Abstract
Selenium (Se) supplementation may decrease the severity of ulcerative colitis (UC) through the activation of genes responsible for immune modulation. The present research was aimed to assess the effect of Se supplementation on the expression of silent information regulator 1 (SIRT1) and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) in UC patients. In a double-blind randomized parallel clinical trial, 100 patients with mild-to-moderate active UC met inclusion criteria and divided into 2 groups of treatment (50 patients received selenomethionine [200 µg daily]) and placebo (50 patients received placebo [1 capsule daily]) for 10 weeks. The expression rates of SIRT1 and PGC-1α were examined in the peripheral blood mononuclear cell (PBMC) using the real-time polymerase chain reaction. There was no considerable difference in the mean of baseline demographic and clinical characteristics between groups. Also, there were no significant differences in total energy intake, macronutrients, and micronutrients between groups. The SIRT1 gene expression in the Se group was significantly increased compared to the placebo (p < 0.001). An increase in the expression of the PGC-1α gene in the Se group was not statistically significant. It seems that Se supplementation caused a significant decrease in the inflammatory response of the colon by a significant increase in the expression of the SIRT1 gene.
Keywords: Selenium, Ulcerative colitis, SIRT1, PGC-1alpha protein, Gene expression
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) with higher distribution amongst 30–40 years old adults [1]. Epidemiological surveys revealed the high annual incidence of UC amongst the people in Europe (24.3 per 100,000 individuals), Asia, and the Middle East (6.3 per 100,000 individuals) [2]. UC is mostly recognized with bowel movements, diarrhea, rectal bleeding, local inflammation, abdominal pain, urgency, and incontinence [1,2]. Nevertheless, many factors play significant portions in the pathogenesis of UC, but the roles of nutritional factors may be more prominent [3].
Selenium (Se) is a vital trace element that exists in both organic and inorganic forms with considerable catalytic and antioxidant effects [4]. The therapeutic roles of Se have been mainly attributed to its presence in selenoproteins, as the 21st amino acid, selenocysteine, which is responsible for modulating pathways in inflammation [5]. Several epidemiological studies have shown low Se concentrations in patients who suffered from UC and IBD [6,7]. Thus, Se deficiency in IBD patients was accompanied by increased severity of the UC and IBD [6,8].
Se supplementation has been specified to protect toward tissue damage in experimental UC by adjusting the expression of genes involved in the mitochondrial cell death regulation [9]. According to the hypothesis, Se supplementation may act as a protective factor against inflammation by adjusting the expression of some kinds of genes involved in the immune responses [10]. In the study, Li et al. [11] found that treatment with Se nanoparticles reduced cell apoptosis through an increase of mRNA levels of silent information regulator 1 (SIRT1) in acute kidney injury models.
SIRT1 is NAD(+)-dependent histone deacetylases involved in the regulation of antioxidant defense factors, inflammation, and cell apoptosis [12,13]. Furthermore, SIRT1 can play a vital role in increasing the survival of inflammatory cells, including colon cells, in colitis [14]. On the other hand, human and experimental studies have shown that SIRT1 was down-regulated in UC patients or IBD models [14,15,16,17], and treatment with SIRT1 activators significantly alleviates the symptoms of colitis [15,17]. Studies have demonstrated that SIRT1 is an important factor in the activation of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) [18].
PGC-1α protein is an activator in the cell nucleus which controls numerous biological activities, such as oxidation, mitochondrial biogenesis, and inflammation [18]. PGC-1α like SIRT1 has decreased in colitis patients as well as Se deficient experimental colitis [19,20]. Increasing the expression of PGC-1α by increasing antioxidant enzymes can protect intestinal cells against oxidative stress and reduce the incidence of apoptosis which leads to ameliorating the disease in IBD patients [19].
However, the exact protective effects of these genes in patients with UC is still unknown. According to the high importance of UC, the possible role of Se supplementation in patients with UC, and also probable roles of SIRT1 and PGC-1α genes in the pathogenesis of UC, existing research was carried out to assess the effect of Se supplementation on the expression of SIRT1 and PGC-1α genes on UC patients.
MATERIALS AND METHODS
Ethical consideration
The study was ethically confirmed by the ethical council of the Iran University of Medical Sciences (IR.IUMS.REC.1397.688) and registered in the Iranian Center for Clinical Trials (No. IRCT20091114002709N51). All included patients signed an informed consent form, and the method and benefits of the study were explained.
Study population
The present multicenter randomized, placebo-controlled, double-blind study was conducted on 100 patients with mild-to-moderate active UC who were referred to 3 medical centers in Tehran, Iran (Rasoul Akram Hospital, Imam Khomeini Hospital, and gastrology clinic of Iran medical university) between April 2019 and May 2020. Patients over 18 years of age with a confirmed histological and endoscopic diagnosis of UC, body mass index (BMI) of 18.5–30 kg/m2 were selected. In the study, active mild to moderate UC was determined by a Simple Clinical Colitis Activity Index score of ≥ 5 and < 12. Exclusion criteria of the study were use of non-steroidal anti-inflammatory drugs and corticosteroids, the use of anti-tumor necrosis factor (TNF) agents, the use of multivitamin-mineral, omega-3 fatty acids, polyphenolic and antioxidant supplements and also changing the type or dose of medication over the past month. Besides patients with other diseases (cancer, renal, liver or cardiovascular disease, and diabetes mellitus), pregnant or lactating women were excluded.
Interventions
Patients were randomly divided into 2 groups according to permuted block randomization design generated via the www.Randomization.com website. Patients were given 1 selenomethionine capsule contained 200 µg/day (21st-Century Company, Tempe, AZ, USA) or placebo for 10 weeks. Placebo capsules contained rice flour and were prepared by the Faculty of Pharmacy, Shahid Beheshti at University of Medical Sciences, Tehran, Iran. The size and color of placebos were completely similar to Se capsules, also packaged likely to Se boxes. All intervention packs were marked by numbers (1–100) to concealment of randomization codes. The randomization list and numbered packing of intervention were performed by a person not involved in the study. The intervention packs were placed in the laboratory and given to the patients by the secretary according to the code numbers. Patients and all study personnel were blinded to treatment assignment throughout the study. The patient an adherence was assessed based on peel count at the end of the study, and adherence rate of ≤ 85% has been reported as non-adherence and excluded.
Measurements
At the first and 10th weeks of study, anthropometric measurements, dietary intake, and physical activity were assessed in each patient. Dietary intake by 3-day food record and physical activity by the International Physical Activity Questionnaire were evaluated. Patients were asked to continue their daily regular diet and physical activity during the study without any change. Furthermore, to an assessment of gene expression, a peripheral blood sample (10 mL) was obtained after 10–12 h of fasting at the first and the end of the intervention.
RNA extraction and cDNA synthesis
Buffy coat samples of blood white cells were separated by centrifugation (Shimadzu, Kyoto, Japan). RNA was extracted using RNX-plus Sinacolon Kit (CinnaGen Co., Tehran, Iran) according to the protocol of the producing company. The RNA concentrations were determined by Nanodrop device (NanoDrop, Wilmington, DE, USA). All RNA samples had a 260:280 absorbance ratio between 1.9 and 2.1. Then, cDNA was synthesized using SinaClon first-strand cDNA synthesis kit (CinnaGen Co.) according to the protocol of the producing company. Extracted cDNA samples were subjected to quantification by NanoDrop device (Thermo Scientific,, USA), qualification (2% agarose gel), and purity checking (A260/A280). The cDNA was stored at −80°C until subsequent analysis.
Gene expression
Real-time polymerase chain reaction (PCR) was carried out to determine the levels of SIRT1 and PGC-1α genes expression in extracted cDNA samples. GAPDH was used as the housekeeping gene. Table 1 describes a list of primers and housekeeping gene used in the real-time PCR reaction [21]. Real-time PCR thermocycler (Rotor-Gene 6000; QIAGEN, Germantown, MD, USA) was used to assess the expression rates of the SIRT1 and PGC-1α genes in examined groups using SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. The real-time PCR reaction was performed by 5 µL of SinaSYBR Blue HS-qPCR Mix (2×) (Sinaclone, Tehran, Iran), 1 µL of extracted cDNA, 0.25 μL of each 10 μM forward and reverse primers, and 3.5 µL sterile distilled water, making a total volume of 10 μL. Forty cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 20 seconds were run after the denaturation of DNA at 95°C for 5 minutes. The melting curve analysis was conducted from 55°C to 99°C with a 0.2-second interval. The data were analyzed according to the delta-delta Ct (ΔΔCt) method and were normalized to SIRT1 and PGC-1α expression in each sample.
Table 1. Primers and housekeeping gene used in the real-time polymerase chain reaction.
| Target gene | Oligonucleotide primers (5′-3′) |
|---|---|
| SIRT1 | F: TAGTAGGCGGCTTGATGGTAATC |
| R: GGTTCTTCTAAACTTGGACTCTGG | |
| PGC-1α | F: GTCAACATTCAAAGCAGCAGAGAG |
| R: GACACATAATCATTACCTACTGGAAGC | |
| GAPDH | F: GAAGGTGAAGGTCGGAGTCAAC |
| R: CAGAGTTAAAAGCAGCCCTGGT |
F, forward primer; R: reverse primer; SIRT1, silent information regulator 1; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase
Statistical analysis
Statistical analysis was performed using STATA v.14 (StataCorp, College Station, TX, USA). Data of 3-day food records were analyzed via Nutritionist IV software (v.4.1; First Data Bank Division, The Hearst Corporation, New York, NY, USA). The assessing normality of data distribution was performed by histograms and the Shapiro-Wilks test. Baseline characteristics were compared among the 2 intervention groups using an independent sample t-test for continuous data and a χ2 test for ordinal data. Intention to treat analysis was performed to address missing data. To remove the impact of the confounding factors, an analysis of analysis of variance/analysis of covariance models was used. The p value was considered to be statistically significant when 0.05. The magnitude of the treatment effect is presented as mean difference, standardized mean difference (SMD), and 95% confidence interval (CI).
RESULTS
Study population
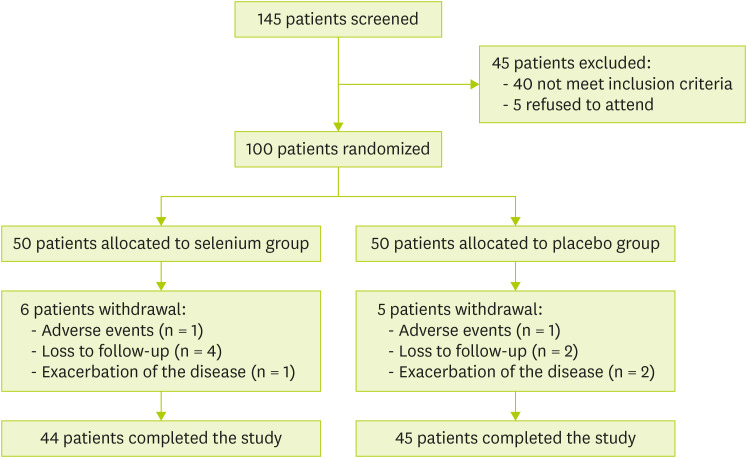
One-hundred eligible patients (50 patients in each group) took part in the randomized controlled trial. Eighty-nine patients completed the study (44 patients in Se group and 45 patients in the placebo group) and eleven patients lost to follow up. Figure 1 shows the withdrawal reasons of the patients.
Figure 1. PRISMA flow diagram for study selection.
The baseline demographic and clinical characteristics contain sex, age, BMI, waist circumference (WC), duration of UC, and extension of disease in both groups were summarized in Table 2. The mean age in Se and placebo groups was 34.5 and 37.9, respectively. There was no considerable difference in mean of weight, BMI, and WC between groups at the beginning of the study. The median duration of disease in Se and placebo groups was 5 (3–10) and 6 (3–10) years, respectively. Although the number of patients with rectosigmoid colitis (10 vs. 16) were higher in the placebo group, there was no significant difference between other colitis groups. Eighty-two percent of patients had used mesalamine in the Se group vs. 85% in the placebo group (p > 0.05). Also, there was no significant difference in mean (standard deviation) of anthropometric indices such as weight (69.5 [0.8] vs. 69.8 [0.8]) and BMI (24.1 [0.2] vs. 24.2 [0.3]) between Se and placebo at the end of the study (p > 0.05).
Table 2. Baseline demographics and clinical characteristics of the study patients.
| Characteristics | Selenium (n = 50) | Placebo (n = 50) | p value | |
|---|---|---|---|---|
| Age | 34.5 ± 11.2 | 37.9 ± 10.8 | NS* | |
| Sex (M/F) | 16/34 | 20/30 | NS† | |
| Weight | 69.2 ± 12.1 | 70.3 ± 11.1 | NS* | |
| BMI | 24.4 ± 3.4 | 24.3 ± 3.3 | NS* | |
| WC | 82.5 ± 10.8 | 83.6 ± 12.8 | NS* | |
| Smoking | 13 (26) | 12 (24) | NS† | |
| Alcohol | 8 (16) | 6 (12) | NS† | |
| Duration of disease (yr) | 5 (3–10) | 6 (3–10) | NS‡ | |
| Extension of disease | NS† | |||
| Proctitis | 18 (36) | 15 (30) | ||
| Left-sided | 18 (36) | 17 (34) | ||
| Extensive | 4 (8) | 2 (4) | ||
| Rectosigmoid | 10 (20) | 16 (32) | ||
| Medicines used | NS† | |||
| Mesalamine | 41 (82) | 43 (86) | ||
| Mesalamine + immunosuppressants | 9 (18) | 7 (14) | ||
Values are presented as mean ± standard deviation, number (%), or median (interquartile range).
NS, not significant.
*According to independent t-test; †According to χ2 test; ‡According to Mann-Whitney.
Dietary intakes and physical activity levels
The mean dietary intakes and physical activity levels are displayed in Table 3. There were no significant differences in total energy, macronutrients, and micronutrients intake between 2 groups at the beginning and also at the end of the study. The mean daily Se intakes were 58.67 ± 15.40 µg/day in Se group and 57.93 ± 13.76 µg/day in the placebo group at the beginning of the study (Table 3).
Table 3. Dietary intake and physical activity of the study patients.
| Variables/groups | Selenium (n = 50) | Placebo (n = 50) | p value* | |
|---|---|---|---|---|
| Energy intake (kcal/day) | ||||
| Pre | 1,794.2 ± 208.8 | 1,834.2 ± 207.6 | 0.594 | |
| Post | 1,819.3 ± 212.7 | 1,828.3 ± 262.7 | 0.262 | |
| Carbohydrate intake (g/day) | ||||
| Pre | 243.5 ± 27.3 | 254.0 ± 38.8 | 0.719 | |
| Post | 251.9 ± 52.4 | 249.8 ± 36.6 | 0.625 | |
| Protein (g/day) | ||||
| Pre | 71.1 ± 22.8 | 62.1 ± 9.8 | 0.554 | |
| Post | 70.7 ± 10.6 | 66.5 ± 10.6 | 0.314 | |
| Fat (g/day) | ||||
| Pre | 73.1 ± 18.32 | 69.1 ± 18.2 | 0.264 | |
| Post | 72.9 ± 20.1 | 70.2 ± 14.7 | 0.385 | |
| Selenium (µg/day) | ||||
| Pre | 58.6 ± 15.4 | 57.9 ± 13.7 | 0.900 | |
| Post | 59.4 ± 14.4 | 59.4 ± 15.9 | 0.997 | |
| Physical activity (MET.h/day) | ||||
| Pre | 27.2 ± 11.9 | 28.7 ± 9.3 | 0.691 | |
| Post | 27.6 ± 12.1 | 28.9 ± 10.0 | 0.708 | |
Values are presented as mean ± standard deviation.
*According to independent t-test.
SIRT1 and PGC-1α genes expression
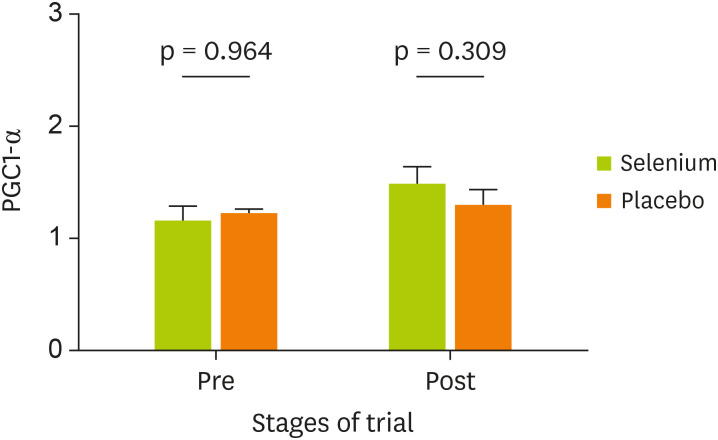
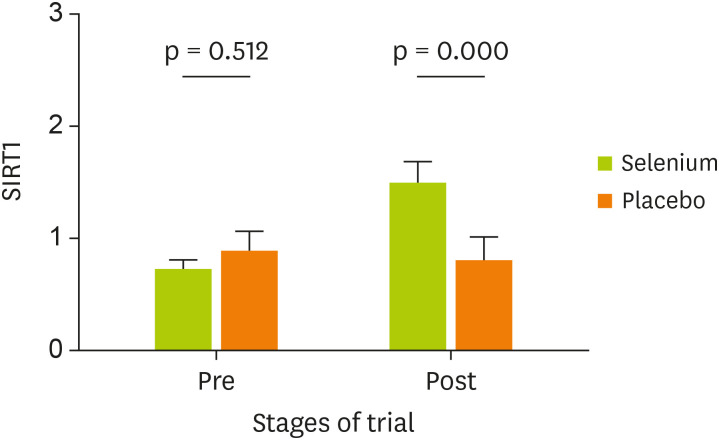
Table 4 indicates the comparison of PGC-1α and SIRT1 gene expression in the 2 groups. Findings revealed that the expression of SIRT1 in the group of UC patients treated with Se has been significantly increased compared with placebo (p < 0.001) (SMD, 0.86; 95% CI, 0.45–1.27). After adjustment for study confounders (the baseline value, age, and colitis groups), the difference between Se and placebo remained statistically significant (p < 0.001). Whereas an increase in the expression of PGC-1α gene after Se treatment was not statistically significant between groups at the end of the study (p = 0.146) (SMD, 0.29; 95% CI, −0.10–0.68). The effect of Se supplementation on gene expression of PGC-1α and SIRT1 was shown in Figures 2 and 3, respectively.
Table 4. Comparison of SIRT1 and PGC-1α expression in pre and post intervention by the study groups in according to different models.
| Gene | Time point | Selenium (n = 50) | Placebo (n = 50) | Mean difference (95% CI) | Cohen's d (95% CI) | p value | Adjusted R2 | Adjusted p value‡ | Adjusted p value§ |
|---|---|---|---|---|---|---|---|---|---|
| SIRT1 | Pre | 0.863 ± 0.149 | 0.972 ± 0.285 | - | - | 0.512* | - | - | - |
| Post | 1.573 ± 0.111 | 0.896 ± 0.109 | 0.67 (0.36–0.98) | 0.86 (0.45–1.27) | < 0.001† | 0.15 | < 0.001 | < 0.001 | |
| PGC-1α | Pre | 1.102 ± 0.866 | 1.110 ± 0.131 | - | - | 0.964* | - | - | - |
| Post | 1.352 ± 0.102 | 1.141 ± 0.100 | 0.21 (−0.07–0.49) | 0.29 (−0.10–0.68) | 0.146† | 0.42 | 0.148 | 0.149 |
Data presented as mean ± standard deviation.
PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; SIRT1, silent information regulator 1; CI, confidence interval; ANOVA, analysis of variance; ANCOVA, analysis of covariance.
*Calculated based on independent t-test; †Calculated based on ANOVA model; ‡Adjusted for potential covariate (the baseline value, age) (calculated based on ANOVA/ANCOVA model); §Adjusted for potential covariate (the baseline value, colitis groups) (calculated based on ANOVA/ANCOVA model).
Figure 2. Effect of Se supplementation on gene expression of PGC-1α (data presented as 2−ΔΔCt).
Se, selenium; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; ΔΔCt, delta-delta Ct.
Figure 3. Effect of Se supplementation on gene expression of SIRT1 (data presented as 2−ΔΔCt).
Se, selenium; SIRT1, silent information regulator 1; ΔΔCt, delta-delta Ct.
DISCUSSION
According to our knowledge, this is the first clinical trial to investigate the effects of Se supplementation on expression of genes involved in the inflammatory response in UC patients. The study findings revealed that the expression of SIRT1 gene was significantly increased after Se supplementation. Also, according to our results, the Se supplementation was a large and considerable effect on up-regulating the expression of SIRT1 gene, (SMD, 0.86; 95% CI, 0.45–1.27). This finding may show the therapeutic effect of Se supplementation in UC patients through the up-regulating expression of SIRT1.
SIRT1 is an essential metabolic sensor that controls a diversity of biological procedures such as apoptosis, metabolism, stress response, mitochondrial biogenesis, and inflammation in response to environmental conditions/ oxidants [22,23]. The SIRT1 physiological roles are mostly mediated through some transcription factors such as nuclear factor-κB (NF-κB) and PGC-1α [24]. The various beneficial functions of SIRT1 in metabolism and inflammation have been demonstrated in different diseases such as IBD [25]. It has been documented that the change in SIRT1 is very important as controllers of chronic inflammation [26].
Studies proved that SIRT1 can influence the regulation of intestinal inflammation and tissue homeostasis in UC model [15,16,27]. Since down-regulation the expression of SIRT1 upraise the concentrations of the pro-inflammatory cytokines that are involved in UC pathogenesis activation of SIRT1 caused a significant reduction in the symptoms of the disease in IBD [14,15].
Treatment with SIRT1 activators is beneficial in improving cell function and inflammatory responses [28]. Finding a recent study shows that treatment with curcumin and resveratrol exert protective functions on the colitis model via up-regulating the expression of SIRT1 and inhibition of the pro-inflammatory cytokines [29]. In another study, Se nanoparticle supplementation effectively elevated expression of SIRT1 and thus, improved cell survival and also, decreased cell apoptosis in kidney cells of diabetic rats [30]. SIRT1 may represent anti-inflammatory functions by several mechanisms [28].
NF-κB is a major transcription factor that regulates the transcription of proinflammatory genes [31], and chronically active in several inflammatory diseases, such as UC and IBD [32]. Because activation is responsible for the production of proinflammatory cytokines like TNF-α and interleukin-6 [24]. It has been reported that SIRT1 can interact with numerous subunit of NF-κB complex and deacetylate the transcription factor, whereby suppresses NF-κB activity and reduces transcription of proinflammatory genes [24,25]. In our study, the expression of SIRT1 was increased by Se in mild to moderate UC patients, suggesting that the anti-inflammation effects of Se may be due to the upreguation of SIRT1 signaling activation.
Other studies show that resveratrol may indirectly activate SIRT1 by cyclic-adenosine monophosphate signaling that causes activation of 5′ AMP-activated protein kinase (AMPK)/ SIRT1 pathway' [33]. AMPK and SIRT1 have been presented to play some similar functions, such as the ability to react to nutrient and stress status, like mitochondrial biogenesis, and regulate the important transcriptional factor activity such as PGC-1α [26,34].
On the other hand, studies have shown that the SIRT1 is an important factor in the activation of PGC-1α protein via deacetylating in several lysine sites of PGC-1α [18,35]. Regulation of normal mitochondrial function needs PGC-1α activity [36]. In stressful circumstances, PGC-1α is an effective stimulator of mitochondrial turnover and antioxidant effects [19]. Researches revealed that an increase in the expression of PGC-1α caused a significant increase in the levels of antioxidant enzymes such as catalase and superoxide dismutase, and selenoproteins are able to prevent oxidative stress in the intestinal cells and increase their survival and reduce apoptosis [37]. It has also recently been shown that the expression of PGC-1α is reduced in people with severe UC and colon cancer [18]. Moreover, PGC-1α in the gut can prevent the development of colitis and maintains the continuity of intestinal epithelial cells [18,37]. Some studies demonstrate Se deficiency reduced PGC-1α expression in mice [38] and therapeutic induction of SIRT1 in UC mice increases PGC-1α expression [39].
As the expression of the SIRT1 was significantly increased after Se supplementation than that of PGC-1α, it seems that the Se supplementation had higher effects on the SIRT1 gene expression. There is no evidence about the impact of Se on the expression of PGC-1α gene, but studies have shown that resveratrol increases PGC-1α expression by increasing the induction of SIRT1 [40,41]. In our study, an increase in the expression of the PGC-1α gene was not significant. The main reason for this matter is unknown. Possibly, increasing the duration of Se supplementation may effectively increase PGC-1α expression. Thus, further investigations are required to found the exact effects of Se on the expression of PGC-1α.
Anti-inflammatory and protective roles of Se supplements in the IBD have been determined previously [42,43,44]. Additionally, Se is a key component of antioxidant enzymes, such as glutathione peroxidase, that exerts anti-inflammatory effects by several pathways [33,45,46]. The present study shows probably Se as a SIRT1 activator can modulate inflammation in UC patients. Nevertheless, the fundamental mechanisms are not well recognized and the exact role of Se in SIRT1 and PGC-1α activation needs further investigation.
The present study revealed the favorable increasing effect of Se supplementation on the expression of SIRT1 for the first time. This is probably considered as a therapeutic window in colitis patients, since Se may resemble an anti-TNF-α agent, infliximab and led to a rising in SIRT1 expression that conversely decreases TNF-α levels [17]. However, the lack of evaluation of other transcriptional and translational factors such as NF-κB that may affect through SIRT1 are the limitations of this research. Therefore, it is suggested that for better understanding, the effect of Se on the mechanisms and transcription factors associated with SIRT1 in the future experimental and clinical studies be investigated.
CONCLUSION
This is the first report of the effect of Se supplements on the SIRT1 and PGC-1α gene expression in patients with UC. It seems that the Se caused a significant decrease in the inflammatory response due to the overexpression of the SIRT1 gene. However, further surveys are required to found the exact role of the Se in the controlling of UC through the expression of the SIRT1 gene.
ACKNOWLEDGMENTS
The authors would like to thank from the Iran University of Medical Sciences, Tehran, Iran for its financial supports and also from all patients who were participated in this study. Also, authors thank from National Reference Laboratory Applied Study Diagnosis, Tehran, Iran for their valuable help in the conductance of the trial.
Footnotes
Funding: This article was extracted from the approved academic research project supported by the Iran University of Medical Sciences, Tehran, Iran (IRCT20091114002709N51).
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, Ziade N, Hashash JG. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65:100851. doi: 10.1016/j.disamonth.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Mokhtar NM, Nawawi KN, Verasingam J, Zhiqin W, Sagap I, Azman ZA, Mazlan L, Hamid HA, Yaacob NY, Rose IM, Den EL, Wan MS, Raja Ali RA. A four-decade analysis of the incidence trends, sociodemographic and clinical characteristics of inflammatory bowel disease patients at single tertiary centre, Kuala Lumpur, Malaysia. BMC Public Health. 2019;19:550. doi: 10.1186/s12889-019-6858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun J, Xu CT, Pan BR. Epidemiology and gene markers of ulcerative colitis in the Chinese. World J Gastroenterol. 2009;15:788–803. doi: 10.3748/wjg.15.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2008;13:102–108. doi: 10.1007/s12199-007-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 6.Castro Aguilar-Tablada T, Navarro-Alarcón M, Quesada Granados J, Samaniego Sánchez C, Rufián-Henares JÁ, Nogueras-Lopez F. Ulcerative colitis and Crohn's disease are associated with decreased serum selenium concentrations and increased cardiovascular risk. Nutrients. 2016;8:780. doi: 10.3390/nu8120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudva AK, Shay AE, Prabhu KS. Selenium and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2015;309:G71–7. doi: 10.1152/ajpgi.00379.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short SP, Pilat JM, Williams CS. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic Biol Med. 2018;127:26–35. doi: 10.1016/j.freeradbiomed.2018.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tirosh O, Levy E, Reifen R. High selenium diet protects against TNBS-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition. 2007;23:878–886. doi: 10.1016/j.nut.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Wang Q, Deng G, Liu Y, Wei B, Liu X, Bao W, Wang Q, Wu S. Porous Se@SiO2 nanospheres attenuate cisplatin-induced acute kidney injury via activation of Sirt1. Toxicol Appl Pharmacol. 2019;380:114704. doi: 10.1016/j.taap.2019.114704. [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43:1589–1598. doi: 10.1007/s10753-020-01242-9. [DOI] [PubMed] [Google Scholar]
- 13.Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A, Altucci L. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics. 2016;8:61. doi: 10.1186/s13148-016-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso R, Marafini I, Franzè E, Stolfi C, Zorzi F, Monteleone I, Caprioli F, Colantoni A, Sarra M, Sedda S, Biancone L, Sileri P, Sica GS, MacDonald TT, Pallone F, Monteleone G. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014;7:1467–1479. doi: 10.1038/mi.2014.35. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M, Mohapatra J, Wagh A, Patel HM, Pandey D, Kadam S, Argade A, Deshpande SS, Shah GB, Chatterjee A, Jain MR. Involvement of TACE in colon inflammation: a novel mechanism of regulation via SIRT-1 activation. Cytokine. 2014;66:30–39. doi: 10.1016/j.cyto.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-κB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devi K, Singh N, Jaggi AS. Dual role of sirtuin 1 in inflammatory bowel disease. Immunopharmacol Immunotoxicol. 2020;42:385–391. doi: 10.1080/08923973.2020.1790595. [DOI] [PubMed] [Google Scholar]
- 18.D'Errico I, Salvatore L, Murzilli S, Lo Sasso G, Latorre D, Martelli N, Egorova AV, Polishuck R, Madeyski-Bengtson K, Lelliott C, Vidal-Puig AJ, Seibel P, Villani G, Moschetta A. Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci U S A. 2011;108:6603–6608. doi: 10.1073/pnas.1016354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham KE, Vincent G, Sodhi CP, Novak EA, Ranganathan S, Egan CE, Stolz DB, Rogers MB, Firek B, Morowitz MJ, Gittes GK, Zuckerbraun BS, Hackam DJ, Mollen KP. Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) protects against experimental murine colitis. J Biol Chem. 2016;291:10184–10200. doi: 10.1074/jbc.M115.688812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai H, Nie T, Zhang Y, Chen Y, Tao J, Lin T, Ge T, Li F, Li H. Selenium deficiency-induced damage and altered expression of mitochondrial biogenesis markers in the kidneys of mice. Biol Trace Elem Res. 2020 doi: 10.1007/s12011-020-02112-z. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 21.Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, Tanbakooei S, Shidfar F. The effects of curcumin supplementation on oxidative stress, sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020;14:77–82. doi: 10.1016/j.dsx.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sands BE, Joshi S, Haddad J, Freudenberg JM, Oommen DE, Hoffmann E, McCallum SW, Jacobson E. Assessing colonic exposure, safety, and clinical activity of SRT2104, a novel oral SIRT1 activator, in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. 2016;22:607–614. doi: 10.1097/MIB.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vachharajani VT, Liu T, Wang X, Hoth JJ, Yoza BK, McCall CE. Sirtuins link inflammation and metabolism. J Immunol Res. 2016;2016:8167273. doi: 10.1155/2016/8167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Sasso G, Ryu D, Mouchiroud L, Fernando SC, Anderson CL, Katsyuba E, Piersigilli A, Hottiger MO, Schoonjans K, Auwerx J. Loss of Sirt1 function improves intestinal anti-bacterial defense and protects from colitis-induced colorectal cancer. PLoS One. 2014;9:e102495. doi: 10.1371/journal.pone.0102495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan X, Wen JJ, Koo SJ, Liang LY, Garg NJ. SIRT1-PGC1α-NFκB pathway of oxidative and inflammatory stress during Trypanosoma cruzi infection: benefits of SIRT1-targeted therapy in improving heart function in Chagas disease. PLoS Pathog. 2016;12:e1005954. doi: 10.1371/journal.ppat.1005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Xue H, Zhao G, Qiao C, Sun X, Pang C, Zhang D. Curcumin and resveratrol suppress dextran sulfate sodium‑induced colitis in mice. Mol Med Rep. 2019;19:3053–3060. doi: 10.3892/mmr.2019.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar GS, Kulkarni A, Khurana A, Kaur J, Tikoo K. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem Biol Interact. 2014;223:125–133. doi: 10.1016/j.cbi.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991;88:4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang K, Li YF, Lv Q, Li XM, Dai Y, Wei ZF. Bergenin, acting as an agonist of PPARγ, ameliorates experimental colitis in mice through improving expression of SIRT1, and therefore inhibiting NF-κB-mediated macrophage activation. Front Pharmacol. 2018;8:981. doi: 10.3389/fphar.2017.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 36.Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speckmann B, Walter PL, Alili L, Reinehr R, Sies H, Klotz LO, Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1α with FoxO1a and hepatocyte nuclear factor 4α transcription factors. Hepatology. 2008;48:1998–2006. doi: 10.1002/hep.22526. [DOI] [PubMed] [Google Scholar]
- 38.He S, Guo X, Tan W, Su X, Li J, Pan W, Qiu H. Effect of selenium deficiency on phosphorylation of the AMPK pathway in rats. Biol Trace Elem Res. 2016;169:254–260. doi: 10.1007/s12011-015-0427-z. [DOI] [PubMed] [Google Scholar]
- 39.Kaistha A, Levine J. Inflammatory bowel disease: the classic gastrointestinal autoimmune disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:328–334. doi: 10.1016/j.cppeds.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vargas-Robles H, Castro-Ochoa KF, Citalán-Madrid AF, Schnoor M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo . World J Gastroenterol. 2019;25:4181–4198. doi: 10.3748/wjg.v25.i30.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddavide R, Rotolo O, Caruso MG, Stasi E, Notarnicola M, Miraglia C, Nouvenne A, Meschi T, De' Angelis GL, Di Mario F, Leandro G. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018;89:60–75. doi: 10.23750/abm.v89i9-S.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bitiren M, Karakilcik AZ, Zerin M, Ozardali I, Selek S, Nazligül Y, Ozgonul A, Musa D, Uzunkoy A. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol Trace Elem Res. 2010;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- 45.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speckmann B, Steinbrenner H. Selenium and selenoproteins in inflammatory bowel diseases and experimental colitis. Inflamm Bowel Dis. 2014;20:1110–1119. doi: 10.1097/MIB.0000000000000020. [DOI] [PubMed] [Google Scholar]