Abstract
The ongoing COVID-19 pandemic is the most trending and talked topic across the World. From its point of origin in Wuhan, China to clinical laboratory at NIH, a mere six-month-old SARS-CoV-2 virus is keeping the clinicians, and scientists busy at various fronts. However, COVID-19 is an emerging and evolving disease and each day brings in more data, new figures, and findings from the field of clinical practice. The role of hematologists has been increasingly recognized during the current pandemic because of several reasons. Most important of them are the characteristic hematological findings of COVID-19 patients that also have prognostic implications and that were not seen in other viral infections. The treatment of hematological complications in COVID-19 patients is very challenging given the critical care setting. There are interim and limited guidelines thus far due to the novelty of the disease. As this remains to be a quite fluid situation, all the appropriate medical societies including the major hematology bodies are proposing initial and interim guidelines (e.g. ASH guideline). This puts a hematologist on consult service in a dubious position where, he/she must tailor the recommendations on case to case basis. The purpose of this review is to provide the background context about the impact of COVID-19 on the blood system and to summarize the current interim guidelines to manage the associated hematological issues in COVID-19 infection.
Keywords: Pandemic, Hematology, Coronavirus, Consultation, COVID-19
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been recently recognized as a new human infectious virus [1]. Starting early December 2019 as a local outbreak, now coronavirus disease (COVID-19) has turned to a pandemic status with more than 13,800,016 cases and 589,114deaths (as on July 16, 2020) [2]. The clinical continuum of COVID-19 varies from asymptomatic form to critically ill requiring intensive care. Recent studies have found a specific trend of laboratory abnormalities in patients with COVID-19. The initial reports noted the following common hematological changes lymphopenia (82.1%), thrombocytopenia (36.2%), and leukopenia (33.7%) [3]. Similarly, coagulopathies with prolonged prothrombin time, and D dimer elevation have been observed in patients with more critical clinical picture [4]. It is expected that we will have to learn how to live with coronavirus infection risk for the foreseeable future. It is very likely that patients with various clinical pictures will need to be evaluated by a hematologist and thus we feel this review of known hematological abnormalities associated with COVID-19 is going to be important for routine clinical practice [5]. Multiple hematologic parameters in COVID-19 patients can predict prognosis as well as severity of illness thus may help with proper triage of patients within the hospital.
Research on COVID-19 disease is evolving and data emerges rapidly. Hence, with changing dynamics driven by no previous experience on SARS-CoV-2 infection and at the same time growing body of data, which seems to vary from region to region slightly, it is difficult to formulate universal guidelines for hematological concerns in patients with COVID-19. The principal responsibilities during such hematology-oncology consultations are still to review the patients' records and assist the treating physicians with the latest institutional, specialty societies, and federal government recommendations. Most importantly it is crucial for any healthcare worker to protect themselves appropriately as dictated by the specific situation and local guidelines. Using telehealth methods whenever feasible and appropriate and using PPE when physical exam absolutely needs to take place (to identify a rare physical finding during an exam that cannot be detected otherwise) in cases where suspicion for COVID-19 is high is therefore an important aspect of care.
Herein, we discuss the most common COVID-19 associated hematological abnormalities, possible pathophysiology, and current literature which could help the hematology specialists while consulting on these patients.
2. Thrombosis and hemostasis and COVID-19
2.1. Evaluating risk for thrombotic disease
2.1.1. Pathogenesis
Acute infectious process can be associated with an increased risk for venous thrombo-embolic events. So far, the exact incidence of various thrombotic disease is unclear, but the preliminary reports have pointed towards a prothrombotic state in COVID-19 patients [6]. Thrombotic complications have thus emerged in COVID-19 patients as an important aspect of their clinical presentation and some form of anticoagulation is being used in many COVID-19 treatment protocols. The possible mechanisms could be systemic inflammatory response, stasis, and SARS-CoV-2 – angiotensin converting enzyme (ACE2) binding and direct endothelial cell damage [7].
2.1.2. Current literature
There are various risk assessment tools that one can use to identify hospitalized medical patients who are at increased risk for venous thromboembolism (VTE). One such VTE risk tool, Padua Prediction Score was recently utilized by Xu et al. who found that 23 out of 138 (16.67%) patients with COVID-19 had at high risk for VTE [8]. Critically ill patients (15/23 patients) were noted to have double the risk to develop thrombosis. Improved prediction scores also showed a higher risk of bleeding while on VTE prophylaxis in 9 out of 138 (6.52%) patients. Contrary to prediction, thrombotic events were noted only in 4 out of 138 patients (2.9%), 3 of them were critically ill. Upon treatment for VTE, one critically ill patient also suffered a massive hemorrhage. Postmortem findings of variable degrees of hemorrhagic pulmonary infarction, occlusion, and micro thrombosis formation have also been noted by Luo et al. [9]. Cui et al. did their study exclusively in 81 intensive care unit (ICU) patients with COVID-19. 20 out of the 81 patients (25%) developed VTE, of which 8 patients ultimately died. They found D-dimers >1.5 μg/mL to a good prognostic indicator to develop VTE in critically ill patients [10].
2.1.3. Role of anticoagulants (prophylactic)
Based on the increased risk of thrombosis the current consensus is to consider judicious use of anticoagulants for VTE prophylaxis in all hospitalized patients especially the ones who are critically ill [11]. Another group of researchers suggests using low molecular weight heparin (LMWH) as it might have inhibitory potential against SARS-CoV-2 replication [12,13]. This is based on the observation of FXa and FIIa induced activation of SARS-CoV-2 Spike protein which promotes infectivity. Recently, Kim et al. reported a potential link between host cell surface glycosaminoglycan (GAGs) and SARS-CoV-2 viral entry into the cell [14]. They further indicated heparan interaction with the GAG-binding motif that could potentially block the SARS-CoV-2 pathogenesis. Hence the use of anticoagulation especially during the early phase of viremia might inhibit SARS-CoV-2 replication. Tang et al. studied 449 patients with severe COVID-19, 99 of them received LMWH for 7 days or more [15]. In patients with sepsis-induced coagulopathy (SIC score) ≥4, the 28-day mortality of heparin users was lower than nonusers (40.0% vs 64.2%, P = 0.029), or D-dimer >6-fold of upper limit of normal (32.8% vs 52.4%, P = 0.017). The consensus is to use prophylactic LMWH unless contraindicated in all COVID-19 (even in non-critically ill) patients. Usual contraindications to using anticoagulation are active bleeding or platelet count less than 20,000 [16].
-
•
If CrCl > 30, Inj. LMWH 40 mg Subcutaneous daily
-
•
If CrCl < 30 or acute kidney injury: Heparin 5000 units Subcutaneous three times daily.
2.1.4. Role of antiplatelets (dipyridamole, prophylactic)
Recently, observational studies mostly from China have explored the utility of dipyridamole in patients with COVID-19 [12]. Dipyridamole, a phosphodiesterase inhibitor, have been found to have in vitro anti-SARS-CoV-2 activity, suppress inflammation, promote mucosal healing, and has an adjunctive role in increasing circulating lymphocytes and platelet counts and decreasing D-dimer levels. However, these observations need further confirmation in subsequent larger studies.
2.1.5. Therapeutic anticoagulation in COVID-19
While consulting on COVID-19 patients, there could be two common scenarios about the need for therapeutic anticoagulation. One, a patient on therapeutic anticoagulation [pulmonary embolism (PE), venous thromboembolism (VTE), atrial fibrillation, prosthetic valves] gets diagnosed with COVID-19, and second, a hospitalized COVID-19 patient develops thromboembolism requiring therapeutic anticoagulation. In both the scenarios, choosing the proper anticoagulation is a tough task as there is no clear anticoagulation of choice due to lack of data. We found that different institutes formulated their own guidelines and choose between heparin infusion versus LMWH versus Direct Oral Anticoagulants (DOACs).
-
•
Unfractionated Heparin (UFH): UFH comes with an added advantage of its better anti-inflammatory action than the other two and can be stopped and reverted immediately if needed to so. However, it has its own challenge in the form of frequent activated partial thromboplastin time (aPTT) monitoring which may be practically overwhelming for the staff. In addition, aPTT monitoring may not be a correct test for dose titration because of its elevated values in critically ill patients even without UFH use or due to Lupus-like inhibitors. In such special scenarios, cases, monitoring of unfractionated heparin might require anti-Xa activity checks.
-
•
Low molecular weight Heparin (LMWH): To date, most of the data on anticoagulation in COVID-19 patients is with LMWH which might give it an upper hand for practical purposes over UFH unless patients have CrCl <30 or acute kidney injury.
-
•
Oral anticoagulants (Warfarin and DOACs): During the current pandemic, we have witnessed that a few guidelines have been formulated keeping in mind the practical issues. One such context is using DOACs in COVID-19 patients considering it is an oral drug, easy to dispense with no need of frequent laboratory draws minimizing exposure risk to the nurses, and less workload overall. Multiple investigational, or off-label used medications are currently being tried in COVID-19 patients, but there might be potential drug-drug interactions with DOACs. For instance, an investigational drug, sarilumab (IL-6 receptor blockade) interacts with apixaban and rivaroxaban, hence, clinicians might consider not to use both the drugs at the same time. Similarly, increased doses of warfarin may be required while with sarilumab. Due to so many uncertainties involved while using oral anticoagulants, many centers are preferring them in following sequence LMWH>UFH > Warfarin/DOACs (Table 1 ) [17,18].
-
•
Empirical Therapeutic-intensity anticoagulation: Once acquired, SARS-CoV-2 infection initiates a complex systemic inflammatory response. Activation of host defense mechanisms including the coagulation cascade and complement system prompts thromboinflammation or immunothrombosis. The severity of this phenomenon correlates with the severity of the critical illness of the patients. Postmortem series have confirmed evidence of multi-organ and disseminated microvascular thrombosis in various organs [19,20]. This has served as a platform for a few ongoing clinical trials to try prophylactic high-intensity prophylaxis in critically ill patients with COVID-19 with no known thrombosis. Few medical centers are using full-dose anticoagulation through their institutional protocol but so far, no data support it for the prevention of microvascular thrombosis in patients suffering from severe COVID-19 infection [21]. As per the latest ASH guidelines (Version 3.0; last reviewed June 23, 2020), therapeutic-dose heparin should only be limited to clinical trials than empiric use [22].
Table 1.
Various anticoagulants, advantages, and disadvantages with their use in COVID-19.
| Anticoagulation use in COVID-19 patients | |||
|---|---|---|---|
| Section A | |||
| Anticoagulants drugs | Pros/benefits | Cons/disadvantages | |
| UFH | • Can be stopped immediately | • Less efficient than LMWH | |
| • Predictable response | • Current studies mostly done on LMWH | ||
| • Anti-inflammatory effect | • Needs frequent lab draws (for therapeutic only) | ||
| • Can be used in acute renal failure | • Needs anti-Xa levels rather than aPTT as later also elevates in COVID-19 patients | ||
| LMWH | • Shorter half life | • Cannot be used if CrCl <30 or acute kidney injury. | |
| • Most available studies on COVID-19 used LMWH | • Lesser anti-inflammatory activity than UFH | ||
| • No need of frequent lab draws (both for prophylactic and therapeutic use) | |||
| DOACs | • Oral pill | • Almost no experience | |
| • Less chance of exposure to COVID-19 patients due to easy dispensing and no need of frequent lab draws | • Multiple drug interactions possible | ||
| Section B | |||
| Common covid-19 scenarios | Recommendations | Indications | |
| B.1 Anticoagulation for Prophylaxis | • If CrCl >30, Inj. LMWH 40 mg Subcutaneous daily | -All hospitalized patients (including non-critically ill). | |
| ▪ Contraindications: [1] Active bleeding [2] platelet count <25 × 109/L [3] Fibrinogen <0.5 g/L | |||
| • If CrCl <30 or acute kidney injury: Heparin 5000 units Subcutaneous three times daily. | ▪ Close monitoring advised in severe renal impairment. | ||
| • Mechanical thromboprophylaxis, only when chemical treatment is contraindicated | ▪ An abnormal PT or APTT is not a contraindication | ||
| B.2 Anticoagulation for therapeutic purposes | |||
| B.2.1 | A COVID-19 patient already on oral anticoagulants at the time of admission | • Switch to therapeutic dose of LMWH (preferred over UFH due to reasons mentioned in section A) | Known history of thrombosis or other indications requiring therapeutic anticoagulation. |
| • Fondaparinux preferred in patient has a history of HIT | Caution: To hold anticoagulation temporarily if platelet count is <30–50 × 109/L or if the fibrinogen is <1.0 g/L | ||
| • Mechanical thromboprophylaxis, only when chemical treatment is contraindicated | |||
| B.2.2 | A COVID-19 patient who develop acute DVT/PE during hospital stay | • LMWH is preferred (preferred over UFH due to reasons mentioned in section A) | Acute thrombosis |
| • UFH only is used only If CrCl <30 or acute kidney injury | |||
| B.3 Empirical therapeutic anticoagulation | • Not recommended | Not indications so far, under study | |
| B.4 Use of tPA for therapeutic anticoagulation | • Not recommended | Not indications so far | |
APTT: Activated partial thromboplastin time CAC: Coagulopathy associated with COVID-19, DIC: Disseminated intravascular coagulation, DOACs: FFP: Fresh frozen plasma, HIT: Heparin induced thrombocytopenia, LMWH: Low molecular weight heparin, PT: Prothrombin time, PCC: Prothrombin complex concentrate, tPA: tissue plasminogen activator, UFH: Unfractionated heparin. This table has been adopted based on the recommendations by ASH and ISTH combined (please visit websites for their individual recommendations).
There are anecdotal reports of transient benefits of using tissue plasminogen activator (tPA) in critically ill patients with Acute respiratory distress syndrome (ARDS) [23]. However, there is not enough data to suggest using more innovative therapies like tPA outside of a clinical trial and most centers recommend against using them [22].
2.2. Coagulopathy in patients with COVID-19: Coagulopathy associated with COVID-19 (CAC) vs disseminated intravascular coagulation (DIC)
In general, patients with impending multiorgan failure who develop disseminated intravascular coagulation (DIC) have the worst prognosis. In DIC, one can see various combinations of exaggerated thrombotic and/or fibrinolytic phase [24,25]. Studies so far have suggested that coagulopathy associated with COVID-19 (CAC) is predominantly a pro-thrombotic DIC with elevation in D-dimer levels, and fibrinogen levels, and a decrease in anti-thrombin levels. A critical evaluation of coagulogram of patients with COVID-19 has shown a spectrum of the coagulopathic pattern from CAC to frank DIC [24]. The clinical implications of these coagulopathy perturbations are pulmonary congestion with microvascular thrombosis and micro occlusion with an increased rate of thromboembolic events, central line thrombosis, and strokes.
Elevation in the D-dimers is a very commonly observed laboratory abnormalities in COVID-19 patients. Guan et al. in their study on 1099 hospitalized COVID-19 patients found elevated D-dimers (≥0.5 mg/L) as an indicator of severe illness [3]. Tang et al. reported D-dimers as a mortality predictor with the median value of 2.12 μg/ml (range 0.77–5.27 μg/ml) in the non-survivors as compared to 0.61 μg/ml (range 0.35–1.29 μg/ml) in the survivors [4]. Another significant observation by Huang et al. was that a higher at admission median D-dimers were associated with an increased chance of requiring critical care support (2·4 vs 0.5 mg/L, p = 0·0042) [26]. Based on these findings, D-dimer has become a reliable marker of prognosis, hospital mortality, and need for ICU level care [3,15,26].
Though not significant, modestly prolonged prothrombin time (PT) was noted in non-survivors (15.5 s) than in survivors (13.6 s) by Huang et al. [26]. Rovina et al. suggested in their small study (57 patients) the use of measurement of soluble urokinase plasminogen activator (suPAR) within first 24 h that can be predictive of severe respiratory failure and identify patients at high risk early. The urokinase plasminogen activator receptor (uPAR) that is bound on the endothelium is cleaved as SARS-CoV-2 activates endothelial cells [27]. The endothelial activation is an early step in COVID process. While this parameter of endothelial activation has been described in several settings predicting kidney injury or progression of sepsis it may not be readily available as a routine test and we need to see further studies in COVID-19 patients as well.
2.3. Managing coagulopathy in COVID-19
As discussed, patients can be anywhere in the spectrum of coagulopathy from mild to severe, and from CAC to DIC. The nature of the disease, viral load, and spread in the body, cytokine storm, etc. are a few of the many factors that help to assess the actual patient's situation. The American Society for Hematology (ASH) recommends regular monitoring of platelet count, PT/aPTT, D-dimer, and fibrinogen levels to guide how aggressive critical care needs to be and when to scale up or scale down the level of care.
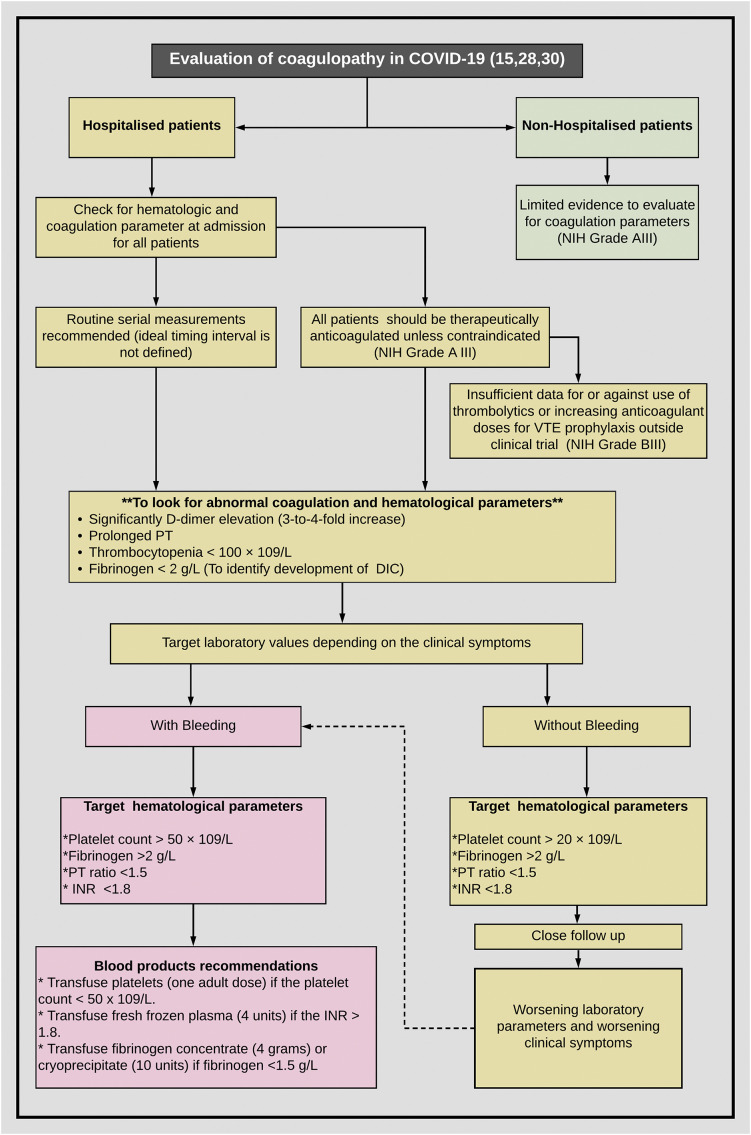
So far, there are no high-quality data to guide management for coagulopathy in patients with COVID-19. Therefore, both International Society of Thrombosis and Hemostasis (ISTH) and ASH suggest managing coagulopathy in COVID-19 just like any in other non-COVID-19 patient, that primarily includes treating the underlying disease [15,28]. Unfortunately, for COVID-19, we do not have any specific treatment to mitigate the viremia and organ dysfunction. Current literature from clinical experience with COVID-19 patients suggests bleeding not be a major concern [22,24,29] ASH committee strongly suggests a tailored approach and clinical judgment on case to case basis while deciding for the use of blood components (Fig. 1 ). Based on the recommendations by ASH and ISTH, Table 1 describes the various types of anticoagulants and the possible scenarios for using them [15,28,30](Table 1).
Fig. 1.
Approach to coagulopathy in a patient with COVID-19. Depending on clinical symptoms of bleeding (left section in red) vs no bleeding (right section in yellow), target value threshold for platelet count and coagulogram parameters are variable (adapted from ASH and ISTH). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.4. Significance of antiphospholipid antibodies in COVID-19
Antiphospholipid antibodies are classically discussed in context to their role in laboratory diagnosis of antiphospholipid syndrome. However, these antibodies are well known to rise transiently in various infections (tuberculosis, syphilis, Human immunodeficiency virus, hepatitis C) or other auto-immune disorders [[31], [32], [33], [34], [35]] Recently, few cases of COVID-19 with positive antiphospholipid antibodies have been reported [33,34]. Harzallah et al. from France reported that out of 56 patients with COVID-19, 25 cases (45%) had lupus anticoagulant (LAC) positive while anti-cardiolipin antibody (aCL) or aβ2GPI were detected in only 5 out of 50 tested patients [34]. No clinical information regarding the thromboembolic phenomenon in these patients was available in the report. Zhang et al. reported three patients with COVID-19 who were evaluated for cerebral infarctions and peripheral limb ischemia with digit discoloration. All three patients had positive aCL and anti–β2-glycoprotein I (aβ2GPI) [but no lupus anticoagulant was detected [33]. Mao et al. reported 4 cases of ischemic stroke in their 214 patient's data [36]. Similarly, Li et al. reported an incidence of stroke as 5% among hospitalized patients [37]. Large vessel stroke in 5 COVID-19 patients younger than 50 yrs. was recently reported by Oxley et al. [25]. None of these three studies evaluated their patients for the presence of antiphospholipid antibodies. Hence, so far significance of antiphospholipid antibodies in precipitating ischemic/thrombotic events in COVID-19 patients is unknown and needs further evaluation.
3. Platelet abnormalities and COVID-19
Abnormal platelet count is often associated with a viral illness and that is not COVID-19 specific [[38], [39], [40], [41]]. Similarly, the importance of low platelet in prognostication is well described in multiple studies in critically ill patients. Various patterns such as low nadir platelet count, or rapid decline in platelet count have proven significance based on several studies [42,43]. The evidence so far has confirmed a low platelet count as an important prognostic indicator of severity and mortality in COVID-19 patients [44]. While thrombocytopenia may be associated with significant bleeding events, a reactive thrombocytosis could be associated with heightened hypercoagulability or/and thrombogenesis [45,46].
3.1. Thrombocytopenia
Thrombocytopenia is the one of the most common reasons hematologists are being consulted on critically ill patients. Unfortunately, a thrombocytopenia in an ICU patient is often multifactorial and thus it is often difficult to pinpoint one and only factor. Similarly, in COVID-19 as well, combination of various factors could be responsible for low platelets. Underlying liver issues, drug side effects, heparin-related thrombocytopenia (HIT), primary hematological disease (Immune thrombocytopenia, Thrombotic thrombocytopenic purpura etc.), impending DIC, viral infection and overt inflammatory response are few of the many such causes [39,47].
3.1.1. Pathophysiology
Studies from previous viral outbreaks might help to understand the possible mechanism behind SARS-CoV-2 induced thrombocytopenia [44,48].
Few of the potential mechanisms proposed are:
-
➢
Endothelial damage → platelet activation, aggregation, and thrombosis (predominantly in lungs) → consumptive thrombocytopenia.
-
➢
Deranged platelet defragmentation from mature megakaryocytes due to pulmonary capillary bed morphologic alternation → reduced release in the peripheral circulation.
-
➢
Robust auto-immune response against platelets → platelet destruction.
-
➢
Virus directly infecting the hematopoietic stem cells, megakaryocytes, and platelets (via CD13 or CD66a) → apoptosis.
3.1.2. Prognostic value of thrombocytopenia
As discussed previously thrombocytopenia especially the declining trend has been consistently studied in many studies as a poor indicator of survival. Lippi et al. in their recent meta-analysis of 9 studies and 1779 COVID-19 patients studied thrombocytopenia in COVID-19 infections [44]. Their meta-analysis showed that thrombocytopenia was associated with increased severity of illness, and dismal survival. They however admitted their study had limitations including arbitrary cutoff for low platelet count in various studies thereby making interpretation difficult. Qu et al. also reported a high platelet to lymphocyte ratio as an independent prognostic factor for prolonged hospitalization and a more pronounced cytokine storm in COVID-19 patients [49].
3.2. Managing immune thrombocytopenic purpura (ITP) in COVID-19
3.2.1. Newly diagnosed ITP (Outpatient)
Treatment options for newly diagnosed ITP remains the same. Rather, the frequency of lab draws, and office visits should be minimized as much as possible. COVID-19 patients who need urgent platelet count increase (e.G. major bleeding or wet purpura or platelet count), Intravenous immune globulin (IVIg) therapy (1 g/kg for 1–2 days), and oral thrombopoietic (TPO) agents like eltrombopag or avatrombopag. Both IVIg and TPO agents have the advantage of not being immunosuppressive agents unlike other ITP drugs like steroids, and cyclosporine.
3.2.2. Hospitalized patient with ITP and COVID-19
If a patient is critically ill, extremely thrombocytopenic to less than 20,000/μl, treatment with IVIg and oral thrombopoietic (TPO) agents (eltrombopag or avatrombopag) may be considered. If a patient is already on a TPO agent from before, the trial of dose increment or adding a second TPO agent could be considered. A trial of short-term steroids (1–5 days) has not been studied in COVID-19, hence it is difficult to recommend for or against it [41].
3.2.3. Chronic ITP
As of now, there is no evidence of increased incidence of COVID-19 in known ITP patients [38]. Patients with stable disease and receiving lower doses of immunosuppressants do not need any modification. However, patients receiving higher doses of steroids may benefit by attempting to replace with TPO agents and/or IVIg. Rituximab may need to be possibly avoided.
3.3. Secondary thrombocytosis
An increased platelet count has also been seen in association with respiratory viral illnesses. Based on previous studies, the stand of secondary thrombocytosis (ST) as a prognostic marker in viral diseases is controversial with contrasting results for and against both [50,51]. As of now, only a few studies so far have discussed ST in patients with COVID-19. Chen at al identified thrombocytosis in a small proportion ~ 4% of cases [52]. Further significance is yet to be studied. Both ASH and ISTH have no specific recommendations with regards to ST. From the hematology consult angle, in our opinion we can say that ST is something that should be kept in mind, the increase in the number of platelets counts per se may not matter unless there is clinical evidence of thrombotic state. Previous studies showed increased circulating cytokines, such as thrombopoietin, interleukin 6/8/1a, and tumor necrosis factor as responsible agents to cause ST [53,54]. Hence, to our assumption, once the cytokine storm subsides, the platelet counts are expected to go down.
4. White blood cells abnormalities and COVID-19
4.1. Leukocytosis and leukopenia
A recent metanalysis by Zhu et al. included 38 studies involving 3062 COVID-19 patients [55]. With regards to leukocyte counts, most patients had normal leukocytes counts (69.7%), followed by leukopenia (25.9%), and leukocytosis (12.6%). Another meta-analysis showed that leukocytosis was identified more (11.4%) often in patients with severe illness than with mild to moderate 4.8% disease [odds ratio, 2.54; 95% confidence interval, 1.43–4.52] [56].
Worsening leukocytosis in COVID-19 infected patients may suggest bacterial infection or superinfection. Any rising leukocytosis is a red flag and a prompt evaluation for bacterial superinfection or bacterial resistance if the patient is on antibiotics already. The clinical importance of rising white blood cells (WBC) was documented by Li et al. showing that a WBC >10.0 × 109/L (adjusted HR 2.0; 95% CI 1.3–3.3) was associated with increased risk of death in severe cases [57]. A similar observation was presented by Lippi et al. who noted that patients with severe disease had only a mild increased in WBC count (Weighted mean difference, WMD: 0.41 × 109/L), as opposed the clinically significant increase in WBC count (WMD: 4.15 × 109/L) in severe patients who died of COVID-19 disease [56]. To summarize in patients with severe COVID-19 a significant increase in WBC counts may predict clinical worsening and poor outcomes. We suggest that rising WBC in patients with COVID-19 is reviewed by infectious disease specialists as well as hematologists.
4.1.1. Neutrophilia in COVID-19: prevalence and prognostic value
4.1.1.1. Current literature and prognostic implications
Wu et al. in their study on 201 patients with COVID-19 reported that 84 patients (41.8%) developed ARDS, and 44 (52.4%) out of those 84 patients died [58]. They reported neutrophilia to be associated with the development of ARDS (HR, 1.14; 95% CI, 1.09–1.19), and progression from ARDS to death (HR, 1.08; 95% CI, 1.01–1.17). There is enough evidence to suggest that patients with severe COVID-19 have dysregulation of the immune response that allows viremia and subsequent hyper inflammation and cytokine storm. Worsening neutrophilia has been noted in severe COVID-19 as an expression of the exaggerated cytokine response and hyper inflammation in COVID-19 [26,56,59,60]. For example, Fan et al. from in their study of 67 patients with COVID-19 found neutrophilia to be common in patients requiring intensive care as compared to those who did not require [11.6 vs 3.5 × 109/L] [61]. Neutrophilia may also indicate a secondary bacterial infection in COVID-19 patients as discussed earlier [26]. COVID-19 patients have also been found to have circulating CD14 + CD16+ monocyte population that augments the overall IL-6 productions and that is not usually present in healthy individuals.
4.1.1.2. Neutrophil extracellular traps (NETs)
Neutrophils are considered as the first line of defense of anyone's immune system. Neutrophils act via engulfment of microbes, secretion of anti-microbials, and formation of Neutrophil Extracellular Traps (NETs) [62]. The international research consortium termed the NETwork is working in NETs since the time of its discovery back in 2004 [62,63]. Since then, aberrant NET formation and subsequent NETosis activation pathway are under investigation for their possible role in NET-associated host damage, which includes the development of Acute Respiratory Distress Syndrome (ARDS), blood clots, mucous secretions in respiratory airways, and cytokine production. Recently, Barnes et al. from the NETwork group hypothesized the convincing role of NETs towards organ damage and mortality in COVID-19 [64]. According to them, critical symptoms of severe COVID-19 could be a consequence of overactive neutrophils. Detection of neutrophil infiltration of the pulmonary capillaries and trachea in the autopsy samples supports the hypothesis of NET induced pathogenesis in COVID-19. As of now, ongoing trials are studying the molecules (e.g. colchicine and anakinra) targeting NETs to treat COVID-19 (ClinicalTrials.gov identifiers: NCT04324021, NCT04330638, NCT02735707, NCT04326790, NCT04328480, NCT04322565, NCT04322682).
4.2. Role of granulocyte colony-stimulating factor (G-CSF) during COVID-19 pandemic
Use of Granulocyte colony-stimulating factor (G-CSF) during COVID-19 pandemic can be discussed in two scenarios [1] as a support of patients with pneumonia, and [2] for febrile neutropenia (FN) related to malignancy or post chemotherapy. The use of G-CSF in non-neutropenic patients suffering from pneumonia has been explored in the past. The hypothesis is that G-CSF could promote anti-inflammatory cytokines that could the downmodulate the inflammatory response within the lung microenvironment and eventually improvement in lung function [65]. Chen et al. did a Cochrane database systemic analysis to explore the role of G-CSF in addition to antibiotics for the treatment of non-neutropenic adults with pneumonia [66]. The review involving six studies with a total of 1984 patients with pneumonia did not find any evidence supporting the routine use of G-CSF for pneumonia. Similarly, randomized, double-blind studies, did not find any evidence supporting G-CSF use in addition to standard care for severe sepsis or septic shock [67,68].
Granulocyte–macrophage colony-stimulating factor (GM-CSF) is known to have pleiotropic effect in alveolar macrophage homeostasis, and lung inflammation [69]. Recent studies have found higher levels of circulating GM-CSF-expressing leukocytes in patients with COVID-19 [70]. Based on the animal studies, clinical trials are underway to study if inhibition of GM-CSF could be beneficial to maintain lung homeostasis, reduce the hyperinflammation, and help in the lung pathogen clearance [71,72]. Contrarily, the benefit of administering the GM-CSF to patients with COVID-19 is also an ongoing debate. Most of the data are from mouse model which claim that GM-CSF administered mice had improved repair of injured lung tissue and modulation of innate and adaptive immune responses towards pathogen clearance [[73], [74], [75]]. Hence, there is no clear evidence to recommend for or against GM-CSF and the ongoing clinical trials might be helpful to guide us.
FN is one of the serious treatment-related toxicities of chemotherapy for cancer. It is associated with a mortality rate ranging from 2% to 21% [76]. While G-CSF in FN has not shown any mortality benefit it reduces the hospital stay, which can be beneficial especially during current COVID-19 due to limited resources. However, at the same time there are reports which have shown that G-CSF-induced neutropenia recovery coincides with respiratory deterioration due to acute lung injury or ARDS [77]. As per both European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO), the benefit of using G-CSF outweighs the risk of respiratory deterioration for patients with high risk of febrile neutropenia. Due to lack of data of management of patients with FN and active COVID-19, careful clinical judgment is necessary on case by case basis [78,79].
4.3. Lymphopenia in COVID-19: prevalence and prognostic value
Lymphopenia is one of the common hematological findings in viral illnesses and other immunological disorders. It has been well described during previous coronavirus outbreaks as well.
Most of the studies from the beginning of the pandemic have found lymphopenia in a significant patient population [7,26,80]. Huang et al. reported lymphopenia in 26 (63%) of the 41 hospitalized patients with COVID-19 [26]. A patient pooled study was recently conducted by Guan et al. that involved 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in 30 provinces from China. The study showed that 83.2% of the patients in the study had lymphopenia at presentation. Furthermore, lymphopenia emerged as a prognostic marker for COVID-19 [81]. A recent meta-analysis found 35–75% of patients to have lymphopenia, likely more so in non-survivors [56,61]. Similarly, Fan et al. shared their experience of 67 patients with COVID-19 from Singapore and confirmed a severe lymphopenia of <0.6 × 109/L as a poor prognostic marker with a likely requirement of ICU stay [61].
Contrast to adults, so far, in children, lymphopenia has been noted much less commonly. Henry et al. in their meta-analysis of 66 cases identified lymphopenia only in 3% of patients [82]. Could this be the difference in the outcome of COVID-19 where normal lymphocyte count could be a surrogate for good function of the immune system that helps to clear the infection without overt cytokine storm and hyper inflammation? Once again this is where hematologists' role is important whether reviewing peripheral blood smear, lymphocyte subset analysis from a hemogram or flow cytometry may lead to further infectious disease workup or intensified management of supportive care measures [83]. Patients with severe COVID-19 and high levels of inflammatory cytokines had a higher level of CD69, CD38 and CD44 expression on CD4+ and CD8+ T lymphocytes suggestive of activation, but these T lymphocytes were also exhausted as documented by a higher percentage of Tim3, PD-1 as well as NKG2A positive subsets thus perhaps providing insight into possible pathogenesis of progression of the disease. In patients with severe COVID-19, co-expression of interferon (IFN)- gamma and GM-CSF was noted on pathogenic CD4+ T cells.
4.4. Eosinopenia in COVID-19: prevalence and prognostic value
Eosinophils are known for their role in host defense against infections, immunological and allergic disorders, and various hematological disorders and cancers [84]. Eosinopenia, has been also studied as an early marker of increased mortality in critically ill patient's medical intensive care units [85]. The probable explanation could be eosinophilic sequestration into the inflammation sites or suppressed bone marrow leading to reduced generation of eosinophils. Unlike lymphopenia or thrombocytopenia, only a few studies have so far found eosinopenia to a significant level in COVID-19 patients. Qian et al. in their retrospective, multi-center case study reported that 47 out of 91 (51.65%) COVID-19 patients had eosinopenia (<0.02109 × /L) [86]. In a literature review on “COVID-19 and eosinophils”, Lippi et al. found three studies, all from China that included 294 patients, 75 of whom (25.5%) had a severe COVID-19 [82,[86], [87], [88], [89]]. This pooled analysis did not find eosinophil count to be of clinical significance between patients with or without the severe disease. Based on these preliminary results, in our opinion the diagnostic value of eosinopenia in COVID-19 is still unclear. However, eosinophil count is an easy and very cost-effectiveness test. Larger studies should help to establish the significance of eosinophils COVID-19 patients.
5. Markers of systemic inflammation
In recent years, many simple elements reported in the routine laboratory results have been investigated for their role to predict systemic inflammation. Some of these biomarkers are; Neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-C-reactive protein ratio (LCR), platelet-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, neutrophil CD64 expression, mean cell volume of neutrophils and monocytes, delta neutrophil index immature granulocyte fraction, and monocyte distribution width (MDW). So far, except for a handful of studies, we do not have any good data on any of them for their usability in the COVID-19 pandemic [56,90].
5.1. Neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-C-reactive protein ratio (LCR)
One step ahead to neutrophilia, and lymphopenia, now researchers have also investigated the other established inflammatory markers like neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-C-reactive protein ratio (LCR). Rangel et al. in their meta-analysis on prognostic utility of NLR and LCR involving six studies and a total of 828 patients that included 407 patients had severe disease [49.15%] [90]. They found NLR values to be significantly elevated (SMD = 2.404, 95% CI = 0.98–3.8 2) and LCR values to be significantly decreased (SMD = −0.912, 95% CI = −1.275 to −0.5 50) in patients with COVID-19 with severe disease. This finding suggests that NLR and LCR values can serve as a predictor of clinical severity in COVID-19.
5.2. Other inflammatory biomarkers
Several other biomarkers have been studied to assess for their clinical application in the assessment of COVID-19 severity [91].
Inflammatory cytokines and chemokines levels like tumor necrosis factor-alpha (TNF-α), interferon-γ-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), chemokine (C-C motif) ligand 3 (CCL-3) and interleukins (IL) (IL-2, IL-6, IL-7, IL-10) [except IL-1 and IL-8] have been found to be significantly associated with severity and lactate dehydrogenase (LDH). worse outcome [88,92].
Among biochemical markers, C reactive protein (CRP) levels, ferritin levels, procalcitonin, and lactate dehydrogenase (LDH) have been shown to predict poor outcome [[93], [94], [95]]. Ruan et al. found a significant difference in median CRP values between non-survivors (125 mg/L) versus survivors (40 mg/L) [95]. Similarly, they also found a significant elevation (p < 0·001) of serum ferritin levels in non- survivors (mean value: 1297.6 ng/ml) versus in survivors (mean value 614.0 ng/ml).
Florid combination of unresolving fever, worsening pancytopenia, hyperferritinemia, and cytokine storm in a subset of critically ill patients with COVID-19 prompted to explore the possibility of SARS-CoV-2 virus-induced to secondary hemophagocytic lymphohistiocytosis (sHLH) [59]. HLH, a fulminant cytokine storm syndrome is often an under-recognized entity and report by Karakike et al. suggested it to be in the rage of 3.7–4.3% of sepsis cases [96]. Tocilizumab, a recombinant humanized monoclonal antibody against the human IL-6 receptor is currently approved for chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome [97]. Early studies from China showed the benefit of tocilizumab administration in patients with severe or critical illnesses [98,99]. Currently, the Food and Drug Administration's (FDA) has approved this drug for the phase III trial study which would provide us more data regarding this indication. From the hematologist's perspective, all consults for suspected sHLH should be thoroughly investigated and COVID-19 might be considered as a potential trigger. As of now, in the United States, use of tocilizumab in critically ill COVID-19 cases with raised IL-6 levels is based on compassionate ground and no recommendations could be given [100].
6. Red blood cell abnormalities and COVID-19
6.1. Anemia
Like other hematological parameters, hemoglobin levels and its significance has also been studied in COVID-19 patients. Studies conducted in the past on hospitalized patients with pneumonia have found low hemoglobin to be independently associated with increased mortality [101,102]. On similar lines, COVID-19 pneumonia and its association with hemoglobin have been studied during the current pandemic as well [3,103,104]. A recent metanalysis by Lippi et al. including four studies comprised of 1210 COVID-19 patients. Out of 1210 patients, 224 (18.5%) patients had severe disease [105]. Except for the study by Yang et al., rest all studies found hemoglobin value to be significantly lower in COVID-19 patients with the severe disease than in those with mild cases [104,105]. Although, not looked in detail so far in any of the above-mentioned studies, it is expected that multiple factors would be contributing to anemia in COVID-19 patients who are critically ill and/or in intensive care. Basics remain the same while consulting for anemia in patients with COVID-19, that is to look for [1] Loss of blood due to phlebotomy and bleeding [2] Decreased erythropoiesis secondary to bone marrow suppression [3] Increased destruction of RBCs, or [4] nutritional deficiency [82,106,107].
Based on the meta-analysis outcome by Lippi et al., serial monitoring of hemoglobin in critically ill patients might give a hint towards the direction of the clinical progression [105]. So far, we do not have any literature if blood transfusions and keeping a set target of hemoglobin threshold has any prognostic outcome in these patients.
6.2. Morphological anomalies of circulating blood cells
We have mentioned the importance of peripheral smear film (PBF) examination as one of the easiest, cheapest, basic, and quickly available tests already several times. We believe that the value of a peripheral smear needs no attestation. Hematologists and hematopathologists have noted a few interesting observations in the peripheral smear of the patients with COVID-19 that could point towards the diagnosis. Foldes et al. reported their common observation of atypical lymphocytes, most prominent of them were lymphoplasmacytoid lymphocytes [108]. They concluded with a remark that in the appropriate clinical scenario while awaiting the COVID-19 PCR test results, presence of plasmacytoid lymphocytes in the PBF supports a provisional clinical diagnosis. Similarly, Mitra et al. reported an unusual finding of leukoerythroblastosis in a 46-year-old previously healthy COVID-19 positive female that disappeared once the patient improved [83]. Zini et al. from Italy shared their observation while examining the PBF of 249 patients with COVID-19 admitted to their hospital [109]. They found many noticeable features in the neutrophilic, lymphocytic, and platelet lineage. They often noted hyperchromatic, giant, vacuolated platelets with pseudopodia formations. The early symptomatic phase was characterized by a pronounced granulocytic reaction with immature, dysmorphic, and apoptotic-degenerated neutrophils. Few days into the treatment for COVID-19, morphological features of lymphocyte activation were the most impressive changes. These morphological changes at various stages of COVID-19 disease could be related to the viremia, and subsequent immune response leading to cytokine storm and hyperinflammation. Though these changes do not hold much significance in terms of prognostic value, these observations might help study the linkage between the hematopoietic system and pathogenesis and evolution of COVID-19 disease.
6.3. Hemoglobinopathy and COVID-19 susceptibility
Patients with sickle cell disease (SCD) are at increased risk of infections. In general, SCD patients have underlying cardiopulmonary co-morbidities that predisposes them to respiratory illnesses more than the general healthy population [110]. Another serious complication of SCD is acute chest syndrome (ACS), which is often provoked by a respiratory infection like viral illnesses. Previous studies during the 2009 H1N1 influenza pandemic found SCD patients to be at risk for developing more complications [111,112]. The SARS-CoV-2 virus seems to also to have the potential to trigger such an attack. Also, to remember the possible immune-compromising effects of hydroxycarbamide (hydroxyurea), a cytotoxic agent often used in SCD patients. So far, only a few cases of SCD patients developing ACS secondary to COVID-19 have been reported [113]. Patients were successfully managed with different drug combinations including analgesics, fluids, exchange transfusions, and tocilizumab. Considering ACS as a very close clinical mimicker of COVID-19 pneumonia, Nur et al. recommended including SARS-CoV-2 polymerase chain reaction (PCR) testing in the evaluation of any SCD case presenting with vaso-occlusive crisis symptoms [114].
Unlike SCD, patients with thalassemia have comparatively a lower risk of lung infections but, an overall compromised cardiopulmonary system, diabetes, and hemochromatosis secondary to severe iron overload increase the vulnerability to complications of the virus. Another concern of hypoadrenalism in thalassemia secondary to iron overload may merit using rescue steroids as a stress dose [115]. Concerns over chelation related side effects also merit discussion on case to case basis. Deferiprone carries the risk of reversible agranulocytosis and neutropenia while deferoxamine is known for its association with bacterial infections (notably Yersinia and Klebsiella).
6.4. Blood groups types and COVID-19 susceptibility
Individual's susceptibility to infections based on their blood groups has always been a matter of epidemiological investigations for immunohematology. Many blood groups can facilitate the infectious process by acting as receptors thereby facilitating cell invasion and evasion of host defense. One of the classical observations is a correlation between ABO type and norovirus infection [32,116]. Hutson et al. in their study reported blood group A or group O individuals to have the highest susceptibility to norovirus infection, whereas group B individuals were asymptomatic or resistant [116]. So far, we lack a well-designed study for the current COVID-19 pandemic, but preliminary reports from China suggest that group A individuals have the highest propensity and groups O individuals have the least propensity to catch SARS-CoV-2 infection [117]. Zhao et al. did not ascertain any reasoning for this differential susceptibility but proposed that presence of anti-A antibody in group B and O individuals might have some role but that needs further confirmation [118].
7. Hypogammaglobulinemia and COVID-19
Hypogammaglobulinemia is a condition with reduced immunoglobulins in the serum, which can be a primary or secondary phenomenon [119]. The primary antibody deficiency syndromes are extremely rare and hence, malnutrition, plasma cell, or B cell-directed therapy as in patients with myeloma, lymphoma, patients on immunosuppression after a stem cell transplant or after chimeric antigen receptor (CAR) T cells remain the most common causes of hypogammaglobulinemia worldwide [120]. The experience of COVID-19 with hypogammaglobulinemia remains limited due to lack of IgG levels testing hence our understanding so far is relying on individual case reports or institutional experience. Quinti et al. reported their experience of seven patients with primary antibody deficiencies (PADs) and COVID-19 infection (five with common variable immune deficiencies [CVIDs] and 2 with agammaglobulinemia) [120]. They found that as compared to CVIDs, the patients with agammaglobulinemia had mild symptoms, a shorter duration, and did not require immune-modulating drug blocking IL-6, with an overall favorable outcome. It was postulated that in the absence of B cells (in patients with agammaglobulinemia), the lack of B-cell–derived IL-6 levels resulted in attenuation in the level of inflammation and cytokine storm. A similar observation was noted by Soresina et al. who reported two COVID-19 patients with X-linked agammaglobulinemia (XLA), both recovered without the need for intensive care [121]. Given the limited data, we can only speculate that the immunosuppressed state caused by inborn defects leading to hypogammaglobulinemia maybe perhaps protective. It is possible that in patients with severe COVID-19 aberrant B-cell signaling is detrimental. This theory is further supported by preliminary reports showing that BTK inhibitors were able to curtail inflammatory responses in patients with COVID-19 [122]. BTK inhibitors create a pharmacologically acquired “absence” of B cells or B cell signaling, which essentially resembles Bruton's agammaglobulinemia [123]. On the other hand, profound secondary hypogammaglobulinemia (< 400 mg/dL) in multiple myeloma patients (n = 54) was associated with more likely hospitalization and mortality in a study by Wang et al. So it appears that the risk factor may not be hypogammaglobulinemia itself, but rather how a specific patient becomes hypogammaglobulinemic in terms of COVID-19 severity and outcome [124].
8. Immunosuppression in hematological malignancies
The preliminary observations from China and Europe suggested that COVID-19 patients with cancer overall faced much higher morbidity and mortality. This has led to the initial wave of recommendations of delaying therapy cycles, switching to less myelosuppressive regimens, and delaying stem cell transplantations (and related immunosuppression) whenever clinically acceptable [[125], [126], [127]]. More recently single-institution studies seem to suggest however that immunosuppression and anti-cancer therapy can be delivered safely as long as aggressive non-pharmacological intervention (NPI) protocols are followed to prevent COVID-19 transmission. Besides these aggressive NPI protocol individualized approach is paramount as various factors like the type of cancer, stage, and prognosis, comorbidities (hypertension, diabetes remain the key risk factors for COVID-19 outcome across all diseases), performance status, treatment options, the local situation of the pandemic as it relates to the availability of ICU level of care and blood product supply in case of complications, patient's preference, and specialists' experience play important role in deciding the approach [128,129]. And at least until further studies are available case to case basis evaluation is most likely going to be the prevalent approached, which may be modified as the interim recommendations by hematology and oncology societies may be changed based on data available from larger cohorts of patients such as ASH, ASCO, and CIBMTR databases [14,25,[130], [131], [132]].
9. Conclusion and future considerations
COVID-19 has emerged as an unparalleled health care crisis that represents a challenge for all medical specialties by its pervasive presence and by its novel clinical symptomatology and syndromes. The role of a hematology expert is paramount in managing clotting conditions, associated cytopenias. Furthermore, hematologists with expertise in handling Cytokine release syndrome (CRS) that occurs in patients receiving cellular therapies (CAR T cells) or hematopoietic stem cell transplants may provide key insights into the appropriate management of cytokine storm associated with severe COVID-19. Last, but not least as many patients may present to the hospital in the presymptomatic phase a unique constellation of hematological abnormalities that are recognized by hematology specialists may thus lead to proper COVID diagnosis and in turn prevent potential unnecessary SARS-CoV-2 exposures and transmissions.
9.1. Practice points
-
•
Use telehealth for consultations on COVID-19 positive patients. Use proper PPE in rare situations when patient's physical exam needs to be performed and such consult should be the last task for the day.
-
•
COVID-19 patients have high risk of thrombosis and all hospitalized patients should receive prophylactic anticoagulation.
-
•
Hematologists (with transplant background) may offer valuable input for management of COVID-19 related cytokine storm.
9.2. Research agenda
-
•
Role of therapeutic anticoagulation in critically ill patients needs further studies.
-
•
Significance of morphological anomalies of circulating blood cells in COVID-19.
-
•
Significance of antiphospholipid antibodies and predisposition to clot formation in COVID-19.
Funding
None.
Ethical statement
The article doesn't contain the participation of any human being and animal.
Patient consent
Not applicable.
Author contributions
Kamal Kant Sahu: Conceptualization; Methodology; Resources; Writing - original draft; Writing - review & editing.
Jan Cerny: Conceptualization; Methodology; Project administration; Resources; Supervision; Writing - original draft; Writing - review & editing.
Declaration of Competing Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
References
- 1.Sahu K.K., Mishra A.K., Lal A. COVID-2019: update on epidemiology, disease spread and management. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2020 Apr;16:90(1). doi: 10.4081/monaldi.2020.1292. [DOI] [PubMed] [Google Scholar]
- 2.COVID-19 Map - Johns Hopkins Coronavirus Resource Center. 2020 Jul 16. https://coronavirus.jhu.edu/map.html Available from:
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu K.K., Siddiqui A.D. From Hematologist’s desk: the effect of COVID-19 on the blood system. Am J Hematol. 2020;95(8):E213–E215. doi: 10.1002/ajh.25849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahu K.K., Mishra A.K., Lal A. Comprehensive update on current outbreak of novel coronavirus infection (2019-nCoV) Ann Transl Med. 2020 Mar;8(6) doi: 10.21037/atm.2020.02.92. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7186600/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J., Wang L., Zhao L., Li F., Liu J., Zhang L. Risk assessment of venous thromboembolism and bleeding in COVID-19 patients. In review. 2020 Mar.. https://www.researchsquare.com/article/rs-18340/v1 Available from: [DOI] [PMC free article] [PubMed]
- 9.Luo W., Yu H., Gou J., Li X., Sun Y., Li J. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) 2020 Feb 27. https://www.preprints.org/manuscript/202002.0407/v1 [cited 2020 Jul 16]; Available from:
- 10.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A.K., Sahu K.K., Lal A., Sargent J. Mechanisms of stroke and the role of anticoagulants in COVID-19 [published online ahead of print, 2020 Jun 26] J Formos Med Assoc. 2020;119(11):1721–1722. doi: 10.1016/j.jfma.2020.06.026. Epub 2020 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X., Li Z., Liu S., Chen Z., Zhao Z., Huang Y. Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction. 2020 Feb 29. 2020 Jul 16. https://europepmc.org/article/ppr/ppr115150 Available from:
- 13.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 2020;181:104873. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost JTH. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates S.M., Ginsberg J.S. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351(3):268–277. doi: 10.1056/NEJMcp031676. [DOI] [PubMed] [Google Scholar]
- 17.Hematology Brigham and Women's Hospital COVID-19 Clinical Guidelines. 2020 Jul 16. https://5f0d01e605ca8259a8da5fd0--covid-protocols.netlify.app/protocols/therapeutics/ Available from:
- 18.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonzogni A., Previtali G., Seghezzi M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40(9):2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mondal R., Lahiri D., Deb S., Bandyopadhyay D., Shome G., Sarkar S. COVID-19: are we dealing with a multisystem vasculopathy in disguise of a viral infection? J Thromb Thrombolysis. 2020 Jul 5;50:567–579. doi: 10.1007/s11239-020-02210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treatment and Testing Guidelines from ID UPHS COVID-19 Learning Perelman School of Medicine at the University of Pennsylvania. 2020 Jul 16. https://www.med.upenn.edu/uphscovid19education/treatment-guidelines/ Available from:
- 22.COVID-19 Resources – Hematology.org. 2020 Jun 16. https://www.hematology.org/covid-19 Available from:
- 23.Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost JTH. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt F.C.F., Manolov V., Morgenstern J., Fleming T., Heitmeier S., Uhle F. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ASCO Survey on COVID-19 in Oncology (ASCO) Registry. ASCO; 2020 Jul 16. https://www.asco.org/asco-coronavirus-information/coronavirus-registry Available from: [Google Scholar]
- 26.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovina N., Akinosoglou K., Eugen-Olsen J., Hayek S., Reiser J., Giamarellos-Bourboulis E.J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han H., Yang L., Liu R., Liu F., Wu K., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med CCLM. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 30.Antithrombotic Therapy Coronavirus Disease COVID-19. COVID-19 Treatment Guidelines. 2020 Jul 17. https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ Available from:
- 31.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of COVID-19 in the Young. N Engl J Med. 2020 14;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uthman I.W., Gharavi A.E. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020 23;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost JTH. 2020 Apr;23 doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhibar D.P., Sahu K.K., Varma S.C., Kumari S., Malhotra P., Mishra A.K. Intra-cardiac thrombus in antiphospholipid antibody syndrome: an unusual cause of fever of unknown origin with review of literature. J Cardiol Cases. 2016;14(5):153–156. doi: 10.1016/j.jccase.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Li M., Wang M. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study [published online ahead of print, 2020 Jul 2] Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. Epub 2020 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.K., Jeon J.-S., Kim J.W., Kim G.-Y. Correlation between abnormal platelet count and respiratory viral infection in patients from Cheonan, Korea. J Clin Lab Anal. 2016;30(3):185–189. doi: 10.1002/jcla.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamad H., Sahu K.K., Dunn S., Milla L., Caffery A., Islam N. Rifampin induced thrombotic thrombocytopenic purpura. Indian J Hematol Blood Transfus. 2020 Jan 10 doi: 10.1007/s12288-019-01249-9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahu K.K., Varma S.C. Cortical vein thrombosis in a case of idiopathic thrombocytopenic purpura. Platelets. 2015;26(4):374–375. doi: 10.3109/09537104.2014.898180. [DOI] [PubMed] [Google Scholar]
- 41.Sahu K.K., Siddiqui A.D., Rezaei N., Cerny J. Challenges for Management of Immune Thrombocytopenia during COVID-19 pandemic. J Med Virol. 2020 Jul;3 doi: 10.1002/jmv.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burunsuzoğlu B., Saltürk C., Karakurt Z., Öngel E.A., Takır H.B., Kargın F. Thrombocytopenia: a risk factor of mortality for patients with Sepsis in the intensive care unit. Turk Thorac J. 2016;17(1):7–14. doi: 10.5578/ttj.17.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderschueren S., De Weerdt A., Malbrain M., Vankersschaever D., Frans E., Wilmer A. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28(6):1871–1876. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 44.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta Int J Clin Chem. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra A.K., Sahu K.K., George A.A., Sargent J., Lal A. Cerebrovascular events in COVID-19 patients. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2020 Jun 10;90(2) doi: 10.4081/monaldi.2020.1341. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A.K., Lal A., Sahu K.K., George A.A., Sargent J. Letter to the editor regarding “neurological impact of coronavirus disease (COVID-19): practical considerations for the neuroscience community” [published online ahead of print, 2020 May 17] World Neurosurg. 2020;142:533–534. doi: 10.1016/j.wneu.2020.05.089. Published online 2020 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhibar D.P., Sahu K.K., Dhir V., Singh S. Immune thrombocytopenia as a presenting manifestation of tuberculosis- challenge in resource constraint settings. J Clin Diagn Res JCDR. 2016;10(10):OD01–2. doi: 10.7860/JCDR/2016/20911.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang M., Ng M.H.L., Li C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review) Hematol Amst Neth. 2005;10(2):101–105. doi: 10.1080/10245330400026170. [DOI] [PubMed] [Google Scholar]
- 49.Qu R., Ling Y., Zhang Y., Wei L., Chen X., Li X. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533–1541. doi: 10.1002/jmv.25767. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vlacha V., Feketea G. Thrombocytosis in pediatric patients is associated with severe lower respiratory tract inflammation. Arch Med Res. 2006;37(6):755–759. doi: 10.1016/j.arcmed.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Indolfi G., Catania P., Bartolini E., Azzari C., Massai C., Poggi G.M. Incidence and clinical significance of reactive thrombocytosis in children aged 1 to 24 months, hospitalized for community-acquired infections. Platelets. 2008;19(6):409–414. doi: 10.1080/09537100802233107. [DOI] [PubMed] [Google Scholar]
- 52.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaser A., Brandacher G., Steurer W., Kaser S., Offner F.A., Zoller H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 54.Hsu H.C., Tsai W.H., Jiang M.L., Ho C.H., Hsu M.L., Ho C.K. Circulating levels of thrombopoietic and inflammatory cytokines in patients with clonal and reactive thrombocytosis. J Lab Clin Med. 1999;134(4):392–397. doi: 10.1016/s0022-2143(99)90154-3. [DOI] [PubMed] [Google Scholar]
- 55.Zhu J., Ji P., Pang J., Zhong Z., Li H., He C. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 Apr;15 doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. 2020 25;58(7):1063–1069. doi: 10.1515/cclm-2020-0240. [DOI] [PubMed] [Google Scholar]
- 57.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020 28;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 62.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 63.Brenner S.R. Erythropoietin-induced hemoglobin subunit beta may stimulate innate immune RNA virus pattern recognition, suppress reactive oxygen species, reduce ACE2 viral doorway opening, and neutrophil extracellular traps against COVID-19 [published online ahead of print, 2020 Jul 9] J Med Virol. 2020 doi: 10.1002/jmv.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore T.A., Standiford T.J. The role of cytokines in bacterial pneumonia: an inflammatory balancing act. Proc Assoc Am Physicians. 1998;110(4):297–305. [PubMed] [Google Scholar]
- 66.Cheng A.C., Stephens D.P., Currie B.J. Granulocyte-Colony stimulating factor (G-CSF) as an adjunct to antibiotics in the treatment of pneumonia in adults. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD004400.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Cavaillon J.-M., Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12(3):151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 68.Murata A. Granulocyte colony-stimulating factor as the expecting sword for the treatment of severe sepsis. Curr Pharm Des. 2003;9(14):1115–1120. doi: 10.2174/1381612033454982. [DOI] [PubMed] [Google Scholar]
- 69.Lang F.M., Lee K.M.-C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol. 2020 Jun;23 doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020 Mar 13;7(6):998–1002. doi: 10.1093/nsr/nwaa041. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7108005/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamilton J.A. GM-CSF in inflammation. J Exp Med. 2020;217(1) doi: 10.1084/jem.20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton J.A., Cook A.D., Tak P.P. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov. 2016 29;16(1):53–70. doi: 10.1038/nrd.2016.231. [DOI] [PubMed] [Google Scholar]
- 73.Unkel B., Hoegner K., Clausen B.E., Lewe-Schlosser P., Bodner J., Gattenloehner S. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Invest. 2012;122(10):3652–3664. doi: 10.1172/JCI62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rösler B., Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy? Mol Cell Pediatr. 2016;3(1):29. doi: 10.1186/s40348-016-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subramaniam R., Hillberry Z., Chen H., Feng Y., Fletcher K., Neuenschwander P. Delivery of GM-CSF to protect against influenza pneumonia. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skoetz N., Bohlius J., Engert A., Monsef I., Blank O., Vehreschild J.-J. Prophylactic antibiotics or G(M)-CSF for the prevention of infections and improvement of survival in cancer patients receiving myelotoxic chemotherapy. Cochrane Database Syst Rev. 2015;12 doi: 10.1002/14651858.CD007107.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlin L., Darmon M., Thiéry G., Ciroldi M., de Miranda S., Lefebvre A. Respiratory status deterioration during G-CSF-induced neutropenia recovery. Bone Marrow Transplant. 2005;36(3):245–250. doi: 10.1038/sj.bmt.1705037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ESMO Cancer Patient Management During the COVID-19 Pandemic. 2020 Jun 16. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic Available from:
- 79.ESMO Supportive Care Strategies During the COVID-19 Pandemic. 2020 Jul 16. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/supportive-care-in-the-covid-19-era Available from:
- 80.Sahu K.K., Mishra A.K., Lal A. Trajectory of the COVID-19 pandemic: chasing a moving target. Ann Transl Med. 2020;8(11):694. doi: 10.21037/atm-20-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahu K.K., Cerny J. Managing patients with hematological malignancies during COVID-19 pandemic. Expert Rev Hematol. 2020 Jul;12:1–7. doi: 10.1080/17474086.2020.1787147. [DOI] [PubMed] [Google Scholar]
- 82.Henry B.M., Lippi G., Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020 25;58(7):1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 83.Mitra A., Dwyre D.M., Schivo M., Thompson G.R., Cohen S.H., Ku N. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95(8):999–1000. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sahu K.K., Malhotra P., Khadwal A. Hypereosinophilia in acute lymphoblastic leukemia: two cases with review of literature. Indian J Hematol Blood Transfus. 2015;31(4):460–465. doi: 10.1007/s12288-014-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abidi K., Belayachi J., Derras Y., Khayari M.E., Dendane T., Madani N. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med. 2011;37(7):1136–1142. doi: 10.1007/s00134-011-2170-z. [DOI] [PubMed] [Google Scholar]
- 86.Qian G.-Q., Yang N.-B., Ding F., Ma A.H.Y., Wang Z.-Y., Shen Y.-F. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM Mon J Assoc Phys. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 89.Lippi G., Henry B.M. Eosinophil count in severe coronavirus disease 2019. QJM Mon J Assoc Phys. 2020;113(7):511–512. doi: 10.1093/qjmed/hcaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020 Apr;3 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China [published online ahead of print, 2020 Mar 16] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. ciaa270. [DOI] [Google Scholar]
- 93.Sahu K.K., Kumar R. Current perspective on pandemic of COVID-19 in the United States. J Family Med Prim Care. 2020;9(4):1784–1791. doi: 10.4103/jfmpc.jfmpc_424_20. Published 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra A.K., Lal A., Sahu K.K., Sargent J. Cardiovascular factors predicting poor outcome in COVID-19 patients [published online ahead of print, 2020 May 28] Cardiovasc Pathol. 2020;49:107246. doi: 10.1016/j.carpath.2020.107246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in Sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.England J.T., Abdulla A., Biggs C.M., Lee A.Y.Y., Hay K.A., Hoiland R.L. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020 May;15:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020 19;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sahu K.K., Kumar R. Preventive and treatment strategies of COVID-19: from community to clinical trials. J Fam Med Prim Care. 2020;9(5):2149. doi: 10.4103/jfmpc.jfmpc_728_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reade M.C., Weissfeld L., Angus D.C., Kellum J.A., Milbrandt E.B. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med. 2010;10:15. doi: 10.1186/1471-2466-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He Y., Li M., Mai C., Chen L., Zhang X., Zhou J. Anemia and Low albumin levels are associated with severe community-acquired pneumonia in pregnancy: a case-control study. Tohoku J Exp Med. 2019;248(4):297–305. doi: 10.1620/tjem.248.297. [DOI] [PubMed] [Google Scholar]
- 103.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 Mar;3 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lippi G., Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116–117. doi: 10.1016/j.htct.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hadadi A., Mortezazadeh M., Kolahdouzan K., Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol. 2020;92(7):915–918. doi: 10.1002/jmv.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aydemir D., Ulusu N.N. Is glucose-6-phosphate dehydrogenase enzyme deficiency a factor in Coronavirus-19 (COVID-19) infections and deaths? Pathog Glob Health. 2020;114(3):109–110. doi: 10.1080/20477724.2020.1751388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Foldes D., Hinton R., Arami S., Bain B.J. Plasmacytoid lymphocytes in SARS-CoV-2 infection (Covid-19) Am J Hematol. 2020;95(7):861–862. doi: 10.1002/ajh.25834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zini G., Bellesi S., Ramundo F., d’Onofrio G. Morphological anomalies of circulating blood cells in COVID-19. Am J Hematol. 2020;95(7):870–872. doi: 10.1002/ajh.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bundy D.G., Strouse J.J., Casella J.F., Miller M.R. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125(2):234–243. doi: 10.1542/peds.2009-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Inusa B., Zuckerman M., Gadong N., Afif M., Arnott S., Heath P. Pandemic influenza a (H1N1) virus infections in children with sickle cell disease. Blood. 2010;115(11):2329–2330. doi: 10.1182/blood-2009-12-260836. [DOI] [PubMed] [Google Scholar]
- 112.George A., Benton J., Pratt J., Kim M.-O., Kalinyak K.A., Kalfa T.A. The impact of the 2009 H1N1 influenza pandemic on pediatric patients with sickle cell disease. Pediatr Blood Cancer. 2011;57(4):648–653. doi: 10.1002/pbc.23030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sahu K.K., Siddiqui A.D., Cerny J. Managing sickle cell patients with COVID-19 infection: the need to pool our collective experience. Br J Haematol. 2020 May;23 doi: 10.1111/bjh.16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nur E., Gaartman A.E., van Tuijn C.F.J., Tang M.W., Biemond B.J. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19) Am J Hematol. 2020;95(6):725–726. doi: 10.1002/ajh.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy N.B.A., Telfer P., Eleftheriou P., de la Fuente J., Drasar E., Shah F. Protecting vulnerable patients with inherited anaemias from unnecessary death during the COVID-19 pandemic. Br J Haematol. 2020;189(4):635–639. doi: 10.1111/bjh.16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hutson A.M., Atmar R.L., Marcus D.M., Estes M.K. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J Virol. 2003;77(1):405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu Y., Feng Z., Li P., Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin Chim Acta Int J Clin Chem. 2020;509:220–223. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao J., Yang Y., Huang H., Li D., Gu D., Lu X. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. medRxiv. 2020 Mar 27 2020.03.11.20031096. [Google Scholar]
- 119.Cook G., John Ashcroft A., Pratt G., Popat R., Ramasamy K., Kaiser M. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020;190(2):e83–e86. doi: 10.1111/bjh.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211–213. doi: 10.1016/j.jaci.2020.04.013. (e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Focà E. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover [published online ahead of print, 2020 Apr 22] Pediatr Allergy Immunol. 2020;31(5):565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Treon S.P., Castillo J.J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19–infected patients. Blood. 2020;135(21):1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020 Jun 5;5(48) doi: 10.1126/sciimmunol.abd0110. https://immunology.sciencemag.org/content/5/48/eabd0110 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. medRxiv. 2020 Jul 16 doi: 10.1101/2020.06.04.20122846v2. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sahu K.K., Jindal V., Siddiqui A.D. Managing COVID-19 in patients with Cancer: a double blow for oncologists. JCO Oncol Pract. 2020;16(5):223–225. doi: 10.1200/OP.20.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sahu K.K., Siddiqui A.D., Cerny J. COVID-19 pandemic and impact on hematopoietic stem cell transplantation [published online ahead of print, 2020 May 4] Bone Marrow Transplant. 2020:1–3. doi: 10.1038/s41409-020-0913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sahu K.K., Jindal V., Siddiqui A.D., Cerny J. Facing COVID-19 in the hematopoietic cell transplant setting: a new challenge for transplantation physicians. Blood Cells Mol Dis. 2020;83:102439. doi: 10.1016/j.bcmd.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sahu K.K., Mishra A.K., Martin K., Chastain I. COVID-19 and restrictive lung disease: a deadly combo to trip off the fine balance. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2020 Jun 29;90(2) doi: 10.4081/monaldi.2020.1346. [DOI] [PubMed] [Google Scholar]
- 129.Jindal V., Sahu K.K., Gaikazian S., Siddiqui A.D., Jaiyesimi I. Cancer treatment during COVID-19 pandemic. Med Oncol. 2020;37(7):58. doi: 10.1007/s12032-020-01382-w. Published 2020 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pourroy B., Tournamille J.F., Bardin C., Slimano F., Chevrier R., Rioufol C. Providing oncology pharmacy services during the coronavirus pandemic: French Society for Oncology Pharmacy (Société Francaise de Pharmacie Oncologique [SFPO]) guidelines. JCO Oncol Pract. 2020 Jun;15 doi: 10.1200/OP.20.00295. [DOI] [PubMed] [Google Scholar]
- 131.Data Resources: Publications, Slides and Reports. 2020 Jul 16. https://www.cibmtr.org/Data/Resources/Pages/index.aspx Available from:
- 132.Slimano F., Baudouin A., Zerbit J., Toulemonde-Deldicque A., Thomas-Schoemann A., Chevrier R. Cancer, immune suppression and coronavirus Disease-19 (COVID-19): need to manage drug safety (French Society for Oncology Pharmacy [SFPO] guidelines) Cancer Treat Rev. 2020;88:102063. doi: 10.1016/j.ctrv.2020.102063. [DOI] [PMC free article] [PubMed] [Google Scholar]