Abstract
Recent decades have been marked by enormous progress in the field of synthesis and chemistry of 5‐(hydroxymethyl)furfural (HMF), an important platform chemical widely recognized as the “sleeping giant” of sustainable chemistry. This multifunctional furanic compound is viewed as a strong link for the transition from the current fossil‐based industry to a sustainable one. However, the low chemical stability of HMF significantly undermines its synthetic potential. A possible solution to this problem is synthetic diversification of HMF by modifying it into more stable multifunctional building blocks for further synthetic purposes.
Keywords: 5-(hydroxymethyl)furfural, renewable building blocks, plant biomass, carbohydrates, sustainable chemistry, biorefining
Green, renewable, versatile: 5‐(Hydroxymethyl)furfural is a known platform chemical for the transition to sustainable chemical industry. Achilles heel of HMF is its low chemical stability. Here we describe the possibilities of HMF diversification into more stable multifunctional derivatives as a means of increasing the competitiveness of HMF‐based biorefining.

1. Introduction
Intensive use of fossil hydrocarbons as an affordable source of high‐energy fuels, chemicals and materials dates from the middle of the 19th century. It has enormously accelerated the rates of industrial growth and may be considered as the root cause of high living standards in many countries. To this day, despite several decades of the development of renewable resources, only approximately 10 % of chemicals and less than 2 % of organic polymers are obtained from non‐fossil feedstocks. [1] At the current rates of carbon consumption, the easily accessible fossil reserves will be depleted within the next several decades. [2] Overcoming the oil and gas dependence is one of the most significant challenges for chemical science and industry. Transition to the carbon‐neutral economy driven by renewable resources is a pressing need for modern society and the main focus of numerous recent investigations in the field of green and sustainable chemistry. [3]
Plant biomass, with its 75 % content of polysaccharides (predominantly hemicellulose and cellulose), is one of the largest sources of renewable carbon. The complex chemical composition of plant biomass underlies the great potential of this material as a source of low‐molecular‐weight compounds, which may be obtained by catalytic or biocatalytic conversion. Although the prices of carbohydrates are comparable with the prices of refined petroleum products, [4] chemical conversion of carbohydrates is still largely neglected. This is primarily due to the differences in the properties of plant biomass and fossil hydrocarbons associated with the different oxygen content. Hyperfunctionalization of carbohydrates (too high saturation with polar and highly reactive hydroxyl groups) significantly limits selective modification of the carbohydrate part of biomass and complicates purification of the resulting products. Key limiting factors for the large‐scale commercialization of the plant biomass processing are as follows:
The necessity of redevelopment (setting up new plants for biomass conversion as well as the infrastructure for the transportation and storage).
High E‐factors of the biomass conversion processes due to the inefficient utilization of all components of biomass and by‐products of its processing, and, as a result, additional costs for waste disposal.
Reputation risks associated with insufficient knowledge of the consumer properties of many innovative biobased products.
In 2010, the top 14 renewable “platform chemicals” (including C1‐C6 alcohols, sugars, acids, furfurals, and biohydrocarbons) as key templates for the sustainable chemical industry of the 21st century were identified. [5] The tremendous synthetic potential of the furanic platform chemicals (furfural, 5‐hydroxymethylfurfural (HMF), and 2,5‐furandicarboxylic acid (FDCA)) explains the unprecedented scale of research in the field of furan chemistry. [6] The Brønsted‐ or Lewis‐acid‐catalyzed dehydration of common hexose carbohydrates provides a versatile approach to C6 furanic derivatives (Scheme 1). [7] The platform chemical HMF which contains three different highly reactive functional centers (aldehyde, hydroxymethyl group and furan ring) can be afforded from the most common hexoses (cellulose, glucose, sucrose or fructose) as starting substrates.
Scheme 1.

Lewis‐acid‐catalyzed conversion of aldohexose carbohydrates into C6 furfurals.
Due to its enormous synthetic and commercial potential, HMF has been dubbed a “sleeping giant” of sustainable chemistry. [8] The flip side of the high reactivity of HMF is instability: it is highly prone to side processes such as hydrolysis into levulinic acid and humification.[ 7d , 9 ] The use of carbohydrates modified at the C6 hydroxyl group leads to significant increase in selectivity of the conversion process due to the direct formation of the C6 furanic derivatives without unstable HMF as an intermediate (Scheme 1).[ 7c , 7d , 10 ] Another problem is the high oxygen content of the HMF molecule, which complicates its isolation and purification. These obstacles impede the full‐fledged industrial production of the HMF‐derived products.
The diversity of functionalities in one molecule creates enormous opportunities for the application of HMF as a synthetic platform for the production of biofuel candidates, advanced materials, and valuable chemicals (Scheme 2).[ 3a , 7b , 11 ] The oxidation or reduction of functional groups in HMF is a general approach to access the key biobased furanic monomers with symmetric structure, such as 2,5‐bis(hydroxymethyl)furan (BHMF), 2,5‐bis(aminomethyl)furan (BAMF), 2,5‐diformylfuran (DFF), 2,5‐furandicarboxylic acid and its esters (FDCA, FDME, FDEE).[ 11f , 12 ] The most commercially important derivative of HMF – FDCA – is a biobased alternative to terephthalic acid for the production of polyesters and polyamides. [13] Despite considerable efforts to promote large‐scale industrial production of HMF, FDCA and PEF (polyethylene furanoate, a 100 % biobased polymer obtained by polycondensation of FDCA with bioethylene glycol), it is still maintained on a pilot‐scale only (several hundred tons per year). [14] Thus, despite the high synthetic potential and the trend towards commercialization, the HMF‐based biorefining is still far from real practical impact.[ 8 , 14b ]
Scheme 2.

(A) Traditional approach to HMF‐based biorefining through direct derivatization of HMF. (B) Biorefining based on HMF diversification to multifunctionalized building blocks (the scope of this review). The key polyfunctional HMF‐derived building blocks are shown in green to emphasize their importance; the same color code is used in the schemes below.
A possible way to increase the competitiveness of the HMF‐based biorefining is diversification of HMF into other (more stable) multifunctional chemical building blocks as bio‐derived analogs of the traditional base and commodity chemicals (Scheme 2). This approach is aimed at circumventing the direct use of HMF, with its high oxygen content and instability, to obtain a variety of valuable HMF derivatives, including monomers, fine and specialty chemicals. This minireview highlights representative examples of the recently developed approaches for obtaining multifunctional HMF derivatives containing three or more different reaction centers. These valuable derivatives can be obtained from HMF directly or in few stages. We survey the main strategies used for the chemical modification of substituents (affording non‐symmetrical (AB‐type) furanic products) or the furan ring itself and discuss the most important derivatives. Although many of the recent and comprehensive reviews have covered the synthetic potential of HMF and some related derivatives for production of biofuels, monomers and chemicals,[ 7b , 11a , 11b , 15 ] a dedicated survey on the literature focused on the synthetic approaches to the multifunctional derivatives of HMF containing three or more different functional groups has not been previously reported. Of course, we want to emphasize that Minireview format is not aimed for exhaustive and comprehensive literature coverage due to size limitations. Rational selection of most perspective HMF‐derived polyfunctional building blocks based on the recent literature and analysis of their synthetic potential represent the general aim of this Minireview.
2. AB‐Type Furanic Building Blocks
2.1. 5‐Chloromethylfurfural (CMF)
High reactivity of the hydroxyl group in HMF results from stability of the corresponding furan carbocation [15a] and makes HMF an efficient alkylating agent. Relevant examples include the acid‐catalyzed self‐etherification of HMF [16] and the benzylation of aromatic hydrocarbons by Friedel‐Crafts reactions. [17] Modification of the hydroxyl functionality in HMF into a better leaving group provides additional functionalization opportunities. Among halide and sulfonate derivatives of HMF, 5‐chloromethylfurfural (CMF) is probably the most studied. CMF is considered as a new platform chemical and a reasonable alternative to HMF due to its lower polarity and higher stability with retention of the high synthetic potential.[ 15p , 18 ] An important advantage of CMF over HMF is the possibility of preparation directly from the most abundant carbohydrate raw materials (cellulose or crude biomass) in high yields of up to 80 %. [19]
The most important HMF derivatives can be obtained directly from CMF in good yields and under mild conditions (Scheme 3). Simple heating of CMF in DMSO (the Kornblum oxidation) affords furanic monomer DFF in a yield of 81 %..[ 19c , 20 ] Another promising monomer, FDCA, can be obtained with the use of stronger oxidizing agents, such as concentrated nitric acid. [19c] The platform chemical levulinic acid (LA) can be obtained in high yields by hydrolysis of CMF. [21] The possibility of straightforward selective reduction of the chloromethyl group to methyl (leading to formation of the promising biofuel candidates DMF or MF) makes CMF a valuable platform for biofuels. [22]
Scheme 3.

CMF‐based synthesis of key HMF derivatives.
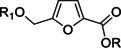
Synthetic potential of CMF as multifunctional platform for a wide range of fine and specialty chemicals has been highlighted in several reviews.[ 15p , 18 , 23 ] Synthesis of versatile polyfunctionalized building blocks by modification of CMF is shown in Scheme 4. Since the chloromethyl group in CMF is poorly susceptible to oxidation, selective transformation of the aldehyde group to carboxyl is easier to perform. Carboxyl derivative 1 can be obtained from CMF on Au‐catalysts. [24] Ester 2 can be obtained by using a two‐stage methodology (reaction of tert‐butyl peroxide with CMF followed by reaction with alcohol via the formation of acid chloride as an intermediate). [20] Reaction of CMF acetal with sodium cyanide followed by acid‐catalyzed deprotection leads to product 3 containing nitrile and aldehyde groups at the furan ring. [23b] This compound is of interest as an N‐containing polyfunctional C7 building block.
Scheme 4.

Synthesis of multifunctional building blocks from CMF.
Due to the presence of good leaving group, CMF can react with various S, [23a] O,[ 19a , 19c , 25 ] or P [26] nucleophiles, as well as enter Friedel‐Crafts reactions. [20] Amination of CMF with liquid ammonia affords furanic derivative 4 containing both aldehyde and primary amine functions. [24] Polyfunctional compound 5, obtained by reacting CMF with sodium azide, can be selectively modified at the aldehyde or azide functional groups. Product 6, which contains a combination of triple bond and azide fragment, can be obtained by reacting 5 with the Ohira‐Bestmann reagent. Product 6 makes a strong candidate platform for “click” chemistry due to the capability of entering the Cu‐catalyzed [3+2] cycloaddition at both azide and alkyne fragments. [27]
Although chemical reactivity of CMF makes this building block attractive for several applications, a care should be taken considering possible formation of Cl‐containing wastes. At both stages, CMF synthesis and further functionalization/usage, careful planning is required to avoid contamination of the environment with Cl‐containing by‐products and wastes. Recycling of Cl or a closed Cl loop are highly desirable approaches.
2.2. Building Blocks Obtained by Modification of the Aldehyde Group
2.2.1. HMFCA and Esters
The aldehyde group in HMF is one of the key components responsible for its high synthetic potential. Selective oxidation of the aldehyde group with formation of HMFCA and its esters under the action of molecular oxygen or other oxidants can be achieved on metal catalysts (Au, [28] Ag, [29] Pt, [30] Mo, [31] or Mg/Ce [32] ). A significant drawback of this approach is side oxidation of the hydroxymethyl group, which leads to formation of FFCA esters or FDCA.[ 30 , 33 ] Organocatalytic oxidative esterification of HMF in the presence of alcohols catalyzed by imidazolium carbenes [34] or sodium cyanide (the Corey‐Gilman‐Ganem oxidation) [35] can be used to avoid over‐oxidation and afford the desired HMFCA esters in good yields. HMFCA may also be obtained from HMF through Cannizzaro reaction; [36] however, an obvious drawback of this approach is stoichiometric formation of a 2,5‐bis(hydroxymethyl)furan as a by‐product. Biocatalytic oxidation of HMF into HMFCA is also good option which overcomes many limitations of other methods. [37]
HMFCA and its esters attract much interest as monomers and multifunctional building blocks (Scheme 5). The presence of reactive groups in HMFCA ensures its high synthetic potential, which, however, may be difficult to realize due to the possibility of side processes of self‐condensation. One possible way to solve this problem is to modify the hydroxyl group into halogen or sulfonate containing intermediates (7 and 8) for further production, for example, of thiol ethers and amines. [38] Furanic monomer, FDCA monoester 9, can be obtained by oxidation of HMFCA esters. [39] The furan ring in HMFCA may also be selectively modified, e. g. hydrogenated into tetrahydrofuran‐containing product 10. [40] Unlike HMF, HMFCA and its esters can enter Diels‐Alder cycloadditions. With ethylene as a dienophile, this reaction results in formation of functional para‐xylene derivatives 11 produced by deoxygenation of the Diels‐Alder adducts. [41] A recently published Ru‐catalyzed alkenylation of HMFCA with acrylates proceeds with or without decarboxylation (depending on the reaction temperature) to afford product 12 or 13, respectively. [42]
Scheme 5.

HMFCA as a multifunctional building block.
2.2.2. N‐Containing Building Blocks
Some of the polyfunctional nitrogen‐containing building blocks can be obtained by modification of the aldehyde group in HMF by using standard methods involving nitrile 15, [43] oxime 16, [44] imines 17, [45] or its O‐substituted derivatives (Scheme 6). [46] Synthesis of nitrone 14 in a yield of 76 % by reacting HMF with nitrobenzene in the presence of hydrogen and a Pt/C catalyst has been reported. [47] Classical methods of reductive amination with borohydrides as reducing agents afford furanic amines 18 via formation of imine intermediates. [48] The same outcome can be achieved with more environmentally friendly transition metal‐catalyzed[ 27 , 45a , 45e , 49 ] or biocatalyzed [50] methodologies.
Scheme 6.
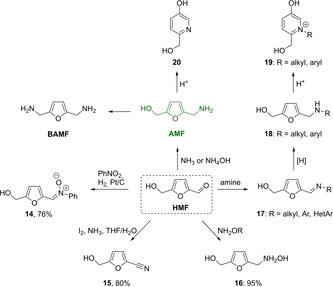
Aminated polyfunctional derivatives of HMF and AMF.
5‐(Aminomethyl)‐2‐furanmethanol (AMF) containing both hydroxymethyl and aminomethyl groups at the furan ring is one of the most important aminated HMF‐derived building blocks with enormous synthetic potential. AMF can be prepared in good yields by reductive amination of HMF with ammonia in the presence of hydrogen by using either transition metal catalysts[ 45e , 49a , 49b , 49f , 51 ] or biocatalytic methods.[ 45e , 50 , 52 ] Amination of AMF affords important monomer 2,5‐bis(aminomethyl)furan (BAMF) [49a] or other furanic amines including biologically active compounds. [53] Recyclization of AMF or its N‐substituted derivatives in the presence of mineral acids, affording pyridinol 20 or pyridinoxonium salts 19, respectively, has been widely applied for the synthesis of bioactive compounds.[ 9 , 48a , 51f , 54 ]
2.2.3. Building Blocks Obtained by C−C Coupling
A variety of carbon‐extension reactions may be used to access >C6 building blocks from HMF (Scheme 7). Classical nucleophilic additions to the aldehyde group (aldol‐type reactions, [55] Grignard reactions[ 55d , 56 ] or other organometallic additions [57] ) yielding polyfunctionalyzed furanic alcohols 21 are predominantly carried out with the use of protective groups at the hydroxyl substituent. However, some of these reactions may preserve free hydroxymethyl groups.[ 55a , 55d , 58 ] Allylic alcohol‐substituted derivatives 22 can be obtained via N‐heterocyclic carbene‐catalyzed Baylis‐Hilman reaction. [59]
Scheme 7.

Building blocks obtained from HMF by C−C coupling.
The simplest acetylenic derivative 23 with high potential as a platform for polymer syntheses can be afforded in excellent yields by using the Ohira‐Bestmann reagent and potassium carbonate as a base. [60] Methods for producing furfuryl ketones 24–26 from HMF using Stetter reaction, [61] interaction with diazomethane [62] or direct metal‐catalyzed C−H functionalization [63] of the aldehyde group are also available.
Significant number of studies focused on polyfunctional furfurylidenes as promising precursors for materials synthesis. Ethylene derivative 27 and carboxylic acid derivatives 28, as well as its phosphorus‐containing analog 29, can be prepared from HMF in good yields by using Wittig reaction[ 51e , 64 ] or Knoevenagel condensation. [65]
Furfurylidenes 30 obtained by aldol condensation of HMF and its derivatives with ketones represent important and easily accessible building blocks for various syntheses of fuels and chemicals (Scheme 8).[ 65a , 66 ] Complete hydrogenation of furfurylidene ketones is a promising approach for the production of the high energy density drop‐in alkane biofuels 31.[ 66c , 66e , 67 ] Partial hydrogenation with or without ring‐opening affords a wide range of synthetic intermediates 32 and 33.[ 66c , 66d , 66e , 68 ] Importantly, some of furfurylidenes can be prepared in high yields directly from carbohydrates without HMF isolation. [69]
Scheme 8.

Synthetic potential of the HMF‐derived furfurylidene ketones.
3. Building Blocks Obtained by Modification of the Furanic Ring
3.1. Hydrogenative Ring‐Opening
Transition metal‐catalyzed hydrolytic ring‐opening in BHMF (where water simultaneously acts both as a catalyst and as a solvent) in the presence of hydrogen provides a route to several C6 aliphatic and cyclic ketones with high platform potential: HHD, HCPN, HCPL and MCP (Scheme 9). The acid‐catalyzed HHD formation in the presence of Ru, Ir or Rh−Re catalysts affords up to 85 % yields with high selectivities.[ 15b , 70 ] Cyclic derivatives HCPN [71] or HCPL[ 71c , 72 ] can be formed by aldol condensation of HHD under acidic conditions through generation of HHED followed by reduction with hydrogen, or as a result of Piancatelli rearrangement of BHMF. [15b] In the presence of Amberlyst, HHED undergoes acetalization to pyranone 34, which can be isolated in a yield of 53 %. [73]
Scheme 9.

(A) Products of hydrogenative hydrolytic ring‐opening in BHMF. (B) Synthetic potential of HHD.
The potential of HHD as a biobased platform chemical has been highlighted in a recent review. [15b] Under basic conditions, HHD undergoes dehydration into cyclopentenone derivative MCP. [74] In the presence of amines, HHD can undergo cyclization into 2‐hydroxymethyl pyrroles 35 in good yields. [75] In addition, HHD can be easily reduced to triol 36 on Ru catalysts.[ 71a , 76 ]
3.2. Oxidative Ring‐Opening
Some highly functionalized building blocks can be obtained by the oxidative ring‐opening in HMF or its derivatives (Scheme 10). In the case of O‐substituted HMF or BHMF, formation of various linear and cyclic products is possible depending on the type of oxidant and reaction conditions. For example, carboxylic acids 37 and 38 can be obtained from sugar‐substituted HMF via singlet‐oxygen oxidation in methanol solution or reaction with aqueous hydrogen peroxide, respectively.[ 10c , 77 ] Reacting anhydrous MCPBA, singlet oxygen, or dimethyldioxirane with disubstituted derivatives of BHMF (40 c) gives cis‐ene‐diones 41 c [78] which show high potential as building blocks. Compounds 41 c may be isomerized into trans‐isomers 42 c or reduced to 1,4‐diketones 43 c which can be further utilized in syntheses of substituted pyrroles 44 and thiophenes 45 by reactions with the Lawesson reagent and amines, respectively.[ 78a , 79 ]
Scheme 10.

(A) General routes to polyfunctional building blocks via oxidation of HMF. (B) Potential of compounds 41 and 46 as precursors for the synthesis of heterocycles.
Oxidation of unsubstituted or mono‐substituted BHMF (40 a,b) affords dehydropyranones 46 via reversible acetalization of en‐diones 41 a,b (the Achmatowich rearrangement).[ 78b , 80 ] Cyclization of 41 b or recyclization of 46 into pyrazines 47 b,c or pyrroles 48 b by reactions with hydrazine or amines, respectively (Scheme 10) is widely applied in natural compound syntheses.[ 78a , 81 ]
Oxidation of O‐substituted HMF with singlet oxygen or MCPBA in aprotic solvents leads to versatile butenolide derivatives 39 as mixtures of stereoisomers.[ 10c , 81d ] Synthetic potential of the HMF‐derived polyfunctional building blocks 39 is shown in Scheme 11. Acids 50 a,b were obtained with good yields by zinc‐mediated reduction of the open form of butenolide derivatives (49 a,b) in acetic acid under ultrasonication. [82] Reduction of 39 a with sodium borohydride affords a versatile building block HBO. [83] Diels‐Alder reactions of 49 b (the opened form of 39 b) with various dienes lead to polyfunctionalized bicyclic or cyclic products 51–53 with high diastereoselectivity. [84]
Scheme 11.

Butenolide 39 a as a multifunctional building block.
3.3. Arene Building Blocks
Due to the presence of the strong π‐accepting aldehyde group, HMF and other furfurals poorly interact with dienophiles in Diels‐Alder reactions. No efficient reactions of dienophiles with HMF or its derivatives containing aldehyde group at the furan ring (DFF, FFA, CMF) have been published. However, Diels‐Alder reactions may be carried out upon transformation of the aldehyde substituent into a weaker π‐acceptor substituent, such as hydroxymethyl, acetal, hydrazone, oxime or carboxyl. This strategy has found broad application in materials science and medicinal chemistry (see Table 1 and references therein).[ 11c , 48b , 85 ] In situ deoxygenation of Diels‐Alder adducts formed from functionalized furanic products and ethylene is one of the mainstream routes to biobased para‐xylene derivatives 54 (entries 1, 6, 12, 17 and 19) towards biobased plastics. Aromatic building blocks 56 and saturated polysubstituted tricyclic products 57 can be obtained via the formation of Diels‐Alder adducts 55 by reactions with various dienophiles: alkenes (entries 3–5, 7, 8, 14 and 15), alkynes (entries 9, 10 and 16), or benzyne (entries 2, 11, 13, 18 and 20).
Table 1.
General synthetic routes to functional aromatic and aliphatic products via Diels‐Alder reactions of HMF derivatives with various dienophiles.
|
| |||
|---|---|---|---|
|
No |
Furanic diene |
Dienophile |
Product: yield |
|
1 |
|
ethylene |
54 a: 0 % [41] |
|
2 |
benzyne |
55 b: 0 % [86] |
|
|
3 |
|
maleic anhydride |
56 a: 13 % [87] |
|
4 |
maleimide |
56 b: 87 % [88] |
|
|
5 |
N‐Et maleimide |
56 c: ∼100 % [89] |
|
|
6 |
|
ethylene |
54 c: <1 % [41] |
|
7 |
fumaronitrile |
56 d: 12 % [90] |
|
|
8 |
maleimide |
||
|
9 |
hexafluorobut‐2‐yne |
56 f: 87 % [92] |
|
|
10 |
dimethyl acetylenedicarboxylate |
||
|
11 |
benzyne |
55 h: 74 % [94] |
|
|
12 |
|
Ethylene[a] |
54 d: 2 % [84] |
|
13 |
benzyne |
55 e: 0 % [86] |
|
|
14 |
|
acrylonitrile |
|
|
15 |
|
maleic anhydride |
|
|
16 |
methyl propiolate |
56 m: R=OBn; 48 % [87] |
|
|
17 |
|
ethylene |
|
|
18 |
benzyne |
55 n: R=Me, R1=Ac; 72 %; [96] 56 n: R=Me, R1=Ac; 82 %;[96],[b] |
|
|
19 |
|
ethylene |
54 h: R=H; 0 % [41] 54 i: R =Me; 36 % [97] 54 j: R=Et; 59 % [98] |
|
20 |
benzyne |
55 o: R=H; 0 %; [86] 55 p: R=Me; 91 %; [99] 56 p: R=H; 89 %;[99],[c] |
|
[a] Formed in situ from ethanol. [b] Final yield after two‐stage methodology including reduction of the double bond in 55 n followed by deoxygenation. [c] Obtained from 55 p by two‐stage methodology including reduction of the double bond followed by deoxygenation.
Hydrothermal recyclization of HMF (where water simultaneously acts both as a reagent and as a solvent) in the presence of various Lewis acids as catalysts affords 1,2,4‐benzene triol (BTO) in yields of up to 54 % (Scheme 12). [100] This compound represents an important bioderived aromatic building block for the synthesis of fuels, chemicals and materials.[ 100a , 100b ] Hydrodeoxygenation of BTO into hydroquinone proceeds under various conditions with the maximum yield of 53 % achieved on a Rh/Al2O3 catalyst. [101] Amination of BTO with aqueous ammonia under acidic conditions affords 1‐amino‐resorcinol (ARCL) in a 34 % yield. [102] Under oxidizing conditions, BTO undergoes dimerization into polyhydroxylated products 58 or 59 in neutral or acidic water media, respectively.[ 100a , 103 ]
Scheme 12.

Hydrothermal recyclization of HMF into BTO and its synthetic potential.
3.4. Polysubstituted Furanic Building Blocks
A reliable access to polysubstituted furanic building blocks by substitution at the furan ring may significantly increase the synthetic potential of the HMF‐based chemicals. However, few examples of the furan CH‐functionalization in HMF and its derivatives can be found in the literature (Schemes 13 and 14). Reactions of magnesiated FDEE with various electrophiles give trisubstituted furans 60 in good yields (Scheme 13). [104]
Scheme 13.

Synthesis of trisubstituted furanic building blocks by CH‐functionalization of FDCA esters or monoamides.
Scheme 14.

Ru‐catalysed CH‐activation of HMF‐derived imines. TBS=tert‐butyldimethylsilyl. Bnep=boronic acid neopentylglycol ester. BA=benzylideneacetone. a) Contain 2‐(piperidin‐1‐yl)ethyl as a directing group. PMP=p‐methoxyphenyl.
A ruthenium‐catalyzed CH‐activation of HMF‐derived imines 63 and 64, containing a TBS protective group at the hydroxyl and an aminated directing group, with alkenes (the Murai reaction) followed by deprotection yields alkylated products 66 or acylated (68) products (Scheme 14a). [105] Carbonylative version of this reaction affording C3‐acylated products 68 with good yields was very recently reported. [106] In similar catalytic systems, an HMF‐derived substrate 64, [107] as well as some FDCA monoamides, [108] can be modified into arylated products 67–69 or silyl derivatives 70 by reactions with the corresponding ArBnep reagents or vinyl silanes, respectively (Scheme 14b,c).
4. Conclusions
HMF, a bioderived platform chemical, is of key importance as multifunctional building block for sustainable chemical industries in the post‐oil era. A plethora of studies on the use of HMF as a synthetic intermediate for the production of biofuels, materials and fine chemicals (including solvents, pharmaceuticals and other highly valuable products) have been published over the past 20 years. However, HMF is not an ideal platform for the use in all of the proposed synthetic applications. In our opinion, HMF has the greatest impact as a platform for the production of polymeric materials. As an example, a straightforward sequence from HMF to 3D printing material was demonstrated. [109] A number of other applications of HMF‐derived polymeric materials were also studied.[ 12a , 110 ]
The low stability and high oxygen content limit the use of HMF as a platform for biofuels and complicate direct access from HMF to certain fine chemicals. Among multifunctional HMF derivatives, it may be anticipated that CMF and furfurylidene ketones have the high potential for the development of biofuels and fuel additives. Diketones obtained from HMF by reductive or oxidative ring‐opening show high performance in the construction of heterocycles (including aromatic systems). The highly accessible furanic AB‐type products (mostly HMFCA), as well as non‐furanic HMF derivatives, may be considered as building blocks for fine and specialty chemicals. Some HMF derivatives hold great promise for special applications in the syntheses of industrially relevant compounds. For example, monoamine BAF may be used as a platform chemical for bioderived amines. Diels‐Alder cycloadditions with HMF‐derived functionalized substrates, as well as modifications of BTO, may be considered as key routes to biobased aromatics. We believe that synthetic potential of many HMF‐derived building blocks is still untapped. Preparation of multifunctional HMF‐derived building blocks from carbohydrates or crude biomass and further study of their synthetic potential will pay off. Efforts in this direction will facilitate access from HMF to higher‐value‐added products and ultimately increase the competitiveness of HMF‐based biorefining.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Konstantin Galkin is a research fellow in the Laboratory of Metal‐Complex and Nanoscale Catalysts at the N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences (Moscow). In 2009, he graduated from the D. I. Mendeleev University of Chemical Technology of Russia, and, in 2014, he received his Ph.D. in organic chemistry at the A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences. His research areas of interest include utilization of renewable resources for the production of fine chemicals and dynamic materials.

Biographical Information
Valentine Ananikov received his Ph.D. degree in 1999, habilitation in 2003, and in 2005, he was appointed Professor and Laboratory Head of the ND Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences. In 2008 he was elected as a Member of Russian Academy of Sciences, in 2018 elected as a Member of European Academy (Academia Europaea) and in 2019 elected as Academician of Russian Academy of Sciences. He was the recipient of a medal from the Russian Academy of Sciences (2000), the Russian State Prize for Outstanding Achievements in Science and Technology (2004), an award from the Science Support Foundation (2005), the Balandin Prize for outstanding achievements in the field of catalysis (2010), a Liebig Lectureship from the German Chemical Society (2010), Organometallics Distinguished Author Award Lectureship by American Chemical Society (2016), and Reaxys Award Russia (2019). His scientific interests focus on catalysis, the development of new sustainable methods, and molecular complexity.

Acknowledgements
This work was supported by the Russian Science Foundation (RSF Grant No. 17‐13‐01176‐p).
K. I. Galkin, V. P. Ananikov, ChemistryOpen 2020, 9, 1135.
Contributor Information
Dr. Konstantin I. Galkin, Email: kgalkin@emtc.ru
Prof. Valentine P. Ananikov, Email: val@ioc.ac.ru.
References
- 1.
- 1a. De Clercq R., Dusselier M., Sels B. F., Green Chem. 2017, 19, 5012–5040; [Google Scholar]
- 1b. Clark J. H., Deswarte F. E. I., in Introduction to Chemicals from Biomass (Eds.: Clark J. H., Deswarte F. E. I.), Wiley, Chichester, 2008, pp. 1–20. [Google Scholar]
- 2. Palkovits R., Wright W. R. H., in Chemical Energy Storage (Ed.: Schlögl R.), De Gruyter, Berlin, 2012, pp. 59–86. [Google Scholar]
- 3.See, for example:
- 3a. Gerardy R., Debecker D. P., Estager J., Luis P., Monbaliu J. M., Chem. Rev. 2020, 120, 7219–7347; [DOI] [PubMed] [Google Scholar]
- 3b. Ko C.-H., Yang B.-Y., Lin L.-D., Chang F.-C., Chen W.-H., J. Cleaner Prod. 2020, 254, 119914; [Google Scholar]
- 3c. Zang G., Shah A., Wan C., J. Cleaner Prod. 2020, 260, 120837; [Google Scholar]
- 3d. Sarkar M., Sarkar B., J. Cleaner Prod. 2020, 262, 121200; [Google Scholar]
- 3e. Saini S., Chandel A. K., Sharma K. K., J. Cleaner Prod. 2020, 268, 122357; [Google Scholar]
- 3f. Theppitak S., Hungwe D., Ding L., Xin D., Yu G., Yoshikawa K., Appl. Energy 2020, 272, 115264. [Google Scholar]
- 4.
- 4a. Tuck C. O., Pérez E., Horváth I. T., Sheldon R. A., Poliakoff M., Science 2012, 337, 695–699; [DOI] [PubMed] [Google Scholar]
- 4b. Huber G. W., Iborra S., Corma A., Chem. Rev. 2006, 106, 4044–4098; [DOI] [PubMed] [Google Scholar]
- 4c. Lichtenthaler F. W., Acc. Chem. Res. 2002, 35, 728–737. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Werpy T., Petersen G., Top Value Added Chemicals from Biomass, Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, National Renewable Energy Laboratory, U. S. Department of Energy, Washington, D. C., 2004; [Google Scholar]
- 5b. Bozell J. J., Petersen G. R., Green Chem. 2010, 12, 539. [Google Scholar]
- 6.
- 6a. Li X., Jia P., Wang T., ACS Catal. 2016, 6, 7621–7640; [Google Scholar]
- 6b. Caes B. R., Teixeira R. E., Knapp K. G., Raines R. T., ACS Sustainable Chem. Eng. 2015, 3, 2591–2605; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c. Teong S. P., Yi G., Zhang Y., Green Chem. 2014, 16, 2015; [Google Scholar]
- 6d. Lange J. P., van der Heide E., van Buijtenen J., Price R., ChemSusChem 2012, 5, 150–166; [DOI] [PubMed] [Google Scholar]
- 6e. Chheda J. N., Román-Leshkov Y., Dumesic J. A., Green Chem. 2007, 9, 342–350; [Google Scholar]
- 6f. Lichtenthaler F. W., Peters S., C. R. Chim. 2004, 7, 65–90. [Google Scholar]
- 7.
- 7a. Mika L. T., Cséfalvay E., Németh A., Chem. Rev. 2018, 118, 505–613; [DOI] [PubMed] [Google Scholar]
- 7b. van Putten R. J., van der Waal J. C., de Jong E., Rasrendra C. B., Heeres H. J., de Vries J. G., Chem. Rev. 2013, 113, 1499–1597; [DOI] [PubMed] [Google Scholar]
- 7c. Galkin K. I., Ananikov V. P., ChemSusChem 2019, 12, 185–189; [DOI] [PubMed] [Google Scholar]
- 7d. Galkin K. I., Krivodaeva E. A., Romashov L. V., Zalesskiy S. S., Kachala V. V., Burykina J. V., Ananikov V. P., Angew. Chem. Int. Ed. 2016, 55, 8338–8342; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 8478–8482. [Google Scholar]
- 8. Galkin K. I., Ananikov V. P., ChemSusChem 2019, 12, 2976–2982. [DOI] [PubMed] [Google Scholar]
- 9. Gomes R. F. A., Mitrev Y. N., Simeonov S. P., Afonso C. A. M., ChemSusChem 2018, 11, 1612–1616. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. van der Klis F., van Haveren J., van Es D. S., Bitter J. H., ChemSusChem 2017, 10, 1460–1468; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Gavilà L., Esposito D., Green Chem. 2017, 19, 2496–2500; [Google Scholar]
- 10c. Brust A., Lichtenthaler F. W., Green Chem. 2013, 15, 1368; [Google Scholar]
- 10d. Heguaburu V., Franco J., Reina L., Tabarez C., Moyna G., Moyna P., Catal. Commun. 2012, 27, 88–91; [Google Scholar]
- 10e. Tuteja J., Nishimura S., Ebitani K., Bull. Chem. Soc. Jpn. 2012, 85, 275–281; [Google Scholar]
- 10f. Martin D., Lichtenthaler F. W., Tetrahedron: Asymmetry 2006, 17, 756–762; [Google Scholar]
- 10g. Hamada K., Yoshihara H., Suzukamo G., Chem. Lett. 1982, 11, 617–618. [Google Scholar]
- 11.
- 11a. Kucherov F. A., Romashov L. V., Galkin K. I., Ananikov V. P., ACS Sustainable Chem. Eng. 2018, 6, 8064–8092; [Google Scholar]
- 11b. Kong Q.-S., Li X.-L., Xu H.-J., Fu Y., Fuel Process. Technol. 2020, 209, 106528; [Google Scholar]
- 11c. Hu X., Zeng T., Husic C. C., Robb M. J., J. Am. Chem. Soc. 2019, 141, 15018–15023; [DOI] [PubMed] [Google Scholar]
- 11d. Rosenboom J. G., Hohl D. K., Fleckenstein P., Storti G., Morbidelli M., Nat. Commun. 2018, 9, 2701; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11e. Jenkins R. W., Moore C. M., Semelsberger T. A., Chuck C. J., Gordon J. C., Sutton A. D., ChemSusChem 2016, 9, 922–931; [DOI] [PubMed] [Google Scholar]
- 11f. Rajendran S., Raghunathan R., Hevus I., Krishnan R., Ugrinov A., Sibi M. P., Webster D. C., Sivaguru J., Angew. Chem. Int. Ed. 2015, 54, 1159–1163; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 1175–1179; [Google Scholar]
- 11g. Buntara T., Noel S., Phua P. H., Melian-Cabrera I., de Vries J. G., Heeres H. J., Angew. Chem. Int. Ed. 2011, 50, 7083–7087; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 7221–7225. [Google Scholar]
- 12.
- 12a. Delidovich I., Hausoul P. J., Deng L., Pfutzenreuter R., Rose M., Palkovits R., Chem. Rev. 2016, 116, 1540–1599; [DOI] [PubMed] [Google Scholar]
- 12b. Gandini A., Lacerda T. M., Carvalho A. J., Trovatti E., Chem. Rev. 2016, 116, 1637–1669; [DOI] [PubMed] [Google Scholar]
- 12c. Bao L., Sun F. Z., Zhang G. Y., Hu T. L., ChemSusChem 2020, 13, 548–555; [DOI] [PubMed] [Google Scholar]
- 12d. Arias P. L., Cecilia J. A., Gandarias I., Iglesias J., López Granados M., Mariscal R., Morales G., Moreno-Tost R., Maireles-Torres P., Catal. Sci. Technol. 2020, 10, 2721–2757; [Google Scholar]
- 12e. Motagamwala A. H., Won W., Sener C., Alonso D. M., Maravelias C. T., Dumesic J. A., Sci. Adv. 2018, 4, eaap9722; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12f. Dijkman W. P., Groothuis D. E., Fraaije M. W., Angew. Chem. Int. Ed. 2014, 53, 6515–6518; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 6633–6636. [Google Scholar]
- 13.
- 13a. Sajid M., Zhao X., Liu D., Green Chem. 2018, 20, 5427–5453; [Google Scholar]
- 13b. Zhang Z., Deng K., ACS Catal. 2015, 5, 6529–6544; [Google Scholar]
- 13c. Knoop R. J. I., Vogelzang W., van Haveren J., Van Es D. S., J. Polym. Sci. Part A 2013, 51, 4191–4199; [Google Scholar]
- 13d. de Jong E., Dam M. A., Sipos L., Gruter G. J. M., in Biobased Monomers, Polymers, and Materials, Vol. 1105, American Chemical Society, 2012, pp. 1–13. [Google Scholar]
- 14.
- 14a. Thoma C., Konnerth J., Sailer-Kronlachner W., Solt P., Rosenau T., van Herwijnen H. W. G., ChemSusChem 2020, 3544–3564; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14b. Wojcieszak R., Itabaiana I., Catal. Today 2020, 354, 211–217; [Google Scholar]
- 14c. Hwang K.-R., Jeon W., Lee S. Y., Kim M.-S., Park Y.-K., Chem. Eng. J. 2020, 390, 124636; [Google Scholar]
- 14d. Fei X., Wang J., Zhu J., Wang X., Liu X., ACS Sustainable Chem. Eng. 2020, 8, 8471–8485. [Google Scholar]
- 15.
- 15a. Lewkowski J., Arkivoc 2005, 2001, 17; [Google Scholar]
- 15b. Wozniak B., Tin S., de Vries J. G., Chem. Sci. 2019, 10, 6024–6034; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15c. Xu C., Paone E., Rodriguez-Padron D., Luque R., Mauriello F., Chem. Soc. Rev. 2020, 49, 4273–4306; [DOI] [PubMed] [Google Scholar]
- 15d. Rosatella A. A., Simeonov S. P., Frade R. F. M., Afonso C. A. M., Green Chem. 2011, 13, 754; [Google Scholar]
- 15e. Kong X., Zhu Y., Fang Z., Kozinski J. A., Butler I. S., Xu L., Song H., Wei X., Green Chem. 2018, 20, 3657–3682; [Google Scholar]
- 15f. Hu L., Lin L., Wu Z., Zhou S., Liu S., Renewable Sustainable Energy Rev. 2017, 74, 230–257; [Google Scholar]
- 15g. Fan W., Verrier C., Queneau Y., Popowycz F., Curr. Org. Synth. 2019, 16, 583–614; [DOI] [PubMed] [Google Scholar]
- 15h. Hu L., Xu J., Zhou S., He A., Tang X., Lin L., Xu J., Zhao Y., ACS Catal. 2018, 8, 2959–2980; [Google Scholar]
- 15i. Xia H., Xu S., Hu H., An J., Li C., RSC Adv. 2018, 8, 30875–30886; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15j. Zang H., Wang K., Zhang M., Xie R., Wang L., Chen E. Y. X., Catal. Sci. Technol. 2018, 8, 1777–1798; [Google Scholar]
- 15k. Gupta K., Rai R. K., Singh S. K., ChemCatChem 2018, 10, 2326–2349; [Google Scholar]
- 15l. Liu B., Zhang Z., ChemSusChem 2016, 9, 2015–2036; [DOI] [PubMed] [Google Scholar]
- 15m. Pal P., Saravanamurugan S., ChemSusChem 2019, 12, 145–163; [DOI] [PubMed] [Google Scholar]
- 15n. Singh S. K., Asian J. Org. Chem. 2018, 7, 1901–1923; [Google Scholar]
- 15o. Chernyshev V. M., Kravchenko O. A., Ananikov V. P., Russ. Chem. Rev. 2017, 86, 357–387; [Google Scholar]
- 15p. Mascal M., ACS Sustainable Chem. Eng. 2019, 7, 5588–5601. [Google Scholar]
- 16. Shinde S., Rode C., Catal. Commun. 2017, 88, 77–80. [Google Scholar]
- 17.
- 17a. Ryabukhin D. S., Zakusilo D. N., Kompanets M. O., Tarakanov A. A., Boyarskaya I. A., Artamonova T. O., Khohodorkovskiy M. A., Opeida I. O., Vasilyev A. V., Beilstein J. Org. Chem. 2016, 12, 2125–2135; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b. Iovel I., Mertins K., Kischel J., Zapf A., Beller M., Angew. Chem. Int. Ed. 2005, 44, 3913–3917; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 3981–3985. [Google Scholar]
- 18. Mascal M., ChemSusChem 2015, 8, 3391–3395. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Mascal M., Nikitin E. B., Angew. Chem. Int. Ed. 2008, 47, 7924–7926; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 8042–8044; [Google Scholar]
- 19b. Mascal M., Nikitin E. B., ChemSusChem 2009, 2, 859–861; [DOI] [PubMed] [Google Scholar]
- 19c. Brasholz M., von Kanel K., Hornung C. H., Saubern S., Tsanaktsidis J., Green Chem. 2011, 13, 1114–1117. [Google Scholar]
- 20. Dutta S., Wu L., Mascal M., Green Chem. 2015, 17, 3737–3739. [Google Scholar]
- 21. Mascal M., Nikitin E. B., Green Chem. 2010, 12, 370–373. [Google Scholar]
- 22. Dutta S., Mascal M., ChemSusChem 2014, 7, 3028–3030. [DOI] [PubMed] [Google Scholar]
- 23.
- 23a. Romashov L. V., Ananikov V. P., Org. Biomol. Chem. 2016, 14, 10593–10598; [DOI] [PubMed] [Google Scholar]
- 23b. Chang F., Dutta S., Becnel J. J., Estep A. S., Mascal M., J. Agric. Food Chem. 2014, 62, 476–480; [DOI] [PubMed] [Google Scholar]
- 23c. Saska J., Li Z., Otsuki A. L., Wei J., Fettinger J. C., Mascal M., Angew. Chem. Int. Ed. 2019, 58, 17293–17296. [DOI] [PubMed] [Google Scholar]
- 24.M. N. Masuno, D. A. Hirsch-Weil, R. L. Smith, J. A. Bissell II, (Micromidas, Inc), WO2015175528 (A1), 2015.
- 25. Kang E.-S., Hong Y.-W., Chae D. W., Da W. C., Kim B., Kim B., Kim Y. J., Cho J. K., Kim Y. G., ChemSusChem 2015, 8, 1179–1188. [DOI] [PubMed] [Google Scholar]
- 26.J. P. Klein, (Empire Technology Development LLC), WO2015137923 (A1), 2015.
- 27. Karlinskii B., Romashov L., Galkin K., Kislitsyn P., Ananikov V., Synthesis 2019, 51, 1235–1242. [Google Scholar]
- 28.
- 28a. Kegnaes S., Mielby J., Mentzel U. V., Jensen T., Fristrup P., Riisager A., Chem. Commun. 2012, 48, 2427–2429; [DOI] [PubMed] [Google Scholar]
- 28b. Gupta N. K., Nishimura S., Takagaki A., Ebitani K., Green Chem. 2011, 13, 824. [Google Scholar]
- 29. Schade O. R., Kalz K. F., Neukum D., Kleist W., Grunwaldt J.-D., Green Chem. 2018, 20, 3530–3541. [Google Scholar]
- 30. Liu Y., Ma H.-Y., Lei D., Lou L.-L., Liu S., Zhou W., Wang G.-C., Yu K., ACS Catal. 2019, 9, 8306–8315. [Google Scholar]
- 31. Zhang Z., Liu B., Lv K., Sun J., Deng K., Green Chem. 2014, 16, 2762. [Google Scholar]
- 32. Ambreen N., Wirth T., Eur. J. Org. Chem. 2014, 2014, 7590–7593. [Google Scholar]
- 33.
- 33a. Han G., Jin Y. H., Burgess R. A., Dickenson N. E., Cao X. M., Sun Y., J. Am. Chem. Soc. 2017, 139, 15584–15587; [DOI] [PubMed] [Google Scholar]
- 33b. Hopf H., Abhilash K., Synlett 2009, 2009, 3349–3351; [Google Scholar]
- 33c. Yang Z.-Y., Wen M., Zong M.-H., Li N., Catal. Commun. 2020, 139, 105979. [Google Scholar]
- 34.
- 34a. Brandolese A., Ragno D., Di Carmine G., Bernardi T., Bortolini O., Giovannini P. P., Pandoli O. G., Altomare A., Massi A., Org. Biomol. Chem. 2018, 16, 8955–8964; [DOI] [PubMed] [Google Scholar]
- 34b. Mohlmann L., Ludwig S., Blechert S., Beilstein J. Org. Chem. 2013, 9, 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kozlov K. S., Romashov L. V., Ananikov V. P., Green Chem. 2019, 21, 3464–3468. [Google Scholar]
- 36.
- 36a. Chacón-Huete F., Messina C., Chen F., Cuccia L., Ottenwaelder X., Forgione P., Green Chem. 2018, 20, 5261–5265; [Google Scholar]
- 36b. Subbiah S., Simeonov S. P., Esperança J. M. S. S., Rebelo L. P. N., Afonso C. A. M., Green Chem. 2013, 15, 2849. [Google Scholar]
- 37.
- 37a. Knaus T., Tseliou V., Humphreys L. D., Scrutton N. S., Mutti F. G., Green Chem. 2018, 20, 3931–3943; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37b. Qin Y.-Z., Li Y.-M., Zong M.-H., Wu H., Li N., Green Chem. 2015, 17, 3718–3722. [Google Scholar]
- 38.
- 38a.M. J. Pedersen, C. M. Pedersen, (Københavns Universitet), WO2019170204 (A1), 2019;
- 38b.R. V. J. Chari, W. C. Widdison, S. D. Wilhelm, (Immunogen Inc), US2016058882 (A1), 2016;
- 38c.J. B. J. Summers, S. K. Davidsen, M. L. Curtin, H. R. Heyman, G. S. Sheppard, L. Xu, G. M. J. Carrera, R. B. Garland, (Abbott Laboratories), US5486525 (A), 1996;
- 38d. Haworth W. N., Jones W. G. M., J. Chem. Soc. 1944, 667. [Google Scholar]
- 39. Schmuck C., Machon U., Eur. J. Org. Chem. 2006, 2006, 4385–4392. [Google Scholar]
- 40. Talaga P., Matagne A., Klitgaard H., Bioorg. Med. Chem. Lett. 2001, 11, 1313–1316. [DOI] [PubMed] [Google Scholar]
- 41. Pacheco J. J., Davis M. E., Proc. Natl. Acad. Sci. USA 2014, 111, 8363–8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li F., Li X., Gong T., Fu Y., ChemCatChem 2019, 11, 5124–5130. [Google Scholar]
- 43. Baliani A., Bueno G. J., Stewart M. L., Yardley V., Brun R., Barrett M. P., Gilbert I. H., J. Med. Chem. 2005, 48, 5570–5579. [DOI] [PubMed] [Google Scholar]
- 44. Osayamen E., Lett. Org. Chem. 2007, 4, 306–308. [Google Scholar]
- 45.
- 45a. Zhu M.-M., Tao L., Zhang Q., Dong J., Liu Y.-M., He H.-Y., Cao Y., Green Chem. 2017, 19, 3880–3887; [Google Scholar]
- 45b. Han W., Wang J., Li X., Zhou L., Yang Y., Tang M., Ge H., Catal. Commun. 2019, 124, 86–91; [Google Scholar]
- 45c. van Schijndel J., Canalle L., Molendijk D., Meuldijk J., Synlett 2018, 29, 1983–1988; [Google Scholar]
- 45d. Zhang B., Guo X.-W., Liang H., Ge H., Gu X., Chen S., Yang H., Qin Y., ACS Catal. 2016, 6, 6560–6566; [Google Scholar]
- 45e. Chen W., Sun Y., Du J., Si Z., Tang X., Zeng X., Lin L., Liu S., Lei T., J. Chem. Technol. Biotechnol. 2018, 93, 3028–3034. [Google Scholar]
- 46. Bahta M., Lountos G. T., Dyas B., Kim S. E., Ulrich R. G., Waugh D. S., T. R. Burke, Jr. , J. Med. Chem. 2011, 54, 2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cisneros L., Serna P., Corma A., Angew. Chem. Int. Ed. 2014, 53, 9306–9310; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 9460–9464. [Google Scholar]
- 48.
- 48a. Ren H., Wu C., Ding X., Chen X., Shi F., Org. Biomol. Chem. 2012, 10, 8975–8984; [DOI] [PubMed] [Google Scholar]
- 48b. Firth J. D., Craven P. G., Lilburn M., Pahl A., Marsden S. P., Nelson A., Chem. Commun. 2016, 52, 9837–9840; [DOI] [PubMed] [Google Scholar]
- 48c. Cukalovic A., Stevens C. V., Green Chem. 2010, 12, 1201. [Google Scholar]
- 49.
- 49a. Komanoya T., Kinemura T., Kita Y., Kamata K., Hara M., J. Am. Chem. Soc. 2017, 139, 11493–11499; [DOI] [PubMed] [Google Scholar]
- 49b. Yuan H., Li J. P., Su F., Yan Z., Kusema B. T., Streiff S., Huang Y., Pera-Titus M., Shi F., ACS Omega 2019, 4, 2510–2516; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49c. Roylance J. J., Choi K.-S., Green Chem. 2016, 18, 5412–5417; [Google Scholar]
- 49d. Chieffi G., Braun M., Esposito D., ChemSusChem 2015, 8, 3590–3594; [DOI] [PubMed] [Google Scholar]
- 49e. García-Ortiz A., Vidal J. D., Climent M. J., Concepción P., Corma A., Iborra S., ACS Sustainable Chem. Eng. 2019, 7, 6243–6250; [Google Scholar]
- 49f. Karve V. V., Sun D. T., Trukhina O., Yang S., Oveisi E., Luterbacher J., Queen W. L., Green Chem. 2019, 22, 368–378. [Google Scholar]
- 50. Dunbabin A., Subrizi F., Ward J. M., Sheppard T. D., Hailes H. C., Green Chem. 2017, 19, 397–404. [Google Scholar]
- 51.
- 51a. Li H., Guo H., Su Y., Hiraga Y., Fang Z., Hensen E. J. M., Watanabe M., R. L. Smith, Jr. , Nat. Commun. 2019, 10, 699; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51b. Chandra D., Inoue Y., Sasase M., Kitano M., Bhaumik A., Kamata K., Hosono H., Hara M., Chem. Sci. 2018, 9, 5949–5956; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51c. Jagadeesh R. V., Murugesan K., Alshammari A. S., Neumann H., Pohl M. M., Radnik J., Beller M., Science 2017, 358, 326–332; [DOI] [PubMed] [Google Scholar]
- 51d. Chatterjee M., Ishizaka T., Kawanami H., Green Chem. 2016, 18, 487–496; [Google Scholar]
- 51e. Deng D., Kita Y., Kamata K., Hara M., ACS Sustainable Chem. Eng. 2018, 7, 4692–4698; [Google Scholar]
- 51f. Müller C., Diehl V., Lichtenthaler F. W., Tetrahedron 1998, 54, 10703–10712; [Google Scholar]
- 51g. Xie C., Song J., Hua M., Hu Y., Huang X., Wu H., Yang G., Han B., ACS Catal. 2020, 10, 7763–7772. [Google Scholar]
- 52.T. Haas, J. C. Pfeffer, K. Faber, M. Fuchs, (Evonic Degussa GMBH), WO2012171666 (A1).
- 53.
- 53a. Honig M., Plihalova L., Spichal L., Gruz J., Kadlecova A., Voller J., Svobodova A. R., Vostalova J., Ulrichova J., Dolezal K., Strnad M., Eur. J. Med. Chem. 2018, 150, 946–957; [DOI] [PubMed] [Google Scholar]
- 53b.D. De Roulet, R. Devita, (Mitokinin LLC), WO2015123365 (A1), 2015;
- 53c. Frydman B., Ojea M. I., Tetrahedron Lett. 1998, 39, 4765–4768; [Google Scholar]
- 53d. Holfinger M. S., Conner A. H., Holm D. R., Hill C. G., J. Org. Chem. 1995, 60, 1595–1598. [Google Scholar]
- 54.
- 54a. Sowmiah S., Veiros L. F., Esperanca J. M., Rebelo L. P., Afonso C. A., Org. Lett. 2015, 17, 5244–5247; [DOI] [PubMed] [Google Scholar]
- 54b. Villard R., Robert F., Blank I., Bernardinelli G., Soldo T., Hofmann T., J. Agric. Food Chem. 2003, 51, 4040–4045; [DOI] [PubMed] [Google Scholar]
- 54c. Elming N., Clauson-Kaas N., Anderson E. P., Eliasson N. A., Thorell B., Acta Chem. Scand. 1956, 10, 1603–1605. [Google Scholar]
- 55.
- 55a. Zeng R., Zhang G., Zheng J., Zhou H., Wang Y., Huang C., Hu W., Ou S., J. Agric. Food Chem. 2020, 68, 384–389; [DOI] [PubMed] [Google Scholar]
- 55b. Koh P. F., Loh T. P., Green Chem. 2015, 17, 3746–3750; [Google Scholar]
- 55c. Zhu L., Liu Y., Ma R., Tong R., Angew. Chem. Int. Ed. 2015, 54, 627–632; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 637–642; [Google Scholar]
- 55d. Rajmohan R., Gayathri S., Vairaprakash P., RSC Adv. 2015, 5, 100401–100407; [Google Scholar]
- 55e. McNelis B. J., Sternbach D. D., MacPhail A. T., Tetrahedron 1994, 50, 6767–6782. [Google Scholar]
- 56.
- 56a. Vares L., Bredihhin A., Luiga S., Synthesis 2016, 48, 4181–4188; [Google Scholar]
- 56b. Ghosh A. K., Li J., Org. Lett. 2011, 13, 66–69; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56c. Griggs N. D., Phillips A. J., Org. Lett. 2008, 10, 4955–4957; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56d. Barriga S., Fuertes P., Marcos C. F., Rakitin O. A., Torroba T., J. Org. Chem. 2002, 67, 6439–6448. [DOI] [PubMed] [Google Scholar]
- 57.
- 57a. Cheng K., Kelly A. R., Kohn R. A., Dweck J. F., Walsh P. J., Org. Lett. 2009, 11, 2703–2706; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57b. Chen L., Xu H. H., Yin B. L., Xiao C., Hu T. S., Wu Y. L., J. Agric. Food Chem. 2004, 52, 6719–6723. [DOI] [PubMed] [Google Scholar]
- 58. Wang Q., Guerrero V. V., Ghosh A., Yeu S., Lunn J. D., Shantz D. F., J. Catal. 2010, 269, 15–25. [Google Scholar]
- 59.
- 59a. Wang L., Tan J.-N., Ahmar M., Queneau Y., C. R. Chim. 2019, 22, 615–620; [Google Scholar]
- 59b. Tan J.-N., Ahmar M., Queneau Y., RSC Adv. 2015, 5, 69238–69242; [Google Scholar]
- 59c. Yu C., Hu L., J. Org. Chem. 2002, 67, 219–223. [DOI] [PubMed] [Google Scholar]
- 60. Romashov L. V., Ananikov V. P., Chem. Asian J. 2017, 12, 2652–2655. [DOI] [PubMed] [Google Scholar]
- 61. El-Hajj T., Martin J.-C., Descotes G., J. Heterocycl. Chem. 1983, 20, 233–235. [Google Scholar]
- 62. Ramonczai J., Vargha L., J. Am. Chem. Soc. 1950, 72, 2737–2737. [Google Scholar]
- 63.
- 63a. Lei C., Zhu D., Tangcueco V. I. T., Zhou J. S., Org. Lett. 2019, 21, 5817–5822; [DOI] [PubMed] [Google Scholar]
- 63b. Sharma U. K., Gemoets H. P. L., Schröder F., Noël T., Van der Eycken E. V., ACS Catal. 2017, 7, 3818–3823; [Google Scholar]
- 63c. Nicklaus C. M., Minnaard A. J., Feringa B. L., de Vries J. G., ChemSusChem 2013, 6, 1631–1635. [DOI] [PubMed] [Google Scholar]
- 64.
- 64a. Han M., Liu X., Zhang X., Pang Y., Xu P., Guo J., Liu Y., Zhang S., Ji S., Green Chem. 2017, 19, 722–728; [Google Scholar]
- 64b. Fumagalli T., Sello G., Orsini F., Synth. Commun. 2009, 39, 2178–2195; [Google Scholar]
- 64c. Mouloungui Z., Delmas M., Gaset A., Synth. Commun. 2006, 14, 701–706; [Google Scholar]
- 64d. Mouloungui Z., Delmas M., Gaset A., Synth. Commun. 2006, 15, 491–494. [Google Scholar]
- 65.
- 65a. Muthusamy K., Lalitha K., Prasad Y. S., Thamizhanban A., Sridharan V., Maheswari C. U., Nagarajan S., ChemSusChem 2018, 11, 2453–2463; [DOI] [PubMed] [Google Scholar]
- 65b. D′Onofrio F., Piancatelli G., Nicolai M., Tetrahedron 1995, 51, 4083–4088. [Google Scholar]
- 66.
- 66a. Chang H., Motagamwala A. H., Huber G. W., Dumesic J. A., Green Chem. 2019, 21, 5532–5540; [Google Scholar]
- 66b. Rojas-Buzo S., García-García P., Corma A., Green Chem. 2018, 20, 3081–3091; [Google Scholar]
- 66c. Song H. J., Deng J., Cui M. S., Li X. L., Liu X. X., Zhu R., Wu W. P., Fu Y., ChemSusChem 2015, 8, 4250–4255; [DOI] [PubMed] [Google Scholar]
- 66d. Pupovac K., Palkovits R., ChemSusChem 2013, 6, 2103–2110; [DOI] [PubMed] [Google Scholar]
- 66e. Sutton A. D., Waldie F. D., Wu R., Schlaf M., L. A. Silks, 3rd , Gordon J. C., Nat. Chem. 2013, 5, 428–432. [DOI] [PubMed] [Google Scholar]
- 67.
- 67a. Bohre A., Saha B., Abu-Omar M. M., ChemSusChem 2015, 8, 4022–4029; [DOI] [PubMed] [Google Scholar]
- 67b. Xia Q. N., Cuan Q., Liu X. H., Gong X. Q., Lu G. Z., Wang Y. Q., Angew. Chem. Int. Ed. 2014, 53, 9755–9760; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 9913–9918. [Google Scholar]
- 68.
- 68a. Li X.-L., Zhang K., Jiang J.-L., Zhu R., Wu W.-P., Deng J., Fu Y., Green Chem. 2018, 20, 362–368; [Google Scholar]
- 68b. Li S., Chen F., Li N., Wang W., Sheng X., Wang A., Cong Y., Wang X., Zhang T., ChemSusChem 2017, 10, 711–719; [DOI] [PubMed] [Google Scholar]
- 68c. Xu Z., Yan P., Xu W., Liu X., Xia Z., Chung B., Jia S., Zhang Z. C., ACS Catal. 2014, 5, 788–792; [Google Scholar]
- 68d. Waidmann C. R., Pierpont A. W., Batista E. R., Gordon J. C., Martin R. L., Pete Silks L. A., West R. M., Wu R., Catal. Sci. Technol. 2013, 3, 106–115; [Google Scholar]
- 68e. Julis J., Hölscher M., Leitner W., Green Chem. 2010, 12, 1634; [Google Scholar]
- 68f. Qian Y., Li Z.-J., Du X.-L., Zhang Q., Zhao Y., Liu Y.-M., Cao Y., Green Chem. 2020, 22, 850–859. [Google Scholar]
- 69. Wrigstedt P., Keskiväli J., Perea-Buceta J. E., Repo T., ChemCatChem 2017, 9, 4244–4255. [Google Scholar]
- 70.
- 70a. Ramos R., Grigoropoulos A., Griffiths B. L., Katsoulidis A. P., Zanella M., Manning T. D., Blanc F., Claridge J. B., Rosseinsky M. J., J. Catal. 2019, 375, 224–233; [Google Scholar]
- 70b. Fujita S., Nakajima K., Yamasaki J., Mizugaki T., Jitsukawa K., Mitsudome T., ACS Catal. 2020, 10, 4261–4267. [Google Scholar]
- 71.
- 71a. Zhang S., Ma H., Sun Y., Luo Y., Liu X., Zhang M., Gao J., Xu J., Green Chem. 2019, 21, 1702–1709; [Google Scholar]
- 71b. Li X., Deng Q., Zhang L., Wang J., Wang R., Zeng Z., Deng S., Appl. Catal. A 2019, 575, 152–158; [Google Scholar]
- 71c. Ramos R., Grigoropoulos A., Perret N., Zanella M., Katsoulidis A. P., Manning T. D., Claridge J. B., Rosseinsky M. J., Green Chem. 2017, 19, 1701–1713; [Google Scholar]
- 71d. Xu Y.-J., Shi J., Wu W.-P., Zhu R., Li X.-L., Deng J., Fu Y., Appl. Catal. A 2017, 543, 266–273; [Google Scholar]
- 71e. Perret N., Grigoropoulos A., Zanella M., Manning T. D., Claridge J. B., Rosseinsky M. J., ChemSusChem 2016, 9, 521–531; [DOI] [PubMed] [Google Scholar]
- 71f. Ohyama J., Kanao R., Ohira Y., Satsuma A., Green Chem. 2016, 18, 676–680; [Google Scholar]
- 71g. Ohyama J., Kanao R., Esaki A., Satsuma A., Chem. Commun. 2014, 50, 5633–5636; [DOI] [PubMed] [Google Scholar]
- 71h. Deng Q., Gao R., Li X., Wang J., Zeng Z., Zou J.-J., Deng S., ACS Catal. 2020, 10 7355–7366. [Google Scholar]
- 72. Ohyama J., Ohira Y., Satsuma A., Catal. Sci. Technol. 2017, 7, 2947–2953. [Google Scholar]
- 73. Gelmini A., Albonetti S., Cavani F., Cesari C., Lolli A., Zanotti V., Mazzoni R., Appl. Catal. B 2016, 180, 38–43. [Google Scholar]
- 74.
- 74a. Wozniak B., Spannenberg A., Li Y., Hinze S., de Vries J. G., ChemSusChem 2018, 11, 356–359; [DOI] [PubMed] [Google Scholar]
- 74b. Duan Y., Zheng M., Li D., Deng D., Ma L.-F., Yang Y., Green Chem. 2017, 19, 5103–5113. [Google Scholar]
- 75. Wozniak B., Li Y., Hinze S., Tin S., de Vries J. G., Eur. J. Org. Chem. 2018, 2018, 2009–2012. [Google Scholar]
- 76. Wozniak B., Li Y., Tin S., de Vries J. G., Green Chem. 2018, 20, 4433–4437. [Google Scholar]
- 77. Marisa C., Ilaria D. S., Marotta R., Roberto A., Vincenzo C., J. Photochem. Photobiol. A 2010, 210, 69–76. [Google Scholar]
- 78.
- 78a. Lichtenthaler F. W., Brust A., Cuny E., Green Chem. 2001, 3, 201–209; [Google Scholar]
- 78b. Adger B. M., Barrett C., Brennan J., McKervey M. A., Murray R. W., J. Chem. Soc. Chem. Commun. 1991, 1553. [Google Scholar]
- 79. Okada T., Sakaguchi K., Shinada T., Ohfune Y., Tetrahedron Lett. 2011, 52, 5744–5746. [Google Scholar]
- 80.
- 80a. Falenczyk C., Pölloth B., Hilgers P., König B., Synth. Commun. 2014, 45, 348–354; [Google Scholar]
- 80b. Achmatowicz O., Burzyńska M. H., Tetrahedron 1982, 38, 3507–3513. [Google Scholar]
- 81.
- 81a. Wood J. M., Furkert D. P., Brimble M. A., Org. Biomol. Chem. 2016, 14, 7659–7664; [DOI] [PubMed] [Google Scholar]
- 81b. Kamalov M., Harris P. W., Wood J. M., Brimble M. A., Chem. Commun. 2015, 51, 9475–9478; [DOI] [PubMed] [Google Scholar]
- 81c. Geng H. M., Stubbing L. A., Chen J. L., Furkert D. P., Brimble M. A., Eur. J. Org. Chem. 2014, 2014, 6227–6241; [Google Scholar]
- 81d. Yuen T.-Y., Eaton S. E., Woods T. M., Furkert D. P., Choi K. W., Brimble M. A., Eur. J. Org. Chem. 2014, 2014, 1431–1437; [Google Scholar]
- 81e. Woods T. M., Kamalov M., Harris P. W., Cooper G. J., Brimble M., Org. Lett. 2012, 14, 5740–5743; [DOI] [PubMed] [Google Scholar]
- 81f. Brimble M., Geng H., Chen J., Furkert D., Jiang S., Synlett 2012, 23, 855–858; [Google Scholar]
- 81g. Snider B. B., Grabowski J. F., Tetrahedron Lett. 2005, 46, 823–825. [Google Scholar]
- 82. Cottier L., Descotes G., Eymard L., Rapp K., Synthesis 1995, 1995, 303–306. [Google Scholar]
- 83. Alibés R., Font J., Mulá A., Ortuño R. M., Synth. Commun. 1990, 20, 2607–2615. [Google Scholar]
- 84.S. Berard, C. Vallee, S. Maury, D. Delcroix, M. Jacquin, (IFP Energies Nouvelles), WO2018015112 (Al), 2018.
- 85.
- 85a. Shen X., Liu X., Wang J., Dai J., Zhu J., Ind. Eng. Chem. Res. 2017, 56, 8508–8516; [Google Scholar]
- 85b. Galkin K. I., Kucherov F. A., Markov O. N., Egorova K. S., Posvyatenko A. V., Ananikov V. P., Molecules 2017, 22; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85c. Gupta P., Singh S. K., Pathak A., Kundu B., Tetrahedron 2002, 58, 10469–10474. [Google Scholar]
- 86.M. P. Sibi, S. Sermadurai, N. Zimmermann, E. Serum, G. Ma, R. Moorthy, K. Kalliokoski, (NDSU Research Foundation), WO2016022943 (A2), 2016.
- 87.M. Crockatt, J. H. Urbanus, (Nederlandse Organisatie Voor Toegepast-Natuurwetenschappelijk On-Derzoek TNO), WO2017146581 (A1), 2017.
- 88. Higson S., Subrizi F., Sheppard T. D., Hailes H. C., Green Chem. 2016, 18, 1855–1858. [Google Scholar]
- 89.P. M. Konst, J. H. Urbanus, (Nederlandse Organisatie Voor Toegepast-Natuurwetenschappelijk On-Derzoek TNO), WO2017204634 (A1), 2017.
- 90. Cammidge A. N., Cook M. J., Harrison K. J., McKeown N. B., J. Chem. Soc. Perkin Trans. 1 1991, 3053. [Google Scholar]
- 91. Kucherov F. A., Galkin K. I., Gordeev E. G., Ananikov V. P., Green Chem. 2017, 19, 4858–4864. [Google Scholar]
- 92. Chambers R. D., Roche A. J., Rock M. H., J. Chem. Soc. Perkin Trans. 1 1996, 1095. [Google Scholar]
- 93. Andreu C., Villarroya J.-P., García-Gastaldi A., Medio-Simón M., Server-Carrió J., Varea T., Tetrahedron: Asymmetry 1998, 9, 3105–3114. [Google Scholar]
- 94. Yen A., Choo K. L., Yazdi S. K., Franke P. T., Webster R., Franzoni I., Loh C. C. J., Poblador-Bahamonde A. I., Lautens M., Angew. Chem. Int. Ed. 2017, 56, 6307–6311; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 6404–6408. [Google Scholar]
- 95.E. Muller, B. Thota, (Rhodia Operations), WO2018229237 (A1), 2018.
- 96. Serum E. M., Sutton C. A., Renner A. C., Dawn D., Sibi M. P., Pure Appl. Chem. 2019, 91, 389–396. [Google Scholar]
- 97. Orazov M., Davis M. E., Chem. Sci. 2016, 7, 2264–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ogunjobi J. K., Farmer T. J., McElroy C. R., Breeden S. W., Macquarrie D. J., Thornthwaite D., Clark J. H., ACS Sustainable Chem. Eng. 2019, 7, 8183–8194. [Google Scholar]
- 99. Serum E. M., Selvakumar S., Zimmermann N., Sibi M. P., Green Chem. 2018, 20, 1448–1454. [Google Scholar]
- 100.
- 100a. Cai T., Deng Q., Peng H., Zhong J., Gao R., Wang J., Zeng Z., Zou J.-J., Deng S., Green Chem. 2020, 22, 2468–2473; [Google Scholar]
- 100b. Kumalaputri A. J., Randolph C., Otten E., Heeres H. J., Deuss P. J., ACS Sustainable Chem. Eng. 2018, 6, 3419–3425; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100c. Luijkx G. C. A., van Rantwijk F., van Bekkum H., Carbohydr. Res. 1993, 242, 131–139. [Google Scholar]
- 101. Hansen C. A., Frost J. W., J. Am. Chem. Soc. 2002, 124, 5926–5927. [DOI] [PubMed] [Google Scholar]
- 102. Lantz R. L., Michel E., Bull. Soc. Chim. Fr. 1961, 2402–2408. [Google Scholar]
- 103. Randolph C., Lahive C. W., Sami S., Havenith R. W. A., Heeres H. J., Deuss P. J., Org. Process Res. Dev. 2018, 22, 1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Knochel P., Piller F., Synthesis 2011, 2011, 1751–1758. [Google Scholar]
- 105. Pezzetta C., Veiros L. F., Oble J., Poli G., Chem. Eur. J. 2017, 23, 8385–8389. [DOI] [PubMed] [Google Scholar]
- 106. Sala R., Roudesly F., Veiros L. F., Broggini G., Oble J., Poli G., Adv. Synth. Catal. 2020, 362, 2486–2493. [Google Scholar]
- 107. Siopa F., Ramis Cladera V.-A., Afonso C. A. M., Oble J., Poli G., Eur. J. Org. Chem. 2018, 2018, 6101–6106. [Google Scholar]
- 108. Ravasco J., Monteiro C. M., Siopa F., Trindade A. F., Oble J., Poli G., Simeonov S. P., Afonso C. A. M., ChemSusChem 2019, 12, 4629–4635. [DOI] [PubMed] [Google Scholar]
- 109. Kucherov F. A., Gordeev E. G., Kashin A. S., Ananikov V. P., Angew. Chem. Int. Ed. 2017, 56, 15931–15935; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16147–16151. [Google Scholar]
- 110. Zhang D., Dumont M.-J., J. Polym. Sci. Part A 2017, 55, 1478–1492. [Google Scholar]