Abstract
The novel coronavirus was recognised in December 2019 and caught humanity off guard. The virus employs the angiotensin‐converting enzyme 2 (ACE2) receptor for entry into human cells. ACE2 is expressed on different organs, which is raising concern as to whether these organs can be infected by the virus or not. The testis appears to be an organ enriched with levels of ACE2, while the possible mechanisms of involvement of the male reproductive system by SARS‐CoV‐2 are not fully elucidated. The major focus of the present studies is on the short‐term complications of the coronavirus and gains importance on studying the long‐term effects, including the possible effects of the virus on the male reproductive system. The aim of this review was to provide new insights into different possible mechanisms of involvement of male gonads with SARS‐CoV‐2 including investigating the ACE2 axis in testis, hormonal alterations in patients with COVID‐19, possible formation of anti‐sperm antibodies (ASA) and subsequently immunological infertility as a complication of SARS‐CoV‐2 infection. Finally, we suggest measuring the sperm DNA fragmentation index (DFI) as a determiner of male fertility impairment in patients with COVID‐19 along with other options such as sex‐related hormones and semen analysis. Invasion of SARS‐CoV‐2 to the spermatogonia, Leydig cells and Sertoli cells can lead to sex hormonal alteration and impaired gonadal function. Once infected, changes in ACE2 signalling pathways followed by oxidative stress and inflammation could cause spermatogenesis failure, abnormal sperm motility, DNA fragmentation and male infertility.
Keywords: ACE2 receptor, anti‐sperm antibody, male gonadal function, SARS‐CoV‐2, sperm DNA fragmentation index
1. INTRODUCTION
Since the first emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), also known as novel coronavirus (2019‐nCoV), in Wuhan, China, in December 2019 (Tan et al., 2020), the virus has spread rapidly worldwide and, therefore, has been officially declared a pandemic outbreak with more than 10 million confirmed cases and 499,000 deaths according to the WHO report (Situation Report, 2020). Pneumonia with fever seems to be the most common presentation of SARS‐CoV‐2 infection (Guan et al., 2020; Wang, Hu et al., 2020). SARS‐CoV‐2 was also detected in specimens of faeces and a small percentage of blood and urine samples, which raises the question of whether or not the virus can be transmitted by the aforementioned body fluids (Peng et al., 2020; Song et al., 2020; Wang, Xu et al., 2020). Full‐genome sequencing has resulted in approximately 80% sequencing similarity between SARS‐CoV‐2 and SARS epidemic viruses (Gralinski & Menachery, 2020; Lu et al., 2020). Moreover, studies revealed that SARS‐CoV‐2 and SARS‐CoV share the same binding receptor named angiotensin‐converting enzyme 2 (ACE2) to enter the host cell (Hoffmann et al., 2020). In addition to being abundantly expressed in type II alveolar cells of the lungs, ACE2 also has significantly high expression levels in organs such as the small intestine, kidney and testes. There are concerns regarding the probability of these organs being possible targets for SARS‐CoV‐2‐induced infection (Fan et al., 2020; Wang & Xu, 2020).
Spermatogonia, Leydig cells and Sertoli cells are the three main types of testicular cells that express high levels of ACE2 receptor protein on their surface membrane. Theoretically, any cell expressing ACE2 can be a target of SARS‐CoV‐2 (Fan et al., 2020). Furthermore, a previous study has shown that testicular damage, orchitis and sterility are possible complications of SARS‐CoV (Xu et al., 2006). Recently, a case report study showed that orchiepididymitis in a 14‐year‐old boy seemed to be a complication of COVID‐19 (Gagliardi et al., 2020). Notably, higher expression of ACE2 in male gonads seems to be associated with younger age and, as a result, provides evidence that younger patients are more vulnerable to gonadal involvement by SARS‐CoV‐2 (Shen et al., 2020). Therefore, since the mechanism of involvement of different organs by SARS‐CoV‐2 is not fully understood, all the potential complications of the virus are based on assumptions and further research should be conducted to investigate the effect of SARS‐CoV‐2 on the male reproductive system and probable infertility. However, it should be taken into account that every systemic inflammatory process with fever tends to interfere with spermatogenesis and could possibly affect fertility (Hedger, 2011).
The major focus of recent studies on SARS‐CoV‐2 is mainly on the short‐term complications and acute phase of the virus; however, statistics have revealed that a considerable number of the infected population survive the disease (Lai et al., 2020). Moreover, studies on the long‐term complications of the virus, such as the possibility of male infertility, are scarce. This issue points out the importance of further investigation of the male reproductive system as a potential target of SARS‐CoV‐2. There are many unknown aspects of the coronavirus effect on the male reproductive system. Considering the fact that the testis is highly enriched in ACE2 receptors (Fan et al., 2020) and its vulnerability to SARS‐CoV‐2 invasion, detectable changes in semen analysis, alteration in sex hormones balance and, most importantly, anti‐sperm antibodies (ASA) formation and sperm DNA fragmentation are considered to play a major role in male infertility. The main purpose of this review was to challenge the aforementioned key points and to identify the possibility of SARS‐CoV‐2 targeting the male reproductive system, subsequently forming ASA and changing the sperm DFI, semen analysis and sex hormones.
2. MATERIALS AND METHODS
A categorised and comprehensive search in literature published in PubMed, Embase, Web of Science, Scopus and Cochrane Library Databases was conducted in accordance with the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses).
Literature reviews, both published and peer‐reviewed of existing literature in English, were studied from online publications between the years 2000 to 2020. Additional sources were identified from citations of retrieved literature, and there was no limitation on sample size. Published reports of experiences with the SARS‐CoV‐2 have increased greatly in the past months, but to date, no randomized trials regarding possible treatments were identified. Most reports relating to reproduction or male infertility involved small cohorts, case reports and editorials. Similarly, prior publications of other coronaviruses were limited in number. We reviewed all appropriate titles of all reports including male gonadal function, infertility or reproductive tissues. Papers were considered that contained information relating to male reproduction, the presence of virus in reproductive tissues, effects on gametes, fertilisation outcomes or related complications. Full‐text articles by author teams were included, reviewed and evaluated. The authors acknowledge that the number of publications about the novel SARS‐CoV‐2 is increasing at an exponential rate, and there may be bias towards the reporting of positive findings. We used a broad inclusive search strategy so as not to miss a formative contribution, and a comprehensive search was conducted by two experienced information specialists. Search phrases used for different databases strategy included the following: "severe acute respiratory syndrome coronavirus 2", "2019 nCoV", "SARS‐CoV‐2", "coronavirus", "COVID‐19", "reproductive system", "fertility", "infertility", "germ cells", "gamete", "spermatogonia", "spermatogenesis" "spermatozoa", "spermatozoan", "testis", "Sertoli cells", "Leydig cells", "Androgen", "steroidogenesis", "spermiogenesis", "spermiation", "development", "fertilization", "gonadal function", "sex hormones", "angiotensin‐converting enzyme 2 receptor", "ACE2", "anti‐sperm antibodies", "ASA", "sperm DNA fragmentation index", "DFI", and "semen analysis".
The search revealed 76 manuscripts after removal of duplicates and sixty‐five manuscripts related to the reproductive system and coronaviruses. Small cohorts, case reports, guidelines, comments on guidelines and editorials were retrieved. After exclusion, 55 manuscripts were included in the review based on relevance and new data.
3. RESEARCH FINDINGS
3.1. Role of ACE2‐angiotensin‐(1–7)‐MAS receptor axis in spermatogenesis and male gonadal involvement by SARS‐CoV‐2
The novel SARS‐CoV‐2 mainly employs an ACE2 receptor to enter and infect human cells similar to SARS‐CoV (Zhang et al., 2020). It is implicit that co‐expression of ACE2 and transmembrane protease serine 2 (TMPRSS2) is essential for involvement of a tissue by SARS‐CoV‐2, and a recent study has shown that the testis has a high co‐expression of ACE2 and TMPRSS2 (Ren et al., 2020).
One recent study demonstrated that the testis is the most ACE2‐enriched organ in the body with Leydig and Sertoli cells appearing to have significantly higher expression levels of ACE2 compared to alveolar type II (AT2) cells. ACE2‐positive spermatogonia appear to form a remarkable portion of all the spermatogonia in the testis, and the genes involved in spermatogenesis are impaired in ACE2‐positive spermatogonia, while these genes are fully functioning in ACE2‐negative spermatogonia. Therefore, ACE2‐positive spermatogonia in infected patients appear to have impaired spermatogenesis (Fan et al., 2020). Possible involvement of Sertoli cells by the coronavirus can lead to impairment of the blood–testis barrier that is also known as the Sertoli cell seminiferous epithelium barrier, which is formed by special junctions between Sertoli cells and preserves spermatogenesis from cytotoxic agents of the blood, while this barrier prevents autoimmune responses that directly results in preserving spermatogenesis (Archana et al., 2019). As a result, there is a great possibility of both direct and indirect male gonadal involvement by the coronavirus and subsequent gonadal dysfunction and deterioration in the spermatogenesis cycle (Figure 1).
FIGURE 1.
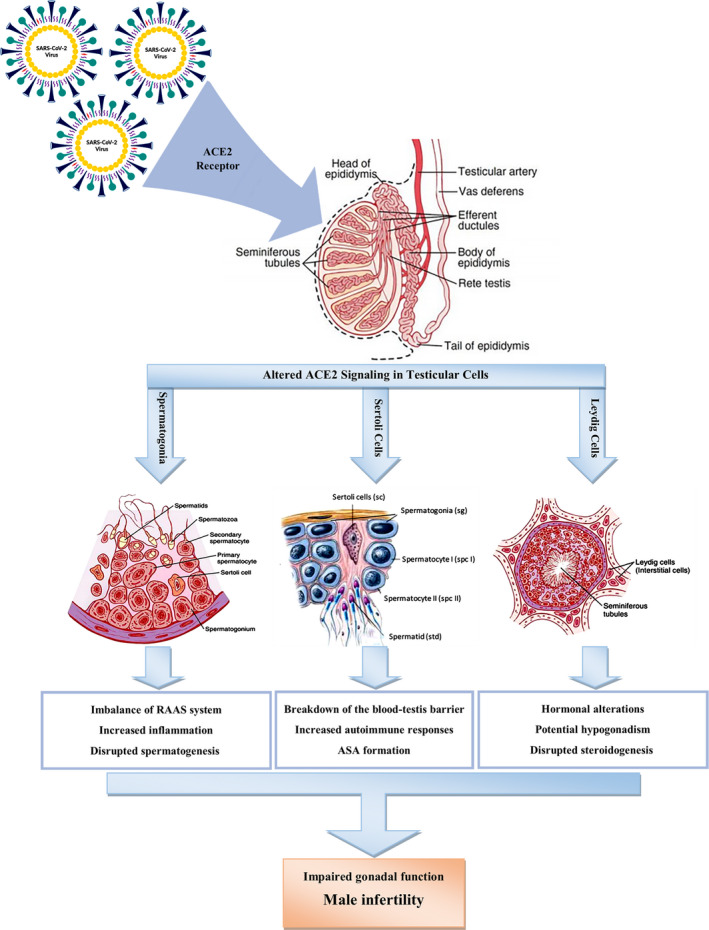
Possible effects of SARS‐CoV‐2 on the function of main types of testicular cells by an altered ACE‐2 signalling pathway. ACE2: angiotensin‐converting enzyme‐2; ASA: anti‐sperm antibody
New studies have provided a better understanding of the function of ACE2 and its impacts on the male reproductive system (Hermann et al., 2018; Jan et al., 2017; Wang & Xu, 2020). ACE2 is the homolog of angiotensin‐converting enzyme (ACE), and it can play the counter‐regulator role of ACE by converting angiotensin‐II, the main product of ACE, into angiotensin‐(1–7) which acts as a vasodilator (Douglas et al., 2004; Pencheva et al., 2015). Angiotensin‐II is a component of the renin–angiotensin system (RAS) that regulates sperm motility and function in addition to controlling blood pressure (Gianzo et al., 2016). ACE has two isoforms including somatic and germinal ACE (g ACE). Somatic ACE (sACE) is expressed in many tissues including Sertoli and Leydig cells, and germinal ACE (gACE) is exclusively found in spermatozoa and spermatids (Douglas et al., 2004). Another study has revealed that gACE knockout mice turn out to be sterile, while ACE2‐null mice showed no fertility impairment (Pan et al., 2013). In spite of recent studies in which they did not demonstrate sterility in mice with impaired function of ACE2, the role of ACE2 in decreasing the Ang‐II level seems to modulate sperm maturation and function; hence, potential male infertility cannot be denied (Douglas et al., 2004; Pan et al., 2013).
Furthermore, recent studies have shown that in the male reproductive tract, the ACE2/Ang‐(1–7)/Mas axis may activate sperm motility through the PI3K/AKT pathway (Tsuji et al., 2020; Valdivia et al., 2020). In general, the ACE2/ Ang (1–7)/Mas activates PI3K/AKT signalling, and the PI3K/AKT signalling is thought to be associated with host protection in several diseases by improving oxidative stress and inflammation (Figure 2).
FIGURE 2.
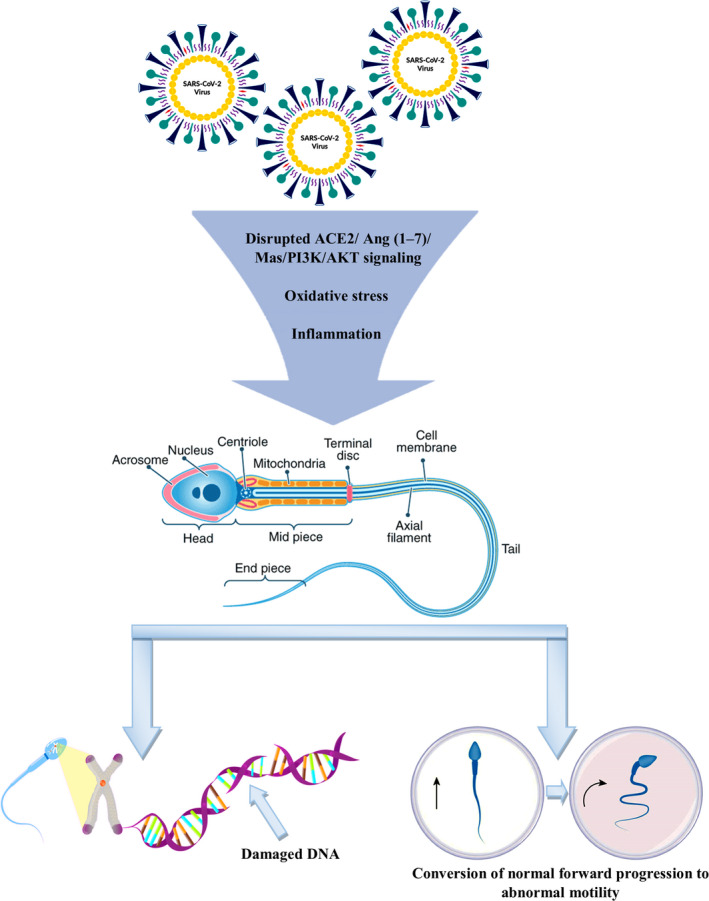
Possible mechanisms of SARS‐CoV‐2 infection induced sperm DNA damage, abnormal motility and male infertility through disruption of ACE2/Ang (1–7)/Mas/PI3K/AKT signalling axis and systemic oxidative stress and inflammation. ACE2, angiotensin‐converting enzyme‐2; Ang, angiotensin; PI3K/AKT, phosphatidylinositol‐3‐kinase and protein kinase B
SARS‐CoV‐2 infection decreased ACE2 expression and its functional number from the membrane surface; therefore, the ACE2‐angiotensin‐(1–7)‐Mas receptor axis tends towards angiotensin‐II actions and will have devastating effects on sperm motility and gonadal function. Overall, the aforementioned points raise the question of whether or not SARS‐CoV‐2 can target the male reproductive system through ACE2 receptors leading to the idea that more studies should be conducted to answer this question.
3.2. Possible inflammatory and oxidative stress‐related mechanisms induced by SARS‐CoV‐2 in male reproductive system
The outbreak of SARS‐CoV in 2003 was accompanied by different complications such as inflammation in male sexual organs. A study by Xu et al. that investigated testis autopsies obtained from six fatal cases diagnosed with SARS‐CoV discovered that all six cases had orchitis. The study further discovered that SARS‐CoV‐induced orchitis had led to extensive destruction of testicular tissue, germ cell destruction and subsequent seminiferous tubule necrosis (Xu et al., 2006). There is strong evidence of sex‐related hormonal change in viral orchitis, which can disrupt spermatogenesis (Barták, 1973; Ku et al., 1999). The laboratory tests of these cases detected antibody deposition in testicular tissue but viral genome of SARS‐CoV could not be extracted from the tissue samples. This implies that orchitis induced by SARS‐CoV was probably due to an immunological response rather than the direct effect of the virus (Xu et al., 2006).
Yang et al. (2020) reported on the pathological changes in 12 testis samples from patients who died of COVID‐19 where they found no evidence of SARS‐CoV‐2 virus in the testes in the majority of the cases using RT‐PCR and electron microscopy. However, there was significant injury to Sertoli cells and seminiferous tubules, Leydig cells loss and mild inflammatory infiltrates in the interstitium. These findings can provide evidence‐based guidance for indirect testicular damage during the COVID‐19 course.
The blood–testis barrier elevates the immune status in testes and preserves male gonads from immune responses. Overproduction of inflammatory cytokines induced by viral infections can lead to autoimmune responses and infiltration of leucocytes, subsequently disrupt spermatogenesis and interfere with sex‐related hormones secretion (Hedger & Meinhardt, 2003; Xu et al., 2006). A previous study in rodents with autoimmune orchitis showed that an increase in the serum level of IL‐6 and its receptor, a cytokine that regulates inflammation and immune response, could be associated with pathogenesis of autoimmune orchitis (Rival et al., 2006). It has been reported that more severe cases of COVID‐19 are accompanied by a high level of IL‐6, and its receptors are highly expressed in testicular cells (Ren et al., 2020; Shen et al., 2020). This can justify the probable male gonadal involvement and a subsequent possibility of orchitis as a complication of SARS‐CoV‐2.
The location of the testis prepares an environment with a temperature lower than the body's core temperature for the development of germ cells. SARS‐CoV‐2 infections, however, are usually accompanied by inflammation and symptoms such as fever and every inflammatory process with fever and can interfere with spermatogenesis (Hedger, 2011; Xu et al., 2006) (Figure 1).
Thus, based on information given above and the fact that SARS‐CoV‐2 and SARS‐CoV share the same receptor and have genetic content similarity, it can be suggested that male gonadal involvement, impaired spermatogenesis and consequential fertility impairment can be a complication of SARS‐CoV‐2 (Gralinski & Menachery, 2020; Lu et al., 2020; Zhang et al., 2020).
3.3. Hormonal alterations in infected patients with SARS‐CoV‐2
A recent study has aimed to measure the serum level of androgens and gonadotropins in male patients diagnosed with COVID‐19 to evaluate whether or not SARS‐CoV‐2 leads to hormonal alterations and male gonadal dysfunction. This study demonstrated a significant increase in serum LH levels but no remarkable change in testosterone and FSH levels. Subsequently, a notable decrease in T/LH and FSH/LH was noted in COVID‐19 patients compared to healthy individuals. LH stimulates Leydig cells to produce testosterone and Sertoli cells to secrete inhibin B, which has a negative feedback on FSH. Therefore, this study implies that SARS‐CoV‐2 tends to impair the function of Leydig cells more than Sertoli cells (Ma et al., 2020). Moreover, a remarkable increase in serum prolactin (PRL) was seen in infected patients. Although high levels of PRL are associated with different aetiologies such as drugs and stress, hyperprolactinemia may suppress the pituitary gland and subsequently decrease the gonadotropins (Brown et al., 2019). It can be assumed that SARS‐CoV‐2 can interfere with male gonadal function; however, the mentioned study measured the hormones in the acute phase of the disease (Ma et al., 2020). Long‐term follow‐up and observation of the hormonal status of these patients are of great importance and point out the importance of further prospective long‐term studies of sex‐related hormonal change in patients with SARS‐CoV‐2 as an indicator of male gonadal function.
3.4. Potential formation of anti‐sperm antibodies due to SARS‐CoV‐2 infection
It has been shown that antigens expressed on spermatozoa are foreign to the immune system, and ASA can be formed as a result of different aetiologies such as breakdown of blood–testis barrier, orchitis and genital tract inflammation (Archana et al., 2019; Jalal et al., 2004; Marconi & Weidner, 2017). As mentioned before, overproduction of cytokines which regulate immune response such as IL‐6 induced by a viral infection can result in leucocyte infiltration in testis interstitium and consequently cause an autoimmune response and form ASA (Hedger & Meinhardt, 2003; Mahmudpour et al., 2020; Rival et al., 2006). Several studies have provided evidence of correlation between the presence of ASA in serum and genital tract secretions and increased chance of infertility (Marshburn & Kutteh, 1994; Restrepo & Cardona‐Maya, 2013). A meta‐analysis has revealed that ASA leads to negative effects on sperm motility and concentration (Cui et al., 2015). Furthermore, 13% of infertility cases seem to be associated with high levels of ASA and, in another study, high levels of ASA in infertile couples could interfere with in vitro fertilisation results (Nagy et al., 1995; Sinisi et al., 1993).
Considering the abovementioned points, it is suggested that there is a possibility of ASA formation and subsequent infertility problems in patients with COVID‐19. First, since there is a high expression of ACE2 on Sertoli cells and these cells form the blood–testis barrier, Sertoli cells’ involvement with SARS‐CoV‐2 can lead to a breakdown of the blood–testis barrier. The blood–testis barrier preserves the spermatozoa from immune responses, which can be a consequence of ASA formation. Therefore, a breakdown of blood–testis barrier and a subsequent development of ASA can lead to male fertility impairment (Archana et al., 2019; Fan et al., 2020). Second, orchitis was found to be a complication of SARS‐CoV, and by considering the fact that SARS‐CoV and SARS‐CoV‐2 share the same receptor and their genome content is similar, it can be suggested that possible orchitis and a subsequent ASA formation in COVID‐19 patients can lead to male fertility impairment (Gralinski & Menachery, 2020; Lu et al., 2020; Xu et al., 2006; Zhang et al., 2020). Third, high expression of ACE2 on testis and spermatogonia makes the male reproductive system a potential target of infection and inflammation by SARS‐CoV‐2, and since genital tract infection/inflammation can lead to development of ASA, male infertility due to ASA and the subsequent immune response should be taken into consideration (Fan et al., 2020; Marconi & Weidner, 2017). Thus, we suggest that development of ASA, a subsequent immune response and immunological infertility can be complications of SARS‐CoV‐2; therefore, further studies on ASA formation and male gonadal involvement by SARS‐CoV‐2 are recommended.
3.5. Sperm DFI as a promising determiner of male infertility
The prevalence of male infertility and insufficiency of conventional diagnosing options have led to an urgent requirement for seeking new assessment options to predict the chances of fertility. Semen analysis, as a conventional diagnostic option, used to play a crucial role in predicting male fertility but recent studies have found that normal semen parameters cannot guarantee fertility (Cissen et al., 2016; Esteves, 2014). Sperm DFI has gained clinical importance recently and is a fertility testing technique which measures the amount of damaged genetic content within spermatozoa with a threshold value of 20%–30% as mentioned in different studies. Naturally, mature spermatozoa need DNA integrity to maintain the ability of fertilisation. The breakage of DNA is mainly a result of apoptosis and excessive reactive oxygen species (ROS), which is associated with different aetiologies including drugs, smoking, high testicular temperature and infection and can lead to increased DFI and possibly increasing the chances of infertility (Chamley & Clarke, 2007; Cissen et al., 2016; Evenson & Wixon, 2006; Lewis et al., 2008; Masjedi et al., 2020; Santi et al., 2018; Sergerie et al., 2005).
Recent studies have shown that infection by different microorganisms can result in increased DFI (Tangal et al., 2018; Zeyad et al., 2018). A study by Boeri et al. investigated the potential impact of human papillomavirus (HPV) on sperm DNA integrity in subfertile men. Notably, HPV‐positive specimens showed a significantly higher rate of sperm DNA fragmentation (Boeri et al., 2019). The results of this study were in agreement with several other studies that investigated the association between infection with different microorganisms such as Chlamydia trachomatis, Mycoplasma and hepatitis C virus (HCV) and a remarkable increase in sperm DNA fragmentation (Gallegos et al., 2008; La Vignera et al., 2012).
Considering the fact that DFI is a promising option to evaluate the chances of infertility and the results of the abovementioned studies suggesting a correlation between the increase of DFI and infection with different microorganisms (Lewis et al., 2008), it is suggested that the addition of DFI to conventional diagnosing options such as semen analysis can play a crucial role in investigating the possibility of male fertility impairment due to SARS‐CoV‐2 infection.
There are two main reasons why increased sperm DFI can be expected in patients with COVID‐19. First, male gonadal involvement and a subsequent inflammation in testes are possible complications of SARS‐CoV‐2 (Fan et al., 2020; Ma et al., 2020; Ren et al., 2020; Rival et al., 2006; Xu et al., 2006). Inflammation can lead to excessive production of ROS, and this product can lead to sperm DNA damage (Anifandis et al., 2020; Evenson & Wixon, 2006; Homa et al., 2019; Karimi et al., 2020). Moreover, SARS‐CoV‐2 infection causes psychological stress, which is a major cause of systemic oxidative stress (Li et al., 2020). In one study, rise in the level of sperm DNA damage was noticed in a patient with influenza (Evenson et al., 2000). Oxidative stress‐mediated mechanisms of male infertility are widely documented, as oxidative stress can affect semen quality and disrupt sperm functions and motility, intracellular oxidative damage to spermatozoa by lipid peroxidation of sperm membrane, sperm DNA damage and inducing apoptotic pathways in spermatozoa (Figure 2). Moreover, high testicular temperature due to fever induced by SARS‐CoV‐2 infection can increase the level of sperm DFI (Evenson & Wixon, 2006). Overall, this points out the importance of employing sperm DFI as a determiner of male infertility in patients with SARS‐CoV‐2 infection.
4. CONCLUSION
Few studies have investigated possible male fertility impairment in patients with COVID‐19. Moreover, these studies mainly focus on the acute phase effects and complications of the virus rather than the long‐term effects. As a result, we suggest future studies should include a long‐term follow‐up of patients with COVID‐19 and employ sperm DFI as a promising determiner of male infertility along with other options such as semen analysis and sex‐related hormones in the investigation of male infertility. Furthermore, the possibility of the male reproductive system involvement induced by SARS‐CoV‐2 and formation of ASA and a subsequent immunological infertility in patients with COVID‐19 should be taken into consideration.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors wish to thank native editors of the Native English Edit Company (London, UK) and Radan English Edit manager, the exclusive representative of this company in Iran, for the valuable comments in editing this manuscript. This study was supported by the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences (Academic grant number: 99‐01‐18‐23731).
Haghpanah A, Masjedi F, Alborzi S, et al. Potential mechanisms of SARS‐CoV‐2 action on male gonadal function and fertility: Current status and future prospects. Andrologia.2021;53:e13883. 10.1111/and.13883
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [repository name e.g. “figshare”] at http://doi.org/, reference number [reference number].
REFERENCES
- Anifandis, G. , Messini, C. I. , Daponte, A. , & Messinis, I. E. (2020). COVID‐19 and fertility: A virtual reality. Reproductive BioMedicine Online, 41(2), 157–159. 10.1016/j.rbmo.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archana, S. S. , Selvaraju, S. , Binsila, B. K. , Arangasamy, A. , & Krawetz, S. A. (2019). Immune regulatory molecules as modifiers of semen and fertility: A review. Molecular Reproduction and Development, 86(11), 1485–1504. 10.1002/mrd.23263 [DOI] [PubMed] [Google Scholar]
- Barták, V. (1973). Sperm count, morphology and motility after unilateral mumps orchitis. Journal of Reproduction and Fertility, 32(3), 491–494. 10.1530/jrf.0.0320491 [DOI] [PubMed] [Google Scholar]
- Boeri, L. , Capogrosso, P. , Ventimiglia, E. , Pederzoli, F. , Cazzaniga, W. , Chierigo, F. , Pozzi, E. , Clementi, M. , Viganò, P. , Montanari, E. , Montorsi, F. , & Salonia, A. (2019). High‐risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Human Reproduction, 34(2), 209–217. 10.1093/humrep/dey348 [DOI] [PubMed] [Google Scholar]
- Brown, R. S. E. , Khant Aung, Z. , Phillipps, H. R. , Barad, Z. , Lein, H.‐J. , Boehm, U. , Szawka, R. E. , & Grattan, D. R. (2019). Acute suppression of LH secretion by prolactin in female mice is mediated by kisspeptin neurons in the arcuate nucleus. Endocrinology, 160(5), 1323–1332. 10.1210/en.2019-00038 [DOI] [PubMed] [Google Scholar]
- Chamley, L. W. , & Clarke, G. N. (2007). Antisperm antibodies and conception. Seminars in Immunopathology, 29(2), 169–184. 10.1007/s00281-007-0075-2 [DOI] [PubMed] [Google Scholar]
- Cissen, M. , Wely, M. V. , Scholten, I. , Mansell, S. , Bruin, J. P. D. , Mol, B. W. , Braat, D. , Repping, S. , & Hamer, G. (2016). Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: A systematic review and meta‐analysis. PLoS One, 11(11), e0165125. 10.1371/journal.pone.0165125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, D. , Han, G. , Shang, Y. , Liu, C. , Xia, L. , Li, L. , & Yi, S. (2015). Antisperm antibodies in infertile men and their effect on semen parameters: A systematic review and meta‐analysis. Clinica Chimica Acta, 444, 29–36. 10.1016/j.cca.2015.01.033 [DOI] [PubMed] [Google Scholar]
- Douglas, G. C. , O'Bryan, M. K. , Hedger, M. P. , Lee, D. K. , Yarski, M. A. , Smith, A. I. , & Lew, R. A. (2004). The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology, 145(10), 4703–4711. 10.1210/en.2004-0443 [DOI] [PubMed] [Google Scholar]
- Esteves, S. C. (2014). Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. International Brazilian Journal of Urology, 40(4), 443–453. 10.1590/s1677-5538.Ibju.2014.04.02 [DOI] [PubMed] [Google Scholar]
- Evenson, D. P. , Jost, L. K. , Corzett, M. , & Balhorn, R. (2000). Characteristics of human sperm chromatin structure following an episode of influenza and high fever: A case study. Journal of Andrology, 21(5), 739–746. [PubMed] [Google Scholar]
- Evenson, D. P. , & Wixon, R. (2006). Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology, 65(5), 979–991. 10.1016/j.theriogenology.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Fan, C. , Li, K. , Ding, Y. , Lu, W. L. , & Wang, J. (2020). ACE2 expression in kidney and testis may cause kidney and testis damage after 2019‐nCoV infection. medRxiv. 10.1101/2020.02.12.20022418. [DOI] [PMC free article] [PubMed]
- Gagliardi, L. , Bertacca, C. , Centenari, C. , Merusi, I. , Parolo, E. , Ragazzo, V. , & Tarabella, V. (2020). Orchiepididymitis in a boy with COVID‐19. The Pediatric Infectious Disease Journal, 39(8), e200–e202. 10.1097/INF.0000000000002769 [DOI] [PubMed] [Google Scholar]
- Gallegos, G. , Ramos, B. , Santiso, R. , Goyanes, V. , Gosálvez, J. , & Fernández, J. L. (2008). Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma . Fertility and Sterility, 90(2), 328–334. 10.1016/j.fertnstert.2007.06.035 [DOI] [PubMed] [Google Scholar]
- Gianzo, M. , Muñoa‐Hoyos, I. , Urizar‐Arenaza, I. , Larreategui, Z. , Quintana, F. , Garrido, N. , Subirán, N. , & Irazusta, J. (2016). Angiotensin II type 2 receptor is expressed in human sperm cells and is involved in sperm motility. Fertility and Sterility, 105(3), 608–616. 10.1016/j.fertnstert.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Gralinski, L. E. , & Menachery, V. D. (2020). Return of the coronavirus: 2019‐nCoV. Viruses, 12(2), 135. 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. U. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , Liu, L. , Shan, H. , Lei, C.‐L. , Hui, D. S. C. , Du, B. , Li, L.‐J. , Zeng, G. , Yuen, K.‐Y. , Chen, R.‐C. , Tang, C.‐L. , Wang, T. , Chen, P.‐Y. , Xiang, J. , … Zhong, N.‐S. (2020). Clinical characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger, M. P. (2011). Toll‐like receptors and signalling in spermatogenesis and testicular responses to inflammation‐A perspective. Journal of Reproductive Immunology, 88(2), 130–141. 10.1016/j.jri.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger, M. P. , & Meinhardt, A. (2003). Cytokines and the immune‐testicular axis. Journal of Reproductive Immunology, 58(1), 1–26. 10.1016/s0165-0378(02)00060-8 [DOI] [PubMed] [Google Scholar]
- Hermann, B. P. , Cheng, K. , Singh, A. , Roa‐De La Cruz, L. , Mutoji, K. N. , Chen, I.‐C. , Gildersleeve, H. , Lehle, J. D. , Mayo, M. , Westernströer, B. , Law, N. C. , Oatley, M. J. , Velte, E. K. , Niedenberger, B. A. , Fritze, D. , Silber, S. , Geyer, C. B. , Oatley, J. M. , & McCarrey, J. R. (2018). The mammalian spermatogenesis single‐cell transcriptome, from spermatogonial stem cells to spermatids. Cell Reports, 25(6), 1650–1667.e1658. 10.1016/j.celrep.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , Nitsche, A. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa, S. , Vassiliou, A. , Stone, J. , Killeen, A. , Dawkins, A. , Xie, J. , Gould, F. , & Ramsay, J. (2019). A comparison between two assays for measuring seminal oxidative stress and their relationship with sperm DNA fragmentation and semen parameters. Genes, 10(3), 236. 10.3390/genes10030236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal, H. , Bahadur, G. , Knowles, W. , Jin, L. , & Brink, N. (2004). Mumps epididymo‐orchitis with prolonged detection of virus in semen and the development of anti‐sperm antibodies. Journal of Medical Virology, 73(1), 147–150. 10.1002/jmv.10544 [DOI] [PubMed] [Google Scholar]
- Jan, S. Z. , Vormer, T. L. , Jongejan, A. , Röling, M. D. , Silber, S. J. , de Rooij, D. G. , Hamer, G. , Repping, S. , & van Pelt, A. M. M. (2017). Unraveling transcriptome dynamics in human spermatogenesis. Development, 144(20), 3659–3673. 10.1242/dev.152413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, Z. , Pakfetrat, Z. , Roozbeh, J. , & Janfeshan, S. (2020). Toll‐like receptor‐2 mediates systemic inflammation in gentamicin‐induced rat nephrotoxicity. Clininical and Experimental Pharmacology and Physiology, 47(9), 1584–1590. 10.1111/1440-1681.13334 [DOI] [PubMed] [Google Scholar]
- Ku, J. H. , Kim, Y. H. , Jeon, Y. S. , & Lee, N. K. (1999). The preventive effect of systemic treatment with interferon‐alpha2B for infertility from mumps orchitis. BJU International, 84(7), 839–842. 10.1046/j.1464-410x.1999.00273.x [DOI] [PubMed] [Google Scholar]
- La Vignera, S. , Condorelli, R. A. , Vicari, E. , D'Agata, R. , & Calogero, A. E. (2012). Sperm DNA damage in patients with chronic viral C hepatitis. European Journal of Internal Medicine, 23(1), e19–e24. 10.1016/j.ejim.2011.08.011 [DOI] [PubMed] [Google Scholar]
- Lai, C. C. , Shih, T. P. , Ko, W. C. , Tang, H. J. , & Hsueh, P. R. (2020). Severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) and Coronavirus Disease‐2019 (COVID‐19): The epidemic and the challenges. International Journal of Antimicrobial Agents, 55(3), 105924. 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. E. M. , Agbaje, I. , & Alvarez, J. (2008). Sperm DNA tests as useful adjuncts to semen analysis. Systems Biology in Reproductive Medicine, 54(3), 111–125. 10.1080/19396360801957739 [DOI] [PubMed] [Google Scholar]
- Li, R. , Yin, T. , Fang, F. , Li, Q. , Chen, J. , Wang, Y. , Hao, Y. , Wu, G. , Duan, P. , Wang, Y. , Cheng, D. , Zhou, Q. I. , Zafar, M. I. , Xiong, C. , Li, H. , Yang, J. , & Qiao, J. (2020). Potential risks of SARS‐CoV‐2 infection on reproductive health. Reproductive BioMedicine Online, 41(1), 89–95. 10.1016/j.rbmo.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. O. , Wu, H. , Wang, W. , Song, H. , Huang, B. , Zhu, N. A. , Bi, Y. , Ma, X. , Zhan, F. , Wang, L. , Hu, T. , Zhou, H. , Hu, Z. , Zhou, W. , Zhao, L. I. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel Coronavirus: Implications for virus origins and receptor binding. Lancet, 395(10224), 565–574. 10.1016/s0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Xie, W. , Li, D. , Shi, L. , Mao, Y. , Xiong, Y. , Zhang, M. (2020). Effect of SARS‐CoV‐2 infection upon male gonadal function: A single center‐based study. medRxiv. 10.1101/2020.03.21.20037267 [DOI]
- Mahmudpour, M. , Roozbeh, J. , Keshavarz, M. , Farrokhi, S. , & Nabipour, I. (2020). COVID‐19 cytokine storm: The anger of inflammation. Cytokine, 133, 155151. 10.1016/j.cyto.2020.155151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi, M. , & Weidner, W. (2017). Site and risk factors of antisperm antibodies production in the male population. In Immune Infertility (pp. 133–147). Springer. [Google Scholar]
- Marshburn, P. B. , & Kutteh, W. H. (1994). The role of antisperm antibodies in infertility. Fertility and Sterility, 61(5), 799–811. 10.1016/S0015-0282(16)56687-4 [DOI] [PubMed] [Google Scholar]
- Masjedi, F. , Keshtgar, S. , Zal, F. , Talaei‐Khozani, T. , Sameti, S. , Fallahi, S. , & Kazeroni, M. (2020). Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. The Journal of Steroid Biochemistry and Molecular Biology, 197, 105521. 10.1016/j.jsbmb.2019.105521 [DOI] [PubMed] [Google Scholar]
- Nagy, Z. P. , Verheyen, G. , Liu, J. , Joris, H. , Janssenswillen, C. , Wisanto, A. , Devroey, P. , & Van Steirteghem, A. C. (1995). Andrology: Results of 55 intracytoplasmic sperm injection cycles in the treatment of male‐immunological infertility. Human Reproduction, 10(7), 1775–1780. 10.1093/oxfordjournals.humrep.a136172 [DOI] [PubMed] [Google Scholar]
- Pan, P. P. , Zhan, Q. T. , Le, F. , Zheng, Y. M. , & Jin, F. (2013). Angiotensin‐converting enzymes play a dominant role in fertility. International Journal of Molecular Sciences, 14(10), 21071–21086. 10.3390/ijms141021071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencheva, M. , Koeva, Y. , & Atanassova, N. (2015). Protective role of germinal angiotensin I converting enzyme (gACE) for sperm and fertilization. Acta Morphologica Et Anthropologica, 22, 167–175. [Google Scholar]
- Peng, L. , Liu, J. , Xu, W. , Luo, Q. , Deng, K. , Lin, B. , & Gao, Z. (2020). Novel Coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. 10.1101/2020.02.21.20026179 [DOI] [PMC free article] [PubMed]
- Ren, X. , Wei, X. , Li, G. , Ren, S. , Chen, X. , Zhang, T. , Zhang, X. , Zhongwen, L. , You, Z. , Wang, S. , Qin, C. , & Song, N. & Wang, Z. (2020). Multiple expression assessments of ACE2 and TMPRSS2 SARS‐CoV‐2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID‐19. bioRxiv. 10.1101/2020.05.08.083618 [DOI] [PMC free article] [PubMed]
- Restrepo, B. , & Cardona‐Maya, W. (2013). Antisperm antibodies and fertility association. Actas Urológicas Españolas (English Edition), 37(9), 571–578. 10.1016/j.acuroe.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Rival, C. , Theas, M. S. , Guazzone, V. A. , & Lustig, L. (2006). Interleukin‐6 and IL‐6 receptor cell expression in testis of rats with autoimmune orchitis. Journal of Reproductive Immunology, 70(1–2), 43–58. 10.1016/j.jri.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Santi, D. , Spaggiari, G. , & Simoni, M. (2018). Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management – Meta‐analyses. Reproductive Biomedicine Online, 37(3), 315–326. 10.1016/j.rbmo.2018.06.023 [DOI] [PubMed] [Google Scholar]
- Sergerie, M. , Laforest, G. , Bujan, L. , Bissonnette, F. , & Bleau, G. (2005). Sperm DNA fragmentation: Threshold value in male fertility. Human Reproduction, 20(12), 3446–3451. 10.1093/humrep/dei231 [DOI] [PubMed] [Google Scholar]
- Shen, Q. , Xiao, X. , Aierken, A. , Yue, W. , Wu, X. , Liao, M. , & Hua, J. (2020). The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS‐CoV‐2 infection. Journal of Cellular and Molecular Medicine, 24, 9472–9477. 10.1111/jcmm.15541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinisi, A. A. , Finizio, B. D. , Pasquali, D. , Scurini, C. , D'apuzzo, A. , & Bellastella, A. (1993). Prevalence of antisperm antibodies by SpermMARtest in subjects undergoing a routine sperm analysis for infertility. International Journal of Andrology, 16(5), 311–314. 10.1111/j.1365-2605.1993.tb01197.x [DOI] [PubMed] [Google Scholar]
- Situation report, WHO (2020). Coronavirus disease (COVID‐19) outbreak situation. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Song, C. I. , Wang, Y. , Li, W. , Hu, B. , Chen, G. , Xia, P. , Wang, W. , Li, C. , Diao, F. , Hu, Z. , Yang, X. , Yao, B. , & Liu, Y. (2020). Absence of 2019 novel Coronavirus in semen and testes of COVID‐19 patients. Biology of Reproduction, 103(1), 4–6. 10.1093/biolre/ioaa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, W. , Zhao, X. , Ma, X. , Wang, W. , Niu, P. , Xu, W. , & Wu, G. (2020). A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Weekly, 2(4), 61–62. [PMC free article] [PubMed] [Google Scholar]
- Tangal, S. , Taşçı, Y. , Pabuçcu, E. G. , Çağlar, G. S. , Haliloğlu, A. H. , & Yararbaş, K. (2018). DNA fragmentation index and human papilloma virus in males with previous assisted reproductive technology failures. Turkish Journal of Urology, 45(1), 12–16. 10.5152/tud.2018.96393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji, A. , Murakami, M. , & Matsuda, S. (2020). COVID‐19, an infertility risk? Clinical Obstetrics, Gynecology and Reproductive Medicine, 6, 1. 10.15761/cogrm.1000291 [DOI] [Google Scholar]
- Valdivia, A. , Cortés, L. , Beitia, M. , Totorikaguena, L. , Agirregoitia, N. , Corcostegui, B. , Casis, L. , Matorras, R. , Irazusta, J. , & Agirregoitia, E. (2020). Role of Angiotensin‐(1–7) via MAS receptor in human sperm motility and acrosome reaction. Reproduction, 159(3), 241–249. 10.1530/rep-19-0274 [DOI] [PubMed] [Google Scholar]
- Wang, D. , Hu, B. O. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , Wang, B. , Xiang, H. , Cheng, Z. , Xiong, Y. , Zhao, Y. , Li, Y. , Wang, X. , & Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus‐Infected pneumonia in Wuhan, China. The Journal of the American Medical Association (JAMA), 323(11), 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Xu, Y. , Gao, R. , Lu, R. , Han, K. , Wu, G. , & Tan, W. (2020). Detection of SARS‐CoV‐2 in different types of clinical specimens. The Journal of the American Medical Association (JAMA), 323(18), 1843–1844. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , & Xu, X. (2020). scRNA‐seq profiling of human testes reveals the presence of ACE2 receptor, a target for SARS‐CoV‐2 infection, in spermatogonia. Leydig and Sertoli Cells. Cells, 9(4), 920. 10.3390/cells9040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Qi, L. , Chi, X. , Yang, J. , Wei, X. , Gong, E. , Peh, S. & Gu, J. (2006). Orchitis: A complication of severe acute respiratory syndrome (SARS). Biology of Reproduction, 74(2), 410–416. 10.1095/biolreprod.105.044776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Chen, S. , Huang, B. O. , Zhong, J.‐M. , Su, H. , Chen, Y.‐J. , Cao, Q. , Ma, L. , He, J. , Li, X.‐F. , Li, X. , Zhou, J.‐J. , Fan, J. , Luo, D.‐J. , Chang, X.‐N. , Arkun, K. , Zhou, M. , & Nie, X. (2020). Pathological findings in the testes of COVID‐19 patients: Clinical implications. European Urology Focus, 6(5), 1124–1129. 10.1016/j.euf.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyad, A. , Hamad, M. F. , & Hammadeh, M. E. (2018). The effects of bacterial infection on human sperm nuclear protamine P1/P2 ratio and DNA integrity. Andrologia, 50(2), 10.1111/and.12841 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [repository name e.g. “figshare”] at http://doi.org/, reference number [reference number].


