Abstract
In the context of the coronavirus disease 2019 pandemic, we aimed to systematically address the global seasonal patterns of human coronavirus (HCoV) infections. We identified relevant articles from MEDLINE, EMBASE, and CINAHL Plus as of May 11, 2020. The main outcomes were the peak months of HCoV infections each year and the months during which more than 5% of positive respiratory specimen tests were attributable to HCoV. Of 707 articles reviewed, 22 met the inclusion criteria. The annual percentage of HCoV infections reached a peak in February globally. We found a higher HCoV positivity rate among studies that tested only children (median: 5.9%, range: 0.9%–18.4%), compared with other studies of adults alone (median: 5.2%, range: 3.3%–7.1%) or the entire population (median: 1.9%, range: 0.2%–8.1%). We found the largest global peak of HCoV during the winter season, with the highest rate of positivity among children.
Keywords: climate, coronavirus, epidemic, seasonality, weather
Periodic surge in disease incidence corresponding to seasons is characteristic of many infectious diseases [1]. Although the mechanisms underlying seasonal occurrence remain poorly understood, recognition of periodic oscillation is of public health importance in the view of resource allocation and setting.
Human coronaviruses (HCoVs) are common causes of acute respiratory infections, leading to a wide range of disease severity. The 2 emergent zoonotic HCoV infections, namely, severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV), have not established sustained community transmission in the past. However, the zoonotic SARS-CoV-2 continues its rampant spread across the globe, causing more than 6 million cases of coronavirus disease 2019 (COVID-19) and 390 000 deaths, as of June 7, 2020 [2].
Predicting the fate of SARS-CoV-2 remains a challenge because of its unique virulence, transmissibility, and genetic adaptation. To develop timely and effective countermeasures to combat COVID-19, information about the periodicity of the common endemic HCoV subtypes (HCoV-OC43, -HKU1, -229E, and -NL63) may be useful to inform public health strategies considering that SARS-CoV-2 may become an endemic seasonal virus. The aim of this report is to systematically collate and evaluate the existing evidence describing seasonal patterns in the incidence of nonzoonotic HCoV globally.
METHODS
This study was performed in accordance with PRISMA guidelines [3]. Our research question was formulated in accordance with PICO (P = population, I = intervention, C = comparison, and O = outcome): how does the incidence of coronavirus (O) among adults and children (P) vary by season (I)? We searched MEDLINE (beginning in 1946), EMBASE (beginning in 1947), and CINAHL Plus (beginning in 1937) to find relevant articles through May 11, 2020 without language restrictions. We used the following Medical Subject Heading terms in exploring MEDLINE: coronavirus AND (season OR climate OR weather). Each term was modified to an appropriate thesaurus term suitable for other databases. In all databases, we used narrower terms of each broad thesaurus term by activating the “explode” function. We also manually screened references of relevant articles to find additional articles.
Inclusion criteria were as follows: a prospective or retrospective study that included HCoV-OC43, -HKU1, -229E, or -NL63; ≥1 consecutive year study period; and clearly reported proportions of coronavirus infections of study populations in each month or season. Studies that used multiple-year data were retrieved as presented. We excluded studies on SARS-CoV-1 or -2 and MERS-CoV because occurrences of these viruses were observed in situations carefully controlled with special isolation precautions unlike for other HCoVs. Because the purpose of this study was to evaluate seasonality of HCoV infections, we excluded studies that lacked information about the proportion of HCoV infections per month or season. We also excluded studies that reported the number of HCoV cases but not the number of specimens.
Two investigators (S.P. and Y.J.C.) independently performed study selection. Articles written in other languages than English were translated using the Google Translator (https://translate.google.com/) before reviewing full texts. We quantified the interrater agreement for study selection using the Cohen’s kappa coefficient. Two investigators (S.P. and Y.L.) extracted the data from study articles using a uniform method. From eligible studies, we collated the following information: author, year, country, study design, study population, subtypes of virus, participants’ age, observation period, sample size, positive proportion of HCoV tests, months with ≥5% positive HCoV tests, and peak months, or month of year when the incidences are at their highest, of HCoV infections. Information on peak months and months with ≥5% positive tests were aggregated from consecutive annual data. If the data collection period was <12 months, data were combined with those collected during the previous or following year. For example, if a study collected data from May to December 2005 and from January to December 2006, data were combined into a single observation over 20 months for measurement.
Two investigators (S.P. and Y.L.) assessed the risk of study bias using a modified Hoy’s tool [4]. We used 9 items to evaluate validity of each study. Items with high risk of bias (answered as "no") were assigned a score of 1; and items with low risk of bias (answered as "yes") were assigned a score of zero. External validity was assessed by 4 items: study’s target population was similar to the national population; study’s sampling frame was similar to the target population; study used random selection to collect participants; and study minimized nonresponse bias by obtaining response rate ≥75%. Internal validity was assessed by 5 items: study directly collected data from individual participants or specimens; study used an acceptable case definition; diagnostic tool had validity and reliability; study used the same mode of data collection for all participants; and study reported numerators and denominators of the variables of interest. We classified studies as being at low (0–1), moderate (2–4), or high (5–9) risk of bias.
Patient Consent Statement
This systematic review is exempt from ethics approval and patient consent from the Hallym University Institutional Review Committee because the study collected and synthesized data from aggregated, anonymized data in the public domain.
RESULTS
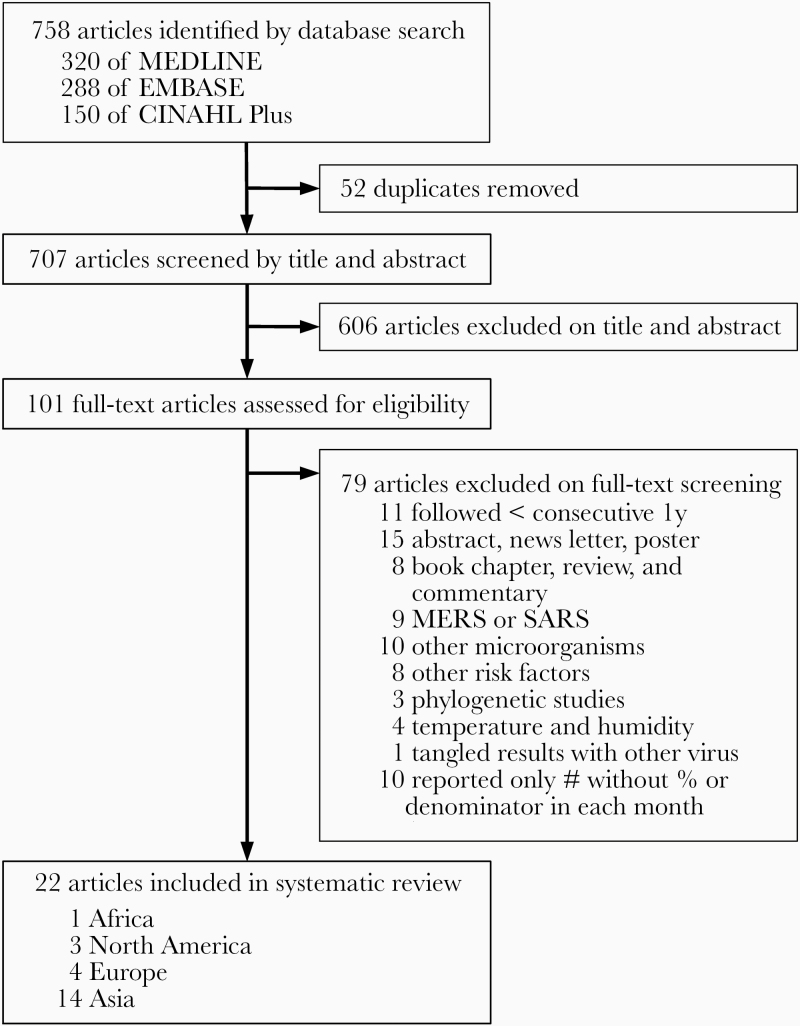
Our search identified 758 articles and conference abstracts (Figure 1). Fifty-two duplicates were removed. After screening titles and abstracts, we excluded 606 studies that did not meet eligibility criteria for this systematic review. We then reviewed full text of 101 articles and excluded another 79 studies that were ineligible. We ultimately included 22 studies with 979 400 specimens from 12 countries in the systematic review. Interrater agreement for study selection was good (Cohen’s kappa = 0.917, 97.1% agreement).
Figure 1.
Study selection. MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome.
The included studies were published between 2005 and 2020 (Table 1) [5–26]. More than half of the studies (14 of 22) were performed in Asia [6–19]. Only 1 study followed participants longitudinally and collected specimens from them periodically [25]. Two studies collected respiratory specimens from cohorts [23, 25]. Two studies used surveillance samples [21, 26]. Sixteen studies reported HCoV infection by screening all 4 subtypes of virus [5, 7, 9–11, 13, 14, 16–22, 24, 25]. The range of proportions of positive specimens was 0.2%–18.4% (median: 4.45%) (Table 2). We classified 1, 21, and 0 studies as having low, moderate, and high risk of bias, respectively (Supplementary Table 1).
Table 1.
Studies Included in the Systematic Review
| Study, year | Country | Population | HCoV subtype | Age |
|---|---|---|---|---|
| Africa | ||||
| Brini Khalifa et al [5], 2019 | Tunisia | ARI patients | O/H/N/E | 0–12 months |
| North America | ||||
| Dominguez et al [24], 2009 | USA | ARI patients | O/H/N/E | Children |
| Killerby et al [26], 2018 | USA | Surveillance samples | O/N/E | 0–96 years |
| Galanti et al [25], 2019 | USA | Healthy individuals | O/H/N/E | 0–63 years |
| Europe | ||||
| van der Zalm et al [23], 2009 | Netherlands | Healthy individuals | O/N/E | Birth–1 year |
| Gaunt et al [21], 2010 | UK | Surveillance samples | O/H/N/E | Children, adults |
| Jevsnik et al [22], 2016 | Slovenia | ARI patients | O/H/N/E | Children, adults |
| De Conto et al [20], 2019 | Italy | ARI patients | O/H/N/E | 2 days–14 years |
| Asia | ||||
| Ren et al [13], 2019 | China | ARI patients | O/H/N/E | 14–97 years |
| Xin et al [15], 2012 | China | ARI patients | N | 29 days–15.9 years |
| Feng et al [9], 2014 | China | ARI patients | O/H/N/E | Children, adults |
| Zeng et al [17], 2018 | China | ARI patients | O/H/N/E | Children |
| Zhang et al [18], 2018 | China | ARI patients | O/H/N/E | 1 days–103 years |
| Zhao et al [19], 2019 | China | ARI patients | O/H/N/E | 1 months–14 years |
| Chiu et al [8], 2005 | Hong Kong | ARI patients | O/N/E | Children, adults |
| Yip et al [16], 2016 | Hong Kong | ARI patients | O/H/N/E | 22 days–95 years |
| Matoba et al [12], 2015 | Japan | ARI patients | O/N/E | Unclear |
| Al-Khannaq et al [6], 2016 | Malaysia | ARI patients | N/E | Adults |
| Toh et al [14], 2019 | Malaysia | ARI patients | O/H/N/E | Children, adults |
| Al-Romaihi et al [7], 2020 | Qatar | ARI patients | O/H/N/E | 0–14 years |
| Kim et al [10], 2016 | South Korea | ARI patients (COPD) | O/H/N/E | Adults |
| Ko et al [11], 2017 | South Korea | ARI patients | O/H/N/E | 0–91 years |
Abbreviation: ARI, acute respiratory infection; COPD, chronic obstructive pulmonary disease; E, human coronavirus (HCoV)-229E; H, HCoV-HKU1; N, HCoV-NL63; O, HCoV-OC43; UK, United Kingdom; USA, United States of America.
Table 2.
Positive Proportion of Human Coronavirus (HCoV) Tests, Months with ≥5% Positive HCoV Tests, and Peak Months of HCoV Infections in Included Studies
| Study | Observation Period | na | Positive, % | Positive ≥5% | Peak Month |
|---|---|---|---|---|---|
| Africa | |||||
| Brini Khalifa et al [5] | September 2013–December 2014 | 515 | 18.4 | All year round | April–June |
| North America | |||||
| Dominguez et al [24] | December 2004–November 2005 | 1683 | 5.0 | January–May | January |
| Killerby et al [26] | July 2014–December 2015 | 854 575 | 0.2 | January–March, December | February |
| Galanti et al [25] | October 2016–April 2018 | 4215 (214) | 4.5 | January–March, May, July, November–December | May |
| Europe | |||||
| van der Zalm et al [23] | October 2003–September 2006 | 668 (305) | 7.6 | January–April, August, November–December | December |
| Gaunt et al [21] | July 2006–December 2007 | 11 661 (7383) | 2.3 | February, March | February |
| January 2008–June 2009 | None | February | |||
| Jevsnik et al [22] | October 2009–December 2010 | 718, 156 | 8.1, 1.9 | February–April, December | February |
| January–October 2011 | January–April | February | |||
| De Conto et al [20] | October 2012–December 2013 | 2892 (2575) | 9.1 | November, January–March | February |
| January–December 2014 | January–June, August, December | March | |||
| January–September 2015 | January, March | January | |||
| Asia | |||||
| Ren et al [13] | May 2005–December 2006 | 4335 | 0.7 | None | October |
| January–December 2007 | 2214 | 0.5 | June | June | |
| January 2008–April 2009 | 1847 | 1.9 | None | April | |
| Xin et al [15] | April 2006–March 2008 | 878 | 0.9 | None | September |
| Feng et al [9] | January–December 2009 | 28 369 | 1.4 | None | March |
| January–December 2010 | None | February | |||
| January–December 2011 | None | November | |||
| January 2012–September 2013 | None | September | |||
| Zeng et al [17] | July 2009–December 2010 | 11 399 | 4.3 | June, August–October, December | October |
| January–December 2011 | February, April, June, October–November | April | |||
| January–December 2012 | January, March–August | April | |||
| January–December 2013 | May–September | July | |||
| January–December 2014 | January | January | |||
| January 2015–June 2016 | September, June | September | |||
| Zhang et al [18] | July 2010–June 2015 | 13 048 | 2.3 | None | February |
| Zhao et al [19] | May 2008–March 2014 | 700 | 10.7 | All year round | Spring (March–May) |
| Chiu et al [8] | August 2001–August 2002 | 581 | 4.4 | February, July, November– December | November |
| Yip et al [16] | September 2008–December 2009 | 8275 | 0.9 | None | December |
| January–December 2010 | None | December | |||
| January–December 2011 | None | May = September | |||
| January–December 2012 | None | January = November | |||
| January 2013–August 2014 | February | February | |||
| Matoba et al [12] | January–December 2010 | 4342 | 7.6 | January–March, October– November | January |
| January–December 2011 | January–March, July–August | January | |||
| January–December 2012 | January–April, June, November–December | December | |||
| January–December 2013 | January–March | January | |||
| Al-Khannaq et al [6] | March 2012–February 2013 | 2060 | 3.3 | July–August, October | July |
| Toh et al [14] | June 2017–May 2018 | 599 | 1.0 | None | August–September = October–November = November–December = December–January |
| Al-Romaihi et al [7] | January–December 2012 | 1846 | 8.3 | None | February |
| January–December 2013 | 2081 | 6.0 | None | February | |
| January–December 2014 | 2901 | 5.9 | None | May | |
| January–December 2015 | 4614 | 5.1 | None | January | |
| January–December 2016 | 5314 | 5.0 | None | December | |
| January–December 2017 | 14 190 | 5.6 | March, November–December | December | |
| Kim et al [10] | January 2010–December 2012 | 477 | 7.1 | January–April, June, November–December | April |
| Ko et al [11] | January–December 2013 | 3467 | O/H: 4.1, N/E: 1.7 | Unclear | Unclear |
| January–December 2014 | December | December | |||
| January–December 2015 | February | February |
aIf the number of participants and specimen were different, the number of participants is shown in the parenthesis.
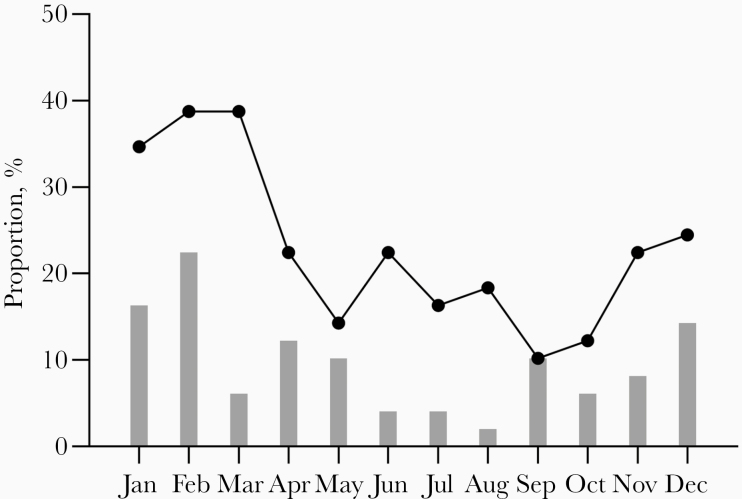
Figure 2 depicts the proportions of HCoV infections peaked in the winter season (February, 22.4%; January, 16.3%; and December, 14.3%). A small number of studies reported peaks during summer months (June, 4.1%; July, 4.1%; and August, 2.0%) [5, 6, 13, 14, 17]. Around 38.8% of observation periods showed that more than 5% of specimens tested positive for HCoVs in February and March [5, 7, 8, 10–12, 16, 17, 19–26]. Around 10 to 22% of observation periods showed that more than 5% of specimens had positive test results during fall (September, 10.2%; October, 12.2%; and November, 22.4%) [5–8, 10, 12, 17, 19, 20, 23, 25]. Some research that studied children only showed higher HCoV positivity rate (median: 5.9%, range: 0.9%–18.4%) [5, 7, 15, 17, 19, 20, 23, 24] than the studies of adults alone (median: 5.2%, range: 3.3%–7.1%) [6, 10] or the general populations (median: 1.9%, range: 0.2%–8.1%) [8, 9, 11, 13, 14, 16, 18, 21, 22, 25, 26].
Figure 2.
Monthly distribution of human coronavirus (HCoV) infection worldwide. The bar indicates the proportion of the peaks in HCoV infection per observation period. The line indicates the proportion of studies that reported more than 5% of specimens that tested positive for HCoV.
DISCUSSION
This review describes the incidence and proportion of common nonzoonotic HCoV among various populations, providing a snapshot of the global seasonality of this virus. We found a consistent winter peak of HCoV incidence in the northern hemisphere, with modest decline in the summer months. Various factors may contribute to the seasonal occurrence of HCoV, as in other respiratory viruses. Human factors, such as behaviors and activities that are closely associated with proximity of humans to one another, may contribute in seasonal cycles in virus transmission [27]. Environmental factors such as humidity have been noted with improving duration of viral survival in the environment, and thus they may increase the chance of indirect transmission of viruses [28]. However, these descriptions carry an important caveat of societal changes over the past centuries with industrial revolutions that relocated outdoor agricultural workplaces into indoor factories and offices, while moving human lifestyle away from nature and outdoor climate [29]. In the context of urbanization, a consistent thermal comfort zone could be maintained indoors, causing even further disconnection from daily and seasonal outdoor climate fluctuations. Nonetheless, it is important to address the current questions related to seasonality of HCoV in light of the COVID-19 pandemic. The apparent seasonality of HCoV across the globe suggests that this phenomenon might be mined to produce improved understanding of transmission of COVID-19 and improve public health intervention.
The observed seasonal variation of HCoV rates may have resulted from a dynamic interaction of factors as in other respiratory viruses [30]. The typical winter peak seasonality of respiratory viruses is highly dependent on geographic location and climate. In northern and southern hemisphere temperate regions, an annual seasonal pattern is predictably limited to a few months during winter [31]. It has been suggested that differences in host susceptibility, environmental factors, and population behavior are potential determinants for seasonal incidence of respiratory virus infections [32]. Other authors have found that low temperatures during winter prolong the stability of viruses in fomites [33]. Nevertheless, none of these associations have been proven to be causal for incidences. Regardless of the cause, identifying the seasonal pattern of HCoV is important when planning strategies to mitigate the spread of SARS-CoV-2. Our finding indicates that the seasonality of HCoV peaks during winter in the northern hemisphere across the globe, as with other respiratory viruses [34].
Most studies included in this review reported the percentage of HCoV detection in patients presenting with acute respiratory infections. As expected, considerable variation was found in the percentage of HCoV infections reported by the included studies. In Norway, among children hospitalized with respiratory tract infection, HCoV was responsible for 9.1% of all infections [35]. Among 1404 children in Mexico with community-acquired pneumonia from 2010 to 2013, HCoV accounted for 7.2% of infections [36]. Among 219 respiratory specimens from patients admitted to pediatric hematology and oncology department in Turkey, HCoV accounted for 14.8% of all respiratory viruses [37]. Several factors such as case definition, study setting, population structure, and differences in the host and pathogen across the globe may have contributed to the observed variability and should be considered when interpreting the study result. Another notable finding was that HCoV positivity rates in studies that exclusively tested children were higher than in studies of the general population, which is consistent with findings from other respiratory viruses [34]. It is in line with a recent cohort study from Michigan that showed the highest infection frequency of HCoV in children <5 years (18 per 100 person-years), with little variation in older age groups (range, 7 to 11 per 100 person-years) [38]. These findings may not be generalizable to SARS-CoV-2 given the unclear virologic properties of SARS-CoV-2; however, like other respiratory viruses, HCoV infects people of all ages, both in adults and children.
Our review has potential limitations. First, by comparing proportions of individuals who tested positive for HCoV, rather than the total number of HCoV cases, the observed seasonal patterns may have been driven by periodic changes in other respiratory pathogens. Moreover, we did not distinguish between temporal trends of the annual and seasonal occurrences in studies that presented aggregated data on given seasons [7]. However, we found similar results when we compared differences in the total number of HCoV cases. Second, we did not directly assess factors that may lead to higher numbers of HCoV cases. Seasonality may or may not have a direct causal relationship with HCoV transmissibility, but if it does, it can be challenging to detect such an association because epidemiological data represent case events, not transmission events. Several studies have investigated the role of geospatial factors (ie, latitude) and societal factors (ie, population density) on the seasonal occurrence of respiratory viruses [29]. Our findings may not be generalizable because the only available published data were mostly from countries in the northern hemisphere. Yet, given the wide range of variations in environments within the studied areas (ie, the United States, China), these factors were not included in our analysis. Finally, previous studies have shown the difference in seasonality between alpha-coronavirus (ie, HCoV-229E) and beta-coronavirus (ie, HCoV-OC43); therefore, our finding may not be directly extrapolated as a general characteristic of SARS-CoV-2 [39, 40]. Nonetheless, the lack of population immunity against SARS-CoV-2 in the setting of social distancing makes the temporal trend of COVID-19 largely unpredictable. Although it would be challenging to estimate the seasonality of SARS-CoV-2 at this time, it is plausible that if there are overlapping virus-specific characteristics with other HCoVs, our finding may add a piece of information in regard to public health preparedness.
CONCLUSIONS
This systematic review reveals that common HCoVs tend to spread during the winter months across the globe, with higher positivity rate in children compared with adults. If the virologic properties of SARS-CoV-2 on seasonality of transmission is similar to general HCoV, it may be possible to estimate the months most likely to be at higher risk of spread of COVID-19, allowing for public health preparedness and response efforts.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was funded by Hallym University Research Fund, 2019 (HRF-201912-0009).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Fisman DN. Seasonality of infectious diseases. Annu Rev Public Health 2007; 28:127–43. [DOI] [PubMed] [Google Scholar]
- 2. Nickbakhsh S, Ho A, Marques DFP, et al. Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019. J Infect Dis 2020; 16:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65:934–9. [DOI] [PubMed] [Google Scholar]
- 5. Brini Khalifa I, Hannachi N, Guerrero A, et al. Demographic and seasonal characteristics of respiratory pathogens in neonates and infants aged 0 to 12 months in the Central-East region of Tunisia. J Med Virol 2019; 91:570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Khannaq MN, Ng KT, Oong XY, et al. Diversity and evolutionary histories of human coronaviruses NL63 and 229E associated with acute upper respiratory tract symptoms in Kuala Lumpur, Malaysia. Am J Trop Med Hyg 2016; 94:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Romaihi HE, Smatti MK, Al-Khatib HA, et al. Molecular epidemiology of influenza, RSV, and other respiratory infections among children in Qatar: a six years report (2012–2017). Int J Infect Dis 2020; 95:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis 2005; 40:1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng L, Li Z, Zhao S, et al. Viral etiologies of hospitalized acute lower respiratory infection patients in China, 2009–2013. PLoS One 2014; 9:e99419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HC, Choi SH, Huh JW, et al. Different pattern of viral infections and clinical outcomes in patient with acute exacerbation of chronic obstructive pulmonary disease and chronic obstructive pulmonary disease with pneumonia. J Med Virol 2016; 88:2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko DH, Hyun J, Kim HS, et al. Analysis of respiratory viral infections detected using multiplex real-time PCR in Hwaseong, Korea from 2013 to 2015. Clin Lab 2017; 63:1003–7. [DOI] [PubMed] [Google Scholar]
- 12. Matoba Y, Abiko C, Ikeda T, et al. Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Jpn J Infect Dis 2015; 68:138–41. [DOI] [PubMed] [Google Scholar]
- 13. Ren L, Gonzalez R, Xu J, et al. Prevalence of human coronaviruses in adults with acute respiratory tract infections in Beijing, China. J Med Virol 2011; 83:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toh TH, Hii KC, Fieldhouse JK, et al. High prevalence of viral infections among hospitalized pneumonia patients in equatorial Sarawak, Malaysia. Open Forum Infect Dis 2019; 6:ofz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xin C, Yong ZZ, Yan L, Dong ZX. Human coronavirus NL63 in hospitalized children with respiratory infection: a 2-year study from Chongqing, China. Indian Pediatr 2012; 49:825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yip CC, Lam CS, Luk HK, et al. A six-year descriptive epidemiological study of human coronavirus infections in hospitalized patients in Hong Kong. Virol Sin 2016; 31:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng ZQ, Chen DH, Tan WP, et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis 2018; 37:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang SF, Tuo JL, Huang XB, et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010–2015 in Guangzhou. PLoS One 2018; 13:e0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y, Lu R, Shen J, et al. Comparison of viral and epidemiological profiles of hospitalized children with severe acute respiratory infection in Beijing and Shanghai. BMC Infect Dis 2016; 19:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Conto F, Conversano F, Medici MC, et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3-year hospital-based survey in Northern Italy. Diagn Microbiol Infect Dis 2019; 94:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaunt ER, Hardie A, Claas EC, et al. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 2010; 48:2940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jevšnik M, Steyer A, Pokorn M, et al. The role of human coronaviruses in children hospitalized for acute bronchiolitis, acute gastroenteritis, and febrile seizures: a 2-year prospective study. PLoS One 2016; 11:e0155555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Zalm MM, Uiterwaal CS, Wilbrink B, et al. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J 2009; 28:472–6. [DOI] [PubMed] [Google Scholar]
- 24. Dominguez SR, Robinson CC, Holmes KV. Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 2009; 81:1597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galanti M, Birger R, Ud-Dean M, et al. Longitudinal active sampling for respiratory viral infections across age groups. Influenza Other Respir Viruses 2019; 13:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Killerby ME, Biggs HM, Haynes A, et al. Human coronavirus circulation in the United States 2014–2017. J Clin Virol 2018; 101:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dushoff J, Plotkin JB, Levin SA, Earn DJ. Dynamical resonance can account for seasonality of influenza epidemics. Proc Natl Acad Sci U S A 2004; 101:16915–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dowell SF, Ho MS. Seasonality of infectious diseases and severe acute respiratory syndrome-what we don’t know can hurt us. Lancet Infect Dis 2004; 4:704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neiderud CJ. How urbanization affects the epidemiology of emerging infectious diseases. Infect Ecol Epidemiol 2015; 5:27060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol 2020; 7:83–101. [DOI] [PubMed] [Google Scholar]
- 31. Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 2001; 7:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect 2012; 18:946–54. [DOI] [PubMed] [Google Scholar]
- 33. Lowen AC, Steel J. Roles of humidity and temperature in shaping influenza seasonality. J Virol 2014; 88:7692–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heimdal I, Moe N, Krokstad S, et al. Human coronavirus in hospitalized children with respiratory tract infections: a 9-year population-based study from Norway. J Infect Dis 2019; 219:1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong-Chew RM, García-León ML, Noyola DE, et al. Respiratory viruses detected in Mexican children younger than 5 years old with community-acquired pneumonia: a national multicenter study. Int J Infect Dis 2017; 62:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aydin Köker S, Demirağ B, Tahta N, et al. A 3-year retrospective study of the epidemiology of acute respiratory viral infections in pediatric patients with cancer undergoing chemotherapy. J Pediatr Hematol Oncol 2019; 41:e242–6. [DOI] [PubMed] [Google Scholar]
- 38. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis 2020; 222:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cabeça TK, Granato C, Bellei N. Epidemiological and clinical features of human coronavirus infections among different subsets of patients. Influenza Other Respir Viruses 2013; 7:1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vabret A, Mourez T, Gouarin S, et al. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis 2003; 36:985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.