Abstract
Background
We describe the epidemiological, clinical, and prognostic aspects of 177 tularemia cases diagnosed at the National Reference Center for rickettsioses, coxiellosis, and bartonelloses between 2008 and 2017.
Methods
All patients with a microbiological diagnosis of tularemia made in the laboratory were included. Clinical and epidemiological data were collected retrospectively from clinicians in charge of patients using a standardized questionnaire. Diagnostic methods used were indirect immunofluorescence serology, real-time polymerase chain reaction (PCR), and universal PCR targeting the 16S ribosomal ribonucleic acid gene.
Results
The series included 54 females and 123 males (sex ratio, 2.28; mean age, 47.38 years). Eighty-nine (50.2%) were confirmed as having tularemia on the basis of a positive Francisella tularensis PCR or seroconversion, and 88 (49.8%) were considered as probable due to a single positive serum. The regions of France that were most affected included Pays de la Loire (22% of cases), Nouvelle Aquitaine (18.6% of cases), and Grand Est (12.4% of cases). Patients became infected mainly through contact with rodents or game (38 cases, 21.4%), through tick-bites (23 cases, 12.9%), or during outdoor leisure activities (37 cases, 20.9%). Glandular and ulceroglandular forms were the most frequent (109 cases, 61.5%). Two aortitis, an infectious endocarditis, a myocarditis, an osteoarticular infection, and a splenic hematoma were also diagnosed. Tularemia was discovered incidentally in 54.8% of cases. Seventy-eight patients were hospitalized, and no deaths were reported.
Conclusions
Our data suggest that in an endemic area and/or in certain epidemiological contexts, tularemia should be sought to allow an optimized antibiotic therapy and a faster recovery.
Keywords: case series, diagnosis, France, Francisella tularensis, tularemia
This case series presents 177 patients with tularemia in France between 2008 and 2017: glandular and ulceroglandular forms were the most frequent. Two aortitis, an infectious endocarditis, a myocarditis, an osteoarticular infection, and a splenic hematoma were diagnosed.
Tularemia is a zoonotic disease caused by Francisella tularensis. This facultative intracellular Gram-negative bacillus was isolated for the first time from flying squirrels in Tulare County in the United States by McCoy and Chapin [1]. Tularemia is endemic in North America [2], Asia [3], and Europe [4–8].
Francisella tularensis subspecies tularensis (type A), almost restricted to North America, is responsible for the most severe diseases. The lethality may be as high as 30% in untreated pulmonary forms [9]. In contrast, F tularensis subspecies holarctica (type B) is widely distributed in the Northern Hemisphere but also in southern Australia, and it is associated with a lethality rate of <1%. Clinical signs vary according to the geographical area, season, and thus mode of contamination [6, 7, 10–13]. Six clinical forms are classically recognized: the ulceroglandular (ulcer associated with lymphadenopathy) and glandular (lymphadenopathy) forms, upon skin inoculation after animal contact (most often lagomorphs or small rodents) or by arthropod bite; the oculoglandular (conjunctivitis) form after conjunctival inoculation; the oropharyngeal (sore throat) form upon ingestion of contaminated water or food [14]; and 2 systemic diseases, including the pneumonic (pneumonia) and the typhoidal forms (mimicking symptoms of typhoid). Frequents complications of lymphadenopathies are suppurations and even skin fistulas. Other complications such as sepsis or meningitis [15] are more rarely reported and usually occur in patients with comorbidities and/or immunosuppression.
Current knowledge of tularemia comes from data reported in highly endemic areas such as North America [11, 16] and Northern Europe [10]. In contrast, few data exist for Western Europe [17–19].
In this study, we present the epidemiological, clinical, diagnostic, and treatment data of 177 patients diagnosed with tularemia in the French reference center for rickettsioses, coxiellosis, and bartonelloses in Marseille, France, from January 1, 2008 to December 31, 2017.
METHODS
Definition of Tularemia Cases
All patients with a suspected diagnosis of tularemia were considered to be confirmed when they exhibited compatible clinical findings and at least (1) a positive real-time polymerase chain reaction (RT-PCR) and/or 16S ribosomal ribonucleic acid (rRNA) PCR for F tularensis, (2) seroconversion, or (3) a 4-fold increase in immunofluorescence serological titers, as previously described [20]. Culture was carried out only on PCR-positive samples. Patients with compatible clinical findings and a single positive serological test were considered probable cases.
Patients and Clinical Samples
The present study is a retrospective analysis of epidemiological and clinical data from patients with tularemia. Data had been prospectively collected since 2008 using an anonymized and standardized questionnaire and stored on a secured computer by the French reference center for tularemia.
Patient Consent Statement
The present study was validated by the Ethics Committee of the “Institut Hospitalo Universitaire” (IHU) Méditerranée Infection under reference 2017-029. The consent form to be signed by patients was sent to the clinicians, who obtained the signature before transmitting the data.
Mode of Transmission
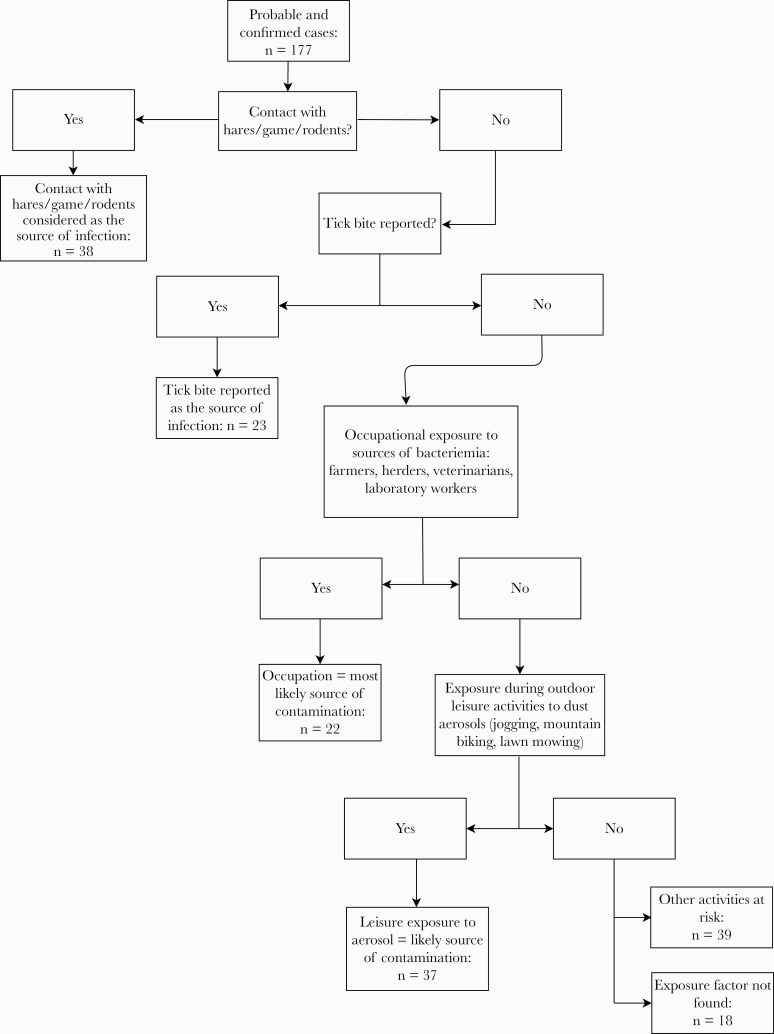
We assigned the most likely source of contamination according to the following scheme already used by Maille and Vaillant [18]: (1) any patient infected with F tularensis who reported direct contact with hares during the month before the onset of symptoms would have been infected by this exposure; (2) a tick bite reported during the month before the onset of symptoms would be the route of contamination for any patient unless the patient also reported direct contact with hares; (3) an occupational exposure during the month before the onset of symptoms would constitute a circumstance of contamination unless the patient also reported direct contact with hares or a tick bite; (4) recreational activities resulting in exposure to aerosols or dust in the forest during the month before the onset of symptoms would be circumstances of contamination unless the patient reported direct contact with hares, tick bite, or occupational exposure.
Laboratory Diagnosis
Serology was performed using indirect immunofluorescence. Immunoglobulin G and M titers were measured. For this purpose, a formalin-inactivated antigen prepared in-house from a biovar I strain of F tularensis subspecies holarctica was used as previously described [21].
When lymph node biopsy specimens were available, a F tularensis-specific RT-PCR assay targeting the yqaB gene (Ftul0541F, Ftul0541R, and Ftul0541P) was also used for the detection of the bacterium, as previously described [21]. A patient with hip arthritis was detected positive using a broad-range PCR assay followed by sequencing the 16S RNA gene with the fD1 and rP2 primers was used [21].
Outcome
Therapeutic success was defined as the resolution of symptoms (fever, abscess) 1 month after appropriate antibiotics (fluoroquinolone, tetracycline, or aminoglycoside) had been discontinued. We considered therapeutic failure to be the persistence of symptoms and/or the occurrence of complication(s) despite appropriate antibiotic.
Surgery was performed either as a diagnostic and/or a curative procedure. Diagnostic surgery was performed in patients presenting with enlarged lymph nodes of unknown or uncertain etiology. Curative surgery was performed in patients with a complicated form such as abscess, prosthesis infection, or aortitis.
Statistics
The Fisher’s exact test was used to compare the number of therapeutic failures with doxycycline and fluoroquinolones and to assess the correlation between immunosuppression and the need for surgical treatment as well as the correlation between immunosuppression and the occurrence of therapeutic failure. An analysis of variance test was carried out to investigate statistical correlations between the occurrence of therapeutic failure or the need for surgical treatment and the time between the onset of symptoms and effective antibiotic therapy.
RESULTS
Confirmed and Probable Cases
A total of 251 patients were included. For 68 patients, the laboratory criteria were not fulfilled. Another 6 patients that had been infected abroad were excluded. Overall, 89 confirmed and 88 probable cases (total 177 patients) were included in the study (Supplementary Figure 1).
Epidemiological Data
The male/female sex ratio was 2.28 (54 female and 123 male). The mean age of patients was 47.38 ± 17.8 years (range, 2 to 89 years).
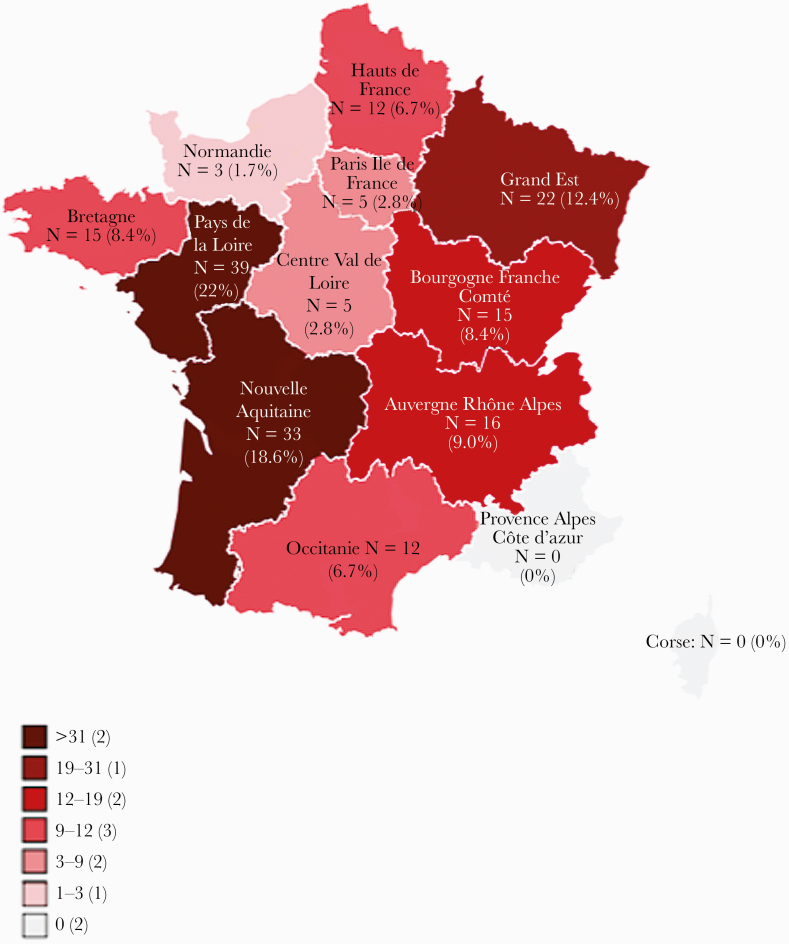
A total of 173 cases were sporadic, and 4 cases were clustered in 2 groups: 2 were household cases, and 2 were patients infected during an orientation race in a forest. The geographical distribution of infected patients is depicted in Figure 1.
Figure 1. .
Geographical distribution of tularemia cases.
Exposure factors are detailed in Figure 2. The most common risk factor was a contact with hares, game (including boar, deer, and doe), or rodents (21.5%), followed by exposure to aerosols (20.9%). No specific exposure factor was identified in 18 patients. Contact with hares, game, or rodents occurred more often in the winter season, whereas tick bites were mostly reported in summer (Supplementary Figure 2).
Figure 2. .
Source of exposure (game include boar, deer, and doe).
Clinical Data
Significant Background
Thirteen of 142 patients (9.15%) were immunocompromised: 8 patients had diabetes (one type I and 7 type II). Four patients were on immunosuppressive therapy: 1 on mycophenolate mofetil, 1 on methotrexate, and 2 on antitumor necrosis factor α. The 13th patient suffered from liver cirrhosis. One patient had a vascular prosthesis (Tables 1 and 2).
Table 1. .
Comparison of the Data in Our Series With 2 Other French Series
| Data | Maurin et al [19] | Mailles et al [18] | Our Series |
|---|---|---|---|
| Number of patients included | 101 | 433 | 177 |
| Mean age | 51.7 | 49 | 47.38 |
| Sex ratio | 1.2 | 1.8 | 2.28 |
| Hunters | 16 (15.8%) | 52 (12%) | 39 (21.7) |
| Incubation time (days) | 5.5 | NR | 9.28 |
| Glandular forms | 24 (23.7%) | 200 (46%) | 48 (27.1%) |
| Ulceroglandular forms | 34 (33.7%) | 113 (26%) | 61 (34.5%) |
| Pleuropulmonary forms | 10 (10%) | 42 (10%) | 32 (18%) |
| Oropharyngeal forms | 17 (16.8%) | 25 (6%) | 9 (5.0%) |
| Oculoglandular forms | 4 (4%) | 8 (2%) | 4 (2.3%) |
| Typhoidal forms | 9 (8.9%) | 45 (10%) | 14 (7.9%) |
| Hospitalization | 30 (29.7%) | 188 (43%) | 78 (53.4%) |
| Complications | NR | 20 | 60 (40.8) |
| Death | 1 | 2 | 0 |
| Confirmed cases | 94 | 130 | 89 |
| Strain isolation | 16 | 30 | 0 |
| PCR | 39 | 75 | 78 |
| Serologies (seroconversion, titer ×4) | 39 | 11 | 11 |
| Probable cases | 7 | 303 | 88 |
| Cases in common with our series | 3 | 63 |
Abbreviations: NR, not reported; PCR, polymerase chain reaction.
Table 2. .
Clinical Data of 177 Patients in France (2008–2017): Comparison With Data From 5 Previously Reported Case Seriesa
| Data | Ref [11] | Ref [8] | Ref [22] | Ref [7] | Ref [19] | Our Study |
|---|---|---|---|---|---|---|
| Country | USA | Sweden | Turkey | Spain | France | France |
| Number of patients (M/F) | 88 (3.5) | 234 (1.05) | 205 (0.55) | 142 (0.59) | 101 (1.37) | 177 (2.28) |
| Study period | 1949–1979 | 2000–2004 | 1988–1998 | 1997–1998 | 2006–2010 | 2008–2017 |
| Age ranges (means) | 2–82 (NI) | 1–88 (48.3) | 5–75 (NI) | 14–82 (52) | 5–95 (52) | 2–89 (47.4) |
| Main sources of infection | Hares | Mosquitos | Water | Hares | Hares | Hares |
| Fever | 85 | 83 | 66 | 90.8 | 67.3 | 85.0 |
| Headache | 45 | 32 | 15 | 9.9 | NA | 13.4 |
| Myalgia | 31 | NA | 6 | 25.4 | 20.8 | 25.5 |
| Arthralgia | 15 | NA | NA | 14.8 | 16.8 | 21.2 |
| Cough | 38 | 25 | NA | 24 | 5.9 | 9.2 |
| Sore throat | 15 | 3 | 58.5 | 16.9 | 15.8 | 7.0 |
| Nausea and/or vomiting | 17 | 23 | NA | 24 | 5.9 | 2.8 |
| Diarrhea | 10 | 9 | NA | 2.1 | 3.9 | 6.4 |
| Inoculation lesion | 59 | 88 | NA | 61.4 | 31.7 | 35.6 |
| Secondary dermatological lesion | 5.7 | 29.9 | 14 | 17.6 | 2.9 | 11.9 |
| Lymphadenopathy | 86 | 91.9 | 85 | 69.7 | 69.3 | 87.8 |
| Pharyngitis | 23.8 | NA | 29 | 16.2 | 19.8 | 4.2 |
| Conjunctivitis | NA | NA | 2 | NA | 2.9 | 3.5 |
| Hepatosplenomegaly | 16 | NA | 2 | NA | 2.9 | 5.6 |
| Worsening of condition | NA | NA | NA | 38.7 | 3.9 | 33.8 |
Abbreviations: M/F, male/female sex ratio; NA, no data available; Ref, reference.
aAll data are given as percentages unless otherwise specified.
The average incubation time of tularemia, calculated from 49 patients’ data, was 9.28 ± 9.7 days (range, 1 to 43 days). Supplementary Figure 3 represents the distribution of the incubation times, which mostly ranged from 1 to 5 days. In our series, 7 patients exhibited unusually long incubation delays, ranging from 16 to 43 days.
Clinical Forms
Sixty-one (34.5%), 48 (27.1%), 32 (18%), 14 (7.9%), 9 (5.0%), and 4 (2.3%) patients presented with an ulceroglandular, glandular, pleuropulmonary, typhoidal, oropharyngeal, or oculoglandular forms, respectively. We identified 9 (5.1%) atypical forms, which are presented in Supplementary Tables 1 and 2: a combined oculoglandular and ulceroglandular form, a prethyroid abscess, an ulceroglandular form associated with aortitis, a pleuropulmonary form associated with aortitis, a pleuropulmonary form associated with myocarditis, an aortic endocarditis, a total hip prosthesis infection, a pleuropulmonary form complicated by a splenic hematoma, and a pleuropulmonary form complicated by pericarditis.
The hip prosthesis infection occurred in a 49-year-old immunocompromised patient with cirrhosis. While drunk and walking outside, this male patient fell and suffered both femoral neck fracture and skin lacerations. He had a total hip replacement. In addition to hepatorenal decompensation of his cirrhosis with hepatic encephalopathy, the immediate aftermath of surgery was complicated by a hematoma of the hip that had to be surgically drained. 16S rRNA PCR testing of a hematoma sample revealed the presence of F tularensis. No culture was performed. The evolution of the illness was favorable with a 3-month treatment with doxycycline followed by a 1-stage exchange arthroplasty and additional antibiotic therapy with ciprofloxacin and gentamicin for 3 months.
The case of splenic hematoma occurred in a 73-year-old male with a pleuropulmonary form of tularemia. This non-immunocompromised patient had a history of pericarditis, hypothyroidism, and coronary stent placement. The diagnosis of tularemia was confirmed by PCR detection of F tularensis deoxyribonucleic acid in a mediastinal lymph node. A spontaneous splenic hematoma developed during hospitalization for tularemia as a complication of a moderate splenomegaly. The evolution of the illness was favorable after 21 days of doxycycline treatment.
We also diagnosed tularemia in a patient who developed a prethyroid lodge abscess without having any evident history of exposure to F tularensis. While performing a mediastinoscopy for a mediastinal lymph node biopsy, the surgeon unexpectedly drained an abscess from the prethyroid chamber. The diagnosis of tularemia was made by F tularensis-specific PCR from the abscess pus. The evolution of the illness was favorable after surgical drainage and doxycycline therapy.
Symptoms
The patients’ symptoms are summarized in Table 2. The main symptoms included fever (85.0%), general impairment (33.3%), chills (26.4%), myalgia (25.5%), sweats (25.0%), and arthralgia (21.2%).
Location of Lymphadenopathies
The upper limbs were the most frequent location, in 56 patients (31.6%), followed by the chest (46, 30.0%), lower limbs (38, 21.4%), neck (31, 17.5%), and abdomen (2, 1.1%). Twenty-nine patients (16.4%) had enlarged nodes in various anatomical sites.
Inoculation Skin Lesions
Twenty-nine patients (17.0%) exhibited an inoculation lesion on the upper limbs, 23 (13.5%) on the lower limbs, 5 (2.9%) on the trunk, 3 (1.7%) on the scalp, and 1 (0.6%) patient had both upper limb and facial ulcers.
Secondary Dermatological Lesions (All Dermatological Manifestations After the Initial Inoculation Ulcer)
Nineteen patients had secondary dermatological lesions, including 7 with lymphangitis ranging from the primary skin lesion to an adenopathy, 5 had a rash (location and type not specified), 3 had nodosum, 1 had cellulitis, 1 had erythema multiforme, 1 had pustules, and 1 had livedo reticularis.
Microbiological Results
Initial Presumed Diagnosis
For 67 of 148 patients (45.2%) for whom a suspected diagnosis was specified, tularemia was the main diagnostic request. In the other cases, the main suspected etiologies were as follows: cat scratch disease (44, 27.7%), Q fever (19, 12.8%), rickettsiosis (12, 7.9%), Whipple’s disease (3, 2.0%), ehrlichiosis (2, 1.3%), and anaplasmosis (1, 0.7%).
Confirmation of Tularemia Diagnosis
Six cases (6.9%) were confirmed by seroconversion and 5 (5.8%) by a 4-fold rise in antibody titers between 2 serum samples. Confirmed diagnosis of tularemia was obtained in 78 patients (86%) by PCR testing of lymph node (64), skin (6), lung (3) or aorta biopsies (2), bronchoalveolar lavage (1), and joint (1) fluid. In addition, F tularensis was identified by 16S rRNA PCR sequencing of a bacterial strain that could not be routinely identified. Cultures were performed for 59 of the 78 PCR-proven specimens, and 6 (1%) were positive.
Blood Tests
Blood test results are summarized in Supplementary Table 3. Twenty-two of 91 (24.1%) patients had leukocytosis, 1.1% had leukopenia, 31.3% had liver cytolysis, 6.7% had thrombocytopenia, and 4.0% had thrombocytosis. The mean C-reactive protein rate was 81.6 ± 60.5 mg/L (range, 4 to 248).
Evolution
Seventy-eight (53.4%) patients were hospitalized. Sixty (40.5%) developed complications, including lymph node abscesses (39), fistulizations (14), persistent asthenia (2), aortitis (2), and 1 case each of myocarditis, pericarditis, and splenic hematoma.
Antibiotic Therapy
One hundred seventy-two patients received an antibiotic therapy active against F tularensis, including 118 who received 1 antibiotic. The most commonly prescribed antibiotics were doxycycline (106 patients) and an oral fluoroquinolone (42 patients). Two patients were administered a fluoroquinolone and doxycycline. Table 3 and Supplementary Table 4 specify the effectiveness of treatments known to be active against tularemia. The mean duration of antibiotic therapy was 16 (minimum 7, maximum 28, standard deviation [SD] 4.5) days for doxycycline and 21 (minimum 7, maximum 84, SD 18.3) days for fluoroquinolones. There were more therapeutic failures for fluoroquinolones (ciprofloxacin, levofloxacin, ofloxacin, and unspecified fluoroquinolones, 14 of 43 [33%]) than for doxycycline (9 of 91 [9.9%], P = .02). The mean duration between the onset of symptoms and the onset of effective antibiotics was 38.6 days (range, 5 to 71; SD 16.7) for patients who presented with a therapeutic failure versus 42.5 days (range, 1 to 175; SD 33.4) for patients who recovered (P = .64).
Table 3. .
Antibiotic Therapy Administred and Therapeutic Failure Rates
| Antibiotic Therapya | Total Number of Patients on Antibiotic Therapy | |
|---|---|---|
| N (%) | TF/* (%) | |
| Doxycycline, 200 mg/d | 104 (59) | 9 /91 (9.9) |
| Ciprofloxacin, 500 mg ×2/d | 21 (12) | 7/21 (33) |
| Unspecified fluoroquinolone and dosage | 11(6.2) | 4/9 (44) |
| Ofloxacin, 200 mg ×2/d | 5 (2.8) | 1/5 (20) |
| Levofloxacin, 500 mg/d | 4 (2.3) | 2/4 (50) |
| Doxycycline, 200 mg/d + IV amikacin | 2 (1.1) | 0/2 (0) |
| Ciprofloxacin, 750 mg ×2/d + IV gentamicin + doxycycline, 200 mg/d | 1 (0.56) | 0/1 (0) |
| Unspecified fluoroquinolone + IV gentamicin | 1 (0.60) | 0 (0) |
| Levofloxacin, 500 mg ×2/d + doxycycline, 200 mg/d | 1 (0.60) | 0/1 (0) |
| Ofloxacin, 400 mg ×2/d | 1 (0.60) | 0/1 (0) |
| IV amikacin | 1 (0.60) | 0/1 (0) |
| IV gentamicin | 1 (0.60) | 0/1 (0) |
| No antibiotic active on Francisella tularensis | 13 (7.3) | 0/12 (0) |
| No data | 11 (6.2) | 0/1 (0) |
Abbreviations: IV, intravenous; mg/d, milligram/day; N, number of patients; TF, therapeutic failure; ×2/d, 2 times per day.
aIf not specified, antibiotics were administred orally.
*, Number of patients for whom data are available.
In contrast, 13 patients recovered without any effective antibiotic therapy, including 4 who benefited from lymph node excision, 6 who received an ineffective empirical antibiotic treatment, and 3 who neither received antibiotics nor underwent surgery. Their characteristics are specified in Supplementary Table 5. None of the 13 patients was immunocompromised. There were 4 pleuropulmonary forms, 3 oropharyngeal forms, 3 glandular forms, 2 typhoidal forms, and 1 oculoglandular form.
Surgery
Eighty patients (51.6%) underwent surgery: 31 (20%) had a lymph node puncture, 47 had lymph node removal (30.3%), 1 (0.6%) had an aortic allograft, and 1 (0.6%) had a total hip prosthesis replacement (Supplementary Table 4).
Thirty-four patients (21.9%) underwent surgery before effective antibiotic therapy, 17 patients (10.9) underwent surgery after effective antibiotic therapy, and 8 patients (5.1%) underwent surgery while undergoing antibiotic therapy. For 21 patients, the date of surgery was unknown. Data about surgery were not available for 22 patients.
For patients who underwent surgery, the mean delay between onset of symptoms and effective antibiotic therapy was longer (52.1 days, range 7 to 175, SD 37.9) than for those who did not have surgery (mean 32.1 days, range 1 to 87, SD 19.5, P = .006). In immunocompromised patients, 5 of 13 patients (38.5%) benefited from surgery, compared with 21 of 127 (16.6%) in immunocompetent patients (P = .06).
DISCUSSION
We describe 177 cases of tularemia diagnosed in the French reference center for rickettsioses, coxiellosis, and bartonelloses from January 1, 2008 to December 31, 2017. During this period, the French reference center for tularemia in Grenoble recorded 317 cases and Santé Publique France recorded 611 cases. Among our 177 cases, 3 were previously described in a published series by the French reference center for tularemia [19] and 44 cases were included in a study published by Santé Publique France, the French national public health agency [18]. Table 1 compares these 3 French series.
The most affected regions were “Nouvelle Aquitaine,” “Pays de la Loire”, and “Grand Est” (Figure 1), as previously described [18, 19]. The annual distribution of cases is similar to that described in the literature [19]: tick-borne contamination occurred more often in summer, whereas contamination during hunting or contact with infected animals occurred more often in winter. The mean incubation time was 9.28 days, which is longer than that usually described (3 to 5 days [16]). As expected, the most common forms were ulceroglandular (34.5%) and glandular (27.1%). These forms had a significantly higher therapeutic failure rate than the other forms (19.2% versus 7.6%, P < 10–2). We also diagnosed rare forms (Supplementary Tables 1 and 4), including 1 case each of hip prosthesis infection, splenic hematoma, and prethyroid lodge abscess. To date, few cases of joint prosthetic infections, affecting the knees [23, 24] or hips, [25] have been reported. We assume that the hip prosthesis infection complicated a skin wound contamination with F tularensis. In contrast, we could not find any previous case of spontaneous splenic hematoma complicating a splenomegaly, or prethyroid abscess, in the course of tularemia. However, a case of parapharyngeal abscess was reported in the literature [26].
In addition, we diagnosed 2 cases of F tularensis aortitis, 1 of which had previously been published [27], and 1 case of pericarditis complicating a pleuropulmonary form. Other cases of pericarditis have been described in the literature [28], including 1 previously diagnosed in our laboratory in 2007 [29]. We also diagnosed a case of F tularensis aortic endocarditis [30]. Only 4 confirmed cases of F tularensis endocarditis have been described to date [30–32]. We also described a probable case of myocarditis complicating a pleuropulmonary form. Indeed, the diagnosis was made on the basis of positive serology and histological findings observed in a mediastinal lymphadenopathy biopsy. To date, only 2 cases of F tularensis myocarditis have been described in the literature [33, 34].
Seventy-eight of the 177 patients (53.4%) required hospitalization. The clinical signs exhibited by patients were not specific (Table 2). In our series, the majority of patients had fever (85%), lymphadenopathy (72.2%), and inoculation lesions (36%). During our study, we did not find a statistical relationship between the time to introduce effective treatment and the occurrence of therapeutic failures. The diagnosis was obtained in 99 patients (56%) by serology and in 78 patients (44%) by PCR.
The antibiotic that was most commonly used in our series was doxycycline (administered 106 times as monotherapy), followed by a fluoroquinolone alone (42 patients). In our series, the observed therapeutic failure rates for doxycycline and fluoroquinolones (9.9% and 32.5%, respectively) were high. It was previously demonstrated that the earlier effective antibiotic therapy is administered to tularemia patients, the less often therapeutic failure occurs [35, 36]. We observed long delays before antibiotic therapy onset, which may explain such high therapeutic failure rates. However, the therapeutic failure rate was significantly higher for fluoroquinolones (P = .002), which are currently recommended with doxycycline as first-line treatment in the less severe cases [37] by the World Health Organization [38]. In their series, Rojas-Moreno [39] described in their retrospective analysis of 17 patients no case of therapeutic failure under doxycycline for a mean duration of 21 days in addition to surgical treatment. Pérez-Castrillón [7] et al and Eliasson et al [10] both described more therapeutic failures with doxycycline than with fluoroquinolones (42.8% and 3% therapeutic failures with doxycycline, respectively, and 4.5% and no therapeutic failure with fluoroquinolones). In our series, among the 42 patients on fluoroquinolone alone, only 11 patients received ciprofloxacin 500 mg 2 times per day (Table 3). We acknowledge the fact that the high failure rate observed for fluoroquinolones may at least in part be explained by the heterogeneity in molecule, dosage, and treatment duration used.
Due to its clinical and pathological presentation, tularemia can be confused with cat scratch disease or tuberculosis [40, 41]. In our series, the suspected diagnosis by clinicians was tularemia in only 67 patients (45.2%) and Bartonella infection in 44 patients (27.7%). Our data suggest that tularemia is probably underdiagnosed in France due to its unspecific clinical presentation and insufficient knowledge of the disease by clinicians. We suggest that tularemia should systematically be considered among the differential diagnoses of enlarged lymph nodes or prolonged fever in patients with outdoor activities.
CONCLUSIONS
Tularemia is endemic in France in the Nouvelle Aquitaine, Grand Est, and Pays de la Loire regions. This infection is probably underdiagnosed. Although the ulceroglandular form was the most common, we identified rare forms of the disease such as splenic hematoma and osteoarticular infection. In endemic areas and in a consistent epidemiological context, diagnosing this disease allows optimized patient management.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the clinicians who provided the patients’ clinical informations.
Author contributions. A. D.-C. and P.-E. F. analyzed the data; A. D.-C. and P.-E. F. wrote the manuscript; F. D. performed the statistical analysis; A. H., M. M., T. G., G. M.-B., T. K., and J.-P. T. provided information on many patients; S. E. and D. R. critically revised the manuscript.
Financial support. The study was funded by the Mediterranee Infection Foundation and the French National Research Agency under Reference ANR-10-IAHU-03.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. McCoy Georges W, Chapin Charles W. Further observations on a plague-like disease of rodents with a preliminary note on the causative agent, bacterium tularense. J Infect Dis 1912; 10:61–72. [Google Scholar]
- 2. Petersen JM, Carlson JK, Dietrich G, et al. Multiple Francisella tularensis subspecies and clades, tularemia outbreak, Utah. Emerg Infect Dis 2008; 14:1928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chitadze N, Kuchuloria T, Clark DV, et al. Water-borne outbreak of oropharyngeal and glandular tularemia in Georgia: investigation and follow-up. Infection 2009; 37:514–21. [DOI] [PubMed] [Google Scholar]
- 4. Hauri AM, Hofstetter I, Seibold E, et al. Investigating an airborne tularemia outbreak, Germany. Emerg Infect Dis 2010; 16:238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantardjiev T, Ivanov I, Velinov T, et al. Tularemia outbreak, Bulgaria, 1997–2005. Emerg Infect Dis 2006; 12:678–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reintjes R, Dedushaj I, Gjini A, et al. Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg Infect Dis 2002; 8:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pérez-Castrillón JL, Bachiller-Luque P, Martín-Luquero M, et al. Tularemia epidemic in northwestern Spain: clinical description and therapeutic response. Clin Infect Dis 2001; 33:573–6. [DOI] [PubMed] [Google Scholar]
- 8. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am 2006; 20:289–311, ix. [DOI] [PubMed] [Google Scholar]
- 9. Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev 2002; 15:631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eliasson H, Bäck E. Tularaemia in an emergent area in Sweden: an analysis of 234 cases in five years. Scand J Infect Dis 2007; 39:880–9. [DOI] [PubMed] [Google Scholar]
- 11. Evans ME, Gregory DW, Schaffner W, McGee ZA. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 1985; 64:251–69. [PubMed] [Google Scholar]
- 12. Helvaci S, Gedikoğlu S, Akalin H, Oral HB. Tularemia in Bursa, Turkey: 205 cases in ten years. Eur J Epidemiol 2000; 16:271–6. [DOI] [PubMed] [Google Scholar]
- 13. Feldman KA, Enscore RE, Lathrop SL, et al. An outbreak of primary pneumonic tularemia on Martha’s Vineyard. N Engl J Med 2001; 345:1601–6. [DOI] [PubMed] [Google Scholar]
- 14. Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis 2016; 16:113–24. [DOI] [PubMed] [Google Scholar]
- 15. Gangat N. Cerebral abscesses complicating tularemia meningitis. Scand J Infect Dis 2007; 39:258–61. [DOI] [PubMed] [Google Scholar]
- 16. Matyas BT, Nieder HS, Telford SR 3rd. Pneumonic tularemia on Martha’s Vineyard: clinical, epidemiologic, and ecological characteristics. Ann N Y Acad Sci 2007; 1105:351–77. [DOI] [PubMed] [Google Scholar]
- 17. Pilo P, Johansson A, Frey J. Identification of Francisella tularensis cluster in central and western Europe. Emerg Infect Dis 2009; 15:2049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mailles A, Vaillant V. 10 years of surveillance of human tularaemia in France. Euro Surveill 2014; 19:20956. [DOI] [PubMed] [Google Scholar]
- 19. Maurin M, Pelloux I, Brion JP, et al. Human tularemia in France, 2006–2010. Clin Infect Dis 2011; 53:2006–10. [DOI] [PubMed] [Google Scholar]
- 20. Institut de Veille Sanitaire (InVS). [Tularémie: définition de cas. Saint-Maurice: InVS]. Available at: https://www.formulaires.service-public.fr/gf/cerfa_12214.do. Accessed 11 October 2020.
- 21. Gouriet F, Levy PY, Samson L, et al. Comparison of the new InoDiag automated fluorescence multiplexed antigen microarray to the reference technique in the serodiagnosis of atypical bacterial pneumonia. Clin Microbiol Infect 2008; 14:1119–27. [DOI] [PubMed] [Google Scholar]
- 22. Angelakis E, Roux V, Raoult D, Rolain JM. Real-time PCR strategy and detection of bacterial agents of lymphadenitis. Eur J Clin Microbiol Infect Dis 2009; 28:1363–8. [DOI] [PubMed] [Google Scholar]
- 23. Cooper CL, Van Caeseele P, Canvin J, Nicolle LE. Chronic prosthetic device infection with Francisella tularensis. Clin Infect Dis 1999; 29:1589–91. [DOI] [PubMed] [Google Scholar]
- 24. Chrdle A, Trnka T, Musil D, et al. Francisella tularensis periprosthetic joint infections diagnosed with growth in cultures. Am Soc Microbiol 2019; 57:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rawal H, Patel A, Moran M. Unusual case of prosthetic joint infection caused by Francisella Tularensis Case Reports 2017; 2017:bcr-2017-221258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koc S, Gürbüzler L, Yaman H, et al. Tularaemia presenting as parapharyngeal abscess: case presentation. J Laryngol Otol 2012; 126:535–7. [DOI] [PubMed] [Google Scholar]
- 27. Briere M, Kaladji A, Douane F, et al. Francisella tularensis aortitis. Infection 2016; 44:263–5. [DOI] [PubMed] [Google Scholar]
- 28. Adams CW. Tularemic pericarditis; report of two cases and review of literature. Dis Chest 1958; 34:632–9. [PubMed] [Google Scholar]
- 29. Landais C, Levy PY, Habib G, Raoult D. Pericardial effusion as the only manifestation of infection with Francisella tularensis: a case report. J Med Case Rep 2008; 2:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaci R, Alauzet C, Selton-Suty C, et al. Francisella tularensis endocarditis: two case reports and a literature review. Infect Dis (Lond) 2017; 49:128–31. [DOI] [PubMed] [Google Scholar]
- 31. Tancik CA, Dillaha JA. Francisella tularensis endocarditis. Clin Infect Dis 2000; 30:399–400. [DOI] [PubMed] [Google Scholar]
- 32. Salit IE, Liles WC, Smith C. Tularemia endocarditis from domestic pet exposure. Am J Med 2013; 126:e1. [DOI] [PubMed] [Google Scholar]
- 33. Franco S, Prieto JM, Balaguer I, et al. Infection due to Francisella tularensis, myocarditits ant dilated myocardiopathy. Enferm Infecc Microbiol Clin 2010; 28:752–3. [DOI] [PubMed] [Google Scholar]
- 34. Frischknecht M, Meier A, Mani B, et al. Tularemia : an experience of 13 cases including a rare myocarditis in a referral center in Eastern Switzerland (Central Europe) and a review of the literature. Infection 2019; 47:683–95. [DOI] [PubMed] [Google Scholar]
- 35. Meric M, Willke A, Finke EJ, et al. Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia. APMIS 2008; 116:66–73. [DOI] [PubMed] [Google Scholar]
- 36. Gozel MG, Engin A, Altuntas EE, et al. Evaluation of clinical and laboratory findings of pediatric and adult patients with oropharyngeal tularemia in Turkey: a combination of surgical drainage and antibiotic therapy increases treatment success. Jpn J Infect Dis 2014; 67:295–9. [DOI] [PubMed] [Google Scholar]
- 37. Caspar Y, Hennebique A, Maurin M. Antibiotic susceptibility of Francisella tularensis subsp. holarctica strains isolated from tularaemia patients in France between 2006 and 2016. J Antimicrob Chemother 2018; 73:687–91. [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization. WHO Guidelines on tularaemia. World Health Organization; 2007. Available at: https://apps.who.int/iris/handle/10665/43793. [Google Scholar]
- 39. Rojas-Moreno C, Bhartee H, Vasudevan A, et al. Tetracyclines for treatment of tularemia: a case series. Open Forum Infect Dis 2018; 5:ofy176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yildirim Ş, Turhan V, Karadenizli A, et al. Tuberculosis or tularemia? A molecular study in cervical lymphadenitis. Int J Infect Dis 2014; 18:47–51. [DOI] [PubMed] [Google Scholar]
- 41. Turhan V, Berber U, Haholu A, et al. Differential diagnosis of cervical lymphadenitis mimicking malignancy due to tularemia: our experiences. Indian J Pathol Microbiol 2013; 56:252–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.