 | 5 | |
| 2 | 2 | 1 | 3 | 1 | |
| Histology | adenocarcinoma | 9 | 7 | 6 | 4 |
| squamous cell | 6 | 5 | 5 | 1 | |
| adenosquamous | 0 | 1 | 0 | 0 | |
| NOS | 1 | 1 | 2 | 3 | |
| PD-L1 IHC* | positive | 5 | 6 | 2 | 5 |
| negative | 3 | 2 | 2 | 2 | |
| unknown | 7 | 6 | 9 | 1 | |
| EGFR | wild | 10 | 9 | 9 | 7 |
| uncommon | 1 | 0 | 0 | 0 | |
| exon19 deletion | 1 | 1 | 0 | 0 | |
| unknown | 3 | 4 | 4 | 1 | |
| ALK | wild | 11 | 10 | 9 | 7 |
| Unknown | 4 | 4 | 4 | 1 | |
| Stage | IIIB | 1 | 0 | 0 | 0 |
| IV | 14 | 14 | 13 | 8 | |
| Drug | nivolumab | 15 | 13 | 8 | 3 |
| Atezolizumab | 0 | 1 | 3 | 0 | |
| Pembrolizumab | 0 | 0 | 2 | 5 | |
Cases positive for PD-L1 had 1% or more tumor cells expressing PD-L1 by immunohistochemistry.
Abbreviation : NOS, not otherwise specified
Table 2:
General statistics of small RNA sequencing results
| Sample | Whole plasma | Extracellular vesicle (EV) | ||||
|---|---|---|---|---|---|---|
| Non-responders (mean) |
Responder (mean) |
p-Value | Non-responders (mean) |
Responder (mean) |
p-Value | |
| Raw read count | 7,681,934 | 6,552,515 | 0.165 | 5,716,147 | 5,361,041 | 0.63 |
| Total number of read mapped to miRNA | 4,477,728 | 3,587,555 | 0.171 | 740,376 | 580,498 | 0.525 |
| miRNA with 1 or more mapped read | 1,099 | 981 | 0.046* | 678 | 629 | 0.334 |
| miRNA with 10 or more mapped reads | 548 | 478 | 0.030* | 342 | 321 | 0.477 |
Represents statistically significant difference.
From the small RNA sequencing results, 32 miRNAs in whole plasma (Figure 1A and Table 3) and 7 miRNAs in EVs (Figure 1B, and Table 3) showed significant concentration differences between responders and non-responders. Some of these miRNAs, such as the miR-21 and miR-200 family members (Table 3), are known to be associated with different types of cancers14. Prior studies have examined the circulating miRNA in NSCLC patients to identify diagnostic markers. Of these, seven miRNAs (miR-21-3p, miR-24-1-3p, miR-30d-3p, miR-200c-3p, miR-28-5p, miR-22-5p, miR-191-3p) previously shown to be elevated in NSCLC patient plasma compared to healthy controls5 , were lower in the anti-PD-1/PD-L1 pre-treatment plasma of responders compared to non-responders in our study. The miRNAs identified in the few prior EV/exosomal miRNA studies did not overlap with miRNAs identified here. This is not a surprise since none of the previous studies focused on the differences between responders and non-responders of anti-PD-1 immunotherapy.
Figure 1. Circulating miRNAs showed concentration differences between responders and non-responders.
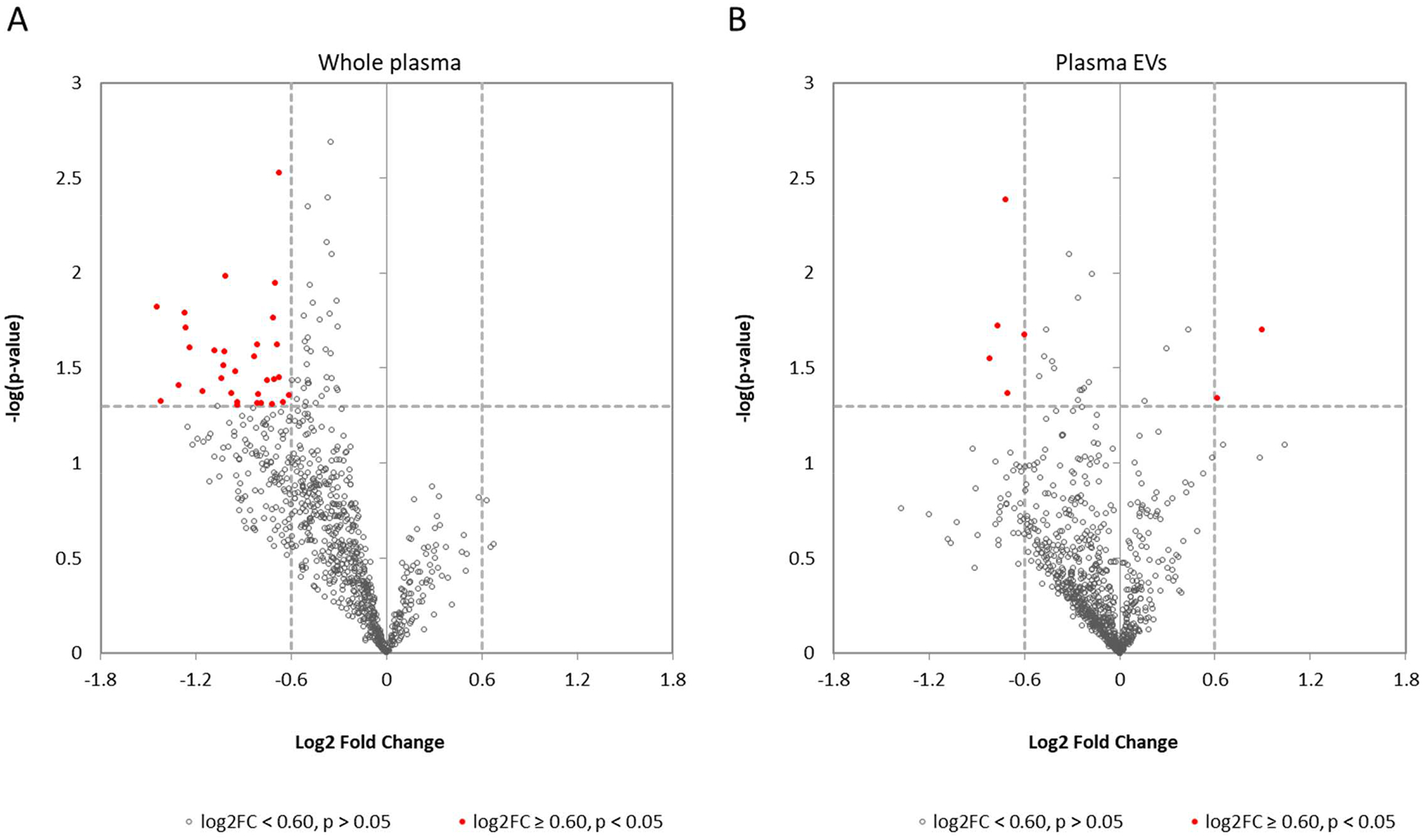
(A) Volcano plot identifying miRNAs that have significant concentration differences (red dots) between responders and non-responders in whole plasma. (B) Volcano plot showing miRNAs that with significant concentration differences (red dots) between responders and non-responders in plasma EVs.
Table 3:
List of miRNAs showing concentration differences between responder and non-responder
| miRNA | Source | log2 RPM non-responder | log2 RPM responder | Log2FC | P-value |
|---|---|---|---|---|---|
| miR-548am-5p | whole plasma | 4.20 | 2.30 | −1.9 | 0.008924 |
| miR-200a-3p | whole plasma | 4.25 | 2.80 | −1.45 | 0.015159 |
| miR-4707-3p | whole plasma | 3.43 | 2.01 | −1.42 | 0.047221 |
| miR-335-3p | whole plasma | 6.09 | 4.78 | −1.31 | 0.039141 |
| miR-429-3p | whole plasma | 3.11 | 1.84 | −1.27 | 0.016288 |
| miR-200b-3p | whole plasma | 5.09 | 3.82 | −1.26 | 0.019483 |
| miR-191-3p | whole plasma | 4.70 | 3.47 | −1.24 | 0.024736 |
| miR-1277-3p | whole plasma | 5.70 | 4.54 | −1.15 | 0.042013 |
| miR-200c-3p | whole plasma | 6.14 | 5.06 | −1.08 | 0.02577 |
| miR-28-5p | whole plasma | 7.96 | 6.93 | −1.04 | 0.035919 |
| miR-3120-3p | whole plasma | 3.78 | 2.75 | −1.02 | 0.030878 |
| miR-152-3p | whole plasma | 7.93 | 6.92 | −1.02 | 0.026069 |
| miR-335-5p | whole plasma | 10.43 | 9.42 | −1.01 | 0.010409 |
| miR-199a-1-3p | whole plasma | 12.17 | 11.19 | −0.98 | 0.043257 |
| miR-22-5p | whole plasma | 7.45 | 6.50 | −0.95 | 0.033056 |
| miR-30e-3p | whole plasma | 6.09 | 5.15 | −0.94 | 0.049463 |
| miR-33a-5p | whole plasma | 3.72 | 2.78 | −0.94 | 0.047688 |
| miR-556-5p | whole plasma | 2.53 | 1.71 | −0.83 | 0.027636 |
| miR-21-3p | whole plasma | 6.90 | 6.08 | −0.82 | 0.023739 |
| miR-30d-3p | whole plasma | 3.60 | 2.79 | −0.81 | 0.048456 |
| miR-130b-5p | whole plasma | 6.46 | 5.65 | −0.81 | 0.043705 |
| miR-24-1-3p | whole plasma | 12.85 | 12.06 | −0.79 | 0.048364 |
| miR-3138-3p | whole plasma | 2.80 | 2.05 | −0.75 | 0.036909 |
| miR-548ax-5p | whole plasma | 2.20 | 1.48 | −0.72 | 0.049165 |
| miR-6791-3p | whole plasma | 2.26 | 1.55 | −0.71 | 0.017305 |
| miR-1287-5p | whole plasma | 3.25 | 2.54 | −0.71 | 0.036516 |
| miR-3074-5p | whole plasma | 2.14 | 1.44 | −0.7 | 0.011355 |
| miR-103a-1-3p | whole plasma | 11.31 | 10.62 | −0.69 | 0.023826 |
| miR-21-5p | whole plasma | 14.29 | 13.62 | −0.67 | 0.035532 |
| miR-130b-3p | whole plasma | 8.45 | 7.77 | −0.67 | 0.002988 |
| miR-186-5p | whole plasma | 10.84 | 10.19 | −0.65 | 0.047882 |
| miR-660-3p | whole plasma | 2.87 | 2.25 | −0.61 | 0.043853 |
| miR-1246-5p | plasma EVs | 1.71 | 2.61 | 0.9 | 0.019986 |
| miR-1296-5p | plasma EVs | 3.02 | 2.20 | −0.82 | 0.02823 |
| miR-4707-3p | plasma EVs | 1.42 | 0.65 | −0.77 | 0.019005 |
| miR-1229-3p | plasma EVs | 1.35 | 0.64 | −0.71 | 0.004144 |
| miR-874-3p | plasma EVs | 2.59 | 1.89 | −0.7 | 0.043249 |
| miR-378c-5p | plasma EVs | 0.64 | 1.26 | 0.62 | 0.045665 |
| miR-1468-5p | plasma EVs | 1.71 | 2.61 | −0.6 | 0.021257 |
Based on the miRNA levels in circulation and concentration changes between responders and non-responders, several miRNAs, including miR-199a-3p, miR-200c-3p, miR-21–5p, miR-28–5p and miR-30e-3p were selected for qRT-PCR verification (Table 3 and Figure 2A). We were able to verify the changes of these selected miRNAs in plasma as shown in Figure 2B. For the affected miRNAs in EVs, due to the low miRNA concentration and the lack of commercially available reagents for the miRNAs identified, we could not perform qRT-PCR verification.
Figure 2. qRT-PCR verification of selected plasma miRNAs.
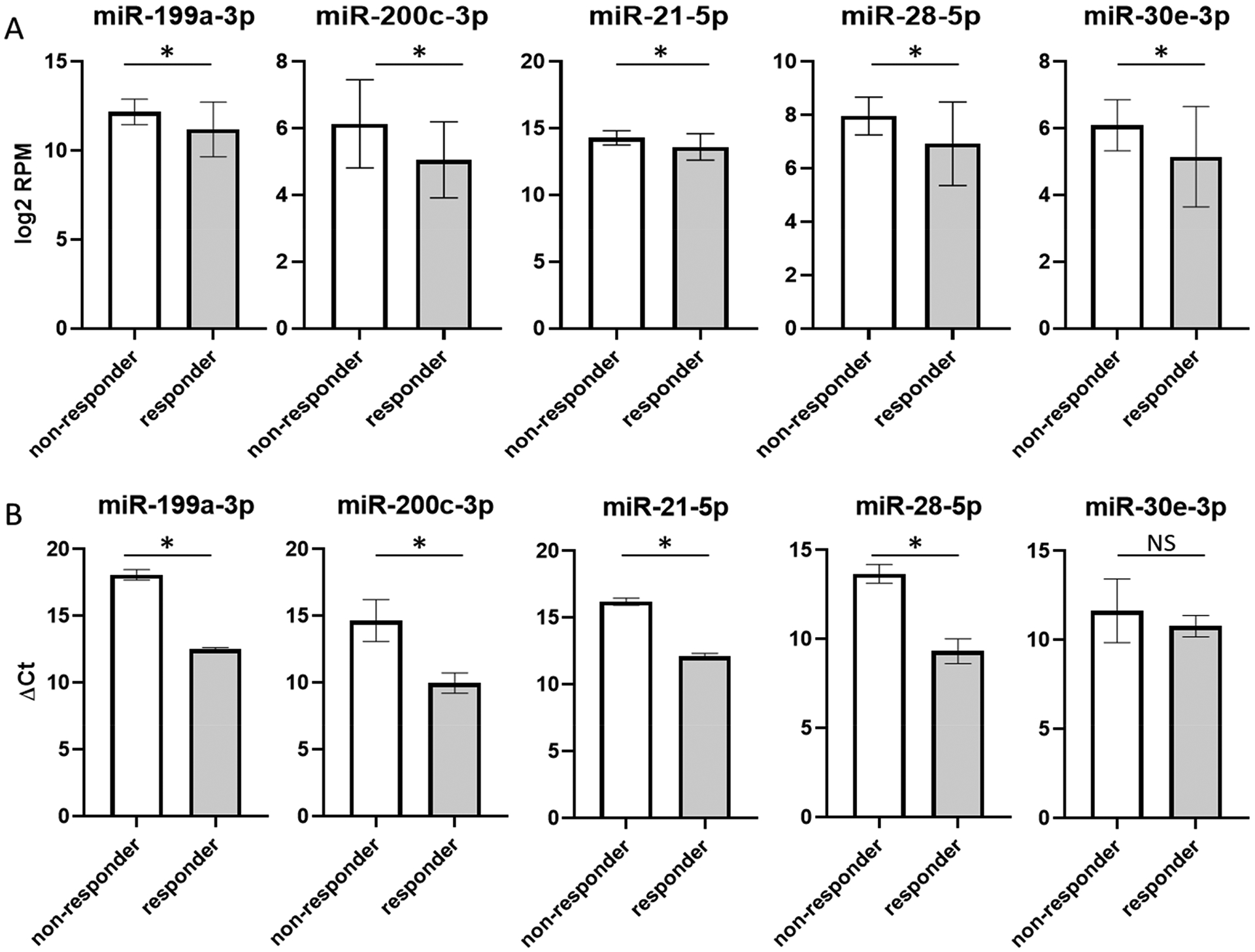
(A) Selected miRNAs chosen for follow up verification. All showed decreased concentration in responders. Y-axis represents miRNA concentration in log2 value of Read Per Million processed reads. (B) qRT-PCR verification results. Y-axis represents miRNA concentration in ΔCt values. White bars are non-responders, and grey bars are responders. Asterisk sign indicates statistically significant change (p < 0.05) and NS indicates the changes are not statistically significant.
Using information from experimental validated mRNA targets for miRNAs, a set of gene targets for the anti-PD-1/PD-L1 therapeutic response associated miRNAs were identified and used for pathway analysis. Results from gene enrichment analysis suggested that these miRNAs are involved in many cancer and immune system related pathways (based on Kyoto Encyclopedia of Genes and Genomes (KEGG) database) (Supplementary Figure 1).
To validate the immunotherapy response associated miRNAs identified in cohort 1, we performed qRT-PCR on samples from a separate cohort (cohort 2) with the same set of miRNAs used in cohort 1 (miR-199a-3p, miR-200c-3p, miR-21–5p, miR-28–5p and miR-30e-3p) (Figure 3A). The miR-200c-3p, miR-21–5p, and miR-28–5p levels were statistically significantly decreased in responders in cohort 2. The miR-199a-3p level was decreased in responders and the concentration change was well within our cutoff level, but the p-value was > 0.05. As observed in cohort 1, the miR-30e-3p was not validated by qRT-PCR in cohort 2.
Figure 3. Validation of selected plasma miRNAs in cohort 2.
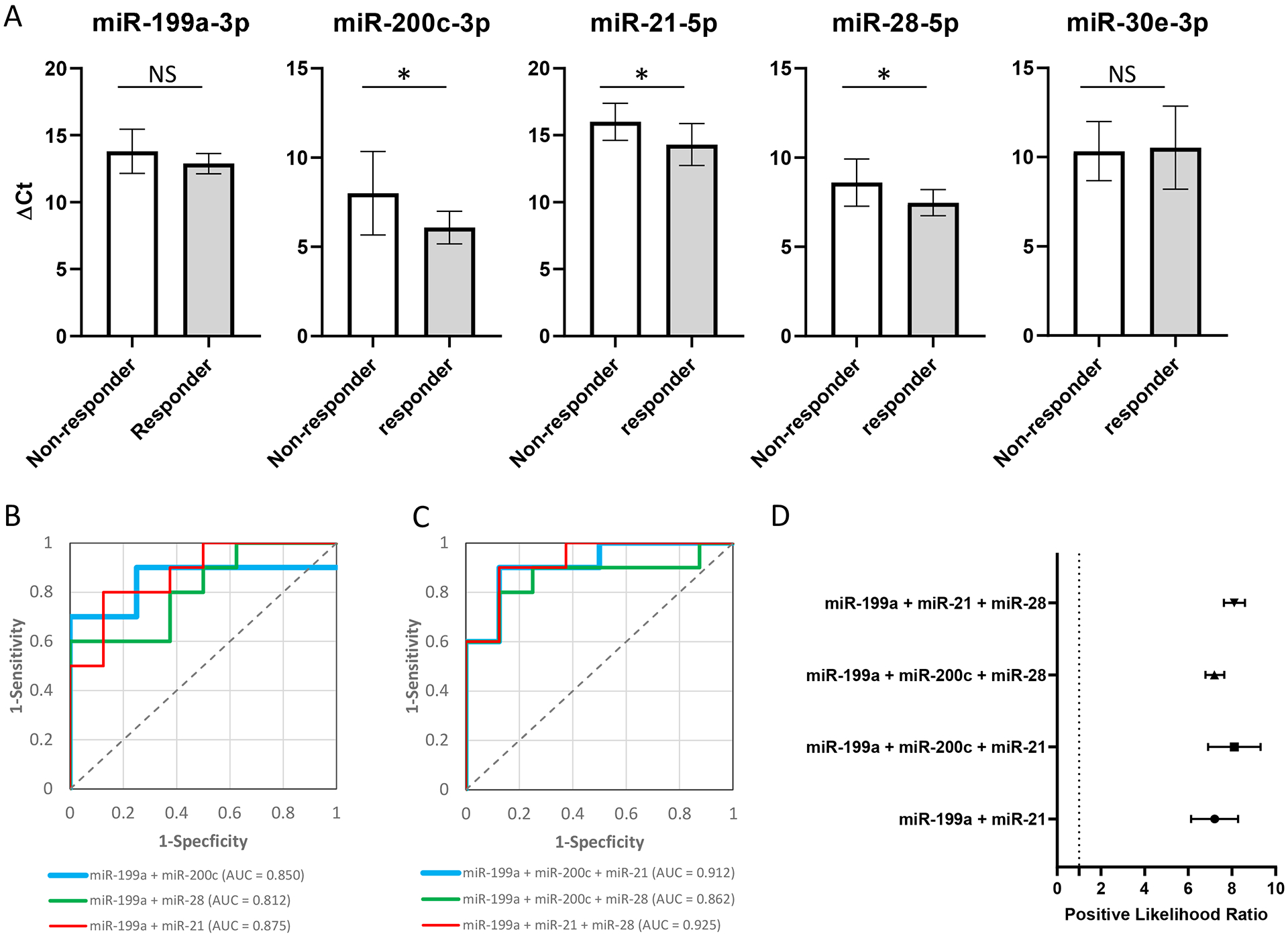
(A) qRT-PCR validation results. Y-axis represents miRNA concentration in ΔCt values. White bars are non-responders, and grey bars are responders. Asterisk sign indicates statistically significant change (p < 0.05) and NS indicates the changes are not statistically significant. (B), (C) ROC (receiver operator characteristic) curves to calculate diagnostic accuracy with combinations of two or three plasma miRNAs (B and C, respectively). The AUC (area under the curve) was calculated for each ROC curve. (D) Positive likelihood ratios were calculated with the plasma miRNA combinations with the four best AUC values (miR-199a-5p + miR-21–5p, the 3 miRNAs combinations).
To assess the ability of using the concentration of specific circulating miRNAs to predict patient’s response to anti-PD-1 immunotherapy, different combinations of either 2 or 3 miRNAs were tested based on the qRT-PCR data. The AUC (area under the curve) of a ROC (receiver operator characteristic) curve (sensitivity vs specificity) was calculated, and several panels of either 2 or 3 miRNAs showed good performance (Figures 3B and C). The highest performing set was a combination of 3 miRNAs: miR-199a-3p, miR-21–5p, and miR-28–5p, which gave an AUC of 0.925 with the best positive likelihood ratio (Figure 3D). The AUC and positive likelihood ratio were also calculated for PD-L1 IHC in the patients with PD-L1 IHC data in cohort 1 and 2, which were 0.575 (Supplementary Figure 2), and 1.257 (CI: 0.713–2.217), respectively. This suggests that the combination miRNA biomarker panel perform better than PD-L1 IHC at predicting response to immunotherapy.
Levels of some of these miRNAs have also been associated with clinical outcomes in NSCLC patients. Elevated miR-21–5p and miR-30d-3p are associated with poor outcomes and reduced survival15, 16, and miR-21–5p and miR-24–3p were significantly decreased in post-operative sera compared with levels in paired pre-operative sera16. This suggests the lower levels of those miRNAs in patients might result in less perturbation of cancer related pathways, resulting in a more robust therapeutic response with PD-1/PD-L1 immunotherapy. Because the EV miRNAs associated with therapeutic response are poorly studied with very few validated targets, functional enrichment analysis cannot be reliably performed. However, these EV miRNAs represent new candidate miRNAs associated with NSCLC and immunotherapy and should be studied further.
When investigating predictive biomarkers for treatment response, it is important to distinguish predictive biomarkers from prognostic biomarkers. Based on the encouraging results of this study, further study is warranted to determine the involvement of therapeutic response biomarkers in disease prognosis, and establish the utility of these potential biomarkers.
To compare the predictive value of our miRNA biomarkers with the only approved biomarker for immunotherapy response, we performed an AUC analysis using PD-L1 tumor percentage as determined by IHC. The PD-L1 IHC data for some of the patients could not be obtained because their PD-L1 status was not in their medical record and FFPE samples were not available for testing in some of the patients. However, for the number of samples that were available, the AUC calculated for PD-L1 IHC in our study was compatible with a previous report17. In addition, our study also lacks information on the tumor mutation burden (TMB), which is limitation of this study.
In conclusion, circulating miRNAs in whole plasma and miRNAs encapsulated in EVs are differentially expressed in IO responders vs. IO non-responders and could have potential as predictive biomarkers for anti PD-1/PD-L1 treatment response. Further investigation of circulating RNAs including EV encapsulated RNAs are warranted to find more accurate biomarkers to predict benefit from anti-PD-1/PD-L1 therapy.
Supplementary Material
Acknowledgements:
We appreciate the technical support by Dr. Mohammed Rahman, and support for analyzing clinical data by Dr. Michael Smith.
This work was supported by a Lilly Oncology Fellowship from The Japanese Respiratory Society (TS), an alumni scholarship from the Juntendo University School of Medicine (TS), a research fellowship from Uehara Memorial Foundation (TS), a Pelotonia postdoctoral fellowship (TS), DoD W81XWH-16-1-0301 and W911NF-17-2-0086 (KW), NIH grants U01HL126496-02, R56HL133887, U01CA213330 and R01DA040395 (KW), Grant from Bristol-Myers Squibb (DPC), and James Thoracic Oncology Center Fund (DPC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
No conflict relevant to this work
Dr. Shukuya reports personal fees from Astrazeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, Taiho Pharma, Daiichi-Sankyo, Oho Pharmaceutical, and Bristol-Myers Squibb, grants and personal fees from MSD outside the submitted work. Dr. Carbone reports personal fees from Abbvie, personal fees from Adaptimmune, personal fees from Agenus, personal fees from Amgen, personal fees from Ariad, personal fees from AstraZeneca (AZ), personal fees from Biocept, personal fees from Boehringer Ingelheim, grants and personal fees from Bristol Myers-Squibb (BMS), personal fees from Celgene, personal fees from Clovis, personal fees from EMD Serono, personal fees from Foundation Medicine, personal fees from Genentech/Roche, personal fees from Gritstone, personal fees from Guardant Health, personal fees from Helsinn, personal fees from Humana, personal fees from Incyte, personal fees from Inivata, personal fees from Inovio, personal fees from Janssen, personal fees from Kyowa Kirin, personal fees from Merck, personal fees from Merck Sharp Dohme (MSD), personal fees from Novartis, personal fees from Palobiofarma, personal fees from Pfizer, personal fees from prIME Oncology, personal fees from Stemcentrx, personal fees from Takeda, personal fees from Teva, personal fees from Nexus Oncology, outside the submitted work.
References
- 1.Shukuya T, Carbone DP. Predictive Markers for the Efficacy of Anti-PD-1/PD-L1 Antibodies in Lung Cancer. J Thorac Oncol. 2016;11(7):976–988. doi: 10.1016/j.jtho.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleinovink JW, Marijt KA, Schoonderwoerd MJA, van Hall T, Ossendorp F, Fransen MF. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology. 2017;6(4). doi: 10.1080/2162402X.2017.1294299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zappasodi R, Merghoub T, Wolchok JD. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell. 2018;33(4):581–598. doi: 10.1016/j.ccell.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PD, Morelli AE. Regulation of Immune Responses by Extracellular Vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghai V, Lee I, Wang K. Circulating miRNAs as Tumor Biomarkers In: Dammacco F, Silvestris F, eds. Oncogenomics. Academic Press; 2019:191–206. doi: 10.1016/B978-0-12-811785-9.00013-2 [DOI] [Google Scholar]
- 6.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38(20):7248–7259. doi: 10.1093/nar/gkq601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukuya T, Mori K, Amann JM, et al. Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1/PD-L1 Antibodies. J Thorac Oncol. 2016;11(11):1927–1939. doi: 10.1016/j.jtho.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etheridge A, Wang K, Baxter D, Galas D. Preparation of Small RNA NGS Libraries from Biofluids In: Extracellular RNA. Methods in Molecular Biology. Humana Press, New York, NY; 2018:163–175. doi: 10.1007/978-1-4939-7652-2_13 [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Kim T-K, Baxter D, et al. sRNAnalyzer-a flexible and customizable small RNA sequencing data analysis pipeline. Nucleic Acids Res. 2017;45(21):12140–12151. doi: 10.1093/nar/gkx999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu S-D, Lin F-M, Wu W-Y, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011;39(Database issue):D163–169. doi: 10.1093/nar/gkq1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 13.Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–14893. doi: 10.1073/pnas.1408301111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Shen H, Qiu C, et al. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. European Journal of Cancer. 2013;49(3):604–615. doi: 10.1016/j.ejca.2012.09.031 [DOI] [PubMed] [Google Scholar]
- 16.Le H-B, Zhu W-Y, Chen D-D, et al. Evaluation of dynamic change of serum miR-21 and miR-24 in pre- and post-operative lung carcinoma patients. Med Oncol. 2012;29(5):3190–3197. doi: 10.1007/s12032-012-0303-z [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Stein JE, Rimm DL, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019. July 18. doi: 10.1001/jamaoncol.2019.1549. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


