Abstract
Mutations in genes affecting primary cilia cause ciliopathies, a diverse group of disorders often affecting skeletal development. This includes Jeune syndrome or asphyxiating thoracic dystrophy (ATD), an autosomal recessive skeletal disorder. Unraveling the responsible molecular pathology helps illuminate mechanisms responsible for functional primary cilia. We identified two families with ATD caused by loss‐of‐function mutations in the gene encoding adrenergic receptor kinase 1 (ADRBK1 or GRK2). GRK2 cells from an affected individual homozygous for the p.R158* mutation resulted in loss of GRK2, and disrupted chondrocyte growth and differentiation in the cartilage growth plate. GRK2 null cells displayed normal cilia morphology, yet loss of GRK2 compromised cilia‐based signaling of Hedgehog (Hh) pathway. Canonical Wnt signaling was also impaired, manifested as a failure to respond to Wnt ligand due to impaired phosphorylation of the Wnt co‐receptor LRP6. We have identified GRK2 as an essential regulator of skeletogenesis and demonstrate how both Hh and Wnt signaling mechanistically contribute to skeletal ciliopathies.
Keywords: asphyxiating thoracic dystrophy, GRK2, hedgehog, smoothened, Wnt
Subject Categories: Development & Differentiation; Genetics, Gene Therapy & Genetic Disease; Musculoskeletal System
This study identifies GRK2 as a regulator of human skeletogenesis. Loss of GRK2 deregulates the function of two major morphogens in the bone ‐ Hedgehog and canonical Wnt signaling, and manifests in autosomal recessive skeletal ciliopathy syndrome, asphyxiating thoracic dystrophy or Jeune syndrome.
The paper explained.
Problem
Herein, we identified patients with Jeune syndrome or asphyxiating thoracic dystrophy (ATD), a genetically heterogeneous disorder that is both multisystemic disorder and often associated with lethality. In two independent families, we identified homozygosity for a loss‐of‐function variant and biallelic variants predicting loss of functional protein. Because of this novel finding that links GRK2 to the skeletal development, we focused on identification of the molecular mechanisms underlying this disorder since loss of Grk2 in mice is an early embryonic lethal.
Results
We found that the loss of GRK2 leads to specific changes in the bone that indicated impaired function of two major regulators of bone development, both Hedgehog and Wnt signaling. We indeed found that loss of GRK2 in patient's cells and model cell lines led to deregulation of these two pathways, suggesting in part the molecular mechanisms underlying this phenotype.
Impact
Development skeletal disorders, including ATD, are often severe, lethal syndromes with no cure or treatment options. Identification of the molecular pathogenesis of the disease therefore expands our understanding of the genetic heterogeneity associated with this disorder, provides families with reproductive options, and uncovers the role of GRK2 in skeletogenesis.
Introduction
A single primary cilium protrudes from nearly every post‐mitotic vertebrate cell, and cilia sense and transduce a vast array of extracellular cues. Cilia utilize intraflagellar transport (IFT), a bidirectional system that builds and maintains the cilium while also facilitating protein entry, exit and trafficking through the organelle. IFT is governed by a large multimeric protein complex with two main subcomplexes, IFT‐A and IFT‐B. The anterograde IFT is driven by the kinesin motor KIF3 and mediates transport from the base to the tip of cilia, while retrograde IFT is driven by the dynein‐2 motor and transports cargo from the tip to the base of the cilium (Kozminski et al, 1993). Vertebrate primary cilia act as signaling centers for the Hedgehog (Hh) family of morphogens and also orchestrate a variety of other cell signaling cascades (Gerhardt et al, 2016).
Mutations in genes affecting primary cilia structure or function cause ciliopathies, a group of pleiotropic disorders. Among these disorders is a subset with profound abnormalities in the skeleton, termed skeletal ciliopathies. Asphyxiating thoracic dystrophy (ATD) or Jeune syndrome is considered an autosomal recessively inherited skeletal ciliopathy characterized by a long narrow chest, shortened long bones, and occasional polydactyly. Other affected organs can include the brain, retina, lungs, liver, pancreas, and kidneys. Characteristic radiographic findings include handlebar shaped clavicles, short horizontal ribs, shortened appendicular bones with irregular metaphyseal ends, a small pelvis with a trident shaped acetabular roof, and brachydactyly with cone‐shaped epiphyses. Other skeletal ciliopathies with overlapping features include short rib polydactyly syndrome (SRPS), Ellis‐van Creveld dysplasia (EVC), and cranioectodermal dysplasia (CED). ATD is clinically and radiographically most similar to the perinatal‐lethal SRPS. However, while ATD is not uniformly lethal, long‐term survivors often have multi‐organ system complications. Mutations in genes affecting ciliogenesis or the IFT process produce a broad phenotypic spectrum of skeletal ciliopathies, and there is significant allelic and locus heterogeneity. Many of the genes mutated in this spectrum of disorders encode IFT‐A proteins and their motors: the cytoplasmic dynein‐2 motor heavy chain, DYNC2H1 (MIM 603297), WDR34 (MIM 615633), WDR60 (MIM 615462), WDR19 (also known as IFT144) (MIM 614376), IFT140 (MIM 266920), WDR35 (also known as IFT121) (MIM 614091), TTC21B (also known as IFT139) (MIM 612014), IFT43 (MIM 614068), TCTEX1D2 (MIM 617353), IFT122 (NIM 606045), IFT140 (MIM 614620), and DYNC2LI1 (MIM 617083) (Gilissen et al, 2010; Walczak‐Sztulpa et al, 2010; Arts et al, 2011; Bredrup et al, 2011; Davis et al, 2011; Perrault et al, 2012; Huber et al, 2013; McInerney‐Leo et al, 2013; Schmidts et al, 2013, 2015; Taylor et al, 2015). Mutations also have been reported in the genes encoding IFT‐B members: IFT80 (MIM 611263), IFT52 (MIM 617094), IFT81 (MIM 605489), and IFT172 (MIM 615630) (Beales et al, 2007; Halbritter et al, 2013; Duran et al, 2016; Zhang et al, 2016). Additional locus heterogeneity in these disorders results from mutations in the genes that encode the centrosomal kinase NEK1 (MIM 604588), MAP kinase family member ICK (MIM 612325), CEP290 (MIM 6101142), KIAA05866 (MIM 610178), and C21ORF2 (Thiel et al, 2011; Alby et al, 2015; Paige Taylor et al, 2016; McInerney‐Leo et al, 2017), as well as two proteins involved in planar cell polarity, INTU (MIM 610621) and FUZ (MIM 610622) (Toriyama et al, 2016; Zhang et al, 2018); three centrosomal proteins, EVC (NIM 604831), EVC2 (NIM 607261) and CEP120 (NIM 613446) (Ruiz‐Perez et al, 2000; Galdzicka et al, 2002; Shaheen et al, 2015); and the nuclear membrane protein LBR (NIM 600024) (Zhang et al, 2018).
In an effort to identify additional ATD genes and increase our understanding of ciliary function in skeletal development, exome sequence analysis was carried out in a cohort of patients within the skeletal ciliopathy spectrum, and two families were identified with pathogenic variants in the adrenergic receptor kinase beta 1 gene (ADRBK1 or GRK2). GRK2 is one of seven G protein‐coupled receptor kinases (GRKs), which bind to and rapidly desensitize activated G protein‐coupled receptors (GPCRs). GPCRs localize to the cell membrane where they sense extracellular cues and, through coupling with G proteins, relay signals to the cell. The wide repertoire of GPCR ligands includes photons, peptides, hormones, lipids, and sugars. With over 800 known members, the GPCR family represents the largest and most diverse class of eukaryotic membrane receptors (Fredriksson et al, 2003). In its canonical signaling cascade, GRK2 is activated by protein kinase A and phosphorylates the beta‐adrenergic receptor, desensitizing it, and thus preventing the cells from overstimulation by catecholamines (Hausdorff et al, 1990). In addition to GPCR signaling, the ubiquitously expressed GRK2 participates in many other processes, including regulation of phospholipase C, PI3 kinase, and RAF kinase activity, modulation of cytoskeletal proteins and activation of Smad and NFκB signaling through direct phosphorylation (Evron et al, 2012).
GRK2 also functions in the Hh pathway. Hh signaling is initiated by Hh ligand binding to the Patched 1 (PTCH1) receptor, followed by phosphorylation‐dependent accumulation of Smoothened (SMO) GPCR at the cell membrane (Drosophila) or in the primary cilia (vertebrates). This results in activation of the Hh gene expression through degradation of the GLI2/GLI3 transcriptional repressors and production of GLI activators (Forbes et al, 1993; Van den Heuvel & Ingham, 1996; Dai et al, 1999; Sasaki et al, 1999; Aza‐Blanc et al, 2000; Wang & Holmgren, 2000; Haycraft et al, 2005; Rohatgi et al, 2007; Tukachinsky et al, 2010; Su et al, 2011). The ciliary cAMP levels appear to have a central role in this process, regulating activity of protein kinase A (PKA) that is critical for formation of GLI repressors (Humke et al, 2010; Tuson et al, 2011; Mukhopadhyay et al, 2013; Niewiadomski et al, 2014). In this context, GRK2 acts as a positive regulator of Hh signaling, necessary for maximal Hh response in both Drosophila and vertebrates (Jia et al, 2004; Maier et al, 2014; Li et al, 2016); yet, the molecular mechanisms are not fully understood. GRK2 was shown to phosphorylate SMO C‐tail, leading to ciliary accumulation of SMO and Hh pathway activity (Chen et al, 2011); the former has been disputed and appears cell context dependent (Zhao et al, 2016; Pusapati et al, 2018). GRK2‐mediated SMO phosphorylation was shown to induce β‐arrestin (ARRB) recruitment, leading to KIF3A‐dependent cilia accumulation of SMO (Chen et al, 2004; Kovacs et al, 2008); however, later studies showed normal cilia accumulation of SMO in ARRB knock‐out cells (Pal et al, 2016; Desai et al, 2020). Ligand‐dependent removal of the Hh pathway inhibitor GPR161 was shown to depend on GRK2 activity (Pal et al, 2016; Pusapati et al, 2018). More recently, the GRK2 activity was shown necessary for SMO to sequester the catalytic subunit of PKA which in turn can no longer phosphorylate GLI3 in order to produce the GLI3 repressor (Niewiadomski et al, 2014; preprint: Happ et al, 2020).
Despite the limited understanding of the complex role of GRK2 in Hh signaling, its depletion by RNA interference in cell cultures clearly inhibits Hh activity (Meloni et al, 2006; Chen et al, 2010, 2011; Maier et al, 2014), and the Grk2−/− NIH3T3 do not respond to Hh stimulation as they fail to degrade GLI3 repressor and to activate Hh gene expression (Zhao et al, 2016; Pusapati et al, 2018). This effect is recapitulated by morpholino‐mediated knock‐down of grk2 and in the maternal‐zygotic grk2 mutant zebrafish embryos (Philipp et al, 2008; Evron et al, 2011; Zhao et al, 2016). Loss of grk2 in zebrafish results in a curved body axis, U‐shaped body somites and severe cyclopia (Zhao et al, 2016), phenocopying the smo mutant (Chen et al, 2001). In contrast, the Grk2 mouse knock‐out shows milder phenotypes, at least in the neural tube patterning, yet is lethal around midgestation (Jaber et al, 1996; Philipp et al, 2008). This lethality was associated with developmental heart defects (Jaber et al, 1996; Matkovich et al, 2006), yet the timing did not allow for studying the effect of Grk2 loss on later developing tissues and organs such as skeleton. Herein, we demonstrate that pathogenic variants in GRK2 produce ATD and modulate both Hh and Wnt signaling, demonstrating that GRK2 is an essential regulator of skeletogenesis.
Results
Loss of GRK2 results in ATD
The first proband (R05‐365A) was born at 38 weeks to second‐cousin parents. Prenatal ultrasound showed shortened limbs with a lag of approximately 8–9 weeks from the estimated due date. The pregnancy was complicated by ascites and hydrops fetalis that arose in the third trimester. The proband was delivered at term and had a very small chest with underlying pulmonary insufficiency. Additionally, she had low muscle tone, an atrial septal defect, hypoplastic nails, but no polydactyly. Radiographic findings included long narrow clavicles, short horizontal bent ribs with lack of normal distal flare, short humeri, mesomelia with bending of the radii, short femora and tibiae with broad metaphyses, diminished mineralization, and no endochondral ossification delay (Fig 1A and C). She expired 5 days after birth. The findings compared to characteristic ATD are delineated in Table 1.
Figure 1. Asphyxiating thoracic dystrophy (ATD) probands R05‐365A and Cmh001543‐01.
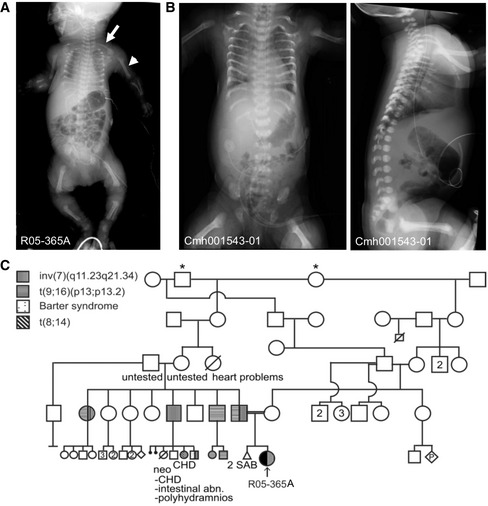
-
AAP radiograph demonstrates characteristic findings of ATD in the R05‐365A proband. Note the shortened humeri (closed arrowhead) and elongated clavicles (arrow).
-
BRadiographs of the Cmh001543‐01 proband showing similar findings.
-
CFamily R05‐365A pedigree; * indicates common ancestors. CHD, congenital heart disease, SAB, spontaneous abortion. Abn, abnormalities.
Table 1.
Clinical and radiographic phenotype of ATD and the R05‐365A and Cmh001543‐01 and ‐02 cases
| Clinical and Radiographic Phenotype | ATD/Jeune syndrome | R05‐365A | Cmh001543‐01 and ‐02 |
|---|---|---|---|
| Autosomal recessive | + | + | + |
| Retinal insufficiency (age dependent) | + | − | − |
| Pulmonary insufficiency/hypoplasia | + | + | + |
| Polycystic liver disease/hepatic fibrosis (age dependent) | + | − | − |
| Cystic kidneys/chronic renal failure (age dependent) | + | − | − |
| Congenital heart defect | + | + | − |
| Ascites | + | + | − |
| Lethality due to pulmonary hypoplasia | + | + | + |
| Long narrow thorax | + | + | + |
| Short horizontal ribs | + | + | + |
| Handlebar clavicles | + | − | + |
| Small pelvis | + | + | + |
| Hypoplastic iliac wings (infancy) | + | + | + |
| Short long bones | + | + | + |
| Irregular metaphyses | + | + | + |
| Short fibulae (relative) | + | + | Unknown |
| Short ulnae (relative) | + | + (bowed) | Unknown |
| Brachydactyly | + | Unknown | Unknown |
| Cone‐shaped epiphyses (childhood) | + | Unknown | Unknown |
| Occasional polydactyly | + | − | − |
R05‐365A, International Skeletal Dysplasia Registry Number, +, present, −, absent.
In the second family, the elder of the two affected female siblings (proband Cmh001543‐01) was delivered at 39 weeks’ gestational age by cesarean section. The pregnancy was complicated by exposure to cannabis and the antiseizure medication, levetiracetam. Prenatal ultrasound at 20 weeks showed shortened long bones and a small chest and evaluation of the chest to abdominal circumference was predictive of lethality. At delivery, the proband was cyanotic with Apgar scores were 31, 75, and 810 and the head circumference was 32.5 cm, birth length 46 cm, and weight 2.82 kg. She was intubated at 12 min of life and initially required high levels of respiratory support and was extubated on day 14 of life, but continued to have chronic respiratory issues. Radiographic findings showed similar findings to the first proband (R05‐365A) and included handlebar clavicles, short horizontal bent ribs with lack of normal distal flare, short humeri, broad irregular metaphyses, diminished mineralization, axial clefts, and odontoid hypoplasia (Fig 1B). She died at 5.5 months of age related to respiratory sequelae due to chronic lung disease. The second affected sibling had similar radiographic findings, but did not require prolonged respiratory support. She had questionable seizure events, but otherwise exhibited a much less severe clinical course and was alive at 12 months of age.
Using exome sequence analyses of the R05‐365A proband, homozygosity for a nonsense variant in exon 6 of GRK2, c. 469 C>T predicting the amino acid change p.R158*, was identified. The pathogenic variant localizes to the G protein signaling (RGS) domain of GRK2 (Fig 2A and C). The pathogenic variant occurred within a 13 Mb block of homozygosity on chromosome 11 and has not been seen in population databases. Detection of GRK2 expression, by RT–PCR of cDNA and Western blot analysis of protein, respectively, demonstrated loss of both GRK2 transcript and protein in cultured patient fibroblasts (Fig 2D and E). The data thus demonstrate that the p.R158* pathogenic variant results in a GRK2 null (GRK2 −/− ) genotype.
Figure 2. Homozygosity and compound heterozygosity for null mutations in GRK2 in ATD .
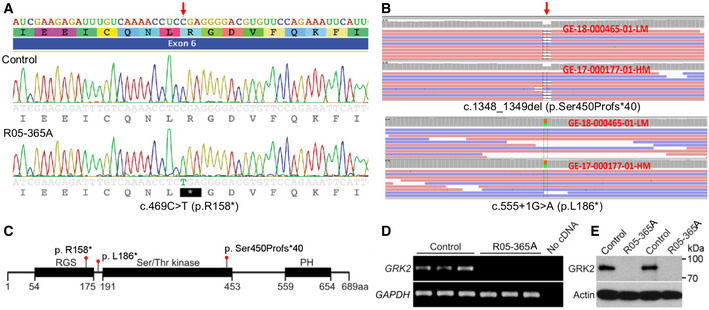
-
ASanger sequence trace showing homozygosity for the c.469C>T mutation, predicting a stop codon in exon 6 of GRK2 (arrow).
-
BExome analysis showing the biallelic changes c. 1348_1349del and c.555 +1G>A. Red arrow points to the place of mutation. Orange and green boxes indicate the reference nucleotide and its substitution, respectively.
-
CDomain composition of GRK2 and the corresponding location of the mutations (RGS, regulator of G protein signaling domain; PH, pleckstrin homology domain).
-
DExpression of GRK2 as measured by RT–PCR in three replicates each of control and R05‐365A fibroblasts, demonstrating absence of GRK2 transcript in ATD cells. GAPDH served as a positive control.
-
ETwo control and ATD fibroblast samples were immunoblotted for GRK2. Note the absence of GRK2 protein in the ATD cells. Actin served as a loading control. The data shown are representative of three independent experiments.
Source data are available online for this figure.
In this second family, exome analysis identified two pathogenic variants, each inherited from an unaffected parent. The maternally inherited variant, c.1348_1349del in exon 16, predicted an amino acid substitution followed by a frameshift, p.Ser450Profs*40. The altered serine residue localizes to the kinase domain. The paternally inherited variant, c.555 +1 G>A, is predicted to lead to exon 7 skipping and introduction of a frameshift in exon 8, p.L186*. This change resides in a region between the RGS and kinase domain of GRK2 (Fig 2B and C). Both of these variants were absent in control databases. These biallelic alterations are predicted to lead to non‐sense‐mediated decay of the transcript and loss of the GRK2 protein. Cell lines were not available from either affected individual in this family.
GRK2 is essential for skeletal growth plate development
Although the early embryonic lethality prevents detailed analyses of the skeleton, the Grk2 −/− mice embryos display growth retardation, suggesting GRK2 role in skeletal development (Jaber et al, 1996; Philipp et al, 2008). The profound skeletal abnormalities observed as a result of loss of GRK2 in the R05‐365A patient suggested that the function of the protein is essential for normal development of the cartilage growth plate. To define the localization of GRK2 during embryonic skeletal development, immunohistochemistry was performed on human cartilage growth plate derived from a control 17‐week gestational age distal femur. GRK2 was present throughout the growth plate and adjacent tissues, with the strongest staining detected in proliferating chondrocytes, primary spongiosum, and perichondrium (Fig 3A).
Figure 3. GRK2 is essential for skeletal growth plate development.
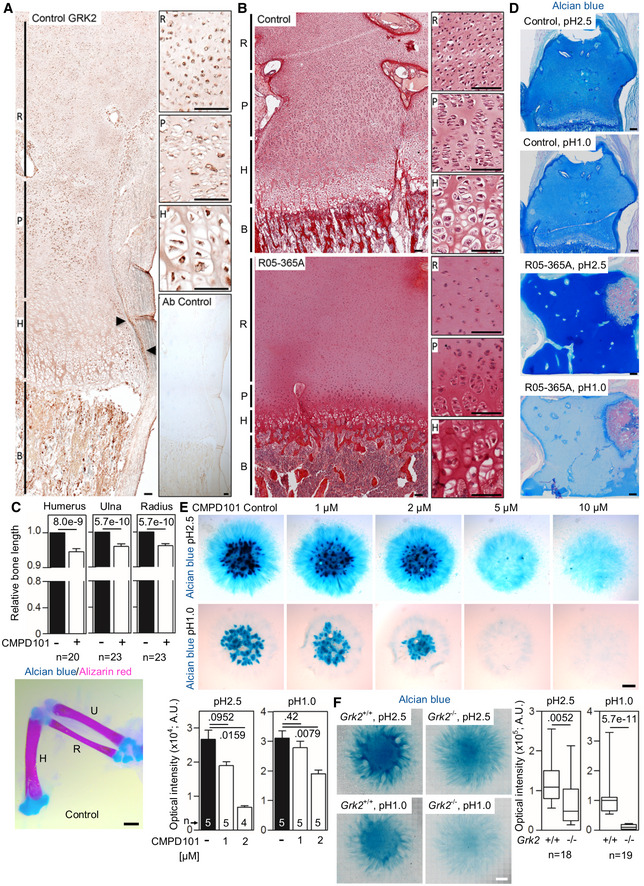
-
AGRK2 immunohistochemistry in a control 17‐week gestational age distal femur growth plate demonstrating widespread expression, particularly in the bone (B), proliferating zone of the growth plate (P), and the perichondrium (arrowheads). Non‐reactive antibody was used as a control (Ab control). R, reserve zone. H, hypertrophic zone. Scale bars, 100 μm.
-
BPicrosirius red staining of the femoral growth plates of a control and the ATD R05‐365A fetus. The control growth plate shows normal architecture with clearly distinguishable reserve (R), proliferating (P) and hypertrophic (H) zones. A profound disruption of growth plate architecture is obvious in the GRK2 −/− growth plate, which shows irregularly shaped reserve zone chondrocytes, a lack of discernable proliferating chondrocytes, and a significantly under‐developed hypertrophic zone. Scale bars, 100 μm.
-
CShortened long bones in the chicken wings injected with CMPD101. The right limb bud was injected with CMPD101, and the left one was left as a control. Note the shortened humerus (H), ulna (U), and radius (R) in the CMPD101‐injected wing. Mean ± SEM. Mann–Whitney U‐test; number of animals is indicated. Scale bar, 150 μm.
-
DAlcian blue staining of the femoral growth plates of a control and the ATD R05‐365A fetus. Note the significantly lesser blue staining of the R05‐365A growth plate stained by alcian blue pH 1.0, suggesting impaired sulfation of the extracellular matrix. Scale bars, 200 μm.
-
EAlcian blue staining of the chicken limb bud micromasses treated with CMPD101. Note the gradual inhibition of the cartilage nodules with increasing concentration of CMPD101, quantified below. Mean ± SEM. Mann–Whitney U‐test; number of micromasses is indicated. A representative experiment of four (pH 2.5) and three (pH 1.0) is shown. Scale bar, 100 μm.
-
FAlcian blue staining of the micromasses generated from Grk2 +/+ and Grk2 −/− NIH3T3 cells. Note the mildly weaker staining by alcian blue pH 2.5 and the nearly absent sulfation stained by alcian blue pH 1.0 in the Grk2 −/− micromasses. Central band, median. Box, 1st‐3rd quartile. Whiskers, 10%‐90% percentile. Mann–Whitney U‐test; number of micromasses is indicated. Scale bar, 1 mm.
Source data are available online for this figure.
To investigate the effects of GRK2 depletion on skeletal development through the cartilage growth plate, we compared the microanatomical appearance of femoral growth plate cartilage between the affected (R05‐365A) individual and a 17‐week fetal control. Compared with control, the affected growth plate architecture was profoundly disturbed, with a markedly expanded reserve zone containing small round chondrocytes, absence of characteristic proliferating chondrocytes, an extremely short hypertrophic zone, small hypertrophic chondrocytes, and a minimal zone of terminal differentiation (Fig 3B). The profound disorganization of the growth plate cartilage underlies the skeletal changes causing ATD and demonstrates that, at the cellular level, the loss of GRK2 affects chondrocyte proliferation and hypertrophic differentiation. To further confirm the role of GRK2 in regulation of bone development, we inhibited GRK2 kinase activity by CMPD101, a chemical inhibitor of GRK2 activity [32]. CMPD101 was injected into the right wing bud of chick embryos at stage HH20‐22, and the effect on skeletal development was determined 10–12 days later. CMPD101 caused no gross abnormalities in the shape of ulna, radius and humerus, but lead to shortening of all three skeletal elements (Fig 3C).
The femoral growth plate cartilage samples available on GRK2 −/− individual (R05‐365A) were stained with alcian blue, to visualize the proteoglycan content of the chondrocyte extracellular matrix. At pH2.5 (proteoglycan staining) (Green & Pastewka, 1974), no significant change was found in the signal between control and R05‐365A growth plate. At pH1.0 (sulfated proteoglycan staining), a lack of signal was found in R05‐365A (Fig 3D). The putative lack of proteoglycan sulfation in the absence of GRK2 was addressed in two independent in vitro models. First, the mesenchymal cultures were established from the wing buds of stage HH20 chicken embryos, and differentiated into the cartilage in a 5‐day micromass culture (Horakova et al, 2014). Treatment with CMPD101 led to a significantly reduced alcian blue staining at both pH2.5 and pH1.0 (Fig 3E; graphs, cartilage nodules staining). Second, the Grk2 −/− NIH3T3 fibroblasts (Pusapati et al, 2018) were differentiated to cartilage in a 7‐day micromass culture and stained with alcian blue to estimate the sulfated proteoglycan content. A significant reduction of alcian blue staining was found at both pH2.5 and pH1.0, with more profound at pH1.0 (Fig 3F). Our data suggest a role of GRK2 in regulation of sulfation of the cartilage extracellular matrix proteoglycans.
GRK2 is not required for ciliogenesis
Because ATD is a ciliopathy, we next examined the affected patient's GRK2 −/− patient fibroblasts for defects in ciliogenesis. Cells were serum starved to produce primary cilia, and immunohistochemistry was performed to visualize the axoneme (acetylated tubulin), ciliary membrane (ARL13B), and basal bodies (pericentrin) (Fig EV1A). No differences in ARL13B or acetylated tubulin staining were found between R05‐365A and control fibroblasts. Because tubulin‐based staining does not visualize ciliary tips, ARL13B signal was used to measure cilia length. We found no significant differences in the average cilia length between control and GRK2 −/− cells (Fig EV1B). Similarly, no differences were seen in the efficiency of ciliogenesis, determined by counting the number of ciliated cells after starvation (Fig EV1C).
Figure EV1. GRK2 is not required for ciliogenesis or IFT .
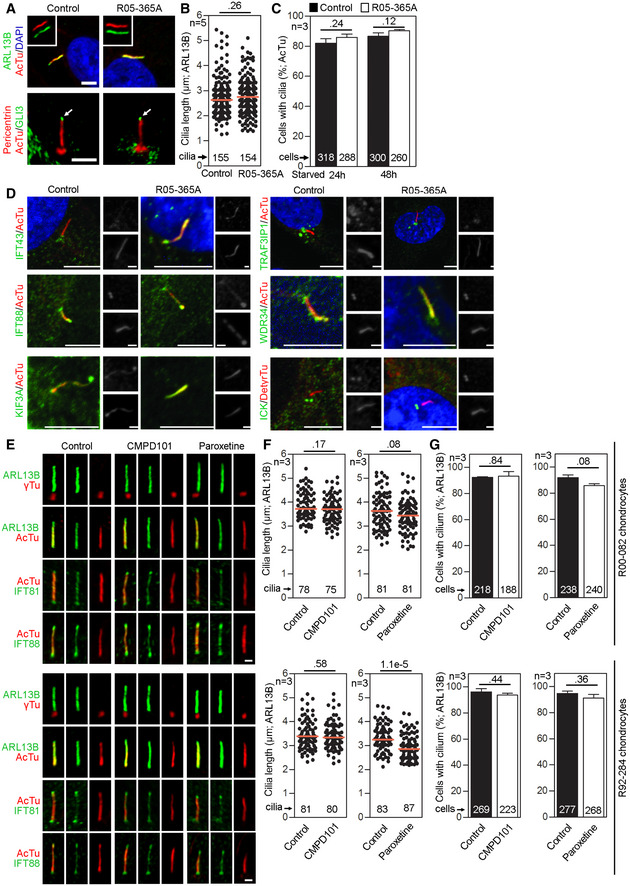
-
A–CPrimary cilia are normal in GRK2 −/− fibroblasts. Cells were serum starved for 24 or 48 h to induce cilia, and (A) immunostained for acetylated tubulin (AcTu, red), ARL13B (green) (upper panel; insets show the individual signals), or AcTu (red), pericentrin (red) and GLI3 (green) (lower panel). Control and GRK2 −/− cilia were positive for AcTu and ARL13B, and showed similar localization of GLI3 to the tips (lower panel, arrows). Acetylated tubulin and pericentrin staining were used to visualize the axoneme and centrioles, respectively. Scale bar, 2 μm. (B) Length distribution of cilia did not differ between control and GRK2 −/− cells. Red bars show medians; dots represent individual cilia. Mann–Whitney U test; number of biological experiments and the total numbers of analyzed cilia are indicated. (C) There was no difference in the efficiency of ciliogenesis between control and GRK2 −/− cells. Mean ± SEM. Welch's t‐test; number of biological experiments and the total numbers of analyzed cells are indicated.
-
DLoss of GRK2 did not affect localization of ciliary and IFT components. Immunofluorescence of serum‐starved (48 hr) control and GRK2 −/− fibroblasts immunostained with AcTu or detyrosinated tubulin (DetyrTu) to mark the cilium (red), and IFT43, IFT88, KIF3A, TRAF3IP1, WDR34, or ICK (green). No significant differences in staining were found between control and GRK2 −/− cilia, suggesting normal IFT. Scale bars, 5 μm and 1 μm (insets).
-
E–GInhibition of GRK2 activity did not affect primary cilia in chondrocytes. (E) Control human R92‐284 and R00‐082 chondrocytes were serum starved in the presence of a GRK2 inhibitor, either 20 μM CMPD101 or 10 μM paroxetine, for 24 h before they were fixed and immunostained for cilia (ARL13B; gamma tubulin, γTu; acetylated tubulin, AcTu) and the retrograde IFT‐B proteins IFT81 or IFT88. Scale bar, 1 μm. (F) Cilia length was measured using the ARL13B signal. Red bar, median. Mann–Whitney U‐test; number of biological experiments and total cilia numbers are indicated. (G) The percentage of ciliated cells was calculated. Mean ± SEM. Welch's t‐test; number of biological experiments and total cell numbers are indicated.
Source data are available online for this figure.
To determine whether loss of GRK2 affected IFT in the normally appearing primary cilia, we compared the distribution of several IFT components between control and GRK2 −/− cilia (Fig EV1D). The immunostaining for IFT43 (IFT‐A complex component), WDR34 (IFT‐A dynein‐2 motor complex member), IFT88 (IFT‐B complex component), TRAF3IP1 (IFT‐B complex component), and KIF3A (IFT‐B kinesin motor component) showed that the IFT components accessed and localized to cilia similarly between control and GRK2 −/− cells. The distribution of ICK (centrosomal kinase) and GLI3 (Hh target transcription factor) in GRK2 −/− cilia also followed the reported normal localization to the basal bodies or the tips of the cilia, respectively (Paige Taylor et al, 2016; Fig EV1A and D).
Finally, we used two chondrocyte cell lines established from control fetal growth plate cartilage (lines R00‐082 and R92‐284), and treated them with chemical inhibitors of GRK2 kinase activity, paroxetine, and CMPD101 (Thal et al, 2012; Pusapati et al, 2018), and examined for number of ciliated cells and for cilia length; no effect on cilia structure, length, or frequency was found (Fig EV1E–G). Together, these data demonstrated that loss of GRK2, or reduction of GRK2 kinase activity, produces no effect on ciliogenesis, ciliary length, or cilia morphology.
GRK2 −/− cells do not respond to Hh pathway activation and fail to traffic Smoothened to cilia
The data shown in Fig EV1 demonstrated that loss of GRK2 did not affect ciliogenesis or localization of IFT components, and thus, we hypothesized that the defect causing ATD is likely to lie in the specialized signaling functions of primary cilia. In vertebrates, the core components of the Hh pathway localize to the primary cilia. In the absence of stimulation, the cilia‐localized Hh receptor PTCH1 prevents SMO from accumulating in cilia (Ingham et al, 1991; Rohatgi et al, 2007). Under this condition, the transcriptional effector of the Hh pathway, GLI3, is proteolytically cleaved into the repressor form, GLI3R. Upon Hh binding to PTCH1, SMO accumulates in primary cilia, resulting in priming of the ciliary GLI3 for degradation. This relieves the GLI3R‐mediated repression on Hh target genes, leading to their transcriptional induction (Dai et al, 1999; Aza‐Blanc et al, 2000; Wang & Holmgren, 2000).
To evaluate the cellular response to Hh pathway activation, control and patient GRK2 −/− fibroblasts were treated with Smoothened agonist (SAG) (Chen et al, 2002), and whole cell extracts were immunoblotted for GLI3. In control cells, SAG treatment resulted in the progressive downregulation of both full‐length GLI3 (GLI3FL) and GLI3R, demonstrating normal processing (Wen et al, 2010). There was no effect of SAG in GRK2 −/− cells, which maintained constant GLI3FL and GLI3R levels throughout the 24 h of SAG treatment (Fig 4A). Because GLI3FL acts as a transcriptional activator, and skeletal ciliopathies often show altered GLI3FL to GLI3R ratios (Murdoch & Copp, 2010), we quantified the amounts of GLI3FL and GLI3R in cells. When treated with SAG, control fibroblasts showed an increased ratio of GLI3FL to GLI3R compared to baseline; this ratio was not increased in GRK2 −/− cells (mean ± SEM, 2.23 ± 0.31 in control vs. 0.94 ± 0.35 in R05‐365A; Mann–Whitney U‐test) (Fig 4B).
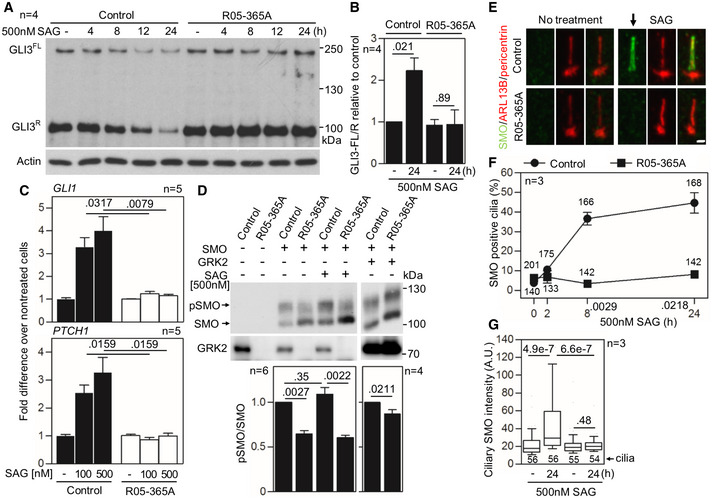
Figure 4. GRK2 −/− cells do not respond to Hh pathway activation and fail to accumulate Smoothened in cilia.
-
A, BDefective GLI3 processing in GRK2 −/− fibroblasts. Cells were serum starved to induce cilia before treatment with the Hh pathway activator SAG (Smoothened agonist), and the whole cell lysates were immunoblotted for GLI3. Note the SAG‐mediated downregulation of both full‐length (FL) and repressor (R) GLI3 in control fibroblasts, demonstrating normal processing. There was no effect of SAG on GLI3 processing in GRK2 −/− cells. A representative experiment of four is shown. (B) Quantitation of the GLI3FL to GLI3R ratio using densitometry. Mean ± SEM. Mann–Whitney U test; number of biological replicates is indicated.
-
CDefective SAG response in GRK2 −/− fibroblasts. Cells were treated with SAG for 24 h. Transcript levels of GLI3 targets GLI1 and PTCH1 were determined by qRT–PCR; GAPDH was used for normalization. Note the SAG‐mediated induction of GLI1 and PTCH1 expression in controls but not in GRK2 −/− cells. Mean ± SEM. Mann–Whitney U‐test; number of biological replicates is indicated.
-
DUnder‐phosphorylation of Smoothened (SMO) in GRK2 −/− fibroblasts. Lysates of SMO‐transfected cells were resolved by the phospho(p)‐shift PAGE and immunoblotted for SMO. The portion of pSMO analyzed by densitometry is shown. GRK2 add‐back demonstrates a rescue of the pSMO in GRK2 −/− fibroblasts. Mean ± SEM. Mann–Whitney U‐test; number of biological replicates is indicated.
-
E–GDefective SMO accumulation in cilia of GRK2 −/− fibroblasts. Cells were treated with SAG and immunostained for SMO, ARL13B, and pericentrin. Note the failure in SMO accumulation in cilia of GRK2 −/− cells (arrow). Scale bar, 2 μm (E). The percentage of SMO‐positive cilia was calculated and plotted. Mean ± SEM. Welch's t‐test; number of biological experiments and the total numbers of analyzed cilia are indicated (F). The intensity of ciliary SMO was analyzed and plotted. Central band, median. Box, 1st‐3rd quartile. Whiskers, 10%‐90% percentile. Mann–Whitney U‐test; number of biological experiments and the total numbers of analyzed cilia are indicated (G).
Source data are available online for this figure.
To test whether reduced GLI3 processing in SAG‐treated GRK2 −/− cells resulted in defective induction of Hh target genes, cells were treated with SAG for 24 h and transcript levels of the GLI3 target genes GLI1 and PTCH1 (Dai et al, 1999; Ågren et al, 2004) were determined by quantitative RT–PCR. Fig 4C demonstrates a potent, SAG‐mediated induction of GLI1 and PTCH1 expression in control cells, in contrast to GRK2 −/− cells, which did not induce GLI1 or PTCH1 expression in response to SAG. These data demonstrate that loss of GRK2 compromises Hh signaling in cells, rendering them unresponsive to Hh pathway activation.
While SMO represents an atypical GPCR, it is phosphorylated by GRKs in a similar way as classic GPCRs. In response to Hh ligands, GRK2 phosphorylates SMO at multiple C‐terminal sites, and this phosphorylation induces conformational changes in SMO which facilitate interactions with ARRB and the kinesin KIF3A that were reported necessary for SMO translocation to cilia (Chen et al, 2004; Meloni et al, 2006; Kovacs et al, 2008). The SMO phosphorylation was inhibited in R05‐365A fibroblasts, as shown by phosphoshift assay (Jia et al, 2004; Zhang et al, 2004; Chen et al, 2011) in cells transfected by murine SMO (Fig 4D). Removal of GRK2‐induced SMO phosphorylation sites has been shown to abolish SMO translocation to primary cilia in response to Hh ligands (Chen et al, 2011). To determine whether this mechanism is relevant in GRK2 −/− cells, we treated the cells with SAG, and the immunocytochemistry was employed using SMO, ARL13B, and pericentrin to visualize SMO translocation into cilia (Fig 4E). In control human fibroblasts, SAG induced progressive accumulation of SMO in cilia, which was observed in nearly 50% of cells at 24 h of SAG treatment. In contrast, no SMO accumulation in primary cilia was observed in GRK2 −/− cells (Fig 4F). This was confirmed by measurement of SMO signal intensity in the cilia; significant accumulation of SMO signal was found in SAG‐treated control cells but not in GRK2 −/− patient cells (Fig 4G).
To further confirm the data obtained with patient GRK2 −/− fibroblasts, we used two chondrocyte cell lines (lines R00‐082 and R92‐284). Inhibition of GRK2 kinase activity in these cells, via treatment with CMPD101 and paroxetine, did not affect ciliogenesis or ciliary length (Fig EV1E–G). To assess SAG response in CMPD101‐ and paroxetine‐treated chondrocytes, the cells were starved for 24 h in the presence of these inhibitors before treatment with SAG for additional 12 h. Cilia were stained by ARL13B and SMO antibodies, and the intensity of SMO signal in cilia was determined. Both GRK2 inhibitors significantly impaired SAG‐mediated SMO accumulation in cilia (Figs 5A and B, and EV2A and B), similar to the findings in the GRK2 −/− patient cells (Fig 4E–G). Also similar to patient cells, the SAG‐mediated increased GLI3FL to GLI3R ratio and upregulated GLI1 expression was inhibited in cells treated with CMPD101 or paroxetine (Figs 5C and D, and EV2C and D). Next, the doxycycline (DOX)‐inducible shRNA expression was used to target GRK2 in control human R00‐082 fibroblasts. Cells were transduced with lentiviral vectors for stable integration, and probed for GRK2 expression after 4 days of DOX induction. Approximately 75% GRK2 knock‐down was achieved with the shRNA, compared to scramble shRNA control (Fig 5E). Treatment of the GRK2 shRNA chondrocytes with SAG showed normal accumulation of SMO in cilia of non‐induced cells, in contrast to DOX‐induced cells that failed to accumulate SMO in cilia (Fig 5F).
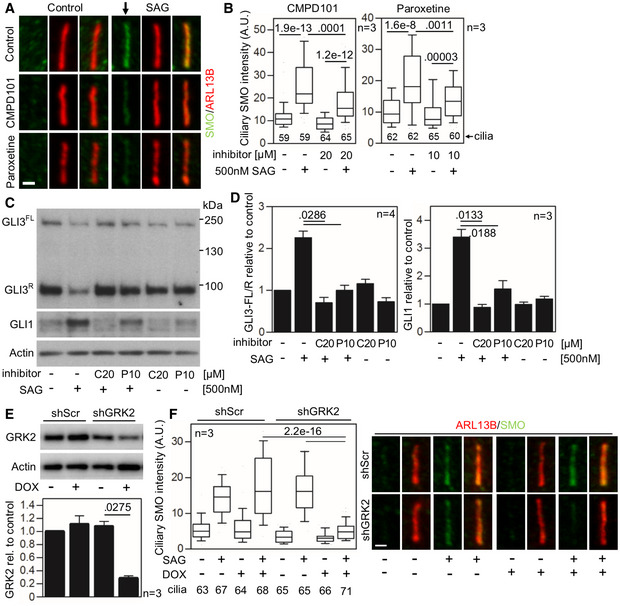
Figure 5. Inhibition of GRK2 activity decreases Smoothened accumulation in cilia and GLI3 processing.
-
A–DDefective SMO cilia accumulation and inhibited Hh signaling in chondrocytes treated with GRK2 inhibitors. Control human R00‐082 chondrocytes were serum starved in the presence of a GRK2 inhibitor, either CMPD101 (C) or paroxetine (P), for 24 h before they were treated with SAG for additional 12 (A, B) or 24 h (C, D). (A,B) Cilia were stained by ARL13B and SMO antibodies, and the intensity of ciliary SMO was analyzed and plotted. Note the impaired SMO accumulation in cilia caused by GRK2 inhibition (black arrow). Central band, median. Box, 1st‐3rd quartile. Whiskers, 10%‐90% percentile. Mann–Whitney U test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bar, 1 μm. (C) Cell lysates were immunoblotted for GLI3 processing and GLI1 upregulation; actin levels served as a loading control. GLI3FL and GLI3R, full‐length, and repressor GLI3 variants, respectively. (D) Quantification of GLI3 and GLI1 levels by densitometry. Note the impaired GLI3 processing and no GLI1 upregulation in cells treated with CMPD101 or paroxetine. Mean ± SEM. Mann–Whitney U‐test (GLI3 ratio) and Welch's t‐test (GLI1 levels); number of biological experiments is indicated.
-
E, FDefective SMO cilia accumulation in chondrocytes with downregulated GRK2. (E) Doxycycline (DOX)‐inducible GRK2 downregulation in control human R00‐082 chondrocytes, tested by Western blot, normalized to actin, and plotted below. Mean ± SEM. Welch's t‐test; number of biological experiments is indicated. shScr, scramble shRNA. (F) Cells pre‐treated with DOX for three days were serum starved, SAG treated, stained, and analyzed as in (A,B), and the intensity of ciliary SMO was analyzed and plotted. Note the impaired SMO accumulation in cilia caused by GRK2 loss. Central band, median. Box, 1st‐3rd quartile. Whiskers, 10%‐90% percentile. Mann–Whitney U test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bar, 1 μm.
Source data are available online for this figure.
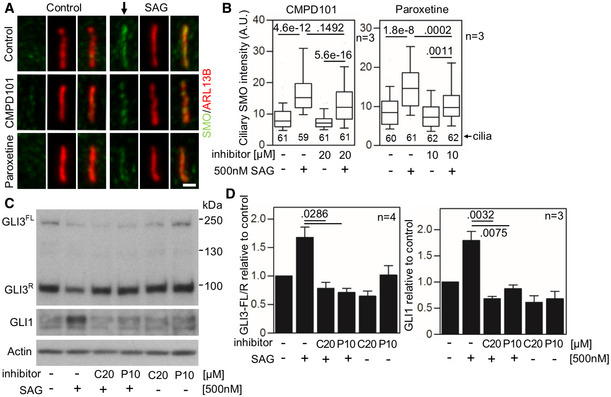
Figure EV2. Inhibition of GRK2 activity decreases SMO accumulation in cilia and GLI activity in the human chondrocytes.
-
A–DDefective SMO cilia accumulation and inhibited Hh signaling in chondrocytes treated with GRK2 inhibitors. Control human R92‐284 chondrocytes were serum starved in the presence of a GRK2 inhibitor, either CMPD101 (C) or paroxetine (P), for 24 h before they were treated with SAG for additional 12 (A, B) or 24 h (C, D). (A, B) Cilia were stained by ARL13B and SMO antibodies, and the intensity of ciliary SMO was analyzed and plotted. Note the impaired SMO accumulation in cilia caused by GRK2 inhibition (black arrow). Central band, median. Box, 1st–3rd quartile. Whiskers, 10%–90% percentile. Mann–Whitney U‐test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bar, 1 μm. (C) Cell lysates were immunoblotted for GLI3 processing and GLI1 upregulation; actin levels served as a loading control. GLI3FL and GLI3R, full‐length and repressor GLI3 variants, respectively. (D) Quantification of GLI3 and GLI1 levels by densitometry. Note the impaired GLI3 processing and no GLI1 upregulation in cells treated with CMPD101 or paroxetine. Mean ± SEM. Mann–Whitney U‐test (GLI3 ratio) and Welch's t‐test (GLI1 levels); number of biological experiments is indicated.
Source data are available online for this figure.
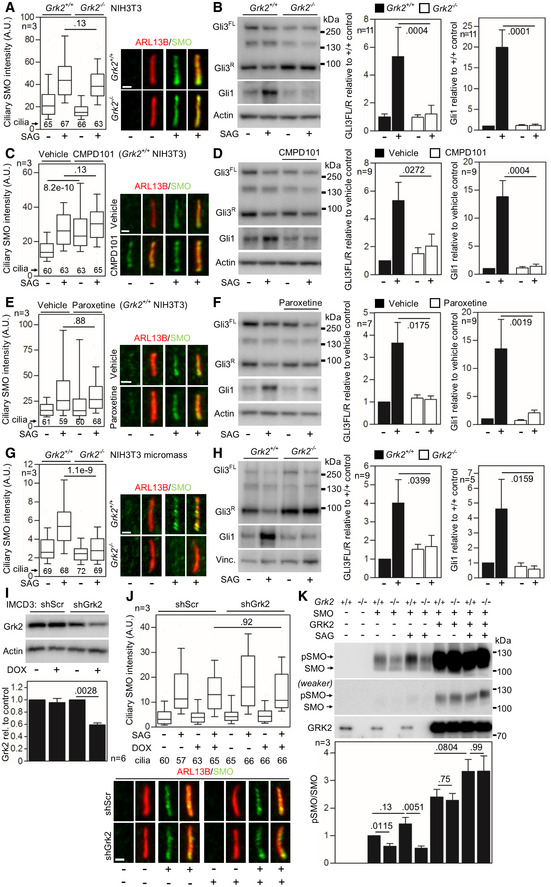
The effect of GRK2 removal, or inhibition of its kinase activity, on SMO accumulation in cilia varied between mouse and human cells. Mouse NIH3T3 Grk2 −/− cells responded to SAG treatment with SMO accumulation in the cilia, while still failing to process Gli3 and induce Gli1 expression (Fig EV3A and B). Similarly, inhibition of GRK2 activity in wild‐type (Grk2 +/+) NIH3T3 cells, by paroxetine or CMPD101, did not prevent SAG‐induced SMO accumulation in the cilia (Fig EV3C and E). Similar data were found in IMCD3 mouse cells, where downregulation of GRK2 expression by DOX‐inducible shRNA did not abolish SMO accumulation in the cilia (Fig EV3I and J). We also looked at the SAG‐mediated SMO accumulation in cilia of NIH3T3 cells differentiated toward chondrocytes in micromass culture (Fig 3F). Surprisingly, the Grk2 −/− NIH3T3 micromass cultures failed to accumulate SMO in cilia, while wild‐type micromasses accumulated SMO in cilia normally (Fig EV3G and H). Regardless of SMO accumulation, all mouse model cell lines had impaired response to SAG, as shown by analyses of GLI3 processing and induction of GLI1 expression (Fig EV3B, D, F and H). Also, the lack of SMO phosphorylation was found in the Grk2 −/− NIH3T3 cells (Fig EV3K), similar human GRK2−/− patient chondrocytes (Fig 4D).
Figure EV3. Loss of Grk2 protein or its activity does not prevent ciliary SMO accumulation in the murine NIH3T3 cells.
-
ASerum‐starved Grk2 +/+ and Grk2 −/− NIH3T3 cells were treated with 500 nM SAG for 4 h; the cells were immunostained for ARL13B and SMO, and the intensity of ciliary SMO was analyzed and plotted. Note the normal ciliary SMO intensity in Grk2 −/− cells. Central band, median. Box, 1st–3rd quartile. Whiskers, 10–90% percentile. Mann–Whitney U test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bars, 1 μm.
-
BSerum‐starved Grk2 +/+ and Grk2 −/− NIH3T3 cells were treated with 500 nM SAG for 10–16 h, and immunoblotted for Gli3 and Gli1. The optical density of the bands was normalized to that of actin, and the Gli3‐FL/R ratios and Gli1 protein levels were plotted. Mean ± SEM. Mann–Whitney U‐test; number of biological experiments is indicated. Gli3FL and Gli3R, full‐length and repressor GLI3 variants, respectively.
-
C–FGrk2 +/+ NIH3T3 cells were serum starved together with 25 μM CMPD101 (C, D) or 1 μM paroxetine (E, F) for 12 h, and treated with 500 nM SAG for 4 hours (C, E) or 12 h (D, F). (C, E) Inhibition of Grk2 activity by neither CMPD101 nor paroxetine prevents the SAG‐induced ciliary SMO accumulation. Central band, median. Box, 1st–3rd quartile. Whiskers, 10%–90% percentile. Mann–Whitney U test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bars, 1 μm. (D, F) CMPD101 and paroxetine inhibit SAG‐mediated Gli3 processing and Gli1 upregulation. Mean ± SEM. Mann–Whitney U test; number of biological experiments is indicated.
-
G, HMicromasses derived from Grk2 +/+ and Grk2 −/− NIH3T3 cells were serum starved, treated, and analyzed as in (C–F). (G) Note the inhibition of ciliary SMO intensity in Grk2 −/− micromasses. Central band, median. Box, 1st–3rd quartile. Whiskers, 10%–90% percentile. Mann–Whitney U‐test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bars, 1 μm. (H) Western blots for Gli3 processing and Gli1 upregulation. Mean ± SEM. Mann–Whitney U‐test; number of biological experiments is indicated. Vinc., vinculin.
-
I, JNormal SMO cilia accumulation in murine IMCD3 cells with downregulated Grk2. (I) Doxycycline (DOX)‐inducible Grk2 downregulation, tested by Western blot, normalized to actin and plotted below. Mean ± SEM. Mann–Whitney U‐test; number of biological experiments is indicated. shScr, scramble shRNA. (J) Cells pre‐treated with DOX for 3 days were serum starved, SAG treated, stained, and analyzed as in (A), and the intensity of ciliary SMO was analyzed and plotted. Note the normal SMO accumulation in cilia in cells with Grk2 downregulation. Central band, median. Box, 1st–3rd quartile. Whiskers, 10%–90% percentile. Mann–Whitney – test; number of biological experiments and the total numbers of analyzed cilia are indicated. Scale bar, 1 μm.
-
KUnder‐phosphorylation of SMO in Grk2 −/− NIH3T3 cells. Lysates of SMO‐transfected cells were resolved by the phospho(p)‐shift PAGE and immunoblotted for SMO. The portion of pSMO analyzed by densitometry is shown. GRK2 add‐back demonstrates a rescue of the pSMO in Grk2 −/− cells. Mean ± SEM. Welch's t‐test; number of biological replicates is indicated.
Source data are available online for this figure.
Loss of GRK2 impairs canonical Wnt signaling
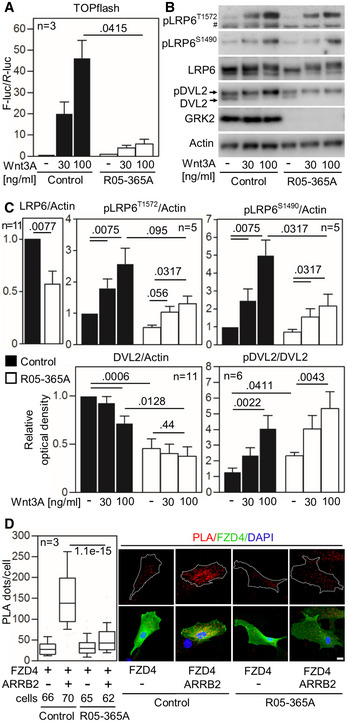
Because GRKs such as GRK5/6 are known to phosphorylate the Wnt co‐receptor LRP6 and thereby activate Wnt signaling (Chen et al, 2009), as Wnt signaling plays a key role in skeletogenesis (Gong et al, 2001; Laine et al, 2013), and as loss of GRK2 produced a profound skeletal disorder, we asked whether canonical (i.e., β‐catenin dependent) Wnt signaling is impaired in GRK2 −/− cells. Control and GRK2 −/− fibroblasts were transfected with the TOPflash luciferase reporter for detection of Wnt/β‐catenin transcriptional activity. Control cells responded to Wnt3A treatment with potent TOPflash trans‐activation, in contrast to GRK2 −/− cells in which the response to Wnt3A was markedly abrogated (Fig 6A). We next focused on the phosphorylation within the intracellular PPPS/TP motifs on LRP6. Ser/Thr phosphorylation in PPPS/TP motifs is a critical event in Wnt/β‐catenin signaling, because it provides binding sites for AXIN1 and GSK3, leading to removal of the two proteins from the β‐catenin destruction complex, and its dissolution (Wolf et al, 2008). Defective TOPflash trans‐activation in GRK2 −/− cells correlated with reduced phosphorylation of LRP6 in response to Wnt3A, as measured with antibodies specific to two of the five phosphorylated PPPS/TP sites on LRP6, S1490, and T1572 (Fig 6B and C). In addition to the diminished phosphorylation of LRP6 in response to Wnt3A, we also detected decreased levels of total LRP6 in GRK2 −/− cells (Fig 6C). Moreover, GRK2 −/− cells showed increased steady‐state phosphorylation of disheveled 2 (DVL2) (Fig 6B and C), an important signaling protein required for Wnt‐induced LRP6 phosphorylation (Bilic et al, 2007), which may suggest that there is compensation for insufficient signal transduction through LRP6 phosphorylation in the absence of GRK2. A similar cellular phenotype has been previously observed in other mutants that affect signal transduction at the level of Wnt receptor complexes (Bryja et al, 2007). Together, these results demonstrated an impaired capacity of GRK2 −/− cells to respond to canonical Wnt pathway activation.
Figure 6. Loss of GRK2 impairs canonical Wnt signaling.
-
AControl and GRK2 −/− fibroblasts were transfected with the TOPflash Firefly (F) luciferase vector together with a control Renilla (R) luciferase vector, and the effect of Wnt3A on TOPflash transcriptional activation was determined by dual‐luciferase assay. Note the impaired response to Wnt3A in GRK2 −/− cells. Mean ± SEM. Welch′s t‐test; number of biological experiments is indicated.
-
B, CLow phosphorylation (p) of Wnt3A co‐receptor LRP6 as determined by Western blot with pS1490‐ and pT1572‐LRP6 specific antibodies, reduced LRP6 and DVL2 expression, and increased steady state phosphorylation of DVL2. A representative experiment of four is shown. #, nonspecific band. (C) Densitometry of Western blot results demonstrating decreased levels of LRP6 in GRK2 −/− cells, lower response of GRK2 −/− cells to Wnt3A by LRP6 phosphorylation at S1490, and tendency toward the similar defect at T1572. Densitometry also showed less of total DVL2 levels, and increased steady state pDVL2 in GRK2 −/− cells. Mean ± SEM. Mann–Whitney U‐test; number of biological experiments is indicated.
-
DLoss of interaction between Frizzled 4 (FZD4) and ß‐Arrestin 2 (ARRB2) in GRK2 −/− fibroblasts. Proximity ligation assay (PLA) between expressed FZD4‐GFP and ARRB2‐Flag showed significantly reduced proximity events between the two proteins in GRK2 −/− cells. White dashed lines outline the transfected cells. Central band, median. Box, 1st–3rd quartile. Whiskers, 10–90% percentile. Mann–Whitney U‐test; number of biological experiments and the total numbers of analyzed cells are indicated. Scale bar, 10 μm.
Source data are available online for this figure.
Next, the mechanism by which GRK2 regulates Wnt signaling was interrogated. In 293T cells transfected with LRP6 and GRK2, no increased LRP6 phosphorylation was detected (unpublished observation), suggesting that GRK2 is not a LRP6 kinase. Next, we focused on ARRB2, which is well‐known partner of GRK2, involved in GRK2‐mediated agonist‐induced desensitization b2‐adrenergic receptors (Goodman et al, 1996). Importantly, ARRB2 is also a component of the canonical Wnt pathway, where it associates with the transmembrane Wnt signaling complex containing LRP6, FZD, and DVL. Importantly, the ARRB2 association with Wnt signaling complex is necessary for proper activation of pathway in response to Wnt ligands (Bryja et al, 2007). We asked whether ARRB2 interaction with FZD is affected by the GRK2 loss. The FZD4 and ARRB2 were expressed in R05‐365A patient cells, and their interaction was probed by proximity ligation assay. The results show a loss of interaction of FZD4 with ARRB2 in the GRK2−/− patient cells, compared to control fibroblasts (Fig 6D).
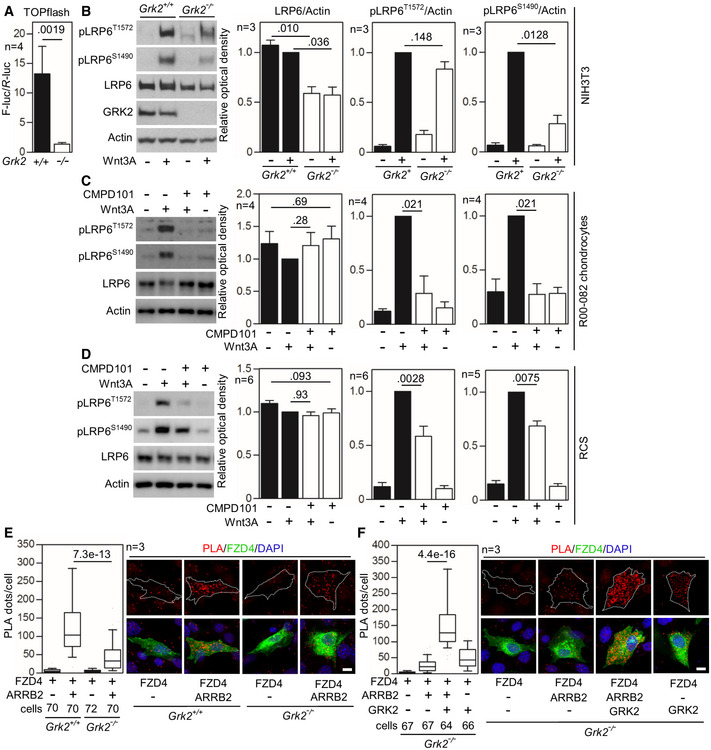
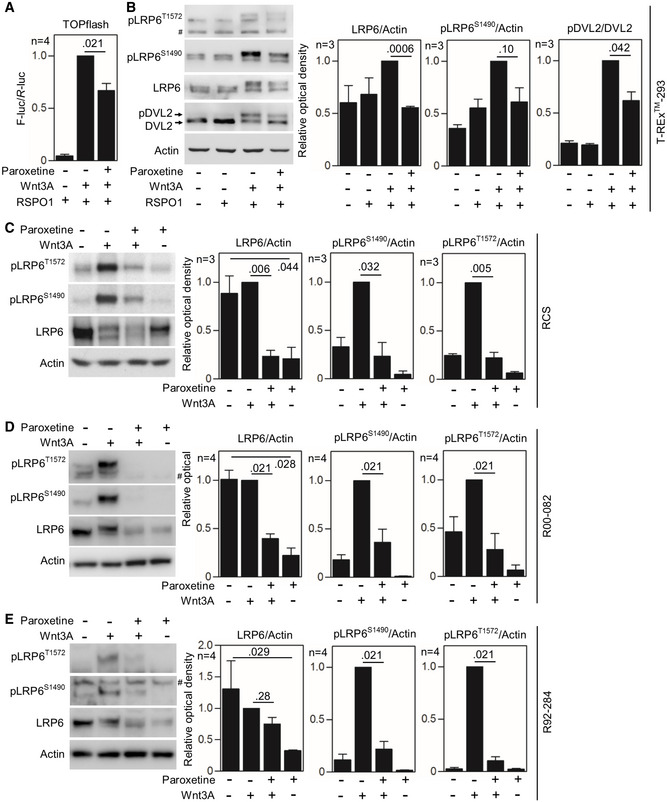
To confirm the data obtained with GRK2 −/− patient fibroblasts, we again used the NIH3T3 Grk2 −/− cells (Pusapati et al, 2018). The Wnt3A‐mediated trans‐activation of TOPflash reporter was significantly downregulated in Grk2 −/− NIH3T3 cells compared to wild‐type controls (Fig 7A). This was accompanied by downregulated total LRP6 protein in Grk2 −/− NIH3T3 cells, and under‐phosphorylation of pLRP6S1490 in response to Wnt3A (Fig 7B). In rat chondrosarcoma (RCS) chondrocytes and control human R00‐082 chondrocytes, treatment with GRK2 inhibitor CPMD101 produced no effect on LRP6 protein expression, but inhibited the Wnt3A‐mediated phosphorylation of LRP6 on T1572 and S1490 (Fig 7C and D). In T‐REx™‐293 cells, RCS chondrocytes, and control human R92‐284 and R00‐082 chondrocytes, inhibition of GRK2 kinase activity by paroxetine caused diminished response to Wnt3A in the TOPflash assay (Fig EV4A), downregulated total LRP6, and impaired LRP6 phosphorylation in response to Wnt3A (Fig EV4B–E). As GRK2 loss in the R05‐365A patient fibroblasts and Grk2 −/− NIH3T3 cells lead to downregulation of the LRP6 protein levels, we aimed to see whether the levels of the Frizzled (FZD) Wnt receptor were also affected in these cells. The Western blots showed that both cell types express FZD5, but not FZD4 or FZD10, and that the loss of GRK2 does not affect FZD5 expression (Fig EV5). Finally, Grk2 −/− NIH3T3 cells showed a significant reduction of FZD4 interaction with ARRB2, when compared to wild‐type (Grk2 +/+) cells (Fig 7E), a similar finding as in GRK2‐/‐ patient cells (Fig 6D). This phenotype was rescued by Grk2 add‐back into the Grk2 −/− NIH3T3 cells (Fig 7F), suggesting that one mechanism how GRK2 regulates Wnt signaling is promotion of ARRB2 association with the Wnt signaling complex.
Figure 7. Inhibition of GRK2 activity inhibits canonical Wnt signaling.
-
AControl (+/+) and Grk2 −/− NIH3T3 cells were transfected with the TOPflash Firefly (F) luciferase vector together with a control Renilla (R) luciferase vector, treated with Wnt3A (100 ng/ml) for 24 h and subjected to dual‐luciferase assay. Note the impaired response to Wnt3A in Grk2 −/− cells. Mean ± SEM. Mann–Whitney U test; number of biological experiments is indicated.
-
BControl (+/+) and Grk2 −/− NIH3T3 cells were treated with 40 ng/ml Wnt3A for 1 h. Note the lower LRP6 expression and inhibited Wnt3A‐induced LRP6 phosphorylation in the Grk2 −/− NIH3T3 cells. Mean ± SEM. Welch's t‐test; number of biological experiments is indicated.
-
C, DLoss of LRP6 phosphorylation in chondrocytes treated with the GRK2 inhibitor CMPD101. Control human R00‐082 chondrocytes (C) and rat chondrosarcoma (RCS) cells (D) were treated with 20 μM CMPD101 overnight and then treated with 100 ng/ml Wnt3A for 1 h. Note the less LRP6 phosphorylation (pS1490‐ and pT1572‐LRP6) in cells treated with CMPD101. Mean ± SEM. Mann–Whitney U test; number of biological experiments is indicated.
-
E, FLoss of interaction between Frizzled 4 (FZD4) and ß‐Arrestin 2 (ARRB2) in Grk2 −/− NIH3T3 cells. (E) Proximity ligation assay (PLA) between expressed FZD4‐GFP and ARRB2‐Flag showed significantly reduced proximity events between the two proteins in Grk2 −/− cells. (F) GRK2 add‐back rescued the interaction. White dashed lines outline the transfected cells. Central band, median. Box, 1st–3rd quartile. Whiskers, 10–90% percentile. Mann–Whitney U test; number of biological experiments and the total numbers of analyzed cells are indicated. Scale bars, 10 μm.
Source data are available online for this figure.
Figure EV4. Inhibition of GRK2 activity inhibits canonical Wnt signaling.
-
AT‐REx™‐293 cells were transfected with the TOPflash Firefly (F) luciferase vector together with a control Renilla (R) luciferase vector, and the effect of Wnt3A on TOPflash transcriptional activation was determined by dual‐luciferase assay. Note the less Wnt3A‐induced TOPflash trans‐activation in cells treated with 20 μM paroxetine. R‐spondin‐1 (RSPO1) was used to block endogenous inhibition of canonical Wnt. Mean ± SEM. Mann–Whitney U‐test; number of biological experiments is indicated.
-
BT‐REx™ 293 cells with GRK2 inhibited by 20 μM paroxetine show lower levels of LRP6 and lower pDVL2/DVL2 ratio, as determined by Western blot and quantified by densitometry. No significant Wnt3A‐induced LRP6‐T1572 phosphorylation was observed in T‐REx™ 293 cells. Mean ± SEM. Welch's t‐test; number of biological experiments is indicated.
-
C–ERat chondrosarcoma (RCS) cells (C), and control R00‐082 (D) and R92‐284 (E) chondrocytes were treated with 20 μM paroxetine overnight and then treated with Wnt3A for 1 h. Note the lower levels of LRP6 and less LRP6 phosphorylation (pS1490‐ and pT1572‐LRP6) in cells treated with paroxetine. #, nonspecific band. Mean ± SEM. Welch's t‐test (C) and Mann–Whitney U test (D, E); number of biological experiments is indicated.
Source data are available online for this figure.
Figure EV5. Frizzled 5 (FZD5) levels are normal in R05‐365A GRK2 −/− fibroblasts and Grk2 −/− NIH3T3 cells.
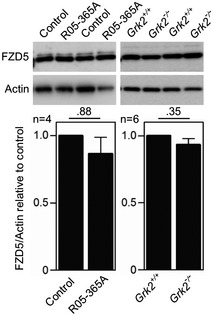
Lysates of R05‐365A GRK2 −/− fibroblasts and Grk2 −/− NIH3T3 cells were analyzed by Western blots for expression levels of FZD5. After normalization to actin, the densitometry revealed no differences between the wild‐type and GRK‐null cells. Mean ± SEM. Mann–Whitney U‐test; number of biological experiments is indicated.Source data are available online for this figure.
Discussion
Loss of GRK2 results in ATD and loss of Hh signaling
We determined that loss of GRK2 in humans results in the ATD phenotype, characterized by marked skeletal abnormalities, an atrial septal defect and significant respiratory impairment. Loss of GRK2 resulted in under‐phosphorylation of SMO and its exclusion from the cilia in cultured patient cells, and in GRK2‐downregulated and GRK2‐inhibited human chondrocytes (Figs 4D–G and 5A, B, E, F, and EV2A and B), demonstrating that GRK2 activity is critical for SMO accumulation in human primary cilia. In addition, there was no transcriptional induction of Hh response genes in the GRK2 −/− cells, so the cells were functionally nonresponsive to Hh signaling (Fig 4C). These data contrast with findings in mouse cells, in which the SMO accumulation was abrogated only at one case, i.e., in Grk2 −/− NIH3T3 cells that were primed toward chondrocytes in micromass culture. In undifferentiated Grk2 −/− NIH3T3 cells, in NIH3T3 cells treated with Grk2 inhibitors (Zhao et al, 2016; Pusapati et al, 2018) or in IMCD3 cells with downregulated Grk2, the SMO accumulated in cilia upon SAG normally, despite its under‐phosphorylation (Fig EV3). This suggests that the SMO ciliary trafficking requires GRK2 in a cell context‐dependent manner, and that, in human skin fibroblasts and cultured chondrocytes, this process is more dependent on the GRK2 activity. Notably, the SAG‐mediated GLI3 activation and GLI1 induction were inhibited in all mouse and human cell models used in this study, suggesting the Hedgehog signaling is impaired in the absence of GRK2 or its activity, regardless of SMO ciliary accumulation (Zhao et al, 2016; Pusapati et al, 2018; preprint: Happ et al, 2020).
According to the current understanding, a massive SMO phosphorylation is necessary for its full activation and for the expression of high‐threshold Hh genes, with the extent of the Hh pathway activity correlating with the SMO phosphorylation status (Jia et al, 2004; Chen et al, 2011). Specifically in vertebrates, Hh ligand binding to its receptor PTCH1 causes CK1α to phosphorylate the proximal regions of the C‐terminal tail of SMO. This leads to a more open conformation of the SMO C‐tail and prompts GRK2 to phosphorylate SMO at several more sites, leading to its full activation (Chen et al, 2004, 2010, 2011; Meloni et al, 2006; Zhao et al, 2007; Kovacs et al, 2008). Because CK1α phosphorylation sites on SMO partially overlap with those of GRK2 (Chen et al, 2011), it is unclear why, in the absence of GRK2, CK1α‐mediated phosphorylation does not partially rescue the SMO signaling. Similarly, why other GRKs cannot phosphorylate SMO in the absence of GRK2 is surprising in that current thinking postulates that all GRKs are able to phosphorylate any of their GPCR targets, albeit with different efficiencies (Gurevich et al, 2012). Future studies will be needed to define the hierarchy of activity of the various molecules that phosphorylate SMO to mediate Hh signal transduction. Interestingly, the distinct phenotype produced by loss of GRK2 suggests that GRK2 has distinct roles, particularly in skeletogenesis.
No gross CNS abnormalities were observed in the GRK2 null individuals. This is surprising since the CNS patterning depends on Hh activity (Ribes & Briscoe, 2009), and the patient's GRK2 −/− skin fibroblasts did not respond to SAG stimulation. The published studies show that deletion of Grk2 in the mouse leads only to mild defects in the neural tube patterning (Philipp et al, 2008), and that loss of both Grk2 and Grk3 is required to fully inhibit the GLI3 processing and subsequently the Hh target gene expression in the murine neural precursor cells (Pusapati et al, 2018). This suggests at least partially redundant functions of Grk2 and Grk3 in cells and tissues expressing both proteins, and could explain the absence of CNS abnormalities in the GRK2 null patients. Unlike neural precursors, the mouse embryonic fibroblasts and NIH3T3 cells do not express Grk3 (Zhao et al, 2016; Pusapati et al, 2018); therefore, genetic ablation of Grk2 alone is sufficient to obtain full inhibition of the Hh pathway in these cells. In line with these observations, the complete inhibition of the SAG‐mediated GLI3 processing and GLI1 and PTCH1 expression in the GRK2 −/− skin fibroblasts suggest they either do not express GRK3 or (less likely) that GRK3 does not contribute to Hh signaling in these cells. It is of note, however, that Grk3 knock‐out mice develop normally (Peppel et al, 1997).
Most IFT mutants causing ATD and SRPS disrupt cilia number and architecture, which impairs Hh signaling as well as other ciliary functions. However, the cilia of GRK2 −/− cells were structurally normal, suggesting that the phenotype is mediated through abnormal cilia function. In control cells, Hh pathway activation results in activation of SMO and degradation of GLI3R, increasing the GLI3FL/GLI3R ratio and inducing expression of downstream target genes (Wang et al, 2007). In GRK2 −/− cells, the level of GLI3R was maintained and target gene expression could not be induced. Loss of Hh signaling and the resulting reduction of growth plate chondrocyte proliferation is likely to be mediated in part through defective or loss of Indian hedgehog (IHH) signaling. Ihh knock‐out mice show severe dwarfism with abnormal axial and appendicular skeletal elements due to the marked reduction of chondrocyte proliferation and poorly organized, smaller hypertrophic zones (St‐Jacques et al, 1999). IHH is produced by post‐mitotic chondrocytes in the early stage of hypertrophic differentiation and regulates proliferation by increasing the expression of the chondrocyte mitogen PTHrP in the perichondrium (Kronenberg, 2006). The defective endochondral ossification resulting from loss of GRK2 is consistent with dependence of growth plate chondrocytes on IHH signaling.
GRK2 in regulation of canonical Wnt signaling
Although there were similarities between the Ihh −/− mouse (St‐Jacques et al, 1999) and GRK2 −/− human growth plates, the latter exhibited a more marked loss of hypertrophic chondrocytes (Fig 3B). This difference suggested that perhaps reduced Hh signaling was not the sole contributor to the cartilage growth plate pathology and mineralization defect. Based on the roles of paralogous GRKs in Wnt signaling, we tested the hypothesis that the human GRK2 −/− cells might have defective Wnt signaling. The impaired transcriptional response to the canonical Wnt ligand, Wnt3A, in GRK2 −/−cells, was accompanied by downregulated LRP6 expression and markedly diminished phosphorylation of PPPS/TP motifs within the LRP6 cytoplasmic tail, demonstrating that defective canonical Wnt signaling also contributes to the ATD phenotype.
In canonical Wnt signaling, the transcriptional activity of β‐catenin is low due to its degradation mediated by the cytoplasmic destruction complex composed of Axin, Adenomatosis polyposis coli (APC), and GSK3. Canonical Wnt ligands including Wnt3A cause dissolution of the destruction complex leading to β‐catenin stabilization and activation of β‐catenin‐dependent transcription (Nusse & Clevers, 2017). Phosphorylation at ser/thr residues in several PPPS/TP motifs within the cytoplasmic tail of Wnt co‐receptor LRP6, mediated by GSK3, is critical events in the cellular response to canonical Wnts, because these motifs sequester GSK3 away from the destruction complex, efficiently eliminating its function (Cselenyi et al, 2008). Some GRKs such as GRK5/6 are also known to activate Wnt signaling via LRP6 phosphorylation (Chen et al, 2009). We found GRK2 unable to phosphorylate LRP6 in cells, suggesting that GRK2 is likely not a LRP6 kinase (unpublished observation). Instead, the loss of GRK2 impaired interaction between FZD4 and ARRB2 (Figs 6D and 7E). In canonical Wnt signaling, ARRB2 promotes formation of a ternary ARRB2‐FZD‐DVL complex, which promotes activation of the Wnt signaling through sequestration of axin away from the β‐catenin destruction complex, leading to dissolution of destruction complex (Bryja et al, 2007; Rosanò et al, 2009). Recruitment of DVL to FZD depends on phosphorylation of the FZD C‐terminus by kinases, including CK1 and GRK2 (Strakova et al, 2018). It is therefore possible that the inhibited response of GRK2‐null cells to Wnt stimulation is due to abolished formation of the FZD‐DVL‐ARRRB2‐axin complex.
Our findings differ from the findings of Wang et al (2009) that indicate that GRK2 negatively regulates Wnt signaling through interactions between GRK2 and APC protein. Their findings suggest that loss of GRK2 would have a positive effect on bone mineralization, in contrast to our findings of a negative impact on bone mineralization based on the radiographic phenotype.
Canonical Wnt signaling is a critical regulator of chondrocyte differentiation and promotes the development of hypertrophic chondrocytes via inhibition of mitogenic PTHrP signaling and induction of hypertrophic chondrocyte proteins such as type X collagen and matrix metalloproteinase 13 (Tamamura et al, 2005; Dong et al, 2006; Guo et al, 2009). In mice, constitutive activation of β‐catenin‐mediated transcription promoted chondrocyte hypertrophy and maturation, leading to an elongated hypertrophic zone. In contrast, β‐catenin inactivation resulted in small hypertrophic zones, disorganized pre‐hypertrophic chondrocytes, and delayed primary ossification (Dao et al, 2012). The similarity of the latter phenotype with the shortened and disorganized hypertrophic cartilage, and poorly developed primary bone observed in the growth plate suggests that the loss of GRK2 negatively affects skeletogenesis, in part mediated by defective canonical Wnt signaling.
GRK2 in skeletogenesis
Loss of GRK2 in humans produces a severe, but not an early embryonic lethal skeletal disorder. By contrast, loss of GRK2 in mice (Grk2 −/− or βark1 −/−) resulted in embryonic lethality between E9 and E15.5 (Jaber et al, 1996). The Grk2 −/− embryos were described as smaller than their wild‐type and heterozygous littermates and had significant hypoplasia of the ventricular myocardium (Jaber et al, 1996). Similarly, conditional Grk2 mice with EIIA‐Cre‐mediated germline deletion of Grk2 exhibited early embryonic lethality at E10.5; embryos were also described as smaller and having hypoplasia of a single ventricle, suggesting impaired or delayed heart development (Matkovich et al, 2006). Conditional ablation of Grk2 in cardiomyocytes using Nkx2.5‐Cre showed normal cardiac development and viability, but the mice did show enhanced baseline contractility and diminished β‐adrenergic receptor‐mediated tachyphylaxis (Matkovich et al, 2006). Thus, the mouse data demonstrated that overall growth and heart development in mice depend on GRK2. One patient had a cardiac defect, but it has not been previously appreciated that GRK2 has a profound effect on skeletogenesis.
In summary, this study demonstrates that loss of GRK2 causes ATD via defects in ciliary signaling rather than as a consequence of a structural defect in primary cilia. We confirmed that GRK2 is a positive regulator of Hh signaling, and that its absence results in inhibition of the GLI3 activity. We also show that loss of GRK2 activity results in defective ciliary accumulation of SMO in human fibroblasts and chondrocytes, and in chondrocytic micromasses produced from murine NIH3T3 cells. We also demonstrated impaired canonical Wnt signaling in GRK2 −/− cells and showed that this defect stems from reduced expression and phosphorylation of LRP6 in response to canonical Wnt ligands. Finally, a severe under‐sulfation of extracellular matrix proteoglycans was found in the ATD cartilage, and in the cartilaginous micromass cultures generated from Grk2‐null or Grk2‐inhibited cells (Fig 3D–F), suggesting that GRK2 regulates proteoglycan metabolism. The maintenance of proteoglycan homeostasis is important for the cartilage growth, as numerous skeletal dysplasias associate with deficient sulfation (Paganini et al, 2020). Apart from organization of the extracellular matrix network, the sulfated proteoglycans also promote the Ihh and TGFβ signaling, which is essential for chondrocyte proliferation and differentiation (Klüppel et al, 2005; Cortes et al, 2009; Gualeni et al, 2010). The mechanism of how GRK2 regulates proteoglycan sulfation is not known, and is opened for future research.
Further investigations should reveal which of the many potential targets of GRK2 are contributing to its role in ATD. Apart from GRK2, mutations in two other ser/thr kinases, ICK and NEK1, are also associated with skeletal ciliopathies (Thiel et al, 2011; Paige Taylor et al, 2016), together revealing an emerging theme that regulation of cell function by ser/thr kinases plays a role in cilia disorders, as well as in skeletal patterning and linear growth.
Materials and Methods
Ascertainment
Affected individuals were ascertained under human subjects’ protocols, and the diagnosis of ATD was made by reviewing clinical records and radiographic images. Each proband was assigned a reference number, R05‐365A and Cmh001543‐01. In the case of R05‐365A, a skin fibroblast culture was established and a section of distal femur was placed in paraffin for histologic analysis. Human subject approval for the affected individual and their unaffected family members was obtained through an approved University of California at Los Angeles human subject protocol that follows principles set forth in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. Human data and samples can be obtained with proper institutional human subjects approval.
Exome analysis
Genomic DNA was isolated from cultured fibroblasts and serum using a kit, according the manufacturer's protocol (Qiagen). Library construction and exome sequencing of DNA from the proband was carried out at the University of Washington Center for Mendelian Genomics and Children's Mercy, Center for Pediatric Genomic Medicine. The exome sequencing libraries were prepared with the NimbleGen SeqCap EZ Exome Library v2.0 kit and sequenced on the Illumina GAIIx platform. Reads were mapped with Novoalign and variants called using the Genome Analysis Toolkit following their Best Practices recommendations. Variants were filtered against public databases and annotated as previously described (Lee et al, 2012). The identified variants in GRK2 were confirmed by Sanger sequence analysis of amplified DNA.
Animal experiments
ISA brown fertilized chicken eggs were obtained from Integra farm (Zabcice, Czech Republic). Chicken embryos were selected randomly for the study. All fertilized eggs without developmental defects were used for the injection. Eggs were incubated in a humidified incubator at 37.8°C. CMPD101 (10 mM; Tocris) was applied into the right wing bud at stage HH20‐22. Micromanipulator (Leica) and a microinjector (Eppendorf) were used to better target the selected area of application. Embryos were collected after 10–12 days of incubation following the treatment. Both right (injected) and left (untreated) wings were fixed in 100% ethanol, stained with Alizarin red/Alcian blue solution, and cleared in KOH/glycerol. Injection of CMPD was performed in 2 independent experiments: 17 embryos, 42 embryos; 18 embryos died shortly after injection, and the skeletal analysis was performed on 27 embryos. Bones that could not be accurately measured due to insufficient quality of the acquired pictures or the bone preparation were excluded from the analysis. Micromasses were established from chicken embryos at 3 independent experiments from approximately 80 embryos for each experiment. Mesenchymal cultures were established from the anterior and posterior parts of forelimb buds of stage HH20‐21 chicken embryos. Wing buds were dissected into Pucks saline A (0.8% NaCl, 0.04% KCl, 0.035% NaHCO3, 0.1% glucose), digested by Dispase II (1 U/ml; Sigma), and filtered to obtain a single cell population. Cells were resuspended at a density of 2 × 107 cells/ml and plated in 10 μl aliquots in 6‐well culture dishes. Cells were left to adhere for 1 h in the incubator before 2 ml of culture media (F12/DMEM supplemented with 10% FBS, penicillin, streptomycin, glutamine, 1% ascorbic acid, and 10 mM β‐glycerol phosphate) containing CMPD101 was added to each well. Micromasses were cultured for 5 days. For Alcian blue staining, micromass cultures were fixed by 4% paraformaldehyde, washed in PBS, and stained overnight with 1% Alcian blue in 0.1 M HCl (pH1.0) or 1% Alcian blue in 3% CH3COOH (pH2.5). Images were taken with a Leica S6D (Leica) microscope, and signal was quantified in Adobe Photoshop 7.0. The analyses were done blinded by a researcher different from the one doing injection. All procedures were performed according to the experimental protocols and rules established by the Laboratory Animal Science Committee of the Institute of Animal Physiology and Genetics (Libechov, Czech Republic).
Cell lines, transfection, micromass culture, shRNA, luciferase reporter assay, and RT–PCR
Control and R05‐365A skin fibroblasts (38 weeks gestation), and R00‐082 (22 weeks), and R92‐284 (22 weeks) control chondrocytes were obtained under an approved University of California at Los Angeles human subjects protocol. Primary cultures of cells isolated from skin or growth plate cartilage were cryopreserved in liquid nitrogen; low passage number cells (<p. 6) were used in the experiments. T‐REx™‐293 cells were purchased from Thermo Scientific. RCS cells were obtained from B. de Crombrugghe (Mukhopadhyay et al, 1995). Grk2 −/− NIH3T3 cells were previously described (Pusapati et al, 2018). Cells were propagated in DMEM media supplemented with 10% FBS and antibiotics (Invitrogen), and starved in 0.5% serum (chondrocytes and human fibroblasts) or in 0.1% FBS (NIH3T3). IMCD3 were obtained from ATCC, propagated in DMEM:F12 (1:1) supplemented with 10% FBS and antibiotics, and starved without serum. All cell lines were routinely tested for mycoplasma infection using DAPI staining. For micromasses, the NIH3T3 cells were seeded in 10 μl aliquots in 6‐well plate at density 1×107 cells/ml and left to adhere for 2 h before 2 ml of culture media (F12/DMEM supplemented with 10% FBS, penicillin, streptomycin, glutamine, 1% ascorbic acid, and 10 mM β‐glycerol phosphate) containing CMPD101 was added to each well. Micromasses were cultured for 7 days, and the medium, supplemented with CMPD101, was changed every other day. For shRNA, the murine IMCD3 and human R00‐082 cell lines with stably integrated, doxycycline‐inducible expression of shRNA targeting GRK2 and the respective scramble controls were generated by lentiviruses. Lentiviral vector containing doxycycline‐inducible U6 promoter and TetRep‐P2A‐Puro‐P2A‐mCherry (Eshtad et al, 2016; Kunova Bosakova et al, 2019) (kindly provided by Mikael Altun) was modified to express shRNA by introducing the following oligonucleotides (mouse‐shGRK2 forward: CCGGGAGATCTTTGACTCCTATATTCTCGAGAATATAGGAGTCAAAGATCTCTTTTTG, mouse‐shGRK2 reverse: AATTCAAAAAGAGATCTTTGACTCCTATATTCTCGAGAATATAGGAGTCAAAGATCTC; mouse‐scrambled forward: CCGGGATGCTAACGTCTTATACTTTCTCGAGAAAGTATAAGACGTTAGCATCTTTTTG, mouse‐scrambled reverse: AATTCAAAAAGATGCTAACGTCTTATACTTTCTCGAGAAAGTATAAGACGTTAGCATC; human‐shGRK2 forward: CCGGGAGGCTGACATGCGCTTCTATCTCGAGATAGAAGCGCATGTCAGCCTCTTTTTG, human‐shGRK2 reverse: AATTCAAAAAGAGGCTGACATGCGCTTCTATCTCGAGATAGAAGCGCATGTCAGCCTC; human‐scrambled forward: CCGGGGATTCAGCTGCACGACTTGTCTCGAGACAAGTCGTGCAGCTGAATCCTTTTTG, human‐scrambled reverse: AATTCAAAAAGGATTCAGCTGCACGACTTGTCTCGAGACAAGTCGTGCAGCTGAATCC; cloned shRNA sequences were verified by Sanger sequencing. Lentiviral particles were generated as described previously (Peskova et al, 2019; Barta et al, 2016) using pMD2.G (Addgene #12259) and psPAX2 (Addgene #12260) (gift from Didier Trono). After transduction, at least 2x104 mCherry‐positive cells were sorted using BD FACSAria™ II (BD Biosciences). Generated cell lines were propagated in the presence of 1 μg/ml puromycin. shRNA expression was induced by 1 μg/ml doxycycline (Invitrogen) for in total four days before the analyses. Wnt3A was obtained from RnD Systems, SAG from EMD Millipore, CMPD101, and paroxetine from Tocris. Cells were transfected by electroporation using the Neon Transfection System (Invitrogen). For the luciferase reporter assays, the μg ratio between the TOPflash luciferase vector (obtained from R. Moon) and the pRL‐TK (Promega) control Renilla vector was 3∶1. The luciferase activity was determined using a Dual‐Luciferase Reporter Assay (Promega). For RT–PCR, total RNA was isolated using the RNeasy Mini Kit (Qiagen) and reverse transcribed with the First Strand cDNA Synthesis Kit (Roche). The following primers were used for RT–PCR: GRK2‐F 5′‐AGAGTGCCACTGAGCATGTC‐3′ (exon 5), GRK2‐R 5′‐CCTGCTTCATCTTGATGCGC‐3′ (exon 9); GAPDH‐F 5′‐AGCCACATCGCTCAGACACC‐3′, GAPDH‐R 5′‐GTACTCAGCGCCAGCATCG‐3′. For qPCR, the following QuantiTect primers (Qiagen) were used: Hs_PTCH1_1_SG (QT00075824), Hs_GLI1_1_SG (QT00060501), and Hs_GAPDH_vb.1_SG (QT02504278).
Western blots
Cells were extracted directly in Laemmli sample buffer (125 mM Tris–HCl pH 6.8, 4% SDS, 5% ß‐mercaptoethanol, 10% glycerol, 0.01% bromphenol blue). Lysates were resolved by SDS–PAGE, transferred onto a PVDF membrane, and visualized by chemiluminescence (Thermo Scientific). For the phosphoshift PAGE, cells were transfected with the pEGFP‐mSMO vector (#25395; Addgene) for 24 h, and lysed on ice for 30 min in RIPA buffer (50 mM Tris–HCl pH7.5, 150 mM NaCl, 5 mM EDTA, 1% IGEPAL CA‐630) with freshly added 1 mM Na3VO4 and protease inhibitors (Roche). The samples were subjected to three freeze/thaw cycles before being loaded on the phosphoshift PAGE gels (6% acrylamide:bis‐acrylamide, 99:1), and the electrophoresis run at constant 100V for 2 h. The following antibodies were used: LRP6 (3395), LRP6S1490 (2568), DVL2 (3216), GLI1 (2643), and actin (3700) (Cell Signaling); actin (sc‐1615, sc‐1616) and GRK2 (sc‐562) (Santa Cruz); GRK2 (G0296) (Sigma); LRP6T1572 (72187) and FZD5 (06‐756; Millipore); GLI3 (AF3690) (RnD Systems); FZD4 (ab83042) and FZD10 (ab83044; Abcam); GFP (50430‐2‐AP, Proteintech); the final dilution was 1:1,000. Band intensities were quantified using ImageJ (http://www.imagej.nih.gov/ij/). In some in‐cell experiment, the researcher analyzing the samples was not informed about the identity of the samples, or the idea of the experiment.
Proximity ligation assay (PLA), immunochemistry, and microscopy
For the PLA, the cells were transfected with these vectors: FZD4‐EGFP (a kind gift from Gunnar Schulte; Bryja et al, 2007), pcDNA3Flag‐ARRB2 and bGRK2 (kind gifs from Robert J. Lefkowitz; Strakova et al, 2018) in a 1:3:3 ratio, fixed 24 h later in paraformaldehyde, permeabilized in 0.1% Triton X‐100, and stained by Duolink® PLA (Sigma) according to manufacturer′s protocol. Rabbit FLAG (1:200; F7425; Sigma) and mouse GFP (1:200; sc‐53882; Santa Cruz) antibodies were used for PLA; the GFP signal was used to identify the co‐transfected cells. PLA counting analysis was done in Fiji (http://fiji.sc/Fiji). For immunocytochemistry, cells were fixed in paraformaldehyde and incubated with the following antibodies: acetylated α‐tubulin (1:100; 32‐2700; Invitrogen), ARL13B (1:300; 17711‐1‐AP; Proteintech), pericentrin (1:1,000; ab4448; Abcam), γ‐tubulin (1:150; 66320‐1‐Ig; Proteintech), GLI3 (1:50; AF3690; RnD Systems), Smoothened (1:50; sc‐166685; Santa Cruz), IFT43 (1:100; sc‐245285; Santa Cruz), IFT81 (1:100; 11744‐1‐AP; Proteintech), IFT88 (1:100; 13967‐1‐AP; Proteintech), KIF3A (1:100; ab11259; Abcam), TRAF3IP (1:100; PA5‐30507; Pierce), WDR34 (1:100; HPA041091; Sigma), and ICK (1:100; HPA001113; Sigma). Secondary antibodies conjugated with AlexaFluor488/594 (1:500; A21202, A21203, A21206, A21207, A11055) were from Invitrogen; the donkey anti‐mouse AlexaFluor405 antibody was from Abcam (1:250; ab175658). Images were taken on a Carl Zeiss LSM700 laser scanning microscope with acquisition using ZEN Black 2012 software. Images were acquired using a 63× oil immersion objective as Z‐stacks with 0.3 μm distance between neighboring z‐sections. Measurements of cilia length in 3D were performed as previously described (Paige Taylor et al, 2016). The intensity of ciliary SMO was analyzed as previously described (Kunova Bosakova et al, 2018). For histology, growth plate samples were fixed with 4% PFA overnight and decalcified for 3 days. After decalcification, samples were embedded in paraffin and 10‐μm sections were prepared. Samples were stained with Picrosirius red for 1 h and hematoxylin for contrast. For immunohistochemistry, sections were treated with citrate buffer for antigen retrieval and quenched using peroxidase solution, both for 10 min each. GRK2 antibody was obtained from Cell Signaling (3982; 1:100). Immunostained sections were developed with the Histostain Plus kit with DAB as the chromogen (Invitrogen). The purified rabbit IgG isotype standard (BD Biosciences) was used as a negative control. Alcian blue staining in the patient growth plates was performed using alcian blue/hematoxylin for 40 min and then processed through a series of washes. Brightness, contrast, and threshold were adjusted in some images for a clear data presentation, always equally through the entire image and the panel of images. Only raw images and blot scans without any adjustments were used for quantitative analyses. In some in‐cell experiment, the researcher analyzing the samples was not informed about the identity of the samples, or the idea of the experiment.
Sample size and statistical analyses
The minimal number of independent biological experiments was set to three. Whenever applicable, technical replicates were done and eventually averaged for every biological experiment. With assays having higher variability, additional biological experiments were undertaken in order to present a clear information. A non‐parametric Mann–Whitney U‐test (for n ≥ 4) and Welch's t‐test (n = 3) were used to calculate the P values that are specified directly in the figure panels. Every figure and figure legends contain information on the sample size, the statistical test used, and the P value.
Author contributions
MBo, SPT, DHC, PK, and DK designed the research; SPT collected the R05‐365A patient data and analyzed primary cilia; ID and JM analyzed the growth plates; EH and MBu produced the animal and micromass experiments; TB produced the lentiviruses and shRNA cell lines; ETR and IT provided the Cmh001543‐01 patient data and contributed to the exome analyses; MBo, SPA, AN, KS, MV, LB, JK, TR, GVP, VB, DAN, MJB, and RR performed the cell experiments; MBo, DHC, PK, and DK wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
For more information
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Acknowledgements
This work was supported by NIH grants RO1 AR066124, R01 AR062651, and RO1 DE019567 to D.K and D.H.C. R.R. was supported by grant NIH/NIGMS GM118082. Sequencing was provided by the University of Washington Center for Mendelian Genomics (UW‐CMG) and was funded by NHGRI and NHLBI grants UM1 HG006493 and U24 HG008956. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NIH Training Grant in Genomic Analysis and Interpretation, T32 HG002536 supported S.P.T. This work was also supported by Ministry of Education, Youth and Sports of the Czech Republic (KONTAKT II LH15231; LTAUSA19030); Agency for Healthcare Research of the Czech Republic (NV18‐08‐00567) and Czech Science Foundation (GA17‐09525S, GA19‐20123S) grants to P.K. Czech Science Foundation (GA18‐17658S, GA17‐16680S) supported V.B. Czech Science Foundation (16‐24043J) supported J.K. A Junior Researcher Award from the Faculty of Medicine, Masaryk University supported M.Bo. I.D. is a Geisman Fellow supported by the Osteogenesis Imperfecta Foundation (OIF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. M.Bu. was supported by the project EXCELLENCE in molecular aspects of the early development of vertebrates from the Operational Programme Research, Development and Education (CZ.02.1.01/0.0/0.0/15_003/0000460), funded by the Ministry of Education, Youth and Sports of the Czech Republic. A.N. is a Brno Ph.D. Talent Scholarship Holder—Funded by the Brno City Municipality.
EMBO Mol Med (2020) 12: e11739
Contributor Information
Deborah Krakow, Email: dkrakow@mednet.ucla.edu.
Pavel Krejci, Email: krejcip@med.muni.cz.
Data availability
The numerical data, uncropped blots, and confocal images for all main figures and EV figures are available on the Journal's web page. This study includes no data deposited in external repositories.
References
- Ågren M, Kogerman P, Kleman MI, Wessling M, Toftgård R (2004) Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli‐binding site. Gene 330: 101–114 [DOI] [PubMed] [Google Scholar]
- Alby C, Piquand K, Huber C, Megarbané A, Ichkou A, Legendre M, Pelluard F, Encha‐Ravazi F, Abi‐Tayeh G, Bessières B et al (2015) Mutations in KIAA0586 cause lethal ciliopathies ranging from a hydrolethalus phenotype to short‐rib polydactyly syndrome. Am J Hum Genet 97: 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Bongers EMHF, Mans DA, van Beersum SEC, Oud MM, Bolat E, Spruijt L, Cornelissen EAM, Schuurs‐Hoeijmakers JHM, de Leeuw N et al (2011) C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet 48: 390–395 [DOI] [PubMed] [Google Scholar]
- Aza‐Blanc P, Lin HY, Ruiz i Altaba A, Kornberg TB (2000) Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development 127: 4293–4301 [DOI] [PubMed] [Google Scholar]
- Barta T, Peskova L, Hampl A (2016) miRNAsong: a web‐based tool for generation and testing of miRNA sponge constructs in silico . Sci Rep 6: 36625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J et al (2007) IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet 39: 727–729 [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang Y‐L, Davidson G, Zimmermann T, Cruciat C‐M, Bienz M, Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled‐dependent LRP6 phosphorylation. Science 316: 1619–1622 [DOI] [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbø M, Filhol E, Bole‐Feysot C et al (2011) Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT‐A gene WDR19. Am J Hum Genet 89: 634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G (2007) Beta‐arrestin is a necessary component of Wnt/beta‐catenin signaling in vitro and in vivo . Proc Natl Acad Sci USA 104: 6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Burgess S, Hopkins N (2001) Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development 128: 2385–2396 [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA (2002) Small molecule modulation of Smoothened activity. Proc Natl Acad Sci 99: 14071–14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ren X‐R, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ (2004) Activity‐dependent internalization of smoothened mediated by ‐Arrestin 2 and GRK2. Science 306: 2257–2260 [DOI] [PubMed] [Google Scholar]
- Chen M, Philipp M, Wang J, Premont RT, Garrison TR, Caron MG, Lefkowitz RJ, Chen W (2009) G protein‐coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J Biol Chem 284: 35040–35048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li S, Tong C, Zhao Y, Wang B, Liu Y, Jia J, Jiang J (2010) G protein‐coupled receptor kinase 2 promotes high‐level Hedgehog signaling by regulating the active state of Smo through kinase‐dependent and kinase‐independent mechanisms in Drosophila . Genes Dev 24: 2054–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, Jiang J (2011) Sonic hedgehog dependent phosphorylation by CK1α and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol 9: e1001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes M, Baria AT, Schwartz NB (2009) Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development 136: 1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E (2008) LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of ‐catenin. Proc Natl Acad Sci 105: 8032–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S (1999) Sonic Hedgehog‐induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 274: 8143–8152 [DOI] [PubMed] [Google Scholar]
- Dao DY, Jonason JH, Zhang Y, Hsu W, Chen D, Hilton MJ, O'Keefe RJ (2012) Cartilage‐specific β‐catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res 27: 1680–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV et al (2011) TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PB, Stuck MW, Lv B, Pazour GJ (2020) Ubiquitin links smoothened to intraflagellar transport to regulate Hedgehog signaling. J Cell Biol 219: e201912104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y‐F, Soung DY, Schwarz EM, O'Keefe RJ, Drissi H (2006) Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol 208: 77–86 [DOI] [PubMed] [Google Scholar]
- Duran I, Taylor SP, Zhang W, Martin J, Forlenza KN, Spiro RP, Nickerson DA, Bamshad M, Cohn DH, Krakow D (2016) Destabilization of the IFT‐B cilia core complex due to mutations in IFT81 causes a Spectrum of Short‐Rib Polydactyly Syndrome. Sci Rep 6: 34232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshtad S, Mavajian Z, Rudd SG, Visnes T, Boström J, Altun M, Helleday T (2016) hMYH and hMTH1 cooperate for survival in mismatch repair defective T‐cell acute lymphoblastic leukemia. Oncogenesis 5: e275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron T, Philipp M, Lu J, Meloni AR, Burkhalter M, Chen W, Caron MG (2011) Growth arrest specific 8 (Gas8) and G protein‐coupled receptor kinase 2 (GRK2) cooperate in the control of smoothened signaling. J Biol Chem 286: 27676–27686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron T, Daigle TL, Caron MG (2012) GRK2: multiple roles beyond G protein‐coupled receptor desensitization. Trends Pharmacol Sci 33: 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes AJ, Nakano Y, Taylor AM, Ingham PW (1993) Genetic analysis of hedgehog signalling in the Drosophila embryo. Development 119: 115–124 [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin L‐G, Schiöth HB (2003) The G‐protein‐coupled receptors in the human genome form five main families. phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63: 1256–1272 [DOI] [PubMed] [Google Scholar]
- Galdzicka M, Patnala S, Hirshman M, Cai J‐F, Nitowsky H, A Egeland J, Ginns E (2002) A new gene, EVC2, is mutated in Ellis–van Creveld syndrome. Mol Genet Metab 77: 291–295 [DOI] [PubMed] [Google Scholar]
- Gerhardt C, Leu T, Lier JM, Rüther U (2016) The cilia‐regulated proteasome and its role in the development of ciliopathies and cancer. Cilia 5: 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG et al (2010) Exome sequencing identifies WDR35 variants involved in sensenbrenner syndrome. Am J Hum Genet 87: 418–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman‐Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D et al (2001) LDL receptor‐related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523 [DOI] [PubMed] [Google Scholar]
- Goodman J, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL (1996) β‐Arrestin acts as a clathrin adaptor in endocytosis of the β2‐ adrenergic receptor. Nature 383: 447–450 [DOI] [PubMed] [Google Scholar]
- Green MR, Pastewka JV (1974) Simultaneous differential staining by a cationic carbocyanine dye of nucleic acids, proteins and conjugated proteins. II. Carbohydrate and sulfated carbohydrate containing proteins. J Histochem Cytochem 22: 774–781 [DOI] [PubMed] [Google Scholar]
- Gualeni B, Facchini M, De Leonardis F, Tenni R, Cetta G, Viola M, Passi A, Superti‐Furga A, Forlino A, Rossi A (2010) Defective proteoglycan sulfation of the growth plate zones causes reduced chondrocyte proliferation via an altered Indian hedgehog signalling. Matrix Biol 29: 453–460 [DOI] [PubMed] [Google Scholar]
- Guo X, Mak KK, Taketo MM, Yang Y (2009) The Wnt/β‐Catenin Pathway Interacts Differentially with PTHrP Signaling to Control Chondrocyte Hypertrophy and Final Maturation. PLoS ONE 4: e6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Tesmer JJG, Mushegian A, Gurevich VV (2012) G protein‐coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther 133: 40–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney‐Leo AM, Krug P, Filhol E, Davis EE et al (2013) Defects in the IFT‐B component IFT172 cause jeune and mainzer‐saldino syndromes in humans. Am J Hum Genet 93: 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ JT, Hedeen DS, Zhu J‐F, Capener JL, Klatt ShawD, Deshpande I, Liang J, Xu J, Stubben SL, Walker MF et al (2020) Smoothened transduces hedgehog signals via activity‐dependent sequestration of 1 PKA catalytic subunits 2 3 Corvin D. bioRxiv 10.1101/2020.07.01.183079 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff WP, Lohse MJ, Bouvier M, Liggett SB, Caron MG, Lefkowitz RJ (1990) Two kinases mediate agonist‐dependent phosphorylation and desensitization of the beta 2‐adrenergic receptor. Symp Soc Exp Biol 44: 225–240 [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin‐Son Y, Zhang Q, Michaud EJ, Yoder BK (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1: e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horakova D, Cela P, Krejci P, Balek L, Moravcova Balkova S, Matalova E, Buchtova M (2014) Effect of FGFR inhibitors on chicken limb development. Dev Growth Differ 56: 555–572 [DOI] [PubMed] [Google Scholar]
- Huber C, Wu S, Kim AS, Sigaudy S, Sarukhanov A, Serre V, Baujat G, Le Quan Sang K‐H, Rimoin DL, Cohn DH et al (2013) WDR34 mutations that cause short‐rib polydactyly syndrome type III/Severe asphyxiating thoracic dysplasia reveal a role for the NF‐κB pathway in cilia. Am J Hum Genet 93: 926–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R (2010) The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev 24: 670–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, Taylor AM, Nakano Y (1991) Role of the Drosophila patched gene in positional signalling. Nature 353: 184–187 [DOI] [PubMed] [Google Scholar]
- Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Lefkowitz RJ, Caron MG, Giros B (1996) Essential role of beta‐adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA 93: 12974–12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Tong C, Wang B, Luo L, Jiang J (2004) Hedgehog signalling activity of smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature 432: 1045–1050 [DOI] [PubMed] [Google Scholar]
- Klüppel M, Wight TN, Chan C, Hinek A, Wrana JL (2005) Maintenance of chondroitin sulfation balance by chondroitin‐4‐sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development 132: 3989–4003 [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ (2008) Beta‐arrestin‐mediated localization of smoothened to the primary cilium. Science 320: 1777–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 90: 5519–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM (2006) PTHrP and skeletal development. Ann N Y Acad Sci 1068: 1–13 [DOI] [PubMed] [Google Scholar]
- Kunova Bosakova M, Varecha M, Hampl M, Duran I, Nita A, Buchtova M, Dosedelova H, Machat R, Xie Y, Ni Z et al (2018) Regulation of ciliary function by fibroblast growth factor signaling identifies FGFR3‐related disorders achondroplasia and thanatophoric dysplasia as ciliopathies. Hum Mol Genet 27: 1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunova Bosakova M, Nita A, Gregor T, Varecha M, Gudernova I, Fafilek B, Barta T, Basheer N, Abraham SP, Balek L et al (2019) Fibroblast growth factor receptor influences primary cilium length through an interaction with intestinal cell kinase. Proc Natl Acad Sci USA 116: 4316–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ et al (2013) WNT1 mutations in early‐onset osteoporosis and osteogenesis imperfecta. N Engl J Med 368: 1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Graham JM, Rimoin DL, Lachman RS, Krejci P, Tompson SW, Nelson SF, Krakow D, Cohn DH (2012) Exome sequencing identifies PDE4D mutations in acrodysostosis. Am J Hum Genet 90: 746–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li S, Han Y, Tong C, Wang B, Chen Y, Jiang J (2016) Regulation of smoothened phosphorylation and high‐level hedgehog signaling activity by a plasma membrane associated kinase. PLoS Biol 14: e1002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier D, Cheng S, Faubert D, Hipfner DR (2014) A broadly conserved G‐protein‐coupled receptor kinase phosphorylation mechanism controls Drosophila smoothened activity. PLoS Genet 10: e1004399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW (2006) Cardiac‐specific ablation of G‐protein receptor kinase 2 redefines its roles in heart development and beta‐adrenergic signaling. Circ Res 99: 996–1003 [DOI] [PubMed] [Google Scholar]
- McInerney‐Leo AM, Schmidts M, Cortés CR, Leo PJ, Gener B, Courtney AD, Gardiner B, Harris JA, Lu Y, Marshall M et al (2013) Short‐Rib Polydactyly and Jeune Syndromes Are Caused by Mutations in WDR60. Am J Hum Genet 93: 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney‐Leo AM, Wheeler L, Marshall MS, Anderson LK, Zankl A, Brown MA, Leo PJ, Wicking C, Duncan EL (2017) Homozygous variant in C21orf2 in a case of Jeune syndrome with severe thoracic involvement: extending the phenotypic spectrum. Am J Med Genet A 173: 1698–1704 [DOI] [PubMed] [Google Scholar]
- Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler‐Reya RJ, Lefkowitz RJ, Caron MG (2006) Smoothened signal transduction is promoted by G protein‐coupled receptor kinase 2. Mol Cell Biol 26: 7550–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay K, Lefebvre V, Zhou G, Garofalo S, Kimura JH, de Crombrugghe B (1995) Use of a new rat chondrosarcoma cell line to delineate a 119‐base pair chondrocyte‐specific enhancer element and to define active promoter segments in the mouse pro‐alpha 1(II) collagen gene. J Biol Chem 270: 27711–27719 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK (2013) The ciliary G‐protein‐coupled receptor Gpr161 negatively regulates the sonic hedgehog pathway via cAMP signaling. Cell 152: 210–223 [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Copp AJ (2010) The relationship between sonic Hedgehog signaling, cilia, and neural tube defects. Birth Defects Res . A Clin Mol Teratol 88: 633–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R (2014) Gli protein activity is controlled by multisite phosphorylation in vertebrate hedgehog signaling. Cell Rep 6: 168–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Clevers H (2017) Wnt/β‐Catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999 [DOI] [PubMed] [Google Scholar]
- Paganini C, Tota CG, Superti‐Furga A, Rossi A (2020) Skeletal dysplasias caused by sulfation defects. Int J Mol Sci 21: 2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige Taylor S, Kunova Bosakova M, Varecha M, Balek L, Barta T, Trantirek L, Jelinkova I, Duran I, Vesela I, Forlenza KN et al (2016) An inactivating mutation in intestinal cell kinase, ICK, impairs hedgehog signalling and causes short rib‐polydactyly syndrome. Hum Mol Genet 25: 3998–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K, Hwang S, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S (2016) Smoothened determines β‐arrestin‐mediated removal of the G protein‐coupled receptor Gpr161 from the primary cilium. J Cell Biol 212: 861–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ (1997) G protein‐coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem 272: 25425–25428 [DOI] [PubMed] [Google Scholar]
- Perrault I, Saunier S, Hanein S, Filhol E, Bizet AA, Collins F, Salih MAM, Gerber S, Delphin N, Bigot K et al (2012) Mainzer‐Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet 90: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskova L, Cerna K, Oppelt J, Mraz M, Barta T (2019) Oct4‐mediated reprogramming induces embryonic‐like microRNA expression signatures in human fibroblasts. Sci Rep 9: 15759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M, Fralish GB, Meloni AR, Chen W, MacInnes AW, Barak LS, Caron MG (2008) Smoothened signaling in vertebrates is facilitated by a G protein‐coupled receptor kinase. Mol Biol Cell 19: 5478–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusapati GV, Kong JH, Patel BB, Gouti M, Sagner A, Sircar R, Luchetti G, Ingham PW, Briscoe J, Rohatgi R (2018) G protein–coupled receptors control the sensitivity of cells to the morphogen Sonic Hedgehog. Sci Signal 11: eaao5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V, Briscoe J (2009) Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb Perspect Biol 1: a002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP (2007) Patched1 regulates hedgehog signaling at the primary cilium. Science 317: 372–376 [DOI] [PubMed] [Google Scholar]
- Rosanò L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A (2009) β‐Arrestin links endothelin A receptor to β‐catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA 106: 2806–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S et al (2000) Mutations in a new gene in Ellis‐van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet 24: 283–286 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui CC, Nakafuku M, Kondoh H (1999) Regulation of Gli2 and Gli3 activities by an amino‐terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126: 3915–3924 [DOI] [PubMed] [Google Scholar]
- Schmidts M, Frank V, Eisenberger T, al Turki S, Bizet AA, Antony D, Rix S, Decker C, Bachmann N, Bald M et al (2013) Combined NGS approaches identify mutations in the intraflagellar transport Gene IFT140 in skeletal ciliopathies with early progressive kidney disease. Hum Mutat 34: 714–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts M, Hou Y, Cortés CR, Mans DA, Huber C, Boldt K, Patel M, van Reeuwijk J, Plaza J‐M, van Beersum SEC et al (2015) TCTEX1D2 mutations underlie Jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport. Nat Commun 6: 7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Schmidts M, Faqeih E, Hashem A, Lausch E, Holder I, Superti‐Furga A, Mitchison HM, Almoisheer A, Alamro R et al (2015) A founder CEP120 mutation in Jeune asphyxiating thoracic dystrophy expands the role of centriolar proteins in skeletal ciliopathies. Hum Mol Genet 24: 1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St‐Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13: 2072–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova K, Kowalski‐Jahn M, Gybel T, Valnohova J, Dhople VM, Harnos J, Bernatik O, Ganji RS, Zdrahal Z, Mulder J et al (2018) Dishevelled enables casein kinase 1–mediated phosphorylation of Frizzled 6 required for cell membrane localization. J Biol Chem 293: 18477–18493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Ospina JK, Zhang J, Michelson AP, Schoen AM, Zhu AJ (2011) Sequential phosphorylation of smoothened transduces graded hedgehog signaling. Sci Signal 4: ra43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M et al (2005) Developmental regulation of Wnt/β‐catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem 280: 19185–19195 [DOI] [PubMed] [Google Scholar]
- Taylor SP, Dantas TJ, Duran I, Wu S, Lachman RS, Bamshad MJ, Shendure J, Nickerson DA, Nelson SF, Cohn DH et al (2015) Mutations in DYNC2LI1 disrupt cilia function and cause short rib polydactyly syndrome. Nat Commun 6: 7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY et al (2012) Paroxetine is a direct inhibitor of g protein‐coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol 7: 1830–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stöss H, Beinder E et al (2011) NEK1 mutations cause short‐Rib polydactyly syndrome type majewski. Am J Hum Genet 88: 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama M, Lee C, Taylor SP, Duran I, Cohn DH, Bruel A‐L, Tabler JM, Drew K, Kelly MR, Kim S et al (2016) The ciliopathy‐associated CPLANE proteins direct basal body recruitment of intraflagellar transport machinery. Nat Genet 48: 648–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukachinsky H, Lopez LV, Salic A (2010) A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu–Gli protein complexes. J Cell Biol 191: 415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuson M, He M, Anderson KV (2011) Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development 138: 4921–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel M, Ingham PW (1996) Smoothened encodes a receptor‐like serpentine protein required for hedgehog signalling. Nature 382: 547–551 [DOI] [PubMed] [Google Scholar]
- Walczak‐Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A et al (2010) Cranioectodermal dysplasia, sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet 86: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA (2000) Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development 127: 3131–3139 [DOI] [PubMed] [Google Scholar]
- Wang C, Rüther U, Wang B (2007) The Shh‐independent activator function of the full‐length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol 305: 460–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gesty‐Palmer D, Fields TA, Spurney RF (2009) Inhibition of WNT signaling by G protein‐coupled receptor (GPCR) kinase 2 (GRK2). Mol Endocrinol 23: 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Lai CK, Evangelista M, Hongo J‐A, de Sauvage FJ, Scales SJ (2010) Kinetics of hedgehog‐dependent full‐length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol 30: 1910–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Palmby TR, Gavard J, Williams BO, Gutkind JS (2008) Multiple PPPS/TP motifs act in a combinatorial fashion to transduce Wnt signaling through LRP6. FEBS Lett 582: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Williams EH, Guo Y, Lum L, Beachy PA (2004) Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc Natl Acad Sci USA 101: 17900–17907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Taylor SP, Nevarez L, Lachman RS, Nickerson DA, Bamshad M, University of Washington Center for Mendelian Genomics Consortium D , Krakow D, Cohn DH (2016) IFT52 mutations destabilize anterograde complex assembly, disrupt ciliogenesis and result in short rib polydactyly syndrome. Hum Mol Genet 25: 4012–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Taylor SP, Ennis HA, Forlenza KN, Duran I, Li B, Sanchez JAO, Nevarez L, Nickerson DA, Bamshad M et al (2018) Expanding the genetic architecture and phenotypic spectrum in the skeletal ciliopathies. Hum Mutat 39: 152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tong C, Jiang J (2007) Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450: 252–258 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lee RTH, Pusapati GV, Iyu A, Rohatgi R, Ingham PW (2016) An essential role for Grk2 in Hedgehog signalling downstream of Smoothened. EMBO Rep 17: 739–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Data Availability Statement
The numerical data, uncropped blots, and confocal images for all main figures and EV figures are available on the Journal's web page. This study includes no data deposited in external repositories.


