Abstract
Inflammation associated with gram‐negative bacterial infections is often instigated by the bacterial cell wall component lipopolysaccharide (LPS). LPS‐induced inflammation and resulting life‐threatening sepsis are mediated by the two distinct LPS receptors TLR4 and caspase‐11 (caspase‐4/‐5 in humans). Whereas the regulation of TLR4 activation by extracellular and phago‐endosomal LPS has been studied in great detail, auxiliary host factors that specifically modulate recognition of cytosolic LPS by caspase‐11 are largely unknown. This study identifies autophagy‐related and dynamin‐related membrane remodeling proteins belonging to the family of Immunity‐related GTPases M clade (IRGM) as negative regulators of caspase‐11 activation in macrophages. Phagocytes lacking expression of mouse isoform Irgm2 aberrantly activate caspase‐11‐dependent inflammatory responses when exposed to extracellular LPS, bacterial outer membrane vesicles, or gram‐negative bacteria. Consequently, Irgm2‐deficient mice display increased susceptibility to caspase‐11‐mediated septic shock in vivo. This Irgm2 phenotype is partly reversed by the simultaneous genetic deletion of the two additional Irgm paralogs Irgm1 and Irgm3, indicating that dysregulated Irgm isoform expression disrupts intracellular LPS processing pathways that limit LPS availability for caspase‐11 activation.
Keywords: autophagy, caspase‐4, IRGM, lipopolysaccharide, noncanonical inflammasome
Subject Categories: Autophagy & Cell Death; Immunology; Microbiology, Virology & Host Pathogen Interaction
Complex regulatory networks calibrate inflammation during an infection. Membrane‐remodeling IRGM proteins act as rheostats attenuating cytosolic sensing of bacterial LPS and protecting against LPS‐induced sepsis.
Introduction
Severe sepsis is an overwhelming microbe‐induced inflammatory response resulting in organ dysfunction and constitutes a major public health concern due to high mortality rates and lack of treatment options (Polat et al, 2017). A chief sepsis‐inducing agent is lipopolysaccharide (LPS, or endotoxin), an abundant building block of the cell wall of all gram‐negative bacteria (Munford, 2008; Simpson & Trent, 2019). Extracellular and endocytosed LPS is sensed by the transmembrane receptor toll‐like receptor 4 (TLR4), which signals to induce the expression of pro‐inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and pro‐interleukin‐1 beta (pro‐IL‐1β) (Rosadini & Kagan, 2017). Cytosolic LPS on the other hand is sensed by the cysteine protease caspase‐11 (caspase‐4 and caspase‐5 in humans), which upon direct binding to LPS oligomerizes and proteolytically activates the cell death‐inducing protein gasdermin D (GSDMD) (Hagar et al, 2013; Kayagaki et al, 2013; Shi et al, 2014, 2015; Liu et al, 2016). GSDMD forms pores in the plasma membrane, causing pyroptosis directly as well as indirectly through potassium efflux‐mediated activation of the canonical NLRP3 inflammasome (Ruhl & Broz, 2015; Shi et al, 2015; Liu et al, 2016). Activated caspase‐1, the defining component of canonical inflammasomes, cleaves numerous substrates including pro‐IL‐1β to produce mature IL‐1β for secretion (Broz & Dixit, 2016; Brewer et al, 2019).
Whereas regulation of TLR4 signaling has been extensively studied, mechanisms that prevent excessive caspase‐11‐induced inflammation are just beginning to be understood (Rathinam et al, 2019). The host‐derived oxidized phospholipid oxPAPC as well as stearoyl lysophosphatidylcholine competitively block binding of LPS to caspase‐11 (Chu et al, 2018; Li et al, 2018). However, oxPAPC was also shown to function as a caspase‐11 agonist in dendritic cells and skews LPS‐primed macrophages toward pro‐inflammatory metabolism (Zanoni et al, 2016, 2017; Di Gioia et al, 2020). Additional known checkpoints on caspase‐11‐triggered inflammation operate downstream from cytosolic LPS sensing and involve inhibition of caspase oligomerization, reduction in membrane lipid peroxidation and membrane repair by the ESCRT III pathway (Kang et al, 2018; Ruhl et al, 2018; Choi et al, 2019).
Similar to the limited number of studies exploring regulatory mechanisms that operate downstream from cytosolic LPS sensing, investigators only recently began to characterize the upstream pathways that control LPS accessibility to the cytosolic compartment (Rathinam et al, 2019). Phagocytosis of bacterial LPS‐replete outer membrane vesicles (OMVs) triggers potent activation of caspase‐11 (Vanaja et al, 2016). The mechanism by which LPS derived from endocytosed OMVs escapes into the cytosol to be detected by caspase‐11 is not understood but depends on the function of interferon (IFN)‐inducible host guanylate binding proteins (Gbps) (Finethy et al, 2017). Gbps are additionally required for fast‐kinetics caspase‐11 activation following cell transfection with purified LPS (Pilla et al, 2014), implying a role for Gbps in caspase‐11 activation downstream from LPS release into the host cell cytosol. In support of this model, we recently demonstrated that human GBP1 binds directly to LPS and aggregates LPS in vitro (Kutsch et al, 2020), indicating a direct role for Gbps in cytosolic LPS recognition.
A second family of dynamin‐like proteins, the Immunity‐related GTPases (IRGs), bind to various intracellular membrane compartments, where they ostensibly modulate membrane dynamics (Pilla‐Moffett et al, 2016), including fusion of autophagosomes with lysosomes (Kumar et al, 2018). IRGs are produced in response to IFNs as well as other immune stimuli including LPS‐TLR4 signaling and mediate several immune programs in response to those agents (Coers, 2013; Pilla‐Moffett et al, 2016). Variants of the human IRG M clade encoding IRGM gene are associated with increased risk for Crohn's Disease, as first demonstrated by two large genome wide association studies (Parkes et al, 2007; Weersma et al, 2009). Subsequent candidate gene association studies expanded the association of human IRGM variants to increased susceptibility to mycobacterial infection (Intemann et al, 2009; King et al, 2011), and most pertinently, poor outcomes from sepsis (Kimura et al, 2014). While these studies linked the IRGM gene to inflammatory diseases, the cellular mechanism by which IRGM suppresses inflammation during infection and the causative genetic alteration in human IRGM disease‐associated alleles remain poorly defined.
The human genome encodes a single IRGM gene, which expresses at least 5 distinct hIRGM splice isoforms. The mouse genome on the other hand encodes 3 paralogous Irgm genes, named Irgm1, Irgm2, and Irgm3 (Bekpen et al, 2005). The exact functional relationship between hIRGM isoforms and their mouse Irgm orthologs is currently not well defined. However, several recent studies demonstrated that hIRGM and mouse Irgm1 commonly regulate cellular processes that include mitochondrial dynamics (Singh et al, 2010; Liu et al, 2013; Henry et al, 2014; Schmidt et al, 2017), autophagic flux (Gutierrez et al, 2004; Singh et al, 2006; Traver et al, 2011) and NLRP3 inflammasome activation (Mehto et al, 2019). The parallels between human and mouse IRGMs extend beyond these cell culture phenotypes: similar to hIRGM, Irgm1 attenuates intestinal inflammation (Liu et al, 2013; Rogala et al, 2018) and promotes host survival during LPS‐induced sepsis (Bafica et al, 2007). Increased mortality among endotoxemic Irgm1 −/− mice is largely due to hyperresponsive TLR4 signaling in these animals, which leads to augmented TNFα production and inflammation (Bafica et al, 2007; Schmidt et al, 2017). Whereas the anti‐inflammatory function of Irgm1 is now firmly established, possible immune‐modulatory functions of the two other Irgm paralogs have remained unexplored.
In this study, we systematically assessed the role of individual Irgm proteins in shaping endotoxemic inflammation and discovered a novel function for Irgm2 as an inhibitor of caspase‐11 activation following LPS ingestion by macrophages. Accordingly, Irgm2‐deficient mice display caspase‐11‐dependent elevated IL‐1β serum levels and more readily succumb to LPS‐induced sepsis. Unexpectedly, the simultaneous deletion of all Irgm paralogs partially restores immune homeostasis, thus revealing inter‐regulatory relationships among Irgm proteins important for balancing inflammation during gram‐negative infections.
Results
Irgm1 and Irgm2 but not Irgm3 limit the production of inflammatory cytokines in response to LPS in vivo
Mice deficient for Irgm1 and Irgm3 were previously reported (Taylor et al, 2000; Collazo et al, 2001). To systematically dissect the physiological function of all three mouse Irgm isoforms, we generated an Irgm2‐deficient mouse strain. Lack of Irgm2 protein expression was confirmed by Western blotting and immunofluorescence (Appendix Fig S1A and B). To profile the in vivo function of individual Irgm isoforms during endotoxemia, we injected wild‐type C57BL/6J (WT), Irgm1 −/− , Irgm2 −/−, and Irgm3 −/− mice with LPS at a concentration of 8 mg/kg bodyweight, collected serum at 4 h post‐injection (hpi), and performed multiplex quantitative serum cytokine measurements. Whereas the LPS‐induced cytokine profile of Irgm3 −/− mice was comparable to WT mice, serum concentrations of several cytokines were elevated in Irgm1 −/− and Irgm2 −/− relative to WT (Figs 1A and EV1). Consistent with the previously reported role for Irgm1 as an attenuator of TLR4 signaling (Bafica et al, 2007), we detected significantly elevated TNFα serum concentrations in LPS‐treated Irgm1 −/− mice. Notably, Irgm2 −/− mice exhibited WT‐like TNFα serum levels but increased serum levels for the inflammasome‐dependent cytokines IL‐1α and IL‐1β (Figs 1A and EV1). To validate our multiplex data and to further asses a potential role for Irgm2 in regulating inflammasome‐dependent cytokine production in vivo, we injected WT, Irgm1 −/−, Irgm2 −/−, and Casp1 −/− Casp11 −/− mice with LPS and assayed serum IL‐18, an additional inflammasome‐processed cytokine, as well as IL‐1β and TNFα concentrations via ELISA. We detected elevated concentrations of IL‐1β and IL‐18 but not of TNFα in the serum of LPS‐injected Irgm2 −/− mice by ELISA (Fig 1B), confirming our initial observations and thus implicating Irgm2 in the suppression of LPS‐induced inflammasome activation in vivo.
Figure 1. Irgm1 and Irgm2 but not Irgm3 limit the production of inflammatory cytokines in response to LPS in vivo .
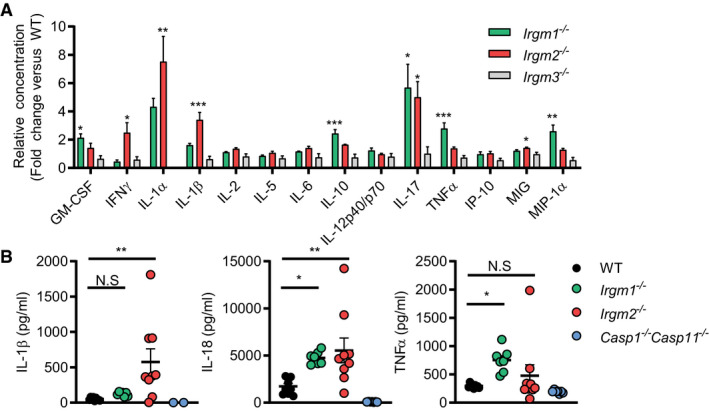
- WT, Irgm1 −/−, Irgm2 −/−, and Irgm3 −/− mice (n = 4 mice/genotype) were injected i.p. with LPS (8 mg/kg). Serum was collected 4 h post‐injection (hpi) and concentration of various cytokines determined via a preconfigured Luminex multiplex panel. Relative concentration (fold change relative mean of WT) is shown for the indicated cytokines (absolute cytokine concentrations of same experiment are shown in Fig EV1).
- WT (n = 9), Irgm1 −/− (n = 7), Irgm2 −/− (n = 9), and Casp1 −/− Casp11 −/− (n = 7) mice were injected i.p. with LPS (8 mg/kg). Serum was collected 4 hpi and concentration of IL‐1β, IL‐18, and TNFα was measured via ELISA.
Figure EV1. Absolute quantification of multiplex cytokine data of PBS‐ and LPS‐treated WT and Irgm‐deficient mouse strains.
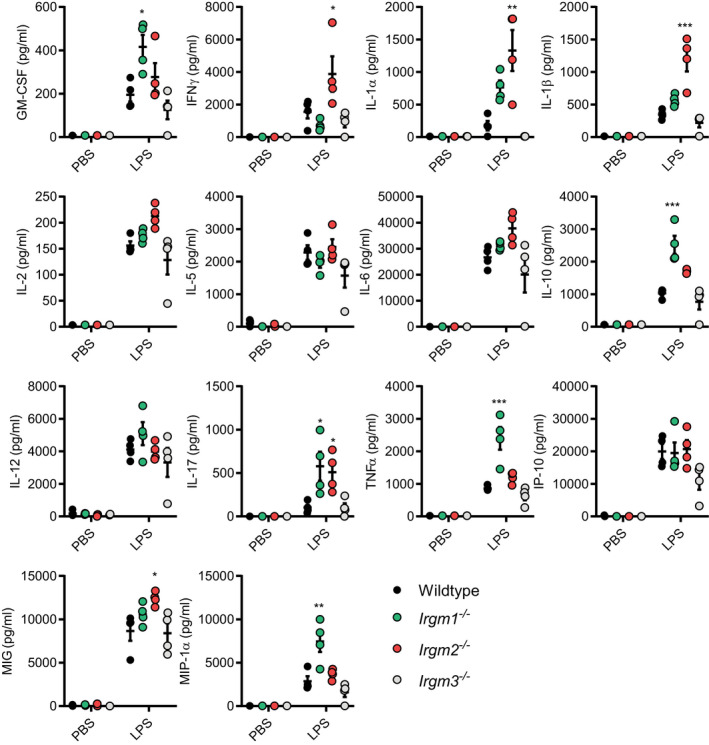
WT, Irgm1 −/−, Irgm2 −/−, and Irgm3 −/− mice were injected i.p. with LPS (8 mg/kg in PBS) or PBS alone. Serum was collected 4 hpi and concentration of indicated cytokines determined via Luminex platform (these data are shown normalized to WT mice in Fig 1A). n = 4 mice/genotype for all groups, except Irgm1 −/− + PBS where n = 3. Data information: Data shown are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 for comparison between WT and indicated genotype by one‐way ANOVA with Dunnett's multiple comparison test.
Irgm2 suppresses inflammasome activation in LPS‐treated macrophages
To define the mechanism by which Irgm2 regulates cytokine production, we employed bone marrow‐derived macrophages (BMMs) as an established tissue culture model to study LPS‐dependent IL‐1β secretion. Inflammasome activation in BMMs and consequential secretion of IL‐1β is dependent on two signals: the first signal is provided by extracellular damage‐ or pathogen‐associated molecular patterns (DAMPs or PAMPs), such as LPS, that induce the expression of inflammasome components and cytokine proforms; cytoplasmic PAMPs or DAMPs act as a second signal to trigger inflammasome assembly (Broz & Dixit, 2016). To provide the first signal, we primed WT, Irgm2 −/−, and Casp1 −/− Casp11 −/− BMMs with LPS. In all genotypes, LPS priming induced WT levels of pro‐IL‐1β and TNFα mRNA and also induced WT‐like TNFα secretion, indicating that TLR4 signaling is unchanged in Irgm2 −/− BMMs (Fig 2A and B). Unexpectedly, 24 h priming with extracellular LPS at 1 μg/ml was sufficient to induce robust secretion of IL‐1β and IL‐18 in Irgm2 −/− but not WT BMMs (Fig 2B). We considered the hypothesis that Irgm2 −/− BMMs no longer required a second signal and would therefore secrete IL‐1β in response to a broad range of first signals. However, we found that among several tested TLR agonists only LPS was sufficient to trigger IL‐1β and IL‐18 secretion in Irgm2 −/− BMMs (Fig 2C), suggesting that extracellular LPS, in the absence of Irgm2 expression, serves as both the first and second signal to activate inflammasomes.
Figure 2. Irgm2 suppresses inflammasome activation in LPS‐treated macrophages.
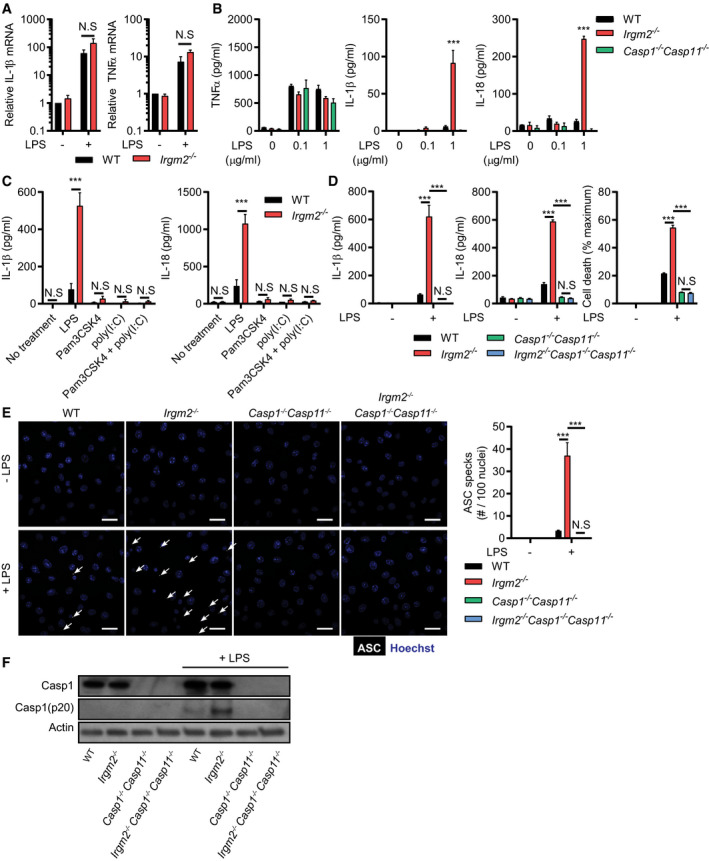
- qPCR measurement of IL‐1β and TNF‐α mRNA levels in WT and Irgm2 −/− BMMs following 8‐h stimulation with LPS (1 μg/ml).
- WT, Irgm2 −/−, and Casp1 −/− Casp11 −/− BMMs were treated for 24 h with LPS at indicated doses and supernatant TNFα, IL‐1β, and IL‐18 was measured by ELISA.
- IFNγ‐primed WT and Irgm2 −/− BMMs were treated with LPS, Pam3CSK4, poly(I:C), or a combination of Pam3CSK4 and poly(I:C) (1 μg/ml for all treatments) for 24 h and cell supernatant IL‐1β and IL‐18 concentrations were assessed by ELISA.
- WT, Irgm2 −/−, Casp1 −/− Casp11 −/−, and Irgm2 −/− Casp1 −/− Casp11 −/− BMMs were treated with LPS (1 μg/ml) and IL‐1β, IL‐18, and LDH release were assessed at 24 h post‐treatment (hpt).
- IFNγ‐primed WT, Irgm2 −/−, Casp1 −/− Casp11 −/− and Irgm2 −/− Casp1 −/− Casp11 −/− BMMs were treated with LPS (5 μg/ml) for 4 h and subsequently stained with anti‐ASC antibody and Hoechst stain (DNA/nuclei). Representative images are shown with white arrows pointing at ASC specks. Number of ASC specks per nuclei was quantified. Scale bars: 20 μm.
- IFNγ‐primed WT, Irgm2 −/−, Casp1 −/− Casp11 −/− and Irgm2 −/− Casp1 −/− Casp11 −/− BMMs were treated with LPS (1 μg/ml) for 24 h and cell lysates and supernatants collected. Protein levels in cell lysates (caspase‐1, and actin) and supernatants (caspase‐1 p20) were visualized via immunoblotting.
To further test the hypothesis that Irgm2 blocks inflammasome‐dependent cellular responses, we monitored pyroptotic cell death in LPS‐primed cells. In congruence with our cytokine data, LPS priming resulted in significantly higher rates of cell death in Irgm2 −/− compared with WT BMMs (Fig 2D). Ectopic expression of Irgm2 in Irgm2 −/− immortalized BMMs (iBMMs) restored cell viability to WT iBMM levels (Appendix Fig S2A and B), ruling out the possibility that the death phenotype is due to bystander mutations associated with the Irgm2 loss‐of‐function allele. Deletion of the inflammatory caspases, caspase‐1 and caspase‐11, restored cell viability and abolished IL‐1β/IL‐18 secretion in LPS‐primed Irgm2 −/− BMMs (Fig 2D), indicating that Irgm2 expression suppresses canonical inflammasome activation by extracellularly supplied LPS. To directly visualize canonical inflammasome formation in BMMs, we stained cells for the canonical inflammasome adapter protein Apoptosis‐associated Speck‐like protein containing a CARD (ASC). During canonical inflammasome assembly, ASC polymerizes into a large bioactive protein complex that is visible as a single large speck per individual cell (Broz & Dixit, 2016). Consistent with our predictions, loss of Irgm2 enhanced LPS‐induced ASC speck formation (Fig 2E) and increased the amount of autoproteolytically processed caspase‐1 p20 (Fig 2F). Collectively, these observations reveal a novel role for Irgm2 in mitigating inflammasome responses.
Irgm2 suppresses caspase‐11 activation in response to LPS and bacterial infections
Because LPS alone is sufficient to trigger inflammasome‐mediated cellular responses in Irgm2 −/− BMMs, we hypothesized that the IFN‐inducible cytosolic LPS sensor caspase‐11 was activated by extracellularly supplied LPS in cells deficient for Irgm2. In support of this hypothesis, we noticed that IFNγ priming accelerated the kinetics of pyroptotic cell death in Irgm2 −/− BMMs (Appendix Fig S3A and B). However, to test our hypothesis directly, we monitored inflammasome‐dependent responses in BMMs obtained from cross‐bred Irgm2 −/− Casp11 −/− mice. Deletion of caspase‐11 in Irgm2 −/− BMMs repressed ASC foci formation, restored cell viability, and abrogated LPS‐elicited IL‐1β/IL‐18 secretion (Fig 3A and B). Together, these data show that extracellularly supplied LPS is able to robustly activate the cytosolic LPS sensor caspase‐11 when cells lack Irgm2 expression.
Figure 3. Irgm2 suppresses caspase‐11 activation in response to LPS and bacterial infections.
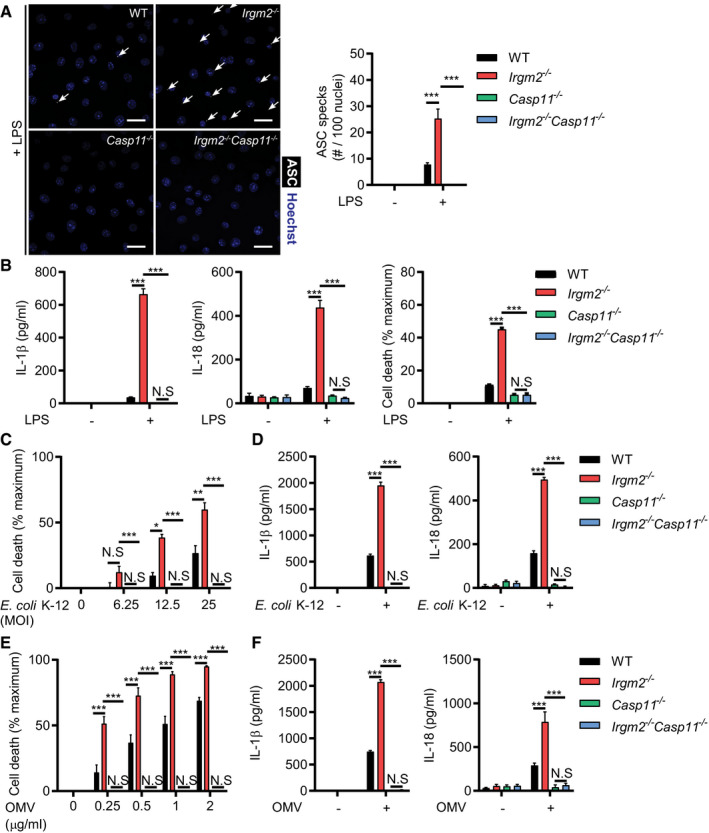
- IFNγ primed WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with LPS (5 μg/ml) for 4 h. Following treatment, cells were stained with anti‐ASC antibody and Hoechst (DNA/nuclei). Representative images of ASC specks (white arrows point at specks) are shown and number of ASC specks per nuclei quantified. Scale bars: 20 μm.
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with LPS (1 μg/ml). IL‐1β, IL‐18, and LDH release were assessed at 24 h post‐treatment (hpt).
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were infected with E. coli K‐12 at indicated MOIs and cell viability assessed via CellTiter‐Glo. Cell death was calculated as a function of relative viability to uninfected cells.
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were infected with E. coli K‐12 (MOI 25) and 24 hpi supernatant IL‐1β and IL‐18 levels were measured by ELISA.
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with OMVs at indicated concentrations for 24 h. Cell viability was assessed via CellTiter‐Glo, and cell death was calculated as a function of relative viability to untreated cells.
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with OMVs (1 μg/ml) and 24 hpt cell supernatant IL‐1β and IL‐18 levels were measured via ELISA.
To determine whether Irgm2‐mediated regulation of caspase‐11 was unique to purified LPS or extended to other caspase‐11‐activating stimuli, we tested whether Irgm2 could regulate the inflammatory response to bacterial infections. We confirmed our previous observations (Finethy et al, 2017) that nonvirulent Escherichia coli K12 induces caspase‐11 activation in BMMs in a dose‐dependent manner, and found that K12‐elicited responses were significantly exacerbated in Irgm2 −/− BMMs (Fig 3C and D). Similarly, we found that Irgm2 lessened caspase‐11 activation in BMMs infected with virulent E. coli species, including adherent invasive E. coli (AIEC) and uropathogenic E. coli (UPEC) (Fig EV2), demonstrating that Irgm2 attenuates caspase‐11 activation in response to both virulent and avirulent E. coli.
Figure EV2. Irgm2 suppresses caspase‐11 activation in response to pathogenic E. coli infections.
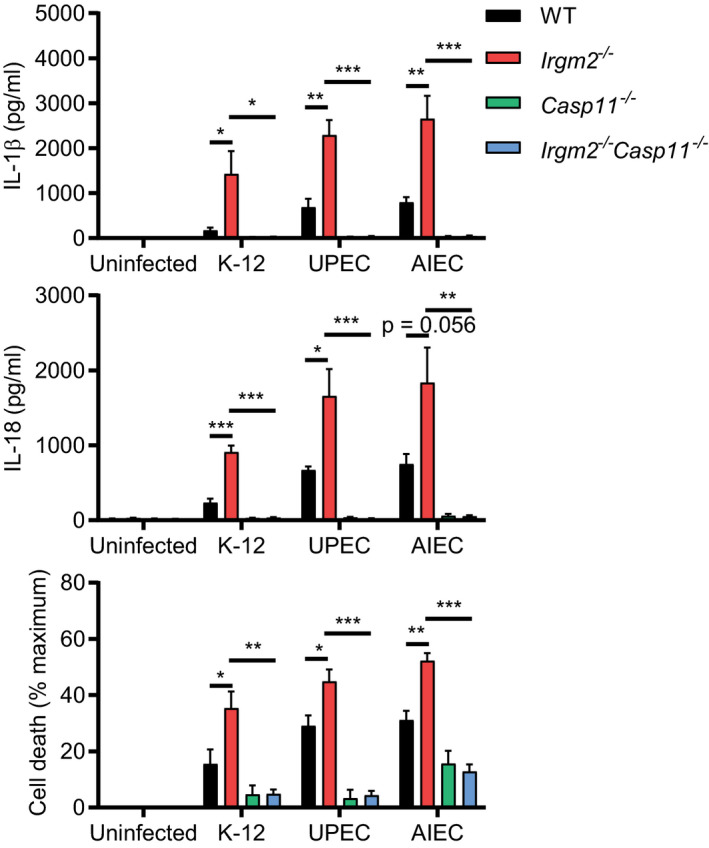
WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were infected with E. coli K‐12, E. coli UPEC, or E. coli AIEC (MOI 25) and IL‐1β, IL‐18, and LDH release were assessed at 24 hpi. Shown are means ± SEM from n = 3 (IL‐1β, IL‐18) or n = 4 (LDH release) independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 for indicated comparisons by one‐way ANOVA with Tukey's multiple comparisons test.
Irgm proteins regulate the subcellular localization and function of members of a second family of IFN‐inducible dynamin‐like GTPase, the Gbps (Traver et al, 2011; Haldar et al, 2013). Gbps colocalize with phagocytosed bacteria expressing virulence‐inducing protein secretion systems (Coers, 2017; Feeley et al, 2017), promote the bacteriolytic destruction of these targeted virulent bacteria, and thereby release PAMPs from lysed bacteria resulting in the activation of cytosolic pattern recognition receptors (Man et al, 2015; Meunier et al, 2015; Liu et al, 2018; Fisch et al, 2019). Although Gbps fail to colocalize with avirulent E. coli, Gbps still promote caspase‐11 activation in BMMs exposed to non‐pathogenic bacteria through a process that may be facilitated by the Gbp‐mediated recognition and processing of bacterially secreted OMVs (Vanaja et al, 2016; Finethy et al, 2017). We therefore asked whether Irgm2 could regulate the inflammatory response to OMVs. As with our infection experiments, we observed that loss of Irgm2 expression resulted in higher rates of cell death across a range of OMV concentrations (Fig 3E) and similarly amplified OMV‐stimulated IL‐1β and IL‐18 secretion (Fig 3F). These data thus define Irgm2 as an inhibitor of LPS‐, OMV‐, and infection‐induced caspase‐11 activation.
Irgm2 deficiency elevates caspase‐11 activity in the absence of chromosome‐3 encoded GBPs
In light of the functional interactions that exist between Gbp and Irgm proteins (Coers et al, 2018), we considered the hypothesis that Irgm2 could attenuate inflammasome activation by acting as a Gbp antagonist. We first monitored expression of Gbp2, a protein required for activation of both the noncanonical caspase‐11 and the canonical absent in melanoma 2 (AIM2) inflammasomes (Man et al, 2017; Gomes et al, 2019). Gbp2 and caspase‐11 were expressed normally in Irgm2 −/− BMMs (Fig 4A). To functionally test whether Irgm2 modulates Gbp2‐dependent activation of AIM2, we infected WT, Aim2 −/−, Gbp chr3−/− (lacking expression of Gbp1, Gbp2, Gbp3, Gbp5, and Gbp7 but still expressing Gbps encoded on chromosome 5), and Irgm2 −/− BMMs with the cytosol‐invading gram‐negative bacterium Francisella novicida. We observed that F. novicida induced AIM2‐mediated cell death in a Gbp‐dependent manner (Fig 4B), thus supporting previous findings that Gbp‐induced bacteriolysis liberates bacterial DNA for recognition by AIM2 (Man et al, 2015; Meunier et al, 2015). Whereas F. novicida‐induced pyroptosis was reduced in Gbp chr3−/− BMMs, cell death remained unchanged in Irgm2 −/− BMMs (Fig 4B), demonstrating that the absence of Irgm2 leaves Gbp‐dependent AIM2 activation undisturbed.
Figure 4. Irgm2 deficiency restores caspase‐11 activity in the absence of chromosome‐3 encoded GBPs.
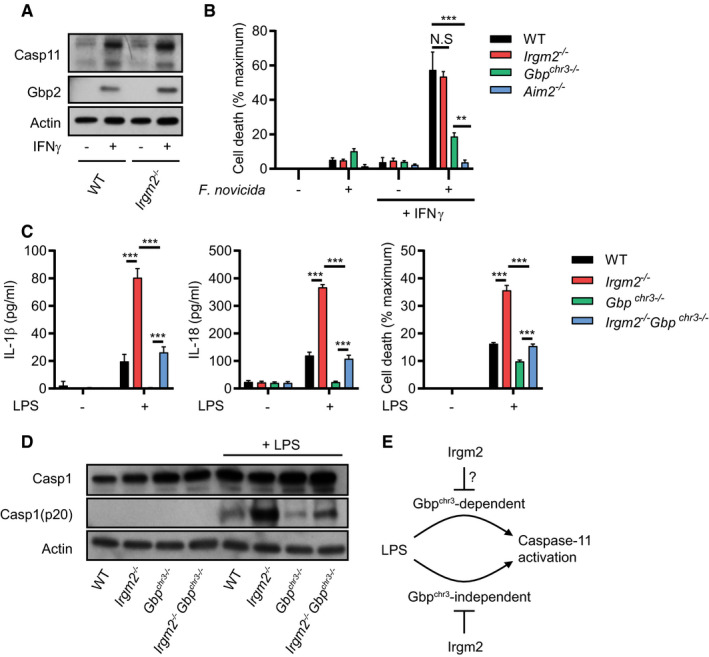
- WT and Irgm2 −/− BMMs were stimulated overnight with IFNγ or left untreated and cell lysates were collected. Lysates were assessed for Casp11, Gbp2, and actin protein levels via immunoblotting.
- IFNγ‐primed and unprimed WT, Irgm2 −/−, Gbp chr3−/− and Aim2 −/− BMMs were infected with Francisella novicida (MOI 10) and LDH release measured at 4 hpi.
- WT, Irgm2 −/−, Gbp chr3−/− and Irgm2 −/− Gbp chr3−/− BMMs were treated with LPS (1 μg/ml). IL‐1β, IL‐18, and LDH release were assessed at 24 hpt.
- IFNγ‐primed WT, Irgm2 −/−, Gbp chr3−/− and Irgm2 −/− Gbp chr3−/− BMMs were treated with LPS (1 μg/ml) for 24 h and cell lysates and supernatants collected. Protein levels in cell lysates (Caspase‐1 and actin) and supernatants (Caspase‐1 p20) were visualized via immunoblotting.
- Model depicting regulation of caspase‐11 activation by Irgm2 and Gbps.
We next considered the hypothesis that Irgm2 more narrowly regulates Gbp functions specifically tailored toward the activation of caspase‐11. To address this hypothesis, we monitored caspase‐11‐dependent activation in BMMs taken from cross‐bred Irgm2 −/− Gbp chr3−/− mice. We found that loss of Irgm2 on a Gbp chr3−/− background led to an increase in cell death, enhanced caspase‐1 processing and elevated IL‐1β/IL‐18 secretion (Fig 4C and D), albeit not to the same level as observed in Irgm2 −/− BMMs. While these data do not necessarily allow us to rule out a potential role for Irgm2 in modulating the GBPchr3‐dependent caspase‐11 pathway, we can conclude that Irgm2 suppresses an additional GBPchr3‐independent caspase‐11 activation pathway (Fig 4E).
Irgm2 interferes with caspase‐11‐dependent but not caspase‐11‐independent NLRP3 activation
Based on a previous report describing Gbp5 as a potential regulator of the NLRP3 inflammasome (Shenoy et al, 2012), we asked whether lack of Irgm2 expression directly impacted NLRP3 activation. We found that treatment of cells with the NLPR3 activator nigericin induced the secretion of equivalent amounts of IL‐1β and comparable levels of cell death in WT and Irgm2 −/− BMMs (Fig 5A). As expected, treatment of cells with the NLRP3 inhibitor MCC950 effectively blocked nigericin‐induced IL‐1β secretion and cell death (Fig 5B). MCC950 treatment of LPS‐activated Irgm2 −/− BMMs on the other hand only blocked IL‐1β secretion but failed to suppress pyroptosis (Fig 5C). Further arguing against a regulatory role for Irgm2 in canonical NLRP3 activation, we found levels of mitochondrial radical oxygen species, known activators of the NLRP3 inflammasome, to be comparable between Irgm2 −/− and WT BMMs under different experimental conditions (Fig EV3A). Overall, our data thus demonstrate that Irgm2 specifically interferes with caspase‐11 and associated NLRP3 activation triggered by LPS treatment.
Figure 5. Irgm2 interferes with caspase‐11‐dependent but not caspase‐11‐independent NLRP3 activation upstream of cytosolic LPS accessibility.
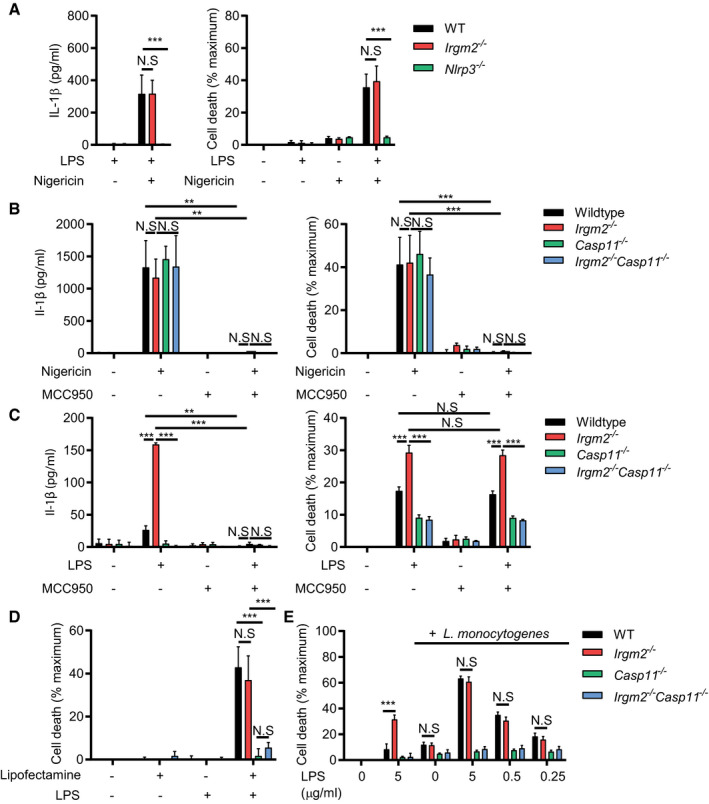
- WT, Irgm2 −/−, and Nlrp3 −/− BMMs were treated with LPS (0.1 μg/ml) for 3 h followed by nigericin for 1 h and IL‐1β/LDH release was measured.
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with LPS (0.1 μg/ml) for 3 h followed by nigericin and/or MCC950 for 1 h and IL‐1β/LDH release was measured.
- WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with LPS (1 μg/ml) and/or MCC950 for 24 h and IL‐1β/LDH release was measured.
- IFNγ‐primed WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were transfected with LPS using lipofectamine LTX and LDH release was measured at 2 hpt.
- IFNγ‐primed WT, Irgm2 −/−, Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were co‐treated with LPS (indicated doses) and Listeria monocytogenes (MOI 5) and LDH release measured at 4 hpt.
Figure EV3. Mitochondrial radical oxygen production and bulk LPS internalization remain unchanged in Irgm2‐deficient macrophages.
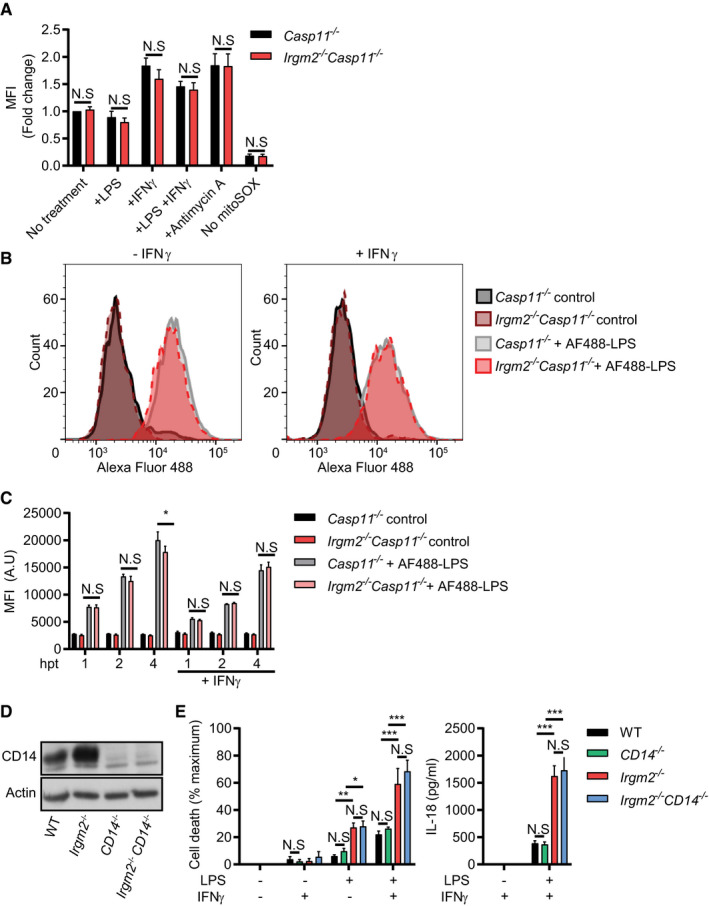
- IFNγ‐primed and unprimed Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with LPS (1 μg/ml) for 3 h, antimycin A for 30 min or were left untreated. Cells were then stained with mitoSOX red and fluorescence measured via flow cytometry. For each experiment, mean fluorescence intensity (MFI) was normalized to untreated Casp11 −/− BMMs.
- IFNγ‐primed and unprimed Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with Alexa Fluor 488‐conjugated LPS or unconjugated LPS (control) for 4 h and Alexa Fluor 488 cell fluorescence measured via flow cytometry. Representative flow cytometry data are depicted.
- IFNγ‐primed and unprimed Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs were treated with Alexa Fluor 488 conjugated LPS or unconjugated LPS (control) for 1, 2, or 4 h and fluorescence measured via flow cytometry. MFI (A.U) = Mean fluorescent intensity (arbitrary units).
- Lysates from WT, CD14 −/−, Irgm2 −/−, and Irgm2 −/− CD14 −/− BMMs were assessed for CD14 and actin protein levels via immunoblotting.
- IFNγ‐primed and unprimed WT, CD14 −/−, Irgm2 −/−, and Irgm2 −/− CD14 −/− BMMs were treated with LPS (1 μg/ml) for 24 h and LDH and IL‐18 release measured.
Direct cytosolic delivery of LPS overcomes Irgm2‐mediated suppression of caspase‐11 activation
We next asked whether Irgm2 could exert its anti‐inflammatory effect simply through the inhibition of LPS ingestion. We therefore monitored the uptake of fluorescently labeled LPS in both unprimed and IFNγ‐primed Casp11 −/− and Irgm2 −/− Casp11 −/− BMMs, choosing caspase‐11‐deficient cells to avoid compounding effects resulting from pyroptotic cell death. We found that lack of Irgm2 expression failed to increase LPS ingestion (Fig EV3B and C). However, deletion of an important mediator of LPS uptake, the glycosylphosphatidylinositol (GPI)‐anchored LPS‐binding protein CD14, did not reduce cell death or IL‐18 secretion in LPS‐treated Irgm2 −/− BMMs (Fig EV3D and E), Because LPS bound to hepatocyte‐secreted high mobility group box 1 (HMGB1) protein is endocytosed by macrophages via the receptor for advanced glycation end products (RAGE) and subsequently released into the host cell cytosol through destabilization of LPS‐containing endosomes (Deng et al, 2018), we also tested whether HMGB1/RAGE expression as well as signaling were altered in Irgm2 −/− BMMs. However, we found protein expression of HMGB1 and RAGE as well as RAGE agonist‐induced TNFα secretion to be comparable between WT and Irgm2 −/− BMMs (Appendix Fig S4A and B). Furthermore, the presence of Irgm2‐deficient BMMs did not increase the rate of LPS‐induced cell death in WT cells during co‐culture (Appendix Fig S4C), ruling out increased secretion of HMGB1 as the mechanisms underlying the enhanced LPS responsiveness of Irgm2 −/− BMMs. Together, these data suggest that a CD14‐ and HMGB1‐independent pathway delivers LPS for caspase‐11 sensing in cultured Irgm2 −/− BMMs.
Having established that Irgm2 −/− BMMs ingest LPS at levels comparable to WT BMMs, we next investigated whether Irgm2 was directly interfering with the recognition of cytosolic LPS by caspase‐11. To do so, we transfected WT, Irgm2 −/−, Casp11 −/−, and Irgm2 −/− Casp11 −/− BMMs with LPS and measured pyroptotic death. As expected, caspase‐11‐deficient BMMs were resistant to LPS‐mediated cell death, whereas WT BMMs succumbed to cytosolically delivered LPS (Fig 5D). Remarkably, we found that cell death was equivalent between LPS‐transfected WT and Irgm2 −/− BMMs (Fig 5D). To further substantiate these findings, we deposited LPS into the host cell cytosol via co‐delivery with the vacuole‐disrupting gram‐positive bacterium Listeria monocytogenes. Using this alternative approach, we again found that WT and Irgm2 −/− BMMs were equally susceptible to LPS‐triggered pyroptotic cell death across a 1‐log range of LPS concentrations (Fig 5E). These data showed that cytosolic delivery of LPS circumnavigates the inhibitory function Irgm2 exerts on caspase‐11.
Atg‐deficient macrophages undergo NLRP3‐independent pyroptosis in response to extracellular LPS
IRGM proteins control membrane fusion events through direct interactions with members of the autophagy‐related protein 8 (Atg8) family (Kumar et al, 2018) and Atg‐deficient macrophages were previously shown to secrete elevated levels of IL‐1β in response to LPS treatment (Saitoh et al, 2008; Harris et al, 2011). In agreement with these studies, we found that LPS‐treated autophagy‐deficient Atg5 −/− and Atg14 −/− BMMs secreted elevated amounts of IL‐1β and IL‐18, formed ASC specks more frequently and underwent cell death more readily when compared to WT BMMs, thus resembling the response we observed in Irgm2 −/− BMMs (Fig EV4A and B). Treatment of Atg5 −/− BMMs with the NLRP3 inhibitor MCC950 blocked LPS‐induced IL‐1β secretion but not pyroptotic cell death (Fig EV4C), thus paralleling our observations made in Irgm2 −/− BMMs. To address whether enhanced LPS accessibility was also primarily responsible for the LPS hypersusceptibility phenotype of Atg‐deficient BMMs, we transfected these cells with LPS and monitored cell death. We found that LPS transfection induced comparable levels of cell death in Atg5 −/−, Atg14 −/−, Irgm2 −/−, and WT BMMs (Fig EV4D), suggesting that Atg5, Atg14, and Irgm2 are likewise involved in blocking LPS accessibility to the cytosolic sensor caspase‐11 rather than the direct regulation of caspase‐11 function itself.
Figure EV4. Autophagy‐related proteins control caspase‐4 activation upstream from cytosolic LPS sensing.
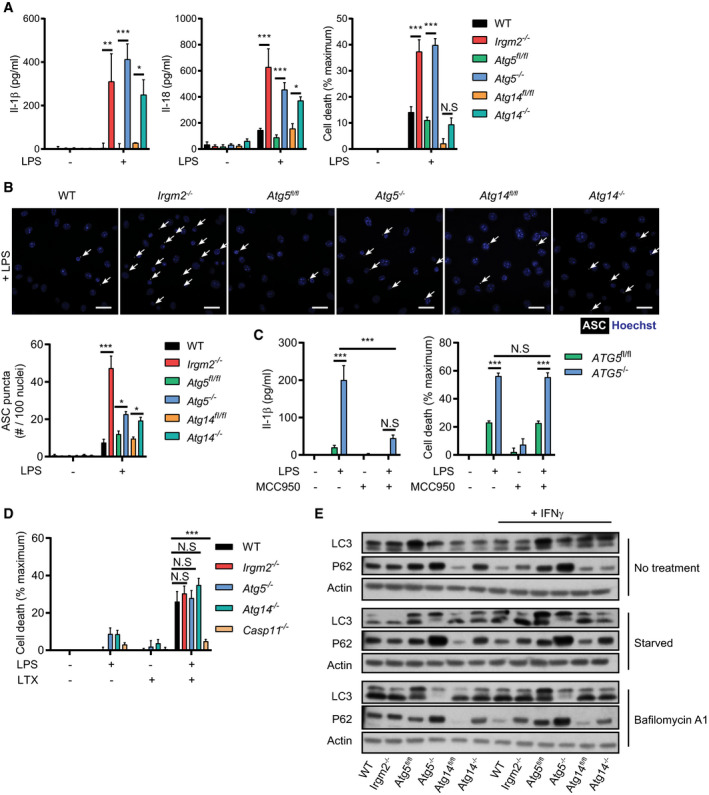
- WT, Irgm2 −/−, Atg5 fl/fl, LysMCre‐Atg5 f/f (Atg5 −/−), Atg14 fl/fl and LysMCre‐Atg14 f/f (Atg14 −/−) BMMs were treated with LPS (1 μg/ml) and IL‐1β, IL‐18, and LDH release were assessed at 24 hpt (n = 4 independent experiments for IL‐1β, IL‐18 and n = 7 independent experiments for LDH release).
- IFNγ‐primed BMMs of the indicated genotypes were treated with LPS (5 μg/ml) for 4 h and subsequently stained with anti‐ASC antibody and Hoechst stain (DNA/nuclei). Representative images are shown with white arrows pointing at ASC specks. Number of ASC specks per nuclei was quantified. Scale bars: 20 μm. (n = 3 independent experiments, >200 nuclei counted for each condition/replicate)
- Atg5 fl/f and LysMCre‐Atg5 f/f (Atg5 −/−) BMMs were treated with LPS (1 μg/ml) and/or MCC950 for 24 h and IL‐1β/LDH release was measured (n = 3 independent experiments).
- IFNγ‐primed BMMs of the indicated genotypes were transfected with LPS using lipofectamine LTX and LDH release measured 2 hpt (n = 5 independent experiments).
- IFNγ‐primed BMMs of the indicated genotypes were stimulated overnight with IFNγ or left untreated. Cells were then starved for 2 h in HBSS or were treated with Bafilomycin A1 (100 nM), and cell lysates were collected. Lysates were assessed for LC3, p62, and actin protein levels via immunoblotting. Image is representative of n = 3 independent experiments.
Because Atg5 −/−, Atg14 −/−, and Irgm2 −/− BMMs display comparable phenotypes, we asked whether the observed LPS sensitization of Irgm2 −/− BMMs could be the result of a defect in canonical autophagy. To test this hypothesis, we monitored LC3 lipidation and autophagic flux‐dependent degradation of the ubiquitin‐binding adapter protein p62 in WT, Irgm2‐, Atg5‐, and Atg14‐deficient BMMs. LC3 lipidation and p62 degradation were comparable in WT and Irgm2 −/− BMMs, essentially excluding a prominent role for Irgm2 in modulating canonical autophagy (Fig EV4E), and instead suggesting a possible role for Irgm2 in a noncanonical autophagy‐related pathway. This model is further supported by findings reported in the accompanying manuscript by Meunier and colleagues (Eren et al, 2020), which shows that Irgm2 interacts with the Atg8 protein Gabarapl2 to attenuate caspase‐11 activation.
Irgm2 protects against caspase‐11‐driven septic shock during endotoxemia
Because cytosolic sensing of LPS by caspase‐11 promotes septic shock in a mouse model (Hagar et al, 2013; Kayagaki et al, 2013), we hypothesized that loss of Irgm2 would increase susceptibility to an LPS challenge in vivo. To test our hypothesis, WT, Irgm2 −/−, Casp11 −/−, and Irgm2 −/− Casp11 −/− mice were intraperitoneally injected with 8 mg/kg bodyweight LPS and monitored for 48 h. As predicted, Irgm2 −/− mice displayed decreased survival rates relative to WT mice and loss of caspase‐11 restored survival in Irgm2‐deficient and WT animals alike (Fig 6A). Similarly, deletion of caspase‐11 dramatically reduced IL‐1β and IL‐18 serum levels in LPS‐challenged Irgm2 −/− mice (Fig 6B). These findings demonstrate that Irgm2 blocks caspase‐11‐dependent inflammatory responses initiated by circulating serum LPS.
Figure 6. Irgm2 protects against caspase‐11‐driven septic shock during endotoxemia.
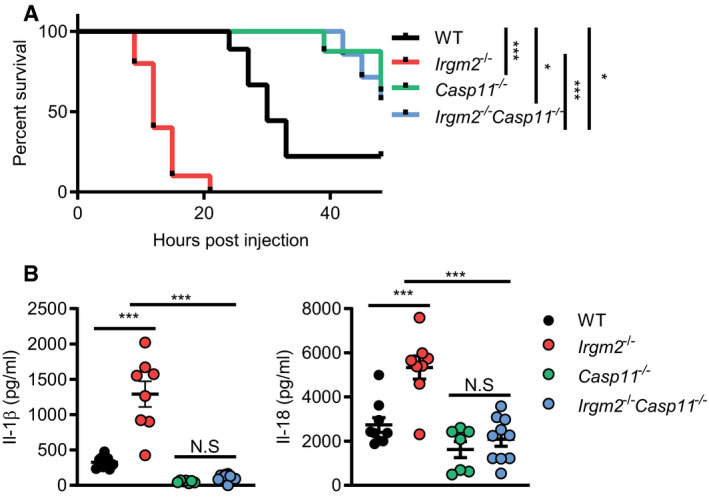
- WT (n = 9), Irgm2 −/− (n = 10), Casp11 −/− (n = 7), and Irgm2 −/− Casp11 −/− (n = 8) mice were injected i.p. with LPS (8 mg/kg). Morbidity and mortality were observed for 48 h at 3‐h intervals.
- WT (n = 9), Irgm2 −/− (n = 8), Casp11 −/− (n = 7), and Irgm2 −/− Casp11 −/− (n = 10) mice were injected i.p. with LPS (8 mg/kg). Serum was collected 4 hpi and concentration of IL‐1β, and IL‐18 was measured by ELISA. Data shown are from 2 pooled experiments.
Immune homeostasis is partially restored in mice deficient for all Irgm paralogs
We previously provided evidence in favor of a model in which distinct Irgm isoforms have antagonistic relationships with each other. For example, we showed that T cell homeostasis disrupted in Irgm1 −/− animals was restored through the simultaneous deletion of Irgm3, a second Irgm family member (Feng et al, 2008; Henry et al, 2009; Coers et al, 2011). To determine whether the disturbance of these inter‐regulatory relationships among Irgm proteins could contribute to the elevated inflammatory state of LPS‐treated Irgm2 −/− cells and mice, we generated a novel mouse strain deficient for the expression of all 3 Irgm isoforms, henceforth referred to as the panIrgm −/− strain (Fig EV5A). We found that lack of Irgm1 and Irgm3 expression in panIrgm −/− BMMs substantially moderated the inflammatory response of Irgm2‐deficient macrophages exposed to extracellular LPS or E. coli: specifically, we observed that panIrgm −/− BMMs secreted significantly less IL‐1β and were more resistant to pyroptotic cell death than Irgm2 −/− BMMs (Fig 7A and B). Nonetheless, panIrgm −/− BMMs remained more responsive to LPS and E. coli than WT BMMs under IFNγ‐primed conditions (Fig 7A and B). Restoring Irgm1 expression in BMMs obtained from a panIrgm −/−‐derived Irgm2 −/− Irgm3 −/− recombinant backcross (Fig EV5A) again reverted the panIrgm −/− phenotype and restored elevated LPS‐induced cell death and IL‐1β secretion, as seen in Irgm2 −/− BMMs (Fig 7A and B). Cell death and IL‐1β secretion in Irgm3 −/− BMMs was comparable to WT (Fig 7A and B). Together, these observations indicate that the expression of endogenous Irgm1 is required for the hyperinflammatory state of Irgm2‐deficient macrophages.
Figure EV5. PanIrgm −/− mice lacking expression of Irgm1/m2/m3 display improved viability during endotoxemia compared with Irgm2 −/− mice.
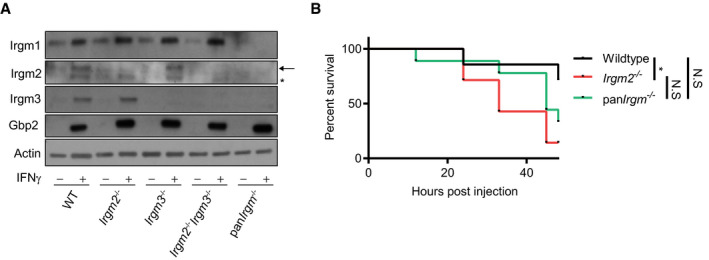
- WT, Irgm2 −/−, Irgm3 −/−, Irgm2 −/− Irgm3 −/−, and panIrgm −/− BMMs were stimulated overnight with IFNγ or left untreated and cell lysates were collected. Lysates were assessed for Irgm1, Irgm2, Irgm3, Gbp2, and actin protein levels via immunoblotting (arrow = band of interest, * = nonspecific band).
- WT (n = 7), Irgm2 −/− (n = 7), and panIrgm −/− (n = 9) mice were injected i.p. with LPS (2 mg/kg bodyweight). Morbidity and mortality were observed for 48 h at 3 h intervals. *P < 0.05, for indicated comparisons by log‐rank Mantel‐Cox test. N.S, non‐significant.
Source data are available online for this figure.
Figure 7. Restoring expression of endogenous Irgm1 is sufficient to hypersensitize panIrgm −/−, macrophages for caspase‐11 activation.
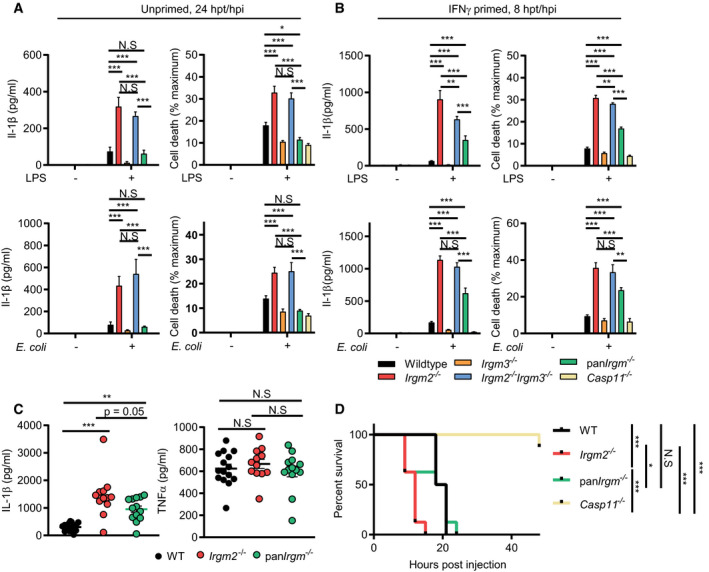
- WT, Irgm2 −/−, Irgm3 −/−, Irgm2 −/− Irgm3 −/−, panIrgm −/−, and Casp11 −/− BMMs were treated with LPS (1 μg/ml) or infected with E. coli K‐12 (MOI 25) and IL‐1β, and LDH release were assessed at 24 hpt (n = 3 independent experiments).
- IFNγ‐primed WT, Irgm2 −/−, Irgm3 −/−, Irgm2 −/− Irgm3 −/−, panIrgm −/−, and Casp11 −/− BMMs were treated with LPS (1 μg/ml) or infected with E. coli K‐12 (MOI 25) and IL‐1β, and LDH release were assessed at 8 hpt/hpi (n = 3 independent experiments).
- WT (n = 15), Irgm2 −/− (n = 12), and panIrgm −/− (n = 13) mice were injected i.p. with LPS (8 mg/kg). Serum was collected 4 hpi and concentration of IL‐1β and TNFα was measured via ELISA. Data shown are from 3 pooled experiments.
- WT, Irgm2 −/−, panIrgm −/−, and Casp11 −/− mice (n = 8 mice/genotype) were injected i.p. with LPS (8 mg/kg body weight). Morbidity and mortality were observed for 48 h at 3‐h intervals.
Lastly and in agreement with our cell culture observations, we found that deletion of Irgm1 and Irgm3 partially reversed the caspase‐11‐driven hyperinflammatory response of Irgm2‐deficient animals, as evidenced by the intermediate IL‐1β serum levels of endotoxemic panIrgm −/− relative to WT and Irgm2 −/− mice (Fig 7C). TNFα serum levels were comparable across the different genotypes tested (Fig 7C), indicating that deletion of all Irgm isoforms not only partially normalized the sensitized caspase‐11 pathway of Irgm2 −/− mice but also tempered the excessive TLR4 signaling pathway of Irgm1 −/− mice (Bafica et al, 2007; Schmidt et al, 2017). Lastly, we found that the deletion of all Irgm genes increased survival of endotoxemic mice to WT or near WT levels (Figs 7D and EV5B), further supporting a model, according to which dysregulated Irgm isoform expression rather than merely Irgm2 loss‐of‐function drives the pathological inflammation in endotoxemic Irgm2 −/− mice.
Discussion
Infections elicit robust inflammatory responses, which benefit the host by boosting protective antimicrobial immunity. However, dysregulated or overly robust inflammation can cause substantial host morbidity and mortality. Host organisms including humans thus evolved complex regulatory mechanisms to calibrate inflammation so that infections can be contained while collateral damage to infected tissue is minimized (Casadevall & Pirofski, 1999). Here, we define a novel pathway responsible for regulating and minimizing inflammation elicited by the cytosolic LPS sensor caspase‐11. Our studies characterize mouse Irgm proteins as novel rheostats calibrating the sensitivity of cells and whole animals to LPS. We demonstrate that deletion of Irgm2 leads to excessive activation of the noncanonical caspase‐11 inflammasome pathway. We further show that simultaneous deletion of all Irgm paralogs largely restores immune homeostasis in Irgm2‐deficient animals, thus indicating that the disturbance of the Irgm expression network is largely responsible for LPS hyperresponsiveness and inflammation in these animals.
While caspase‐11 is known to sense LPS in the cytosol, how LPS becomes accessible to its cytosolic sensor is not well understood. The majority of “free” LPS is internalized via receptor‐mediated endocytosis or macropinocytosis and then trafficked to the lysosomal compartment for detoxification (Kitchens et al, 1998; Poussin et al, 1998; Deng et al, 2018). How LPS exits membrane‐bound compartments has remained mostly unstudied owing in part to the observation that when WT macrophages are exposed to extracellular LPS in cell culture, only minimal caspase‐11 activation is detectable (Hagar et al, 2013; Kayagaki et al, 2013). Here, we demonstrate that extracellular LPS can potently activate the inflammasome in murine macrophages deficient for Irgm2.
There are a variety of nodes in the LPS uptake and processing pathway, which could potentially be modulated by Irgm2 including (i) lysosomal LPS detoxification, (ii) LPS internalization, and (iii) LPS accessibility. The lysosomal enzyme acyloxyacyl hydrolase (AOAH) deacylates LPS and thereby inactivates LPS as a caspase‐11 and TLR4 agonist. Consequently, deletion of AOAH results in prolonged reactions to LPS in vivo (Lu et al, 2008, 2013). However, AOAH operates with slow kinetics and inactivates only approximately 25% of LPS over a 12‐h period (Ojogun et al, 2009). We therefore predict that even a complete loss of AOAH could only have a minor effect on caspase‐11 activation in the shorter time courses used in this study. A more likely model would predict a role for Irgm2 in LPS internalization. However, arguing against this model, we found that bulk LPS uptake by macrophages was not noticeably changed in Irgm2‐deficient macrophages. As an alternative model, we propose that Irgm2 controls cytosolic accessibility of LPS. As far as we know, no mammalian LPS transmembrane transporter exists. However, a recent report demonstrated that hepatocyte‐secreted HMGB1 binds to circulating serum LPS, promotes LPS uptake into lysosomes and facilitates LPS delivery from lysosomes into the host cell cytosol (Deng et al, 2018). Based in part on the observation that amphipathic HMGB1 can destabilize liposomes at an acidic pH in vitro, it was proposed that HMGB1 disrupts lysosomal membranes and thereby promotes cytosolic leakage of LPS (Deng et al, 2018). In addition to HMGB1 it is also conceivable that amphipathic LPS itself or other host‐derived molecules such as Irgm1 could induce moderate vacuolar destabilization of LPS‐containing compartments. We predict that Irgm2 minimizes membrane damage of LPS‐containing vacuoles and thereby blocks LPS accessibility for caspase‐11. The expression of Irgm2 could promote vacuolar stability through a number of potential mechanisms such as repairing damaged endosomes or sorting LPS‐containing endosomes away from rupture‐prone compartments. Additional studies are necessary to define if and how Irgm2 regulates each of these processes.
While our results reveal a novel function for mouse Irgm2 in controlling caspase‐11 activation, it is unclear whether Irgm2 shares this function with human IRGM. Prior studies of mouse Irgm proteins almost exclusively focused on Irgm1‐deficient mice and revealed shared functions of mouse Irgm1 and human IRGM, especially regarding the regulation of mitochondrial dynamics (Coers et al, 2018). Mitochondrial dysfunction and the associated release of oxidized mitochondrial DNA provide compelling molecular models to account for the increased inflammation observed in Irgm1‐deficient cells and animals, and may also underlie some of the autoinflammation associated with human IRGM disease alleles (Coers et al, 2018; Holley & Schroder, 2020). In addition to controlling mitochondrial remodeling and autophagy, Golgi‐resident human IRGM also regulates Golgi fragmentation in response to viral infections (Hansen et al, 2017). Whether mouse Irgm proteins execute similar functions has not been explored, but the localization of both Irgm1 and Irgm2 to the Golgi Apparatus ((Henry et al, 2014; Martens & Howard, 2006) and Appendix Fig S1) indicates that murine IRGM homologs may play similar roles in shaping Golgi membrane remodeling events. An accompanying study (Eren et al, 2020) shows that the Irgm2‐interaction partner Gabarapl2, another Golgi‐resident protein (Sagiv et al, 2000), cooperates with Irgm2 in mouse macrophages and blocks caspase‐11 activation in both mouse and human macrophages. Thus, the same anti‐inflammatory pathway disrupted in Irgm2 −/− BMMs appears to also operate in human cells. Future studies will be aimed at defining this pathway in detail through the systematic discovery and characterization of the molecular players and their activities, many of which are likely shared between mouse and human macrophages.
Too much or too little inflammation is host detrimental. The mammalian host thus evolved complex regulatory networks, which fine‐tune the inflammatory response during an infection. The identity of many of these regulatory circuits and how they function remain to be determined. Here, we report the discovery of a novel regulatory pathway, which attenuates cytosolic LPS sensing and moderates LPS‐induced inflammation. Future studies will expand our mechanistic understanding of this regulatory pathway and may open up avenues for therapeutic interventions to alleviate disease during gram‐negative bacterial infections.
Materials and Methods
Production of the Irgm2 −/−mouse line
To generate Irgm2 −/− mice, we inserted a loxP site together with a neo marker (floxneo) flanked by two flippase recognition target sites (frt) before the ATG of Irgm2 and a loxP site after the stop codon of Irgm2 using backbone vector pEZ‐Frt‐lox‐DT (Addgene plasmid #11736). We placed this cassette between 5′ and 3′ Irgm2 homology arms. The Irgm2 targeting construct was transfected into C57BL/6‐derived PRX‐B6N ES cells (Primogenix). Properly targeted ES cell clones were identified by PCR screening, confirmed by Southern blotting and used for blastocyst injection. The resulting chimeras were crossed with C57BL/6J mice and germline transmission of targeted ES clones was obtained. We performed Southern blot analysis to confirm the proper targeting in F2 mice. These mice were crossed with a germline flippase‐expressing strain B6(C3)‐Tg(Pgk1‐FLPo)10Sykr/J (Wu et al, 2009) to remove the neomycin cassette. Offspring from this cross harbored a floxed Irgm2 allele (Irgm2 flox). Irgm2 flox mice were crossed with the germline‐expressing Cre strain B6.FVB‐Tg(EIIa‐cre)C5379Lmgd/J (Lakso et al, 1996) to generate Irgm2 −/− mice. Mice were genotyped by PCR using primers Irgm2_543 5′‐ GATCTGAAAGCCTGGACAA‐3′ and Irgm2_542 5′‐ GGGTACACATGGGGTACAGG‐3′ which yield a 1.4 kb (WT) and 0.2 kb (Irgm2 −/−) DNA product.
Production of the panIrgm −/− and Irgm2 −/− Irgm3 −/− mouse lines
To generate panIrgm −/− mice, CRISPR sgRNA‐Cas9 protein ribonucleoprotein complexes were injected into pronuclear‐stage mouse embryos derived from previously reported double‐knockout (Irgm1 −/− Irgm3 −/−) mice on a C57BL/6J genetic background (Henry et al, 2009; Coers et al, 2011). The following pair of sgRNA were used for the injection: sgRNApan1 5′‐GACTGCCGTGAAAGAAGGGG‐3′ and sgRNApan4 5′‐ GGTTGGCTCTATAGGTTTTG‐3′. The genomic Irgm2 locus of panIrgm −/− founder mice was sequenced and a founder line with 925 bp deletion and nonsense mutation was backcrossed to C57BL/6J and then intercrossed to obtained animals homozygous for the deletion alleles of all 3 Irgm paralogs, as confirmed by PCR genotyping and Western blotting. PanIrgm −/− mice were backcrossed to C57BL6/J mice and screened for recombinants by PCR. A Irgm2 −/− Irgm3 −/− recombinant was intercrossed to obtain homozygous mice.
Additional mouse strains used
WT (C57BL/6J) mice were originally purchased from Jackson Laboratories. Irgm1 −/−, Irgm3 −/−, Atg5 f/f, LysMCre‐Atg5 f/f, Atg14 f/f, LysMCre‐Atg14 f/f, Casp1 −/− Casp11 −/−, Casp11 −/− , CD14 −/− , Aim2 −/− , Nlrp3 −/− , and Gbp chr3−/− mice were previously described (Kuida et al, 1995; Moore et al, 2000; Taylor et al, 2000; Collazo et al, 2001; Zhao et al, 2008; Rathinam et al, 2010; Kayagaki et al, 2011; Inoue et al, 2012; Yamamoto et al, 2012; Choi et al, 2014). The mouse strains Irgm2 −/− Casp1 −/− Casp11 −/−, Irgm2 −/− Casp11 −/−, Irgm2 −/− GBP chr3−/−, and Irgm2 −/− CD14 −/− mice were generated through intercrossing of aforementioned mouse lines.
Mouse husbandry and in vivo experiments
All mouse lines were maintained at animal facilities at Duke University Medical Center. Animal protocols were approved by the Institutional Animal Care and Use Committees at Duke University. Mice were between 7 and 14 weeks of age with the exception of the Luminex experiment (age range was 7–20 weeks). Approximately equal numbers of male (n = 122, weight (mean ± SD) = 24.18 ± 2.45 g) and female (n = 105 mice, weight (mean ± SD) = 19.18 ± 1.91 g) mice were used with both sexes utilized in each set of experiments. Mice were injected intraperitoneally with 2 or 8 mg E. coli LPS (O111:B4 LPS; L3024; Sigma)/kg bodyweight or with PBS control, as indicated. For cytokine monitoring, serum was collected 4 h after LPS injection. Cytokine concentrations were measured by ELISA or Luminex Multiplex Assay (Invitrogen Mouse 20‐plex). To determine host susceptibility to endotoxemia, mice were monitored every 3 h for up to 48 h following LPS injection. Mice were considered moribund and euthanized when their body weight dropped below 80% of starting weight, or the mice exhibited convulsions, or uncontrollable shivering, or severe ataxia as indicated by lack of righting response. We used approximately equal numbers of male and female mice for experiments.
Production and culturing of bone marrow‐derived macrophages (BMMs)
Bone marrow‐derived macrophages (BMMs) were generated essentially as previously described (Pilla et al, 2014; Finethy et al, 2017). Briefly, bone marrow was flushed out from mouse femurs and tibia using a 23‐gauge needle and then cultured in RPMI 1640, 20% FBS, 2‐mercaptoethanol, and 14% conditioned media containing macrophage colony‐stimulating factor at 37°C in 5% (vol/vol) CO2 in nontissue culture‐treated dishes for 5–7 days. Differentiated BMMS were harvested, frozen, and then thawed again for use in experiments. Immortalized BMMs (iBMMs) were generated through infection with J2 virus as previously described (Pilla et al, 2014).
Ectopic expression of Irgm2
Irgm2 was inserted into the tetracycline‐inducible vector pInducer20 via Gateway recombination (Meerbrey et al, 2011). To this end, the Irgm2 cDNA was amplified from RNA isolated from IFNγ‐stimulated WT BMMs using the primer pair attB1‐IRGM2‐Forward/attB2‐IRGM‐Reverse, which add both flanking attB‐recombination sites and a 5′‐Kozak consensus sequence. The resulting PCR product was inserted into pDONR221 via Gateway BP recombination, followed by Gateway LR recombination into pInducer20. Viral particles were prepared in HEK‐293T cells and transduced into iBMMs as previously described (Feeley et al, 2017). Expression of Irgm2 was induced by overnight incubation with cell culture medium containing anhydrotetracycline (aTc, 2 μg/ml). Primers: attB1‐Irgm2‐Forward, 5′‐GGGGACAAGTTTGTACAAAAAAGCAGGCTGCCACCATGGAAGAGGCAGTTGAGTCACC‐3′; attB2‐Irgm2‐Reverse, 5′‐GGGGACCACTTTGTACAAGAAAGCTGGGTCCTAAGGATGAGGAATGGAGAGTCTCAGG‐3′
Bacterial culture and OMV production
E. coli K‐12 BW5113 (K12), uropathogenic E. coli CFT073 (UPEC), and E. coli O18:K1:H7 strain C5 (AIEC) were cultured overnight in Luria‐Bertani broth (LB) at 37°C with agitation. Francisella novicida strain U112 (ATTC catalog No. NR‐13) was grown overnight in Brain Heart Infusion (BHI) broth shaking at 37°C. For LPS delivery experiments, Listeria monocytogenes strain DH‐L1039 was grown overnight in BHI at 30°C, as previously reported (Pilla et al, 2014). OMVs were isolated from culture supernatants via ultracentrifugation and ultrafiltration as previously described (Finethy et al, 2017).
Tissue culture infection and treatment protocols
For all infections, bacteria cultures were diluted in cell culture medium to achieve the desired multiplicity of infection (MOI) and then added directly to cells. Cells were then centrifuged at 700 g for 10 min. Following 1‐h incubation at 37°C, gentamicin was added to wells at a final concentration of 100 μg/ml and cells were assayed at indicated time points. Where indicated, cells were primed with 100 U/ml of mouse IFNγ (EMD Millipore) overnight. Ultrapure S. minnesota R595 LPS (List Biologicals) at a concentration of 1 μg/ml was used for all cell culture LPS treatments unless otherwise noted. Where described, cells were treated with 5 μM MCC950 (Invivogen), 1 μg/ml poly(I:C) (Sigma‐Aldrich), 1 μg/ml Pam3CSK4 (Invivogen) alone or in combination with other reagents. Where noted, cells were treated with indicated concentrations of advanced glycation end product‐bovine serum albumin (AGE‐BSA, Sigma‐Aldrich) for 3 h. Unless otherwise mentioned, agonists were diluted in complete cell culture medium and added directly to cells for designated times. For qPCR experiments, cells were treated with LPS or left untreated for 8 h prior to harvesting of RNA. For immunoblot experiments, cells were washed 3 times with PBS before addition of LPS diluted in Opti‐MEM (Gibco). Cell lysates and supernatants were collected 24 h post‐treatment (hpt). To visualize ASC specks, LPS was diluted to a concentration of 5 μg/ml in Opti‐MEM and cells were fixed 4 hpt. To activate the NLRP3 inflammasome, BMMs were first primed with LPS in cell culture medium or left unstimulated for 3 h before cells were treated with 5 μM nigericin (Sigma‐Aldrich) for 1 h. At that time, cell supernatants were collected for analysis by ELISA or LDH release assays. For monitoring autophagy, IFNγ primed and unprimed BMMs were washed with PBS and then incubated for 2 h in cell culture media, media containing Bafilomycin‐A1 (100 nM, Sigma‐Aldrich), or Hank's balanced salt solution (HBSS, Thermo Fisher) prior to harvesting of cell lysates.
Cytoplasmic delivery of LPS
Cells were primed overnight with 100 U/ml of IFNγ prior to LPS treatments. LPS was transfected into BMMs using Lipofectamine LTX with Plus Reagent (Thermo Fisher). BMMs were seeded in 96‐well flat bottom plates at approximately 4 × 105 cells per well. For each well, 375 ng of Lipofectamine LTX and 75 ng of LPS alongside 75 ng of Plus Reagent were each diluted in 2 μl of Opti‐MEM. Following a 10‐min incubation period at room temperature (RT), solutions were mixed and incubated for an additional 30 min at RT before the total reaction volume was brought up to 50 μl with media. Solutions were added to wells and plates centrifuged at 700 g for 10 min. Cells were then incubated at 37°C for indicated times and cytotoxicity assessed via LDH release assay. For co‐delivery with L. monocytogenes, bacteria were grown overnight at 30°C and then diluted to give an MOI of 5 in Opti‐MEM. LPS was added at indicated concentrations and mixtures spun onto cells at 700 g for 10 min. Cells were incubated at 37°C for 1 h, gentamicin was added to a final concentration of 15 μg/ml and cells were returned to the incubator for an additional 3 h before LDH release was assessed.
Fluorescence‐associated cell sorting
For LPS internalization studies, cells were treated with 2.5 μg/ml Alexa Fluor 488‐conjugated LPS (L23351, Thermo Fisher) or unlabeled control LPS for indicated times. Following treatment, cells were washed twice with PBS and collected in PBS containing 2% FBS. For measuring mitochondrial reactive oxygen species, IFNγ primed and unprimed cells were treated with LPS (1 μg/ml) for 3 h, antimycin A (100 μM) for 30 min, or were left untreated. Following treatments, cells were washed twice with PBS, incubated for 30 min with mitoSOX red (5 μM, Invitrogen) in Opti‐MEM, washed thrice with PBS and were collected in PBS containing 2% FBS. Fluorescence intensity data was collected a BD FACSCanto II system (BD Biosciences) and analysis was carried out using FlowJo Software.
Cytotoxicity, viability, and cytokine measurements
LDH release was measured using the CytoTox One homogenous membrane integrity assay (Promega). Cytotoxicity was calculated as a function of LDH release using the following formula: (sample – untreated control)/(100% lysis control – untreated control) × 100. In some instances, cell viability was determined using the CellTiter‐Glo Luminescent Cell Viability Assay (Promega) and changes in viability relative untreated or uninfected cells was used to calculate cell death. Measurements of propidium iodide (PI) uptake were used to assess cell death over time. In these instances, treatments were performed in Opti‐MEM containing 6 μg/ml PI and fluorescence measured at indicated time points. Relative fluorescence was calculated by subtracting PI fluorescence at time of initial treatment. For all cytotoxicity and viability assays, measurements were performed via Enspire 2300 (PerkinElmer) Multilabel Reader.
Cytokine concentrations were determined via enzyme‐linked immunosorbent assay (ELISA) or Luminex Multiplex Assay as indicated in figure legends. ELISAs were performed per manufacturer's instructions. The following ELISA kits were used: IL‐1β Mouse ELISA kit (Thermo Fisher), IL‐18 Mouse ELISA kit (Thermo Fisher), and ELISA MAX Deluxe Set Mouse TNFα (Biolegend). Measurements were performed via Enspire 2300 (PerkinElmer) Multilabel Reader. Luminex Multiplex Assay (Mouse 20‐plex; Invitrogen) was performed in the Immunology Unit of the Regional Biocontainment Laboratory at Duke using a BioPlex 200 instrument.
RNA isolation and quantitative PCR
RNA was isolated using a RNeasy Mini Kit (Qiagen) and reverse transcription accomplished using an iScript cDNA synthesis kit (Bio‐Rad). Quantitative PCR was performed with QuantaFast SYBR Green PCR mix (Qiagen) on a 7500 Fast Real‐Time PCR system (Applied Biosystems). The following primers were used:
mGAPDH F, 5′‐GGTCCTCAGTGTAGCCCAAG‐3′;
mGAPDH R, 5′‐AATGTGTCCGTCGTGGATCT‐3′;
mIL‐1β F, 5′‐GCAACTGTTCCTGAACTCAACT‐3′;
mIL‐1β R, 5′‐ATCTTTTGGGGTCCGTCAACT‐3′;
mTNF F, 5′‐CATCTTCTCAAAATTCGAGTGACAA‐3′;
mTNF R, 5′‐TGGGAGTAGACAAGGTACAACCC‐3′
Immunocytochemistry
For visualizing ASC specks, cells were fixed for 5 min with ice‐cold methanol. Coverslips were then washed 3 times with PBS, and cells were blocked with PBS containing 5% Bovine Serum Albumin (BSA) for 30 min at RT Cells were stained with rabbit anti‐ASC antibody (AG‐25B‐0006; Adipogen; 1:1,000) diluted in blocking buffer overnight at 4°C. Coverslips were then washed three times with PBS containing 5% BSA and 0.1% saponin, and cells were next incubated with anti‐rabbit Alexa Fluor 568‐conjugated secondary antibody (Invitrogen; 1:1,000) and Hoechst for 1 h at RT followed by an additional three washes. For visualizing Gm130 and Irgm2, cells were fixed for 15 min at RT with 4% (wt/vol) paraformaldehyde. Coverslips were washed 3 times with PBS and then blocked with 5% BSA and 0.3 M glycine in PBS‐saponin (0.1%) for 30 min at RT. Cells were stained with mouse anti‐Gm130 (610823; BD Transduction Laboratories; 1:200) and rabbit anti‐Irgm2 (1:500) diluted in blocking buffer for 1 h at RT. Coverslips were then washed 3 times with PBS‐saponin and stained with anti‐mouse Alexa Fluor 488 and anti‐rabbit Alexa Fluor 568‐conjugated secondary antibodies (Invitrogen), and Hoechst for 1 h at RT. Imaging was performed using a Zeiss Axioskop 2 upright epifluorescence microscope, a Zeiss 780 upright confocal microscope, or a Zeiss 880 Airyscan Fast Inverted Confocal. The custom‐made polyclonal rabbit anti‐Irgm2 antibody was raised against the N‐terminal peptide KEAVESPEVKEFEYFS.
Immunoblotting
Whole‐cell lysates were harvested in RIPA lysis buffer (Sigma‐Aldrich) containing 1 × protease inhibitor cocktail (Sigma‐Aldrich). For visualizing cleaved caspase‐1, supernatants were collected and protein concentrated via trichloroacetic acid (TCA) precipitation. Samples were mixed with a 100% (wt/vol) TCA solution in water to a final TCA concentration of 10% and incubated on ice overnight. Samples were then centrifuged at 21,130 g for 10 min. Following centrifugation, supernatant was discarded, and pellets washed twice with ice‐cold acetone. Pellets were air dried and resuspended in 8 M urea. Samples were loaded on 4–20% gradient SDS–PAGE gels and transferred to nitrocellulose or PVDF. Membranes were blocked for 1 h at RT in Tris‐buffered saline‐0.1% Tween 20 (TBST) containing 5% BSA and incubated overnight at 4°C with indicated primary antibodies. Membranes were washed thrice with TBST and then incubated for 1 h at RT with appropriate HRP‐conjugated secondary antibodies. Membranes were washed and visualized on film. The following antibodies and dilutions were used: rabbit anti‐Irm1 1B2 ((Butcher et al, 2005); 1:500), rabbit anti‐Irgm2 (raised against peptides KEAVESPEVKEFEYFS or KPESHDFPKLRETLQKDL; 1:500), rabbit anti‐Irgm3 927B0 ((Taylor et al, 1996); 1:500), rabbit anti‐GBP2 ((Coers et al, 2011); 1:1,000), rabbit anti‐caspase‐1 (AG‐20B‐0042; Adipogen; 1:1,000), mouse anti‐β‐actin (A2228; Sigma‐Aldrich; 1:1,000), rabbit anti‐P62 (PM045; MBL; 1:1,000), rabbit anti‐LC3 (2775; Cell Signaling Technology; 1:500), goat anti‐CD14 (AF982; Novus Biologicals; 1:500), and rat anti‐Casp11 (17D9; Novus Biologicals; 1:500), mouse anti‐HMGB1 (GT348; Invitrogen, 1:1,000), and rat anti‐RAGE (MAB11795; R&D Systems, 1:2,000).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.00 software. Significance was determined using one‐way analysis of variance (ANOVA) or two‐way ANOVA with multiple comparisons tests as indicated in figure legends. Log‐rank Mantel‐Cox test was used for determining significance in survival experiments. *P < 0.5, **P < 0.01, ***P < 0.001. N.S, non‐significant.
Author contributions
RF and JC conceived the project with input from GAT. RF, JD, MK, GW, ASP, AKH, and SL conducted the experiments and acquired data. RF, JD, MK, GW, SL, and JC analyzed data. RF and JC wrote the manuscript. All authors contributed to manuscript reviewing and editing. SH, NO‐R, JM, MJK, SW, and GAT provided reagents. JC and GAT supervised the project and acquired funding related to the project.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 2
Source Data for Figure 4
Acknowledgements
This work was supported by National Institutes of Health grants AI103197 (to J.C.) and AI148243 (to G.A.T and J.C.). We would like to thank Mr. She Zhe for his contributions to the production of the Irgm2 −/− mouse as well as Ms. Cheryl Bock and Mr. Gary Kucera at the Duke Cancer Institute Transgenic Mouse Facility (supported by NIH grant P30 CA014236) for their technical support in generating the Irgm2 −/− as well as the panIrgm −/− mouse lines. We are grateful to Dr. Masahiro Yamamoto for sharing the GBP chr3−/− mice with us. Luminex assay was performed in the Immunology Unit of the Regional Biocontainment Laboratory at Duke, which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6‐AI058607). J.C. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, M.K. is supported by a German Research Foundation (DFG) fellowship, A.K.H. by the Ramalingaswami Fellowship Grant (BT/RLF/Re‐entry/03/2015), Department of Biotechnology (DBT), India, and R.F. was supported by a National Science Foundation (NSF) Graduate Research Fellowship.
EMBO Reports (2020) 21: e50830
See also: E Eren et al and A Linder & V Hornung (November 2020)
Data availability
This study includes no data deposited in external repositories.
References
- Bafica A, Feng CG, Santiago HC, Aliberti J, Cheever A, Thomas KE, Taylor GA, Vogel SN, Sher A (2007) The IFN‐inducible GTPase LRG47 (Irgm1) negatively regulates TLR4‐triggered proinflammatory cytokine production and prevents endotoxemia. J Immunol 179: 5514–5522 [DOI] [PubMed] [Google Scholar]
- Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, Glowalla E, Leptin M, Howard JC (2005) The interferon‐inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol 6: R92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer SM, Brubaker SW, Monack DM (2019) Host inflammasome defense mechanisms and bacterial pathogen evasion strategies. Curr Opin Immunol 60: 63–70 [DOI] [PubMed] [Google Scholar]
- Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16: 407–420 [DOI] [PubMed] [Google Scholar]
- Butcher BA, Greene RI, Henry SC, Annecharico KL, Weinberg JB, Denkers EY, Sher A, Taylor GA (2005) p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect Immun 73: 3278–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L‐a (1999) Host‐pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun 67: 3703–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, Fujita N, Saitoh T, Akira S, Yoshimori T et al (2014) The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin‐like conjugation systems of autophagy. Immunity 40: 924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Kim S, Choi Y, Nielsen TB, Yan J, Lu A, Ruan J, Lee HR, Wu H, Spellberg B et al (2019) SERPINB1‐mediated checkpoint of inflammatory caspase activation. Nat Immunol 20: 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, Monack DM, Dorfleutner A, Stehlik C (2018) The oxidized phospholipid oxPAPC protects from septic shock by targeting the non‐canonical inflammasome in macrophages. Nat Commun 9: 996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers J, Gondek DC, Olive AJ, Rohlfing A, Taylor GA, Starnbach MN (2011) Compensatory T cell responses in IRG‐deficient mice prevent sustained chlamydia trachomatis infections. PLoS Pathog 7: e1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers J (2013) Self and non‐self discrimination of intracellular membranes by the innate immune system. PLoS Pathog 9: e1003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers J (2017) Sweet host revenge: galectins and GBPs join forces at broken membranes. Cell Microbiol 19: e12793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers J, Brown HM, Hwang S, Taylor GA (2018) Partners in anti‐crime: how interferon‐inducible GTPases and autophagy proteins team up in cell‐intrinsic host defense. Curr Opin Immunol 54: 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Woude GF, Sher A, Taylor GA (2001) Inactivation of LRG‐47 and IRG‐47 reveals a family of interferon gamma‐inducible genes with essential, pathogen‐specific roles in resistance to infection. J Exp Med 194: 181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z et al (2018) The endotoxin delivery protein HMGB1 mediates caspase‐11‐dependent lethality in sepsis. Immunity 49: 740–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioia M, Spreafico R, Springstead JR, Mendelson MM, Joehanes R, Levy D, Zanoni I (2020) Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol 21: 42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, Planes R, Bagayoko S, Bordignon P‐J, Chaoui K, Hessel A, Santoni K, Pinilla M, Lagrange B, Burlet‐Schiltz O et al (2020) Irgm2 and Gate‐16 cooperatively dampen Gram‐negative bacteria‐induced caspase‐11 response. EMBO Rep 21: e50829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley EM, Pilla‐Moffett DM, Zwack EE, Piro AS, Finethy R, Kolb JP, Martinez J, Brodsky IE, Coers J (2017) Galectin‐3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems. Proc Natl Acad Sci USA 114: E1698–E1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CG, Zheng L, Jankovic D, Bafica A, Cannons JL, Watford WT, Chaussabel D, Hieny S, Caspar P, Schwartzberg PL et al (2008) The immunity‐related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon‐gamma‐induced cell death. Nat Immunol 9: 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finethy R, Luoma S, Orench‐Rivera N, Feeley EM, Haldar AK, Yamamoto M, Kanneganti TD, Kuehn MJ, Coers J (2017) Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. mBio 8: e01188‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch D, Bando H, Clough B, Hornung V, Yamamoto M, Shenoy AR, Frickel EM (2019) Human GBP1 is a microbe‐specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J 38: e100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MTR, Cerqueira DM, Guimaraes ES, Campos PC, Oliveira SC (2019) Guanylate‐binding proteins at the crossroad of noncanonical inflammasome activation during bacterial infections. J Leukoc Biol 106: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119: 753–766 [DOI] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA (2013) Cytoplasmic LPS activates caspase‐11: implications in TLR4‐independent endotoxic shock. Science 341: 1250–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J (2013) IRG and GBP host resistance factors target aberrant, “non‐self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog 9: e1003414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MD, Johnsen IB, Stiberg KA, Sherstova T, Wakita T, Richard GM, Kandasamy RK, Meurs EF, Anthonsen MW (2017) Hepatitis C virus triggers Golgi fragmentation and autophagy through the immunity‐related GTPase M. Proc Natl Acad Sci USA 114: E3462–E3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Hartman M, Roche C, Zeng SG, O'Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J et al (2011) Autophagy controls IL‐1beta secretion by targeting pro‐IL‐1beta for degradation. J Biol Chem 286: 9587–9597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SC, Daniell XG, Burroughs AR, Indaram M, Howell DN, Coers J, Starnbach MN, Hunn JP, Howard JC, Feng CG et al (2009) Balance of Irgm protein activities determines IFN‐gamma‐induced host defense. J Leukoc Biol 85: 877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SC, Schmidt EA, Fessler MB, Taylor GA (2014) Palmitoylation of the immunity related GTPase, Irgm1: impact on membrane localization and ability to promote mitochondrial fission. PLoS ONE 9: e95021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley CL, Schroder K (2020) The rOX‐stars of inflammation: links between the inflammasome and mitochondrial meltdown. Clin Transl Immunol 9: e01109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, Shinohara ML (2012) Interferon‐beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal 5: ra38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intemann CD, Thye T, Niemann S, Browne EN, Amanua Chinbuah M, Enimil A, Gyapong J, Osei I, Owusu‐Dabo E, Helm S et al (2009) Autophagy gene variant IRGM ‐261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog 5: e1000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G et al (2018) Lipid peroxidation drives gasdermin d‐mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S et al (2011) Non‐canonical inflammasome activation targets caspase‐11. Nature 479: 117–121 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi‐Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A et al (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249 [DOI] [PubMed] [Google Scholar]
- Kimura T, Watanabe E, Sakamoto T, Takasu O, Ikeda T, Ikeda K, Kotani J, Kitamura N, Sadahiro T, Tateishi Y et al (2014) Autophagy‐related IRGM polymorphism is associated with mortality of patients with severe sepsis. PLoS ONE 9: e91522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Lew JD, Ha NP, Lin JS, Ma X, Graviss EA, Goodell MA (2011) Polymorphic allele of human IRGM1 is associated with susceptibility to tuberculosis in African Americans. PLoS ONE 6: e16317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens RL, Wang P, Munford RS (1998) Bacterial lipopolysaccharide can enter monocytes via two CD14‐dependent pathways. J Immunol 161: 5534–5545 [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA (1995) Altered cytokine export and apoptosis in mice deficient in interleukin‐ 1 beta converting enzyme. Science 267: 2000–2003 [DOI] [PubMed] [Google Scholar]
- Kumar S, Jain A, Farzam F, Jia J, Gu Y, Choi SW, Mudd MH, Claude‐Taupin A, Wester MJ, Lidke KA et al (2018) Mechanism of Stx17 recruitment to autophagosomes via IRGM and mammalian Atg8 proteins. J Cell Biol 217: 997–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsch M, Sistemich L, Lesser CF, Goldberg MB, Herrmann C, Coers J (2020) Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J 39: e104926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H (1996) Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA 93: 5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang W, Deng M, Loughran P, Tang Y, Liao H, Zhang X, Liu J, Billiar TR, Lu B (2018) Stearoyl lysophosphatidylcholine inhibits endotoxin‐induced caspase‐11 activation. Shock 50: 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Gulati AS, Cantillana V, Henry SC, Schmidt EA, Daniell X, Grossniklaus E, Schoenborn AA, Sartor RB, Taylor GA (2013) Irgm1‐deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 305: G573–G584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome‐activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BC, Sarhan J, Panda A, Muendlein HI, Ilyukha V, Coers J, Yamamoto M, Isberg RR, Poltorak A (2018) Constitutive interferon maintains GBP expression required for release of bacterial components upstream of pyroptosis and anti‐DNA responses. Cell Rep 24: 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Varley AW, Ohta S, Hardwick J, Munford RS (2008) Host inactivation of bacterial lipopolysaccharide prevents prolonged tolerance following gram‐negative bacterial infection. Cell Host Microbe 4: 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Varley AW, Munford RS (2013) Persistently active microbial molecules prolong innate immune tolerance in vivo . PLoS Pathog 9: e1003339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD (2015) The transcription factor IRF1 and guanylate‐binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 16: 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Place DE, Kuriakose T, Kanneganti TD (2017) Interferon‐inducible guanylate‐binding proteins at the interface of cell‐autonomous immunity and inflammasome activation. J Leukoc Biol 101: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Howard J (2006) The interferon‐inducible GTPases. Annu Rev Cell Dev Biol 22: 559–589 [DOI] [PubMed] [Google Scholar]
- Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE, Herschkowitz JI, Burrows AE, Ciccia A, Sun T et al (2011) The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo . Proc Natl Acad Sci USA 108: 3665–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehto S, Jena KK, Nath P, Chauhan S, Kolapalli SP, Das SK, Sahoo PK, Jain A, Taylor GA, Chauhan S (2019) The Crohn's disease risk factor IRGM limits NLRP3 inflammasome activation by impeding its assembly and by mediating its selective autophagy. Mol Cell 73: 429–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick MS, Kistner A, Rigard M et al (2015) Guanylate‐binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida . Nat Immunol 16: 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Andersson LP, Ingalls RR, Monks BG, Li R, Arnaout MA, Golenbock DT, Freeman MW (2000) Divergent response to LPS and bacteria in CD14‐deficient murine macrophages. J Immunol 165: 4272–4280 [DOI] [PubMed] [Google Scholar]
- Munford RS (2008) Sensing gram‐negative bacterial lipopolysaccharides: a human disease determinant? Infect Immun 76: 454–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojogun N, Kuang TY, Shao B, Greaves DR, Munford RS, Varley AW (2009) Overproduction of acyloxyacyl hydrolase by macrophages and dendritic cells prevents prolonged reactions to bacterial lipopolysaccharide in vivo . J Infect Dis 200: 1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D et al (2007) Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet 39: 830–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J (2014) Guanylate binding proteins promote caspase‐11‐dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci USA 111: 6046–6051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla‐Moffett D, Barber MF, Taylor GA, Coers J (2016) Interferon‐inducible GTPases in host resistance, inflammation and disease. J Mol Biol 428: 3495–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat G, Ugan RA, Cadirci E, Halici Z (2017) Sepsis and septic shock: current treatment strategies and new approaches. Eurasian J Med 49: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussin C, Foti M, Carpentier JL, Pugin J (1998) CD14‐dependent endotoxin internalization via a macropinocytic pathway. J Biol Chem 273: 20285–20291 [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E et al (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VAK, Zhao Y, Shao F (2019) Innate immunity to intracellular LPS. Nat Immunol 20: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogala AR, Schoenborn AA, Fee BE, Cantillana VA, Joyce MJ, Gharaibeh RZ, Roy S, Fodor AA, Sartor RB, Taylor GA et al (2018) Environmental factors regulate Paneth cell phenotype and host susceptibility to intestinal inflammation in Irgm1‐deficient mice. Dis Model Mech 11: dmm031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosadini CV, Kagan JC (2017) Early innate immune responses to bacterial LPS. Curr Opin Immunol 44: 14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Broz P (2015) Caspase‐11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol 45: 2927–2936 [DOI] [PubMed] [Google Scholar]
- Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P (2018) ESCRT‐dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362: 956–960 [DOI] [PubMed] [Google Scholar]
- Sagiv Y, Legesse‐Miller A, Porat A, Elazar Z (2000) GATE‐16, a membrane transport modulator, interacts with NSF and the Golgi v‐SNARE GOS‐28. EMBO J 19: 1494–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M et al (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin‐induced IL‐1beta production. Nature 456: 264–268 [DOI] [PubMed] [Google Scholar]
- Schmidt EA, Fee BE, Henry SC, Nichols AG, Shinohara ML, Rathmell JC, MacIver NJ, Coers J, Ilkayeva OR, Koves TR et al (2017) Metabolic alterations contribute to enhanced inflammatory cytokine production in Irgm1‐deficient macrophages. J Biol Chem 292: 4651–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336: 481–485 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665 [DOI] [PubMed] [Google Scholar]
- Simpson BW, Trent MS (2019) Pushing the envelope: LPS modifications and their consequences. Nat Rev Microbiol 17: 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SB, Davis AS, Taylor GA, Deretic V (2006) Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313: 1438–1441 [DOI] [PubMed] [Google Scholar]
- Singh SB, Ornatowski W, Vergne I, Naylor J, Delgado M, Roberts E, Ponpuak M, Master S, Pilli M, White E et al (2010) Human IRGM regulates autophagy and cell‐autonomous immunity functions through mitochondria. Nat Cell Biol 12: 1154–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Jeffers M, Largaespada DA, Jenkins NA, Copeland NG, Woude GF (1996) Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon gamma. J Biol Chem 271: 20399–20405 [DOI] [PubMed] [Google Scholar]
- Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, Eagleson B, Secrest L, Southon EA, Reid SW et al (2000) Pathogen‐specific loss of host resistance in mice lacking the IFN‐gamma‐inducible gene IGTP. Proc Natl Acad Sci USA 97: 751–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver MK, Henry SC, Cantillana V, Oliver T, Hunn JP, Howard JC, Beer S, Pfeffer K, Coers J, Taylor GA (2011) Immunity‐related gtpase M (IRGM) proteins influence the localization of guanylate‐binding protein 2 (GBP2) by modulating macroautophagy. J Biol Chem 286: 30471–30480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VA (2016) Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase‐11 activation. Cell 165: 1106–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weersma RK, Stokkers PC, Cleynen I, Wolfkamp SC, Henckaerts L, Schreiber S, Dijkstra G, Franke A, Nolte IM, Rutgeerts P et al (2009) Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch‐Belgian cohort. Am J Gastroenterol 104: 630–638 [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang C, Sun H, LeRoith D, Yakar S (2009) High‐efficient FLPo deleter mice in C57BL/6J background. PLoS ONE 4: e8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T et al (2012) A cluster of interferon‐gamma‐inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii . Immunity 37: 302–313 [DOI] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR et al (2016) An endogenous caspase‐11 ligand elicits interleukin‐1 release from living dendritic cells. Science 352: 1232–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Springstead JR, Kagan JC (2017) By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome‐Dependent Phagocyte Hyperactivation. Immunity 47: 697–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG et al (2008) Autophagosome‐independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4: 458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 2
Source Data for Figure 4
Data Availability Statement
This study includes no data deposited in external repositories.


