Abstract
Objective
The main objective of the present study was to investigate the electromyographic (EMG) activity of gluteus medius (Gmed) and gluteus maximus (Gmax) muscles during functional exercises in subjects with chronic ankle instability (CAI) vs healthy controls.
Methods
Seventeen subjects (age, 24.4 ± 2.03 years) with CAI and 17 healthy controls (age, 24.6 ± 2.57 years) were recruited for the present study. For all participants, after testing maximum voluntary isometric contraction of the Gmed and Gmax muscle, EMG activity of these muscles was recorded during functional exercises, such as the Y Balance Test and the single-leg squat with and without Swiss ball.
Results
EMG activity of Gmed and Gmax was found to be significantly (P < .05) reduced during all functional exercises in subjects with CAI when compared with healthy controls. No significant differences (P > .05) were observed in the EMG activity of both muscles across different functional exercises.
Conclusion
Our findings indicate that EMG activity of hip muscles is significantly reduced in CAI subjects, which might give an indication regarding the inclusion of hip muscle strengthening (Gmax and Gmed) in the rehabilitation of CAI. Moreover, Gmed and Gmax muscle activity did not vary during the different functional exercises within each group, which might indicate that activation pattern of these muscles are not sensitive to the type of functional task.
Key Indexing Terms: Ankle, Hip, Joint Instability, Muscles
Introduction
Ankle injuries are common musculoskeletal problems encountered by 10% to 30% of the participating sports population, and 80% of the victims of ankle injury experience recurrence. Recurrence often leads to signs and symptoms such as instability, pain, and reduced overall functionality. Chronic ankle instability (CAI) can be defined as a tendency to “give way” during routine and sports activity, leading to repeated episodes of ankle sprains.1 Occurrence of CAI can be explained by two phenomena: mechanical ankle instability and functional ankle instability (FAI). Mechanical ankle instability is defined as a 5-degree difference in the talar tilt test and a 4-mm side-to-side difference in the anterior drawer test,2 whereas a feeling of “giving way” during functional tasks, combined with negative talar tilt and anterior drawer objective test results, is a defining characteristic of FAI.3 Another reason for development of ankle instability is articular deafferentation, which occurs because of the lesion of mechanoreceptors within the joint capsule and ligaments surrounding the ankle. Furthermore, dynamic stability of the ankle joint is also dependent on the ability of ankle evertors to develop adequate tension during sudden inversion perturbations to prevent ankle sprains.1
Altered motor control as indicated by EMG activity of leg muscles during walking and drop landing has been seen in subjects with CAI.4 During walking, there is a compensatory preactivation of peroneus longus muscle before initial contact, whereas healthy individuals activate peroneus longus muscle after initial contact.5 Balance deficits have been found in subjects with CAI during challenging tasks, such as jump landing and during the execution of functional tasks such as the Star Excursion Balance Test.6 Furthermore, a slower and delayed onset of EMG activity in muscles at the ankle, knee, and hip has been seen during transition from a bipedal to a unipedal stance in subjects with CAI, which might indicate alteration in motor control neural pathways in these subjects.7 CAI subjects build neuromuscular alterations in structures not only around the ankle but also in structures proximal to the ankle, such as changes in postural sway and strength of hip abductor muscles, to compensate for repeated ankle injury.8 Along with balance and strength deficits in proximal joints, CAI has been shown to impair kinematic patterns of the hip and knee joints during gait.9 Hence, this reduced proximal neuromuscular control, especially at the hip, seen in subjects with CAI could result in improper foot placement and further predisposition to ankle injuries, because strength and stability of the hip are important for appropriate gait mechanics and foot position during heel strike.10 Interestingly, a recent study of patients with FAI illustrated that proximal muscles (Gmax and gluteus medius [Gmed]) were faster than the distal muscles (tibialis anterior, peroneus longue) during external perturbations to compensate for the impaired ankle muscle activity, which again highlights the fact that proximal hip muscles are involved in CAI subjects.11
In light of this finding, it is clear that proximally situated hip musculature has a role to play in neuromuscular control of the ankle in CAI subjects and hence leads to speculation that strengthening exercises targeting hip muscles could provide additional benefits to patients experiencing recurring ankle instability. Previous literature has been able to identify rehabilitative exercises, such as single-leg stance and trunk or hip rotation, which lead to greater recruitment of hip muscles in healthy subjects.12 Conversely in CAI subjects, research has mainly focused on studying the neuromuscular alterations pertaining to the ankle musculature with less emphasis on the hip muscles, which are equally necessary to complete a functional task in CAI subjects. Furthermore, exercises used in previous studies13,14 are largely bilateral, which is contrary to the unilateral nature of this musculoskeletal problem. Hence, a thorough analysis is required to further understand the neuromuscular behavior of proximally situated hip muscles during unilateral functional tasks in CAI subjects. A scientific analysis of functional exercises that specifically target the neuromuscular system of subjects with CAI would be of particular interest. The Y Balance Test (YBT) is used for measuring balance and reach deficits in subjects with CAI, and assessing EMG activity of hip muscles during this test might be considered extremely important to understand neuromuscular deficits in these subjects, considering the complexity of this task. Furthermore, EMG analysis of hip muscles during unilateral functional exercises, such as a single-leg squat with and without Swiss ball, might also provide more insight into the neuromuscular strategies used by subjects with CAI to maintain postural stability. It might also provide insight regarding the potential utility of these exercises in the rehabilitation of CAI. This knowledge, if attained properly, may help to broaden the physical therapy approach in the management of CAI. Therefore, the objective of the present study was to investigate the EMG activity of the hip muscles (Gmed and Gmax muscle) during different functional exercises (Y balance and single-leg squat with and without swiss ball) in CAI subjects vs age-matched healthy controls. We hypothesized that those with CAI will demonstrate differential recruitment patterns of hip muscles during different functional exercises compared with their healthy counterparts without CAI.
Methods
Participants
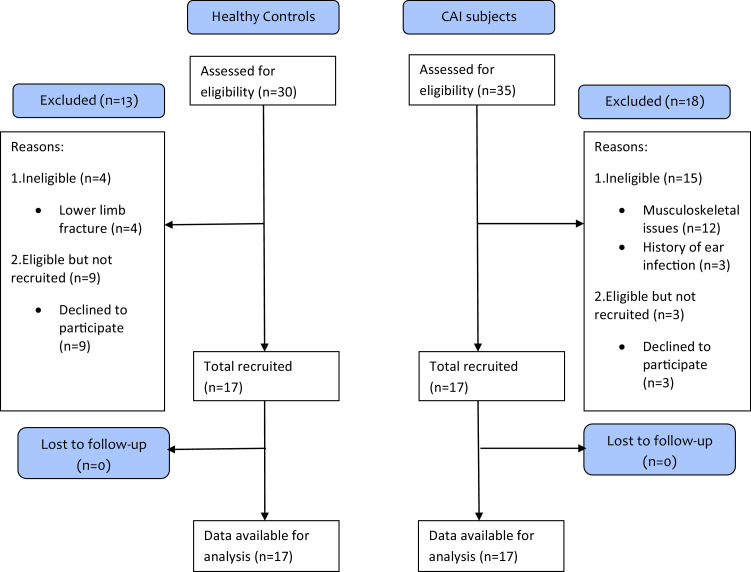
The present study was conducted between January 2018 and May 2018 after obtaining clearance from the Institutional Ethics Committee of Jamia Millia Islamia (A Central University). The sample size for the present study was determined with Software G Power 3.1.9.2 (Franz F; Universitat Kiel, Kiel, Germany) using differences in Gmed muscle activation pattern during double-leg stance and single-leg stance in subjects with CAI and healthy controls during functional exercises from a previous study.7 Thirty-four subjects (17 per group) were found to be necessary based on the effect size of 0.6, α level of 0.05, and power (1-β) of 0.90. Thirty-five subjects with CAI and 30 healthy controls were assessed for eligibility (Fig 1). Seventeen patients with CAI (mean ± standard deviation for age, 24.4 ± 2.03 years; height, 157.8 ± 7.86 cm; weight, 54.9 ± 8.75 kg; body mass index, 22.1 ± 3.37 kg/m2) were recruited by convenience sampling from the physiotherapy center of the university. Of the 30 healthy controls screened, 17 controls (mean ± standard deviation for age, 24.6 ± 2.57 years; height, 159.7 ± 6.87 cm; weight, 57.7 ± 8.93 kg; body mass index, 22.5 ± 3.12 kg/m2) were recruited from the nearby community. Presence of CAI was defined as a history of at least 2 ankle inversion injuries in the same ankle for the past 2 years, which had required a period of protected weight bearing or immobilization along with a complaint of giving way of the ankle during functional activities.15 Subjects were excluded for vestibular disorders; a history of lower limb fracture; ear infection associated with the vestibular system; a history of any lower limb injury other than CAI; and receiving any treatment for CAI, cardiovascular, pulmonary, neuromuscular, or musculoskeletal conditions that are suspected to interfere with motor performance. Study procedures were performed in accordance with the Helsinki Declaration of 1964 after obtaining written informed consent from each participant.
Fig 1.
Flow chart demonstrating participant flow through the study.
Experimental Procedures
After recording basic demographic and anthropometric measures, participants were familiarized with the functional exercises (YBT in anterior, posteromedial, and posterolateral direction; single-leg squat with and without Swiss ball) until they felt confident enough to proceed with the actual measurements. Thereafter, maximum voluntary isometric contraction (MVIC) testing was conducted for Gmed and Gmax muscles on the affected side in CAI subjects and on the dominant side in healthy controls. After MVIC recording, participants were made to perform the functional exercises one by one, and simultaneous recordings of electromyography (EMG) using surface electrodes were done. Lab chart 8 software (AD Instruments, Dunedin, New Zealand) was used as the data acquisition software. After recording EMG during these functional tasks, raw EMG amplitude was normalized by the respective MVIC of each muscle.
Functional Exercises
The YBT began with the participant standing on a functional Y Balance board (Functional Movement Systems, Chatham, VA), with the heel of the dominant lower extremity in healthy individuals and the affected leg in CAI subjects aligned in the center of the grid and the great toe aligned along the anteriorly projecting line. Instructions were given to each participant to shift their weight to the dominant leg (for healthy individuals) and to the affected leg (for CAI subjects), reach the opposite lower extremity as far as possible along the specified line (anterior, posteromedial, posterolateral), and lightly touch the toe of the reaching leg while pushing the reach indicator in the direction being tested and then return to the board. The trial was rated as null if the subject raised the heel of the testing leg off the Y balance board, took some rest at maximal reach distance, could not return to the starting position, or lost balance anytime during the test. Three trials were performed for each reach direction. A metronome was set at 60 beats/min to ensure consistent timing of each YBT trial. Participants were given 2 beats (2 seconds) from initial stance to maximum reach with the reaching leg lightly touching the sliding board on the second beat. They were instructed to come to the starting position from maximum reach to bilateral stance at their own controlled pace. Subjects were asked to keep their stance leg on the ground with their hand on the waist.16 A single-leg squat was performed by standing on the dominant or affected extremity with the hands crossed over the chest. The nondominant extremity was kept in approximately 45° of knee flexion. Thereafter, the squat was performed by the subject without losing his or her balance and without making any contact between uninvolved and involved or dominant stance extremities at any time during the activity. A static position was maintained for 5 seconds; they were then asked to return to a single-leg stance. For single-leg squat with Swiss ball, the entire procedure remained the same as for the single-leg squat. In addition, a Swiss ball was placed on the back of the subject and held in its position against the wall. Each functional exercise was performed 3 times with a rest period of 30 seconds between the repetitions. Surface EMG was recorded for the Gmed and Gmax muscles from the beginning to the end of each functional task, and an average of 3 repetitions was calculated for each exercise.
EMG Recording During Functional Exercises
Skin Preparation and Electrode Placement
Surface EMG tests were conducted in accordance with the Surface Electromyography for the Non-Invasive Assessment of Muscles guidelines.17 EMG was recorded using rectangular silver chloride surface electrodes measuring 44 × 28 × 1 mm (Medico Electrodes International, Noida, Uttar Pradesh, India). Electrodes were placed gently over the area after shaving, abrading, and cleaning the skin. For Gmax, electrodes were placed one third the distance from the second sacral vertebrae to the greater trochanter. For Gmed, electrodes were placed at one third the distance between the iliac crest and the greater trochanter parallel to the muscle fibers. Interelectrode distance was kept to 20 mm. A reference electrode was placed on the posterior superior iliac spine. Electrodes were secured over the skin using micropore tape to minimize their movement during testing.
MVIC Assessment
Measurement of MVIC for each muscle was performed to calculate muscle activity in terms of percentage MVIC during functional exercises. For determining the MVIC of the Gmax, the subject was asked to lie in the prone position with the knee of the dominant leg flexed to 90°. The participant's hip was maintained in a neutral position during testing. The stabilizing strap was positioned above the femoral condyles, and a towel was placed between the strap and the participant's leg for comfort. During MVIC testing, the participant was instructed to contract the buttocks while lifting the dominant leg toward the ceiling against the strap to elicit isometric contraction of the Gmax. For Gmed, participants were asked to perform a maximal contraction of Gmed by abducting their hip against manual resistance offered by the investigator on the lateral thigh above the knee joint. For both the muscles, three maximum isometric holds for 5 seconds each were performed. EMG activity from the middle 3 seconds of the entire 5 seconds for each muscle was used for analysis. MVIC was calculated by computing the arithmetic average of the three MVIC trials for each muscle.18
Signal Analysis
EMG signals were recorded using an analog-to-digital convertor (Lab Chart software version 7). Data collected at a sampling rate of 1000 Hz were recorded with a combined preamplifier gain of 100 to 10000 and with a band-pass filter of 0.8 to 800 Hz. The root mean square value was calculated from the raw EMG signals obtained during the functional exercises. EMG activity of Gmed and Gmax was calculated in terms of percentage MVIC (%MVIC) by normalizing the root mean square value obtained during each functional exercise with the average MVIC obtained for the respective muscle (Gmed or Gmax) during the MVIC testing.
Statistical Analysis
Data were analyzed using SPSS Statistics for Windows, version 17 (SPSS, Chicago, IL). Shapiro-Wilk test was used to verify the normality of variables. When the requirements did not meet the normal distribution, the data were log transformed (height and entire EMG data). The demographic characteristics and criterion measures were compared between the healthy controls and CAI subjects using an independent t test. A 2 × 5 mixed-model analysis of variance was used to analyze differences in muscle activity between the 2 groups (CAI vs healthy control) and differences in muscle activity during 5 functional tasks (group × functional task interaction effect). Again, independent t test was used to locate differences in EMG activity of the hip muscles between the 2 groups during functional exercises. Significance level was set at P ≤ .05 for the study.
Results
Demographic Characteristics
Thirty-four subjects completed this study whose objective was to determine the EMG activity of Gmed and Gmax muscle during functional exercises in healthy and CAI subjects. All participants successfully completed all procedures involved in the present study. Comparison of demographic characteristics between the groups is shown in Table 1.
Table 1.
Comparison of Demographic Characteristics Between Subjects with Chronic Ankle Instability and Healthy Controls
| Variables | HC(n = 17) | CAI(n = 17) | P Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Male/Female (n) | 6/11 | 6/11 | |
| Age (years) | 24.6 ± 2.57 | 24.41 ± 2.03 | .76 |
| Height(cm) | 159.7 ± 6.87 | 157.8 ± 7.86 | .35 |
| Weight(kg) | 57.7 ± 8.93 | 54.9 ± 8.75 | .37 |
| BMI(kg/m2) | 22.5 ± 3.12 | 22.1 ± 3.37 | .67 |
BMI, body mass index; HC, healthy controls; CAI, chronic ankle instability; SD, standard deviation.
EMG Activity of Gluteus Maximus Muscle
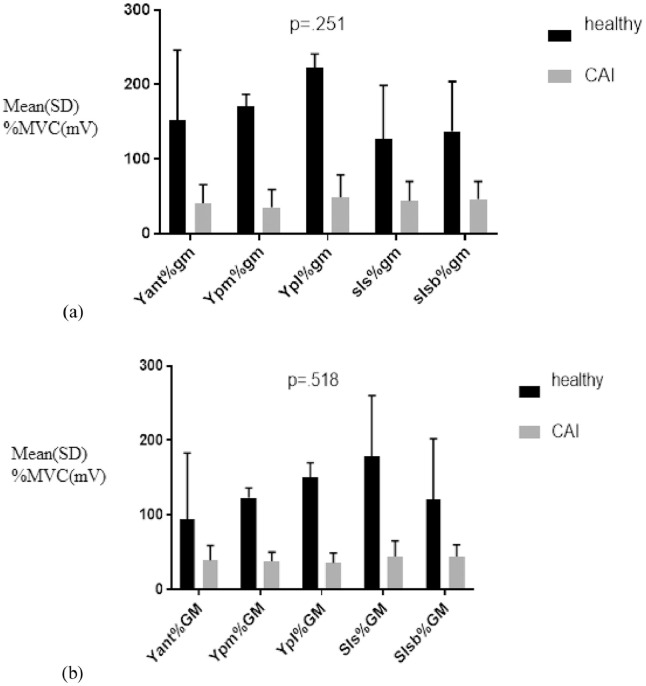
A 2 × 5 mixed-model analysis of variance yielded a significant main effect of group F (1, 32) = 86.24 (P < .001). There was an insignificant main effect for task F (2.335, 32) = 0.181 (P = .866) and for group × task interaction F (2.33, 32) = 0.705 (P = .518; Table 2). These findings signify that the EMG activity of the Gmax muscle was significantly different between patients with CAI and healthy controls; however, the activity did not differ significantly across the functional exercises (Table 3; Fig 2).
Table 2.
Findings of Mixed Model Analysis of Variance Showing Main and Interaction Effects
| Muscle Activity | Main and Interaction Effects | df | F | P Value | η2 |
|---|---|---|---|---|---|
| Gluteus maximus | Group (main effect)a | 1 | 86.24 | <.001 | 0.72 |
| Task (main effect)b | 2.33 | 0.181 | .866 | 0.006 | |
| Group × task (interaction) | 2.33 | 0.705 | .518 | 0.02 | |
| Gluteus medius | Group (main effect) | 1 | 40.40 | <.001 | 0.55 |
| Task (main effect) | 3.72 | 2.06 | .094 | 0.061 | |
| Group × task (interaction) | 3.72 | 1.36 | .254 | 0.041 |
F, Fischer statistic; η2, partial eta square.
Chronic ankle instability vs healthy subjects.
Functional exercises
Table 3.
Comparison of Electromyography Activity of Hip Muscles (Gluteus Medius and Gluteus Maximus Muscle) During Functional Exercises in Healthy Controls vs Subjects With Chronic Ankle Instability
| CAI | Healthy | |||
|---|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | P Value | ES (CI) |
| Gmed (%MVIC) | ||||
| Y ant | 40.5 ± 25.02 | 152.2 ± 94.98 | <.0001 | –1.57 (–2.35 to –0.79) |
| Y pm | 35.2 ± 24.24 | 171.0 ± 169.34 | <.0001 | –1.10 (–1.82 to 0.37) |
| Y pl | 48.9 ± 30.59 | 222.9 ± 192.5 | <.0001 | –1.40 (–2.16 to 0.64) |
| Sls | 43.5 ± 26.77 | 127.4 ± 72.21 | <.0001 | –1.50 (–2.28 to –0.73) |
| Slsb | 45.7 ± 24.13 | 137.6 ± 67.46 | <.0001 | –1.77 (–2.58 to –0.96) |
| Gmax (%MVIC) | ||||
| Y ant | 39.0 ± 20.55 | 93.6 ± 60.19 | <.0001 | –1.19 (–1.92 to –0.45) |
| Y pm | 38.2 ± 12.55 | 123.7 ± 89.59 | <.0001 | –1.31 (–2.05 to –0.56) |
| Y pl | 36.3 ± 13.84 | 150.5 ± 136.74 | <.0001 | –1.15 (–1.88 to –0.42) |
| Sls | 44.3 ± 21.08 | 178.5 ± 203.01 | <.0001 | –0.91 (–1.62 to –0.20) |
| Slsb | 44.3 ± 16.91 | 120.1 ± 82.26 | <.0001 | –1.50 (–2.28 to –0.73) |
CI, confidence interval; ES, effect size; Gmed, gluteus medius; Gmax, gluteus maximus; %MVC, percentage of maximum voluntary contraction; Sls, single-leg squat; Slsb, single-leg squat with swiss ball; Y ant, anterior approach of Y Balance Test; Y pm, posteromedial approach of Y Balance Test; Y pl, posterolateral approach of Y Balance Test.
Fig 2.
(A) Gluteus medius (gm) and (B) Gluteus maximus (GM) electromyographic activity during functional exercises. CAI, chronic ankle instability; Yan%, percent maximum voluntary contraction during anterior; Ypm%, posteromedial approach of Y balance; Ypl%, posterolateral approach of Y balance; Sls%, single-leg squat without Swiss ball; Slsb%, single-leg squat with swiss ball.
EMG Activity of Gluteus Medius Muscle
Findings yielded a significant main effect for group F (1, 32) = 40.40 (P < .001). However, an insignificant main effect for task F (3.72, 32) = 2.06 (P = .061) and group × task interaction was observed for F (3.72, 32) =1.36 (P = .041), which signifies that EMG activity of the Gmed muscle was significantly different between CAI patients and healthy controls (Table 2); however, the activity did not significantly differ across the functional exercises (Table 3; Fig. 2).
Discussion
Main Findings
The purpose of the present study was to determine EMG activity of the Gmed and Gmax muscles in healthy individuals and patients with CAI during the YBT (anterior, posteromedial, posterolateral approach) and during single-leg squat with and without Swiss ball. Results of the present study showed there was a statistically significant difference in the Gmed and Gmax muscle activity between the two groups. The CAI group exhibited lower percent MVIC of the 2 hip muscles in comparison with the healthy group. However, statistically significant differences were not observed for the EMG activity of the 2 muscles during different functional exercises within the groups.
The present study demonstrated significantly lower percent MVIC of the Gmed and Gmax muscles in subjects with CAI compared with healthy individuals. These findings are consistent with the results of Kazemi et al,11 who reported reduced activity of Gmed and Gmax muscles in subjects with CAI and suggested that these subjects probably try to maintain balance by decreasing hip muscle activity to restore some energy, as the human body always seeks minimum energy dissipation. Previous work by Friel et al19 demonstrated significantly reduced hip abduction strength during side-lying hip abduction. The findings of Friel et al19 concur with the findings of Nicholes et al,20 who reported that subjects with CAI showed weakness in hip abduction during isokinetic testing. Moreover, reduced muscle activity observed in the present study and in work by Kazemi et al11 may be the result of the inability of the ankle to provide adequate stability during functional tasks, which is partially compensated by initiation of hip strategy, which helps to prevent the excessive inversion movement at the ankle. The hip strategy model is characterized by decrease in latencies of muscles at the hip when the ankle is perturbated using a horizontal platform. In this model, the ankle is inefficient to provide adequate sensory information regarding the perturbation, and this altered sensory information triggers compensatory responses at more proximal sites, such as the hip joint. Another mechanism is the central set model, wherein the central nervous system perceives the ankle musculature as inadequate in compensating for perturbation and in turn increases the sensitivity to a partially deafferented ankle complex by providing enhanced gamma motor neuron activity to the hip musculature.8 Furthermore, in a study by Feger et al,21 insignificant differences in Gmed activity were observed despite its earlier onset and preactivation during walking in subjects with CAI as compared with healthy controls. These findings are in disagreement with the present findings; which could be accounted for by assessment of muscle activity under different conditions in the present study vs the study by Feger et al.21 In addition, more challenging unilateral tasks used in the present study could elicit reduced recruitment pattern of Gmed in subjects with CAI, contrary to a normal phenomenon of walking in the study by Feger et al.21 However, Feger et al21 observed an earlier onset of the Gmed muscle in subjects with CAI, which might be a compensating strategy to enhance limb stability or to provide functional bracing for ground contact. Previous literature has reported that subjects with CAI have different gait initiation strategies compared with healthy individuals,22 which indicates an alteration in sensorimotor function along with altered alpha motor neuron pool excitability in subjects with CAI, which suggests that the spinal-level motor control mechanism has changed in relation to the decrease in center of pressure excursion during gait.23
Previous literature have also reported the same significant delay for Gmax muscle in CAI subjects during prone hip extension as it was seen for Gmed previously.24 There is the presence of a centralized feed-forward neuromuscular alteration that indicates anticipatory responses to compensate for the intrinsic delay in reflex pathways, and these are intended to minimize the equilibrium disturbances provoked by balance deficits in subjects with CAI. Furthermore, there is a reflex chain of events that occurs in the lower limb after injury because of the pre-existing biomechanical link between proximal and distal lower extremity.25 Pronated feet, which is a common finding in subjects with CAI, has been found to cause internal rotation at the tibia and femur, which consecutively leads to anterior tilting of the pelvis in these subjects.26 This biomechanical link might explain the lower activation of hip muscles demonstrated in our study in subjects with CAI, because skeletal malalignment has been found to alter the angle of action and recruitment of skeletal muscles. According to MacKinnon et al,27 improper foot placements are corrected by synergistic action of the subtalar and hip joint such that small errors in foot placement are compensated by distal adjustments, whereas larger errors are corrected by proximal adjustments at the hip, which might have led to altered or diminished EMG activity of hip muscles observed in the present study.
The present study demonstrated insignificant differences in the activity of hip musculature within both the groups across functional activities. These findings might be partially attributed to the use of closed-chain activity rather than the open-chain position in the present study. Our tasks positions were similar, all using a closed-chain posture and probably posing similar neuromuscular demands on the hip muscles, which might be the reason behind similar EMG activity of hip muscles across the functional exercises. Similarly, insignificant differences were observed for EMG activity of the Gmed muscle during the performance of tasks such as forward lunge, single-limb standing with eyes closed, Star Excursion Balance Test (anterior, posteromedial, posterolateral) approach, and lateral hop tasks in a previous study,4 which again highlights the fact that closed-chain tasks with a balance component in them usually causes similar recruitment of hip muscles in subjects with CAI. In our study, insignificant differences in hip muscle activity during functional exercises within both the groups could be explained by the 2-dimensional design of functional exercises, because previously challenging 3-dimensional tasks, such as rotational squat and rotational lunge, have resulted in notably different muscle activity patterns at the hip in subjects with CAI and healthy controls.28 It has been reported previously that subjects with CAI have a reduced ability to cope with changing task demands, which thus elicits differential muscle activation patterns at the hip during performance of challenging tasks. Moreover, although nonsignificant, the addition of a Swiss ball during single-leg squat increased Gmed activity by 10% in healthy individuals in the present study, which might be a result of greater pelvic stability challenge and neuromuscular control demands offered by the Swiss ball during the single-leg squat.29 In summary, it can be inferred that all the functional exercises assessed in the present study demonstrate statistically similar recruitment of Gmed and Gmax muscles across all functional exercises, suggesting the use of similar neuromuscular behavior during these exercises.
Strength and Limitations
The present study has some limitations, such as the use of surface electrodes for recording EMG activity that might have taken noises from other nearby muscles. In addition, we recorded EMG activity of only the hip muscles. The recording of ankle muscle activity along with the hip might have provided a better understanding of neuromuscular control strategies adopted by subjects with CAI. Moreover, a control group with a previous history of ankle injury but without any recurrent instability should have been taken for in-depth investigation of motor control strategies in patients with ankle sprain with and without CAI. Such an investigation might have given some indications regarding the development of CAI in selected individuals and not all. Despite these limitations, all the procedures were performed as per standard guidelines. The findings of this study are convincing, and they provide indications regarding the involvement and alteration of hip muscle activation patterns in subjects with CAI, and they might be of importance to clinicians working with these patients.
Recommendations for Future Research
Because there is a strong biomechanical link between proximal and distal musculature and joints, investigating EMG activity of abdominal and spinal muscles in subjects with CAI would be of interest; it should be investigated to determine whether there is more proximal involvement in these patients. Investigating hip muscle activation during more challenging functional tasks and prospective studies to determine whether altered activation of Gmed and Gmax develops in relation to CAI are necessary for more conclusive rehabilitation recommendations.
Conclusion
The main findings of this study demonstrate that EMG activation patterns of hip musculature (Gmed and Gmax) are significantly different between subjects with CAI and healthy controls. The activity of these muscles is diminished in subjects with CAI during all functional tasks as compared with healthy individuals without any ankle instability. This finding is the most important one in this study, and it might be of relevance to clinicians with patients with CAI. The inclusion of hip muscle strengthening (Gmed and Gmax) can be considered to evolve the present rehabilitation protocols for CAI. Another important finding of this study indicates that both Gmed and Gmax muscle activity do not vary during functional tasks within each group, which might indicate that the activation pattern of these muscles is not sensitive to the type of functional task in subjects with CAI and in healthy individuals; thus, it could be inferred that any of these exercises can be used for selective recruitment of Gmed and Gmax in subjects with CAI.
Funding Sources and Potential Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): S.F., E.H.
Design (planned the methods to generate the results): S.F., P.B.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): P.B., S.C., E.H.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): S.F., S.C.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): S.F., P.B., D.S.
Literature search (performed the literature search): S.F.
Writing (responsible for writing a substantive part of the manuscript): S.F., P.B., D.S.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): P.B., D.S., E.H.
Practical Applications.
-
•
EMG activity of hip abductors is significantly reduced in subjects with chronic ankle instability (CAI).
-
•
Gluteus maximus and medius strengthening should be incorporated in the rehabilitation of CAI patients.
Alt-text: Unlabelled box
References
- 1.Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg. 1965;47(4):678–685. [PubMed] [Google Scholar]
- 2.Hirai D, Docherty CL, Schrader J. Severity of functional and mechanical ankle instability in an active population. Foot Ankle Int. 2009;0(11):1071–1077. doi: 10.3113/FAI.2009.1071. [DOI] [PubMed] [Google Scholar]
- 3.Mann G, Nyska M, Finsterbush A, Constantini N, Lowe J. Chronic ankle instability, mechanical and functional. The Unstable Ankle. 1st ed. Israel. Human Kinetics. 2002:102–108. [Google Scholar]
- 4.Feger MA, Donovan L, Hart JM, Hertel J. Lower extremity muscle activation in patients with or without chronic ankle instability during walking. J Athl Train. 2015;50(4):350–357. doi: 10.4085/1062-6050-50.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delahunt E, Monaghan K, Caulfield B. Changes in lower limb kinematics, kinetics, and muscle activity in subjects with functional instability of the ankle joint during a single leg drop jump. J Orthop Res. 2006;24(10):1991–2000. doi: 10.1002/jor.20235. [DOI] [PubMed] [Google Scholar]
- 6.Olmsted LC, Carcia CR, Hertel J, Shultz SJ. Efficacy of the star excursion balance tests in detecting reach deficits in subjects with chronic ankle instability. J Athl Train. 2002;37(4):501–506. [PMC free article] [PubMed] [Google Scholar]
- 7.Van Deun S, Staes FF, Stappaerts KH, Janssens L, Levin O, Peers KK. Relationship of chronic ankle instability to muscle activation patterns during the transition from double-leg to single-leg stance. Am J Sports Med. 2007;35(2):274–281. doi: 10.1177/0363546506294470. [DOI] [PubMed] [Google Scholar]
- 8.Beckman SM, Buchanan TS. Ankle inversion injury and hypermobility: effect on hip and ankle muscle electromyography onset latency. Arch Phys Med Rehabil. 1995;76(12):1138–1143. doi: 10.1016/s0003-9993(95)80123-5. [DOI] [PubMed] [Google Scholar]
- 9.Gribble PA, Robinson RH. Alterations in knee kinematics and dynamic stability associated with chronic ankle instability. J Athl Train. 2009;44(4):350–355. doi: 10.4085/1062-6050-44.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerny K. Pathomechanics of stance: clinical concepts for analysis. Phys Ther. 1984;64(12):1851–1859. doi: 10.1093/ptj/64.12.1851. [DOI] [PubMed] [Google Scholar]
- 11.Kazemi K, Arab AM, Abdollahi I, López-López D, Calvo-Lobo C. Electromyography comparison of distal and proximal lower limb muscle activity patterns during external perturbation in subjects with and without functional ankle instability. Hum Mov Sci. 2017;55:211–220. doi: 10.1016/j.humov.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz RJ, Riemann BL, Thompson T. Gluteus medius activity during isometric closed-chain hip rotation. J Sport Rehabil. 2002;11(3):179–188. [Google Scholar]
- 13.Webster KA, Gribble PA. A comparison of electromyography of gluteus medius and maximus in subjects with and without chronic ankle instability during two functional exercises. Phys Ther Sport. 2013;14(1):17–22. doi: 10.1016/j.ptsp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Donovan L, Hart J M, Hertel J. Effects of 2 ankle destabilization devices on electromyography measures during functional exercises in individuals with chronic ankle instability. J Orthop Sports Phys Ther. 2015;45(3):220–232. doi: 10.2519/jospt.2015.5222. [DOI] [PubMed] [Google Scholar]
- 15.Lentell G, Baas B, Lopez D, McGuire L, Sarrels M, Snyder P. The contributions of proprioceptive deficits, muscle function, and anatomic laxity to functional instability of the ankle. J Orthop Sports Phys Ther. 1995;21(4):206–215. doi: 10.2519/jospt.1995.21.4.206. [DOI] [PubMed] [Google Scholar]
- 16.Norris B, Trudelle-Jackson E. Hip-and thigh-muscle activation during the star excursion balance test. J Sport Rehabil. 2011;20(4):428–441. doi: 10.1123/jsr.20.4.428. [DOI] [PubMed] [Google Scholar]
- 17.Hermens HJ, Freriks B, Merletti R. European recommendations for surface electromyography. Roessingh Res Dev. 1999;8(2):13–54. [Google Scholar]
- 18.Hislop H, Avers D, Brown M. 9th ed. Saunders; Philadelphia: 2013. Daniels and Worthingham's Muscle Testing-E-Book: Techniques of Manual Examination and Performance Testing. [Google Scholar]
- 19.Friel K, McLean N, Myers C, Caceres M. Ipsilateral hip abductor weakness after inversion ankle sprain. J Athl Train. 2006;41(1):74–78. [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholas JA, Strizak AM, Veras G. A study of thigh muscle weakness in different pathological states of the lower extremity. Am J Sports Med. 1976;4(6):241–248. doi: 10.1177/036354657600400602. [DOI] [PubMed] [Google Scholar]
- 21.Feger MA, Hart JM, Saliba S, Abel MF, Hertel J. Gait training for chronic ankle instability improves neuromechanics during walking. J Orthop Res. 2018;36(1):515–524. doi: 10.1002/jor.23639. [DOI] [PubMed] [Google Scholar]
- 22.Gribble PA, Hertel J, Denegar CR, Buckley WE. The effects of fatigue and chronic ankle instability on dynamic postural control. J Athl Train. 2004;39(4):321–329. [PMC free article] [PubMed] [Google Scholar]
- 23.Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med. 2010;38(4):829–834. doi: 10.1177/0363546509351562. [DOI] [PubMed] [Google Scholar]
- 24.Bullock Saxton JE. Local sensation changes and altered hip muscle function following severe ankle sprain. Phys Ther. 1994;71(1):17–28. doi: 10.1093/ptj/74.1.17. [DOI] [PubMed] [Google Scholar]
- 25.Rao S, Riskowski JL, Hannan MT. Musculoskeletal conditions of the foot and ankle: assessments and treatment options. Best Pract Res Clin Rheumatol. 2012;26(3):345–368. doi: 10.1016/j.berh.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138(8):613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 27.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26(6):633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 28.Webster KA, Pietrosimone BG, Gribble PA. Muscle activation during landing before and after fatigue in individuals with or without chronic ankle instability. J Athl Train. 2016;51(8):629–636. doi: 10.4085/1062-6050-51.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escamilla RF, Lewis C, Bell D. Core muscle activation during Swiss ball and traditional abdominal exercises. J Orthop Sports Phys Ther. 2010;40(5):265–276. doi: 10.2519/jospt.2010.3073. [DOI] [PubMed] [Google Scholar]