Summary
Lipid composition varies among organelles, and the distinct lipid composition is important for specific functions of each membrane. Lipid transport between organelles, which is critical for the maintenance of membrane lipid composition, occurs by either vesicular or non-vesicular mechanisms. In yeast, ceramide synthesized in the endoplasmic reticulum (ER) is transported to the Golgi apparatus where inositolphosphorylceramide (IPC) is formed. Here we show that a fraction of Tcb3p, a yeast tricalbin protein, localizes to ER-Golgi contact sites. Tcb3p and their homologs Tcb1p and Tcb2p are required for formation of ER-Golgi contacts and non-vesicular ceramide transport. Absence of Tcb1p, Tcb2p, and Tcb3p increases acylceramide synthesis and subsequent lipid droplet (LD) formation. As LD can sequester excess lipids, we propose that tricalbins act as regulators of ceramide transport at ER-Golgi contact sites to help reduce a potentially toxic accumulation of ceramides.
Subject Areas: Cell Biology, Functional Aspects of Cell Biology
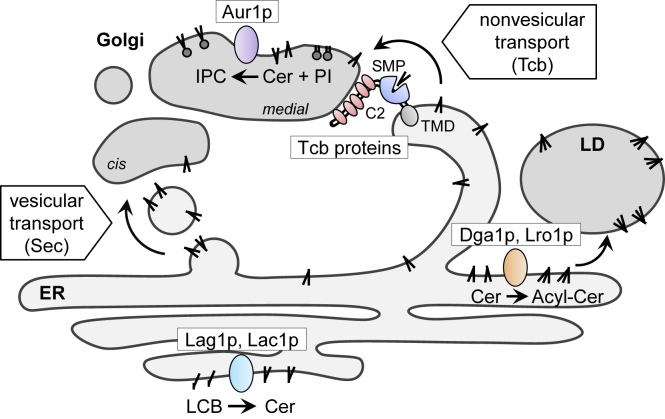
Graphical Abstract
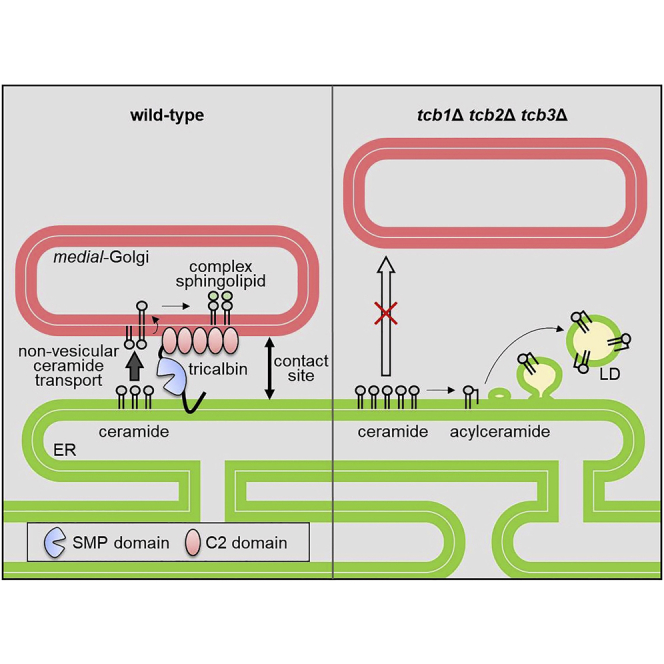
Highlights
-
•
Yeast tricalbin Tcb3p localizes at ER-Golgi contact sites
-
•
Lack of tricalbins reduces ER-Golgi contacts
-
•
Tricalbins regulate non-vesicular ceramide transport
-
•
Tricalbin deletion causes both acylceramide and lipid droplet accumulation
Cell Biology; Functional Aspects of Cell Biology
Introduction
Organelle membranes have unique lipid compositions, which relate to their physical properties, and specific function (Harayama and Riezman, 2018; van Meer et al., 2008). Membrane lipids are not only asymmetrically distributed in the leaflets of bilayers but also organized laterally into distinct domains (Sezgin et al., 2017; Simons and Ikonen, 1997). Such organization of membranes is achieved by transport and sorting of lipids from the endoplasmic reticulum (ER) to other organelles since the ER is the major site of lipid synthesis and the supplier of lipids to organelles (Holthuis and Levine, 2005; Holthuis and Menon, 2014; van Meer et al., 2008). Translocation of lipids across the bilayer and lateral segregation of lipids within a leaflet of membranes are also necessary for proper membrane organization (Holthuis and Levine, 2005; van Meer et al., 2008). Little is known, however, about the molecular mechanisms of lipid movement.
Ceramide is transported from the ER to the Golgi apparatus and incorporated into complex sphingolipids (Funato et al., 2002; Jain and Holthuis, 2017; Perry and Ridgway, 2005; Yamaji and Hanada, 2015). In mammalian cells, the ceramide transport protein (CERT) mediates non-vesicular trafficking of ceramide for sphingomyelin synthesis in the trans-Golgi (Hanada et al., 2003). The budding yeast S. cerevisiae uses both non-vesicular and vesicular ceramide transport pathways for inositolphosphorylceramide (IPC) synthesis (Funato and Riezman, 2001). S. cerevisiae has no CERT homolog, but a recent study reveals that Nvj2p promotes the non-vesicular transfer of ceramide from the ER to the Golgi (Liu et al., 2017). It was proposed that Nvj2p acts as a tether to establish membrane contact sites between the ER and the Golgi because Nvj2p is localized at ER-Golgi membrane contact sites and its overexpression increases ER-Golgi contact sites. ER-to-Golgi vesicular transport of ceramides in yeast is, on the other hand, mediated by COPII-coated vesicles (Funato and Riezman, 2001). This vesicular traffic accounts for 60%–80% of transport of ceramides (Kajiwara et al., 2014; Funato and Riezman, 2001). Previously, we showed that vesicular transport of ceramides is coupled with glycosylphosphatidylinositol (GPI) anchor biosynthesis and proposed that ceramides are transported to the Golgi by the same vesicles used for GPI anchored proteins (GPI-APs) (Kajiwara et al., 2008). In addition, we have shown that oxysterol-binding proteins regulate the vesicular transport of ceramides (Kajiwara et al., 2014).
Perturbation of ceramide transport or sphingolipid metabolism causes an accumulation of ceramides in the ER, leading to cellular dysfunction and death (Eisenberg and Büttner, 2014; Tani and Funato, 2018). One of the protection mechanisms preventing lipotoxicity when ceramide level is transiently increased appears to be to facilitate conversion of ceramides into complex sphingolipids. It is conceivable that degradation of ceramides by ceramidases also plays an important role to reduce the cellular level of ceramides (Ito et al., 2014; Kus et al., 2015; Voynova et al., 2015). In addition, experiments in mammalian cells showed that ceramides can also be converted to acylceramide and consequently incorporated into lipid droplets (LDs) to prevent the toxic accumulation of ceramides (Senkal et al., 2017); however, if a similar pathway exists in yeast has not been demonstrated, although it was proposed (Liu et al., 2017; Voynova et al., 2012).
In this study, we show that a conserved yeast ER membrane protein, tricalbin-3 (Tcb3p), localizes to contact sites between the ER and the Golgi. We also provide the first evidence to suggest that tricalbins are required for formation of ER-Golgi contact sites and non-vesicular transport of ceramides between the ER and Golgi compartments. In addition, we found that ceramide transport defects lead to increased acylceramide synthesis and subsequent LD formation, which may play a role in preventing the toxic accumulation of ceramides. Based on these results, we propose that yeast tricalbins regulate ER contacts with Golgi to mediate nonvesicular ceramide transport, which alleviates ceramide toxicity.
Results
Overexpressed Tcb3-GFP Localizes to ER-Golgi Contact Sites and the Localization Requires the C2 Domains of Tcb3 and Other Tcb Proteins, Tcb1p, and Tcb2p
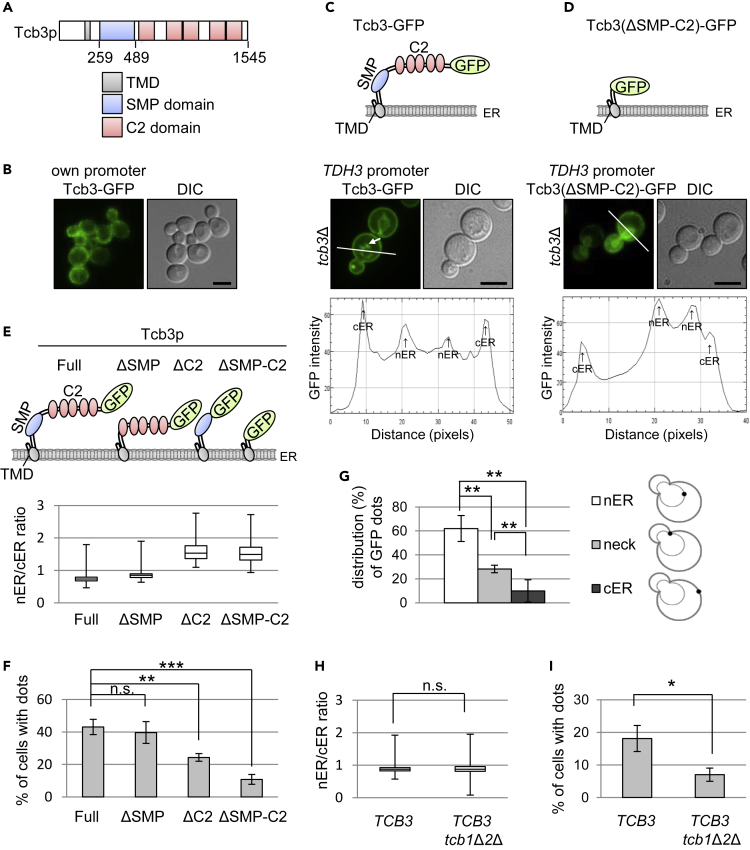
ER-plasma membrane (PM) tethering proteins, tricalbins (Tcb1p, Tcb2p and Tcb3p; yeast orthologs of the extended synaptotagmins, E-Syts), possess a transmembrane domain (TMD), a synaptotagmin-like mitochondrial lipid-binding protein (SMP) domain, and multiple C2 domains (Figure 1A) and localize to cortical ER (cER) (Kopec et al., 2010; Manford et al., 2012; Toulmay and Prinz, 2012). A recent study shows that Tcb3 protein level is upregulated in response to environmental changes, such as sterol depletion (Quon et al., 2018). To unravel the roles of tricalbins, we have re-examined the localization of Tcb3p. C-terminal tagged Tcb3-GFP was overexpressed under the control of the constitutive TDH3 promoter and analyzed by fluorescent microscopy. As reported previously (Toulmay and Prinz, 2012), Tcb3-GFP expressed under the control of the endogenous TCB3 promoter was localized in the cER but not in the perinuclear ER (nER) (Figure 1B), whereas overexpressed Tcb3-GFP was found in both cER and nER (Figure 1C). The TMD is essential for ER localization of Tcb3p because truncated forms of Tcb3p lacking the TMD (ΔTMD; SMP-C2 domains, ΔTMDΔC2; only SMP domain, ΔTMD-SMP; only C2 domains) are distributed in the cytosol or nucleus (see Figure S1). A truncated Tcb3(ΔSMP-C2)-GFP lacking the SMP and C2 domains localized to both the cER and nER (Figure 1D), but the nER/cER signal ratio of Tcb3(ΔSMP-C2)-GFP was higher than that of Tcb3-GFP (Figure 1E). In addition, Tcb3(ΔC2)-GFP lacking only C2 domains showed the same nER/cER ratio as Tcb3(ΔSMP-C2)-GFP, whereas no increase in the nER/cER ratio was seen with Tcb3(ΔSMP)-GFP lacking only SMP domain (Figure 1E). Thus, these results suggest that transmembrane and C2 domains but not SMP domain are required for localization of Tcb3p to the cER.
Figure 1.
Overexpressed Tcb3-GFP Localizes to the Dot-like Domains of Perinuclear ER, and Its Localization Depends on C2 Domains and Tcb1p/Tcb2p
(A) Diagram of domain organization of Tcb3 protein. Amino acid length is indicated. TMD, transmembrane domain; SMP, synaptotagmin-like mitochondrial lipid-binding protein; C2, calcium-dependent lipid-binding domain.
(B) Endogenously expressed Tcb3-GFP localizes to cortical ER (cER) in wild-type cells. Cells expressing Tcb3p tagged with GFP at the C terminus under the control of TCB3 endogenous promoter were grown at 25°C and imaged by differential interference contrast (DIC) and fluorescence microscopy. Scale bar, 5 μm.
(C–G) The C2 domains are required for Tcb3p localization to the dot-like domains of perinuclear ER (nER). tcb3Δ cells overexpressing Tcb3-GFP (C) or Tcb3(ΔSMP.C2)-GFP (D) under TDH3 promoter were grown at 25°C and imaged by DIC and fluorescence microscopy. An arrow in the image in C indicates dot-like structures. Scale bar, 5 μm. Bottom panels are intensity plots along the white line in C and D. nER/cER intensity ratios for Tcb3-GFP, Tcb3(ΔSMP)-GFP, Tcb3(ΔC2)-GFP, and Tcb3(ΔSMP-C2)-GFP (E), percentages of cells with GFP dots (F) were quantified, and distribution of Tcb3-GFP dot was shown (G). The data represent the mean ± standard deviation (SD) of three independent experiments, each based on more than 100 cells. ∗∗p < 0.01 and ∗∗∗p < 0.001 by Student's t-test. n.s., not significant.
(H and I) Tcb1p and Tcb2p are required for formation of Tcb3-GFP-positive dot structures. nER/cER intensity ratios for tcb3Δ and tcb1Δ tcb2Δ tcb3Δ cells expressing Tcb3-GFP under TDH3 promoter (H) and quantification of percentages of cells with GFP dots (I). The data represent mean ± SD of three independent experiments, each based on more than 100 cells. ∗p < 0.05 by Student's t-test. n.s., not significant.
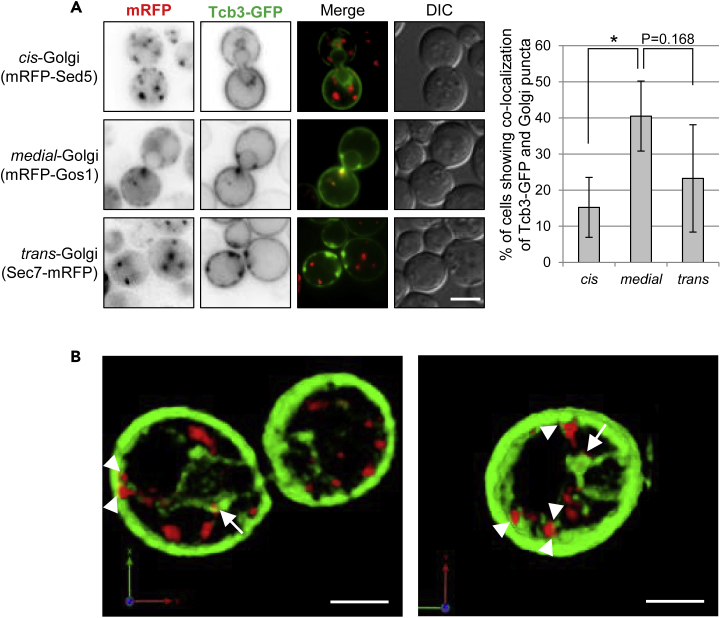
Remarkably, we found that overexpressed Tcb3-GFP localizes to dot-like structures on the ER (Figure 1C). The Tcb3-GFP puncta were decreased in strains expressing Tcb3 (ΔSMP-C2)-GFP and Tcb3(ΔC2)-GFP, although to a lesser extent, but not in strain expressing Tcb3(ΔSMP)-GFP (Figure 1F), indicating that the C2 domains of Tcb3p seem to be involved in the formation of Tcb3-GFP puncta. Tcb3-GFP puncta were preferentially localized in the nER (Figures 1C and 1G), and more importantly, they were found in close proximity to Golgi compartments (Figure 2A). Strikingly, they were enriched in regions in close proximity to the medial-Golgi compartments which contain Gos1p (Matsuura-Tokita et al., 2006; McNew et al., 1998). In parallel, close appositions between the cER and medial-Golgi were also found. Three-dimensional images reconstructed with two-dimensional confocal sections of Tcb3-GFP and mRFP-Gos1 fluorescence by the SCLIM system confirmed that medial-Golgi is located in the proximity of Tcb3p (Figure 2B). In addition, the time-lapse analysis revealed a dynamic behavior of ER-medial-Golgi contact sites (see Figure S2). Thus, these observations suggest that a portion of Tcb3p localizes to ER-Golgi contacts, and the C2 domains of Tcb3p play an important role in the localization of Tcb3p to ER-Golgi contact sites.
Figure 2.
Overexpressed Tcb3-GFP Punctum Preferentially co-localizes with the Medial-Golgi-Resident Marker mRFP-Gos1
(A) tcb3Δ cells expressing Tcb3-GFP and mRFP-Sed5 (cis-Golgi marker) or mRFP-Gos1 (medial-Golgi marker) under TDH3 promoter or Sec7-mRFP (trans-Golgi marker) under ADH promoter were grown at 25°C and imaged by DIC and fluorescence microscopy. The images were taken at 24 z-sections with 0.2-μm parallel intervals. Scale bar, 5 μm. The percentage of cells showing co-localization of Tcb3-GFP and mRFP-Sed5 puncta, mRFP-Gos1 puncta, or Sec7-mRFP puncta was quantified, and the data represent means ± SD of three independent experiments, each based on more than 60 cells. ∗p < 0.05 by Student's t-test.
(B) tcb3Δ cells expressing Tcb3-GFP with mRFP-Gos1 under TDH3 promoter were observed with super-resolution confocal live imaging microscopy (SCLIM). Representative 3D images of whole cells are shown. Arrows in the images indicate nER-medial-Golgi contact sites, and arrowheads point to cER-medial-Golgi contact sites. Scale bar, 2 μm.
Tcb3p physically and functionally interacts with other Tcb proteins, Tcb1p and Tcb2p (Creutz et al., 2004; Tarassov et al., 2008). We asked whether Tcb1p and Tcb2p regulate Tcb3p localization. In cells lacking both Tcb1p and Tcb2p, the nER/cER signal ratio of Tcb3-GFP was unaffected as compared with the control cells (Figure 1H), but the number of cells with puncta (Figure 1I) was significantly reduced. These results suggest that Tcb1p and Tcb2p regulate the localization of Tcb3p to ER-Golgi contact sites but not to cER.
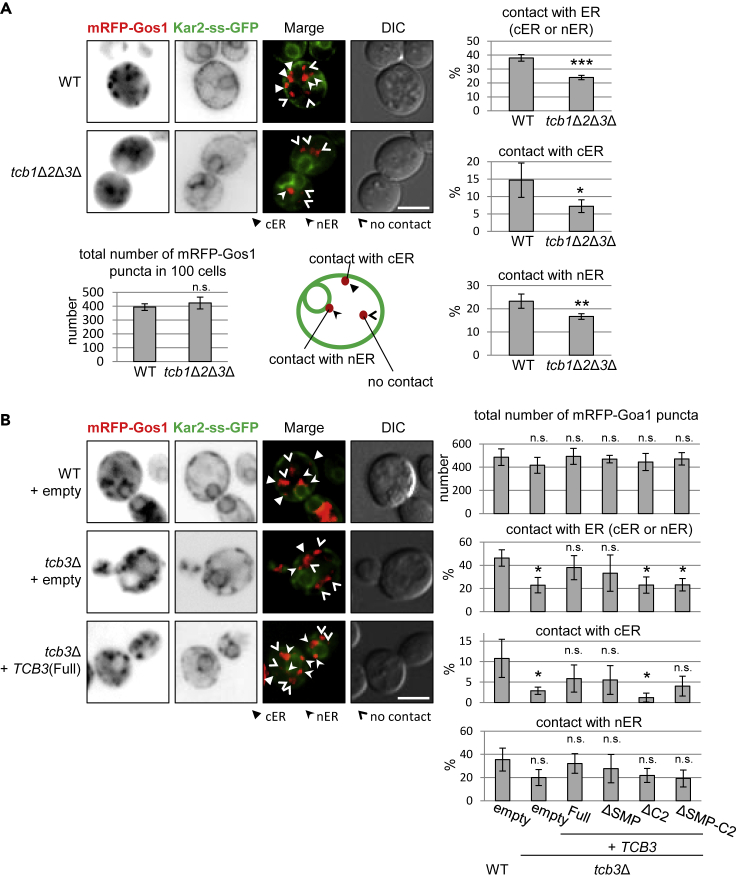
Elimination of Tcb Proteins Reduces ER-Golgi Contacts
As we found that a fraction of Tcb3p localized to ER-Golgi contact sites, this observation raised the possibility that Tcb proteins are required for formation of ER-Golgi contacts. To address this possibility, we assessed contacts between the ER and medial-Golgi by expressing the ER marker Kar2-SS-GFP and mRFP-Gos1. We quantified how often mRFP-Gos1 puncta were associated with the cER or nER. Although the total number of medial-Golgi vesicles containing mRFP-Gos1 in the cells was not affected by deletion of TCB1, TCB2, and TCB3, the number of medial-Golgi vesicles associated with the cER or the nER was significantly decreased in the tcb1Δ tcb2Δ tcb3Δ cells compared with wild-type control cells (Figure 3A). Similar results could be observed in cells lacking only Tcb3p (tcb3Δ cells) (Figure 3B). Tcb3(Full) and Tcb3(ΔSMP) were able to rescue the decrease of ER-Golgi contacts in tcb3Δ cells, whereas neither Tcb3 (ΔSMP-C2) nor Tcb3(ΔC2) restored them (Figure 3B). Collectively, these data suggest that Tcb proteins function as tethers to form ER-medial-Golgi contacts, and C2 domains are necessary to support the function of Tcb proteins.
Figure 3.
C2 Domains of Tcb3 Are Required to Form ER-medial-Golgi Contact
(A) Wild-type and tcb1Δ2Δ3Δ cells expressing mRFP-Gos1 (medial-Golgi marker) and Kar2-ss-GFP (ER marker) were grown at 25°C and imaged by DIC and fluorescence microscopy. Scale bar, 5 μm. The total number of mRFP-Gos1 puncta in 100 cells was quantified. The number of mRFP-Gos1 puncta associated with ER (cER or nER), cER, or nER was also quantified, and the results were expressed as percentage to the total number of mRFP-Gos1 puncta. Experiments were repeated three times; n = 300 cells. The data represent means ± SD of three experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.005 by Student's t-test. n.s., not significant.
(B) tcb3Δ cells expressing mRFP-Gos1 (medial-Golgi marker) and Kar2-ss-GFP (ER marker) with empty, TCB3, TCB3(ΔSMP), TCB3(ΔC2), or TCB3(ΔSMP-C2) plasmid were grown at 25°C and imaged by DIC and fluorescence microscopy. Scale bar, 5 μm. The total number of mRFP-Gos1 puncta and the number of mRFP-Gos1 associated with the ER were quantified as described in (A), and the data represent means ± SD of three independent experiments. ∗p < 0.05 by Student's t-test. n.s., not significant.
Tcb Proteins Are Not Required for COPII-Mediated Vesicular Transport
Although to a limited great extent, Tcb3p was found in close proximity to cis-Golgi (Figure 2A). Since cis-Golgi contacts the ER exit sites (ERES) where COPII-coated buds are formed to mediate vesicular transport to the Golgi (Kurokawa et al., 2014), we tested whether Tcb proteins are needed for efficient vesicular transport of proteins from the ER. In tcb1Δ tcb2Δ tcb3Δ cells, neither the GPI-AP, Gas1p, nor the non-GPI-AP, carboxypeptidase Y (CPY), maturation was affected (see Figure S3A) at 24 or 37°C. As control, sec18-20 mutant cells showed accumulation of immature proteins for both cargo proteins. Furthermore, a triple deletion of the TCB genes did not facilitate the accumulation of immature ER form of cargo proteins in sec18-20 mutant cells. These results suggest that Tcb proteins do not function as regulators of COPII vesicle-dependent transport from the ER. Consistent with this, we found no difference in numbers of puncta of Sec13-mCherry, which marks the ERES, between wild-type and mutant cells (see Figure S3B). In addition, we observed no co-localization of the overexpressed Tcb3-GFP with the puncta of Sec13-mCherry in sec12-4 mutants under conditions that block COPII vesicle budding from the ER (see Figure S3C), which may also support the idea that Tcb proteins do not function in vesicular transport.
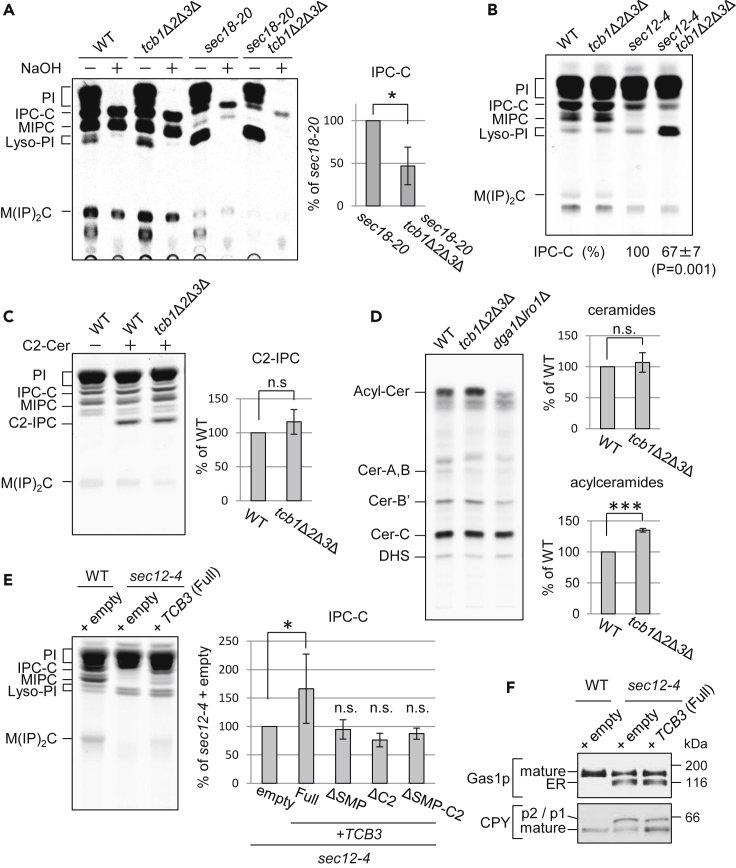
Tcb Proteins Are Required for Non-vesicular Ceramide Transport
The IPC synthase, Aur1p, is localized primarily in the medial-Golgi compartment (Levine et al., 2000). Therefore, this Golgi compartment is likely to be the site that receives and converts ceramides into IPC. Although it is still unknown whether ceramide transfer to the Golgi occurs at ER-medial-Golgi contact sites, an in vitro assay revealed that non-vesicular transport of ceramides requires organelle contact (Funato and Riezman, 2001). Given that Tcb proteins participate in ER-medial-Golgi contact sites, we examined whether they are required for IPC synthesis. As shown in Figures 4A and 4B, IPC synthesis was severely reduced in sec18-20 or sec12-4 mutant cells at non-permissive temperatures, but was unaffected in tcb1Δ tcb2Δ tcb3Δ cells, suggesting that Tcb proteins are dispensable for vesicular transport of ceramides. This is consistent with the results showing normal transport of proteins (see Figure S3A). If Tcb proteins are required for non-vesicular ceramide transport, deletion of TCB genes combined with ER-to-Golgi sec mutations could lead to an additional reduction in IPC synthesis, greater than that in sec-mutant cells. Thus, we examined the effect of TCB gene disruption on IPC synthesis under conditions that block ER-to-Golgi vesicular trafficking of ceramides by sec mutations. When cells were labeled with [3H]myo-inositol, we found that IPC synthesis was approximately 50% lower in sec18-20 tcb1Δ tcb2Δ tcb3Δ mutant cells than in sec18-20 mutant cells (Figure 4A). Similar results were obtained with sec12-4 mutants (Figure 4B). Together, these findings support a role for Tcb proteins in non-vesicular transport of ceramides but not in vesicular transport.
Figure 4.
Tcb Proteins Are Required for Non-vesicular Ceramide Transport and Regulate Acylceramide Levels
(A and B) Cells were grown at 25°C, sifted to 37°C for 20 min (A) or 30°C for 30 min (B), and labeled with [3H]myo-inositol for 1 h (A) or 1.5h (B). Labeled lipids were (+) or were not (−) mildly hydrolyzed with NaOH to deacylate glycerophospholipids and detect base-resistant complex sphingolipids (IPC-C, MIPC and M(IP)2C) and applied to TLC plates using solvent system I. Incorporation (%) of [3H]myo-inositol into IPC-C were quantified, and the percentage of the incorporation (%) in sec18-20 (A) or sec12-4 (B) was determined. Data represent mean ± SD of three independent experiments. ∗p < 0.05 by Student's t-test.
(C) Cells were grown at 25°C, incubated without (−) or with (+) C2-ceramide for 20 min, and labeled with [3H]myo-inositol for 3 h. Labeled lipids were applied to TLC plates using solvent system I. Incorporation (%) of [3H]myo-inositol into C2-IPC was quantified, and the percentage of the incorporation (%) in wild-type cells was determined. Data represent mean ± SD of three independent experiments. n.s., not significant by Student's t-test.
(D) Cells were grown at 25°C and labeled with [3H]DHS for 3h. Labeled lipids were applied to TLC plates using solvent system II. Fractions containing ceramides and acylceramides on the TLC plate were collected by scraping and eluting the lipid extracts from the silica and analyzed by TLC using solvent system III. Incorporation of [3H]DHS into ceramides (Cer-A, B, B′ and (C) or acylceramides was quantified, and the percentage of the total radioactivity (%) in wild-type cells was determined. Data represent mean ± SD of three independent experiments. ∗∗∗p < 0.001 by Student's t-test. n.s., not significant.
(E) sec12-4 cells transformed with empty, TCB3, TCB3(ΔSMP), TCB3(ΔC2), or TCB3(ΔSMP-C2) plasmid were grown at 25°C, sifted to 30°C for 30 min, and labeled with [3H]myo-inositol for 1.5 h and chased for 2 h. Labeled lipids were applied to TLC plates using solvent system I, and the percentage of incorporation (%) of IPC-C in cells transformed with empty plasmid was determined. Data represent mean ± SD of three independent experiments. ∗p < 0.05 by Student's t-test. n.s., not significant.
(A–E) PI, phosphatidylinositol; IPC-C, inositolphosphorylceramide subclasses C; MIPC, Mannosyl inositolphosphorylceramide; Lyso-PI, lysophosphatidylinositol; M(IP)2C, mannosyl di(inositolphosphoryl) ceramide.
(F) Wild-type and the same strains as in (E) were grown at 25°C and sifted to 30°C for 4 h. Cell lysates were prepared and subjected to SDS-PAGE, followed by Western blot analysis.
To exclude the possibility that deletion of TCB genes affects IPC synthesis activity, we analyzed the IPC synthesis activity in the tcb1Δ tcb2Δ tcb3Δ cells in vivo as described previously (Kajiwara et al., 2008). For this purpose, we used C2-ceramide that reaches the Golgi through a diffusion-mediated or an endocytic route when added exogenously to cells and measured the incorporation of exogenous C2-ceramide into C2-IPC. In comparison to wild-type cells, the incorporation of C2-ceramide into C2-IPC was not affected in tcb1Δ tcb2Δ tcb3Δ cells (Figure 4C). Similar results were obtained with sec background strains (the relative incorporation into C2-IPC [%] of sec12-4 tcb1Δ tcb2Δ tcb3Δ to sec12-4 cells was 108%). Phosphatidylinositol (PI) is the substrate for the IPC synthesis enzyme converting C2-ceramide to C2-IPC. If Tcb proteins are involved in the delivery of PI to the site of IPC synthesis, TCB deletion should result in decreased C2-IPC synthesis. This was not the case. In addition, we measured the levels of ceramides in wild-type and tcb1Δ tcb2Δ tcb3Δ cells. The total ceramide levels were not decreased in the tcb mutant cells (Figure 4D). These results suggest that the IPC synthesis defect of the tcb mutant combined with the sec mutation is not due to reduced enzyme activity, impaired delivery of substrate PI, or a ceramide synthesis defect. Thus, we conclude that the problem is in delivery of the ceramide to the IPC synthesis enzyme and thus that Tcb proteins are required for non-vesicular ceramide transport.
We also investigated whether Tcb3p overexpression restores the IPC synthesis defect in sec12-4 mutant cells. As shown in Figure 4E, overexpression of TCB3 in sec12-4 cells partially rescued the IPC synthesis defect. Because overexpression of TCB3 did not suppress the protein transport defects of sec12-4 mutant cells (Figure 4F), it is most likely that the restoration of IPC synthesis by TCB3 overexpression results from stimulation of non-vesicular ceramide transport but not vesicular traffic. In addition, our data revealed that overexpression of mutants lacking the SMP domain, C2 domains, or SMP-C2 domains failed to rescue the IPC synthesis defect in sec12-4 mutant cells (Figure 4E). Collectively, these results suggest that Tcb proteins are involved in non-vesicular ceramide transport and that both SMP and C2 domains of Tcb3p are required to transport ceramide efficiently at ER-Golgi contact sites.
The tcb mutant combined with the sec mutation caused increased levels in lysophosphatidylinositol (lyso-PI) (Figure 4B). Elevated levels of lyso-PI have been often observed in sec mutants such as sec12, sec13, sec16, sec23, sec18, sec6, sec7, and sec14 at restricted temperatures (Figure 4A) (Puoti et al., 1991), suggesting that increase in the lyso-PI level is not specific for TCB deletion and vesicular transport processes may be involved in lyso-PI metabolism. As lysophospholipids facilitate COPII vesicle formation (Melero et al., 2018), the increased level of lyso-PI may function to attenuate the secretion defects of sec mutants in a feedback loop. Another possibility is that tricalbin-mediated ER-Golgi contact sites regulate transport of lyso-PI between the ER and other organelles including the Golgi and the PM or lyso-PI metabolism.
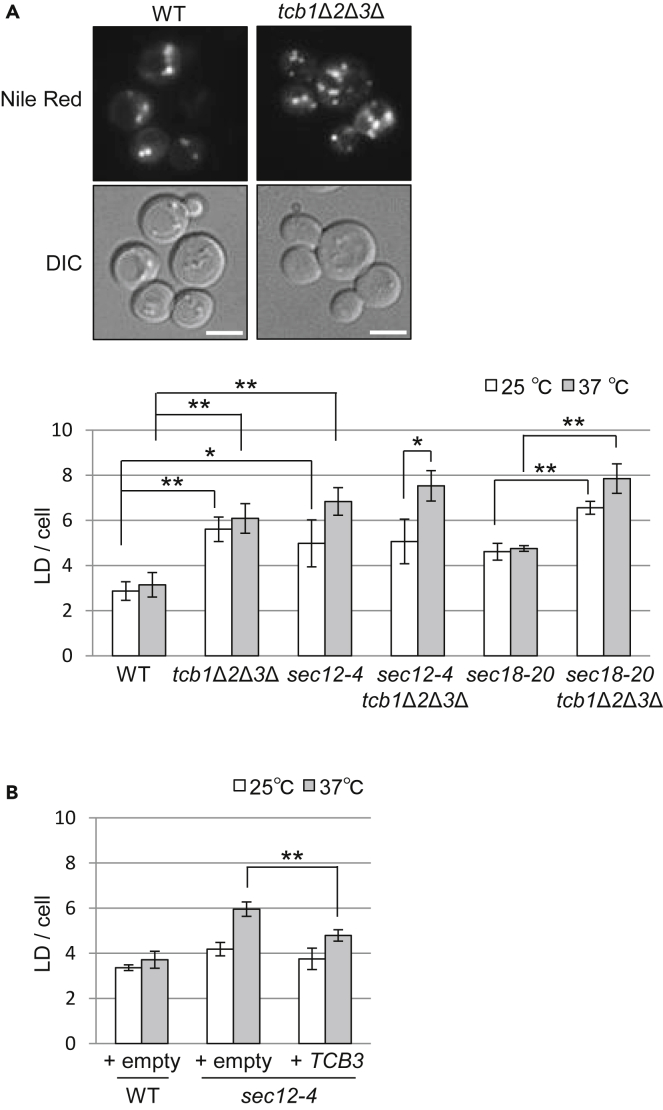
Reduced Ceramide Transport Facilitates LD Formation
Overproduced lipids are converted to neutral forms and sequestered in LDs to alleviate lipotoxicity, resulting in an accumulation of LD (Walther and Farese, 2012). Ceramide is metabolized to acylceramide by acyltransferases Lro1p and Dga1p in S. cerevisiae (Voynova et al., 2012). This has been proposed to be a mechanism for removing excess ceramides from the ER (Liu et al., 2017; Tani and Funato, 2018; Senkal et al., 2017; Voynova et al., 2012). Given that we found that tcb1Δ tcb2Δ tcb3Δ cells accumulated acylceramide (Figure 4D), we explored the possibility that deletion of Tcb proteins or defect of vesicular transport leads to stimulation of LD formation. Cells were stained with Nile Red, a lipophilic dye to visualize LDs (Greenspan et al., 1985; Radulovic et al., 2013), and the number of LDs per cell was quantified. We found that LD formation was increased in sec12-4 or sec18-20 cells (Figure 5A). Lack of Tcb proteins also caused an enhanced LD formation. In addition, the coupling of TCB deletion and sec18 mutation showed a maximal formation of LDs at a non-permissive temperature. These observations suggest that inhibition of ceramide transport or other potential substrates of Tcb proteins facilitates LD formation.
Figure 5.
Inhibition of Ceramide Transport Facilitates LD Formation
(A and B) The same strains as in Figures 4A and 4B (A) and 4E (B) were grown at 25°C and then incubated at 25°C or 37°C for 30 min. Lipid droplets (LDs) were stained with Nile Red and imaged by fluorescence microscopy, and numbers of LDs per cell were counted. The data represent mean ± SD of three independent experiments, each based on more than 100 cells. ∗p < 0.05 and ∗∗p < 0.01 by Student's t-test.
Next, we tested if Tcb3p overexpression reduces LD formation in sec12-4 mutant cells because it partially rescued the IPC synthesis defect in mutant cells (Figure 4E). As shown in Figure 5B, the increased LD formation at a non-permissive temperature in sec12-4 mutant cells was rescued by overexpression of Tcb3p. Thus, these findings indicate that Tcb3p regulates LD formation negatively, consistent with the role of Tcb proteins in non-vesicular ceramide transport. These data also suggest that non-vesicular ceramide transport can alleviate abnormal LD formation caused by defects in vesicular transport. Collectively, these results support the idea that ceramide transport is critical for the regulation of ER ceramide levels and that LDs play an important role to alleviate toxicity caused by accumulation of excess ceramides in the ER, through formation of acylceramide.
Discussion
Although Tcb proteins were initially identified as tethers localized at ER-PM contact sites and involved in regulation of PI4P metabolism (Manford et al., 2012; Toulmay and Prinz, 2012), our results revealed that a fraction of Tcb3p is also localized at contact sites with Golgi compartments and that Tcb3p and other Tcb proteins, Tcb1p and Tcb2p, play an important role in ER-Golgi contact formation (Figure 6). We also provide evidence to suggest roles of Tcb proteins in facilitating non-vesicular ceramide transport and in downregulating LD formation.
Figure 6.
A Model for the Role of Tcb Proteins in Non-vesicular Ceramide Transport and LD Formation
Tcb proteins are required for non-vesicular transport of ceramide at ER-medial-Golgi contact sites. The SMP domains in Tcb proteins may transfer ceramide, and the C2 domains may tether the ER to the medial-Golgi via the interaction with acidic phospholipids. Ceramide transport defects result in ceramide accumulation, which may increase acylceramide formation, thereby facilitating LD formation to alleviate cells from ceramide toxicity.
How might Tcb proteins promote non-vesicular ceramide transport? The SMP domains of Tcb proteins are required to localize proteins containing them to ER-PM contact sites and bind membranes (Toulmay and Prinz, 2012), suggesting that they may function to form membrane contacts. However, lack of the SMP domain in Tcb3p did not impact the number of ER-medial-Golgi contacts (Figure 3B). Because the mutant lacking the SMP domain still failed to synthesize IPC (Figure 4E), it is possible that the SMP domain mediates ceramide exchange between the ER and the Golgi. The SMP domains in the extended synaptotagmin-like proteins, E-Syts, which are mammalian orthologs of Tcb proteins, can bind and transfer lipids between membranes (Schauder et al., 2014; Yu et al., 2016). A large-scale protein-lipid interaction study using nitrocellulose arrays with immobilized lipids implied that Tcb3p binds phytoceramide (Gallego et al., 2010). Hence, the SMP domains in Tcb proteins may transfer ceramide. Alternatively, the SMP domains in Tcb proteins could function in concert with other lipid transfer proteins. As Nvj2 is localized at ER-Golgi contact sites (Liu et al., 2017) and an unknown cytosolic protein is required for non-vesicular ceramide transport (Funato and Riezman, 2001), they are possible candidates with the ability to bind and extract ceramides from the ER and deliver it to the Golgi.
In addition, our results suggest that the C2 domains of Tcb proteins are used to tether the ER to the Golgi because lack of the C2 domains in Tcb3p led to a decreased number of ER-medial-Golgi contact (Figure 3B). This is consistent with a study in mammalian cells, where knockdown of three E-Syts reduces ER-PM contact sites in a cytosolic Ca2+-regulated way, probably mediated by Ca2+-binding C2 domains (Giordano et al., 2013; Min et al., 2007). As C2 domains of Tcb proteins bind acidic phospholipids, including phosphatidylserine and phosphoinositides (Gallego et al., 2010; Schulz and Creutz, 2004), ER-Golgi contacts could take place via the interaction of acidic phospholipids with C2 domains. Thus, we propose that Tcb proteins function as tethers or lipid transfer proteins at ER-Golgi contact sites to drive non-vesicular ceramide transport (Figure 6).
Given that Tcb3p puncta are found in close appositions with the medial-Golgi where the IPC synthase enzyme Aur1p is localized (Levine et al., 2000) and that Tcb3p appears to form a complex with Tcb1p and Tcb2p (Creutz et al., 2004; Tarassov et al., 2008), it is conceivable that Tcb protein complex acts as a functional tethering complex to form ER-medial-Golgi contacts, which can transfer ceramides directly to the medial-Golgi to efficiently feed into IPC synthesis. This is consistent with our data, which showed that Tcb1p and Tcb2p are required for formation of Tcb3p puncta, suggesting that they might be important for Tcb3p function. Interestingly, there was a synthetic effect of SMP deletion to C2 deletion on Tcb3p puncta formation (Figure 1F). As no such effect was observed for ER-Golgi contact formation (Figure 3B), SMP domain may be implicated in protein oligomerization to form stable Tcb protein complexes.
Because endogenous Tcb3p is selectively enriched in the cER even though a small part is localized to small dot-like structures on the nER (see Figure S4A), we wondered what conditions increase the nER localization and cause dot formation of Tcb3p. Nvj2p was shown to become enriched at ER-Golgi contacts when cells were treated with dithiothreitol (DTT), which causes ER stress (Liu et al., 2017). We found that ER stress by treatment with DTT or tunicamycin resulted in an increased number of cells with Tcb3-GFP dots (see Figures S4A and S4B), although the fluorescence intensity (or size) of the Tcb3-GFP dots is much lower than that of Tcb3-GFP dots when overexpressed. Some of the dots co-localize with mRFP-Gos1 (see Figures S4C and S4D). These findings suggest that endogenously expressed Tcb3-GFP becomes enriched at ER-Golgi contacts in response to ER stress. This is also consistent with the model that tricalbins play a role in preventing the accumulation of toxic lipids such as ceramide, which causes ER stress. It is noteworthy that tunicamycin treatment appears to impair the transport of ceramides between the ER and the Golgi (Pittet et al., 2006; Yabuki et al., 2019).
In addition to localization to nER-medial-Golgi contact sites, Tcb3p also localizes to contact sites between the cER and medial-Golgi. Thus, it is possible that ceramide transfer occurs at cER-medial-Golgi contact sites. Further work will be needed to determine the exact site where non-vesicular ceramide transport occurs.
How the impaired transport of ceramide facilitates LD formation is not fully understood, but we propose a model in which abnormal accumulation of ceramides in the ER caused by impaired ceramide transport leads to an elevated acylceramide level through activity of ER-localized Lro1p and Dga1p (Voynova et al., 2012), thereby stimulating LD formation. Indeed, a significant increase of acylceramide levels was observed in the tcb1Δ tcb2Δ tcb3Δ cells compared to wild-type cells (Figure 4D). Similar results were obtained with sec background strains (the relative acylceramide level [%] of sec12-4 tcb1Δ tcb2Δ tcb3Δ to sec12-4 cells was 122%). The levels of triacylglycerides and sterol esters, which are the main components of the lipid droplet core structure, were significantly increased in sec mutants at non-permissive temperatures, but not in the cells lacking all three Tcb proteins (see Figures S5A and S5B); rather, the lack of Tcb proteins led to a reduced level of sterol esters, which might be due to a defect in retrograde transport of sterol from the PM to the ER (Quon et al., 2018). Therefore, these results imply that the enhanced LD formation in tcb1Δ tcb2Δ tcb3Δ cells results from the acylceramide accumulation caused by defect of non-vesicular ceramide transport. We have previously shown that LD formation is facilitated in S. cerevisiae mutant strains defective in GPI anchor synthesis and in GPI anchoring (Kajiwara et al., 2008). Mutant cells of the S. pombe gene homolog of S. cerevisiae CWH43 gene, which is implicated in remodeling of GPI lipid moiety to ceramide (Ghugtyal et al., 2007; Umemura et al., 2007), were also shown to have increased numbers of LDs (Nakazawa et al., 2018). Since these gpi mutants cause IPC synthesis defects (Kajiwara et al., 2008), it is plausible that enhanced LD formation in these mutants results from increased ceramide levels through defects in vesicular transport of ceramides. Moreover, it is noteworthy that overexpression of TCB3 suppressed the enhanced LD formation in sec12-4 mutant cells. Because their overexpression did not affect vesicular protein traffic in the mutant cells, ceramides accumulated in the ER due to vesicular transport defects can likely be bypassed by non-vesicular transport routes. Thus, non-vesicular ceramide transport may play important roles as a defense system to protect cells against the toxic accumulation of ceramide when vesicular traffic is impaired.
In summary, our studies suggest a new function for Tcb proteins in non-vesicular transport of ceramides at ER-Golgi contact sites. Further work is required to elucidate what features of medial-Golgi are recognized by the C2 domains of Tcb proteins, whether the SMP domain transfers ceramides between membranes, to identify the ligand-binding sites within the C2 domains and the SMP domain by biochemical methods using various point mutants and radioactive or photocrosslinkable ligands (Dadsena et al., 2019). Nvj2p has been shown to be an ER-Golgi tethering protein that facilitates the non-vesicular transfer of ceramides to the Golgi complex (Liu et al., 2017). It is an intriguing question whether tricalbins and Nvj2p have cooperative roles in ceramide transport. Also, a cytosolic protein(s) involved in non-vesicular ceramide transport (Funato and Riezman, 2001) still remains to be identified.
Limitation of the Study
Although our study reveals that tricalbins are involved in non-vesicular ceramide transport from the ER to the Golgi, it is unknown whether tricalbins have the ability to bind and extract ceramides from the ER and deliver it to the Golgi. One possibility is that SMP domain of Tcb3p may transfer ceramides. However, we could not show direct binding of SMP domain of Tcb3p to ceramides. Further studies will be required to determine whether tricalbins directly bind to ceramide or to identify other ceramide transfer proteins that may function in concert with tricalbins at ER-Golgi contact sites. We also wish to emphasize that nanometer distances between membranes at membrane contact sites are below the diffraction limit for light microscope. As electron microscopy, especially when correlated with light microscopy, is a powerful technique to analyze ultrastructure which cannot otherwise be seen, it might provide further insight into the role of tricalbins at ER-Golgi contact sites.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Kouichi Funato (kfunato@hiroshima-u.ac.).
Materials Availability
Yeast strains and plasmids generated in this study will be made available on reasonable requests.
Data and Code Availability
The data that support the findings of this study are included in the article and its Supplemental Information, or are available from the corresponding authors on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to Dr. R. Schekman for providing yeast sec mutant strains. This work was supported by the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Scientific Research (KAKENHI), Japan [JP16K07693 and 19H02922 to K.F.], [JP25221103, JP16HD05419, JP17H06420 and JP18H05275 to K.K.], by a JSPS Research Fellowship for Young Scientists, Japan [18J10104 to A.I.], by the Swiss National Science Foundation, Switzerland [310030B_166686 to H.R,], and by the NCCR Chemical Biology funded by the Swiss National Science Foundation, Switzerland [51NF40-160589 to H.R.].
Author Contributions
A.I. and K.F. designed the experiments. A.I., P.S., and K.K. performed the experiments. A.I., P.S., K.K., A.N., H.R, and K.F. analyzed data and wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101603.
Supplemental Information
References
- Creutz C.E., Snyder S.L., Schulz T.A. Characterization of the yeast tricalbins: membrane-bound multi-C2-domain proteins that form complexes involved in membrane trafficking. Cell Mol. Life Sci. 2004;61:1208–1220. doi: 10.1007/s00018-004-4029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadsena S., Bockelmann S., Mina J.G.M., Hassan D.G., Korneev S., Razzera G., Jahn H., Niekamp P., Müller D., Schneider M. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 2019;10:1832. doi: 10.1038/s41467-019-09654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T., Büttner S. Lipids and cell death in yeast. FEMS Yeast Res. 2014;14:179–197. doi: 10.1111/1567-1364.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato K., Riezman H. Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast. J. Cell Biol. 2001;155:949–959. doi: 10.1083/jcb.200105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato K., Vallée B., Riezman H. Biosynthesis and trafficking of sphingolipids in the yeast Saccharomyces cerevisiae. Biochemistry. 2002;41:15105–15114. doi: 10.1021/bi026616d. [DOI] [PubMed] [Google Scholar]
- Gallego O., Betts M.J., Gvozdenovic-Jeremic J., Maeda K., Matetzki C., Aguilar-Gurrieri C., Beltran-Alvarez P., Bonn S., Fernández-Tornero C., Jensen L.J. A systematic screen for protein-lipid interactions in Saccharomyces cerevisiae. Mol. Syst. Biol. 2010;6:430. doi: 10.1038/msb.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghugtyal V., Vionnet C., Roubaty C., Conzelmann A. CWH43 is required for the introduction of ceramides into GPI anchors in Saccharomyces cerevisiae. Mol. Microbiol. 2007;65:1493–1502. doi: 10.1111/j.1365-2958.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- Giordano F., Saheki Y., Idevall-Hagren O., Colombo S.F., Pirruccello M., Milosevic I., Gracheva E.O., Bagriantsev S.N., Borgese N., De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P., Mayer E.P., Fowler S.D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Harayama T., Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018;19:281–296. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- Holthuis J.C., Levine T.P. Lipid traffic: floppy drives and a superhighway. Nat. Rev. Mol. Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- Holthuis J.C., Menon A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510:48–57. doi: 10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- Ito M., Okino N., Tani M. New insight into the structure, reaction mechanism, and biological functions of neutral ceramidase. Biochim. Biophys. Acta. 2014;1841:682–691. doi: 10.1016/j.bbalip.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Jain A., Holthuis J.C.M. Membrane contact sites, ancient and central hubs of cellular lipid logistics. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1450–1458. doi: 10.1016/j.bbamcr.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Ikeda A., Aguilera-Romero A., Castillon G.A., Kagiwada S., Hanada K., Riezman H., Muñiz M., Funato K. Osh proteins regulate COPII-mediated vesicular transport of ceramide from the endoplasmic reticulum in budding yeast. J. Cell Sci. 2014;127:376–387. doi: 10.1242/jcs.132001. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Watanabe R., Pichler H., Ihara K., Murakami S., Riezman H., Funato K. Yeast ARV1 is required for efficient delivery of an early GPI intermediate to the first mannosyltransferase during GPI assembly and controls lipid flow from the endoplasmic reticulum. Mol. Biol. Cell. 2008;19:2069–2082. doi: 10.1091/mbc.E07-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec K.O., Alva V., Lupas A.N. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics. 2010;26:1927–1931. doi: 10.1093/bioinformatics/btq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K., Okamoto M., Nakano A. Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat. Commun. 2014;5:3653. doi: 10.1038/ncomms4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus G., Kabadere S., Uyar R., Kutlu H.M. Induction of apoptosis in prostate cancer cells by the novel ceramidase inhibitor ceranib-2. In Vitro Cell Dev. Biol. Anim. 2015;51:1056–1063. doi: 10.1007/s11626-015-9932-9. [DOI] [PubMed] [Google Scholar]
- Levine T.P., Wiggins C.A., Munro S. Inositol phosphorylceramide synthase is located in the Golgi apparatus of Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:2267–2281. doi: 10.1091/mbc.11.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.K., Choudhary V., Toulmay A., Prinz W.A. An inducible ER-Golgi tether facilitates ceramide transport to alleviate lipotoxicity. J. Cell Biol. 2017;216:131–147. doi: 10.1083/jcb.201606059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford A.G., Stefan C.J., Yuan H.L., Macgurn J.A., Emr S.D. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev. Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K., Takeuchi M., Ichihara A., Mikuriya K., Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- McNew J.A., Coe J.G., Søgaard M., Zemelman B.V., Wimmer C., Hong W., Söllner T.H. Gos1p, a Saccharomyces cerevisiae SNARE protein involved in Golgi transport. FEBS Lett. 1998;435:89–95. doi: 10.1016/s0014-5793(98)01044-8. [DOI] [PubMed] [Google Scholar]
- Melero A., Chiaruttini N., Karashima T., Riezman I., Funato K., Barlowe C., Riezman H., Roux A. Lysophospholipids facilitate COPII vesicle formation. Curr. Biol. 2018;28:1950–1958. doi: 10.1016/j.cub.2018.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S.W., Chang W.P., Südhof T.C. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc. Natl. Acad. Sci. U S A. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa N., Teruya T., Sajiki K., Kumada K., Villar-Briones A., Arakawa O., Takada J., Saitoh S., Yanagida M. The putative ceramide-conjugation protein Cwh43 regulates G0 quiescence, nutrient metabolism and lipid homeostasis in fission yeast. J. Cell Sci. 2018;131:jcs217331. doi: 10.1242/jcs.217331. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Ridgway N.D. Molecular mechanisms and regulation of ceramide transport. Biochim. Biophys. Acta. 2005;1734:220–234. doi: 10.1016/j.bbalip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Pittet M., Uldry D., Aebi M., Conzelmann A. The N-glycosylation defect of cwh8Delta yeast cells causes a distinct defect in sphingolipid biosynthesis. Glycobiology. 2006;16:155–164. doi: 10.1093/glycob/cwj043. [DOI] [PubMed] [Google Scholar]
- Puoti A., Desponds C., Conzelmann A. Biosynthesis of mannosylinositolphosphoceramide in Saccharomyces cerevisiae is dependent on genes controlling the flow of secretory vesicles from the endoplasmic reticulum to the Golgi. J. Cell Biol. 1991;113:515–525. doi: 10.1083/jcb.113.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon E., Sere Y.Y., Chauhan N., Johansen J., Sullivan D.P., Dittman J.S., Rice W.J., Chan R.B., Di Paolo G., Beh C.T., Menon A.K. Endoplasmic reticulum-plasma membrane contact sites integrate sterol and phospholipid regulation. PLoS Biol. 2018;16:e2003864. doi: 10.1371/journal.pbio.2003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M., Knittelfelder O., Cristobal-Sarramian A., Kolb D., Wolinski H., Kohlwein S.D. The emergence of lipid droplets in yeast: current status and experimental approaches. Curr. Genet. 2013;59:231–242. doi: 10.1007/s00294-013-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder C.M., Wu X., Saheki Y., Narayanaswamy P., Torta F., Wenk M.R., De Camilli P., Reinisch K.M. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014;510:552–555. doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T.A., Creutz C.E. The tricalbin C2 domains: lipid-binding properties of a novel, synaptotagmin-like yeast protein family. Biochemistry. 2004;43:3987–3995. doi: 10.1021/bi036082w. [DOI] [PubMed] [Google Scholar]
- Senkal C.E., Salama M.F., Snider A.J., Allopenna J.J., Rana N.A., Koller A., Hannun Y.A., Obeid L.M. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 2017;25:686–697. doi: 10.1016/j.cmet.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E., Levental I., Mayor S., Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Tani M., Funato K. Protection mechanisms against aberrant metabolism of sphingolipids in budding yeast. Curr. Genet. 2018;64:1021–1028. doi: 10.1007/s00294-018-0826-8. [DOI] [PubMed] [Google Scholar]
- Tarassov K., Messier V., Landry C.R., Radinovic S., Serna Molina M.M., Shames I., Malitskaya Y., Vogel J., Bussey H., Michnick S.W. An in vivo map of the yeast protein interactome. Science. 2008;320:1465–1470. doi: 10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- Toulmay A., Prinz W.A. A conserved membrane-binding domain targets proteins to organelle contact sites. J. Cell Sci. 2012;125:49–58. doi: 10.1242/jcs.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura M., Fujita M., Yoko-O T., Fukamizu A., Jigami Y. Saccharomyces cerevisiae CWH43 is involved in the remodeling of the lipid moiety of GPI anchors to ceramides. Mol. Biol. Cell. 2007;18:4304–4316. doi: 10.1091/mbc.E07-05-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynova N.S., Roubaty C., Vazquez H.M., Mallela S.K., Ejsing C.S., Conzelmann A. Saccharomyces cerevisiae is dependent on vesicular traffic between the Golgi apparatus and the vacuole hhen inositolphosphorylceramide synthase Aur1 is inactivated. Eukaryot. Cell. 2015;14:1203–1216. doi: 10.1128/EC.00117-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynova N.S., Vionnet C., Ejsing C.S., Conzelmann A. A novel pathway of ceramide metabolism in Saccharomyces cerevisiae. Biochem. J. 2012;447:103–114. doi: 10.1042/BJ20120712. [DOI] [PubMed] [Google Scholar]
- Walther T.C., Farese R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuki Y., Ikeda A., Araki M., Kajiwara K., Mizuta K., Funato K. Sphingolipid/Pkh1/2-TORC1/Sch9 signaling regulates ribosome biogenesis in tunicamycin-induced stress response in yeast. Genetics. 2019;212:175–186. doi: 10.1534/genetics.118.301874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji T., Hanada K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic. 2015;16:101–122. doi: 10.1111/tra.12239. [DOI] [PubMed] [Google Scholar]
- Yu H., Liu Y., Gulbranson D.R., Paine A., Rathore S.S., Shen J. Extended synaptotagmins are Ca2+-dependent lipid transfer proteins at membrane contact sites. Proc. Natl. Acad. Sci. U S A. 2016;113:4362–4367. doi: 10.1073/pnas.1517259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are included in the article and its Supplemental Information, or are available from the corresponding authors on request.