 A new carbocyclic nucleoside with the salient features of entecavir and aristeromycin identified as the anti-HBV lead candidate.
A new carbocyclic nucleoside with the salient features of entecavir and aristeromycin identified as the anti-HBV lead candidate.
Abstract
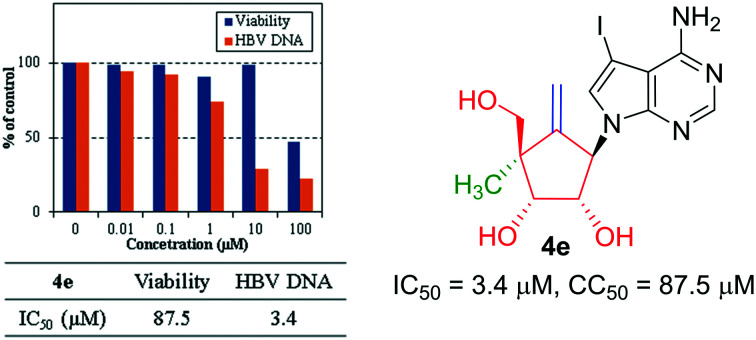
Modified carbocyclic nucleosides (4a–g) constituting 7-deazapurine, 4′-methyl, exocyclic double bond and 2′,3′-hydroxy were synthesized. NOE and X-ray studies of 4c confirmed the α-configuration of 4′-methyl. The anti-HBV assay demonstrated 4e (IC50 = 3.4 μM) without notable cytotoxicity (CC50 = 87.5 μM) as a promising lead for future exploration.
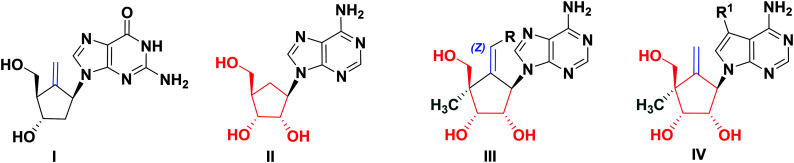
Hepatitis B is one of the common viral diseases. As per estimation, 350 million people are chronically infected with hepatitis B virus (HBV) worldwide and are at the risk of developing liver cancer.1,2 Chronic HBV patients require long-term treatment, which only suppresses the infection and is not efficient in eliminating the virus. Drug-resistant viruses emerging due to the long-term regimen mandates synthesis and efforts to be directed towards finding more potent and less toxic novel anti-HBV agents. Entecavir (I, Fig. 1) has become one of the most prescribed anti-HBV drugs for the treatment of chronically infected patients.3,4 It comprises a carbocyclic framework with a 6′-exo double bond, which seems to be an essential pharmacophore.5 Aristeromycin (II, Fig. 1) is a naturally occurring carbocyclic purine nucleoside and its modified derivatives are reported to exhibit a wide range of pharmacological activities against viral infection, cancer etc.6,7 Recently, 4′-substituted nucleosides have attracted consideration as balapiravir (for HCV) and festinavir (for HIV) reached the advanced phase of drug development.8 Moreover, there are a few reports on 4′-substituted carbocyclic nucleosides analogs (CNAs) in literature.9,10
Fig. 1. Chemical structures entecavir (I), aristeromycin (II), aristeromycin analogs (III), and modified carbocyclic nucleosides (IV) for investigation in the present study.
From the last decade, our group has been involved in the synthesis of biologically significant novel CNAs.11–14 Recently, we described the synthesis of aristeromycin analogs (III, Fig. 1) with novel features: the 6′-exocyclic double bond and 4′-α-methyl group.5 Although none of them demonstrated significant anti-HBV activity, none were strongly cytotoxic (CC50 > 100 μM). These results motivated us for further chemical exploration of this new class of CNAs towards improving their medicinal properties. Recently, base-modified nucleosides containing 7-deazapurine have attracted considerable attention.15,16 Therefore, in this study, we designed a novel class of modified carbocyclic nucleosides (IV, Fig. 1) by replacing the adenine moiety of III with 7-deazapurine. Herein, we report our efforts toward the synthesis, anti-HBV activity, and cytotoxicity profiles of newly designed molecules.
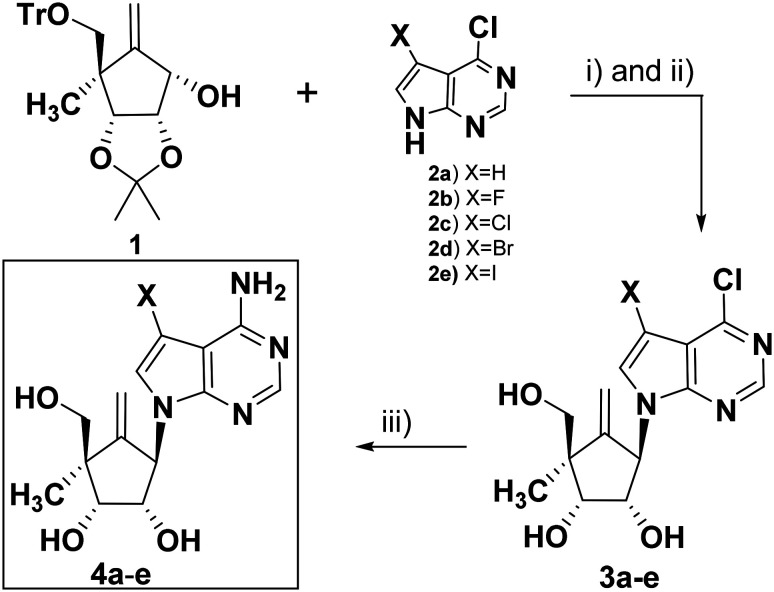
The carbocyclic sugar intermediate (1) as a single diastereomer was achieved in eight synthetic steps from d-ribose with an overall yield of 17–20%.5 The Cl/Br/I group was successfully substituted at the C-7 position of 6-chloro-7-deazapurine (2a) by treating with NCS/NBS/NIS in DMF.12
The fluoro derivative (2b) was obtained by heating 2a with selectfluor in a acetonitrile : acetic acid (80 vol, 5 : 1) mixture at 70 °C.17 The coupling of 1 with 6-chloro-7-deazapurine (2a) or its 7-halo derivatives (2b–e) under Mitsunobu reaction conditions afforded the corresponding protected coupled products. The deprotection was performed under acidic conditions without further purification to yield 3a–e (Scheme 1).
Scheme 1. Synthesis of 4a–e. Reagents and conditions: (i) PPh3, DIAD, THF, 10 °C–rt, 1 h; (ii) TFA : H2O (8 : 2 ratio), rt, 30 min; (iii) NH3 in MeOH, 100 °C, sealed tube, 24 h.
The treatment of 3a–e with methanolic ammonia at 100 °C under a sealed condition yielded the desired carbocyclic nucleosides (4a–e).18
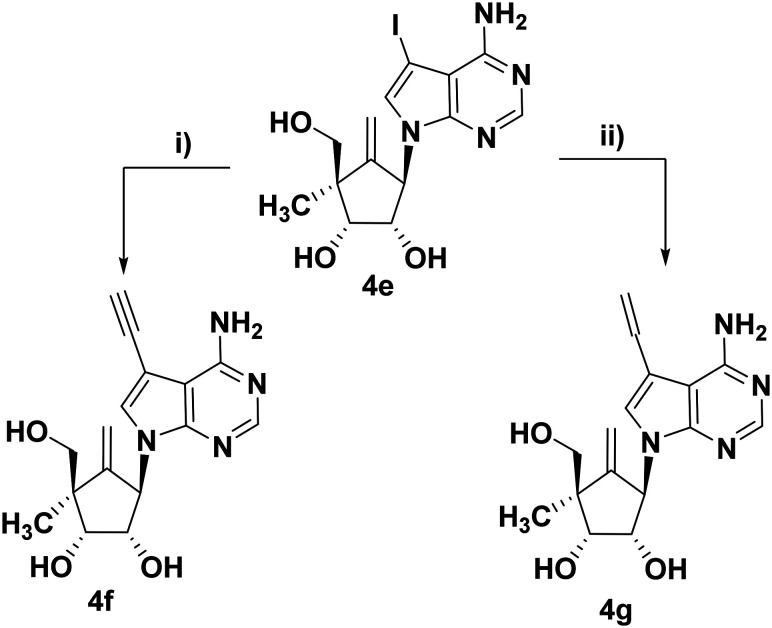
The 7-vinyl/ethynyl analogs of 4a were synthesized from the iodo derivative 4e by palladium-catalyzed cross-coupling reaction to give the desired compounds (4f–g) in good yield (Scheme 2).18 In brief, the 7-ethynyl derivative (4f) was synthesized in two steps from 4e by treating with trimethylsilyl acetylene (TMS-acetylene) and Pd(PPh3)4 in DMF at 50 °C under the sealed condition, followed by treatment with K2CO3 in methanol at ambient temperature. The vinyl derivative (4g) was synthesized from 4e by treating with tributyl vinyl tin and Pd(PPh3)4 in DMF at 110 °C. All compounds and key intermediates were characterized via spectral analyses.
Scheme 2. Synthesis of 7-ethynyl/vinyl derivatives (4f–g). Reagents and conditions: i) a) trimethylsilyl acetylene, CuI, Et3N, Pd(PPh3)4, DMF, 50 °C, 3 h; b) K2CO3, MeOH, rt, 30 min; ii) tri-n-butyl vinyl tin, Pd(PPh3)4, DMF, 110 °C, 3 h.
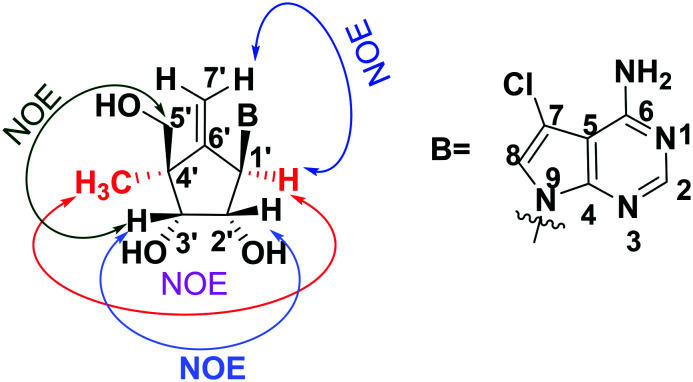
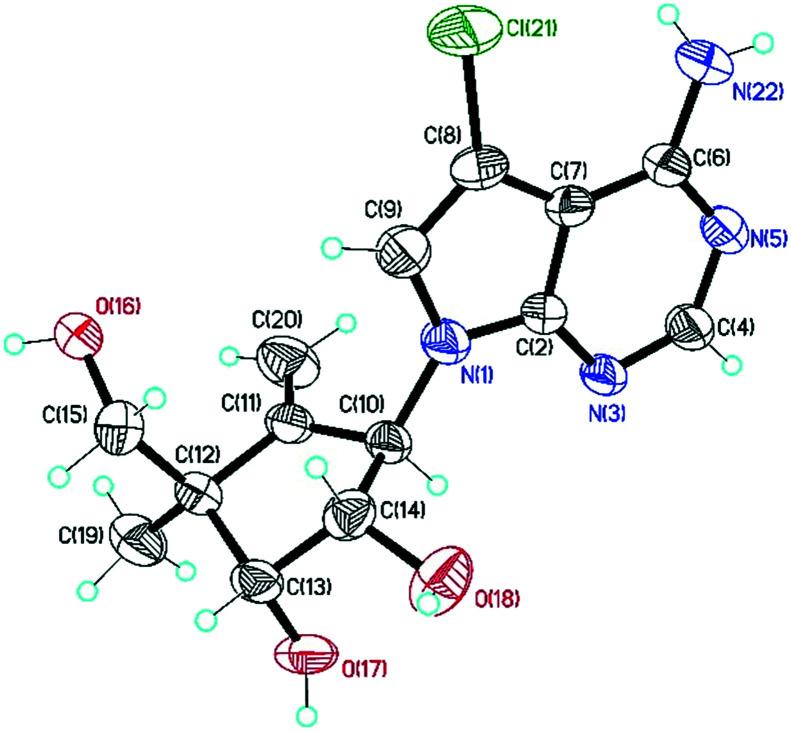
The NOE study of 4c (Fig. 2) indicated the α-configuration of the methyl group at 4′-position in the carbocyclic sugar ring,19 which was further confirmed via single-crystal X-ray diffraction studies (Fig. 3).
Fig. 2. Compound 4c showed NOE between 4′-methyl with 1′-H, as shown in red arrow to demonstrate α-configuration.
Fig. 3. Single-crystal X-ray structure of 4c, showing a thermal displacement ellipsoid (50% probability) plot [CCDC no. ; 1969219].
HepG2.2.15.7 cells (1 × 104 cells per well) were inoculated into a microtiter plate. After incubation for 24 h, the cells were cultured in the presence of various concentrations of test compounds (4a–g). Then, every three days, the culture medium was replaced by a fresh one containing an appropriate concentration of 4a–g.
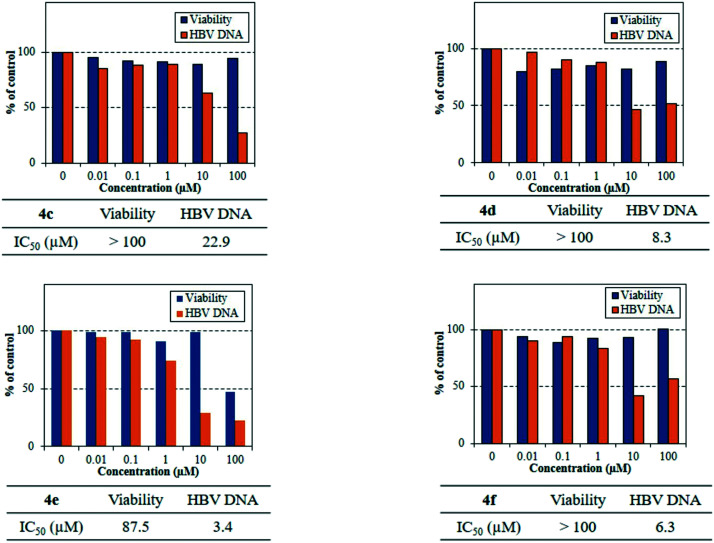
After nine days of incubation, the culture supernatants were collected and examined for HBV DNA levels using real-time PCR. The cells were tested for their viability by the tetrazolium dye method. IC50: 50% effective concentration based on the inhibition of the HBV DNA levels in culture supernatants and CC50: 50% cytotoxic concentration based on the reduction of viable cells was calculated. The results are summarized in Table 1 and Fig. 4. Interestingly, structural modification resulted in a noteworthy antiviral activity (4c–f) with IC50 ranging from 3.4 to 22.9 μM without considerable cytotoxicity. From the above-mentioned results, 4a–b and 4g were inactive towards HBV. It is interesting to note that the introduction of the bulkier halo groups at C-7 of the base (4e) remarkably increased the activity.
Table 1. Anti-HBV and cytotoxicity of 4a–g in HepG2.2.15.7 cells.
| Compound | IC50 (μM) | CC50 (μM) |
| 4a | >100 | >100 |
| 4b | >100 | >100 |
| 4c | 22.9 | >100 |
| 4d | 8.3 | >100 |
| 4e | 3.4 | 87.5 |
| 4f | 6.3 | >100 |
| 4g | >100 | >100 |
| Aristeromycin | >3 | >3 |
| Entecavir | 0.18 (nM) | >100 (nM) |
Fig. 4. The anti-HBV activity of 4c–f. The cell viability and activity are shown in the bar diagram. IC50: 50% effective concentration based on the inhibition of the HBV DNA levels in culture supernatants. CC50: 50% cytotoxic concentration based on the reduction of viable cells.
In summary, we synthesized a new series of modified carbocyclic nucleosides (4a–g) from commercially available starting materials in 10–12 synthetic steps. These compounds were evaluated for their antiviral activity against the hepatitis B virus and cytotoxicity properties in the HepG2.2.15.7 cells. Among the screened compounds, 4e exhibited a noteworthy antiviral activity (IC50 = 3.4 μM) without notable cytotoxicity. From these studies, it is evident that these novel carbocyclic nucleosides might be valuable in designing new drugs for the HBV treatment.
Experimental and spectral data of final compounds
General method for synthesis of 4a–e
A screw-cap vial equipped with a magnetic stirrer bar was charged with NH3 in methanol (7 M, 7 ml) and appropriate 3a–e (0.80 mmol), and then sealed with a screw cap. The vial was heated up to 100 °C and stirred for 24 h. The reaction mixture was concentrated under a reduced pressure, and crude was purified by flash chromatography on a silica gel (230–400 mesh, elution gradient 0–9% MeOH in CH2Cl2).
(1S,2R,3R,5R)-5-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4a): purified yield: 78%, off-white solid, (TLC: Rf 0.2, 10% MeOH in CH2Cl2); [α]20D: +2.73 (c = 0.25, DMSO); mp: 210–220 °C; UV (MeOH) λmax: 274.25 nm; 1H NMR (400 MHz, CD3OD) δ: 1.19 (s, 3H), 3.51 (d, J = 10.8 Hz, 1H), 3.66 (d, J = 10.8 Hz, 1H), 4.01 (d, J = 5.2 Hz, 1H), 4.55 (d, J = 3.2 Hz, 1H), 4.73 (dd, J = 4.4 and 9.6 Hz, 1H), 5.07 (d, J = 3.2 Hz, 1H), 5.50–5.53 (m, 1H), 6.70 (d, J = 3.2 Hz, 1H), 7.27 (d, J = 3.2 Hz, 1H), 8.08 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.2, 62.7, 69.2, 73.5, 74.3, 99.8, 102.0, 107.8, 123.8, 148.3, 149.5, 155.0, 155.3; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C14H19N4O3 [M + H]+: 291.1457, found: 291.1422.
(1S,2R,3R,5R)-5-(4-Amino-5-fluoro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4b): purified yield: 55%, off-white solid, (TLC: Rf 0.2, 10% MeOH in CH2Cl2); [α]20D: –6.43 (c = 0.25, DMSO); mp: 243–247 °C; UV (MeOH) λmax: 280.25 nm; 1H NMR (300 MHz, CD3OD) δ: 1.18 (s, 3H), 3.50 (d, J = 10.8 Hz, 1H), 3.63 (d, J = 10.8 Hz, 1H), 3.98 (d, J = 4.8 Hz, 1H), 4.59 (d, J = 3.2 Hz, 1H), 4.63 (dd, J = 4.5 and 9.6 Hz, 1H), 5.07 (d, J = 3.3 Hz, 1H), 5.47–5.51 (m, 1H), 7.00 (d, J = 2.1 Hz, 1H), 8.02 (s, 1H); 19F NMR (376 MHz, DMSO-d6) δ: –168.25; 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.1, 62.0, 69.1, 73.4, 74.2, 91.9, 105.2, 107.8, 140.3, 146.4, 152.3, 154.2, 155.7; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C14H18FN4O3 [M + H]+: 309.1285, found: 309.1325.
(1S,2R,3R,5R)-5-(4-Amino-5-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4c): purified yield: 80%, off-white solid, (TLC: Rf 0.2, 10% MeOH in CH2Cl2); [α]20D: –30.41 (c = 0.25, DMSO); mp: 226–229 °C; UV (MeOH) λmax: 281.25 nm; 1H NMR (300 MHz, CD3OD) δ: 1.18 (s, 3H), 3.51 (d, J = 11.1 Hz, 1H), 3.65 (d, J = 10.8 Hz, 1H), 3.99 (d, J = 4.8 Hz, 1H), 4.59 (d, J = 2.7 Hz, 1H), 4.68 (dd, J = 4.8 and 9.9 Hz, 1H), 5.08 (d, J = 3.0 Hz, 1H), 5.46–5.51 (m, 1H), 7.24 (s, 1H), 8.05 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.2, 62.4, 69.1, 73.5, 74.2, 99.4, 101.4, 107.9, 120.0, 149.6, 152.2, 154.7, 156.7; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C14H18ClN4O3 [M + H]+: 325.0989, found: 325.1031.
(1S,2R,3R,5R)-5-(4-Amino-5-bromo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4d): purified yield: 75%, off-white solid, (TLC: Rf 0.2, 10% MeOH in CH2Cl2); [α]20D: +6.14 (c = 0.25, DMSO); mp: 228–232 °C; UV (MeOH) λmax: 283.25 nm; 1H NMR (300 MHz, CD3OD) δ: 1.18 (s, 3H), 3.51 (d, J = 11.1 Hz, 1H), 3.65 (d, J = 11.1 Hz, 1H), 3.98 (d, J = 4.5 Hz, 1H), 4.58 (d, J = 2.7 Hz, 1H), 4.69 (dd, J = 4.8 and 9.9 Hz, 1H), 5.08 (d, J = 2.7 Hz, 1H), 5.48–5.51 (m, 1H), 7.30 (s, 1H), 8.04 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.2, 62.5, 69.1, 73.5, 74.2, 85.4, 100.6, 107.9, 122.5, 150.0, 152.0, 154.7, 156.8; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C14H18BrN4O3 [M + H]+: 369.0484, found: 369.0522.
(1S,2R,3R,5R)-5-(4-Amino-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4e): purified yield: 80%, off-white solid, (TLC: Rf 0.2, 10% MeOH in CH2Cl2); [α]20D: +1.92 (c = 0.25, DMSO); mp: 227–228 °C; UV (MeOH) λmax: 290.25 nm; 1H NMR (300 MHz, CD3OD) δ: 1.17 (s, 3H); 3.51 (d, J = 11.1 Hz, 1H), 3.65 (d, J = 10.8 Hz, 1H), 3.99 (d, J = 4.8 Hz, 1H), 4.57 (d, J = 2.4 Hz, 1H), 4.70 (dd, J = 4.8 and 9.9 Hz, 1H), 5.07 (d, J = 3.0 Hz, 1H), 5.40–5.50 (m, 1H), 7.37 (s, 1H), 8.04 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.2, 50.4, 62.6, 69.2, 73.6, 74.3, 102.8, 107.9, 127.9, 150.7, 151.6, 154.8, 157.1; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C14H18IN4O3 [M + H]+: 417.0345, found: 417.0377.
Synthetic procedure for 4f
A suspension of 4e (0.60 mmol), trimethylsilyl acetylene (3.0 mmol), CuI (0.06 mmol), Et3N (3.0 mmol) and (PPh3)4Pd (0.06 mmol) in DMF was stirred at 50 °C under sealed condition for 3 h. The reaction mixture was concentrated under reduced pressure and crude was purified by silica gel (100–200 mesh) column chromatography, elution gradient 0–6% MeOH in CH2Cl2 to afford trimethylsilyl protected compound. The deprotection was carried out by stirring in methanol and K2CO3 (3.0 mmol) at rt for 30 min. The reaction mixture was concentrated under reduced pressure and purified by flash chromatography on silica gel (230–400 mesh), eluting gradient 0–7% MeOH in CH2Cl2.
(1S,2R,3R,5R)-5-(4-Amino-5-vinyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4f): purified yield: 62%, off white solid. (TLC: Rf 0.1, 10% MeOH in CH2Cl2); [α]20D: –2.17 (c = 0.25, DMSO); mp: 183–187 °C; UV (MeOH) λmax: 283.25 nm; 1H NMR (400 MHz, CD3OD) δ: 1.18 (s, 3H), 3.51 (d, J = 10.8 Hz, 1H), 3.66 (d, J = 10.8 Hz, 1H), 3.70 (s, 1H), 3.99 (d, J = 4.4 Hz, 1H), 4.58 (d, J = 2.8 Hz, 1H), 4.72 (dd, J = 4.4 and 10.0 Hz, 1H), 5.08 (d, J = 3.2 Hz, 1H), 5.48–5.44 (m, 1H), 7.48 (s, 1H), 8.06 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.3, 62.8, 69.2, 73.5, 74.2, 77.7, 82.7, 93.0, 102.1, 108.0, 128.5, 150.0, 152.4, 154.8, 157.4; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C16H19N4O3 [M + H]+: 315.1457, found: 315.1418.
Synthetic procedure for 4g
To a suspension of 4e (0.6 mmol), (PPh3)4Pd (0.06 mmol) in anhydrous DMF under argon atmosphere, tri-n-butyl(vinyl)tin (1.8 mmol) was added. The resulting mixture was heated at 110 °C for 3 h under sealed condition. Upon completion of reaction, concentrated the volatile under reduced pressure and crude was purified by flash chromatography on silica gel (230–400 mesh), elution gradient 0–7% MeOH in CH2Cl2.
(1S,2R,3R,5R)-5-(4-Amino-5-ethynyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-(hydroxymethyl)-3-methyl-4-methylenecyclopentane-1,2-diol (4g): purified yield: 60%, off white solid, (TLC: Rf 0.1, 10% MeOH in CH2Cl2); [α]20D: –14.28 (c = 0.25, DMSO); mp: 194–198 °C; UV (MeOH) λmax: 294.25 nm; 1H NMR (300 MHz, CD3OD) δ: 1.19 (s, 3H), 3.52 (d, J = 11.1 Hz, 1H), 3.67 (d, J = 11.1 Hz, 1H), 4.00 (d, J = 4.8 Hz, 1H), 4.57 (d, J = 2.7 Hz, 1H), 4.78 (dd, J = 4.8 and 9.9 Hz, 1H), 5.07 (d, J = 3.3 Hz, 1H), 5.24 (dd, J = 1.5 and 10.8 Hz, 1H), 5.44–5.48 (m, 1H), 5.58 (dd, J = 1.8 and 17.4 Hz, 1H), 7.05 (dd, J = 10.8 and 11.1 Hz, 1H), 7.35 (s, 1H), 8.02 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 18.9, 49.2, 62.3, 69.2, 73.5, 74.1, 100.2, 107.7, 112.1, 113.3, 120.0, 129.2, 151.1, 155.0, 157.5; HRMS (ESI-Orbitrap) m/z: exact mass calculated for C16H21N4O3 [M + H]+: 317.1614, found: 317.1575.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors are thankful to CIF, BIT Mesra, Ranchi, and GVK's analytical team for spectroscopic characterization data. The authors thank Dr. Ashoke Sharon, Department of Chemistry, BIT Mesra, for X-ray structure solution.
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1969219. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0md00059k
References
- Marcellin P., Kutala B. K. Liver Int. 2018;38:2–6. doi: 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- Maucort-Boulch D., de Martel C., Franceschi S., Plummer M. Int. J. Cancer. 2018;142:2471–2477. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- Lam Y.-F., Seto W.-K., Wong D., Cheung K.-S., Fung J., Mak L.-Y., Yuen J., Chong C.-K., Lai C.-L., Yuen M.-F. Clin. Transl. Gastroenterol. 2017;8:e125. doi: 10.1038/ctg.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L. J., Keating G. M. Drugs. 2009;69:1003–1033. doi: 10.2165/00003495-200969080-00005. [DOI] [PubMed] [Google Scholar]
- Samunuri R., Jha A. K., Bal C. Nucleosides, Nucleotides Nucleic Acids. 2019;38:391–399. doi: 10.1080/15257770.2018.1554221. [DOI] [PubMed] [Google Scholar]
- Rawal R. K., Bariwal J., Singh V. Curr. Top. Med. Chem. 2016;16:3258–3273. doi: 10.2174/1568026616666160506145300. [DOI] [PubMed] [Google Scholar]
- Xu G., Kong L., Gong R., Xu L., Gao Y., Jiang M., Cai Y.-S., Hong K., Hu Y., Liu P., Deng Z., Price N. P. J., Chen W. Appl. Environ. Microbiol. 2018;84:e01860-18. doi: 10.1128/AEM.01860-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betson M., Allanson N., Wainwright P. Org. Biomol. Chem. 2014;12:9291–9306. doi: 10.1039/c4ob01449a. [DOI] [PubMed] [Google Scholar]
- Kato K., Suzuki H., Tanaka H., Miyasaka T. Tetrahedron: Asymmetry. 1998;9:911–914. [Google Scholar]
- Gumina G., Chong Y., Choi Y., Chu C. K. Org. Lett. 2000;2:1229–1231. doi: 10.1021/ol005665k. [DOI] [PubMed] [Google Scholar]
- Kasula M., Balaraju T., Toyama M., Thiyagarajan A., Bal C., Baba M., Sharon A. ChemMedChem. 2013;8:1673–1680. doi: 10.1002/cmdc.201300277. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan A., Salim M. T., Balaraju T., Bal C., Baba M., Sharon A. Bioorg. Med. Chem. Lett. 2012;22:7742–7747. doi: 10.1016/j.bmcl.2012.09.072. [DOI] [PubMed] [Google Scholar]
- Kasula M., Samunuri R., Chakravarty H., Bal C., Baba M., Jha A. K., Sharon A. Nucleosides, Nucleotides Nucleic Acids. 2016;35:43–52. doi: 10.1080/15257770.2015.1114126. [DOI] [PubMed] [Google Scholar]
- Kasula M., Toyama M., Samunuri R., Rozy F., Yadav M., Bal C., Jha A. K., Baba M., Sharon A. Bioorg. Med. Chem. Lett. 2016;26:3945–3949. doi: 10.1016/j.bmcl.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Nauš P., Caletková O., Perlíková P., Slavětínská L. P., Tloušťová E., Hodek J., Weber J., DŽubák P., Hajdúch M., Hocek M. Bioorg. Med. Chem. 2015;23:7422–7438. doi: 10.1016/j.bmc.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Perlíková P., Hocek M. Med. Res. Rev. 2017;37:1429–1460. doi: 10.1002/med.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Wang P. A., Song C., Pan Z., Wang Q., Guo X., Yu X., Shen Z., Wang S., Chang J. Bioorg. Med. Chem. Lett. 2010;20:7297–7298. doi: 10.1016/j.bmcl.2010.10.076. [DOI] [PubMed] [Google Scholar]
- Kim H.-J., Sharon A., Bal C., Wang J., Allu M., Huang Z., Murray M. G., Bassit L., Schinazi R. F., Korba B. J. Med. Chem. 2008;52:206–213. doi: 10.1021/jm801418v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.