Abstract
Portal vein thromboses (PVTs) are associated with hepatic decompensation, worse survival, and worse liver transplant outcomes. We evaluated the impact of anticoagulation (AC) and transjugular intrahepatic portosystemic shunting (TIPS) on recanalization and mortality in patients with cirrhosis and PVT. Systematic search of electronic databases was performed. Clinical trials and observational studies that evaluated primary outcomes of recanalization and survival in patients with cirrhosis having PVT treated with AC or TIPS were included. Risk of bias was assessed. Summary odds ratios (ORs) for pooled data from the included studies were generated using a random effects model. A total of 505 studies were screened for inclusion. After review, 7 studies were ultimately included. Data from 327 patients in total were evaluated. Overall, treatment with either AC or TIPS resulted in partial or complete recanalization (OR: 4.56 [95% confidence interval, CI: 2.46-8.47]) but did not significantly impact mortality (OR: 0.57 [95% CI: 0.21-1.57]). The summary OR of AC for recanalization was 6.00 (95% CI: 2.38-15.07). The summary OR of TIPS for recanalization was 3.80 (95% CI: 1.47-9.83). The summary OR of mortality in patients treated with AC for PVT was 0.28 (95% CI: 0.08-0.95). The mortality summary OR was 1.10 (95% CI 0.23-5.16) in patients who underwent TIPS. There was insufficient data to assess complications such as hepatic encephalopathy or bleeding. Both AC and TIPS have a significant effect on recanalization. Anticoagulation appears to have a protective effect on mortality that is not seen with TIPS. More studies with control groups are need.
Keywords: portal vein thrombosis, direct oral anticoagulants, thrombosis, warfarin
Introduction
Patients with cirrhosis are at increased risk of portal vein thrombosis (PVT). This is thought to be related to slow flow in the portal vein from portal hypertension and a prothrombotic state from liver synthetic dysfunction.1,2 Supporting this hypothesis, the prevalence of PVT increases with severity of cirrhosis.3 Prevalence estimates range from 0.6% to 16% in all patients with cirrhosis depending on the stage of disease and associated conditions such as primary liver malignancy.4 Prevalence rates among patients awaiting liver transplant are higher with one study reporting evidence of PVT or prior PVT in more than 30% of patients at the time of transplant.5 In multiple observational studies, PVTs have been associated with worse outcomes in patients with cirrhosis. In patients listed for liver transplantation, PVT worsens overall mortality (odds ratio [OR]: 1.62, 95% confidence interval [CI]: 1.11-2.36) and post-liver transplant survival (hazard ratio: 1.32; P = .02).6–9 Portal vein thrombosis is associated with post-liver transplant hepatic artery thrombosis that itself independently associated with increased posttransplant mortality.10,11
Debate continues over whether PVTs are merely the consequence of advancing portal hypertension or an independent cause of increased mortality in patients with cirrhosis.12 One recent randomized controlled study supports a causal relationship. Villa et al. randomized patients with cirrhosis without PVT to prophylactic anticoagulation (AC) therapy for 12 months and found that participants in the intervention arm had lower rates of PVT, reduced rates of hepatic decompensation, and improved survival.13
As clinical data accumulate to suggest benefit of treatment of PVT and evidence supporting the safety of the use of AC in patients with cirrhosis grows,14,15 several recent societal guidelines advise treatment of PVT with AC and/or transjugular intrahepatic portosystemic shunting (TIPS) in certain scenarios. The European Association for the Study of Liver Diseases recommends consideration of therapeutic AC for at least 6 months as well as consideration of TIPS in liver transplant candidates not responding to AC.16 The 2015 Baveno VI Portal Hypertension Consensus Report suggests AC in liver transplant candidates with PVT and does not discuss the use of TIPS.17 A recent symposium summary focusing on coagulation in liver disease recommended a similar approach to patients with PVT, advising AC in transplant candidates with occlusive PVT and consideration of AC in patients with high-grade PVT even if not eligible for transplant. This symposium characterized TIPS as a “potentially effective” treatment for PVT.18
Several cross-sectional studies and clinical trials have evaluated the efficacy and safety of treatment of PVT in patients having cirrhosis with either AC or TIPS. Both interventions appear promising with relatively good safety profiles and high rates of portal vein recanalization.19 In one meta-analysis, AC improved rates of complete recanalization (OR: 4.16, 95% CI: 1.88-9.30) and had a pooled rate of bleeding of 3.3% (95% CI: 1.1%-6.7%).20 In meta-analyses evaluating TIPS for PVT, recanalization rates of 73% to 79% have been reported, with a pooled rate of hepatic encephalopathy of 25.3% (95% CI: 19.2%-32.6%).21,22 All of these studies showed improved rates of recanalization with intervention (AC or TIPS), but none have compared rates between the 2 interventions, and the TIPS meta-analyses in particular are limited by a lack of control groups. In this meta-analysis, we evaluate the recanalization rate and mortality associated with both AC and TIPS in adult patients with cirrhosis having chronic PVT.
Methods
Data Sources and Searches
The study team investigators systematically searched English-language literature for clinical trials and observational studies evaluating the outcomes of recanalization and survival in patients having cirrhosis with PVT who were treated with therapeutic AC or TIPS. Databases were searched through April 2018 and included PubMed, Medline, Cochrane Central Register of Trials, and Web of Science. Search terms included portal venous thrombosis, portal venous thrombus, PVT, portal vein thrombus, thrombosed portal vein, PVT, cirrhosis, AC, anticoagulant(s), antithrombotic, transjugular intrahepatic shunt(s), transjugular intrahepatic portosystemic shunt(s), and TIPS. References from full-text articles reviewed were examined for any pertinent articles not captured by initial search terms. Investigators additionally reviewed abstract list from recent conferences with the same search terms to ensure inclusion of pertinent studies not yet available in published databases.
Study Selection
Studies that evaluated adult patients with cirrhosis and PVT who underwent treatment with AC or TIPS were included in the analysis. Exclusion criteria included a restriction to malignant or acute PVT, lack of an untreated control group with PVT, or application of both interventions (AC and TIPS) within the same group of patients. Studies investigating PVT in noncirrhotic patients were excluded, as risk factors, epidemiology, and treatment approach of PVT are distinct in patients with and without cirrhosis.23 Search results were reviewed for duplicate entries, and studies from the same institution were reviewed for time of subject enrollment to ensure no patient population was duplicated.
Outcome Measures
Primary outcome assessed was recanalization of portal vein after treatment. Although several studies reported both partial and complete recanalization rates, complete recanalization was used to increase standardization across studies. Secondary outcome was all-cause mortality. There were insufficient data available to assess the rate of bleeding complications or hepatic encephalopathy.
Data Extraction and Quality Assessment
Rayyan QCRI24 was used for the management of electronic database search results. Two authors (C.E.R. and A.G.O.) independently reviewed abstracts and titles of all studies included after the initial search. Discrepancies were resolved by consensus adjudication. Three authors (J.P.E.D., C.E.R., and A.G.O.) independently reviewed the full text of articles included after initial evaluation. Discrepancies were resolved by discussion. Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement formatting was used to display the flowchart of study selection (Figure 1).25,26 Data from included studies were extracted independently by 3 authors (J.P.E.D., C.E.R., and A.G.O.). Extracted data included study author, study publication year, study design, study enrollment period, total number of enrolled patients per study, follow-up period patient characteristics (age, gender, and Model for End Stage Liver Disease score), and outcomes data (recanalization rate and survival). Risk of bias was assessed using the Cochrane Collaboration’s tool for risk of assessing bias for randomized controlled trials and the Newcastle-Ottawa Scale for observational studies.27,28
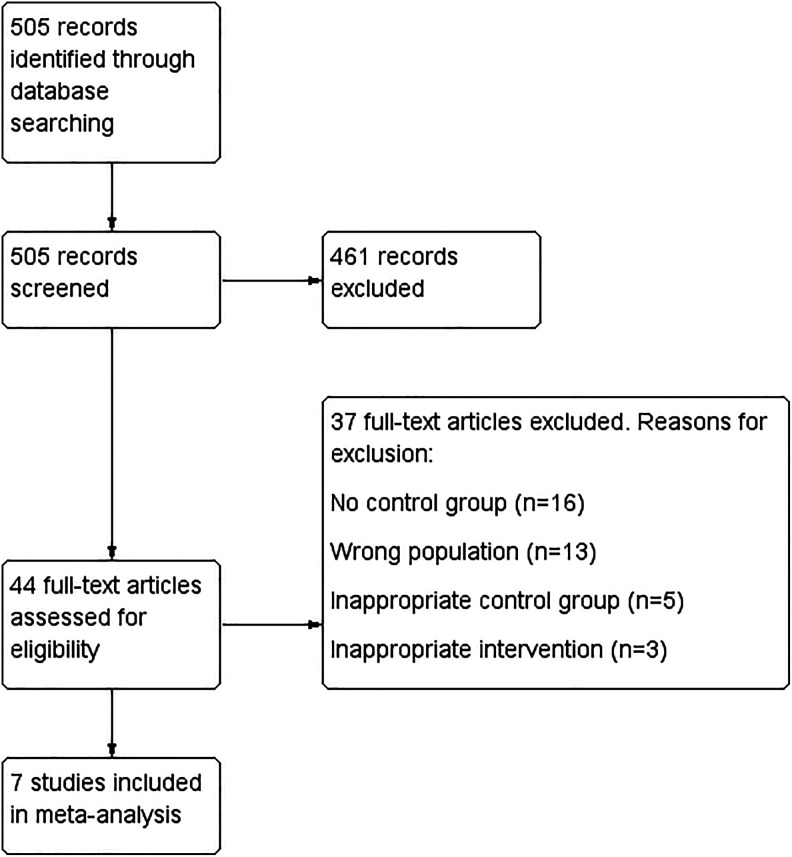
Figure 1.
Flowchart of electronic database search results.
Data Synthesis and Analysis
Review Manager software (Rev-Man version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration; 2014, Copenhagen, Denmark) was used for data synthesis and descriptive and outcomes measures analysis. Pooled ORs were estimated with DerSimonian and Laird random effects models to account for variability in treatment effect among studies.29 Forest plots were used to present study-level and summary-level results. Variability between studies was assessed using both Cochran Q statistic, with P < .10 indicating significant heterogeneity,29 and the Higgin’s I 2 index (considered significant if I 2 > 50%).29 Funnel plots were generated to evaluate for the presence of publication bias. The statistical methods of this study were reviewed by Min-Woong Sohn from the University of Virginia, School of Public Health Sciences.
Results
Selected Studies
In total, 505 studies were identified through the initial database search (Figure 1). After initial screening and application of inclusion criteria, 44 studies were selected for full-text review. After full-text qualitative review and application of exclusion criteria, 7 studies were ultimately included in the final meta-analysis. Studies excluded on full-text review were excluded due to lack of control group, inappropriate control group (eg, patients with cirrhosis without PVT), duplicate intervention (ie, both TIPS and AC), or wrong population (eg, patients without cirrhosis).
Among the included studies, 4 assessed the impact of AC and 3 evaluated the efficacy of TIPS.30–36 All studies assessing AC were observational, while 2 of the TIPS studies were open-label randomized trials.34,35 The Newcastle-Ottawa and Cochrane Risk of Bias Tool results are shown in Supplemental Tables 1 and 2. Neither randomized trial was blinded, and some of the observational cohorts had control groups that were potentially different from intervention groups, including controls drawn from different institutions in different countries36 or obtained from a different time period.33
Study population characteristics are summarized in Tables 1 and 2. In total, the use of AC for PVT was evaluated in 179 patients, while the use of TIPS was evaluated in 148 patients. Follow-up for most studies was between 22 and 30 months. One study, however, had only 3.8 months mean follow-up,31 and 2 studies did not specify mean or median follow-up.32,33
Table 1.
Study Characteristics of Studies Evaluating the Use of Anticoagulation for Treatment of Portal Vein Thrombosis.
| Author and Publication Year | Total Number of Patients Enrolled | Study Design | Study Population | AC | Recanalization Controls, n (%) | Recanalization Intervention, n (%) | Deaths Controls, n (%) | Deaths Intervention, n (%) |
|---|---|---|---|---|---|---|---|---|
| Chen et al (2016)30 | 66 | OC | Adult patient with cirrhosis with PVT | VKA | 4 (25) | 15 (68) | 6 (20) | 0 (0) |
| Chung et al (2014)31 | 28 | OC | Adult patients with cirrhosis with PVT | VKA | 3 (21) | 6 (43) | 4 (29) | 2 (14) |
| Francoz et al (2005)33 | 29 | OC | Adult patients with cirrhosis having PVT on liver transplant waitlist | VKA | 0 (0) | 8 (42) | NR | NR |
| Senzolo et al (2012)36 | 56 | OC | Adult patients with cirrhosis with PVT | LMWH | 1 (5) | 12 (36) | 3 (14) | 2 (6) |
Abbreviations: AC, anticoagulation; LMWH, low-molecular-weight heparin; NR, not reported; OC, observational cohort; PVT, portal vein thrombosis; VKA, vitamin K antagonists.
Table 2.
Study Characteristics of Studies Evaluating the Use of TIPS for Treatment of Portal Vein Thrombosis.
| Author and Publication Year | Total Number of Patients Enrolled | Study Design | Study Population | Intervention | Control | Recanalization Controls, n (%) | Recanalization Intervention, n (%) | Deaths Controls, n (%) | Deaths Intervention, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lv et al (2017)35 | 52 | RCT | Adult patient with cirrhosis with PVT and bleeding esophageal varices | TIPS | EVL + BB | 12 (48) | 19 (79) | 4 (16) | 8 (33) |
| Luo et al (2015)34 | 73 | RCT | Adult patient with cirrhosis with PVT and bleeding esophageal varices | TIPS | EVL + BB | 7 (19) | 24 (65) | 17 (47) | 12 (32) |
| D’Avola et al (2012)32 | 23 | OC | Adult patient with cirrhosis with PVT on liver transplant waitlist | TIPS | No TIPS | 4 (50) | 15 (100) | NR | NR |
Abbreviations: NR, not reported; OC, observational cohort; PVT, portal vein thrombosis; RCT, randomized controlled trial; TIPS, transjugular intrahepatic portosystemic shunting.
Among studies evaluating AC, 3 used vitamin K antagonists (VKAs),30,31,33 and 1 used low-molecular-weight heparin (LMWH).36 Duration of AC ranged from 4 to 12 months.30,31,33,36
Among studies evaluating TIPS, indication for TIPS in 2 studies was history of variceal bleeding.34,35 In these studies, the control group underwent endoscopic variceal ligation and was treated with nonselective β-blockade. Mortality in the control groups of these 2 trials was higher (16%-47%) than that of the control groups in other trials (14%-29%).
Anticoagulation Versus TIPS for PVT: Recanalization of the Portal Vein
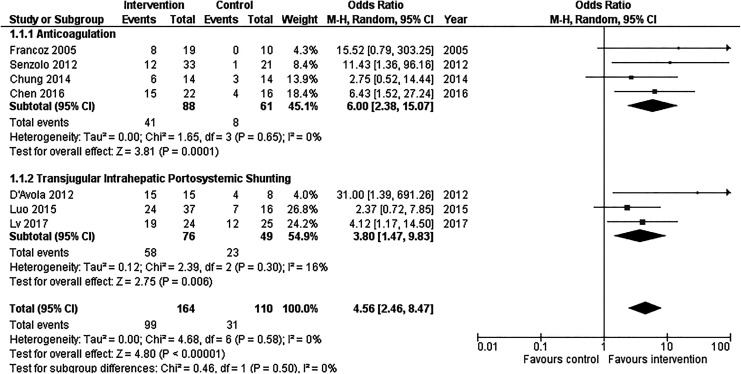
All 7 included studies showed a statistically significant favorable treatment effect with a summary OR of 4.56 (95% CI: 2.46-8.47; Figure 2).30–36 Heterogeneity was not significant among these studies (P = .50, I 2 = 0%). The funnel plot for these studies was not suggestive of publication bias, as more precise studies demonstrated smaller effect sizes (Supplemental Figure 1A).
Figure 2.
Forest plot of included studies demonstrating effect of anticoagulation or transjugular intrahepatic portosystemic shunting (TIPS) on recanalization of portal vein. CI indicates confidence interval; M-H, Mantel-Haenszel.
Among studies that evaluated the efficacy of AC for recanalization, OR ranged from 2.75 to 15.52 with a summary OR of 6.00 (95% CI: 2.38-15.07; Figure 2).30,31,33,36 Heterogeneity among these studies was not significant (P = .65, I 2 = 0%).
Among the 3 studies that evaluated the efficacy of TIPS for recanalization of the portal vein, OR ranged from 2.37 to 31.00 with a summary OR of 3.80 (95% CI: 1.47-9.83; Figure 2).32,34,35 Heterogeneity among these studies was not significant (P = .30, I 2 = 16%).
Anticoagulation Versus TIPS for PVT: Mortality
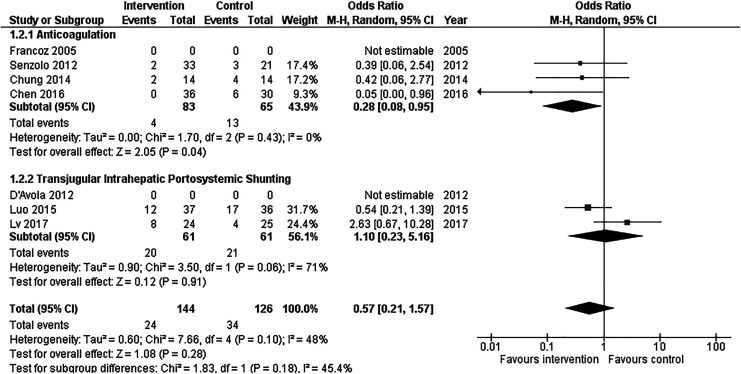
Mortality data were not included for 2 of the studies, one evaluating AC and one evaluating TIPS.32,33 Of the remaining 5 studies, one study30 demonstrated a positive treatment effect on mortality (OR: 0.05 [95% CI: 0.00-0.96]), and the remaining 4 studies demonstrated no significant difference in mortality between intervention and control arms (Figure 3).31,34–36
Figure 3.
Forest plot of included studies demonstrating effect of anticoagulation or transjugular intrahepatic portosystemic shunting (TIPS) on mortality in patients with portal vein thrombosis. CI indicates confidence interval; M-H, Mantel-Haenszel.
Among studies including the impact of AC on mortality in patients with PVT, the summary OR was 0.28 (95% CI: 0.08-0.95; Figure 3). There was no significant heterogeneity among these studies (P = .43, I 2 = 0%).
Among the 2 studies evaluating the impact of TIPS on mortality in patients with PVT, the summary OR was 1.10 (95% CI: 0.23-5.16; Figure 3). There was significant heterogeneity among these studies (P = .06, I 2 = 71%).
The overall summary OR for the impact of treatment with AC or TIPS on mortality in patients with PVT was 0.57 (95% CI: 0.21-1.57). There was no significant heterogeneity among studies in this estimate (P = .10, I 2 = 48%). A funnel plot of these studies was not suggestive of publication bias (Supplemental Figure 1B).
Discussion
Our study quantitatively evaluates the efficacy of AC and TIPS in treatment of PVT in patients with cirrhosis. We found that both treatments improved the rate of recanalization and that AC had a mortality benefit not seen with TIPS. These findings are significant as while multiple current societal guidelines support treatment of PVT, this recommendation is limited to certain populations, currently those patients eligible for liver transplant.16,17 Additionally, prior studies have demonstrated that a proportion of PVTs will spontaneously regress without treatment,37 leading some authors to support a “watchful waiting” approach to avoid risks associated with treatment. At this point, individualized risk and benefit assessment should be performed for each patient, given treatment is certainly not risk-free. Given the increased odds of recanalization and, mortality benefit associated with treatment, however, our study suggests treatment should be considered in a broader population of patients with cirrhosis and PVT. Potentially all patients with cirrhosis and PVT without a clear contraindication should be considered for treatment after variceal prophylaxis. Mounting evidence supporting the safety of AC in patients with cirrhosis argues for this approach as well.14,15,19,38
The survival benefit of AC in patients with PVT is intriguing, particularly given that it was not seen with TIPS. This finding suggests that the treatment of PVT with AC has benefits that extend beyond recanalization of the portal venous system and provides further evidence that PVT may contribute to hepatic decompensation. The relationship between hypercoagulability and fibrosis has been explored in animal models and clinical studies. A hypercoagulable state in cirrhosis is thought to potentially worsen fibrosis by direct activation of hepatic stellate cell protease-activated receptors (PAR)-1 and PAR-2 via thrombin and factor Xa.39 This mechanism is supported by animal studies in which murine models of liver injury using mice lacking PAR-1 receptors showed reduced fibrosis development compared to controls.40 In complementary observational studies, patients with hepatitis C cirrhosis having elevated prothrombin levels or factor V Leiden disease have been found to have higher fibrosis levels than controls.41,42 Furthermore, prior studies in animal models43–47 and humans13,48 suggest AC may have an antifibrinogenic effect and potentially modify the course of chronic liver disease.5,39 In particular, administration of LMWH reduced fibrosis in multiple rat models of liver injury,43,44 while use of both VKA45 and factor Xa inhibitor46 reduced fibrogenesis in murine models. In clinical trials, administration of LMWH to patients with cirrhosis not only reduced rates of PVT formation but also reduced hepatic decompensation and demonstrated a mortality benefit in the only prospective trial concluded to date,13 and administration of warfarin to patients with hepatitis C cirrhosis posttransplant reduced rates of fibrosis 1 year after transplant.48 Taken together, these findings suggest that AC may have a beneficial impact on fibrosis and survival in many patients with cirrhosis. Given the increasing evidence of safety of use of AC in patients with cirrhosis, these data argue that AC should not be withheld from patients with cirrhosis having a compelling indication such as PVT.
For patients with cirrhosis having PVT who are not felt to be candidates for AC, TIPS provides an additional route to recanalization and is a good second-line option. Although the mortality benefit of AC was not seen in patients who underwent TIPS, it should be noted that the TIPS mortality data were somewhat limited, as there was significant heterogeneity among TIPS studies, and mortality data were not provided in all included studies. TIPS did significantly increase rates of recanalization. The mechanism for this is increase in portal vein flow and reduction in portal stasis, a known risk factor for PVT formation.2 In addition to increased rates of recanalization, TIPS reduces portal hypertension so has the additional benefits of reducing both ascites49 and variceal bleeding50,51 and may be a particularly good option for patients with cirrhosis having PVT with high-risk varices or diuretic refractory ascites. Finally, for patients on the liver transplant list, TIPS, even in chronic, cavernomatous PVT, can restore portal vasculature and enable physiologic anastomosis at the time of transplant.52 Avoidance of nonphysiologic anastomoses (eg, jump grafts) at transplant is key, as this improves posttransplant outcomes.53
Our study has some limitations. First, we relied on data from many observational studies, particularly those evaluating AC, as there are limited randomized trials published to date. Additionally, none of the AC studies used direct-acting oral anticoagulants (DOACs) for AC, so application of these results to patients with cirrhosis on DOACs remains limited. Many studies, particularly recent studies, evaluating the use of TIPS for PVT in patients with cirrhosis, were excluded due to lack of a control group. The overall number of patients evaluated was relatively small with 327 patients total included. The mortality data among studies evaluating TIPS were limited to only 2 studies and had significant heterogeneity. Finally, there were insufficient data available to assess known complications of treatment with AC or TIPS, bleeding, and hepatic encephalopathy, so we were unable to quantitatively compare these outcomes.
In summary, our findings support the use of AC to treat PVT and suggest the use of TIPS for PVT treatment warrants more investigation. Our finding that AC has a survival benefit is in line with a growing body of literature, which suggests that AC improves outcomes in patients with cirrhosis, potentially through reduction in activation of PAR on hepatic cells that leads to fibrosis and suggests that AC should not be restricted to only certain subsets of patients with cirrhosis having PVT. In patients with PVT and other indications for TIPS (eg, uncontrolled ascites or variceal bleeding), TIPS will increase the chance of recanalization and is a good therapeutic option. More data are needed before using TIPS as first-line therapy for PVT in patients who do not have uncontrolled portal hypertension, particularly if patients are candidates for AC, given the mortality benefit demonstrated with AC not seen with TIPS. Further randomized studies are needed to better delineate the safety and role of TIPS in treatment of PVT. Currently, DOACs cannot be routinely recommended for PVT, as they have not been specifically addressed by clinical studies; however, these should be evaluated in future trials, given the ease of dosing and relative safety of these agents compared to VKA, the most common agent evaluated in studies published to date. Finally, future studies should include large, randomized, controlled trials that include safety and survival end points in addition to recanalization.
Supplemental Material
Supplemental Material, DavisFigS1FunnelPlots for Anticoagulation and Transjugular Intrahepatic Portosystemic Shunting for Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis by Jessica P. E. Davis, Amy G. Ogurick, Carrie E. Rothermel, Min-Woong Sohn, Nicolas M. Intagliata and Patrick G. Northup in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, TableS1 for Anticoagulation and Transjugular Intrahepatic Portosystemic Shunting for Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis by Jessica P. E. Davis, Amy G. Ogurick, Carrie E. Rothermel, Min-Woong Sohn, Nicolas M. Intagliata and Patrick G. Northup in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, TableS2 for Anticoagulation and Transjugular Intrahepatic Portosystemic Shunting for Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis by Jessica P. E. Davis, Amy G. Ogurick, Carrie E. Rothermel, Min-Woong Sohn, Nicolas M. Intagliata and Patrick G. Northup in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: Informed consent for patient information to be published in this article was not obtained because this manuscript relied on published anonymous data. Jessica P. E. Davis acquired data, performed statistical analysis and interpretation of data, and drafted the article. Amy G. Ogurick and Carrie E. Rothermal acquired data, and revised the manuscript. Min-Woong Sohn performed statistical analysis and interpretation of data, and revised the manuscript. Nicolas M. Intagliata and Patrick G. Northup interpreted the data, and revised the manuscript. All authors gave final approval.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jessica P. E. Davis  https://orcid.org/0000-0002-8918-7806
https://orcid.org/0000-0002-8918-7806
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Janssen HL, Wijnhoud A, Haagsma EB, et al. Extrahepatic portal vein thrombosis: aetiology and determinants of survival. Gut. 2001;49(5):720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stine JG, Wang J, Shah PM, et al. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: a matched case-control study. Liver Int. 2018;38(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeLeve LD, Valla DC, Garcia-Tsao G, American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49(5):1729–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Violi F, Corazza GR, Caldwell SH, et al. Portal vein thrombosis relevance on liver cirrhosis: Italian Venous Thrombotic Events Registry. Int Emerg Med. 2016;11(8):1059–1066. [DOI] [PubMed] [Google Scholar]
- 5. Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21(5):1238–1247. [PubMed] [Google Scholar]
- 6. Englesbe MJ, Schaubel DE, Cai S, Guidinger MK, Merion RM. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010;16(8):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez Castro KI, Porte RJ, Nadal E, Germani G, Burra P, Senzolo M. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation. 2012;94(11):1145–1153. [DOI] [PubMed] [Google Scholar]
- 8. Stine JG, Shah PM, Cornella SL, et al. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: a meta-analysis. World J Hepatol. 2015;7(27):2774–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi X, Dai J, Jia J, et al. Association between portal vein thrombosis and survival of liver transplant recipients: a systematic review and meta-analysis of observational studies. J Gastrointestin Liver Dis. 2015;24(1):51–59. [DOI] [PubMed] [Google Scholar]
- 10. Fujiki M, Hashimoto K, Palaios E, et al. Probability, management, and long-term outcomes of biliary complications after hepatic artery thrombosis in liver transplant recipients. Surgery. 2017;162(5):1101–1111. [DOI] [PubMed] [Google Scholar]
- 11. Stine JG, Pelletier SJ, Schmitt TM, Porte RJ, Northup PG. Pre-transplant portal vein thrombosis is an independent risk factor for graft loss due to hepatic artery thrombosis in liver transplant recipients. HPB (Oxford). 2016;18(3):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61(2):660–667. [DOI] [PubMed] [Google Scholar]
- 13. Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(5):1253–1260. e1251–1254. [DOI] [PubMed] [Google Scholar]
- 14. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393–397. [DOI] [PubMed] [Google Scholar]
- 15. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721–1727. [DOI] [PubMed] [Google Scholar]
- 16. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64(1):179–202. [DOI] [PubMed] [Google Scholar]
- 17. De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. [DOI] [PubMed] [Google Scholar]
- 18. Intagliata NM, Argo CK, Stine JG, et al. Concepts and controversies in haemostasis and thrombosis associated with liver disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost. 2018;118(8):1491–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology. 2017;153(2):480–487.e481. [DOI] [PubMed] [Google Scholar]
- 20. Qi X, De Stefano V, Li H, Dai J, Guo X, Fan D. Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Eur J Int Med. 2015;26(1):23–29. [DOI] [PubMed] [Google Scholar]
- 21. Rodrigues SG, Sixt S, Abraldes JG, et al. Systematic review with meta-analysis: portal vein recanalisation and transjugular intrahepatic portosystemic shunt for portal vein thrombosis. Aliment Pharmacol Ther. 2019;49(1):20–30. [DOI] [PubMed] [Google Scholar]
- 22. Valentin N, Korrapati P, Constantino J, Young A, Weisberg I. The role of transjugular intrahepatic portosystemic shunt in the management of portal vein thrombosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(10):1187–1193. [DOI] [PubMed] [Google Scholar]
- 23. Cruz Ramon V, Chinchilla Lopez P, Ramirez Perez O, et al. Thrombosis of the portal venous system in cirrhotic vs. non-cirrhotic patients. Ann Hepatol. 2018;17(3):476–481. [DOI] [PubMed] [Google Scholar]
- 24. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Int Med. 2015;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPTAD. Assessing risk of bias in included studies In: Julian H, Sally G. (eds) Cochrane Handbook for Systematic Reviews of Interventions. Chichester, United Kingdom: John Wiley & Sons Ltd; 2008:187–241. [Google Scholar]
- 28. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 29. Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28(1):82–89. [DOI] [PubMed] [Google Scholar]
- 31. Chung JW, Kim GH, Lee JH, et al. Safety, efficacy, and response predictors of anticoagulation for the treatment of nonmalignant portal-vein thrombosis in patients with cirrhosis: a propensity score matching analysis. Clin Mol Hepatol. 2014;20(4):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D’Avola D, Bilbao JI, Zozaya G, et al. Efficacy of transjugular intrahepatic portosystemic shunt to prevent total portal vein thrombosis in cirrhotic patients awaiting for liver transplantation. Transplant Proc. 2012;44(9):2603–2605. [DOI] [PubMed] [Google Scholar]
- 33. Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54(5):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo X, Wang Z, Tsauo J, Zhou B, Zhang H, Li X. Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of tips versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal variceal bleeding. Radiology. 2015;276(1):286–293. [DOI] [PubMed] [Google Scholar]
- 35. Lv Y, Qi X, He C, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. 2018;67(12):2156–2168. [DOI] [PubMed] [Google Scholar]
- 36. Senzolo M, M Sartori T, Rossetto V, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32(6):919–927. [DOI] [PubMed] [Google Scholar]
- 37. Qi X, Guo X, Yoshida EM, et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med. 2018;16(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. La Mura V, Braham S, Tosetti G, et al. Harmful and beneficial effects of anticoagulants in patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2018;16(7):1146–1152.e1144. [DOI] [PubMed] [Google Scholar]
- 39. Pant A, Kopec AK, Luyendyk JP. Role of the blood coagulation cascade in hepatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315(2):G171–G176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nault R, Fader KA, Kopec AK, Harkema JR, Zacharewski TR, Luyendyk JP. From the cover: coagulation-driven hepatic fibrosis requires protease activated receptor-1 (PAR-1) in a mouse model of TCDD-elicited steatohepatitis. Toxicol Sci. 2016;154(2):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maharshak N, Halfon P, Deutsch V, et al. Increased fibrosis progression rates in hepatitis C patients carrying the prothrombin G20210A mutation. World J Gastroenterol. 2011;17(45):5007–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright M, Goldin R, Hellier S, et al. Factor V Leiden polymorphism and the rate of fibrosis development in chronic hepatitis C virus infection. Gut. 2003;52(8):1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abdel-Salam OM, Baiuomy AR, Ameen A, Hassan NS. A study of unfractionated and low molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol Res. 2005;51(1):59–67. [DOI] [PubMed] [Google Scholar]
- 44. Abe W, Ikejima K, Lang T, et al. Low molecular weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J Hepatol. 2007;46(2):286–294. [DOI] [PubMed] [Google Scholar]
- 45. Anstee QM, Goldin RD, Wright M, Martinelli A, Cox R, Thursz MR. Coagulation status modulates murine hepatic fibrogenesis: implications for the development of novel therapies. J Thromb Haemost. 2008;6(8):1336–1343. [DOI] [PubMed] [Google Scholar]
- 46. Dhar A, Sadiq F, Anstee QM, Levene AP, Goldin RD, Thursz MR. Thrombin and factor Xa link the coagulation system with liver fibrosis. BMC Gastroenterol. 2018;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vilaseca M, Garcia Caldero H, Lafoz E, et al. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65(6):2031–2044. [DOI] [PubMed] [Google Scholar]
- 48. Dhar ETA, Brown R, Manousou P, et al. Warfarin anticoagulation for liver fibrosis in patients transplanted for hepatitis C (WAFT-C): results at one year. J Hepatol. 2015;62(S2):S268–S269. [Google Scholar]
- 49. Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133(3):825–834. [DOI] [PubMed] [Google Scholar]
- 50. Garcia Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. [DOI] [PubMed] [Google Scholar]
- 51. Holster IL, Tjwa ET, Moelker A, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + beta-blocker for prevention of variceal rebleeding. Hepatology. 2016;63(2):581–589. [DOI] [PubMed] [Google Scholar]
- 52. Thornburg B, Desai K, Hickey R, et al. Pretransplantation portal vein recanalization and transjugular intrahepatic portosystemic shunt creation for chronic portal vein thrombosis: final analysis of a 61-patient cohort. J Vasc Interv Radiol. 2017;28(12):1714–1721.e1712. [DOI] [PubMed] [Google Scholar]
- 53. Hibi T, Nishida S, Levi DM, et al. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg. 2014;259(4):760–766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, DavisFigS1FunnelPlots for Anticoagulation and Transjugular Intrahepatic Portosystemic Shunting for Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis by Jessica P. E. Davis, Amy G. Ogurick, Carrie E. Rothermel, Min-Woong Sohn, Nicolas M. Intagliata and Patrick G. Northup in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, TableS1 for Anticoagulation and Transjugular Intrahepatic Portosystemic Shunting for Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis by Jessica P. E. Davis, Amy G. Ogurick, Carrie E. Rothermel, Min-Woong Sohn, Nicolas M. Intagliata and Patrick G. Northup in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, TableS2 for Anticoagulation and Transjugular Intrahepatic Portosystemic Shunting for Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis by Jessica P. E. Davis, Amy G. Ogurick, Carrie E. Rothermel, Min-Woong Sohn, Nicolas M. Intagliata and Patrick G. Northup in Clinical and Applied Thrombosis/Hemostasis