Abstract
Systemic sclerosis (SSc) is a connective tissue disease characterized by excessive fibrosis, microvasculopathy, and autoimmunity. Endothelial cell (EC) injury and subsequent endothelial cell dysfunction is believed to be an initial event that eventually leads to a vicious pathogenic cycle. This process is further enhanced by defective angiogenesis and vasculogenesis, as the vascular repair machinery does not work properly. Endothelial progenitor cells (EPCs) are functionally and quantitatively insufficient to recover the endothelium in SSc patients. The dysfunctional ECs and EPCs not only trigger the formation of typical vascular lesions, such as progressive intimal fibrosis in small arteries and the loss of capillaries, but also promote a series of inflammatory and profibrotic processes, such as endothelial-mesenchymal transition and recruitment and accumulation of monocytic EPCs with profibrotic properties. These processes together contribute to the accumulation of extracellular matrix in the affected tissue. This review features current insights into the roles of ECs and EPCs in the pathogenesis of SSc.
Keywords: Scleroderma, systemic sclerosis, endothelial cell, progenitor
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by a combination of excessive fibrosis, microvasculopathy, chronic inflammation, and autoimmunity (1). Vascular involvement in patients with SSc mainly affects small arteries and causes reduced blood flow and tissue ischemia, leading to clinical manifestations, such as digital ulcer and pulmonary arterial hypertension (PAH). The vascular pathologies unique to SSc include progressive intimal proliferation and fibrosis in small arteries, along with the loss of capillaries. The mechanism of SSc vasculopathy is not fully understood, but increasing evidence indicates that endothelial injury and subsequent endothelial dysfunction is a primary event that triggers the subsequent formation of typical vascular lesions (2). In addition, vascular recovery barely occurs in SSc patients, which indicates a defective vascular repair process (3). Postnatal vascular repair is mediated through the collaborative effects of two distinct processes: (1) angiogenesis, i.e., the formation of new blood vessels sprouting from preexisting vessels through proliferation and migration of mature endothelial cells (ECs), and (2) vasculogenesis, i.e., the de novo differentiation of mature ECs through recruitment and differentiation of endothelial progenitor cells (EPCs) (4). This results in the activation of inflammatory and fibrotic processes in perivascular lesions, leading to complex vascular remodeling and irreversible structural changes. This review focuses on the roles of two major cell types that contribute to the homeostasis of the vascular system, ECs and EPCs, in the pathogenic processes of vasculopathy and excessive fibrosis in SSc patients.
Role of ECs in the pathogenesis of SSc
EC apoptosis as a trigger
It is believed that endothelial injury is an initial event that eventually leads to EC dysfunction in SSc patients, and can be triggered via a number of different mechanisms, including infection, ischemia-reperfusion reaction caused by the vasospasm resulting from Raynaud’s phenomenon, oxidative stress through abnormal regulation of reactive oxygen species, turbulent blood flow and shear stress, and the imbalance between coagulation and fibrinolysis (5, 6). In this regard, an infection with human cytomegalovirus induces antibodies to recognize an amino acid sequence on the human cytomegalovirus-derived protein UL94, which is homologous to NAG-2, a surface molecule highly expressed on ECs. Antibodies against UL94 peptide have been shown to induce apoptosis of ECs upon engagement with the NAG-2-integrin complex (7). Another potential contributor is anti-endothelial cell antibody (AECA), which is a heterogeneous antibody family that reacts with various cell surface antigens on ECs (8). The mechanisms of AECA-mediated cytotoxicity against ECs include antibody-dependent cell-mediated cytotoxicity (9, 10) and have direct effects through an interaction between the Fas and Fas ligands (11, 12).
Defective angiogenesis
SSc patients represent clinical features that are consistent with insufficient vascular repair, but demonstrate up-regulation of a series of pro-angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), platelet-derived growth factor (PDGF), hepatocyte growth factor, placental growth factor, and CXCL12 (13). Increased levels of circulating VEGF have been reported in SSc patients (14, 15), but it has been shown that increased VEGF isoform is actually anti-angiogenic to VEGF165-b, rather than pro-angiogenic for VEGF165. In contrast, a soluble VEGF receptor (VEGFR)-1 in circulation works as a decoy receptor for VEGF, and is decreased in SSc patients (16). Interestingly, all three forms of VEGFR were upregulated in the skin biopsies of SSc patients (17, 18), suggesting that VEGF system, which plays a central role in the development and maintenance of the blood and lymphatic vascular systems, is totally disrupted in SSc patients. In addition, reduced levels of pro-angiogenic angiopoietin-1 along with increased levels of angiopoietin-2, an antagonist of angiopoietin-1, were also observed in SSc patients. The expression of kallikreins 1, 9, 11, and 12, which are powerful modulators of angiogenesis, was down-regulated in ECs derived from the affected skin of SSc patients (19, 20). Furthermore, circulating anti-angiogenic factors, such as angiostatin, thrombospondin-1, endostatin, angiostatin, platelet factor 4/CXCL4, and pentraxin 3, were increased in SSc patients as compared to healthy controls (13, 21–23). Endostatin was increased in all phases of the disease, while angiostatin levels were elevated only in the late phase and were correlated with the severity of interstitial lung disease (ILD) (24).
Defective responses of ECs to pro-angiogenic factors in SSc patients can be explained, in part, by the down-regulated expression of their receptors and/or impaired intracellular signaling. In fact, the reduced expression of CXCR4, a receptor of CXCL12, has been found in the affected skin of SSc patients, especially in the late stage of the disease (25). The angiogenic transcription factors implicated in the pathogenesis of SSc include the Friend leukemia integration-1 (Fli1) and Fos-related antigen 2 (Fra-2). Fli1 belongs to the Ets family of transcription factors, and acts as a repressor of collagen transcription in the human skin. A sustained down-regulation of Fli1 in the SSc fibroblasts has been correlated with abnormal matrix deposition in SSc-affected skin (26). Although the Fli1 deficiency in ECs promotes migration, proliferation, and survival, it also suppresses tube formation, which suggests that Fli1 deficiency is potentially attributable to the development of both proliferative obliterative vasculopathy and the loss of vessels, which are characteristic of SSc vasculopathy (27). Fra-2 is a member of the multifunctional activator protein 1 family, and mice overexpressing Fra-2 replicate SSc phenotype, including proliferative obliterative vasculopathy (28, 29). Fra-2 expression was upregulated in SSc fibroblasts, and has been potentially correlated with an increase in the profibrotic effects of transforming growth factor-β (TGF-β) and PDGF (30). Fra-2 appears to contribute to the development of microvasculopathy by inducing EC apoptosis and reducing the migration of ECs (31).
Roles of EC interaction with other cell types in promoting fibrosis
Dysfunctional ECs are also involved in promoting tissue fibrosis in SSc patients by cellular interactions with other cells types, including resident cells within the vascular wall and inflammatory cells infiltrated into the affected tissues. Specifically, ECs in the affected tissue recruit and activate skin fibroblasts by inducing mesenchymal-to-mesenchymal transition through the secretion of a connective tissue growth factor, TGF-β (32). On the other hand, dermal fibroblasts derived from SSc patients are known to overexpress matrix metalloproteinase (MMP)-12, which cleaves the urokinase-type plasminogen activator receptor of microvascular ECs, resulting in the failure of ECs to induce an efficient angiogenic program (33). This bi-directional interaction of ECs and fibroblasts synergistically promotes fibrosis and inhibition of angiogenesis. The altered function of microvascular pericytes has also been reported in patients with early SSc, which resulted from interactions with dysregulated ECs (34, 35). In SSc-affected skin, pericytes expressed activation markers, including PDGF receptor β, high molecular weight melanoma-associated antigen, a regulator of G protein signaling 5 (36), and secreted PDGF-BB that recruits and induces proliferation of pericyte progenitors (37). Interestingly, the co-culture of SSc-derived pericytes with microvascular ECs from healthy controls resulted in an increased production of collagen (38).
Endothelial-mesenchymal transition (Endo-MT)
Given the crucial role of myofibroblasts in the pathogenesis of systemic and organ-specific fibrotic diseases, such as SSc and idiopathic pulmonary fibrosis (IPF), extensive research has previously aimed at precisely identifying their cellular origin. Tissue myofibroblasts can originate from various sources, including quiescent resident tissue fibroblasts, pericytes, adipocytes, macrophages, and epithelial cells (39). Moreover, Karasek et al. (40) have demonstrated that ECs are capable of trans-differentiating toward myofibroblasts in a process called endothelial-to-mesenchymal transition (Endo-MT), by which ECs change their morphological features and acquire a myofibroblast-like phenotype. In SSc patients, evidence has shown that Endo-MT plays a role in vascular remodeling and tissue fibrosis (41). This process is mediated primarily through TGF-β, but the detailed intracellular pathways activated by TGF-β have not been entirely elucidated. TGF-β induces Endo-MT through both Smad-dependent and independent pathways, such as c-Abl kinase, protein kinase c–δ, and β-catenin (42). Moreover, various transcriptional regulators, such as Snail1 and Snail2, Twist, and some members of the Zeb family of proteins, are associated with the regulation of TGF-β-induced Endo-MT (43–47). In addition, many other mediators collaboratively promote Endo-MT, as shown below:
Caveolin-1 (CAV1)
CAV1, a major protein component of caveolae, plays an important role in the pathogenesis of tissue fibrosis and various fibrotic diseases by regulating the internalization, transport, and degradation of TGF-β receptors, thereby modulating TGF-β signaling (48, 49). Indeed, the gene and protein expression of CAV1 was decreased in the affected tissues of patients with SSc and SSc-associated ILD and in the lung tissue of patients with IPF (50–52). Del Galdo et al. (50) demonstrated that Cav1−/− mice readily developed pulmonary and skin fibrosis. Restoration of CAV1 function in vitro by supplementing CAV1 with scaffolding domain peptide or overexpression of CAV1 using adenovirus helped to normalize the phenotypes of SSc fibroblasts and suppress TGF-β-induced extracellular matrix production, by inhibiting Smad3 activation and regulation of the c-Jun N-terminal kinase pathway in vitro (50–52). It has also been demonstrated that the in vivo restoration of CAV1 by transfer via adenovirus or administration of a cell-permeable CAV1 peptide prevented bleomycin-induced pulmonary fibrosis and monocrotaline-induced PAH through inhibition of the STAT3 signaling cascade (52, 53). In cultures of pulmonary ECs derived from Cav1−/− mice, Endo-MT occurred spontaneously, as evidenced by the constitutive expression of α-SMA, the high levels of production of type I collagen, and the high expression of Snai1 and Snai2. These observations suggest that CAV1 deficiency may participate in the development of progressive tissue fibrosis and proliferative vasculopathy through the promotion of Endo-MT.
Endothelin-1 (ET-1)
Besides its crucial role in the development of PAH, ET-1 has been implicated in the development of organ fibrosis and is an important trigger of the fibrotic process in SSc (54–56). Recent studies have examined whether ET-1 may also play a role in the development of tissue fibrosis by inducing Endo-MT. For example, EC-derived ET-1 promotes cardiac fibrosis and heart failure in diabetic hearts through the induction of Endo-MT (56). However, ET-1 alone was unable to induce Endo-MT in murine lung EC cultures, but did enhance TGF-β-induced Endo-MT (57). The subsequent study confirmed this finding and showed that cultured human ECs induced Endo-MT in vitro when treated with ET-1 in the presence of TGF-β (57), indicating a potent synergistic effect of TGF-β and ET-1 on Endo-MT. Endo-MT induced by TGF-β and ET-1 primarily involved the Smad pathway, and was blocked by an ET-1 receptor antagonist, macitentan (58).
Notch pathway
Recent studies indicate that Notch pathways contribute to the pathogenesis of SSc and other fibrotic diseases (59–61), and may also be involved in the regulation of Endo-MT (62). The canonical Notch signaling can act in conjunction with TGF-β to induce Endo-MT by activating the expression of Snail and upregulate a subset of genes through recruiting Smad3 to Smad binding sites (63). However, it should be noted that Kaposi’s sarcoma-associated herpesvirus was found to induce Endo-MT via Notch signaling, which was independent of the TGF-β pathway (64).
Wnt pathway
Wnt contains a multigene family of secreted glycoproteins that play important roles during embryogenesis through canonical and non-canonical pathways (65, 66). Recent studies using cultured ECs have demonstrated that canonical Wnt signaling activates Endo-MT pathways (67, 68). On the other hand, the Wnt/β-catenin pathway is involved in the activation of multiple profibrotic steps in SSc pathogenesis (69–72). In fact, increased Wnt activation has been found in skin biopsies from patients with SSc, and Wnt3a-induced myofibroblast differentiation via Smad-dependent autocrine TGF-β signaling has also been observed (70). In addition, the nuclear accumulation of β-catenin in activated fibroblasts was detected in fibroblastic foci in the lungs of patients with SSc-associated ILD (73).
Hypoxia-inducible factor-1α (HIF-1α)
The transcription factor HIF-1α is a key regulator responsible for inducing a number of cellular and molecular responses to hypoxia and is dysregulated in various pathologic conditions, including SSc (74–76). The mechanisms involved in HIF-1α-induced fibrosis are very complex and may affect numerous gene expression changes, interaction with profibrotic factors (such as TGF-β and VEGF), and the induction of Endo-MT (77–79). One study has shown that the important downstream effects of HIF-1α on Endo-MT induction involve a potent activation of Snail that may ultimately lead to the development of cardiac fibrosis (80).
Roles of EPCs in pathogenesis of SSc
Defective vasculogenesis by aberrant EPCs
Since EPCs are defined as circulating primitive cells that contribute to postnatal vasculogenesis (81), many studies have been conducted to clarify the contribution of EPCs to the pathogenesis of various vascular and connective tissues diseases (82). In patients with SSc, we first reported a reduced number of circulating EPCs, compared with age- and sex-matched rheumatoid arthritis patients or healthy individuals (83). The subsequent analyses done by other groups confirmed our finding (84–86), but some showed a comparable or even increased count of EPCs in SSc patients (87–91). It is now known that these contradictory results resulted from differences in experimental protocols used for quantifying EPCs. Circulating EPCs are identified as cells expressing CD34 in combination with CD133 and/or CD309/VEGFR2 by multi-color flow cytometry, but accurate quantification is technically difficult due to the extreme rarity of this population in circulation. To overcome this limitation, flow cytometry was combined with procedures that enrich EPCs, such as sorting of CD34+ cells and lineage-negative cells, in some studies (83, 90). In these circumstances, the European League Against Rheumatism Scleroderma Trials and Research (EUSTAR) proposed recommendations for the standardization of EPC research (92). We have directly compared several different protocols for quantifying circulating EPCs, and confirmed that the EUSTAR recommendations are valid when combined with an accurate quantification technique, which substantially improved the reproducibility of the results (93). Using standardized protocols, circulating EPCs were shown to be reduced in SSc patients in comparison with healthy controls. Recently, circulating lymphatic EPCs, identified by CD34+CD133+VEGFR3+ cells, were also decreased in SSc patients, and the lower counts were associated with the current digital ulcer (94).
In terms of functional properties of EPCs, we previously reported an impaired potential of SSc-derived EPCs to differentiate into mature ECs using in vitro cultures with multiple pro-angiogenic factors (83). Another study utilizing cultured EPCs showed an impaired differentiation potential to ECs in SSc-derived EPCs, as compared to EPCs derived from healthy controls (16). We recently developed a system to evaluate the in vivo differentiation potential of EPCs, using a murine tumor neovascularization model, in which freshly isolated human CD133+ cells are transplanted into the skin of mice in conjunction with syngeneic mouse tumor cells (95). Using this system, the neovascularization capacity of circulating EPCs was impaired in SSc patients, partly due to a deficiency in their vasculogenic ability. Therefore, defects in vasculogenesis observed in SSc patients are mediated through the impaired function of EPCs as well.
Studies examining the potential associations of EPC counts with clinical manifestations consistently reported an association between the presence of the digital ulcer and low EPC counts (85, 86, 88, 90). A recent prospective study revealed that low EPC counts were identified as independent predictors of the occurrence of new digital ulcer during follow-up (96) and were correlated with the late pattern of nailfold capillaroscopic findings (97). In SSc patients, the upregulated expression of MMP-10 in EPC-derived ECs was associated with PAH, and the histologic findings of pulmonary arterial remodeling was suppressed by the blockade of MMP-10 in Fra-2-transgenic mice, a model mimicking the vascular and fibrotic aspects of SSc. These findings together suggest that defective EPC leads to the formation of digital ulcers and other vascular manifestations of SSc.
Currently, little is known about the mechanisms behind decreased numeric and functional aberrations in EPCs in SSc patients. In this regard, Del Papa and colleagues reported an interesting finding, i.e., EPCs in the bone marrow from SSc patients were defective in their ability to proliferate in long-term culture with pro-angiogenic factors, suggesting that EPC precursors were functionally altered before their release into the bloodstream (88). The bone marrow of patients with diffuse cutaneous SSc showed markedly reduced microvascular density and increased fibrosis (98), indicating that the dysregulated microenvironment within the bone marrow may alter the EPC differentiation process. In this regard, we recently found that EPC counts are inversely correlated with the level of circulating pentraxin 3, a multifunctional pattern recognition protein with a capacity to inhibit angiogenesis through suppression of FGF-2 (23). Pentraxin-3 is capable of inhibiting the differentiation of bone marrow stem cells into EPCs in in vitro cultures with FGF-2, indicating that exposure to a high concentration of pentraxin-3 would suppress the FGF2-mediated EPC differentiation in the bone marrow. Finally, EPCs in circulation may be attacked though autoimmune mechanisms. In this regard, Zhu and colleagues found that the sera from SSc patients were able to induce apoptosis of EPCs, which was mediated through the Akt-FOXO3a-Bim pathway (84).
Heterogeneity of EPC subsets
There is a great deal of controversy about the definitions and roles of EPCs in postnatal vascular formation (99). This is primarily because of the technical difficulty in identifying those cells due to their extreme rarity in circulation (100). The utilization of a variety of experimental procedures has resulted in a number of definitions of EPCs in the literature. Nevertheless, it is currently accepted that there are at least two EPC subsets that can be discriminated between, based on their surface antigen expression, proliferation potential, and time of emergence in the cell culture system (101). Endothelial colony-forming cells detected in cultures are lineage-restricted progenitor cells that only give rise to endothelium with a clonogenic expansion potential (101), although their circulating origin has not been identified yet. On the other hand, the cells originally identified as “EPCs” are in fact hematopoietic lineage cells that display pro-angiogenic properties, and are now termed pro-angiogenic hematopoietic cells (101). Pro-angiogenic hematopoietic cells are also heterogeneous cell population, including CD14+ monocytic origin (monocytic EPCs) and CD14− cells positive for CD34, CD133, and CD309 (narrowly defined or conventional EPCs) (102), which were initially termed circulating endothelial progenitors (103). Currently, it is generally accepted that pro-angiogenic hematopoietic cells do not give rise to mature ECs efficiently, rather they work as vascular regenerating and supporting cells (104). Monocytic EPCs especially lack the capacity to proliferate or form tubular structures in the absence of mature ECs. On the other hand, conventional EPCs have typical features of progenitors, including the capacity to proliferate and to differentiate into ECs, however, their efficiency is much lower in comparison with endothelial colony-forming cells. Nevertheless, pro-angiogenic hematopoietic cells, either in a monocytic or conventional subset, are capable of promoting blood vessel formation through multiple mechanisms, including the secretion of a series of pro-angiogenic factors, including VEGF, granulocyte colony-stimulating factor (G-CSF), and stromal cell-derived factor-1 (SDF-1) (105, 106), and differentiation into other elements of the vasculature, such as pericytes and smooth muscle cells. Theoretically, pro-angiogenic hematopoietic cells play a major role in the very early phase of vascular repair by attaching to the denuded vascular endothelium immediately after injury and taking advantage of the large number of ECs in circulation (102). In the following vascular processes, endothelial colony-forming cells and pro-angiogenic hematopoietic cells work in conjunction with platelets and residential ECs to form new blood vessels.
Potential roles of monocytic EPCs in tissue fibrosis
When the number of circulating monocytic EPCs was examined in SSc patients using a culture system developed to enrich this cell population, circulating monocytic EPCs were found to be paradoxically increased in SSc patients as compared to age- and sex-matched healthy controls (107). Intriguingly, monocytic EPCs derived from SSc patients showed enhanced in vitro tubular structure formation compared with the structures seen in healthy controls. Furthermore, in a murine tumor neovascularization model, the transplantation of SSc-derived monocytic EPCs dramatically promoted tumor growth and tumor vessel formation in vivo, indicating that monocytic EPCs derived from SSc patients have enhanced angiogenic activity. The increased number and enhanced pro-angiogenic potency of monocytic EPCs is likely to be a compensatory response to impaired vasculogenesis due to the malfunction of conventional EPCs. Circulating monocytic EPCs are mobilized from the bone marrow and recruited to affected lesions of SSc in response to chemokines such as monocyte chemoattractant protein-1/CCL2 and SDF-1, which are upregulated in the affected skin of SSc patients (108, 109). In addition, the hypoxic condition of the affected tissues of SSc patients are known to stimulate the differentiation of monocytic EPCs through activation of HIF-1α (110). These local stimuli promote the accumulation of functionally altered monocytic EPCs into the affected lesions of SSc. Since monocytic EPCs are capable of differentiating into cells that produce extracellular matrix proteins (111–115), they might participate in the fibrotic process in the affected organs in an CCL2/CCR2-dependent amplification loop (114, 115). In this regard, the fibrotic clinical features in SSc patients were correlated with an increased proportion of CXCR4+ circulating cells with monocytic and endothelial markers, which correspond to monocytic EPCs (116). Interestingly, monocytic EPCs have common phenotypic features of alternatively activated or M2 macrophages, which are appreciated increasingly as important cells that contribute to the pathogenic process of SSc (117). Recent studies have shown that circulating monocytes with combined classically and alternatively activated features are increased in SSc patients (118, 119). Furthermore, in a phase II clinical trial of tocilizumab in early, active patients with diffuse cutaneous SSc, tocilizumab treatment resulted in down-regulation of M2-associated genes in the skin and a sustained reduction of circulating CCL18, a chemokine associated with M2, in association with the improvement of skin sclerosis (120). Therefore, the pathogenic process of SSc is likely triggered by recruitment and accumulation of circulating monocytic EPCs with M2 features into the affected sites, where they acquire profibrotic properties, i.e., the production of a variety of profibrotic growth factors, cytokines, and chemokines to stimulate resident mesenchymal cells, and their own trans-differentiation into extracellular matrix-producing cells.
Summary: A potential link between aberrant EPC/EC and the pathogenic processes of SSc
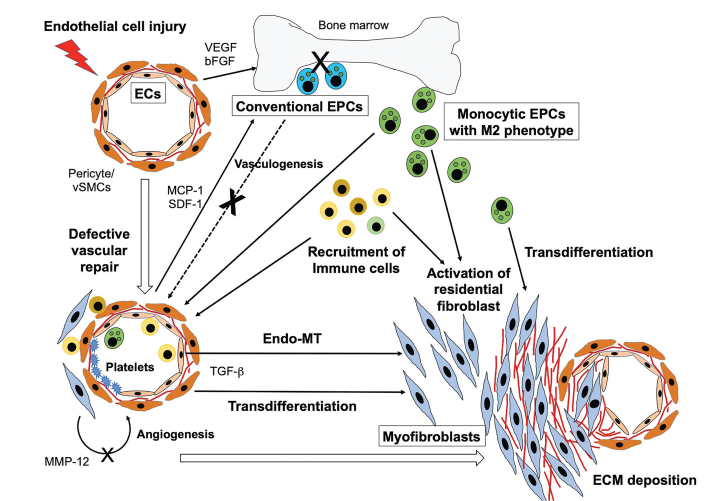
Current insights raise the intriguing hypothesis that ECs and EPCs are directly involved in the pathogenesis of SSc by virtue of participating in two major pathologic aspects of the disease; vascular remodeling and excessive fibrosis (Figure 1). Specifically, in early phase of SSc, a variety of triggers damage the endothelium, leading to subsequent expression of a series of angiogenic factors, growth factors, and chemokines, including VEGF, MCP-1, and SDF-1. Normally, the denuded vessels would be rapidly fixed by a highly regulated angiogenic and vasculogenic process, but, in SSc patients, the vascular repair machinery is impaired, which results in disrupted EC functions. Dysregulated ECs promote excessive fibrosis through the activation of fibroblasts by a direct interaction and their trans-differentiation into myofibroblasts via Endo-MT. Additionally, in compensation for the insufficient vascular repair process, monocytic EPCs with M2 features are recruited into circulation instead and are made to function to enhance angiogenesis. However, this mechanism eventually fails to repair vessels because the local environment suppresses angiogenesis, a process which is mediated primarily by dysregulated ECs. Finally, monocytic EPCs accumulate at affected sites and acquire profibrotic characteristics, which enables them to participate in the progression of excessive fibrosis. Further investigation into the mechanisms underlying dysregulated endothelial homeostasis in the disease process of SSc may be key in dissecting its pathogenesis and developing novel therapeutic strategies.
Figure 1.
Roles of ECs and EPCs in pathogenesis of SSc.
A variety of triggers damage the endothelium, leading to subsequent expression of a series of pro-angiogenic factors, growth factors, and chemokines. Increased levels of these mediators promote recruitment of conventional EPCs from bone marrow, however, the vascular repair machinery is intrinsically impaired, which results in altered EC functions that induce the activation of fibroblasts by a direct interaction and their trans-differentiation into myofibroblasts via Endo-MT. Additionally, in compensation for the insufficient vascular repair process, monocytic EPCs with M2 features are recruited into circulation and are made to accumulate at the affected sites, thereby promoting ECM deposition and tissue fibrosis.
ECs: endothelial cells; vSMCs: vascular smooth muscle cells; EPCs: endothelial progenitor cells; VEGF: vascular endothelial growth factor; FGF-2: basic fibroblast growth factor-2; MCP-1: monocyte chemoattractant protein-1; SDF-1: stromal-derived factor-1; MMP12: matrix metalloproteinase 12; TGF-β: transforming growth factor β; ECM: extracellular matrix.
Main Points.
Endothelial injury and subsequent endothelial dysfunction by a variety of triggers is believed to be an initial event that leads to a vicious pathogenic cycle of SSc.
Vascular repair is mediated through two distinct processes, angiogenesis and vasculogenesis, and endothelial cells (ECs) and endothelial progenitor cells (EPCs) play critical roles in each process, respectively, but contribute collaboratively to blood vessel formation.
In SSc, dysfunctional ECs and EPCs do not only trigger the formation of typical vascular lesions but also promote inflammation and excessive fibrosis through recruitment of monocytic cells with M2 features, activation of fibroblasts, and transdifferentiation into myofibroblasts via Endo-MT.
Acknowledgements
This work was supported by a research grant on intractable diseases from the Japanese Ministry of Health, Labour and Welfare, and a grant from the Japanese Ministry of Education, Science, Sports and Culture.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.O., M.K.; Design - Y.O., M.K.; Analysis and/or Interpretation - Y.O., M.K.; Literature Search - Y.O., M.K.; Writing Manuscript - Y.O., M.K.; Critical Review - Y.O., M.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Rel Disord. 2017;2:137–52. doi: 10.5301/jsrd.5000249. [DOI] [Google Scholar]
- 2.Guiducci S, Giacomelli R, Cerinic MM. Vascular complications of scleroderma. Autoimmun Rev. 2007;6:520–3. doi: 10.1016/j.autrev.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P, Giacomelli R. Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev. 2011;10:590–4. doi: 10.1016/j.autrev.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol. 2006:157–212. doi: 10.1007/3-540-36028-X_6. [DOI] [PubMed] [Google Scholar]
- 5.Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther. 2007;9(Suppl 2):S2. doi: 10.1186/ar2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272. doi: 10.3389/fimmu.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunardi C, Bason C, Navone R, Millo E, Damonte G, Corrocher R, et al. Systemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cells. Nat Med. 2000;6:1183–6. doi: 10.1038/80533. [DOI] [PubMed] [Google Scholar]
- 8.Del Papa N, Conforti G, Gambini D, La Rosa L, Tincani A, D’Cruz D, et al. Characterization of the endothelial surface proteins recognized by anti-endothelial antibodies in primary and secondary autoimmune vasculitis. Clin Immunol Immunopathol. 1994;70:211–6. doi: 10.1006/clin.1994.1031. [DOI] [PubMed] [Google Scholar]
- 9.Marks RM, Czerniecki M, Andrews BS, Penny R. The effects of scleroderma serum on human microvascular endothelial cells. Induction of antibody-dependent cellular cytotoxicity. Arthritis Rheum. 1988;31:1524–34. doi: 10.1002/art.1780311209. [DOI] [PubMed] [Google Scholar]
- 10.Holt CM, Lindsey N, Moult J, Malia RG, Greaves M, Hume A, et al. Antibody-dependent cellular cytotoxicity of vascular endothelium: characterization and pathogenic associations in systemic sclerosis. Clin Exp Immunol. 1989;78:359–65. [PMC free article] [PubMed] [Google Scholar]
- 11.Sgonc R, Gruschwitz MS, Boeck G, Sepp N, Gruber J, Wick G. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000;43:2550–62. doi: 10.1002/1529-0131(200011)43:11<2550::AID-ANR24>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Bordron A, Dueymes M, Levy Y, Jamin C, Leroy JP, Piette JC, et al. The binding of some human antiendothelial cell antibodies induces endothelial cell apoptosis. J Clin Invest. 1998;101:2029–35. doi: 10.1172/JCI2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostmans Y, Cutolo M, Giddelo C, Decuman S, Melsens K, Declercq H, et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun Rev. 2017;16:774–86. doi: 10.1016/j.autrev.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Bielecki M, Kowal K, Lapinska A, Chwiesko-Minarowska S, Chyczewski L, Kowal-Bielecka O. Peripheral blood mononuclear cells from patients with systemic sclerosis spontaneously secrete increased amounts of vascular endothelial growth factor (VEGF) already in the early stage of the disease. Adv Med Sci. 2011;56:255–63. doi: 10.2478/v10039-011-0025-z. [DOI] [PubMed] [Google Scholar]
- 15.Maurer B, Distler A, Suliman YA, Gay RE, Michel BA, Gay S, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis. 2014;73:1880–7. doi: 10.1136/annrheumdis-2013-203535. [DOI] [PubMed] [Google Scholar]
- 16.Avouac J, Wipff J, Goldman O, Ruiz B, Couraud PO, Chiocchia G, et al. Angiogenesis in systemic sclerosis: impaired expression of vascular endothelial growth factor receptor 1 in endothelial progenitor-derived cells under hypoxic conditions. Arthritis Rheum. 2008;58:3550–61. doi: 10.1002/art.23968. [DOI] [PubMed] [Google Scholar]
- 17.Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95:109–16. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 18.Higashi-Kuwata N, Makino T, Inoue Y, Ihn H. Expression pattern of VEGFR-1, −2, −3 and D2–40 protein in the skin of patients with systemic sclerosis. Eur J Dermatol. 2011;21:490–4. doi: 10.1684/ejd.2011.1284. [DOI] [PubMed] [Google Scholar]
- 19.Giusti B, Serrati S, Margheri F, Papucci L, Rossi L, Poggi F, et al. The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum. 2005;52:3618–28. doi: 10.1002/art.21383. [DOI] [PubMed] [Google Scholar]
- 20.Michalska-Jakubus M, Kowal-Bielecka O, Chodorowska G, Bielecki M, Krasowska D. Angiopoietins-1 and -2 are differentially expressed in the sera of patients with systemic sclerosis: high angiopoietin-2 levels are associated with greater severity and higher activity of the disease. Rheumatology (Oxford) 2011;50:746–55. doi: 10.1093/rheumatology/keq392. [DOI] [PubMed] [Google Scholar]
- 21.Rabquer BJ, Koch AE. Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheum Rep. 2012;14:56–63. doi: 10.1007/s11926-011-0219-1. [DOI] [PubMed] [Google Scholar]
- 22.Distler O, Del Rosso A, Giacomelli R, Cipriani P, Conforti ML, Guiducci S, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res Ther. 2002;4:R11. doi: 10.1186/ar596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirai Y, Okazaki Y, Inoue Y, Tamura Y, Yasuoka H, Takeuchi T, et al. Elevated levels of pentraxin 3 in systemic sclerosis: associations with vascular manifestations and defective vasculogenesis. Arthritis Rheumatol. 2015;67:498–507. doi: 10.1002/art.38953. [DOI] [PubMed] [Google Scholar]
- 24.Almeida I, Oliveira Gomes A, Lima M, Silva I, Vasconcelos C. Different contributions of angiostatin and endostatin in angiogenesis impairment in systemic sclerosis: a cohort study. Clin Exp Rheumatol. 2016;34(Suppl 100):37–42. [PubMed] [Google Scholar]
- 25.Mackiewicz Z, Sukura A, Povilenaite D, Ceponis A, Virtanen I, Hukkanen M, et al. Increased but imbalanced expression of VEGF and its receptors has no positive effect on angiogenesis in systemic sclerosis skin. Clin Exp Rheumatol. 2002;20:641–6. [PubMed] [Google Scholar]
- 26.Wang Y, Fan PS, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–9. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- 27.Toyama T, Asano Y, Miyagawa T, Nakamura K, Hirabayashi M, Yamashita T, et al. The impact of transcription factor Fli1 deficiency on the regulation of angiogenesis. Exp Dermatol. 2017;26:912–8. doi: 10.1111/exd.13341. [DOI] [PubMed] [Google Scholar]
- 28.Maurer B, Reich N, Juengel A, Kriegsmann J, Gay RE, Schett G, et al. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann Rheum Dis. 2012;71:1382–7. doi: 10.1136/annrheumdis-2011-200940. [DOI] [PubMed] [Google Scholar]
- 29.Maurer B, Distler JH, Distler O. The Fra-2 transgenic mouse model of systemic sclerosis. Vascul Pharmacol. 2013;58:194–201. doi: 10.1016/j.vph.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Reich N, Maurer B, Akhmetshina A, Venalis P, Dees C, Zerr P, et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. Arthritis Rheum. 2010;62:280–90. doi: 10.1002/art.25056. [DOI] [PubMed] [Google Scholar]
- 31.Maurer B, Busch N, Jungel A, Pileckyte M, Gay RE, Michel BA, et al. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation. 2009;120:2367–76. doi: 10.1161/CIRCULATIONAHA.109.855114. [DOI] [PubMed] [Google Scholar]
- 32.Serrati S, Chilla A, Laurenzana A, Margheri F, Giannoni E, Magnelli L, et al. Systemic sclerosis endothelial cells recruit and activate dermal fibroblasts by induction of a connective tissue growth factor (CCN2)/transforming growth factor beta-dependent mesenchymal-to-mesenchymal transition. Arthritis Rheum. 2013;65:258–69. doi: 10.1002/art.37705. [DOI] [PubMed] [Google Scholar]
- 33.Serrati S, Cinelli M, Margheri F, Guiducci S, Del Rosso A, Pucci M, et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J Pathol. 2006;210:240–8. doi: 10.1002/path.2048. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–23. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajkumar VS, Sundberg C, Abraham DJ, Rubin K, Black CM. Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis and rheumatism. 1999;42:930–41. doi: 10.1002/1529-0131(199905)42:5<930::AID-ANR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452. doi: 10.1371/journal.pone.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem cells. 2008;26:2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 38.Cipriani P, Di Benedetto P, Ruscitti P, Campese AF, Liakouli V, Carubbi F, et al. Impaired endothelium-mesenchymal stem cells cross-talk in systemic sclerosis: a link between vascular and fibrotic features. Arthritis Res Ther. 2014;16:442. doi: 10.1186/s13075-014-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karasek MA. Does transformation of microvascular endothelial cells into myofibroblasts play a key role in the etiology and pathology of fibrotic disease? Medical Hypotheses. 2007;68:650–5. doi: 10.1016/j.mehy.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 41.Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, et al. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Annals Rheum Dis. 2017;76:924–34. doi: 10.1136/annrheumdis-2016-210229. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Jimenez SA. Protein kinase Cdelta and c-Abl kinase are required for transforming growth factor beta induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. 2011;63:2473–83. doi: 10.1002/art.30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–27. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 44.Medici D, Potenta S, Kalluri R. Transforming growth factor-beta2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. 2011;437:515–20. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2012;347:177–86. doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piera-Velazquez S, Jimenez SA. Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair. 2012;5:S7. doi: 10.1186/1755-1536-5-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–87. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gvaramia D, Blaauboer ME, Hanemaaijer R, Everts V. Role of caveolin-1 in fibrotic diseases. Matrix Biol. 2013;32:307–15. doi: 10.1016/j.matbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Miyasato SK, Loeffler J, Shohet R, Zhang J, Lindsey M, Le Saux CJ. Caveolin-1 modulates TGF-beta1 signaling in cardiac remodeling. Matrix Biol. 2011;30:318–29. doi: 10.1016/j.matbio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Galdo F, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, Lisanti MP, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–65. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 52.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114:912–20. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 54.Abraham DJ, Vancheeswaran R, Dashwood MR, Rajkumar VS, Pantelides P, Xu SW, et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. 1997;151:831–41. [PMC free article] [PubMed] [Google Scholar]
- 55.Xu S, Denton CP, Holmes A, Dashwood MR, Abraham DJ, Black CM. Endothelins: effect on matrix biosynthesis and proliferation in normal and scleroderma fibroblasts. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S360–3. doi: 10.1097/00005344-199800001-00101. [DOI] [PubMed] [Google Scholar]
- 56.Leask A. The role of endothelin-1 signaling in the fibrosis observed in systemic sclerosis. Pharmacol Res. 2011;63:502–3. doi: 10.1016/j.phrs.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Wermuth PJ, Li Z, Mendoza FA, Jimenez SA. Stimulation of Transforming Growth Factor-beta1-Induced Endothelial-To-Mesenchymal Transition and Tissue Fibrosis by Endothelin-1 (ET-1): A Novel Profibrotic Effect of ET-1. PLoS One. 2016;11:e0161988. doi: 10.1371/journal.pone.0161988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cipriani P, Di Benedetto P, Ruscitti P, Capece D, Zazzeroni F, Liakouli V, et al. The endothelial-mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor-beta and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J Rheumatol. 2015;42:1808–16. doi: 10.3899/jrheum.150088. [DOI] [PubMed] [Google Scholar]
- 59.Beyer C, Distler JH. Morphogen pathways in systemic sclerosis. Cur Rheumatol Rep. 2013;15:299. doi: 10.1007/s11926-012-0299-6. [DOI] [PubMed] [Google Scholar]
- 60.Beyer C, Dees C, Distler JH. Morphogen pathways as molecular targets for the treatment of fibrosis in systemic sclerosis. Arch Dermatol Res. 2013;305:1–8. doi: 10.1007/s00403-012-1304-7. [DOI] [PubMed] [Google Scholar]
- 61.Distler A, Lang V, Del Vecchio T, Huang J, Zhang Y, Beyer C, et al. Combined inhibition of morphogen pathways demonstrates additive antifibrotic effects and improved tolerability. Ann Rheum Dis. 2014;73:1264–8. doi: 10.1136/annrheumdis-2013-204221. [DOI] [PubMed] [Google Scholar]
- 62.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, et al. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94:910–7. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 63.Fu Y, Chang A, Chang L, Niessen K, Eapen S, Setiadi A, et al. Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J Biol Chem. 2009;284:19452–62. doi: 10.1074/jbc.M109.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gasperini P, Espigol-Frigole G, McCormick PJ, Salvucci O, Maric D, Uldrick TS, et al. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 2012;72:1157–69. doi: 10.1158/0008-5472.CAN-11-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–79. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 67.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–83. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L, Chen L, Zang J, Tang X, Liu Y, Zhang J, et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64:597–610. doi: 10.1016/j.metabol.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Lafyatis R. Connective tissue disease: SSc-fibrosis takes flight with Wingless inhibition. Nat Rev Rheumatol. 2012;8:441–2. doi: 10.1038/nrrheum.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, et al. Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012;64:2734–45. doi: 10.1002/art.34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, et al. beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis. 2012;71:761–7. doi: 10.1136/annrheumdis-2011-200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dees C, Schlottmann I, Funke R, Distler A, Palumbo-Zerr K, Zerr P, et al. The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann Rheum Dis. 2014;73:1232–9. doi: 10.1136/annrheumdis-2012-203194. [DOI] [PubMed] [Google Scholar]
- 73.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, et al. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45:915–22. doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lokmic Z, Musyoka J, Hewitson TD, Darby IA. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol. 2012;296:139–85. doi: 10.1016/B978-0-12-394307-1.00003-5. [DOI] [PubMed] [Google Scholar]
- 76.Haase VH. Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci. 2009;1177:57–65. doi: 10.1111/j.1749-6632.2009.05030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–35. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 78.Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75:1278–87. doi: 10.1038/ki.2009.62. [DOI] [PubMed] [Google Scholar]
- 79.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–20. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM. Snail is a direct target of hypoxia-inducible factor 1alpha (HIF1alpha) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J Biol Chem. 2015;290:16653–64. doi: 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 82.Ferrante A, Guggino G, Di Liberto D, Ciccia F, Cipriani P, Balistreri CR, et al. Endothelial progenitor cells: Are they displaying a function in autoimmune disorders? Mech Ageing Dev. 2016;159:44–8. doi: 10.1016/j.mad.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364:603–10. doi: 10.1016/S0140-6736(04)16853-0. [DOI] [PubMed] [Google Scholar]
- 84.Zhu S, Evans S, Yan B, Povsic TJ, Tapson V, Goldschmidt-Clermont PJ, et al. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation. 2008;118:2156–65. doi: 10.1161/CIRCULATIONAHA.108.787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mok MY, Yiu KH, Wong CY, Qiuwaxi J, Lai WH, Wong WS, et al. Low circulating level of CD133+KDR+cells in patients with systemic sclerosis. Clin Exp Rheumatol. 2010;28:S19–25. [PubMed] [Google Scholar]
- 86.Andrigueti FV, Arismendi MI, Ebbing PC, Kayser C. Decreased numbers of endothelial progenitor cells in patients in the early stages of systemic sclerosis. Microvasc Res. 2015;98:82–7. doi: 10.1016/j.mvr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Del Papa N, Colombo G, Fracchiolla N, Moronetti LM, Ingegnoli F, Maglione W, et al. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004;50:1296–304. doi: 10.1002/art.20116. [DOI] [PubMed] [Google Scholar]
- 88.Del Papa N, Quirici N, Soligo D, Scavullo C, Cortiana M, Borsotti C, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54:2605–15. doi: 10.1002/art.22035. [DOI] [PubMed] [Google Scholar]
- 89.Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25:60–6. [PubMed] [Google Scholar]
- 90.Avouac J, Juin F, Wipff J, Couraud PO, Chiocchia G, Kahan A, et al. Circulating endothelial progenitor cells in systemic sclerosis: association with disease severity. Ann Rheum Dis. 2008;67:1455–60. doi: 10.1136/ard.2007.082131. [DOI] [PubMed] [Google Scholar]
- 91.Nevskaya T, Bykovskaia S, Lyssuk E, Shakhov I, Zaprjagaeva M, Mach E, et al. Circulating endothelial progenitor cells in systemic sclerosis: relation to impaired angiogenesis and cardiovascular manifestations. Clin Exp Rheumatol. 2008;26:421–9. [PubMed] [Google Scholar]
- 92.Distler JH, Allanore Y, Avouac J, Giacomelli R, Guiducci S, Moritz F, et al. EULAR Scleroderma Trials and Research group statement and recommendations on endothelial precursor cells. Ann Rheum Dis. 2009;68:163–8. doi: 10.1136/ard.2008.091918. [DOI] [PubMed] [Google Scholar]
- 93.Kuwana M, Okazaki Y. Quantification of circulating endothelial progenitor cells in systemic sclerosis: a direct comparison of protocols. Ann Rheum Dis. 2012;71:617–20. doi: 10.1136/annrheumdis-2011-200713. [DOI] [PubMed] [Google Scholar]
- 94.Manetti M, Pratesi S, Romano E, Rosa I, Bruni C, Bellando-Randone S, et al. Decreased circulating lymphatic endothelial progenitor cells in digital ulcer-complicated systemic sclerosis. Ann Rheum Dis. 2019;78:575–7. doi: 10.1136/annrheumdis-2018-214240. [DOI] [PubMed] [Google Scholar]
- 95.Kuwana M, Okazaki Y. Brief report: impaired in vivo neovascularization capacity of endothelial progenitor cells in patients with systemic sclerosis. Arthritis Rheumatol. 2014;66:1300–5. doi: 10.1002/art.38326. [DOI] [PubMed] [Google Scholar]
- 96.Avouac J, Meune C, Ruiz B, Couraud PO, Uzan G, Boileau C, et al. Angiogenic biomarkers predict the occurrence of digital ulcers in systemic sclerosis. Ann Rheum Dis. 2012;71:394–9. doi: 10.1136/annrheumdis-2011-200143. [DOI] [PubMed] [Google Scholar]
- 97.Avouac J, Vallucci M, Smith V, Senet P, Ruiz B, Sulli A, et al. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther. 2013;15:R55. doi: 10.1186/ar4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carrai V, Miniati I, Guiducci S, Capaccioli G, Alterini R, Saccardi R, et al. Evidence for reduced angiogenesis in bone marrow in SSc: immunohistochemistry and multiparametric computerized imaging analysis. Rheumatology (Oxford) 2012;51:1042–8. doi: 10.1093/rheumatology/ker447. [DOI] [PubMed] [Google Scholar]
- 99.Watt SM, Athanassopoulos A, Harris AL, Tsaknakis G. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface. 2010;7(Suppl 6):S731–51. doi: 10.1098/rsif.2010.0377.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahaleh B, Guiducci S, Kuwana M. Recent updates in experimental protocols for endothelial cells. J Scleroderma Rel Disord. 2016;1:257–65. doi: 10.5301/jsrd.5000217. [DOI] [Google Scholar]
- 101.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 102.Yamaguchi Y, Kuwana M. Proangiogenic hematopoietic cells of monocytic origin: roles in vascular regeneration and pathogenic processes of systemic sclerosis. Histol Histopathol. 2013;28:175–83. doi: 10.14670/HH-28.175. [DOI] [PubMed] [Google Scholar]
- 103.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. doi: 10.1182/blood.V95.3.952.003k27_952_958. [DOI] [PubMed] [Google Scholar]
- 104.Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol. 2011;50:266–72. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- 106.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 107.Yamaguchi Y, Okazaki Y, Seta N, Satoh T, Takahashi K, Ikezawa Z, et al. Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Res Ther. 2010;12:R205. doi: 10.1186/ar3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Distler O, Pap T, Kowal-Bielecka O, Meyringer R, Guiducci S, Landthaler M, et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet-derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum. 2001;44:2665–78. doi: 10.1002/1529-0131(200111)44:11<2665::aid-art446>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 109.Cipriani P, Franca Milia A, Liakouli V, Pacini A, Manetti M, Marrelli A, et al. Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: Pathogenetic implications. Arthritis Rheum. 2006;54:3022–33. doi: 10.1002/art.22047. [DOI] [PubMed] [Google Scholar]
- 110.Bellik L, Musilli C, Vinci MC, Ledda F, Parenti A. Human mature endothelial cells modulate peripheral blood mononuclear cell differentiation toward an endothelial phenotype. Exp Cell Res. 2008;314:2965–74. doi: 10.1016/j.yexcr.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 111.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.CIR.0000051460.85800.BB. [DOI] [PubMed] [Google Scholar]
- 112.Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–45. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- 113.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–22. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 114.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, et al. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555–63. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 115.Masuda A, Yasuoka H, Satoh T, Okazaki Y, Yamaguchi Y, Kuwana M. Versican is upregulated in circulating monocytes in patients with systemic sclerosis and amplifies a CCL2-mediated pathogenic loop. Arthritis Res Ther. 2013;15:R74. doi: 10.1186/ar4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Campioni D, Lo Monaco A, Lanza F, Moretti S, Ferrari L, Fotinidi M, et al. CXCR4 pos circulating progenitor cells coexpressing monocytic and endothelial markers correlating with fibrotic clinical features are present in the peripheral blood of patients affected by systemic sclerosis. Haematologica. 2008;93:1233–7. doi: 10.3324/haematol.12526. [DOI] [PubMed] [Google Scholar]
- 117.Stifano G, Christmann RB. Macrophage Involvement in Systemic Sclerosis: Do We Need More Evidence? Curr Rheumatol Rep. 2016;18:2. doi: 10.1007/s11926-015-0554-8. [DOI] [PubMed] [Google Scholar]
- 118.Soldano SP, Trombetta ACP, Contini PP, Tomatis VM, Ruaro BM, Brizzolara RP, et al. Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. Ann Rheum Dis. 2018;77:1842–5. doi: 10.1136/annrheumdis-2018-213648. [DOI] [PubMed] [Google Scholar]
- 119.Lescoat A, Ballerie A, Jouneau S, Fardel O, Vernhet L, Jego P, et al. M1/M2 polarisation state of M-CSF blood-derived macrophages in systemic sclerosis. Ann Rheum Dis. 2018;78:e127. doi: 10.1136/annrheumdis-2018-214333. [DOI] [PubMed] [Google Scholar]
- 120.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet. 2016;387:2630–40. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]