Abstract
Background
Iron deficiency (ID) is the leading single-nutrient deficiency in the world. Anaemia is a common outcome of ID that affects half of pregnancies worldwide with serious consequences for child development. Whether haematologic indices and biomarkers of iron status in pregnant women correlate with those of their neonates is unclear. This systematic review evaluated studies comparing haematologic and iron status indices in pregnant women and their newborns/neonates.
Methods
We searched MEDLINE, EMBASE, CINAHL, and Web of Science from database inception until March 2020 for primary studies comparing haematologic and iron status indices between women and their newborns up to 48 h after birth. We summarized the results descriptively and calculated pooled correlation coefficients in mothers and newborns/neonates using the Schmidt-Hunter method. The protocol was registered at PROSPERO International Prospective Register of Systematic Reviews (Registration number: CRD42018093094).
Findings
Sixty-five studies were included. Pooled correlation coefficients for biomarkers of iron status in mothers and newborns/neonates were 0.13 (ferritin), 0.42 (hepcidin), 0.30 (serum/plasma iron), 0.09 (transferrin), 0.20 (transferrin saturation), and 0.16 (total iron binding capacity). Pooled correlation coefficients for haematological indices in mothers and newborns/neonates were 0.15 (haemoglobin), 0.15 (haematocrit), 0.25 (mean cell/corpuscular haemoglobin), 0.22 (mean cell/corpuscular volume).
Interpretation
Maternal biomarkers of iron and haematologic status correlate poorly with those in newborns/neonates. These results underscore a need for alternative approaches to estimate foetal/neonatal iron status and haematological indices.
Funding
MBO and SLB hold Canada Research Chairs, and grants from the Women and Children's Health Research Institute and Canadian Institutes of Health Research.
Research in context.
Evidence before this study
Iron deficiency (ID) is a global health issue, for which the burden on pregnant women and neonates is staggering. Numerous groups worldwide have studied the association between maternal haematological and iron status indices with those of their newborn children, though no clear association has been established.
Added value of this study
This study is the first to consolidate data regarding the correlation of maternal haematological and iron status biomarkers with those of their newborn children. Of the parameters studied, maternal serum iron was most strongly correlated with offspring haematological and iron status indices. Notably, maternal ferritin, often utilized as a primary ID screen assessment, did not show any association with offspring indices.
Implications of all the available evidence
In contrast to the dominant clinical paradigm, only weak correlations exist between maternal and offspring haematological and iron status indices. Consequently, neonatal haematological and iron status should not be estimated based on a maternal indices alone, and more proximal sources (e.g. cord blood) may be more appropriate for these assessments.
Alt-text: Unlabelled box
Introduction
Iron deficiency (ID) is a pervasive global health issue, and represents one of the most treatable and preventable causes of daily-adjusted life-years lost [1]. Yet a worldwide prevalence of ~25%, with a sizeable majority of this burden shouldered by women of reproductive ages and young children, underscores the challenges that impede effective treatment [2]. The complex relationship between ID and anaemia, which varies considerably by subpopulation and geography, makes universal recommendations for treatment of ID challenging[3]. Accurate assessments of maternal, foetal and neonatal iron status are critical for pre- and postnatal interventions that maximize health benefits while minimizing adverse effects. However, recent reviews have highlighted the challenges associated with reliable iron assessments in populations [4] —a task even more complex in pregnant women and in foetuses/neonates.
Since the body prioritizes iron utilization for erythropoiesis, depletion of iron stores and consequent ID can occur in the absence of anaemia [5]. Additionally, ID is believed to underlie only half the cases of anaemia worldwide, while nutrient deficiencies (e.g., folate, vitamin B12, vitamin A), inflammation, inherited disorders (e.g., thalassaemia) and myriad other causes account for the rest [6]. Diagnostic criteria for both anaemia and ID remain contentious in the context of pregnancy, as reviewed by a consortium on behalf of the British Society of Haematology [7]. Cut-offs defining anaemia during pregnancy are based on historically normal Hb values in non-pregnant persons, but do not correlate well with clinical outcomes, resulting in calls to establish evidence-based values [8]. The clinical cut-offs for diagnosis of ID during pregnancy remain even more contentious. Serum ferritin is often used as a clinical index of iron status, yet values used to define ID vary, and validated cut-off values have yet to be established for pregnant women [9]. Moreover, as an acute phase protein, normal serum ferritin values do not exclude the possibility of ID. Alternative indices, such as soluble transferrin receptor (sTfR) levels, may overcome such limitations, but lack established cut-off values and must be validated for use in pregnancy [10]. Altogether, it remains unclear which indices are most applicable for use in pregnancy, on the basis of which indices best reflect the status of mother and developing child.
Foetal blood is rarely collected during pregnancy due to inherent risks to the foetus, making direct assessments of iron status and haematological indices challenging. Moreover, cord blood collected at delivery is not routinely analysed in otherwise healthy pregnancies. Rather, maternal haematological indices, and less frequently biomarkers of iron status, are often used to guide intervention strategies in pregnancy and the neonatal period [11]. However, the prevailing notion that maternal iron status and severity of anaemia is a useful surrogate of foetal iron status has not been validated. A discordance between maternal and foetal indices could have implications for foetal and neonatal health, since ID in pregnancy has been associated with adverse pregnancy outcomes and altered developmental trajectories in the offspring [12]. Indeed, ID at birth may deprive the neonate of critical iron stores needed for optimal growth and development in early postnatal life [13].
This systematic review was undertaken to examine the correlation between maternal and neonatal/neonate biomarkers for iron status and haematologic indices. This review is the first to curate available evidence regarding how these indices correlate in mothers and their neonates, which will help investigators design studies to establish evidence-based clinical cut-offs and guidelines.
Methods
2.1 Design and protocol development
The systematic review and meta-analyses were conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14]. A protocol was registered in the International Prospective Register of Systematic Reviews database (PROSPERO, CRD42018093094).
2.2 Eligibility criteria
To be eligible for inclusion, primary studies needed to include populations of women with no apparent complications in pregnancy (i.e. no gestational hypertension, preeclampsia, and/or gestational diabetes) and report correlation coefficients between iron status and haematological biomarkers in the mother and newborns within 48 h of birth (primary outcome of the review). No exclusions were made based on duration of gestation or alterations in foetal growth reported in the studies. Randomized controlled trials were excluded as their primary focus is on the efficacy/effectiveness of interventions. Review articles, animal studies, case reports, letters to the editor, commentaries, in vitro studies, and articles that did not report or allow for calculation of correlation coefficients were excluded. Exposures of interest were maternal biomarkers of iron status, which included serum ferritin, hepcidin, serum/plasma iron, soluble transferrin receptor [sTfR], transferrin [Tf] levels, transferrin saturation [Tf Sat], total iron binding capacity [TIBC], zinc protoporphyrin levels [ZPP], or maternal haematological indices, which included haemoglobin (Hb), haematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCHb), collected during pregnancy or in the immediate postnatal period (up to 48 h after birth). Primary outcomes of interest were the same biomarkers of iron status or haematological indices collected in newborns or in cord blood (up to 48 h after birth).
2.3 Search strategy and selection criteria
Comprehensive searches in MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science were conducted from database inception up to March 2020. The search strategy was designed by an information specialist (TC) with feedback from content experts (SLB) and methodologists (MBO) using Medical Subject Headings and relevant keywords related to pregnancy, and iron status and haematological indices in the mother and newborns. The full MEDLINE search strategy with keywords and MeSH terms is provided in Supplementary Table 1. Additionally, grey literature and reference lists searches of potentially relevant articles were conducted. Language or publication status restrictions were not applied. After removal of duplicates, the references file was split amongst five pairs of reviewers (MBO and JL, MBO and SR, MBO and OBS, SR and AGW, or OBS and SLB) for independent screening of titles, abstracts and full texts. Disagreements amongst pairs of reviewers were resolved by consensus.
2.4 Data extraction
A standardized tool was designed to extract relevant information from studies. Data relating to names of authors, year and country where studies were conducted, study design, whether studies were conducted in a malaria-endemic setting, sample size, maternal age and maternal/neonate biomarkers were extracted by one of two reviewers (JL or SR) and independently verified by a second reviewer (OBS); discrepancies were resolved by consensus.
2.5 Risk of bias assessment
Two independent reviewers (OBS and AGW) assessed the risk of bias of included studies using the Newcastle-Ottawa Scale (NOS) [15] for observational studies. The NOS evaluates risk of bias in selection of study participants, comparability amongst study groups, ascertainment of exposures, and outcomes assessment. Based on the final NOS score, the risk of bias of each article was graded as either low (selection 3–4 stars, comparability 2 stars, outcome 3 stars), moderate (selection 2 stars, comparability 1 star, outcome 2 stars), or high (selection 1 or 0 stars, comparability 0 stars, outcome 1 or 0 stars)[15]. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) [16] approach was used to rate the body of evidence for each comparison of maternal-neonate iron status and haematological biomarkers. Briefly, evidence from non-randomized studies begins as low-quality evidence but can be downgraded or upgraded according to risk of bias, inconsistency, indirectness, and imprecision of the evidence. Grades of evidence were rated as high, moderate, low, or very low.
2.6 Data analysis and interpretation
Correlation coefficients obtained in individual studies for biomarkers of iron status in mothers and newborns/neonates were pooled using the Schmidt-Hunter random-effects model method [17] and reported with 95% confidence intervals (CI) when three or more studies reported similar biomarkers. Population characteristics and outcome estimates of included studies were narratively synthesized. I2 statistic was used to assess heterogeneity across included studies - an I2 of <26%, 26–74% or >74% indicate low, moderate or high heterogeneity respectively. To account for heterogeneity, sub-group analyses were performed by study design where at least 10 studies were pooled. Funnel plots were used to assess publication bias; an asymmetrical funnel plot suggests evidence of publication bias.
Pooled correlation coefficients were interpreted as follows: very high positive/negative correlation (0.90 to 1.00 or −0.90 to −1.00), high positive/negative correlation (0.70 to 0.89 or −0.70 to −0.89), moderate positive/negative correlation (0.50 to 0.69 or −0.50 to −0.69), low positive/negative correlation (0.30 to 0.49 or −0.30 to −0.49), and negligible correlation (0.00 to 0.29 or 0.00 to −0.29) [18]. Analyses were conducted using StatsDirect version 3 [19] (for meta-analysis of correlation data) and RevMan version 5.3 [20] (for risk of bias summary).
2.7 Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
3.1 Search results
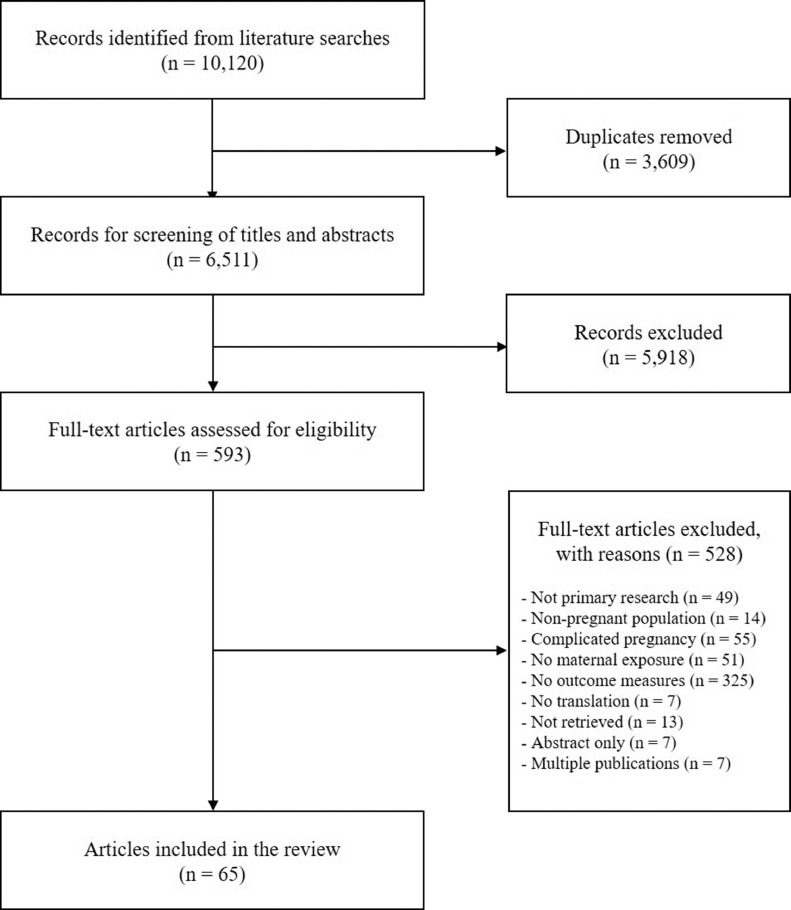
A total of 10,120 references were identified, and after duplicates were removed, 6511 titles and abstracts were screened for relevance, yielding 593 full text articles. A total of 65 studies were included in the review. Detailed study inclusion and exclusion process is presented in Fig. 1. The full list of excluded studies is available upon request.
Fig. 1.
PRISMA flow diagram.
3.2 Characteristics of included studies
Characteristics of included studies are described in Table 1. Studies were published between 1973 and 2019. The majority of studies were cross-sectional: 54 studies [21–74], 9 were prospective cohort studies [75–83], and two were retrospective cohort studies [84,85]. 24 studies were conducted in Asia [21–23,32,35–37,39,40,42,49,50,53,54,57,58,65,66,70,75,76,78,80,83], 18 in Europe [26,28,30,41,43,45,46,48,51,55,56,61,63,68,69,71,79], 8 in North America [44,52,72,73,81,82,85,86], 7 in Africa [24,27,29,31,38,47,59], 6 in South America [25,34,64,67,74,84], and 2 were conducted in Australia and Oceania.[33,60] Thirty-four studies were conducted in malaria-endemic countries [[21], [22], [23], [24], [25],31,32,[34], [35], [36], [37], [38],41,42,47–49,52,53,55,57–60,64–67,70,72,74,76,78,84].
Table 1.
Summary of study characteristics.
| Author, year, location | Study design | Participant characteristics | Methods and timing of data collection | Biomarker analyses | Biomarkers | Correlation r (95%CI)/mean | NOS quality score |
|---|---|---|---|---|---|---|---|
| Adams et al. [21], 1981, Nepal | Cross-sectional | Sample size: 151 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethemoglobin method | Maternal: Hb Newborn: Hb |
r = −0.06 (−0.22, 0.10) | 3 |
| Agrawal et al. [22] 1983, India | Cross-sectional | Sample size: 51 Maternal age (a) : NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord (placental end) at delivery Inflammation biomarkers: NR Factors adjusted:Groups compared had no racial, cultural and environmental differences. |
NR | Maternal: Serum iron Newborn: Serum iron |
r = 0.53 (0.30, 0.70) | 5 |
| Akhter et al. [23] 2010, Bangladesh | Cross-sectional | Sample size: 50 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal antecubital blood postpartum Umbilical cord (placental end) at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethemoglobin for Hb, ELISA for ferritin | Maternal: Ferritin Newborn: Ferritin |
r = −0.94 (−0.97, −0.90) | 4 |
| Maternal: Hb Newborn: Ferritin |
r = 0.48 (0.23, 0.67) | ||||||
| Altinkaynak et al., [41] 1984, Turkey | Cross-sectional | Sample size: 52 Maternal age (a): 26±5.3 Malaria setting: Yes Iron supplementation: Some women |
Maternal peripheral blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Ferritin enzymatic testing kits from Medix Biotech | Maternal: Ferritin Newborn: Ferritin |
r = 0.68 (0.49, 0.80) | 4 |
| Awadallah et al. [75], 2004, Jordan | Prospective cohort | Sample size: 186 Maternal age (a): 27±4.9/ 17–45 Malaria setting: No Iron supplementation: All women |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Haematology cell counter | Maternal: Hb Newborn: Hb |
Maternal (anaemic 10.2 ± 0.6, non-anaemic 12.2 ± 1.2 g/dL); newborn (anaemic: 15.1 ± 2.0; non-anaemic 15.8 ± 2.3 g/dL) | 7 |
| Babay et al. [42] 2002, Saudi Arabia | Cros-sectional | Sample size: 82 Maternal age (a): 26.9 ± 5.79/ 16–40 Malaria setting: Yes Iron supplementation: None |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Radial immunodiffusion for Tf, Coulter Counter ZF6 for haematologic parameters | Maternal: Tf Newborn: Tf |
r = 0.40 (0.20, 0.57) | 6 |
| Maternal: Hb Newborn: Hb |
r = 0.14 (−0.08, 0.35) | ||||||
| Maternal: MCHb Newborn: MCHb |
r = 0.66 (0.52, 0.77) | ||||||
| Maternal: MCV Newborn: MCV |
r = 0.36 (0.16, 0.54) | ||||||
| Basu et al. [76] 2016, India | Prospective cohort | Sample size: 45 Maternal age (a): Anaemic: 25.3 ± 3.7; Non-anaemic: 26.3 ± 3.4 Malaria setting: Yes Iron supplementation: NR |
Maternal peripheral blood postpartum Umbilical cord (placental end) at delivery Inflammation biomarkers: NR Factors_adjusted: NR |
Auto analyser for serum iron, ferritin, TIBC, Tf Sat. ELISA for hepcidin | Maternal: Ferritin Newborn: Ferritin, Hepcidin, Serum iron, Tf Sat, TIBC, Hb |
r = 0.94 (0.89, 0.97), 0.46 (0.19, 0.66), 0.91 (0.84, 0.95), 0.88 (0.69, 0.90), −0.81 (−0.89, −0.68), 0.89 (0.80, 0.94) | 5 |
| Maternal: Hepcidin Newborn: Ferritin, Hepcidin, Serum iron, Tf Sat, TIBC, Hb |
r = 0.37 (0.09, 0.60), 0.72 (0.54, 0.83), 0.37 (0.09, 0.60), 0.33 (0.04, 0.57), −0.24 (−0.50, 0.06), 0.39 (0.11, 0.61) | ||||||
| Maternal: Serum iron Newborn Ferritin, Hepcidin, Serum iron, Tf Sat, TIBC, Hb |
r = 0.91 ( 0.78, 0.93), 0.401 (0.12, 0.62), 0.87 (0.78, 0.93), 0.87 (0.77, 0.92), −0.79 (−0.88, −0.65), 0.81 (0.67, 0.89) | ||||||
| Maternal: Tf Sat Newborn: Ferritin, Hepcidin, serum iron, Tf Sat, TIBC, Hb |
r = 0.90 (0.82, 0.94), 0.35 (0.06, 0.58), 0.81 (0.68, 0.89), 0.84 (0.73, 0.91), 0.80 (0.66, 0.89), 0.84 (0.73, 0.91) | ||||||
| Maternal: TIBC Newborn: Ferritin, Serum iron, Tf Sat, TIBC, ZPP, Hb |
r = −0.84 (−0.91, −0.73), −0.75 (−0.86, −0.59), −0.79 (−0.88, −0.64), 0.80 (0.67, 0.89), −0.404 (−0.62, −0.13), −0.838 (−0.91, −0.72) | ||||||
| Maternal: Hb Newborn: Ferritin, Hepcidin , Serum iron, Tf Sat, TIBC, Hb |
r = 0.92 (0.85, 0.95), 0.556 (0.31, 0.73), 0.88 (0.80, 0.93), 0.87 (0.77, 0.93), −0.78 (−0.87, −0.63), 0.83 (0.70, 0.90) | ||||||
| Best et al. [77] 2016, USA | Prospective cohort | Sample size: 255 Maternal age (a): 17.1 ± 1.1 Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: IL-6 Factors adjusted: NR |
NR | Maternal: Ferritin Newborn: Ferritin |
r = 0.37 (0.26, 0.47) | 6 |
| Maternal: Hepcidin Newborn: Hepcidin |
r = 0.32 (0.21, 0.43) | ||||||
| Maternal: sTfR Newborn: sTfR |
r = 0.23 (0.11, 0.34) | ||||||
| Bratlid et al. [43] 1980, Norway | Cross-sectional | Sample size: 54 Maternal age (a): NR Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Radioimmunoassay for ferritin; method for Hb NR | Maternal: Ferritin Newborn: Ferritin |
r = 0.18 (−0.09, 0.43) | 3 |
| Maternal: Hb Newborn: Hb |
r = 0.33 (0.07, 0.55) | ||||||
| Butte et al. [44] 1982, USA | Cross-sectional | Sample size: 28 Maternal age (a): 16–33 Malaria setting: No Iron supplementation: NR |
Maternal venous blood postpartum Umbilical cord blood at delivery Inflammation biomarkers: None Factors adjusted: NR |
NR | Maternal: Ferritin Newborn: Ferritin |
r = −0.002 (−0.37, 0.37) | 3 |
| Celada et al. [45] Germany | Cross-sectional | Sample size: 64 Maternal age (a): 23±4/ 19–31 Malaria setting: No Iron supplementation: All women |
Maternal venous blood postpartum Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Radioimmunoassay for ferritin | Maternal: Ferritin Newborn: Ferritin |
r = 0.07 (−0.18, 0.31) | 4 |
| Custodio et al. [46] 2005, Portugal | Cross-sectional | Sample size: NR Maternal age (a): 15–39 Malaria setting: No Iron supplementation: NR |
Maternal blood postpartum Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Energy dispersive X-ray fluorescence spectrometry | Maternal: Serum iron Newborn: Serum iron |
r = 0.51 (0.31, 0.67) | 2 |
| Daouda et al. [24] 1991, Niger | Cross-sectional | Sample size: 364 Maternal age (a): 26.0 ± 6.4/ 15–47 Malaria setting: Yes Iron supplementation: None |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
ELISA for ferritin. Microcentrifugation for Ht | Maternal: Ferritin Newborn: Ferritin Maternal: Ht Newborn: Ht |
Maternal mean 41.8 ± 66.1 ug/L; cord mean 127.3 ± 62.9 ug/L | 6 |
| Maternal mean 32.8 ± 5.1%; cord mean 43.0 ± 5.7% | |||||||
| Dapper et al. [47] 2006, Nigeria | Cross-sectional | Sample size: 30 Maternal age (a): 19–40 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood postpartum Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Microcentrifugation for Ht. Cyanmethaemoglobin for Hb. Manual counting chamber for blood cell counts | Maternal: Hb Newborn: Hb |
r = 0.42 (0.08, 0.68) | 4 |
| Maternal: Ht Newborn: Ht |
r = 0.22 (−0.16, 0.53) | ||||||
| Maternal: MCHb Newborn: MCHb |
r = −0.05 (−0.40, 0.32) | ||||||
| Maternal: MCV Newborn: MCV |
r = 0.22 (−0.15, 0.54) | ||||||
| De Sa, [25] 2015, Brazil | Cross-sectional | Sample size: 54 Maternal age (a): 24.5 ± 4.1/ 20–38 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Automatic device for haematologic analyses; ELISA for ferritin | Maternal: Ferritin Newborn: Ferritin |
Maternal mean: 9.6 ± 8.0 ug/L, cord mean: 122.9 ± 62.4 ug/L | 4 |
| Maternal: Hb Newborn: Ht |
r = 0.47 (0.23, 0.66) | ||||||
| Devi et al., [78] 1989, India | Prospective cohort | Sample size: 165 Maternal age (a): 26.8/ 18–42 Malaria setting: Yes Iron supplementation: All women |
Maternal blood postpartum Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanomethaemoglobin method for Hb | Maternal: Hb Newborn: Hb |
r = 0.02 (−0.13, 0.18) | 5 |
| Ek et al., [26] 1982, Norway | Cross-sectional | Sample size: 139 Maternal age (a): NR Malaria setting: No Iron supplementation: All women |
Maternal venous blood postpartum Neonatal capillary blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
NR | Maternal: Hb Newborn: Hb |
r = 0.17 (0.004, 0.33) | 6 |
| El Guindi et al. [84] 2004, Guyana | Retrospective cohort | Sample size: 222 Maternal age (a): Anaemic: 24.9/ non-anaemic 26.7 Malaria setting: Yes Iron supplementation: NR |
Timing of maternal blood collection NR Neonatal blood source NR, collected at delivery Inflammation biomarkers: CRP Factors adjusted: NR |
NR | Maternal: hb Newborn: hb |
anaemic mothers 6.92 g/100 ml; non-anaemic mothers 11.54 g/100 ml; newborns of anaemic mothers 15.04 g/100 ml, newborns of non-anaemic mothers 15.75 g/100ml | 6 |
| El-Farrash et al. [27] 2012, Egypt | Cross-sectional | Sample size: 80 Maternal age (a): Anaemic: 25.2 ± 3.3; Non-anaemic: 26.8 ± 5.0 Malaria setting: No Iron supplementation: None |
Maternal blood postpartum Umbilical cord (placental end) at delivery Inflammation biomarkers: CRP Factors adjusted: CRP |
ELISA for ferritin; Commercial kit for TIBC; Hitachi 917 analyser for serum iron; haematologic indices by Coulter Counter GEN-S | Maternal: Ferritin Newborn: Ferritin, Serum iron, Tf Sat, TIBC, Hb, MCHb, MCV, |
r = 0.47 (0.28, 0.62), 0.39 (0.19, 0.56), 0.430 (0.21, 0.87), −0.39 (−0.56, −0.18), 0.67 (0.52, 0.77), 0.496 (0.31, 0.65), 0.39 (0.19, 0.56) | 5 |
| Maternal: Serum iron Newborn: Ferritin, Serum iron, Tf Sat, TIBC, Hb, MCHb, MCV |
r = 0.345 (0.25, 0.61), 0.45 (0.25, 0.61), 0.44 (0.25, 0.60), −0.36 (−0.54, −0.15), 0.58 (0.42, 0.71), 0.348 (0.14, 0.53), 0.327 (0.12, 0.51) | ||||||
| Maternal: Hb Newborn: Serum iron, Tf Sat, TIBC, Hb, MCV, |
r = 0.48 (0.29, 0.63), 0.51 (0.32, 0.65), −0.46 (−0.62, −0.27), 0.76 (0.65, 0.84), 0.55 (0.37, 0.69), | ||||||
| Erdem et al. [48] 2002, Turkey | Cross-sectional | Sample size: 44 Maternal age (a): NR Malaria setting: Yes Iron supplementation: Unclear |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: Controls matched for age, parity and gestational age |
Automated cytometer for Hb. Chemiluminescence technique for ferritin | Maternal: Ferritin Newborn: Ferritin |
r = 0.52 (0.27, 0.71) | 7 |
| Maternal: Hb Newborn: Hb |
Maternal mean: (anaemic 8.72±0.22; non-anaemic 16.11±0.39); cord mean: (anaemic 11.74±0.24; non-anaemic 16.57± | ||||||
| Esmailnasab et al. [49] 2012, Iran | Cross-sectional | Sample size: 604 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Timing of maternal blood collection NR Timing of umbilical cord collection NR Inflammation biomarkers: NR Factors adjusted: NR |
Cell counter machine | Maternal: Hb Newborn: Hb |
r = 0.14 (0.06, 0.22) | 3 |
| Garcia-Valdes et al. [79] 2015, Spain | Prospective cohort | Sample size: 308 Maternal age (a): Control 30.8 ± 4.3, Overweight 31.8 ± 4.5, Obese = 29.0 ± 4.6 Malaria setting: No Iron supplementation: Some women |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: CRP Factors adjusted: Inflammation |
ELISA | Maternal: Hepcidin Newborn: Hepcidin |
r = 0.70 (0.64, 0.75) | 6 |
| Gaspar et al. [28] 1993, Spain | Cross-sectional | Sample size: 157 Maternal age (a): 29.0 ± 0.5 Malaria setting: No Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Automated haematology cell counter | Maternal: Hb Newborn: Hb, Ht |
r = 0.36 (0.22, 0.49), 0.30 (0.15, 0.44) | 5 |
| Maternal: Ht Newborn: Hb, Ht |
r = 0.37 (0.23, 0.50), 0.33 (0.18, 0.46) | ||||||
| Huang et al. [50] 2017, Taiwan | Cross-sectional | Sample size: 150 Maternal age (a): 28.1 ± 5.2 Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Plasma mass spectrometry | Maternal: Serum iron Newborn: Serum iron |
r = 0.17 (95%CI 0.01, 0.32) | 5 |
| Hussain et al. [51] 1977, UK | Cross-sectional | Sample size: 51 Maternal age (a): 17–38 Malaria setting: No Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Immunoradiometric assay | Maternal: Ferritin Newborn: Ferritin |
r = 0.30 (0.03, 0.53) | 6 |
| Jaime-Perez et al. [52] 2005, Mexico | Cross-sectional | Sample size: 201 Maternal age (a): 23.0 ± 6.5 Malaria setting: Yes Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Automated blood cell counter | Maternal: Hb Newborn: Hb |
Newborns of anaemic 157±17 g/L and non-anaemic 159±14 g/L mothers | 7 |
| Jariwala et al. [53] 2014, India | Cross-sectional | Sample size: 42 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
NR | Maternal: Serum iron Newborn: Serum iron |
r = 0.39 (0.09, 0.62) | 4 |
| Kaneshige et al. [80] 1981, Japan | Prospective cohort | Sample size: 80 Maternal age (a): 20–30 Malaria setting: No Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
NR | Maternal: Ferritin Newborn: Ferritin |
r = 0.75 (0.64, 0.84) | 7 |
| Maternal: Serum iron Newborn: Serum iron |
r = 0.52 (0.22, 0.73) | ||||||
| Katoh et al. [54], 1984, Japan | Cross-sectional | Sample size: NR Maternal age (a): NR Malaria setting: No Iron supplementation: NR |
Maternal blood at delivery Neonatal blood source unclear at delivery Inflammation biomarkers: NR Factors adjusted:NR |
NR | Maternal: Serum iron Newborn: Serum iron, Hb |
r = 0.64 (0.37, 0.81), 0.71 (0.47, 0.85) | 4 |
| Maternal: Hb Newborn: Serum iron, Hb |
r = 0.22 (−0.14, 0.52), 0.68 (0.43, 0.83) | ||||||
| Koc et al. [55] 2006, Turkey | Cross-sectional | Sample size: 188 Maternal age (a): 27±5.8 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Colorimetric method for serum iron, TIBC | Maternal: Serum iron Newborn: Serum iron |
r = 0.13 (−0.02, 0.26) | 4 |
| Maternal: Tf Sat Newborn: Tf Sat |
r = 0.11 (−0.04, 0.24) | ||||||
| Maternal: TIBC Newborn: TIBC |
r = 0.20 (0.06, 0.33) | ||||||
| Kulik-Rechberger et al. [56] 2016, Poland | Cross-sectional | Sample size: 44 Maternal age (a): 27.8 ± 5.5/ 18–42 Malaria setting: No Iron supplementation: NR |
Maternal peripheral blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: CRP Factors adjusted: NR |
ELISA for sTfR and automated analyser for Hb | Maternal: Hb Newborn: sTfR |
r = 0.36 (0.07, 0.59) | 5 |
| Kumar et al. [57] 2008, India | Cross-sectional | Sample size: 75 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethaemoglobin method for Hb. Atomic absorption spectroscopy for serum iron. ELISA for ferritin | Maternal: Ferritin Newborn: Ferritin, Serum iron, Hb |
r = 0.44 (0.24, 0.61), 0.45 (0.24, 0.61), 0.49 (0.29, 0.64) | 7 |
| Maternal: Serum iron Newborn: Serum iron |
r = 0.76 (0.65, 0.84) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.62 (0.45, 0.74) | ||||||
| Lao et al. [58] 1991, China | Cross-sectional | Sample size: 96 Maternal age (a): 27.6 ± 3.6 Malaria setting: Yes Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted:NR |
Dye-binding analysis for serum iron and TIBC; Radioimmunoassay for ferritin; automated Technicon CBC counter for haematologic indices nalysis for serum iron and TIBC. Radioimmunoassay for ferritin |
Maternal: Ferritin Newborn: Ferritin, Serum iron, TIBC |
r = 0.10 (−0.10, 0.29), 0.18 (−0.02, 0.37), 0.22 (0.02, 0.40) | 3 |
| Maternal: Serum iron Newborn: Ferritin, Serum iron, TIBC |
r = 0.126 (−0.07, 0.34), 0.14 (−0.07, 0.33), 0.04 (−0.17, 0.25) | ||||||
| Maternal: TIBC Newborn: Ferritin, Serum iron, TIBC |
r = 0.10 (−0.10, 0.29), 0.18 (−0.02, 0.37), 0.20 (0.06, 0.33) | ||||||
| Maternal: Hb Newborn: Ferritin, Serum iron, TIBC |
r = 0.03 (−0.17, 0.23), 0.14 (−0.07, 0.33), 0.03 (−0.17, −0.23) | ||||||
| Maternal: Ht Newborn: Ferritin, Serum iron, TIBC |
r = −0.034 (−0.17, 0.23), 0.075 (−0.13, 0.27) | ||||||
| Maternal: MCHb Newborn: Ferritin, Serum iron, TIBC |
r = 0.056 (−0.15, 0.25), 0.248 (0.05, 0.43), 0.087 (−0.12, 0.28) | ||||||
| Maternal: MCV Newborn: Ferritin, Serum iron, TIBC |
r = 0.001(−0.20, 0.20), 0.238 (0.04, 0.42), 0.077 (−0.13, 0.27) | ||||||
| Lee et al. [81] 2016, USA | Prospective cohort | Sample size: 255 Maternal age (a): NR Malaria setting: No Iron supplementation: NR |
Timing of maternal blood sampling NR Umbilical cord blood at delivery Inflammation biomarkers: CRP, IL-6 Factors adjusted: NR |
Automated haematology analyser or HemoCue system for Hb; ELISA for sTfR, ferritin; method for hepcidin NR | Maternal: Ferritin Newborn: Ferritin |
r = 0.08 (−0.04, 0.20) | 6 |
| Maternal: Hepcidin Newborn: Hepcidin |
r = 0.13 (0.0073, 0.25) | ||||||
| Maternal: sTfR Newborn: sTfR |
r = 0.18 (0.06, 0.30) | ||||||
| Maternal: Hb Newborn: Hb |
r = −0.08 (−0.20, 0.04) | ||||||
| MacPhail et al. [59] 1980, South Africa | Cross-sectional | Sample size: 103 Maternal age (a): 24.7/16–39 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanomethaemoglobin method for Hb; Colorimetric chromogen for serum iron; radioimmunoassay for ferritin;, coated charcoal assay for unsaturated iron-binding capacity | Maternal: Ferritin Newborn: Ferritin, Serum iron, Tf Sat, TIBC |
r = 0.21 (0.02, 0.39), −0.19 (−0.37, 0.003), −0.11 (0.02, 0.25), 0.20 (0.005, 0.38) | 3 |
| Maternal: Serum iron Newborn: Ferritin, Serum iron, Tf Sat, TIBC |
r = 0.07 (0.02, 0.39), 0.21 (0.02, 0.39), 0.18 (−0.02, 0.36) 0.07 (−0.13, 0.26) |
||||||
| Maternal: Tf Sat Newborn: Ferritin, Serum iron, Tf Sat, TIBC |
r = 0.14 (−0.06, 0.32), 0.09 (−0.11, 0.28), 0.10 (−0.10, 0.29), 0.02 (−0.17, 0.21) | ||||||
| Maternal: TIBC Newborn: Ferritin, Serum iron, Tf Sat, TIBC |
r = 0.18 (−0.01, 0.36), 0.11 (−0.09, 0.30), 0.02 (−0.17, 0.21), 0.10 (−0.10, 0.29) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.40 (0.22, 0.55) | ||||||
| Malcolm et al. [60] 1973, Papua New Guinea | Cross-sectional | Sample size: 98 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal blood postpartum Umbilical cord blood at delivery Inflammation biomarkers: IgM, IgA Factors adjusted: NR |
EEL colorimetry | Maternal: Hb Newborn: Hb |
r = 0.32 (0.13, 0.49) | 2 |
| Mezdoud et al. [29] 2017, Algeria | Cross-sectional | Sample size: 97 Maternal age (a): 31.7 ± 4.7/ 22–42 Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Automated counter for Hb and Ht. Colorimetric method for serum iron. ELISA for ferritin | Maternal: Serum iron Newborn: Serum iron |
r = 0.39 (0.21, 0.55) | 4 |
| Maternal: Tf Sat Newborn: Tf Sat |
r = 0.26 (0.06, 0.44) | ||||||
| Maternal: TIBC Newborn: TIBC |
r = 0.20 (−0.0005, 0.38) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.22 (0.22, 0.02, 0.40) | ||||||
| Maternal: Ht Newborn: Ht |
r = 0.60 (0.46, 0.71) | ||||||
| Milman et al. [62] 1987, Denmark | Cross-sectional | Sample size: 85 Maternal age (a): Median: 27/ 15–38 Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Coulter-S for Hb. Radioimmunoassay for ferritin. | Maternal: Ferritin Newborn: Ferritin |
r = 0.36 (0.16, 0.53) | 5 |
| Maternal: Hb Newborn: Ferritin |
r = −0.31 (−0.49, −0.10) | ||||||
| Milman et al. [61] 1988, Denmark | Cross-sectional | Sample size: 78 Maternal age (a): Median: 27/ 16–38 Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Haematofluorometry | Maternal: ZPP Newborn: ZPP |
r = 0.04 (−0.18, 0.26) | 3 |
| Montemagno et al. [63] 1995, UK | Cross-sectional | Sample size: 64 Maternal age (a): NR Malaria setting: No Fe supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Coulter cell ounter | Maternal: Hb Newborn: Hb |
r = 0.22 (−0.03, 0.44) | 4 |
| Maternal: MCV Newborn: MCV |
r = 0.24 (−0.006, 0.46) | ||||||
| Nemet et al. [30] 1986, Hungary | Cross-sectional | Sample size: 156 Maternal age: Fer <10 µg/l: 26.1 ± 5.0, Fer >20 µg/l: 27.2 ± 4.6 Malaria setting: No Iron supplementation: NR |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Ferrozine colour agent for serum iron and TIBC. Radioimmunoassay for ferritin | Maternal: Ferritin Newborn: Ferritin, Serum iron, Tf Sat, TIBC |
r = 0.15 (−0.007, 0.30), 0.09 (−0.07, 0.24), −0.10 (−0.22, −0.02), −0.17 (−0.32, −0.01), | 3 |
| Maternal: Tf Sat Newborn: Ferritin, serum iron, Tf Sat, TIBC |
r = −0.12 (−0.27, 0.04), 0.04 (−0.12, 0.20), 0.04 (−0.12, 0.20), 0.04 (−0.12, 0.20) | ||||||
| Maternal: TIBC Newborn: Ferritin, serum iron, Tf Sat, TIBC |
r = 0.07 (−0.09, 0.22), 0.07 (−0.09, 0.22), 0.05 (−0.11, 0.21), −0.02 (−0.18, 0.14), | ||||||
| Nhonoli et al. [31] 1975, Tanzania | Cross-sectional | Sample size: 580 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Chromogen for serum iron | Maternal: Serum iron Newborn: Serum iron |
r = 0.59 (0.53, 0.64) | 4 |
| Norimah et al. [32] 2010, Malaysia | Cross-sectional | Sample size: 70 Maternal age (a): 25.6 ± 4.9/ 17–40 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethaemoglobin for Hb. Radioimmunoassay for ferritin | Maternal: Ferritin Newborn: Ferritin, Hb |
r = 0.01 (−0.22, 0.25), 0.06 (−0.18, 0.29) | 5 |
| Maternal: Hb Newborn: Ferritin, Hb |
r = 0.06 (−0.18, 0.29), 0.42 (0.21, 0.60) | ||||||
| Maternal: Ht Newborn: Ferritin, Hb |
r = 0.008 (−0.23, 0.24), 0.23 (−0.007, 0.44) | ||||||
| Paiva Ade et al. [64] 2007, Brazil | Cross-sectional | Sample size: 95 Maternal age (a): anaemic: 22.9; iron deficient: 23.1; control: non-iron deficient: 24.8 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Colorimetric method for serum iron. Turbidimetric method for TIBC. Chemiluminescence for ferritin. Haematofluorometry for ZPP. Method for haematologic indices NR |
Maternal: Ferritin Newborn: Ferritin, Tf Sat, TIBC, ZPP, Hb, MCV |
r = 0.07 (−0.13, 0.27), −0.02 (−0.22, 0.18), −0.02 (−0.22, 0.18), 0.10 (−0.10, 0.30) 0.05 (−0.15, 0.25), 0.12 (−0.08, 0.31) | 7 |
| Maternal: Serum iron Newborn: Ferritin, Serum iron, Tf Sat, TIBC, ZPP, Hb, MCV |
r = 0.04 (0.03, 0.44), 0.26 (0.06, 0.44), 0.20 (−0.001, 0.39), −0.08 (−0.39, 0.09), 0.10 (−0.10, 0.30), 0.00 (−0.20, 0.20), −0.11 (−0.30, 0.09) | ||||||
| Maternal: Tf Sat Newborn: Ferritin, Serum iron, Tf Sat, TIBC, ZPP, Hb, MCV |
r = −0.07 (−0.27, 0.13), 0.22 (0.02, 0.40), 0.23 (0.03, 0.41), −0.23 (−0.41, −0.03), −0.03 (−0.17, 0.23), 0.06 (−0.14, 0.26), −0.04 (−0.24, 0.16) | ||||||
| Maternal: TIBC Newborn: Ferritin, serum iron, Tf Sat, TIBC |
r = 0.20 (−0.0001, 0.39), 0.05 (−0.15, 0.25), −0.12 (0.31, 0.08), 0.42 (0.24, 0.57) | ||||||
| Maternal: ZPP Newborn: Ferritin, serum iron, Tf Sat, TIBC, ZPP, Hb, MCV |
r = −0.23 (−0.41, −0.03), −0.13 (−0.32, 0.07), 0.15 (−0.34, 0.05), 0.17 (−0.03, 0.36), −0.04 (−0.16, 0.24), 0.08 (−0.12, 0.28), 0.08 (−0.12, 0.28) | ||||||
| Maternal: Hb Newborn: Serum iron, Tf Sat, TIBC, ZPP, Hb, MCV |
r = 0.02 (−0.18, 0.22), −0.03 (−0.23, 0.17), 0.01 (−0.19, 0.21), 0.08 (−0.12, 0.28), 0.08 (−0.12, 0.28), 0.03 (−0.17, 0.23) | ||||||
| Pope et al. [33] 2014, Australia | Cross-sectional | Sample size: 91 Maternal age (a): 33.7 ± 4.9 Malaria setting: No Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: CRP Factors adjusted: NR |
Biochemistry analyser for serum iron, TIBC; automated haematology analyser for haematologic indices | Maternal: Ferritin Newborn: Ferritin |
r = −0.05 (−0.25, 0.16) | 4 |
| Maternal: Serum iron Newborn: Serum iron |
r = 0.15 (−0.06, 0.34) | ||||||
| Maternal: Tf Sat Newborn: Tf Sat |
r = 0.12 (−0.09, 0.32) | ||||||
| Maternal: TIBC Newborn: TIBC |
r = −0.18 (−0.37, 0.03) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.02 (−0.19, 0.22) | ||||||
| Maternal: MCHb Newborn: MCHb |
r = 0.07 (−0.14, 0.27) | ||||||
| Maternal: MCV Newborn: MCV |
r = −0.09 (−0.29, 0.12) | ||||||
| Qaiser et al. [65] 2013, Pakistan | Cross-sectional | Sample size: 404 Maternal age (a): 15–45 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Standard coultergram using Beckman Coulter Max M | Maternal: Hb Newborn: Hb |
r = 0.12 (0.02, 0.22) | 6 |
| Maternal: Ht Newborn: Ht |
r = 0.23 (0.14, 0.32) | ||||||
| Maternal: MCHb Newborn: MCHb |
r = 0.17 (0.07, 0.26) | ||||||
| Maternal: MCV Newborn: MCV |
r = 0.30 (0.21, 0.39) | ||||||
| Ramirez-Cardich et al. [34] 2004, Peru | Cross-sectional | Sample size: 36 Maternal age (a): 28.1 ± 1.1 Malaria setting: Yes Iron supplementation: NR |
Maternal blood <12 h prior to delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Microcapillary method | Maternal: Ht Newborn: Ht |
r = −0.57 (−0.76, −0.30) | 5 |
| Rioux et al. [85] 2001, Canada | Retrospective cohort | Sample size: 952 Maternal age (a): NR Malaria setting: No Iron supplementation: NR |
Maternal blood collected at 1st, 2nd and 3rd trimester and 12 hrs postpartum Neonatal blood <48 hrs after delivery Inflammation biomarkers: NR Factors adjusted: NR |
NR | Maternal: Hb Newborn: Hb, Ht |
r = 0.13 (0.07, 0.19), 0.08 (0.02, 0.14) | 5 |
| Maternal: Ht Newborn: Hb, Ht |
r = 0.10 (0.04, 0.16), 0.08 (0.02, 0.14) | ||||||
| Rusia et al. [35] 1996, India | Cross-sectional | Sample size: 100 Maternal age (a): 17–39 Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Automated particle counter for haematologic indices. ELISA for ferritin Method for serum iron NR |
Maternal: Ferritin Newborn: Ferritin |
r = 0.14 (−0.06, 0.32) | 4 |
| Maternal: Serum iron Newborn: Serum iron |
r = 0.44 (0.27, 0.58) | ||||||
| Maternal: Tf Sat Newborn: Tf Sat |
r = 0.30 (0.11, 0.47) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.41 (0.23, 0.56) | ||||||
| Shao et al. [66] 2012, China | Cross-sectional | Sample size: 3891 Maternal age (a): 26.4 ± 3.6/ 20–35 Malaria setting: Yes Iron supplementation: NR |
Timing of maternal blood collection NR Umbilical cord blood at delivery Inflammation biomarkers: CRP Factors adjusted: NR |
Auto analyser for haematologic indices. Chemiluscent assay for ferritin. | Maternal: Ferritin Newborn: Ferritin, Hb |
r = 0.07 (0.04, 0.10), 0.01 (−0.02, 0.04) | 6 |
| Maternal: Hb Newborn: Hb |
r = 0.10 (0.07, 0.13) | ||||||
| Shukla et al. [83] 2019, India | Prospective cohort | Sample size: 163 Maternal age (a): NR Malaria setting: Yes Fe supplementation: NR |
Timing of maternal blood collection NR Venous blood at 14 weeks Inflammation biomarkers: NR Factors adjusted: NR |
NR | Maternal: Ferritin Newborn: Ferritin |
r = 0.23 (0.08, 0.37) | 7 |
| Maternal: Hb Newborn: Hb |
r = 0.23 (0.08, 0.37) | ||||||
| Sichieri et al. [67] 2006, Brazil | Cross-sectional | Sample size: 82 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood; timing NR Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Plasma-atomic emission spectroscopy for serum iron; Automated analyser for Hb and Ht | Maternal: Serum iron Newborn: Serum iron |
r = 0.21 (−0.007, 0.41) | 4 |
| Maternal: Hb Newborn: Hb |
r = 0.04 (−0.18, 0.25) | ||||||
| Maternal: Ht Newborn: Ht |
r = 0.15 (−0.07, 0.36) | ||||||
| Sikorsi et al. [68] 1998, Poland | Cross-sectional | Sample size: 100 Maternal age (a): 15–42 Malaria setting: No Iron supplementation: NR |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Atomic absorption spectroscopy | Maternal: Serum iron Newborn: Serum iron |
r = 0.08 (−0.12, 0.27) | 3 |
| Singla et al. [36] 1978, India | Cross-sectional | Sample size: 85 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
NR | Maternal: Serum iron Newborn: Serum iron |
r = 0.41 (0.22, 0.58) | 5 |
| Maternal: Tf Sat Newborn: Tf Sat |
r = 0.33 (0.12, 0.50) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.73 (0.61, 0.82) | ||||||
| Srivastava et al. [37] 2002, India | Cross-sectional | Sample size: 54 Maternal age (a) NR Malaria setting: Yes Iron supplementation: NR |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Flame atomic absorption spectroscopy | Maternal: Serum iron Newborn: Serum iron |
r = −0.02 (−0.29, 0.25) | 5 |
| Tamura et al. [82] 1999, USA | Prospective cohort | Sample size: 255 Maternal age (a): Mothers of female neonates: 24.6 ± 4.3; mothers of male neonates: 24.1 ± 4.3 Malaria setting: No Iron supplementation: All women |
Maternal blood at 10–36 weeks Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: Maternal race, age, height, prepreg weight and BMI, smoking, alcohol |
Radio-immunoassay | Maternal: Ferritin Newborn: Ferritin |
r = 0.32 (0.21, 0.43) (male babies), r = 0.09 (−0.03, 0.21) (female babies) | 4 |
| Tekinalp et al. [69] 1996, Turkey | Cross-sectional | Sample size: 76 Maternal age (a): NR Malaria setting: Yes Iron supplementation: NR |
Maternal venous blood postpartum Peripheral vein at delivery Inflammation biomarkers: NR Factors adjusted: NR |
ELISA | Maternal: Ferritin Newborn: Ferritin |
r = 0.16 (−0.06, 0.38) (anaemic mothers), r = 0.33 (0.12, 0.52) (non-anaemic mothers) | 3 |
| Terefe et al. [38] 2015, Ethiopia | Cross-sectional | Sample size: 89 Maternal age (a): Median age 23; IQR 21–27 Malaria setting: Yes Iron supplementation: Some women |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: CRP Factors adjusted: NR |
Automated Cobas for ferritin, Automated analyser for haematologic indices | Maternal: Ferritin Newborn: Ferritin, Hb, MCHb, MCV |
r = 0.38 (0.19, 0.55), 0.28 (0.08, 0.46), 0.10 (−0.11, 0.30), −0.05 (−0.26, 0.16) | 6 |
| Maternal: Hb Newborn: Ferritin, Hb, MCHb, MCV |
r = 0.25 (0.04, 0.44), 0.22 (0.01, 0.41), 0.15 (−0.06, 0.35), 0.06 (−0.15, 0.26) | ||||||
| Timilsina et al. [70] 2018, Nepal | Cross-sectional | Sample size: 114 Maternal age (a): 26.0 ± 3.5 Malaria setting: Yes Iron supplementation: All women |
Maternal venous blood when presenting for delivery Umbilical cord 2 min after delivery Inflammation biomarkers: NR Factors adjusted: NR |
Haematology analyser | Maternal: Hb Newborn: Hb |
r = 0.50 (0.34, 0.62) | 6 |
| Maternal: Ht Newborn: Ht |
r = 0.11 (−0.08, 0.29) | ||||||
| Maternal: MCHb Newborn: MCHb |
r = 0.48 (0.32, 0.61) | ||||||
| Maternal: MCV Newborn: MCV |
r = 0.06 (−0.13, 0.24) | ||||||
| Vahlquist et al. [71] 1975, Sweden | Cross-sectional | Sample size: 49 (1 pair of twins included) Maternal age (a): NR Malaria setting: No Iron supplementation: NR |
Timing of maternal blood collection NR Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Radioimmunodiffusion | Maternal: Tf Sat Newborn: Tf Sat |
r = 0.21 (−0.08, 0.46) | 2 |
| Vasquez-Molina et al. [72] 1982, Mexico | Cross-sectional | Sample size: 163 Maternal age (a): NR Malaria setting: Yes Iron supplementation: All women |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethaemoglobin for Hb. Microcapillary for Ht. Chemiluminometric assay for ferritin | Maternal: Ferritin Newborn: Ferritin |
r = 0.14 (−0.01, 0.29) | 5 |
| Maternal: Hb Newborn: Hb |
r = 0.11 (−0.04, 0.26) | ||||||
| Maternal: Ht Newborn: Ht |
r = 0.09 (−0.06, 0.24) | ||||||
| Vobecky et al. [73] 1982, Canada | Cross-sectional | Sample size: 556 Maternal age (a): 26.3 ± 4.2/ 15–43 Malaria setting: No Iron supplementation: NR |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethaemoglobin for Hb. Photometric determination for serum iron; method for Ht NR | Maternal: Serum iron Newborn: Serum iron |
r = −0.03 (95%CI −0.11, 0.05) | 5 |
| Maternal: Hb Newborn: Hb |
r = 0.08 (−0.003, 0.16) | ||||||
| Maternal: Ht Newborn: Ht |
r = 0.14 (0.06, 0.22) | ||||||
| Wong et al. [39] 1990, Singapore | Cross-sectional | Sample size: 72 Maternal age (a): 28.1 ± 4.9/ 16–41 Malaria setting: No Iron supplementation: All women |
Maternal blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
ELISA | Maternal: Ferritin Newborn: Ferritin |
Maternal 17.4 ± 12.5 µg/L; Newborn 142±68.6 µg/L | 4 |
| Wong et al. [40] 1991, Singapore | Cross-sectional | Sample size: 352 Maternal age (a): NR Malaria setting: No Iron supplementation: NR |
Maternal venous blood at delivery Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Rocket immunoelectrophoresis | Maternal: TF Newborn: TF |
r = 0.0064 (−0.10, 0.11) | 2 |
| Yepez et al. [74] 1987, Ecuador | Cross-sectional | Sample size: 84 Maternal age (a): 20.2 ± 3.3 Malaria setting: Yes Iron supplementation: None |
Maternal venous blood during labour Umbilical cord blood at delivery Inflammation biomarkers: NR Factors adjusted: NR |
Cyanmethaemoglobin for Hb. Microcentrifugation for Ht. Colorimetric technique for serum iron. ELISA for ferritin | Maternal: Ferritin Newborn: Hb |
r = 0.25 (0.04, 0.44) | 5 |
| Maternal: Serum iron Newborn: Serum iron |
r = 0.26 (0.05, 0.45) | ||||||
| Maternal: Hb Newborn: Hb |
r = 0.28 (0.07, 0.47) |
a, years; BMI, body mass index; CRP, C-reactive protein; ELISA, enzyme-linked immunoassay; Hb, haemoglobin; Ht, haematocrit; MCHb- mean corpuscular haemoglobin; MCV, mean corpuscular/cell volume; NR, not reported; sTfR, serum/soluble transferrin receptor; Tf, transferrin; Tf Sat, transferrin saturation; TIBC, total iron binding capacity; ZPP, zinc protoporphyrin.
Sample sizes ranged from 28 to 3981 participants and the mean maternal age across studies was 26 years. Fifteen studies[25,26,28,38,39,52,56,59,62,64,66,70,72,76,82] reported that some or all the pregnant women had received iron supplementation during pregnancy.
3.3 Risk of bias of included studies
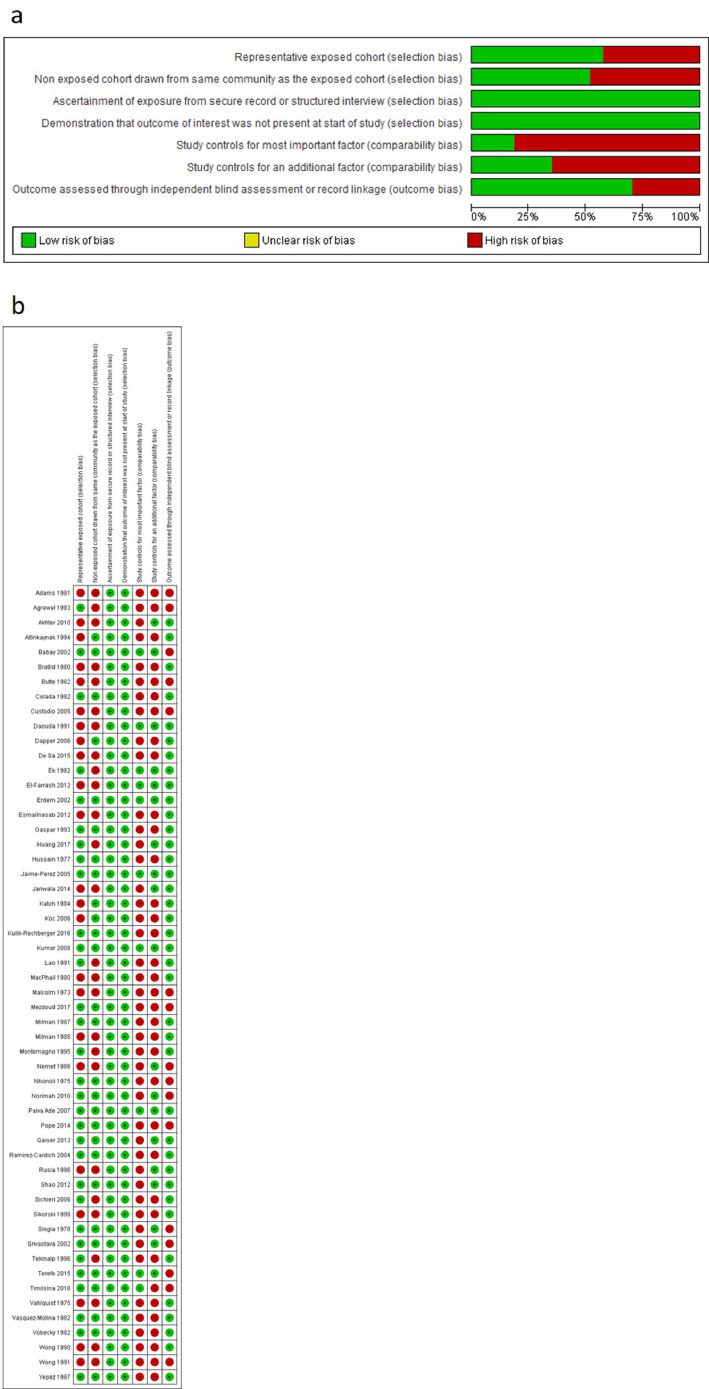
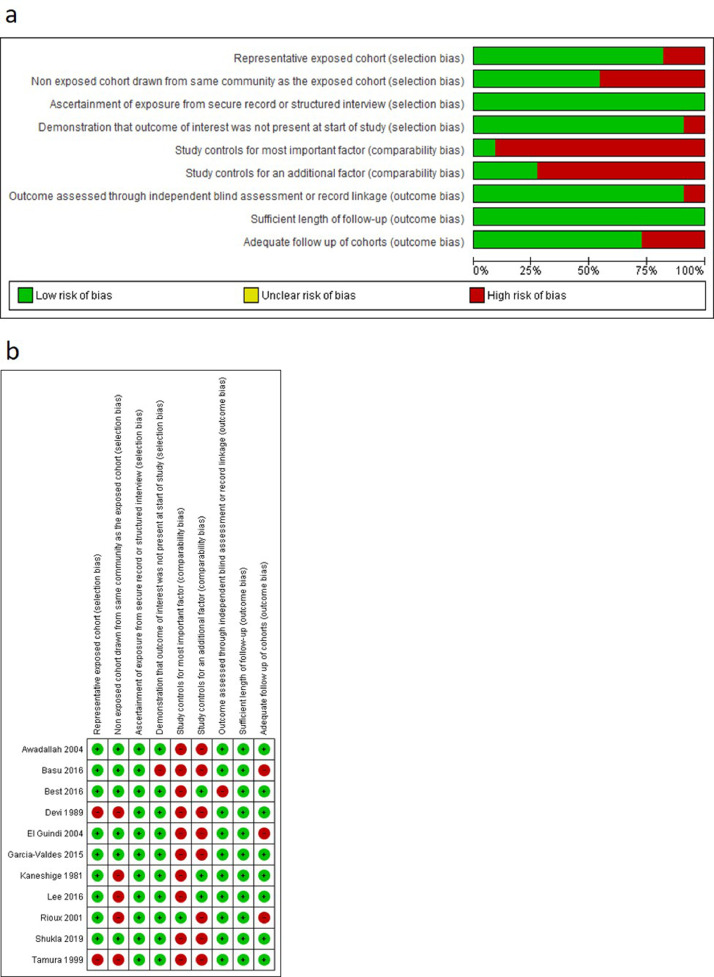
The risk of bias of individual studies is summarised in Fig. 2, Fig. 3. The risk of bias was low in seven studies [48,52,57,64,75,80,83], moderate in 28 studies) [24,26–28,32,34,36–38,42,50,51,56,61,65,66,70,72–74,[76], [77], [78], [79],81,87,84,85] and high in 30 studies [21,23,25,[29], [30], [31],33,35,39–41,[43], [44], [45], [46], [47],49,[53], [54], [55],58–63,67,69,71,82]. Overall, cross-sectional studies had a high risk of bias in the two comparability domains, moderate risk of bias in the domains relating to representativeness of exposed cohort and selection of non-exposed cohort, low risk of bias in domains that related to ascertainment of exposure and demonstration that the outcome of interest was not present at the start of study. Conversely, cohort studies had a high risk of bias in the domains that related to comparability bias (i.e. whether studies controlled for important factors), moderate risk of bias in the domains that assessed selection of non-exposed cohort and loss of cohort to follow-up. Finally, there was a low risk of bias amongst cohort studies in the domains that assessed representativeness of exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at start of study, independent blind assessment of outcome and whether follow-up time was sufficient for outcome to occur. Overall, the quality of the evidence to inform the association between maternal and neonatal iron status and haematologic indices was very low (see Supplementary Table 2 for a Summary of GRADE evidence profile).
Fig. 2.
Risk of bias assessments of included cross-sectional studies. (A) Review author judgments about the risk for each bias item presented as percentages across all included studies. (B) Review author judgments about the risk for each bias item in all included studies.
Fig. 3.
Risk of bias assessments of included cohort studies. (A) Review author judgments about the risk for each bias item presented as percentages across all included studies. (B) Review author judgments about the risk for each bias item in all included studies.
3.4 Maternal iron status and neonatal indices
A summary of correlations between maternal iron status biomarkers and neonatal iron and haematological indices is shown in Table 2.
Table 2.
Summary of Correlations Between Maternal Iron Biomarkers and Neonatal Iron and Haematological Indices.
| Mother | Neonate | Number of Studies and References | Pooled weighted mean correlation coefficient (95% CI)(Schmidt-Hunter) |
|---|---|---|---|
| Ferritin (n = 29) | Ferritin | 27[23,27,30,32,33,35,38,41,[43], [44], [45],48,51,57,58,59,62,64,66,69,72,76,77,[80], [81], [82], [83]] | 0.14 (0.07, 0.20) – Negligible P<0.0001 |
| Hepcidin | 1[76] | Not calculated | |
| Plasma/serum iron | 6[27,30,58,59,64,76] | 0.21 (−0.02, 0.45) – Negligible P = 0.08 |
|
| Tf Sat | 5[27,30,59,64,76] | 0.10 (−0.19, 0.39) – Negligible P = 0.50 |
|
| TIBC | 6[27,30,58,59,64,76] | −0.09 (−0.33, 0.14) – Negligible P = 0.43 |
|
| ZPP | 1[64] | Not calculated | |
| Hb | 8[27,32,38,57,64,66,74,76] | 0.05 (−0.05, 0.15) – Negligible P = 0.32 |
|
| MCHb | 2[27,38] | Not calculated | |
| MCV | 3[27,38,64] | 0.15 (−0.06, 0.35) – Negligible P = 0.16 |
|
| Hepcidin (n = 4) | Ferritin | 1[76] | Not calculated |
| Hepcidin | 4[76,79,77,81] | 0.42 (0.18, 0.66) – Low positive P = 0.001 |
|
| Plasma/serum iron | 1[76] | Not calculated | |
| Tf Sat | 1[76] | Not calculated | |
| TIBC | 1[76] | Not calculated | |
| Hb | 1[76] | Not calculated | |
| Serum Iron (n = 23) | Ferritin | 5[27,58,59,64,76] | 0.33 (0.13, 0.52) – Low positive P = 0.001 |
| Hepcidin | 1[76] | Not calculated | |
| Plasma/serum iron | 23[22,27,29,31,33,[35], [36], [37],46,50,[53], [54], [55],[57], [58], [59],64,67,68,73,74,76,80] | 0.30 (0.19, 0.40) – Low positive P<0.0001 |
|
| Tf Sat | 4[27,59,64,76] | 0.35 (0.12, 0.58) – Low positive P = 0.003 |
|
| TIBC | 5[27,58,59,64,76] | −0.15 (−0.39, 0.09) – Negligible P = 0.22 |
|
| ZPP | 1[64] | Not calculated | |
| Hb | 4[27,54,64,76] | 0.42 (0.09, 0.75) – Low positive P = 0.01 |
|
| MCHb | 1[27] | Not calculated | |
| MCV | 2[27,64] | Not calculated | |
| sTfR (n = 2) | sTfR | 2[77,81] | Not calculated |
| Tf (n = 3) | Tf | 3[42,40,71] | 0.09 (−0.08, 0.26) – Negligible P = 0.28 |
| Tf Sat (n = 9) | Ferritin | 4[30,59,64,76] | 0.07 (−0.23, 0.38) – Negligible P = 0.64 |
| Hepcidin | 1[76] | Not calculated | |
| Plasma/serum iron | 4[30,59,64,76] | 0.18 (−0.05, 0.41) – Negligible P = 0.12 |
|
| Tf Sat | 9[29,30,33,35,36,55,59,64,76] | 0.20 (0.08, 0.31) – Negligible P = 0.001 |
|
| TIBC | 4[30,59,64,76] | 0.06 (−0.22, 0.34) – Negligible P = 0.69 |
|
| ZPP | 1[64] | Not calculated | |
| Hb | 2[64,76] | Not calculated | |
| MCV | 1[64] | Not calculated | |
| TIBC (n = 8) | Ferritin | 5[30,58,59,64,76] | 0.04 (−0.21, 0.29) – Negligible P = 0.75 |
| Serum Iron | 5[30,58,59,64,76] | 0.02 (−0.20, 0.24) – Negligible P = 0.85 |
|
| Tf Sat | 4[30,59,64,76] | −0.09 (−0.34, 0.16) – Negligible P = 0.47 |
|
| TIBC | 8[29,30,33,55,58,59,64,76] | 0.16 (0.01, 0.31) – Negligible P = 0.03 |
|
| ZPP | 2[64,76] | Not calculated | |
| Hb | 1[76] | Not calculated | |
| MCV | 1[64] | Not calculated | |
| ZPP (n = 2) |
Ferritin | 1[64] | Not calculated |
| Plasma/serum iron | 1[64] | Not calculated | |
| Tf | 1[61] | Not calculated | |
| Tf Sat | 1[64] | Not calculated | |
| TIBC | 1[64] | Not calculated | |
| ZPP | 1[64] | Not calculated | |
| Hb | 2[61,64] | Not calculated | |
| MCV | 1[64] | Not calculated |
Hb=haemoglobin; MCHb=mean corpuscular haemoglobin; MCV=mean cell volume; NOS=Newcastle-Ottawa scale;.
sTfR=soluble/serum transferrin receptor; TIBC=total iron binding capacity; Tf=transferrin; Tf Sat=transferrin saturation; ZPP= zinc protoporphyrin.
3.4.1 Ferritin
Thirty studies assessed ferritin levels in pregnant women. Negligible pooled correlation were found for maternal ferritin levels in relation to newborn ferritin, 0.14 (95%CI 0.07, 0.20; n = 27 studies [23,27,30,32,33,35,38,41,43–45,48,51,57–59,62,64,66,69,72,76,77,80–83]; I2=93.7%), serum/plasma iron (0.21: 95%CI −0.02, 0.45; n = 6 studies [27,30,59,64,76]; I2=94.8), Tf Sat (0.10: 95%CI −0.09, 0.39; n = 5 studies [27,30,58,59,64,76]; I2=96.6%), TIBC (−0.09: 95%CI −0.33, 0.14; n = 6 studies[27,30,58,59,64,76]); I2=93.7%), Hb (0.05: 95%CI −0.05, 0.15; n = 8 studies [27,32,38,57,64,66,74,76]; I2=95.4%), and MCV (0.15: 95%CI −0.06, 0.35; n = 3 studies [27,38,64]; I2=77.8% Supplementary Fig. 1a–f). Pooled estimates for correlations between maternal ferritin and newborn hepcidin (0.44; n = 1 study [76]), MCHb (0.5027 and 0.1038; n = 2 studies), and ZPP (0.10; n = 1 study [64]) are not summarised in tables or supplementary figures because they comprise less than three studies. Pooled estimates remained negligible and heterogeneity remained high after sub-group analysis of maternal versus newborn ferritin by study design (cross-sectional [n = 21] versus prospective [n = 6] cohort studies; data available upon request). Similarly, the strength of correlation between maternal and neonatal ferritin remained unchanged after subgroup analysis by timing of maternal blood draw (before birth [n = 21] versus after birth [n = 6]; data available upon request).
3.4.2 Hepcidin
Four studies assessed hepcidin levels in pregnant women.[76,77,79,81] There was a low positive pooled correlation for maternal versus newborn hepcidin (0.42 (95%CI 0.18, 0.66; I2=96.6% Supplementary Fig. 2). Pooled estimates for correlations between maternal hepcidin and newborn ferritin (0.37; n = 1 study [76]), serum iron (0.37; n = 1 study [76]), Tf Sat (0.33; n = 1 study [76]), TIBC (−0.24; n = 1 study [76]), and Hb (0.39; n = 1 study [76]) are not summarised in tables or supplementary figures because they comprise less than three studies.
3.4.3 Serum/ plasma iron
Twenty-three studies assessed serum/plasma iron in pregnant women [22,27,29,31,33,35–37,46,50,[53], [54], [55], 57–59,64,67,68,73,74,76,80]. Low positive pooled correlations were identified for comparisons between maternal serum/plasma iron versus serum ferritin (0.33: 95%CI 0.13, 0.52; n = 5 studies [27,58,59,64,76]); I2=91.8%), newborn serum/plasma iron (0.30: 95%CI 0.19, 0.40; I2=91.8%), Tf Sat (0.35: 95%CI 0.12, 0.58; n = 4 studies [27,59,64,76]; I2=93.2%) and Hb (0.42: 95%CI 0.09, 0.75; n = 4, studies [27,54,64,76]; I2=93.4%) (Supplementary Fig. 3). A negligible pooled correlation was identified between maternal serum/plasma iron and newborn TIBC (−0.15: 95%CI −0.39, 0.09; n = 5 studies [27,58,59,64,76]; I2=91.6%). Pooled estimates for correlations between maternal serum/plasma iron and newborn hepcidin (0.40; n = 1 study [76]), ZPP (0.1; n = 1 study [64]), MCHb (0.35; n = 1 study [27]), and MCV (0.3327 and −0.1164; n = 2 studies) are not summarized in tables or supplementary figures because they comprise less than three studies. Subgroup analysis between maternal and neonatal serum iron concentrations according to the timing of maternal blood draw revealed a negligible correlation [0.28 (95%CI 0.17, 0.39)] when studies that reported maternal blood draw before delivery alone were included (n = 19), but were moderate [0.57 (95%CI 0.38, 0.76)] when studies that reported maternal blood draw after delivery alone (n = 3) were included.
3.4.4 sTfR
Two studies assessed the correlation between maternal and newborn sTfR [77,81]. The correlation coefficients reported in the two studies were negligible (0.2377 and 0.1881), and are not summarized in Tables or Supplementary Figures because they comprise less than three studies.
3.4.5 Tf
Three studies assessed the correlation between maternal Tf levels and newborn Tf levels [40,42,71]. The pooled correlation for maternal versus newborn Tf levels from the three studies was negligible (0.09: 95%CI −0.08, 0.26; I2=83.2%) (Supplementary Fig. 4).
3.4 .6 Tf sat
Nine studies assessed Tf Sat levels in pregnant women.[29,30,33,35,36,55,59,64,76] Negligible pooled correlations were found for maternal versus newborn ferritin (0.07: 95%CI −0.23, 0.38; n = 4 studies [30,59,64,76]; I2=96.5%), serum iron (0.18: 95%CI −0.05, 0.41; n = 4 studies [30,59,64,76]); I2=92.8%), Tf Sat 0.20 (95%CI 0.08, 0.31; n = 9 studies [29,30,33,35,36,55,59,64,76]; I2=85.2%), and TIBC (0.06: 95%CI −0.22, 0.34; n = 4 studies [30,59,64,76]; I2=94.4%) (Supplementary Fig. 5). Pooled estimates of correlation between maternal Tf Sat and newborn hepcidin (0.35; n = 1 study [76]), ZPP (−0.03; n = 1 study [64]), Hb (0.84476 and 0.0664; n = 2 studies), and MCV (−0.04; n = 1 study [64]) are not summarized in tables or supplementary figures because they comprise less than three studies.
3.4.7 TIBC
Eight studies assessed TIBC levels in pregnant women.[29,30,33,55,58,59,64] Negligible pooled correlations were found for maternal TIBC in relation to newborn ferritin (0.04: 95%CI −0.21, 0.29; n = 5 studies [30,58,59,64,76]); I2=94.4%), serum iron (0.02: 95%CI −0.20, 0.24; n = 5 studies [30,58,59,64,76]); I2=91.1%), Tf Sat (−0.09: 95%CI −0.34, 0.16; n = 4 studies [30,59,64,76]; I2=93.1%), and TIBC 0.16 (95%CI 0.01, 0.31; n = 8 studies; I2=88.5%) (Supplementary Fig. 6). Pooled estimates of correlation between maternal Tf Sat and newborn ZPP (−0.40 [76] and 0.1564; n = 2 studies), Hb (−0.838; n = 1 study [76]), and MCV (−0.01; n = 1 study [64]) are not summarized in tables or supplementary figures because they comprise less than three studies.
3.4.8 ZPP
Two studies assessed ZPP levels in pregnant women [61,64]. The correlation between maternal ZPP and the following biomarkers in newborns were reported: ferritin (−0.23; n = 1 study [64]), serum iron (−0.13; n = 1 study [64]), Tf (0.44; n = 1 study [61]), Tf Sat (−0.15; n = 1 study [64]), TIBC (0.17; n = 1 study [64]), ZPP (0.04; n = 1 study [64]), Hb (0.3961 and 0.0864; n = 2 studies), and MCV (0.08; n = 1 study [64]).
3.5 Maternal haematological and neonatal indices
A summary of correlations between maternal haematologic indices and neonatal iron and haematological indices is shown in Table 3.
Table 3.
Summary of correlations between maternal haematological indices and neonatal iron and haematological indices.
| Mother | Neonate | Number of Studies and References | Pooled weighted mean correlation coefficient (95% CI)(Schmidt-Hunter) |
|---|---|---|---|
| Hb (n = 32) | Ferritin | 6[23,32,38,58,62,76] | 0.16 (−0.12, 0.43) – Negligible P = 0.27 |
| Hepcidin | 1[76] | Not calculated | |
| Plasma/serum iron | 5 [25,54,58,64,76] | 0.29 (0.04, 0.54) – Negligible P = 0.02 |
|
| sTfR | 2[56,35] | Not calculated | |
| Tf Sat | 3[27,64,76] | 0.35 (−0.05, 0.75) – Low positive P = 0.09 |
|
| TIBC | 4[25,58,64,76] | −0.22 (−0.52, 0.08) – Negligible P = 0.16 |
|
| ZPP | 1[64] | Not calculated | |
| Hb | 32[21,[26], [27], [28], [29],32,33,35,36,38,42,43,47,49,54,57,59,[63], [64], [65]-67,70,[72], [73], [74],76,78,81,83,85,88] | 0.15 (0.10, 0.20) – Negligible P<0.0001 |
|
| Ht | 3[25,28,85] | 0.13 (0.007, 0.25) – Negligible P = 0.04 |
|
| MCHb | 2[27,38] | Not calculated | |
| MCV | 3[27,38,64] | 0.20 (−0.07, 0.46) – Negligible P = 0.14 |
|
| Ht (n = 13) | Ferritin | 2[32,58] | Not calculated |
| Plasma/serum iron | 1[58] | Not calculated | |
| sTfR | 1[24] | Not calculated | |
| TIBC | 1[58] | Not calculated | |
| Hb | 3[28,32,85] | 0.14 (0.04, 0.25) – Negligible P = 0.01 |
|
| Ht | 10[28,29,34,47,65,67,70,72,73,85] | 0.15 (0.06, 0.23) – Negligible P = 0.001 |
|
| MCV (n = 7) | Ferritin | 1[58] | Not calculated |
| Plasma/serum iron | 1 [58] | Not calculated | |
| TIBC | 1 [58] | Not calculated | |
| MCV | 6[33,42,47,63,65,70] | 0.22 (0.10, 0.33) – Negligible P = 0.0002 |
|
| MCHb (n = 6) | Ferritin | 1[58] | Not calculated |
| Plasma/serum iron | 1[58] | Not calculated | |
| TIBC | 1[58] | Not calculated | |
| MCHb | 5[33,42,47,65,70] | 0.25 (0.08, 0.43) – Negligible P = 0.004 |
Hb=haemoglobin; Ht=haematocrit; MCHb=mean corpuscular haemoglobin; MCV=mean cell volume; NOS=Newcastle-Ottawa scale; sTfR=soluble/serum transferrin receptor; TIBC=total iron binding capacity; Tf=transferrin; Tf Sat=transferrin saturation; ZPP= zinc protoporphyri.
3.5.1 Hb
Thirty-two studies assessed Hb levels in pregnant women. [21,26–29,32,33,35,36,38,42,43,47,49,54,57,59,60,63–67,70,72–74,76,78,81,83,85] There was negligible pooled correlation between maternal Hb and newborn: ferritin (0.16: 95%CI −0.12, 0.43; n = 6 studies [23,32,38,58,62,76]); I2=95.5%), serum iron (0.29: 95%CI 0.04, 0.54; n = 5 studies [25,54,58,64,76]; I2=93.6%), TIBC (−0.22: 95%CI −0.52, 0.08; n = 4 studies [25,58,64,76]; I2=93.3%), Hb (0.15: 95%CI 0.10, 0.20; n = 32 studies [21,26,29,32,33,35,36,38,42,43,47,49,54,57,59,[63], [64], [65], [66], [67],70,72–74,76,78,81,83,85,88], I2=89.1%), Ht (0.13: 95%CI −0.007, 0.25; n = 3, studies [25,28,85]; I2=86.4%), and MCV (0.20: 95%CI −0.07, 0.46; n = 3 studies [27,38,64]; I2=88.6%) (Supplementary Fig. 7). There was a low positive pooled correlation for maternal Hb versus newborn Tf Sat (0.35: 95%CI −0.05, 0.75; n = 3 studies[27,64,76]; I2=96.4%). Pooled estimates of correlations between maternal Hb and newborn hepcidin (0.556; n = 1 study [76]), sTfR (0.29835 and 0.3656; n = 2 studies), ZPP (0.08; n = 1 study [64]), and MCHb (0.56327 and 0.1538; n = 2 studies) are not summarized in tables or supplementary figures because they comprise less than three studies.
Sub-group analysis by study design had no impact on pooled estimates and heterogeneity for maternal versus newborn Hb (data available upon request). Pooled mean differences between newborns of mothers with anaemia (Hb <110 g/L) and without anaemia (≥110 g/L) across 10 studies [22,25,32,35,36,48,52,57,75,76], was −15.26 (95% CI −27.89, −2.63); however, there was very high heterogeneity across these studies (I2=97%). The strength of correlation between maternal and neonatal Hb was negligible (0.14 [95%CI 0.09, 0.19] when only studies in which maternal blood was drawn before delivery were included (n = 25) but upgraded to low (0.30 [95% CI 0.08, 0.53]) when studies in which maternal blood was drawn after delivery were included (n = 6).
3.5.2 Ht
Thirteen studies assessed haematocrit levels in pregnant women [24,28,29,32,34,47,58,65,67,70,72,73,85]. There was negligible pooled correlation between maternal Ht and newborn Hb: (0.14: 95%CI 0.04, 0.25; n = 3, studies [28,32,85]; I2=82.7%) and Ht (0.15 (95%CI 0.06, 0.23; n = 10 studies [28,29,34,47,65,67,70,72,73,85]; I2=85.6%) (Supplementary Fig. 8). Pooled estimates of correlations between maternal Ht and newborn ferritin (−0.0358 and 0.0132; n = 2 studies), serum iron (0.08; n = 1 study [58]), sTfR (0.14; n = 1 study [24]), and TIBC (0.01; n = 1 study [58]) are not summarized in tables or supplementary figures because they comprise less than three studies.
3.5.3 MCHb
Six studies assessed MCHb levels in pregnant women.[33,42,47,58,65,70] A negligible pooled correlation was found for maternal versus newborn MCHb (0.25: 95%CI 0.08, 0.43; n = 5 studies. [33,42,47,65,70]; I2=89.7%) (Supplementary Fig. 9). Pooled estimates of correlations between maternal MCHb and newborn ferritin (0.06; n = 1 study [58]), serum iron (0.25; n = 1 study [58]) and TIBC (0.09; n = 1 study [58]) are not summarized in tables or supplementary figures because they comprise less than three studies.
3.5.4 MCV
Seven studies assessed MCV levels in pregnant women [33,42,47,58,63,65,70]. A negligible pooled correlation was found for maternal versus newborn MCV 0.22 (95%CI 0.10, 0.33; n = 6 studies[33,42,47,63,65,70]; I2=70.1%) (Supplementary Fig. 10). Pooled estimates of correlations between maternal MCV and newborn and ferritin (0.001; n = 1 study [58]), serum iron (0.24; n = 1 study [58]) and TIBC (0.08; n = 1 study [58]) are not summarized in tables or supplementary figures because they comprise less than three studies.
In addition to forest plots showing pooled correlations for maternal versus newborn biomarkers (Supplementary Figs. 1–10), funnel plots to explore publication bias (where n>3 studies) are presented as Supplementary Figs. 11–19. Symmetry was observed in the majority of funnel plots except for studies assessing correlations between maternal ferritin and newborn Hb, maternal versus newborn hepcidin, maternal serum iron versus newborn ferritin, Tf Sat, TIBC and Hb, maternal Tf Sat versus newborn TIBC, maternal TIBC versus newborn ferritin, serum iron and Tf Sat as well as maternal Hb versus newborn TIBC. The asymmetry observed with these biomarker combinations suggests publication bias.
Discussion
This systematic review of 65 studies evaluating the relationships between maternal and neonate haematological/iron status indices showed overall negligible correlations. The negligible correlations between maternal and offspring haematological indices may be attributed, at least in part, to a lack of information regarding the cause of anaemia. Approximately half the cases of anaemia worldwide are attributed to ID [89], with a preponderance of affected people in low- and middle-income countries, where other causes (e.g. infection, nutritional deficiencies) are also prevalent. Thus, assessments of maternal haematological indices must be made not only in conjunction with iron status biomarkers [3], but also screen for other causes (i.e. malaria, inflammation) to improve predictive ability of these indices in neonates. In addition, routine assessment of cord blood haematologic indices may also be warranted to identify at-risk neonates whose mothers may have no abnormalities found in screening.
Advances in the understanding of iron metabolism in pregnancy, and increased assay reliability and availability, has led to relatively recent studies reporting iron biomarkers in maternal and cord/neonatal blood, albeit they are few. Unfortunately, the data was insufficient for sTfR to be assessed. Of those iron biomarkers with at least three qualifying studies, maternal serum iron was the strongest predictor for foetal iron and haematologic indices, albeit these correlations were considered low positive, and varied based on the timing of maternal blood sampling. Despite a paucity of data, it is interesting to note that indices of maternal serum iron transport, including Tf Sat, serum ferritin and TIBC had negligible correlations with newborn indices, underscoring a complex relationship between maternal iron storage and placental/foetal iron delivery. Numerous health agencies, including the World Health Organization, recommend routine screening for ID anaemia in pregnant women, for which serum ferritin is the first-line iron status indicator and serum iron plays an ancillary diagnostic role (i.e. when ferritin assay results are ambiguous) [90].
Recent studies have demonstrated the usefulness of serum hepcidin and ferritin in the assessment of iron status.[4] Hepcidin is a principal regulator of plasma iron concentrations; it inhibits iron efflux from gut enterocytes and reticuloendothelial cells by binding and inhibiting the export channel ferroportin [91]. However, as an acute phase protein like ferritin, corrections for inflammation or the use of composite metrics (e.g. total body iron) that mitigate the confounding effects of inflammation are needed [92], especially due to lability of pro- and anti-inflammatory mediators throughout pregnancy [93]. Notwithstanding, the capacity of maternal total body iron to predict iron status in the foetus and neonate remains an open question and requires validation. The absence of correction for inflammation may explain the lack of maternal ferritin correlations with any foetal iron indices. Interestingly, maternal hepcidin, an important mediator of iron sequestration during infection [94], shows a significant correlation with foetal hepcidin only, accounting for 18% of variance of offspring levels. The extent to which this reflects similar iron stores, inflammation or a coordinated response to infection is unclear and requires further study, especially due to the low number of studies (n = 4) available to generate this correlation.
The present systematic review identifies a clear knowledge gap in the assessment of iron status and anaemia in the foetus and neonate. Notwithstanding the mechanisms governing the interaction between maternal and foetal iron metabolism, the low positive correlations suggest that maternal haematological indices and biomarkers of iron status are poor surrogates of foetal and neonate iron and haematologic status. However, recognition of notable challenges may guide future study design to address these knowledge gaps. First, the conflation between ID and anaemia is an important factor. The underlying cause of anaemia may be important in dictating the relationship between maternal and neonatal haematologic indices; since the foetus is entirely reliant on the mother for iron supply, a more intimate relationship between maternal and neonatal haematologic and iron indices may be expected in cases of ID. Conversely, the cause of anaemia should not be assumed to be ID, as nutrient deficiencies (e.g. folate, vitamin B12, vitamin A), inflammation, and inherited disorders (e.g. thalassaemia) account for approximately half of all cases [89]. Herein, subgroup analyses on correlations between maternal and neonatal indices in ID and non-ID mothers could not be performed, because few studies reported stratified outcomes. Therefore, care should be taken to screen for ID in mothers and cord blood. The assumption that ID is largely the cause of anaemia in many intervention programs has likely contributed to ID and anaemia's intractability as global health problems.
A second notable challenge is the standardization of clinical techniques, screening procedures, and assay reference values. As previously mentioned, acute phase proteins such as ferritin and hepcidin should be measured concurrently with markers of inflammation. It should be noted that while the confounding effects of inflammation on various biomarkers of iron status has been recognised, the problem has not been solved. Notwithstanding, concurrent measures of C-reactive protein, α−1 acid glycoprotein-1, or IL-6 [3,95,96] may help with interpretation and inform further testing. Few studies included in this review reported inflammatory marker results, and although our search strategy excluded studies with known chronic disease or complications of pregnancy, many studies did not explicitly state the inclusion/exclusion criteria of their respective studies, and there it is not clear whether complicated pregnancies were included in the analysis. Even in the absence of pregnancy complications, inflammatory changes associated with pregnancy and subclinical infections may be present and could confound the results [96]. Further, novel indices (e.g. sTfR) suffer from a lack of standardization and consistency between analytical platforms, and thus variations in reference ranges remains an important limitation [97]. Other assays, such as serum iron measurements, may also be confounded by a lack of standardization for post-prandial and diurnal variations, length of fasting prior to testing [98], as well as the timing of maternal blood sampling (pre- versus post-delivery) as revealed in our subgroup analysis, which could reflect the effects of postpartum haemorrhage, amongst other circumstances. Finally, the use of either venous or capillary blood sampling techniques can influence haematological assessments [99], and thus standard techniques to limit outcome variability are needed. Inconsistent or incomplete reporting of variables including fasting, blood collection techniques, and inflammatory status in the included studies furthers the need for validation of results.
There are several study limitations that warrant discussion. Meta-analyses were limited to bivariate analyses of linear relationships; however, consideration of multiple indices in tandem, using more complex (i.e. non-linear) models may provide better predictive ability of neonatal and neonate outcomes. As noted above, consideration of inflammation markers, either as a correction or means to exclude values is likely to improve the predictive ability of various maternal indices; since this information was not readily available, this analysis was beyond the purview of this systematic review. Finally, although multiple electronic databases were searched, relevant articles from grey literature may have been missed.
In conclusion, the results from the present meta-analyses emphasize the lack of strong correlations between haematological indices and iron biomarkers between mother and the newborn child. Adequate iron is critical for optimal growth and development in early infancy; maternal ID may interfere with foetal iron accretion in the late stages of gestation, and thus early identification of neonatal ID and anaemia is important. There results presented herein suggest that neonatal iron status cannot be accurately ascertained by relying on maternal indices alone due to poor correlations between these indices. Therefore, sources of blood more proximal to the neonate (e.g. cord blood) may be more appropriate for assessment of iron and haematologic indices. This strategy could inform neonatal iron supplementation regimens, thereby improving offspring health during this critical period of growth and development.
Contributors
-
•
Study design: SLB, MBO
-
•
Protocol development: SLB, MBO, TC
-
•
Search strategy: TC
-
•
Screening of research results: OBS, MBO, SR, JL, SLB, AGW
-
•
Manuscript writing and editing: OBS, SLB, MBO, AGW
-
•
Database search: TC
-
•
Data analysis: OBS, MBO
Declaration of Competing Interest
Dr. Ospina reports grants from the Women and Children's Health Research Institute, grants and other from Canada Research Chairs (Government of Canada), during the conduct of the study. Dr. Bourque reports grants from the Canadian Institutes of Health Research (Government of Canada), grants from the Women and Children's Health Research Institute, grants and other from Canada Research Chairs (Government of Canada), during the conduct of the study.
Acknowledgments
Funding
MBO and SLB hold Canada Research Chairs and are funded by grants from the Women and Children's Health Research Institute and the Canadian Institutes of Health Research. The funders were not involved in the planning, design, analysis, interpretation of study results, preparation of the manuscript, and submission plan.
Data sharing
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100555.
Appendix. Supplementary materials
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G.A., Finucane M.M., De-Regil L.M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Global Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Centers for Disease Control and Prevention. Assessing the iron status of populations: second edition, including literature reviews. 2007; ISBN:978 92 41596107.
- 4.Pfeiffer C.M., Looker A.C. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(Suppl 6):1606S–1614S. doi: 10.3945/ajcn.117.155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgieff Michael K. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassebaum N.J. The global burden of anemia. Hematol Oncol Clinics. 2016;30(2):247–308. doi: 10.1016/j.hoc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Pavord S., Myers B., Robinson S., Allard S., Strong J., Oppenheimer C. British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588–600. doi: 10.1111/j.1365-2141.2011.09012.x. [DOI] [PubMed] [Google Scholar]
- 8.Pasricha S.R., Colman K., Centeno-Tablante E., Garcia-Casal M.N., Pena-Rosas J.P. Revisiting WHO haemoglobin thresholds to define Anaemia in clinical medicine and public health. Lancet Haematol. 2018;5(2):e60–e62. doi: 10.1016/S2352-3026(18)30004-8. [DOI] [PubMed] [Google Scholar]
- 9.Daru J., Allotey J., Peña‐Rosas J., Khan K. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med. 2017;27(3):167–174. doi: 10.1111/tme.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merlin G., Falcoff E., Aguet M. 125I-labelled human interferons alpha, beta and gamma: comparative receptor-binding data. J Gen Virol. 1985;66(5):1149–1152. doi: 10.1099/0022-1317-66-5-1149. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. Daily iron and folic acid supplementation during pregnancy. 2020; Available at: https://www.who.int/elena/titles/guidance_summaries/daily_iron_pregnancy/en/. Accessed March/05, 2020.
- 12.Georgieff M.K. Iron Deficiency in Pregnancy. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholl T.O. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev. 2011;69(suppl_1):S23–S29. doi: 10.1111/j.1753-4887.2011.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Scale N. Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses2014; .
- 16.Schünemann H., Brozek J., Guyatt G., Oxman A. The GRADE Working Group; 2013. GRADE handbook for grading quality of evidence and strength of recommendations.guidelinedevelopment.org/handbook editor (s) [Google Scholar]
- 17.Field A.P. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. 2001;6(2):161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- 18.Hinkle DE, Wiersma W, Jurs SG, 2003. Applied statistics for the behavioral sciences (Vol. 663). Houghton Mifflin College Division.
- 19.StatsDirect L. StatsDirect; UK: 2005. StatsDirect statistical software. [Google Scholar]
- 20.Cochrane Collaboration RevMan 5.3 user guide. Cochrane Collaborat. 2014;5 [Google Scholar]
- 21.Adams W.H. Correlations and cumulative frequency distributions of maternal and newborn hematologic data. Acta Haematol. 1981;65(4):233–239. doi: 10.1159/000207186. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal R.M., Tripathi A.M., Agarwal K.N. Cord blood haemoglobin, iron and ferritin status in maternal anaemia. Acta Paediatr Scand. 1983;72(4):545–548. doi: 10.1111/j.1651-2227.1983.tb09768.x. [DOI] [PubMed] [Google Scholar]
- 23.Akhter S., Momen M.A., Rahman M.M., Parveen T., Karim R.K. Effect of maternal anemia on fetal outcome. Mymensingh Med J MMJ. 2010;19(3):391–398. [PubMed] [Google Scholar]
- 24.Daouda H., Galan P., Prual A., Sekou H., Hercberg S. Iron status in Nigerian mothers and their newborns. Int J Vitam Nutrit Res. 1991;61(1):46–50. [PubMed] [Google Scholar]
- 25.de Sa S.A., Willner, Duraes Pereira E., de Souza T.A., Teles Boaventura V.R., Blondet dA G. Anemia in Pregnancy: impact on Weight and in the Development of Anemia in Newborn. Nutr Hosp. 2015;32(5):2071–2079. doi: 10.3305/nh.2015.32.5.9186. [DOI] [PubMed] [Google Scholar]
- 26.Ek J. Plasma and red cell folate in mothers and neonates in normal pregnancies. Relation to birth weight. Acta Obstet Gynecol Scand. 1982;61(1):17–20. doi: 10.3109/00016348209156944. [DOI] [PubMed] [Google Scholar]
- 27.El-Farrash R., Ismail E.A., Nada A.S. Cord blood iron profile and breast milk micronutrients in maternal iron deficiency anemia. Pediatr Blood Cancer. 2012;58(2):233–238. doi: 10.1002/pbc.23184. [DOI] [PubMed] [Google Scholar]
- 28.Gaspar M.J., Ortega R.M., Moreiras O. Relationship between iron status in pregnant women and their newborn babies. Investigation in a Spanish population. Acta Obstet Gynecol Scand. 1993;72(7):534–537. doi: 10.3109/00016349309058158. [DOI] [PubMed] [Google Scholar]
- 29.Mezdoud A., Agli A.N., Oulamara H. Relationships between umbilical vein and mother iron status] Nutr Hosp. 2017;34(3):562–567. doi: 10.20960/nh.238. [DOI] [PubMed] [Google Scholar]
- 30.Nemet K., Andrassy K., Bognar K., Czappan P., Stuber A., Simonovits I. Relationship between maternal and neonate iron stores: 1. Full term neonates. Haematologia (Budap) 1986;19(3):197–205. [PubMed] [Google Scholar]
- 31.Nhonoli A.M., Kihama F.E., Ramji B.D. The relation between maternal and cord serum iron levels and its effect on fetal growth in iron deficient mothers without malarial infection. Br J Obstet Gynaecol. 1975;82(6):467–470. doi: 10.1111/j.1471-0528.1975.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 32.Norimah A.K., Poh B.K., Firoozehchian F., Hadipour R., Akaberi A. Haemoglobin and serum ferritin levels in newborn babies born to anaemic Iranian women: a cross-sectional study in an Iranian Hospital. Pak J Nutrit. 2010;9(6):562–566. [Google Scholar]
- 33.Pope B., Hokin B., Grant R. Effect of maternal iron status on the number of CD34+ stem cells harvested from umbilical cord blood. Transfusion. 2014;54(7):1876–1880. doi: 10.1111/trf.12547. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez-Cardich M., Saito M., Gilman R.H. Effect of maternal anemia at high altitude on neonate hematocrit and oxygenation. Am J Trop Med Hygiene. 2004;70(4):420–424. [PubMed] [Google Scholar]
- 35.Rusia U., Flowers C., Madan N., Agarwal N., Sood S.K., Sikka M. Serum transferrin receptor levels in the evaluation of iron deficiency in the neonate. Acta Paediatr Jap. 1996;38(5):455–459. doi: 10.1111/j.1442-200x.1996.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 36.Singla P.N., Chand S., Khanna S., Agarwal K.N. Effect of maternal anaemia on the placenta and the newborn neonate. Acta Paediatr Scand. 1978;67(5):645–648. doi: 10.1111/j.1651-2227.1978.tb17816.x. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava S., Mehrotra P.K., Srivastava S.P., Siddiqui M.K. Some essential elements in maternal and cord blood in relation to birth weight and gestational age of the baby. Biol Trace Elem Res. 2002;86(2):97–105. doi: 10.1385/BTER:86:2:097. [DOI] [PubMed] [Google Scholar]
- 38.Terefe B., Birhanu A., Nigussie P., Tsegaye A. Effect of maternal iron deficiency anemia on the iron store of newborns in ethiopia. Anemia. 2015;2015 doi: 10.1155/2015/808204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong C.T., Saha N. Inter-relationships of storage iron in the mother, the placenta and the newborn. Acta Obstet Gynecol Scand. 1990;69(7–8):613–616. doi: 10.3109/00016349009028705. [DOI] [PubMed] [Google Scholar]
- 40.Wong C.T., Saha N. Lack of influence of maternal and fetal transferrin phenotypes and concentrations on normal fetal growth. Biol Neonate. 1991;59(3):156–160. doi: 10.1159/000243338. [DOI] [PubMed] [Google Scholar]
- 41.Altinkaynak S., Alp H., Bastem A., Selimoglu M., Energin M. Serum ferritin and hemoglobin levels of mothers and their newborns.Erratum appears in Turk J Pediatr 1995 Oct-Dec;37(4):439] Turk J Pediatr. 1994;36(4):289–293. [PubMed] [Google Scholar]
- 42.Babay Z.A., Addar M.H., Warsy A.S., El-Hazmi M. The inter-relationship hematological parameters between Saudi newborns and parents. Saudi Med J. 2002;23(8):943–946. [PubMed] [Google Scholar]
- 43.Bratlid D., Moe P.J. Hemoglobin and serum ferritin levels in mothers and neonates at birth. Eur J Pediatr. 1980;134(2):125–127. doi: 10.1007/BF01846030. [DOI] [PubMed] [Google Scholar]
- 44.Butte N.F., Calloway D.H., Proteins vitamin A. carotene, folacin, ferritin and zinc in Navajo maternal and cord blood. Biol Neonate. 1982;41(5–6):273–278. doi: 10.1159/000241562. [DOI] [PubMed] [Google Scholar]
- 45.Celada A., Busset R., Gutierrez J., Herreros V. Maternal and cord blood ferritin. Helv Paediatr Acta. 1982;37(3):239–244. [PubMed] [Google Scholar]
- 46.Custodio P.J., Carvalho M.L., Nunes F., Pedroso S., Campos A. Direct analysis of human blood (mothers and newborns) by energy dispersive X-ray fluorescence. J Trace Elements Med Biol. 2005;19(2–3):151–158. doi: 10.1016/j.jtemb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Dapper D.V., Didia B.C. Haemorheological parameters of umbilical cord blood of Nigerian newborns: correlations with maternal parameters. West Afr J Med. 2006;25(3):226–230. doi: 10.4314/wajm.v25i3.28283. [DOI] [PubMed] [Google Scholar]
- 48.Erdem A., Erdem M., Arslan M., Yazici G., Eskandari R., Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Maternal-Fetal Neonatal Med. 2002;11(5):329–332. doi: 10.1080/jmf.11.5.329.332. [DOI] [PubMed] [Google Scholar]
- 49.Esmailnasab N., Afkhamzadeh A., Delpisheh A. Prevalence of maternal anemia and its association with hemoglobin levels of newborn babies. Arch Dis Child. 2012;2):A218. doi: 10.1136/archdischild-2012-302724.0757. [DOI] [Google Scholar]
- 50.Huang S.H., Weng K.P., Lin C.C., Wang C.C., Lee C.T., Ger L.P., Wu M.T. Maternal and umbilical cord blood levels of mercury, manganese, iron, and copper in southern Taiwan: a cross-sectional study. J Chinese Med Assoc JCMA. 2017;8(7):442–451. doi: 10.1016/j.jcma.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Hussain M.A., Gaafar T.H., Laulicht M., Hoffebrand A.V. Relation of maternal and cord blood serum ferritin. Arch Dis Child. 1977;5(10):782–784. doi: 10.1136/adc.52.10.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaime-Perez J., Herrera-Garza J., Gomez-Almaguer D. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2005;3(5):598–602. doi: 10.1016/j.arcmed.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Jariwala M., Suvarna S., Kiran Kumar G., Amin A., Udas A.C. Study of the concentration of trace elements Fe, Zn, Cu, Se and their correlation in maternal serum, cord serum and colostrums. Ind J Clin Biochem. 2014;2(2):181–188. doi: 10.1007/s12291-013-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katoh K. Variations and correlation of hematological and serum iron values in pregnant and parturient women, neonates and neonates: with special reference to feto-maternal transfusion and neonateile hemoglobin values] Nippon Ika Daigaku Zasshi - J Nippon Med School. 1984;51(3):362–375. doi: 10.1272/jnms1923.51.362. [DOI] [PubMed] [Google Scholar]
- 55.Koc A., Kocyigit A., Soran M., Demir N., Sevinc E., Erel O., Mil Z. High frequency of maternal vitamin B12 deficiency as an important cause of neonateile vitamin B12 deficiency in Sanliurfa province of Turkey. Eur J Nutr. 2006;45(5):291–297. doi: 10.1007/s00394-006-0598-7. [DOI] [PubMed] [Google Scholar]
- 56.Kulik-Rechberger B., Kosciesza A., Szponar E., Domosud J. Hepcidin and iron status in pregnant women and full-term newborns in first days of life. Ginekol Pol. 2016;87(4):288–292. doi: 10.17772/gp/62202. [DOI] [PubMed] [Google Scholar]
- 57.Kumar A., Rai A.K., Basu S., Dash D., Singh J.S. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121(3):e673–e677. doi: 10.1542/peds.2007-1986. [DOI] [PubMed] [Google Scholar]
- 58.Lao T.T., Loong E.P., Chin R.K., Lam C.W., Lam Y.M. Relationship between newborn and maternal iron status and haematological indices. Biol Neonate. 1991;60(5):303–307. doi: 10.1159/000243421. [DOI] [PubMed] [Google Scholar]
- 59.MacPhail A.P., Charlton R.W., Bothwell T.H., Torrance J.D. The relationship between maternal and neonate iron status. Scand J Haematol. 1980;25(2):141–150. doi: 10.1111/j.1600-0609.1981.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 60.Malcolm L.A., Booth P.B. Haematological relationships between New Guinean mothers and their neonates. Med J Aust. 1973;2(19):886–889. doi: 10.5694/j.1326-5377.1973.tb76613.x. [DOI] [PubMed] [Google Scholar]
- 61.Milman N., Graudal N., Nielsen O.J., Agger A.O. Cord serum erythropoietin in 90 healthy newborn term neonates: relationship to blood gases and iron status markers. Int J Hematol. 1996;64(3–4):197–201. doi: 10.1016/0925-5710(96)00485-9. [DOI] [PubMed] [Google Scholar]
- 62.Milman N., Ibsen K.K., Christensen J.M. Serum ferritin and iron status in mothers and newborn neonates. Acta Obstet Gynecol Scand. 1987;66(3):205–211. doi: 10.3109/00016348709020748. [DOI] [PubMed] [Google Scholar]
- 63.Montemagno R., Soothill P.W., Karim S.A., Rodeck C.H. Fetal haemoglobin concentration and mean red cell volume are not related to the maternal values at 18-25 weeks' gestation. Early Hum Dev. 1995;41(1):11–14. doi: 10.1016/0378-3782(94)01604-n. [DOI] [PubMed] [Google Scholar]
- 64.Paiva Ade A., Rondo P.H., Pagliusi R.A., Latorre Mdo R., Cardoso M.A., Gondim S.S. Relationship between the iron status of pregnant women and their newborns. Rev Saude Publica. 2007;41(3):321–327. doi: 10.1590/s0034-89102007000300001. [DOI] [PubMed] [Google Scholar]
- 65.Qaiser D.H., Sandila M.P., Omair A., Ghori G.M. Correlation of routine haematological parameters between normal maternal blood and the cord blood of healthy newborns in selected hospitals of Karachi. Jcpsp, J College Phys Surgeons - Pak. 2013;23(2):128–131. doi: 10.2013/JCPSP.128131. [DOI] [PubMed] [Google Scholar]
- 66.Shao J., Lou J., Rao R. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–2009. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sichieri R., Fonseca V.M., Hoffman D., Trugo N.M., Moura A.S. Lack of association between iron status at birth and growth of preterm neonates. Rev Saude Publica. 2006;40(4):641–647. doi: 10.1590/s0034-89102006000500013. [DOI] [PubMed] [Google Scholar]
- 68.Sikorski R., Paszkowski T., Milart P., Radomanski T., .J, Szkoda J. Intrapartum levels of trace metals in maternal blood in relation to umbilical cord blood values: lead, iron, copper, zinc. Int J Gynaecol Obstetr. 1988;26(2):213–221. doi: 10.1016/0020-7292(88)90265-2. [DOI] [PubMed] [Google Scholar]
- 69.Tekinalp G., Oran O., Gurakan B., Saracel M., Erdem G., Yurdakok M., Gurgey A. Relationship between maternal and neonatal iron stores. Turk J Pediatr. 1996;38(4):439–445. [PubMed] [Google Scholar]
- 70.Timilsina S., Karki S., Gautam A., Bhusal P., Paudel G., Sharma D. Correlation between maternal and umbilical cord blood in pregnant women of Pokhara Valley: a cross sectional study. BMC Pregnan Childb. 2018;18(1):70. doi: 10.1186/s12884-018-1697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vahlquist A., Rask L., Peterson P.A., Berg T. The concentrations of retinol-binding protein, prealbumin, and transferrin in the sera of newly delivered mothers and children of various ages. Scandinav J Clin Lab Invest. 1975;35(6):569–575. [PubMed] [Google Scholar]
- 72.Vasquez-Molina M., Corral-Terrazas M., Apezteguia M.A., Carmona-Sawasky J., Levario-Carrillo M. Relationship between maternal and neonatal iron stores] Salud Publica Mex. 2001;43(5):402–407. [PubMed] [Google Scholar]
- 73.Vobecky J.S., Vobecky J., Shapcott D., Demers P.P., Cloutier D., Blanchard R., Fisch C. Biochemical indices of nutritional status in maternal, cord, and early neonatal blood. Am J Clin Nutr. 1982;36(4):630–642. doi: 10.1093/ajcn/36.4.630. [DOI] [PubMed] [Google Scholar]
- 74.Yepez R., Calle A., Galan P. Iron status in Ecuadorian pregnant women living at 2,800m altitude: relationship with neonate iron status. Int J Vitam Nutrition Res. 1987;57(3):327–332. [PubMed] [Google Scholar]
- 75.Awadallah S.M., Abu-Elteen K., Elkarmi A.Z., Qaraein S.H., Salem N.M., Mubarak M.S. Maternal and cord blood serum levels of zinc, copper, and iron in healthy pregnant Jordanian Women. J Trace Elem Exp Med. 2004;17(1):1–8. doi: 10.1002/jtra.10032. [DOI] [Google Scholar]
- 76.Basu S., Kumar N., Srivastava R., Kumar A. Maternal and cord blood hepcidin concentrations in severe iron deficiency anemia. Pediatri Neonatol. 2016;5(5):413–419. doi: 10.1016/j.pedneo.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Best C.M., Pressman E.K., Cao C. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans. FASEB J. 2016;3(10):3541–3550. doi: 10.1096/fj.201600069R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devi S.B., Singh K.J., Devi Y.L., Singh W.G. Maternal and neonatal anthropometric and hematological parameters in Manipuri population. Indian Pediatr. 1989;2(7):673–677. [PubMed] [Google Scholar]
- 79.Garcia-Valdes L., Campoy C., Hayes H., Florido J., Rusanova I., Miranda M.T., McArdle H.J. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015;3(4):571–578. doi: 10.1038/ijo.2015.3. [DOI] [PubMed] [Google Scholar]
- 80.Kaneshige E. Serum ferritin as an assessment of iron stores and other hematologic parameters during pregnancy. Obstetr Gynecol. 1981;5(2):238–242. [PubMed] [Google Scholar]
- 81.Lee S., Guillet R., Cooper E.M. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;7(1):42–48. doi: 10.1038/pr.2015.183. [DOI] [PubMed] [Google Scholar]
- 82.Tamura T., Hou J., Goldenberg R.L., Johnston K.E., Cliver S.P. Gender difference in cord serum ferritin concentrations. Biol Neonate. 1999;7(6):343–349. doi: 10.1159/000014114. [DOI] [PubMed] [Google Scholar]
- 83.Shukla A.K., Srivastava S., Verma G. Effect of maternal anemia on the status of iron stores in neonates: a cohort study. J Family Commun Med. 2019;2(2):118–122. doi: 10.4103/jfcm.JFCM_115_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Guindi W., Pronost J., Carles G., Largeaud M., El Gareh N., Montoya Y., Arbeille P. Severe maternal anemia and pregnancy outcome. J Gynecol Obstet Biol Reprod. 2004;3(6):506–509. doi: 10.1016/s0368-2315(04)96563-5. [DOI] [PubMed] [Google Scholar]
- 85.Rioux M.F., Michaud J. Maternal anemia in the southeast and northeast regions of New Brunswick and the impact on hematological parameters and the growth of the newborn] Canad J Dietetic Pract Res. 2001;6(2):70–75. [PubMed] [Google Scholar]
- 86.Wrzesniak M., Zalewska M., Krolik M., Bizon A., Milnerowicz H. The effect of transferrin sialylation on foetal development during pregnancy. FEBS J. 2014;1:339. doi: 10.1111/febs.12919. [DOI] [Google Scholar]
- 87.Agarwal R.K., Verma I.C., Ghai O.P. Correlation of cord & maternal transferrin with gestational age. Indian J Med Res. 1985;8:24–28. [PubMed] [Google Scholar]
- 88.Lindsay R.S., Nelson S.M., Walker J.D., Greene S.A., Milne G., Sattar N., Pearson D.W. Programming of adiposity in offspring of mothers with type 1 diabetes at age 7 years. Diabetes Care. 2010;3(5):1080–1085. doi: 10.2337/dc09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kassebaum N.J. The global burden of anemia. Hematology/Oncology Clinics. 2016;3(2):247–308. doi: 10.1016/j.hoc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 90.World Health Organization, Centers for disease control and prevention assessing iron status populations second edition, including literature reviews.; Available at: https://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en/. Accessed August/10, 2020.
- 91.Nemeth E., Tuttle M.S., Powelson J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;30(5704):2090–2093. doi: 10.1126/science.1104742. 1104742 [pii] [DOI] [PubMed] [Google Scholar]
- 92.Dignass A., Farrag K., Stein J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int J Chronic Dis. 2018;201 doi: 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Graham C., Chooniedass R., Stefura W.P. In vivo immune signatures of healthy human pregnancy: inherently inflammatory or anti-inflammatory. PLoS ONE. 2017;1(6) doi: 10.1371/journal.pone.0177813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nemeth E., Valore E.V., Territo M., Schiller G., Lichtenstein A., Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;10(7):2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 95.Namaste S.M., Rohner F., Huang J. Adjusting ferritin concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;10(Suppl 1):359S–371S. doi: 10.3945/ajcn.116.141762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee S., Guillet R., Cooper E.M., Westerman M., Orlando M., Pressman E., O'Brien K.O. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr. 2014;14(10):1524–1532. doi: 10.3945/jn.114.191445. [DOI] [PubMed] [Google Scholar]
- 97.Infusino I., Braga F., Dolci A., Panteghini M. Soluble transferrin receptor (sTfR) and sTfR/log ferritin index for the diagnosis of iron-deficiency anemia. A meta-analysis. Am J Clin Pathol. 2012;13(5):642–649. doi: 10.1309/AJCP16NTXZLZFAIB. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen L.T., Buse J.D., Baskin L., Sadrzadeh S.H., Naugler C. Influence of diurnal variation and fasting on serum iron concentrations in a community-based population. Clin Biochem. 2017;5(18):1237–1242. doi: 10.1016/j.clinbiochem.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Orkin S.H., Nathan D.G., Ginsburg D., Look A.T., Fisher D.E., Lux S. Elsevier Health Sciences; 2008. Nathan and Oski's hematology of infancy and childhood e-book. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.