Prevalence of Campylobacter in raw poultry remains a major food microbiological safety challenge. Novel mitigation strategies are required to ensure the safety and quality of poultry products. Active food packaging can control pathogens without directly adding antimicrobials into the food matrix and extend the food’s shelf life. The functionalized absorbing pad with ZnO NPs developed in this study was able to inactivate C. jejuni in raw chicken meat and keep the meat free from C. jejuni contamination during shelf life without any observed migration of nanoparticles. The controllable conversion of immobilized ZnO NPs to free Zn2+ makes this approach safe and eco-friendly and paves the way for developing a novel intervention strategy for other high-risk foods. Our study applied nanotechnology to exploit an effective approach for Campylobacter control in raw chicken meat products.
KEYWORDS: Campylobacter, antimicrobial packaging, food safety, metal oxide nanoparticles, poultry
ABSTRACT
Zinc oxide nanoparticles (ZnO NPs) are regarded as a safe and stable antimicrobial that can inactivate bacteria by several potential working mechanisms. We aimed to incorporate ZnO NPs into packaging material to control Campylobacter in raw chicken meat. ZnO NPs were first incorporated into three-dimensional (3D) paper tubes to identify the lethal concentration against Campylobacter jejuni, which was selected as the working concentration to develop 2D functionalized absorbing pads by an ultrasound-assisted dipping technique. The functionalized pad was placed underneath raw chicken meat to inactivate C. jejuni and the predominant chicken microbiota at 4°C within 8 days of storage. Immobilized ZnO NPs at 0.856 mg/cm2 reduced C. jejuni from ∼4 log CFU/25 g raw chicken meat to an undetectable level after 3 days of storage. Analysis by inductively coupled plasma-optical emission spectroscopy showed that the Zn level increased from 0.02 to 0.17 mg/cm2 in treated raw chicken meat. Scanning electron microscopy validated the absence of nanoparticle migration onto raw chicken meat after treatment. Inactivation of C. jejuni was associated with the increase of lactic acid produced by Lactobacillus in raw chicken meat in a pH-dependent manner. Less than 5% of Zn2+ was released from ZnO NPs at neutral pH, while up to 88% was released when the pH was <3.5 within 2 days. Whole-transcriptome sequencing (RNA-Seq) analysis demonstrated a broad effect of ZnO NPs on genes involved in various cellular developmental processes as annotated by gene ontology. Taken together, the results indicate that functionalized absorbing pads inactivated C. jejuni in raw chicken meat by immobilized ZnO NPs along with the controllable released Zn2+.
IMPORTANCE Prevalence of Campylobacter in raw poultry remains a major food microbiological safety challenge. Novel mitigation strategies are required to ensure the safety and quality of poultry products. Active food packaging can control pathogens without directly adding antimicrobials into the food matrix and extend the food’s shelf life. The functionalized absorbing pad with ZnO NPs developed in this study was able to inactivate C. jejuni in raw chicken meat and keep the meat free from C. jejuni contamination during shelf life without any observed migration of nanoparticles. The controllable conversion of immobilized ZnO NPs to free Zn2+ makes this approach safe and eco-friendly and paves the way for developing a novel intervention strategy for other high-risk foods. Our study applied nanotechnology to exploit an effective approach for Campylobacter control in raw chicken meat products.
INTRODUCTION
Campylobacter jejuni colonizes the chicken intestine early in the life of the birds (1, 2) and is routinely detected in chicken carcasses (3). Numerous reports in Europe and North America demonstrate that about 70 to 90% of commercial raw chicken meat is Campylobacter positive (4, 5). Recent epidemiology studies indicated that poultry is the main reservoir and route of transmission of campylobacteriosis to humans (6). Campylobacter-contaminated poultry products have been ranked as the number one pathogen-food combination among 14 foodborne pathogens in 12 different food categories based on significant health burdens on consumers and negative impacts on the economy (7).
High Campylobacter load is a challenge to the poultry industry. Classical intervention strategies on farms (8) or in slaughterhouses (9) have limited impacts on the reduction of Campylobacter loads due to the complexity of both systems. Effective and sustainable Campylobacter control is highly challenging due to the large number of birds, high microbial load and diversity, and rapid horizontal transmission of this microbe within and between batches (10). Nevertheless, Berrang and coworkers isolated Campylobacter from the skin of chicken carcasses even before evisceration or any contact with the internal organs (11), indicating that other parts and fluids (e.g., feathers, blood, and/or water) can also be involved in cross-contamination by this bacterium. Many other factors, including the farm and plant design, waste management, water quality, and washing systems, can have a significant influence on the prevalence of Campylobacter in the final poultry and food products as well.
Campylobacter is an obligately microaerobic bacterium, but it can survive throughout raw poultry meat production from the slaughterhouses to the retail outlets (12). Campylobacter organisms are frequently detected from raw chicken meat at a level as high as 4 to 6 log CFU/chicken carcass (13–15). In addition, the attachment of C. jejuni to the contact surfaces can lead to the development of biofilms that extend its survival outside hosts; but even this fact does not fully explain the long-term survival of culturable cells in processing plants (16). In response to processing conditions, such as extreme pH, temperature fluctuation, and starvation, C. jejuni can enter the viable-but-nonculturable (VBNC) state, which cannot be detected by the conventional plating assay (17). A recent study identified several emerging hyper-aerotolerant C. jejuni clones isolated from raw chicken meat with a potential impact on human Campylobacter infections (18). Generally, the intervention strategies used in the poultry industry are not sufficient to overcome the safety concerns associated with this microorganism (9).
Antimicrobial treatments for raw chicken meat show a significant reduction in Campylobacter (up to 5 log CFU/ml) in the laboratory (19) but have a minimal effect (0.5 to 1.5 log CFU/ml) in large processing plants (13). The U.S. Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) has proposed several antimicrobial agents for the control of Campylobacter in poultry slaughterhouses (20). The efficacies of many approved antimicrobials (e.g., acidified sodium, chlorite, cetylpyridinium, chlorine, chlorine dioxide, peroxyacetic acid, and trisodium phosphate) on the reduction of Campylobacter in poultry processing plants have been reported in several review articles (9, 21). For example, single or combined antimicrobial agents with heat and/or cold shock(s) reduce Campylobacter loads by 1 to 2 log CFU/ml of carcasses rinse. Although the use of commercial antimicrobials may not be effective in reducing Campylobacter, they are still widely used to try to prevent cross-contamination (20). To date, there is no effective control strategy, recommendation, or guideline that can be applied to eliminate or at least significantly reduce Campylobacter in raw chicken meat (12). The use of commercial antimicrobial agents in the poultry industry is limited due to their low inactivation effect on pathogens, chemical residues that constitute potential risks to humans, equipment corrosion, large water consumption, and high cost (15).
Active antimicrobial packaging is an effective control method to enhance the safety of some high-risk food products, such as raw meat and fresh produce. It is usually applied for quality preservation, shelf life extension, and food safety, with a major focus on the control of food freshness and inhibition of spoilage organisms (22). The study of integrating antimicrobials into the packaging material to inactivate pathogenic bacteria in foods is still in its infancy. One example is the use of immobilized bacteriophages to control Escherichia coli O104:H4 in alfalfa sprouts and Listeria monocytogenes in cantaloupes and ready-to-eat meat (23). The stability of antimicrobials in the packaging material is a major concern due to the slow dehydration of paper during shelf life (24). To overcome this limitation, absorbing pads have started to be used under raw meat to absorb moisture and fluids so as to maintain the quality and freshness of red meat, poultry, and fish. However, meat juice (e.g., chicken juice) is not only a rich source of nutrients for the survival and growth of microorganisms but has also been found to enhance the surface attachment and biofilm formation of foodborne pathogens, including C. jejuni (25). The area under the packed meat is difficult to reach by conventional active packaging approaches, such as the combination of modified atmosphere packaging (MAP) and aerosolized antimicrobial treatments. Alternative antimicrobials are thus required to develop novel active packaging techniques to be used in the agri-food industry.
Nanotechnology improves the functionality and physiochemical properties of materials at the nanoscale level and generates sustainable industrial applications. Metal oxide nanoparticles (NPs) are potential candidates for food packaging applications due to their antimicrobial effect and stability under harsh conditions. ZnO is a stable antimicrobial agent that inactivates bacterial cells by several mechanisms involving different chemical species, such as Zn2+ and reactive oxygen species (ROS) (26). It is generally recognized as safe by the U.S. Food and Drug Administration (21CFR182.8991) (27). A few studies indicated that C. jejuni was more susceptible to ZnO NPs than other major foodborne pathogens (28, 29). For instance, 0.005% (wt/vol) ZnO NPs could eliminate >8 log CFU/ml of C. jejuni within 3 h, whereas an 8- to 16-fold-higher concentration was required to inactivate E. coli O157:H7 and Salmonella enterica (29). The electrostatic force between ZnO NPs (positively charged) and bacterial cell wall (negatively charged) leads to the destabilization and disruption of bacterial outer membranes. In addition, the semiconductive property of ZnO allows the generation of ROS that can attack different cytoplasmic and extracytoplasmic targets. A previous study confirmed that treating C. jejuni with ZnO NPs could lead to significant overexpression of several oxidative stress response genes, including katA and ahpC, as well as the disruption of bacterial cell membrane (29).
No single control method is yet known to fully address Campylobacter contamination in the poultry industry. Controlling Campylobacter in the end production of raw chicken meat is a promising strategy that might replace or at least improve ineffective mitigation strategies being used. The objective of this study was to investigate the antimicrobial efficacy of an innovative functionalized absorbing pad by immobilized ZnO NPs to control C. jejuni in raw chicken meat.
RESULTS
Minimal bactericidal concentrations and inhibition zones of immobilized ZnO NPs against C. jejuni.
Three-dimensional (3D) paper tubes and two-dimensional (2D) absorbing pads were designed and developed, as shown in Fig. 1. The 3D paper tubes were designed to determine the minimal bactericidal concentrations (MBCs) of the immobilized ZnO NPs against C. jejuni in broth. Immobilized ZnO NPs in the paper tubes had a concentration-dependent bactericidal effect against C. jejuni F38011 after 3 h of incubation at 37°C under microaerobic conditions (see Fig. S1 in the supplemental material). For example, immobilized ZnO NPs at 25 ppm could reduce ∼90% of the bacterial population, and 50 ppm could inactivate >99% of the bacterial population (i.e., 2.61 log reduction). Immobilized ZnO NPs at 100 ppm reduced the bacterial population to an undetectable level (>8-log reduction). Thus, 100 ppm of ZnO NPs was identified as the MBC, according to its definition as the lowest concentration that leads to no observed bacterial growth in broth medium (30). In contrast, immobilized ZnO NPs at 3.12, 6.25, and 12.5 ppm did not significantly (P > 0.05) affect C. jejuni viability compared to the negative control. The MBC of immobilized ZnO NPs against C. jejuni in 3D paper tubes provided a quantitative prerequisite to further generate the effective functionalized absorbing pads. A diffusion assay was then used to test the effect of functionalized absorbing pads on inactivating C. jejuni F38011 grown on MH agar medium according to a previously established method (31). The functionalized absorbing pads showed clear inhibition zones against bacteria at all concentrations tested (i.e., 102, 105, and 108 CFU/plate), as shown in Fig. S2. Taken together, these data show that the functionalized paper products with ZnO NPs were able to effectively inactivate C. jejuni in a pure culture.
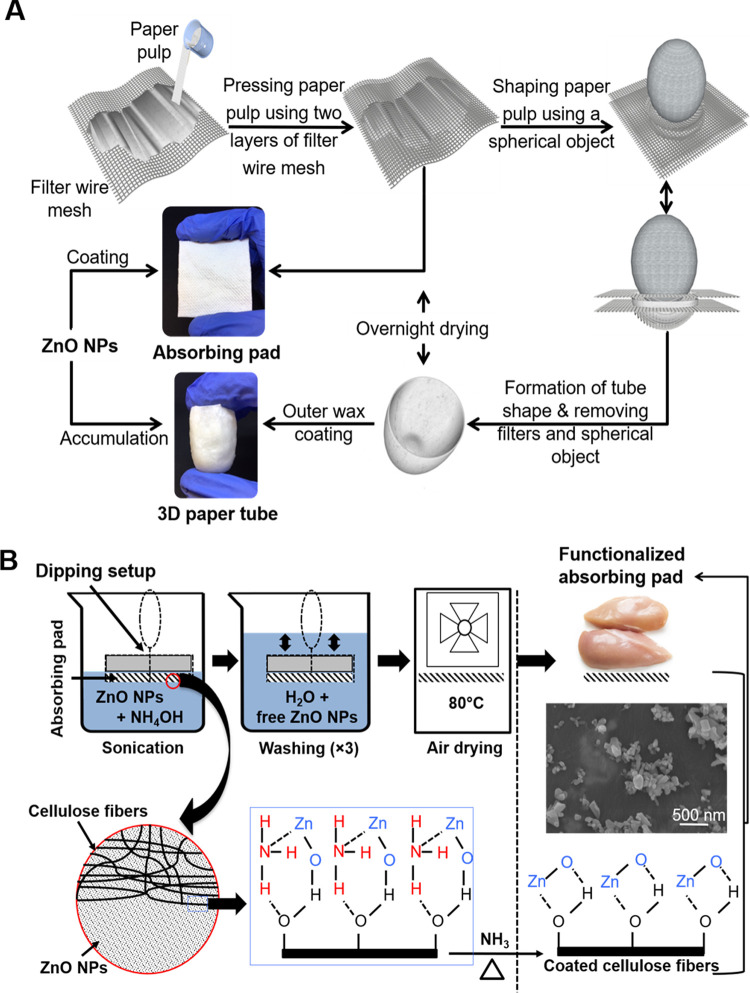
FIG 1.
Schematic diagrams of the development of 2D absorbing pads and 3D paper tubes (A) and functionalization of the absorbing pads by ZnO nanoparticles (B). The 3D paper tubes were functionalized by filling them with diluted ZnO NP suspensions and allowing them to dry at 22°C for 24 h. ZnO NPs attached to the cellulose fibers were observed and confirmed by scanning electron microscopy (SEM).
Quantification and visualization of ZnO NPs in the functionalized absorbing pads.
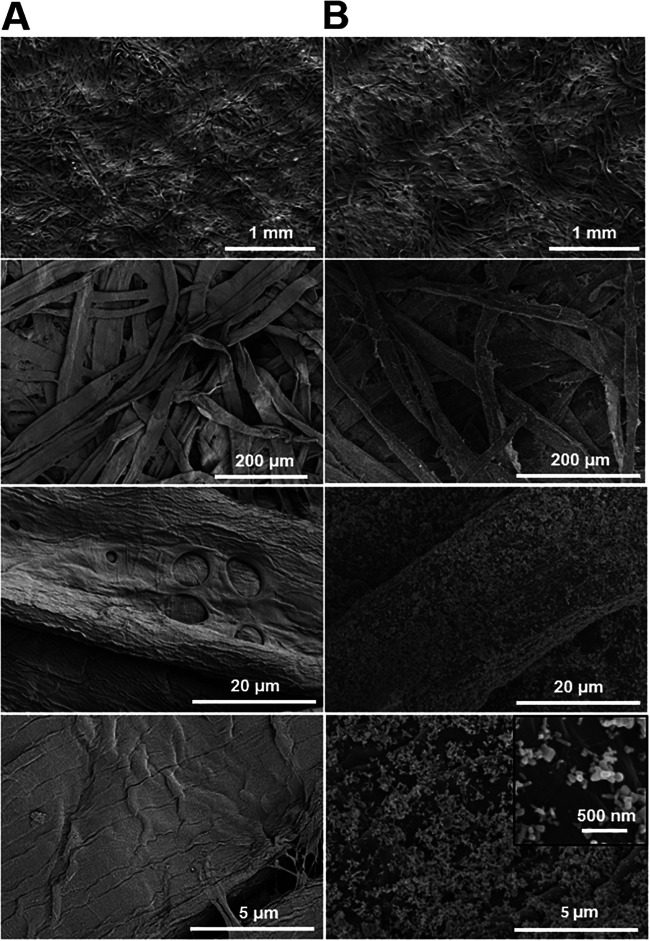
The final Zn concentrations in the two absorbing pads were separately determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) to be 0.856 ± 0.082 mg/cm2 and 0.075 ± 0.012 mg/cm2. Scanning electron microscopy (SEM) images suggested that ZnO NPs were successfully used to coat the surface of individual cellulose fibers and formed a thin layer around individual cellulose fibers after 5 min of sonication-assisted dipping (Fig. 2), including small individual nanoparticles with different shapes (mainly irregular and spherical shapes). In addition, unattached free ZnO NPs were not observed in the cavities between the coated cellulose fibers. In contrast, smooth and uncoated surfaces of cellulose fibers were observed for the uncoated group (Fig. 2). Taken together, ICP-OES analysis and SEM imaging confirmed that a certain percentage of ZnO NPs successfully coated the surface of individual cellulose fibers and formed the functionalized absorbing pads.
FIG 2.
Representative scanning electron microscopic (SEM) images of uncoated absorbing pads (A) and absorbing pads coated with immobilized ZnO nanoparticles at 0.856 mg/cm2 (B). Coating was conducted using a sonication/ultrasound-assisted dipping technique for 5 min, followed by immersion in distilled water for three washes and drying at 80°C for 45 min. SEM accelerating voltage was 2.0 kV, and the magnification ranged from ×250 to ×5,000 (n = 7).
Inactivation of C. jejuni cocktail on raw chicken meat by the functionalized absorbing pads.
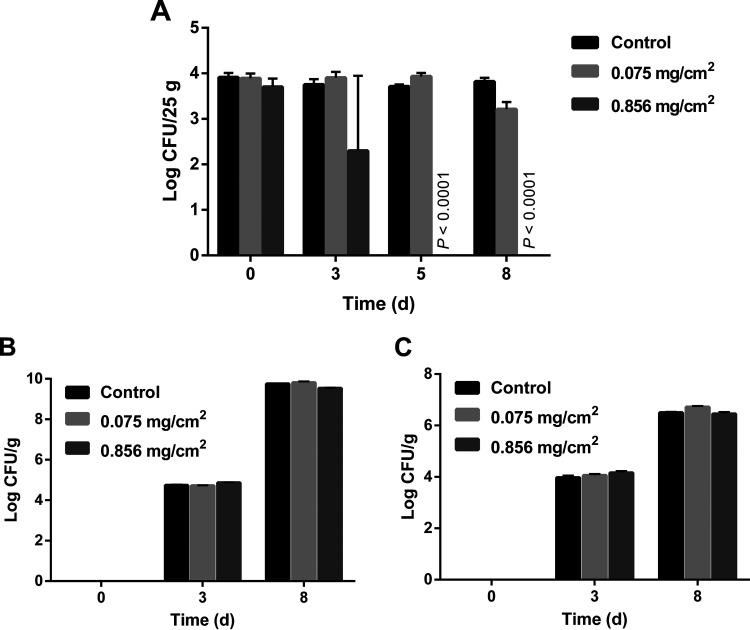
We investigated the effects of functionalized absorbing pads on inactivating C. jejuni-contaminated raw chicken meat at both refrigeration and abuse temperatures (i.e., 4°C and 7°C). The initial inoculum of C. jejuni cocktail (i.e., 4 log CFU/25 g raw chicken meat) did not increase in raw chicken meat during 8-day storage at 4°C in any of the treated or untreated groups (Fig. 3). C. jejuni on the untreated raw chicken meat was able to remain viable during the storage at 4°C for 8 days (Fig. 3A) regardless of cold stress and the presence of a relatively high level of competitive bacteria in the raw chicken meat microbiota, such as Lactobacillus and psychrotrophs (Fig. 3B and C). As shown in Fig. 3A, the functionalized absorbing pads with ZnO NPs at 0.075 mg/cm2 did not have a significant antimicrobial effect (P > 0.05) against C. jejuni after 3 and 5 days of storage but resulted in a reduction of ∼0.5 log CFU/25 g raw chicken meat at day 8 (P ≤ 0.001). In comparison, the functionalized absorbing pads with ZnO NPs at 0.856 mg/cm2 resulted in a reduction of 1.45 log CFU/25 g raw chicken meat after 3 days, followed by a decrease to an undetectable level (≤500 cells) after 5 and 8 days of storage. As confirmed by plating assay, no native Campylobacter bacteria were detected in raw chicken meat samples at all tested temperatures and time points (data not shown). The total counts of other predominant microbiota, including the undefined Lactobacillus and psychrotrophic bacteria, were 4 to 5 log CFU/g after 3 days and 6 to 9 log CFU/g after 8 days (Fig. 3B and C). No interaction between the predominant microbiota and functionalized absorbing pads was observed at all time points at 4°C (Fig. 3B and C). In addition, no reduction of C. jejuni in raw chicken meat products was observed after 24 h of storage at 7°C (Fig. S3A). The predominant microbiota in raw chicken meat grew rapidly at 7°C within 24 h and was not affected by the functionalized absorbing pads (Fig. S3B and C). No further time points were investigated due to the high level of psychrotrophs (∼6 log CFU/g) after only 24 h of incubation at this abuse storage temperature, as the cutoff value of microbial spoilage was identified as 7 log CFU/g in raw chicken (32). Altogether, a significant reduction of the C. jejuni cocktail by the functionalized absorbing pads was observed after 5 days at 4°C.
FIG 3.
Counts of Campylobacter jejuni cocktail (F38011, Human 10, 1173, and ATCC 33560) (A), Lactobacillus (B), and psychrotrophs (C) on raw chicken breasts stored at 4°C with or without functionalized absorbing pads, including immobilized ZnO nanoparticles at 0.075 and 0.856 mg/cm2, for 8 days. Campy-Cefex was used as the plating assay for enumerating C. jejuni, and plates were incubated at 42°C under microaerobic conditions. DeMan, Rogosa and Sharpe agar (MRS) and tryptic soy agar (TSA) were used in the plating assay to enumerate Lactobacillus and psychrotrophs separately, and the plates were incubated aerobically at 30°C (48 h) and 7°C (72 h), respectively. The limit of detection was determined to be 500 CFU/25 g raw chicken meat. Data were analyzed by using one-way ANOVA, followed by the post hoc Tukey’s test for multiple comparisons.
The antimicrobial effect of the functionalized absorbing pads with immobilized ZnO NPs at 0.856 mg/cm2 on individual C. jejuni strains was determined to be strain dependent (Fig. S4). In particular, the clinical strains (F38011 and Human 10) were reduced to undetectable levels after 3 days at 4°C, whereas the other two strains isolated from agri-foods (ATCC 33560 and 1173) were identified to be more tolerant to the treatment by the functionalized absorbing pads. In addition, the bovine isolate ATCC 33560 was more susceptible to immobilized ZnO NPs in the functionalized pads than strain 1173, isolated from chicken, with a variation of ∼1.5 log CFU/25 g raw chicken meat. Taken together, these results show that immobilized ZnO NPs (0.856 mg/cm2) in the functionalized pads were able to eliminate two C. jejuni strains after 3 days (Fig. S4) and inactivate all four tested strains after 5 days on raw chicken meat (Fig. 3A).
Migration of Zn from the functionalized absorbing pads to raw chicken meat.
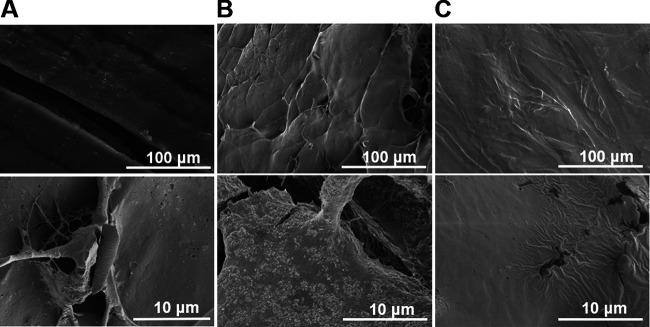
Studies were conducted to determine whether Zn would migrate from the functionalized absorbing pads to raw chicken meat. Zn levels in the treated raw chicken meat continuously increased from 0.02 mg/cm2 to 0.41 mg/cm2 after 8 days at 4°C, as determined by using ICP-OES (Fig. 4). SEM images of the treated raw chicken meat surface were collected to investigate the presence of ZnO NPs after 5 days of treatment with the functionalized absorbing pads containing ZnO NPs at 0.856 mg/cm2. During this time period, the Zn level increased from 0.02 to 0.22 mg/cm2 (Fig. 4) and all C. jejuni strains were reduced to undetectable levels (Fig. 3A). No ZnO NPs were observed on the surface of raw chicken meat exposed to the functionalized absorbing pads for 5 days (Fig. 5C), which was similar to the result for the negative-control group (i.e., untreated raw chicken meat [Fig. 5A]). In comparison, clear accumulation of ZnO NPs on the surface of raw chicken meat was observed for the positive control, which was artificially spiked with 0.22 mg/cm2 of ZnO NPs (Fig. 5B). In conclusion, no ZnO NPs were observed in raw chicken meat using the functionalized absorbing pads, while Zn ion levels increased and C. jejuni bacteria decreased to an undetectable level.
FIG 4.
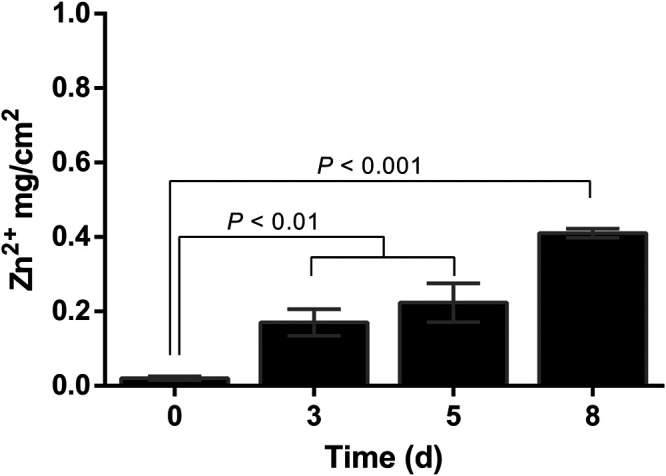
Quantification of Zn ion in treated raw chicken meat with functionalized absorbing pads containing immobilized ZnO NPs at 4°C for up to 8 days. Treated raw chicken pieces were spiked with ZnO NPs to generate calibration curves for quantification. Standards and samples were incinerated at 550°C for 2.5 h to remove the organic contents. Samples were then analyzed using inductively coupled plasma-optical emission spectroscopy (ICP-OES). The results are presented as means ± standard deviations (SD) from three independent experiments. Data were analyzed by using one-way ANOVA, followed by the post hoc Tukey’s test for multiple comparisons.
FIG 5.
Representative scanning electron microscopic (SEM) images of raw chicken meat samples, including an untreated sample (A), a sample spiked with ZnO nanoparticles at 0.22 mg/cm2 (B), and a sample treated with the functionalized absorbing pads containing immobilized ZnO nanoparticles at 0.856 mg/cm2 for 5 days (C). All samples were lyophilized for 24 h before imaging. SEM accelerating voltage was 2.0 kV, and the magnification ranged from ×250 to ×5,000 (n = 7).
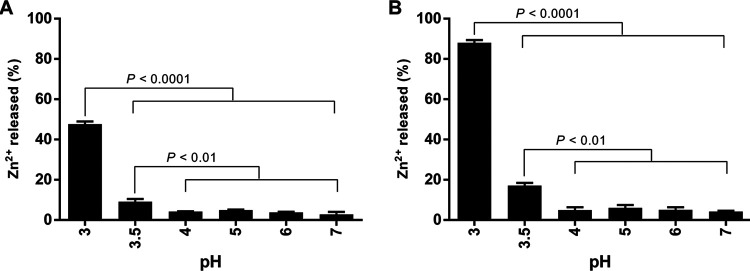
Release of Zn ions at different pH levels.
Considering that there was a correlation between Zn2+ level and antimicrobial effect of ZnO NP-functionalized pads, we then investigated the release of Zn2+ from ZnO NPs at various pHs (i.e., neutral to acidic pH) and 4°C. There was a minor release (2.8% to 6.0%) of Zn2+ at pHs of 4, 5, 6, and 7 after 24 h and 48 h of storage at 4°C (Fig. 6). A pH of 3.5 was identified as the cutoff value for the significant release of Zn2+ (P < 0.01). The largest release of Zn2+ (i.e., 47% and 88% after 24 h and 48 h, respectively) was observed at a pH of 3. The release of Zn2+ could be controlled at different pHs in the presence of lactic acid and by refrigeration.
FIG 6.
Quantification of Zn ions released by ZnO nanoparticles in the dialysis devices at pH 3 to 7 at 4°C. The pH levels were adjusted using 10% (vol/vol) lactic acid. Dialysate samples of Zn ions were collected and then analyzed by using inductively coupled plasma-optical emission spectroscopy (ICP-OES) after 24 h (A) and 48 h (B). The results are presented as means and SD from three independent experiments. Data were analyzed by using one-way ANOVA, followed by the post hoc Tukey’s test for multiple comparisons.
Whole-transcriptome sequencing analysis.
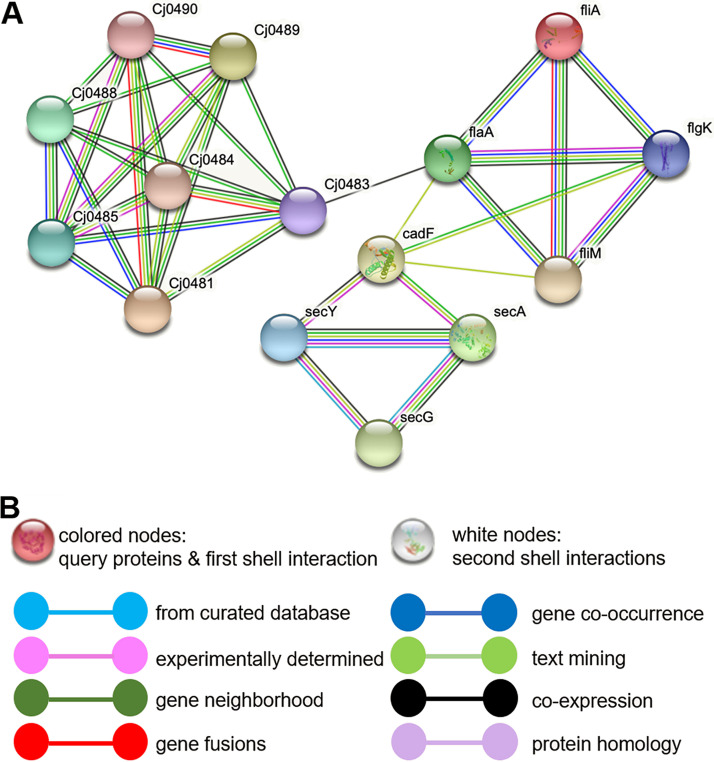
To investigate the antimicrobial mechanism, we performed whole-transcriptome sequencing (RNA-Seq). This enabled us to identify the Cj0131, Cj0132, Cj1339c, Cj0942c, Cj0944c, Cj0946, Cj1409, and Cj1626c genes, which are differentially expressed in response to ZnO NPs treatment. These differentially expressed genes are involved in many cellular developmental processes, as annotated by gene ontology, including the synthesis of outer membrane proteins, flagellum synthesis, heat and oxidative stress response, and ion transport, as well as the metabolism of amino acids and fatty acids (Table 1). Among these differentially expressed genes, genes responsible for the integral of outer and plasma membrane, including Cj0944c, Cj1626c, Cj0942c, and Cj0946, are among the most upregulated ones. This observation suggests a potential antimicrobial mode of ZnO NPs by targeting the C. jejuni outer and plasma membrane. Interaction network analysis further reveals that a possible consequence of impacting cell membrane synthesis is that both flagella and transmembrane transport are affected, because the genes involved are either functionally or structurally related (Fig. 7). We also noticed that genes involved in the synthesis of amino acids (Cj0130 and Cj0134) and lipids (Cj0132 and Cj1409) are significantly underexpressed, indicating a low intracellular anabolic activity.
TABLE 1.
Differentially expressed genes in C. jejuni treated with ZnO NPs (100 ppm)
| Gene group and tag | GO term and/or function | Fold change |
|---|---|---|
| Overexpressed genes | ||
| Cj1339c | GO:0071973; flagellum motility | 2.60 |
| Cj0944c | Periplasmic protein | 33.36 |
| Cj1626c | Periplasmic protein | 8.85 |
| Cj0942c | GO:0065002; intracellular protein transmembrane transport | 3.75 |
| Cj0946 | Membrane lipoproteins and porins | 20.97 |
| Cj0422c | Broad regulatory functions | 2.21 |
| Cj1387c | Broad regulatory functions | 2.78 |
| Underexpressed genes | ||
| Cj0128c | GO:0046854; phosphatidylinositol phosphorylation | −2.27 |
| Cj0129c | GO:0043165; outer membrane assembly | −3.22 |
| Cj0131 | GO:0016021; putative peptidase M23 protein | −3.88 |
| Cj0132 | GO:0009245; lipid biosynthesis | −3.88 |
| Cj1409 | GO:0006633; fatty acid biosynthesis | −2.04 |
FIG 7.
Interaction network of genes associated with outer membrane synthesis in C. jejuni F38011 (A). Genes are represented by nodes, and interaction sources are indicated by various colors (B). The network was built with medium confidence (interaction score, 0.400) in the STRING database (61).
DISCUSSION
Campylobacter control in the poultry industry is challenging (9). So far, no commercially available methods can be effectively applied to reduce Campylobacter load during poultry meat production (8). Advanced biosecurity systems might be the only option to prevent the presence of Campylobacter in poultry farms. However, this approach is costly compared to the controls at the endpoint of the farm-to-fork pathway (i.e., poultry meat), as bacterial transmission can occur via multiple routes, such as the environment, food processing chain, water, and direct contact (8). In addition, poultry carcasses collected from different farms might have distinct Campylobacter contamination levels but end up in the same poultry processing plant, where the Campylobacter-positive birds can pollute the food contact surfaces, equipment, and water system, leading to cross-contamination of clean batches of poultry carcasses. The current available antimicrobial agents can achieve only a 1- to 2-log-CFU/ml reduction of Campylobacter in poultry plants (9, 21). Due to inefficient Campylobacter control measures, the incidence of human campylobacteriosis via the consumption of contaminated chicken products has remained unchanged during the past decade (6, 33). Even with the use of available intervention strategies and fast/sensitive detection methods, Campylobacter-free raw chicken meat at the retail level may be unattainable (34). However, mitigation of Campylobacter in postchilling poultry products can remarkably reduce campylobacteriosis risk. For example, a risk assessment study demonstrated that reducing Campylobacter by 3 log CFU/g of chicken intestinal contents in slaughterhouses or by 1 log CFU/carcass in raw chicken end products can reduce Campylobacter infection risks by 50 to 90% (35). Thus, a new generation of antimicrobials and mitigation strategies need to be investigated to enhance the safety of raw chicken meat, especially at the endpoint of food processing chains.
Nanotechnology offers a variety of novel approaches to improve the quality and safety of foods. Application of metal oxide nanoparticles in foods is still in its infancy due to the general health concerns of nanomaterials in agri-food products on the part of different stakeholders, such as consumers, regulators, and the food industry (36). ZnO NPs should not be added directly to food products, but they can be incorporated into food packaging materials for the inactivation of foodborne pathogens. In the current study, we developed an innovative antimicrobial packaging as an alternative strategy to reduce C. jejuni in raw chicken meat at the retail level. This novel strategy provides consumers protection from Campylobacter-contaminated raw chicken meat at the final stage of the food processing chain, regardless of the contamination levels of chicken in farms or processing plants. Considering the potential migration of ZnO NPs from food packaging materials onto raw chicken meat, we immobilized ZnO NPs at minimum lethal concentrations against C. jejuni and also monitored the presence of ZnO NPs on the surface of the treated raw chicken meat.
Two types of paper-based active packaging (i.e., 3D tubes and 2D pads) were developed for the inactivation of C. jejuni. First, the broth dilution method was performed to identify the MBC of immobilized ZnO NPs in 3D paper tubes. The identified MBC (i.e., 100 ppm) of the functionalized 3D tubes was selected to develop the 2D functionalized absorbing pads, achieving a final surface area concentration of ZnO NPs of 0.856 mg/cm2. The attachment efficiency of ZnO NPs onto 2D functionalized absorbing pads was only 10% by using the ultrasound-assisted dipping technique. This relatively low attachment efficiency was mainly due to the repeated washing steps (i.e., three washes) for the removal of any unattached ZnO NPs (Fig. 1B). This washing step was of great importance because the absence of unattached nanoparticles ensured no migration of free ZnO NPs from the functionalized absorbing pads to raw chicken meat. C. jejuni strain F38011 was selected as the representative isolate for antimicrobial testing of both 3D and 2D functionalized paper products (Fig. S1 and S2). This strain was recovered from a human with severe clinical disease and has been determined to effectively colonize the gastrointestinal tract of chickens (37, 38). To investigate the impact of immobilization on the antimicrobial activity of ZnO NPs, we compared the antimicrobial efficacy of immobilized ZnO NPs in 3D paper tubes with free ZnO NPs in solution. Previous studies reported that 100 ppm of free ZnO NPs could cause a ≥8 log CFU/ml reduction of C. jejuni after only 3 h treatment under microaerobic conditions with constant shaking at 37°C (39) or under static conditions at 42°C (29). ZnO NPs immobilized in 3D paper tubes showed antimicrobial efficacy comparable to that of free ZnO NPs at the same concentration and under the same incubation conditions. Given that immobilization did not influence the antimicrobial effect of ZnO NPs, we immobilized ZnO NPs in 2D functionalized absorbing pads at the MBC level (0.856 mg/cm2) for further food packaging application.
Functionalized absorbing pads with immobilized ZnO NPs were used for the inactivation of C. jejuni in raw chicken meat. According to a ZnO NP quantification assay using ICP-OES, the final concentration of immobilized ZnO NPs on the functionalized absorbing pads was 0.075 mg/cm2 after dipping the absorbing pads in 1,000 ppm of ZnO NPs suspension. This absorbing pad showed a limited inhibition zone when it was used to inactivate C. jejuni by using the diffusion assay. It also showed limited antimicrobial effect against a cocktail of four C. jejuni strains on the surface of raw chicken meat products. Specifically, <1 log CFU/25 g raw chicken meat reduction was obtained after 8 days of treatment at 4°C. A few factors could contribute to the reduced antimicrobial effect of this absorbing pad against C. jejuni in raw chicken meat. First, other tested C. jejuni strains (i.e., Human 10, ATCC 33560, and 1173) could be more tolerant to ZnO NPs than C. jejuni F38011. Second, complex chicken matrices (e.g., lipids and proteins in blood and chicken juice) and/or native chicken microbiota might be protective to C. jejuni, thereby reducing the antimicrobial effect of ZnO NPs. In fact, C. jejuni does not grow below 30°C but remains viable, performs respiration, and responds normally to chemotaxis and aerotaxis, even at 4°C (40). This is consistent with our results, as the C. jejuni level did not decline in raw chicken meat for up to 8 days if the meat was not treated with the immobilized ZnO NPs. This finding highlights the need for an effective intervention strategy to control C. jejuni in raw chicken meat during cold-chain transportation and storage.
To achieve sufficient inactivation of C. jejuni in raw chicken meat, functionalized absorbing pads with a higher concentration of immobilized ZnO NPs dipped in 10,000 ppm of ZnO NPs suspension were developed. The final concentration of the immobilized ZnO NPs was determined to be 0.856 mg/cm2. This level of immobilized ZnO NPs showed clear inhibition zones on C. jejuni lawns regardless of the initial concentrations of this microorganism.
The functionalized absorbing pads at both ZnO NP concentrations (0.075 and 0.856 mg/cm2) were used to test the antimicrobial effect of immobilized ZnO NPs against a cocktail of C. jejuni on the surface of raw chicken meat. The immobilized ZnO NPs at 0.856 mg/cm2 were able to cause a 4-log reduction in the bacterial count for the C. jejuni strains in raw chicken meat after 3 days of treatment at 4°C, which was equivalent to a 5.60-log reduction of this microbe per 1 kg of raw chicken meat. This contamination level is the maximum level of Campylobacter reported in raw chicken meat so far (14, 15, 41). It is noteworthy that the interaction between the immobilized ZnO NPs and C. jejuni was relatively slow and was time and strain dependent. A significant reduction (P < 0.0001) was observed after 3 days for two clinical strains (F38011 and Human 10). In contrast, the other two food-isolated strains (ATCC 33560 and 1173) were more resistant to the immobilized ZnO NPs (Fig. S4). This could be due to the ecology and adaptation and/or different membrane surface charges of various strains (42). A significant reduction in C. jejuni (P < 0.05) was observed with all the strains tested after 5 days of storage at 4°C (Fig. 3A).
Although the antimicrobial effect of ZnO NPs is well known, the antimicrobial mechanism has not been fully investigated yet. Several previous studies reported that Zn2+ had a minor contribution to the antimicrobial effect of ZnO NPs due to its low dissociation (26, 43). However, the antimicrobial role of Zn2+ depends on several factors, including environmental conditions (e.g., pH), exposure time, UV irradiation, presence of other substances or microorganisms, and physiochemical properties of nanoparticles (e.g., size, shape, porosity, and concentration). For example, a recent study investigated the antimicrobial interaction between E. coli and ZnO NPs, along with their released compounds, such as Zn2+ and ROS (43). Polyvinyl chloride (PVC) film coated with ZnO NPs was able to induce a potent antimicrobial affect even without direct contact with bacterial cells. Unlike free ZnO NPs, ZnO NPs coating the PVC films did not induce any outer membrane damage (29), but they disrupted the intracellular integrity of carboxyfluorescein-filled liposomes and caused leakage of K+. The release of Zn2+ did not reduce bacterial populations, while ROS played a role in the lethality of the immobilized ZnO NPs at neutral pH within a 3-h treatment. However, the authors did not consider the antimicrobial effect of ZnO NPs at different pH levels. In another study, several microorganisms, including E. coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans, were reported to be sensitive to ZnO NPs, and the contribution of Zn2+ to their effects varied from 15% to 100% (26). This study also determined that the release rate of Zn2+ was more consistent in buffered solution than distilled water within 24 h. The dissolution profiles of ZnO NPs varied based on the particle characteristics and morphologies (e.g., size, surface area, and type). Smaller nanoparticles released a larger amount of Zn2+. Altogether, the contribution of Zn2+ to the overall antimicrobial effect of ZnO NPs is multifactorial.
The correlation between time/pH and the release of Zn2+ from ZnO NPs was previously studied in simulated uterine solution (44). The contribution of Zn2+ to the overall antimicrobial effect of ZnO NPs was negligible at neutral pH, whereas a greater contribution of Zn2+ was obtained at a lower pH (44). The release of Zn2+ at an acidic pH was due to the reaction between ZnO and protons (ZnO + 2H+ → Zn2+ + H2O) (44). This chemical dissociation may control antimicrobial delivery, such as the release of Zn2+, in food packaging applications. Zn2+ was identified to have potent antimicrobial activity (45) and could treat several bacterial infectious diseases, such as pneumonia, diarrhea, and prostatitis (45, 46). Bacterial cells maintain the intracellular concentration of Zn2+ at a low level (10−4 M) even when they are grown in Zn2+-rich environments, such as soil, oysters, and red meat (47). The antimicrobial effect of Zn2+ is mainly a result of unspecific binding to the intracellular proteins and enzymes that are involved in protein synthesis and important metabolic pathways (48). These include proteins that are involved in DNA transcription and translation, sigma factor proteins, tRNA synthesis proteins, RNA polymerases, and ribosomal proteins. As far as we know, Zn2+ release from ZnO NPs immobilized in food packaging materials has not been investigated.
In the current study, we observed a slow antimicrobial effect of immobilized ZnO NPs against C. jejuni on the surface of raw chicken meat. The level of Zn increased in chicken meat over time, as determined by using ICP-OES (Fig. 4), but no nanoparticles were observed by using SEM (Fig. 5). Thus, we hypothesized that the increase of lactic acid generated by Lactobacillus in the packaging system, including raw chicken meat, chicken juice, and functionalized absorbing pads, allowed the slow release of Zn2+, which eventually led to the inactivation of C. jejuni after 3 to 5 days at 4°C (Fig. 3A). An additional experiment showed a significant and controllable release of Zn2+ from ZnO NPs in the presence of lactic acid at different pH levels (Fig. 6). Such a controlled release of Zn2+ not only improves the inactivation speed against Campylobacter in raw chicken meat but also expands the applications of ZnO NP-functionalized absorbing pads to control other more tolerant pathogenic and spoilage microorganisms in agri-food commodities. It is noteworthy that the immobilized ZnO NPs might also partially disrupt the bacterial cell wall and cell membrane via electrostatic interactions and thus allow the accumulation of extracellular Zn2+ in the cells.
ZnO NPs can strongly bind to bacterial cells and disrupt cell membranes (49). This can be associated with the release of Zn2+, which can accumulate inside bacterial cells. Our RNA-Seq analysis indicated that ZnO NPs alter the regulation of genes with broad functions, which is consistent with previous studies (48, 50–53). In addition, downregulation of intracellular anabolism, such as the synthesis of amino acids and lipids, illustrates potential antimicrobial mechanisms that require further studies.
Conclusions.
We developed a novel functionalized absorbing pad that enabled the reduction of C. jejuni in raw chicken meat from ∼4 log CFU/25 g raw chicken meat to an undetectable level after 3 days at 4°C. No migration of ZnO NPs from the functionalized absorbing pad to raw chicken meat was detected, while Zn level increased during raw chicken meat storage. We also identified that the presence of lactic acid was associated with a significant increase in the release of Zn ions from the functionalized absorbing pad to raw chicken meat. This novel approach has great potential to be applied in the poultry industry to improve the safety of raw chicken meat product.
MATERIALS AND METHODS
Chemical reagents and bacterial strains.
ZnO NPs (size, 40 to 100 nm; surface area, 12 to 24 m2/g) were obtained from Alfa Aesar (Haverhill, MA). Spruce-pine-fir softwood kraft paper sheets were donated from the Paper and Pulp Centre at The University of British Columbia (Vancouver, BC). Two clinical C. jejuni isolates (F38011 and Human 10), one bovine fecal isolate (ATCC 33560), and one chicken isolate (1173) were routinely cultivated on Mueller-Hinton (MH) agar (Oxoid, Nepean, ON, Canada) plates supplemented with 5% defibrinated sheep blood (Alere, Stittsville, ON, Canada) for 48 h. Fresh bacterial cultures were prepared by suspending C. jejuni colonies in MH broth with constant shaking for 18 h at 37°C under microaerobic conditions (85% N2, 10% CO2, 5% O2). Equal volumes of C. jejuni cultures were combined as a cocktail at an initial concentration of 1 × 109 CFU/ml.
Development and antimicrobial functionalization of 3D paper tubes and 2D absorbing pads.
Three-dimensional (3D) paper tubes and two-dimensional (2D) absorbing pads were designed and developed, as shown in Fig. 1A. Briefly, a spruce-pine-fir softwood kraft paper sheet was cut into smaller sheets before being soaked in distilled water for 10 min to allow the paper to adequately absorb water. The paper sheets were then mixed with water and blended for 10 min using a blender to obtain a homogeneous paper pulp. Then, water was removed from the homogenized pulp by squeezing and drying at 22°C for overnight. The dry paper pulp (4 g) was immersed in distilled water again to form a homogenous mixture, which was subsequently pressed between two wire mesh filters to remove the excess water and obtain paper sheets with a surface area of 25 cm2 (5 by 5 cm). This paper sheet was then removed from the wire meshes and dried at 22°C for overnight. To make 3D paper tubes, the paper pulp between the wire meshes was shaped using a spherical object before removing the meshes and allowing it to dry. Finally, the outsides of all paper tubes were coated with wax to make them water resistant and able to hold the bacterial liquid culture.
A ZnO NP suspension was prepared at a concentration of 10,000 ppm and then placed in a fixed power sonicator (model FS110; Fisher Scientific, Ottawa, ON, Canada) for 10 min. The pH was adjusted to 8 by using 0.1 M NH4OH to make it consistent with the alkalinity of the paper (54). Twofold serial dilutions from 100 to 1 ppm of ZnO NP aqueous suspensions were individually added to different paper tubes. The negative control was a paper tube without the addition of ZnO NPs. The tubes were kept at 22°C overnight to dry and allow the absorption between ZnO NPs and cellulose fibers via electrostatic attraction and hydrogen bonding (55). Next, 3D tubes were washed three times with distilled water to remove any free ZnO NPs and then dried at 80°C for 45 min.
ZnO NPs were also immobilized on the 2D absorbing pads according to the protocol described in a previous study, with some modifications (54). Briefly, two different ZnO NP suspensions at concentrations of 1,000 ppm and 10,000 ppm were separately prepared under sonication and pH adjustment conditions as described in reference 54. A spruce-pine-fir softwood kraft paper sheet was used to make flat thick (2 mm) paper sheets as the substrates to generate functionalized absorbing pads. Specifically, paper substrates were placed facedown on ZnO NP suspensions (Fig. 1B). Paper substrates were dipped under sonication for 5 min, followed by immersion in distilled water three times to remove the free nanoparticles. The coated papers were detached from the dipping setup and dried at 80°C for 45 min. By using ZnO NP suspensions at 1,000 ppm and 10,000 ppm, two different concentrations of ZnO NPs in the functionalized absorbing pads were generated.
Testing the antimicrobial effect of functionalized 3D paper tubes and 2D absorbing pads against C. jejuni F38011.
Both functionalized paper products (i.e., functionalized 3D tubes and 2D absorbing pads) were sterilized by autoclave for 15 min at 121°C before antimicrobial testing. The functionalized 3D tubes were filled with 1.5 ml of C. jejuni F38011 culture at early stationary phase (1 × 108 CFU/ml) and incubated with constant shaking (175 rpm) at 37°C under microaerobic conditions. Microbial counts were determined after 3 h of incubation by plating assay, and the minimum bactericidal concentration (MBC) of immobilized ZnO NPs was consequently identified. In addition, the growth of C. jejuni F38011 was examined with either ZnO NP-coated or uncoated absorbing pads using the diffusion assay (31). Three concentrations (1 × 102, 1 × 105, and 1 × 108 CFU/ml) of C. jejuni F38011 (1 ml) at early stationary phase were separately streaked onto the entire surface of MH agar plates supplemented with 5% defibrinated sheep blood and dried for 30 min at 22°C. Both coated and uncoated absorbing pads (∼1 cm2) were then applied to the surface of C. jejuni-inoculated plates and incubated at 37°C for 48 h under microaerobic conditions until C. jejuni lawns developed. Visual comparison of the size of inhibition zones was carried out to determine the antimicrobial effect of the functionalized absorbing pads.
Investigating the antimicrobial effect of functionalized absorbing pads to control a C. jejuni cocktail in raw chicken meat.
Boneless raw chicken breasts were obtained from local grocery stores in Vancouver (BC, Canada) and used immediately after their arrival in the laboratory. Chicken breasts were cut into pieces of 25 g, aseptically mixed in a sterile container, and aged at 4°C overnight to obtain an evenly distributed microbiota on the raw chicken meat surface. The chicken pieces were mixed several times during overnight refrigeration. Each piece of raw chicken breast was then placed in a sterile petri dish and inoculated with a cocktail of four C. jejuni strains (i.e., F38011, Human 10, ATCC 33560, and 1173). The initial inoculum of C. jejuni was adjusted to 4 log CFU/25 g raw chicken meat, which is the level of this microbe commonly observed in raw chicken meat (14, 15, 41). Inoculated raw chicken samples were separately treated by absorbing pads with ZnO NPs and a negative control (i.e., absorbing pad with no ZnO NPs). Two different concentrations of immobilized ZnO NPs in the absorbing pads (0.075 and 0.856 mg/cm2) were selected against the C. jejuni cocktail used to spike raw chicken meat at 4°C. One nonspiked and untreated control group was also included to track the native Campylobacter load in raw chicken meat samples. After treatment, absorbing pads were aseptically removed, and raw chicken meat samples were immersed in 225 ml of phosphate-buffered saline (PBS, pH 7.4), followed by a manual massage for 10 min. Raw chicken rinses were serially diluted and plated on Campy-Cefex agar (56), DeMan, Rogosa, and Sharpe agar (MRS) (Fisher Scientific, Ottawa, ON, Canada), and tryptic soy agar (TSA) (VWR, Mississauga, ON, Canada) plates, followed by incubation at 42°C, 30°C and 7°C, respectively. Bacterial counts were enumerated for C. jejuni and Lactobacillus after 72 h of incubation and psychrotrophic bacteria after 3 to 7 days of incubation. When necessary, up to 10 ml of raw chicken meat rinse was directly plated on 10 Campy-Cefex agar plates (1 ml each) to improve the limit of detection of the plating assay. Each biological sample was tested at least in triplicate.
Testing the antimicrobial effect of functionalized absorbing pads against individual C. jejuni strains in raw chicken meat.
Chicken breasts were decontaminated to remove the native microbiota before testing the effect of functionalized absorbing pads on each individual Campylobacter strain. Briefly, raw chicken breasts were soaked in 3% (vol/vol) hydrogen peroxide for 2 min with constant shaking (100 rpm). Samples were air dried for 2 min and soaked in 98% (vol/vol) ethanol for another 1 min following the same procedure. A sterilized knife was then used to remove the outer layer of chicken and cut the raw chicken meat into pieces of 5 g or 5 cm2 (2.236 cm by 2.236 cm). Random portions of decontaminated chicken meat (5 g) were separately placed in stomacher bags with 45 ml of PBS and then manually massaged for 10 min. Chicken rinses were plated on TSA and incubated at 37°C for at least 48 h to confirm the absence of native microbiota. Four strains of C. jejuni (i.e., F38011, Human 10, ATCC 33560, and 1173) were inoculated individually onto the surface of meat samples and then treated with either coated or uncoated absorbing pads for 3 days at 4°C. Samples were collected after 0 days and 3 days of storage. Absorbing pads were removed, and meat samples were placed in 45 ml of PBS and then manually massaged for 10 min. These samples were plated on MH agar plates with 5% defibrinated sheep blood. Each sample was tested at least in triplicate.
Determination of Zn levels in both functionalized absorbing pads and treated raw chicken meat.
Zn levels in both functionalized absorbing pads and treated raw chicken meat were quantified according to the protocol described in a previous study (57), with modifications. Briefly, ZnO NPs were used to spike 4 cm2 (equivalent to 0.2 g) of absorbing pads at concentrations of 0, 50, 250, 625, 1,250, and 2,500 ppm to generate a calibration curve for quantification. Similarly, ZnO NPs were used to spike 5 cm2 (equivalent to 5 g) of raw chicken meat at concentrations of 0, 0.1, 0.2, 1, 2, 10, 20, 50, and 400 ppm to quantify the migration level of Zn in raw chicken meat. Samples of both groups (i.e., functionalized absorbing pads and treated raw chicken meat) were separately transferred to ceramic crucibles and incinerated at 550°C for 2.5 h. The samples obtained were placed in 10 ml of 6 N HCl and diluted by adding 30 ml of double-distilled water. The remaining ashes were removed by filtration through Whatman no. 42 paper (Millipore Sigma, Oakville, ON, Canada). The supernatant was collected and analyzed using ICP-OES (Varian model 725ES spectrometer; Agilent Technologies, Inc., USA) to quantify Zn levels. Three batches of either functionalized absorbing pads or raw chicken samples were processed individually and then tested by ICP-OES.
Electron microscopy was conducted to determine if ZnO NPs coated the surfaces of functionalized absorbing pads or migrated to the treated raw chicken meat using a Hitachi-S4700 field emission scanning electron microscope (SEM) (Hitachi, Tokyo, Japan). Raw chicken samples were cut into ∼0.5-cm slices, stored at −80°C for overnight, and then lyophilized at −53°C by using a 12-liter console freeze-dryer (Labconco, Kansas City, MO) until complete dryness. Both samples were cut into ∼1-cm2 pieces and sputter coated with platinum to get an electron-conductive surface. The raw chicken samples used as the negative control for SEM were processed without the treatment of functionalized absorbing pads or without the addition of ZnO NPs, and the positive control was raw chicken meat spiked with ZnO NPs at 0.22 mg/cm2. Images were collected at an accelerating voltage of 2.0 kV and a magnification range of ×50 to ×50,000.
Determination of the release of Zn2+ at different pH levels.
As lactic acid is the key metabolite produced by native raw chicken meat microbiota (e.g., Lactobacillus), we used lactic acid to precisely adjust the pHs under different release conditions. Different pHs (i.e., 3, 3.5, 4, 5, 6, and 7) were prepared in 200 ml of deionized water and adjusted by 10% (vol/vol) lactic acid. Five milliliters at each pH was used to prepare ZnO NP suspensions by sonication for 10 min and then placed in a floating dialysis device with a molecular weight cutoff of 8 to 10 kDa (Spectra-Por Float-A-Lyzer G2; Millipore Sigma, Oakville, ON, Canada). Each dialysis device was placed against 195 ml of deionized water with lactic acid at different pHs. ZnO NP suspensions were dialyzed for 48 h in the dark with stirring at 160 rpm and at 4°C. The dialysates were collected after 24 h and 48 h and then analyzed using ICP-OES to quantify the level of the released of Zn2+. Each sample was tested at least in triplicate.
Whole-transcriptome sequencing analysis of C. jejuni under ZnO NP treatment.
The whole-transcriptome profile of C. jejuni F38011 after ZnO NP treatment was investigated. An adjusted concentration of an overnight culture (108 CFU/ml) was prepared in fresh MH broth and incubated for 2 h before ZnO NP treatment (100 ppm). C. jejuni cells were either treated with ZnO NPs or untreated at 37°C under microaerobic conditions with constant shaking at 175 rpm for 15 min. The bacterial cultures were then placed on ice, and the pellets were collected by centrifugation at 8,000 × g for 3 min at 4°C. Extraction of total RNA from either treated or untreated C. jejuni cells was conducted using a GENEzol TriRNA Pure kit, according to the manufacturer’s protocol, with modifications (Froggabio, North York, ON, Canada). Briefly, bacterial cultures were treated with lysozyme, GENEzol reagent, and DNase for total RNA extraction, homogenization, and purification, respectively. Each sample was then separately loaded into a spin column for purifying the extracted RNA. A Nanodrop1000 spectrophotometer (Thermo Fisher, MA, USA), gel electrophoresis, and Agilent bioanalyzer RNA Nano Chip (Agilent Technologies, Santa Clara, CA) were used to assess both quantity and quality of the purified RNA.
We then conducted a genome-wide transcriptome analysis of ZnO NP treatment against C. jejuni along with a negative control (untreated cells). The cDNA library was constructed, and RNA-Seq was run on the HiSeq 2500 system (Illumina, CA). Sequencing length was 75 bp, and each library yielded in an average of 29.4 million reads. RNA-Seq raw data were processed according to a previous study (58), and the reads were mapped to the reference genome of C. jejuni NCTC 11168 of >1,600 genes (59). CLC genomics workbench software was used for mapping transcripts to the reference genome, calculation of differentially expressed genes, and visualization of the whole-transcriptomic profile (CLCBio, Cambridge, MA, USA). Genes were considered differentially expressed at a cutoff value between 0.05 and 2 log fold change. The Gene Ontology (GO) term tools (http://geneontology.org) was used to define the biological processes or molecular functions of altered genes (60).
Statistical analysis.
Prism6 (version 6.01; GraphPad Software Inc., San Diego, CA) was used for graph generation and statistical analysis. Data were analyzed using one-way analysis of variance (ANOVA), followed by a post hoc Tukey’s test for multiple comparisons. P values were adjusted at 0.05 or lower. All experiments were conducted at least in triplicate.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Natural Sciences and Engineering Research Council of Canada in the form of a Discovery Grant (NSERC RGPIN-2019-03960) and a Discovery Accelerator Grant (PGPAS-2019-00024) and by Genome British Columbia/Genome Canada (SIP021) to X.L. M.H. received a 5-year scholarship from King Saud University. L.M. received an International Doctoral Fellowship from the University of British Columbia.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Beery JT, Hugdahl MB, Doyle MP. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol 54:2365–2370. doi: 10.1128/AEM.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanker S, Lee A, Sorrell TC. 1990. Horizontal transmission of Campylobacter jejuni amongst broiler chicks: experimental studies. Epidemiol Infect 104:101–110. doi: 10.1017/s0950268800054571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newell DG, Fearnley C. 2003. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol 69:4343–4351. doi: 10.1128/aem.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorgensen F, Bailey R, Williams S, Henderson P, Wareing DR, Bolton FJ, Frost JA, Ward L, Humphrey TJ. 2002. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int J Food Microbiol 76:151–164. doi: 10.1016/S0168-1605(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 5.Willis WL, Murray C. 1997. Campylobacter jejuni seasonal recovery observations of retail market broilers. Poult Sci 76:314–317. doi: 10.1093/ps/76.2.314. [DOI] [PubMed] [Google Scholar]
- 6.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. 2015. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batz MB, Hoffmann S, Morris JG. 2012. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot 75:1278–1291. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- 8.Wagenaar JA, French NP, Havelaar AH. 2013. Preventing Campylobacter at the source: why is it so difficult? Clin Infect Dis 57:1600–1606. doi: 10.1093/cid/cit555. [DOI] [PubMed] [Google Scholar]
- 9.Oyarzabal OA. 2005. Reduction of Campylobacter spp. by commercial antimicrobials applied during the processing of broiler chickens: a review from the United States perspective. J Food Prot 68:1752–1760. doi: 10.4315/0362-028X-68.8.1752. [DOI] [PubMed] [Google Scholar]
- 10.Marotta F, Garofolo G, Di Donato G, Aprea G, Platone I, Cianciavicchia S, Alessiani A, Di Giannatale E. 2015. Population diversity of Campylobacter jejuni in poultry and its dynamic of contamination in chicken meat. BioMed Res Int 2015:859845. doi: 10.1155/2015/859845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrang ME, Dickens JA, Musgrove MT. 2000. Effects of hot water application after defeathering on the levels of Campylobacter, coliform bacteria, and Escherichia coli on broiler carcasses. Poult Sci 79:1689–1693. doi: 10.1093/ps/79.11.1689. [DOI] [PubMed] [Google Scholar]
- 12.Tresse O, Alvarez-Ordóñez A, Connerton IF. 2017. Editorial: about the foodborne pathogen Campylobacter. Front Microbiol 8:1908. doi: 10.3389/fmicb.2017.01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashor MP, Curtis PA, Keener KM, Sheldon BW, Kathariou S, Osborne JA. 2004. Effects of carcass washers on Campylobacter contamination in large broiler processing plants. Poult Sci 83:1232–1239. 2004/08/03. doi: 10.1093/ps/83.7.1232. [DOI] [PubMed] [Google Scholar]
- 14.Stern NJ, Robach MC. 2003. Enumeration of Campylobacter spp. in broiler feces and in corresponding processed carcasses. J Food Prot 66:1557–1563. doi: 10.4315/0362-028X-66.9.1557. [DOI] [PubMed] [Google Scholar]
- 15.Kim SA, Park SH, Lee SI, Owens CM, Ricke SC. 2017. Assessment of chicken carcass microbiome responses during processing in the presence of commercial antimicrobials using a next generation sequencing approach. Sci Rep 7:43354. doi: 10.1038/srep43354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanning I, Jarquin R, Slavik M. 2008. Campylobacter jejuni as a secondary colonizer of poultry biofilms. J Appl Microbiol 105:1199–1208. doi: 10.1111/j.1365-2672.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson DN, Davis B, Tirado SM, Duggal M, van Frankenhuyzen JK, Deaville D, Wijesinghe MAK, Tessaro M, Trevors JT. 2009. Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie Van Leeuwenhoek 96:377–394. doi: 10.1007/s10482-009-9378-8. [DOI] [PubMed] [Google Scholar]
- 18.Oh E, McMullen L, Jeon B. 2015. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front Microbiol 6:1263. doi: 10.3389/fmicb.2015.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Garner LJ, McKee SR, Bilgili SF. 2018. Effectiveness of several antimicrobials used in a postchill decontamination tank against Salmonella and Campylobacter on broiler carcass parts. J Food Protection 81:1134–1141. doi: 10.4315/0362-028X.JFP-17-507. [DOI] [PubMed] [Google Scholar]
- 20.Food Safety and Inspection Service. 2019. 7120.1, Revision 51, safe and suitable ingredients used in the production of meat, poultry, and egg products, July 7, 2019. United States Department of Agriculture Food Safety and Inspection Service, Washington, DC. [Google Scholar]
- 21.Capita R, Alonso-Calleja C, Garcia-Fernandez MC, Moreno B. 2002. Trisodium phosphate (TSP) treatment for decontamination of poultry. Food Sci Technol Int 8:11–24. doi: 10.1177/1082013202008001118. [DOI] [Google Scholar]
- 22.Panea B, Ripoll G, González J, Fernández-Cuello Á, Albertí P. 2014. Effect of nanocomposite packaging containing different proportions of ZnO and Ag on chicken breast meat quality. J Food Engineering 123:104–112. doi: 10.1016/j.jfoodeng.2013.09.029. [DOI] [Google Scholar]
- 23.Lone A, Anany H, Hakeem M, Aguis L, Avdjian A-C, Bouget M, Atashi A, Brovko L, Rochefort D, Griffiths MW. 2016. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. Int J Food Microbiol 217:49–58. doi: 10.1016/j.ijfoodmicro.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Anany H, Chen W, Pelton R, Griffiths MW. 2011. Biocontrol of Listeria monocytogenes and Escherichia coli O157: H7 in meat by using phages immobilized on modified cellulose membranes. Appl Environ Microbiol 77:6379–6387. doi: 10.1128/AEM.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown HL, Reuter M, Salt LJ, Cross KL, Betts RP, van Vliet AHM. 2014. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl Environ Microbiol 80:7053–7060. doi: 10.1128/AEM.02614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger M-A. 2014. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf A Physicochem Eng Aspects 457:263–274. doi: 10.1016/j.colsurfa.2014.05.057. [DOI] [Google Scholar]
- 27.The United States Food and Drug Administration. 2016. Code of Federal Regulations, title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.8991.
- 28.Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. 2012. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed (Lond) 7:6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Y, He Y, Irwin PL, Jin T, Shi X. 2011. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol 77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 31.Gittard SD, Perfect JR, Monteiro-Riviere NA, Wei W, Jin C, Narayan RJ. 2009. Assessing the antimicrobial activity of zinc oxide thin films using disk diffusion and biofilm reactor. Applied Surface Science 255:5806–5811. doi: 10.1016/j.apsusc.2009.01.009. [DOI] [Google Scholar]
- 32.Ellis DI, Broadhurst D, Kell DB, Rowland JJ, Goodacre R. 2002. Rapid and quantitative detection of the microbial spoilage of meat by Fourier transform infrared spectroscopy and machine learning. Appl Environ Microbiol 68:2822–2828. doi: 10.1128/aem.68.6.2822-2828.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. 2016. Foodborne Diseases Active Surveillance Network (FoodNet). https://www.cdc.gov/foodnet/reports/prelim-data-2016.html.
- 34.Havelaar AH, Mangen M-JJ, de Koeijer AA, Bogaardt M-J, Evers EG, Jacobs-Reitsma WF, van Pelt W, Wagenaar JA, de Wit GA, van der Zee H, Nauta MJ. 2007. Effectiveness and efficiency of controlling Campylobacter on broiler chicken meat. Risk Anal 27:831–844. doi: 10.1111/j.1539-6924.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 35.European Food Safety Authority. 2011. Scientific opinion on Campylobacter in broiler meatproduction: control options and performance objectives and/or targets at different stages of the foodchain. EFSA J 9:2105. doi: 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 36.McClements DJ, Xiao H. 2017. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci Food 1:6. doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Loughlin JL, Eucker TP, Chavez JD, Samuelson DR, Neal-McKinney J, Gourley CR, Bruce JE, Konkel ME. 2015. Analysis of the Campylobacter jejuni genome by SMRT DNA sequencing identifies restriction-modification motifs. PLoS One 10:e0118533. doi: 10.1371/journal.pone.0118533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klena JD, Gray SA, Konkel ME. 1998. Cloning, sequencing, and characterization of the lipopolysaccharide biosynthetic enzyme heptosyltransferase I gene (waaC) from Campylobacter jejuni and Campylobacter coli. Gene 222:177–185. doi: 10.1016/S0378-1119(98)00501-0. [DOI] [PubMed] [Google Scholar]
- 39.Hakeem MJ, Asseri KA, Ma L, Chou KC, Konkel ME, Lu X. 2019. A novel mathematical model for studying antimicrobial interactions against Campylobacter jejuni. Front Microbiol 10:1038. doi: 10.3389/fmicb.2019.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hazeleger WC, Wouters JA, Rombouts FM, Abee T. 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl Environ Microbiol 64:3917–3922. doi: 10.1128/AEM.64.10.3917-3922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luber P, Bartelt E. 2007. Enumeration of Campylobacter spp. on the surface and within chicken breast fillets. J Appl Microbiol 102:313–318. doi: 10.1111/j.1365-2672.2006.03105.x. [DOI] [PubMed] [Google Scholar]
- 42.Howard SL, Jagannathan A, Soo EC, Hui JPM, Aubry AJ, Ahmed I, Karlyshev A, Kelly JF, Jones MA, Stevens MP, Logan SM, Wren BW. 2009. Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens. Infect Immun 77:2544–2556. doi: 10.1128/IAI.01425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Y, Zhang L, Wen D, Ding Y. 2016. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater Sci Eng C Mater Biol Appl 69:1361–1366. doi: 10.1016/j.msec.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Xie C. 2006. Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Colloids Surf B Biointerfaces 47:140–145. doi: 10.1016/j.colsurfb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 45.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho Y-H, Lee S-J, Lee JY, Kim SW, Lee CB, Lee WY, Yoon MS. 2002. Antibacterial effect of intraprostatic zinc injection in a rat model of chronic bacterial prostatitis. Int J Antimicrobial Agents 19:576–582. doi: 10.1016/S0924-8579(02)00115-2. [DOI] [PubMed] [Google Scholar]
- 47.Eide DJ. 2006. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Cerasi M, Ammendola S, Battistoni A. 2013. Competition for zinc binding in the host-pathogen interaction. Front Cell Infect Microbiol 3:108. doi: 10.3389/fcimb.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. 2013. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc 135:1438–1444. doi: 10.1021/ja309812z. [DOI] [PubMed] [Google Scholar]
- 50.Chivers PT. 2007. A galvanizing story-protein stability and zinc homeostasis. J Bacteriol 189:2953–2954. doi: 10.1128/JB.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell EA, Greenwell R, Anthony JR, Wang S, Lim L, Das K, Sofia HJ, Donohue TJ, Darst SA. 2007. A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Mol Cell 27:793–805. doi: 10.1016/j.molcel.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller WT, Hill KAW, Schimmel P. 1991. Evidence for a “cysteine-histidine box” metal-binding site in an Escherichia coli aminoacyl-tRNA synthetase. Biochemistry 30:6970–6976. doi: 10.1021/bi00242a023. [DOI] [PubMed] [Google Scholar]
- 53.Scrutton MC, Wu CW, Goldthwait DA. 1971. The presence and possible role of zinc in RNA polymerase obtained from Escherichia coli. Proc Natl Acad Sci U S A 68:2497–2501. doi: 10.1073/pnas.68.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghule K, Ghule AV, Chen B-J, Ling Y-C. 2006. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem 8:1034–1041. doi: 10.1039/b605623g. [DOI] [Google Scholar]
- 55.Zhao T, Doyle MP. 2006. Reduction of Campylobacter jejuni on chicken wings by chemical treatments. Int J Food Protection 69:762–767. doi: 10.4315/0362-028X-69.4.762. [DOI] [PubMed] [Google Scholar]
- 56.Oyarzabal OA, Macklin KS, Barbaree JM, Miller RS. 2005. Evaluation of agar plates for direct enumeration of Campylobacter spp. from poultry carcass rinses. Appl Environ Microbiol 71:3351–3354. doi: 10.1128/AEM.71.6.3351-3354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song X, Li R, Li H, Hu Z, Mustapha A, Lin M. 2014. Characterization and quantification of zinc oxide and titanium dioxide nanoparticles in foods. Food Bioprocess Technol 7:456–462. doi: 10.1007/s11947-013-1071-2. [DOI] [Google Scholar]
- 58.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 60.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. 2000. Gene ontology: tool for the unification of biology. Nat Genet 25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. 2015. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.