Abstract
CUDC-907 is a novel dual-acting inhibitor of phosphoinositide 3-kinase (PI3K) and histone deacetylase (HDAC). In this study, we aimed to explore the anticancer effects of CUDC-907 on human breast cancer cells. Our results showed that CUDC-907 effectively inhibited breast cancer cell proliferation. Flow cytometry analysis revealed that CUDC-907 induced cell cycle arrest and apoptosis in breast cancer cells. The combined treatment of CUDC-907 and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resulted in a marked increase in apoptosis and cleavage of caspase-8, −9 and poly (ADP-ribose) polymerase (PARP) in breast cancer cells. CUDC-907 enhanced expressions of death receptor 5 (DR5), reduced the levels of anti-apoptotic molecules XIAP, Bcl-2 and Bcl-xL. Knockdown of DR5 abrogated apoptosis induced by the combination of CUDC-907 and TRAIL in breast cancer cells. CUDC-907 increased the phosphorylation of JNK and p38 MAPK. JNK inhibitor pretreatment attenuated CUDC-907-induced upregulation of DR5. In summary, CUDC-907 shows potent cytotoxicity against breast cancer cells and facilitates TRAIL-mediated apoptosis through DR5 upregulation. The combination of CUDC-907 and TRAIL may be a promising therapeutic approach in the treatment of breast cancer.
Keywords: CUDC-907, Breast cancer, TRAIL, Apoptosis, DR5, MAPK
Introduction
Breast cancer is the most commonly diagnosed and the second leading cause of cancer-related deaths among women (Scimeca et al. 2019). Over the past several decades, significant advances have been made in the understanding of the molecular pathology and therapeutic approaches in breast cancer, which have led to a decrease in breast cancer mortality rates (Nahleh et al. 2019). However, there were still approximately 70,700 deaths of breast cancer in China in 2015 (Chen et al. 2016). For breast cancer, the treatment modalities mainly include surgery, chemotherapy, radiotherapy and endocrine therapy. As an effective means of treating cancer patients, chemotherapy can increase survival and alleviate symptoms of breast cancer. However, the use of chemotherapeutic drugs is frequently limited because of drug-resistance and serious drug-induced side effects (Li et al. 2018). Therefore, it is imperative to identify and characterize more effective agents or new therapeutic strategies to achieve enhanced anticancer efficacy.
The phosphatidylinositol 3-kinases (PI3Ks) are a family of lipid kinases that catalyze phosphorylation of phosphoinositide at the 3-OH position of the inositol ring. PI3K signaling contributes to a variety of biological processes that are critical in mediating multiple cellular functions, including cellular survival, proliferation and migration in different physiological and pathological conditions (Weinberg 2016). Aberrant alterations and activations of the PI3K pathway have been linked with breast cancer tumorigenesis, drug resistance and clinical outcome (Zheng et al. 2018). Therefore, specific targeting of PI3K signaling could be a reasonable strategy in the treatment of various human cancers including breast cancer. Small molecule inhibitors of PI3K have exhibited promising activities and impressive results in breast cancer clinical trials (McRee et al. 2018; Rodon et al. 2018).
Histone deacetylases (HDACs) belong to a family of enzymes that catalyze the removal of acetyl groups from lysine residues in the amino terminal tail of histones, making the surrounding DNA less accessible to transcription factors (Yuan et al. 2009). Given that histone modification modulates gene expression, it is not surprising that aberrant expression of HDACs is associated with a wide variety of human cancers and correlates with poor prognosis (Vancurova et al. 2018). Accumulating evidences have revealed that HDACs inhibitors exert profound antiproliferative or pro-apoptotic activities in many different types of tumor, and a variety of established inhibitors of HDAC are being tested in clinical trials of all phases (Li and Seto 2016; Singh et al. 2011).
As an orally bioavailable small-molecule inhibitor, CUDC-907 has shown broad anticancer activities in hematologic and solid tumors (Kotian et al. 2017; Chen et al. 2019). It also enhanced radiosensitivity by inhibiting radiation-induced DNA repair pathways in gliomas (Pal et al. 2018). In the current study, we detected cytotoxic effect of CUDC-907 in breast cancer cells and explored the potential role of it on the TRAIL-induced apoptosis. Our data indicated that CUDC-907 inhibited cell proliferation, triggered DNA damage, cell cycle arrest and apoptosis in breast cancer cells. Moreover, CUDC-907 enhanced TRAIL-induced apoptosis via upregulating DR5 expression, which may allow us to develop a promising therapeutic strategy for cancer treatment.
Materials and methods
Cell lines
Human MCF-7 and MDA-MB-231 breast cancer cell line were obtained from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Gibco, USA) and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Reagents and antibodies
CUDC-907 was purchased from Selleck (Shanghai, China). TRAIL was purchased from R&D system (Minneapolis, USA). JNK Inhibitor SP600125 was purchased from Merck chemicals (Darmstadt, Germany). P38 MAPK inhibitor SB203580 was obtained from TopScience (Shanghai, China). Anti-p21, anti-p53, anti-ERK1/2, anti-p-ERK1/2, anti-p38, anti-cyclinB1, anti-p70S6K, anti-Cdc2, anti-Bcl-2, anti-XIAP, anti-Bcl-xL and anti-Bax antibodies were purchased from Santa cruz. Anti-cyclinD1 and anti-γ-H2AX antibodies were obtained from Abcam (Shanghai, China). Anti-p-p70S6K, anti-Cdc25C, anti-p-p38, anti-Cdc25C and anti-p-Cdc25C antibodies were purchased from Cell Signaling Technology (Shanghai, China). Anti-p-Akt antibody was purchased from Merck Millipore (Darmstadt, Germany). Anti-p27, anti-Akt, and anti-p-JNK antibodies were purchased from BD Biosciences (San Jose, USA). Anti-β-Actin antibody, goat anti-mouse and anti-rabbit IgG HRP conjugated secondary antibodies were purchased from Sigma-Aldrich (Shanghai, China).
Cell viability assay
Cell viability was measured by Dojindo’s Cell Counting Kit 8 (CCK-8) assay. Briefly, cells were seeded into 96-well plates at a density of 1 × 104. After overnight incubation, cells were treated with either CUDC-907 alone or in combination of TRAIL as described in individual experiments. After the indicated time, CCK-8 was added to each well and incubated at 37 °C for 1 h. The absorbance of samples (450 nm) was determined using a microplate reader (iMark, Bio-Rad, USA). Experiments were repeated independently three times.
Cell cycle analysis
Cells were seeded at densities of 6 × 105 per well in 6-well plates and incubated with different doses of CUDC-907 for 24 h. Cells were then collected and washed with 1 × PBS. Next, cells were fixed in 70% ice-cold ethanol for 24 h and incubated with RNase A (Sigma-Aldrich) for 30 min at 37 °C. Cellular DNA was stained by propidium iodide (Solarbio, Beijing, China) for 15 min at room temperature. The number of cells at each stage of the cell cycle was analyzed by FACSCalibur flow cytometry and CellQuest analysis software (Becton-Dickinson, CA, USA).
Cell apoptosis detection
Cell apoptosis levels were measured using the FITC-Annexin V/PI Staining Detection Kit (KeyGen BioTECH, Nanjing, China) with flow cytometry. Briefly, cells (6 × 105) in 6-well plates were treated with indicated doses of CUDC-907 for 24 h. Then the cells were collected, washed with PBS, and resuspended in 100 μL binding buffer containing FITC-conjugated Annexin V and PI. After incubation under dark conditions for 15 min, cells were then analyzed using BD FACSCalibur flow cytometer.
Western blotting analysis
Cells treated with DMSO or indicated concentrations of CUDC-907 were washed with cold PBS and lysed on ice in RIPA lysis buffer (containing phosphatase and protease inhibitors) for 20 min. Total proteins were denatured at 95 °C for 5 min. Equal amounts of the total proteins were separated by SDS-PAGE gels and transferred to polyvinyldifluoride (PVDF) membranes (Millipore). The membranes were blocked with 5% non-fat milk for 1 h at room temperature and then incubated overnight at 4 °C with primary antibodies. After three washes with PBS, the membranes were incubated with secondary antibodies for 1 h. The signals were detected by Western Chemiluminescent HRP Substrate (ECL) solution (Millipore). The results shown are representative of at least three experiments.
Immunofluorescence assay
Cells were fixed with cold 100% methanol for 20 min at 4 °C. The fixed cells were then permeabilized with 0.1% Triton X-100 and subsequently blocked with 3% BSA for 30 min at room temperature. Next, cells were incubated with primary monoclonal antibodies against γ-H2AX. After washing, cells were incubated with Alexa-488-conjugated secondary antibodies (Thermo Fisher Scientific, USA) for 1 h at RT. The nuclei were stained with DAPI. Fluorescence images were acquired using an inverted fluorescence microscope (Ti-S, Nikon, Japan).
RNA interference
Cells (5 × 105) were seeded per well in 6-well plates. After 24 h incubation, 20 nM DR5 small interfering RNA (sc-40237; Santa cruz) or control siRNA (sc-37007; Santa cruz) were transfected into cells using Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific) according to manufacturer’s instructions. After 48 h transfection, the cells were treated with TRAIL and/or CUDC-907 for another 24 h and then harvested. The knockdown efficiency of DR5 was confirmed by Western blotting analysis.
Statistical analysis
Values represent the means±SD of at least 3 independent experiments. Statistical analysis was performed using SPSS software, p < 0.05 was considered statistically significant.
Results
CUDC-907 downregulates the PI3K downstream pathway in human breast cancer cells
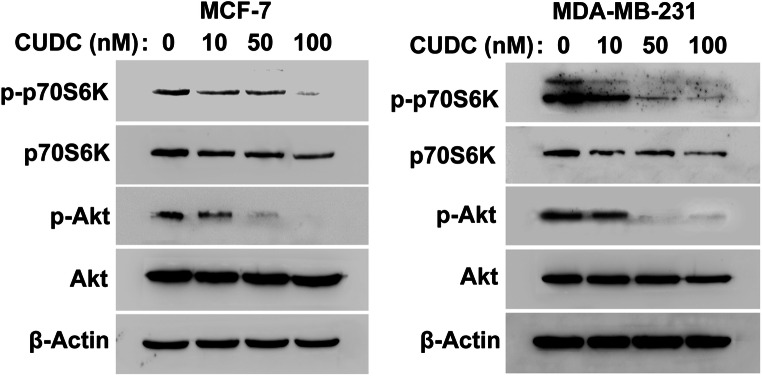
As a dual small-molecule inhibitor of PI3K and HDAC, CUDC-907 has potently inhibited the activity of Akt in thyroid cancer (Kotian et al. 2017). Therefore, we detected the effect of CUDC-907 on the phosphorylation of Akt and p70S6K in breast cancer cells. As shown in Fig. 1, exposure to CUDC-907 for 24 h markedly reduced the phosphorylation of Akt and p70S6K in both MCF-7 and MDA-MB-231 cells. Interestingly, CUDC-907 also downregulated the total levels of p70S6K in breast cancer cells (Fig. 1).
Fig. 1.
CUDC-907 downregulates the PI3K downstream pathway. Cells were treated with CUDC-907 (10, 50, 100 nM) for 24 h. The expression of Akt, p-Akt, p70S6K and p-p70S6K was analyzed using Western blotting analysis. β-Actin was used as a loading control
CUDC-907 inhibits the cell viability of human breast cancer cells
To evaluate the antiproliferative effects of CUDC-907 in human breast cancer cells, we assessed the cell viability by performing CCK-8 assay. As shown in Fig. 2, after 24 h of exposure to the indicated concentrations of CUDC-907, cell viability was decreased significantly in both MCF-7 and MDA-MB-231 cells.
Fig. 2.
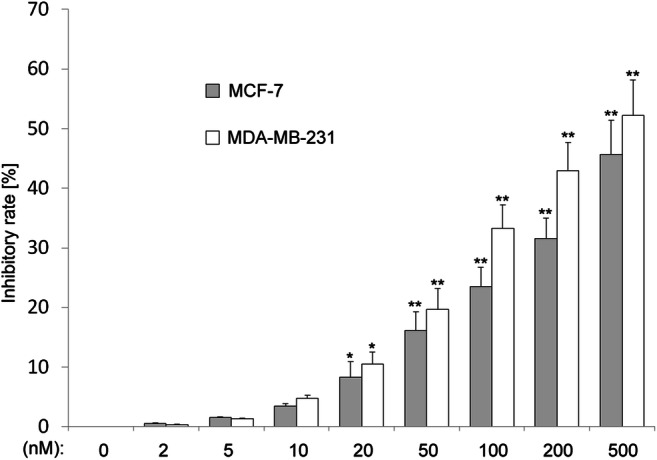
CUDC-907 inhibits cell viability in human breast cancer cells. Cell Counting Kit-8 assay of breast cancer cells treated with increasing dose of CUDC-907 for 24 h. Mean ± SD, n = 3. *indicates that P < 0.05 and **indicates that P < 0.01 versus control group (0 nM CUDC-907)
CUDC-907 induces DNA damage in breast cancer cells
Previous report indicated that CUDC-907 induced DNA damage in acute myeloid leukemia cells (Li et al. 2019). We then tested whether CUDC-907 could induce DNA damage in breast cancer cells. Cells were treated with CUDC-907 for 24 h, and a well-known DNA damage marker γ-H2AX was detected by immunofluorescence assay. As shown in Fig. 3a, exposure to CUDC-907 increased γ-H2AX foci formation in both MCF-7 and MDA-MB-231 cells.
Fig. 3.
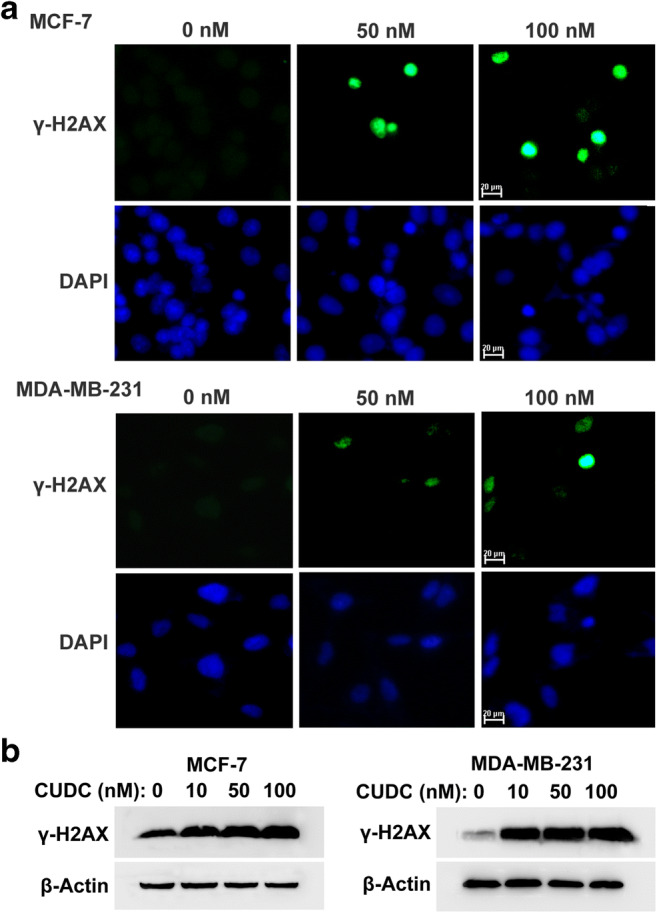
CUDC-907 induces DNA damage response in breast cancer cells. a CUDC-907 treatment increased γ-H2AX foci formation. Cells were treated with CUDC-907 for 24 h, and then fixed 20 min for immunofluorescence analysis of γ-H2AX foci. Nuclei were visualized by DAPI. b Representative Western blotting results showing γ-H2AX expression after incubation with CUDC-907 for 24 h in breast cancer cells. β-Actin was used as a loading control
Meanwhile, the expressions of γ-H2AX were detected by Western blotting analysis. As shown in Fig. 3b, treatment with CUDC-907 induced the γ-H2AX expression in both MCF-7 and MDA-MB-231 cells. These results indicated that CUDC-907 could induce DNA damage in breast cancer cells.
CUDC-907 causes G2/M arrest in breast cancer cells
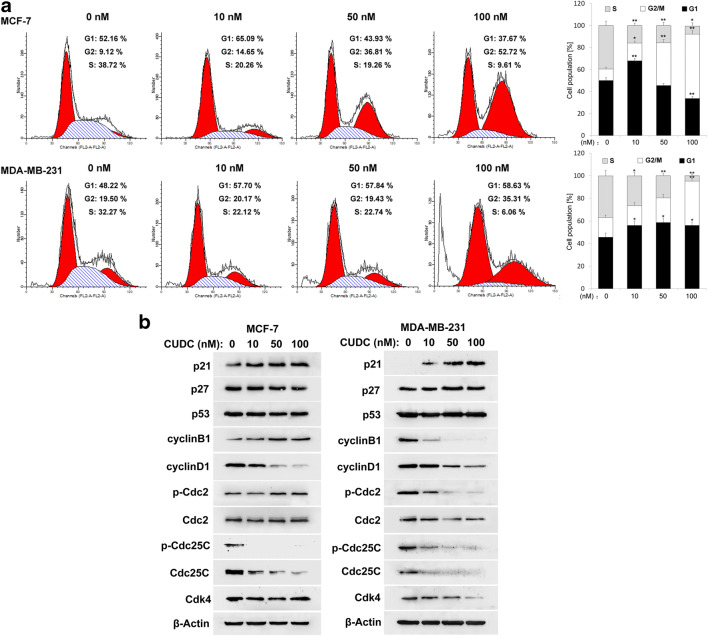
DNA damage may result in cell cycle arrest and apoptosis. We then performed flow cytometry to assess the effect of CUDC-907 on cell cycle distribution in breast cancer cells. As shown in Fig. 4a, CUDC-907 treatment induced cell cycle arrest in G2/M phase in MCF-7 cells, which was accompanied by a progressive decrease of G1 phase and S phase cell population. It should be mentioned that when MDA-MB-231 cells were treated with CUDC-907 for 24 h, the proportion of cell in both G1 and G2/M phase was increased significantly (Fig. 4a).
Fig. 4.
The effects of CUDC-907 on the cell cycle distribution in breast cancer cells. a Cell cycle profile analyses in MCF-7 and MDA-MB-231 cells treated with CUDC-907 for 24 h. Each bar corresponds to the mean ± SD for three independent experiments. *indicates that P < 0.05 and **indicates that P < 0.01 versus control group (0 nM CUDC-907). b Cells were treated with CUDC-907 for 24 h, and the expressions of cell cycle related proteins were measured by Western blotting analysis. β-Actin was used as a loading control
To elucidate the mechanism underlying CUDU-907-induced cell cycle arrest, the expressions of cell cycle-related protein were examined by Western blotting analysis. As shown in Fig. 4b, after treatment with CUDC-907 for 24 h, the levels of the Cdc25C, p-Cdc25C and cyclinD1 were decreased in both MCF-7 and MDA-MB-231 cells, and the expressions of p21 were upregulated. The levels of cyclinB1, p-Cdc2 and Cdk4 were reduced in MDA-MB-231 cells. However, in CUDC-907-treated MCF-7 cells, cyclinB1 and p-Cdc2 were upregulated, and Cdk4 remained unchanged. In MCF-7 cells, p27 levels were downregulated upon CUDC-907 treatment, whereas it was unchanged in MDA-MB-231 cells. Additionally, the expressions of p53 expression were unaffected by exposure to CUDC-907 in breast cancer cells (Fig. 4b).
CUDC-907 triggers cell apoptosis in human breast cancer cells
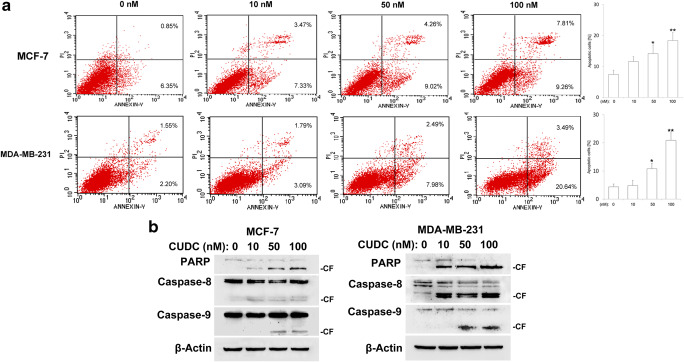
To confirm cell apoptosis contributes to the inhibition of cell viability upon CUDC-907 treatment, Annexin V-FITC and propidium iodide (PI) double staining was used to detect the apoptosis in breast cancer cells. Our results revealed that higher concentrations of CUDC-907 triggered cell apoptosis in both MCF-7 and MDA-MB-231 cells (Fig. 5a).
Fig. 5.
CUDC-907 triggers cell apoptosis in breast cancer cells. a Following treatment with CUDC-907 for 24 h, cells were stained with Annexin V-FITC/PI and then analyzed by flow cytometry. Each bar corresponds to the mean ± SD for three independent experiments. *indicates that P < 0.05 and **indicates that P < 0.01 versus control group (0 nM CUDC-907). b Western blotting analysis of cleaved caspase-8, −9 and PARP in breast cancer cells. β-Actin was used as a loading control
Next, we evaluated the cleavage of caspase-8, caspase-9 and PARP by Western blotting analysis. As shown in Fig. 5b, treatment with CUDC-907 for 24 h clearly induced the cleavage of caspase-8, −9 and PARP in MCF-7 cells. Likewise, similar events also occurred in MDA-MB-231 cells (Fig. 5b).
CUDC-907 enhances TRAIL-mediated apoptosis in human breast cancer cells
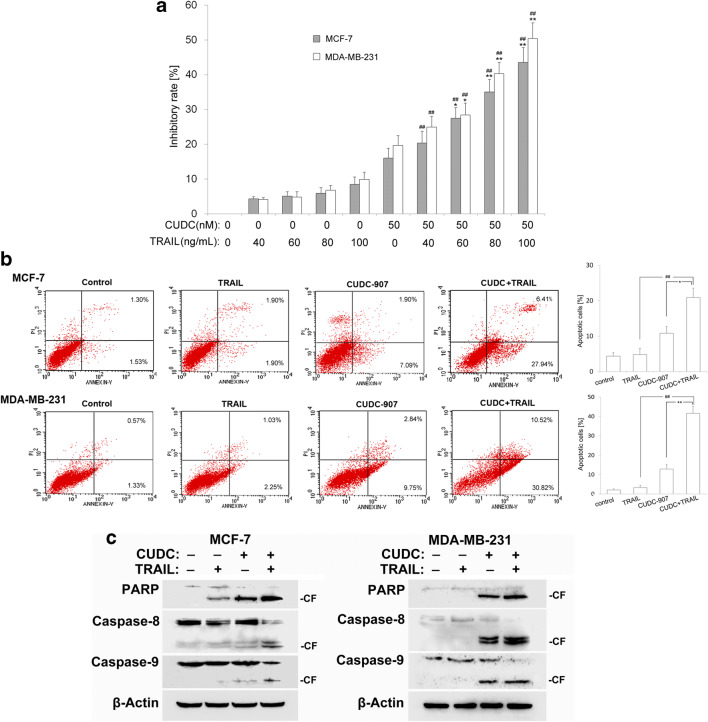
TRAIL is well known for its ability to preferentially induce cancer cell-specific apoptosis without causing adverse effects in normal cells (Lim et al. 2015). However, inherent and acquired resistance of tumor to TRAIL-induced apoptosis limits its therapeutic applicability. We then investigated whether CUDC-907 could sensitize TRAIL-mediated cytotoxic and apoptotic effects in breast cancer cells. Both MCF-7 and MDA-MB-231 cells were treated with CUDC-907 alone (50 nM), TRAIL alone (40, 60, 80, 100 ng/mL), and combined treatment with CUDC-907 and TRAIL. CCK-8 results showed that CUDC-907 sensitized 60–100 ng/mL TRAIL-mediated cytotoxicity in both MCF-7 and MDA-MB-231 cells (Fig. 6a).
Fig. 6.
CUDC-907 enhances TRAIL-mediated apoptosis in breast cancer cells. a Cytotoxic effect of CUDC-907 (50 nM) combined with TRAIL (40–100 ng/mL) on MCF-7 and MDA-MB-231 cells analyzed by CCK-8 assay at 24 h. Each bar corresponds to the mean ± SD for three independent experiments. *indicates that P < 0.05 and **indicates that P < 0.01 versus CUDC-907 group (50 nM); ## indicates that P < 0.01 versus TRAIL group (40–100 ng/mL). b Induction of cell apoptosis was detected by flow cytometry. The combination of CUDC-907 (50 nM) and TRAIL (80 ng/mL) significantly increased the cell apoptosis compared to each drug alone. Mean ± SD, n = 3. *indicates that P < 0.05 and **indicates that P < 0.01 versus CUDC-907 group (50 nM); ## indicates that P < 0.01 versus TRAIL group (80 ng/mL). c The expression of apoptosis related proteins was detected by Western blotting analysis. β-Actin was used as a loading control
Moreover, the effects of CUDC-907 on TRAIL-mediated apoptosis were detected by Annexin V-FITC and PI staining. We found that co-treatment with CUDC-907 and TRAIL synergistically increased the apoptotic population compared with either CUDC-907 or TRAIL alone (Fig. 6b). We then assessed whether CUDC-907 could increase TRAIL-induced activation of caspase-8, −9 and consequent PARP cleavage. Our studies showed that co-treatment with CUDC-907 and TRAIL markedly increased the cleavage of caspase-8, −9 and PARP cleavage (Fig. 6c). These results suggest that CUDC-907 may be a novel potent TRAIL sensitizer in breast cancer.
Upregulation of DR5 by CUDC-907 contributes to the TRAIL-induced cell death
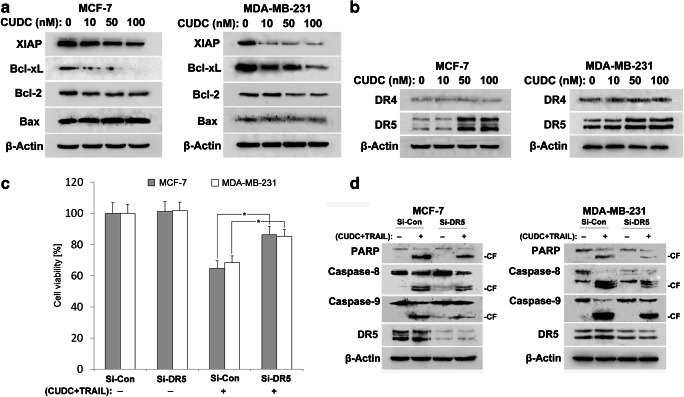
To gain further insight into CUDC-907 enhancement of TRAIL-induced apoptosis, we evaluated the expression of X-linked inhibitor of apoptosis protein (XIAP) and Bcl-2 family members. Western blotting results showed that the expressions of anti-apoptotic proteins including XIAP, Bcl-xL and Bcl-2 were suppressed upon CUDC-907 treatment in both MCF-7 and MDA-MB-231 cells (Fig. 7a), which may account, at least in part, for the synergistic effect of CUDC-907 treatment. No change of Bax levels was observed in CUDC-907-treated cells.
Fig. 7.
CUDC-907 synergistically sensitizes breast cancer cells to TRAIL partially depend on DR5. a Cells were treated with the indicated concentrations of CUDC-907 for 24 h. The protein levels of XIAP, Bcl-xL, Bcl-2 and Bax were determined by Western blotting analysis. β-Actin was used as a loading control. b Expressions of DR4 and DR5 were determined using Western blotting analysis. c Knockdown of DR5 inhibits cytotoxicity after co-treatment with CUDC-907 and TRAIL. Following transfection with DR5 siRNA (Si-DR5) and control siRNA (Si-Con), cells were co-treated with CUDC-907 (50 nM) and TRAIL (80 ng/mL), cell cytotoxicity was examined by CCK-8 assay. Each bar corresponds to the mean ± SD for three independent experiments. *P < 0.05 as compared to control siRNA group. d The expression of DR5 and cleavage of caspase-8, −9 and PARP were measured by Western blotting assay. β-Actin was used as a loading control
Next, we examined the effect of CUDC-907 on the death receptors (DR4 and DR5) expression; because downregulation of death receptors account for TRAIL resistance in certain cancer cell lines (Yuan et al. 2018).We confirmed that CUDC-907 treatment upregulated the expression of DR5 in both MCF-7 and MDA-MB-231 cells. However, the levels of DR4 remained constant upon CUDC-907 treatment (Fig. 7b).
We then verified whether DR5 contributed to the induction of cytotoxicity by co-treatment with CUDC-907 and TRAIL. Breast cancer cells were transfected with DR5 siRNA or control siRNA, and then treated with the combination of CUDC-907 and TRAIL for 24 h. CCK-8 results indicated that transfection with siRNA against DR5 significantly abolished the cytotoxic effects of CUDC-907 and TRAIL (Fig. 7c). Moreover, DR5 knockdown attenuated the cleavage of caspase-8, −9 and PARP induced by CUDC-907 plus TRAIL treatment (Fig. 7d). These results suggest that DR5 upregulation contributes to the sensitization of TRAIL-induced apoptosis by CUDC-907 co-treatment.
Inhibition of the JNK abrogates CUDC-907-mediated DR5 upregulation
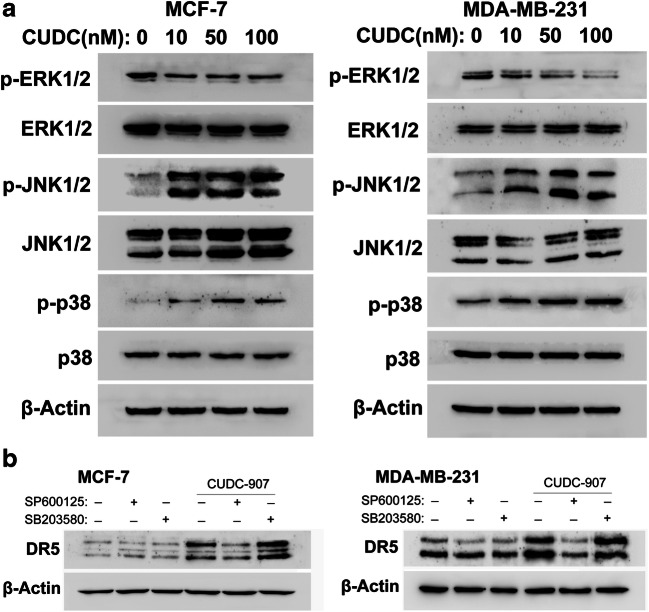
There is increasing evidence that MAPK pathways are involved in cellular responses to small molecule inhibitors treatment, and MAPK activation is critical for the upregulation of DR5 (Shlyakhtina et al. 2017). Accordingly, we examined whether MAPK pathway contributed to the upregulation of DR5 by CUDC-907. After cells were exposure to CUDC-907 for 24 h, the protein expressions of p-ERK, p-JNK and p-p38 MAPK were checked. Our results showed the phosphorylation of ERK was decreased, and the expression levels of p-JNK and p-p38MAPK were increased in CUDC-907-treated cells (Fig. 8a).
Fig. 8.
Activation of JNK is essential for CUDC-907-enhanced DR5 expression in breast cancer cells. a Expressions of p-ERK, p-JNK and p-p38 MAPK were measured in breast cancer cells treated with various concentrations of CUDC-907 for 24 h. b Cell pretreated with or without SP600125 (20 μM) and SB203580 (20 μM) for 1 h, and subsequently treated with or without CUDC-907 (50 nM) for 24 h. The expression of DR5 was measured by Western blotting analysis. β-Actin was used as a loading control
We further performed Western blotting analysis in the presence of SP600125 (JNK inhibitor) and SB203580 (p38 MAPK inhibitor) in CUDC-907-treated cells. As shown in Fig. 8b, pretreatment with the JNK inhibitor SP600125 significantly abrogate the CUDC-907-induced DR5 upregulation in both MCF-7 and MDA-MB-231 cells. However, p38 MAPK inhibitor treatment did not affect CUDC-907-induced DR5 expression (Fig. 8b).
Discussion
It is now widely accepted that PI3K signaling pathway is one of the most frequently activated intracellular pathways in human cancers, which contribute to the tumor progression and poor prognosis. Therefore, inhibitors targeting this pathway have shown some therapeutic promise in clinical studies (McKenna et al. 2018). As a dual PI3K and HDAC inhibitor, CUDC-907 showed greater growth inhibition and proapoptotic activity than single-target PI3K or HDAC inhibitors targeting cancer cells (Qian et al. 2012). CUDC-907 also downregulated Myc protein levels and suppressed the growth and survival of Myc-altered or Myc-dependent cancer cells (Sun et al. 2017).
Based on these reports, we examined the anticancer activity of CUDC-907 on the human breast cancer cells in this study. Our results showed that CUDC-907 induced cell cycle arrest and DNA damage in both MCF-7 and MDA-MB-231 cells. The accuracy of cell cycle progression is controlled by the coordinated activity of the cyclin-dependent kinases (CDK) and regulatory cyclins. In response to DNA damage, cell cycle checkpoint (G1/S, intra-S and G2/M) transitions become active to provide time for cells to repair DNA damage (Wang et al. 2018). The G2/M phase transition is primarily dependent on cyclinB1/Cdc2 activity, it can be activated by Cdc25C or inhibited by p21 and p27 (Jang et al. 2018). In our study, exposure of breast cancer cells to CUDC-907 mainly induced cell cycle arrest in G2/M phase, which correlated with the upregulation of the cyclin-dependent kinase inhibitor p21, and a significant reduction of cyclinD1, Cdc2 and Cdc25C. In addition, CUDC-907 could trigger apoptosis of breast cancer cells by increased the cleavage of caspase-8, −9 and PARP, this is in agreement with previous studies reporting CUDC-907-induced apoptosis in thyroid cancer cell lines (Kotian et al. 2017).
On the other hand, in the present study, CUDC-907 sensitizes TRAIL-mediated apoptosis in human breast cancer cells. As a famous potential anticancer agent, TRAIL is considered as a promising anticancer chemotherapy due to its ability to induce cancer-specific apoptosis but less toxicity in normal cells (Selvarajoo 2017). Although both TRAIL and agonistic antibodies to TRAIL receptors are currently in clinical trials for cancer therapy, resistance of tumor cells to apoptosis is still one of the major hurdles for its application in the clinical setting (von Karstedt et al. 2017). Much effort has focused on combination therapies to establish effective therapeutic strategies to overcome TRAIL resistance in cancer. Thus, we explored the ability of CUDC-907 to enhance TRAIL-triggered apoptosis in breast cancer cells.
Our results showed that CUDC-907 upregulated the expression of DR5 and downregulated cell survival proteins including XIAP, Bcl-xL and Bcl-2, which may help to explain CUDC-907 sensitizing TRAIL activity at a cellular mechanistic level. We are particularly interested in the role of DR5 in the synergistic effect and the mechanism accounting for the upregulation of DR5. To signal cell death, TRAIL trimerizes and binds to its receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), thereby recruiting the intracellular signaling components with Fas-associated death domain (FADD) (Abdulghani and El-Deiry 2010). FADD then recruits pro-caspase-8 and caspase-10 to form a death-inducing signaling complex (DISC). Activated caspases-8 transfers the apoptosis signal to executioners of apoptosis via the intrinsic or extrinsic apoptotic pathways (Yuan et al. 2018). Downregulation of death receptors in cancer cells is associated with TRAIL resistance, and small molecules targeting death receptors are increasingly gaining attention (Das et al. 2017; Carlsten et al. 2018).
We found that CUDC-907 dramatically induced the levels of DR5, but not DR4, in breast cancer cells. However, it was not clear whether elevated expression of DR5 was responsible for the TRAIL-induced apoptosis. Then, we knocked down the expression of DR5 by small interfering RNA and studied the effects on caspase activation after co-treatment with CUDC-907 and TRAIL. We found that knockdown of DR5 abrogated cytotoxicity and apoptosis induced by the combination of TRAIL and CUDC-907. Therefore, we speculated that one of mechanisms of CUDC-907-mediated TRAIL sensitization in breast cancer cell is upregulation of DR5.
Several interrelated mechanisms have been proposed for the induction of DR5, including activation of MAPK signaling pathways (Liu et al. 2017; Wu et al. 2017). To examine whether MAPK pathways are responsible for CUDC-907-induced upregulation of DR5, we detected the effect of CUDC-907 on the phosphorylation of MAPK family members in breast cancer cells. We found that CUDC-907 increased the phosphorylation of JNK and p38 MAPK. However, pretreatment of cells with JNK inhibitor SP600125, but not the p38 MAPK inhibitor SB203580, attenuated CUDC-907-induced DR5 upregulation, which provided the underlying molecular mechanism for CUDC-907-induced DR5 was dependent of JNK activation.
In conclusion, CUDC-907 inhibits the cell proliferation and sensitizes breast cancer cells to TRAIL-induced cell death. CUDC-907 increases the death receptor DR5 via activation of JNK. The use of combination therapy focused upon the CUDC-907 and TRAIL may be a potentially good therapeutic strategy for human breast cancer patients.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No 81703039; No 81570158); Shandong Provincial Natural Science Foundation, China (No ZR2018MH026).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhao-Jun Li and Ya-Jun Hou contributed equally to this work.
Contributor Information
Hong-Rong Fei, Email: hrfei@sdfmu.edu.cn.
Feng-Ze Wang, Email: fzwang@sdfmu.edu.cn.
References
- Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets. 2010;14:1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- Carlsten M, Namazi A, Reger R, Levy E, Berg M, St Hilaire C, Childs RW. Bortezomib sensitizes multiple myeloma to NK cells via ER-stress-induced suppression of HLA-E and upregulation of DR5. Oncoimmunology. 2018;8:e1534664. doi: 10.1080/2162402X.2018.1534664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. C A Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Chen Y, Peubez C, Smith V, Xiong S, Kocsis-Fodor G, Kennedy B, Wagner S, Balotis C, Jayne S, Dyer MJS, Macip S. CUDC-907 blocks multiple pro-survival signals and abrogates microenvironment protection in CLL. J Cell Mol Med. 2019;23:340–348. doi: 10.1111/jcmm.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Tripathi N, Siddharth S, Nayak A, Nayak D, Sethy C, Bharatam PV, Kundu CN. Etoposide and doxorubicin enhance the sensitivity of triple negative breast cancers through modulation of TRAIL-DR5 axis. Apoptosis. 2017;22:1205–1224. doi: 10.1007/s10495-017-1400-4. [DOI] [PubMed] [Google Scholar]
- Jang JY, Kang YJ, Sung B, Kim MJ, Park C, Kang D, Moon HR, Chung HY, Kim ND. MHY440, a novel topoisomerase Ι inhibitor, induces cell cycle arrest and apoptosis via a ROS-dependent DNA damage signaling pathway in AGS human gastric cancer cells. Molecules. 2018;24:96. doi: 10.3390/molecules24010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotian S, Zhang L, Boufraqech M, Gaskins K, Gara SK, Quezado M, Nilubol N, Kebebew E. Dual inhibition of HDAC and tyrosine kinase signaling pathways with CUDC-907 inhibits thyroid cancer growth and metastases. Clin Cancer Res. 2017;23:5044–5054. doi: 10.1158/1078-0432.CCR-17-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Allen JE, Prabhu VV, Talekar MK, Finnberg NK, El-Deiry WS. Targeting TRAIL in the treatment of cancer: new developments. Expert Opin Ther Targets. 2015;19:1171–1185. doi: 10.1517/14728222.2015.1049838. [DOI] [PubMed] [Google Scholar]
- Li M, Wang Y, Wei F, An X, Zhang N, Cao S, Ren B, Zhang X, Ren X. Efficiency of cytokine-induced killer cells in combination with chemotherapy for triple-negative breast cancer. J Breast Cancer. 2018;21:150–157. doi: 10.4048/jbc.2018.21.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PC, Lu G, Deng Y, Wang CD, Su XW, Zhou JY, Chan TM, Hu X, Poon WS. Inhibition of NF-κB pathway and modulation of MAPK signaling pathways in glioblastoma and implications for lovastatin and tumor necrosis factor-related apoptosis inducing ligand (TRAIL) combination therapy. PLoS One. 2017;12:e0171157. doi: 10.1371/journal.pone.0171157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Su Y, Madlambayan G, Edwards H, Polin L, Kushner J, Dzinic SH, White K, Ma J, Knight T, Wang G, Wang Y, Yang J, Taub JW, Lin H, Ge Y. Antileukemic activity and mechanism of action of the novel PI3K and histone deacetylase dual inhibitor CUDC-907 in acute myeloid leukemia. Haematologica. 2019;104:2225–2240. doi: 10.3324/haematol.2018.201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Seto E (2016) HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 6. 10.1101/cshperspect.a026831 [DOI] [PMC free article] [PubMed]
- McKenna M, McGarrigle S, Pidgeon GP. The next generation of PI3K-Akt-mTOR pathway inhibitors in breast cancer cohorts. Biochim Biophys Acta Rev Cancer. 2018;1870:185–197. doi: 10.1016/j.bbcan.2018.08.001. [DOI] [PubMed] [Google Scholar]
- McRee AJ, Marcom PK, Moore DT, Zamboni WC, Kornblum ZA, Hu Z, Phipps R, Anders CK, Reeder-Hayes K, Carey LA, Weck KE, Perou CM, Dees EC. A phase I trial of the PI3K inhibitor buparlisib combined with capecitabine in patients with metastatic breast cancer. Clin Breast Cancer. 2018;18:289–297. doi: 10.1016/j.clbc.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahleh Z, Botrus G, Dwivedi A, Jennings M, Nagy S, Tfayli A. Bevacizumab in the neoadjuvant treatment of human epidermal growth factor receptor 2-negative breast cancer: a meta-analysis of randomized controlled trials. Mol Clin Oncol. 2019;10:357–365. doi: 10.3892/mco.2019.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Kozono D, Yang X, Fendler W, Fitts W, Ni J, Alberta JA, Zhao J, Liu KX, Bian J, Truffaux N, Weiss WA, Resnick AC, Bandopadhayay P, Ligon KL, DuBois SG, Mueller S, Chowdhury D, Haas-Kogan DA. Dual HDAC and PI3K inhibition abrogates NFκB- and FOXM1-mediated DNA damage response to Radiosensitize pediatric high-grade Gliomas. Cancer Res. 2018;78:4007–4021. doi: 10.1158/0008-5472.CAN-17-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M, Zifcak B, Ma AW, DellaRocca S, Borek M, Zhai HX, Cai X, Voi M. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012;18:4104–4113. doi: 10.1158/1078-0432.CCR-12-0055. [DOI] [PubMed] [Google Scholar]
- Rodon J, Pérez-Fidalgo A, Krop IE, Burris H, Guerrero-Zotano A, Britten CD, Becerra C, Schellens J, Richards DA, Schuler M, Abu-Khalaf M, Johnson FM, Ranson M, Edenfield J, Silva AP, Hackl W, Quadt C, Demanse D, Duval V, Baselga J. Phase 1/1b dose escalation and expansion study of BEZ235, a dual PI3K/mTOR inhibitor, in patients with advanced solid tumors including patients with advanced breast cancer. Cancer Chemother Pharmacol. 2018;82:285–298. doi: 10.1007/s00280-018-3610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca M, Urbano N, Bonfiglio R, Duggento A, Toschi N, Schillaci O, Bonanno E. Novel insights into breast cancer progression and metastasis: a multidisciplinary opportunity to transition from biology to clinical oncology. Biochim Biophys Acta Rev Cancer. 2019;1872(1):138–148. doi: 10.1016/j.bbcan.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Selvarajoo K. A systems biology approach to overcome TRAIL resistance in cancer treatment. Prog Biophys Mol Biol. 2017;128:142–154. doi: 10.1016/j.pbiomolbio.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Shlyakhtina Y, Pavet V, Gronemeyer H. Dual role of DR5 in death and survival signaling leads to TRAIL resistance in cancer cells. Cell Death Dis. 2017;8:e3025. doi: 10.1038/cddis.2017.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BN, Zhou H, Li J, Tipton T, Wang B, Shao G, Gilbert EN, Li Q, Jiang SW. Preclinical studies on histone deacetylase inhibitors as therapeutic reagents for endometrial and ovarian cancers. Future Oncol. 2011;7:1415–1428. doi: 10.2217/fon.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Atoyan R, Borek MA, Dellarocca S, Samson ME, Ma AW, Xu GX, Patterson T, Tuck DP, Viner JL, Fattaey A, Wang J. Dual HDAC and PI3K inhibitor CUDC-907 downregulates MYC and suppresses growth of MYC-dependent cancers. Mol Cancer Ther. 2017;16:285–299. doi: 10.1158/1535-7163.MCT-16-0390. [DOI] [PubMed] [Google Scholar]
- Vancurova I, Uddin MM, Zou Y, Vancura A. Combination therapies targeting HDAC and IKK in solid tumors. Trends Pharmacol Sci. 2018;39:295–306. doi: 10.1016/j.tips.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17:352–366. doi: 10.1038/nrc.2017.28. [DOI] [PubMed] [Google Scholar]
- Wang J, Chang L, Lai X, Li X, Wang Z, Huang Z, Huang J, Zhang G. Tetrandrine enhances radiosensitivity through the CDC25C/CDK1/cyclin B1 pathway in nasopharyngeal carcinoma cells. Cell Cycle. 2018;17:671–680. doi: 10.1080/15384101.2017.1415679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MA. RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes. Anti-Cancer Drugs. 2016;27:475–487. doi: 10.1097/CAD.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Yang Y, Deng G, Ma L, Wei G, Zheng G, Han X, He D, Zhao Y, He J, Cai Z, Yu R. Vernodalol enhances TRAIL-induced apoptosis in diffuse large B-cell lymphoma cells. Mol Carcinog. 2017;56:2190–2199. doi: 10.1002/mc.22672. [DOI] [PubMed] [Google Scholar]
- Yuan X, Gajan A, Chu Q, Xiong H, Wu K, Wu GS. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018;37:733–748. doi: 10.1007/s10555-018-9728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Rezai-Zadeh N, Zhang X, Seto E. Histone deacetylase activity assay. Methods Mol Biol. 2009;523:279–293. doi: 10.1007/978-1-4939-2474-5_7. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhang M, Zhang L, Ding X, Li W, Lu S. HSPC159 promotes proliferation and metastasis by inducing epithelial-mesenchymal transition and activating the PI3K/Akt pathway in breast cancer. Cancer Sci. 2018;109:2153–2163. doi: 10.1111/cas.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]