Abstract
In this study, analytical profiling of the bevacizumab (BVZ) biosimilars (N = 3) approved in India were evaluated for charge heterogeneity, isoelectric focusing, aggregation and in vitro potency analysis. The charge variants were characterized using high performance cation-exchange chromatography (CEX-HPLC), capillary zone electrophoresis (CZE) and capillary isoelectric focusing (cIEF). cIEF was also used for estimation of isoelectric point (pI value). In addition, aggregate analysis was done using size exclusion high performance chromatography (SEC-HPLC). The cell-based inhibition of proliferation assay using HUVEC cells, indirect ELISA and Western blot were performed for in vitro biological activity. In addition of cell-based cytotoxicity assay was also performed and found no cytotoxic effect on both HuT78 and WIL2S cells by bevacizumab biosimilars. The significant variations in acidic (p < 0.0001) and basic variants (p < 0.0001), pI value (p = 0.0035), aggregates (p = 0.0306) of biosimilars were found as compared to innovator product; however, cell-based potency analysis (p = 0.6047) and indirect ELISA (p = 0.1611) have shown no significant difference in the biological activity. The banding patterns of all biosimilars in western blot were found similar to the innovator product. The comparatively higher basic variants in the biosimilars were attributing to the high pI value of biosimilars to that of innovator product, although these variations were not affecting the biological activity of the biosimiars. This is a unique study, wherein the independent analysis by a National Control Laboratory (NCL) will not only help the National Regulatory Authority (NRA) to assess the quality and consistency in manufacturing of BVZ biosimilars marketed in India but also facilitate the uptake of BVZ biosimilars, and sustainable access to new medicines against the anti-angiogenic therapy.
Keywords: Bevacizumab, Biosimilars, VEGF, CZE, CEX-HPLC, cIEF, Bioassay
Introduction
Therapeutic proteins have made great progress owing to their curative potential against several oncological, autoimmune and inflammatory diseases, wherein therapeutic monoclonal antibodies (mAbs) have emerged as most important class of these biotherapeutics (Lai et al. 2010; He et al. 2010). Monoclonal antibodies are primarily targeted to critical pathways and growth factors involved in cancer pathogenesis and inflammation. In 1975, the first mAb was produced by Milstein and Kohler by hybridoma technology. Later, advancement in Recombinant DNA (rDNA) technology, laid the foundation of monoclonal antibodies production (Cristina et al. 2013) and in 1986, the first therapeutic monoclonal antibody, Orthoclone OKT3 was approved commercially. Since then, as of 2017, 68 therapeutic monoclonal antibodies have been approved by FDA (Singh et al. 2018). At the current rate of approval of 4 products per year, approx. 70 products will be in market by 2020 and the Global Monoclonal Antibody Therapeutics Market is expected to grow at a CAGR of 7.40% to reach a market value of USD 165.2 billion by 2026 (Lu et al. 2020).
Bevacizumab (BVZ) or Avastin® (Genentech & Roche) is a recombinant humanized mAb that is directed at the human vascular endothelial growth factor (VEGF). Vascular endothelial growth factor is widely expressed throughout the human body and predominantly carried by blood platelets (Verheul et al. 1997; Ecker et al. 2015). Vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen in vitro and an angiogenic inducer in vivo. BVZ has been approved in the European Union and United States as a component of combination therapy for the treatment of colorectal cancer, non-squamous non-small-cell lung cancer, renal cell carcinoma, as well as platinum resistant recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancers (Ferrara and Henzel 1989). Furthermore, VEGF is implicated in intraocular neovascularization associated with diabetic retinopathy and age-related macular degeneration (Penn et al. 2008). There has been intense interest in developing biosimilar agents for cost savings for healthcare and to the global access to this biologic.
Biosimilars are therapeutic proteins that are similar to originator protein therapeutics but are obtained using a different bioprocess. The two products can vary due to the cell line in which the monoclonal antibody is expressed, from small changes in the purification process or from a different composition of the final formulation. As a growing number of biosimilars have been introduced to the market, robust and reliable analytical techniques to confidently evaluate similarities and differences between the biosimilar and its originator are required.
For the same purpose, a good number of biosimilars have been developed in Indian subcontinent and are being marketed effectively. But, prior to market release, these biosimilars are required to undergo a stringent scrutiny in context of their structural and functional characterisation by the National Regulatory Authority (NRA) in India. Furthermore, independent assessment of batch release parameters by National Control Laboratory (NCL) add on to NRA confidence in product quality and manufacturers production consistency. In the present study, 03 indigenously manufactured BVZ biosimilars (03 batches of each biosimilar) approved in India, were characterised for comparative analytical profiling to innovator product (02 batches), using charge variants analysis (by CEX-HPLC, CZE and cIEF), pI value (cIEF), size heterogeneity (by SEC-HPLC) and biological activity by inhibition of proliferation assay using human umbilical vein endothelial cells (HUVEC).
Materials and methods
Materials and equipment
6-Amino-caproic acid (EACA), hydroxypropyl methyl cellulose (HPMC), Triethylenetetramine (TETA), Arginine, Iminodiaetic acid, Phosphoric acid and Sodium Hydroxide were purchased from Sigma-Aldrich. Pre-cut bare fused silica capillary, Peptide marker, cIEF gel, Neutral Capillary, SDS-MW Gel buffer, SDS-MW Sample buffer, Internal Standard (10 KDa), Acidic wash (0.1 N HCL), Basic wash (0.1 N NaOH) were purchased from Beckman Coulter. Potassium dihydrogen phosphate (KH2PO4) was purchased from Rankem. Potassium Chloride and Sodium Chloride were procured from Merck. Column was purchased from TOSOH Lifescience. ProPac™ WCX-10 Column and urea were purchased from Fisher Scientific. Pharmalyte of pH range 3–10 was purchased from GE Healthcare. Glacial Acetic Acid, MES Hydrate Buffer, Dipotassium hydrogen phosphate (K2HPO4) were purchased from SRL. Human endothelial–SFM Basal growth medium, Trypan Blue and Resazurin salt were purchased from Sigma, Gentamicin Sulphate was purchased from Gibco. HUVEC cell line was purchased from Lonza.
CZE, cIEF and CE-SDS was done on Beckman Coulter PA 800 plus CE instrument equipped with UV detector and 32 Karat software, whereas CEX and SEC tests were performed on Waters e2695 equipped with UV/Vis detector 2489 and Empower software. HUVEC cells were counted in automated cell counter, BioRad. The bioassay plates were read in SpectraMax Plate Reader Gemini and analysed the data in Parallel Line Assay (PLA) software.
Three indigenously manufactured BVZ biosimilars (03 batches of each biosimilar) and innovator product (02 batches) approved in India, were characterised for comparative analytical profiling, as listed in Table 1. The samples have been assigned codes at random, and not representing the order of listing in Table 1 to maintain the confidentiality.
Table 1.
List of approved Bevacizumab biosimilars used in this study
| S. no. | Product | Manufacturer/importer | Batches analysed |
|---|---|---|---|
| 1 | Avastin (Innovator) | M/s Roche Products (India) Pvt. Ltd., Mumbai, MH, India | 02 |
| 2 | BevaciRel | M/s Reliance life Sciences, Mumbai, MH, India | 03 |
| 3 | Bevatas | M/s Intas Pharmaceutical Ltd., Ahmedabad, GJ, India | 03 |
| 4 | Cizumab | M/s Hetero Biopharma Ltd, Hyderabad, TS, India | 03 |
Method
Cation-exchange chromatography (CEX-HPLC)
Charge variants were determined using weak cation Propac WCX-10 column (4.0 × 250 mm, 10 µm). Mobile phase A was 20 mM MES pH-5.5 and Mobile Phase B was 20 mM MES with 250 mM NaCl. The concentration of Mobile phase B was 5% and linear gradient was run over 60 min followed by the constant flow of 95% of mobile phase B for 2.0 min and linear gradient of 95–5% of Mobile Phase B was run over 0.2 min. Sample was prepared to 2.5 mg/ml with Mobile Phase A and injection volume was 50 µl. The temperature of column was set at 30 °C and sample at 5 °C ± 3. The peak was detected at 280 nm. The column was first equilibrated for 60 min in initial condition (Du et al. 2012).
Capillary zone electrophoresis (CZE)
The separation was performed in 50 μm ID bare fused silica capillary of 50.2 cm total length and 40 cm effective length. The separation/wash buffer contained 0.05% HPMC, 380 mM EACA and 1.9 mM TETA. All the samples were prepared by diluting the samples with Milli-Q water to 1 mg/ml. Prior to each separation, the capillary was first rinsed with 0.1 N HCL for 5 min followed by the rinsing with separation buffer at 50.0 psi for 10 min. Separation was achieved by injecting samples at 0.5 psi for 10.0 s, separation voltage was set at 30.0 kV for 40 min. Sample was detected at 214 nm by UV detector with data rate 4 Hz and 0.17 min ramp (Witmer et al. 2003; Moritz et al. 2017).
Capillary isoelectrofocusing (cIEF)
Sample was diluted to 1 mg/ml with milli-Q water to which 3 M urea cIEF gel, pharmalyte (3–10), pI marker, 500 mM arginine (Cathodic stabilizer), and 200 mM (Iminodiacetic acid) was added. Before separation column was conditioned by chemical mobilizer (350 mM Acetic Acid) at 50 psi for 5 min followed by rinsing with DDI water for 2 min and thereafter by cIEF gel for 5 min. Separation was achieved by injecting sample at 25 psi for 99.9 s; sample was first focussed, by 200 mM Phosphoric acid and 300 mM Sodium Hydroxide, in capillary for 15 min at 25 kV under normal polarity, 0.17 ramp. Followed by the separation of sample by chemical immobilization (350 mM Acetic Acid) at 30.0 kV for 30 min under normal polarity, 0.17 ramp. The temperature of cartridge was set 20 °C and sample temperature was set at 10 °C. Detection was done at 280 nm by UV detector with data rate 2 Hz (Beckman Coulter PA800 plus Manual).
Size exclusion chromatography (SEC-HPLC)
Size variants were determined by TSK G3000SWXL (7.8 × 300 mm, 5 µm) column. Mobile phase was prepared by adding 10.5 g of dipotassium hydrogen phosphate, 19.1 g of potassium dihydrogen phosphate 18.6 g of potassium chloride in 1000 ml of Milli-Q water, pH range within 6.2 ± 0.1. Sample was diluted to 10 mg/ml with Mobile Phase. Injection volume was 20 µl. The flow rate was 0.5 ml/min and run time was 30 min. The temperature of column was set at 25 °C and sample at 5 °C ± 3. The peak was detected at 280 nm. The column was first equilibrated for 60 min with mobile phase (USP 39 NF 34 < 129 > 2016).
Inhibition of cell proliferation assay
This assay is based on the ability of BVZ to inhibit rhVEGF induced proliferation of HUVEC, by blocking rhVEGF binding to its receptor. Briefly, 100 µl of 0.05 × 105 HUVEC cells/ml were seeded in 96 well plates and were kept in incubator (37 °C, 5% CO2) for 4–6 h. Reference standard, positive control and sample were diluted to 0.5 mg/ml and further serial dilutions were prepared for desired concentrations in dilution block. 100 µl of each dilution of reference standard, positive control, and sample was transferred into 96 well assay plate. The negative or untreated control were kept, wherein in place of drug (bevacizumab), 100 µl of assay medium added per well in 96 well plate which were labelled as VEGF controls. rhVEGF stock was diluted to 100 ng/ml and 100 µl was added to all the dilutions or control wells and mixed nicely. The reaction mix was incubated for 120–150 min at 37 ± 1 °C and was transferred to wells of the 96 wells plate, wherein cells were seeded. The plates were incubated for 72 h in incubator at 37 ± 1 °C with 5 ± 1 °C CO2. On completion of incubation, 25 µl of Alamar blue reagent was added to wells of the plate and plates were incubated for 6 h in incubator. The plates were read at 530 nm excitation and 590 nm emission on florescence plate reader to obtain Relative Florescence Units (RFU) (Wang et al. 2004).
Cytotoxicity assay
The cytotoxicity of the biosimilars and innovator was evaluated by alamar blue [Resazurin; Sigma-Aldrich, St. Louis, MO] assay. In this study, two cell line (HuT78 and WIL-2S) were used. In brief, HuT78 and WIL-2S (2 × 104 cells/well) were seeded in 96-well cell culture plates (Greiner Bio-One, Frickenhausen, Germany). In other dilution plates, innovator bevacizumab and biosimilars were serially diluted from 10,000 ng/ml to 156 ng/ml and incubated with cells for 72 h at 37 °C in humidified atmosphere of 5% CO2. Triton-X (0.1%) was used as positive control. In negative control, no drug was added. On third day, 25 µl of Alamar blue reagent was added to wells of the plate and plates were incubated for 4 h in incubator. The plates were read at 530 nm excitation and 590 nm emission on florescence plate reader (Molecular Devices) to obtain Relative Florescence Units (RFU) (Wang et al. 2004).
Indirect ELISA
The VEGF dissolved in coating buffer (0.05 M bicarbonate, pH 7.4) with final concentration of 12.5 ng/ml and incubated in 96-well plates (100 μl/well) overnight at 4 °C. After washing by 0.01 M PBS with 0.05% Tween-20 (PBST, pH 7.4) three times, the plates were blocked with 2% BSA (200 μl/well) at 37 °C for 2 h and washed with PBST. Bevacizumab biosimilars and innovator diluted with PBS from 200 ng/ml to 0.78 ng/ml were added to each well (100 μl/well) followed by incubation at 37 °C for 1 h and washed. The goat anti-mouse IgG-HRP secondary antibody diluted at 1:40,000 with PBS was added into each well (100 μl/well) and incubated at 37 °C for 1 h and washed. Then OPD substrate solution was added (50 μl/well) and incubated at 37 °C for 10 min. The reaction was terminated by 2 N sulfuric acid solution (50 μl/well), and the optical density (OD) was measured at 492 nm by Micro-plate Reader (Tecan).
Western blot analysis
Western blot analysis was used to establish the binding capacity of bevacizumab biosimilars with VEGF, Twenty micrograms of bevacizumab biosimilars and innovator were equally loaded in duplicate lane of each biosimilars on SDS-PAGE gel in reducing conditions. Proteins were then transferred to nitrocellulose membranes (GE Healthcare, Buckinghamshire, UK). The membranes were blocked with 3% nonfat milk in 0.1% TBS-Tween solution, and then incubated with VEGF concentration of 12.5 ng/ml at 4 °C overnight, then anti-VEGF antibody use for secondary (SAB3300042, Sigma-Aldrich), after that for detection, horseradish peroxidase-conjugated secondary antibodies (Invitrogen, Carlsbad, USA) was used. The blots were then developed using a DAB. The blot was digitized with a flatbed scanner.
Statistical analysis
All the assays were performed in three independent experiments. In inhibition of proliferation assay, the analysis of dose–response curve data was performed using a four-parameter logistic (4PL) model:
where y denotes the assay response, x is the concentration, α is the upper asymptote, δ is the difference between upper and lower asymptotes, β is the slope factor and γ is the EC50 (50% effective concentration). The PLA 2.1 software has been used for analysis of 4PL analysis. GraphPad Prism software was used to plotting the graph, performing the one-way ANOVA, Dunnet test. In Dunnet test, multiple comparisons were done between group mean analytical profiles of different batches of each biosimilar to innovator product. For multiple comparison of any analytical profile, the mean value of all the batches of respective biosimilar and innovator has been considered. Microsoft Excel has also been used for plotting line graphs.
Results
Analysis of charge variants
In absence of any pharmacopoeial monograph on bevacizumab (BVZ), the orthogonal techniques have been used in this study including capillary electrophoresis (CZE, cIEF) and cation-exchange chromatography (CEX) coupled with diode array detection used for the analysis of charge variants in various batches of BVZ biosimilars and innovator product. The charge variants of mAbs are generated by common modifications such as oxidation, deamidation, isomerization, amination, cyclization, glycation, and the presence of C-terminal lysine (Joshi and Rathore 2020). Depending on the locations and types of modification, charged isoforms may adversely impact on biological activities thus it is important to identify and limit these charge variants in the product.
CEX-HPLC
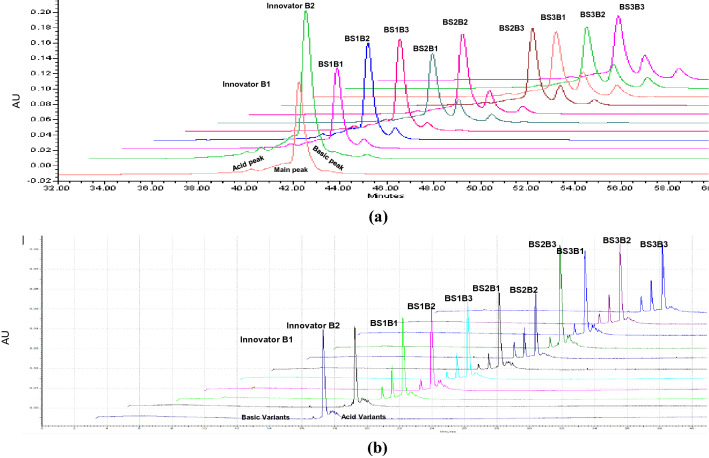
Based on the results of CEX-HPLC, the peaks of the ion exchange profiles were typically identified as three distinct variants, first and late peaks were called acidic and basic variants, respectively. Acidic species are the variants that elute earlier than the main peak from CEX, while basic species are the variants that elute later than the main peak from CEX (Fig. 1a). The acidic variant was relatively high in Innovator as compared to biosimilars, while basic variants were found higher in biosimilars. The percentage of each variant along with main peak is tabulated in Table 2.
Fig. 1.
Acid and base variants of the bevacizumab biosimilars and its innovator characterized by CEX-HPLC and CZE. a Chromatographic profiles obtained from a CEX-HPLC shown in expanded scale for the biosimilars batches and innovator of bevacizumab. b Charge variants of bevacizumab biosimilars and innovator profile obtained by CZE analysis, high peaks are main peak and acid as well as base peaks are side by side to the main peak. Acid and basic variant peak as well as main peak of all biosimilars batches and innovator have shown in this figure as BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3
Table 2.
Comparison of charge variants of bevacizumab biosimilars analysed by CEX-HPLC, cIEF and CZE
| Technique | Innovator | Biosimilar 1 | Biosimilar 2 | Biosimilar 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | Mean ± 2SD | BS1B1 | BS1B2 | BS1B3 | Mean ± 2SD | BS2B1 | BS2B2 | BS2B3 | Mean ± 2SD | BS3B1 | BS3B2 | BS3B3 | Mean ± 2SD | |
| % Acidic variants | |||||||||||||||
| CEX-HPLC | 24.59 | 25.6 | 25.0 ± 1.4 | 19.02 | 19.94 | 19.74 | 19.4 ± 0.9 | 14.49 | 13.37 | 13.85 | 13.9 ± 1.1 | 15.16 | 14.29 | 12.39 | 13.9 ± 2.8 |
| cIEF | 21.73 | 21.2 | 21.4 ± 0.7 | 20.8 | 21.92 | 19.45 | 21.3 ± 2.4 | 12.62 | 9.73 | 12.12 | 11.4 ± 3.0 | 10.59 | 11.82 | 8.88 | 10.4 ± 2.9 |
| CZE | 22.42 | 23.78 | 23.1 ± 1.9 | 21.7 | 21.11 | 24.05 | 21.4 ± 3.1 | 20.01 | 14.47 | 15.17 | 16.5 ± 6.0 | 14.07 | 14.54 | 12.3 | 13.6 ± 2.3 |
| Mean | 22.91 | 23.52 | 23.2 ± 0.8 | 20.50 | 20.99 | 21.08 | 20.7 ± 0.6 | 15.70 | 12.52 | 13.71 | 13.9 ± 3.2 | 13.27 | 13.55 | 11.19 | 12.6 ± 2.5 |
| % Main peak | |||||||||||||||
| CEX-HPLC | 71.87 | 68.96 | 70.4 ± 4.1 | 72.28 | 71.11 | 72.29 | 71.6 ± 1.3 | 58.33 | 62.36 | 64.84 | 61.84 ± 6.5 | 53.86 | 54.62 | 56.32 | 54.9 ± 2.5 |
| cIEF | 74.82 | 74.93 | 74.8 ± 0.1 | 70.3 | 68.65 | 72.91 | 69.4 ± 4.2 | 60.04 | 66.25 | 66.11 | 64.1 ± 7.0 | 56.16 | 55.24 | 58.12 | 56.5 ± 2.9 |
| CZE | 76.16 | 71.9 | 74.0 ± 6.0 | 71.95 | 71.94 | 69.06 | 71.9 ± 3.3 | 52.97 | 64.32 | 64.76 | 60.6 ± 13.3 | 52.97 | 54.18 | 54.98 | 54.0 ± 2.0 |
| Mean | 74.28 | 71.93 | 73.1 ± 3.3 | 71.51 | 70.56 | 71.42 | 71.0 ± 1.0 | 64.31 | 65.23 | 62.22 | 62.2 ± 8.8 | 54.33 | 54.68 | 56.47 | 55.1 ± 2.2 |
| % Basic variants | |||||||||||||||
| CEX-HPLC | 3.53 | 5.44 | 4.4 ± 2.7 | 8.2 | 8.84 | 7.97 | 8.5 ± 0.9 | 27.17 | 24.26 | 21.31 | 24.2 ± 5.8 | 30.97 | 31.06 | 31.29 | 31.1 ± 0.3 |
| cIEF | 3.45 | 3.87 | 3.6 ± 0.5 | 8.9 | 9.43 | 7.65 | 9.1 ± 1.8 | 27.34 | 24.02 | 21.77 | 24.3 ± 5.6 | 32.87 | 32.93 | 33.01 | 32.9 ± 0.1 |
| CZE | 1.42 | 4.32 | 2.8 ± 4.1 | 6.34 | 6.94 | 6.89 | 6.6 ± 0.6 | 27.02 | 21.2 | 19.8 | 22.6 ± 7.6 | 32.94 | 31.27 | 32.69 | 32.3 ± 1.8 |
| Mean | 2.8 | 4.54 | 3.6 ± 2.4 | 7.81 | 8.40 | 7.50 | 8.1 ± 0.9 | 27.17 | 23.16 | 20.96 | 23.7 ± 6.2 | 32.26 | 31.75 | 32.33 | 32.1 ± 0.6 |
BS1 biosimilar 1, BS2 biosimilar 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3, SD standard deviation
CZE
In the obtained electropherogram, the basic and acidic variants were resolved and comparable to the results of CEX-HPLC (Fig. 1b). The details of percentage area of acidic variants, and basic variants were mentioned in Table 2. In this study, acidic variants were found in the range of 13–23% in bevacizumab innovator and its biosimilars, while basic variants varied from 3 to 32%. The results of CZE are summarised in Table 2.
cIEF
In this study, acidic and basic variants of biosimilars were also analysed by cIEF and showed similar pattern of variations as exhibited by CEX-HPLC and CZE (Fig. 3a, Table 2).
Fig. 3.
cIEF electropherograms of bevacizumab biosimilars as compared to innovator has shown in this figure. a It shows electropherogram of cIEF of biosimilars and innovator, which depicted all basic and acidic peaks. b It shows difference between biosimilar groups versus innovator on pI values. c This bar graph shows mean pI values of biosimilars and innovator. BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3
The mean acidic, basic and min peak variants analysed by CEX-HPLC, CZE and cIEF summarised in Table 2. Dunnet test was performed on acidic, basic and main peak percentage area of all the batches of each biosimilar versus innovator product batches. The graphical presentations of the charge variants of biosimilars and innovator product are depicted in Fig. 2.
Fig. 2.
In this figure percent acid variant, basic variant and main peak of biosimilars have shown, in addition, comparison of these variants with innovator also showed. a It shows difference between biosimilar groups versus innovator on acidic variants. b Percent acidic variants in biosimilars and innovator. c It shows difference between biosimilar groups versus innovator on basic variants. d Percent acidic variants in Biosimilars and innovator. e It shows difference between biosimilar groups versus innovator on acidic variants. f Percent acidic variants in biosimilars and innovator
Determination of pI value by cIEF
The isoelectric points (pI value) of the bevacizumab biosimilars and Innovator product were also determined using cIEF. The pI values were found between 7.81 and 8.14 (Table 3) for all biosimilars and innovator product. Although in Dunnet test, it was found a significant difference (p = 0.0035) between biosimilars and innovator (Fig. 3b) in pI value; however, the observed differences among the biosimilars and innovator were lower than 0.4 pI units confirming comparability of charge heterogeneity among the products (Fig. 3c).
Table 3.
Isoelectric point (pI) estimation by cIEF
| Parameter | Innovator | Biosimilar 1 | Biosimilar 2 | Biosimilar 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | BS1B1 | BS1B2 | BS1B3 | BS2B1 | BS2B2 | BS2B3 | BS3B1 | BS3B2 | BS3B3 | |
| pI of main peak | 7.99 | 7.97 | 7.97 | 7.94 | 7.93 | 7.93 | 7.81 | 7.94 | 8.07 | 8.14 | 8.11 |
BS1 biosimilar 1, BS2 biosimilar 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3
Aggregate analysis by SEC-HPLC
The aggregate profiling of bevacizumab samples was done by SEC-HPLC and typical chromatograms are shown in Fig. 4a. The percent aggregates in biosimilars were less than that of innovator (Table 4). The variations in percent aggregates were found as significant difference (p = 0.0306) between biosimilars and innovator (Fig. 3b). The difference in percentage area of aggregates in the different batches of bevacizumab biosimilars and innovator product was found less than 1.5 (Table 4, Fig. 4c).
Fig. 4.
SEC overlay for bevacizumab biosimilars and innovator samples. a It shows electropherogram of SEC of biosimilars and innovator, which depicted aggregates and fragment peaks. b It shows difference between biosimilar groups versus innovator on aggregates. c This bar graph shows mean percent aggregates values of biosimilars and innovator. BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3
Table 4.
Aggregate analysis by SEC-HPLC
| Parameter | Innovator | Biosimilar 1 | Biosimilar 2 | Biosimilar 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | BS1B1 | BS1B2 | BS1B3 | BS2B1 | BS2B2 | BS2B3 | BS3B1 | BS3B2 | BS3B3 | |
| Aggregates | 3.13 | 3.31 | 2.02 | 2.85 | 2.06 | 2.38 | 2.13 | 1.81 | 2.27 | 2.62 | 2.3 |
| Main peak | 96.73 | 96.47 | 97.06 | 96.99 | 97.77 | 97.49 | 97.76 | 98.09 | 97.6 | 97.28 | 97.61 |
| Fragments | 0.14 | 0.22 | 0.16 | 0.16 | 0.17 | 0.13 | 0.1 | 0.1 | 0.13 | 0.1 | 0.09 |
BS1 biosimilar 1, BS2 biosimilar 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3
Biological activity by inhibition of cell proliferation (IOP) assay
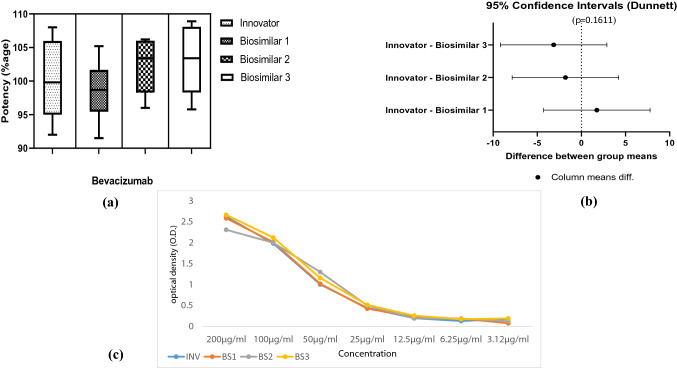
The biological functions that contribute to the clinical efficacy of bevacizumab are mediated by the neutralization of VEGF through the Fab domain. The potency was measured by inhibition of proliferation in human umbilical vein endothelial cells (HUVEC). The results of IOP assay are shown in Fig. 5a. The IOP assay demonstrates that bevacizumab biosimilars with higher potency observed with no significant difference (p = 0.6047) in all batches of biosimilars to that of innovator product, wherein the potency values were found to be lying well within the permissible limit of 80–125% as per the various national and international guidelines (Fig. 5a). The differences in the potency values were also due to the variability within the bioassay as standard error exhibited in various bioassays found to be ranging from 1.41 to 11.77%. Furthermore, analysis by Dunnet test revealed that there is no significant difference in the IOP of biosimilars as compared to innovator (Fig. 5b).
Fig. 5.
Anti-proliferative assay of HUVEC cells by inhibition of VEGF using bevacizumab biosimilars, and innovator products. a Bar graph is showing the percent potency of bevacizumab biosimilars and innovator samples using cell-based assay. b It shows difference between biosimilar groups versus innovator on cell-based potency. c This graph shows cell proliferation of HUVEC in the presence of bevacizumab biosimilars and innovator using alamar blue assay, VEGF without drug was used as control. INV innovator, BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3
Cytotoxicity assay
The toxicity of the innovator and biosimilars which were used in this study evaluated on both HuT78 and WIL-2S cells on dose dependent manner using alamar blue assay. The innovator and biosimilars did not exhibit any cytotoxic effect on used cell line, while Triton-X (0.1%) which used as positive control showed significant cytotoxic effect (Fig. 6).
Fig. 6.
Cell viability was determined by alamar blue assay. The graph represents the cytotoxicity profile of innovator and bevacizumab biosimilarst against a HuT78 cells b WIL-2 s cells at different concentrations (5000–78 ng/ml) on 72 h incubation. Results are expressed as a relative fluorescence unit (RFU) versus concentrations with control ± SEM from at least three independent experiments. INV innovator, BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3
Binding assay of bevacizumab on VEGF by ELISA
In most of the bevacizumab biosimilars studies, the antibody drug concentrations were measured using an indirect ELISA against coated VEGF, which could only measure the free instead of the total antibody drugs. In this study, we reported the binding potency of bevacizumab biosimilars using ELISA in a VEGF non-competitive manner. In this experiment result exhibited that binding pattern of bevacizumab biosimilars on different concentrations is similar and is shown in Fig. 7. Potency of binding assay was also calculated with all biosimilars and innovator and found in the range of 100–120%, which were also shown in Fig. 7.
Fig. 7.
Binding and specificity of bevacizumab biosimilars used to capture VEGF. Commercial VEGF from Sigma-Aldrich was used to coat ELISA plates at 12.5 μg/ml prior to binding dilutions of bevacizumab biosimilars and innovator after detection with TMB, reaction was observed by absorption using spectrophotometer at 490 nm. a Bar graph is showing the percent potency of bevacizumab biosimilars and Innovator samples using VEGF ELISA. b It shows difference between biosimilar groups versus innovator based on VEGF ELISA. c graph showing binding (OD) versus concentrations of bevacizumab biosimilars and innovator in VEGF ELISA. BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3
Western blot of bevacizumab biosimilars
Western blot analysis showed the binding capacity of bevacizumab biosimilars with VEGF. Western blot of all biosimilars and innovator with VEGF shown in Fig. 8 and found that all bevacizumab biosimilars have almost equal capacity to bind with VEGF on blot, which give confirmation to the result of binding assay by ELISA.
Fig. 8.
Western blot analysis was performed on biosimilars of bevacizumab (30 µg). a Marker (lane 1), innovator (lane 2, 3), biosimilar 1 (lane 4, 5, 6), biosimilars 2 (lane 7, 8, 9). b Marker (lane 1), innovator (lane 2, 3), biosimilars 3 (lane 4, 5, 6). The blots were probed with VEGF and further incubated with VEGF Mouse monoclonal Antibody (Product # MA5-13,182, 2 µg/ml) and detected by DAB using Goat anti-Mouse IgG (H + L) Secondary Antibody, HRP conjugate (1:10,000 dilutions). Bands were observed at both 50 KDa and 25 KDa, respectively. INV innovator, BS1 biosimilar 1, BS2 biosimilars 2, BS3 biosimilar 3, B1 batch 1, B2 batch 2, B3 batch 3
Discussion
National Control Laboratory (NCL) serves as an important element in providing effective support for a Drug Regulatory Authority by assuring the quality, safety and efficacy of biotherapeutics. National Institute of Biologicals (NIB) (NOIDA, India) being an NCL is committed towards quality evaluation of various biologicals being marketed in India. NIB is involved in the quality control evaluation of a plethora of biologicals including mAbs, either manufactured in India or imported in India. In this respect, the batches of various biosimilars including bevacizumab are forwarded to NIB by Central Drugs Standard Control Organization (CDSCO) (New Delhi, India) for quality evaluation.
Unlike chemical drugs, biologics specifically therapeutic monoclonal antibodies (mAbs) are highly complex and large molecules. These large complex molecules are subjected to undergo various post-translational modifications during manufacturing process, such as glycosylation and lysine truncation. A number of chemical modifications can also happen during purification and storage such as oxidation or deamination. Acid and basic variants can be formed by chemical and enzymatic modifications during secondary modification of proteins, with the acidic fractions typically consisting of deamidated, sialylated, and glycated forms and the basic fractions consisting of oxidated and C-terminal lysine variants (Visser et al. 2013). Sialic acid has been commonly reported to contribute to the formation of acidic species. Acidic contents of recombinant IgG1 antibodies expressed in CHO cell lines also contain higher levels of sialic acid (Khawli et al. 2010; Weitzhandler et al. 1998). The distribution of charge variants of therapeutic monoclonal antibodies can adversely affect their stability and biological activity, thereby mandating analysis of the charge variant in various regulatory guidelines. Furthermore, aggregation is one of the major degradation pathways that affect the quality and efficacy of protein therapeutics. It is known that protein aggregation can induce immunogenicity and aggregation can also induce the production of anti-drug antibodies (ADAs) which can result in the loss of activity or cause adverse effects upon administration (Ratanji et al. 2014). Therefore, it is important to monitor the amount of aggregation and the charge variants during the production as well as throughout the life cycle of a biotherapeutic to ensure their quality, safety and efficacy.
In this study the charge heterogeneity among the innovator and biosimilars were characterized and confirmed by orthogonal techniques, i.e., chromatography (CEX-HPLC) as well capillary electrophoresis (CZE, cIEF). The mean acidic variants were higher in innovator product in comparison to the biosimilars; however, this is reverse in case of basic variants (Fig. 2). In Dunnet test, the charge variants in biosimilars were have found to have significant difference (p < 0.0001) to that of innovator product; however, the difference in the biological activity of biosimilars and innovator product was non-significant both in HUVEC proliferation assay (p = 0.6047) and VEGF ELISA (p = 0.1611). The western blot study have also shown similar banding pattern in all biosimilars to that of innovator product. The product related impurities, i.e., aggregate profile of all the biosimilars were found to be on the lower side to that of innovator product.
In the previous studies, it has been reported that the acidic variants showed slightly lower potencies (Rodwell et al. 1986; Zhao et al. 2016). The acidic variants are formed by glycosylation with sialic acid or uronic acid, or by deamination of asparagine or glutamine, or by pyroglutamate formation from glutamine and glutamate, whereas the basic variants are formed by formation of Fc-1 lysine or Fc-2 lysine, or by the noncyclization of N-terminal glutamine, or by succinimide formation from aspartic acid. (Liu et al. 2019). Therefore, the slight higher potency values of various biosimilars to that of innovator product might be due to the comparatively low acidic variants and higher basic variants. Furthermore, is has also been reported that the acidic species are variants with lower apparent isoelectric points (pI) and basic species are variants with higher apparent pI (Liu et al. 2019). This might be responsible for the lower pI value of the innovator product attributing to higher acidic variants in comparison to higher pI value of biosimilars attributed because of the higher basic variants.
The differences in the charge variants are observed during routine mAb manufacturing or process changes. Khawli et al. (2010) demonstrated that charge variants of IgG do not affect the in vitro potency, FcRn binding affinity or the PK properties in rats. Similar results have been shown by Yan-Yan Zhao et al. (2016), wherein the deliberate modification of the pI of an antibody by one unit generated no noticeable differences in the pharmacokinetic parameters and demonstrated that the charge heterogeneity of the bevacizumab biosimilar exhibited no influence on the in vitro potency and PK profile.
Therefore, considering the totality of the evidence the biosimilars were found to similar to that of the Innovator product. Although, stringent quality evaluation strategies must be followed in spite of the various regulatory guidelines and each batch should undergo strict independent quality evaluation by NCL initially till the establishment of consistency of a biotherapeutic manufacturer as well as random sampling for surveillance testing must be done to assess the variations throughout the life cycle of these highly complex macromolecules to ensure quality, safe and efficacy of biosimilars.
Conclusion
We have demonstrated comparison of bevacizumab biosimilars and its innovator product by physicochemical characterization for charge variants, pI value, aggregate analysis and bioassay. In this study, it has been found that the higher basic variants and lower acidic variants of the bevacizumab biosimilars in comparison to innovator product, exhibited no adverse effect on the in vitro biological activity. The reported methods can be further considered for inclusion the Indian Pharmacopoeial Monograph on Bevacizumab to have a wider introduction of orthogonal techniques in bevacizumab characterization to ensure uniform quality in biopharmaceutical drug development and throughout the life cycle of these highly complex macromolecules.
Acknowledgements
Our study team is thankful to CDSCO for providing the bevacizumab samples for quality evaluation. Study was fully funded by National Institute of Biologicals (Ministry of Health and Family Welfare), (Grant no. NIB-TAL) Government of India.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
Footnotes
Anu Prakash and Nripendra N. Mishra have equal first author.
References
- Cristina M, Pranchevicius S, Vieira TR. Production of recombinant immunotherapeutics for anticancer treatment: The role of bioengineering. Bioengineered. 2013;4:305–312. doi: 10.4161/bioe.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Yi, Walsh A, Ehrick R, Xu W, May K, Liu H. Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. MAbs. 2012;4:578–585. doi: 10.4161/mabs.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echko MM, Dozier SK (2010) Recombinant antibody technology for the production of antibodies without the use of animals People for the Ethical Treatment of Animals (PETA). AltTox.org, September 15
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14. doi: 10.4161/19420862.2015.989042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;425:540–547. doi: 10.1016/j.bbrc.2012.08.021. [DOI] [PubMed] [Google Scholar]
- He Y, Lacher NA, Hou W, Wang Q, Isele C, Starkey J, Ruesch M. Analysis of identity, charge variants, and disulfide isomers of monoclonal antibodies with capillary zone electrophoresis in an uncoated capillary column. Anal Chem. 2010;82:3222–3230. doi: 10.1021/ac9028856. [DOI] [PubMed] [Google Scholar]
- Hong G, Bazin-Redureau MI, Scherrmann JM. Pharmacokinetics and organ distribution of cationized colchicine-specific IgG and Fab fragments in rat. J Pharm Sci. 1999;88:147–153. doi: 10.1021/js970335n. [DOI] [PubMed] [Google Scholar]
- Joshi S, Rathore AS. Assessment of structural and functional comparability of biosimilar products: trastuzumab as a case study. BioDrugs. 2020;34:209–223. doi: 10.1007/s40259-020-00404-3. [DOI] [PubMed] [Google Scholar]
- Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yan J, et al. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. Mabs. 2010;2:613–624. doi: 10.4161/mabs.2.6.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Wang R, Chen X, Tang D, Hu Y, Cai J, Zhang Q, Hu H. Emerging trends and new developments in monoclonal antibodies: a scientometric analysis (1980–2016) Hum Vaccin Immunother. 2010;13:1388–1397. doi: 10.1080/21645515.2017.1286433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Valente J, Lin S, Chennamsetty N, Qiu D, Bolgar M. Cyclization of N-terminal glutamic acid to pyro-glutamic acid impacts monoclonal antibody charge heterogeneity despite its appearance as a neutral transformation. J Pharm Sci. 2019;108:3194–3200. doi: 10.1016/j.xphs.2019.05.023. [DOI] [PubMed] [Google Scholar]
- Lu RM, Hwang YC, Hwang I, Liu J, Lee CC, Tsai HZ, Li HJ, Han-Chung D. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz B, Locatelli V, Niess M, Bathke A, Kiessig S, Entler B, et al. Optimization of capillary zone electrophoresis for charge heterogeneity testing of biopharmaceuticals using enhanced method development principles. Electrophoresis. 2017;38:3136–3146. doi: 10.1002/elps.201700145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam WS, Prabhu S, Zheng Y, Subramanyam M, Wang Y-MC. Pharmacokinetic, pharmacodynamic and immunogenicity comparability assessment strategies for monoclonal antibodies. Trends Biotechnol. 2010;28:509–516. doi: 10.1016/j.tibtech.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11:99–109. doi: 10.3109/1547691X.2013.821564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell JD, Alvarez VL, Lee C, Lopes AD, Goers JW, King HD, et al. Site-specific covalent modification of monoclonal antibodies: in vitro and in vivo evaluations. Proc Natl Acad Sci USA. 1986;83:26326. doi: 10.1073/pnas.83.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Narula G, Rathore AS. Should charge variants of monoclonal antibody therapeutics be considered critical quality attributes? Electrophoresis. 2016;37(17–18):2338–2346. doi: 10.1002/elps.201600078. [DOI] [PubMed] [Google Scholar]
- Singh S, Kumar NK, Dwiwedi P, Charan J, Kaur R, Sidhu P, Chugh VK. Monoclonal antibodies: a review. Curr Clin Pharmacol. 2018;13:85–99. doi: 10.2174/1574884712666170809124728. [DOI] [PubMed] [Google Scholar]
- USP 39 NF 34 <129> (2016) Analytical Procedures for Recombinant Therapeutic Monoclonal Antibodies/Biological Tests
- Verheul HM, Hoekman K, Luykx-de BS, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187–2190. [PubMed] [Google Scholar]
- Visser J, Feuerstein I, Stangler T, Schmiederer T, Fritsch C, Schiestl M. Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab. BioDrugs. 2013;27:495–507. doi: 10.1007/s40259-013-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- Weitzhandler M, Farnan D, Horvath J, Rohrer JS, Slingsby RW, Avdalovic N, Pohl C. Protein variant separations by cation-exchange chromatography on tentacle-type polymeric stationary phases. J Chromatogr A. 1998;828:365–372. doi: 10.1016/S0021-9673(98)00521-4. [DOI] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GFJM, Van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retinal Eye Res. 2003;22:1–29. doi: 10.1016/S1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Wang N, Liu WH, Tao WJ, Liu LL, Shen ZD. Charge variants of an avastin biosimilar isolation, characterization, in vitro properties and pharmacokinetics in rat. PLoS ONE. 2016;11:e0151874. doi: 10.1371/journal.pone.0151874. [DOI] [PMC free article] [PubMed] [Google Scholar]