Abstract
Objective
To assess the effects of exposures to food cues and stress on hunger and food intake and examine whether cue responses differ by weight status.
Methods
In a laboratory-based, experimental study, participants (N=138) were exposed to stress, neutral, and food cues delivered using an individualized script-driven imagery task on three days. After each cue exposure, participants ate high- and low-calorie snack foods ad-libitum (Food Snack Test; FST). Hunger was measured by visual analog scales.
Results
Food cues elicited significantly greater increases in hunger compared to neutral and stress stimuli. Cue-induced hunger did not differ by weight status. Participants consumed a similar number of total calories across stimuli. In response to food cue provocation, participants with obesity consumed 81.0±4.0% of calories from high-calorie foods, which was significantly greater than participants with normal weight (63.5±3.6%; p=0.001). After the stress cue, participants with obesity consumed 81.4±4.0% of calories from high-calorie foods which was significantly more than participants of normal weight (70.2±3.6%; p=0.04). Energy intake from high-calorie foods did not differ by weight status after the neutral cue.
Conclusions
Among individuals with obesity, exposure to food and stress cues shifted consumption to high-calorie snack foods within a well-controlled experimental setting.
Keywords: Food intake, hunger, obesity, stress
Introduction
Obesity, defined as a body mass index (BMI)≥30 kg/m2, is a global epidemic that greatly increases the burden of chronic diseases.1 Obesity is caused by overconsumption of calories relative to energy expenditure.2 The modern obesogenic environment, filled with cues to consume high-calorie and energy-dense foods, has been implicated as a major driver of overweight and obesity.3
Food cues are discrete food stimuli or food-related contexts/situations that evoke learned food-related memories and food-related conditioned responses. Cues may include responses to external stimuli, such as seeing food advertisements, or to stress, a complex and multidimensional response to a real or perceived disruption in homeostasis.4 In Pavlovian classical conditioning models, repeated pairings of an initially neutral or non-food cue (e.g., objects, stress) with an unconditioned stimulus (e.g., reward from consuming high-calorie foods), leads to the acquisition of an association between the cue and unconditioned stimuli, thereby resulting in a conditioned cue.5 Once an association is learned, the cues associated with the food and food intake may elicit the same set of physiological (e.g., salivation), psychological (e.g., hunger), and behavioral responses (e.g., food intake) thereby resulting in conditioned responses. For example, individuals may learn to cope with stressful life events by consuming high-calorie foods.
Overeating of high-calorie foods in the ubiquitous food cue environment and in the context of stressful life events may promote hunger, caloric intake, and weight gain. Stressful life events are discrete experiences that disrupt an individual’s usual activities causing emotional strain or tension. Stressful events may cause a person to feel anxious (i.e., feelings of fear, worry, or unease).6 Eating in response to cues such as seeing food and to stress is associated with self-reported consumption of nutrient dense foods,7–9 particularly high-fat and high-sugar snack foods.10–12 However, these results have been not been consistently shown, possibly due to recall and reporting biases and differences in the severity, intensity, duration, and nature of cues examined (e.g., episodic (acute) life events versus chronic stress), or intraindividual differences.13–15 Laboratory studies allow for a more precise and well-controlled measure of the relationship between food cues and eating behaviors, and also greater examination of intraindividual differences that drive variations in food cue responses.16 For example, in our preliminary study using a controlled, 3-day laboratory experiment of individualized scripted imagery cues, we found that compared to neutral-relaxing cues, food cues significantly increased high-calorie food craving and intake.17 The stress cue condition, relative to the neutral condition, increased high-calorie food craving and intake in participants with overweight/obese but not those with normal weight.17 In another study, a speech stress performance task did not significantly alter overall intake of food compared to a neutral control; however, compared to non-emotional eaters in the neutral condition, emotional eaters in the stress task had increased intake of sweet-fatty foods.18 Participants with high dietary restraint tend to eat more under stress, whereas intake is typically the same or lower in unrestrained eaters.16 However, other factors associated with responses to food cues have yet to be fully elucidated.
Enhanced responses to food cues are likely an important risk factor for obesity, and cue-elicited responses may be stronger in individuals with overweight and those with obesity.19 Food cues may override homeostatic food signals and promote unhealthy food choices in people with obesity.20 For example, after viewing and smelling a cued food (pizza), individuals with overweight displayed greater salivation and desire for cued and non-cued foods than individuals who were lean21 Compared to participants with normal weight, those with obesity demonstrated increased attention to food images in a fed state.22 Yet, the relationships of different types of food cues, food choice, and weight status are not fully understood.
The present study examined whether the effects of an experimental paradigm using brief, imagined cue exposure to personal, favorite food or to personal, discrete stressful events separately and differentially increased hunger, anxiety, and food intake compared to neutral cues. We also examined whether cue responses of hunger and food intake differ by weight status. We hypothesized that food and stress cues would elicit greater increases in hunger, subjective anxiety, and proportion of intake from high-calorie foods compared to neutral cues. We also hypothesized that participants with obesity would have greater cue responses compared to individuals of normal weight including greater increases in hunger and intake of high-calorie snack foods. Finally, we hypothesized that hunger levels after cue exposure will be associated with food intake across BMI groups.
Methods
Participants
Participants included 138 healthy adults recruited via advertisements placed online or in local newspapers and magazines. Major inclusion criteria were: 18–45 years of age; BMI <40 kg/m2; and hemoglobin A1C ≤5.7%. Major exclusion criteria were: currently dieting; meeting DSM-IV-TR dependence criteria for substance use disorders;23 pregnant; lactating; on birth control; peri or postmenopausal; current psychiatric disorders; or major medical conditions. All participants gave written and oral consent. The Human Investigation Committee at the Yale University School of Medicine approved this study.
General Procedures
Potential participants completed an initial screening over the telephone or in person to determine eligibility. Following screening, eligible individuals met with study staff for an intake session to obtain informed consent and conduct baseline assessments, including height and weight. After the intake session, participants were scheduled for a session to conduct other baseline assessments, a physical examination, and an imagery script development session.
Three individualized scripts were created for stress, favorite food, and neutral cues based on previously validated methods and structured manualized procedures.17,24–27 Personalized imagery scripts were created in a structured clinical interview using scene development questionnaires that asked for details based on participants’ recent life events. The standard, manualized procedure has been used to provoke personal experiences and asks about stimulus contexts and physiological, subjective, experiential, and cognitive details relating to the situation.27 The stress imagery script was based on a personal event that made the participant “sad, mad, or upset, and with little control over the situation in the moment.” The situation was used if participants rated the perceived stress as an 8 or above on a scale of 1 (not at all stressful) to 10 (the most stress they felt in the past year). The favorite food imagery script was based on a situation that included participants’ favorite food-related stimuli and subsequent intake of those foods. The neutral-relaxing imagery script was based on the subject-identified non-physiologically arousing and non-food related relaxing situation. Data from the scene construction questionnaire were used to develop structured scripts of similar length across conditions (examples are provided in Supplement 1), and recorded onto an audiotape to be played in the laboratory sessions. Individuals also participated in a structured, guided progressive relaxation and mental imagery training in a 40-minute session prior to experimental sessions on Day 1, as outlined in the imagery script manual.27 If the participant met inclusion/exclusion criteria, he/she was scheduled for a 3-day laboratory experiment, run across consecutive days, where he/she was presented with the three personalized, 5-minute imagery conditions, one per day, in a randomized and counterbalanced order. Staff and participants were masked to presentation order.
Laboratory Sessions and Measures
Participants arrived at the Stress Center at noon and were provided a standard, healthy lunch at 12:30 pm and then refrained from further food and drink after this time. Laboratory sessions were initiated at 2:00 pm. On each testing day, participants were brought to the testing room at 2:00 pm. After settling into a sitting position on a relaxing armchair, participants had a 45-minute adaptation period during which they were instructed to practice progressive relaxation. Following the relaxation period, participants were provided with headphones and given the following instructions for the imagery procedures, “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”. The length of each script was approximately 5 minutes. Imagery vividness was rated after each script by asking participants to “Please rate how clearly you were able to imagine the scene” on scale ranging from “not clearly at all” (scored 0) to “extremely clear” (rated 10).
Food snack test (FST)
After each cue exposure, participants ate, ad libitum, from a multi-item tray of high-calorie snack foods (i.e., chips, cookies, popcorn, and pudding or brownies) and low-calorie snack foods (i.e., carrots and grapes) (see Figure 1 and 2 for timing of FST). The buffet included 3000 calories (500 cal bowls of each snack food). Participants were left alone in the room and were provided with the instructions: “There are 6 bowls of different snacks on the tray. We will leave these here for the next hour and you can eat as much or as little as you like during the next 30 minutes.” After that time, the food snack tray was removed and the amount of each snack consumed was measured. Each food was weighed before and after the session to determine the amount eaten and was used to calculate the caloric intake. The Cronbach’s alpha for the total calories was 0.90 across days and 0.89 for the high-calorie foods.
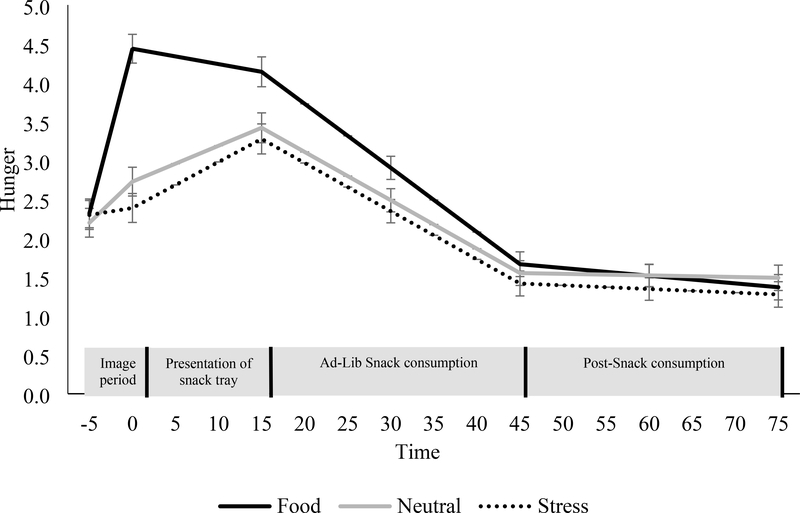
Figure 1. Changes in hunger for the food, stress, and neutral cues during the image period, presentation of the snack tray, food consumption, and post-consumption period.
Mean ± SE. Hunger was assessed using visual analog scales ranging from “not at all” (scored 0) to “extremely high” (scored 10). Analyses are adjusted for day of condition.
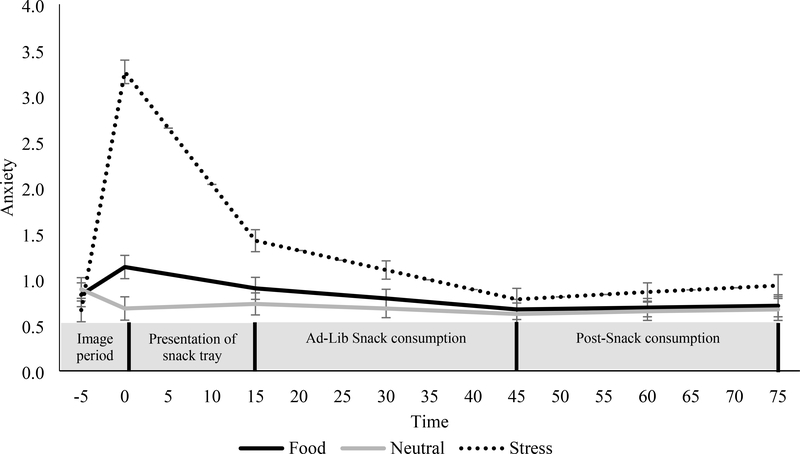
Figure 2. Changes in anxiety for the food, stress, and neutral cues during the image period, presentation of the snack tray, food consumption, and post-consumption period.
Mean ± SE. Anxiety was assessed using visual analog scales ranging from “not at all” (scored 0) to “extremely high” (scored 10). Analyses are adjusted for day of condition.
Hunger and anxiety
Measures for hunger and anxiety were collected at the same 7 time-points during each session: the baseline period/pre-imagery (−5 minutes); post-imagery/prior to the presentation of the snack tray (0 minutes); after presentation of the snack tray (15 minutes); and every 15 minutes during the 30-minute FST (30, 45) and post-FST (60, 75 minutes). Hunger and anxiety were assessed using visual analog scales ranging from 0 (not at all) to 10 (extremely high). Internal consistency, measured by coefficient alpha, was excellent for the scales: for the hunger and anxiety scales were 0.86 and 0.95, respectively, during the food cue condition; 0.89, and 0.88 in the stress condition; and 0.87 and 0.93 during the neutral condition.
Imagery Vividness Ratings
Vividness of imagery was assessed immediately following the imagery period, using a visual analog scale ranging from 0 (not at all) to 10 (extremely clearly). This was measured as a manipulation check to ensure successful and similar food, stress and neutral cue provocation. In previous work we have consistently reported high levels of internal consistency and reliability for stress and food cue ratings post-imagery (see 15, 22–25).
Statistical Analysis
Differences in demographics by weight status (i.e., normal weight=BMI<25.0 kg/m2, overweight=25.0 kg/m2≤BMI<30.0 kg/m2, or obese=BMI≤30 kg/m2) were compared using one-way ANOVAs and chi-square tests. We examined the effects of the brief exposure to food, stress, or neutral cues using a series of piecewise, linear mixed models. The primary eating outcome was the proportion of energy consumed from high-calorie foods (i.e., chocolate chip cookies, chocolate brownies, popcorn, and potato chips). We conducted exploratory analyses based on the total calories ingested and proportion of energy consumed from high-calorie sweets (i.e., chocolate chip cookies, chocolate brownies) as well as energy density (kcal/g). The within-subjects factors of imagery condition (stress, food cue, and neutral) and time-points were the fixed effects with day of condition included as a covariate. The primary comparisons were food versus neutral cue and stress versus neutral cue. BMI status (normal weight, overweight, or obese) was added to models to examine whether cue responses differed by weight status. Pearson product-moment correlation analyses were conducted to assess the relationship of pre-FST hunger and the total food intake consumed during the FST, proportion of energy from sweet foods, proportion of energy from high-calorie sweet foods, and energy density. Analyses were conducted using SPSS software (version 25). Statistical significance was considered as a two-tailed p<0.05.
Results
Participants
Participants (N=138) had a mean ± standard deviation (SD) age of 27.7 ± 6.7 years, and mean BMI of 27.0 ± 4.7 kg/m2 with 31.9% overweight and 29.7% obese. The sample was 52.2% female, and 42.8% white, 31.9% black and 25.3% other. Age, gender, and race did not differ significantly based on BMI class (ps=0.09, 0.23, and 0.23, respectively). Imagery vividness was rated similarly across imagery conditions (p=0.22). The mean±SD vividness rating for food, neutral, and stress imagery were 8.8±1.2, 8.5±1.5, and 8.5±1.7, respectively. Males consumed a significantly greater total number calories than females in the food cue (611.6±31.7 vs 367.0±31.0 kcal, p<0.001), stress (614.6±31.8 vs 369.9±31.0, p<0.001), and neutral conditions (610.4±31.6 vs 365.7±31.1, p<0.001). Males and females did not differ significantly in the proportion of high-calorie foods, energy from sweet foods, or energy-density of foods consumed.
Hunger
As shown in Figure 1, hunger scores increased significantly more after the food cue condition than the neutral and stress condition (ps<0.001). The stress condition produced less of an increase in hunger compared to the neutral condition (p=0.04). After the presentation of the FST food tray, hunger increased more in the neutral condition than in the food cue condition (p<0.001) but was no different than the stress condition (p=0.34). During the food consumption period, hunger declined more in the food cue condition than the neutral condition (p=0.003). The decline in the stress condition did not differ from the neutral condition. Within each condition, BMI status did not have a significant effect on changes in hunger during the imagery, presentation of the FST, or food consumption period.
Anxiety
Figure 2 demonstrates the increase in anxiety after the stress condition, which was significantly greater than the food cue and neutral conditions (ps<0.001). The increase in anxiety was significantly greater in the food cue than the neutral condition (p=0.002). After the FST presentation, levels of anxiety decreased in the stress condition, which was more than the food cue and neutral conditions (ps<0.001). During the food consumption period, anxiety declined more in the stress condition than in the neutral and food condition (p=0.001, 0.01, respectively). Within each condition, BMI status did not have a significant effect on changes in anxiety during the imagery, presentation of the FST, or food consumption period.
Food Consumption
The total calories consumed after the neutral stimuli did not differ significantly from the calories consumed after the food stimuli and stress stimuli (Table 1). The total weight of food consumed, energy density, and percent of energy from high-calorie foods and from sweet foods did not differ significantly between stimuli conditions (Table 1). Relative to the neutral condition, 48.6% of participants consumed more calories in the food cue condition and 50.7% of participants consumed more calories in the stress condition.
Table 1.
Estimated mean±SE food consumption by stimuli type
| P-Value |
||||||
|---|---|---|---|---|---|---|
| Food Cue | Stress | Neutral | Food Cue vs Neutral | Stress vs Neutral | Food Cue vs Stress | |
| Total energy intake (kcal) | 479.9±28.9 | 484.1±28.9 | 484.3±28.9 | 0.91 | 0.99 | 0.92 |
| Total food intake (g) | 213.2±11.1 | 210.9±11.0 | 215.8±11.1 | 0.87 | 0.76 | 0.88 |
| Proportion of energy from high-calorie foods (%) | 73.8±2.2 | 77.0±2.2 | 75.2±2.2 | 0.68 | 0.55 | 0.32 |
| Proportion of energy from sweet foods (%) | 64.5±2.2 | 63.6±2.2 | 60.9±2.2 | 0.24 | 0.38 | 0.77 |
| Proportion of energy from high-calorie, sweet foods (%) | 45.2±2.4 | 46.9±2.4 | 43.9±2.4 | 0.70 | 0.38 | 0.63 |
| Energy density (kcal/g) | 2.3±0.1 | 2.4±0.1 | 2.3±0.1 | 0.80 | 0.38 | 0.26 |
Note: Values shown are means ± SE adjusted for day of condition. For each variable, the three conditions were compared using pair-wise comparisons.
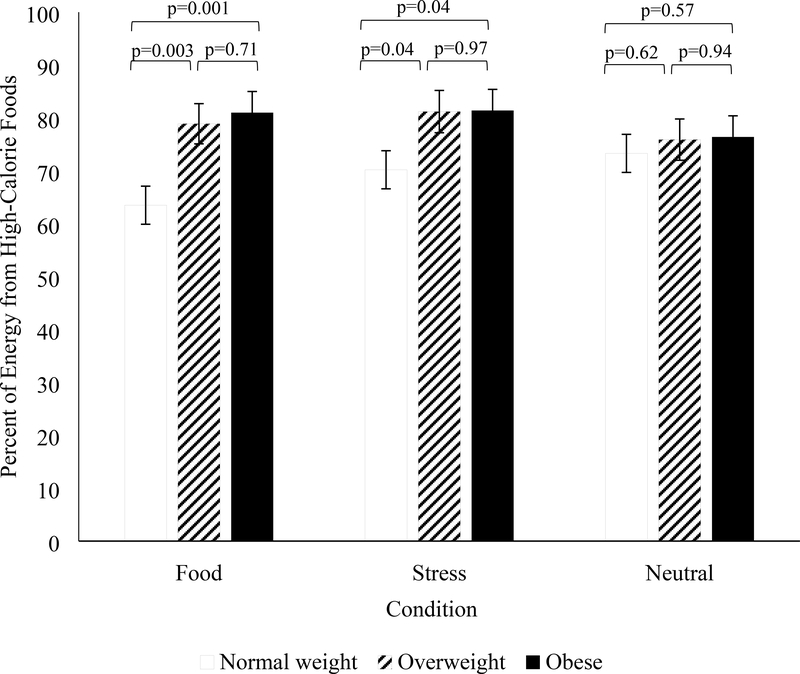
Figure 3 presents energy intake in the FST in each condition by BMI group. In response to the food cue condition, participants with normal weight consumed 63.5 ± 3.6% of energy from high-calorie foods, which was significantly less compared to participants with overweight (78.9 ± 3.8%; p=0.003) and those with obesity (81.0 ± 4.0%; p=0.001; Figure 3). The percent of energy consumed from high-calorie foods did not differ among participant who were overweight and those who were obese. After the stress condition, participants with normal weight consumed 70.2 ± 3.6% of energy from high-calorie foods, which was significantly less compared to participants with overweight (81.2 ± 4.0%; p=0.04) and those with obesity (81.4 ± 4.0%; p=0.04; Figure 3). Participants who were overweight did not differ from those who were obese. Percent of energy from high-calorie foods did not differ by BMI after the neutral cue.
Figure 3. Percent of energy from high-calorie foods for participants with normal weight, overweight, and obesity during each condition.
Mean ± SE. Analyses are adjusted for day of condition.
In the food cue condition, the energy density of the FST among participants with obesity was significantly greater than those with normal weight (Table 2). The energy density of the FST among those with overweight was not significantly different from individuals with normal weight or those with obesity. Participants with obesity consumed a higher percent of calories from high-calorie, sweet foods compared to those with normal weight. After the stress condition, the energy density of the FST was significantly higher among participants with obesity compared to those with normal weight. There were no significant differences between groups in the neutral conditions. The proportion of energy from high-calorie sweet foods did not differ by weight status in the stress or neutral conditions. Total calories and grams of food did not differ significantly by BMI class in the food, neutral, or stress stimuli. The proportion of participants who ate more, relative to the neutral condition, in the food or stress condition did not differ by BMI status.
Table 2.
Estimated mean±SE food consumption by stimuli type and weight status
| Food Cue | Stress | Neutral | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-Value | P-Value | P-Value | |||||||||||||||
| NW | OW | OB | NW vs OW | NW vs OB | OW vs OB | NW | OW | OB | NW vs OW | NW vs OB | OW vs OB | NW | OW | OB | NW vs OW | NW vs OB | OW vs OB |
| Total energy intake (kcal) | |||||||||||||||||
| 418.8±47.8 | 511.9±50.3 | 519.6±53.0 | 0.18 | 0.16 | 0.92 | 461.0±47.4 | 500.2±51.2 | 495.3±53.0 | 0.58 | 0.63 | 0.95 | 477.4±47.8 | 497.5±50.5 | 478.6±53.1 | 0.77 | 0.99 | 0.80 |
| Total food intake (g) | |||||||||||||||||
| 208.0±18.3 | 231.9±19.5 | 199.2±20.3 | 0.37 | 0.75 | 0.25 | 217.0±18.2 | 206.6±19.4 | 208.0±20.4 | 0.70 | 0.74 | 0.96 | 212.1±18.4 | 219.1±19.4 | 217.2±20.3 | 0.79 | 0.85 | 0.95 |
| Proportion of high-calorie foods (%) | |||||||||||||||||
| 63.5±3.6 | 78.9±3.8 | 81.0±4.0 | 0.003 | 0.001 | 0.71 | 70.2±3.6 | 81.2±4.0 | 81.4±4.0 | 0.04 | 0.04 | 0.97 | 73.3±3.6 | 75.9±3.9 | 76.4±4.0 | 0.62 | 0.57 | 0.94 |
| Proportion of energy from sweet foods (%) | |||||||||||||||||
| 62.4±3.5 | 63.2±3.7 | 68.7±3.9 | 0.87 | 0.23 | 0.31 | 64.5±3.5 | 64.4±3.7 | 61.8±3.9 | 0.98 | 0.61 | 0.64 | 67.5±3.5 | 53.6±3.7 | 60.7±3.9 | 0.01 | 0.20 | 0.19 |
| Proportion of energy from high-calorie, sweet foods (%) | |||||||||||||||||
| 37.2±4.0 | 47.6±4.2 | 52.5±4.4 | 0.07 | 0.01 | 0.42 | 44.0±4.0 | 49.2±4.2 | 48.0±4.4 | 0.37 | 0.50 | 0.85 | 47.7±4.0 | 38.2±4.2 | 45.3±4.4 | 0.10 | 0.68 | 0.25 |
| Energy density (kcal/g) | |||||||||||||||||
| 1.9±0.2 | 2.3±0.2 | 2.7±0.2 | 0.09 | 0.001 | 0.10 | 2.2±0.2 | 2.5±0.2 | 2.6±0.2 | 0.09 | 0.04 | 0.73 | 2.3±0.1 | 2.3±0.2 | 2.3±0.2 | 0.92 | 0.79 | 0.88 |
Note: NW=normal weight. OW=overweight. OB=obese. Values shown are means ± SE adjusted for day of condition. For each variable, the three conditions were compared using pair-wise comparisons.
Pre-Food Snack Test Hunger and Food Intake
In the total sample, pre-FST hunger scores were correlated with subsequent total energy intake in the food cue (p=0.002; Table 3), stress (p=0.01), and neutral condition (p=0.03). When stratified by BMI class, pre-FST hunger predicted food intake in participants with obesity (ps<0.05; Table 3) but not those with normal weight or those with overweight. Pre-FST hunger scores were not significantly correlated with proportion of energy from high calorie or sweet foods, proportion of energy intake from high-calorie sweet foods, or energy density in any of the conditions (Table 3). Secondary post-hoc analyses controlling for sex yielded similar statistical conclusions as above.
Table 3.
Correlations between pre-FST hunger and food intake in participants with normal weight, overweight, and obese
| Food Cue | Stress | Neutral | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | NW | OW | OB | Total | NW | OW | OB | Total | NW | OW | OB | |
| Total energy intake (kcal) | 0.27** | 0.25 | 0.20 | 0.37* | 0.25** | 0.10 | 0.10 | 0.50** | 0.20* | 0.22 | 0.05 | 0.33* |
| Total food intake (g) | 0.30** | 0.37** | 0.17 | 0.34* | 0.20* | 0.12 | 0.15 | 0.31 | 0.23** | 0.39** | 0.01 | 0.20 |
| Proportion of high-calorie foods (%) | 0.15 | 0.13 | 0.15 | 0.21 | 0.14 | 0.02 | 0.19 | 0.30 | 0.16 | 0.10 | 0.14 | 0.30 |
| Proportion of energy from sweet foods (%) | −0.09 | −0.13 | −0.14 | 0.03 | 0.08 | 0.04 | 0.08 | 0.12 | −0.04 | −0.22 | 0.001 | 0.21 |
| Proportion of energy from high-calorie, sweet foods (%) | 0.04 | 0.04 | −0.09 | 0.18 | 0.12 | 0.01 | 0.16 | 0.22 | 0.04 | −0.03 | 0.01 | 0.23 |
| Energy density (kcal/g) | 0.12 | 0.12 | 0.21 | 0.06 | 0.15 | 0.05 | 0.14 | 0.27 | 0.06 | −0.07 | 0.15 | 0.14 |
Note: NW=normal weight. OW=overweight. OB=obese.
p<0.05
p<0.01.
Discussion
This study is one of the first to compare different types of cues and how they influence hunger and food snack consumption in a Food Snack Test. Relative to the stress and neutral conditions, food cue exposure significantly increased hunger. Compared to the neutral condition, 48.6% of participants consumed more calories in the food cue condition. Similar to previous self-report studies that have found that 35–60% of people report eating more calories when stressed,28–30 we found that 50.7% consumed more calories in the stress condition relative to the neutral condition. Consistent with our hypothesis, among individuals with obesity, exposure to both the food cue and the stress conditions shifted consumption to greater high-calorie and energy-dense snack foods within a well-controlled experimental setting. Higher cue-induced hunger was associated with greater subsequent intake of total snack foods, particularly for participants with obesity. These results extend previous research by demonstrating that obesity is associated with increased reactivity to food cues and stress, which contributes to intake of high-calorie and energy-dense foods.19,21,22 Snack food consumption contributes 23% of daily dietary energy among adults in the US.31 Decreasing consumption of high-calorie snacks in response to food cues and stress may help to improve nutrition and weight among people with obesity.
Relative to the neutral condition, the food cue condition significantly increased subjective feelings of hunger. These data are consistent with prior findings that rewarding food stimuli increase motivation and wanting of food.17,32,33 Food cues may exert their effects on the experience of hunger through psychological processes such as conditioning and cue reactivity.33 Research also supports that physiological processes contribute to cue-motivated behaviors.33 For example, food cues can activate cephalic responses, signals initiated by the central nervous system to prepare the gastrointestinal tract to process the anticipated nutrients, which can result in feelings of hunger.32,34 Interventions that decrease food cue responsiveness or exposure may help to decrease perceptions of hunger such as mindfulness, environmental changes including limiting high-calorie foods in the home or keeping it out of sight, and policy initiatives to reduce advertisements for high-calorie foods.
Food and stress cue reactivity were more pronounced in those with obesity relative to those with normal weight. Compared to participants with normal weight, among individuals with obesity, food and stress cues each shifted preferences and choice of foods towards high-calorie and energy-dense food items. The percent of calories from high-calorie foods did not differ after the neutral cue across weight groups, demonstrating the specificity of the effects for food and stress cues on food snack intake. These findings are consistent with previous findings of differences in food-cue response between individuals with and without obesity.35 Findings from fMRI studies show that overweight and obese individuals, in comparison with normal-weight individuals, have greater responses to visual food cues, particularly in response to energy dense cues, in reward-related brain areas (e.g., insula and orbitofrontal cortex).35 Similar changes in palatable food intake have been reported in preclinical models during stress. When rodents are exposed to stress, they eat a greater portion of their daily calories from highly-palatable food.36,37 These findings are also consistent with previous research suggesting that stress may not involve greater consumption of food per se but selective consumption favoring palatable and high-calorie foods.10,29,38 External food cues can signal what to eat and how much of a food to consume, leading to alterations in consumption patterns. Collectively, the current study’s findings suggest that modifying one’s exposure and response to food cues and to stress might represent specific targets for interventions to decrease consumption of high-calorie foods among people with obesity.
Among individuals with obesity, the stress condition increased high-calorie food intake. However, there was no evidence that stress, relative to the neutral condition, resulted in increased feelings of hunger in individuals with obesity. This indicates that stress may have less influence on subjective feelings of hunger, and alternate physiologic and metabolic factors may be contributing to the stress-related increases in high-calorie food intake. It is also possible that individuals with obesity were using intake of high-calorie food helps to cope with negative feelings or to dampen stress following a stressor exposure.15 Our results, taken together with previous studies,15,39,40 indicate that individuals with obesity increase preference and consumption of high-calorie “comfort foods” possibly as a way to reduce the stress response and improve mood.
It is important to note that despite differences in feelings of hunger between the food cue and other conditions, and the shift towards higher consumption of energy dense foods during food cue and stress conditions in the obesity group, total caloric intake and the percent of energy from high-calorie foods were similar across conditions. While higher subjective hunger was significantly associated with greater total caloric intake across conditions, especially in the obesity group, we did not find evidence that stress altered food selection towards eating a greater proportion from high-calorie foods in the overall sample. These findings are contrary to previous studies using self-report measures7,38,41 and studies using laboratory measures in college aged females.42,43 It is possible that these differences are related to the type of cue exposures used. This study is unique because it used a highly salient cue that was personalized to each individual, whereas other studies have used standardized stimuli sets. Further study is needed to examine whether the salience of the food cue is an important determinant in driving cue-responsiveness across the weight spectrum. It is also possible that differences were due to variations in sample characteristics, particularly since we found that weight status was an important factor related to cue response.
This study is strengthened by the well-controlled laboratory design with a precise measure of food intake and standardized tasks, personalized to each individual, to assess cue reactivity. The sample was also racially diverse. Limitations of this study include that participants were relatively healthy without pre-diabetes or metabolic syndrome. Stress, food cues, and eating behaviors were measured in a laboratory setting and may differ from more naturalistic situations. Given the ubiquitous nature of food cues, a future direction would be to examine the effects of stress cues paired with and without food cues on appetite and eating behaviors. It is possible that the stress cue described was not conditioned with food intake, in particular for those with normal weight. Future research could ask participants to describe stress scenarios that were specifically linked to changes in food intake. Stress scenarios focused on specific, personalized stressful life events, and further study is needed to assess the effects of chronic stress. This study also focused on snack consumption and further research is needed to examine whether findings generalize to meals. This study looked at a single food snacking session each day. Additional research is needed to examine whether food cues and stress increase the frequency of snacks consumed. Future longitudinal studies are necessary to examine whether changes in food and stress cue responsivity contribute to long-term changes in weight.
Nonetheless, this laboratory-based study found that individuals with obesity consumed a greater proportion of calories from high-calorie foods relative to those of normal weight in response to food cues and stress. The present results are congruent with previous work and specifically identify greater vulnerability to food cues and stress in people with obesity, in that exposures to food cues and stress result in a shift in consumption to high-calorie and energy-dense foods in those with obesity. Results suggest that interventions that decrease cue reactivity to food and stress may help reduce intake of high-calorie foods among individuals with obesity.
Supplementary Material
Study Importance.
What is already known about this subject?
Exposures to energy-dense foods and stressful events are associated with self-reported consumption of high-calorie foods.
These findings, however, are limited by recall and other biases.
What are the new findings in your manuscript?
The present study assessed the effects of exposures to food cues and stress on hunger and food intake and examined whether cue responses differ by weight status.
The present study examined whether the effects of an experimental paradigm using brief imagined cue exposure to personal favorite food or to personal discrete stressful events separately and differentially increased hunger, anxiety, and food intake compared to neutral cues.
Among individuals with obesity, exposure to food and stress cues shifted consumption to high-calorie and energy-dense snack foods within a well-controlled experimental setting.
How might your results change the direction of research or the focus of clinical practice?
Findings suggest interventions focused on reducing cue reactivity to food and stress may help decrease intake of high-calorie foods among individuals with obesity.
Acknowledgments
Funding: This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease/NIH R01-DK099039 to RS. AMC was supported, in part, by the National Institute of Nursing Research/NIH under Award Number K23NR017209.
Disclosures: AMC reports grants and consulting fees from Shire Pharmaceuticals and Weight Watchers Inc., outside the submitted work. CMG reports consulting fees from Sunovion and Weight Watchers, and book royalties from Guilford Press and Taylor and Francis Publishers, outside the submitted work.
References
- 1.GBD Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. NEJM. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38(4):267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravussin E, Ryan DH. Three New Perspectives on the Perfect Storm: What’s Behind the Obesity Epidemic? Obesity. 2018;26(1):9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 5.Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87(6):532. [PubMed] [Google Scholar]
- 6.Herman CP, Polivy J, Lank CN, Heatherton TF. Anxiety, hunger, and eating behavior. J Abnorm Psychol. 1987;96(3):264. [DOI] [PubMed] [Google Scholar]
- 7.Ng DM, Jeffery RW. Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychology. 2003;22(6):638. [DOI] [PubMed] [Google Scholar]
- 8.McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosomatic Medicine. 1990. [DOI] [PubMed] [Google Scholar]
- 9.Barrington WE, Beresford SA, McGregor BA, White E. Perceived stress and eating behaviors by sex, obesity status, and stress vulnerability: findings from the vitamins and lifestyle (VITAL) study. Journal of the Academy of Nutrition and Dietetics. 2014;114(11):1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zellner DA, Loaiza S, Gonzalez Z, et al. Food selection changes under stress. Physiology & Behavior. 2006;87(4):789–793. [DOI] [PubMed] [Google Scholar]
- 11.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. Journal of Psychosomatic Research. 2000;48(2):195–202. [DOI] [PubMed] [Google Scholar]
- 12.Cleobury L, Tapper K. Reasons for eating ‘unhealthy’snacks in overweight and obese males and females. Journal of Human Nutrition and Dietetics. 2014;27(4):333–341. [DOI] [PubMed] [Google Scholar]
- 13.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta‐analytic review. Obesity Reviews. 2016;17(2):159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiology and Health. 2014;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macht M How emotions affect eating: a five-way model. Appetite. 2008;50(1):1–11. [DOI] [PubMed] [Google Scholar]
- 16.Geiker NRW, Astrup A, Hjorth MF, Sjödin A, Pijls L, Markus CR. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obesity Reviews. 2018;19(1):81–97. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R, Gu P, Hart R, Guarnaccia J. Food craving, cortisol and ghrelin responses in modeling highly palatable snack intake in the laboratory. Physiology & Behavior. 2019;208:112563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosomatic Medicine. 2000;62(6):853–865. [DOI] [PubMed] [Google Scholar]
- 19.Schachter S Obesity and eating. Science. 1968. [Google Scholar]
- 20.Cohen DA. Obesity and the built environment: changes in environmental cues cause energy imbalances. International Journal of Obesity. 2009;32(S7):S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferriday D, Brunstrom J. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. International Journal of Obesity. 2011;35(1):142. [DOI] [PubMed] [Google Scholar]
- 22.Castellanos EH, Charboneau E, Dietrich MS, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. International Journal of Obesity. 2009;33(9):1063. [DOI] [PubMed] [Google Scholar]
- 23.Association AP. Diagnostic criteria from dsM-iV-tr. American Psychiatric Pub; 2000. [Google Scholar]
- 24.Sinha R Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14(1):84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jastreboff AM, Potenza MN, Lacadie C, Hong KA, Sherwin RS, Sinha R. Body mass index, metabolic factors, and striatal activation during stressful and neutral-relaxing states: an FMRI study. Neuropsychopharmacology. 2011;36(3):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress-and food cue–induced food craving in obesity: association with insulin levels. Diabetes Care. 2013;36(2):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha R, Tuit K. Imagery script development procedures manual. Charleston, SC: CreateSpace; 2012. [Google Scholar]
- 28.Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Annals of the New York Academy of Sciences. 2004;1032(1):208–210. [DOI] [PubMed] [Google Scholar]
- 29.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiology & Behavior. 1999;66(3):511–515. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein SE, Shide DJ, Rolls BJ. Changes in food intake in response to stress in men and women: psychological factors. Appetite. 1997;28(1):7–18. [DOI] [PubMed] [Google Scholar]
- 31.Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. Journal of the Academy of Nutrition and Dietetics. 2015;115(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schüssler P, Kluge M, Yassouridis A, Dresler M, Uhr M, Steiger A. Ghrelin levels increase after pictures showing food. Obesity. 2012;20(6):1212–1217. [DOI] [PubMed] [Google Scholar]
- 33.Bilman E, van Kleef E, van Trijp H. External cues challenging the internal appetite control system—Overview and practical implications. Critical Reviews in Food Science and Nutrition. 2017;57(13):2825–2834. [DOI] [PubMed] [Google Scholar]
- 34.Smeets PA, Erkner A, De Graaf C. Cephalic phase responses and appetite. Nutrition Reviews. 2010;68(11):643–655. [DOI] [PubMed] [Google Scholar]
- 35.Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Frontiers in Nutrition. 2014;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Packard AE, Ghosal S, Herman JP, Woods SC, Ulrich-Lai YM. Chronic variable stress improves glucose tolerance in rats with sucrose-induced prediabetes. Psychoneuroendocrinology. 2014;47:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. [DOI] [PubMed] [Google Scholar]
- 38.Groesz LM, McCoy S, Carl J, et al. What is eating you? Stress and the drive to eat. Appetite. 2012;58(2):717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubé L, LeBel JL, Lu J. Affect asymmetry and comfort food consumption. Physiology & Behavior. 2005;86(4):559–567. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman HR, Wurtman JJ, Chew B. Changes in mood after carbohydrate consumption among obese individuals. The American Journal of Clinical Nutrition. 1986;44(6):772–778. [DOI] [PubMed] [Google Scholar]
- 41.Finch LE, Tomiyama AJ. Comfort eating, psychological stress, and depressive symptoms in young adult women. Appetite. 2015;95:239–244. [DOI] [PubMed] [Google Scholar]
- 42.Habhab S, Sheldon JP, Loeb RC. The relationship between stress, dietary restraint, and food preferences in women. Appetite. 2009;52(2):437–444. [DOI] [PubMed] [Google Scholar]
- 43.Royal JD, Kurtz JL. I ate what?! The effect of stress and dispositional eating style on food intake and behavioral awareness. Personality and Individual Differences. 2010;49(6):565–569. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.