Abstract
Inflammation and autonomic dysfunction are common findings in chronic and end-stage kidney disease and contribute to a markedly increased risk of mortality in this patient population. The cholinergic anti-inflammatory pathway (CAP) is a vagal neuro-immune circuit that upholds the homoeostatic balance of inflammatory activity in response to cell injury and pathogens. CAP models have been examined in preclinical studies to investigate its significance in a range of clinical inflammatory conditions and diseases. More recently, cervical vagus nerve stimulation (VNS) implants have been shown to be of potential benefit for patients with chronic autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease. We have previously shown that dialysis patients have a functional CAP ex vivo. Here we review the field and the potential role of the CAP in acute kidney injury and chronic kidney disease (CKD) as well as in hypertension. We also present a VNS pilot study in haemodialysis patients. Controlling inflammation by neuroimmune modulation may lead to new therapeutic modalities for improved treatment, outcome, prognosis and quality of life for patients with CKD.
Keywords: acute kidney injury, cholinergic anti-inflammatory pathway, chronic kidney disease, dialysis, inflammation, vagus nerve stimulation

INTRODUCTION
More than 850 million individuals are estimated to have chronic kidney disease (CKD) [1–3]. Patients with CKD are increasing in numbers largely due to an ageing population but also the cumulative incidence of diabetic kidney disease and obesity [4, 5]. Cardiovascular disease (CVD) is a major comorbid condition in CKD and accounts for the increased morbidity and mortality in the CKD population in addition to infections and malignancies [6, 7]. The survival rate of incident dialysis is lower than that of patients with several types of solid organ cancer, while the survival rate of patients >70 years of age is similar to that of pancreas and lung cancer patients [8]. CKD is currently the 10th leading cause of death in high-income countries and is expected to be the 5th leading cause of death in the world in 2040 [7]. Inflammation in combination with autonomic dysfunction can significantly contribute to or trigger non-communicable chronic diseases that represent the bulk of the global burden of disease [9]. Important examples are hypertension, myocardial infarction, type 2 diabetes mellitus, heart failure, CKD and rheumatoid arthritis (RA) [9–12].
In this review we will highlight chronic inflammation and autonomic dysfunction in patients with CKD. It also includes a concise description of the cholinergic anti-inflammatory pathway (CAP) and potential applications for neuroimmune modulation in the emerging field of bioelectronic medicine. Furthermore, we elaborate on the potential role of the CAP in the context of acute kidney injury (AKI) and CKD and present a vagus nerve stimulation (VNS) pilot study in haemodialysis (HD).
Chronic inflammation in CKD
Inflammation is linked to a significantly increased mortality rate in CKD, end-stage kidney disease (ESKD) and dialysis treatment [13–17]. Chronic inflammation, reflected by elevated levels of pro-inflammatory cytokines, is highly associated with predominantly CVD in this population [6, 18]. Underlying diseases, lifestyle factors and age contribute to increased inflammatory activity in CKD [7, 19–21]. Other factors related to a decrease in glomerular filtration rate (GFR) and ESKD, such as reduced elimination of cytokines and metabolic acidosis, promote and maintain inflammation in addition to compromised immune responses and an impaired performance of neutrophils and lymphocytes [18, 22–26]. Several of these circumstances provide an increased susceptibility and an amplified risk of infections, while others are more strongly associated with CKD progression, comorbidity and mortality [18, 27]. Malnutrition and protein wasting in CKD are explained in part by depression and anorexia associated with circulating cytokines in the brain and suppressed levels of anabolic hormones, but are also driven by persistent inflammation [28–30]. Moreover, inflammation upholds anaemia in CKD patients, which is further amplified by a decreased synthesis of erythropoietin (EPO) and a lower responsiveness to EPO in the bone marrow [31].
Autonomic dysfunction
Cardiovascular autonomic dysfunction is a frequent finding in CKD and ESKD and has repeatedly been shown to be associated with poor outcome [32–36]. Autonomic dysfunction, in research and in clinical applications, is commonly measured by heart rate variability (HRV), an assessment of the physiological variation in the time interval between heartbeats. Cardiac activity is controlled by the autonomic nervous system and both heart rate and cardiac output are influenced by the efferent vagus nerve [37]. HRV is analysed by means of a time domain, frequency domain and non-linear variables [38]. Standard deviation of RR intervals (SDNN) is used as an overall estimate of autonomic function. The square root of the squared mean difference between adjacent RR intervals (RMSSD) is predominantly influenced by vagus tone [39]. Low-frequency (LF) power mainly reflects the sympathetic tone, whereas high-frequency (HF) power correlates more with the parasympathetic tone [40]. HRV is also significantly influenced by sex, age, physical fitness, clinical comorbidities, smoking and medication [41–44]. Additional factors linked to decreased HRV are metabolic (diabetes, early stages of glucose intolerance and metabolic syndrome), endocrine (cortisol) and barometric (orthostatic) [45–47]. Reduced HRV is associated with adverse outcomes in hypertension, systemic inflammation, depression and CKD, but also an increased risk of sudden death [48–53]. Inflammatory markers such as C-reactive protein (CRP), tumour necrosis factor (TNF) and interleukin (IL)-6 have been reported to be associated with low HRV in several studies [54, 55]. In inflammatory autoimmune diseases, e.g. RA and systemic lupus erythematosus, autonomic dysfunction and a reduction in vagal nerve activity are common findings [56, 57]. The association between decreased HRV and CKD, ESKD and different dialysis modalities has been demonstrated in several studies [58–60]. All in all, correlations between inflammatory cytokine levels and reduced variation of HRV have been suggested to reflect a reduced vagal tone and, as a consequence, impaired functionality of the CAP [50].
THE CHOLINERGIC ANTI-INFLAMMATORY PATHWAY
The autonomic nervous system maintains homoeostasis in essential organs and tissue functions. The hypothalamus, the limbic system, the medulla and other central functions are constantly processing sensory information ascending from the external and internal milieu. Descending signals in both regulatory sympathetic and parasympathetic nerve bundles deliver regulatory actions in target organs. In situations requiring very rapid adaptation, the target organ is controlled via reflexes [61]. The parasympathetic vagus nerve is a mixed nerve with ∼80% sensory afferent fibres and 20% efferent motor fibres [62].
In 2002, Tracey described the concept of the CAP and the inflammatory reflex [63]. This groundbreaking discovery was preceded by several studies, one of them describing the role of efferent vagal influence on inflammation in a model of acute arthritis. Intracerebral or intravenous administration of the cytokine inhibitor CNI-1493 (Semapimod) resulted in decreased inflammation. Bilateral cervical vagotomy abrogated the anti-inflammatory effect, demonstrating the importance of an intact vagus for CAP activity. However, electrical stimulation of the transected peripheral vagus also attenuated acute inflammation, as did local administration of acetylcholine, indicating several possible mechanisms and approaches for the anti-inflammatory effect of the efferent vagus [64]. Another study on human macrophages in a sepsis model showed decreased TNF when exposed to acetylcholine or electrical VNS [65]. Ever since these seminal papers were published, continued research in the field has delineated many of the mechanisms and substances involved in this neuroimmune pathway.
The following section includes a brief outline reviewing the current understanding of anatomical and biochemical components, properties and functions of the CAP gained from preclinical studies. Resident cells from the immune system produce cytokines, damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) in the event of cell injury, inflammation or infection [66, 67]. Antigen-presenting cells introduce DAMPs and PAMPs to pattern recognition receptors (toll-like receptors) expressed on afferent sensory vagal nerve endings [68]. Afferent vagal nerves also express receptors for cytokines and molecules released from local immune cells, e.g. IL-1 receptors, receptors for histamine and serotonin [69, 70]. Signals generated by activated receptors propagate to the nodose ganglia and are then forwarded to cell bodies located in the tractus solitarius in the brain stem. From here, intermediate fibres transfer the sensory information to be further processed in the central nervous system [71]. However, fibres also convey signals directly to the dorsal motor nucleus (DMN). In the DMN, the efferent motor vagus nerve originates and its posterior trunk ends in the celiac plexus, where the sympathetic splenic nerve is activated [72–76].
The splenic nerve subsequently releases norepinephrine (NE) near a small population of T cells found in the red pulp and the marginal zone of the spleen [77]. Beta-adrenergic receptors (ARs) on these cells induce production of the enzyme choline acetyl transferase and hence these specialized T cells are called choline acetyltransferase (ChAT) cells. Activated ChAT cells synthesize and release the neurotransmitter acetylcholine that binds to an α7nACh receptor on macrophages [77–84]. Activated receptors lead to a decrease in intracellular signalling, which reduces the production and release of cytokines. Released NE in the spleen also inhibits B cell migration and antibody production [74, 78, 81, 82]. Figure 1 briefly summarizes the current understanding of the CAP and Figure 2 displays a simplified figure of the efferent vagus, the inflammatory reflex, the role of the spleen and ChAT cell activity.
FIGURE 1.
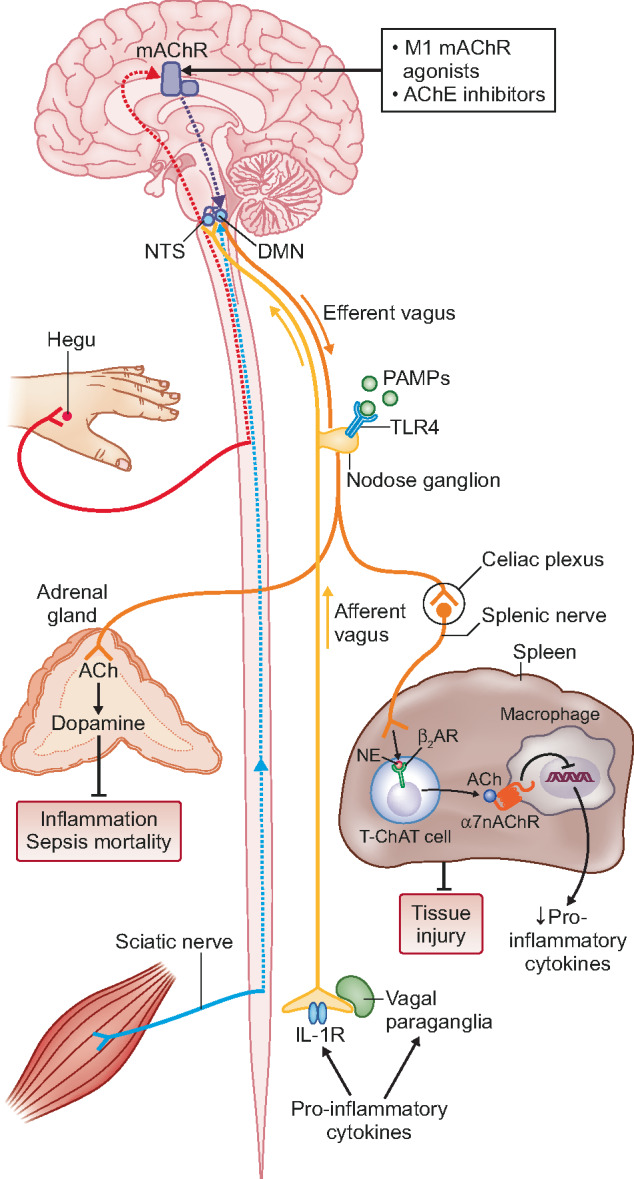
The CAP. Pro-inflammatory cytokines (IL-1, TNF) as well as PAMPs and DAMPs stimulate the sensory afferent vagus nerve endings. A signal is referred to the nucleus tractus solitarius via the nodose ganglion. The dorsal motor nucleus of the vagus is the orgin of the efferent motor vagus, which is activated by intermediate neurons. From here, the signal is spread to the celiac plexus, where the splenic nerve begins. Splenic nerve terminals are found in the vicinity of T cells and B cells in the red pulp and marginal zone in the spleen. Specialized T cells with the enzyme ChAT are activated by NE that binds to ARs of the ChAT T cell. Activated ChAT cells results in production and release of acetylcholine (ACh). ACh binds to the α7nAChR on macrophages and other immune cells and release of TNF is inhibited. Stimulation of muscarinic acetylcholine receptors (mAChR) or acetylcholinesterase (AChE) inhibitor in the central nervous system also activates the CAP. Acupuncture at the Hegu point causes activation of brain mAChR, resulting in activation the CAP [139]. Figure reprinted with permission from Nature Science.
FIGURE 2.
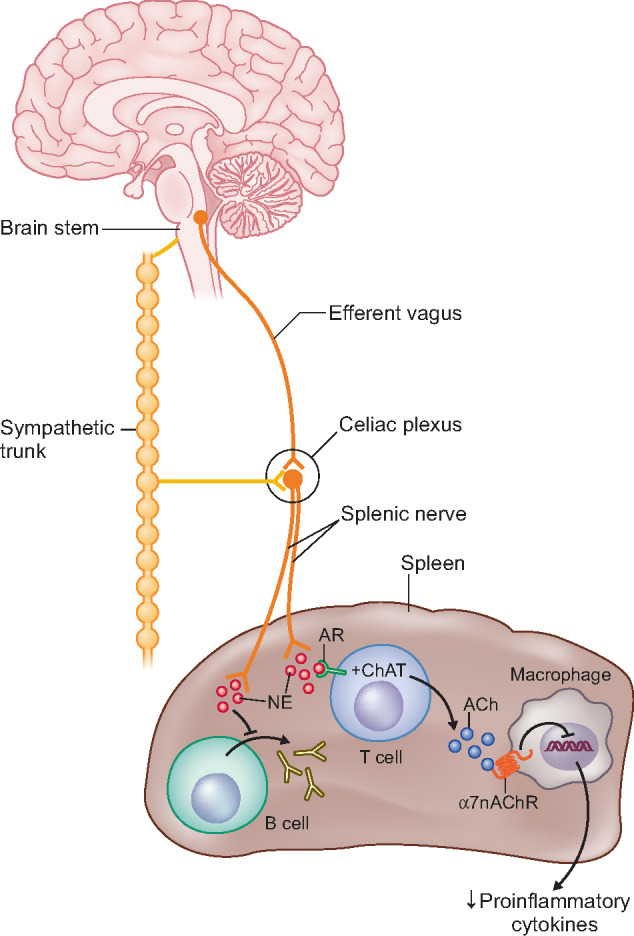
The inflammatory reflex. The celiac plexus receives efferent signals from both the efferent vagus nerve and the sympathetic trunk. Efferent signals from the vagus nerve activate the splenic nerve to release NE that interacts with ARs on ChAT T cells. ChAT T cells release acetylcholine that binds to α7nAChR on macrophages and inhibits further release of pro-inflammatory cytokines. Figure reprinted with permission from Wiley & Sons.
Modulation of CAP
Neuromodulation by VNS was recorded in the late 1800s when Corning observed that mechanical stimulation of the cervical vagal region suppressed epileptic seizure activity [85]. However, as the field of medical therapeutics in epilepsy developed, this type of intervention was considered obsolete until a renewed interest emerged for resistant cases. Cervical VNS, with an implanted wire electrode and a battery-driven stimulator, is currently approved for the treatment of drug-resistant epilepsy and severe depression [86–88]. Transcutaneous electrical VNS of the cervical vagus is approved for treatment of migraine and cluster headaches in Europe since 2013 and by the US Food and Drug Administration (FDA) in 2017 [89]. Transcutaneous electrical VNS to the outer ear has been approved for drug-resistant epilepsy in Europe since 2011 [90] and has also been used in clinical trials for pain and depression [91, 92].
Since the CAP was first described in the early 2000s, CAP models have been examined in preclinical studies in order to investigate the significance of the CAP in a range of clinical inflammatory conditions and diseases. The CAP regulates endotoxin-induced sepsis, experimental pancreatitis and experimental ileus, which has been further validated after vagotomy in these models [65, 93, 94]. Vagotomy in an arthritis model did not eliminate the symptoms, but the severity of the disease was attenuated [95].
It is well established that inflammation is a significant component of most medical conditions, which has placed treatment modalities such as VNS in a broader perspective. Electrical stimulation of the vagus nerve is the most common method to modulate the CAP in animal models. However, modulation of the CAP can also be accomplished by pharmacological means. CNI-1493 inhibits macrophage activation and GTS-21 (dimethoxybenzylidene anabaseine), a selective alpha7 nicotinic acetylcholine (α7nACh) receptor, has been used in preclinical studies. GTS-21 has been shown to improve survival and reduce TNF levels compared with controls in a model of endotoxaemia [96]. As of yet, there is not an established clinical application for either of these drugs. Galantamine, which is licensed for treatment of dementia, is a centrally acting choline esterase inhibitor. It is known to activate the CAP and has been shown to reduce systemic TNF levels after intravenous administration in a model of endotoxaemia. However, this effect was dependent on both an intact vagus nerve and the presence α7nACh receptors [84].
CAP and the possibility of modulating this circuit are potentially of great interest in CVD. Electrical VNS in models of heart failure has demonstrated beneficial effects on left ventricular (LV) function, LV remodelling and long-term survival [97, 98]. In the INOVATE-HF study of 707 patients with chronic heart failure, New York Heart Association (NYHA) functional Class III and ejection fraction ≤40% were studied. Patients were randomized to VNS treatment with a cervical implantable device or medical treatment only. The study population was followed for a mean of 16 months. Mortality in the VNS-treated group did not differ from controls, although NYHA class, physical performance and quality of life improved in the VNS-treated group [99]. A model of cardiac ischaemia–reperfusion injury (IRI) showed that VNS, dispensed before IRI, did not affect the LV function or the extent of myocardial injury [100]. However, in another study, VNS was administered for 24 h after induced ischaemia. Animals treated with VNS showed a substantial improvement in LV function, LV dilatation and less widespread myocardial injury at 8 weeks follow-up compared with controls [101]. VNS for hypertension has been discussed in the literature, but research has been lacking in both preclinical and clinical studies.
Renal denervation (RDN) is an antihypertensive treatment aimed at reducing sympathetic nerve activity in the kidneys through catheter-based radiofrequency ablation of the renal arteries. This approach was evaluated in patients with resistant hypertension in the European Symplicity-2 study. The primary endpoint, office blood pressure (BP) reduction (≥10 mmHg) at 6 months after denervation, was achieved in 80–90% of patients without altering renal function [102]. However, the Symplicity-3 study comparing RDN with a sham procedure failed to demonstrate a significant BP effect [103]. However, a recent publication of 3-year follow-up data from the Global SYMPLICITY Registry indicates that RDN is beneficial for patients with hypertension and CKD (eGFR <60 mL/min/1.73 m2). Patients with CKD had a 3.7 mL/min/1.73 m2 decline in eGFR compared with baseline, in contrast to 7.1 mL/min/1.73 m2 in the non-CKD group [104]. The reason for the beneficial effect on GFR after RDN is likely due to the positive effect of lowering BP in CKD patients, although other mechanisms cannot be excluded.
The RDN treatment provided us with an opportunity to study the effect on inflammation of a potential altered balance in the autonomic nervous system in favour of enhanced parasympathetic tone while reducing sympathetic tone in a prospective manner. Ten patients treated with RDN were analysed for TNF, IL-1 and IL-10 and lipopolysaccharide (LPS)-stimulated cytokine release before RDN, 24 h after and at 3- and 6-months follow-up. Pro-inflammatory cytokines decreased significantly and IL-10, an anti-inflammatory cytokine, increased 1 day after RDN. However, the effect was not sustained during follow-up, and at 6 months the cytokine levels were back at baseline [105]. In another prospective study by Zaldivia et al., monocyte activation and monocyte platelet aggregation decreased 3 months after RDN. Pro-inflammatory markers remained at lower levels after 3 and 6 months, but the effect also diminished with time [106]. Even though RDN may still be an option for BP reduction in a select group of patients, inflammation as reflected by cytokine release is thus not likely to significantly improve with this treatment. However, it may be of interest to assess whether long-term stimulation of the CAP in randomized controlled studies could more effectively reduce inflammation in resistant hypertension.
CAP AND AUTOIMMUNE DISEASE
Recent clinical studies based on experimental preclinical data have shown that cervical VNS implants may be beneficial for patients with chronic autoimmune diseases. Koopman et al. performed a study where 17 patients with RA received 60 s VNS once daily using an implantable device. Levels of TNF and RA disease scores [28-joint Disease Activity Score for Rheumatoid Arthritis with CRP (DAS28-CRP)] improved with VNS daily followed for up to 84 days. At day 28, all patients were off VNS, subsequently leading to a significant increase in TNF and DAS28-CRP. When VNS was resumed, these outcome measures improved again [107, 108]. However, it should be pointed out that in vitro–produced cytokines may not necessarily reflect the in vivo inflammatory cytokine balance in RA, which is a systemic chronic inflammatory disease primarily affecting the joints. Still, it has been shown that serum IL-1, IL-6, TNF and IL-17 are elevated in RA patients as compared with controls [109]. Interestingly, circulating IL-6 and IL-17 in RA patients have been shown to correlate with depression symptoms, which is a condition also known to be associated with reduced HRV [51, 109, 110]. Furthermore, the use of an IL-6 inhibitor in RA seems to have a more significant clinical effect on patients with higher levels of circulating IL-6 [111].
In 2016, Bonaz et al. [112] reported a 6-month VNS follow-up of an experimental study on patients with Crohn’s disease. An implanted cervical device delivering continuous VNS for 6 months achieved both decreased CRP and calprotectin and, moreover, demonstrated improvement of clinical symptoms and endoscopic remission in five of seven patients . Later, an application of VNS was reported from an experimental intestine inflammation model. A VNS electrode was attached to the abdominal part of the vagus nerve and stimulation resulted in lower CRP, normal stool and improved histology, suggesting that localized VNS therapy may be applicable in limited and regional inflammation [114]
CAP AND AKI
AKI is of major importance in hospitalized and critically ill patients since AKI is linked to an increased risk of chronic renal failure and mortality [114–116]. The pathophysiological mechanisms of AKI and subsequent inflammation are determined by functional and morphological changes in the kidney [114, 115, 117, 118]. Sepsis is reported to be the most common cause of AKI, although ischaemia, hypovolaemia and the use of nephrotoxic substances are often culprits as well [119]. Neuromodulation of the CAP in AKI has been investigated in several preclinical studies, primarily in models of sepsis-induced AKI and renal IRI. Administration of cholinergic substances such as nicotine or the selective a7nAChR agonist GTS-21 prior to induced renal IRI by clamping the renal arteries reduced both the loss of renal function and tubular necrosis in this model [120]. Furthermore, the presence of TNF and leucocytes in the kidney was mitigated by the administration of nicotine and GTS-21. However, when GTS-21 was given after induced ischaemia and reperfusion, it did not have the same beneficial effect on prevention of renal function loss [121]. GTS-21 and nicotine administration in sepsis-induced AKI has furthermore been shown to attenuate kidney injury and abolish local expression of TNF and systemic inflammation [122]. Electric VNS in renal IRI models has also demonstrated a substantial reduction of both renal injury and systemic inflammation [123]. In the same study, it was further established that the beneficial effect of VNS regarding renal injury and inflammation was abrogated by splenectomy before IRI, which parallels the diminished protective effect of splenectomy before VNS in sepsis-induced AKI [77].
Recent studies have investigated how localized pulsed ultrasound (US) to the spleen could modulate the CAP. A renal IRI model was used to administer pulsed US for ∼5 min 24 h before inducing IRI by clamping. In the treated animals there was a reduction of local accumulation of immune cells in the kidneys compared with controls. The role of the CAP was further confirmed by sham-treatment and splenectomy or α7nACh receptor depletion [124]. When using the same US protocol in sepsis-induced AKI models it was found that adoptive transfer of splenocytes from US-exposed mice to naive mice induced a renal protective effect from IRI [125].
CAP AND CKD
Alterations of the autonomic function and parasympathetic tone have been identified previously in CKD. Zoccali et al. meticulously investigated this by using non-invasive methods as well as atropine injections to assess changes in BP and heart rate in dialysis patients as compared with controls [126]. Reduced vagal tone in end-stage CKD is associated with negative implications for outcome [36]. In dialysis patients, elevated circulating levels of inflammatory markers are also associated with poor prognosis [14, 18]. There are currently no established or effective treatment strategies to reduce chronic inflammation in this population. The functionality of the CAP or the anti-inflammatory potential of stimulating the CAP in CKD and ESKD has not yet been thoroughly explored.
We have corroborated data from previous studies that cytokine levels are elevated in both HD and peritoneal dialysis (PD) patients. Baseline CRP, TNF, IL-1 and IL-6 were significantly increased, whereas the anti-inflammatory IL-10 was significantly lower in patients compared with controls [123]. In an ex vivo LPS whole blood model, we also showed that in dialysis patients, pro-inflammatory cytokine levels increased significantly more than in healthy controls. The addition of the cholinergic analogue GTS-21 to the LPS-stimulated samples, used in order to mimic the inflammatory reflex, resulted in a reduction of TNF to similar levels in both groups. IL-6 attenuation was comparable to TNF, whereas the IL-1b pattern was similar but remained significantly higher in patients. IL-10, an anti-inflammatory cytokine, increased after adding GTS-21 in a dose-dependent manner, but only in patients. Results in HD and PD patients did not differ, suggesting that dialysis modality was not critical for cytokine response in this model. HRV measurements validated results from previous studies demonstrating autonomic dysfunction in dialysis patients. We concluded that dialysis patients, despite autonomic dysfunction and an underlying dysregulated cytokine response, have a functional CAP [127].
VNS pilot study
Being mindful of differing origins and pathophysiological backgrounds regarding chronic inflammation in CKD as compared with autoimmune disease, we nevertheless aspired to explore the potential of neuroimmune modulation in CKD patients. We decided to perform a pilot study with the aim of investigating if short-term VNS, using a minimally invasive method, could improve inflammatory cytokine levels and alter HRV, in particular vagal tone, in HD patients. We decided against using an implantable VNS device in dialysis patients, which could, in our view, be problematic in a patient population with a ubiquitous risk of infectious complications.
Material and methods
We recruited 12 HD patients (7 males, 5 females, age range 47–86 years) at the Dialysis Department at Karolinska University Hospital, Huddinge, Sweden. Inclusion criteria comprised a stable general condition, stable dialysis treatment, no clinically significant signs of active infection or inflammation and no diagnosed psychiatric disease. Patients were also included regardless of medications and dialysis vintage. All patients received HD 3–4 times per week and, on average, typically 4 h per session. None of the patients had a history of frequent complications during dialysis, e.g. recurrent symptomatic BP reduction or leg cramps. Six patients were previously transplanted, of which two were still treated with low-dose immunosuppressive medication: one with tacrolimus and prednisolone and the other with tacrolimus only. Two patients were treated with long-term antibiotics: one because of osteitis with an infusion of vancomycin 500 mg intravenously 3 times weekly and the other patient with flucloxacillin 750 mg orally daily as urinary tract infection prophylaxis. A summary of patient characteristics including age, gender, dialysis vintage, blood chemistry (baseline TNF, IL-1 and IL-6) and the most common medications are found in Table 1.
Table 1.
Patient and baseline characteristics (N = 12)
| Characterisitcs | Values |
|---|---|
| Sex (female), % | 38 |
| Age (years) | 60 (49–82) |
| Dialysis vintage (months) | 96 (16–233) |
| P-hsCRP (mg/mL) | 6 (0.4–44.6) |
| B-Hb (g/L) | 106 (97–113) |
| B-WBCs (×109) | 6.4 (4.8–10.2) |
| B-monocytes (×109) | 00.7 (0.4–1.0) |
| B-lymphocytes (×109) | 1.4 (0.8–1.9) |
| S-TNF (pg/mL) | 20.3 (14.2–77.4)a |
| S-IL-1b (pg/mL) | 1.3 (0.3–9.2)a |
| S-IL-10 (pg/mL) | 1.2 (0.8–2.6)a |
| Medications, n | |
| β-blocker | 6 |
| α-blocker | 1 |
| Calcium channel antagonist | 5 |
| ACE/ARB inhibitor | 6 |
| Furosemide | 4 |
| Statin | 3 |
Values presented as median (10th–89th percentiles).
References: hsCRP <3 mg/L; B-Hb 117–153 g/L (female), 134–170 g/L (male); B-WBCs 3.5–8.8 × 109/L; B-monocytes 0.1–1.0 × 109/L; B-lymphocytes 1.0–1.4 × 109; S-TNF <12 pg/mL; S-IL-1b <5 pg/mL; S-IL-10 <5 pg/mL.
aBaseline values before stimulation in the LPS model.
undefined
P: plasma; hsCRP: high-sensitivity CRP; B: blood; S: serum.
The study protocol included treatment with a minimally invasive oscillating device prior to dialysis 3 times a week for 4 weeks. We used a device that was originally developed for rhinitis and subsequently used in a migraine study [128–131].
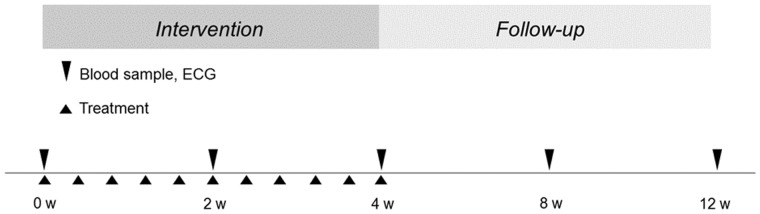
Blood samples and HRV were collected at baseline and after 2 and 4 weeks of intervention. Follow-up blood samples and HRV were also scheduled at 8 and 12 weeks (Figure 3). The study protocol was approved by the local ethics committee (EPN, Stockholm) and informed consent was obtained from each patient.
FIGURE 3.
Study protocol. Timetable for blood samples, electrocardiogram (ECG) and intervention treatment. Blood sampling and ECG at baseline, after 2 and 4 weeks during the intervention period and at 8 and 12 weeks of follow-up.
The minimally invasive oscillating device was a single-use plastic tube with an inflatable tip made of thin latex. Inside the tip, a soft thin plastic pin was anchored to the edge of a plastic tube (Figure 4a). The tip was lubricated with water and inserted in the left nostril. The tube was secured in position by a headband (Figure 4b) and was then connected to a portable electrical energy unit and inflated with room air. When reaching a pressure of 95 mbar the soft pin inside the tip started to oscillate at a frequency of 68 Hz. The oscillations generated vibrations to the nostril mucosa. Each treatment lasted for 10 min. The choice of side, pressure, frequency and duration of treatment was decided in discussions with the designer based on prior studies using the same device [128–131]. The decision to administer the treatment for 10 min was also a practical decision to not disturb routine dialysis treatment and to potentially compensate for not administrating VNS daily.
FIGURE 4.
The minimally invasive treatment device. A plastic tube with inflatable tip of thin latex. (A) The tube is inserted in the left nostril and (B) secured in position by a specially designed headband [129]. Images reprinted with permission from Headache.
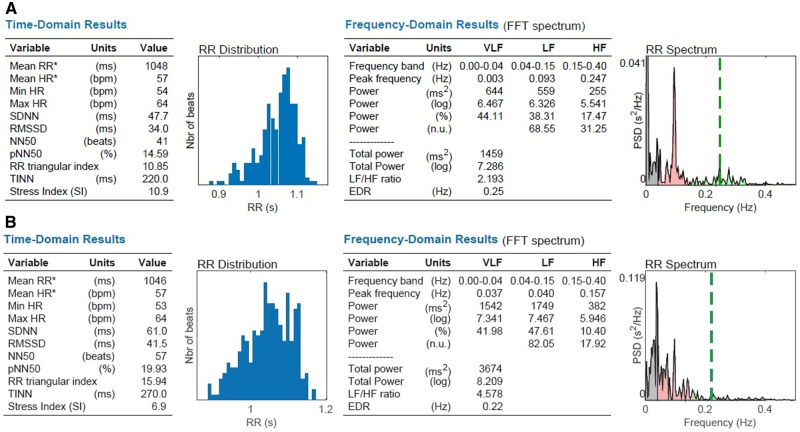
The VNS equipment was tested on healthy volunteers prior to initializing the intervention to safeguard a potential influence on vagus nerve activity. Treatment for 10 min resulted in augmentation of HRV variables, which supported an increased vagal activity exemplified in Figure 5.
FIGURE 5.
HRV in a healthy volunteer (A) before and (B) after 10 min of treatment. Augmentation of time domain variables SDNN (47.7–61.0 ms), RMSSD (34–41.5 ms) and frequency domain variables LF (559–1749 ms2) and HF (255–382 ms2).
HRV
HRV during the study was measured for 20 min using a custom-made device designed by Z-Health Technologies, Borås, Sweden. The HRV analysis generated data for time and frequency domain parameters according to European Task Force for HRV measurements [38]. A quiet examining room outside the Dialysis Department was used and the patient was in a supine position in a bed.
Cytokine and routine blood sample analysis
Blood samples were analysed for CRP, haemoglobin (Hb), white blood cell (WBC) count and TNF, IL-1 and IL-10. High-sensitivity TNF, IL-1 and IL-10 were analysed on an Immulite 1000 Analyser using enzyme-linked immunosorbent assays (Siemens Healthcare Diagnostics, Los Angeles, CA, USA). CRP, Hb and WBC count were analysed by using routine methods at the Department of Clinical Chemistry at Karolinska University Hospital.
Whole blood assay
Whole blood was stimulated with LPS 10 and 100 ng/mL and GTS-21 90 µmol/L using a method previously described and used in our study of CAP functionality in dialysis patients [127]. After 4 h incubation at 37°C on a rocking platform, plasma was collected by centrifugation (2600 g, 20 min, 18°C, Eppendorf centrifuge 5804 R) and frozen at −80°C pending cytokine analyses.
Statistical analysis
All values were expressed as median (10th, 90th percentile) or percentage, as appropriate. A P-value <0.05 was statistically significant. Comparisons between more than two groups were assessed with a non-parametric Kruskal–Wallis analysis of variance (ANOVA) test. The statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
All patients completed the 4-week intervention period of the protocol without interruptions. During follow-up, however, four patients were affected by either infection or dialysis access surgery. The intervention procedure was generally well tolerated and there were no minor or major adverse events.
Cytokine levels
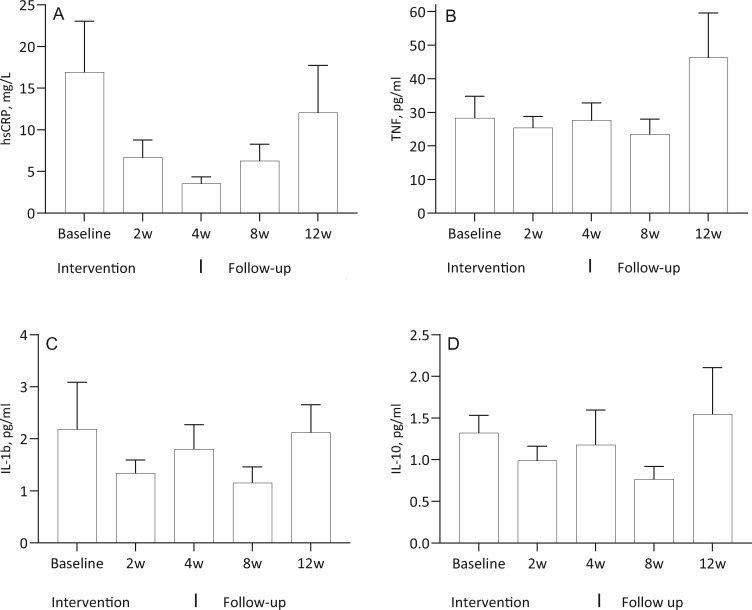
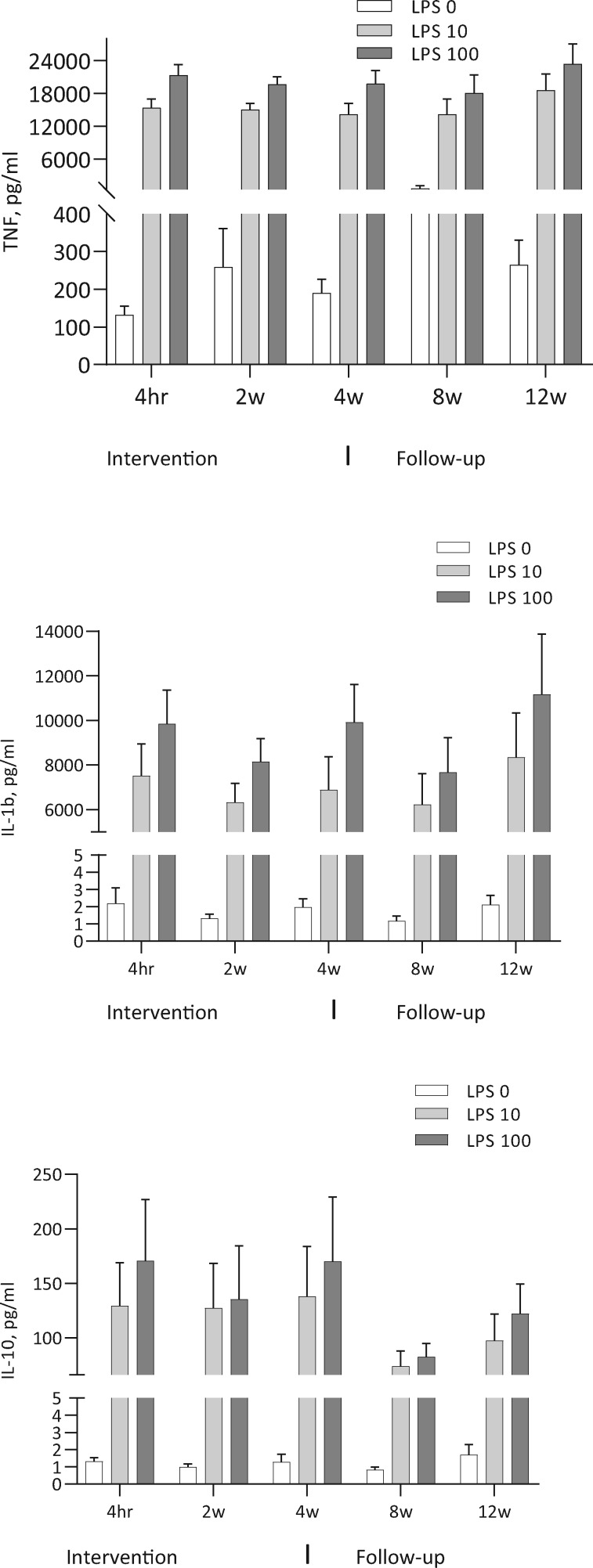
The levels of CRP and unstimulated cytokines did not change significantly during the study: CRP (ANOVA P = 0.61), IL-1 (P = 0.63) and IL-10 (P= 0.37) (Figure 6). The reduction of TNF and IL-1 as well as the increase in IL-10 did not reach statistical sigificance in the LPS-stimulated whole blood assay (Figure 7). In the presence of the cholinergic analogue GTS-21 there was a further ∼50% reduction of cytokine expression, as shown for TNF in Figure 8. We also analysed separately the eight patients who completed the study and follow-up without any hurdles. Although the TNF decrease in LPS-stimulated samples was more pronounced during treatment in these cases, the results again did not reach statistical significance (Figure 9). The IL-1 result was similar to TNF and IL-10 did not show any clear pattern (data not shown).
FIGURE 6.
N = 12. Unstimulated levels of hsCRP, TNF, IL-1b and IL-10 at baseline, at 2 and 4 weeks during the intervention and at 8 and 12 weeks of follow-up.
FIGURE 7.
N = 12. Levels of TNF, IL-1 and IL-10 in the whole blood model at baseline, at 2 and 4 weeks during the intervention and at 8 and 12 weeks of follow-up. Whole blood stimulated with LPS 10 and 100 ng/mL.
FIGURE 8.
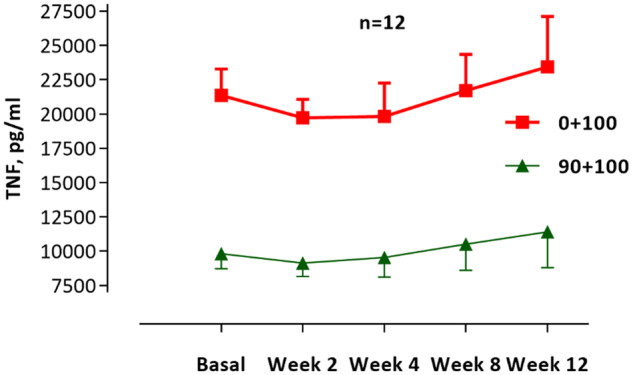
N = 12. Levels of TNF in the whole blood model stimulated with LPS 100 ng/mL (red line) and the reduction with cholinergic analogue GTS-21 90 μmol/mL (green line). Results shown for 2 and 4 weeks during the intervention and 8 and 12 weeks of follow-up.
FIGURE 9.
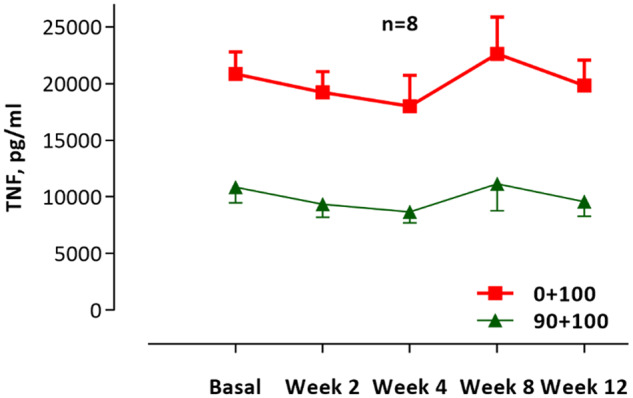
N = 8. Levels of TNF for eight patients that went through the study without obstacles. Whole blood model stimulated with LPS 100 ng/mL (red line) and the reduction with GTS-21 90 μmol/mL (green line). Results shown for 2 and 4 weeks during the intervention and 8 and 12 weeks of follow-up.
HRV
Three patients had a cardiac pacemaker or implantable cardioverter defibrillator and thus it was not possible to analyse for HRV variables. None of the remaining nine patients showed any significant change in HRV during the study and follow-up (data not shown).
Additional findings
Outside the protocol, three of four patients with insulin-dependent diabetes mellitus reported a lowering of insulin doses of ∼25% during the study. The fourth patient had only a small dose (4 units) of long-acting insulin at night. Approximately 3–6 months after finishing the study, the patients had resumed their original insulin dosing schedules. Another example of effects observed outside the protocol was better sleep among several of the participants. This was manifested as a reduction in the use of sleeping medication. Furthermore, several subjects reported an improvement in general mood as well as alertness.
Summary of the pilot study
To our knowledge, this is the first intervention study with the aim of modulating vagus nerve activity as an anti-inflammatory approach in dialysis patients. After 4 weeks of VNS treatment 3 times a week, there were no significant changes in unstimulated CRP, IL-1 and IL-10. In the LPS-stimulated ex vivo whole blood model, changes in levels of TNF, IL-1 and IL-10 during treatment did not reach statistical significance. However, cytokine reduction was more pronounced when adding a cholinergic analogue to the model, which suggests that there may be a further potential for cholinergic modulation in patients. This was, however, a small pilot study with a limited number of patients. There was also no control group. Furthermore, the treatment was not administered daily, which was a disadvantage for the study design and is likely to have hampered the study and thereby potentially influenced the results. The VNS device we used had not previously been applied in a study focusing on inflammatory response. Thus it may not have been effective enough despite the HRV results in healthy volunteers and reported influence on autonomic nervous system activity when used in a study on migraines [130]. However, we did not record any significant changes in HRV in patients during this study.
Studies using cervical vagus implants have demonstrated significant inhibition of cytokine production and disease activity in RA and inflammatory bowel disease [107, 132]. One may hypothesize that daily sessions, prolonged treatment time at each session or a longer protocolized treatment period could have improved outcomes regarding inflammatory response and alterations in HRV in the current pilot study.
DISCUSSION
The notion that the immune system and the nervous system are two different entities without any interaction is no longer true, as there is uncontested evidence of the opposite. Moreover, the immune system is a prerequisite for function of the nervous system and vice versa. The parasympathetic vagus nerve has, through a brain- and spleen-integrated mechanism, been found to be involved in the regulation of inflammation via the CAP [63]. This pathway has been investigated in clinical studies of inflammatory diseases. We and others have shown that vagal tone is significantly decreased and associated with inflammatory markers in RA, IBD and dialysis patients [ 127, 133, 134]. Recent and ongoing research further expands the prospects of modulating the CAP and future therapeutic possibilities.
Chronic low-grade inflammation is a risk factor per se in CKD and is associated with increased morbidity and mortality. Specific treatment to address this problem is lacking.
We have previously shown that dialysis patients have a functional CAP ex vivo [127]. However, in our pilot VNS study we could not demonstrate significant alterations in cytokine levels. Nevertheless and interestingly, three of four diabetic patients had a 25% reduction of insulin doses. In a recently published randomized, double-blind, placebo-controlled study, galantamine, which is known to activate the CAP, was given to patients with metabolic syndrome [135]. Galantamine treatment for 12 weeks was shown to alleviate inflammation, alter HRV and lower plasma insulin and insulin resistance (homoeostasis model assessment of insulin resistance) compared with placebo. These results suggest that the link between chronic inflammation and insulin resistance is treatable. Our findings regarding a reduction in insulin doses in the pilot study may reflect a similar phenomenon.
The reports of enhanced sleep and in some cases also general mood were not anticipated. However, we cannot exclude that these effects were secondary to the attention given to patients during the study. Questionnaires such as the36-item Short Form Health Survey and EuroQol 5-dimensions are directed towards physical status and activities in daily life. These questionnaires do not include detailed questions describing more subjective experiences and mood changes. In future studies, perhaps more granular Patient-Reported Outcome Measures and Patient-Reported Experience Measures could be important to assess in studies aimed at modulating the CAP. We propose that our short-term pilot study may serve as a first step to potentially utilize VNS in this patient group.
The minimally invasive solution employed in this study was a feasible approach in dialysis patients, albeit not effective enough. However, for future studies it would be a prerequisite and a considerable advantage to administer daily VNS to patients with a portable non-invasive tool such as a transcutaneous device. However, a standardized instrument with proven efficacy for stimulating parasympathetic activity is essential. Such a device would potentially enable patients to manage treatment themselves at home on non-dialysis days. A recent pilot study by Addorisio et al. utilized a vibrotactile device for VNS with vibrations to the cymba choncha of the outer ear. Both healthy subjects and patients with RA were treated, which resulted in modulation of peripheral blood cytokine levels in healthy subjects and a lowering of TNF levels and disease activity in patients [136].
Potential VNS applications in nephrology may not be limited to the dialysis population. Hoeger et al. have interestingly shown that vagal stimulation in brain dead donor rats had the ability to decrease chronic allograft nephropathy in recipients [137]. This would be an example of the CAP contributing to adoptive transfer of immune cell modulation, protecting against trauma caused by surgery as well as inflammation due to rejection. Finally, a substantial number of CKD patients suffer from autoimmune disease with renal involvement, where effective treatment is lacking or associated with side effects. The therapeutic possibilities in the growing field of bioelectronic medicine as shown in RA and IBD may lead to new approaches for treating and maintaining remission in inflammatory kidney diseases with or without reduced renal function.
Conducting an intervention study in patients with CKD or dialysis presents various challenges. These patients often have comorbidities that can lead to acute and unexpected changes in the patient's condition. Unfortunately it is far too common that CKD patients are often not included in studies for the above reasons [138]. In addition, it can be difficult to motivate patients to participate in studies that, by being time-consuming, affect their quality of life. The small number of recruited study patients in our pilot study also reflects how much dialysis treatment interferes with daily life. We nevertheless believe that this patient group could potentially benefit from novel non-invasive therapies such as VNS, perhaps starting with patients treated with home dialysis modalities.
CKD and associated comorbidities contribute to years of decreased quality of life for patients. Furthermore, its share of both the national and international health economy is substantial. These factors are a challenge to address on a population basis, but also in individual patients. Dialysis treatment needs to improve and better tools should be used to monitor and reduce chronic inflammation. In CKD and pre-dialysis patients, other types of interventions are pertinent but should also focus on inflammation. New interventional studies with a focus on the CAP in the field of bioelectronic medicine may, in the future, be of substantial benefit in AKI and CKD as well as in associated inflammatory conditions [134].
FUNDING
The pilot study was supported by grants from the Fund for Renal Research, Karolinska Institutet Funds, the Swedish Society of Medicine, Westman Research Fund and Stockholm County Council (ALF project). Annette Bruchfeld was supported by the Stockholm County Council (clinical research appointment).
CONFLICT OF INTEREST STATEMENT
The content of this review has not been published previously but is based partly on our previous clinical and laboratory findings, as stated in the article.
M.H. has no conflicts of interest to report. A.B. reports personal fees from Chemocentryx, AstraZeneca and MSD/Merck, outside the submitted work.
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham R et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019; 96: 1048–1050 [DOI] [PubMed] [Google Scholar]
- 2. Jager KJ, Kovesdy C, Langham R et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 3.ERA-EDTA. The hidden epidemic: worldwide, over 850 million people suffer from kidneys diseases. http://web.era-edta.org/uploads/180627-press-era-asn-isn.pdf
- 4. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1260–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas B, Matsushita K, Abate KH, et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 2017; 28: 2167–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1151–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naylor KL, Kim SJ, McArthur E et al. Mortality in incident maintenance dialysis patients versus incident solid organ cancer patients: a population-based cohort. Am J Kidney Dis 2019; 73: 765–776 [DOI] [PubMed] [Google Scholar]
- 9. Gidron Y, Deschepper R, De Couck M et al. The vagus nerve can predict and possibly modulate non-communicable chronic diseases: introducing a neuroimmunological paradigm to public health. J Clin Med 2018; 7: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walston JD. Chronic inflammation In: Halter JB, Ouslander JG, Studenski S, High KP, Asthana S, Supiano MA (eds). Hazzard’s Geriatric Medicine and Gerontology. 7th edn New York: McGraw-Hill Education, 2017 [Google Scholar]
- 11. Reisner HM. Cell injury, cell death, and aging In:Reisner HM. (ed). Pathology: A Modern Case Study. New York: McGraw-Hill Education, 2015 [Google Scholar]
- 12. Westman M, Saha S, Morshed M et al. Lack of acetylcholine nicotine alpha 7 receptor suppresses development of collagen-induced arthritis and adaptive immunity. Clin Exp Immunol 2010; 162: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snaedal S, Heimburger O, Qureshi AR et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis 2009; 53: 1024–1033 [DOI] [PubMed] [Google Scholar]
- 14. Honda H, Qureshi AR, Heimburger O et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 2006; 47: 139–148 [DOI] [PubMed] [Google Scholar]
- 15. Zoccali C, Tripepi G, Mallamaci F. Dissecting inflammation in ESRD: do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients. J Am Soc Nephrol 2006; 17(12 Suppl 3): S169–S173 [DOI] [PubMed] [Google Scholar]
- 16. Zimmermann J, Herrlinger S, Pruy A et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 1999; 55: 648–658 [DOI] [PubMed] [Google Scholar]
- 17. Dekker MJ, Marcelli D, Canaud BJ et al. Impact of fluid status and inflammation and their interaction on survival: a study in an international hemodialysis patient cohort. Kidney Int 2017; 91: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 18. Stenvinkel P, Ketteler M, Johnson RJ et al. IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia–the good, the bad, and the ugly. Kidney Int 2005; 67: 1216–1233 [DOI] [PubMed] [Google Scholar]
- 19. Franceschi C, Capri M, Monti D et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128: 92–105 [DOI] [PubMed] [Google Scholar]
- 20. Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care 2003; 6: 295–299 [DOI] [PubMed] [Google Scholar]
- 21. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ori Y, Bergman M, Bessler H et al. Cytokine secretion and markers of inflammation in relation to acidosis among chronic hemodialysis patients. Blood Purif 2013; 35: 181–186 [DOI] [PubMed] [Google Scholar]
- 23. Platten M, Youssef S, Hur EM et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA 2009; 106: 14948–14953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glorieux GL, Dhondt AW, Jacobs P et al. In vitro study of the potential role of guanidines in leukocyte functions related to atherogenesis and infection. Kidney Int 2004; 65: 2184–2192 [DOI] [PubMed] [Google Scholar]
- 25. Glorieux G, Helling R, Henle T et al. In vitro evidence for immune activating effect of specific AGE structures retained in uremia. Kidney Int 2004; 66: 1873–1880 [DOI] [PubMed] [Google Scholar]
- 26. Aveles PR, Criminacio CR, Goncalves S et al. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin Pract 2010; 116: c294–c299 [DOI] [PubMed] [Google Scholar]
- 27. Allon M, Depner TA, Radeva M et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol 2003; 14: 1863–1870 [DOI] [PubMed] [Google Scholar]
- 28. Taraz M, Taraz S, Dashti-Khavidaki S. Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: a review of literature. Hemodial Int 2015; 19: 11–22 [DOI] [PubMed] [Google Scholar]
- 29. Mahesh S, Kaskel F. Growth hormone axis in chronic kidney disease. Pediatr Nephrol 2008; 23: 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stenvinkel P, Heimburger O, Paultre F et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 1999; 55: 1899–1911 [DOI] [PubMed] [Google Scholar]
- 31. Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res 1998; 18: 555–559 [DOI] [PubMed] [Google Scholar]
- 32. Grassi G, Quarti-Trevano F, Seravalle G et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 2011; 57: 846–851 [DOI] [PubMed] [Google Scholar]
- 33. Masuo K, Lambert GW, Esler MD et al. The role of sympathetic nervous activity in renal injury and end-stage renal disease. Hypertens Res 2010; 33: 521–528 [DOI] [PubMed] [Google Scholar]
- 34. Zoccali C, Mallamaci F, Tripepi G et al. Norepinephrine and concentric hypertrophy in patients with end-stage renal disease. Hypertension 2002; 40: 41–46 [DOI] [PubMed] [Google Scholar]
- 35. Grassi G, Seravalle G, Dell’Oro R et al. Sympathetic mechanisms, organ damage, and antihypertensive treatment. Curr Hypertens Rep 2011; 13: 303–308 [DOI] [PubMed] [Google Scholar]
- 36. Oikawa K, Ishihara R, Maeda T et al. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol 2009; 131: 370–377 [DOI] [PubMed] [Google Scholar]
- 37. ChuDuc H, NguyenPhan K, NguyenViet D. A review of heart rate variability and its applications. APCBEE Proc 2013; 7: 80–85 [Google Scholar]
- 38. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93: 1043–1065 [PubMed] [Google Scholar]
- 39. Ciccone AB, Siedlik JA, Wecht JM et al. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 2017; 56: 674–678 [DOI] [PubMed] [Google Scholar]
- 40. Kuo CD, Chen GY. Heart rate variability standards. Circulation 1998; 98: 1589–1590 [PubMed] [Google Scholar]
- 41. Murgia F, Melotti R, Foco L et al. Effects of smoking status, history and intensity on heart rate variability in the general population: the CHRIS study. PLoS One 2019; 14: e0215053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 2010; 141: 122–131 [DOI] [PubMed] [Google Scholar]
- 43. Roche F, Xuong AN, Court-Fortune I et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol 2003; 26: 669–677 [DOI] [PubMed] [Google Scholar]
- 44. Lieb DC, Parson HK, Mamikunian G et al. Cardiac autonomic imbalance in newly diagnosed and established diabetes is associated with markers of adipose tissue inflammation. Exp Diabet Res 2012; 2012:878760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ernst G. Heart-rate variability-more than heart beats? Front Public Health 2017; 5: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sassi R, Cerutti S, Lombardi F et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. EP Europace 2015; 17: 1341–1353 [DOI] [PubMed] [Google Scholar]
- 47. Dimova R, Tankova T, Kirilov G et al. Endothelial and autonomic dysfunction at early stages of glucose intolerance and in metabolic syndrome. Horm Metab Res 2020; 52: 39–48 [DOI] [PubMed] [Google Scholar]
- 48. La Rovere MT, Bigger JT Jr, Marcus FI et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet 1998; 351: 478–484 [DOI] [PubMed] [Google Scholar]
- 49. Huikuri HV, Ylitalo A, Pikkujamsa SM et al. Heart rate variability in systemic hypertension. Am J Cardiol 1996; 77: 1073–1077 [DOI] [PubMed] [Google Scholar]
- 50. Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 2011; 269: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Birkhofer A, Schmidt G, Forstl H. Heart rate variability and depression. Arch Gen Psychiatry 2006; 63: 1052. [DOI] [PubMed] [Google Scholar]
- 52. Ranpuria R, Hall M, Chan CT et al. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol Dial Transplant 2007; 23: 444–449 [DOI] [PubMed] [Google Scholar]
- 53. Weber CS, Thayer JF, Rudat M et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol 2010; 109: 201–211 [DOI] [PubMed] [Google Scholar]
- 54. Singh P, Hawkley LC, McDade TW et al. Autonomic tone and C-reactive protein: a prospective population-based study. Clin Auton Res 2009; 19: 367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cooper TM, McKinley PS, Seeman TE et al. Heart rate variability predicts levels of inflammatory markers: evidence for the vagal anti-inflammatory pathway. Brain Behav Immun 2015; 49: 94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Louthrenoo W, Ruttanaumpawan P, Aramrattana A et al. Cardiovascular autonomic nervous system dysfunction in patients with rheumatoid arthritis and systemic lupus erythematosus. QJM 1999; 92: 97–102 [DOI] [PubMed] [Google Scholar]
- 57. Goldstein RS, Bruchfeld A, Yang L et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med 2007; 13: 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chandra P, Sands RL, Gillespie BW et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant 2012; 27: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Genovesi S, Bracchi O, Fabbrini P et al. Differences in heart rate variability during haemodialysis and haemofiltration. Nephrol Dial Transplant 2007; 22: 2256–2262 [DOI] [PubMed] [Google Scholar]
- 60. Mylonopoulou M, Tentolouris N, Antonopoulos S et al. Heart rate variability in advanced chronic kidney disease with or without diabetes: midterm effects of the initiation of chronic haemodialysis therapy. Nephrol Dial Transplant 2010; 25: 3749–3754 [DOI] [PubMed] [Google Scholar]
- 61. Waxman SG. The Autonomic Nervous System. In: Clinical Neuroanatomy. 28 ed. New York: McGraw-Hill Education, 2017 [Google Scholar]
- 62. Strominger NL, Demarest RJ, Laemle LB. Autonomic nervous system. In: Noback’s Human Nervous System, 7th edn Structure and Function. Totowa, NJ: Humana Press, 2012: 343–361 [Google Scholar]
- 63. Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–859 [DOI] [PubMed] [Google Scholar]
- 64. Borovikova LV, Ivanova S, Nardi D et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci 2000; 85: 141–147 [DOI] [PubMed] [Google Scholar]
- 65. Borovikova LV, Ivanova S, Zhang M et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462 [DOI] [PubMed] [Google Scholar]
- 66. Saeed RW, Varma S, Peng-Nemeroff T et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 2005; 201: 1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85: 1–17 [DOI] [PubMed] [Google Scholar]
- 68. Sundman E, Olofsson PS. Neural control of the immune system. Adv Physiol Educ 2014; 38: 135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Steinberg BE, Silverman HA, Robbiati S et al. Cytokine-specific neurograms in the sensory vagus nerve. Bioelectron Med 2016; 3: 7–17 [PMC free article] [PubMed] [Google Scholar]
- 70. Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron 2009; 64: 28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pavlov VA, Ochani M, Gallowitsch-Puerta M et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA 2006; 103: 5219–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tewfik TL. Vagus nerve anatomy. emedicine. Medscape 2017. https://emedicine.medscape.com/article/1875813-overview
- 73. Guyenet PG, Stornetta RL, Bochorishvili G et al. C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol 2013; 305: R187–R204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rosas-Ballina M, Olofsson PS, Ochani M et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334: 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rosas-Ballina M, Ochani M, Parrish WR et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA 2008; 105: 11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vijayaraghavan S, Karami A, Aeinehband S et al. Regulated extracellular choline acetyltransferase activity—the plausible missing link of the distant action of acetylcholine in the cholinergic anti-inflammatory pathway. PLoS One 2013; 8: e65936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huston JM, Ochani M, Rosas-Ballina M et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 2006; 203: 1623–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Olofsson PS, Katz DA, Rosas-Ballina M et al. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol Med 2012; 18: 539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vida G, Pena G, Kanashiro A et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J 2011; 25: 4476–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huston JM, Rosas-Ballina M, Xue X et al. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol 2009; 183: 552–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Olofsson PS, Rosas-Ballina M, Levine YA et al. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 2012; 248: 188–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reardon C, Duncan GS, Brustle A et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci USA 2013; 110: 1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang H, Yu M, Ochani M et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003; 421: 384–388 [DOI] [PubMed] [Google Scholar]
- 84. Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med 2008; 14: 567–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lanska DJ. J.L. Corning and vagal nerve stimulation for seizures in the 1880s. Neurology 2002; 58: 452–459 [DOI] [PubMed] [Google Scholar]
- 86. Bonaz B, Picq C, Sinniger V et al. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 2013; 25: 208–221 [DOI] [PubMed] [Google Scholar]
- 87. Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 2018; 11: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 2017; 14: 716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lendvai IS, Maier A, Scheele D et al. Spotlight on cervical vagus nerve stimulation for the treatment of primary headache disorders: a review. J Pain Res 2018; 11: 1613–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bauer S, Baier H, Baumgartner C et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimulat 2016; 9: 356–363 [DOI] [PubMed] [Google Scholar]
- 91. Nicholson WC, Kempf MC, Moneyham L et al. The potential role of vagus-nerve stimulation in the treatment of HIV-associated depression: a review of literature. Neuropsychiatr Dis Treat 2017; 13: 1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Usichenko T, Laqua R, Leutzow B et al. Preliminary findings of cerebral responses on transcutaneous vagal nerve stimulation on experimental heat pain. Brain Imag Behav 2017; 11: 30–37 [DOI] [PubMed] [Google Scholar]
- 93. van Westerloo DJ, Giebelen IA, Florquin S et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 2006; 130: 1822–1830 [DOI] [PubMed] [Google Scholar]
- 94. The F, Cailotto C, van der Vliet J et al. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br J Pharmacol 2011; 163: 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van Maanen MA, Stoof SP, Larosa GJ et al. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis 2010; 69: 1717–1723 [DOI] [PubMed] [Google Scholar]
- 96. Pavlov VA, Ochani M, Yang LH et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Critic Care Med 2007; 35: 1139–1144 [DOI] [PubMed] [Google Scholar]
- 97. Sabbah HN, Rastogi S, Mishra S et al. Long-term therapy with neuroselective electric vagus nerve stimulation improves LV function and attenuates global LV remodelling in dogs with chronic heart failure. Eur J Heart Fail Suppl 2005; 4: 166–167 [Google Scholar]
- 98. Li M, Zheng C, Sato T et al. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109: 120–124 [DOI] [PubMed] [Google Scholar]
- 99. Gold MR, Van Veldhuisen DJ, Hauptman PJ et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol 2016; 68: 149–158 [DOI] [PubMed] [Google Scholar]
- 100. Nederhoff MGJ, Fransen DE, Verlinde S et al. Effect of vagus nerve stimulation on tissue damage and function loss in a mouse myocardial ischemia-reperfusion model. Auton Neurosci 2019; 221: 102580. [DOI] [PubMed] [Google Scholar]
- 101. Uemura K, Zheng C, Li M et al. Early short-term vagal nerve stimulation attenuates cardiac remodeling after reperfused myocardial infarction. J Card Fail 2010; 16: 689–699 [DOI] [PubMed] [Google Scholar]
- 102. Esler MD, Krum H, Schlaich M et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 2012; 126: 2976–2982 [DOI] [PubMed] [Google Scholar]
- 103. Bakris GL, Townsend RR, Liu M et al. Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3. J Am Coll Cardiol 2014; 64: 1071–1078 [DOI] [PubMed] [Google Scholar]
- 104. Mahfoud F, Bohm M, Schmieder R et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J 2019; 40: 3474–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hilderman M, Qureshi AR, Abtahi F et al. The cholinergic anti-inflammatory pathway in resistant hypertension treated with renal denervation. Mol Med 2019; 25: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zaldivia MT, Rivera J, Hering D et al. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 2017; 69: 323–331 [DOI] [PubMed] [Google Scholar]
- 107. Koopman FA, Chavan SS, Miljko S et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA 2016; 113: 8284–8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Koopman FA, Maanen MA, Vervoordeldonk MJ et al. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med 2017; 282: 64–75 [DOI] [PubMed] [Google Scholar]
- 109. Li YC, Chou YC, Chen HC et al. Interleukin-6 and interleukin-17 are related to depression in patients with rheumatoid arthritis. Int J Rheum Dis 2019; 22: 980–985 [DOI] [PubMed] [Google Scholar]
- 110. Jangpangi D, Mondal S, Bandhu R et al. Alteration of heart rate variability in patients of depression. J Clin Diagn Res 2016; 10: CM04–CM06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Boyapati A, Schwartzman S, Msihid J et al. High serum interleukin-6 is associated with severe progression of rheumatoid arthritis and increased treatment response differentiating sarilumab from adalimumab or methotrexate in a post hoc analysis. Arthritis Rheumatol 2020; doi: 10.1002/art.41299 [DOI] [PMC free article] [PubMed]
- 112. Bonaz B Sinniger V Hoffmann D et al. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil 2016; 28: 948–953 [DOI] [PubMed] [Google Scholar]
- 113. Payne SC, Furness JB, Burns O et al. Anti-inflammatory effects of abdominal vagus nerve stimulation on experimental intestinal inflammation. Front Neurosci 2019; 13: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Uchino S, Kellum JA, Bellomo R et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818 [DOI] [PubMed] [Google Scholar]
- 115. Hoste EA, Bagshaw SM, Bellomo R et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423 [DOI] [PubMed] [Google Scholar]
- 116. Izawa J, Uchino S, Takinami M. A detailed evaluation of the new acute kidney injury criteria by KDIGO in critically ill patients. J Anesth 2016; 30: 215–222 [DOI] [PubMed] [Google Scholar]
- 117. Pakula AM, Skinner RA. Acute kidney injury in the critically Ill patient: a current review of the literature. J Intensive Care Med 2016; 31: 319–324 [DOI] [PubMed] [Google Scholar]
- 118. Dellepiane S, Marengo M, Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care 2016; 20: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Med Inflamm 2009; 2009:137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yeboah MM, Xue X, Duan B et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int 2008; 74: 62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yeboah MM, Xue X, Javdan M et al. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am J Physiol Renal Physiol 2008; 295: F654–F661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chatterjee PK, Yeboah MM, Dowling O et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One 2012; 7: e35361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Inoue T, Abe C, Sung SS et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest 2016; 126: 1939–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gigliotti JC, Huang L, Ye H et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 2013; 24: 1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gigliotti JC, Huang L, Bajwa A et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J Am Soc Nephrol 2015; 26: 2470–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zoccali C, Ciccarelli M, Maggiore Q. Defective reflex control of heart rate in dialysis patients: evidence for an afferent autonomic lesion. Clin Sci (Lond) 1982; 63: 285–292 [DOI] [PubMed] [Google Scholar]
- 127. Hilderman M, Qureshi AR, Al-Abed Y et al. Cholinergic anti-inflammatory pathway activity in dialysis patients: a role for neuroimmunomodulation? Clin Kidney J 2015; 8: 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Juto A, Juto AJ, von Hofsten P et al. Kinetic oscillatory stimulation of nasal mucosa in non-allergic rhinitis: comparison of patient self-administration and caregiver administration regarding pain and treatment effect. A randomized clinical trial. Acta Otolaryngol 2017; 137: 850–855 [DOI] [PubMed] [Google Scholar]
- 129. Juto JE, Hallin RG. Kinetic oscillation stimulation as treatment of acute migraine: a randomized, controlled pilot study. Headache 2015; 55: 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li TQ, Wang Y, Hallin R et al. Resting-state fMRI study of acute migraine treatment with kinetic oscillation stimulation in nasal cavity. Neuroimage Clin 2016; 12: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Juto JE, Axelsson M. Kinetic oscillation stimulation as treatment of non-allergic rhinitis: an RCT study. Acta Otolaryngol 2014; 134: 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bonaz B, Sinniger V, Hoffmann D et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil 2016; 28: 948–953 [DOI] [PubMed] [Google Scholar]
- 133. Bruchfeld A, Goldstein RS, Chavan S et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med 2010; 268: 94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol 2016; 594: 5781–5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hanes WM, Olofsson PS, Kwan K et al. Galantamine attenuates type 1 diabetes and inhibits anti-insulin antibodies in nonobese diabetic mice. Mol Med 2015; 21: 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Addorisio ME, Imperato GH, de Vos AF et al. Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectron Med 2019; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hoeger S, Fontana J, Jarczyk J et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol Dial Transplant 2014; 29: 544–549 [DOI] [PubMed] [Google Scholar]
- 138. Zoccali C, Blankestijn PJ, Bruchfeld A et al. Children of a lesser god: exclusion of chronic kidney disease patients from clinical trials. Nephrol Dial Transplant 2019; 34: 1112–1114 [DOI] [PubMed] [Google Scholar]
- 139. Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci 2017; 20: 156–166 [DOI] [PubMed] [Google Scholar]