Abstract
Background
Real‐world data enables evaluation of immune checkpoint inhibitor (ICI) use in advanced melanoma management. We examined characteristics and outcomes of ICI‐treated patients with advanced melanoma and organ dysfunction (baseline and emergent).
Materials and Methods
This retrospective observational study used electronic health records derived from a nationwide data set to examine advanced melanoma patients treated with first‐line ICIs (2011–2018). Clinical characteristics, real‐world time to treatment discontinuation (rwTTD), and overall survival (OS) were analyzed for patients with normal organ function and those with organ dysfunction prior to ICI initiation. Patients with emergent dysfunction in the 90 days following ICI initiation were identified, and potentially associated characteristics were explored.
Results
Of 2,407 patients included, 1,884 and 1,717 had evaluable renal and hepatic laboratory values, respectively. Patients with baseline renal dysfunction (2.4%) were older and more frequently male, and less frequently treated with ICI combinations, than patients with normal renal function. Patients with baseline hepatic dysfunction (2.8%) were similar to patients with normal hepatic function regarding demographics and treatments received. Patients with baseline organ dysfunction displayed shorter rwTTD and OS. Among patients with normal baseline organ function, 4.6% and 7.4% developed renal and hepatic dysfunction within 90 days of ICI initiation, respectively; this was associated with combination ICI treatment.
Conclusion
Patients with advanced melanoma and baseline organ dysfunction frequently receive ICI treatment but have poorer clinical outcomes than patients with normal organ function. Among patients with normal renal and hepatic function at ICI initiation, emergent organ dysfunction rates in this real‐world cohort are similar to those reported in clinical trials.
Implications for Practice
Real‐world data provide an opportunity to understand treatment patterns, toxicity, and clinical outcomes among patients treated outside of clinical trials. This study confirms that patients with advanced melanoma and baseline renal or hepatic dysfunction are being treated with ICI therapy more frequently as monotherapy than in combination therapy. For those real‐world patients with normal baseline organ function, emergent renal and hepatic dysfunction are both more common in patients treated with combination versus ICI monotherapy.
Keywords: Melanoma, Immunotherapy, Renal impairment, Liver impairment
Short abstract
Evidence shows improved survival for patients with advanced melanoma treated with the CTLA‐4 inhibitor, ipilimumab, and PD‐1 inhibitors. This article examines characteristics and outcomes of patients with advanced melanoma and organ dysfunction treated with immune checkpoint inhibitors, for patients with both baseline dysfunction and treatment‐emergent toxicity, using a dataset of real world patients.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of cancer. In advanced melanoma, randomized controlled trials (RCTs) have demonstrated improved progression‐free and overall survival with the CTLA‐4 inhibitor, ipilimumab, and programmed cell death protein 1 inhibitors nivolumab and pembrolizumab [1, 2, 3], leading to their recommendation as first‐line systemic therapy within the U.S. [4]. Since their first approval in 2011, the adoption of ICIs into clinical practice has been rapid. A recent study of treatment patterns in advanced melanoma in the U.S. found that by mid‐2016, among patients receiving any first‐line systemic therapy, 81% of patients received an ICI‐based regimen [5]. This figure rose to 97% for patients with BRAF wild‐type advanced melanoma [5].
Cancer treatment options and outcomes may be impacted by the presence of comorbid illness. Comorbidities are common in patients diagnosed with cancer [6, 7], represent a competing risk for death, and present challenges to delivering optimal cancer therapies [6, 7, 8, 9]. Evidence of renal or hepatic dysfunction, in particular, may impact treatment choices because of concerns over decreased drug clearance and increased toxicity [7, 8, 10]. Historically, patients with evidence of baseline moderate or severe renal or hepatic dysfunction have been excluded from most clinical trials, including multiple pivotal trials informing ICI drug approvals [11, 12, 13, 14, 15, 16]. However, pharmacokinetic data suggest that ICIs do not undergo renal or hepatic clearance or metabolism [17]. Given the high efficacy of these agents and the limited treatment options available in advanced melanoma, the knowledge gap regarding the clinical profile of ICI therapies in patients with baseline renal and hepatic dysfunction is highly relevant, and additional information is needed.
Although not directly toxic to the liver and kidneys, ICI treatments are also known to give rise to immune‐related adverse events (irAEs) impacting these vital organs, potentially limiting the duration of ICI therapy [18, 19, 20]. The pooled rate for any immune‐related renal dysfunction reported in RCTs of ICIs is approximately 2%, whereas the rates of hepatic dysfunction ranged between 1% and 17%, dependent on specific ICI treatment and/or combination [21, 22, 23]. However, recent small observational studies have suggested that the real‐world incidence of irAEs may be higher than previously estimated in RCTs, reporting occurrences of renal toxicities at rates that range from 10% to 29% [24]. Larger cohorts of patients from real‐world data can provide greater insights into the occurrences of irAEs with ICIs [25]. In this study, we aimed to use a large national data set to examine the characteristics and clinical outcomes of patients with advanced melanoma treated with ICI, considering both baseline and emergent renal and hepatic dysfunction.
Subjects, Materials, and Methods
Data Source
Our retrospective observational study used the Flatiron Health database, which is a nationwide, longitudinal, demographically and geographically diverse deidentified database derived from electronic health record (EHR) data [26]. At the time of data delivery, this database covered over 280 cancer clinics (community oncology and academic centers) at over 800 geographically diverse sites of care, representing over 2.1 million patients with active cancer. The data comprise both structured data (e.g., laboratory values), which undergo harmonization, and unstructured data, which are collected via technology‐enabled chart abstraction from physicians’ notes and other unstructured documents (e.g., biomarker reports), as described previously [27]. Institutional review board approval with waiver of informed consent was obtained prior to study conduct.
Study Cohort
Patients were included for analysis if they had a structured diagnosis code for melanoma (International Classification of Diseases [ICD]‐9 172 or ICD‐10 C43 or D03) and two clinic encounters documented in the database between January 1, 2011, and August 31, 2018, on distinct days (Fig. 1). Advanced melanoma was defined as American Joint Committee on Cancer stage III/IV at initial diagnosis on or after January 1, 2011, or a first locoregional or distant recurrence on or after January 1, 2011, as verified via abstraction of unstructured documents. Patients were excluded if they had noncutaneous melanoma or lacked relevant unstructured documents in the database for manual review. All patients additionally received one of the following standard‐of‐care ICI regimens as their first‐line treatment for advanced melanoma: (a) ipilimumab monotherapy, (b) ipilimumab plus nivolumab combination treatment, (c) nivolumab, or (d) pembrolizumab. First‐line treatment was identified as the first systemic antineoplastic therapy administered after the date of advanced melanoma diagnosis [27].
Figure 1.
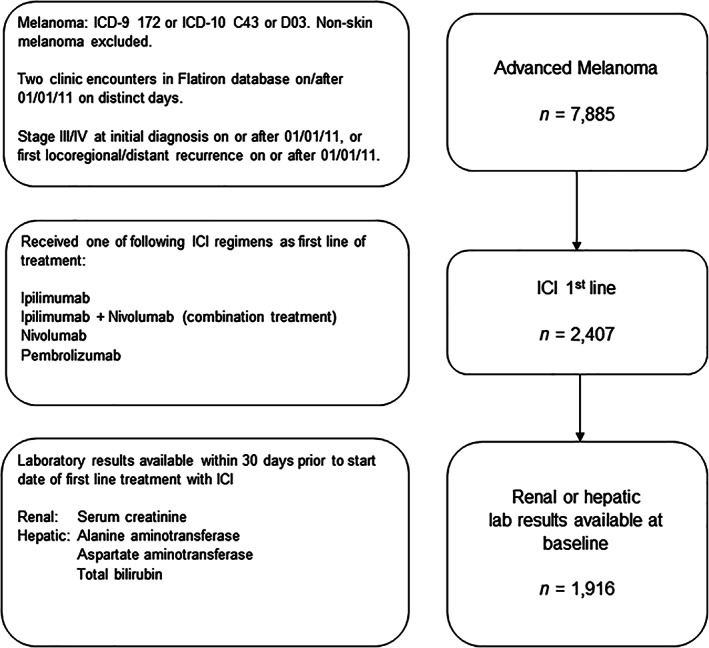
Flow diagram depicting inclusion of patients for analysis. Abbreviations: ICD, International Classification of Diseases; ICI, immune checkpoint inhibitor.
Patients were required to have at least one evaluable laboratory result up to 30 days prior to the start of first‐line ICI treatment for inclusion in the assessment of baseline renal or hepatic function. An evaluable laboratory result required a relevant laboratory name, test date, test result with standardized units, and, for hepatic function laboratory values, an upper limit of normal (ULN). For inclusion in the evaluation of emergent dysfunction, patients were required to have both normal organ function at baseline (a laboratory result of dysfunction grade ≤1; Table 1), and at least one subsequent evaluable renal or hepatic laboratory value up to 90 days after first‐line ICI treatment commencement.
Table 1.
Classification of renal or hepatic dysfunction based on laboratory values
| Organ (dys)function classification | Renal | Hepatic |
|---|---|---|
| “Normal” organ function (Grade ≤1 CTCAE) | Creatinine ≤1.5× ULN | Total bilirubin ≤1.5× ULN; and AST or ALT <3× ULN |
| Moderate dysfunction (Grade 2 CTCAE) | Creatinine >1.5× to 3× ULN | Total bilirubin >1.5× to 3× ULN; or AST or ALT >3× to 5× ULN |
| Severe dysfunction (Grade >2 CTCAE) | Creatinine >3× ULN | Total bilirubin >3× ULN; or AST or ALT >5× ULN |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal range.
Variable and Outcome Definitions
We compared clinical and demographic characteristics in patients with and without baseline renal or hepatic dysfunction. Demographic characteristics included sex and age at time of ICI treatment, whereas clinical characteristics included stage at diagnosis, Eastern Cooperative Oncology Group performance status (ECOG PS) score at time of ICI treatment, lactate dehydrogenase (LDH) elevation (≥2× ULN), BRAF biomarker status, treatment setting (academic vs. community), and first‐line ICI regimen received. For all patients, the start date of ICI treatment (baseline) was recorded as the date of first administration or noncancelled order from structured data during first‐line ICI therapy.
Renal and hepatic dysfunction were assessed based on evaluable laboratory results and were graded according to the Common Terminology Criteria for Adverse Events, v4.03. Renal function was assessed based on serum creatinine values, and hepatic function was assessed based on alanine aminotransferase, aspartate aminotransferase, and bilirubin values, as per Table 1. Emergent dysfunction was determined based on the highest renal or hepatic lab value recorded in the follow‐up period (up to 90 days following ICI treatment).
Outcomes of interest included real‐world time to treatment discontinuation (rwTTD) [28] and overall survival (OS). rwTTD was calculated using the Kaplan‐Meier approach such that the event was the last administration or noncancelled order within first‐line ICI therapy if one of the following censoring conditions were met: (a) subsequent line of therapy commenced, (b) death, or (c) gap of more than 90 days between the patient's last administration or noncancelled order and their last date of EHR‐documented activity (e.g., clinic visit, laboratory order, treatment administration). OS was calculated from the start of first‐line ICI regimen. Otherwise, the patient's last administration or noncancelled order was leveraged as the censor date. OS was examined using composite date of death values derived from unstructured EHR data, external commercial sources, and U.S. Social Security Death Index data, as described previously [29]. Patients without a date of death were censored at their last visit (per structured data).
Statistical Analysis
For the comparison of characteristics among patients with renal or hepatic organ dysfunction versus normal organ function at baseline, characteristics were tabulated by organ function category and described using proportions (categorical variables) or median values accompanied by interquartile ranges (IQR; numerical). Tests for differences between patients with organ dysfunction versus normal organ function were performed using the χ2 test (categorical) or Kruskal‐Wallis test (numerical). Comparisons of outcomes (rwTTD and OS) between patients with organ dysfunction versus normal organ function were made by calculating median values accompanied by 95% confidence intervals (CIs) and constructing unadjusted Kaplan‐Meier curves based on the data; differences between the curves were tested using the log‐rank test. Exploratory adjusted analyses were performed to examine associations between organ dysfunction and rwTTD and OS; Cox proportional hazards models included the following a priori‐selected categorical variables: organ function, age (<70 years, ≥70 years), stage at diagnosis (unknown; 0, I, or II; III or IV); ICI treatment regimen (monotherapy, combination therapy).
For the analysis of emergent organ dysfunction post‐treatment start, we calculated the proportion of patients, among those with evidence of normal organ function at baseline, who subsequently developed (a) renal or (b) hepatic dysfunction within 90 days of treatment initiation. To examine potential associations between patient characteristics, treatment regimen and development of grade 2+ emergent dysfunction (yes or no), adjusted odds ratios (aOR) were estimated from a multivariable logistic regression model, which included the following a priori‐selected categorical variables based on clinical relevance: age (<70 years, ≥70 years), sex (male, female), stage at diagnosis (unknown, 0/I/II, III/IV), practice type (academic, community), and ICI treatment regimen (monotherapy, combination therapy). A supplementary analysis repeated the analysis with the ICI treatment regimen “monotherapy” category broken down by individual drug. A sensitivity analysis was also conducted that excluded patients with a greater‐than‐90‐day gap between their advanced diagnosis date and their first structured visit, as these patients may have received first‐line therapy outside of the network.
In consideration of external validity, as our study design required patients to have evaluable renal and/or hepatic laboratory tests at baseline, we examined the difference in patient characteristics, rwTTD, and OS between patients with and without evaluable laboratory data. All analyses were performed using R version 3.3.2. The threshold for statistical significance was set to p < .05.
Results
Overall Study Population
A total of 2,407 patients with advanced melanoma were identified. Patients had a median age of 69.0 years (IQR, 59.0‐78.0) at diagnosis. Most patients were male (68.6%) and treated at a community oncology practice (87.0%). ECOG performance status at baseline was available more than half the time (53.6%) and most often recorded as either 0 or 1 (44.3%). Melanoma was diagnosed with distant metastatic disease in 30.2% of patients. Among patients who had evidence of a BRAF mutation test result available prior to treatment initiation (80.5% of total), 24.8% were mutation positive. First‐line treatment included ipilimumab monotherapy, pembrolizumab monotherapy, nivolumab monotherapy, and ipilimumab and nivolumab combination therapy, in 30.8%, 27.5%, 21.1%, and 20.5% of patients, respectively. From the overall cohort, 1,916 patients had either an evaluable renal or a hepatic laboratory result at baseline with a median follow‐up time of 29.1 months (95% CI, 27.9–31.0).
Organ Dysfunction at Baseline
Renal
Among patients with evaluable baseline renal or hepatic results, 1,884 had baseline renal laboratory results available up to 30 days prior to ICI treatment start date. Among these, 46 (2.4%) were found to have moderate or severe renal dysfunction at baseline, most of them categorized as having moderate dysfunction (39/46 [84.8%]). Patients with baseline moderate or severe renal dysfunction were more frequently male and older than patients with normal baseline renal function (Table 2). Combination therapy (ipilimumab and nivolumab) was selected as first‐line therapy in 6.5% of all patients with baseline renal dysfunction, in comparison to 21% of patients with normal renal function at baseline (Table 2).
Table 2.
Characteristics of patients by renal function and by hepatic function at start of first‐line immune checkpoint inhibitor treatment
| Baseline renal function | Baseline hepatic function | |||||
|---|---|---|---|---|---|---|
| Patient characteristic | Normal (n = 1,838) | Moderate or severe dysfunction (n = 46) | p value | Normal (n = 1,669) | Moderate or severe dysfunction (n = 48) | p value |
| Sex, n (%) | .004 | .696 | ||||
| Female | 584 (31.8) | 5 (10.9) | 514 (30.8) | 13 (27.1) | ||
| Male | 1,254 (68.2) | 41 (89.1) | 1,155 (69.2) | 35 (72.9) | ||
| Median age a (IQR), yr | 69.0 (59.0–77.8) | 78.5 (72.2–82.0) | <.001 | 69.0 (59.0–78.0) | 66.0 (61.0–75.5) | .493 |
| Clinical characteristics, n (%) | ||||||
| Stage at initial diagnosis | .068 | .839 | ||||
| Stage 0 | 8 (0.4) | 0 (0.0) | 8 (0.5) | 0 (0.0) | ||
| Stage I | 155 (8.4) | 0 (0.0) | 135 (8.1) | 2 (4.2) | ||
| Stage II | 344 (18.7) | 9 (19.6) | 312 (18.7) | 9 (18.8) | ||
| Stage III | 412 (22.4) | 11 (23.9) | 362 (21.7) | 9 (18.8) | ||
| Stage IV | 545 (29.7) | 10 (21.7) | 497 (29.8) | 18 (37.5) | ||
| Unknown | 374 (20.3) | 16 (34.8) | 355 (21.3) | 10 (20.8) | ||
| ECOG PS a | .466 | .351 | ||||
| 0 | 450 (24.5) | 10 (21.7) | 436 (26.1) | 13 (27.1) | ||
| 1 | 373 (20.3) | 8 (17.4) | 353 (21.2) | 12 (25.0) | ||
| 2+ | 156 (8.5) | 7 (15.2) | 152 (9.1) | 7 (14.6) | ||
| Missing | 859 (46.7) | 21 (45.7) | 728 (43.6) | 16 (33.3) | ||
| Tumor characteristics, n (%) | ||||||
| BRAF | .307 | .909 | ||||
| Mutation − | 1,043 (56.7) | 29 (63.0) | 953 (57.1) | 30 (62.5) | ||
| Mutation + | 473 (25.7) | 7 (15.2) | 425 (25.5) | 12 (25.0) | ||
| No evidence of testing in EHR prior to treatment | 267 (14.5) | 8 (17.4) | 234 (14.0) | 5 (10.4) | ||
| Unknown or indeterminate | 55 (3.0) | 2 (4.3) | 57 (3.4) | 1 (2.1) | ||
| Treatment characteristics, n (%) | ||||||
| Practice type | .446 | .225 | ||||
| Academic | 297 (16.2) | 5 (10.9) | 168 (10.1) | 2 (4.2) | ||
| Community | 1,541 (83.8) | 41 (89.1) | 1,501 (89.9) | 46 (95.8%) | ||
| Regimen | .086 | .887 | ||||
| ipilimumab | 575 (31.3) | 15 (32.6) | 528 (31.6) | 14 (29.2) | ||
| ipi. + nivolumab | 386 (21.0) | 3 (6.5) | 339 (20.3) | 12 (25.0) | ||
| nivolumab | 361 (19.6) | 13 (28.3) | 332 (19.9) | 9 (18.8) | ||
| pembrolizumab | 516 (28.1) | 15 (32.6) | 470 (28.2) | 13 (27.1) | ||
At index date, defined as first‐line therapy start date.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; EHR, electronic health record; IQR, interquartile range; ipi, ipilimumab.
Figure 2 presents Kaplan‐Meier curves of the rwTTD of ICI treatment (Fig. 2A) and OS (Fig. 2B) for patients with baseline normal and abnormal renal function. Estimates of the median rwTTD and OS are presented in Table 3, overall and by ICI regimen. For rwTTD, the log‐rank test indicated a difference in discontinuation times for moderate or severe renal dysfunction versus normal renal functional status (p = .033; Fig. 2A). A decrease in median OS was observed for patients with renal dysfunction versus patients with normal renal functional status at baseline (22.1 months [95% CI, 19.9, 25.5] vs. 8.2 months [95% CI, 4.2–12.9]; Fig. 2B; Table 3); we note, however, that this result does not involve adjustment for age or other underlying factors that may influence survival probability. Similar results in outcomes were observed in the sensitivity analysis restricted to patients with structured activity within 90 days of their advanced diagnosis date. Supplemental online Table 1 includes exploratory results of associations between renal function and rwTTD and OS. Following adjustment for age, stage at diagnosis, and treatment regimen, renal dysfunction was associated with decreased OS (p < .001) versus normal renal function.
Figure 2.
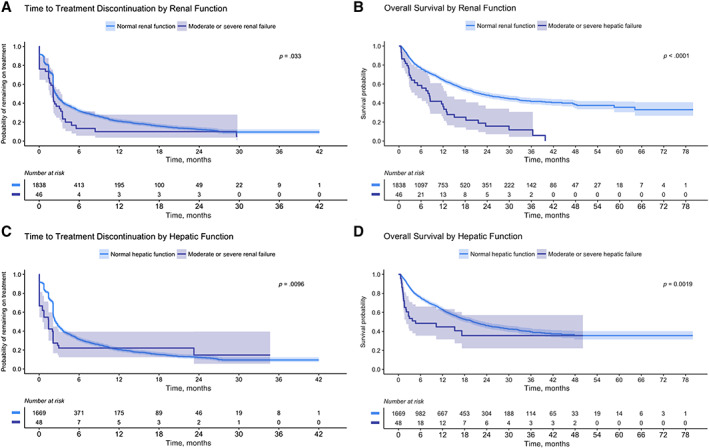
Time‐to‐event results by baseline renal functional status and by baseline hepatic functional status. (A): Real‐world time to treatment discontinuation (rwTTD) of first‐line immune checkpoint inhibitor (ICI) treatment and (B) overall survival (OS) by baseline renal functional status. (C): Time to ICI treatment discontinuation (rwTTD) and (D) OS by baseline hepatic functional status.
Table 3.
Median time‐to‐event results by baseline renal function according to treatment regimens, rwTTD and OS
| Normal | Moderate or severe dysfunction | |||
|---|---|---|---|---|
| Outcome and treatment regimen | Events/n | Median (95% CI), mo | Events/n | Median (95% CI), mo |
| rwTTD of ICI | ||||
| All | 1,299/1,838 | 2.5 (2.3–2.8) | 37/46 | 2.1 (1.6–3.2) |
| Ipilimumab | 545/575 | 2.1 (2.1–2.1) | 14/15 | 2.1 (1.0–3.1) |
| Ipi + nivolumab | 246/386 | 4.0 (3.3–5.5) | 1/3 | 29.6 (n/a–n/a) |
| Nivolumab | 195/361 | 6.6 (5.5–8.5) | 9/13 | 3.3 (0.03–n/a) |
| Pembrolizumab | 313/516 | 5.5 (4.7–7.5) | 13/15 | 2.1 (1.5–n/a) |
| OS | ||||
| All | 738/1,838 | 22.1 (19.9–25.5) | 35/46 | 8.2 (4.2–12.9) |
| Ipilimumab | 345/575 | 15.9 (14.0–18.5) | 13/15 | 8.4 (3.1–n/a) |
| Ipi + nivolumab | 125/386 | 29.4 (20.3 –n/a) | 1/3 | 29.9 (n/a–n/a) |
| Nivolumab | 109/361 | 33.0 (19.6–n/a) | 8/13 | 8.5 (2.6–n/a) |
| Pembrolizumab | 159/516 | 28.8 (23.2–n/a) | 13/15 | 5.0 (2.8–n/a) |
For the median estimate, n/a occurred when <50% of the cohort had the event of interest. For 95% CI bounds, this occurred either when there were too few events or because the upper confidence limit is <50%.
Abbreviations: CI, confidence interval; Ipi, ipilimumab; OS, overall survival; n/a, result not calculable; rwTTD, real‐world time to treatment discontinuation.
Among the 39 patients with moderate (vs. severe) renal dysfunction at baseline, 34 had at least one evaluable renal laboratory value within the first 30 days of treatment. One of these patients was found to have developed a “severe” renal dysfunction status during this time.
Hepatic
There were 1,717 patients with evaluable baseline hepatic test results. A total of 48 (2.8%) were found to have moderate or severe hepatic dysfunction, with 39 (81%) classified as having moderate dysfunction. Patients with baseline moderate or severe hepatic dysfunction were more likely to have an elevated LDH level compared with patients with normal hepatic function (45.8% versus 20.1%, p < .001) but otherwise appeared similar. We note that it was not possible in this study to discern whether raised LDH levels signified hepatic dysfunction or melanoma tumor burden. Combination ICI therapy was first‐line therapy in 25% of patients with baseline hepatic dysfunction during our study period (Table 2).
Figure 2C and D present Kaplan‐Meier curves of the rwTTD of ICI treatment (Fig. 2C) and OS (Fig. 2D) for patients with baseline normal hepatic function and hepatic dysfunction. Estimates of the median rwTTD and OS are presented in Table 4, overall and by ICI regimen. For rwTTD, patients with hepatic dysfunction at baseline were found to discontinue ICI sooner (median, 1.4 months; 95% CI, 0.5–2.1) than patients with normal hepatic function (median, 2.5 months; 95% CI, 2.3–2.8; Fig. 2C; Table 4). For OS, a decrease in median OS was observed for patients with hepatic dysfunction at baseline versus patients with normal hepatic function (4.7 months: 95% CI, 2.0–n/a vs. 20.3 months: 95% CI, 18.0–23.5; Fig. 2D; Table 4). As with renal function, these findings were comparable to those seen in the sensitivity analysis in which patients were required to have structured activity within 90 days of their advanced diagnosis date. Supplemental online Table 2 includes exploratory results of associations between hepatic function and rwTTD and OS. Following adjustment for age, stage at diagnosis, and treatment regimen, hepatic dysfunction was associated with decreased OS (p = .001) versus normal hepatic function.
Table 4.
Median time‐to‐event results by baseline hepatic function for all treatment regimens and individual treatment regimens, rwTTD and OS
| Normal | Moderate or severe dysfunction | |||
|---|---|---|---|---|
| Outcome and treatment regimen | Events/n | Median (95% CI), mo | Events/n | Median (95% CI), mo |
| rwTTD of ICI | ||||
| All | 1,299/1,669 | 2.5 (2.3–2.8) | 37/48 | 1.4 (0.5–2.1) |
| Ipilimumab | 502/528 | 2.1 (2.1–2.1) | 13/14 | 0.4 (0.0–n/a) |
| Ipi + nivolumab | 214/339 | 4.0 (3.3–5.6) | 9/12 | 0.7 (0.0–n/a) |
| Nivolumab | 182/332 | 6.2 (5.3–8.3) | 7/9 | 0.5 (0.0–n/a) |
| Pembrolizumab | 289/470 | 5.5 (4.6–7.1) | 6/13 | n/a (1.4–n/a) |
| OS | ||||
| All | 694/1,669 | 20.3 (18.0–23.5) | 25/48 | 4.7 (2.0–n/a) |
| Ipilimumab | 326/528 | 14.4 (12.2–16.7) | 10/14 | 2.5 (1.6–n/a) |
| Ipi + nivolumab | 110/339 | 29.4 (19.9–n/a) | 6/12 | 17.2 (2.1–n/a) |
| Nivolumab | 104/332 | 33.0 (18.2–n/a) | 5/9 | 1.0 (0.8–n/a) |
| Pembrolizumab | 154/470 | 27.8 (20.7–n/a) | 4/13 | n/a (4.7–n/a) |
For the median estimate, n/a occurred when <50% of the cohort had the event of interest. For 95% CI bounds, this occurred either when there were too few events or because the upper confidence limit is <50%.
Abbreviations: CI, confidence interval; Ipi, ipilimumab; OS, overall survival; n/a, result not calculable; rwTTD, real‐world time to treatment discontinuation.
Among the 39 patients with moderate (vs. severe) hepatic dysfunction at baseline, 35 had at least one evaluable hepatic laboratory result within the first 30 days of treatment. Five of these patients were found to have developed a hepatic dysfunction status classified as “severe” during this time.
Emergent Organ Dysfunction
Renal
There were 1,778 patients with normal renal function at baseline and further evaluable test results within 90 days following first‐line treatment start. Among these patients, 82 (4.6%) had evidence of moderate or severe renal dysfunction. Multivariable logistic regression model results identified that older age (70+) and male sex were positively associated with emergent renal dysfunction, whereas combination therapy (ipilimumab + nivolumab), versus monotherapy (ipilimumab, nivolumab or pembrolizumab), was positively associated with this outcome (aOR 2.47; 95% CI, 1.48–4.12; Table 5). Associations for treatment regimens were also considered; in this instance, relative to ipilimumab monotherapy, combination therapy was strongly associated with a higher likelihood of emergent renal dysfunction (supplemental online Table 3). Patients may have received additional lines of treatment (ICI or otherwise) between ICI initiation and identification of emergent organ dysfunction.
Table 5.
Multivariable odds ratios (OR) and 95% confidence intervals (CI) for associations between patient characteristics, treatment regimen (dichotomized as monotherapy/combination therapy)†, and risk of emergent renal dysfunction (top) or hepatic dysfunction (bottom)
| Characteristic or treatment regimen | Total | Events, n (% total) | Adjusted OR a (95% CI) | p value |
|---|---|---|---|---|
| Emergent renal dysfunction (82 events/1,778 total) | ||||
| Age | ||||
| <70 yr | 935 | 36 (3.9) | Ref | |
| 70+ yr | 843 | 46 (5.5) | 1.67(1.03–2.70) | .039 |
| Sex | ||||
| Female | 569 | 16 (2.8) | Ref | |
| Male | 1,209 | 66 (5.5) | 1.92(1.17–3.14) | .012 |
| State at diagnosis | ||||
| III/IV | 930 | 41 (4.4) | Ref | |
| 0/I/II | 486 | 25 (5.1) | 1.14(0.74–1.76) | .541 |
| Unknown | 362 | 16 (4.4) | 1.00(0.65–1.54) | .996 |
| Practice type | ||||
| Academic practice | 292 | 14 (4.8) | Ref | |
| Community | 1,486 | 68 (4.6) | 0.85(0.33–2.21) | .746 |
| Treatment regimen b | ||||
| Monotherapy | 1,402 | 53 (3.8) | Ref | |
| Combination therapy | 376 | 29 (7.7) | 2.47(1.48–4.12) | <.001 |
| Emergent hepatic dysfunction (119 events/1,616 total) | ||||
| Age | ||||
| <70 yr | 831 | 79 (9.5) | Ref | |
| 70+ yr | 785 | 40 (5.1) | 0.60(0.39– 0.93) | .023 |
| Sex | ||||
| Female | 503 | 39 (7.8) | Ref | |
| Male | 1,113 | 80 (7.2) | 0.96(0.66– 1.40) | .821 |
| Stage at diagnosis | ||||
| III/IV | 836 | 68 (8.1) | Ref | |
| 0/I/II | 435 | 32 (7.4) | 0.96(0.63– 1.45) | .836 |
| Unknown | 345 | 19 (5.5) | 0.69(0.42– 1.13) | .145 |
| Practice type | ||||
| Academic practice | 165 | 10 (6.1) | Ref | |
| Community | 1,451 | 109 (7.5) | 1.23(0.66– 2.29) | .506 |
| Treatment regimen b | ||||
| Monotherapy | 1,288 | 75 (5.8) | Ref | |
| Combination therapy | 328 | 44 (13.4) | 2.17(1.46–3.23) | <.001 |
Models adjusted for all variables listed.
Note: Patients may have received second‐line ICI or other relevant treatment subsequent to treatment start and prior to identification of emergent dysfunction.
Ref, reference.
Hepatic
There were 1,616 patients with normal baseline hepatic function and further evaluable results within 90 days of first‐line treatment start. Among these patients, 119 (7.4%) had evidence of hepatic dysfunction. Older age was negatively associated with emergent hepatic dysfunction. As with emergent renal dysfunction, combination therapy, versus monotherapy, was positively associated with emergent hepatic dysfunction (aOR 2.17; 95% CI, 1.46–3.23; Table 5). Relative to ipilimumab monotherapy, combination therapy was positively associated with emergent hepatic dysfunction, whereas pembrolizumab monotherapy was negatively associated with this outcome (supplemental online Table 3).
External Validity
Patients who had evaluable laboratory values at baseline (renal: n = 1,884; hepatic: n = 1,717) were compared with those who were excluded from the study because of the lack of such data (renal: n = 523, hepatic: n = 690). Patients with available renal laboratory values had a higher proportion of patients treated in the academic setting (16.0% vs. 2.1%, p < .001). A difference in melanoma stage at initial diagnosis was also observed (p = .018); there was a higher proportion of patients with stage IV or unknown staging among those patients who did not have available test results (supplemental online Table 4). There was a clinically insignificant difference in rwTTD in patients with available versus unavailable renal laboratory values (2.1 months; 95% CI, 1.6–3.2 vs. 2.5 months; 95% CI, 2.3–2.8); however, patients with available renal laboratory values experienced a shorter OS (8.2 months; 95% CI, 4.2–12.9 vs. 22.1 months; 95% CI, 19.9–25.5). In contrast, patients who had hepatic laboratory values available, versus unavailable, were less likely to be treated in the academic setting (9.9% vs. 20.7%, p < .001). However, they had identical rwTTD and OS results as those observed in the comparison of patients with evaluable renal laboratory results availability versus those without evaluable results.
Discussion
Using a large real‐world data set, this study demonstrates that some patients with melanoma with baseline moderate or severe organ dysfunction are receiving first‐line ICI therapy and among those with normal baseline function, the rates of emergent organ dysfunction are similar to those previously reported ranges from clinical trials. Patients with renal dysfunction at baseline were older, more commonly male, less likely to receive combination ICI therapy first‐line, and had shorter OS compared with patients with normal baseline renal function. Patients with hepatic dysfunction had similar baseline patient characteristics, with the exception of LDH, to those with normal hepatic function but discontinued treatment sooner and had shorter OS. Few patients with baseline moderate renal or hepatic dysfunction, and who had relevant laboratory results within the first 30 days of ICI treatment, had results indicating severe organ dysfunction during this time. Interestingly, despite sharing a common underlying immune‐mediated pathology and higher risk in patients treated with combination ICI therapy, older age and being male was associated with the development of renal dysfunction, whereas younger age was associated with the development of hepatic dysfunction.
This study contributes substantially to the observations described in recent case reports and small case series on the clinical experience of patients with organ dysfunction at baseline treated with ICIs [12, 17]. The recent American Society of Clinical Oncology (ASCO) Clinical Practice Guideline on the management of irAEs in patients treated with ICIs states that ICI use appears to be safe in patients with baseline renal impairment from a nonimmune basis (e.g., old age, hypertension) but does not comment on patients with baseline hepatic impairment [30]. Our study, which includes 46 patients with baseline renal dysfunction and 48 patients with baseline hepatic dysfunction, is the first to report estimates for median rwTTD and OS for such patients. rwTTD, although less self‐evident than OS, may serve as a proxy of toxicity, disease progression, or patient or physician preference to otherwise discontinue treatment [31].
Our findings of shorter rwTTD and OS among patients with organ dysfunction at baseline may reflect the presence of liver metastases, particularly in the case of hepatic dysfunction. The findings may alternatively reflect competing risks from underlying comorbidities, earlier discontinuation of systemic therapy in patients with underlying organ dysfunction, toxicity, and/or ICI effectiveness in the setting of organ dysfunction. As such, causation may not be determined. Because of low numbers of observations for patients with organ dysfunction, adjusted models may be unreliable. However, our exploratory analyses suggested that organ dysfunction was associated with decreased rwTTD and OS following adjustment for age, stage at diagnosis, and treatment regimen. We note also that these analyses suggested that monotherapy, versus combination therapy, was associated with shorter rwTTD; in the interpretation of these results, we caution that the monotherapy group is heterogeneous and included treatment with ipilimumab. Regardless, the reporting of these outcomes provides information on the experience of these understudied populations relevant for optimally informed treatment decisions for patients and providers.
Our study also contributes to the understanding of the prevalence of emergent renal and hepatic dysfunction post ICI treatment. Similar to ICI phase III trial safety findings, we identified a higher prevalence of real‐world emergent hepatic dysfunction (7.4%) than emergent renal dysfunction (4.6%) at 90 days post–ICI treatment [32]. Our real‐world results also echo phase III trial findings that combination ICI therapy versus monotherapy is associated with increased toxicity overall and for our specific outcomes [33]. Our findings are limited by our restriction of follow‐up of 90 days post baseline; a retrospective case series of 13 patients found that renal toxicity following ICI was diagnosed at a median of 91 days post initiation (range, 21–245), and the time‐to‐onset of hepatotoxicity following ICI treatment has been estimated at 42–84 days [30]. However, our follow‐up period was chosen with the intention of capturing organ dysfunction likely to be associated with ICI treatment as opposed to an unrelated emergent dysfunction; as such, our results for emergent organ dysfunction represent conservative estimates. We were also unable to identify whether the emergent organ dysfunction observed in our study is related to ICI toxicity or underlying morbidity, including the development of liver metastases, or additional patient factors. ASCO's clinical practice guideline on irAE management following ICI notes that addressing hepatotoxicity in ICI‐treated patients requires the evaluation and ruling out of other potential contributory factors. Regarding renal toxicity, the guidelines also note that emerging data [24] suggest higher incidence rates (9.9%–29%) of acute kidney injury following ICI use than those quoted in initial studies, but it is important to note that the definition of renal dysfunction is more expansive and includes electrolyte disturbances rather than measurable increase in blood urea nitrogen or creatinine [30].
Despite capturing data from a large number of patients treated at numerous geographically diverse practices across the U.S. over a span of 7.5 years, this study has some significant limitations. We only included patients with advanced melanoma who received ICI treatment in the first‐line according to available records, which may have led to underascertainment of the prevalence of patients with baseline organ dysfunction in the general advanced melanoma population. Furthermore, our results reflect only patients for whom laboratory tests were available prior to first‐line treatment initiation. Although this included most patients, those for whom laboratory tests were not available appeared to have higher median rwTTD and OS, indicating potential presence of selection bias. We also lacked certain clinical variables; cancer staging information was available only for the time of initial diagnosis (as opposed to the time of treatment) and did not include substage information or site of metastasis. Regarding our organ dysfunction measures, we did not have information on the specific manifestation, etiology, or treatment of the organ dysfunction, and, particularly regarding hepatic dysfunction, we were unable to distinguish between metastatic disease versus underlying hepatic comorbidity. We also note that some patients may have received additional drug treatment in the 90 days following initial first‐line immunotherapy; however, given the relatively short time frame of follow‐up we expect this to be an uncommon event. More generally, given the retrospective observational nature of our data, our cohort may not be fully representative of the U.S. patient population, and differences in practice may lead to variation in capture of clinical results.
Overall outcomes for patients with advanced melanoma have made great strides since 2011, when both vemurafenib and ipilimumab were introduced, and further increases in OS have been detected since the U.S. Food and Drug Administration (FDA) approval of pembrolizumab and nivolumab. In spite of these marked changes in the treatment armamentarium, patients with comorbidities have little guidance for how these novel therapies may affect their outcomes at the outset of therapy. Such patients are less likely to be offered the opportunity to participate in clinical trials generally [33]. As of March 2019, the FDA has produced draft guidance to support the inclusion of patients with organ dysfunction, or prior or concurrent malignancies, in cancer clinical trial eligibility criteria, which may improve the representativeness of future cancer trial data [34]. In the interim, real‐world data may provide much‐needed information on currently excluded population subgroups. Furthermore, as noted by Khoja et al. in their review of ICI‐induced irAEs, previous studies have observed that the severity and frequency of ICI‐related toxicities are usually worse in a real‐world population when compared with clinical trial patient samples [35].
Our study is an example of how data sets including real‐world patients may generate clinically meaningful information more rapidly than other study designs and provide important outcomes for the effectiveness of a given therapy in patients with specific comorbidities. The recent development of large real‐world data sets has the potential to shift the paradigm dramatically and provide valuable real‐time insight to supplement clinical trials in informing optimal clinical care.
Conclusion
Our large retrospective study using real‐world U.S. EHR data provides important information that complements clinical trial information, including the use of ICI in patients with advanced melanoma with renal and hepatic dysfunction at baseline and their subsequent outcomes and the development of renal and hepatic dysfunction among patients with normal kidney and liver function at diagnosis. Although our data and study design are subject to several limitations and potential biases associated with observational research, our results contribute to the understanding of ICI use and patient outcomes for an important subgroup of patients often excluded from traditional clinical trials and add to the growing body of literature examining the incidence of adverse events post ICI treatment.
Author Contributions
Conception/design: Susan Spillane, Shrujal Baxi, Aracelis Z. Torres, David Lenis, Andrew Freedman, Angela Mariotto, Elad Sharon
Provision of study material or patients: Shrujal Baxi, Aracelis Z. Torres, David Lenis
Collection and/or assembly of data: Shrujal Baxi, Aracelis Z. Torres, David Lenis
Data analysis and interpretation: Susan Spillane, Shrujal Baxi, Aracelis Z. Torres, David Lenis, Andrew Freedman, Angela Mariotto, Elad Sharon
Manuscript writing: Susan Spillane, Shrujal Baxi, Aracelis Z. Torres, David Lenis, Andrew Freedman, Angela Mariotto, Elad Sharon
Final approval of manuscript: Susan Spillane, Shrujal Baxi, Aracelis Z. Torres, David Lenis, Andrew Freedman, Angela Mariotto, Elad Sharon
Disclosures
Shrujal Baxi: Flatiron Health (E, OI), Roche (OI); Aracelis Z. Torres: Flatiron Health (E, OI), Roche (OI); David Lenis: Flatiron Health (E‐former). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supplementary Information
Acknowledgments
This study was supported by Flatiron Health Inc., which is an independent subsidiary of the Roche group, and the United States National Cancer Institute (NCI). Dr. Spillane was supported by the Intramural Research Program of the NCI (Cancer Prevention Fellowship Program) and the Health Research Board of Ireland [CPFP‐2016–1] during the course of this work.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schadendorf D, Hodi FS, Robert C et al. Pooled analysis of long‐term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open‐label phase 3 study (KEYNOTE‐006). Lancet 2017;390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 4. Clinical NCCN Practice Guidelines in Oncology. Cutaneous melanoma: Version 2.2019. National Comprehensive Cancer Network; 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. [DOI] [PubMed] [Google Scholar]
- 5. Whitman ED, Liu FX, Cao X, Diede SJ, Haiderali A, Abernethy AP. Treatment patterns and outcomes for patients with advanced melanoma in US oncology clinical practices. Future Oncol 2018;15:459–471. [DOI] [PubMed] [Google Scholar]
- 6. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer Clin 2016;66:337–350. [DOI] [PubMed] [Google Scholar]
- 7. Edwards BK, Noone AM, Mariotto AB et al. Annual Report to the Nation on the status of cancer, 1975‐2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast or prostate cancer. Cancer 2014;120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lameire NH, Flombaum CD, Moreau D et al. Acute renal failure in cancer patients. Ann Med 2005;37:13–25. [DOI] [PubMed] [Google Scholar]
- 9. Lee L, Cheung WY, Atkinson E et al. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J Clin Oncol 2011;29:106–117. [DOI] [PubMed] [Google Scholar]
- 10. Beumer JH, Ding F, Tawbi H et al. Effect of renal dysfunction on toxicity in three decades of cancer therapy evaluation program–sponsored single‐agent phase I studies. J Clin Oncol 2016;34:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim ES, Bruinooge SS, Roberts S et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herz S, Höfer T, Papapanagiotou M et al. Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer. 2016;67:66–72. [DOI] [PubMed] [Google Scholar]
- 13. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 16. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanz BA, Pollack MH, Johnpulle R et al. Safety and efficacy of anti‐PD‐1 in patients with baseline cardiac, renal, or hepatic dysfunction. J Immunother Cancer 2016;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voskens CJ, Goldinger SM, Loquai C et al. The price of tumor control: An analysis of rare side effects of anti‐CTLA‐4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Velasco G, Je Y, Bossé D et al. Comprehensive meta‐analysis of key immune‐related adverse events from CTLA‐4 and PD‐1/PD‐L1 inhibitors in cancer patients. Cancer Immunol Res 2017;5:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michot JM, Bigenwald C, Champiat S et al. Immune‐related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016;54:139–48. [DOI] [PubMed] [Google Scholar]
- 21. Cortazar FB, Marrone KA, Troxell ML et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reynolds K, Thomas M, Dougan M. Diagnosis and Management of Hepatitis in Patients on Checkpoint Blockade. The Oncologist 2018;23:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manohar S, Kompotiatis P, Thongprayoon C et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta‐analysis. Nephrol Dial Transplant 2019;34:108–117. [DOI] [PubMed] [Google Scholar]
- 24. Wanchoo R, Karam S, Uppal NN et al. Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol 2017;45:160–169. [DOI] [PubMed] [Google Scholar]
- 25. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 26. Flatiron Health EHR‐derived database . 2018. Available at https://flatiron.com/real‐world‐evidence/. Access January 21, 2020.
- 27. Khozin S, Abernethy AP, Nussbaum NC et al. Characteristics of real‐world metastatic non‐small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. The Oncologist 2018;23:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart M, Norden AD, Dreyer N et al. An exploratory analysis of real‐world end points for assessing outcomes among immunotherapy‐treated patients with advanced non‐small‐cell lung cancer. JCO Clin Cancer Inform 2019;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curtis MD, Griffith SD, Tucker M et al. Development and validation of a high‐quality composite real‐world mortality endpoint. Health Serv Res 2018;53:4460–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pazdur R. Endpoints for assessing drug activity in clinical trials. The Oncologist 2008;13(suppl 2):19–21. [DOI] [PubMed] [Google Scholar]
- 32. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 33. Unger JM, Hershman DL, Fleury ME et al. Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol 2019;5:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cancer clinical trial eligibility criteria : Patients with organ dysfunction or prior or concurrent malignancies U.S. Food and Drug Administration. 2019. Available at http://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/cancer‐clinical‐trial‐eligibility‐criteria‐patients‐organ‐dysfunction‐or‐prior‐or‐concurrent. Accessed July 2, 2019.
- 35. Khoja L, Day D, Wei‐Wu Chen T et al. Tumour‐ and class‐specific patterns of immune‐related adverse events of immune checkpoint inhibitors: A systematic review. Ann Oncol 2017;28:2377–2385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supplementary Information


