Abstract
Previous research has shown that exposure to volatile anesthetics can induce acute neuroinflammation and neuroapoptopsis in neonatal rodents and that these events can lead to cognitive dysfunction at later stages. Isoflurane and sevoflurane are two of the most popular anesthetics used in the field of pediatrics. However, the relative impact of these two anesthetics on the developing brain at distinct time points after the induction of anesthesia has not been compared. In the present study, we exposed 7-day-old mice to clinically equivalent doses of isoflurane (1.5%) and sevoflurane (2.5%) for 4 h and then investigated consequential changes in the brains of these mice at six different time points. We analyzed the levels of proteins that are directly related to neuroapoptosis, neuroinflammation, synaptic function, and memory, in the brains of neonatal mice. Exposure of neonatal mice to isoflurane and sevoflurane resulted in acute neuronal apoptosis. Our analysis observed significant levels of neuroinflammation and changes in the expression levels of proteins associated with both synaptic transmission and memory in mice from the isoflurane group but not the sevoflurane group. Our results therefore indicate that isoflurane and sevoflurane induce differential effects in the brains of neonatal mice.
Subject terms: Developmental biology, Neuroscience, Molecular medicine
Introduction
Every year, millions of children are given general anesthesia so that they can undergo surgical procedures1, 2. A large retrospective study previously showed that children under the age of 4 years, who received repeated episodes of general anesthesia, were associated with an incidence of reading, writing, and mathematical learning disabilities, that was twice as high as those who had not been exposed to anesthesia3. Children exposed to anesthesia prior to 3 years-of-age were associated with a higher risk of deficits in language and abstract reasoning when they reached an age of 10 years4, 5. In addition, studies have revealed that the exposure of newborn animals to inhaled anesthetics can lead to a rapid and significant increase in acute neuroinflammation and neuroapoptosis, along with cognitive dysfunction at subsequent stages6–9. Therefore, serious concerns have been raised about the potential impacts of inhaled anesthetics on children. However, two large clinical trials, the General Anaesthesia and Awake-Regional Anaesthesia (GAS) trial and the Pediatric Anesthesia Neurodevelopment Assessment (PANDA) study, both showed that a single brief exposure to general anesthesia in early infancy did not affect neurodevelopmental outcomes during a given follow-up period10, 11. The GAS and PANDA studies involved neuropsychological tests that were performed at the ages of 5 and 10, respectively. However, cognitive and behavioral changes may not be obvious until later in life, as indicated by several preclinical studies8. Furthermore, the results derived from the GAS and PANDA studies cannot be extrapolated to conditions involving repeated or prolonged exposure to anesthesia. The potential neurotoxicity of anesthesia under these conditions has yet to be elucidated. It is likely that preclinical studies can provide a far better understanding of neurotoxicity relating to anesthesia.
Isoflurane and sevoflurane are two commonly used pediatric anesthetics. Therefore, it is of vital importance that we investigate their potential neurotoxicity. Both isoflurane and sevoflurane have been shown to induce neuroapoptosis in neonatal mice immediately after exposure12. Isoflurane has also been reported to induce greater levels of neuroapoptosis than sevoflurane in the brains of neonatal mice13. However, the short- and long-term effects of isoflurane and sevoflurane on apoptosis, inflammation, and synaptic protein levels, in the brains of newborn mice have yet to be investigated. Furthermore, very few studies have compared the effects of isoflurane and sevoflurane on the brains of neonatal mice at different time points following anesthesia, particularly with regards to differences over the long-term. Therefore, this study intended to investigate the relative effects of isoflurane and sevoflurane on apoptosis, neuroinflammation, and the synaptic levels of key proteins associated with memory, at different time points (from 0 h to 28 days) in the brains of postnatal day 7 mice mouse brain following 4 h of anesthesia.
Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines for the ethical treatment of animals. Every effort was made to minimize the number of animals used in the experiments. Breeding pairs of C57BL6/J mice were obtained from the SLAC Laboratory Animal Company (Shanghai, China) and housed on a 12:12 light/dark cycle with ad libitum access to food and water. All experiments included both male and female pups; the ratio of males to females was 3:2.
Exposure to anesthetics
On postnatal day 7 (P7), littermate mouse pups were randomly divided into three groups: (1) 4 h exposure to 100% oxygen (control); (2) 4 h exposure to 1.5% isoflurane in 100% oxygen; and (3) 4 h exposure of 2.5% sevoflurane in 100% oxygen. Groups of mice were exposed to the different treatments in specialized chambers. These doses represented 1 minimum alveolar concentration (MAC) of the two anesthetics, and had been validated in a pilot study, as described previously9. During anesthesia, we used a warming blanket to maintain rectal temperature at 38 ± 1 °C. Pups were allowed to wake up and recover on the warming blanket before they were returned to cages with their own mothers. None of the mice died during anesthesia. We sacrificed five mice from each group at 0 h, 4 h, 24 h, 7 days, 14 days, and 28 days, after exposure to anesthetic. We then removed the brains from these mice and snap-froze the cerebral cortices and hippocampi from the left hemisphere in dry ice; the samples were then stored at − 80 °C to await analysis. The right hemispheres were fixed with 4% paraformaldehyde, post-fixed overnight at 4 °C, and then dehydrated in 30% sucrose. Sagittal sections (30 µm thick) were then prepared on a cryostat and stored in glycol anti-freeze solution (glycerol and ethylene glycol in 0.1 M phosphate buffered saline [PBS]) at − 20 °C to await analysis.
Western blotting
Frozen brain tissues were homogenized in ice-cold buffer with protease inhibitors containing 50 mM Tris–HCl (pH 7.4), 2.0 mM EGTA, 2 mM Na3VO4, 50 mM NaF, 20 mM β-glycerophosphate, 0.5 mM AEBSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 4 μg/ml pepstatin A. Extracted total protein was then separated by gel electrophoresis and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Following transfer, membranes were blocked in 5% skimmed milk in 0.1% tris-buffered saline/Tween-20 (TBST) for 1 h, and then incubated overnight with primary antibodies at 4 °C. The following morning, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Positive antibody binding was then visualized by the ECL-PLUS system with enhanced chemiluminescent (ECL, Pierce, and Rockford, USA). Signal intensity was then analyzed with Image Lab (BIO-RAD, Hercules, CA).
Enzyme-linked immunosorbent assays (ELISAs)
Frozen brain tissue was homogenized in ice-cold buffer with protease inhibitors. Brain homogenates were diluted (1:5) prior to ELISA and protein concentration was determined by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA). Levels of IL-1β, IL-6, and TNF-α, were then determined using specialized ELISA kits (Boster Biological Technology, Wuhan, China) in accordance with the manufacturer’s instructions.
Immunofluorescence staining
Free floating sections of brain tissue were incubated for 20 min with 0.3% Triton X-100 at room temperature, washed in PBS, and blocked with 5% normal goat serum in PBS and 0.1% Triton X-100 for 30 min. Sections were then incubated overnight with primary antibodies at 4 °C. The following morning, sections were incubated with an appropriate secondary antibody for 1 h in darkness. We used normal goat serum in the absence of primary antibody as a negative control. After several washes with PBS, the sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and then visualized using an epifluorescence microscope.
Antibodies and reagents
The primary antibodies used in this study are listed in Table 1. For western blotting, we purchased horseradish peroxide (HRP)-conjugated anti-mouse and anti-rabbit secondary antibodies from Invitrogen (Carlsbad, CA). For immunofluorescence, we acquired a goat anti-rabbit secondary antibody (Alexa Fluor 488, Life Technologies, Darmstadt, Germany) and a goat anti-mouse secondary antibody (Alexa Fluor 594, Life Technologies, Darmstadt, Germany). Other chemicals were purchased from Sigma (St. Louis, MO, USA).
Table 1.
The primary antibodies used in this study.
| Antibody | Source |
|---|---|
| Cleaved-caspase3 | Cell Signaling Technology, Danvers, MA |
| Caspase-3 | Cell Signaling Technology, Danvers, MA |
| Cleaved-PARP | Abcam, Cambridge, MA |
| PARP | Cell Signaling Technology, Danvers, MA |
| Caspase-9 | MBL, Woburn, MA |
| Caspase-12 | Cell Signaling Technology, Danvers, MA |
| IL-1β | Cell Signaling Technology, Danvers, MA |
| IL-6 | Cell Signaling Technology, Danvers, MA |
| PSD-95 | Cell Signaling Technology, Danvers, MA |
| Synaptophysin | Millipore, Temecula, CA, USA |
| Synapsin 1 | Santa Cruz Biotechnology, Santa Cruz, CA |
| PKA-Cα | Cell Signaling Technology, Danvers, MA |
| p-CREB (Ser-133) | Cell Signaling Technology, Danvers, MA |
| CREB | Cell Signaling Technology, Danvers, MA |
| GFAP | Millipore, Temecula, CA, USA |
| Iba1 | Wako Chemical, Richmond, VA, USA |
| β-actin | Hua’an, Hangzhou, China |
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). All comparisons between mice in the anesthesia group and mice in the control group were carried out with the Student’s t-test. P values < 0.05 were considered to be statistically significant.
Results
Neuronal apoptosis in neonatal mice following exposure to isoflurane or sevoflurane
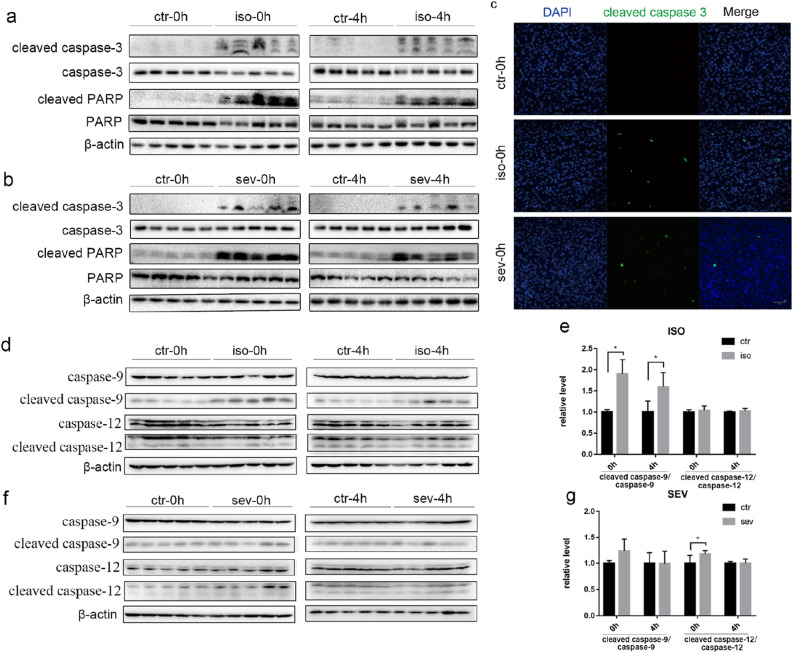
In order to evaluate the effect of isoflurane and sevoflurane on neuronal apoptosis, we investigated the activation of two markers of apoptosis, caspase-3 and poly ADP-ribose polymerase (PARP), in the brains of neonatal mice. Immediately after exposure to anesthetic for 4 h, we observed significantly increased expression levels of cleaved caspase-3 in mice from both the isoflurane and sevoflurane groups when compared with the control group (Fig. 1). The activation of caspase-3 lasted for at least 4 h. Similar changes were also observed for cleaved PARP in mice from both the isoflurane and sevoflurane groups (Fig. 1a,b). Immunofluorescence staining further confirmed that the number of activated caspase-3-positive cells was significantly increased in both of the anesthetic groups immediately after exposure (Fig. 1c). However, at 24 h, 7 days, 14 days, and 28 days, after exposure to anesthetic, we were unable to detect an apoptotic response in any of the groups, as evidenced by the absence of any positive signals for cleaved caspase-3 or cleaved PARP on western blotting.
Figure 1.
Neuronal Apoptosis in Neonatal Mice following Exposure to Isoflurane or Sevoflurane. (a, b, d, f) Brain homogenates from neonatal mice sacrificed at 0 h and 4 h after exposure to anesthesia were analyzed by western blotting using antibodies against cleaved caspase-3, caspase-3, cleaved PARP, PARP, cleaved caspase-9, caspase-9, cleaved caspase-12, caspase-12 and β-actin. Full-length blots are presented in Supplementary Fig. 3. (c) Representative immunofluorescence staining of the brain cortex from three groups immediately after treatment (scale bar, 50 μm). (e, g) Densitometric quantification of western blots after normalization to β-actin levels. Values are presented as mean ± SD. *P < 0.05 vs. Control. n = 5 per group. Ctr control, iso isoflurane, sev sevoflurane.
To explore the possible mechanisms underlying the anesthetic-induced apoptosis, we examined the activation of caspase-9, which plays an important part in the mitochondrial pathway of apoptosis14. The level of cleaved caspase-9 was significantly higher than that of the control group at 0 h and 4 h after exposure to isoflurane (Fig. 1d,e), indicating the involvement of mitochondrial pathway in isoflurane-induced apoptosis. However, there were no significant differences in the level of active caspase-9 between the sevoflurane and control group (Fig. 1f,g). We also checked the activation of caspase-12, which is a major mediator of endoplasmic reticulum (ER) stress-induced apoptosis15. The level of cleaved caspase-12 was higher than that of the control group at 0 h after exposure to sevoflurane (Fig. 1f,g), implying the involvement of ER stress in sevoflurane-induced apoptosis. However, no differences in cleaved caspase-12 were observed between the isoflurane and control group (Fig. 1d,e). These results suggest different apoptotic pathways involved in anesthetic-induced apoptosis.
Inflammatory responses in neonatal mice following exposure to isoflurane or sevoflurane
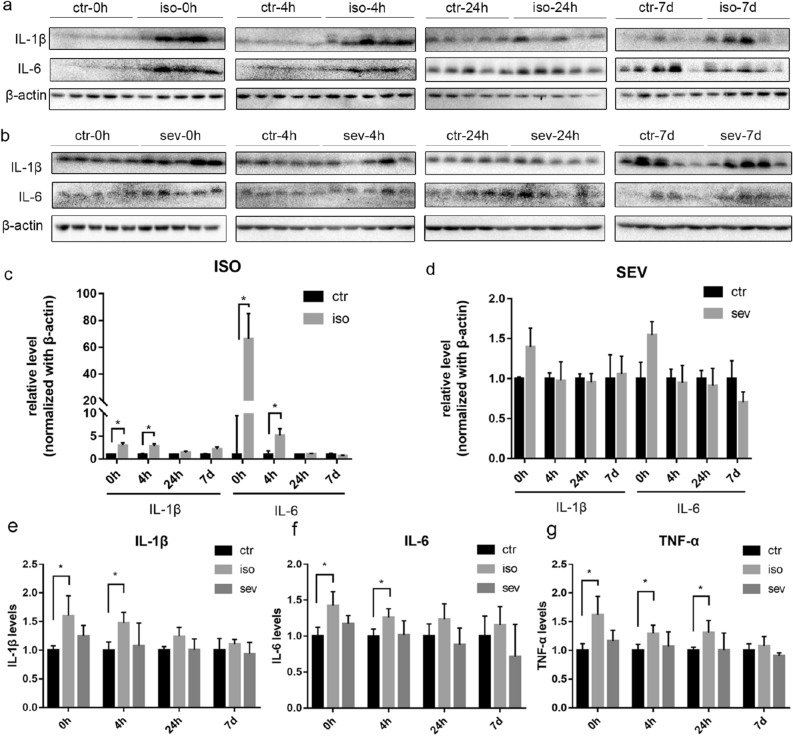
In order to investigate the effects of isoflurane and sevoflurane on neuroinflammation in neonatal mice, we determined the expression levels of several proinflammatory factors (IL-1β, IL-6, and TNF-α). Western blotting and ELISA both showed that the levels of IL-1β and IL-6 levels were significantly higher than the control group at 0 h and 4 h after exposure to isoflurane; these levels had returned to the normal range when tested at 24 h and 7 days after exposure. The levels of TNF-α were significantly higher than those of the control group at 0 h, 4 h, and 24 h, after exposure to isoflurane; levels of TNF-α had returned to the normal range when tested 7 days after exposure to isoflurane (Fig. 2). However, we failed to detect any significant increases with regards to the levels of IL-1β, IL-6, or TNF-α, at any time points after exposure to sevoflurane. We also investigated the activation of glial fibrillary acidic protein (GFAP) and ionized calcium binding adapter molecule 1 (Iba1); these are known markers of astroglia and microglia, respectively. No significant differences were detected between the isoflurane or sevoflurane groups when compared with the control group (Supplementary Fig. 1, 2).
Figure 2.
Inflammatory Responses in Neonatal Mice following Exposure to Isoflurane or Sevoflurane. (a, b) Brain homogenates from neonatal mice sacrificed at 0 h, 4 h, 24 h, and 7 days, after exposure to anesthesia were analyzed by western blotting with antibodies against IL-1β, IL-6, and β-actin. Full-length blots are presented in Supplementary Fig. 4. (c, d) Densitometric quantification of blots after normalization to β-actin level. The levels of IL-1β (e), IL-6 (f), and TNF-α (g), were investigated by ELISA. The relative levels of these proteins are shown after normalization to the control. Values are presented as mean ± SD. *P < 0.05 vs. Control. n = 5 per group. Ctr control, iso isoflurane, sev sevoflurane.
Differential changes in the synaptic proteins of neonatal mice exposed to isoflurane or sevoflurane
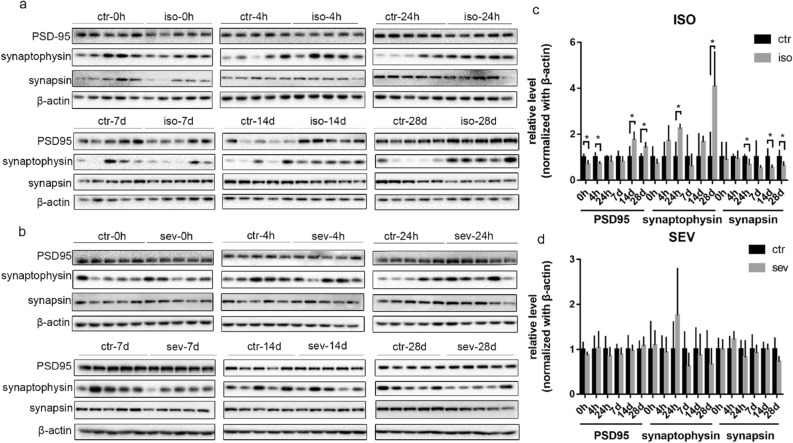
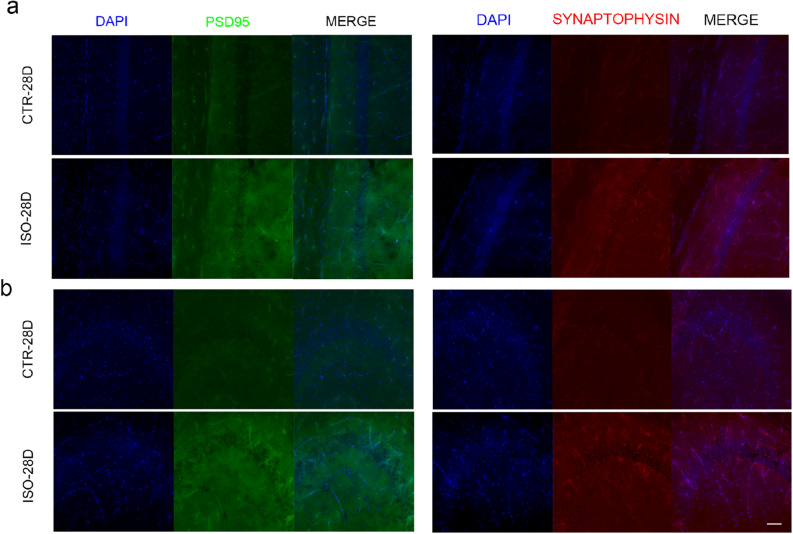
In order to investigate the effects of isoflurane and sevoflurane on the expression of synaptic proteins, we determined the levels of PSD-95, synaptophysin, and synapsin at several time points (0 h, 4 h, 24 h, 7 days, 14 days, and 28 days) after 4 h of anesthetic exposure; these are post-, pre-, and pre-synaptic markers, respectively. The levels of PSD-95 were significantly reduced at 0 h and 4 h after isoflurane exposure as compared to the control group, but were increased at 14 days and 28 days (Fig. 3). After exposure to isoflurane, the levels of synaptophysin remained unchanged at 0 h, 4 h, 7 days, and 14 days, but were higher than the control group at 24 h and 28 days after exposure. In contrast, the levels of synapsin were downregulated when tested 14 days and 28 days after exposure to isoflurane. The increased levels of PSD-95 and synaptophysin on day 28 after exposure to isoflurane were also confirmed by immunofluorescence analysis (Fig. 4). However, no significant changes were identified in the sevoflurane group with respect to the levels of any of the synaptic proteins tested.
Figure 3.
Differential changes in the Levels of Key Synaptic Proteins in Neonatal Mice Exposed to Isoflurane or Sevoflurane. (a, b) Brain homogenates from neonatal mice sacrificed at 0 h, 4 h, 24 h, 7 days, 14 days, and 28 days, after exposure to anesthesia were analyzed by western blotting using antibodies against PSD-95, synaptophysin, synapsin, and β-actin. Full-length blots are presented in Supplementary Fig. 5. (c, d) Densitometric quantification of western blots after normalization to β-actin levels. Values are presented as mean ± SD. *P < 0.05 vs. Control. n = 5 per group. Ctr control, iso isoflurane, sev sevoflurane.
Figure 4.
Immunofluorescence staining of PSD-95 and Synaptophysin in Neonatal Mice following Exposure to Isoflurane or Sevoflurane. Representative immunofluorescence staining for PSD-95 (green) and synaptophysin (red) in CA1 (a) and CA3 (b) of the hippocampus from the two groups on day 28 after exposure to anesthetics (scale bar, 50 μm). The density of PSD-95 and synaptophysin immunostaining in the hippocampus was greater in the isoflurane group than in the control group. Cell nuclei were stained with DAPI (blue). n = 5 per group. Ctr control, iso isoflurane, sev sevoflurane.
The expression of memory-associated proteins in the brains of neonatal mice exposed to isoflurane or sevoflurane
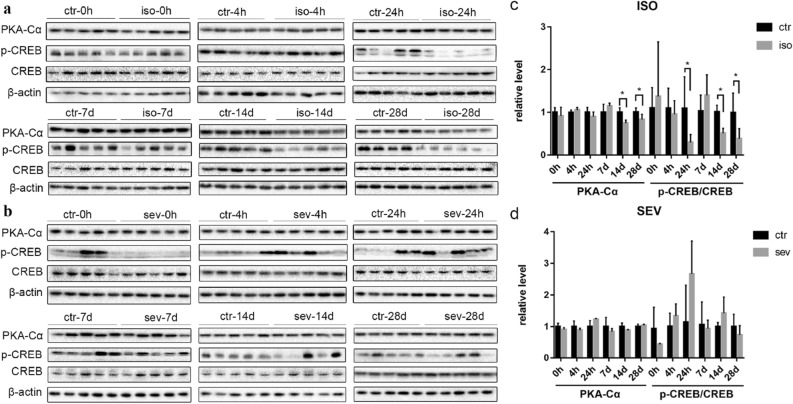
In order to investigate the effects of both volatile anesthetics on memory, we investigated the expression levels of PKA-Cα, the cAMP responsive element binding protein (CREB), and p-CREB, the activated form of CREB. There were no significant differences in the levels of PKA-Cα or p-CREB when compared between the isoflurane group and the control group (Fig. 5) at 0 h, 4 h, 24 h, or 7 days, after anesthesia. However, the levels of PKA-Cα in the isoflurane group were significantly lower than the control group on days 14 and 28 after exposure; these observations were also consistent with the reduced ratio of p-CREB to CREB at these two time points. In contrast, exposure to sevoflurane did not have any significant effect on the levels of any of these proteins at any of the time points.
Figure 5.
The Expression of Memory-Associated Proteins in Neonatal Mice Exposed to Isoflurane or Sevoflurane. (a, b) Brain homogenates from neonatal mice sacrificed at 0 h, 4 h, 24 h, 7 days, 14 days, and 28 days, after exposure to anesthetics were analyzed by western blotting using antibodies against PKA-Cα, p-CREB, CREB, and β-actin. Full-length blots are presented in Supplementary Fig. 6. (c, d) Densitometric quantification of western blots after normalization to β-actin levels. Values are presented as mean ± SD. * P < 0.05 vs. Control. n = 5 per group. Ctr control, iso isoflurane, sev sevoflurane.
Discussion
For many years, concerns have been raised over the potential for anesthetics to induce neurological abnormalities. Some recent clinical studies have indicated that a single, brief exposure to general anesthesia during infancy might not necessarily lead to neurodevelopmental disabilities10, 11. However, lengthy or repeated exposure to anesthetics may have a deleterious impact on brain development16. Preclinical studies could provide us with valuable mechanistic understanding of the neurotoxicity associated with anesthesia. In the present study, we investigated the effects of isoflurane and sevoflurane on neuroapoptopsis, neuroinflammation, synaptic proteins, and memory-associated proteins, at distinct time points after anesthesia. We observed a significant increase in neuroapoptosis at 0 h and 4 h after exposure to both isoflurane and sevoflurane. There was also an enhancement in neuroinflammation at 0 h and 4 h post-anesthesia in the isoflurane group but not in the sevoflurane group. We also observed significant changes in the levels of synaptic proteins at different time points in the isoflurane group; however, in the sevoflurane group, we only observed a reduction in the levels on day 28 post-anesthesia. Furthermore, the expression levels of p-CREB and PKA-Cα were reduced at 14 days and 28 days post-anesthesia in the isoflurane group but not in the sevoflurane group.
Neuronal apoptosis plays an indispensable role in brain development; however, the over-activation of this process could affect neural development and thus contribute to behavioral changes later in life. Several preclinical studies have consistently reported that inhaled anesthetics are associated with acute neuroapoptosis in the developing brain12, 17. In order to investigate whether isoflurane and sevoflurane exert differential impacts on neuroapoptosis, we determined the levels of several apoptosis markers at six different time points after anesthesia. We found that caspase-3, the key biomarker of apoptosis, was upregulated at 0 h and 4 h after exposure to both isoflurane and sevoflurane; these findings concurred with those published previously12, 17. Cleaved PARP is the main cleavage target of caspase-3. Consistently, we observed the upregulation of PARP at 0 h and 4 h after exposure to both isoflurane and sevoflurane. However, the upregulation of these two markers of apoptosis was not, detected by western blotting at 24 h after anesthesia, thus indicating that neuroapoptosis only occurred during the acute phase after anesthesia and subsequently returned to normal levels. Several previous preclinical studies have also reported that the inhalation of these two anesthetics can lead to neuronal apoptosis12, 17. Some studies have also indicated that isoflurane might induce more robust apoptosis than sevoflurane13, 17. In the current study, we did not compare the specific extent of neuroapoptosis; however, it is clear that apoptosis is a common phenomenon when neonatal mice are exposed to isoflurane and sevoflurane. Additionally, we found the activation of caspase-9 by isoflurane but not sevoflurane, indicating the involvement of mitochondrial apoptotic pathway in isoflurane-induced apoptosis. Procaspase-9 combines with cytochrome-c, which is released from the mitochondria, to form an apoptosome, resulting in the cleavage and activation of caspase-9. Cleaved caspase-9 then induced the activation of caspase-3, leading to apoptosis14. On the other hand, the activation of caspase-12 was observed in neonatal mice exposed to sevoflurane but not isoflurane. Excessive ER stress can trigger apoptosis through the activation of caspase-12, which is localized to ER, and is specifically activated by ER stress15. Active caspase-12 can activate caspase-3, independently of the activation of caspase-9, leading to apoptosis18. On the other hand, active caspase-12 can also trigger the activation of caspase-9, leading to the activation of caspase-315. Here, we found the activation of caspase-12 but not caspase-9 in mice exposed to sevoflurane, suggesting a caspase-9-independent manner in sevoflurane-induced ER stress. Collectively, the current study indicates that isoflurane and sevoflurane induced apoptosis through different mechanisms.
Neuroinflammation during early brain development is associated with a wide range of neuropsychiatric diseases, including autism, Alzheimer’s disease, and schizophrenia19. IL-6, IL-1β, and TNF-α, are the major proinflammatory cytokines and are known to influence neuronal differentiation, proliferation, and neurogenesis20. IL-6 has also been shown to be associated with spatial learning and memory impairment21. Therefore, we investigated the expression of IL-6, IL-1β, and TNF-α, in neonatal mice exposed to anesthetic. Following exposure to isoflurane, we found that mice exhibited transient increases in the levels of IL-1β, IL-6, and TNF-α. A previous study also showed that exposure to 1.8% isoflurane for 4 h induced an increase in the expression of proinflammatory cytokines (TNF-α and IL-1β) in the brains of P7 rats22. Surprisingly, we did not observe an increase in the levels of these proinflammatory cytokines at any time points in neonatal mice exposed to sevoflurane. Previous studies also found that a single exposure of neonatal mice to 3% sevoflurane for 2 h or 6 h did not increase the levels of IL-6 or/and TNF-α in the brain23, 24. On the other hand, however, repeated exposure (3% sevoflurane, 2 h daily for 3 days), led to a notable increase in the levels of IL-6, IL-1β, and TNF-α, in the brains of neonatal mice immediately after anesthesia23, 25, 26. This discrepancy may be related to the different doses of anesthetics used, thus indicating that repeated episodes of anesthesia are far more neurotoxic and that the neurotoxicity of sevoflurane in neonatal mice is both dose- and time-dependent. Collectively, our study indicates that isoflurane can cause acute neuroinflammation in the brain of neonatal mice while sevoflurane does not.
Synapsin and synaptophysin are synaptic vesicle proteins. Synapsin regulates the exocytosis of synaptic vesicles while synaptophysin regulates the endocytosis of synaptic vesicles. During synaptic development, these two proteins play key roles in the maturation of presynaptic function27. PSD-95 is important for the conversion of synaptic function from a silent state to a fully functional state28 and is also a good marker for post-synaptic development. Therefore, to investigate the effects of isoflurane and sevoflurane on synaptic function, we investigated the expression of synapsin, synaptophysin, and PSD-95, in neonatal mice. We found that the expression levels of PSD-95 and synaptophysin were increased in the brains of neonatal mice exposed to isoflurane, while the levels of synapsin were reduced. We did not observe any changes in the expression of synaptic proteins when neonatal mice were exposed to sevoflurane. Although a previous study reported the reduced expression of PSD-95 at P8 and P31 after multiple exposures to sevoflurane at P829, our current study suggests that a single exposure to sevoflurane during the early development may have little effect on the expression of synaptic proteins.
As an important nuclear protein, CREB is widely expressed in the hippocampus and cortex of rodents and plays an important role in synaptic formation, neurogenesis, learning, and memory30. The activation of CREB can lead to the transcription of gene products involved in synaptic plasticity and cognitive function, such as brain derived neurotrophic factor (BDNF), thereby enhancing memory formation. CREB is activated via the phosphorylation of Ser-133 by PKA-Cα31. In the present study, we observed reduced levels of PKA-Cα and p-CREB on days 14 and 28 after exposure to isoflurane. This observation is consistent with a previous study that reported the inactivation of CREB by isoflurane, thus resulting in the loss of dendritic outgrowth and the reduced expression of proteins that are essential for memory and cognitive functions in the brains of P7 mice, including BDNF and c-fos32. In contrast, in our present study, we found that the levels of PKA-Cα and p-CREB remained unchanged following exposure to sevoflurane. These results suggest that a single exposure to isoflurane, but not sevoflurane, may induce the inactivation of CREB in the developing brain, and that this process might underlie the cognitive impairments that are associated with anesthesia.
Several possible mechanisms have been proposed to explain the neurotoxicity induced by volatile anesthetics. For example, previous reports have suggested that volatile anesthetics could induce abnormal calcium release from the endoplasmic reticulum via the excessive activation of IP3 receptors, thus leading to cell apoptosis. Isoflurane is known to exhibit a greater potency to disrupt intracellular calcium homeostasis than sevoflurane33–35. The anesthetic-induced suppression of synaptic signaling can also lead to apoptosis by directly activating the intrinsic apoptotic cascade36. Furthermore, anesthetics could block normal neurotransmission by binding to gamma-aminobutyric acid (GABAA) receptors, thus leading to synaptic deprivation. Alterations in GABAA signaling could also lead to pro-inflammatory effects of anesthesia in the developmental brain, which might also trigger the extrinsic apoptotic cascade36. It is possible that isoflurane and sevoflurane have differential potentials for initiating these cascades. In the current study, we detected the activation of caspase-9 in isoflurane exposed neonatal mice, indicating the involvement of mitochondrial apoptotic pathway. It has been shown that aberrant activation of caspase-9 led to dystrophy of neurites and synaptic-memory deficits, which could be rescued by caspase-9 inhibition37. Therefore, caspase-9 mediated downstream events might also attribute to the changes to synaptic proteins and memory-associated proteins induced by isoflurane. However, further studies are needed to elucidate the specific mechanisms that underline these effects.
The present study has several limitations that should be considered. First, we did not investigate behavioral changes that may have been induced by the two different anesthetics. Cognitive and behavioral abnormalities have been widely investigated by previous studies. Given that the main aim of the current study was to compare the biochemical effects of isoflurane and sevoflurane exposure at different time points, we did not perform any behavioral tests. Second, it has been suggested that translating the results of preclinical studies that involve the mouse model to humans might be misleading. A recent study, employing single-nucleus RNA sequencing to classify cell types in the brains of humans and mice found that two thirds of the 9748 genes evaluated were differentially expressed between these two species38. However, it is obvious that ethical considerations create a major limitation with regards to carrying out research on human newborns. We firmly believe that preclinical studies involving mice can still yield valuable information relating to the mechanisms underlying neurotoxicity induced by exposure to anesthetics.
Conclusions
In conclusion, we found both isoflurane and sevoflurane can induce neuroapoptosis through different pathways in neonatal mice. However, only mice exposed to isoflurane showed significant levels of neuroinflammation and the abnormal expression of proteins associated with synaptic function and memory. Our study provides clear evidence for the differential effects of isoflurane and sevoflurane on the brains of neonatal mice. Collectively, our findings enhance our understanding of the potential problems associated with the use of these volatile anesthetics in pediatric medicine.
Supplementary information
Abbreviations
- Cleaved-PARP
Cleaved poly ADP-ribose polymerase
- PARP
Poly-ADP-ribose polymerase
- IL-6
Interleukin-6
- IL-1β
Interleukin-1 beta
- TNF-α
Tumor necrosis factor alpha
- PSD-95
Post synaptic density protein 95
- CREB
CAMP response element binding protein
- p-CREB
Phosphorylated cAMP response element binding protein
- PKA-Cα
CAMP-dependent protein kinase A catalytic subunits alpha
- MAC
Minimum alveolar concentration
- ELISA
Enzyme-linked immunosorbent assay
- SD
Standard deviation
- GFAP
Glial fibrillary acidic protein
- Iba1
Ionized calcium binding adapter molecule 1
- BDNF
Brain-derived neurotrophic factor
- GABA
G-aminobutyric acid
- ctr
Control
- iso
Isoflurane
- sev
Sevoflurane
Author contributions
Z.S., F.Z., and C.Y. conceived the overall project and experimental design. Z.S. acquired data from western blotting, ELISAs, prepared the materials required for all experiments, and drafted the manuscript. F.Z. was responsible for all animal experiments, including management, anesthesia, and immunofluorescence, and drafted the manuscript. Thus, Z.S. and F.Z. contributed equally to this study. H.J., Z.Y., L.C., and S.T. helped to process data derived from western blotting and ELISA. L.Z.Y., L.K., and L.Z.R. acquired experimental recordings, preserved samples, and dealt with deliveries. C.Y. and Z.B. helped to revise the draft manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Reference numbers: 81520108010 and 81870826), the Zhejiang Provincial Natural Science Foundation of China (Reference numbers: LY18H090004 and LY17H090003) and Guangdong Provincial Key S&T Program (2018B030336001).
Data availability
All relevant data for this study are included in the manuscript/supplementary files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuai Zhao and Ziqi Fan.
Contributor Information
Yanxing Chen, Email: chenyanxing@zju.edu.cn.
Baorong Zhang, Email: brzhang@zju.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-76147-6.
References
- 1.Anand KJS, Soriano SG. Anesthetic agents and the immature brain: Are these toxic or therapeutic? Anesthesiology. 2004;101:527–530. doi: 10.1097/00000542-200408000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Li G, DiMaggio C. Anesthesia and neurodevelopment in children: Time for an answer? Anesthesiology. 2008;109:757–761. doi: 10.1097/ALN.0b013e31818a37fd. [DOI] [PubMed] [Google Scholar]
- 3.Wilder RT, Barbaresi WJ, Weaver AL. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ing C, DiMaggio C, Whitehouse A, Hegarty KM. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2019;130:476–485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X, et al. Sevoflurane affects oxidative stress and alters apoptosis status in children and cultured neural stem cells. Neurotox Res. 2018;33:790–800. doi: 10.1007/s12640-017-9827-5. [DOI] [PubMed] [Google Scholar]
- 6.Loepke AW, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth. Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 7.Yon J-H, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, Xue Z, Cang J. Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci. Bull. 2012;28:499–508. doi: 10.1007/s12264-012-1260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z-Q, et al. Duration-dependent regulation of autophagy by isoflurane exposure in aged rats. Neurosci. Bull. 2015;31:505–513. doi: 10.1007/s12264-015-1549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCann ME, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664–677. doi: 10.1016/S0140-6736(18)32485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun LS, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Istaphanous GK, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 13.Liang G, et al. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J. Biol. Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Y, et al. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2006;281:16016–16024. doi: 10.1074/jbc.M601299200. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Drug safety communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children. https://www.fda.gov/Drugs/DrugSafety/ucm554634.htm (2017). Accessed 5 September 2017.
- 17.Kodama M, et al. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 18.Hitomi J, et al. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci. Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]
- 19.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Basu A. Inflammation: A new candidate in modulating adult neurogenesis. J. Neurosci. Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 21.Braida D, et al. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav. Brain Res. 2004;153:423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, et al. Glycyrrhizin attenuates isoflurane-induced cognitive deficits in neonatal rats via its anti-inflammatory activity. Neuroscience. 2016;316:328–336. doi: 10.1016/j.neuroscience.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Shen X, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, et al. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naïve and Alzheimer Disease transgenic mice. Anesthesiology. 2010;112:1404–1416. doi: 10.1097/ALN.0b013e3181d94de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y, et al. Tanshinone IIA attenuates sevoflurane neurotoxicity in neonatal mice. Anesth. Analg. 2017;124:1244–1252. doi: 10.1213/ANE.0000000000001942. [DOI] [PubMed] [Google Scholar]
- 26.Ji M-H, et al. Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology. 2015;46:155–164. doi: 10.1016/j.neuro.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 28.Beique J-C, et al. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Dong Y, Zhou C, Zhang Y, Xie Z. Anesthetic sevoflurane reduces levels of hippocalcin and postsynaptic density protein 95. Mol. Neurobiol. 2015;51:853–863. doi: 10.1007/s12035-014-8746-1. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno M, et al. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav. Brain Res. 2002;133:135–141. doi: 10.1016/S0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 31.Wadzinski BE, et al. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol. Cell. Biol. 1993;13:2822–2834. doi: 10.1128/MCB.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen T, Sen N. Isoflurane-induced inactivation of CREB through histone deacetylase 4 is responsible for cognitive impairment in developing brain. Neurobiol. Dis. 2016;96:12–21. doi: 10.1016/j.nbd.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Wei H. Anesthetic neurotoxicity: Apoptosis and autophagic cell death mediated by calcium dysregulation. Neurotoxicol. Teratol. 2017;60:59–62. doi: 10.1016/j.ntt.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, et al. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Li K, Yao S, Li Z, Liu S. Different effects of isoflurane and sevoflurane on cytotoxicity in primary cortical neurons of rats. Chin. Med. J. Peking. 2008;121:341–346. doi: 10.1097/00029330-200802020-00012. [DOI] [PubMed] [Google Scholar]
- 36.Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: An update. Br. J. Anaesth. 2013;110:i53–i72. doi: 10.1093/bja/aet054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamayev R, Akpan N, Arancio O, Troy CM, D’Adamio L. Caspase-9 mediates synaptic plasticity and memory deficits of Danish dementia knock-in mice: Caspase-9 inhibition provides therapeutic protection. Mol. Neurodegen. 2012;7:60. doi: 10.1186/1750-1326-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodge RD, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573:61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data for this study are included in the manuscript/supplementary files.