Abstract
Background
The open-label, randomised Phase 2 AVATAXHER study (NCT01142778) demonstrated that early PET assessment identified HER2-positive breast cancer patients who responded poorly to neoadjuvant docetaxel plus trastuzumab. Adding neoadjuvant bevacizumab for PET-predicted poor-responders improved pathological complete response (pCR) rates (43.8% vs 24.0%). We investigated long-term study outcomes.
Methods
Patients were treated in three groups. All patients initially received two cycles of standard neoadjuvant therapy with [¹⁸F]-FDG PET conducted before each cycle. Those with ≥70% change in the maximum standardised uptake value (∆SUVmax) received four further cycles of standard neoadjuvant therapy (PET responders). PET-predicted poor-responders (∆SUVmax <70%) were randomised (2:1) to neoadjuvant therapy with (Group A) or without (Group B) bevacizumab for cycles 3–6. All patients received one further cycle of trastuzumab before surgery plus adjuvant trastuzumab (11 cycles).
Findings
142 patients were randomized and treated (PET responders, n = 69; Group A, n = 48; Group B, n = 25). 5-year disease-free survival rates were 90.5% (95% CI: 80.0–95.6%) in PET responders, 90.2% (95% CI: 75.9–96.2%) in Group A, and 76.0% (95% CI: 54.2–88.4%) in Group B. However, no difference was observed between randomised arms in a sensitivity analysis. During adjuvant therapy, the incidence of Grade ≥3 (Group A: 25.6%; Group B 12.5%) and serious adverse events (Group A: 18.6%; Group B 12.5%) was higher in Group A vs Group B, but with no apparent effect on cardiac events.
Interpretation
In patients with HER2-positive breast cancer, an intervention based on early PET assessment and improvement of pCR does not modify disease-free survival.
Funding
Roche France.
Keywords: Her-2 positive breast cancer, Bevacizumab, Neoadjuvant, Positron emission tomography, Early pet assessment, Δsuvmax, pathological complete response, Long-term follow-up
Research in context.
Evidence before this study
Prior to initiating the AVATAXHER study, PubMed, Web of Knowledge, and ClinicalTrials.gov were searched using the terms ‘neoadjuvant’, ‘HER-positive’, and ‘breast cancer’ to identify studies that investigated the use of 18F-PET to predict response to neoadjuvant treatment. No relevant publications or studies were identified in the original search, limited to articles published between May 1, 2007 and May 1, 2012. Consequently, the AVATAXHER study was initiated. The primary analysis of AVATAXHER demonstrated that early PET assessment can identify patients with early-stage HER2-positive breast cancer who will respond poorly to standard docetaxel plus trastuzumab neoadjuvant therapy, and that adding bevacizumab to standard neoadjuvant therapy in PET-predicted poor responders improved the pathological complete response (pCR) rate at surgery (from 24.0% [95% CI: 9.4–45.1%] to 43.8% [95% CI: 29.5–58.8%]).
Added value of this study
This pre-planned 5-year follow-up analysis showed that the improved pCR rate in PET-predicted poor responders observed in the AVATAXHER study did not translate into improved long-term outcomes. The 5-year disease-free survival rates were 90.5% (95% CI: 80.0–95.6%) in the PET-predicted responders (∆SUVmax ≥70%), 90.2% (95% CI: 75.9–96.2%) in PET-predicted poor responders who received additional neoadjuvant bevacizumab, and 76.0% (95% CI: 54.2–88.4%) in PET-predicted poor responders who received standard neoadjuvant therapy. However, a sensitivity analysis discarded any significant difference between the two randomised arms.
Implications of all the available evidence
While the results of this exploratory study require confirmation in a phase III trial, the AVATAXHER study showed the value of early PET to identify patients with HER2 positive early breast cancer that are unlikely to respond to standard neoadjuvant therapy; however, adding bevacizumab to docetaxel plus trastuzumab neoadjuvant therapy in these patients does not improve long-term outcomes.
Alt-text: Unlabelled box
1. Introduction
Trastuzumab with/without pertuzumab anti-HER2 therapy plus taxane-containing chemotherapy is a standard of care in the neoadjuvant treatment of women with early stage HER2-positive breast cancer [1,2] with the aim of improving pathological complete response (pCR) rates and facilitating surgery. Several studies have shown positron emission tomography (PET) to be an early predictor of pCR under neoadjuvant therapy in early breast cancer, [3], [4], [5] with recent evidence that it may also have a role in predicting long-term outcomes in these patients [6,7].
The AVATAXHER study was an open-label, randomised, phase II study that investigated the prognostic value of early PET assessment in patients with early-stage HER2-positive breast cancer [8]. The study also investigated the addition of bevacizumab, which has shown synergistic effects with trastuzumab in animal models, [9] to docetaxel plus trastuzumab neoadjuvant treatment. Clinical trials in HER2 positive breast cancer also conclude that the association of bevacizumab and trastuzumab may be synergistic and promising, but these trials are small and their conclusions have to be confirmed in larger trials [10], [11], [12]. The results of the AVATAXHER study demonstrated two key findings: first, that early PET assessment identified patients predicted to respond poorly to docetaxel plus trastuzumab neoadjuvant treatment – in patient receiving docetaxel plus trastuzumab only, the negative predictive value of ∆SUVmax for predicting pCR was 75.0%. Second, the addition of bevacizumab to neoadjuvant therapy in PET-predicted poor-responders improved pCR rates (from 24.0% to 43.8%) [8]. These results suggest a new role for PET in the early identification of non-responders to neoadjuvant docetaxel plus trastuzumab therapy and the activity of bevacizumab in this setting.
While pCR is recognised by both the FDA and EMA as a surrogate for favourable outcome in breast cancer clinical trials, improvements in pCR rates have not consistently translated into long-term outcome benefit in breast cancer trials, [13], [14], [15], [16] possibly due to differences in study design, breast cancer subtype, patient characteristics and treatment administration / sequencing [14]. Improvements in pCR observed after the addition of bevacizumab to neoadjuvant chemotherapy in patients with HER2-negative breast cancer [17], [18], [19] have largely not been associated with improved long-term outcomes [18,[20], [21], [22]]. However, one recent study in patients with HER2-positive locally advanced breast cancer treated with neoadjuvant trastuzumab plus bevacizumab found a pCR of 46% and 5-year recurrence-free survival and overall survival of 79.9% and 90.8%, respectively [23]. Breast cancer subtype, dose modification according to response, and treatment sequencing may therefore all influence outcome.
Here we report the results of a pre-defined 5-year outcome analysis of the AVATAXHER study, which investigated whether the improved pCR rate observed with the addition of bevacizumab to neoadjuvant docetaxel plus trastuzumab in PET-predicted poor-responders would translate into improved long-term outcome in HER2-positive breast cancer patients. The study also provided further insight into the tolerance of bevacizumab added to neoadjuvant docetaxel plus trastuzumab followed by adjuvant trastuzumab.
2. Patients and methods
2.1. Study design and participants
Full details of the design of the AVATAXHER study and inclusion criteria have been reported previously [8]. The full study protocol is available in the appendix. The study was an exploratory open-label, non-comparative, randomised phase II trial conducted in four stages (Fig. 1, Fig. 2). The primary endpoint, reported previously, [8] was the centrally assessed pathological complete response rate, defined as complete disappearance of tumour in the breast and the axilla according to the Chevallier classification. In brief, the study population comprised women with early stage HER2-positive, histologically confirmed breast cancer (T2/T3 and NX/N0/N1) with ECOG PS 0–2, who were scheduled to receive neoadjuvant therapy and were amenable to receiving [¹⁸F]- fluorodeoxyglucose (FDG) PET imaging prior to cycles 1 and 2.
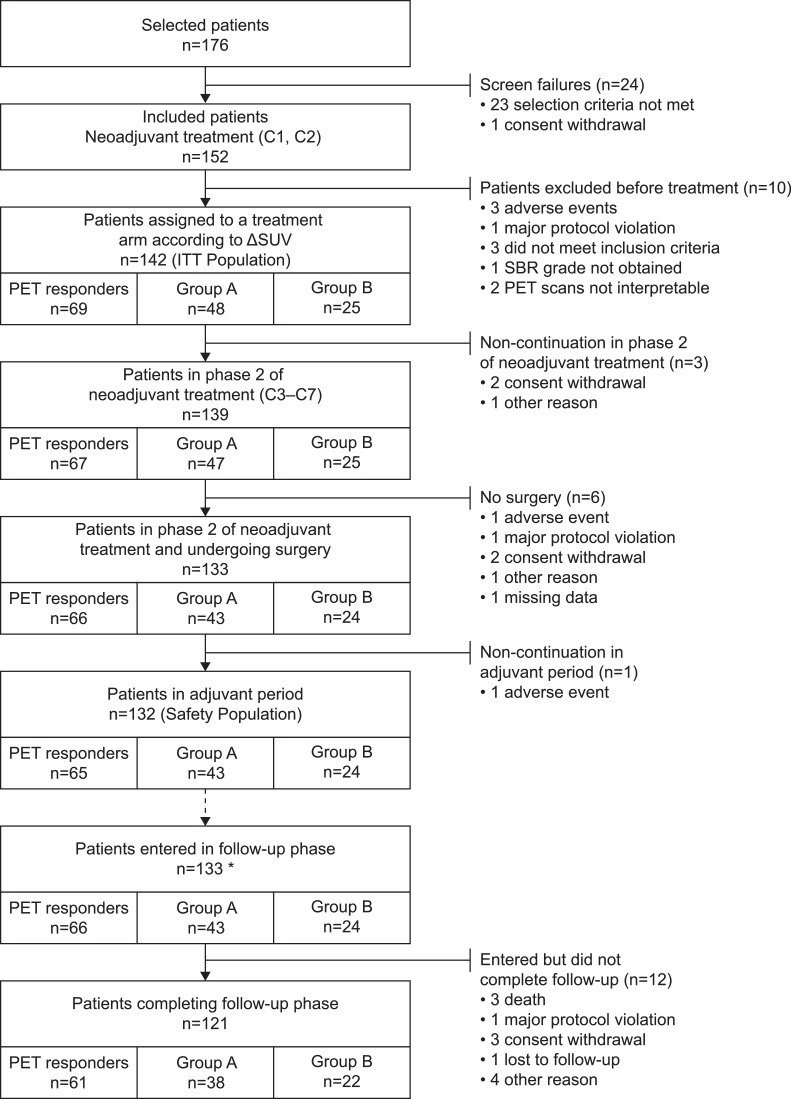
Fig. 1.
Patient flow chart. * 123 patients who completed adjuvant phase and entered in follow up phase plus 10 patients who discontinued treatment but entered in follow-up phase.
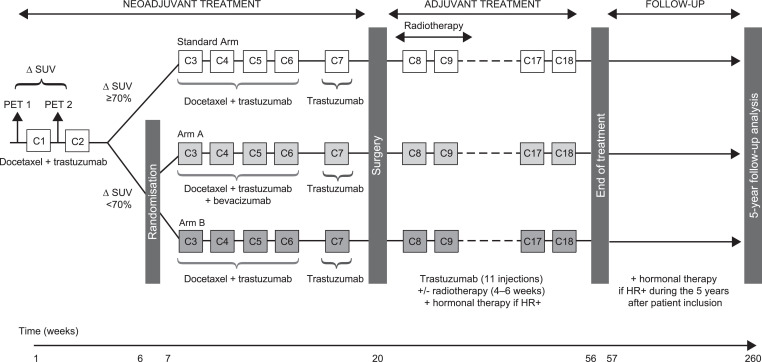
Fig. 2.
AVATAXER study design.
Patients were recruited between May 19, 2010 and October 1, 2012 from 26 oncology centres in France. All enroled patients provided written informed consent before screening procedures that were specific for this study. The study was approved by the central Ethics Committee (Comité de Protection des Personnes) for all participating centres and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study is registered with ClinicalTrials.gov (NCT01142778) and EudraCT (2009-013410-26).
2.2. Treatment
In Stage 1, all patients initially received two cycles of neoadjuvant docetaxel (100 mg/m²) plus trastuzumab (8 mg/kg in cycle 1, and 6 mg/kg thereafter), both administered intravenously (i.v.) every 3 weeks (q3w). No surgical axilla staging was allowed before neoadjuvant therapy. [¹⁸F]-FDG PET was performed before and after the first cycle of neoadjuvant therapy (within −7 days of cycle 1 and −3 days of cycle 2) and the change in maximum standardised uptake value (∆SUVmax) was calculated. In Stage 2, patients with a ∆SUVmax ≥70% continued to receive standard docetaxel and trastuzumab therapy for cycles 3–6 (PET responders group), whereas PET-predicted poor-responders (∆SUVmax <70%) were randomised (2:1) to receive either four cycles of docetaxel and trastuzumab with (Group A) or without (Group B) bevacizumab (15 mg/kg i.v. q3w). One cycle of trastuzumab alone (cycle 7) was administered to all patients before surgery, which was performed after cycle 7 and ≥4 weeks after the last infusion of cycle 6. Following surgery, in Stage 3, patients received 11 cycles of adjuvant trastuzumab q3w with or without radiotherapy (4–6 weeks according to the site's standard practice) plus hormonal therapy in hormone receptor positive patients. Stage 4 consisted of a 5-year post-treatment follow-up during which hormonal therapy was permitted in hormone receptor positive patients only.
2.3. Randomisation and masking
Randomisation methods have been described in full previously [8]. Investigators and patients were aware of group assignment.
2.4. 5-year follow-up study endpoints and statistical analyses
2.4.1. Follow-up
Patients were assessed during follow-up at biannual clinic visits with assessment of left ventricular ejection fraction (LVEF), chest X-ray, and abdominal ultrasound, as well as yearly mammography.
2.4.2. Efficacy outcomes
Long-term efficacy outcomes were secondary endpoints of the AVATAXHER study and comprised disease-free survival (DFS), distant disease-free survival (DDFS), local relapse-free interval (LRFI), and OS. DFS was defined as time from first administration of neoadjuvant treatment to local recurrence, local recurrence in the ipsilateral breast following lumpectomy, regional recurrence, occurrence of distant metastases, controlateral breast cancer, second primary cancer (other than squamous or basal cell carcinoma of the skin, melanoma in situ, carcinoma in situ of the cervix, colon carcinoma in situ, or lobular carcinoma in situ of the breast), or death from any cause. LRFI was defined as the time from first administration of neoadjuvant treatment to local recurrence in the ipsilateral or controlateral breast following lumpectomy. DDFS was defined as time to distant recurrence following first administration of neoadjuvant treatment. OS was defined as time from first administration of neoadjuvant treatment to death from any cause.
2.4.3. Safety outcomes
Safety during the adjuvant phase was analysed in the subset of patients from the safety population (N=132) who received at least one dose of trastuzumab during the adjuvant phase. For safety analyses, patients were grouped according to study group. Safety outcomes included incidence of adverse events (AEs) up to 28 days after the last treatment administration. AEs were coded according to the MedDRA guidelines and their intensity graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4•0. Cardiac safety was assessed according to the New York Heart Association classification.
2.4.4. Statistics
Determination of the sample size for the AVATAXHER study has been reported previously [8]. Efficacy analyses were conducted using the intention-to-treat (ITT) population (i.e. all patients assigned to treatment arm according to ∆SUVmax and randomised). Time-to-event measures were analysed by the Kaplan–Meier method. Median values for survival parameter estimates at 5 years were calculated with 95% CI. Patients lost to follow up were censored at the date of last assessment of the event. For patients alive at study end (premature discontinuation or final visit) censoring for OS was the last known date alive. Additional censoring was applied for LRFI (at first date of regional occurrence, occurrence of distant metastases, second primary cancer, or death without evidence of recurrence) and DDFS (at first date of local or regional recurrence, second primary cancer, contralateral cancer, or death from any cause).
A post-hoc sensitivity analysis for DFS was conducted using alternative censoring methods. In this analysis, patients who stopped treatment early (for example, due to consent withdrawal) were considered treatment failures.
All data analyses were performed using SAS version 9•4. This manuscript adheres to CONSORT reporting guidelines.
2.5. Role of the funding source
The funder of the study (Roche France) supplied trastuzumab and bevacizumab and had a role in the design and conduct of the study; collection, management, and analysis of data (including statistical analyses); interpretation of findings;. The corresponding author had full access to all the data and the final responsibility to submit for publication. All authors were involved in the critical review of the manuscript during its development and approved the final version for submission.
3. Results
3.1. Initial analysis
Overall, 176 patients with HER2-positive breast cancer were screened of which 152 patients were included and received two cycles of neoadjuvant therapy (Fig. 1). Ten patients were excluded prior to treatment arm assignment. In total, 142 patients were assigned to a treatment group (ITT population). The baseline and demographic characteristics of the ITT population have been reported previously; [8] patients were well balanced between groups with respect to tumour characteristics that could affect pCR.
Of the 142 patients in the ITT population, 69 (48.6%) were PET-predicted responders and continued to receive standard docetaxel plus trastuzumab. The remaining 73 (51.4%) patients were PET-predicted poor-responders and were randomised (2:1) to docetaxel, trastuzumab, and bevacizumab (n=48; Group A) or docetaxel plus trastuzumab (n=25; Group B). One hundred and thirty-nine (97.9%) patients underwent phase 2 of neoadjuvant treatment, 133 patients underwent surgery, 128 underwent radiotherapy, 132 patients entered the adjuvant period, and 133 patients entered the long-term follow-up phase (123 patients who completed the adjuvant phase and 10 patients who discontinued treatment but entered the follow-up phase).
Initial mean (SD) SUVmax of the affected breast after the first PET scan was lower for predicted poor-responders in Group A and B (6•68 [4•21] and 7•19 [4•87], respectively) compared with PET responders (10•09 [5•06]). Central review determined a pCR rate of 53•6% (37/69; 95% CI 41•2–65•7) for PET responders. In poor-responders, the addition of bevacizumab to cycles 4–6 of neoadjuvant chemotherapy increased the proportion of patients in Group A achieving a pCR to 43•8% (21/48; 95% CI 29•5–58•8) compared with 24% (6/25; 95% CI 9•4–45•1) for patients in Group B receiving neoadjuvant docetaxel plus trastuzumab alone [8].
3.2. Patient disposition during follow-up analysis
In total, 121/142 patients completed the 5-year follow-up period. The reasons for premature study discontinuation for the 21 patients who did not reach the 5-year follow-up are shown in Table 1.
Table 1.
Reasons for premature study discontinuation of the study (N = 21).
| PET responders (N = 69) |
Group A (N = 48) |
Group B (N = 25) |
Total (N = 142) |
|
|---|---|---|---|---|
| Premature end-of-study | 8 (11.6%) | 10 (20.8%) | 3 (12.0%) | 21 (14.8%) |
| Reasons for discontinuation | ||||
| n | 7 | 10 | 3 | 20 |
| Consent withdrawal | 1 (14.3%) | 5 (50.0%) | 0 | 6 (30.0%) |
| Death | 0 | 2 (20.0%) | 1 (33.3%) | 3 (15.0%) |
| Major protocol deviation | 1 (14.3%) | 1 (10.0%) | 0 | 2 (10.0%) |
| Lost to follow up | 1 (14.3%) | 1 (10.0%) | 0 | 2 (10.0%) |
| Other reason | 4 (57.1%)* | 1 (10.0%)† | 2 (66.7%)‡ | 7 (35.0%) |
| Missing data | 1 | 0 | 0 | 1 |
Blood glucose not measured at baseline and PET n°2 not performed as per Protocol (n = 1), discovery of a non-inclusion criterion (1.5 months after inclusion; n = 1), bone metastasis diagnosed later (n = 1), and patient diagnosed M1 after the PET scan and withdrawn at Sponsor's request (n = 1);.
Patient moved abroad (n = 1);.
Patient included in another clinical trial (n = 2).
After a median (range) follow-up of 5.2 years (range 0.1 to 6.5 years), three patients (two in Group A, one in Group B) had died, three patients (one in Group A, two in Group B) experienced local relapse, and seven patients (four in the PET responders group, three in Group B) experience distant relapse.
3.2.1. Disease-free survival
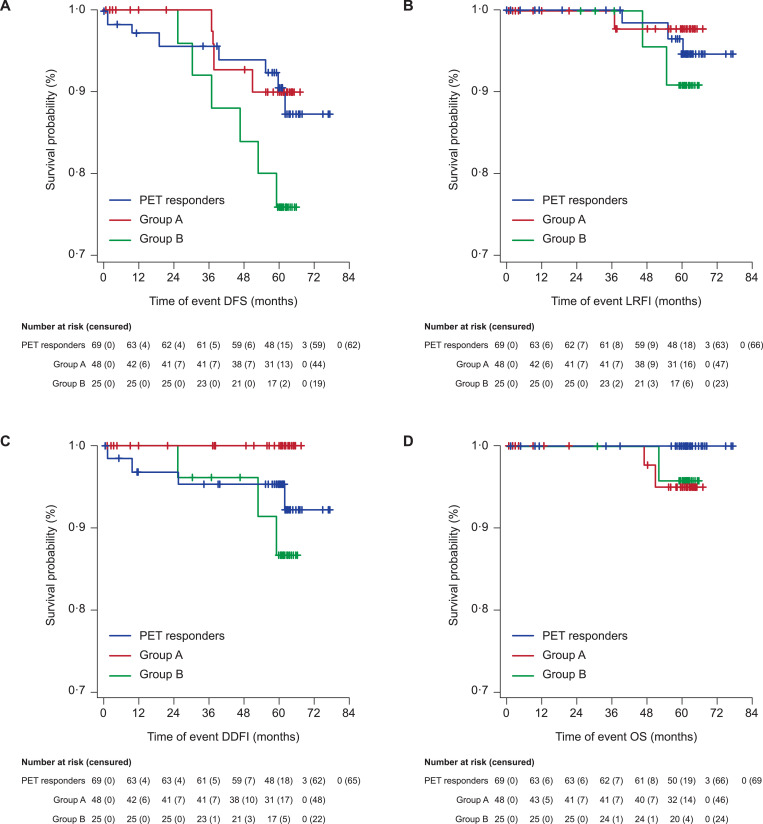
At 5-year follow-up, 17 DFS events were reported: four (8.3%) in Group A, six (24.0%) in Group B, and seven (10.1%) in the PET responders. The DFS rates at 5 years were 90.5% (95% CI: 80.0–95.6%) in PET responders, 90.2% (95% CI: 75.9–96.2%) in Group A and 76.0% (95% CI: 54.2–88.4%) in Group B (Table 2 and Fig. 3A). Median DFS was not reached in all arms.
Table 2.
Analysis of time-to-event efficacy endpoints.
| PET responders | Group A | Group B | |
|---|---|---|---|
| (N = 69) | (N = 48) | (N = 25) | |
| DFS | |||
| Number of events, n (%) | 7 (10·1) | 4 (8·3) | 6 (24·0) |
| Number of censored observations, n (%) | 62 (89·9) | 44 (91·7) | 19 (76·0) |
| 5-year DFS rate,% (95% CI)* | 90·5 (95·6, 80·0) | 90·2 (96·2, 75·9) | 76·0 (88·4, 54·2) |
| LRFI | |||
| Number of events | 3 (4·3) | 1 (2·1) | 2 (8·0) |
| Number of censored observations, n (%) | 66 (95·7) | 47 (97·9) | 23 (92·0) |
| 5-year LRFI rates,% (95% CI)* | 94·8 (98·3, 84·6) | 97·6 (99·7, 83·9) | 90·9 (97·6, 68·3) |
| DDFI | |||
| Number of events | 4 (5·8) | 0 (0·0) | 3 (12·0) |
| Number of censored observations, n (%) | 65 (94·2) | 48 (100·0) | 22 (88·0) |
| 5-year DDFI rates,% (95% CI)* | 95·5 (98·5, 86·7) | 100 (100, 100) | 86·9 (95·6, 64·5) |
| OS | |||
| Number of events | 0 (0·0) | 2 (4·2) | 1 (4·0) |
| Number of censored observations, n (%) | 69 (100·0) | 46 (95·8) | 24 (96·0) |
| 5-year OS rates,% (95% CI)* | 100 (100, 100) | 95·1 (98·7, 81·7) | 95·8 (99·4, 73·9) |
Derived from Kaplan-Meier estimates.
Fig. 3.
Kaplan-Meier curves for (A) DFS, (B) LRFI, (C) DDFI, and (D) OS events. Y axes of the figures have been expanded.
3.2.2. Local-relapse-free interval, distant disease-free interval, and overall survival
Overall, LRFI, DDFI, and OS events were scarce and the median value for these long-term efficacy outcomes not reached in all arms. The 5-year estimates for LRFI, DDFI, and OS are shown in Table 2 and Fig. 3B–D. One (2.1%) LRFI event was reported in Group A, two (8.0%) events in Group B, and three (10.1%) events in the PET responders. Seven DDFI events were reported in total: three (12.0%) events in Group B and four (5.8%) events in the PET responders. For OS, two (4.2%) events were reported in Group A, one (4.0%) event in Group B, and no events in the PET responders.
3.2.3. Post hoc sensitivity analysis of disease-free survival
There was no apparent difference in DFS between Group A and Group B in the sensitivity analysis of DFS where patients who discontinued the study early (for reasons given in Table 1) were considered to be treatment failures. The 5-year DFS rates were 82.4 (95% CI: 71.1–89·6) for PET responders, 74.8 (59.9–84.9) for Group A, and 76.0 (54.2–88.4) for Group B (Supplementary Table 1 and Supplementary Figure 1).
3.3. Adverse events during the adjuvant stage
A total of 132 patients received at least one dose of trastuzumab during the adjuvant phase. Overall, 913 emergent AEs were reported during the adjuvant phase in 131/132 (99.2%) patients (Table 3). No patient died due to an AE or discontinued trastuzumab due to an AE during the adjuvant phase. The most common AEs were radiation skin injury (91 events in 88 patients, 66.7%), arthralgia (36 events in 34 patients, 25.8%), hot flush (31 events in 31 patients, 23.5%), and lymphocele (29 events in 28 patients, 21.2%). Most events were mild or moderate in intensity (95•8%); 30 (3.6%) events were Grade 3 and 5 (0.6%) were Grade 4. In total, 3.9% of emergent AEs were judged to be treatment related and 2.5% of emergent AEs were assessed as serious. The only serious AE occurring in >2% of patients overall was postoperative wound infection. The proportion of patients that experienced a serious AE during the adjuvant period was slightly higher in Group A (18.6%) compared with Group B (12.5%) and PET responders (7.7%). Four serious AEs (two events of impaired healing, one post-operative wound infection, and one localised infection) occurring in three (7.0%) patients in Group A were considered related to bevacizumab.
Table 3.
Summary of treatment emergent adverse events (preferred terms) during the adjuvant stage.
| PET responders | Group A | Group B | |
|---|---|---|---|
| (N = 65) | (N = 43) | (N = 24) | |
| Any AE, n (%) | 64 (98·4) | 43 (100) | 24 (100) |
| AEs occurring in ≥20% of patients in at least one group | |||
| Radiation skin injury | 46 (70·8) | 23 (53·5) | 19 (79·2) |
| Arthralgia | 14 (21·5) | 12 (27·9) | 8 (33·3) |
| Lymphocele | 11 (16·9) | 10 (23·3) | 7 (29·2) |
| Hot flash | 16 (24·6) | 7 (16·3) | 8 (33·3) |
| Asthenia | 8 (12·3) | 6 (14·0) | 5 (20·8) |
| Myalgia | 9 (13·8) | 3 (7·0) | 5 (20·8) |
| Any Grade ≥3 AE | 11 (16·9) | 11 (25·6) | 3 (12·5) |
| Grade ≥3 AE occurring in ≥4% of patients in at least one group | |||
| Postoperative wound infection | 0 | 3 (7·0) | 0 |
| Lymphocele | 1 (1·5) | 2 (4·7) | 0 |
| Radiation skin injury | 4 (6·2) | 1 (2·3) | 1 (4·2) |
| Post procedural infection | 0 | 0 | 1 (4·2) |
| Cholelithiasis | 0 | 0 | 1 (4·2) |
| Any serious AE | 5 (7·7) | 8 (18·6) | 3 (12·5) |
| Postoperative wound infection | 0 | 3 (7·0) | 0 |
| Impaired healing | 0 | 2 (4·7) | 0 |
| Localised infection | 0 | 1 (2·3) | 0 |
| Lung infection | 0 | 1 (2·3) | 0 |
| Mycoplasma infection | 0 | 1 (2·3) | 0 |
| Arthralgia | 0 | 1 (2·3) | 0 |
| Fibromyalgia | 0 | 1 (2·3) | 0 |
| Lymphocele | 0 | 1 (2·3) | 0 |
| Device related infection | 1 (1·5) | 0 | 0 |
| Post procedural infection | 0 | 0 | 1 (4·2) |
| Breast prosthesis removal | 0 | 0 | 1 (4·2) |
| Arrhythmia | 1 (1·5) | 0 | 0 |
| Palpitations | 1 (1·5) | 0 | 0 |
| Bronchogenic cyst | 1 (1·5) | 0 | 0 |
| Pancreatitis | 0 | 0 | 1 (4·2) |
| Cholelithiasis | 0 | 0 | 1 (4·2) |
| Breast cancer | 1 (1·5) | 0 | 0 |
| Syncope | 1 (1·5) | 0 | 0 |
Data show the number of patients (percent out of patients in that group) who experienced each of the listed adverse event (AE).
Three (2.3%) patients, all PET responders, experienced four emergent cardiac events during the adjuvant period: arrhythmia in two patients, palpitations in one patient, and supraventricular extrasystoles in one patient. All events were Grade ≤2 and one event of arrhythmia and one event of palpitations were assessed as serious. No patient developed heart failure or had new LVEF decrease during the study.
4. Discussion
The AVATAXHER study showed that early PET assessment identified patients with HER2-positive breast cancer who will responded poorly to neoadjuvant docetaxel plus trastuzumab therapy, and that the addition of bevacizumab to neoadjuvant therapy improved pCR rates in PET-predicted poor responders [8]. The pre-planned 5-year follow-up analysis reported here shows that the improvement in pCR translated into numerically greater DFS rates; however, the study was not powered to detect a significant difference between treatment arms in this secondary study endpoint, and a difference in DFS between patients treated with or without neoadjuvant bevacizumab was not confirmed in the DFS sensitivity analysis.
Adding bevacizumab to neoadjuvant chemotherapy has been shown to improve pCR rates in patients with HER2-negative breast cancer in the ARTemis, [24] GeparQuinto, [18,25] and NSABP B-4017,21 studies, as well as in meta-analyses, [26] but in general, these improvements in pCR have not translated into improved long-term outcomes. The NSABP B-40 trial found that the addition of bevacizumab to neoadjuvant and adjuvant chemotherapy significantly improved pCR in patients with HER2-negative, hormone receptor positive tumours [17] and this improvement in pCR was associated with higher OS but not DFS [21]. Adding bevacizumab anthracycline- and/or taxane-based chemotherapy did not significantly improve invasive DFS or OS in the phase III BEATRICE trial in patients with early triple-negative breast cancer [27]. Interestingly, a phase II study in HER2-positive breast cancer (NSABP FB-5 trial) showed that the addition of bevacizumab to treatment resulted in similar pCR rates to chemotherapy plus trastuzumab alone with high 5-year recurrence-free survival and OS rates [23]. In the present study, the PET-predicted poor-responders who received neoadjuvant bevacizumab had a similar DFS rate at 5-years to PET-predicted responders (90.2% and 90.5%, respectively) which was higher than that of the PET-predicted poor-responders who did not receive neoadjuvant bevacizumab (76.0%). However, these DFS data were not compared statistically in this phase II trial and despite the large numerical difference in DFS rates observed between the PET-predicted poor-responders treated with or without neoadjuvant bevacizumab, there was a wide overlapping of confidence intervals. When patients who discontinued the study early (predominantly due to patients consent withdrawal) were considered as treatment failures, there was no difference in DFS between patients treated with or without neoadjuvant bevacizumab. This does not distract from the main novel finding of the AVATAXHER study which was that PET could be used to identify early those patients who responded poorly to a homogenous anthracycline-free treatment regimen. The DFS rates in PET responders observed in this study compare favourably with those reported in contemporary studies of patients with HER2-positive breast cancer treated with trastuzumab-containing therapy [2,[28], [29], [30]].
We used change of SUVmax as the PET metric in this study to facilitate the comparison of PET data obtained from different study centres in this multicentre study; this parameter has been shown to be robust and correlate well with pCR [31]. However, other PET variables such as SUVpeak have also been used as predictors of neoadjuvant chemotherapy response and prognosis in breast cancer. At 6-year follow-up, SUVmax on early PET was shown to independently correlate with OS in patients with breast cancer receiving neoadjuvant chemotherapy [32]. In patients with operable HER2-positive and triple negative breast cancer, the SUVmax determined by PET after neoadjuvant chemotherapy predicted pCR and significantly correlated with recurrence-free survival and recurrence [6]. Volume-based metabolic PET variables have also been shown to be good predictors of both neoadjuvant chemotherapy response and prognosis in locally advanced breast cancer [33]. Together, these data suggest that the use of PET in neoadjuvant therapy can predict long-term outcomes for breast cancer patients.
The tolerance profile of study treatments was judged clinically to be acceptable in the adjuvant stage of the AVATAXHER study. The addition of bevacizumab to neoadjuvant chemotherapy resulted in a greater overall number of Grade ≥3 AEs observed during the adjuvant period in Group A compared to Group B, with most common Grade ≥3 AE observed in Group A being postoperative wound infection. This increase in Grade 3/4 toxicities is as expected from previously reported studies in HER2-negative breast cancer in which increases in cases Grade 3/4 neutropenia were reported [26,34]. Previous studies have suggested that the addition of bevacizumab to treatment may be associated with increased cardiac events [35]. The AVATAXHER study was an open-label study, which could influence the reporting of AEs; however, the incidence of cardiac events during the adjuvant period was low: all events were Grade ≤2 and occurred in the PET responders. Similarly, long-term follow-up of the HERA trial reported a low incidence of cardiac events, the majority of which were reversible, in HER2-positive breast cancer patients treated with adjuvant trastuzumab [36].
This trial has several other limitations. Events for LRFI, DDFI, and OS were scarce across all three treatment arms. Furthermore, the treatment regimen used in the AVATAXHER study are of limited relevance to clinical practice today. The subsequent approval of pertuzumab and trastuzumab emtansine (T-DM1) changed the paradigm for neoadjuvant treatment of breast cancer, and while anthracycline-free chemotherapy regimens were being explored at the time of the AVATAXHER study design, the standard combination in neoadjuvant therapy comprises anthracyclines and taxanes. In addition, the acceptability of the tolerability of the treatment regimen was based on clinician judgement whereas the inclusion of patient-reported quality of life measures could have provided insight into the patient's opinion of the tolerability of the regimen. Finally, as this was a Phase II study using a highly selected patient population, the results reported here are not generalisable to other populations.
In conclusion, early PET can identify patients with HER2-positive breast cancer who will responded poorly to neoadjuvant therapy and that the addition of bevacizumab to neoadjuvant docetaxel plus trastuzumab therapy improves the pCR rate. This improvement in pCR rate does not translate into long-term improvements in DFS.
Declaration of Competing Interest
BC reports personal fees and non-financial support from Roche Laboratories France, personal fees from Bristol Myers Laboratories France, and personal fees from AstraZeneca Laboratories.
JYP declares research funding received by his institution from Roche and Menarini Silicon Biosystems; honoraria received by his institution from Roche and Sanofi; and personal fees from Roche, Novartis, AstraZeneca, Pfizer, Amgen, Ipsen, Lilly, and Genomic Health.
TP is a member of scientific boards for Novartis, Lilly and Pfizer.
TB reports personal fees and non-financial support from Roche, Novartis, AstraZeneca, and Pfizer; and personal fees from Seattle Genetics, outside the submitted work.
DC reports honorarium from Roche, Pfizer, Novartis, Sanofi, and AstraZeneca.
GP acted as consultant for Chugai, Novartis and Shire (Takeda), with remunerations received by his institution. GP's research team received grants for academic studies from Amgen, Genzyme (Sanofi), Lilly, Merck, Novartis, and Roche Pharma.
AF and JD are full-time employees of Roche Pharma France.
KK, J-MF, and PG report personal fees from Roche during the conduct of the study.
LA reports personal fees and non-financial support from Roche Laboratories France and Bristol Myers Laboratories France, and personal fees from AstraZeneca Laboratories and Merck.
M-AM-R, FLD, P-FD, M-PC, CB, J-BP, GT, and AB-R declare no conflicts of interest.
Acknowledgments
Role of the funding source
The funder of the study (Roche France) supplied trastuzumab and bevacizumab and had a role in the design and conduct of the study; collection, management, and analysis of data (including statistical analyses); interpretation of findings; and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit for publication. All authors were involved in the critical review of the manuscript during its development and approved the final version for submission.
Acknowledgements
This study was sponsored by Roche France. We thank the patients and their families for their participation in this study, the study management team and staff at individual study sites, Isabelle Josse (Senior Statistician, Aixial, Boulogne-Billancourt, France) for performing the statistical analyses, and Bernard Asselain (Department of Statistics, Institut Curie, Paris, France) for study design discussion. Editorial assistance for the preparation of this article was provided by Jamie Ashman and Megan Christian of Prism Ideas, funded by Roche France.
Author contributions
BC, J-YP, M-AM-R, J-MF, TP, FLD, P-FD, TB, DC, CB, and J-BP recruited and managed patients in the study and participated in data collection.
BC, J-YP, DC, GP, M-PC, GT, LA, PG, KK, and AB-R participated in protocol development. BC, J-YP, JD, AF (from Roche), and LA analysed and interpreted the data.
BC, AF (from Roche), JD, JA, and MC prepared the report.
All authors were given the opportunity to comment on the draft report and saw and approved the final version.
Data sharing statement
ROCHE, the sponsor of the study, and Dr Coudert, the principal investigator, agree to the principle of data sharing with third parties. Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) will be available as the study protocol, statistical analysis plan, clinical study report, and analytic code. Roche has already accepted to share the individual data with the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analysis group.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100566.
Appendix. Supplementary materials
References
- 1.Buzdar A.U., Ibrahim N.K., Francis D. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Gianni L., Pienkowski T., Im Y.H. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 3.Humbert O., Riedinger J.M., Vrigneaud J.M. 18F-FDG PET-derived tumor blood flow changes after 1 cycle of neoadjuvant chemotherapy predicts outcome in triple-negative breast cancer. J Nucl Med. 2016;57:1707–1712. doi: 10.2967/jnumed.116.172759. [DOI] [PubMed] [Google Scholar]
- 4.Garcia Garcia-Esquinas M.A., Arrazola G.J., Garcia-Saenz J.A. Predictive value of PET-CT for pathological response in stages II and III breast cancer patients following neoadjuvant chemotherapy with docetaxel. Rev Esp Med Nucl Imagen Mol. 2014;33:14–21. doi: 10.1016/j.remn.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Gebhart G., Gamez C., Holmes E. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54:1862–1868. doi: 10.2967/jnumed.112.119271. [DOI] [PubMed] [Google Scholar]
- 6.Akimoto E., Kadoya T., Kajitani K. Role of (18)F-PET/CT in predicting prognosis of patients with breast cancer after neoadjuvant chemotherapy. Clin Breast Cancer. 2018;18:45–52. doi: 10.1016/j.clbc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Groheux D., Martineau A., Teixeira L. (18)FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017;19:3. doi: 10.1186/s13058-016-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coudert B., Pierga J.Y., Mouret-Reynier M.A. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial. Lancet Oncol. 2014;15:1493–1502. doi: 10.1016/S1470-2045(14)70475-9. [DOI] [PubMed] [Google Scholar]
- 9.Scheuer W., Friess T., Hasmann M. Enhanced antitumour effect by combination of HER2-targeting antibodies with bevacizumab in a human breast cancer xenograft model. Eur J Cancer Suppl. 2006;4:66. [Google Scholar]
- 10.Zhao M., Pan X., Layman R. A Phase II study of bevacizumab in combination with Trastuzumab and docetaxel in HER2 positive metastatic breast cancer. Invest New Drugs. 2014;32:1285–1294. doi: 10.1007/s10637-014-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianni L., Romieu G.H., Lichinitser M. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 12.Martin M., Makhson A., Gligorov J. Phase II study of bevacizumab in combination with trastuzumab and capecitabine as first-line treatment for HER-2-positive locally recurrent or metastatic breast cancer. Oncologist. 2012;17:469–475. doi: 10.1634/theoncologist.2011-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Untch M., Fasching P.A., Konecny G.E. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 14.Cortazar P., Zhang L., Untch M. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 15.Ohzawa H., Sakatani T., Niki T., Yasuda Y., Hozumi Y. Pathological responses and survival of patients with human epidermal growth factor receptor 2-positive breast cancer who received neoadjuvant chemotherapy including trastuzumab. Breast Cancer. 2014;21:563–570. doi: 10.1007/s12282-012-0424-4. [DOI] [PubMed] [Google Scholar]
- 16.Korn E.L., Sachs M.C., McShane L.M. Statistical controversies in clinical research: assessing pathologic complete response as a trial-level surrogate end point for early-stage breast cancer. Ann Oncol. 2016;27:10–15. doi: 10.1093/annonc/mdv507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bear H.D., Tang G., Rastogi P. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Minckwitz G., Eidtmann H., Rezai M. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 19.Sikov W.M., Berry D.A., Perou C.M. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earl H.M., Hiller L., Dunn J.A. Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): an open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:656–666. doi: 10.1016/S1470-2045(15)70137-3. [DOI] [PubMed] [Google Scholar]
- 21.Bear H.D., Tang G., Rastogi P. Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16:1037–1048. doi: 10.1016/S1470-2045(15)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikov W.M., Berry D.A., Perou C.M. Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/- carboplatin and/or bevacizumab in triple-negative breast cancer: outcomes from CALGB 40603 (Alliance) Cancer Res. 2016;76 Abstract S2-05. [Google Scholar]
- 23.Smith J.W., Buyse M.E., Rastogi P. Epirubicin with cyclophosphamide followed by docetaxel with trastuzumab and bevacizumab as neoadjuvant therapy for HER2-positive locally advanced breast cancer or as adjuvant therapy for HER2-positive pathologic stage III breast cancer: a phase II trial of the NSABP Foundation Research Group, FB-5. Clin Breast Cancer. 2017;17:48–54. doi: 10.1016/j.clbc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Earl H.M., Hiller L., Dunn J. Disease-free and overall survival at 3.5 years for neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide, for women with HER2 negative early breast cancer: aRTemis Trial. Ann Oncol. 2017;28:1817–1824. doi: 10.1093/annonc/mdx173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Minckwitz G., Loibl S., Untch M. et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto)†. Ann Oncol. 2014;25:2363–2372. doi: 10.1093/annonc/mdu455. [DOI] [PubMed] [Google Scholar]
- 26.Cao L., Yao G.Y., Liu M.F., Chen L.J., Hu X.L., Ye C.S. Neoadjuvant bevacizumab plus chemotherapy versus chemotherapy alone to treat non-metastatic breast cancer: a meta-analysis of randomised controlled trials. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0145442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell R., Brown J., Parmar M. Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann Oncol. 2017;28:754–760. doi: 10.1093/annonc/mdw665. [DOI] [PubMed] [Google Scholar]
- 28.Giacchetti S., Hamy A.S., Delaloge S. Long-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 status. Eur J Cancer. 2017;75:323–332. doi: 10.1016/j.ejca.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Guiu S., Liegard M., Favier L. Long-term follow-up of HER2-overexpressing stage II or III breast cancer treated by anthracycline-free neoadjuvant chemotherapy. Ann Oncol. 2011;22:321–328. doi: 10.1093/annonc/mdq397. [DOI] [PubMed] [Google Scholar]
- 30.von Minckwitz G., Rezai M., Fasching P.A. et al. Survival after adding capecitabine and trastuzumab to neoadjuvant anthracycline-taxane-based chemotherapy for primary breast cancer (GBG 40–GeparQuattro) Ann Oncol. 2014;25:81–89. doi: 10.1093/annonc/mdt410. [DOI] [PubMed] [Google Scholar]
- 31.Groheux D., Majdoub M., Sanna A. Early Metabolic Response to Neoadjuvant Treatment: FDG PET/CT Criteria according to Breast Cancer Subtype. Radiology. 2015;277:358–371. doi: 10.1148/radiol.2015141638. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Ibrahim N.K., Yan Y., Wong S.T., Wang H., Wong F.C. Complete metabolic response on interim (18)F-fluorodeoxyglucose positron emission tomography/computed tomography to predict long-term survival in patients with breast cancer undergoing neoadjuvant chemotherapy. Oncologist. 2017;22:526–534. doi: 10.1634/theoncologist.2016-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Vicente A.M., Perez-Beteta J., mo-Salas M. Predictive and prognostic potential of volume-based metabolic variables obtained by a baseline (18)F-FDG PET/CT in breast cancer with neoadjuvant chemotherapy indication. Rev Esp Med Nucl Imagen Mol. 2018;37:73–79. doi: 10.1016/j.remn.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Han R., Wang G., Zhang Y., Zhao X. [Efficacy of neoadjuvant chemotherapy combined with bevacizumab versus neoadjuvant chemotherapy alone for Her2-negative breast cancer: a meta-analysis of randomized controlled clinical trials] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016;45:379–386. doi: 10.3785/j.issn.1008-9292.2016.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choueiri T.K., Mayer E.L., Je Y. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 36.de Azambuja E., Procter M.J., van Veldhuisen D.J. et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32:2159–2165. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.